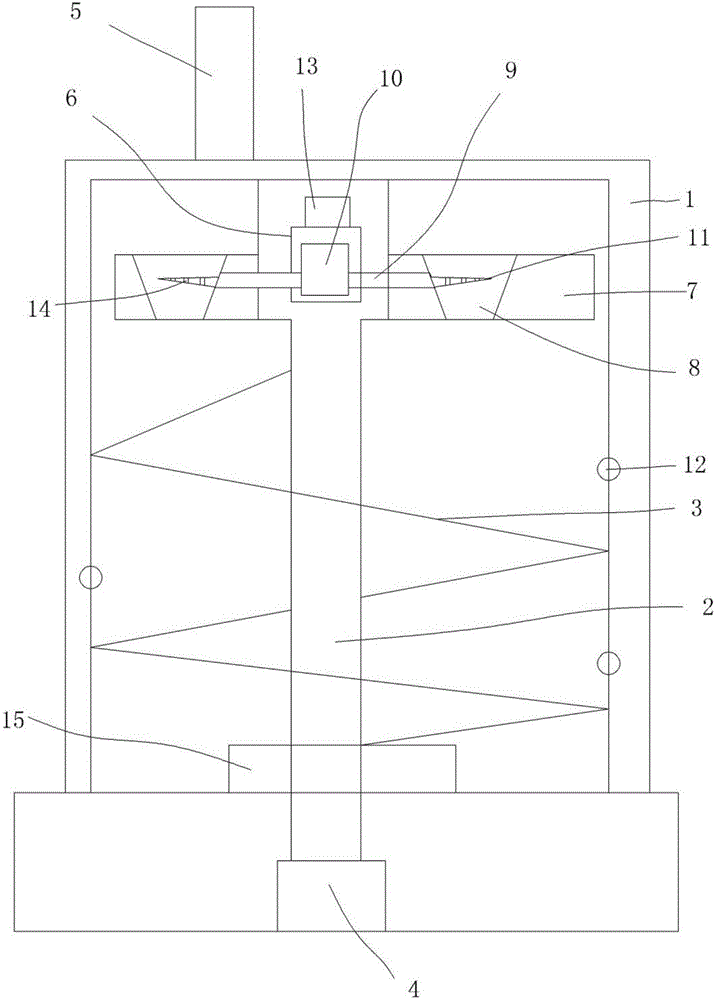
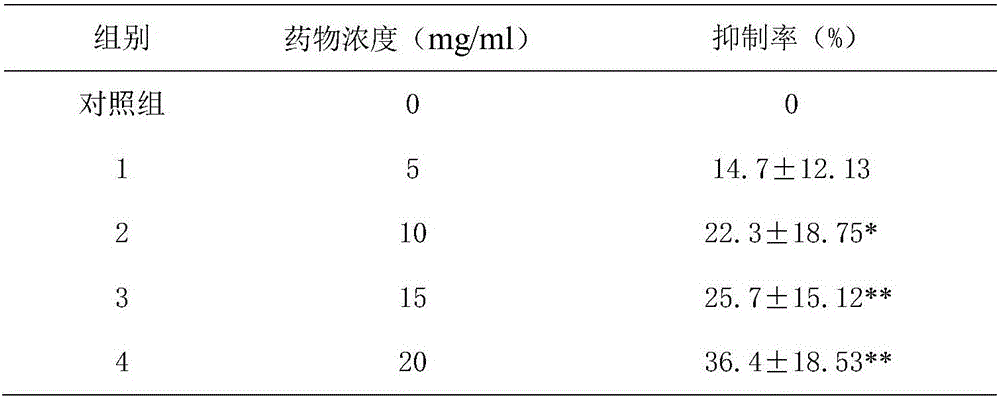
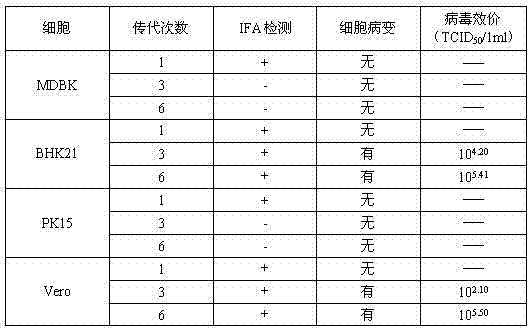
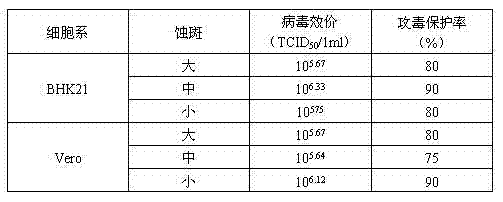
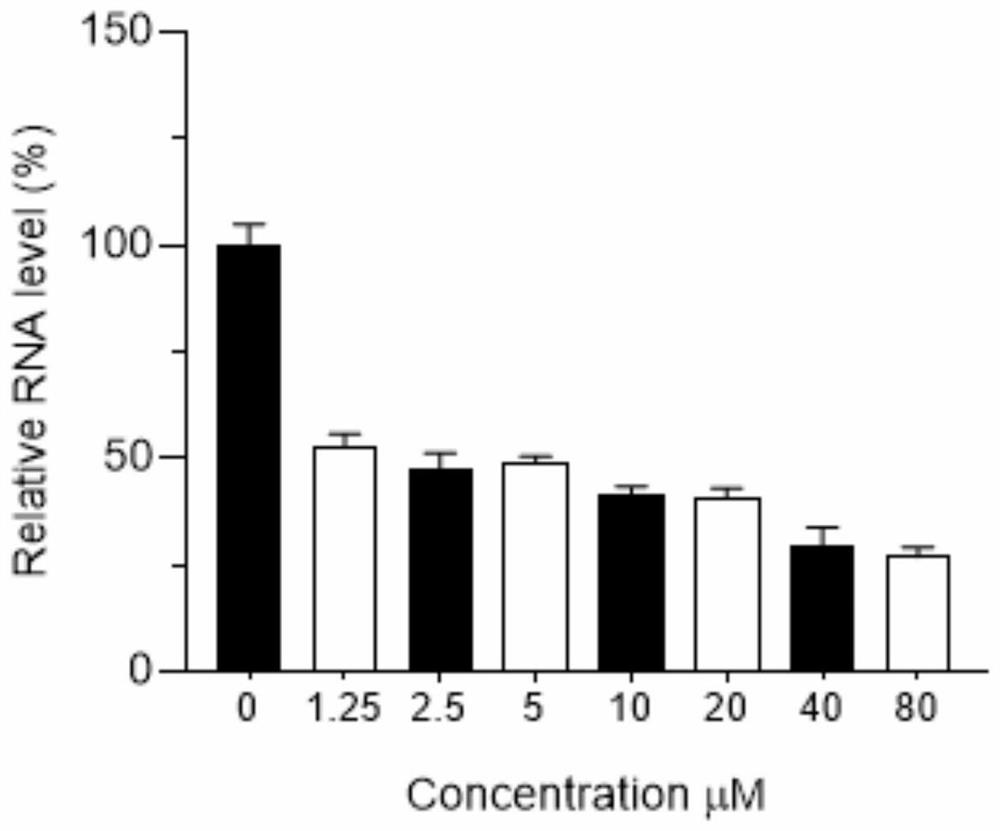
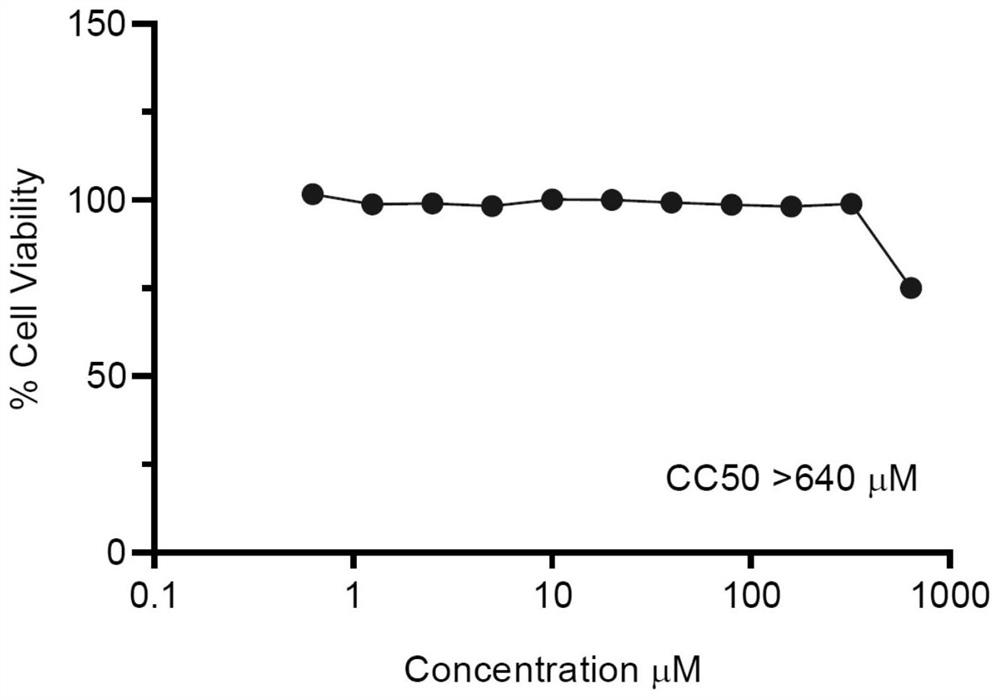
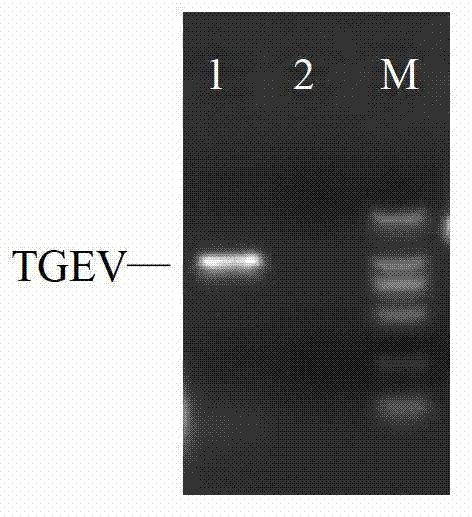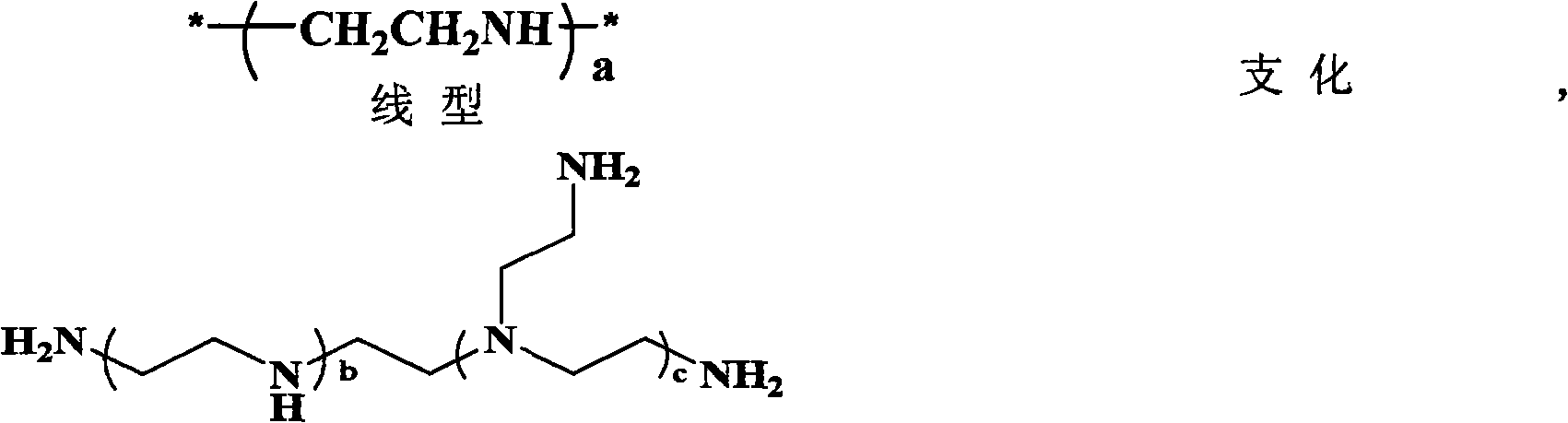Patents
Literature
56 results about "African Green Monkey" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
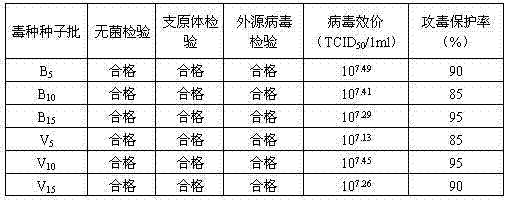
A medium-sized diurnal primate that is omnivorous, Chlorocebus sabaeus has a life span of 10 years in the wild and 25 years in captivity. The African green monkey has been in use in scientific research since the 1950s and its tissues are used to produce vaccines for polio and smallpox. Additionally Vero cells, an African green monkey kidney epithelial cell line, are widely used in immunology and infectious disease research.
