Purification method for rabies virus inactivated vaccine
A technology of rabies virus and rabies vaccine, which is applied in the field of purification of rabies virus inactivated vaccine, can solve the problems of virus yield and expanded production that have not been further explored, and achieve the effects of improving consistency, avoiding dilution, and sufficient operating space
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
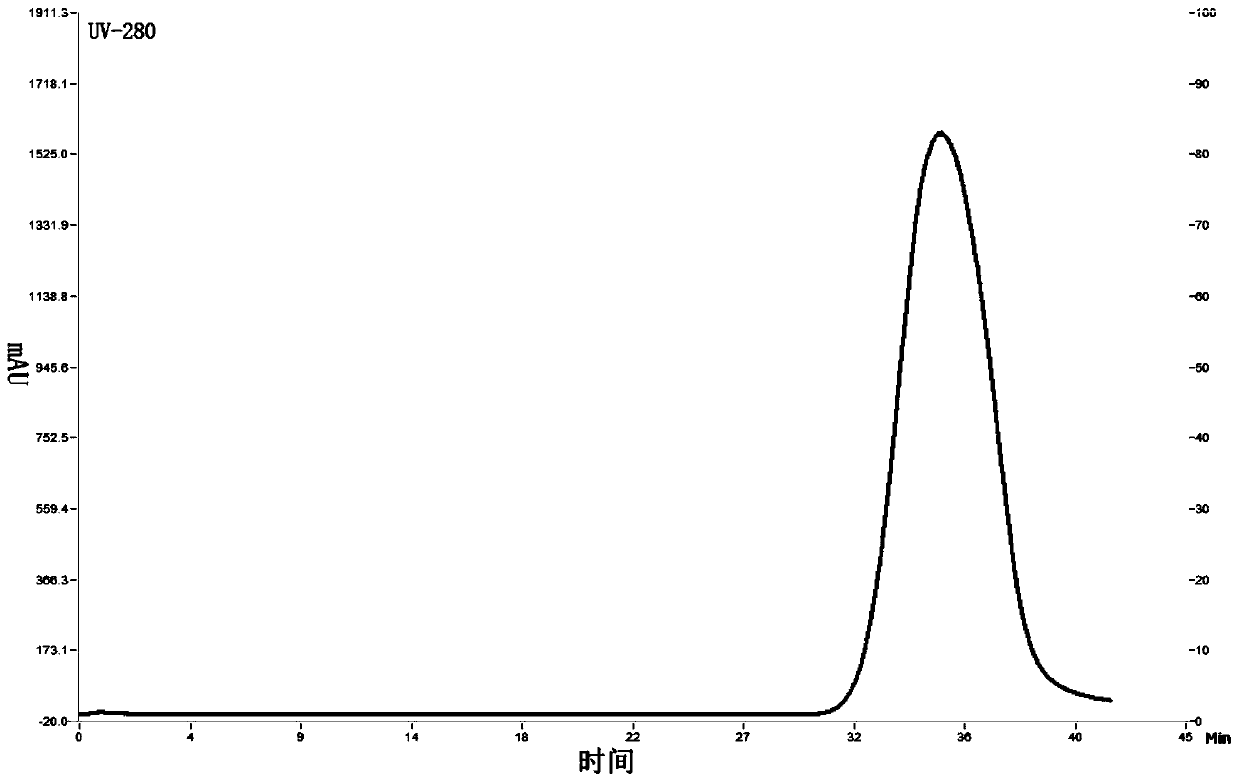
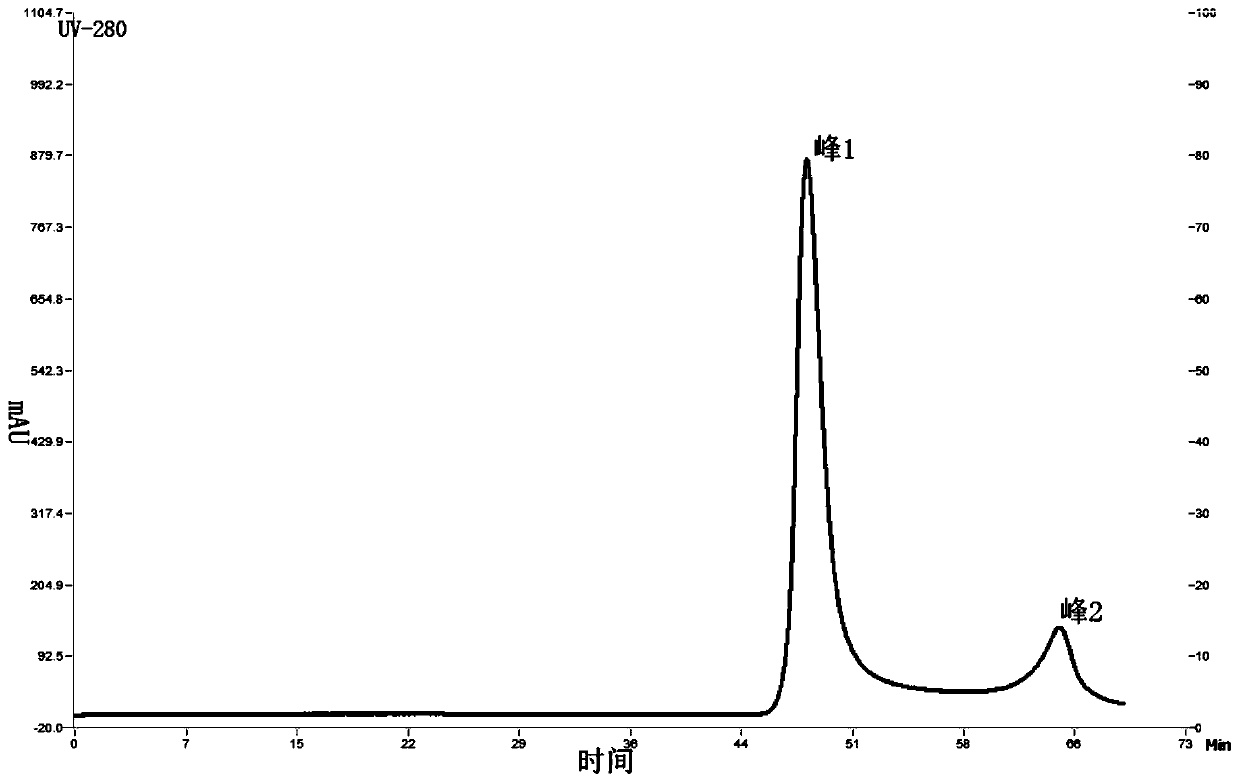
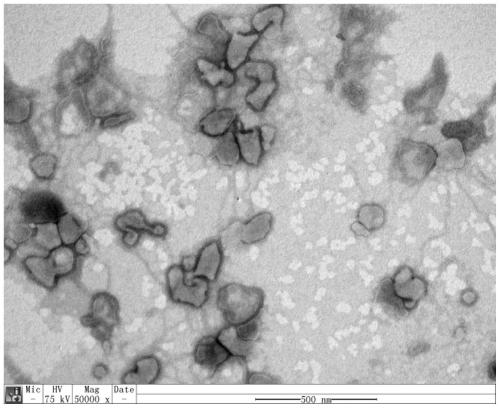
[0062] Embodiment 1, production and chromatographic purification of rabies vaccine
[0063] 1. Cells and viruses
[0064] Vero cells: from the American Type Culture Collection (ATCC CCL-81), with a passage of 134 generations. The rabies vaccine laboratory of our company will pass the passage, establish the main cell bank and the working cell bank, and the cell generation for production shall not exceed 150 generations.
[0065] Rabies virus strain CTN-1V strain: purchased from China Institute for the Control of Pharmaceutical and Biological Products, the 23rd generation. The rabies vaccine laboratory of the company has established a main seed bank and a working seed bank. The batches of working seeds used for production do not exceed 35 generations, and the virus titer is 7.5-8.0LgLD50 / ml.
[0066] 2. Vero cell culture
[0067] (1) Take one or several cell tubes from the same batch in the working cell bank, resuscitate and amplify the cells until they are inoculated with th...
Embodiment 2
[0091] Embodiment 2, the effect evaluation of purified vaccine
[0092] 1. Detection of the nature of the vaccine
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com