Application of proanthocyanidins in the preparation of anti-Zika virus drugs
A Zika virus and proanthocyanidin technology, which can be used in antiviral agents, resistance to vector-borne diseases, pharmaceutical formulations, etc., can solve the problem that other compounds have no anti-Zika virus activity, and achieve good medicinal prospects and antiviral activity. Strong and safe effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Anti-Zika virus activity test based on Q-PCR
[0041] 1. Experimental materials
[0042] (1) Cell line: African green monkey kidney cells (vero cell line) donated by Jin Xia's research group
[0043] (2) Virus strain: Zika virus strain (Microbial Virus Collection Center, Wuhan Institute of Virology, Chinese Academy of Sciences)
[0044] (3) Main reagents: TRNzol lysate (Tiangen), chloroform, isopropanol, 75% ethanol, reverse transcription kit (ReverTraAce qPCR RT, Yoyobo), real-time fluorescent quantitative PCR kit (SYBR Green Realtime PCRMasterMix, Toyobo, Japan)
[0045] (4) Main instruments: Eppendorfcentrifuge 5424R cryogenic centrifuge, Applied Biosysytems PCR instrument, Applied Biosysytems quantistudio 6Flex, Haier refrigerator
[0046] 2. Experimental method
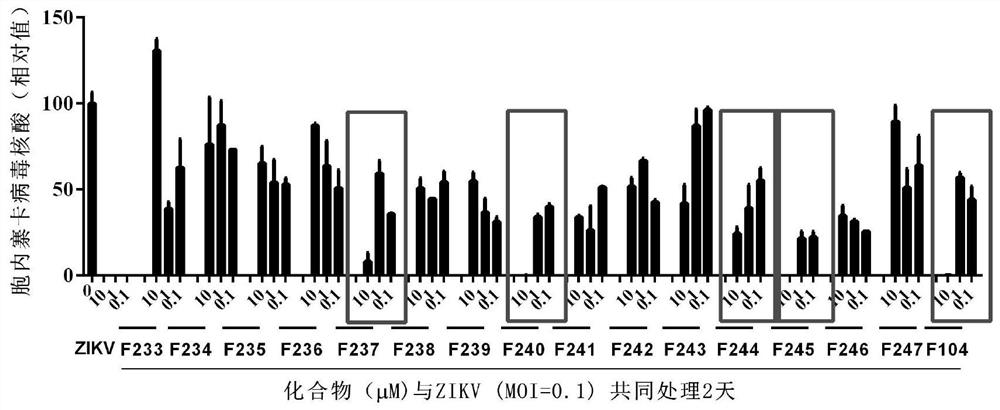
[0047] (1) Compounds and Zika virus co-treated African green monkey kidney cells
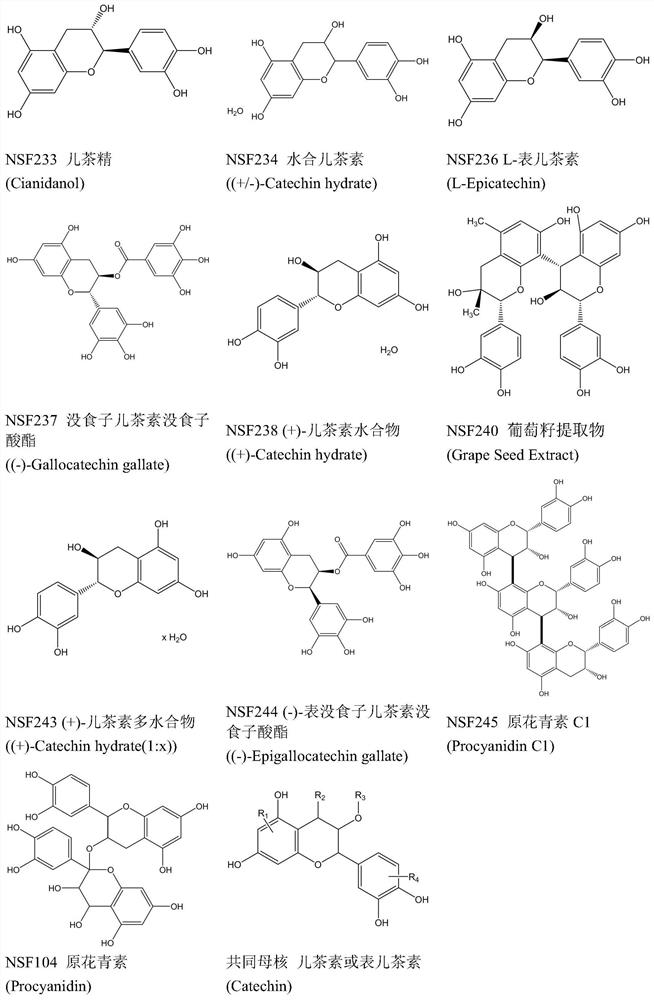
[0048] 4μL 1×10 6 16 compounds (see figure 1 listed) mix well and process 4×10 at the same time 4 Afric...
