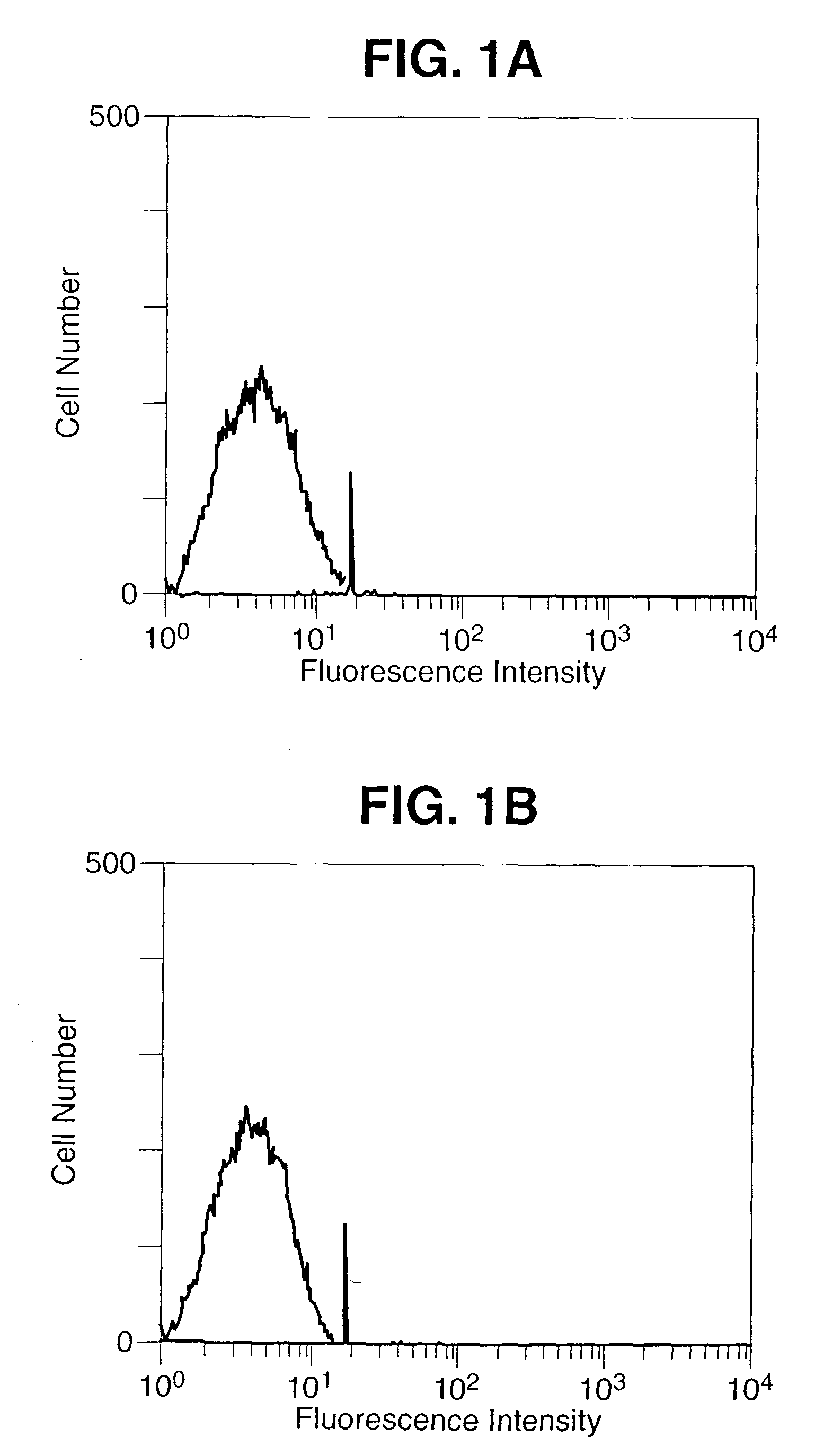
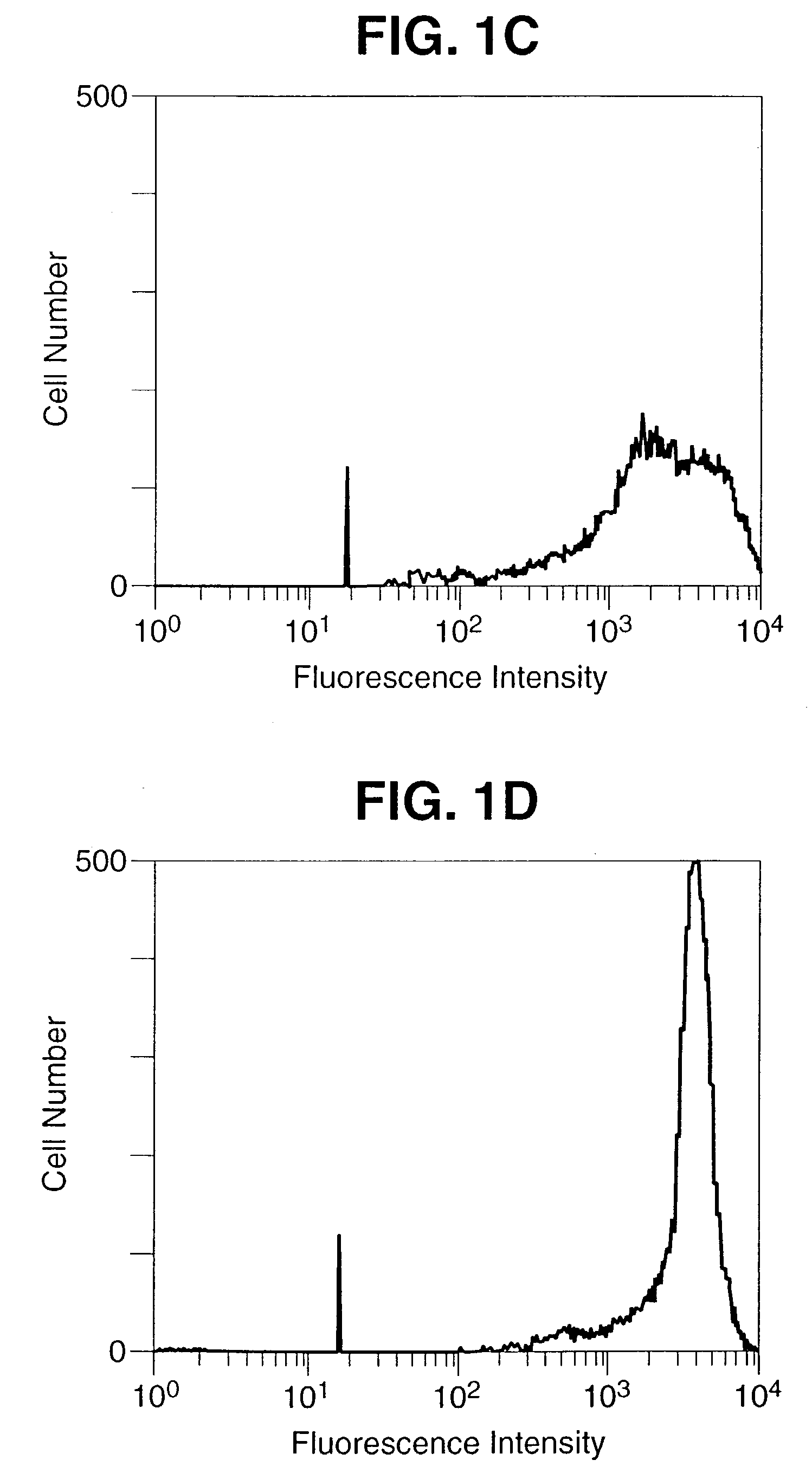
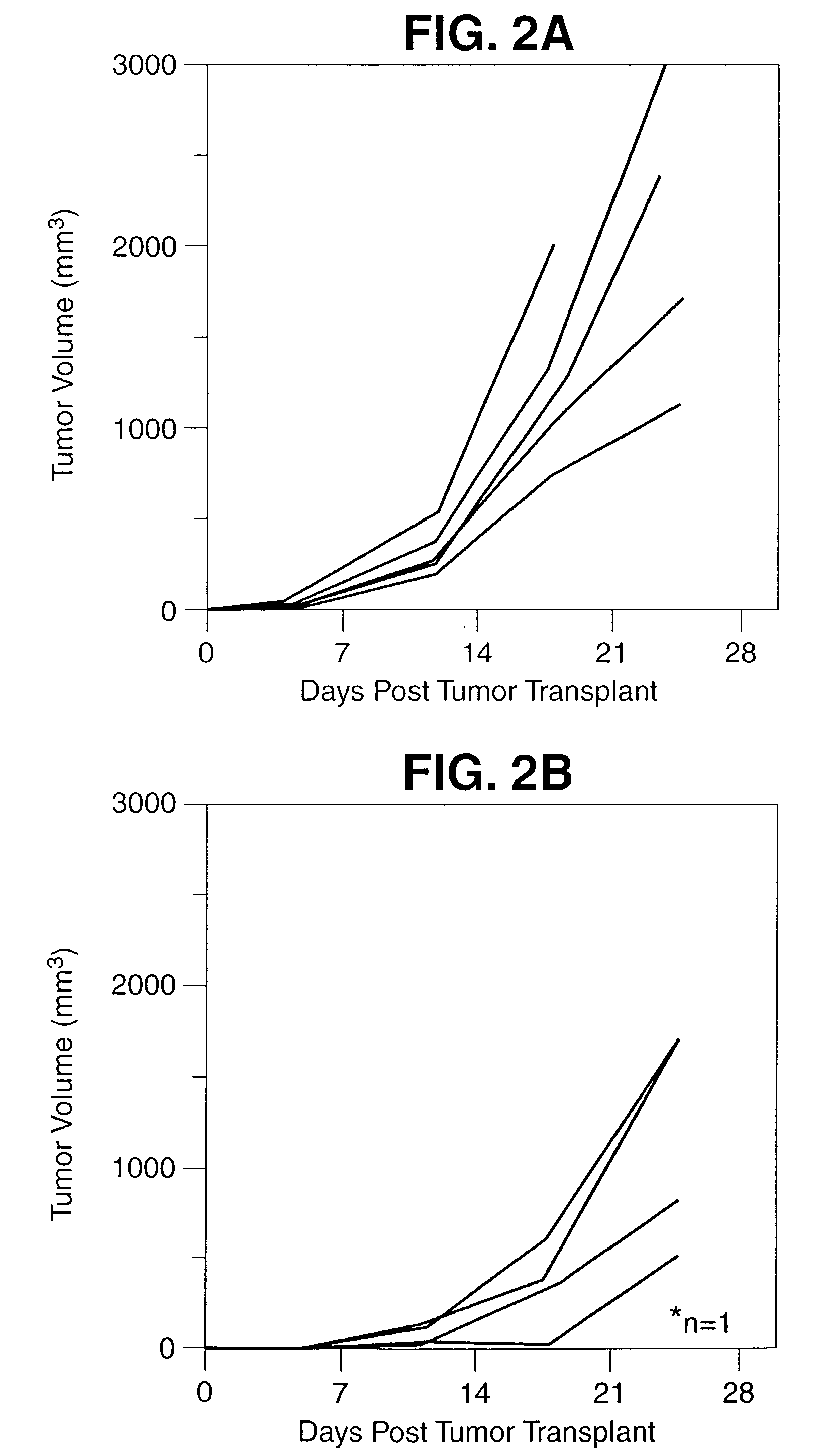
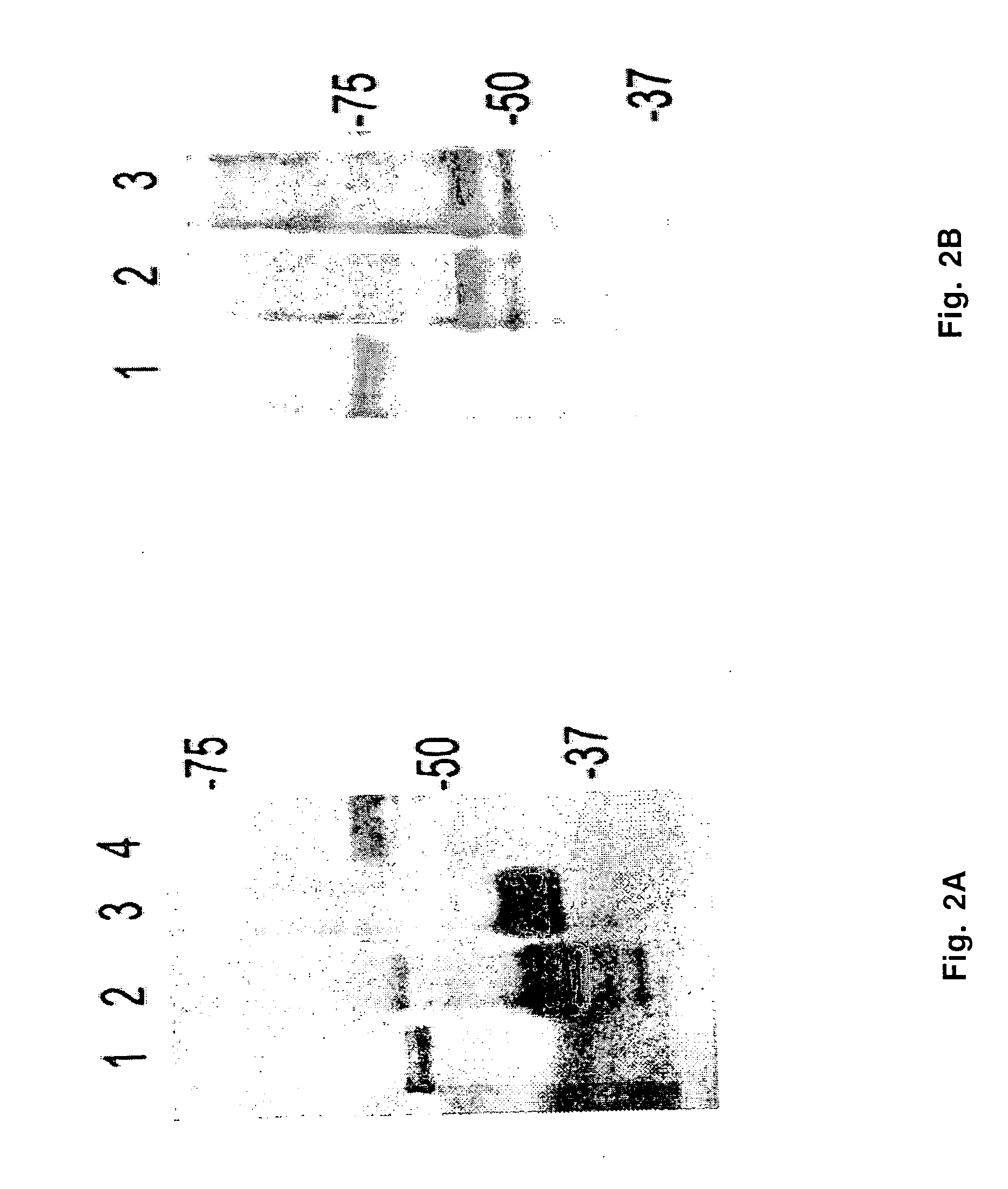
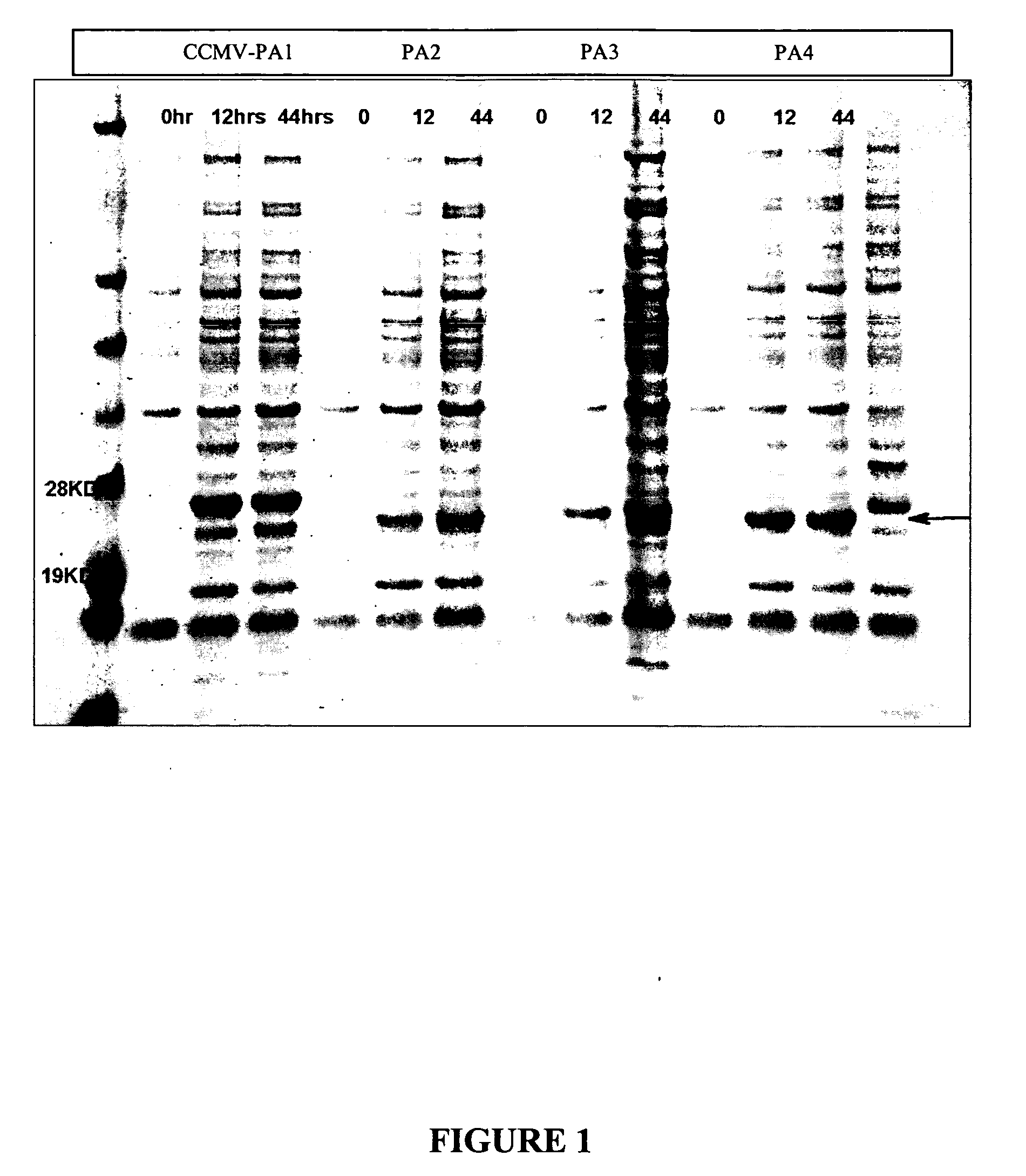
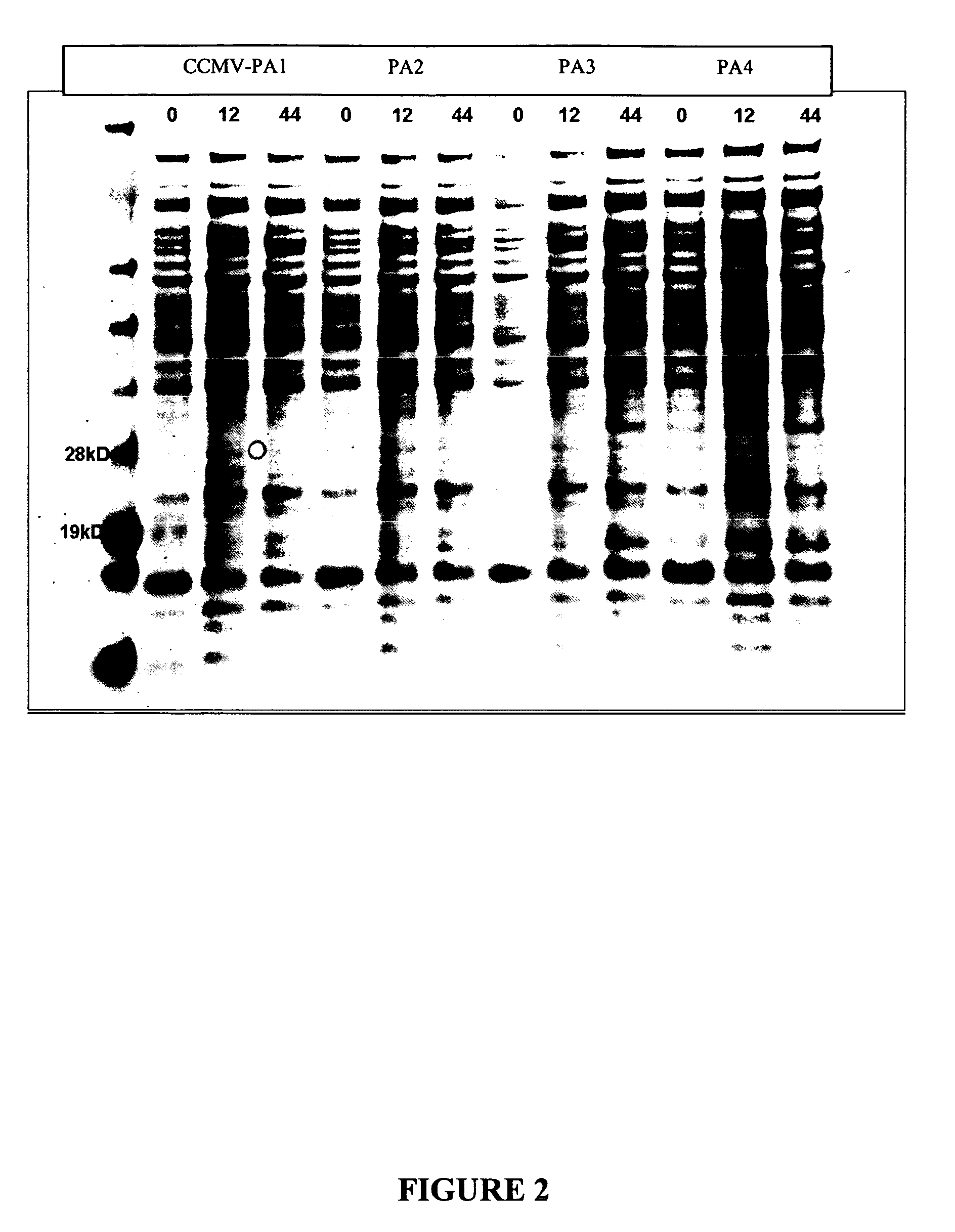
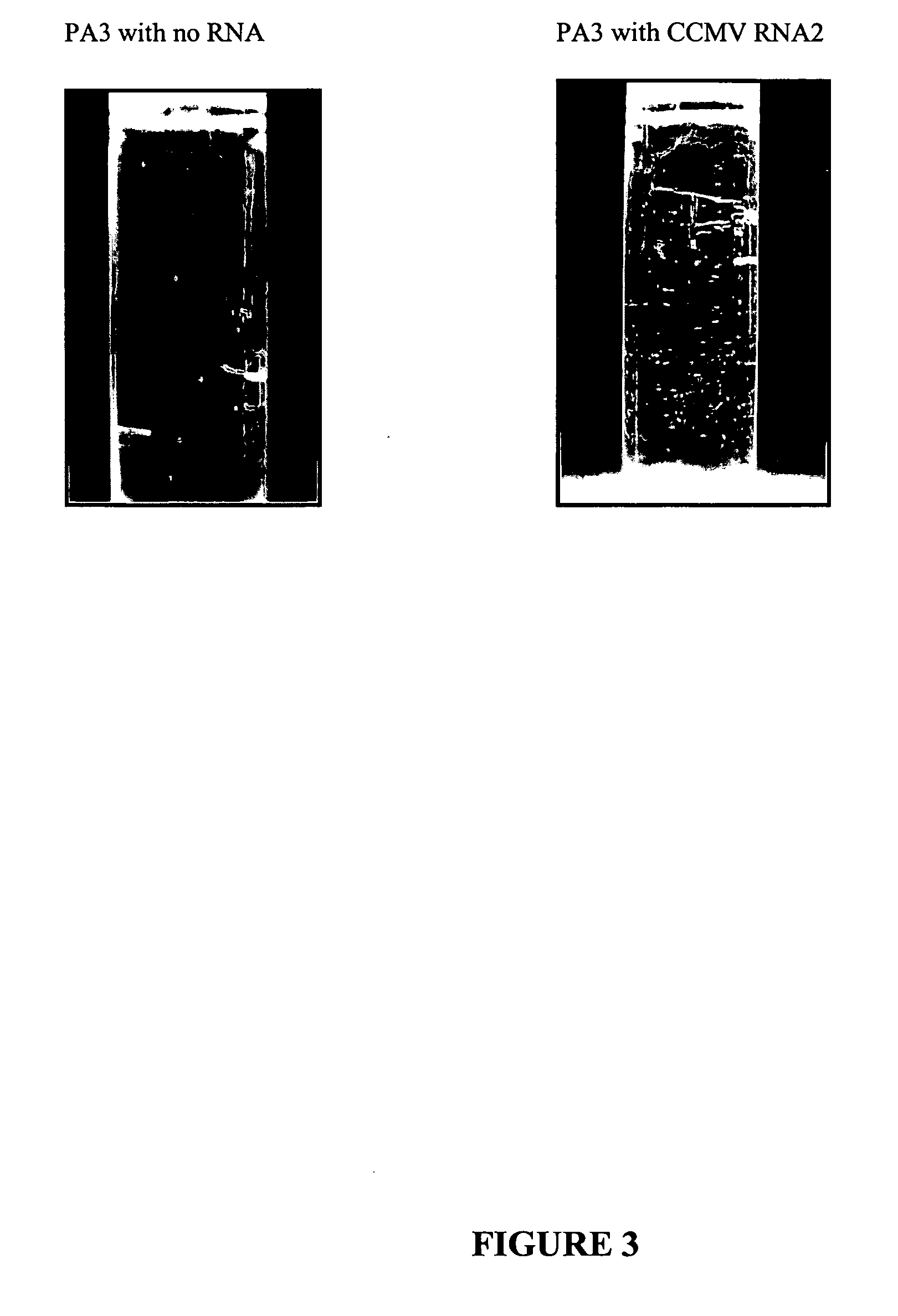
Patents
Literature
164 results about "Immunologic Reactions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immunologic response - a bodily defense reaction that recognizes an invading substance (an antigen: such as a virus or fungus or bacteria or transplanted organ) and produces antibodies specific against that antigen.
