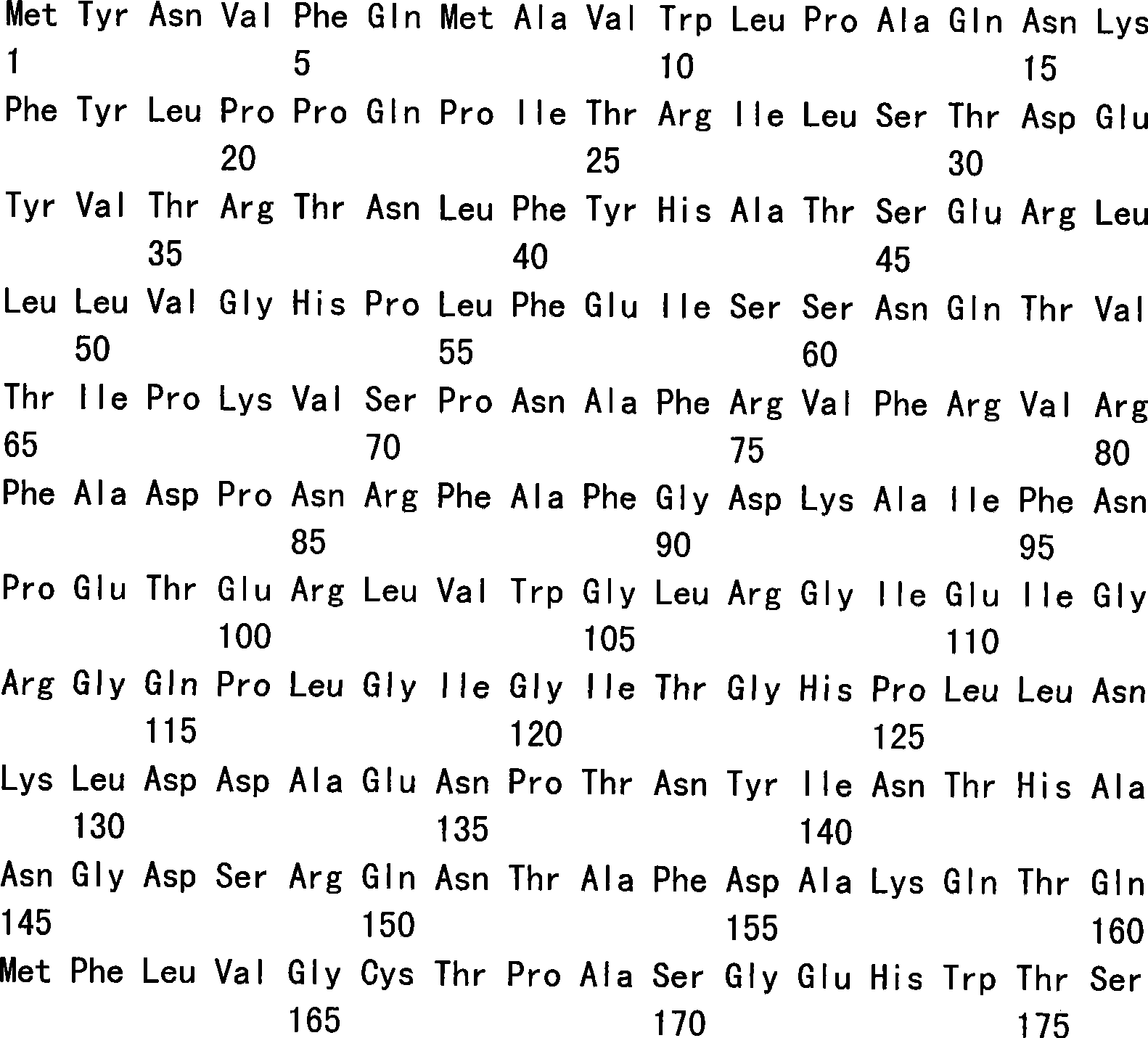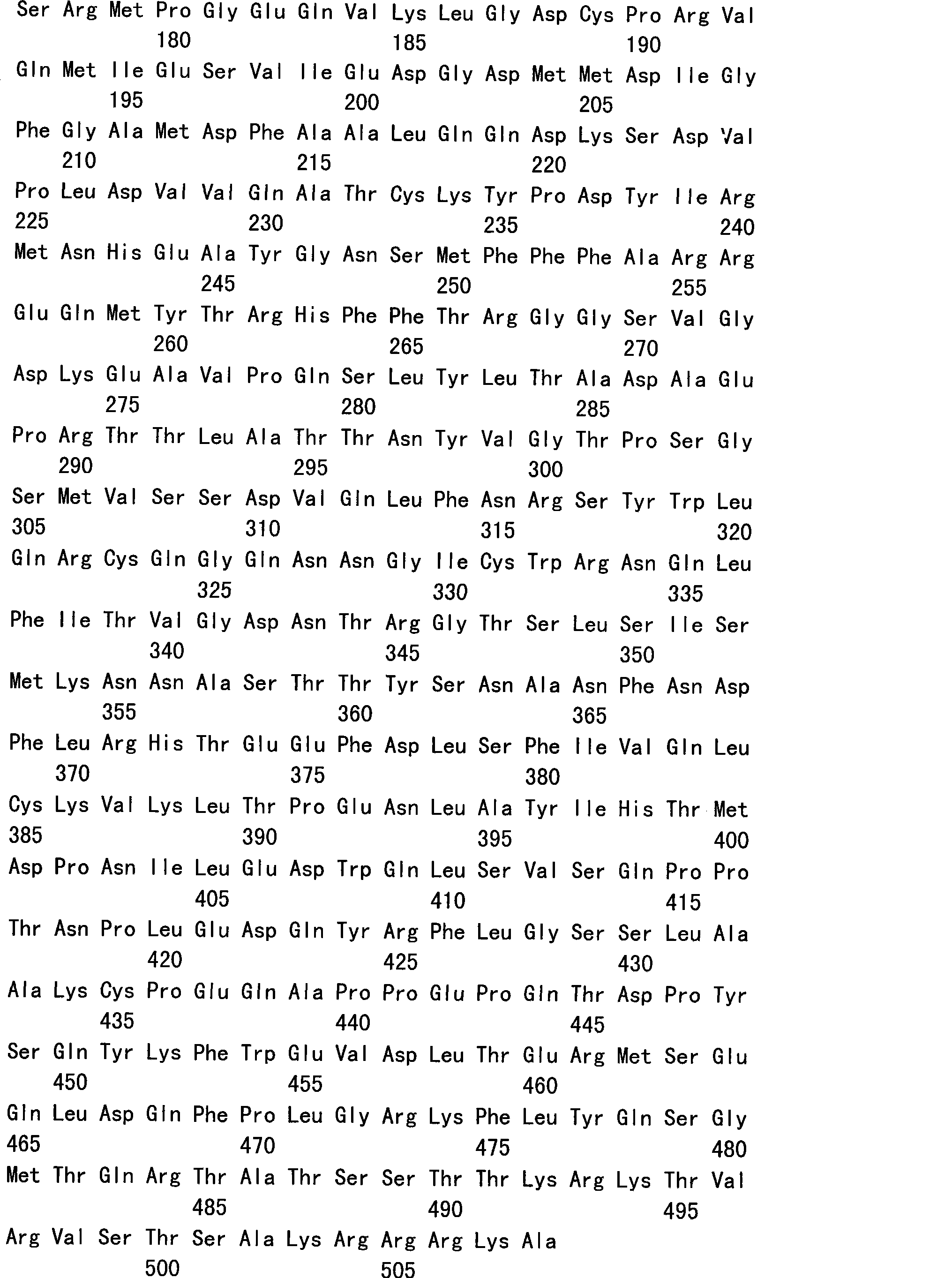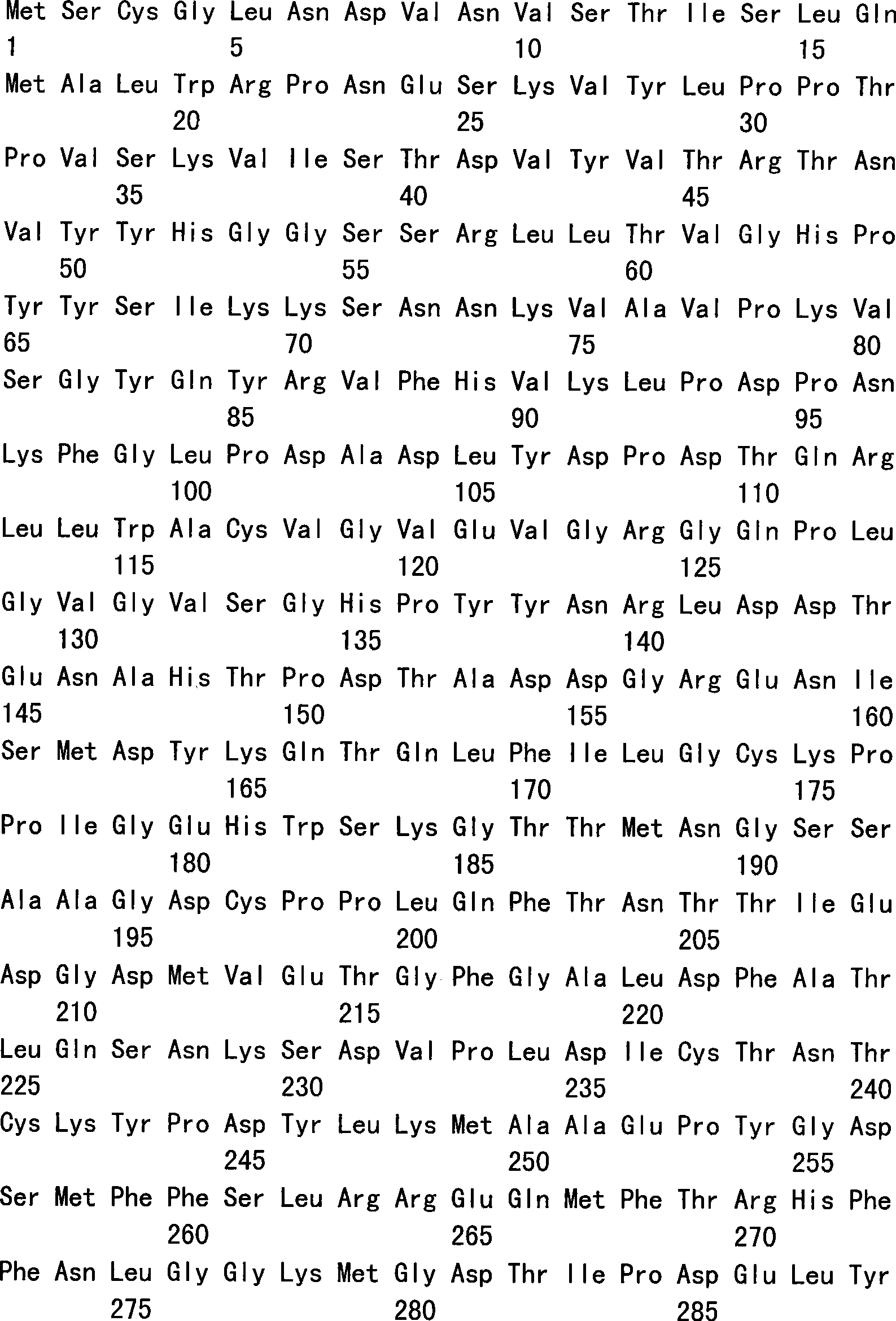Patents
Literature
236 results about "Papilloma virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
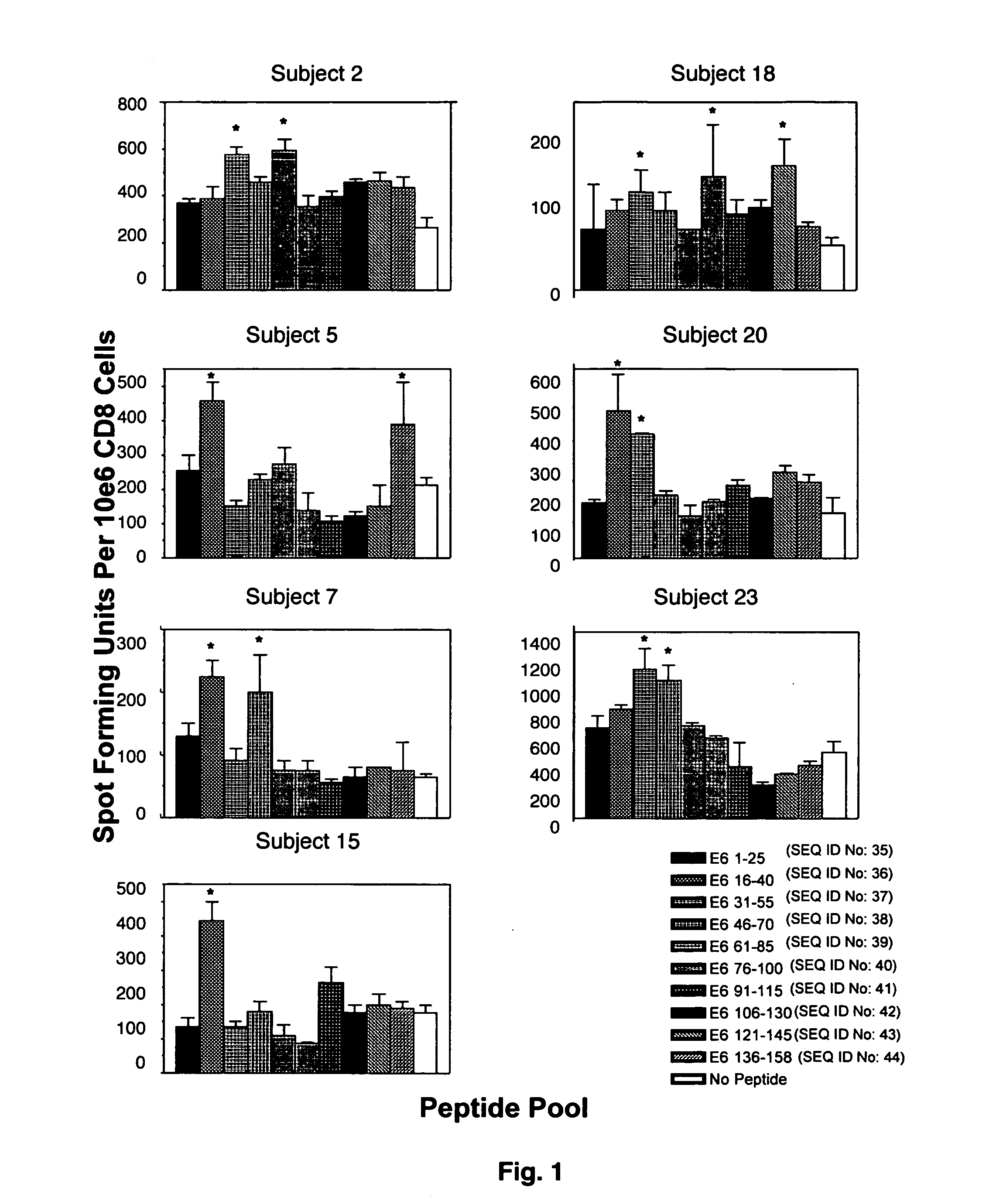
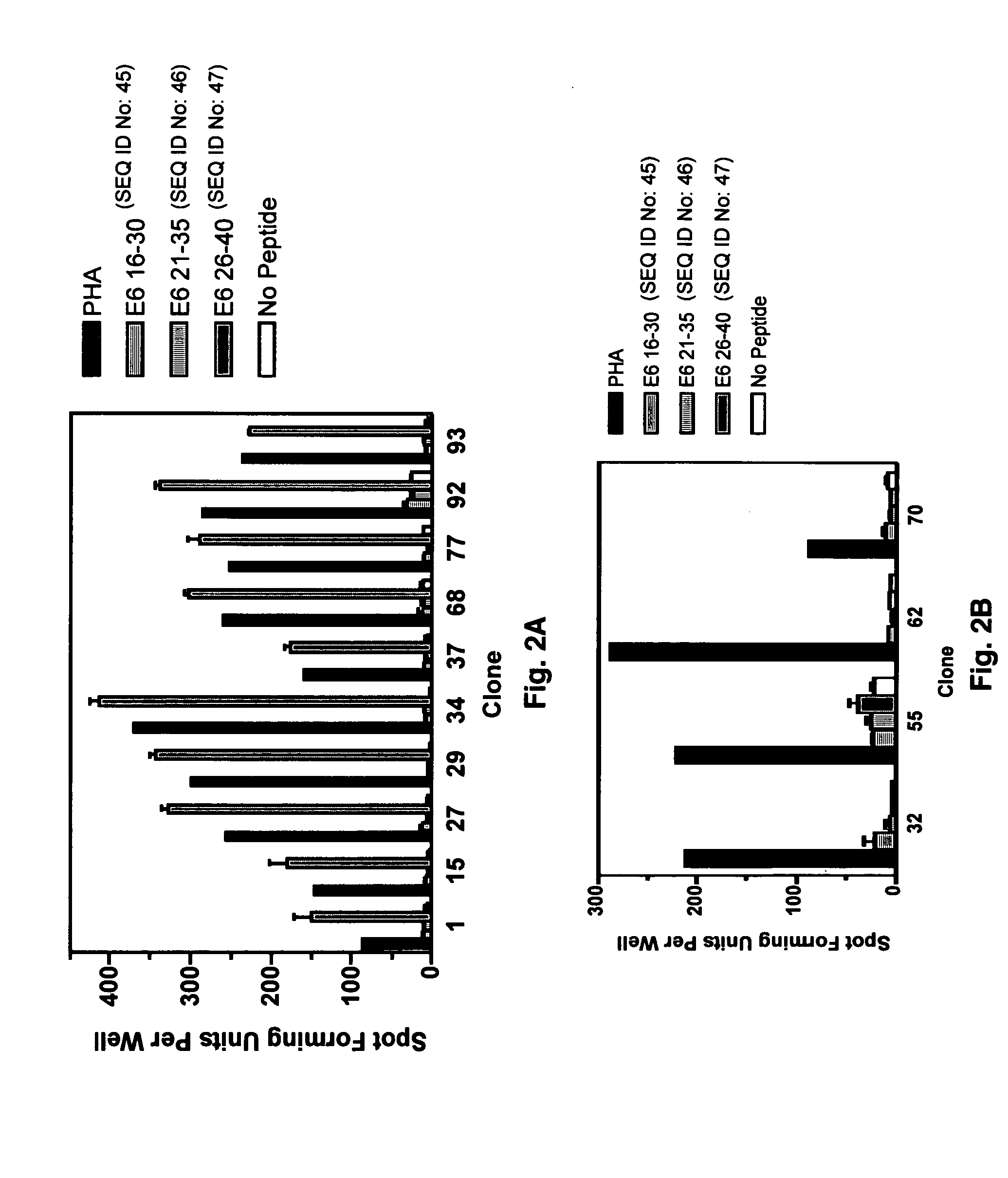
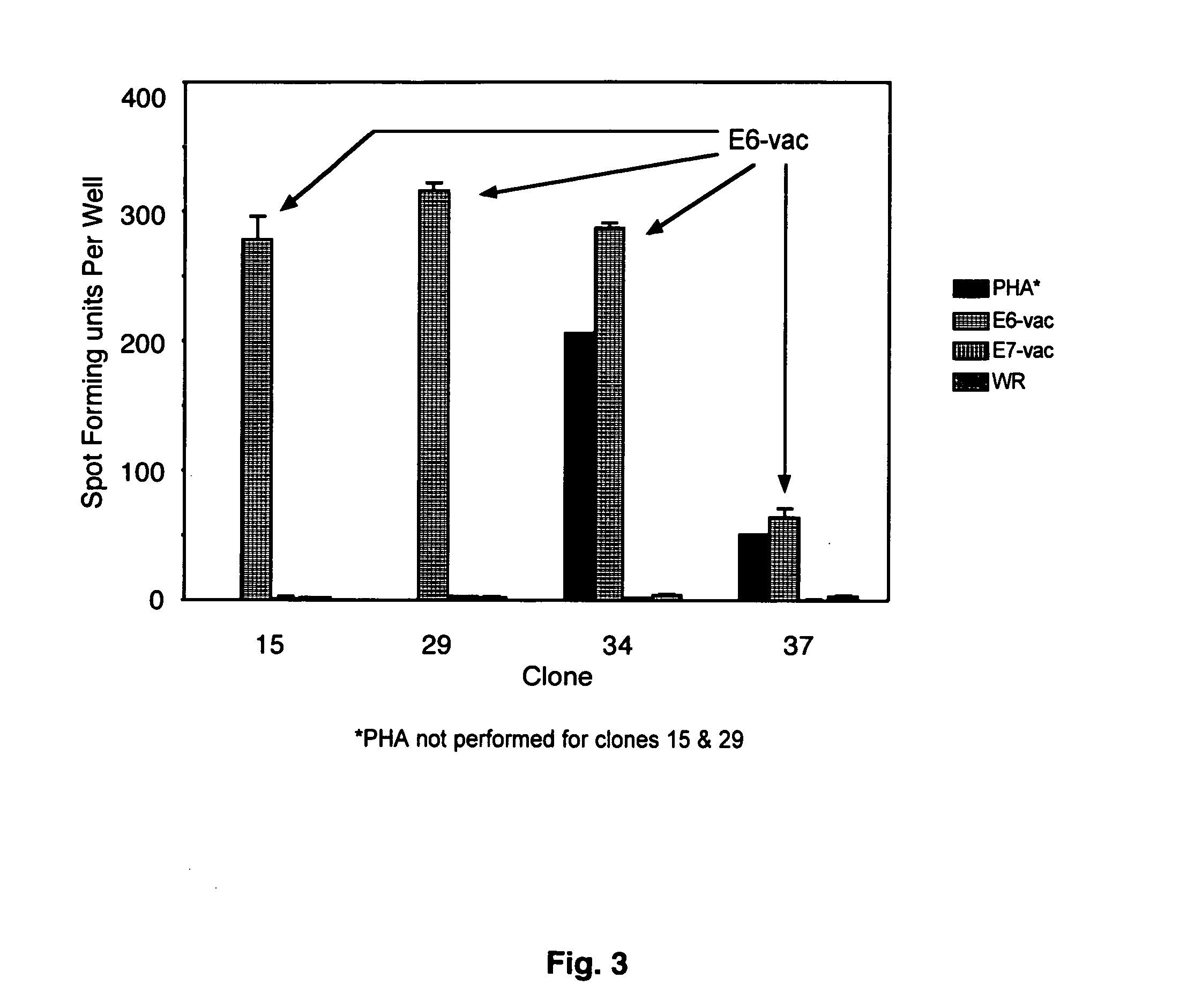
CD8 T cell epitopes in HPV 16 E6 and E7 proteins and uses thereof
InactiveUS20060182763A1Peptide/protein ingredientsViral antigen ingredientsHuman papillomavirusDendritic cell
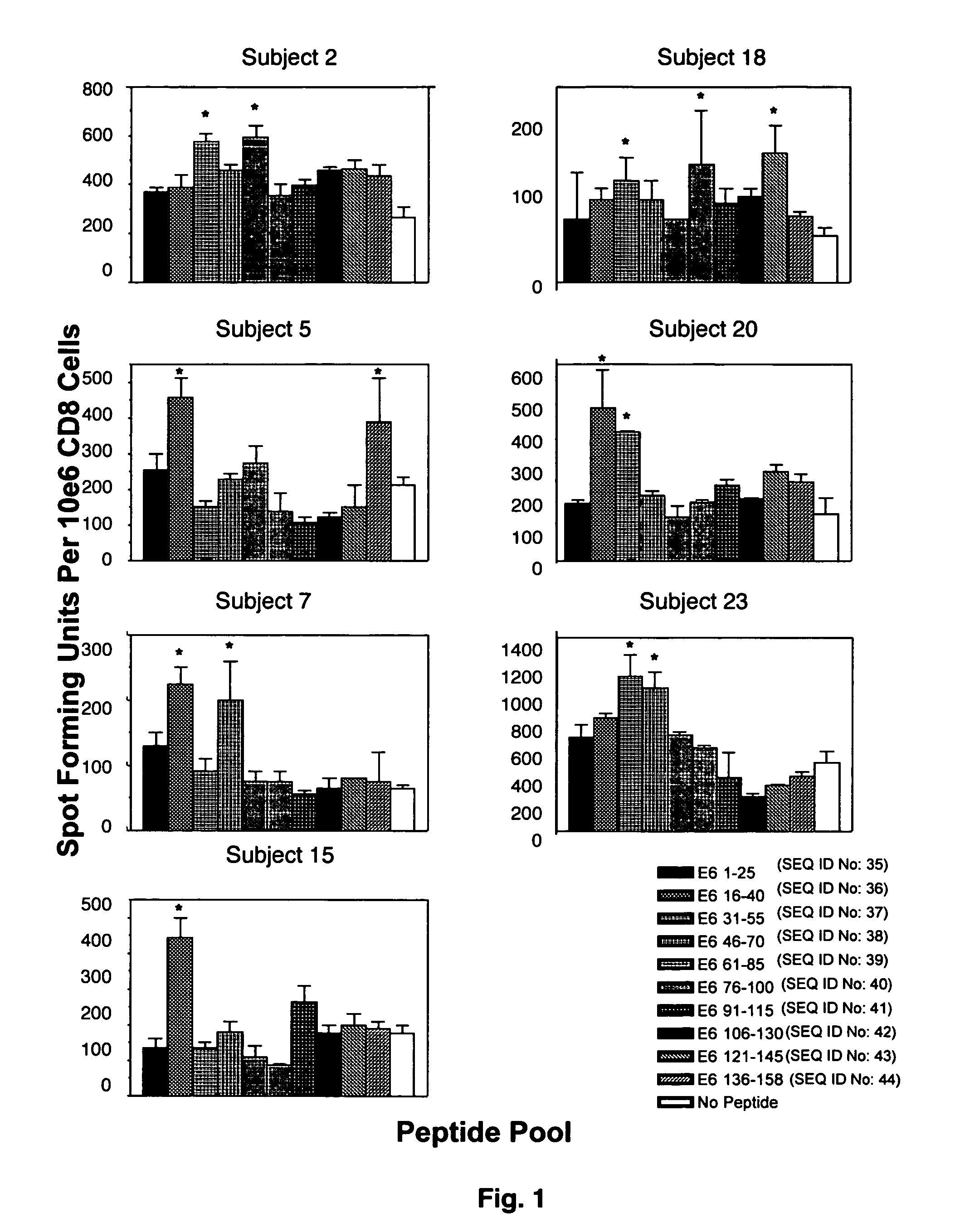
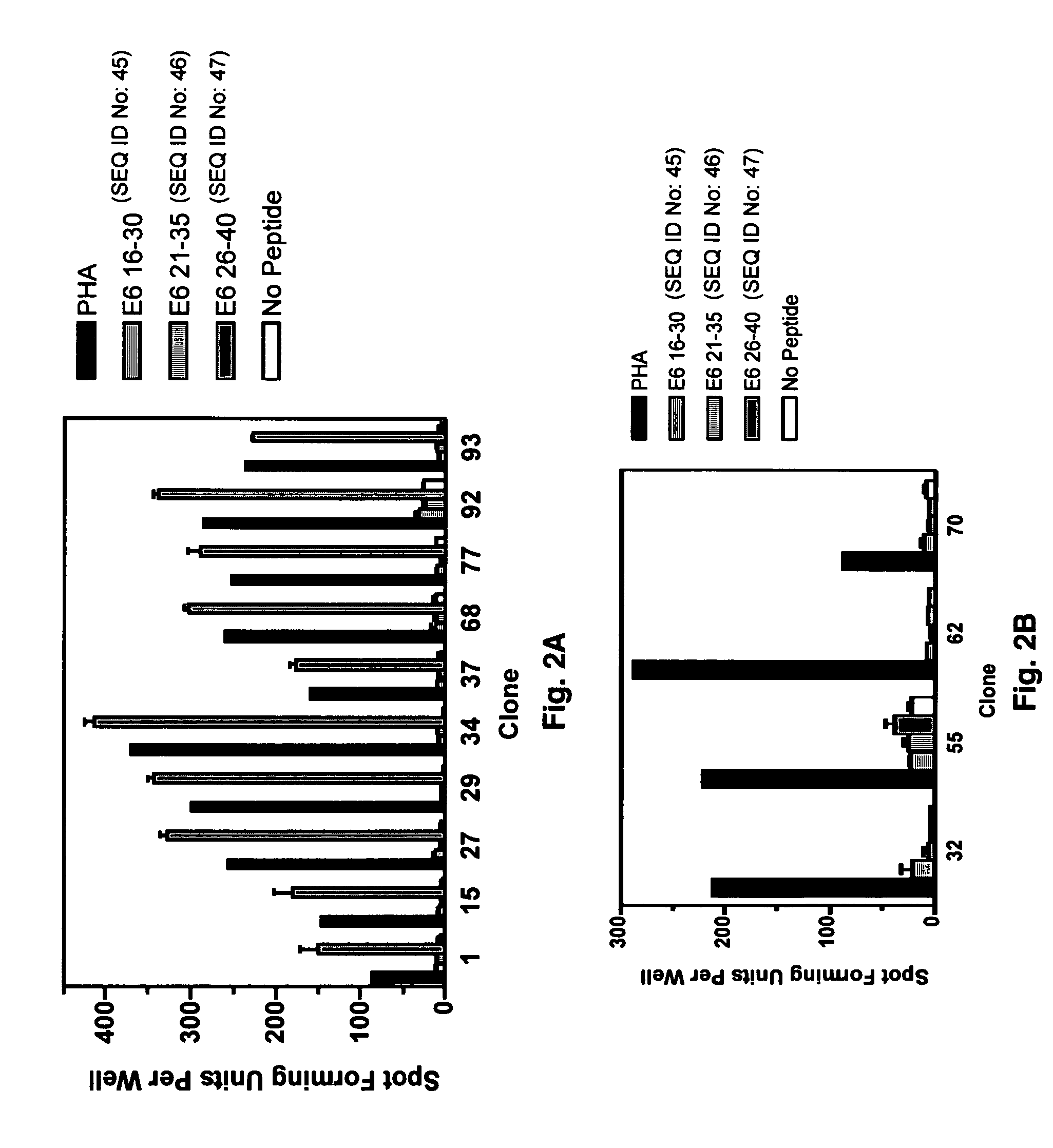
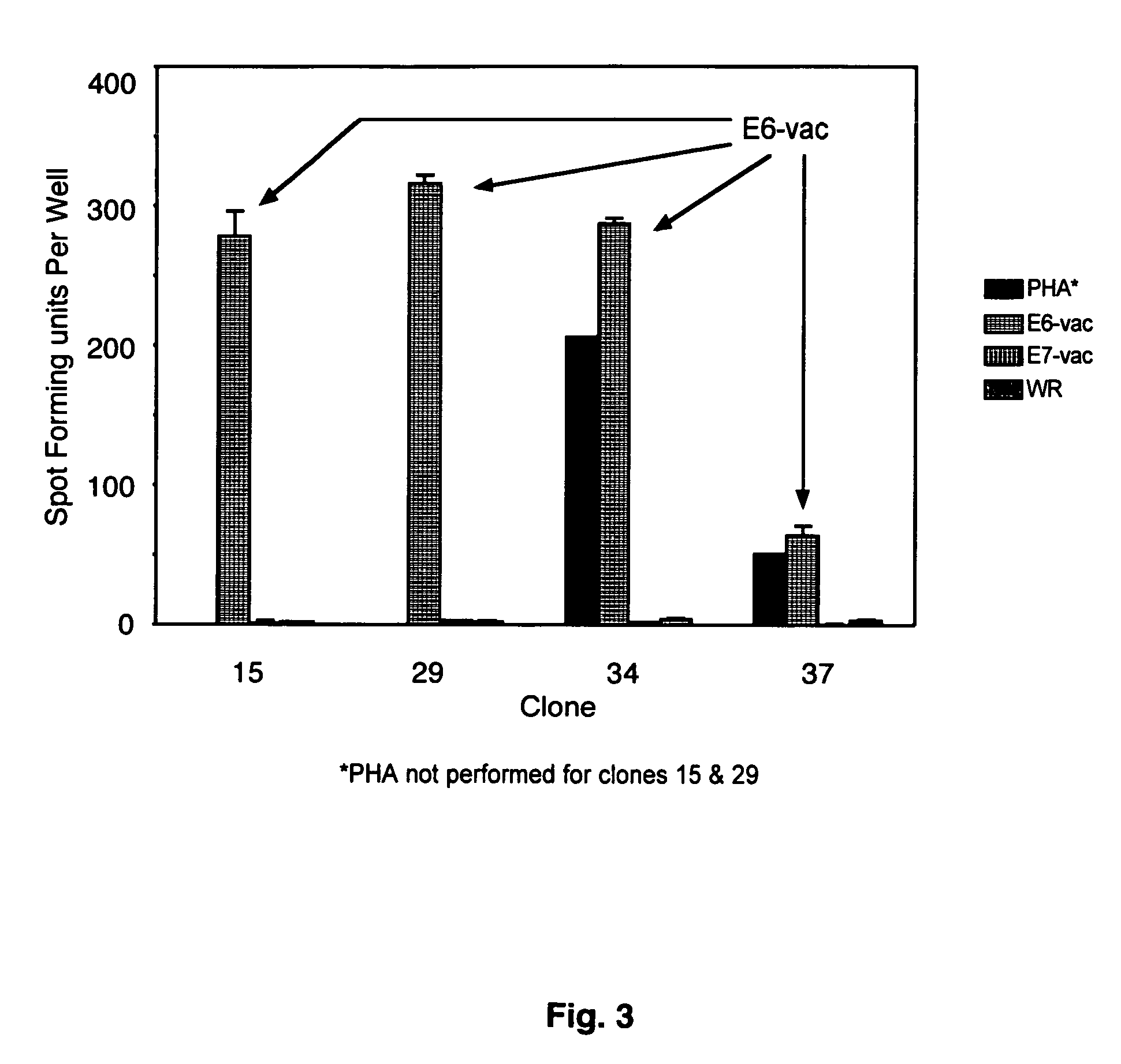
The present invention is directed to the examination of the pattern of immunodominant CD8 T cell epitopes in the E6 and E7 protein of Human Papillomavirus (HPV) and its further characterization in terms of its amino acid sequence and HLA restriction. These epitopes are identified based on their ability to induce strong CD8 T cell response and therefore, are important as sources of antigens for dendritic cell immunotherapy to treat cervical cancer. The present invention contemplates identifying a number of similar epitopes restricted by a wide variety of HLA types so that they can be used in concert to develop a preventative vaccine, which can be used for general population.