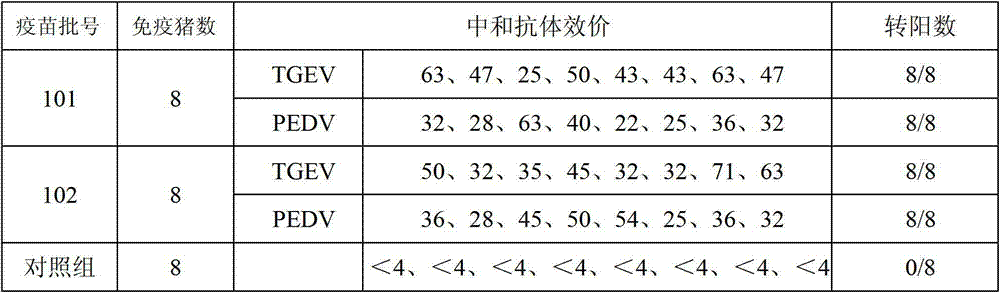
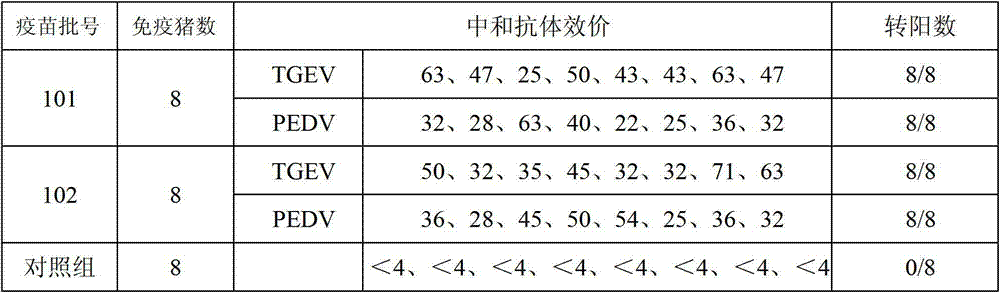
Porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine and preparation method thereof
The invention relates to a porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine and a preparation method of the porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine. The porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine is prepared by performing virus amplification on a swine testicular cell line (ST cells) or an African green monkey kidney cell line (Vero cells) by using a self-attenuated and preserved transmissible gastroenteritis virus SD / L strain and a self-attenuated and preserved porcine epidemic diarrhea virus LW / L strain, and carrying out the steps of harvesting, uniformly mixing, freeze-drying and the like. The porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine can effectively prevent two diseases namely swine transmissible gastroenteritis and epidemic diarrhea.
Owner:QILU ANIMAL HEALTH PROD
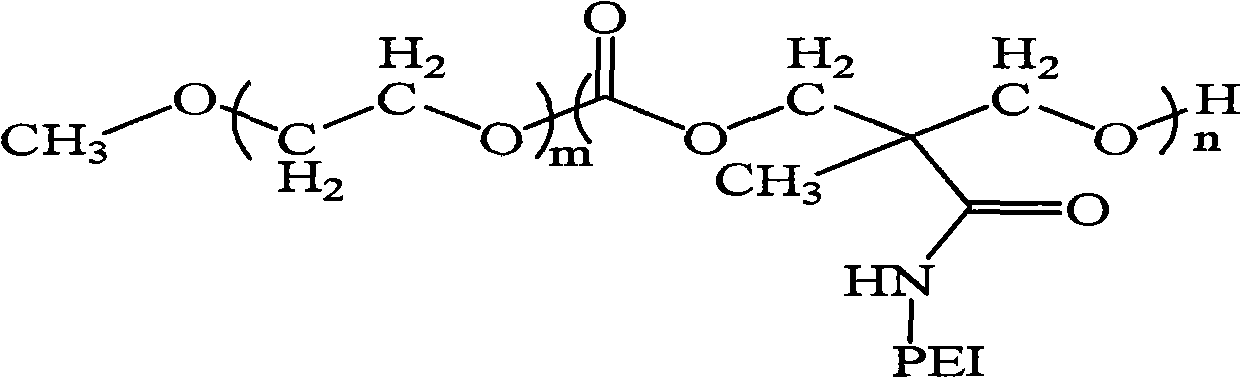
Polyethylene glycol monomethyl ether-poly 2-methyl-carboxyl propylene carbonate graft polyethyleneimine copolymer, preparation method thereof and application thereof
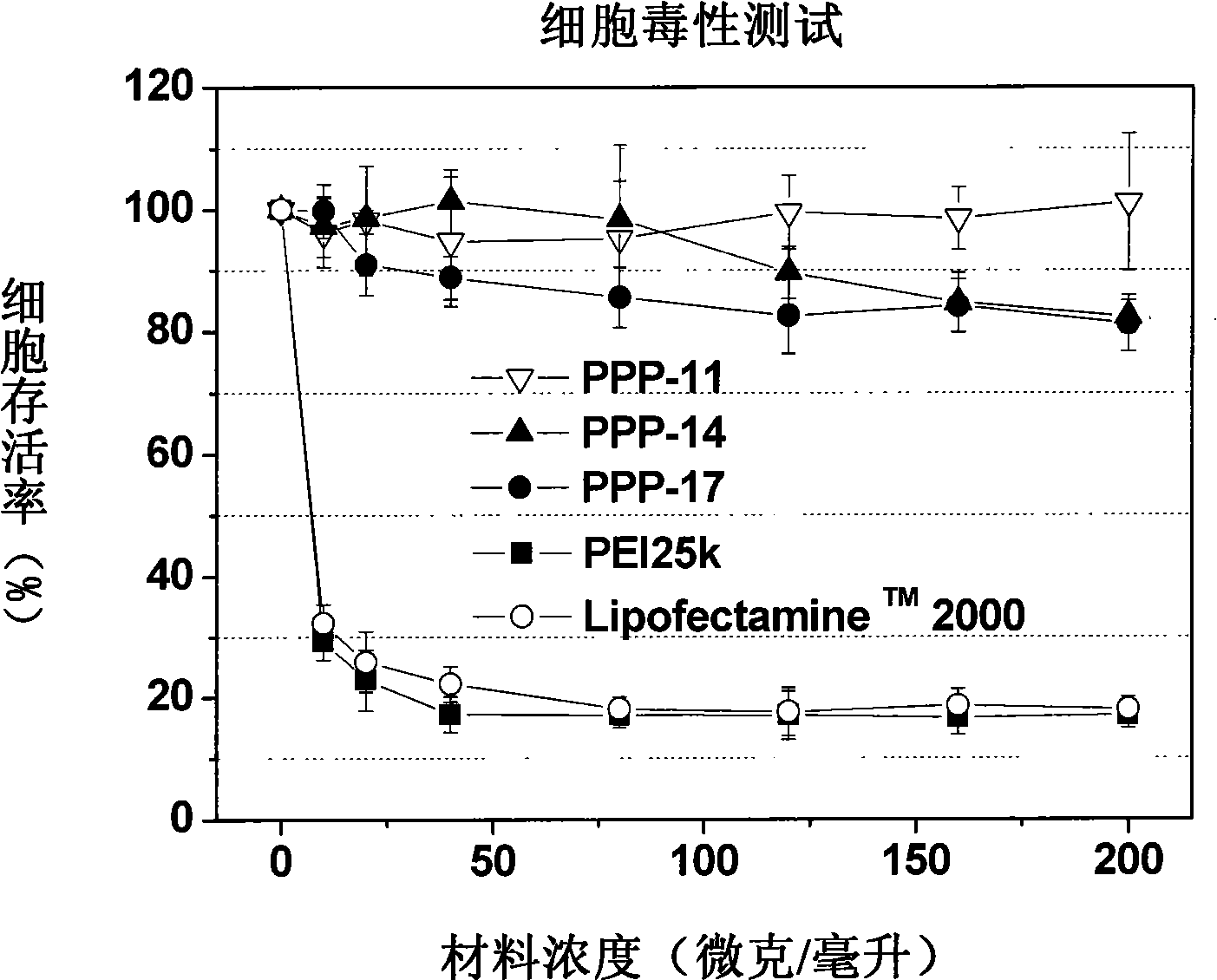
The invention relates to polyethylene glycol monomethyl ether-poly 2-methyl-carboxyl propylene carbonate graft polyethyleneimine copolymer, a preparation method thereof and application thereof. The preparation method comprises the step of directly condensing carboxyl in the polyethylene glycol monomethyl ether-poly 2-methyl-carboxyl propylene carbonate graft polyethyleneimine segmented copolymer with amino in polyethyleneimine to form the graft copolymer. The copolymer is a polycation gene carrier, integrates the advantages of polyethylene glycol, Makrolan and polyethyleneimine, and has high transfection efficiency, wherein the highest transfection efficiency to the medium luciferase plasmid of african green monkey kidney cell is 14 times of that of American Invitrogen biological company transfection reagent Lipofetamine<TM>2000, can effectively antagonize the inhibiting effect of blood serum to the transfection, and has less cell toxicity, wherein consistency thereof is not higher than 200 microgramme / ml and cell survival rate is more than 80%.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Coxsackie virus A16-type virus strain and applications thereof
ActiveCN102533671AStable titerImproving immunogenicitySerum immunoglobulinsMicroorganism based processesSequence analysisImmunogenicity
The invention provides a coxsackie virus A16-type virus strain. The collection number of the coxsackie virus A16-type virus strain is CGMCC No.5372, wherein CGMCC refers to China General Microbiological Culture Collection Center. The virus is a 20-face stereoscopic symmetrical sphere under observation through an electron microscope, and the diameter of the virus is 23-30nm. VP1 conserved region sequence analysis and mass spectrum analysis are respectively performed on the virus strain, and a result shows a CA16 virus. The CA16 virus can be efficiently proliferated in Vero cells (African green monkey kidney cells), and the virus titer can reach 6.61g CCID50 / ml. Moreover, the virus strain has no external pollution, better immunogenicity and a good effect.
Owner:SINOVAC BIOTECH
Method for preparing double yolk antibody of porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus
InactiveCN101845095AStrong specificityStrong targetingEgg immunoglobulinsImmunoglobulins against virusesPig farmsYolk
The invention discloses a method for preparing a double yolk antibody of a porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus. The method comprises the following steps of: performing porcine transmissible gastroenteritis virus multiplication on porcine kidney cells (PK15); performing porcine epidemic diarrhea virus multiplication on African green monkey kidney cells (Vero); emulsifying the two cell cultures used as antigen with an oil emulsion adjuvant to prepare immunogen, namely, mixing the two kinds of viruses in a ratio of (1-3):(1-3) to prepare the immunogen; immunizing non-immunologic laying hens; and obtaining the double yolk antibody which can prevent and treat porcine transmissible gastroenteritis and porcine epidemic diarrhea based on the collection and purification of the yolk. When the double yolk antibody is used for curing experimental pigs, the clinical symptoms in the experiment are obviously reduced compared with a control group, and the death rate of the experimental group is obviously lower than that of the control group. The double yolk antibody has obvious preventing and treating functions when applied in a pig farm with high incidence rate of the porcine transmissible gastroenteritis and the porcine epidemic diarrhea.