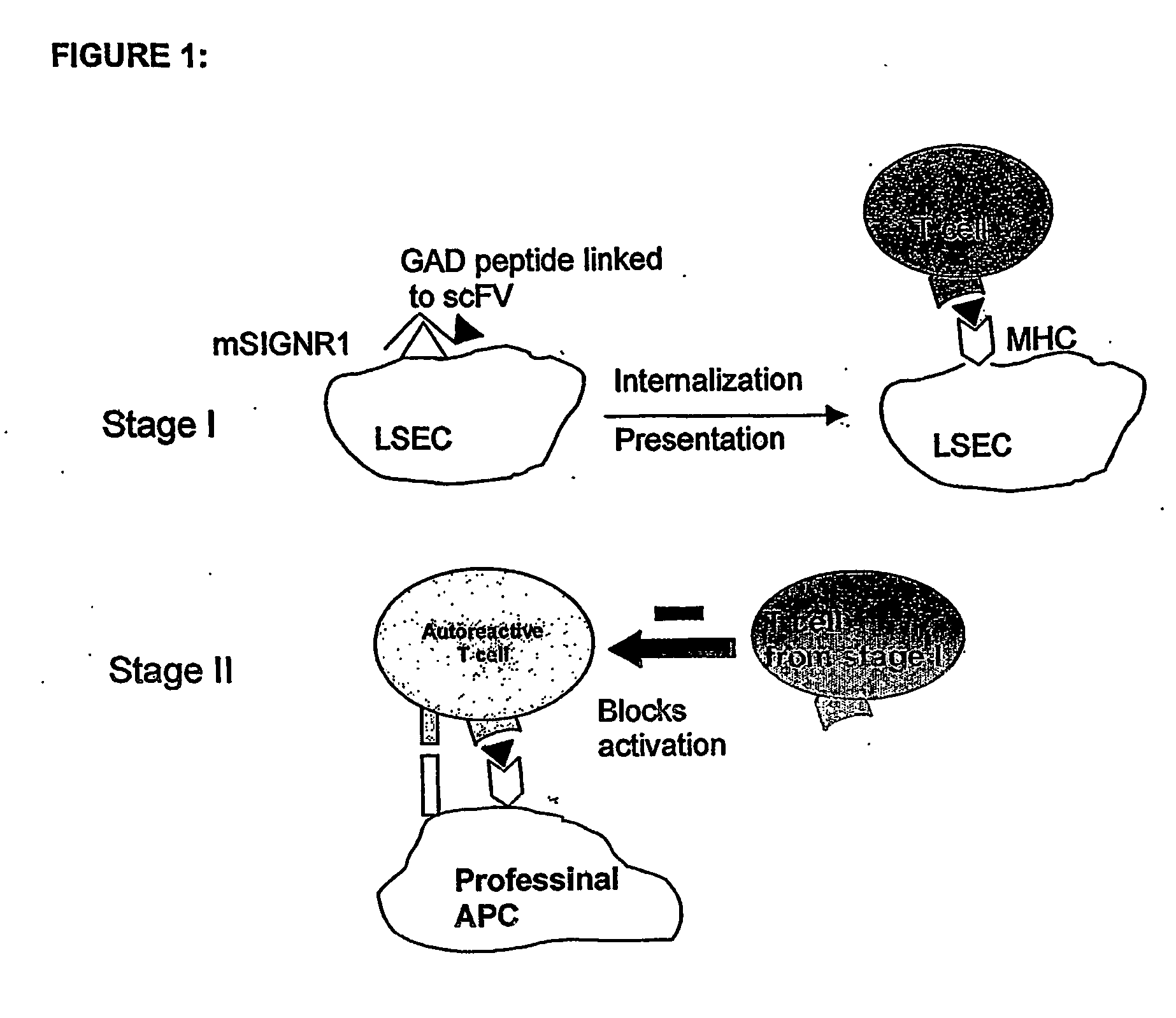
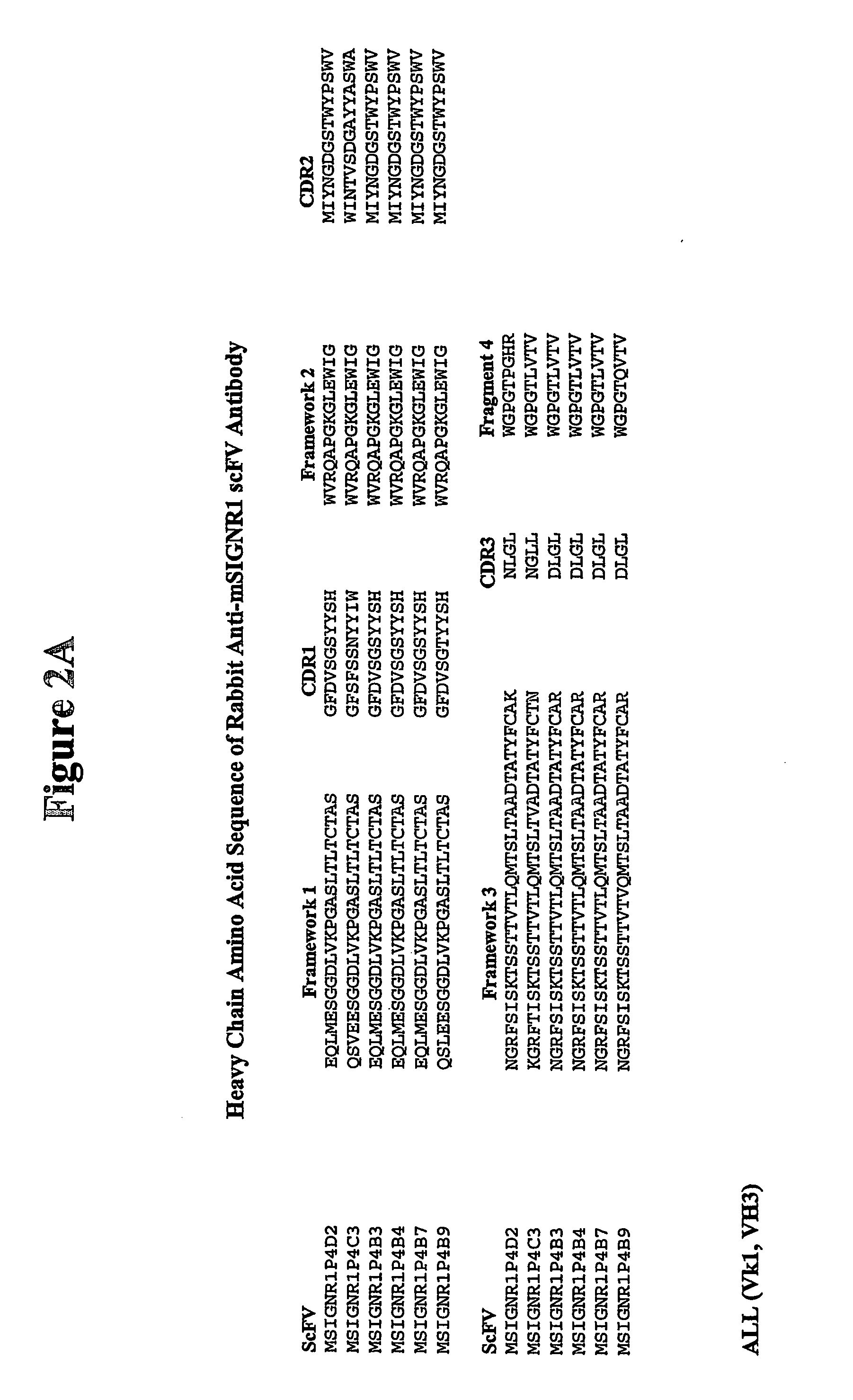
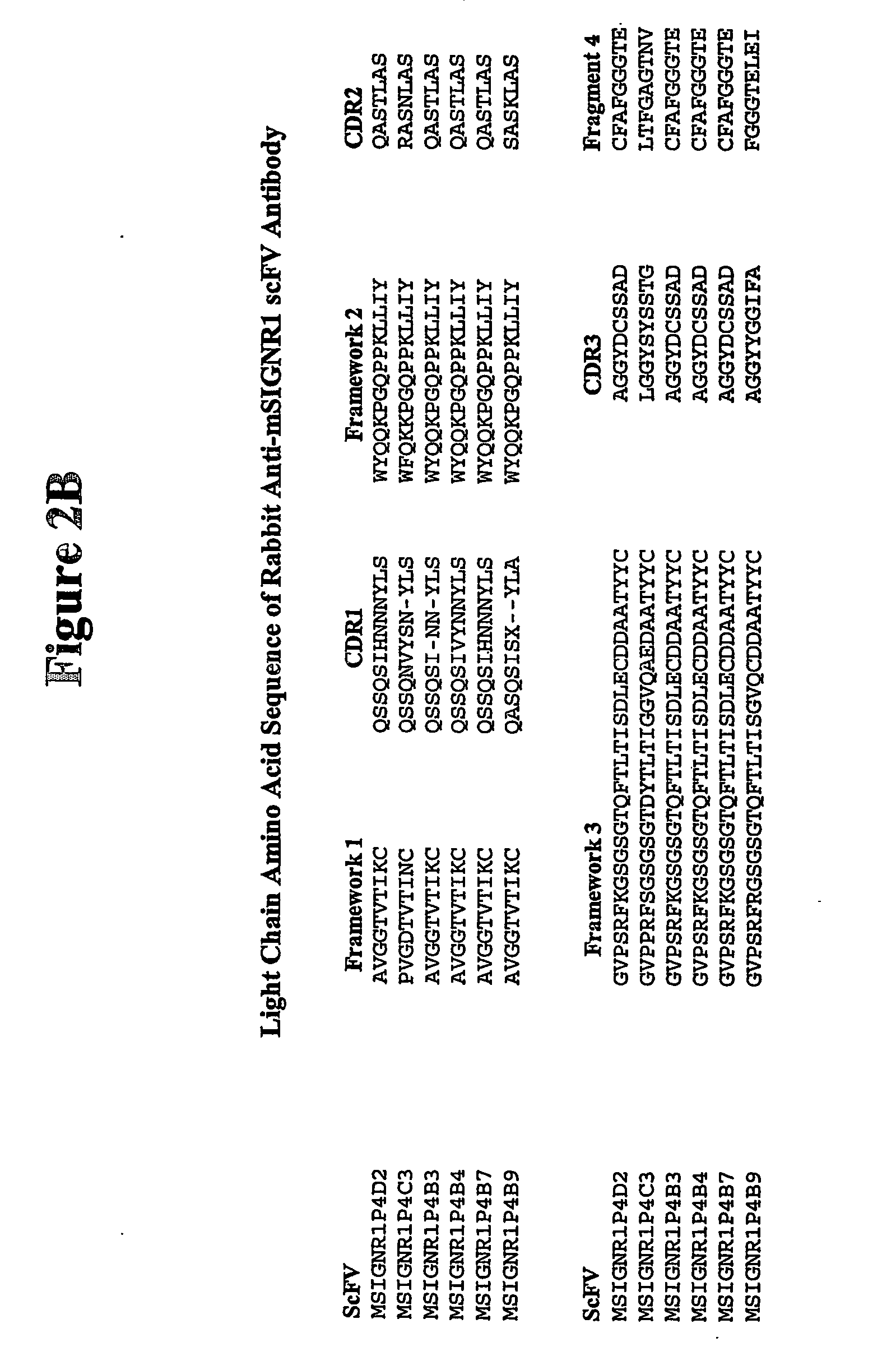
Method of treating autoimmune disease by inducing antigen presentation by tolerance inducing antigen presenting cells
InactiveUS20060257412A1Prevent proliferationAvoid enteringMetabolism disorderAntibody mimetics/scaffoldsAntigenAutoimmune condition
Antibodies to antigen presenting cells may be utilized to interfere with the interaction of the antigen presenting cell and immune cells, including T cells. Peptides may be linked to said antibodies thereby generating an immune response to such peptides. Preferably peptides linked to the antibodies are associated with autoimmunity.