Owner:BIOVENTURES LLC
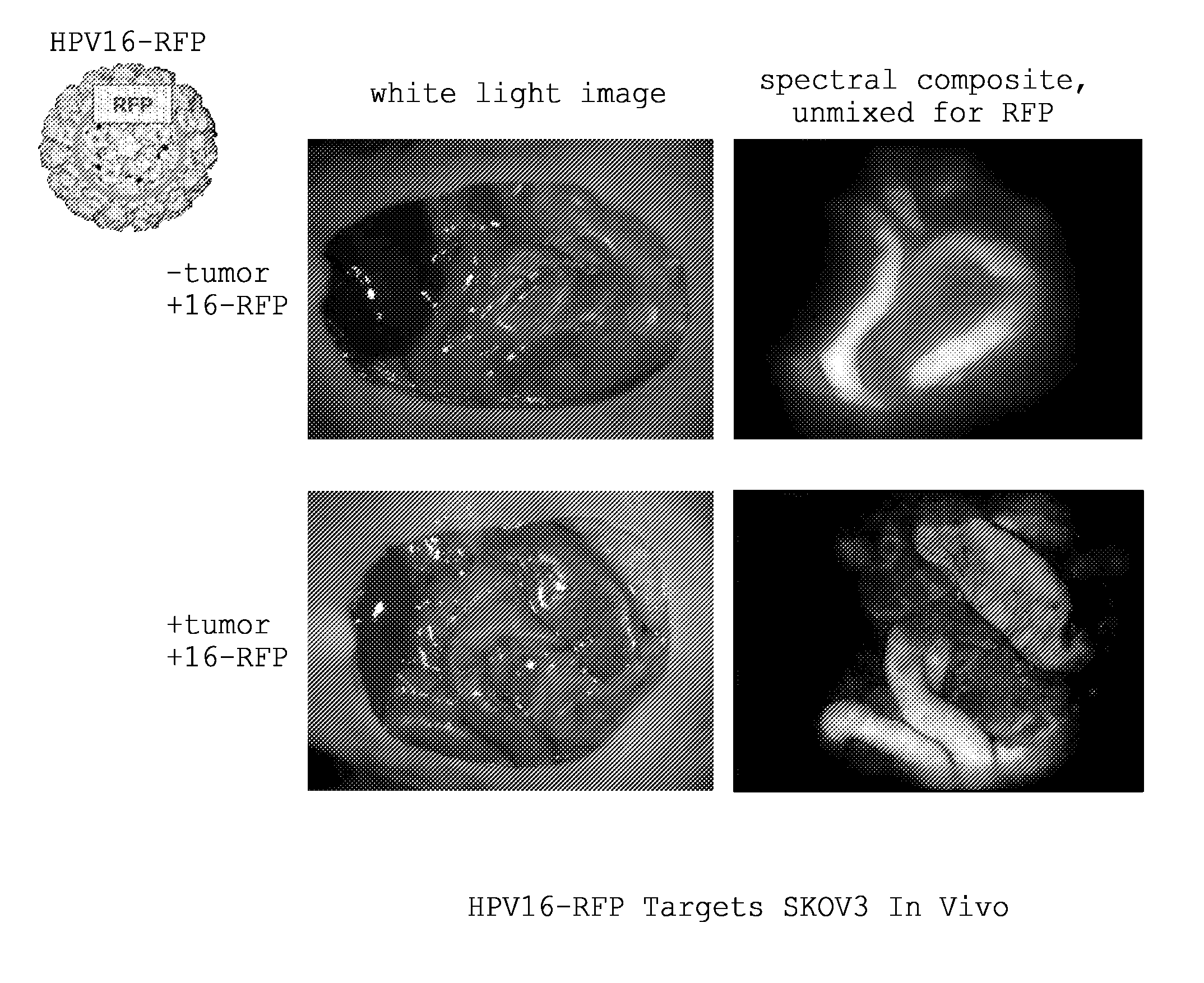
Papillomavirus pseudoviruses for detection and therapy of tumors
ActiveUS20100135902A1Ultrasonic/sonic/infrasonic diagnosticsCompounds screening/testingCancer cellCancer therapy
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Synthetic human papillomavirus genes
InactiveUS7001995B1Easy to insertEasy to removeBiocideGenetic material ingredientsPolynucleotide VaccinesHuman papillomavirus
Synthetic DNA molecules encoding papillomavirus proteins are provided. The codons of the synthetic molecules are codons preferred by the projected host cell. The synthetic molecules may be used as a polynucleotide vaccine which provides effective immunoprophylaxis against papillomavirus infection through stimulation of neutralizing antibody and cell-mediated immunity.
Owner:MERCK SHARP & DOHME CORP
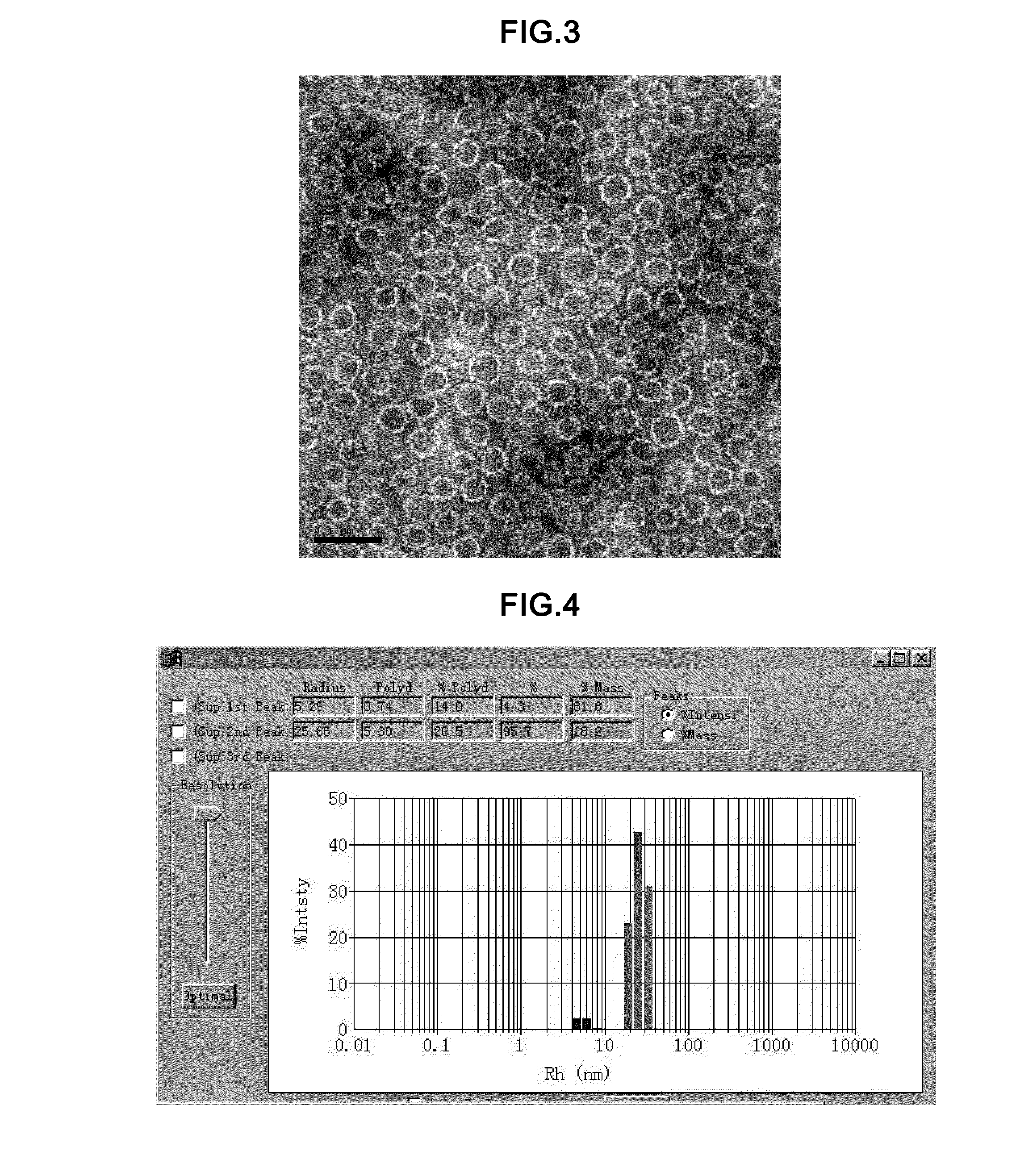
In vitro method for disassembly/reassembly of papillomavirus virus-like particles (VLPs)
InactiveUS6261765B1Stabilize VLPsHigh strength conditionNanotechMicroencapsulation basedEpitopeDiagnostic agent
A method of disassembly / reassembly of papillomavirus VLPs is provided. The resultant VLPs have enhanced homogeneity, present conformational, neutralizing PV epitopes, and therefore are useful prophylactic and diagnostic agents. Further, these VLPs can be used to encapsulate desired moieties, e.g., therapeutic or diagnostic agents, or "marker" DNAs, and the resultant VLPs used as in vivo delivery vehicles or as pseudovirions for evaluating vaccine efficacy.
Owner:MEDIMMUNE LLC
Methods of diagnosing cervical cancer
The invention provides reagents and methods for detecting pathogen infections in human samples. This detection utilizes specific proteins to detect the presence of pathogen proteins or abnormal expression of human proteins resulting from pathogen infections. Specific methods, compositions and kits are disclosed herein for the detection of oncogenic Human papillomavirus E6 proteins in clinical samples.
Owner:ARBOR VITA CORP
Papillomavirus pseudoviruses for detection and therapy of tumors
ActiveUS8394411B2Compounds screening/testingUltrasonic/sonic/infrasonic diagnosticsCancer cellCancer therapy
Owner:UNITED STATES OF AMERICA
Screening for papilloma viruses
InactiveUS7135281B2Microbiological testing/measurementImmunoglobulins against virusesHuman papilloma virus infectionMalignancy
The invention relates to a method of screening for precursor lesions which can lead to cervical malignancy, methods of detecting and typing human papilloma virus infections, and reagents of use in these methods.