Owner:PU LIKE BIO ENG
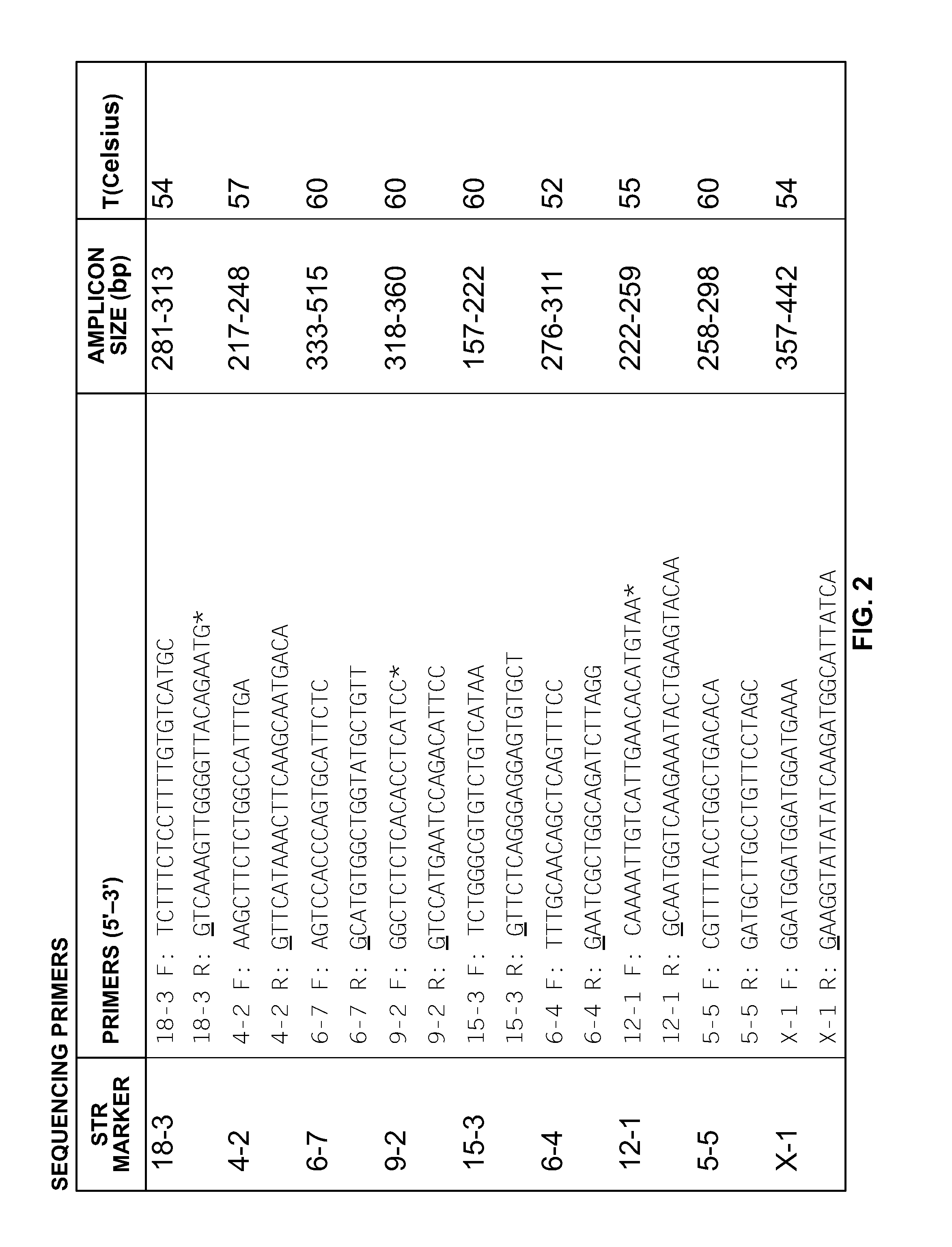
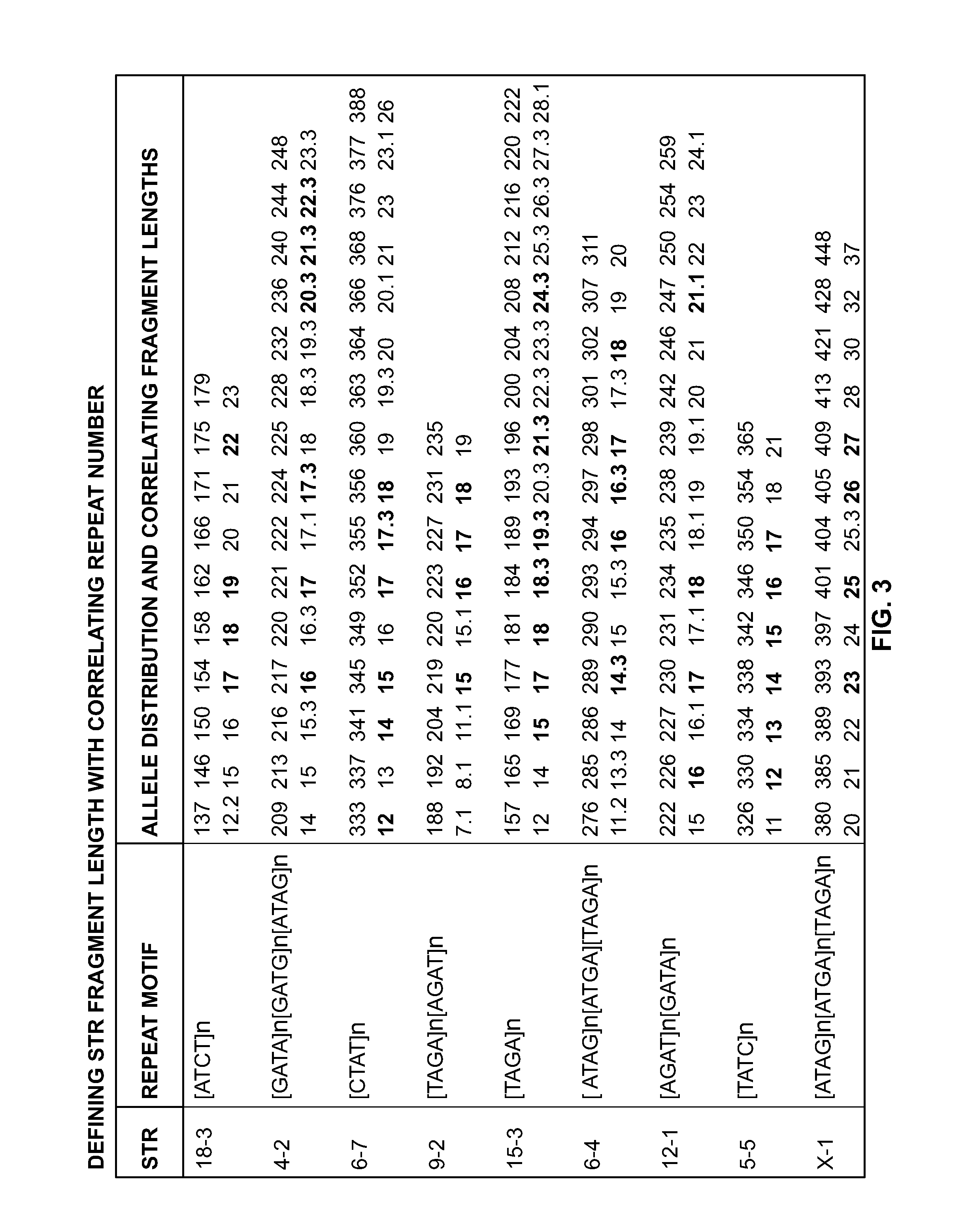
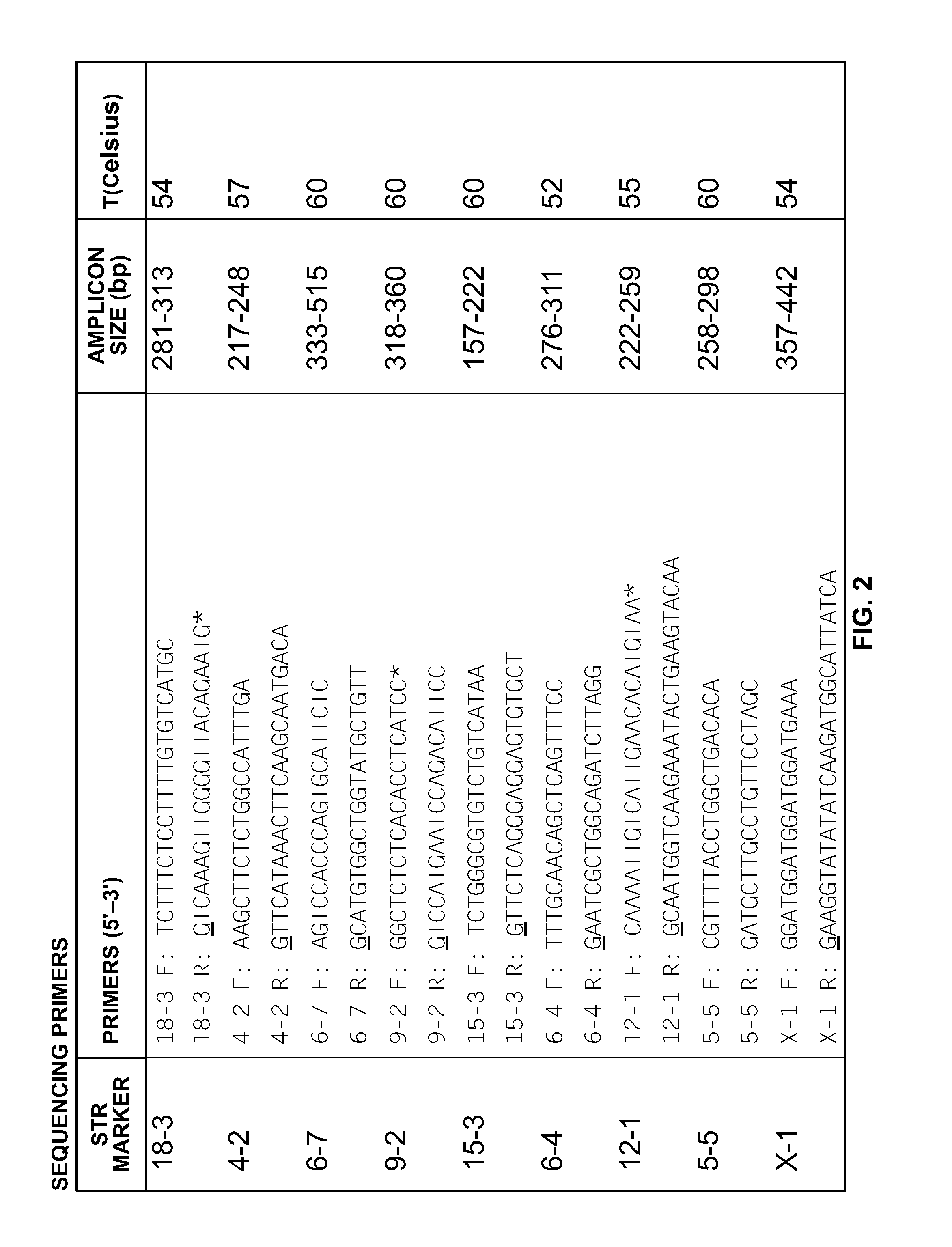
Mouse cell line authentication
ActiveUS20140066322A1High passage numberNucleotide librariesMicrobiological testing/measurementGenotypeTandem repeat
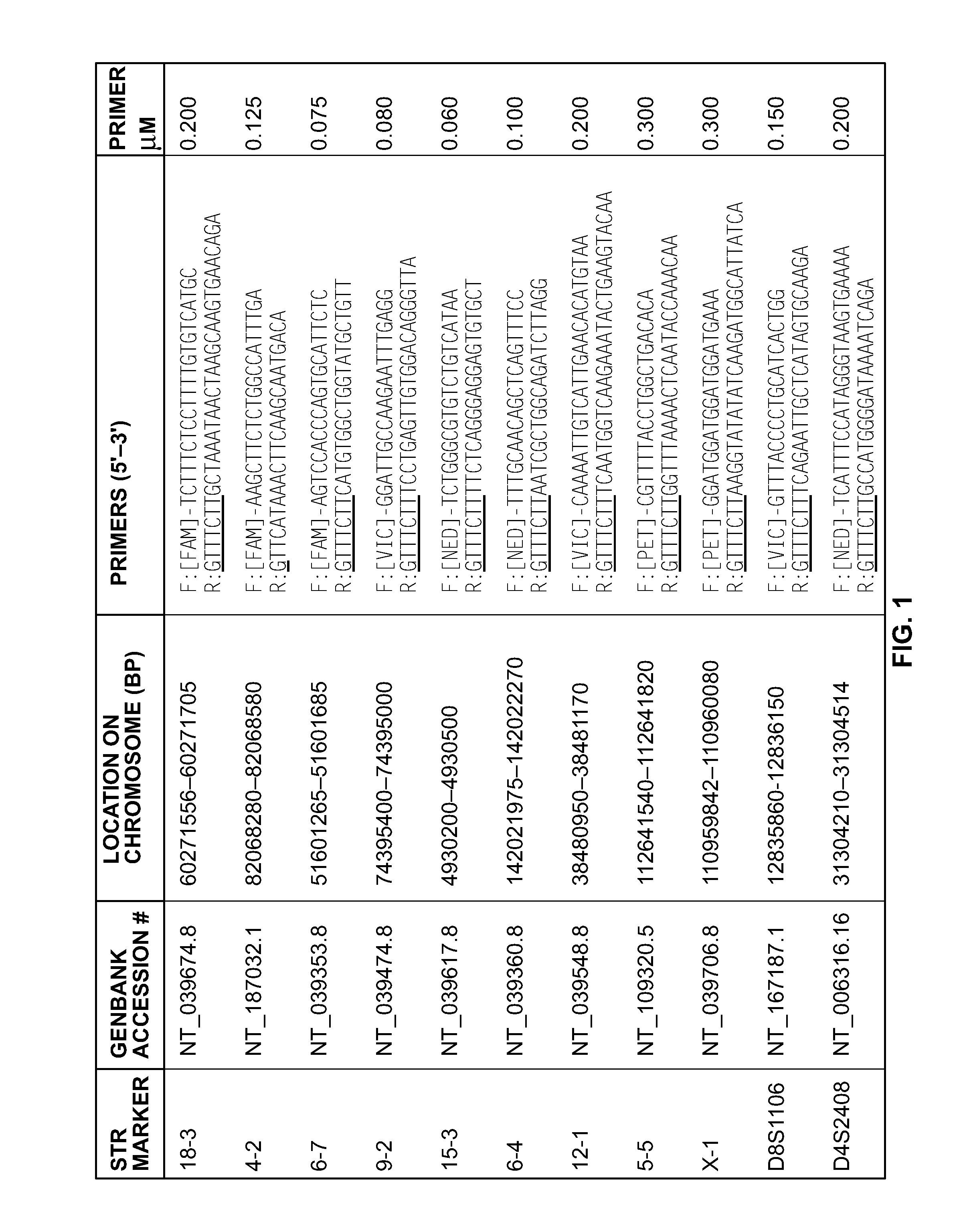
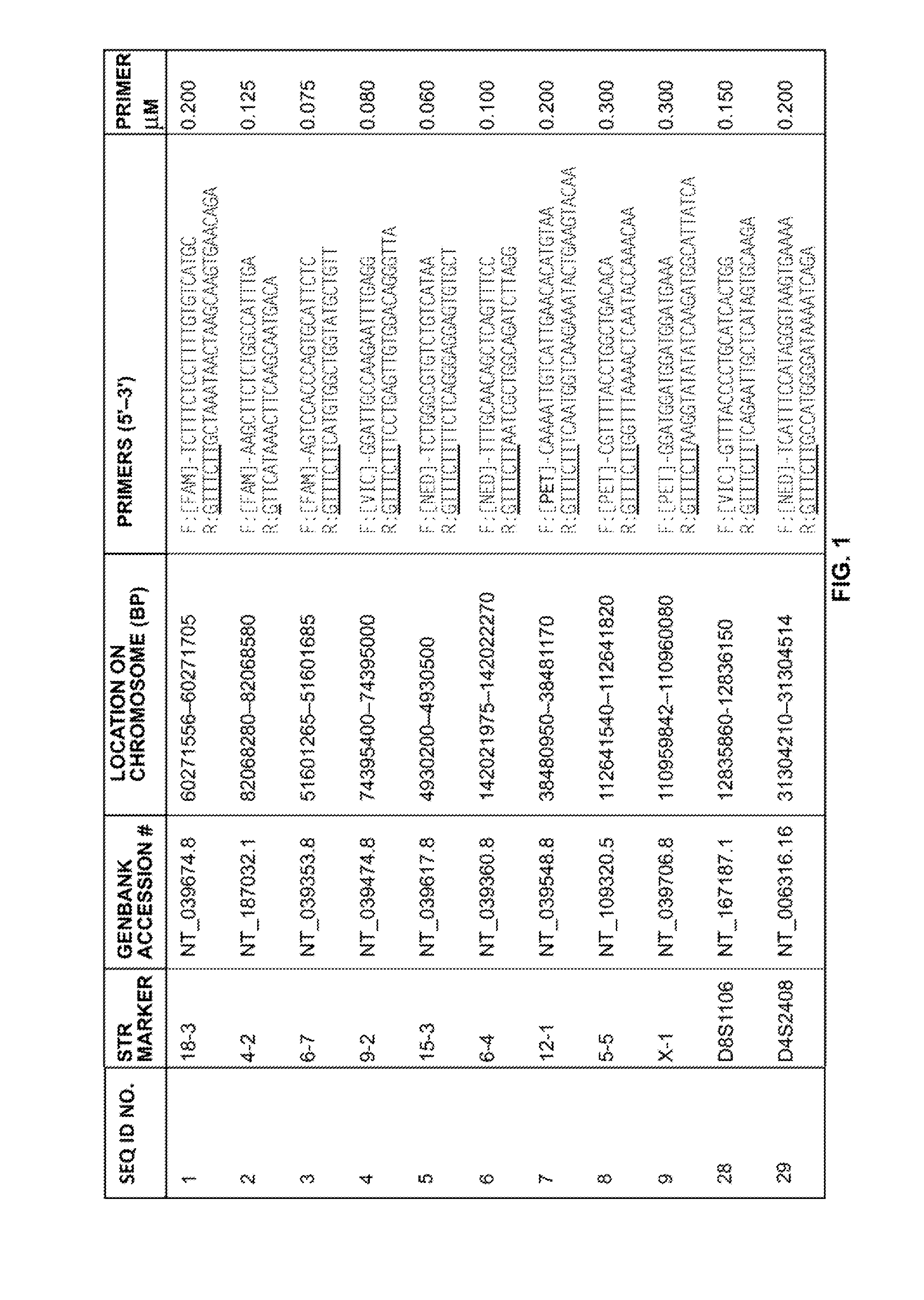
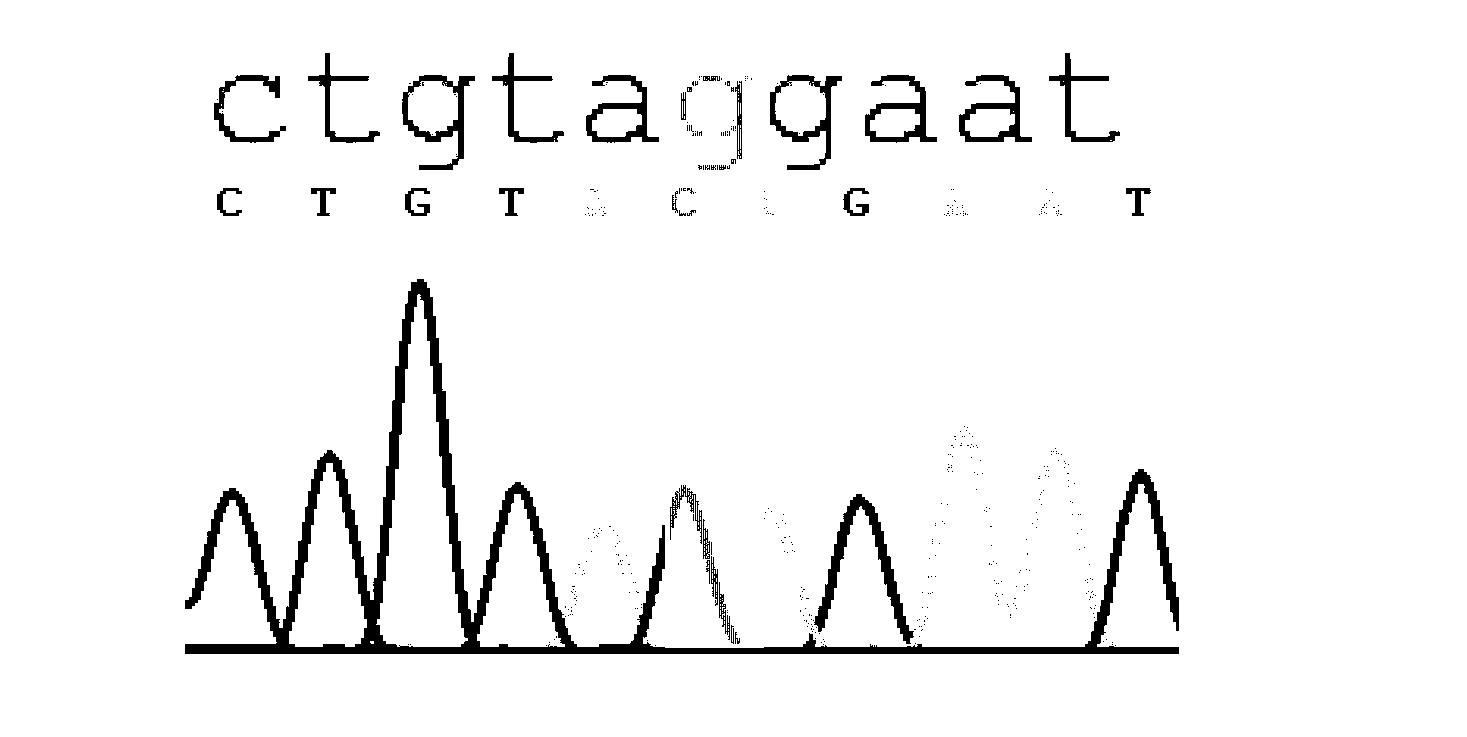
A multiplex polymerase chain reaction assay that targets nine tetranucleotide short tandem repeat (STR) markers in the mouse genome. Unique profiles were obtained from seventy-two mouse samples that were used to determine the allele distribution for each STR marker. Correlations between allele fragment length and repeat number were determined with DNA Sanger sequencing. Genotypes for L929 and NIH3T3 cell lines were shown to be stable with increasing passage numbers as there were no significant differences in fragment length with samples of low passage when compared to high passage samples. In order to detect cell line contaminants, primers for two human STR markers were incorporated into the multiplex assay to facilitate detection of human and African green monkey DNA. This multiplex assay is the first of its kind to provide a unique STR profile for each individual mouse sample and can be used to authenticate mouse cell lines.
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF COMMERCE THE NAT INST OF STANDARDS & TEHCNOLOGY
Duck hemorrhagic oophoritis inactivated vaccine production method by using cell line and product thereof
ActiveCN102488893AImprove adaptabilityHigh potencyViral antigen ingredientsInactivation/attenuationVaccine ProductionHamster
A duck hemorrhagic oophoritis inactivated vaccine production method by using a cell line and a product thereof belong to the field of biotechnology. According to the method provided by the invention, the cell line is selected from hamster kidney cell line or African green monkey kidney cell line, and the preparation of the vaccine is accomplished by the following steps of: (1) breeding strain, (2) establishing virus seed group, (3) preparing cell venom, (4) inactivating virus, (5) washing and concentrating cell venom, (6) emulsifying and the like. The method provided by the invention has characteristics of simple and stable production process, easy operation and high security. The prepared duck hemorrhagic oophoritis inactivated vaccine has advantages of high quality, high yield, low cost, small inter-batch difference, good safety and high immune efficacy.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD +1
Enterovirus type 71 (EV71) infectious clone and construction method
ActiveCN102212554AIncrease abundanceHigh abundance and high qualityMicroorganism based processesViruses/bacteriophagesEscherichia coliEnterovirus
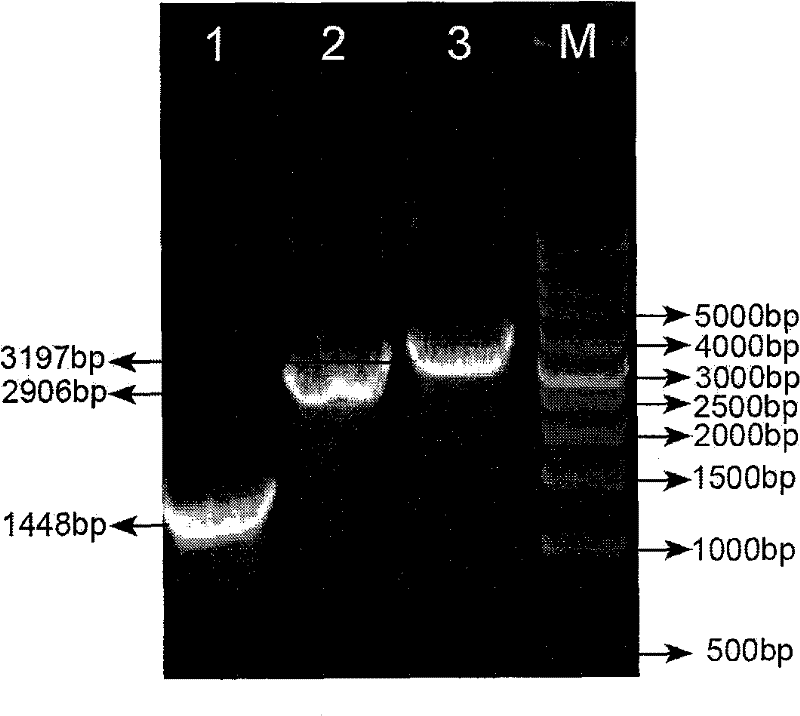
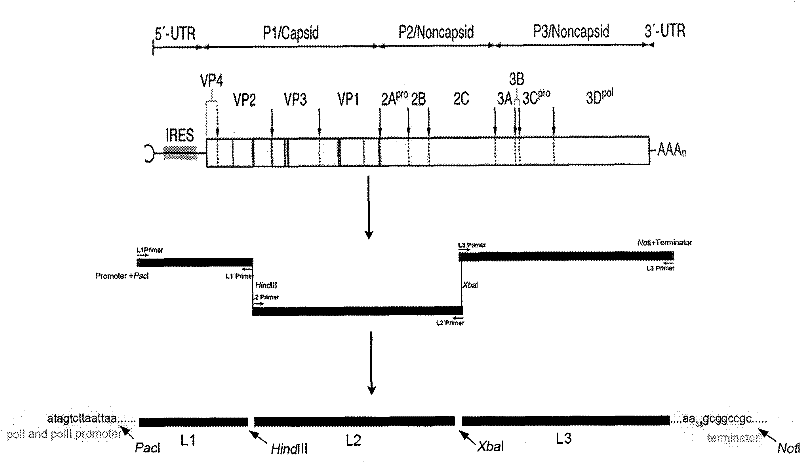
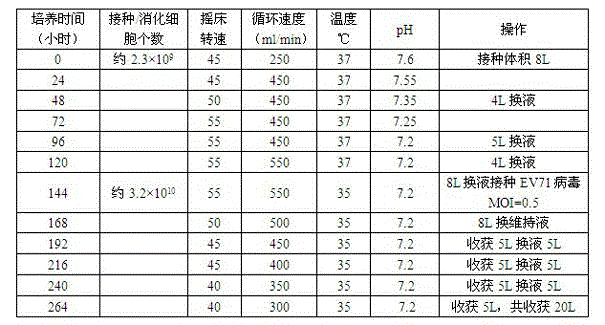