Owner:ALEXION PHARMA INC
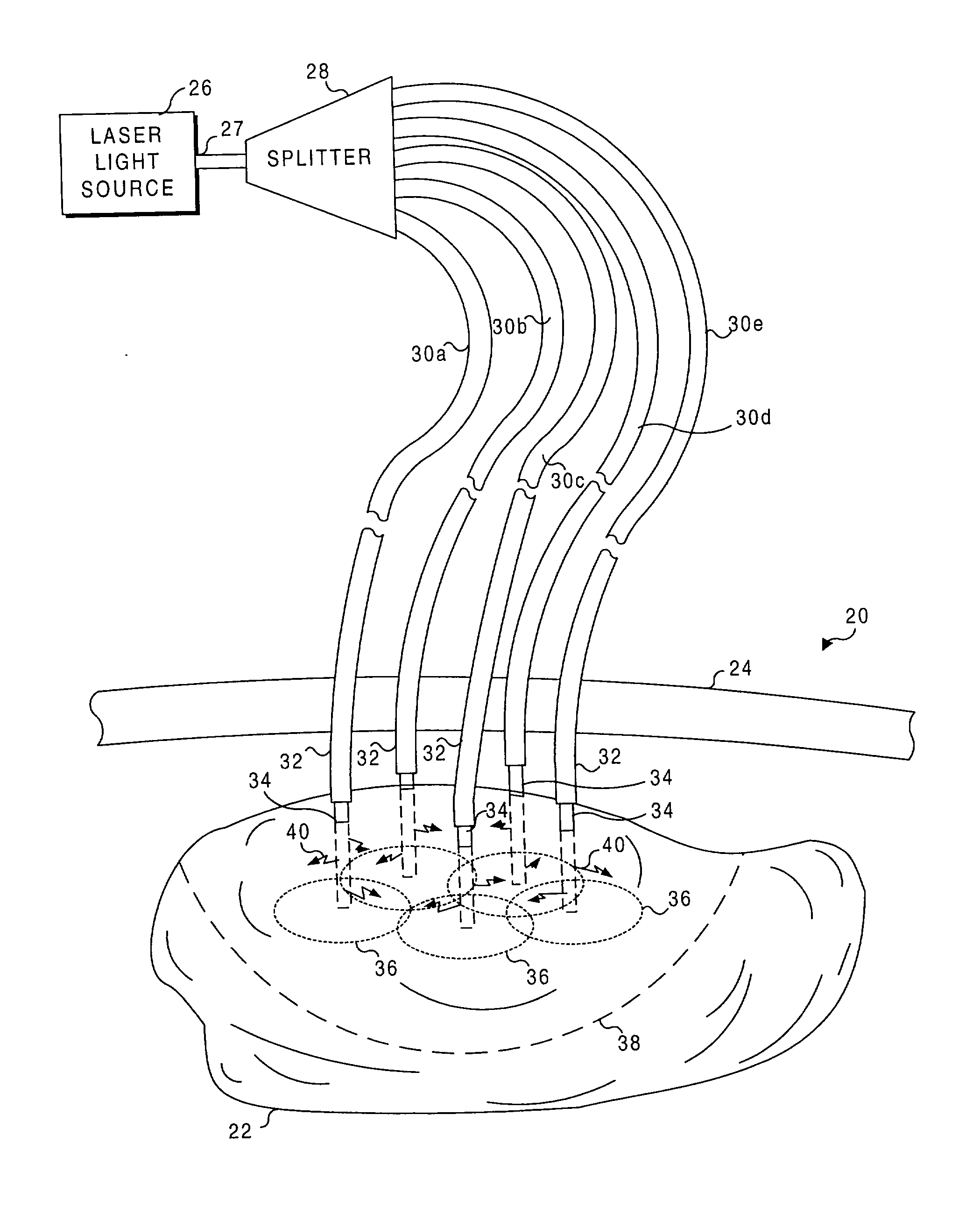
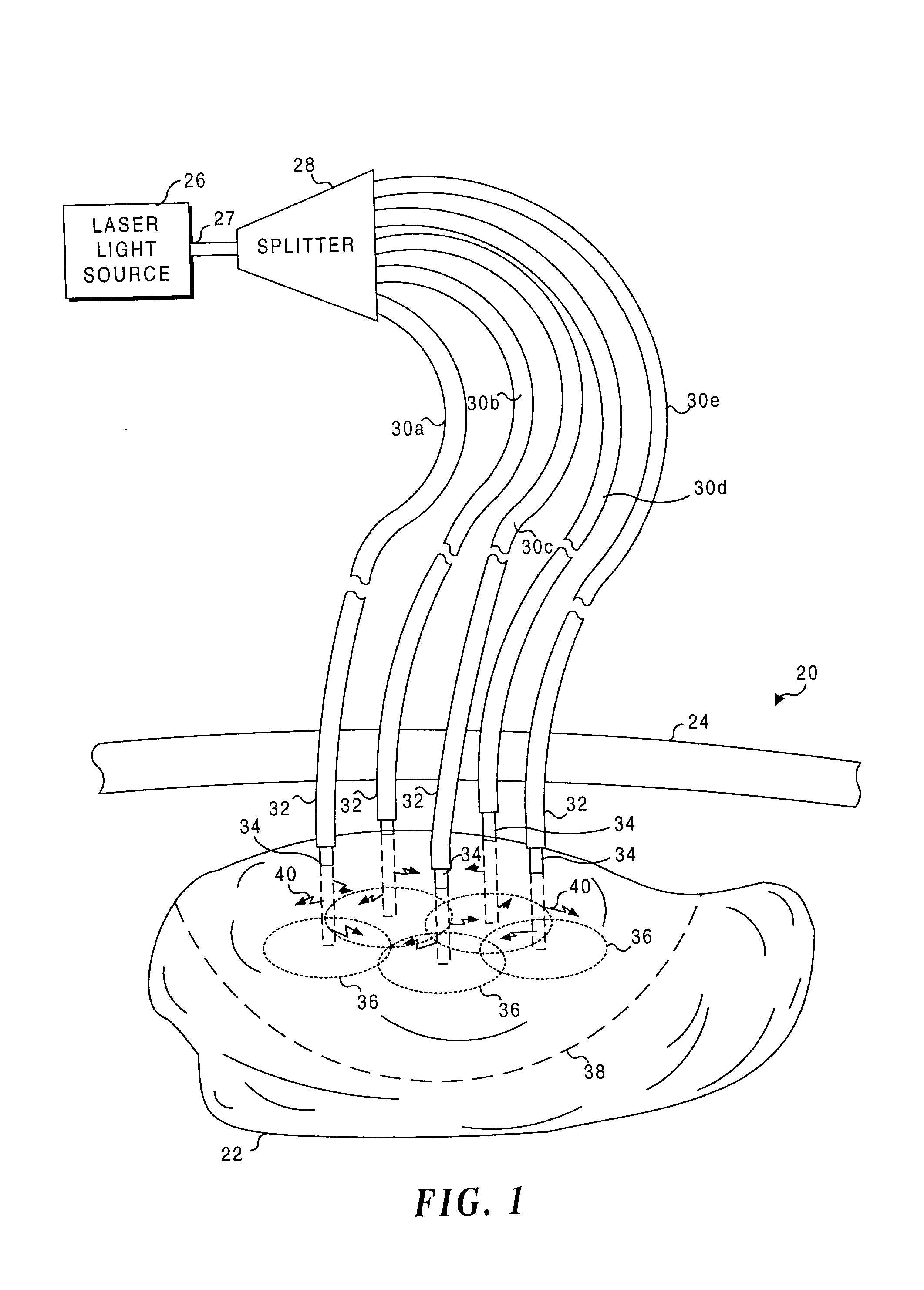
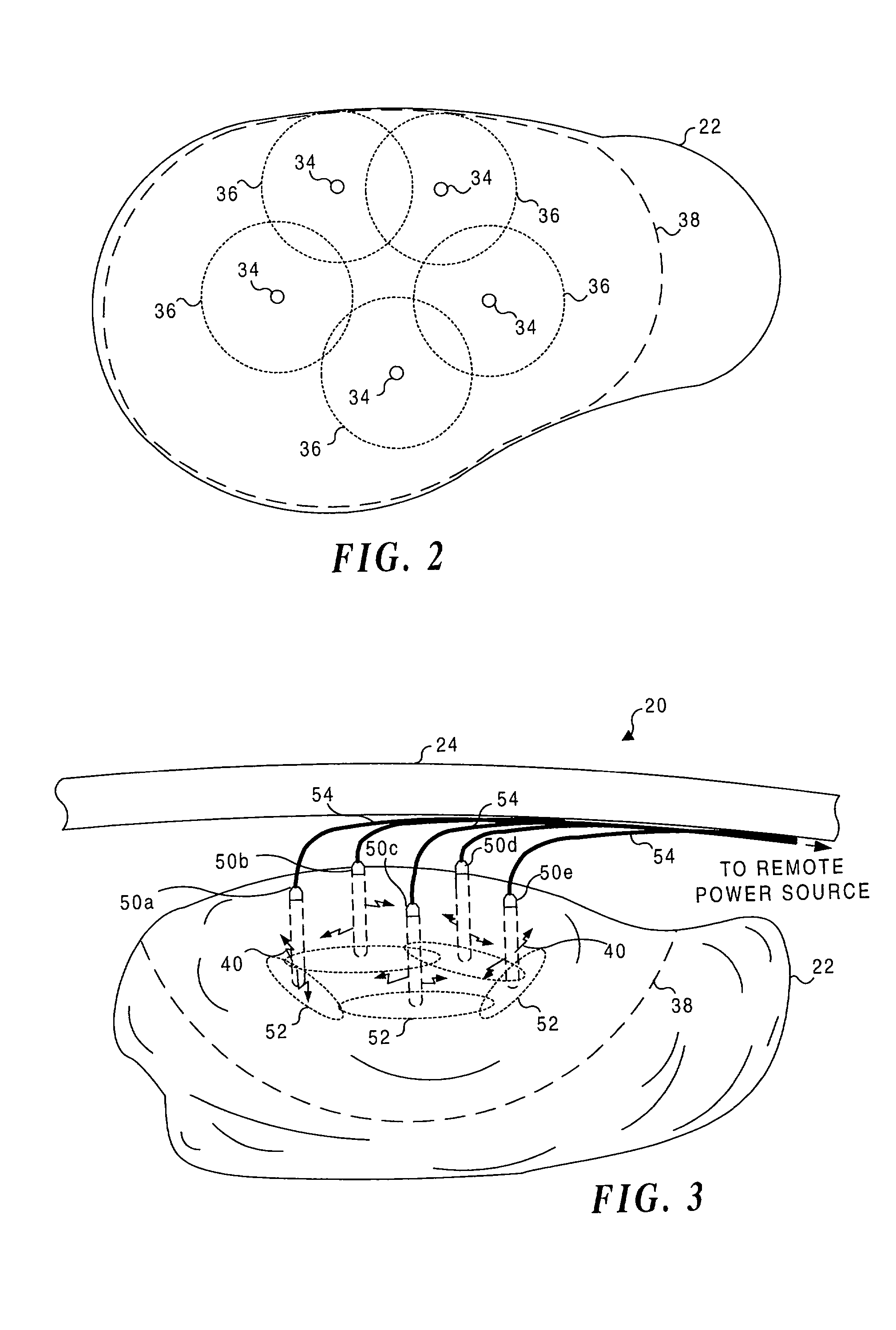
Application of light at plural treatment sites within a tumor to increase the efficacy of light therapy
Light is administered during photodynamic therapy (PDT) for an extended period of time at a plurality of sites distributed within the abnormal tissue of a tumor. A clinical study has shown that a substantially greater volume of abnormal tissue in a tumor is destroyed by the extended administration of light therapy from a plurality of probes than would have been expected based upon the teaching of the prior art. In this process, a plurality of light emitting optical fibers or probes are deployed in a spaced-apart array. After a photoreactive agent is absorbed by the abnormal tissue, the light therapy is administered for at least three hours. The greater volume of necrosis in the tumor is achieved due to one or more concomitant effects, including: the inflammation of damaged abnormal tissue and resultant immunological response of the patient's body; the diffusion and circulation of activated photoreactive agent outside the expected fluence zone, which is believed to destroy the abnormal tissue; a retrograde thrombosis or vascular occlusion outside of the expected fluence zone; and, the collapse of the vascular system that provides oxygenated blood to portions of the tumor outside the expected fluence zone. In addition, is possible that molecular oxygen diffusing and circulating into the expected fluence zone is converted to singlet oxygen during the extended light therapy, causing a gradient of hypoxia and anoxia that destroys the abnormal tissue outside the expected fluence zone.
Owner:LIGHT SCI ONCOLOGY
Retinal pigment epithelial cell cultures on amniotic membrane and transplantation
InactiveUS7824671B2Promotes growth and differentiationMaintenance of morphological appearanceBiocideSenses disorderSurgical GraftRetinal pigment epithelial cell
The present invention relates to a composition for implantation in the subretinal space of an eye, the composition including amniotic membrane, which may be cryopreserved human amniotic membrane, and a plurality of retinal pigment epithelial (RPE) cells or RPE equivalent cells present at the amniotic membrane. The amniotic membrane may be intact, epithelially denuded, or otherwise treated. The invention includes the use of amniotic membrane for the culturing of RPE cells thereon, forming a surgical graft for replacement of Bruch's membrane as a substrate, and for the transplanting of RPE cells to the subretinal space. The composition does not elicit immunological reactions to alloantigens or to RPE specific autoantigens; and exerts anti-inflammatory, and angiogentic, and anti-scarring effects. The invention includes methods and kits for making or using composites including amniotic membrane and RPE cells. Also disclosed is a device for harvesting RPE cells.
Owner:TISSUETECH INC
Recombinant avipox virus
The present invention provides a method for inducing an immunological response in a vertebrate to a pathogen by inoculating the vertebrate with a synthetic recombinant avipox virus modified by the presence, in a non-essential region of the avipox genome, of DNA from any source which encodes for and expresses an antigen of the pathogen. The present invention further provides a synthetic recombinant avipox virus modified by the insertion therein of DNA from any source, and particularly from a non-avipox source, into a non-essential region of the avipox genome.
Owner:HEALTH RES INC
Method for analyzing acridine ester labeled antigen or acridine ester labeled antibody
InactiveCN101609090AReduce consumptionProduct quality and safetyChemiluminescene/bioluminescenceBiological testingChemical structureAntigen
The invention relates to a chemiluminescent immunoassay method, in particular to a method for analyzing an acridine ester labeled antigen or an acridine ester labeled antibody and an immunoassay kit prepared by same. An acridine ester label is provided with a special luminescent group in a chemical structure, and the special group directly participates in luminescent reaction in the analyzing process of luminescent immunization; the substance does not have background luminescence usually, can be used for detecting a sample with low concentration or trace concentration in the reaction and is a luminescent agent with high luminescent efficiency; and molecules of acridine ester I and acridine ester II and acridine amide III can be combined with an antibody (antigen) to generate the labeled antibody with high chemiluminescent activity and immunological reaction specificity.
Owner:BEIJING ELCOTEQ BIO TECH
Enhanced immune response to an antigen by a composition of a recombinant virus expressing the antigen with a recombinant virus expressing an immunostimulatory molecule
InactiveUS20050063993A1Enhance immune responseAntibacterial agentsFungiAntigenPathogenic microorganism
The present invention is a composition of recombinant virus which has incorporated into its genome or portion thereof a gene encoding an antigen to a disease causing agent and a recombinant virus which has incorporated into its genome or portion thereof a gene encoding an immunostimulatory molecule(s) for the purpose of stimulating an immune response against the disease causing agent. Methods of treatment of diseases such as cancer and diseases caused by pathogenic microorganisms is provide using the composition.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
P153 and P156 antigens for the immunodiagnosis of canine and human ehrlichioses and uses thereof
Sequences encoding two immunoreactive glycoproteins were cloned from Ehrlichia canis (p153 gene) and Ehrlichia chaffeensis (p156 gene). These two glycoproteins are species-specific immunoreactive orthologs that are useful as subunit vaccines and for serologic and molecular diagnostics for E. canis and E. chaffeensis.
Owner:RES DEVMENT FOUND
Proteases producing an altered immunological response and methods of making and using the same
The present invention provides novel protein variants that exhibit reduced immunogenic responses, as compared to the parental proteins. The present invention further provides DNA molecules that encode novel variants, host cells comprising DNA encoding novel variants, as well as methods for making proteins less allergenic. In addition, the present invention provides various compositions that comprise these proteins that are less immunogenic than the wild-type proteins.
Owner:GENENCOR INT INC
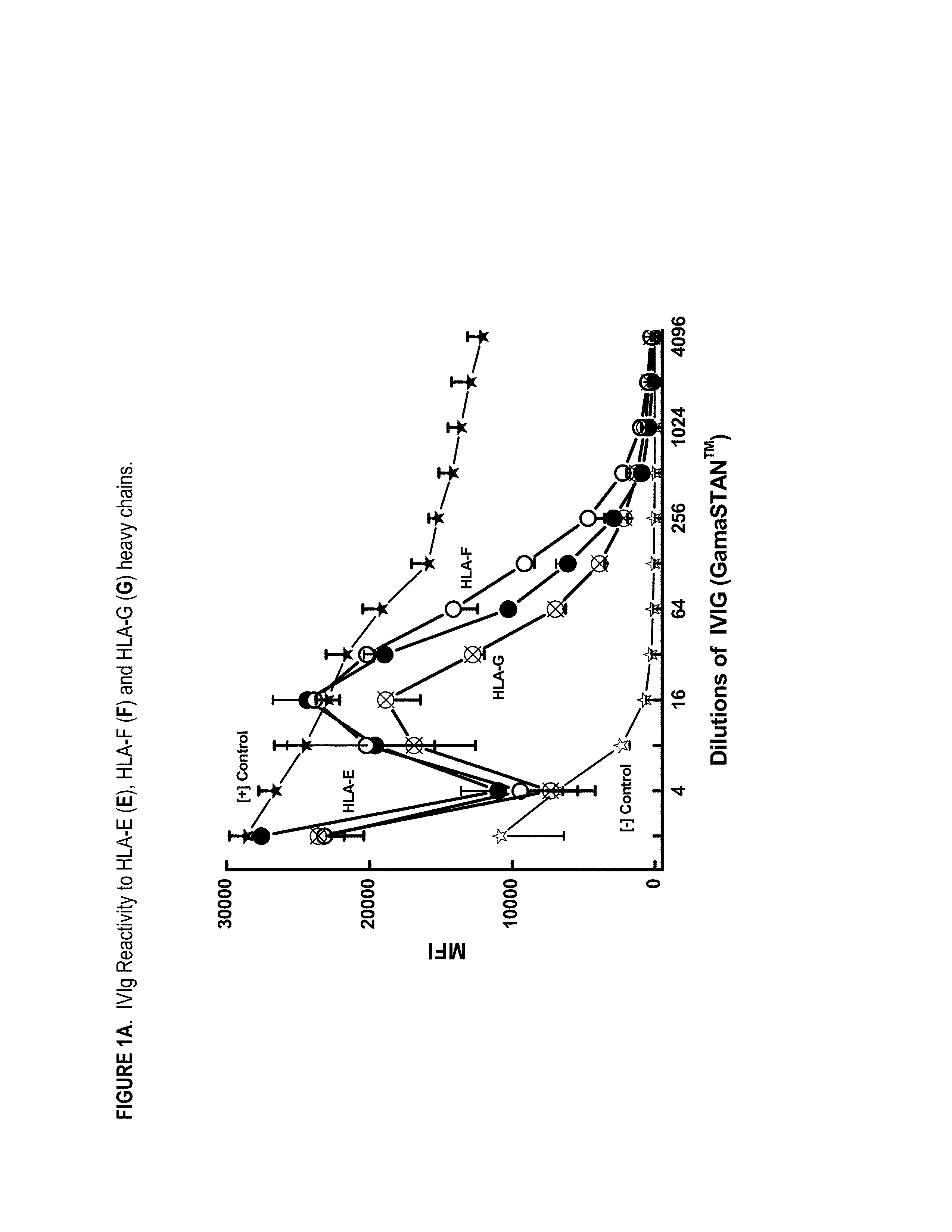
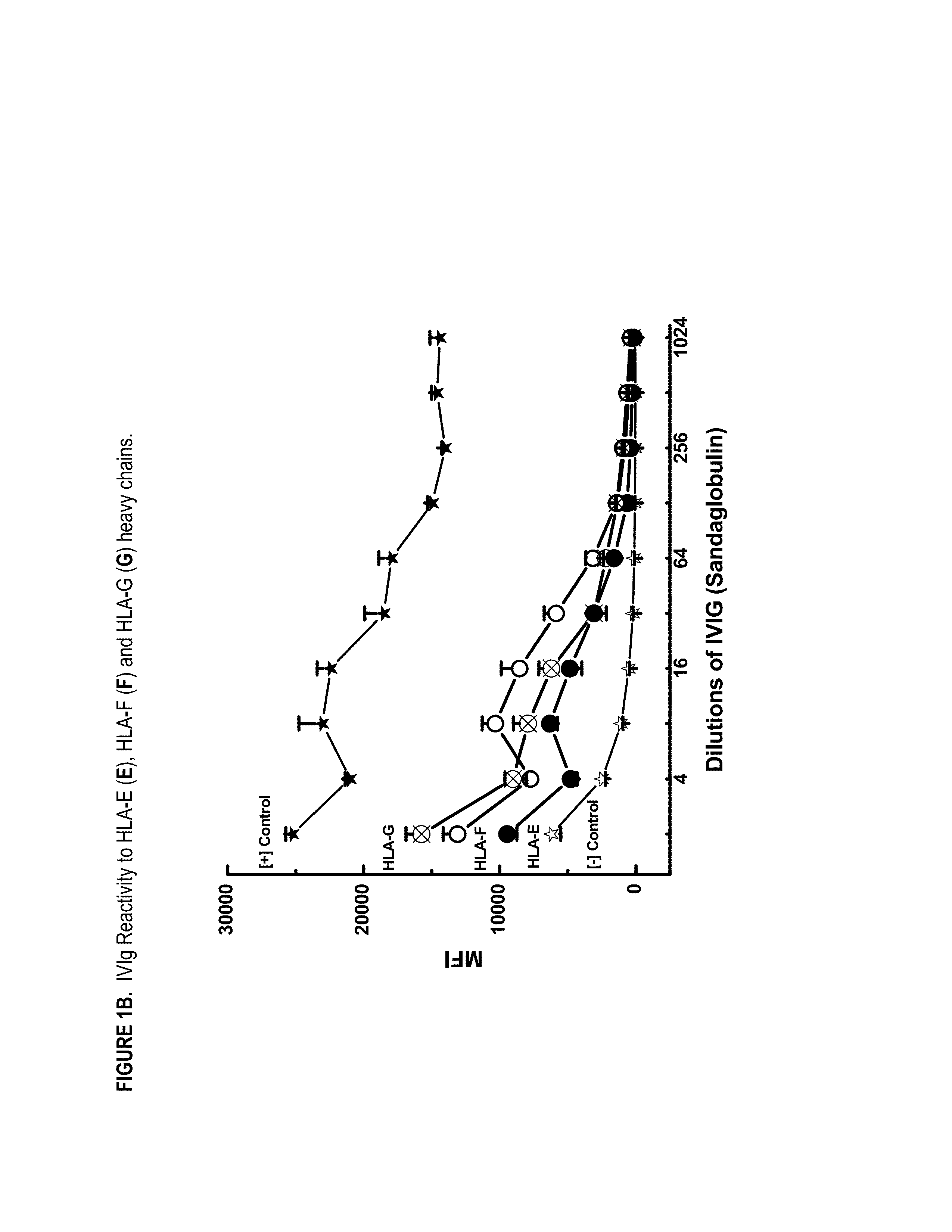
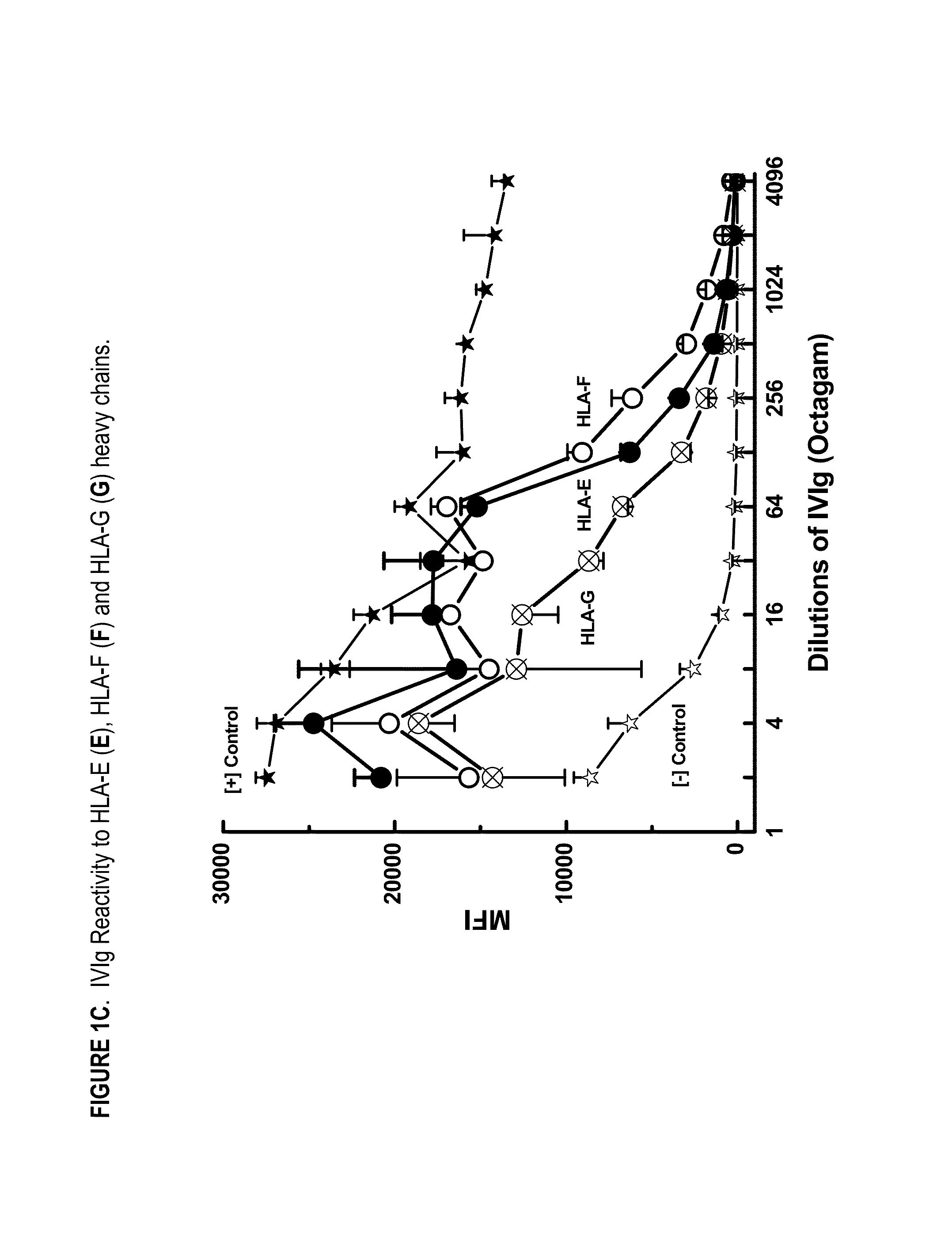
ANTI-HLA CLASS-Ib ANTIBODIES MIMIC IMMUNOREACTIVITY AND IMMUNOMODULATORY FUNCTIONS OF INTRAVENOUS IMMUNOGLOBULIN (IVIg) USEFUL AS THERAPEUTIC IVIg MIMETICS AND METHODS OF THEIR USE
InactiveUS20130177574A1Market price thereof has been risingMinimizing IVIg related side effectAntipyreticAnalgesicsDiseaseAntigen
Provided herein are compositions comprising anti-HLA-Ib antibodies as IVIg mimetics and methods for using the same for the prevention, treatment, therapy and / or amelioration of inflammation induced diseases and allograft rejection. In certain embodiments, the anti-HLA-Ib antibodies (monoclonal antibodies or mixed monoclonal antibodies, recombinant or chimeric or humanized or human antibodies) strongly mimic IVIg in immunoreactivity to HLA class Ia (HLA-A, HLA-B and HLA-Cw) and Ib antigens (HLA-E, HLA-F and HLA-G). In certain embodiments, the anti-HLA-Ib antibodies (monoclonal or mixed monoclonal antibodies; recombinant, chimeric, humanized or human antibodies) strongly mimic IVIg in immunomodulatory or immunosuppressive activities. While anti-HLA-Ib mAbs can be used to restore anti-tumor activities of CD8+ T cells and Natural killer cells by passive therapy in cancer patients, methods are also provided herein to induce production of polyclonal anti-HLA-Ib antibodies in cancer patients for restoring anti-tumor activities of CD8+ T cells and NK cells, by active specific immunotherapy.