Owner:MEDICAL RESEARCH COUNCIL
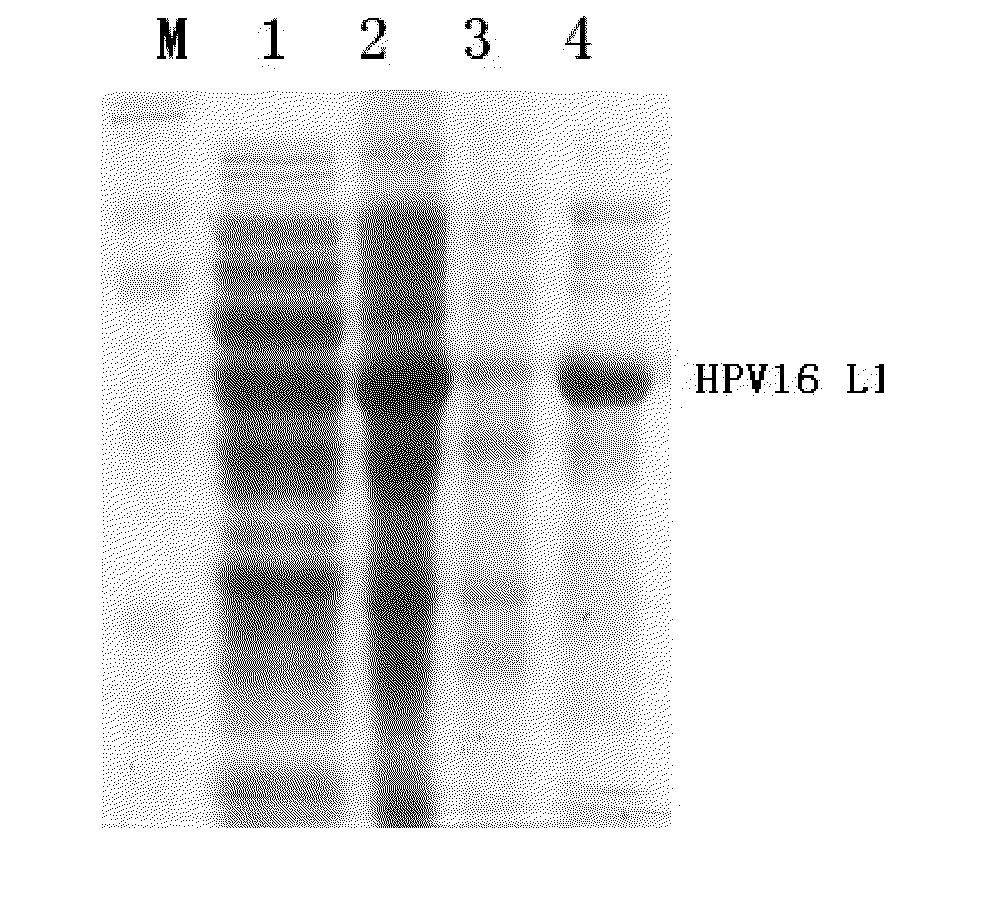
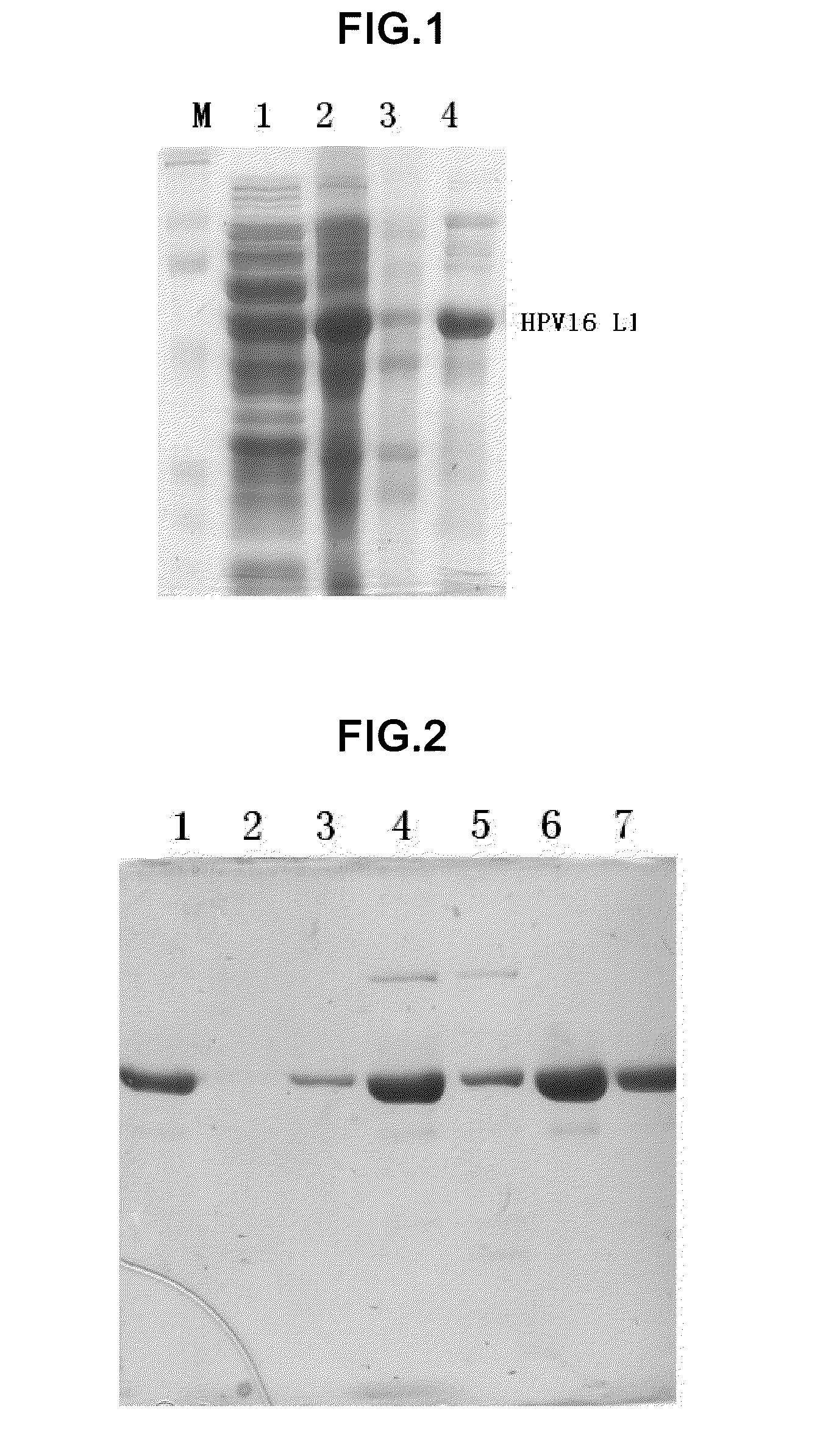
Shorten human papilloma virus 16 type L1 protein
The invention relates to a truncated human papillomavirus type 16 L1 protein, virus-like particles composed of the protein, a vaccine containing the virus-like particles, and an application thereof in preventing cervical cancer.
Owner:XIAMEN INNOVAX BIOTECH +1
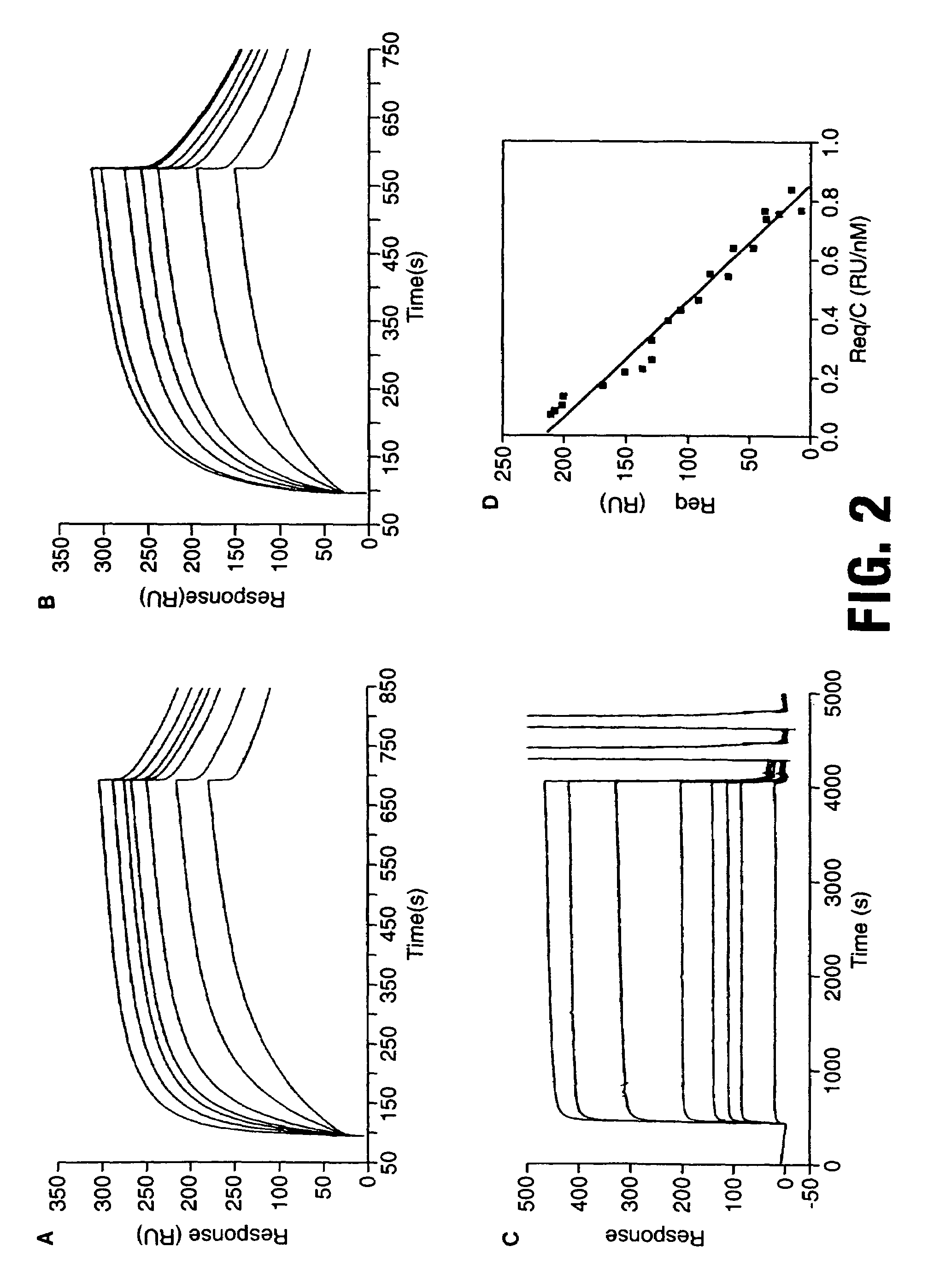
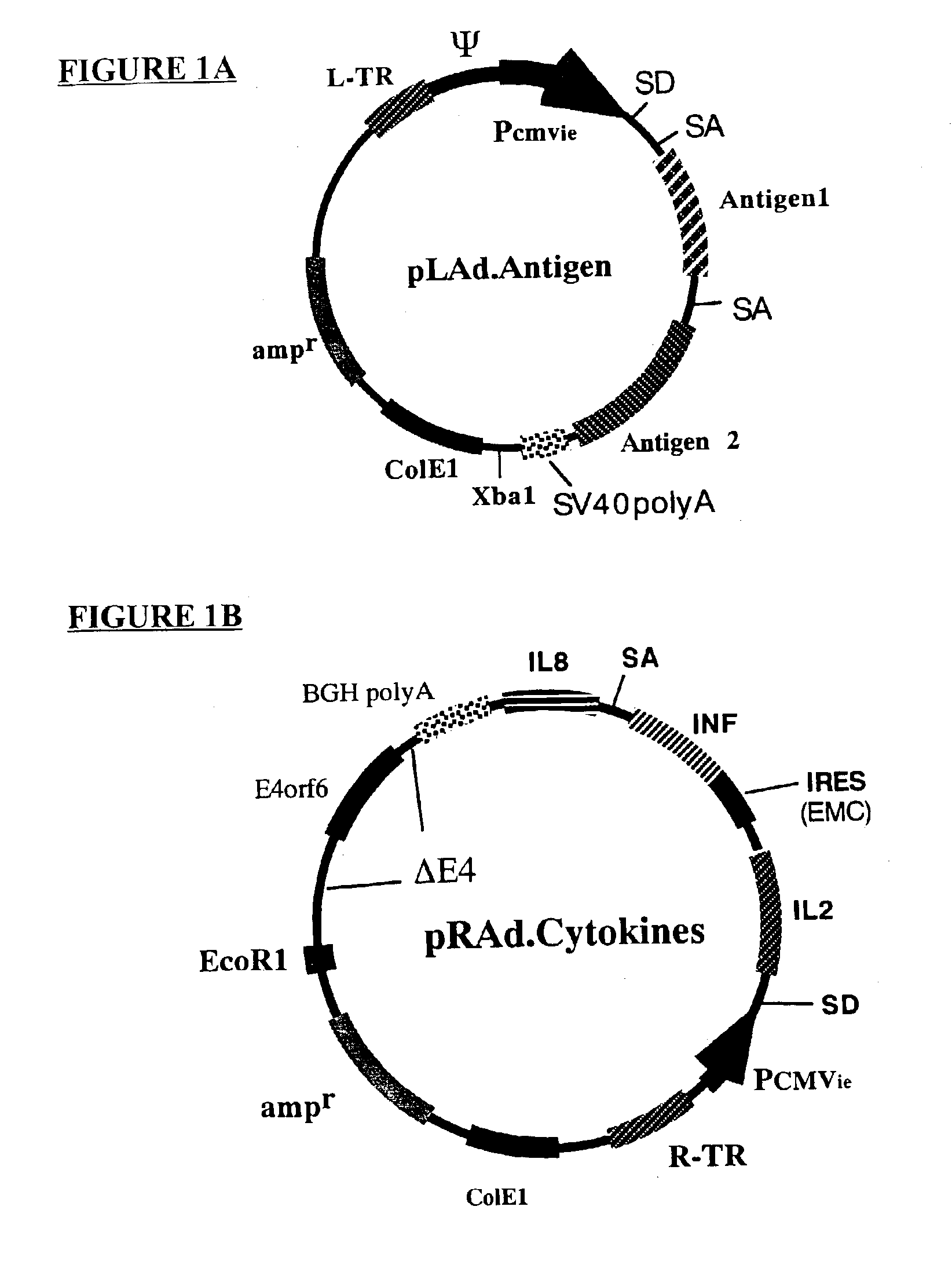
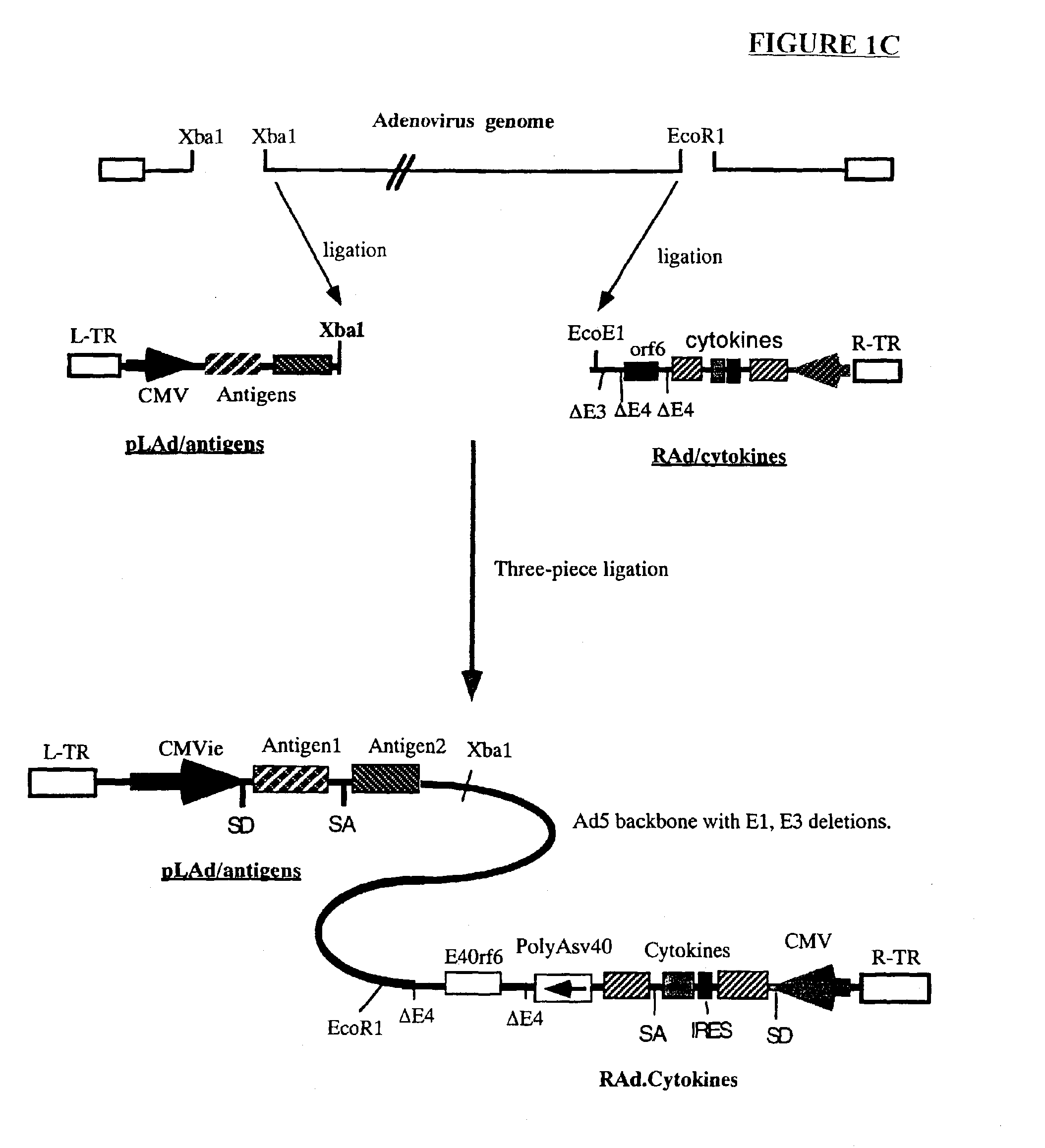
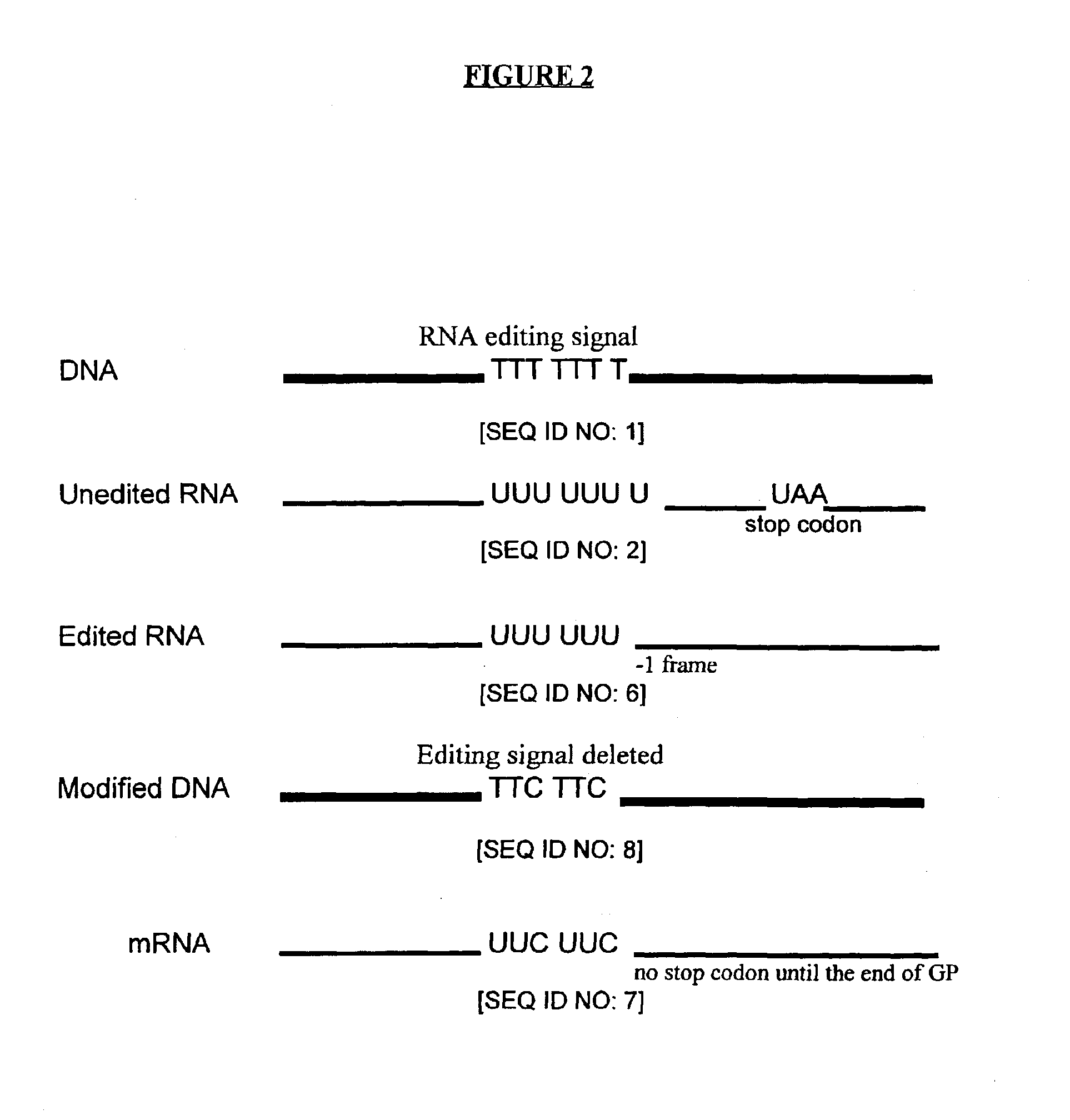
Composition and method for stimulating immune response to pathogen using complex adenoviral vector
InactiveUS6964762B2Improving immunogenicityStrong immune responseSsRNA viruses negative-senseAntibacterial agentsHeterologousProgenitor
Genetic vaccines and methods are provided for enhancing the immunity of a host such as a human to one or more pathogens. In one aspect, a method of enhancing the immunity of a host to a pathogen is provided. The method comprises administering to the host a recombinant virus comprising an antigen sequence that is heterologous to a native progenitor of the recombinant adenovirus and encodes a viral antigen from a pathogenic virus, expression of which is under the transcriptional control of a first promoter; and a cytokine sequence that is heterologous to the native progenitor of the recombinant adenovirus and encodes a cytokine, expression of which is under the transcriptional control of a second promoter. Expression of the antigen and cytokine sequences elicits an immune response directed against the viral antigen upon infection of the host by the recombinant virus. The method can be used for immunizing a host against a wide variety of pathogen viruses, such as HIV, Ebola virus, Marburg virus, hepatitis B virus, hepatitis C virus, influenza virus, human simplex virus, human papilloma virus and respiratory syncytial virus.
Owner:GENPHAR INC
Papilloma virus-like particles, fusion proteins as well as processes for their production
InactiveUS20060153864A1Peptide/protein ingredientsAntibody mimetics/scaffoldsPurification methodsProphylactic vaccination
The invention relates to the recombinant production of proteins as well as VLPs which are suitable as a vaccine for therapeutic and prophylactic vaccination. The invention also relates to processes for the production and purification of recombinant papilloma virus proteins and fusion proteins.
Owner:LOYOLA UNIV OF CHICAGO
Human papilloma virus 18 L1 (HPV18L1) polynucleotide sequence and its expression vector, host cell and use
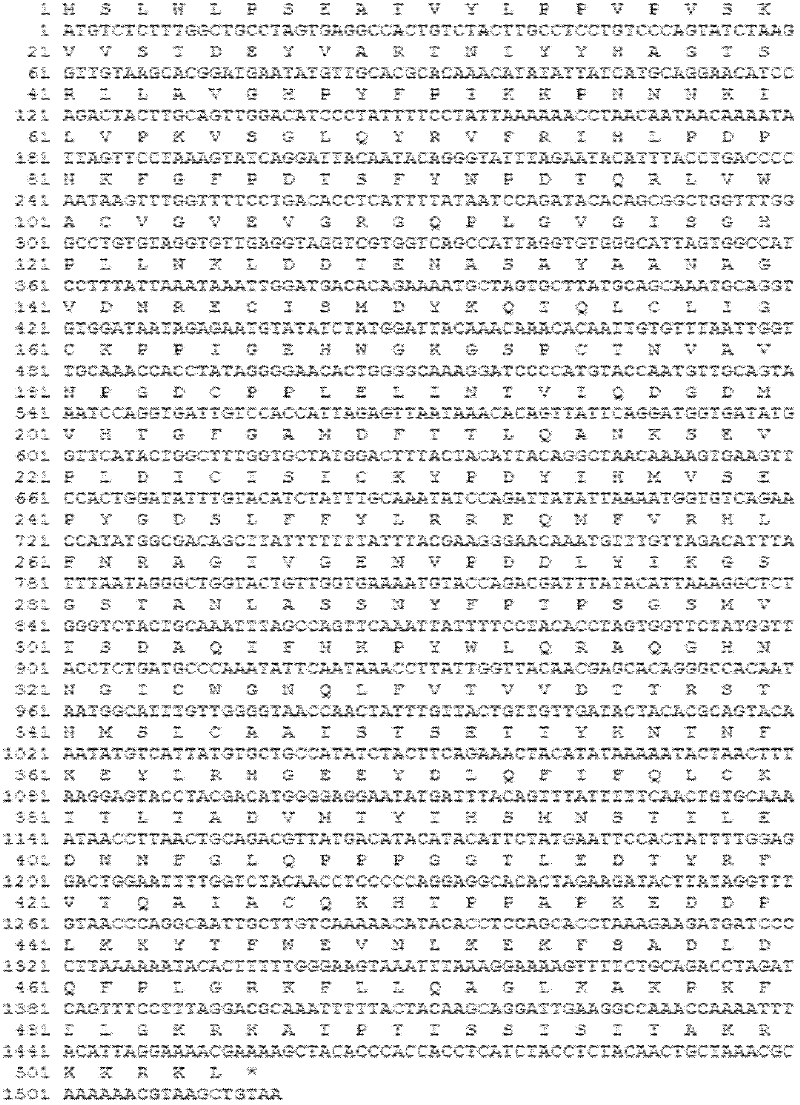
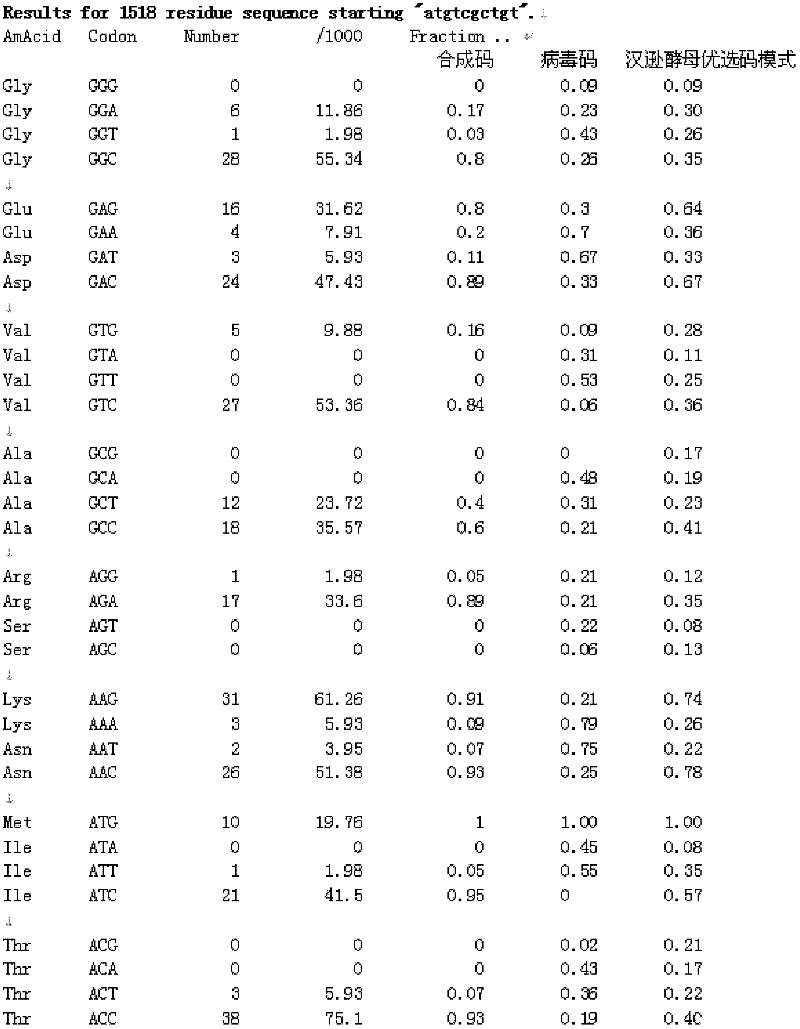
ActiveCN102719453AHigh yieldLow costFungiViral antigen ingredientsSynthetic nucleotideNucleotide sequencing