The invention discloses enterovirus type 71 (EV71) infectious clone and a construction method thereof. The construction method of the EV71 infectious clone comprises the following steps of: extracting RNA serving as a template from an EV17 strain, and amplifying three fragments L1, L2 and L3 by using the following three pairs of primers respectively; connecting the enzyme digested fragment L1 and a eukaryotic expression vector, transforming the connected fragment L1 and eukaryotic expression vector to escherichia coli to obtain a sub-cloned plasmid PL1, performing enzyme digestion, connection and transformation on the plasmid PL1 and the fragment L2 to obtain a plasmid PL2, and performing enzyme digestion, connection and transformation on the plasmid PL2 and the fragment L3 to obtain a full genome cDNA cloned recombinant plasmid containing the fragments L1, L2 and L3; and transfecting the recombinant plasmid to host cells of African green monkey kidney cells to obtain the EV71 infectious clone.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
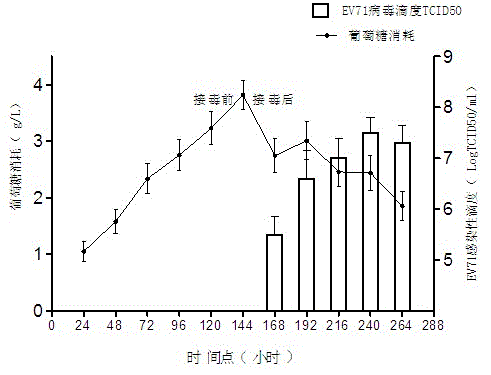
Optimized process method for amplifying enterovirus type 71 by use of bioreactor
ActiveCN103215233AOptimize culture conditionsOptimization parametersMicroorganism based processesViruses/bacteriophagesEnterovirusHydrolysate
The invention relates to the field of biotechnology, and in particular relates to an optimized process method for amplifying the enterovirus type 71. The method adopts a poly-fiber paper scrap as a carrier and amplifies an African green monkey kidney cell by use of a bioreactor so as to establish a whole process flow for copying and amplifying the enterovirus type 71. Under a condition of completely adapting to an enterovirus type 71 copying and amplifying system, the method adopts a DMEM (dulbecco's minimum essential medium) containing 10% of serum in a cell culture stage; after virus inoculation and adsorption for 24 hours, the method adopts a DMEM containing 3-5% of serum; 0.5% of lactoalbumin hydrolysate is added by use of a serum-free medium in a virus harvesting stage, and 2g / L of glucose is supplemented every 24 hours; and on the basis of reducing the later-period purifying difficulty and adapting to the requirements for a biological product, the optimized process realizes a higher virus titer. The method provided by the invention has good repeatability and efficient enterovirus type 71 amplifying process, and can be used for amplifying the enterovirus type 71 by use of a bioreactor by taking a poly-fiber paper scrap as a carrier.
Owner:ZHEJIANG PUKANG BIOTECH
Cell strain for producing goatpox vaccine
InactiveCN104694457AImprove adaptabilityLow risk of recoveryMicrobiological testing/measurementMicroorganism based processesGreen monkeyEngineering
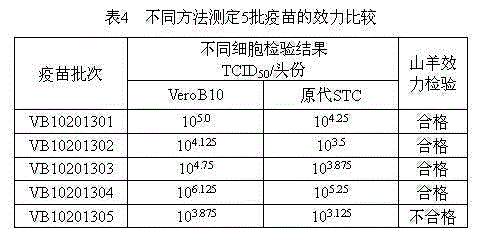
The invention relates to the field of vaccine preparation, and particularly relates to a cell strain for producing a goatpox vaccine. The cell strain is an African green monkey kidney cell Vero B10 and is collected at China General Microbiological Culture Collection Center with the collection number of CGMCC No. 9705. Compared with a primary cell production method, a preparation method of the cell strain has the advantages that a goatpox virus strain AV41 is cultivated by using a VeroB10 cell, good in virus adaptability and low in strong recurrence risk; a passage cell vaccine is superior to a primary cell vaccine in terms of virus yield, uniformity, pureness and the like; the cost is low, and animals are not needed; and the safety of the passage cell vaccine is superior to that of the primary cell vaccine, and the immunity efficiency of the passage cell vaccine is not lower than that of the primary cell vaccine.