Owner:RAVINDRANATH MEPUR DR
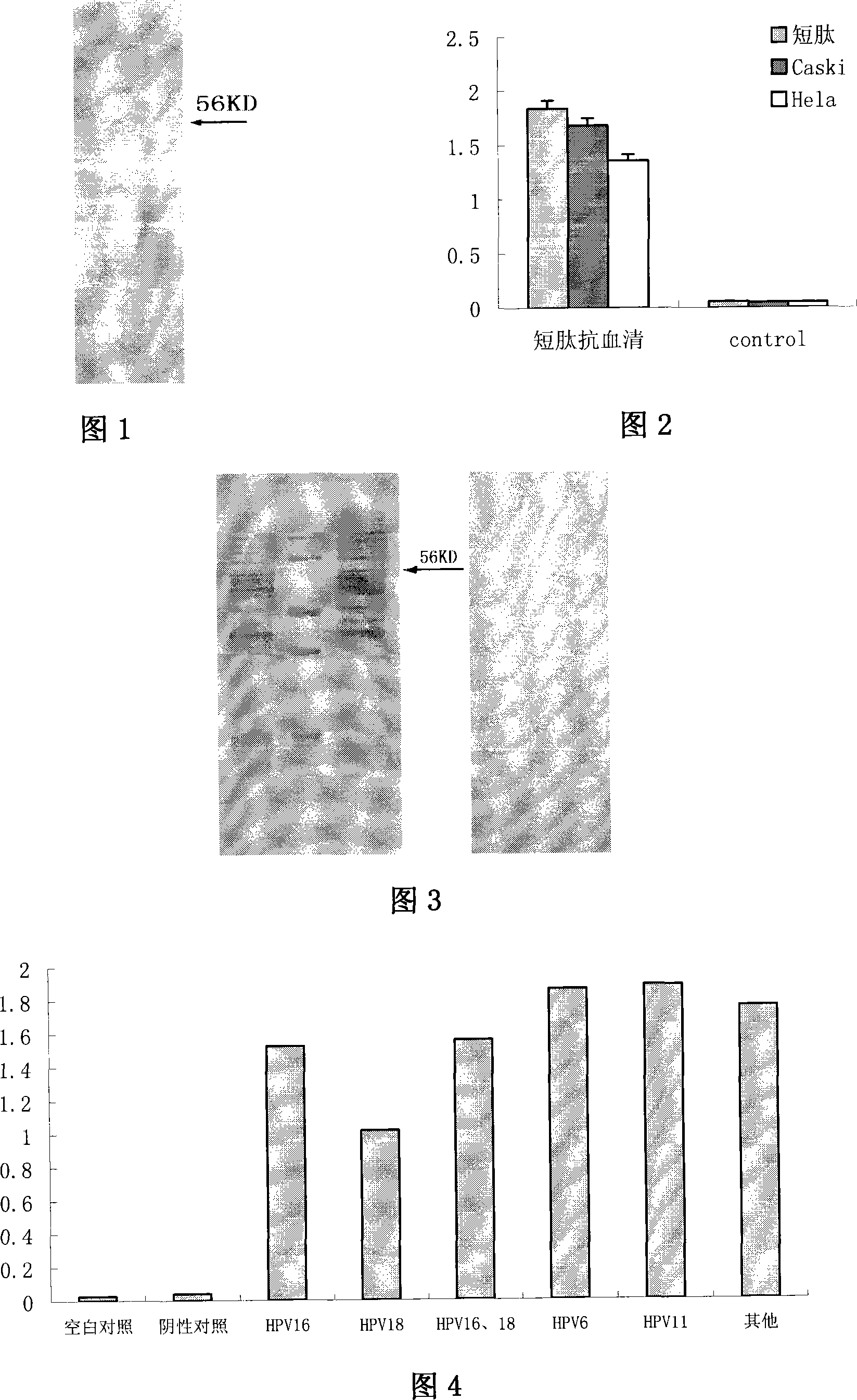
Human papillomavirus shell protein L1 short peptide and application thereof
InactiveCN101186636ASerum immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsAlphapapillomavirusCoat protein
The invention relates to a polypeptide, in particular to a human papilloma virus coat protein L1 short peptide and relative application. The invention is characterized in that the sequence of the human papilloma virus coat protein L1 short peptide is N-EVNLKEKFSADLDQFPLGRKFLLQAGLKAK-C. The invention can simultaneously induce human papilloma virus coat protein L1 short peptide with immunity reaction on high risk type and low risk type, which can induce and form the antibody for various human papilloma virus (HPV) and coat protein (HPV L1), to check various HPV or HPV L1, while the antibody can be used in biological pharmaceutical engineering, to purify and prepare various HPV or HPV L1.
Owner:CHINA THREE GORGES UNIV
Optical waveguide immunosensor and detection method thereof
InactiveCN101846674ASimple structureAccurate Immune Response DetectionColor/spectral properties measurementsBiological testingMagnetic beadAntibody antigen reactions
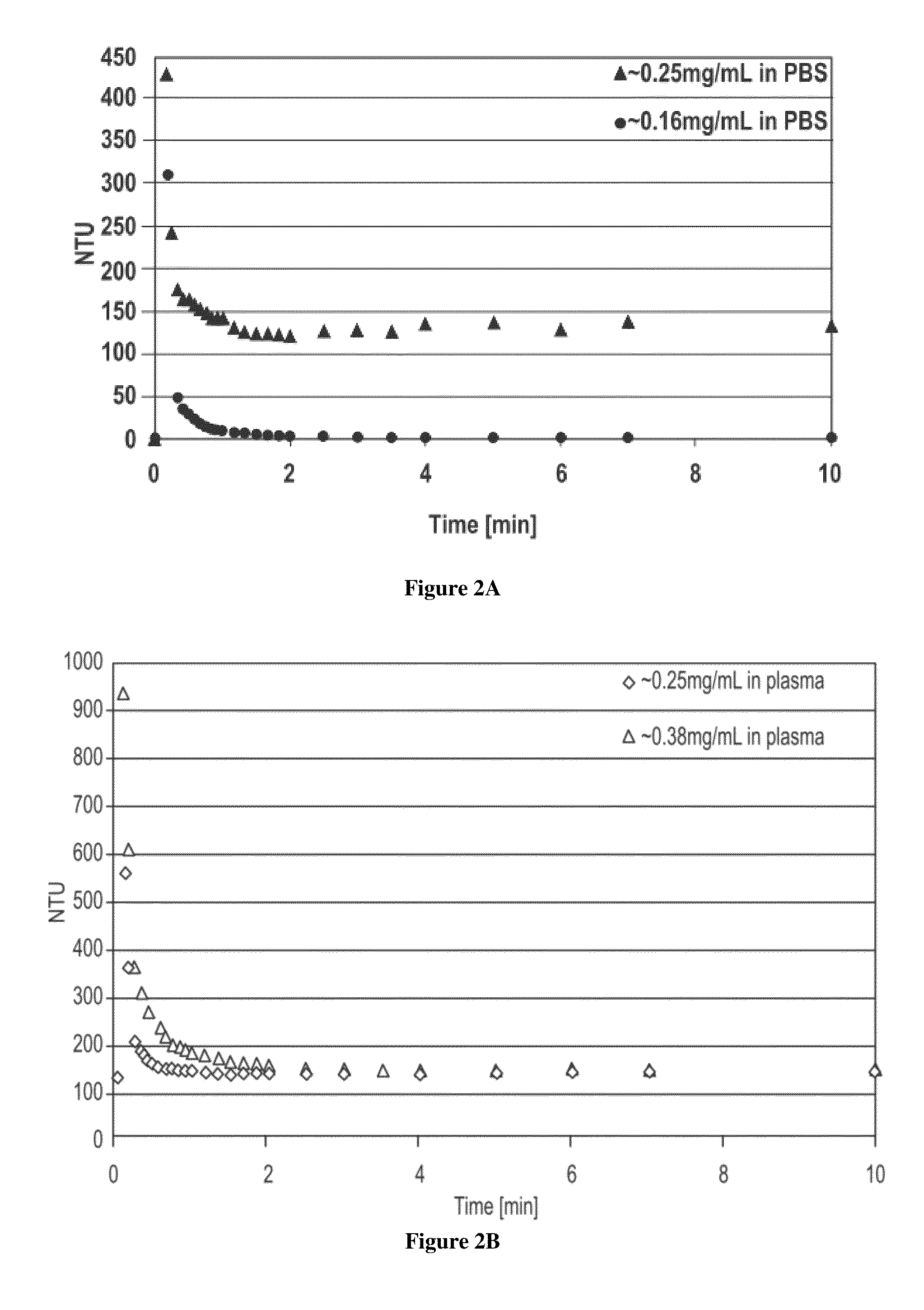
The invention discloses an optical waveguide immunosensor and a detection method thereof. The optical waveguide immunosensor basically comprises an optical waveguide resonator cavity, a magnetic needle and an immunologic reaction tank. The method comprises the following steps of: carrying out surface specificity modification to nano magnetic particles; capturing a substance to be detected by utilizing an antibody-antigen reaction; orderly limiting the nano magnetic particles on the surface of the circular or disc-shaped optical waveguide resonator cavity by utilizing the magnetic needle; sensing refraction index change before and after an immunologic reaction by utilizing an evanescent field; and analyzing corresponding resonance spectrum change to acquire the content of the substance to be detected in a sample. The optical waveguide immunosensor has simple structure. Based on the circular or disc-shaped optical waveguide resonator cavity with compact structure, the nano particles after immunologic specificity modification are absorbed to the optical waveguide surface by the magnetic needle to measure the offset of a resonance frequency and accurately and sensitively detect the immunologic reaction, and the device can be repeatedly used so that the invention has favorable application prospects.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH
Mucosal immunization to prevent prion infection
Vaccines against prion disease eliciting a humoral immune response when administered mucosally are described. The vaccines comprise a prion protein, a prion protein fragment, or a non-amyloidogenic prion protein homolog and an adjuvant suitable for inducing a humoral immune response after mucosal administration. Suitable adjuvants include cholera toxin subunit B, heat-labile enterotoxin and aluminum hydroxide. Alternatively, the vaccine comprises a vector encoding a prion protein, fragment, or homolog in an attenuated Salmonella host. The vaccines can be used to prevent or treat prion disease in humans and other mammals.
Owner:NEW YORK UNIV
Pharmaceutical composition for treatment and/or prophylaxis of cancer
ActiveUS20140178373A1Reduce usageUseful in treatmentNervous disorderSugar derivativesCancer preventionCancer cell
An object of the present invention is to prepare an antibody that targets CAPRIN-1 specifically expressed on the surface of cancer cells and is superior in antitumor activity to conventional antibodies and to provide use of the antibody as a therapeutic and / or preventive agent for cancer. The present invention provides use of an antibody targeting an identified cancer antigenic protein specifically expressed on the surface of cancer cells as a therapeutic and / or preventive agent for cancer, specifically, a pharmaceutical composition for treatment and / or prevention of cancer, comprising as an active ingredient an antibody or a fragment thereof which has immunological reactivity with a CAPRIN-1 protein, the antibody or the fragment thereof comprising a heavy chain variable region comprising amino acid sequences represented by SEQ ID NOs: 5, 6, and 7 and a light chain variable region comprising amino acid sequences represented by SEQ ID NOs: 9, 10, and 11.
Owner:TORAY IND INC
Lipid and Nitrous Oxide Combination as Adjuvant for the Enhancement of the Efficacy of Vaccines
InactiveUS20090010964A1Improve actionStimulate immune responseAntibacterial agentsBacterial antigen ingredientsAntigenAdjuvant
The invention provides for a method of enhancing immunological responses to an antigen in a vaccine formulation, and for a vaccine formulation that provides for an enhanced immunological response to an antigen. In the method and formulation the antigen is administered with an adjuvant which adjuvant comprises a solution of nitrous oxide gas in a pharmaceutically acceptable carrier solvent for the gas and which adjuvant includes at least one fatty acid or ester or other suitable derivative thereof selected from the group consisting of oleic acid, linoleic acid, alpha-linolenic acid, gamma-linolenic acid, arachidonic acid, eicosapentaenoic acid [C20: 5ω3], decosahexaenoic acid [C22: 6ω3], ricinoleic acid and derivatives thereof selected from the group consisting of the C1 to C6 alkyl esters thereof, the glycerol-polyethylene glycol esters thereof and the reaction product of hydrogenated natural oils composed largely of ricinoleic acid based oils, such as castor oil with ethylene oxide.