The invention relates to a human papilloma virus 18 L1 (HPV18L1) polynucleotide sequence and its expression vector, host cell and use and especially relates to an amino acid sequence of an HPV L1 capsid protein, a synthetic nucleotide sequence for coding the amino acid sequence, a recombinant expression vector containing the synthetic nucleotide sequence, and a hansenula polymorpha expression host strain containing the synthetic nucleotide sequence. The invention also relates to a use of an HPV18L1 protein composed of the amino acid sequence and derivatives of the amino acid sequence in preparation of vaccines. Through modification of a nucleotide sequence of a gene of an HPV18L1 wide-type virus, a recombinant HPV18L1 capsid protein can be highly expressed in a hansenula polymorpha expression system and thus hansenula polymorpha expression system-based industrial production of an HPV18L1 capsid protein is realized. Compared with the existing eukaryotic expression systems, the HPV18L1 polynucleotide sequence and its expression vector and host cell have advantages of higher yield and lower cost.
Owner:天津昕因达生物技术有限公司
Human papillomavirus shell protein L1 short peptide and application thereof
InactiveCN101186636ASerum immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsAlphapapillomavirusCoat protein
The invention relates to a polypeptide, in particular to a human papilloma virus coat protein L1 short peptide and relative application. The invention is characterized in that the sequence of the human papilloma virus coat protein L1 short peptide is N-EVNLKEKFSADLDQFPLGRKFLLQAGLKAK-C. The invention can simultaneously induce human papilloma virus coat protein L1 short peptide with immunity reaction on high risk type and low risk type, which can induce and form the antibody for various human papilloma virus (HPV) and coat protein (HPV L1), to check various HPV or HPV L1, while the antibody can be used in biological pharmaceutical engineering, to purify and prepare various HPV or HPV L1.
Owner:CHINA THREE GORGES UNIV
Methods for detecting papillomavirus DNA in blood plasma and serum
InactiveUS7183053B2Assess prognosisAntibody mimetics/scaffoldsMicrobiological testing/measurementDiseaseAlphapapillomavirus
This invention relates to the detection of extracellular papillomavirus DNA in blood plasma or serum from a human or animal. In particular, the invention relates to the detection, identification, evaluation, or monitoring of neoplastic, premalignant or malignant disease associated with a papillomavirus. The invention thereby provides methods for the identification of individuals at risk for, or having, cervical dysplasia, cervical intraepithelial neoplasia, or cervical cancer.