Embodiment 2
[0066] Embodiment two: IC of 5 kinds of compounds with antiviral activity 50 calculate
[0067] 1. Experimental materials
[0068] (1) Main reagents: the same as in Example 1.
[0069] (2) main instrument: with embodiment one.
[0070] 2. Experimental method
[0071] (1) Five compounds screened out in Example 1 and Zika virus were simultaneously treated with African green monkey kidney cells
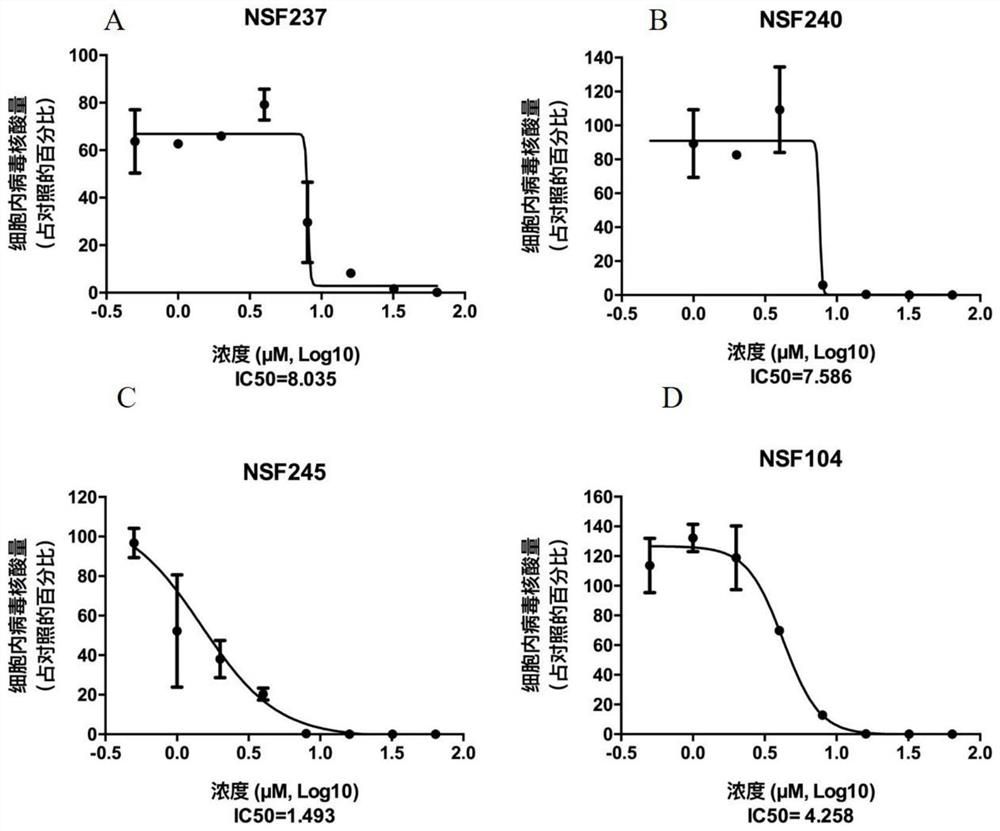
[0072] 1) 4μL 1×10 6 Zika virus (MOI=0.1) was mixed with five compounds of different doses (0, 0.5, 1, 2, 4, 8, 16, 32, 64 μ M) (see image 3 listed) mix well and process 4×10 at the same time 4 African green monkey kidney cells, the supernatant was discarded after 48 hours, and the cells were harvested.
[0073] (2) Intracellular RNA extraction and reverse transcription, real-time quantitative Q-PCR.
[0074] Concrete method is the same as embodiment one.
[0075] 3. Experimental results
[0076] The results were calculated by Graphpad software, the IC of 5 compounds including...
Embodiment 3
[0077] Embodiment 3: Cytotoxicity experiment
[0078] 1. Experimental materials
[0079] (1) Main reagent: CellTiter-Glo reagent (Promega)
[0080] (2) Main instrument: multifunctional microplate reader (Thermo)
[0081] 2. Experimental method
[0082] (1) 2×10 cells spread in 96-well plate 4 Vero cells were treated with five compounds at different concentrations (0, 0.5, 1, 2, 4, 8, 16, 32, 64 μM) for 2 days, and the cell viability was detected using CellTiter-Glo reagent (Promega): Add 100 μL of CellTiter-Glo detection reagent to each cell culture well, place it on a shaker at room temperature for 20 minutes, and use a white microwell luminescence quantifier to detect the fluorescence value.
[0083] 3. Experimental results
[0084] Such as Figure 4 Shown: except the compound NSF245 which has obvious cytotoxicity at a higher dose, the other three compounds have no obvious cytotoxicity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com