Owner:SHANDONG LVDU BIO SICIENCE & TECH
Capsules capable of activating blood and stopping pains and preparation method of capsules
InactiveCN106074658AIncreased yield of ferulic acidSimple structureHeavy metal active ingredientsHydroxy compound active ingredientsAlcohol contentGround beetle
Owner:南京多宝生物科技有限公司
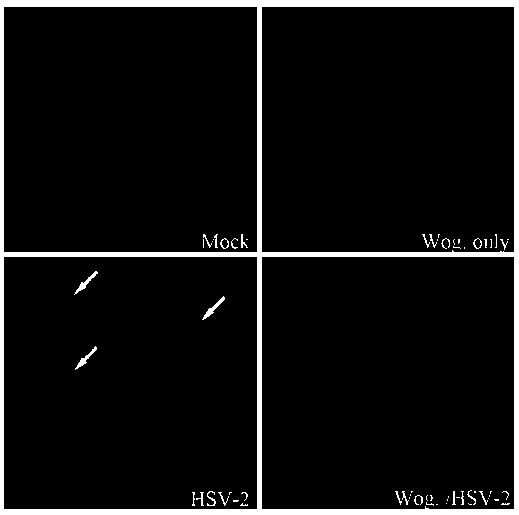
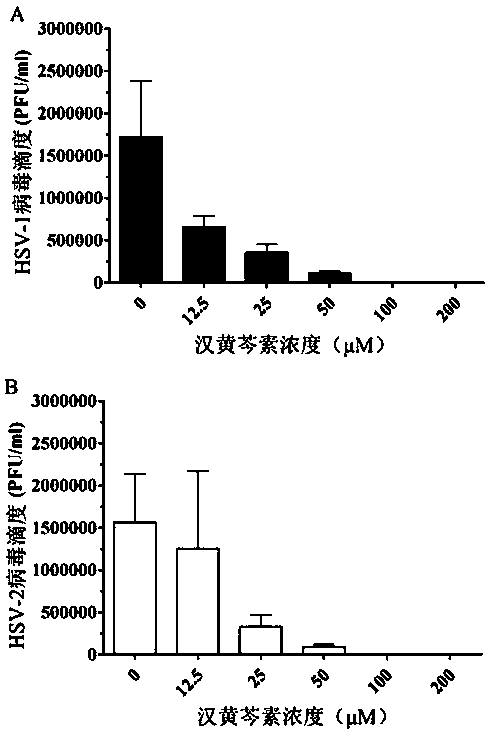
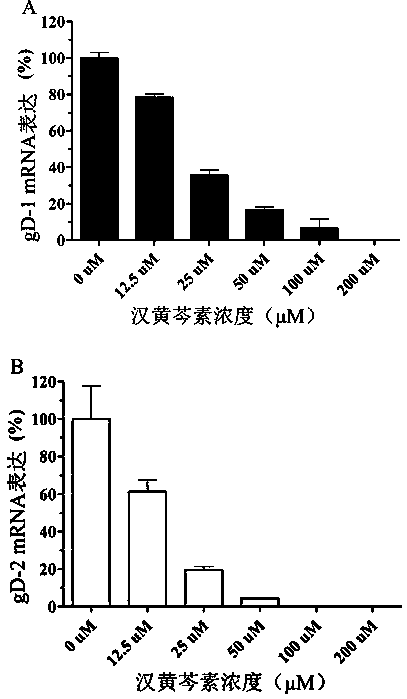
Application of wogonin in preparing medicine for resisting herpes simplex virus
InactiveCN107714689AInhibition of replicationAvoid spreadingOrganic active ingredientsAntiviralsHigh activityWogonin
The invention belongs to the field of pharmacy, and relates to application of wogonin in preparing medicine for resisting a herpes simplex virus. An experiment verifies that wogonin has a high activity of resisting the herpes simplex virus in an in-vitro experiment and can inhibit the expression of virus structure protein gD in mRNA transcription and the protein level; an in-vitro toxicity experiment of wogonin finds that the flavone type compound has low toxicity to cells of the human and African green monkeys and can obtain a high treatment index; therefore, wogonin has potential value as novel medicine resisting the herpes simplex virus and can be applied to preparing the medicine for resisting the herpes simplex virus.
Owner:常州市武进人民医院
Yixuanning capsule and preparation method thereof
InactiveCN106075125ASimple structureEasy to operateHeavy metal active ingredientsDigestive systemOysterFlos chrysanthemi
The invention provides a preparation method of a Yixuanning capsule. The preparation method comprises the following steps of taking 20g of fried fructus xanthii, 15g of flos chrysanthemi, 15g of bile arisaema, 15g of radix scutellariae, 15g of caulis bambusae in taeniam, 20g of calcined oyster shell, 10g of fructus crataegi, 15g of pericarpium citri reticulatae, 15g of radix paeoniae alba, 15g of ferrosic oxide, 20g of poria cocos and 20g of fructus lycii; adding water of which the weight is ten times that of the twelve medicinal materials; adopting specific concentrating and drying equipment. The content of total glucosides of radix paeoniae alba in the prepared Yixuanning capsule is high; the Yixuanning capsule is found to be applied in preparation of medicines for suppressing african green monkey kidney Vero-E6 cell proliferation.
Owner:南京多宝生物科技有限公司
Duck hemorrhagic oophoritis inactivated vaccine production method by using cell line and product thereof
ActiveCN102488893BImprove adaptabilityHigh potencyViral antigen ingredientsInactivation/attenuationVaccine ProductionHamster
A duck hemorrhagic oophoritis inactivated vaccine production method by using a cell line and a product thereof belong to the field of biotechnology. According to the method provided by the invention, the cell line is selected from hamster kidney cell line or African green monkey kidney cell line, and the preparation of the vaccine is accomplished by the following steps of: (1) breeding strain, (2) establishing virus seed group, (3) preparing cell venom, (4) inactivating virus, (5) washing and concentrating cell venom, (6) emulsifying and the like. The method provided by the invention has characteristics of simple and stable production process, easy operation and high security. The prepared duck hemorrhagic oophoritis inactivated vaccine has advantages of high quality, high yield, low cost, small inter-batch difference, good safety and high immune efficacy.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD +1
Method for producing a virus from an african green monkey kidney cell line
InactiveUS6025182AAvoid developmentSupport growthSsRNA viruses negative-senseSsRNA viruses positive-senseMicrobial agentViral Vaccine
A method for producing novel African Green Monkey Kidney (AGMK) cell lines is taught. These cell lines which are free of viable adventitious microbial agents are useful as substrates for viruses and for the preparation of viral vaccines.