Owner:PENN STATE RES FOUND
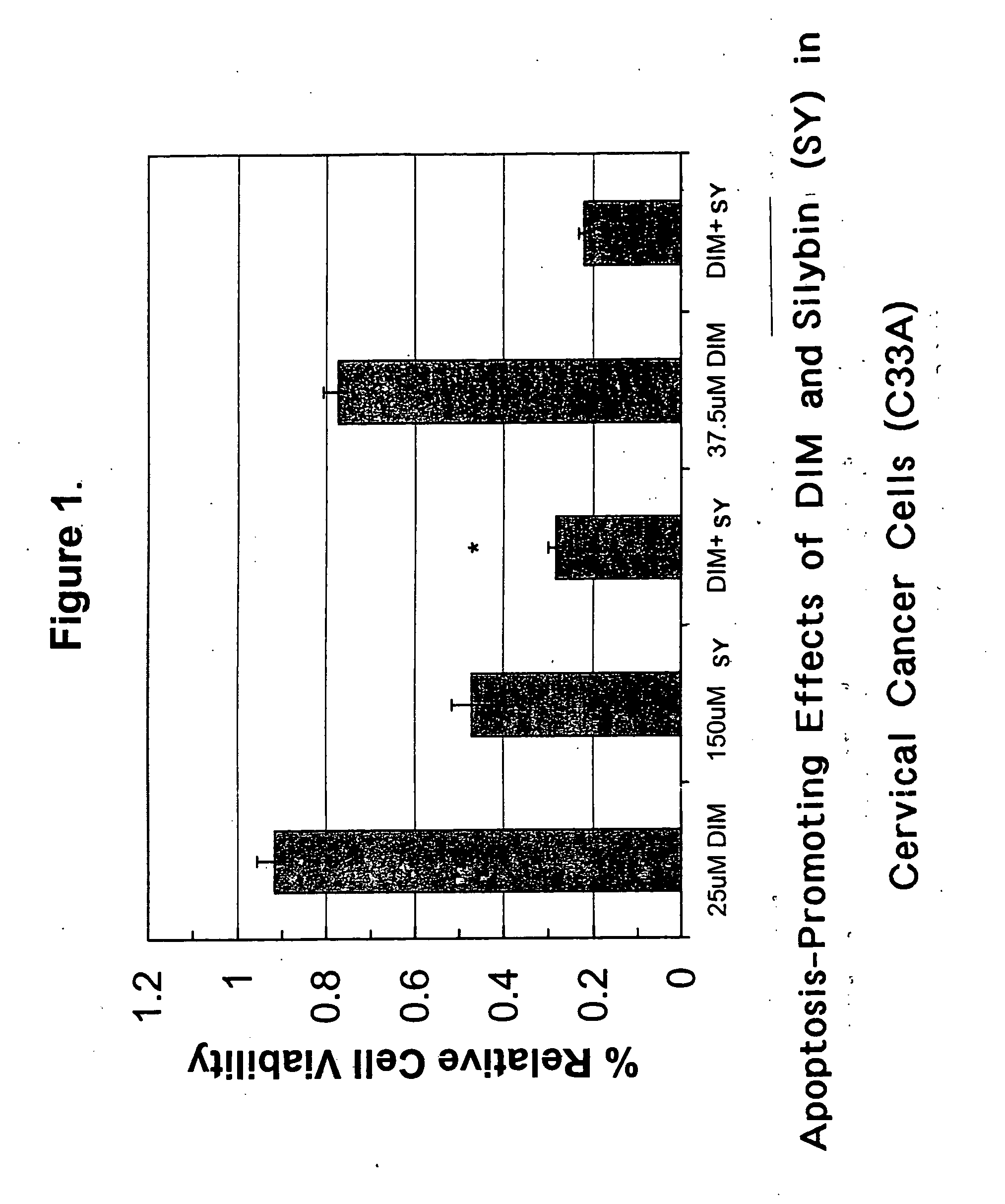
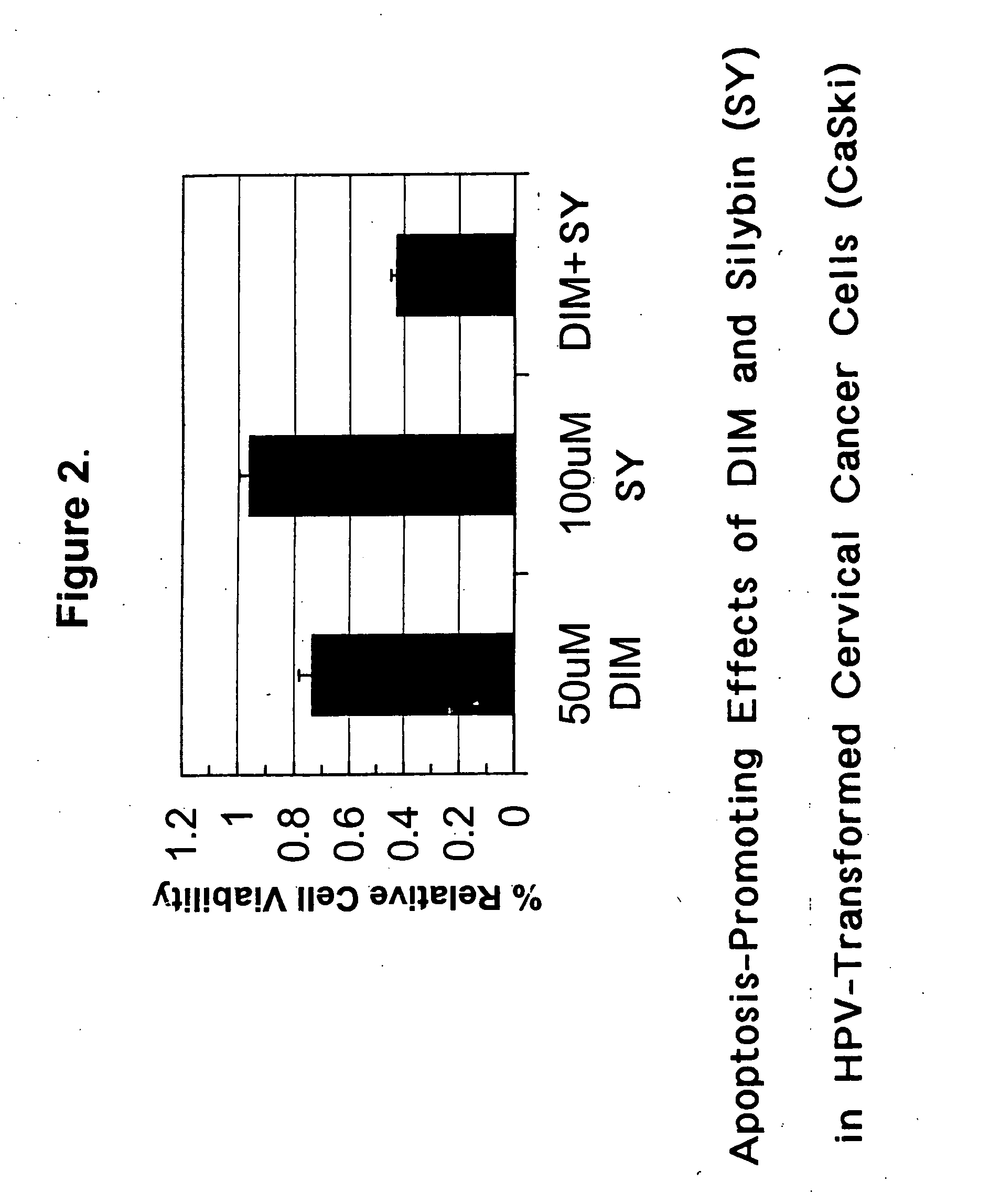
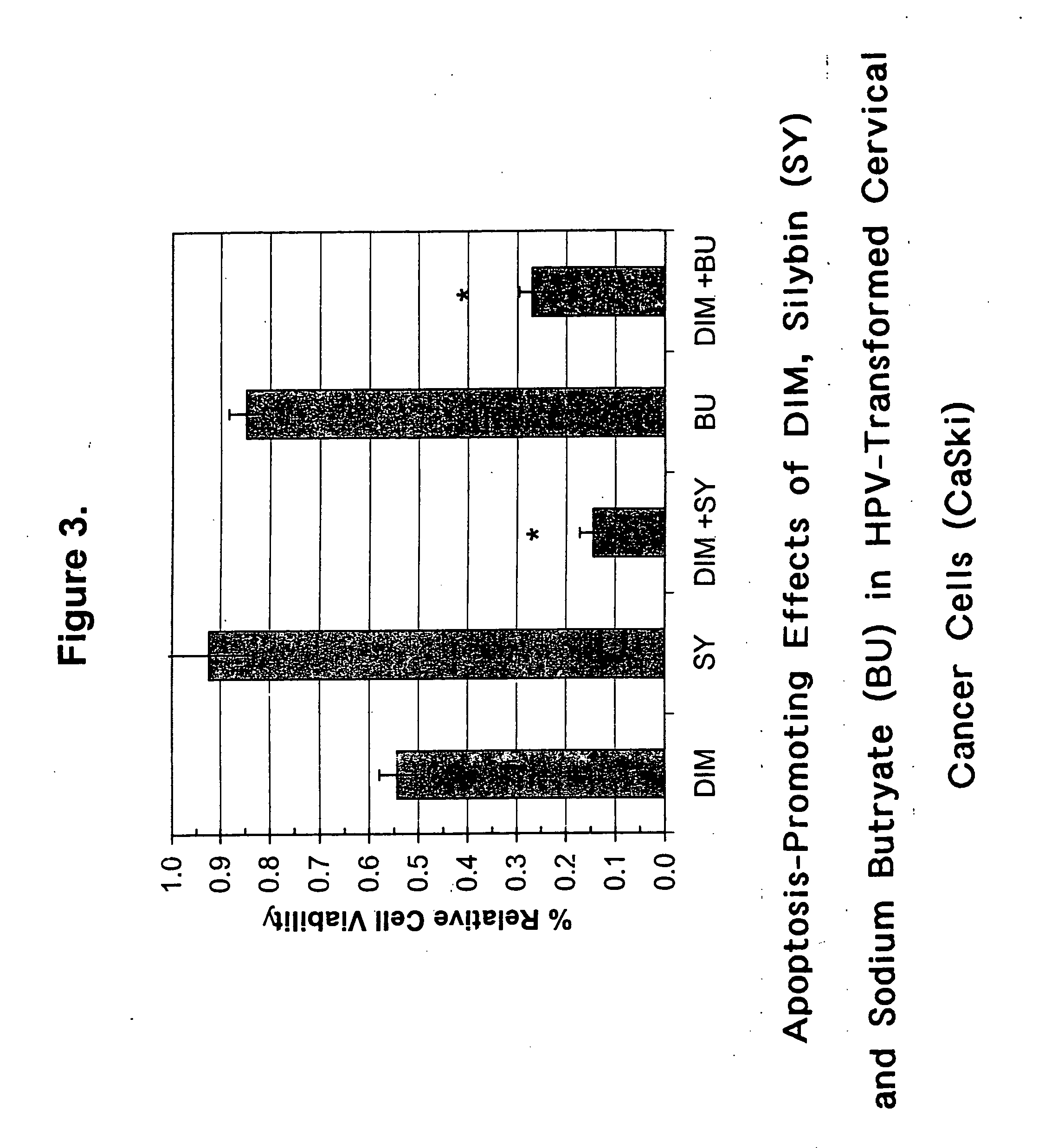
Combined use of cruciferous indoles and chelators for the treatment of papilloma virus-related conditions
InactiveUS20050063903A1Reduce the amount requiredBiocideHeavy metal active ingredientsYarnNylon material
This abstract describes eliminating the need for cutting up manufactured synthetic filament polymer yarns (for examples, nylon and Kevlar) into wool and linen lengths and respinning these again into yarns approaching wool and linen like wear properties in clothing and other textiles at significant reductions in production costs accomplished by using laser pierced holes in the plates of and adding continuous wave or pulsed sonic generators to the rear of spinneret housings through which viscous polymer fluids flow both of which produce surface irregularities in the yarns (longitudinal and circumferential ridges in valleys) in the spun continuous filaments approaching wear properties of natural wool and linen.
Owner:BIORESPONSE
HPV16L1 polynucleotide sequence and expression vector, host cell and application thereof
InactiveCN102586287AHigh yieldLow costFungiViral antigen ingredientsHuman papillomavirusSynthetic nucleotide
The invention relates to an HPV16L1 polynucleotide sequence, and an expression vector, a host cell and application of the HPV16L1 polynucleotide sequence. The HPV16L1 polynucleotide sequence comprises an amino acid sequence of recombinant human papillomavirus (HPV) L1 capsid protein, a synthetic nucleotide sequence coding the amino acid sequence, and a recombinant expression vector and a hansenula polymorpha expression host strain comprising the nucleotide sequence. The invention also relates to the application of HPV16L1 protein consisting of the amino acid sequence and derivatives of the HPV16L1 polynucleotide sequence in preparing vaccine. According to the invention, by transforming the nucleotide sequence of HPV16L1 wild type virogene, the recombinant HPV16L1 capsid protein in a hansenula polymorpha system is efficiently expressed, and the HPV16L1 capsid protein can be industrially produced by using the hansenula polymorpha expression system; and compared with the conventional other eukaryotic expression systems, the hansenula polymorpha expression system has the advantages of high yield, low cost and the like.
Owner:王昌华
Nucleic acid detection kit for human papilloma virus, use method and application thereof
ActiveCN105506173AAvoid false negativesIncrease throughputMicrobiological testing/measurementMicroorganism based processesLower riskFluorescence
The invention discloses a nucleic acid detection kit for human papilloma virus, a use method and an application thereof. The kit includes a nucleic acid amplification reagent comprising a primer pair and a probe corresponding to the primer pair. The use method of the kit includes the following steps: 1) extracting nucleic acids from a sample; 2) preparing the reagent; 3) performing PCR amplification; and 4) performing fluorescent detection to a PCR amplification reaction product at 65-72 DEG C, and determining infection type of the HPV according to the changes on the Ct value of a target amplification curve and the Ct value of an internal standard amplification curve. The kit can be used for detecting low-risk and high-risk human papilloma virus and for typing the human papilloma virus. The kit can be used for calculating relative viral load of the HPV, can avoid pollution, can increase treatment throughput of samples, and can avoid missing detection.
Owner:SUZHOU SYM BIO LIFESCI CO LTD
CD8 T cell epitopes in HPV 16 E6 and E7 proteins and uses thereof
The present invention is directed to the examination of the pattern of immunodominant CD8 T cell epitopes in the E6 and E7 protein of Human Papillomavirus (HPV) and its further characterization in terms of its amino acid sequence and HLA restriction. These epitopes are identified based on their ability to induce strong CD8 T cell response and therefore, are important as sources of antigens for dendritic cell immunotherapy to treat cervical cancer. The present invention contemplates identifying a number of similar epitopes restricted by a wide variety of HLA types so that they can be used in concert to develop a preventative vaccine, which can be used for general population.
Owner:BIOVENTURES LLC
Truncated l1 protein of human papillomavirus type 11
ActiveUS20100291141A1Reduced expression levelLow costVirus peptidesAntiviralsVirus-like particleAlphapapillomavirus
The invention relates to a truncated L1 protein of the Human Papillomavirus Type 11, a virus-like particle consisting of the protein, a vaccine comprising said virus-like particle, and the use of the vaccine in the prevention of condyloma acuminatum or HPV infections.
Owner:XIAMEN INNOVAX BIOTECH +1
Truncated human papilloma virus 18 type L1 protein
The invention relates to a truncated human papilloma virus 18 type L1 protein, and viruslike particles consisting of the truncated human papilloma virus 18 type L1 protein, vaccine containing the viruslike particles and the application thereof in preventing cervical cancer.
Owner:XIAMEN INNOVAX BIOTECH +1
Nucleotide analogs
ActiveUS9493493B2Cosmetic preparationsInorganic phosphorous active ingredientsCyclic nucleotideVirology
Disclosed herein, inter alia, are acyclic nucleotide analogs and methods of using an acyclic nucleotide analog for treating and / or ameliorating a papillomavirus infection.
Owner:RGT UNIV OF CALIFORNIA
Truncated l1 protein of human papillomavirus type 16
ActiveUS20100255031A1Difficult to applyReduced expression levelBacteriaPeptide/protein ingredientsVirus-like particleCervical cancer
The invention relates to a truncated L1 protein of the Human Papillomavirus Type 16, a virus-like particle consisting of the protein, a vaccine comprising said virus-like particle, and the use of the vaccine in the prevention of cervical cancer.