Owner:EXHAUSTO +1
Construction of live attenuated Shigella vaccine strains that express CFA/I antigens (cfaB and CfaE) and the B subunit of heat-labile enterotoxin (LTB) from enterotoxigenic E.coli
ActiveUS20070237791A1Reduce intrusionBacterial antigen ingredientsBacteriaHeterologousMucosal Immune Responses
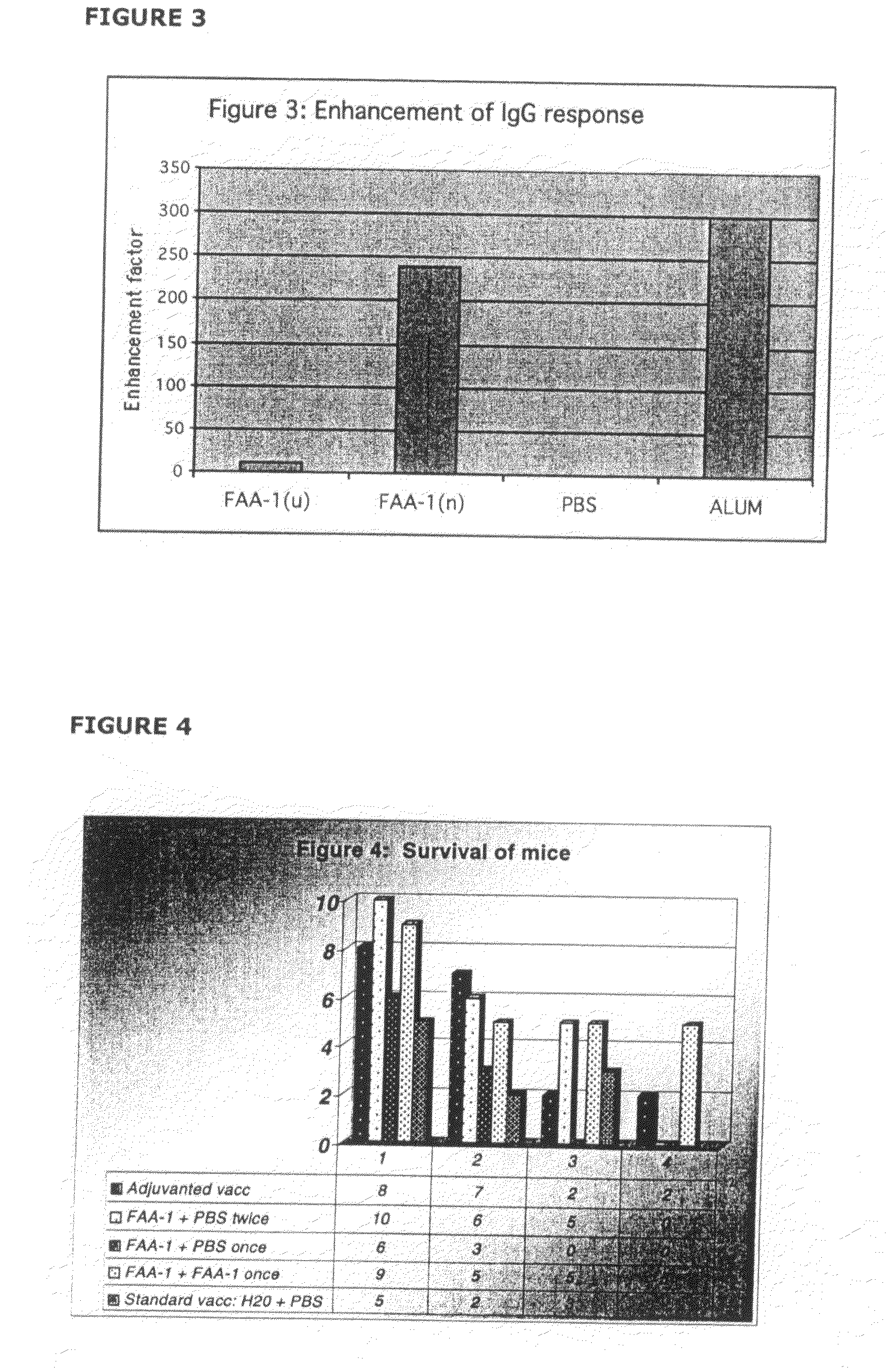
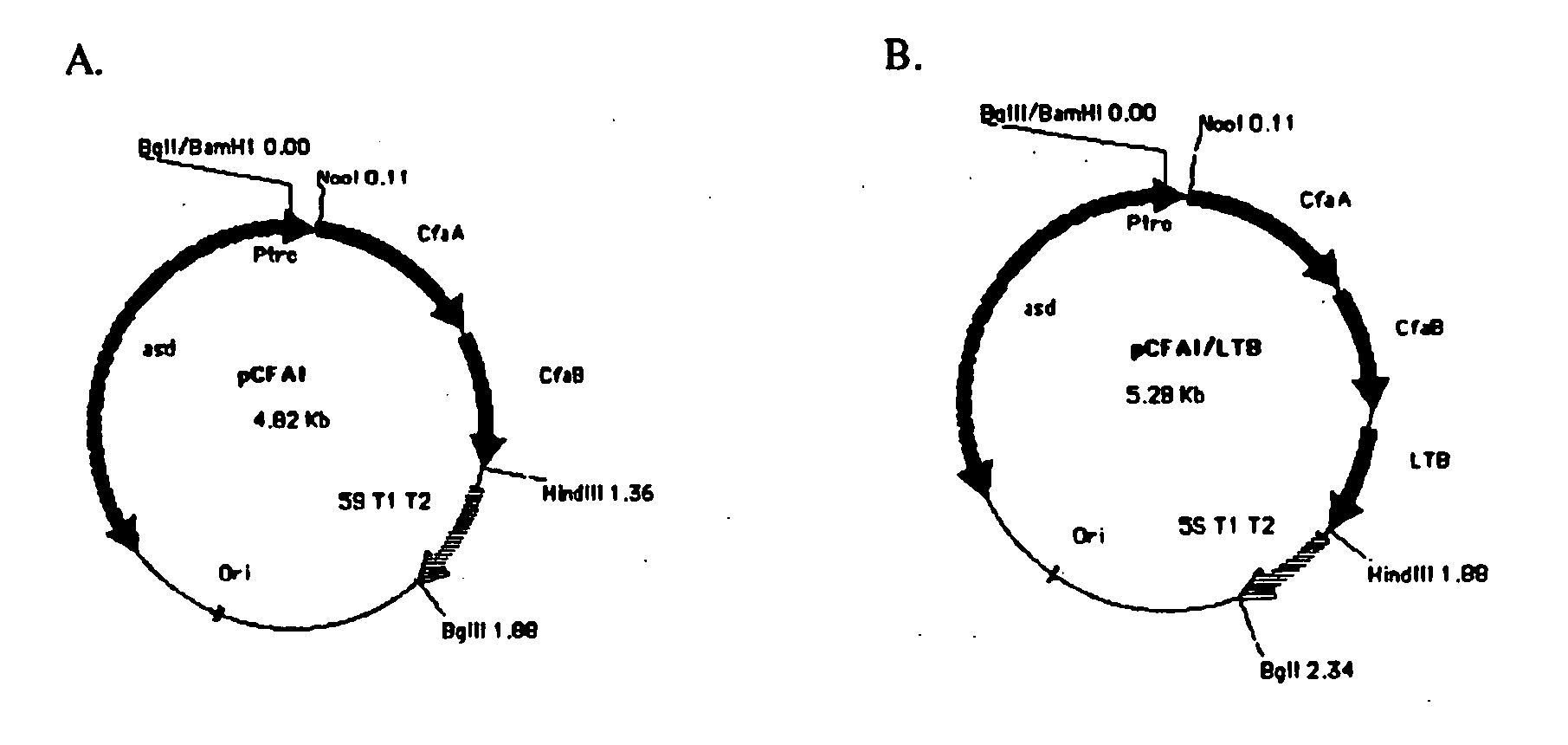
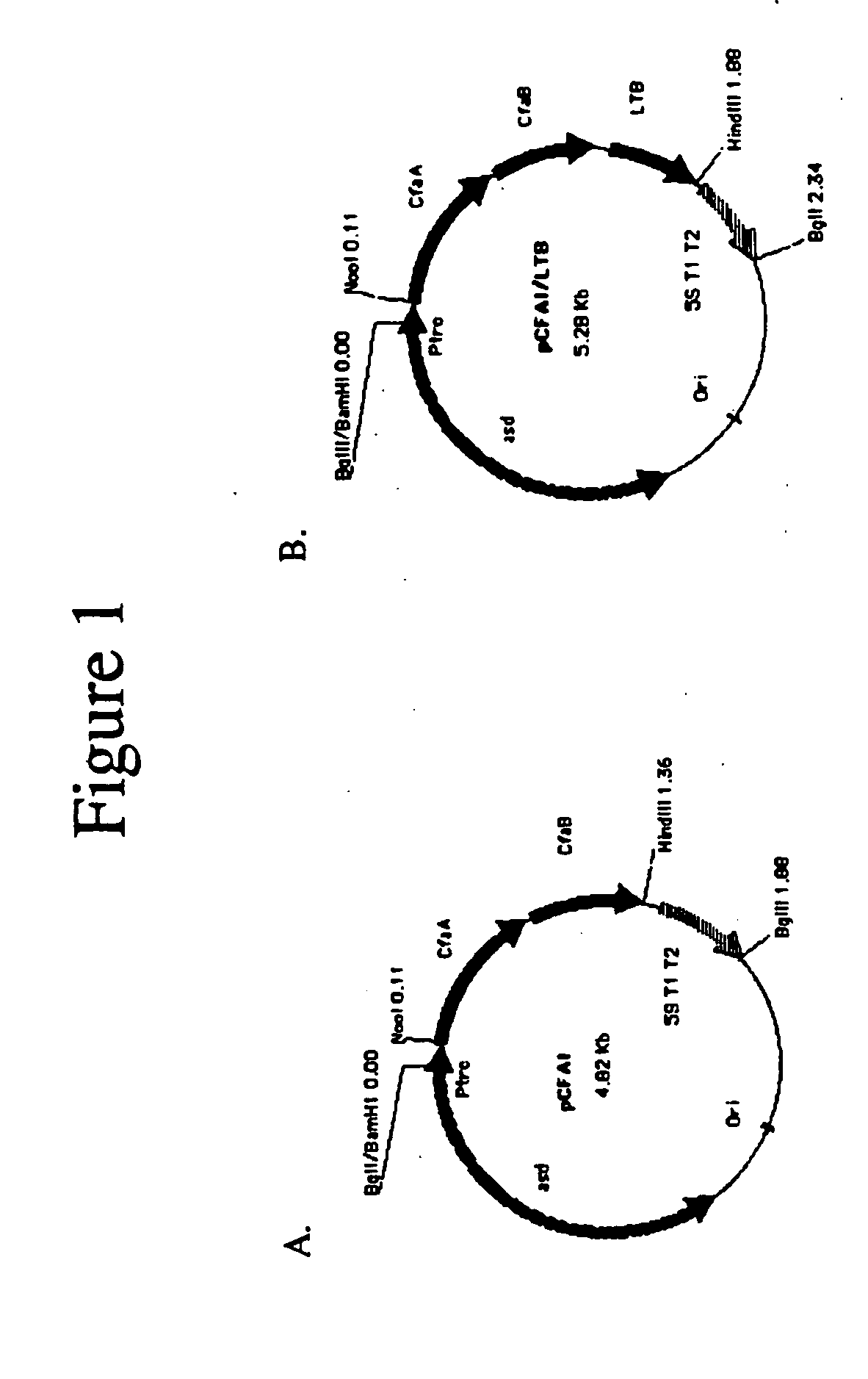
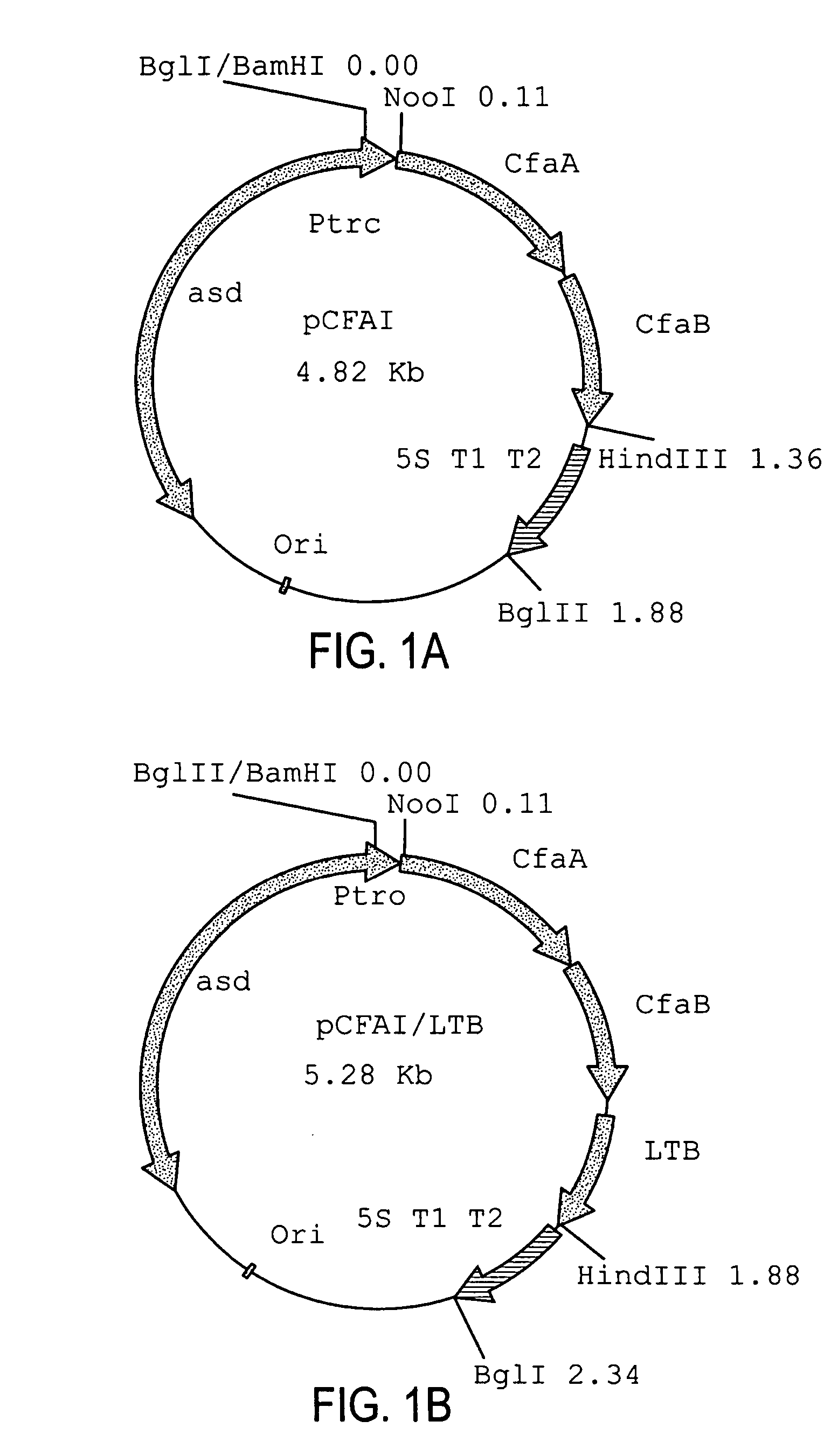
With the goal of creating a combination vaccine against Shigella and other diarrheal pathogens we have constructed a prototype vaccine strain of Shigella flexneri 2a (SC608) that can serve as a vector for the expression and delivery of heterologous antigens to the mucosal immune system. SC608 is an asd derivative of SC602, a well-characterized vaccine strain, which has recently undergone several phase 1 and 2 trials for safety and immunogenicity. Using non-antibiotic asd-based plasmids, we have created novel constructs for the expression of antigens from enterotoxigenic E. coli (ETEC), including CFA / I (CfaB and CfaE) and the B-subunit from heat-labile enterotoxin (LTB) in Shigella vaccine strain SC608. Heterologous protein expression levels and cellular localization are critical to immune recognition and have been verified by immunoblot analysis. Following intranasal immunization (SC608(CFAI) and SC608(CFAI / LTB) of guinea pigs, serum IgG and IgA immune responses to both the Shigella LPS and ETEC antigens can be detected by ELISA. In addition, ELISPOT analysis for ASCs from cervical lymph nodes and spleen showed similar responses. All vaccine strains conferred high levels of protection against challenge with wild-type S. flexneri 2a using the Sereny test. Furthermore, serum from guinea pigs immunized with SC608 expressing CfaB and LTB contained antibodies capable of neutralizing the cytological affects of heat-labile toxin (HLT) on Chinese Hamster Ovary (CHO) cells. These initial experiments demonstrate the validity of a multivalent invasive Shigella strain that can serve as a vector for the delivery of pathogen-derived antigens.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Production of multivalent virus like particles
InactiveUS20070041999A1Easily controlQuick cutSsRNA viruses positive-sensePeptide/protein ingredientsPopulationFusion peptide
The present invention is directed to the production and in vitro assembly of recombinant viral capsid proteins into virus like particles. In particular, the present invention provides rapid, scalable, and cost efficient methods for the production of multivalent virus like particles utilizing separate populations of capsid fusion peptides containing differing antigenic peptide inserts that are combined in vitro to produce homogenous populations of multivalent virus like particles. The virus like particles produced according to the present invention can be utilized to induce an immunological response in human or animal.
Owner:PFENEX
Reagent box for detecting nature of third type hepatitis virus antibody by using chemiluminescence method
InactiveCN101551397AHigh sensitivitySuit one's needsChemiluminescene/bioluminescenceBiological testingSubstance amountAntigen
The present invention discloses a reagent box for detecting nature of third type hepatitis virus antibody by using chemiluminescence method which includes an immunoreaction titration micropore plate and a detecting reagent, the immunoreaction titration micropore plate is a non-transparent polystyrol micropore plate; the detecting reagent includes third type hepatitis viruse antigen, enzyme label antihuman IgG, irradiancy substrates A liquor and irradiancy substrates B liquor. The reagent box uses chemiluminescence immune analysis method for detecting third type hepatitis virus antibody, the irradiancy substrate reacts chemically and releases mass energy by using enzyme catalysis irradiancy substrates for generating an excitation state intermediate body. When the excitation state intermediate body returns to a stable basic state, the excitation state intermediate body can transmit light quantum, utilize an irradiancy signal measurement instrumentation for measuring light quantum yield, the light quantum yield has a direct proportion with a waited-measured substance amount in sample. The method has high sensitivity, high precision and wide linear range; the reagent box has low manufacturing cost which can reduce patient burden and satisfy clinical requirement.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Functional peptide segment of epididymis protease inhibitors and uses thereof
InactiveCN101402674AImproving immunogenicityStimulationImmunoglobulins against animals/humansAntibody medical ingredientsEpididymal protease inhibitorBlood plasma
The invention discloses an epididymis protease inhibitor which is a functional peptide segment of Eppin. The inhibitor is any one selected from amino acid sequences shown from SEQ ID No.1 to SEQ ID No.7 and has advantages of single component, simple structure and strong pertinence of immunoreaction inspired; polypeptide with at least 80 percent of sequences the same as the functional peptide segment of Eppin can be used for constructing immunogen which can improve the immunogenicity of the functional peptide segment of Eppin, stimulate an organism to generate a specific antibody, reduce Eppin level in blood plasma and induce high-efficient, safe and reversible antifertility effect. The immunogen of the epididymis protease inhibitor can form a medicament composite with medically acceptable carriers, and the composite can be used for preparing male contraception vaccines, therefore, the epididymis protease inhibitor has broad application prospect.
Owner:ARMY MEDICAL UNIV
Oncolytic virus construction body, oncolytic virus and application thereof
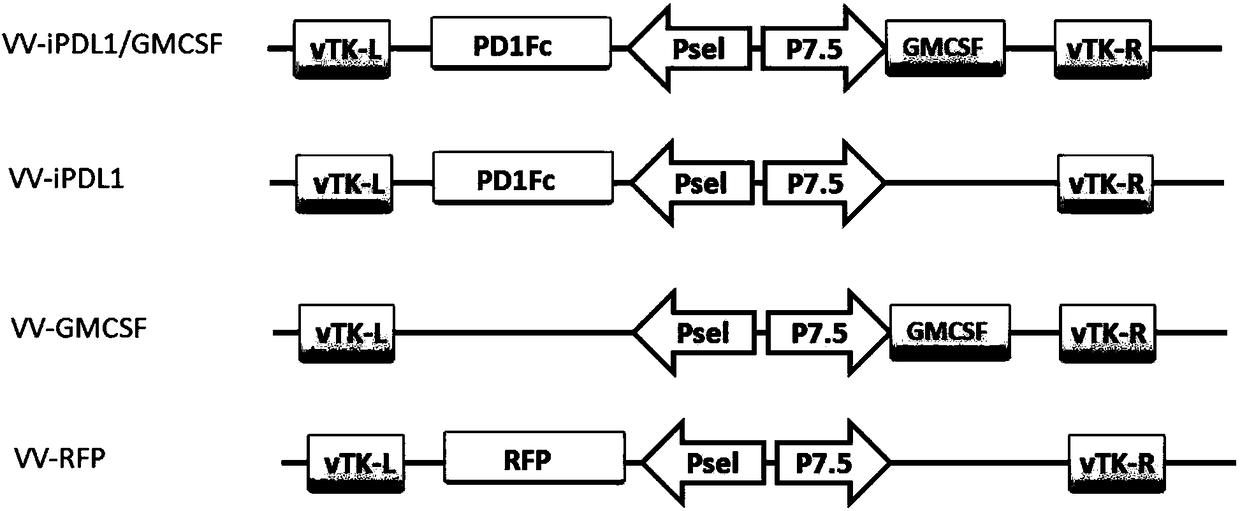
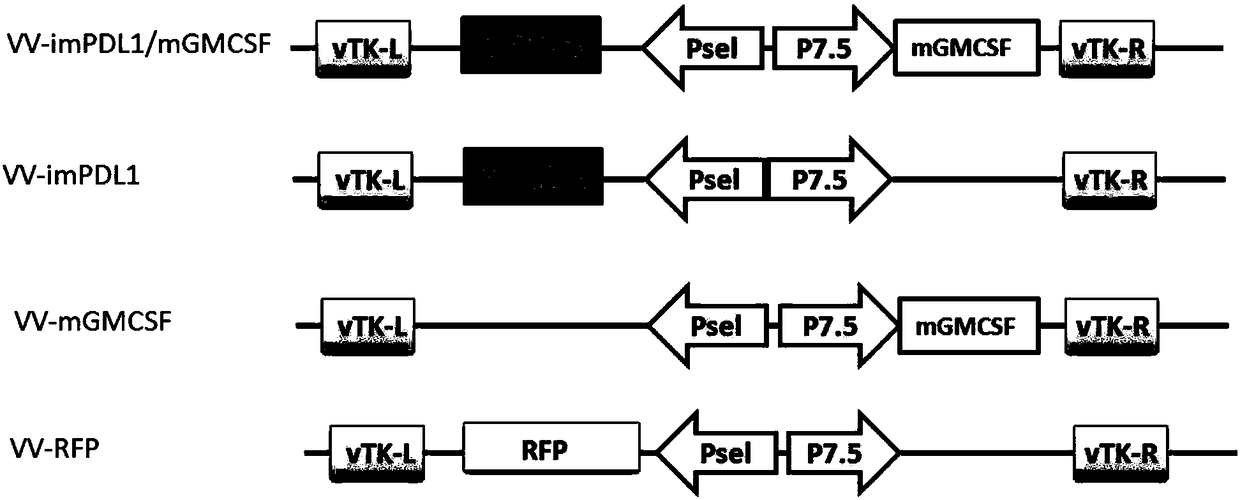
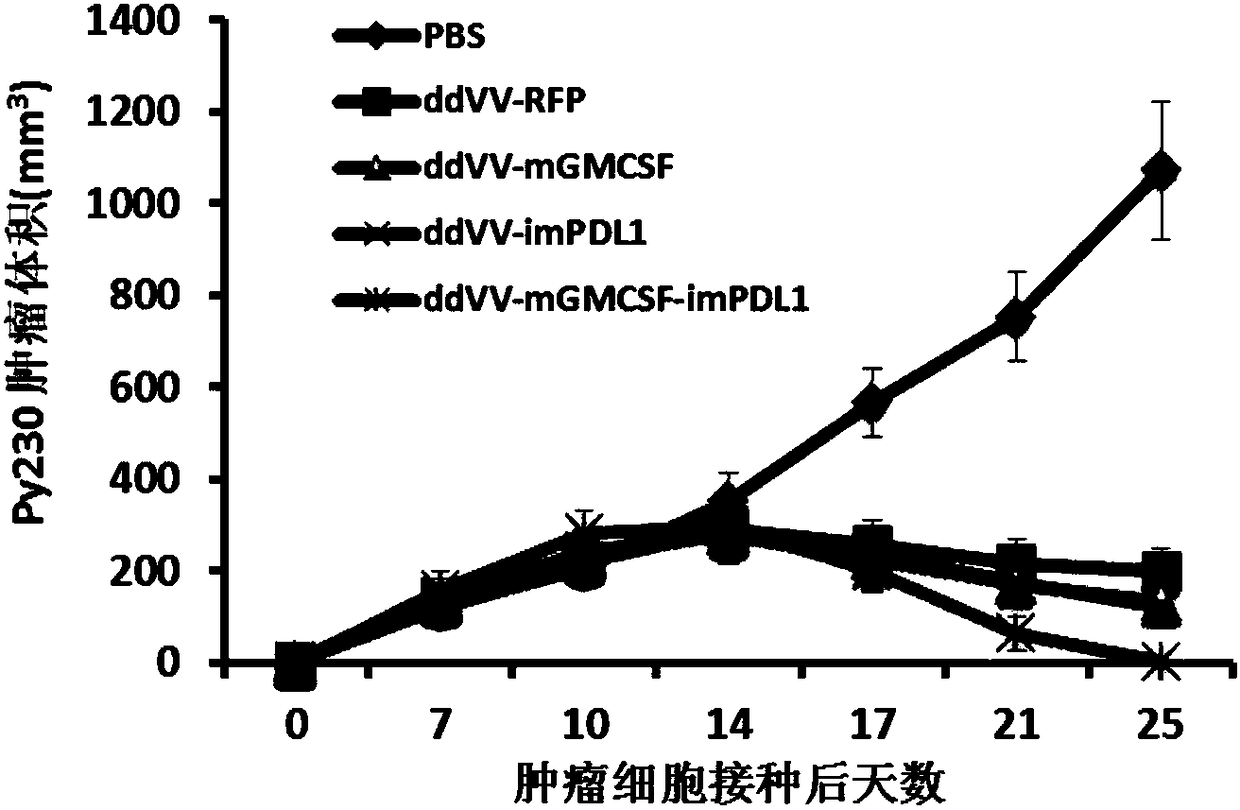
InactiveCN108728488APrevent proliferationSignificant effectAntibody mimetics/scaffoldsUnknown materialsOncolytic adenovirusTumor specific
The invention provides an oncolytic virus construction body, an oncolytic virus and application thereof. The oncolytic virus construction body comprises a first nucleic acid molecule and a second nucleic acid molecule, wherein the first nucleic acid molecule encodes a secreted fusion protein molecule; the secreted fusion protein molecule is used for inhibiting immune checkpoints; and the second nucleic acid molecule encodes an immunostimulant molecule. According to the embodiment of the invention, the construction body encodes fusion protein and immunostimulant molecules for inhibiting the immune checkpoints and can inhibit immunosuppression mechanism mediated by the immune checkpoints and immunoreactions which causes individual tumor specificity so as to realize generalized and effectivetumor cell killing.
