Human papillomavirus shell protein L1 short peptide and application thereof
A human papillomavirus, coat protein technology, applied in the field of human papillomavirus coat protein L1 short peptide, can solve the problems of unsuitable storage, unpredictable CINI/II natural regression, and inability to distinguish persistent infection or reinfection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] (a) Synthesis of HPV L1 short peptide
[0071] In this embodiment, based on the protein sequence of HPV16 L1 aa448-477 segment, the peptide length is 30 amino acids, and the sequence is: EVNLKEKFSADLDQFPLGRKFLLQAGLKAK.
[0072] Beijing Saibaisheng Gene Technology Co., Ltd. was entrusted to synthesize the short peptide of this sequence in Genemed Synthesis Inc. of the United States by conventional methods.
[0073] (b) Preparation of antiserum
[0074] After dissolving the synthetic short peptide in PBS, add Furend's complete adjuvant 1:1, emulsify and immunize Japanese long-eared white rabbits and Balb / c mice with doses of 25ug and 10ug, respectively, three times in total. On the 14th day of the last immunization, the animals were sacrificed, and the rabbit antiserum and mouse antiserum against the short peptide were collected.
Embodiment 2
[0076] Western Blot detection of reactivity of anti-short peptide antiserum to recombinant HPV16 L1
[0077] Sf9 cells expressing recombinant HPV16 L1 were obtained from the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences.
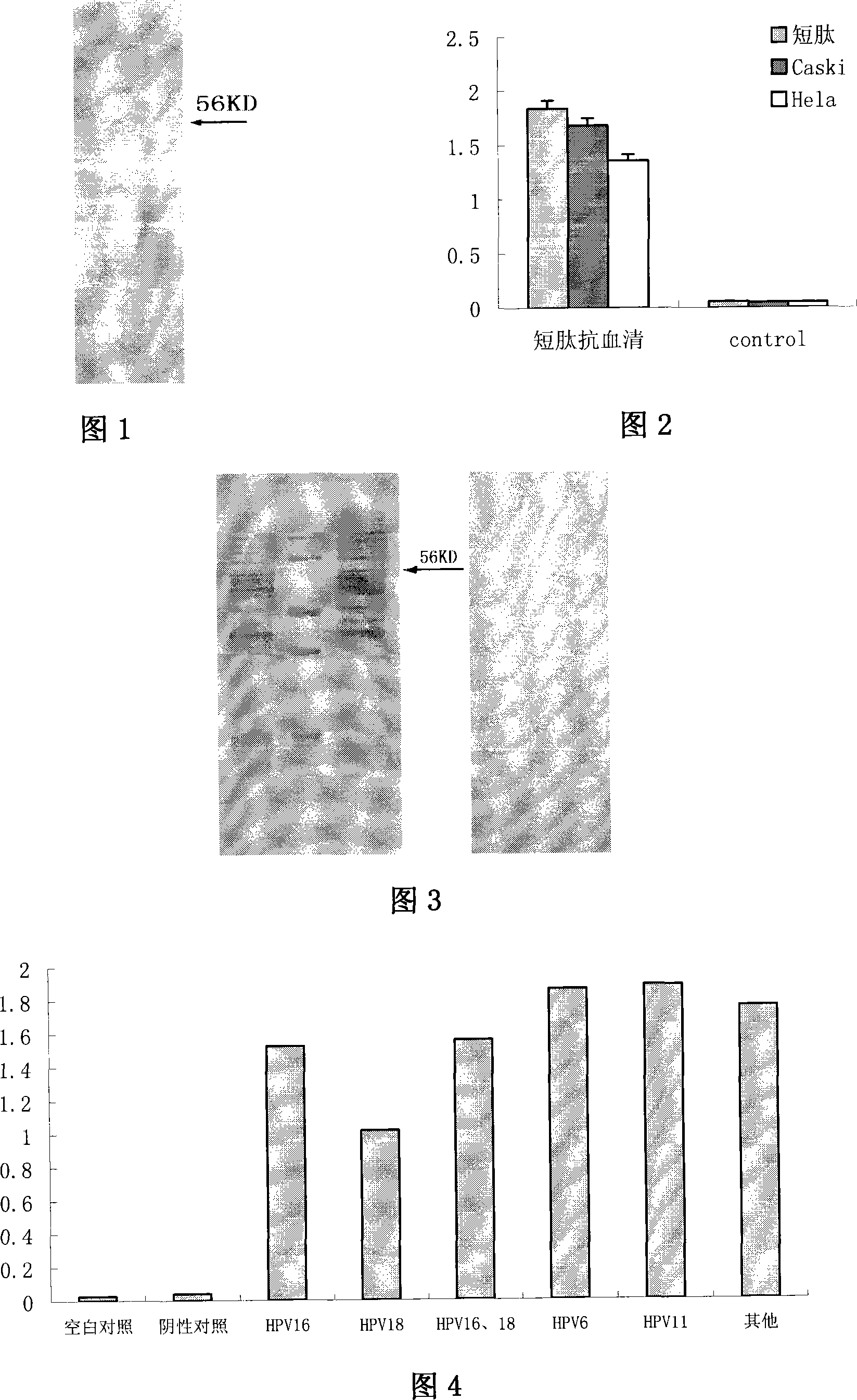
[0078] Perform polyacrylamide gel electrophoresis on the lysate of sf9 cultured cells expressing recombinant HPV16 L1, transfer the gel after electrophoresis to a nitrocellulose membrane, and then use rabbit or mouse anti-short peptide antiserum as the primary antibody to conduct polyacrylamide gel electrophoresis reaction. As a result, the short peptide immune antiserum of mice and rabbits could cause a specific reaction band to appear at the position of 56KD on the electrophoresis lane of the lysate of sf9 cultured cells expressing recombinant HPV16 L1 (Fig. 1).