Owner:XIAMEN UNIV +1
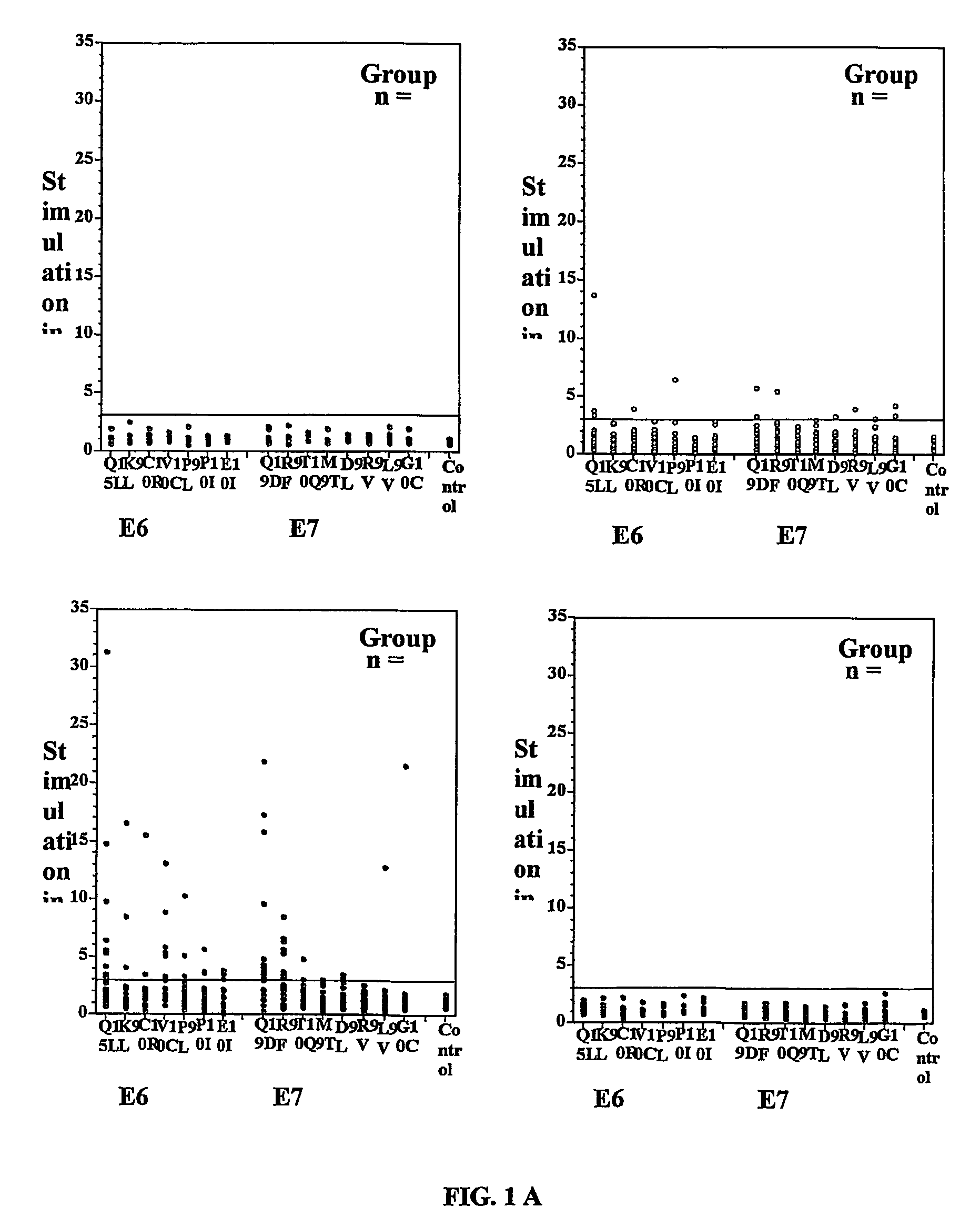
Methods and compositions relating to HPV-associated pre-cancerous and cancerous growths, including CIN
InactiveUS7410758B2Reduce riskImproved prognosisPeptide/protein ingredientsMicrobiological testing/measurementCervical intraepithelial neoplasiaOncology
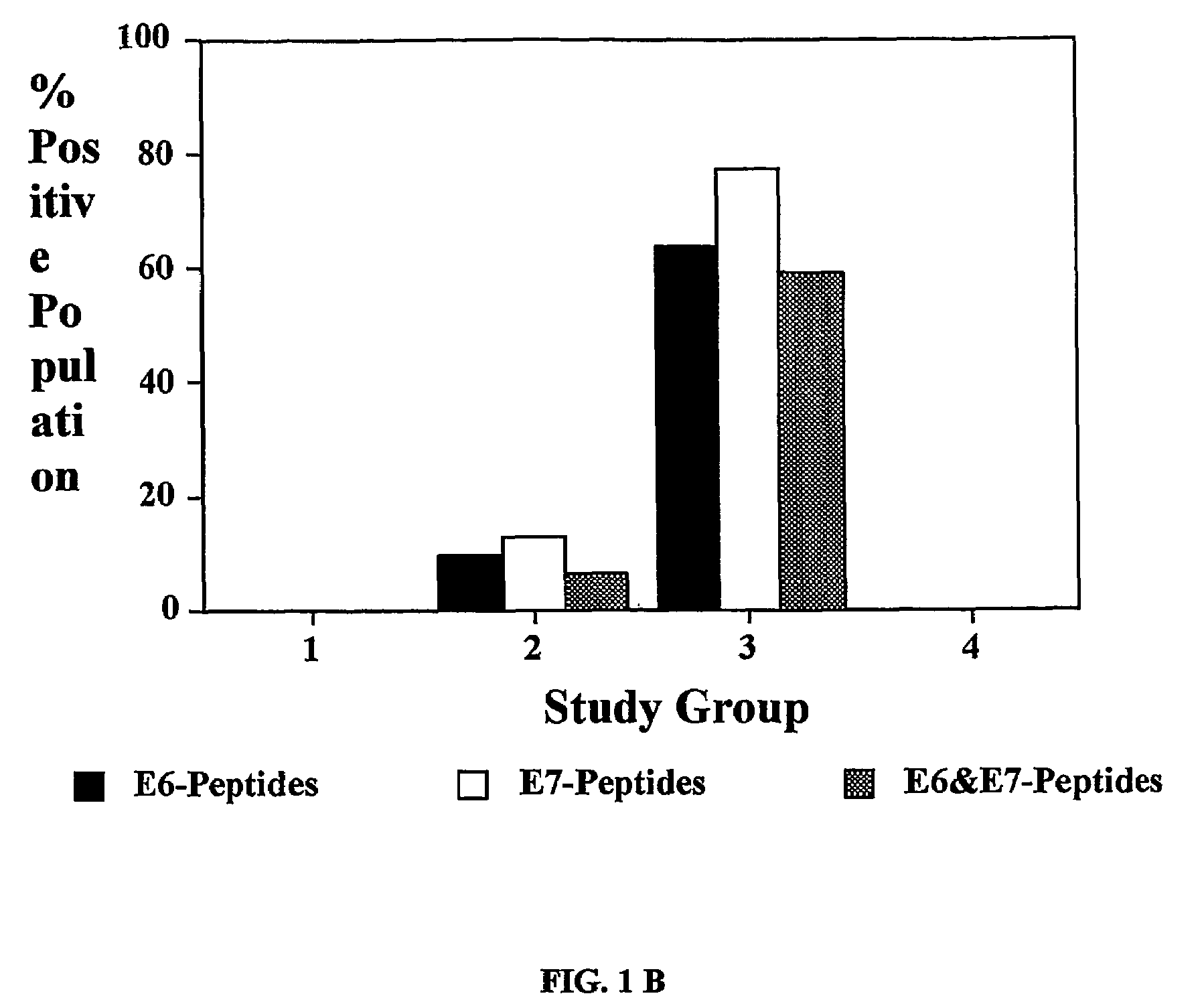
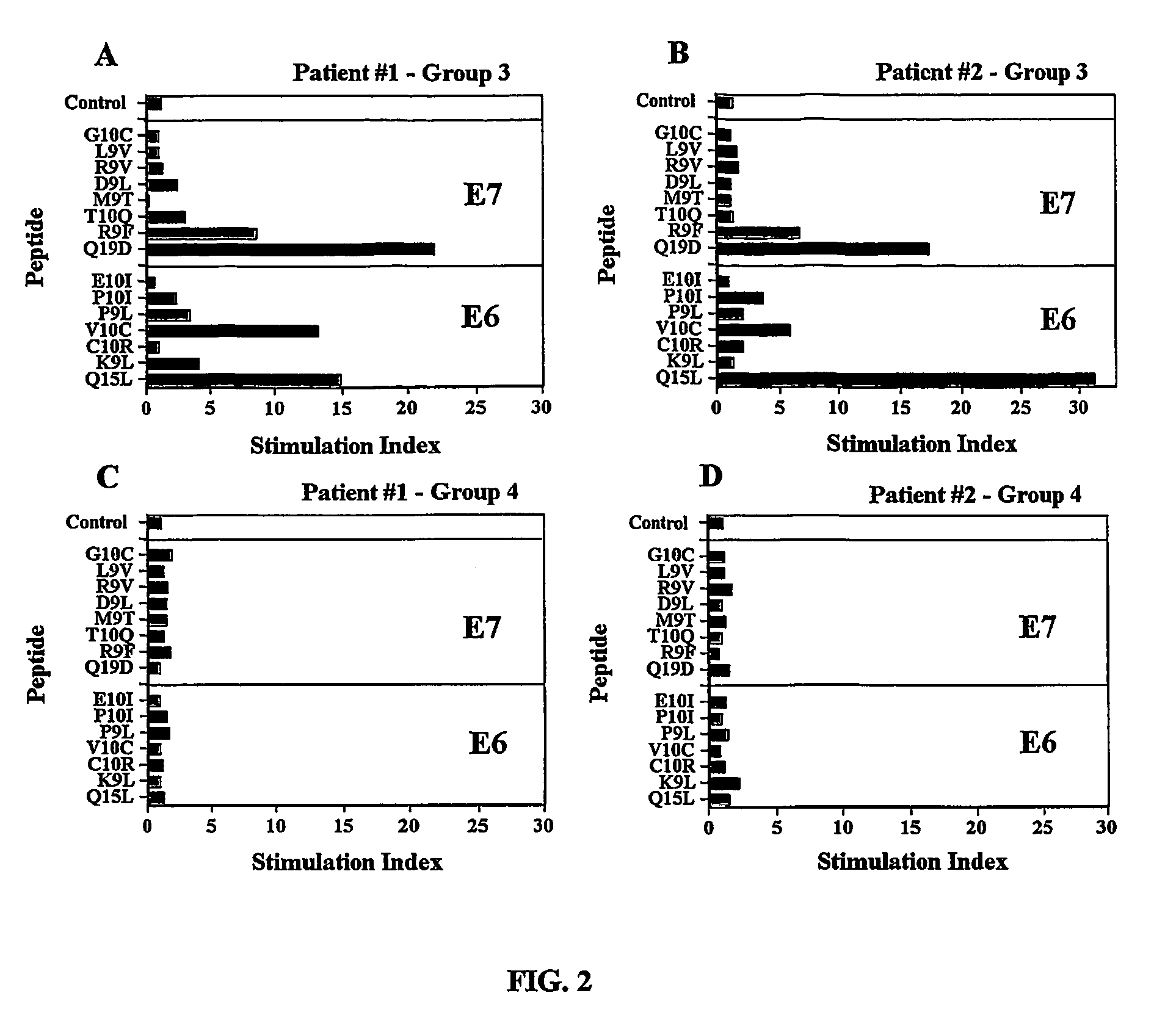
The present invention concerns the use of E6 and / or E7 peptides from human papilloma virus (HPV) to evaluate a cell-mediated response in a patient infected with HPV to determine the prognosis for that patient with respect to the development or recurrence of pre-cancerous or cancerous growths, including cervical intraepithelial neoplasia (CIN).
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Modification sequence of recombinant human mammilla tumor virus L1 capsid protein
InactiveCN101481407AStructural stability has no appreciable effectStructural Stability EffectsViral antigen ingredientsAntiviralsAntigenHuman papillomavirus
The invention relates to the prevention and treatment field of human papillomavirus infection, in particular to an amino acid modification sequence of recombinant human papillomavirus (HPV) L1 capsid protein, a nucleotide sequence encoding the amino acid modification sequence, and a carrier and a transformant containing the nucleotide sequence; and the invention further relates to application of an HPV L1 protein polymer consisting of the amino acid modification sequence to preparing vaccines, pharmaceutical compositions and diagnostic antigen or diagnostic antibodies. In the invention, an HPV L1 protein wild-type sequence is modified to prevent forming of a disulfide bond in the HPV L1 protein, the modification has no significant effect on the structural stability of the recombinant HPV L1 protein, but significantly improves the efficiency of purification process, and directly reduces the reagent consumption, thus effectively lowering the industrial production cost and having great economical benefit.
Owner:马润林 +1
In vitro method for disassembly/reassembly of papillomavirus virus-like particles (VLPS), homogeneous vlp and capsomere compositions produced by said methods; use thereof as vehicle for improved purification, and delivery of active agents
InactiveUS6962777B1Stabilize VLPsIncrease ionic strengthMicrobiological testing/measurementVirus peptidesEpitopeDiagnostic agent
A method of disassembly / reassembly of papillomavirus VLPs is provided. The resultant VLPs have enhanced homogeneity, present conformational, neutralizing PV epitopes, and therefore are useful prophylactic and diagnostic agents. Further, these VLPs can be used to encapsulate desired moieties, e.g., therapeutic or diagnostic agents, or “marker” DNAs, and the resultant VLPs used as in vivo delivery vehicles or as pseudovirions for evaluating vaccine efficacy.
