Charge modified lactoferrin and carrageenin combination medicine and preparation method thereof
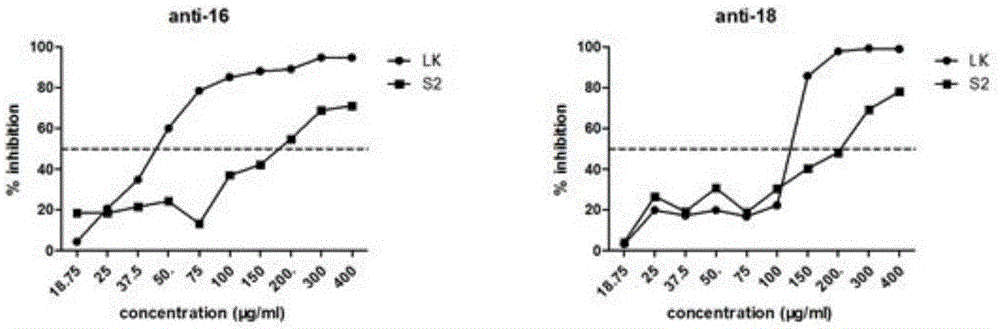
A technology of lactoferrin and charge modification, which is applied in the direction of drug combination, pharmaceutical formula, peptide/protein composition, etc., can solve the problems of high price, unquantitative research, and unclear curative effect, and achieve high negative conversion rate and good curative effect , Ease of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of charge-modified lactoferrin
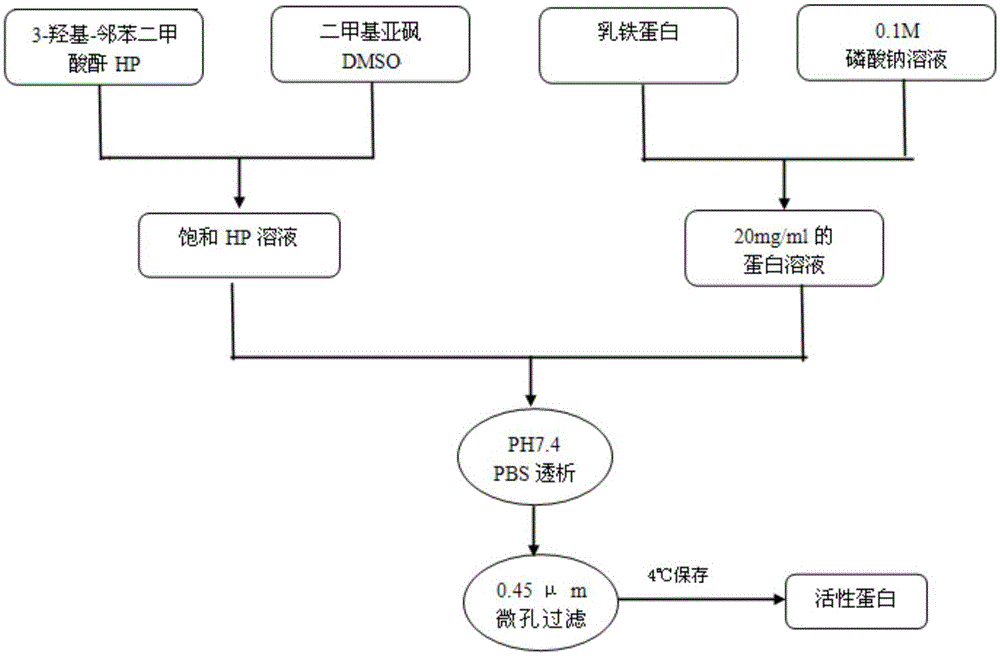
[0033] Put 1.65 (0.8-16.5) parts by weight of 3-hydroxy-phthalic anhydride (HP) in a 10mL small beaker, add an appropriate amount of dimethyl sulfoxide (DMSO), and stir at 200-500rpm in a magnetic stirrer, At the same time, DMSO was added dropwise to about 10 (5-100) parts by weight to prepare a saturated HP solution; under stirring at 200-500 rpm, 2 g of lactoferrin was dissolved in 100 mL of 0.1 M sodium phosphate buffer solution to prepare a 20 mg / mL solution. Protein solution; then take saturated HP solution and 20mg / mL protein solution and mix at a ratio of 1:100, stir for 15-30min to mix evenly, and adjust the pH to 10 with phosphoric acid or sodium phosphate, let it stand at 25°C for 1h, and use pH7.4 dialyzed with PBS, and sterilized by 0.45 μm membrane filtration to obtain charge-modified lactoferrin, specifically as figure 1 shown.