Owner:NOVAVAX
Mouse cell line authentication
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF COMMERCE THE NAT INST OF STANDARDS & TEHCNOLOGY
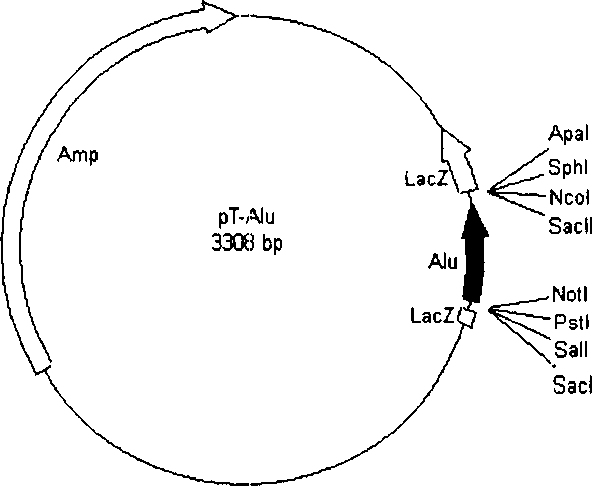
Alu sequence of Africa green monkey for detecting content of residual DNA in SARS vaccine
ActiveCN1690224AEffective monitoring of residual DNA contentMicrobiological testing/measurementGreen monkeyDNA
The invention relates to the African green monkey Alu sequence using to detect the residual content of DNA of the vaccine against SARS, and to the detection method with the related sequence.
Owner:SINOVAC BIOTECH
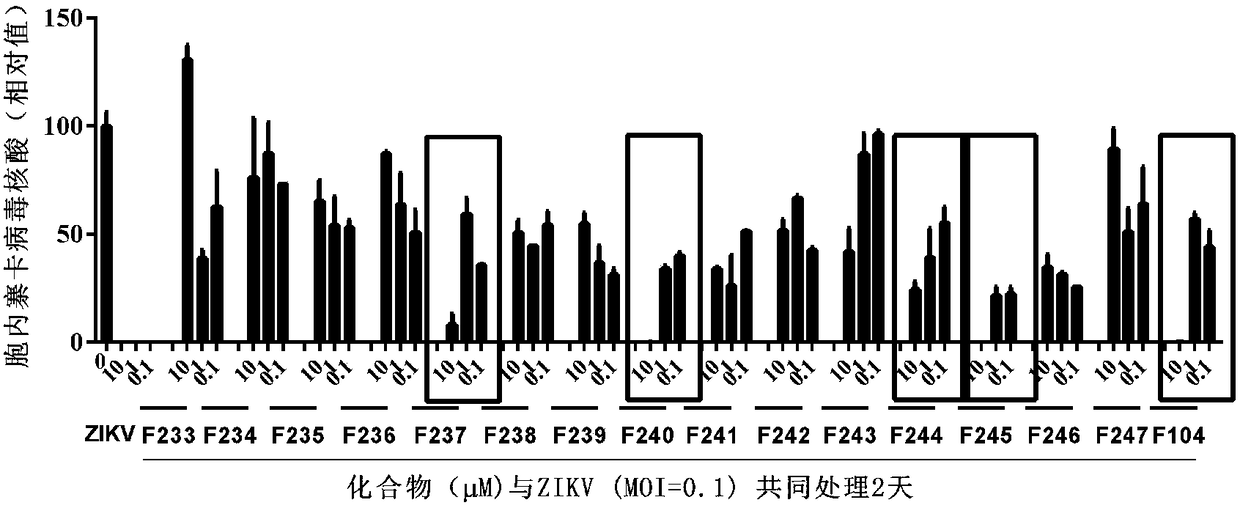
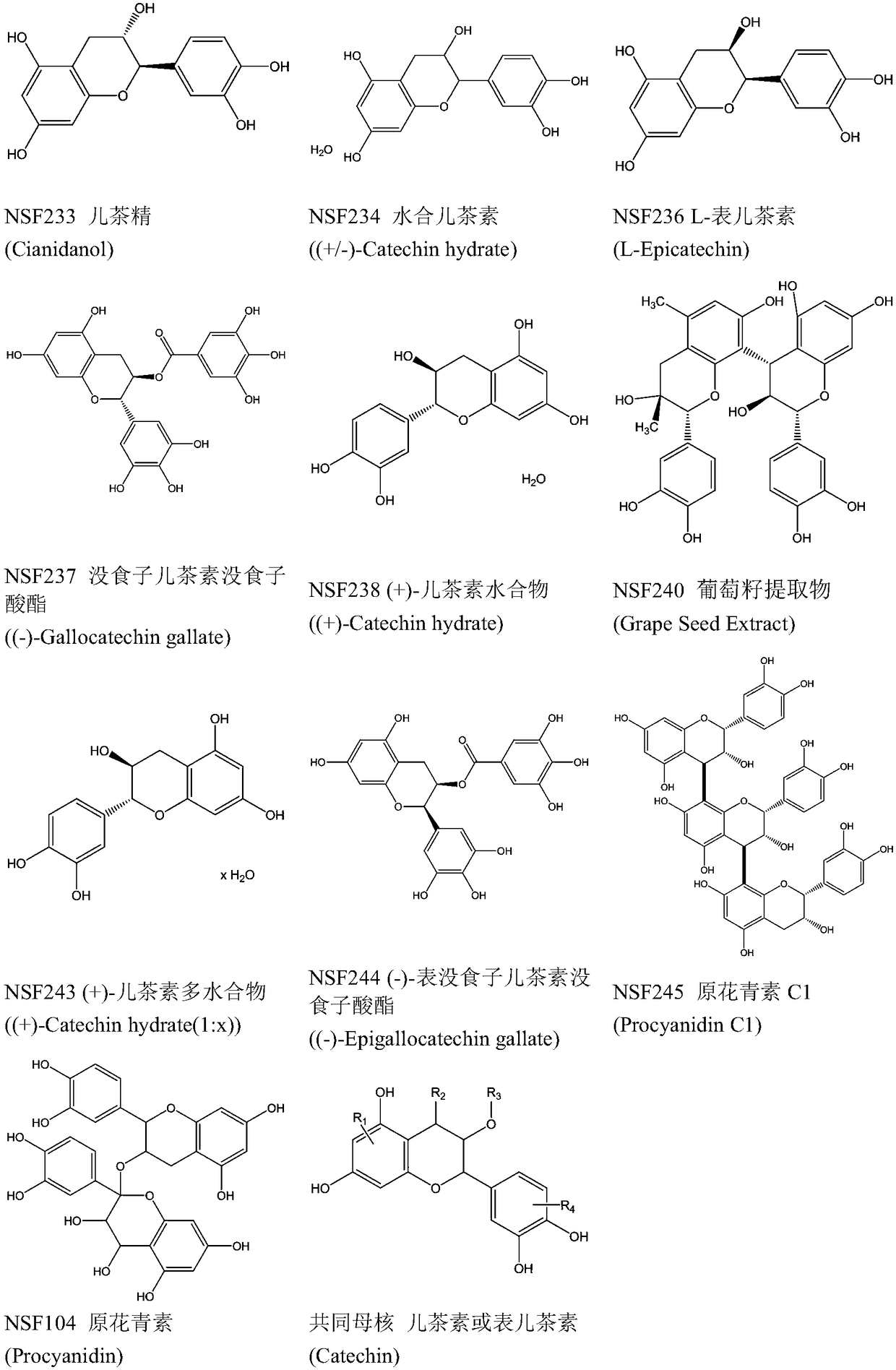
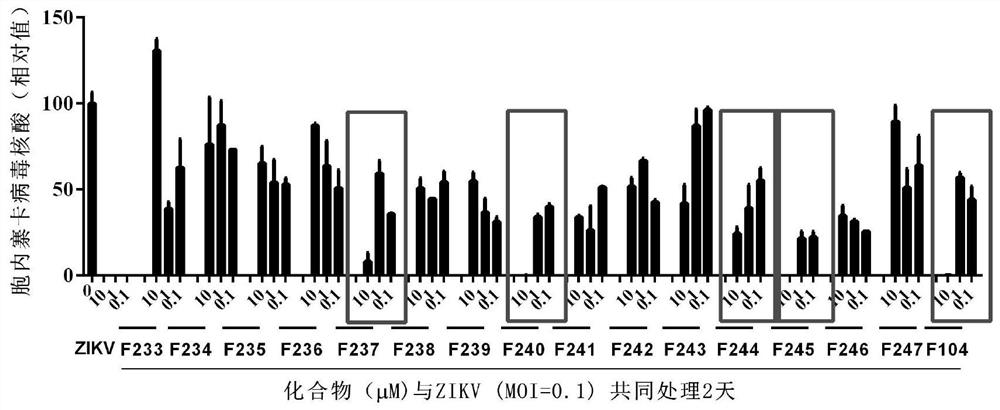
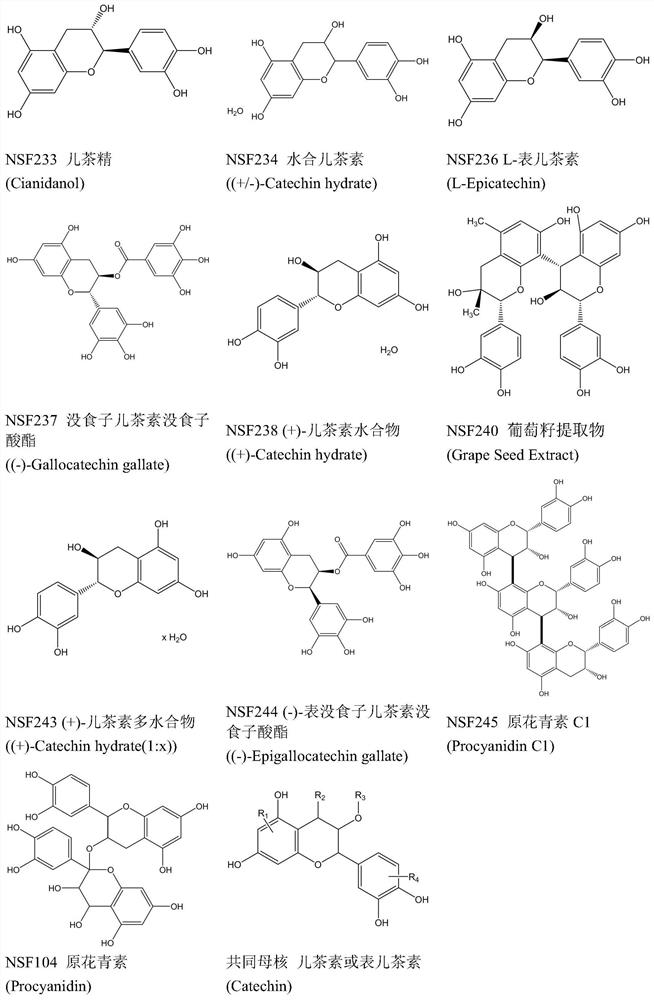
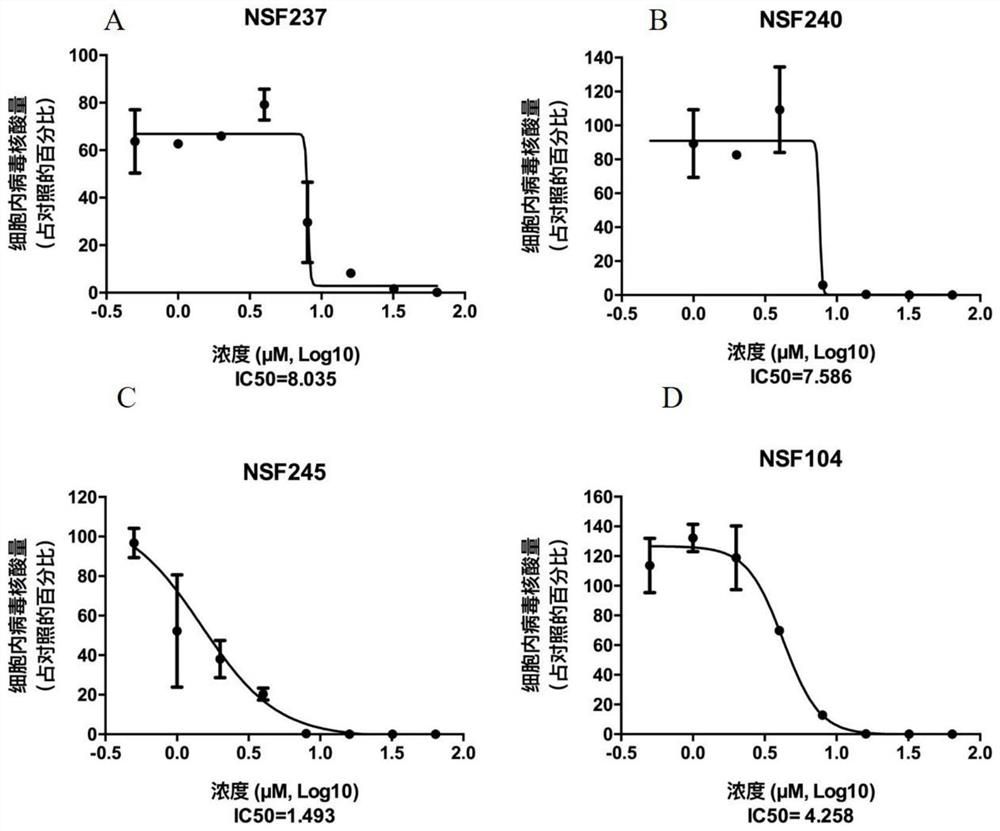
Application of proanthocyanidin compounds to preparation of drug against anti-Zika virus
ActiveCN109394755AHas anti-Zika virus activityStrong antiviral activityOrganic active ingredientsAntiviralsHuman gliomaCytotoxicity
The invention provides application of proanthocyanidin compounds shown by the formula (I) as shown in the specification or a pharmaceutically acceptable salt thereof to preparation of a drug against Zika virus, and provides the novel application of the proanthocyanidin compounds shown by the formula (I) in the specification or the pharmaceutically acceptable salt thereof in the pharmaceutical field. The compounds can effectively inhibit the expansion of Zika virus, and can be used as a lead compound to develop the drug for preventing and treating Zika virus infection. Active compounds have nosignificant cytotoxicity at the inhibitor dose and have a common parent nucleus structure, but the parent nucleus structure has no antiviral activity, wherein, the active compounds, namely proanthocyanidins, are found to have anti-Zika virus activity in African green monkey kidney cells and human glioma cells. The compounds have important application prospects.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +2
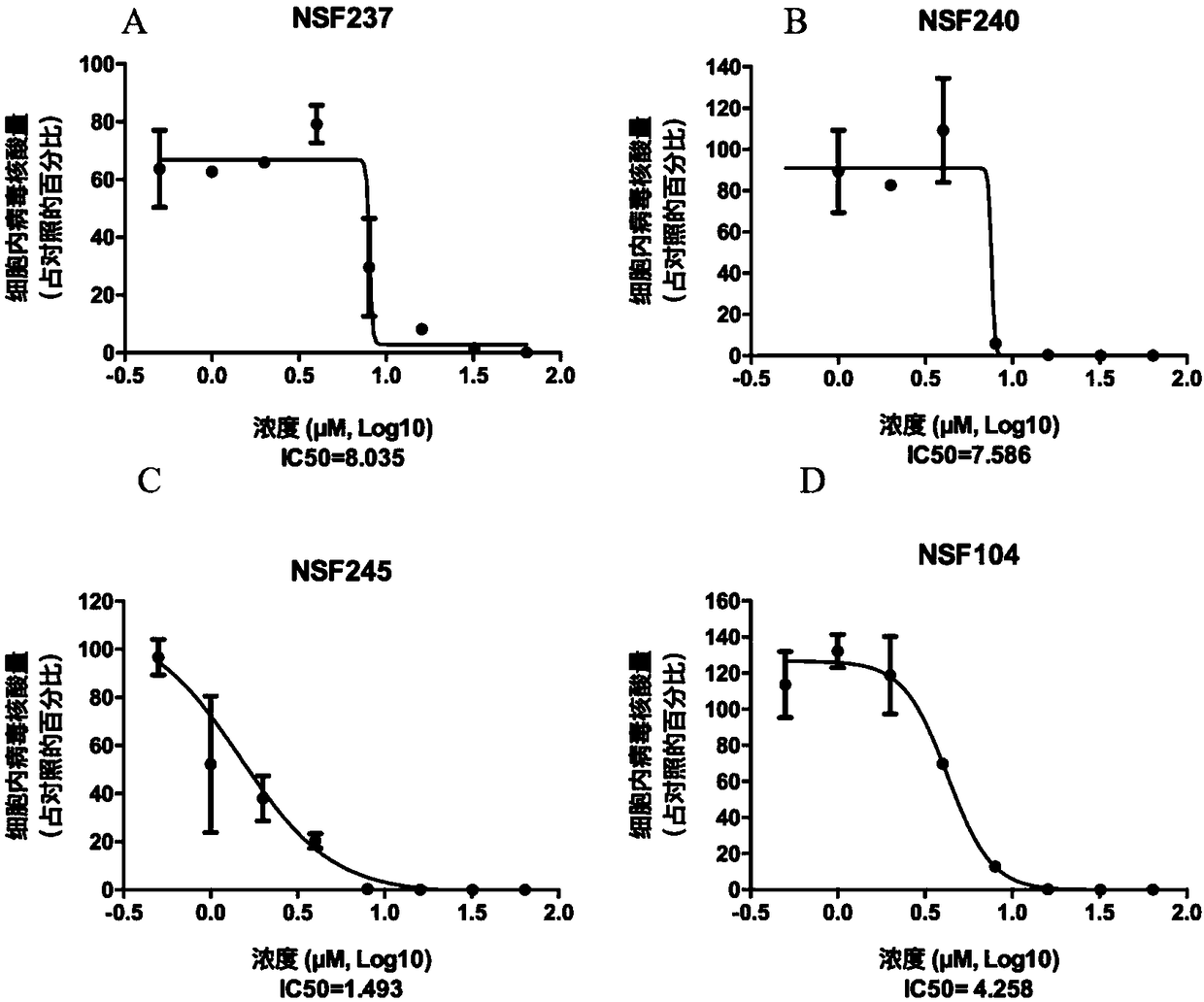
HO-1 and HO-1 inducer for inhibiting PRRS virus (PRRSV) infection as novel blocker
ActiveCN103463626AOrganic active ingredientsPeptide/protein ingredientsHighly pathogenicAlveolar macrophage
The invention provides a method for inhibiting PRRSV infection of cells by adopting heme oxygenase 1 (HO-1) for inhibiting the PRRSV infection of the cells and an HO-1 inducer cobalt protoporphyrin (CoPP) and hemin as a blocker. The expression is substantially increased after the infection of highly-pathogenic PRRSV, and the expression is substantially decreased after the infection of classic PRRSV. The expression is substantially increased after a PRRSV-permissive cell African green monkey kidney cell line is infected with the highly-pathogenic PRRSV, and the expression is substantially decreased after the PRRSV-permissive cell African green monkey kidney cell line is infected with classic PRRSV. The CoPP and the hemin are used to induce the HO-1 expression of Marc-145 and pig alveolar macrophage (PAM). Results show that each of the CoPP and the hemin substantially induces the HO-1 expression and inhibits the PRRSV infection of the Marc-145 and the PAM cells.
Owner:NORTHWEST A & F UNIV
Application of vitamin B12 in preparation of medicine for resisting novel coronavirus SARS-CoV-2
The invention belongs to the technical field of medicines, and relates to a novel application of vitamin B12 in pharmacy, in particular to an application of vitamin B12 in preparation of a medicine for resisting novel coronavirus. Experiments prove that the vitamin B12 can inhibit infection of the novel coronavirus on in-vitro cell culture Vero-E6 (African green monkey kidney cells), new application of the vitamin B12 in treatment of the novel coronavirus caused by the novel coronavirus is developed, and a new medicine is provided for treatment and control of the novel coronavirus.