Embodiment 3
[0080] ELISA detection and Western Blot detection of the reactivity of anti-short peptide antiserum to cultured cells containing HPV virus
[0081] Caski cells containing HPV16 virus and Hela cells containing HPV18 virus were purchased from the China Cell Collection Center of Wuhan University. Imported HRP-labeled and fluorescent-labeled secondary antibodies were purchased from Beijing Zhongshan Biotechnology Co., Ltd. (sigma products).
[0082] The lysates of Caski cultured cells containing HPV16 virus and Hela cultured cells containing HPV18 were coated on plastic plates, and the anti-serum of rabbit or mouse anti-short peptide was used as the primary antibody to react with them. As a result, the anti-serum against short peptides of mice and rabbits could make Caski cultured cells and Hela cultured cell lysates coated plastic plates show positive reaction (Fig. 2).
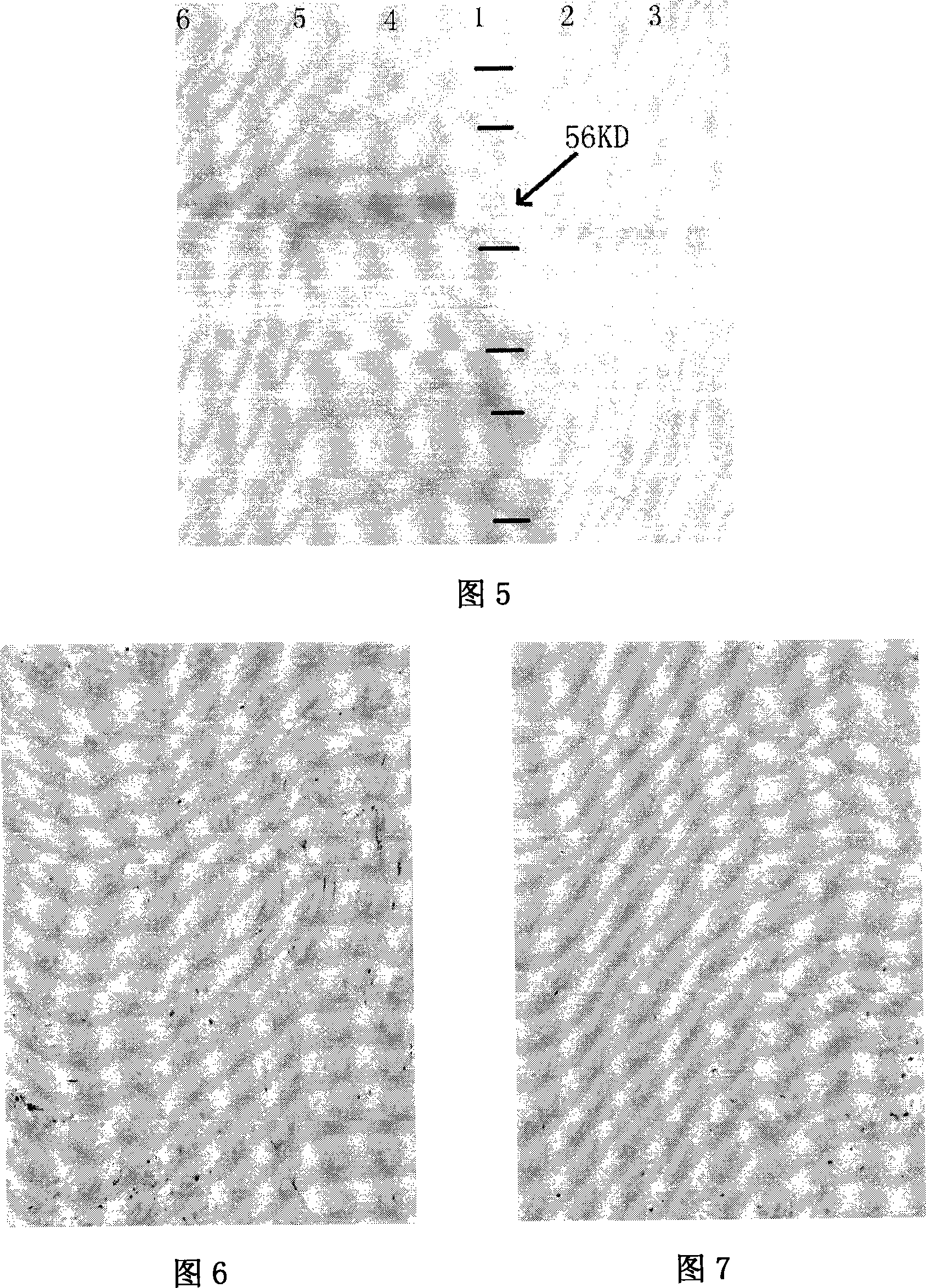
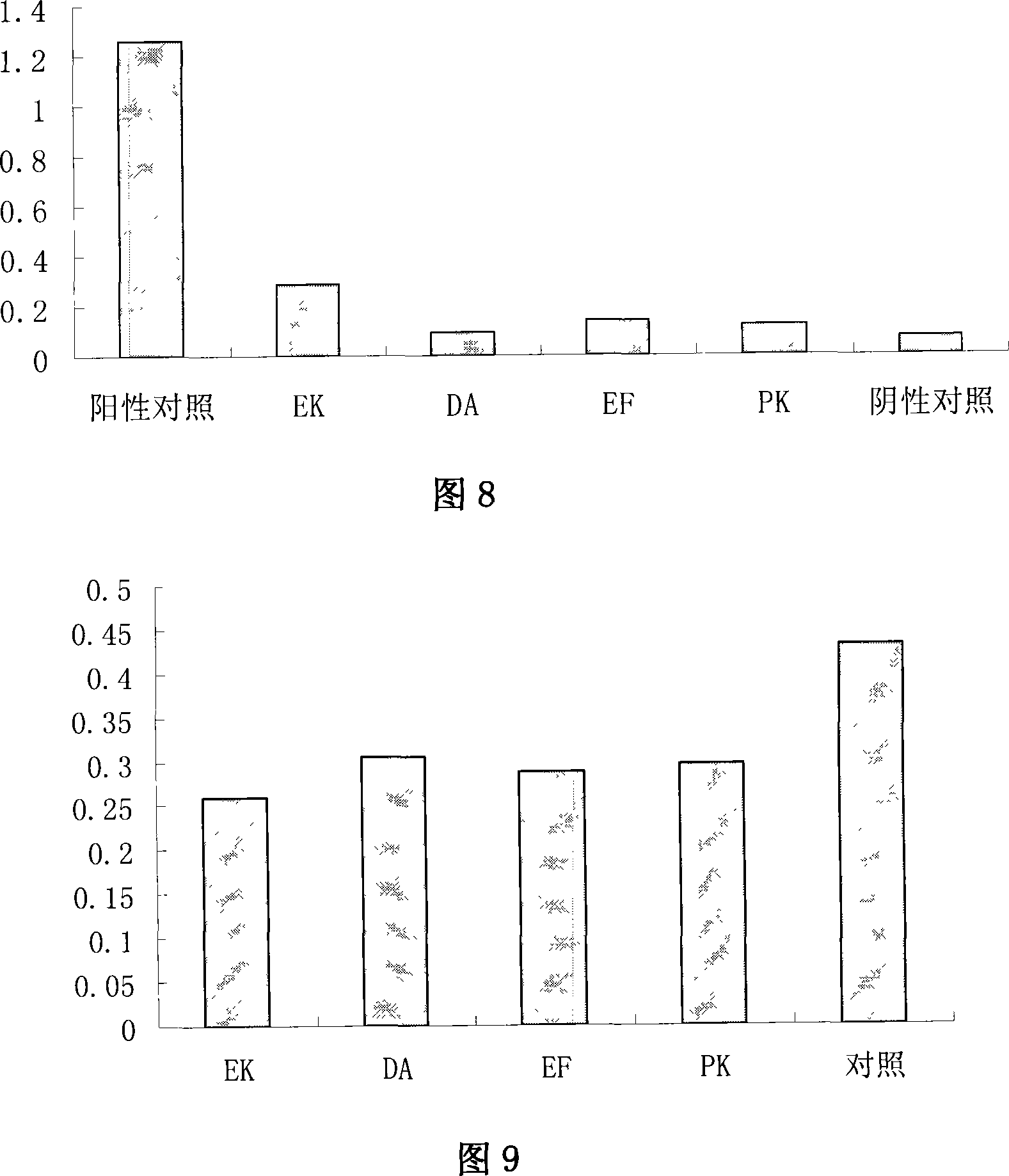
[0083] Perform polyacrylamide gel electrophoresis on the lysate of Caski cultured cells containing HPV16 vir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com