Owner:LIFESEQ LTDRP
Preparation and detection method for ELISA kit detecting Fumonisins
InactiveCN103792356ASimple and fast operationSensitive detectionBiological material analysisAntigenElisa kit
The invention relates to a preparation and detection method for an ELISA kit detecting Fumonisins. The ELISA kit has the characteristics of sensitive, accurate and fast detection, simple operation and strong specificity, and is suitable for detection of a large number of samples. The kit includes: a Fumonisins antigen coated enzyme label plate, a Fumonisins standard substance, a Fumonisins antibody working solution, a Fumonisins enzyme labeled secondary antibody working solution, a substrate solution A, a substrate solution B, a terminating solution, a concentrated sample dilution solution and a concentrated washing solution. The principle of the Fumonisins detection kit is solid-phase indirect competitive enzyme-linked immunosorbent assay reaction. The extracted sample, the enzyme labeled secondary antibody working solution, and the antibody working solution are added into corresponding enzyme labeled holes, after incubation for a period of time, the substrate solution A and the substrate solution B are added into a washing plate, and under the action of enzyme, the holes can present a blue color. Then the terminating solution is added, and the color changes to yellow from blue. The color depth and the content of Fumonisins in the standard substance or the sample are in an inverse proportion relationship. This method can be directly used for detecting Fumonisins in maize.
Owner:JIANGSU WISE SCI & TECH DEV
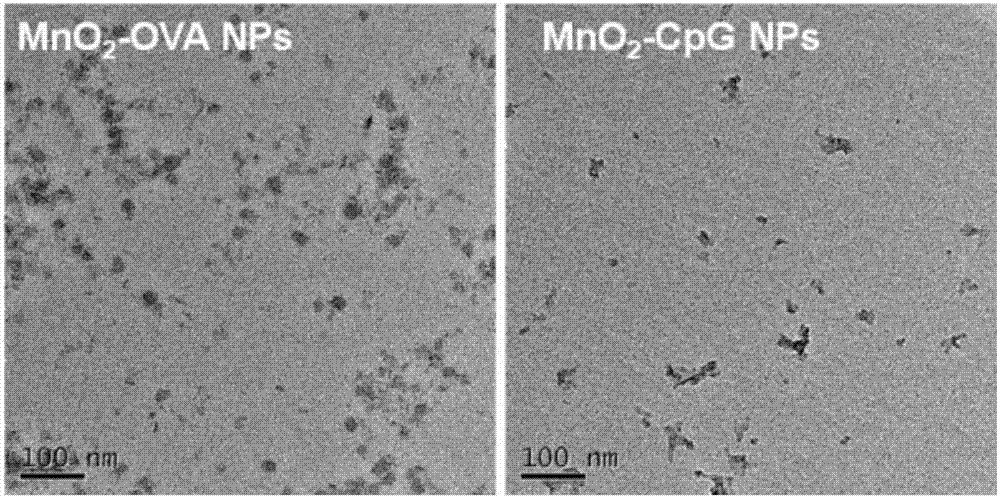
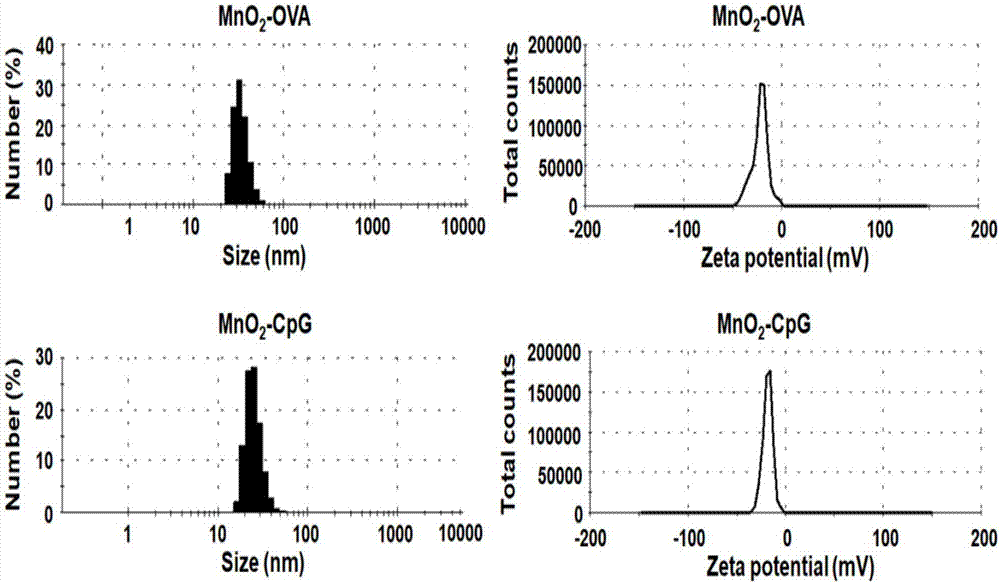
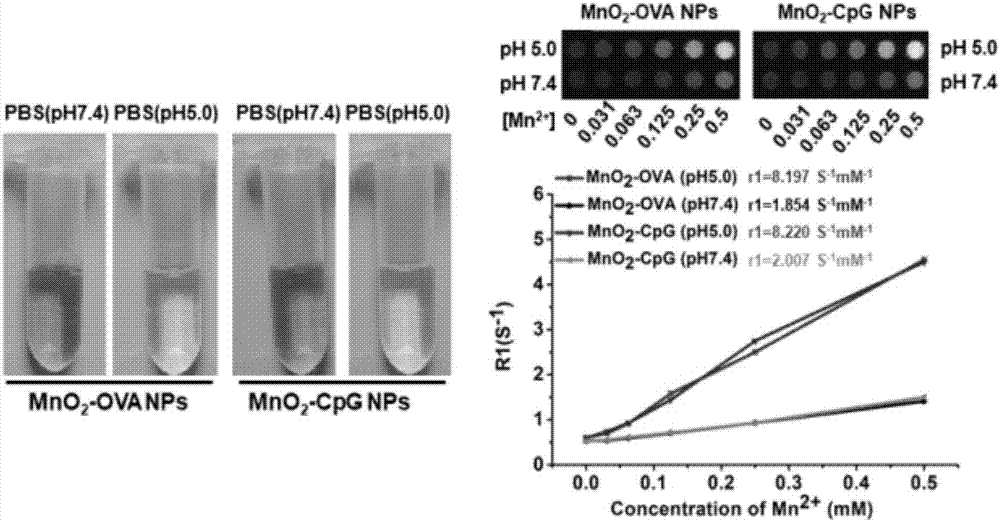
Manganese dioxide nanometer adjuvant and preparation method and application thereof
PendingCN107456575AIncrease intakeImproving immunogenicityAntibody medical ingredientsAgainst vector-borne diseasesStimulantLysosome
The invention provides a manganese dioxide nanometer material. The manganese dioxide nanometer material comprises manganese dioxide nanoparticles and carried matter, wherein the carried matter is compounded onto the surface of the manganese dioxide nanoparticle; the carried matter comprises oligonucleotide CpG and / or antigen; the antigen comprises protein antigen and / or polypeptide antigen. The provided manganese dioxide nanometer material has the advantages that the immunostimulation function of an immunologic adjuvant CpG is greatly improved, the immunity protection function of vaccine is improved, and the inherent defect of effective improving of immune reaction by a large amount of CpG because the oligonucleotide CpG is used as an immunologic stimulant and cannot easily enter the body lymph glands is solved; the manganese dioxide nanometer material is used as a vaccine adjuvant, and can carry the antigen, especially the protein antigen, so that the intake on the antigen by the immune cells in the lymph glands is effectively improved, and the immunogenicity of the antigen is improved; the nanometer adjuvant can be biologically degraded, and can be gradually degraded into manganese ions (Mn<2+>) under the weak acid environment, such as lysosome, and the manganese ions are finally discharged out of the human body.
Owner:SUZHOU INNOVATIVE BIOMATERIALS & PHARM CO LTD
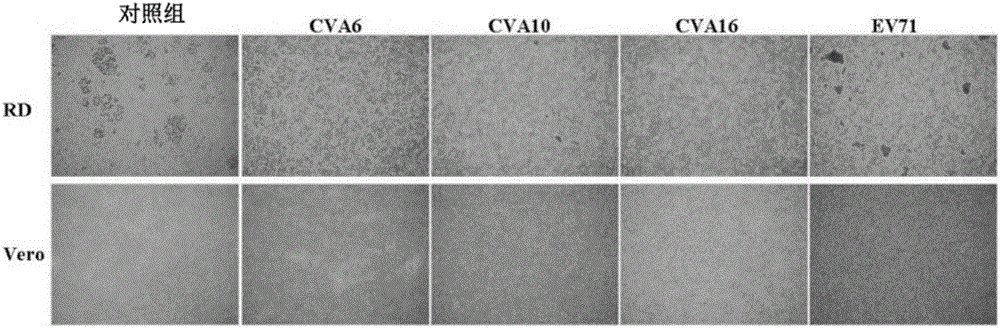
Viral particles as immunogens against enterovirus infection and production thereof
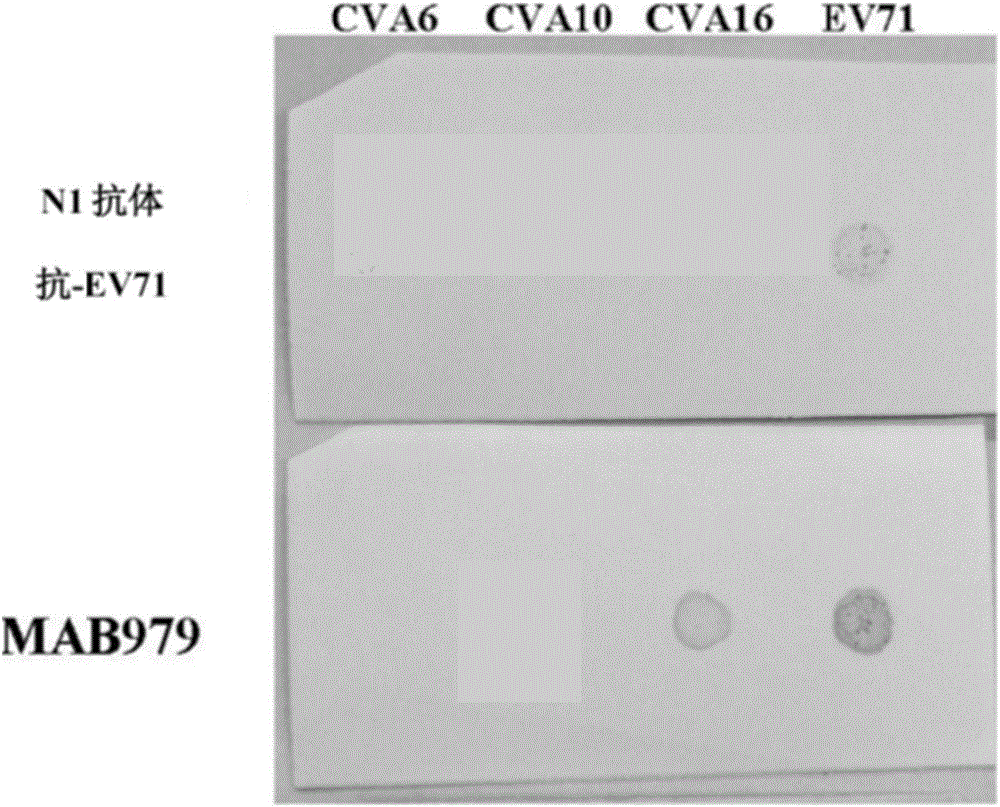
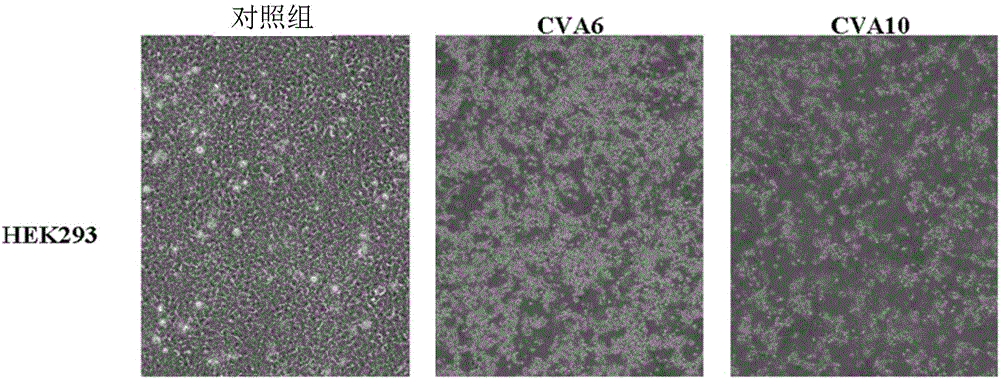
The present invention relates to viral particles as immunogens against enterovirus infection and a method of producing the same. Specifically, the present invention features that human embryo kidney 293 (HEK 293) cells are used to produce viral particles of Enterovirus A, particularly Coxsackievirus A6 (CVA6) particles or Coxsackievirus A10 (CVA10) particles or both and optionally additional viral particles of other Enterovirus A e.g. Coxsackievirus A16 (CVA16) and / or Enterovirus A71 (EV71). The yield of the viral particles in HEK 293 cells is unexpectedly high and effective to induce an immune response against enterovirus infection, especially CVA6 and CVA10. The present invention also relates to an immunogenic composition against enterovirus infection for human use comprising the viral particles as described herein and a method of preventing enterovirus infection or a disease as caused, particularly Hand-Foot-Mouth diseases (HFMD), by administering the immunogenic composition to a subject in need thereof.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH +1
Magnetic bead preparing method and application
ActiveCN104538168AHigh magnetic contentRich surface chargeMagnetsInductances/transformers/magnets manufactureCross-linkFluorescence
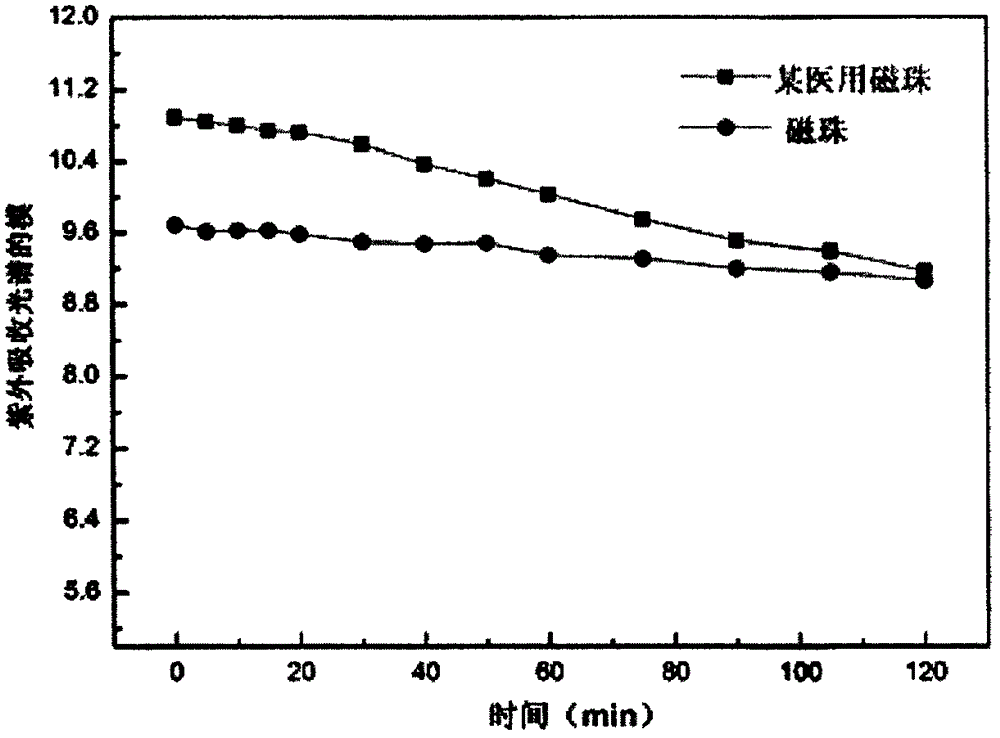
The invention discloses a magnetic bead preparing method and application, and belongs to the technical field of magnetic material preparing. The preparing method comprises the steps that firstly, magnetic nanoparticles with the surface functionalization are prepared, then, at least two magnetic nanoparticles with the surface functionalization are connected through a cross-linking agent, and a magnetic bead with the magnetic content of 70 %-98 % is prepared. The problems that the magnetic content of an existing magnetic bead is low, to achieve fast separation, the size of the magnetic bead is greatly increased, and consequently the suspension stability is reduced are solved. In addition, for the captured component biological reaction, particularly for the immunoreaction, the steric hindrance caused by the small magnetic bead is obviously reduced, the capturing capability and the analyzing and detecting sensitivity are improved, the magnetic bead can be better applied to the biological medicine separation and analysis, and the magnetic bead is particularly suitable for separation of cells, bacteria, viruses, DNA / RNA, protein and the like in biological body fluid or reaction fluid and the clinical biochemistry fast automatic detection based on chemiluminiscence electrochemical luminescence and fluorescence.