Embodiment 2
[0034] Embodiment 2: combination medicine
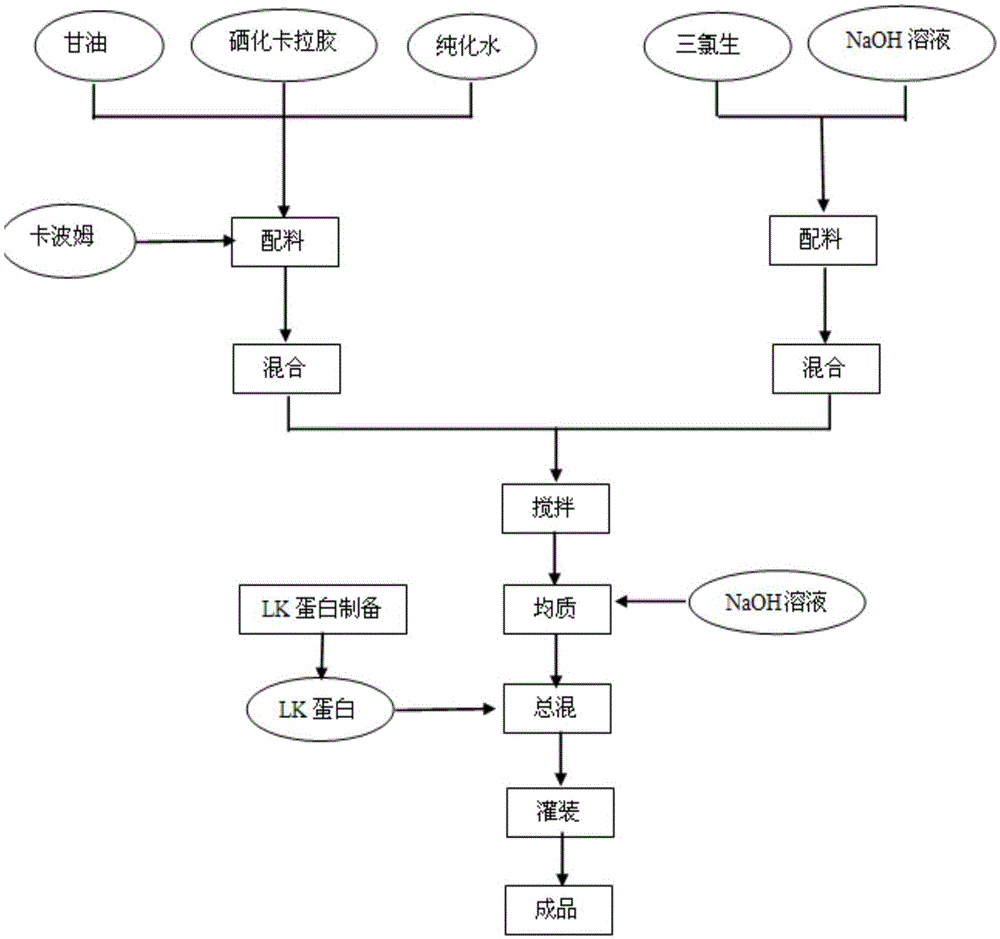
[0035] The combined medicine consists of 0.01 parts by weight of LK protein (charge-modified lactoferrin), 1.5 parts by weight of carrageenan and auxiliary materials, a total of 100 parts by weight. The auxiliary materials include 5 parts by weight of carbomer, 50 parts by weight of glycerin, 0.1 parts by weight of ethylparaben, the combined medicine is a kind of gel, suppository and dressing.
Embodiment 3
[0036] Embodiment 3: the preparation method of combination medicine
[0037] S1: Weigh each raw material according to the proportion, stir 0.5g carbomer in water at 80°C until fully swollen, add 0.15g carrageenan, stir until completely dissolved, and filter and sterilize through a 0.22μm membrane while hot to obtain 1 No. solution;
[0038] S2: Dissolve 0.01g of ethylparaben in water, then add 5g of glycerol, filter and sterilize through a 0.22μm membrane to obtain No. 2 solution;
[0039] S3: Add No. 2 solution to No. 1 solution under agitation and mix evenly, add 0.8 g of triethanolamine, adjust the pH to 5.5, and obtain No. 3 solution;
[0040] S4: Dissolve 0.001g of LK protein in an appropriate amount of water with low-speed stirring at room temperature, and filter and sterilize through a 0.22 μm membrane to obtain solution No. 4;
[0041] S5: under stirring at a certain temperature, add No. 4 solution into No. 3 solution and mix evenly, and adjust the pH to 5.5 with steri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com