Owner:FUDAN UNIV
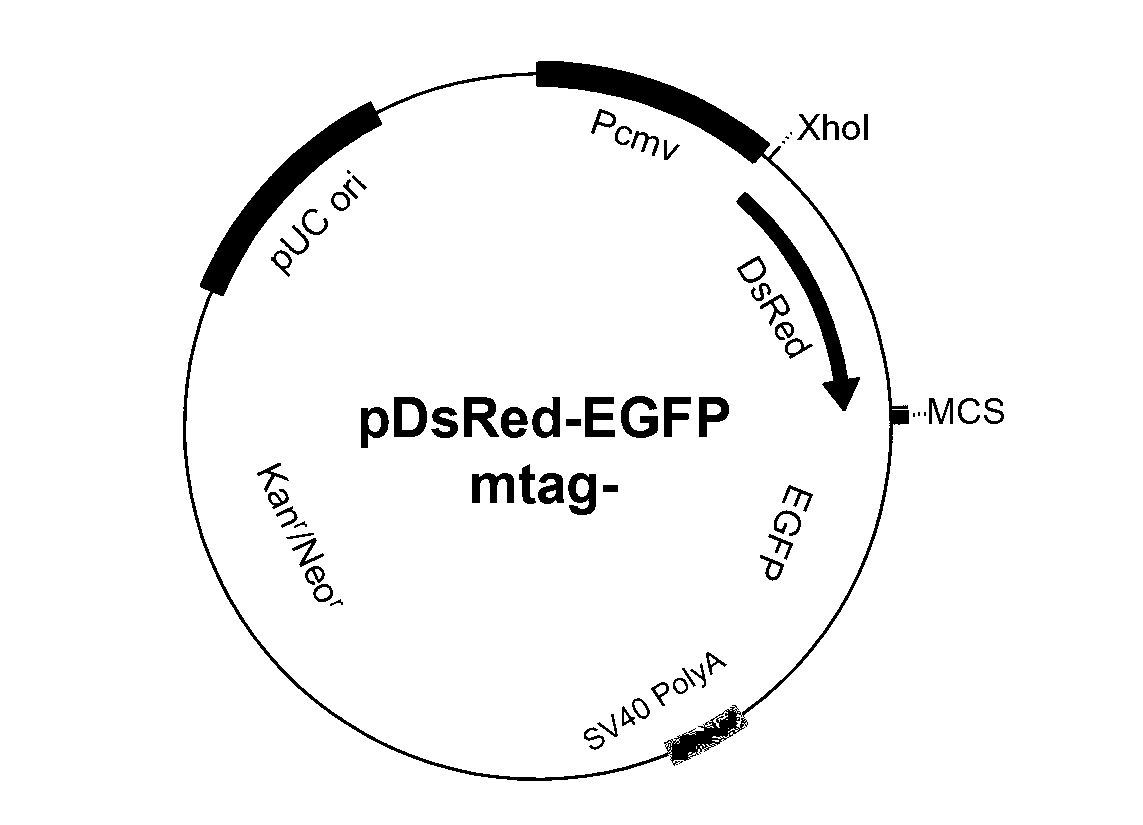
Method for establishing finite cell strain for screening read-though promoting drug
The invention discloses a eukaryote expression vector for expressing a green fluorescent protein (EGFP) and a red fluorescent protein (DsRed); a mutant contains a premature termination condon in a 445 amino acid position of the green fluorescent protein EGFP; and the expression of the green fluorescent protein EGFP can be effectively stopped by a mutational site. A constructed double fluorescent expression vector and a mutant thereof are introduced into an African green monkey kidney cell Cos-7 and treated by a read-though promoting drug PTC124; expression intensities of the green fluorescent protein and the red fluorescent protein are detected by using a flow cytometry; and the read-though promoting efficiency of the read-though promoting drug to the vector can be efficiently detected. In addition, different nonsense mutations and upstream and downstream correlation sequences can be introduced into a multiple clone site of the expression vector, so that targeted drugs for diseases initiated by the different nonsense mutations can be specifically screened.
Owner:SHANXI UNIV
Method for producing an adapted virus population from an African green monkey kidney cell line
InactiveUS6117667ARestricted and narrow tissue culture host rangeAvoid developmentSsRNA viruses negative-senseSsRNA viruses positive-senseEnterovirusSerial passage
The invention described herein provides a method for selecting a novel African Green monkey kidney (AGMK) cell substrate, its cultivation and serial passage and its subsequent characterization. The invention provides a method for the use of the cell substrate in the isolation, growth and serial passage of a large number of viruses, particularly rotaviruses, enteroviruses, respiratory viruses and hepatitis A virus. The invention provides a method for the utilization of this AGMK cell substrate for the production of live and killed virus vaccines.
Owner:NOVAVAX
Promoter-like gene for efficiently promoting and expressing heterologous protein and application thereof
ActiveCN107475257AImprove practicalityImprove abilitiesAcyltransferasesVector-based foreign material introductionNucleotideProtein C
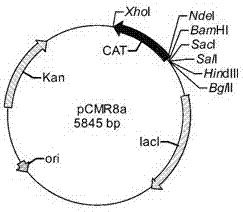
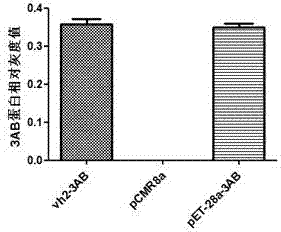
The invention discloses a constitutive promoter-like gene VP2 which is found from a eukaryotic cell African green monkey kidney cell (Vero) and can be used for promoting heterologous protein expression in a prokaryotic cell, and a truncating sequence Vh2 of the constitutive promoter-like gene VP2. A nucleotide sequence of the promoter-like gene VP2 is shown as SEQ ID NO: 1; the nucleotide sequence of the truncating sequence is shown as 60bp to 107bp in SEQ ID NO: 1; the gene is constructed to form an expression reporter gene chloramphenicol acetyl transferase (CAT) gene recombinant vector for screening; the screened vector and a gene segment to be screened are recombined, and a gene library is constructed; a promoter-like sequence is obtained by a recombinant strain containing a promoter-like gene sequence through a method for screening the resistance of chloramphenicol; the sequence can be used for efficiently starting and expressing heterologous protein in the prokaryotic cell.
Owner:KUNMING UNIV OF SCI & TECH
Application of proanthocyanidins in the preparation of anti-Zika virus drugs
ActiveCN109394755BHas anti-Zika virus activityStrong antiviral activityOrganic active ingredientsAntiviralsHuman gliomaGreen monkey
The invention provides application of proanthocyanidin compounds shown by the formula (I) as shown in the specification or a pharmaceutically acceptable salt thereof to preparation of a drug against Zika virus, and provides the novel application of the proanthocyanidin compounds shown by the formula (I) in the specification or the pharmaceutically acceptable salt thereof in the pharmaceutical field. The compounds can effectively inhibit the expansion of Zika virus, and can be used as a lead compound to develop the drug for preventing and treating Zika virus infection. Active compounds have nosignificant cytotoxicity at the inhibitor dose and have a common parent nucleus structure, but the parent nucleus structure has no antiviral activity, wherein, the active compounds, namely proanthocyanidins, are found to have anti-Zika virus activity in African green monkey kidney cells and human glioma cells. The compounds have important application prospects.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +2
Optimized technical method for proliferation of Coxsackie virus A16
InactiveCN106222146AGood value for moneyReduce the difficulty of purificationSsRNA viruses positive-senseFiberHydrolysate
The invention discloses an optimized technical method for proliferation of Coxsackie virus A16. The method includes: taking flaky fibers as carriers, proliferating African green monkey kidney cells in a bioreactor, adopting an 8v / v% serum containing DMEM (Dulbecco modified eagle medium) culture medium in a cell culture phase, changing into a serum-free DMEM culture medium in 5-6 days of cell culture, adopting a 3-5% serum containing DMEM culture medium after virus inoculation and adsorption, adopting a 0.5w / v% lactoalbumin hydrolysate added serum-free culture medium in a virus harvesting phase, statically re-adsorbing for 3h, continuing to culture, and replenishing 2.0g / L glucose about every 24h. The method is high in repeatability and stability, high virus titer can be achieved, and subsequent purification difficulty can be lowered while requirements of biological products are met. In addition, the method is high in application value and applicable to bioreactor based Coxsackie virus A16 proliferation with flaky fiber carriers.
Owner:ZHEJIANG UNIV
Optimized process method for amplifying enterovirus type 71 by use of bioreactor
ActiveCN103215233BOptimize culture conditionsOptimization parametersMicroorganism based processesViruses/bacteriophagesEnterovirusHydrolysate
The invention relates to the field of biotechnology, and in particular relates to an optimized process method for amplifying the enterovirus type 71. The method adopts a poly-fiber paper scrap as a carrier and amplifies an African green monkey kidney cell by use of a bioreactor so as to establish a whole process flow for copying and amplifying the enterovirus type 71. Under a condition of completely adapting to an enterovirus type 71 copying and amplifying system, the method adopts a DMEM (dulbecco's minimum essential medium) containing 10% of serum in a cell culture stage; after virus inoculation and adsorption for 24 hours, the method adopts a DMEM containing 3-5% of serum; 0.5% of lactoalbumin hydrolysate is added by use of a serum-free medium in a virus harvesting stage, and 2g / L of glucose is supplemented every 24 hours; and on the basis of reducing the later-period purifying difficulty and adapting to the requirements for a biological product, the optimized process realizes a higher virus titer. The method provided by the invention has good repeatability and efficient enterovirus type 71 amplifying process, and can be used for amplifying the enterovirus type 71 by use of a bioreactor by taking a poly-fiber paper scrap as a carrier.
Owner:ZHEJIANG PUKANG BIOTECH
Porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine and preparation method thereof
The invention relates to a porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine and a preparation method of the porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine. The porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine is prepared by performing virus amplification on a swine testicular cell line (ST cells) or an African green monkey kidney cell line (Vero cells) by using a self-attenuated and preserved transmissible gastroenteritis virus SD / L strain and a self-attenuated and preserved porcine epidemic diarrhea virus LW / L strain, and carrying out the steps of harvesting, uniformly mixing, freeze-drying and the like. The porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine can effectively prevent two diseases namely swine transmissible gastroenteritis and epidemic diarrhea.
Owner:QILU ANIMAL HEALTH PROD
A semi-perfusion culture method for duck Tembusu virus
ActiveCN105861452BPromote divisionIncrease the number ofSsRNA viruses positive-senseRecovery/purificationPerfusion CultureVirus multiplication
The invention discloses a semi-pouring flow type culture method for the duck Tembusu virus. The culture method comprises the steps that firstly, a cell culture device is used for carrying out virus reproduction, wherein the cell culture device containing a carrier is subjected to autoclaved sterilization, a culture solution is added, and African green monkey kidney cells are inoculated in a sterile mode; secondly, the culture solution is replaced, wherein the glucose content in the culture solution and the pH value are measured every day, and the culture solution is replaced; thirdly, virus inoculation is carried out, wherein allantoic fluid containing the duck Tembusu virus is inoculated, and virus multiplication culture is carried out; fourthly, the culture solution is supplemented, wherein crude virus liquor is collected, and then the fresh culture solution with the equal amount is supplemented to continue culture; fifthly, preservation is carried out, wherein the crude virus liquor collected in the fourth step is subjected to multigelation 1-5 times at minus 100-minus 20 DEG C, cell fragments are extracted through centrifuging after multigelation, filtering is carried out, and virus liquor is obtained and preserved at minus 20 DEG C. By means of the semi-pouring flow type culture method, the production efficiency of the virus can be improved, and crude virus liquor can be continuously gained multiple times every time each batch of cells are cultured.