Owner:SUZHOU UNIV
Methods And Compositions For Reducing Pain, Inflammation, And/Or Immunological Reactions Associated With Parenterally Administering A Primary Therapeutic Agent
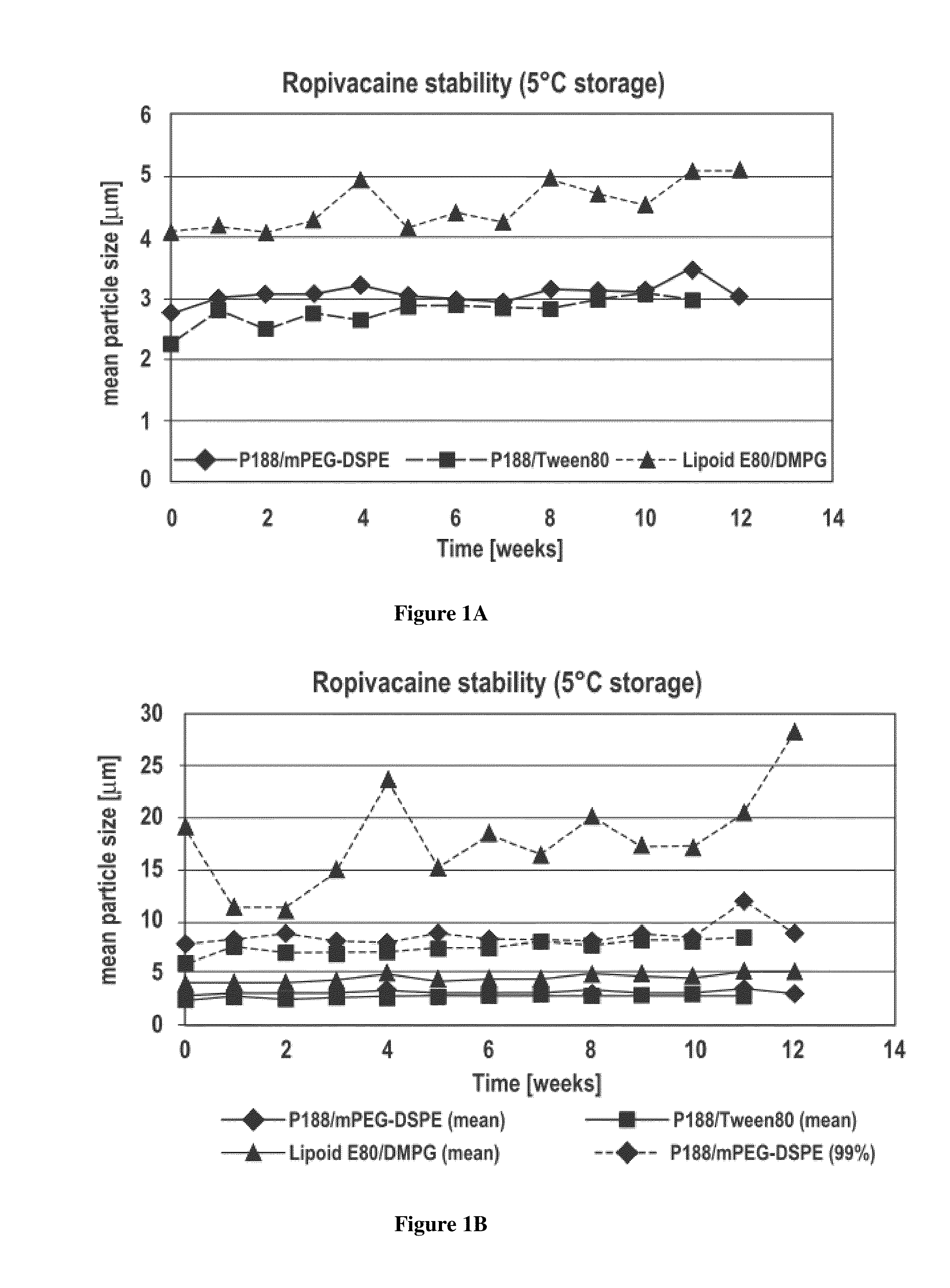
Disclosed herein are methods and pharmaceutical compositions for reducing the pain associated with parenterally administering a therapeutic agent. The methods and compositions comprise a dispersion comprising microparticles of an analgesic agent in an amount effective to reduce the pain, inflammation, and / or immunological reaction associated with parenterally administering a primary therapeutic agent, wherein the microparticles of the analgesic agent have an effective particle size of less than 20 micrometers.
Owner:BAXTER INT INC +1
Methods of evaluating a test agent in a diseased cell model
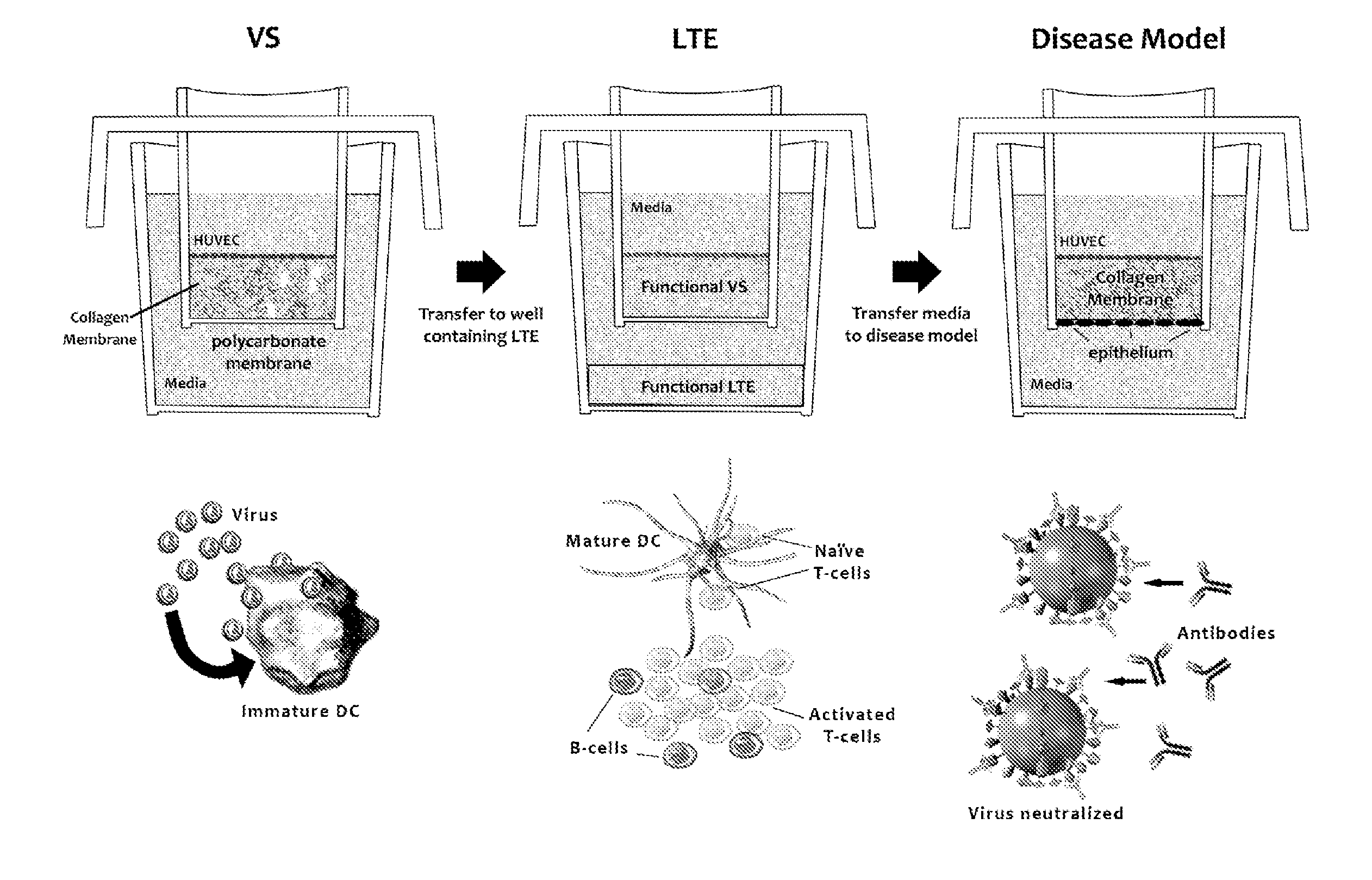
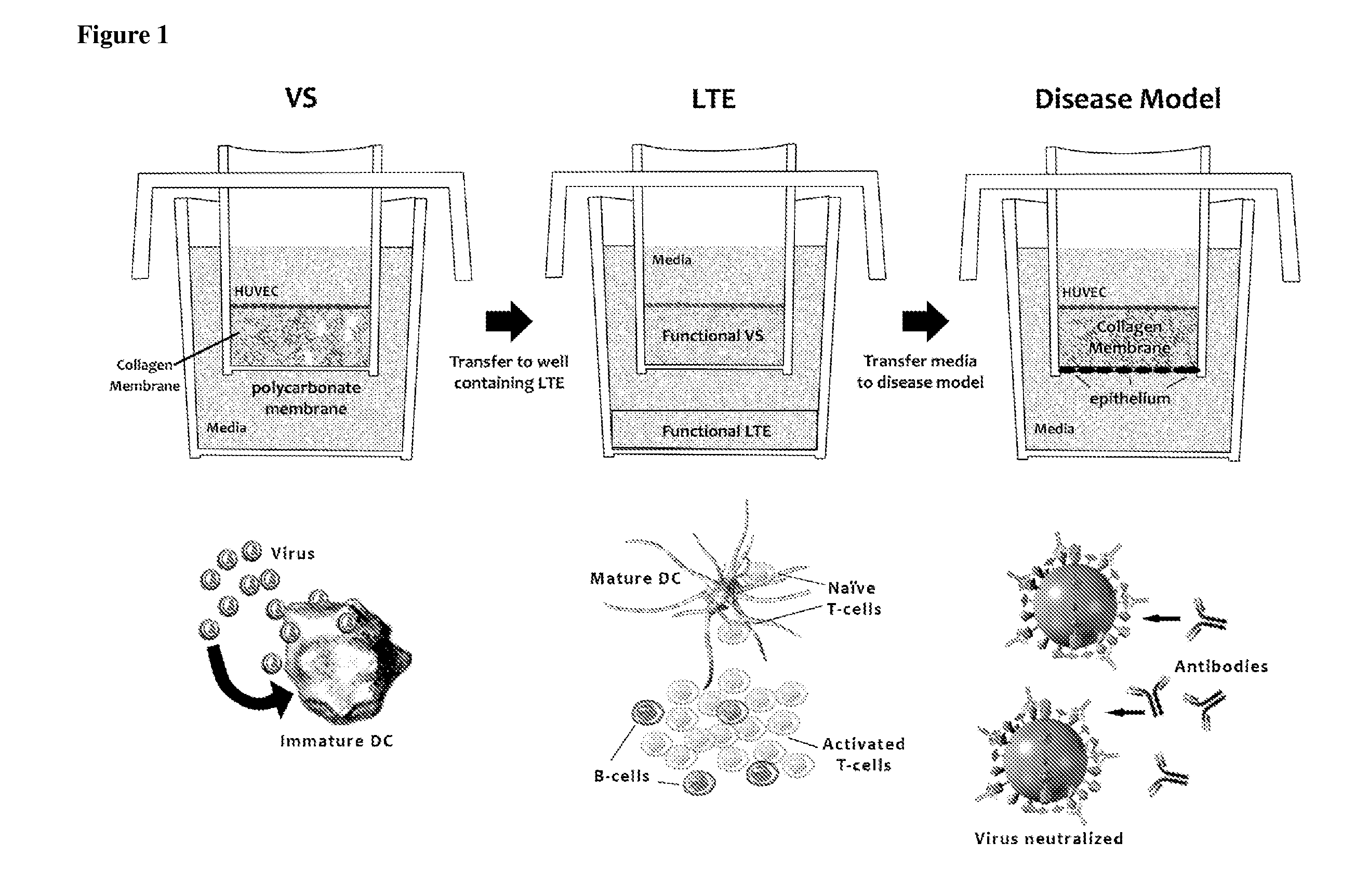
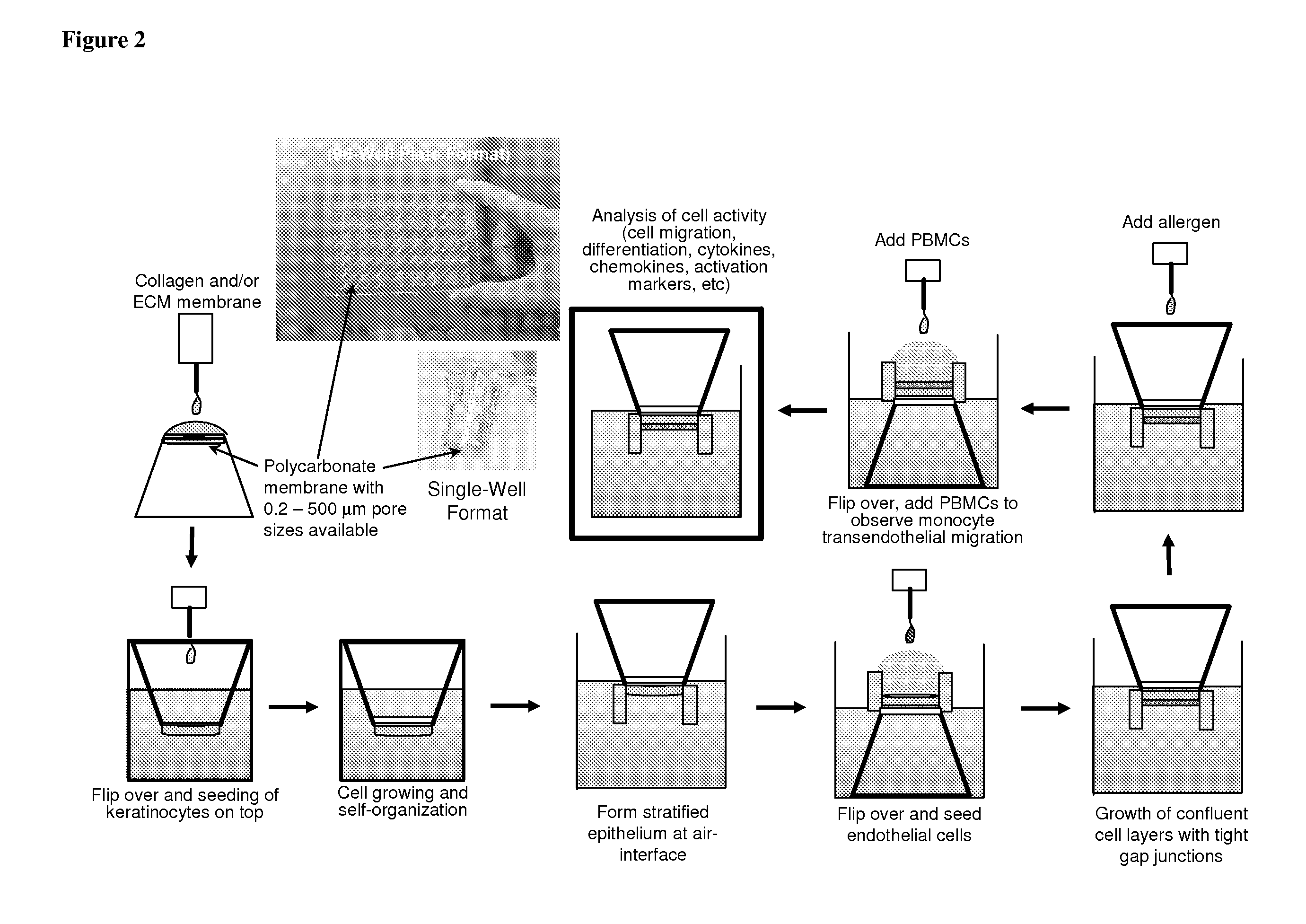
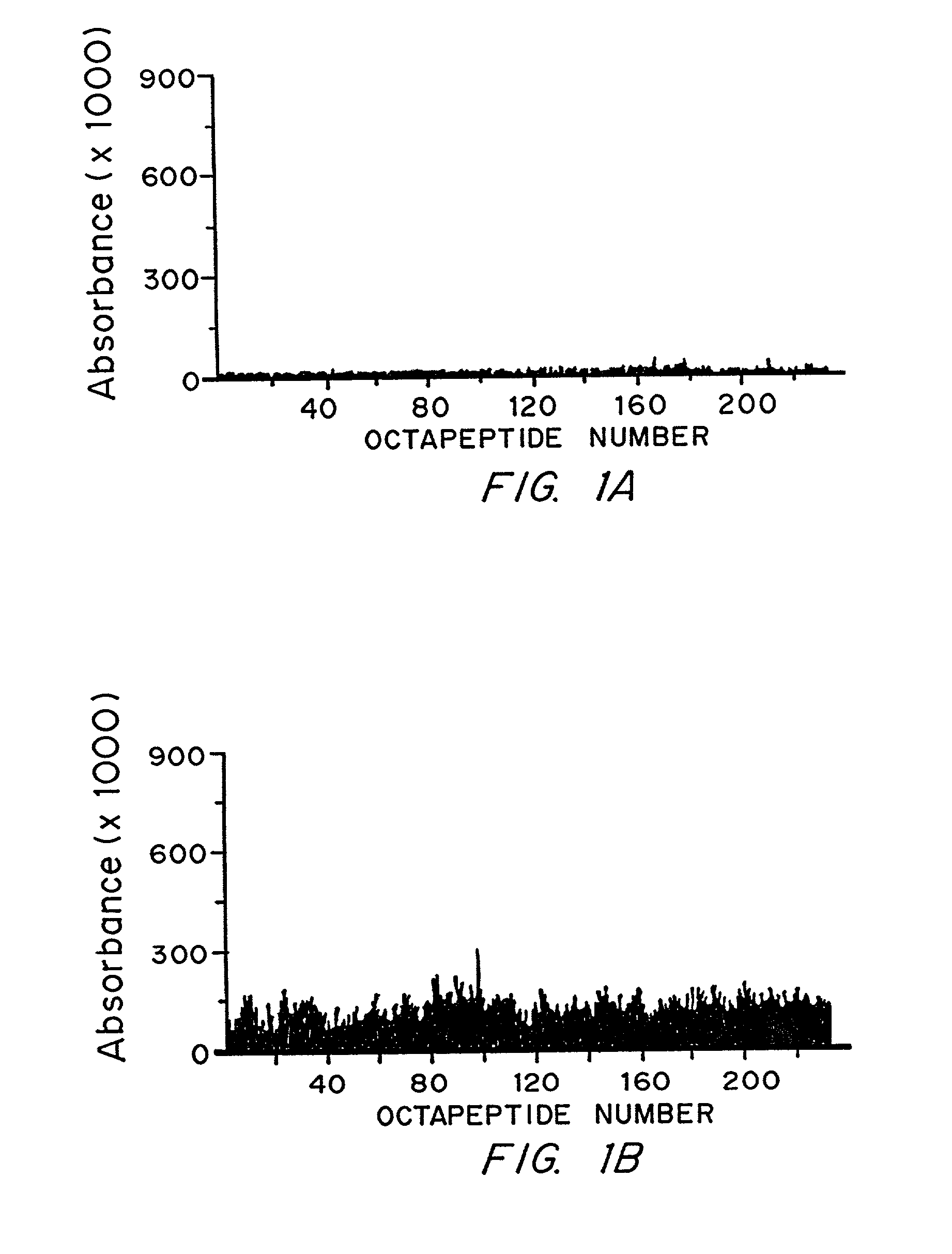
ActiveUS20100279277A1Effective therapyAvoid problemsEpidermal cells/skin cellsSkeletal/connective tissue cellsArtificial immune systemTest agent
The present invention relates to methods of constructing an integrated artificial immune system that comprises appropriate in vitro cellular and tissue constructs or their equivalents to mimic the tissues of the immune system in mammals. The artificial immune system can be used to test the efficacy of vaccine candidates and other materials in vitro and thus, is useful to accelerate vaccine development and testing drug and chemical interactions with the immune system, coupled with disease models to provide a more complete representation of an immune response.