Owner:MEDIMMUNE LLC
Application of polypeptide in preparing preparations for preventing and treating human papilloma virus infection
PendingCN111375051AImprove securityNo inhibitory effectPeptide/protein ingredientsSuppositories deliveryHuman papilloma virus infectionInfectious Disorder
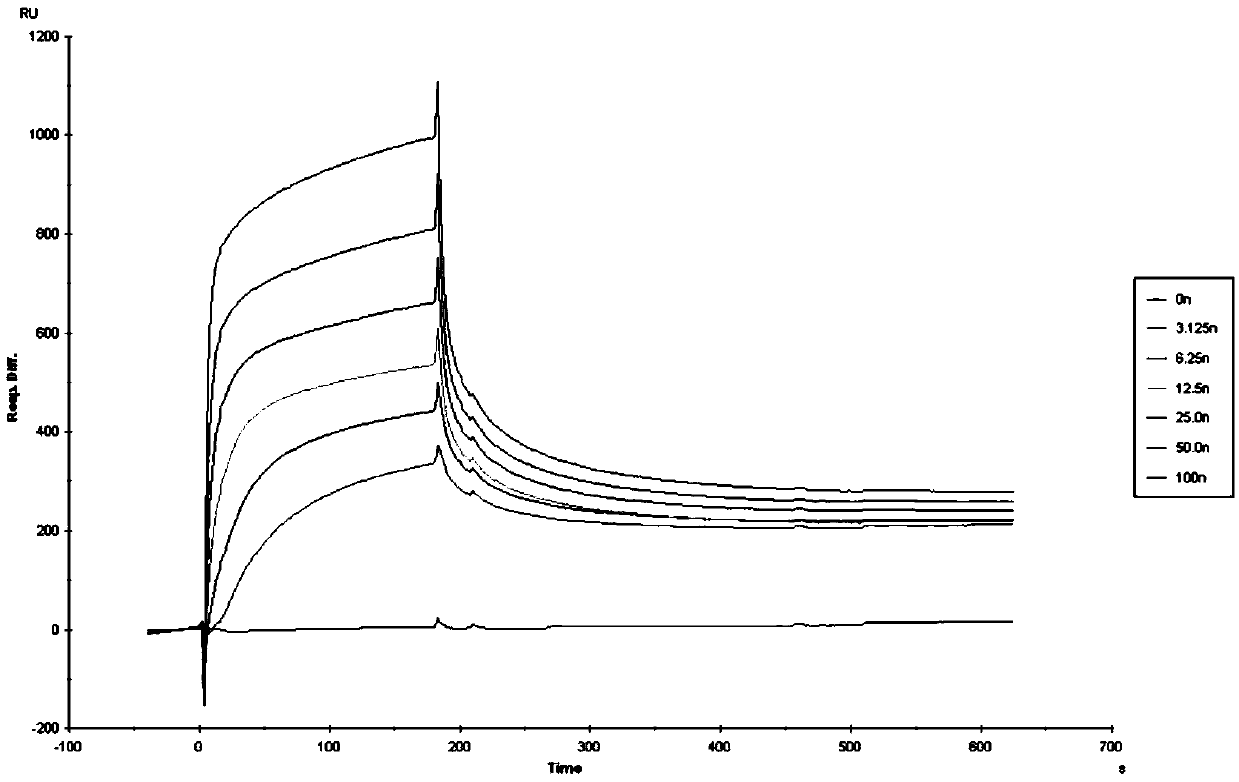
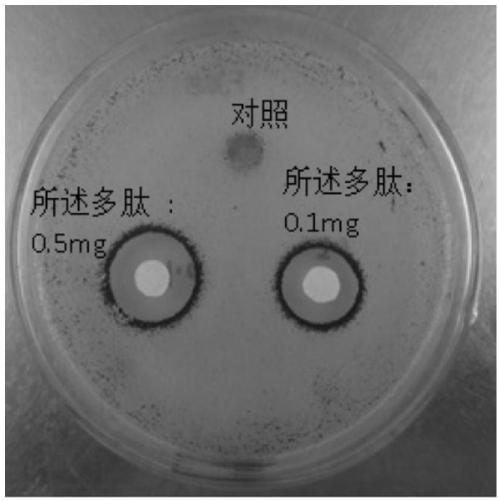
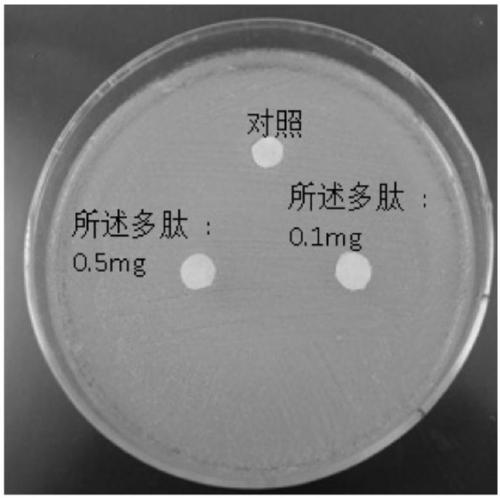
The invention discloses an application of polypeptide in preparing preparations for preventing and treating human papilloma virus infection, and belongs to the field of infectious disease treatment. Apolypeptide preparation for preventing and treating human papilloma virus infection is also provided; and the polypeptide preparation takes the polypeptide with effective dose as active ingredients and is prepared by adding pharmaceutically acceptable subsidiary materials or auxiliary components. The polypeptide can be used for the treatment and prevention of human papilloma virus infection, so that the polypeptide is significant in effect; and the polypeptide has no inhibition effects on the normal flora of female genital tracts, so that the polypeptide is good in safety.
Owner:GENLOCI BIOTECH
Treatment of virally induced lesions
InactiveUS20100204318A1Reduce retard resolve clear eliminateReduce, retard, resolve, clear or eliminate cutaneous lesions or papulesBiocideOintment deliveryCutaneous lesionVirus
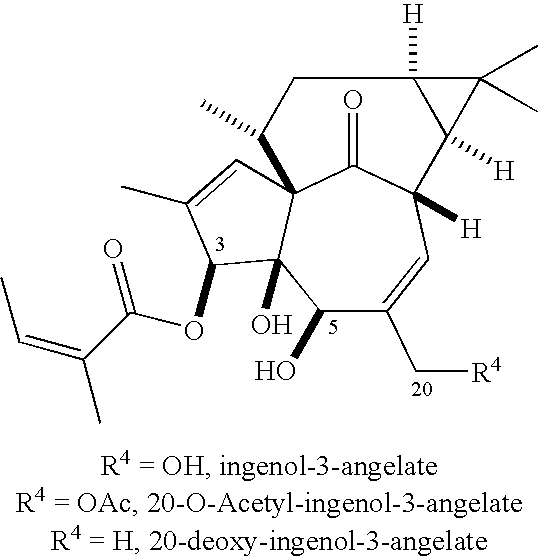
The present invention relates generally to the treatment of cutaneous lesions containing cells infected by a virus, as well as compositions for the treatment of such lesions. More specifically, the invention relates to the use of ingenol compounds, particularly ingenol angelates, in treating lesions caused by infection with a papilloma virus, such as a mammalian papilloma virus, in particular a Human Papilloma Virus.
Owner:LEO LAB +1
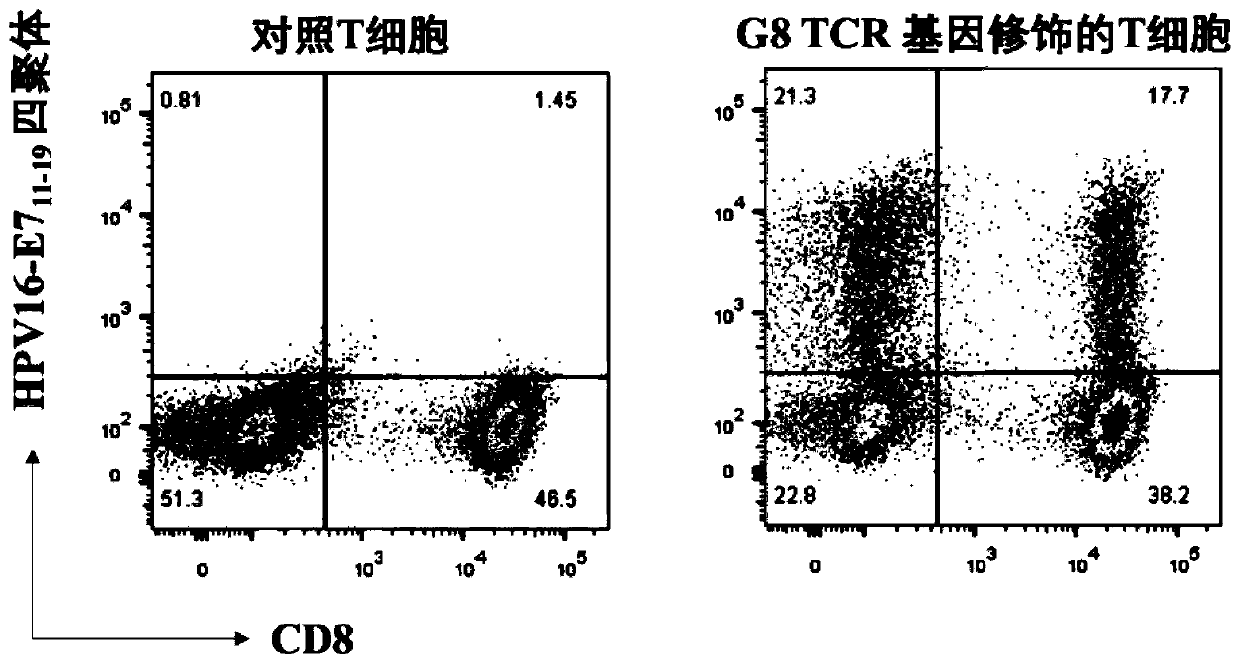
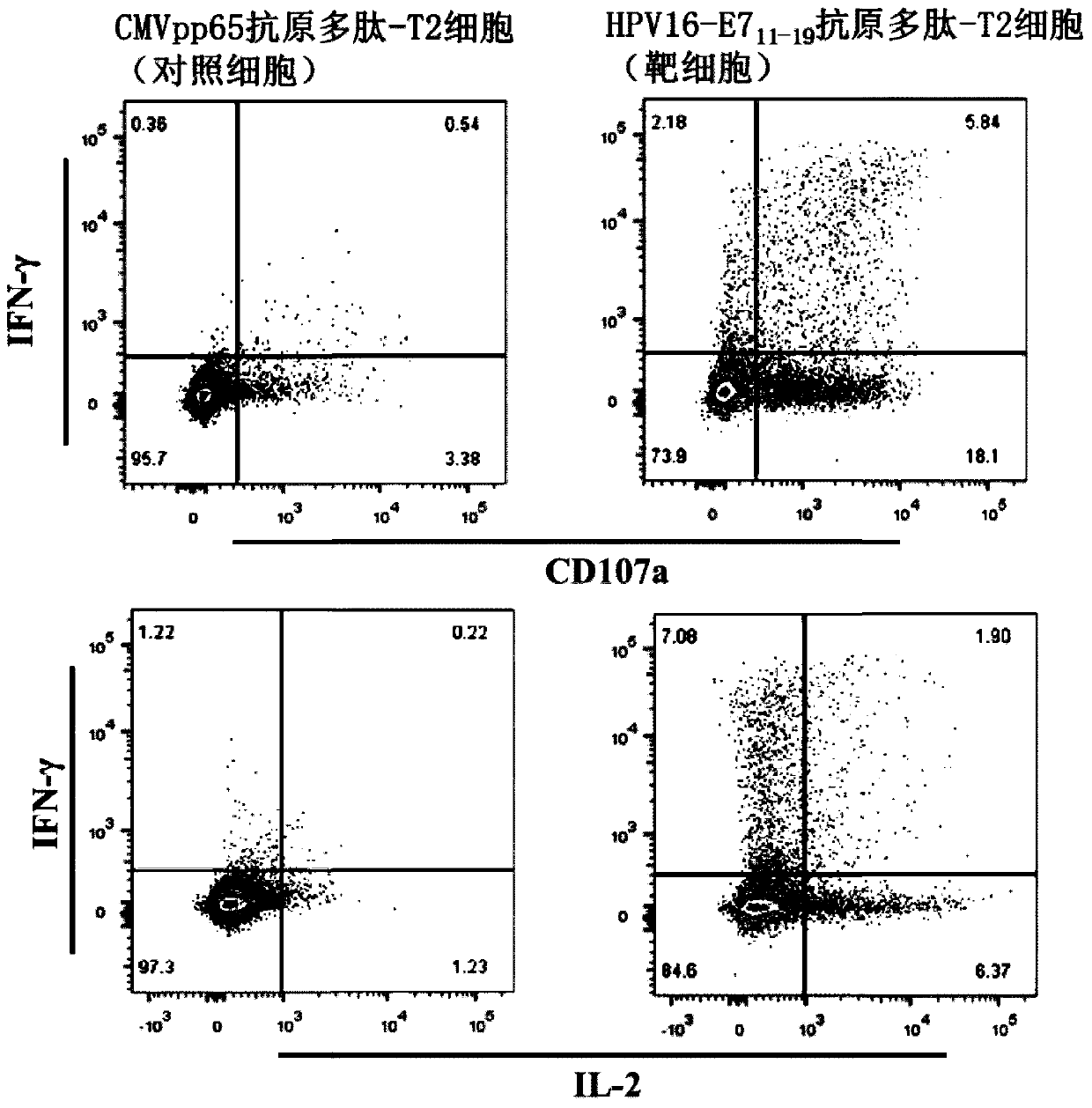
TCR for identifying human papilloma virus HPV16-E7 antigen
ActiveCN110357952AImmunoglobulin superfamilyMammal material medical ingredientsAntigenT-Cell Antigen Receptors
The invention relates to the technical field of T cell antigen receptors, in particular to TCR capable of specifically identifying and combining with a human papilloma virus antigen HPV16-E7, a nucleotide sequence encoding the TCR or nucleic acid molecules of a complementary sequence of the nucleotide sequence, a carrier containing the nucleic acid molecules, cells transducing the nucleic acid molecules or carrier, a drug composition containing the TCR, the nucleic acid molecules, the carrier or the cells which are taken as active ingredients and application of the TCR, nucleic acid molecules,carrier, cells and drug composition which are used for preparing drugs for treating tumors or virus infection respectively.
Owner:深圳市因诺转化医学研究院 +1
DNA coding for a peptide of a papilloma virus main capside protein and use thereof
PCT No. PCT / EP95 / 01697 Sec. 371 Date Jun. 14, 1996 Sec. 102(e) Date Jun. 14, 1996 PCT Filed May 4, 1995 PCT Pub. No. WO95 / 30754 PCT Pub. Date Nov. 16, 1995This invention relates to a DNA encoding a peptide of a papilloma virus major capsid protein. Furthermore, this invention deals with a papilloma virus genome containing such a DNA. In addition, this invention concerns proteins encoded by the papilloma virus genome and virus-like particles as well as antibodies directed thereagainst and the use thereof and the use thereof in diagnosis, treatment and vaccination.
Owner:DEUTES KREBSFORSCHUNGSZENT STIFTUNG DES OFFENTLICHEN RECHTS
Novel expression vectors and uses thereof
InactiveUS20050026137A1Increase the number ofAvoiding a severe drawbackGenetic material ingredientsVirus peptidesEpstein-Barr Virus Nuclear AntigensOrigin of replication
The present invention relates to novel vectors, to DNA vaccines and gene therapeutics containing said vectors, to methods for the preparation of the vectors and DNA vaccines and gene therapeutics containing the vectors, and to therapeutic uses of said vectors. More specifically, the present invention relates to novel vectors comprising (a) an expression cassette of a gene of a nuclear-anchoring protein, which contains (i) a DNA binding domain capable of binding to a specific DNA sequence and (ii) a functional domain capable of binding to a nuclear component and (b) a multimerized DNA sequence forming a binding site for the anchoring protein, and optionally (c) one or more expression cassettes of a DNA sequence of interest. In particular the invention relates to vectors that lack a papilloma virus origin of replication. The nuclear-anchoring protein might be the E2 protein of Bovine Papilloma Virus type 1 or Epstein-Barr Virus Nuclear Antigen 1. The invention also relates to vectors that lack an origin of replication functional in a mammalian cell. The invention further relates to methods for expressing a DNA sequence of interest in a subject.
Owner:FIT BIOTECH OY PLC
6,11 type human papillomavirus fluorescence quantitative PCR detection method and kit thereof
InactiveCN101676408AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceMicrobiology
The invention relates to a 6,11 type human papillomavirus fluorescence quantitative PCR detection method and kit thereof and PCR amplification primer and probe sequence for 6,11 type nucleotide segments of human papillomavirus. The primer sequence comprises: primer pair of human papillomavirus 6,11 type general upstream primer and general downstream primer, upstream primer complementation sequenceor downstream primer complementation sequence, also comprises primer sequences obtained in range of upstream primer extending towards 5'end by 10 basic groups and the upstream primer extending towards 3'end and 5'end by 10 basic groups. The probe sequence comprises: fluorescent probe: SEQ ID NO:3 and its complementation sequence, and probe sequence obtained in range of the extending SEQ ID NO:3 and its complementation sequence towards 3'end and 5'end by 10 basic groups. The method is used for quantitative detection of 6,11 type human papillomavirus with real clinical application value.
Owner:YANGTZE RIVER PHARMA GRP BEIJING HAIYAN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com