Owner:GUANGDONG HAID GROUP +1
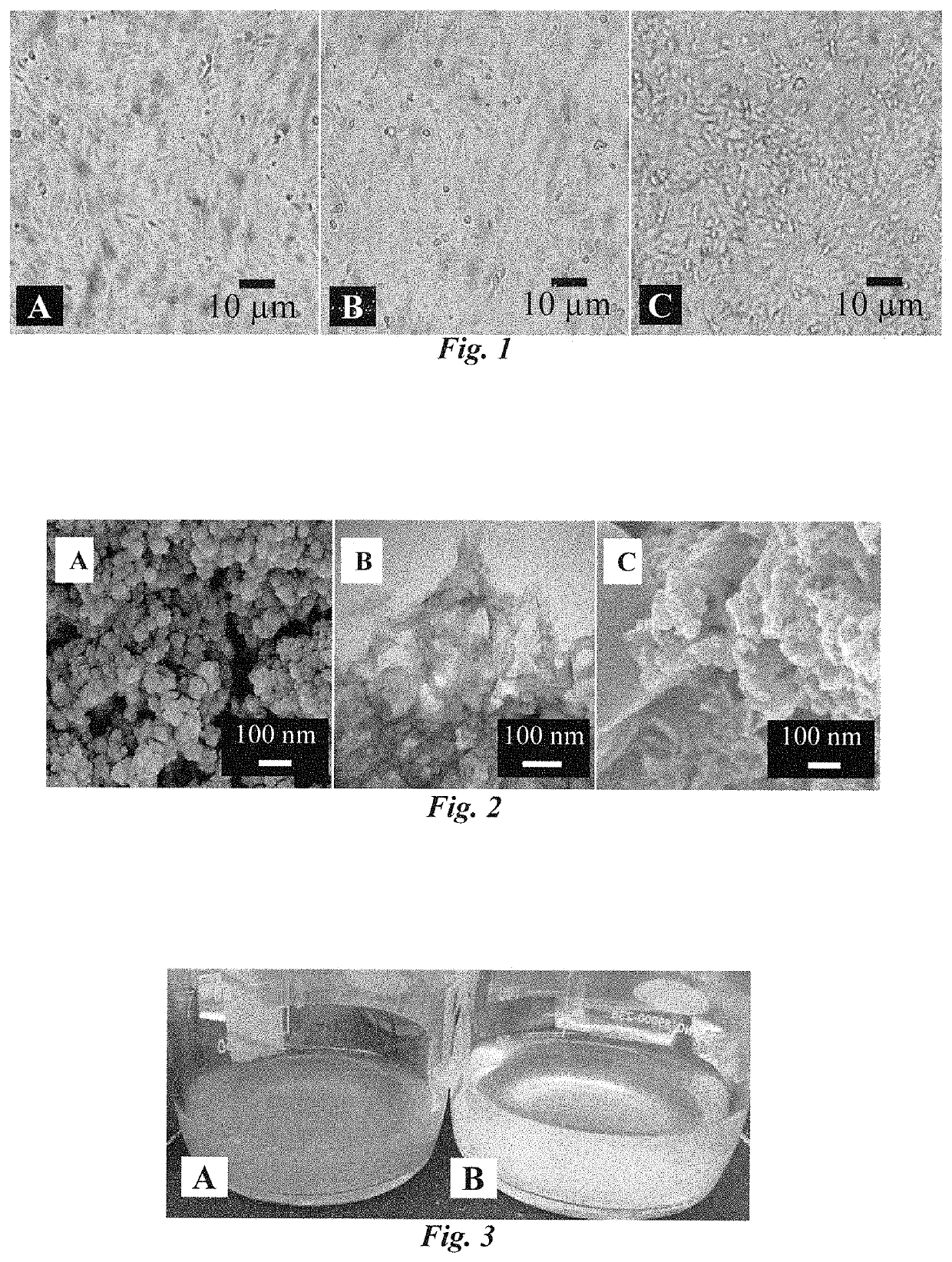
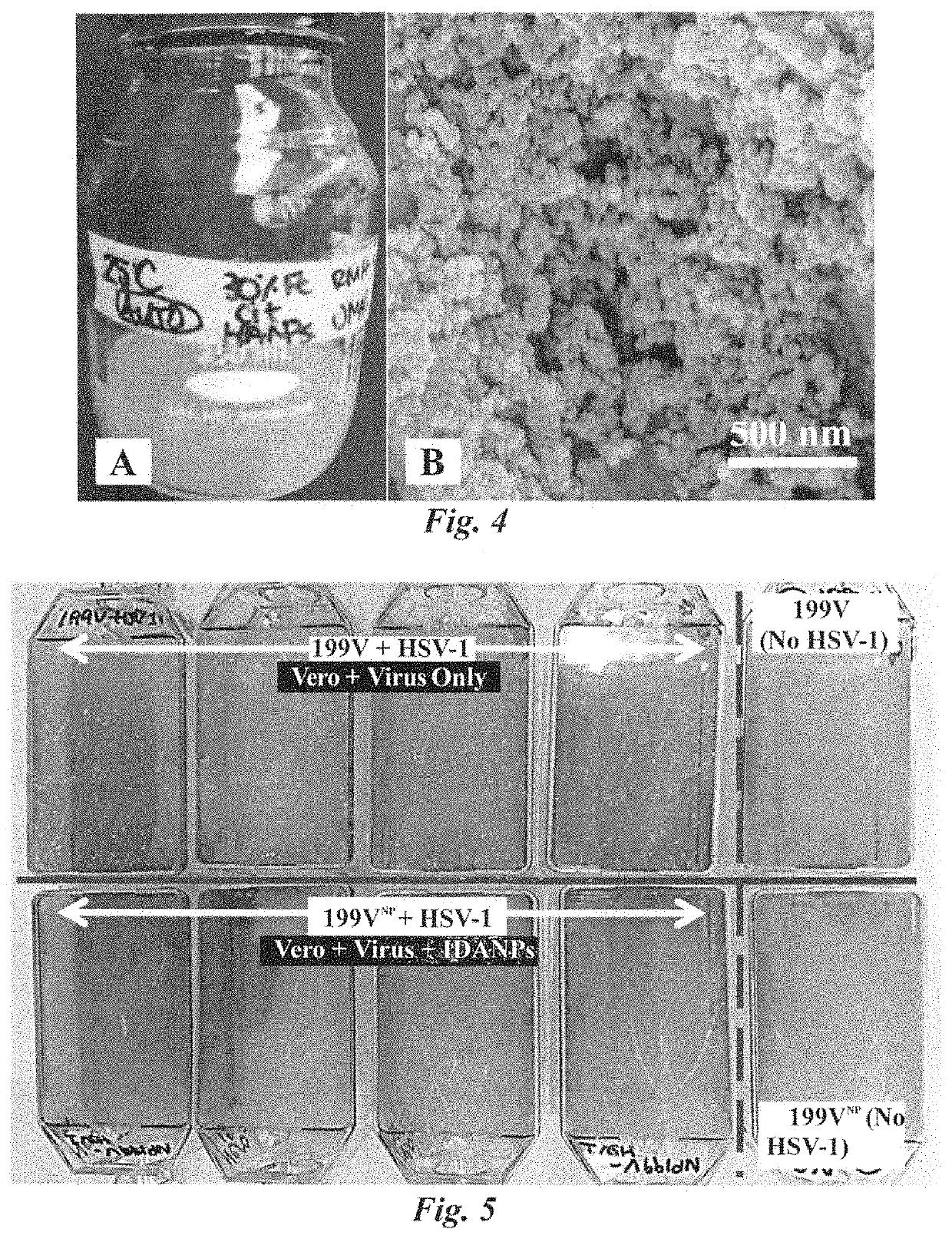
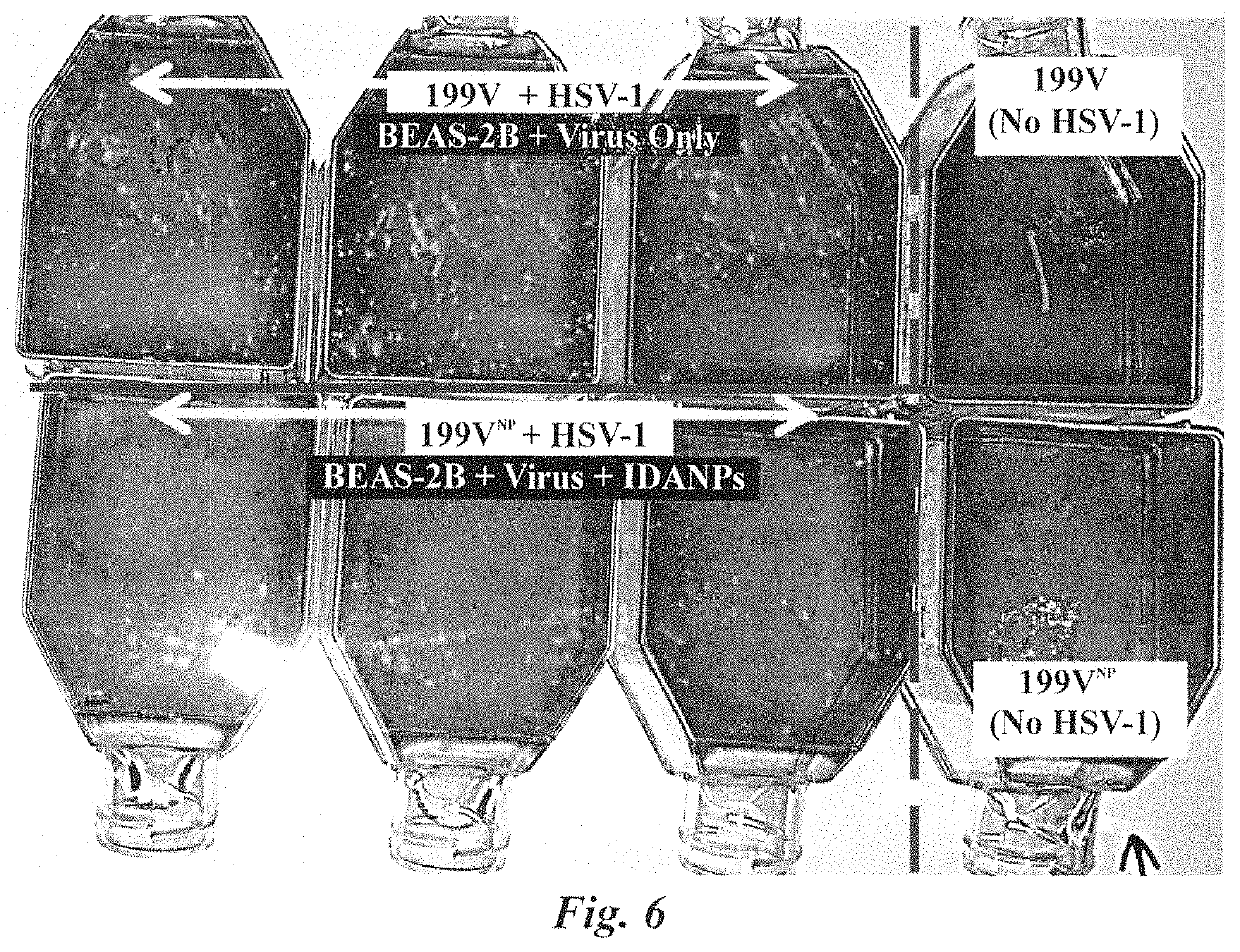
Antiviral composition and applications of iron-doped apatite nanoparticles
ActiveUS10532070B2NanotechInorganic phosphorous active ingredientsBronchial epitheliumBiocompatibility
Iron-doped apatite nanoparticles (IDANPs) are useful for the prevention, treatment, or alleviation of signs or symptoms associated with viral activation or infection. IDANPs have demonstrated a significant influence over herpes simplex virus 1 (HSV-1) infection of two mammalian cell lines. Specifically, IDANPs decreased HSV-1 infection of African Green Monkey kidney epithelial (Vero) cells by 84% and HSV-1 infection of human lung bronchus (BEAS-2B) cells by 71%. IDANPs consist of hydroxyapatite (HA) doped with iron. HA is a mineral known to be biocompatible and analogous to the inorganic constituent of mammalian bone and teeth and has been approved by the Food and Drug Administration (FDA) for many applications in medicine and dentistry. Lactate Dehydrogenase (LDH) and XTT (2,3-Bis 2-methoxy-4-nitro-5-sulfophenyl-2H-tetrazolium-5-carboxanilide inner salt) cytotoxicity assays revealed that IDANPs are largely non-toxic to Vero, BEAS-2B, and human cervical cancer (HeLa) cells lines. HSV-1 afflicted individuals in the United States have been estimated as high as ⅔ the population. Because IDANPs dramatically decrease HSV-1 infection and are largely non-toxic, their application as an antiviral agent is evident. Further, although iron(III) alone has been shown to diminish replication of deoxyribonucleic acid (DNA)- and ribonucleic acid (RNA)-containing viruses, IDANP cytotoxicity studies indicate that encasement and delivery of iron within an apatite unit cell structure diminishes significantly, and in some cases eliminates, cytotoxicity posed by the introduction of iron(III) alone.
Owner:GREGORY JESSICA M +3
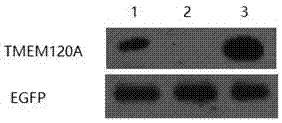
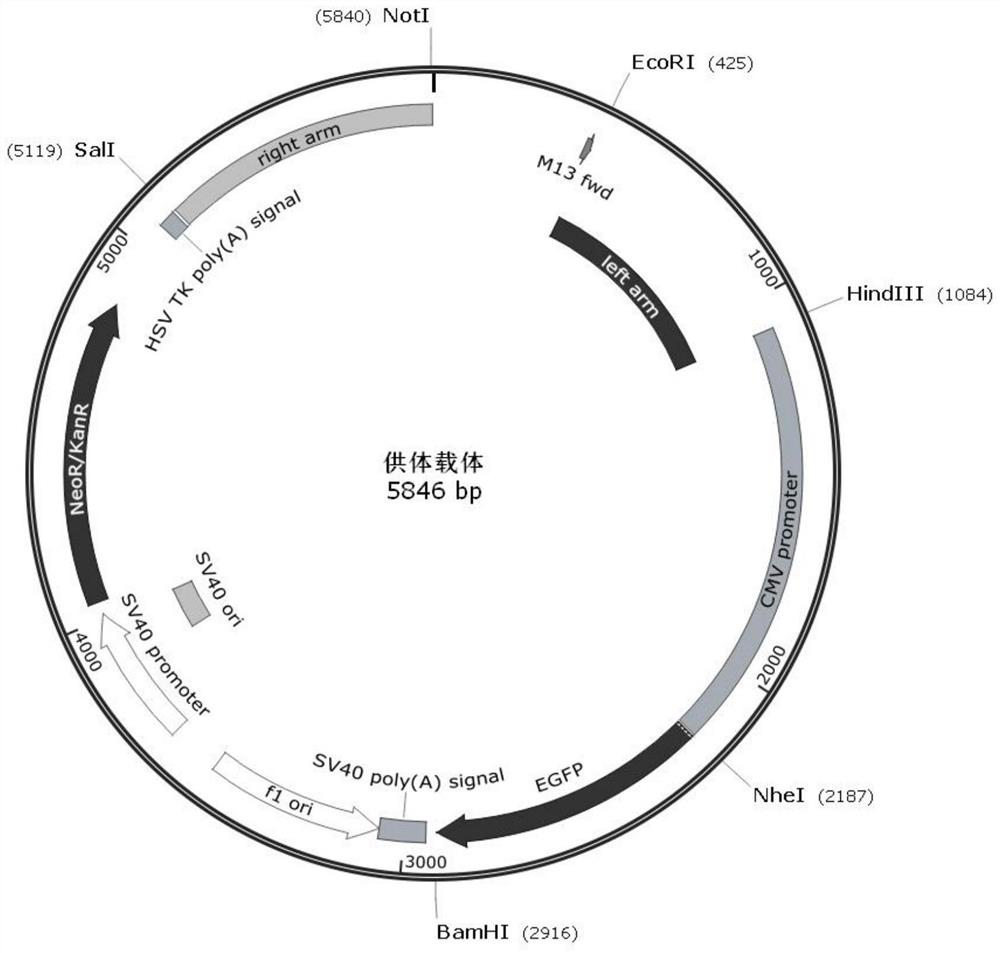
A homologous recombination vector expressing egfp, recombinant cell and its preparation method and application
The invention provides a homologous recombination vector expressing eGFP, which belongs to the field of genome editing technology. The homologous recombination vector expressing eGFP includes a vector backbone and an insert segment, and the insert segment includes a left homology arm, a CMV promoter, An eGFP expression frame and a right homology arm; the nucleotide sequence of the left homology arm is shown in SEQ ID No.1; the nucleotide sequence of the right homology arm is shown in SEQ ID No.2. After the homologous recombination vector described in the present invention and the CRISPR / Cas9 targeting vector are co-transfected into vero cells, the vero cells are screened by G418 to obtain vero cells that integrate eGFP at a specific point and stably express eGFP. The cell construction method described in the present invention supports site-specific integration and stable expression of foreign genes.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Coxsackie virus A16-type virus strain and applications thereof
ActiveCN102533671BGenetic stabilityStable titerSerum immunoglobulinsMicroorganism based processesSequence analysisVirus strain
The invention provides a coxsackie virus A16-type virus strain. The collection number of the coxsackie virus A16-type virus strain is CGMCC No.5372, wherein CGMCC refers to China General Microbiological Culture Collection Center. The virus is a 20-face stereoscopic symmetrical sphere under observation through an electron microscope, and the diameter of the virus is 23-30nm. VP1 conserved region sequence analysis and mass spectrum analysis are respectively performed on the virus strain, and a result shows a CA16 virus. The CA16 virus can be efficiently proliferated in Vero cells (African green monkey kidney cells), and the virus titer can reach 6.61g CCID50 / ml. Moreover, the virus strain has no external pollution, better immunogenicity and a good effect.
Owner:SINOVAC BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com