Owner:SANOFI PASTEUR VAX DESIGN
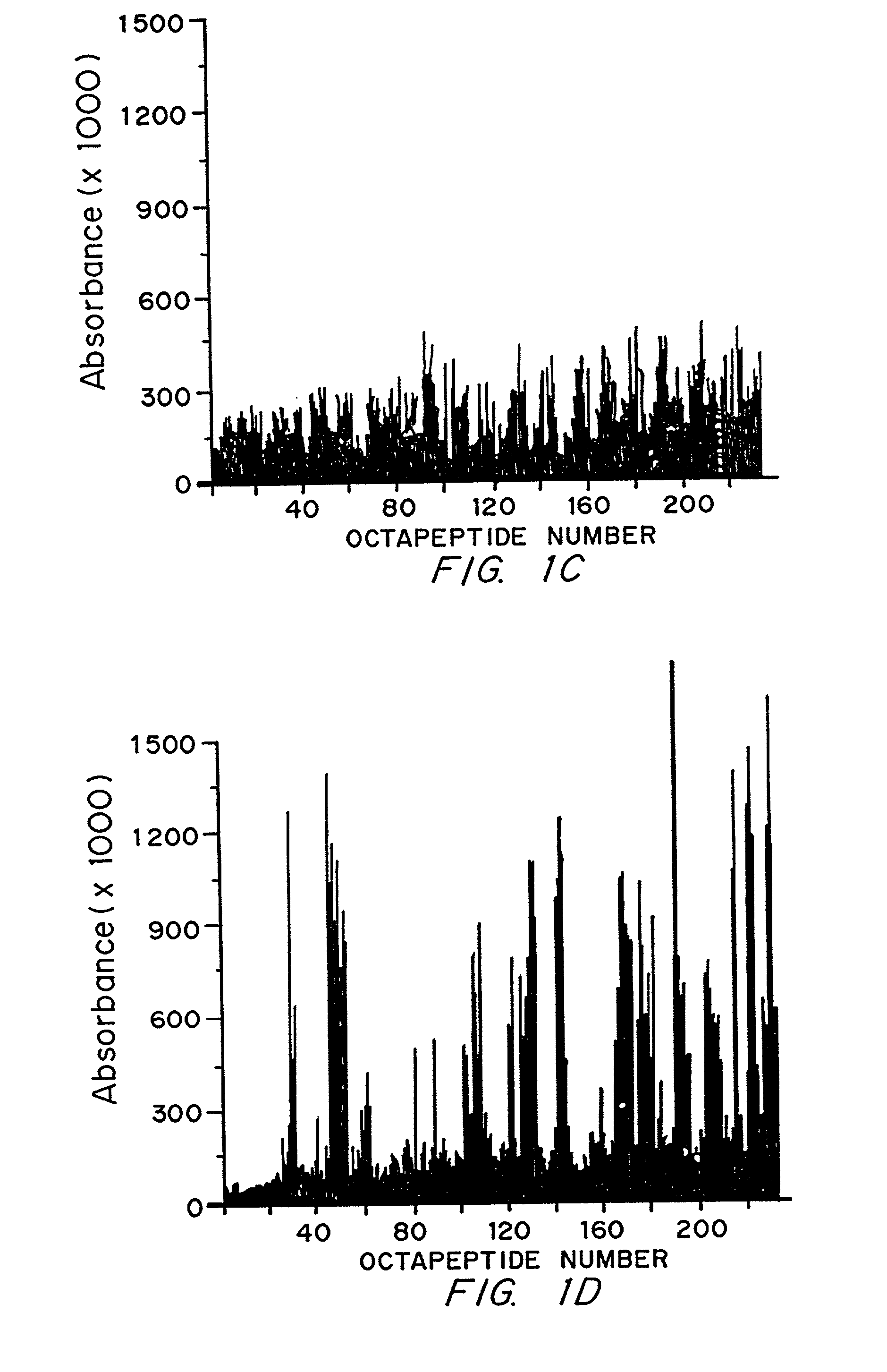
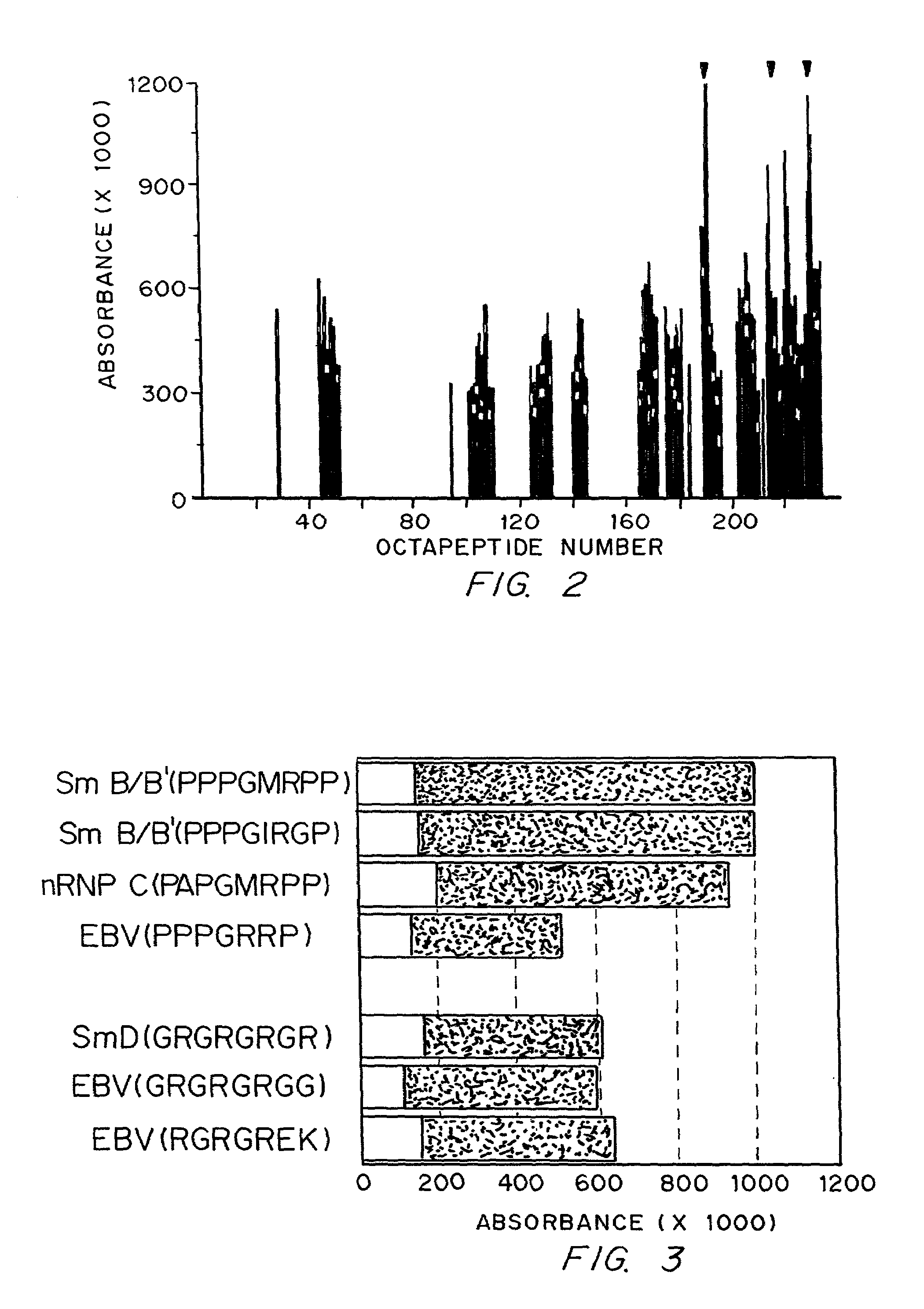
Diagnostics and therapy of epstein-barr virus in autoimmune disorders
InactiveUS7192715B2Encourage autoimmunityVirusesPeptide/protein ingredientsAutoimmune conditionAutoimmune responses
Data consistent with autoimmune disease being caused by Epstein-Barr virus are shown. Based on this evidence, an effective vaccine would prevent the autoimmune disease in those vaccinated, modified or administered so that the vaccine is not itself capable of inducing autoimmune disease. In the case of anti-Sm, structures to be avoided in an Epstein-Barr virus-derived vaccine have been identified. Differences have been identified in the immune responses to Epstein-Barr infection between individuals who develop a specific autoimmune disease and those who do not. These differences are used to distinguish those who are at greater risk for developing specific autoimmune diseases from those who are a lesser risk. Assuming Epstein-Barr virus causes autoimmune disease and that Epstein-Barr virus remains latent in the patient for life, reactivation of the virus from the latent state is important in generating or maintaining the autoimmune response that culminates in autoimmune disease. Cells infected with latent virus may also encourage autoimmunity. Based on the understanding that reactivation or latency are important to produce or sustain autoimmunity, then therapies directed against Epstein-Barr virus will also be effective therapies for the autoimmune manifestations of disease for which Epstein-Barr virus is responsible.
Owner:HARLEY JOHN B +1
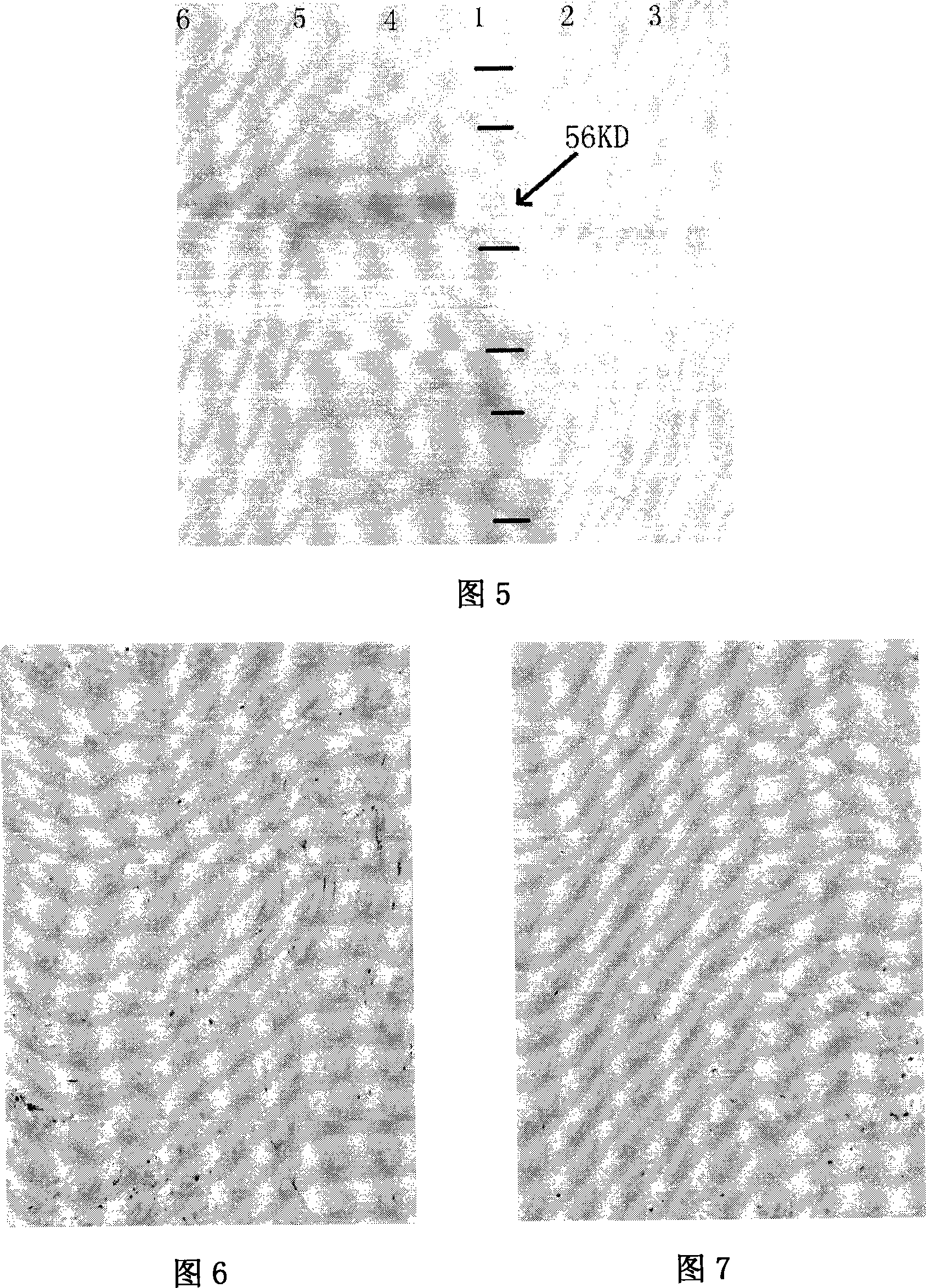
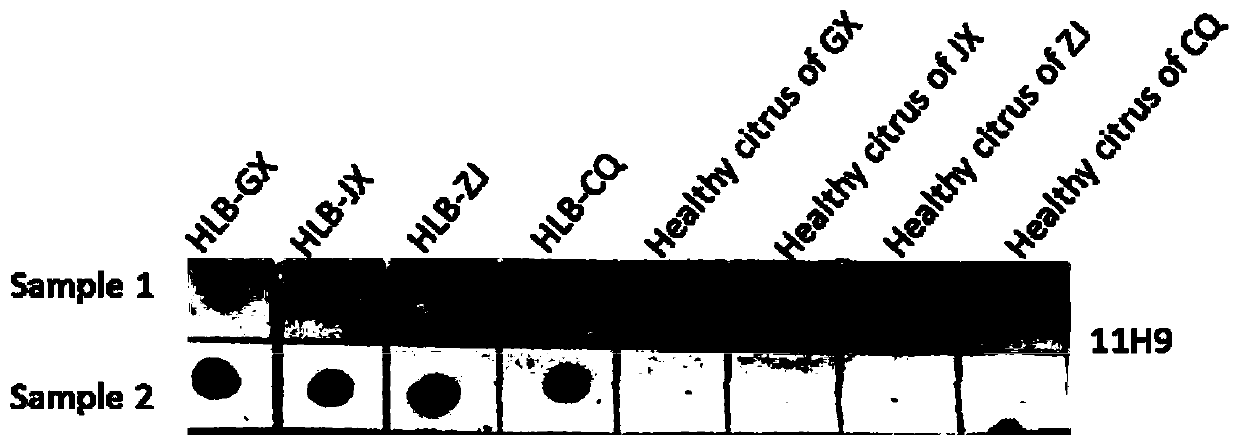
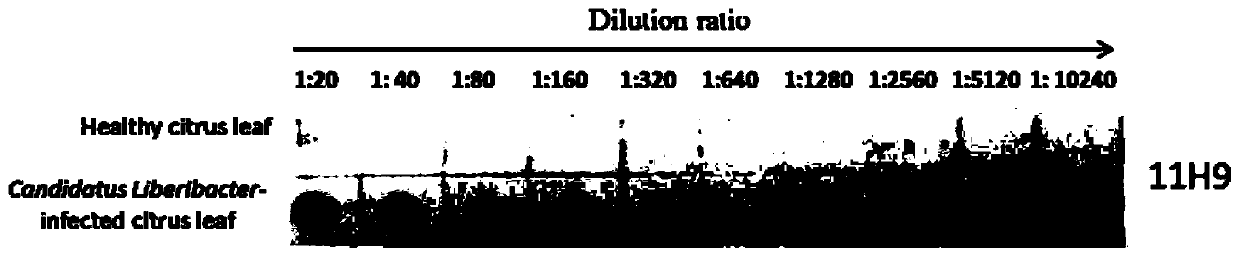
Hybridoma cell strain secreting monoclonal antibody resistant to Candidatus Liberibacter asiaticus pathogens and application of monoclonal antibody
ActiveCN109897829AAccurate detectionSensitive detectionImmunoglobulins against bacteriaMicroorganism based processesAntibody typesBALB/c
The invention discloses a hybridoma cell strain secreting a monoclonal antibody resistant to Candidatus Liberibacter asiaticus pathogens and an application of the monoclonal antibody. A Candidatus Liberibacter asiaticus vein crude extract is taken as an antigen to immunize a BALB / c mouse, one hybridoma cell strain 11H9 capable of secreting the monoclonal antibody resistant to Candidatus Liberibacter asiaticus pathogens is obtained by cell fusion, screening and cloning, and the preservation number of the hybridoma cell strain is CGMCC No.17285. Indirect ELISA valence of monoclonal antibody ascites secreted by the cell strain reaches 10<-7>, the type and the subclass of the antibody are IgG1 and kappa light chains, and the monoclonal antibody has a specific reaction with Candidatus Liberibacter asiaticus pathogen protein of 56 kDa in sick leaves and avoids immunoreaction with healthy leaves. ACP-ELISA, dot-ELISA and Tissue blot-ELISA detection methods for detecting the Candidatus Liberibacter asiaticus pathogens are established on the basis of the monoclonal antibody11H9, wherein sensitivity of the ACP-ELISA and dot-ELISA methods for detecting the Candidatus Liberibacter asiaticus vein crude extract reaches 20480-fold dilution and 10240-fold dilution (w / v,g / mL). The material and technical support is provided for diagnosis and detection of Candidatus Liberibacter asiaticus, epidemiological analysis, sterile seedling production and scientific prevention and control through establishment of the preparation and detection methods of the monoclonal antibody resistant to the Candidatus Liberibacter asiaticus pathogens.
Owner:ZHEJIANG UNIV
Reaction container for chemical analysis with the controlled surface property
InactiveUS20050047970A1Improve reliabilityControlled surface propertyAnalysis using chemical indicatorsPharmaceutical containersSolventCritical surface
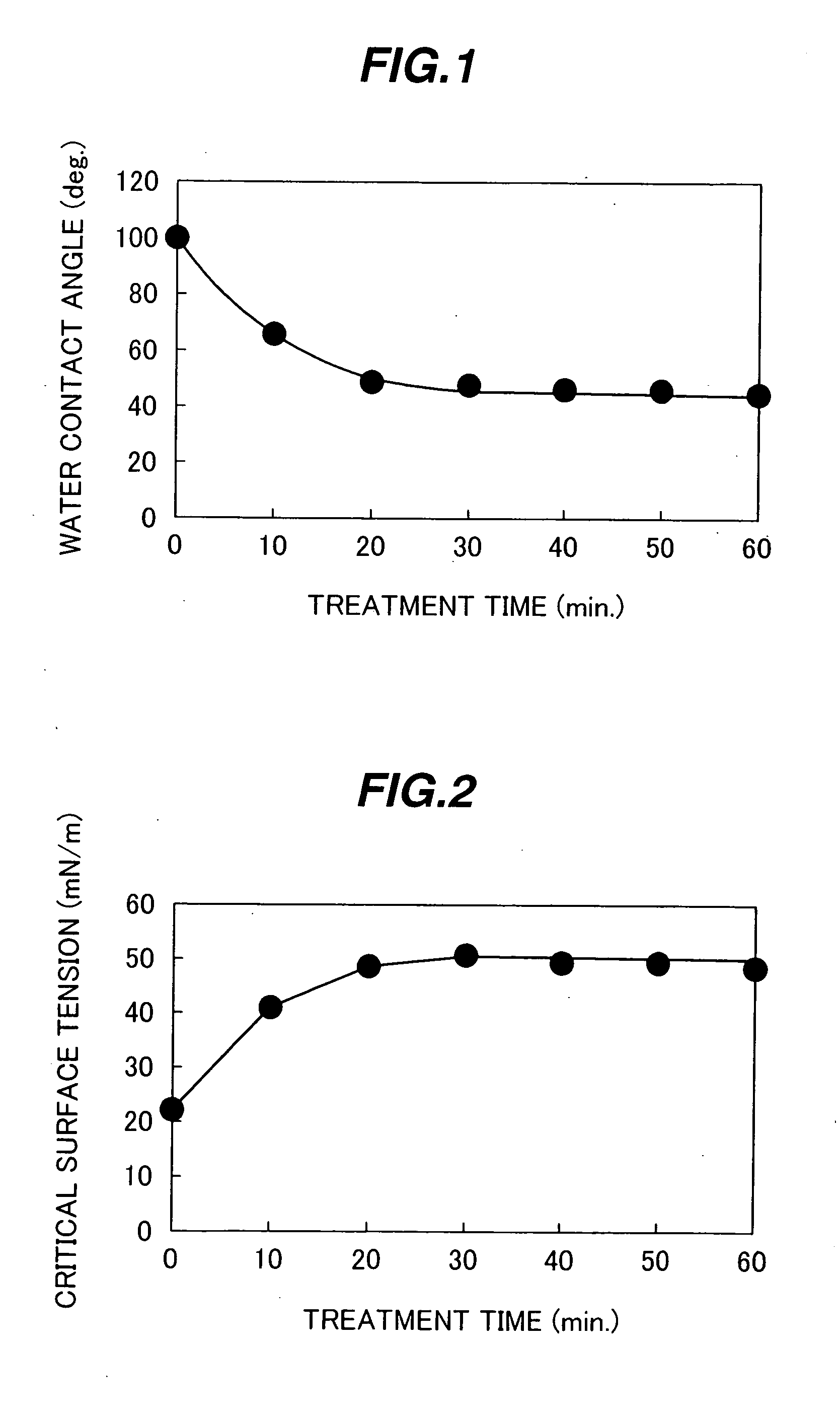
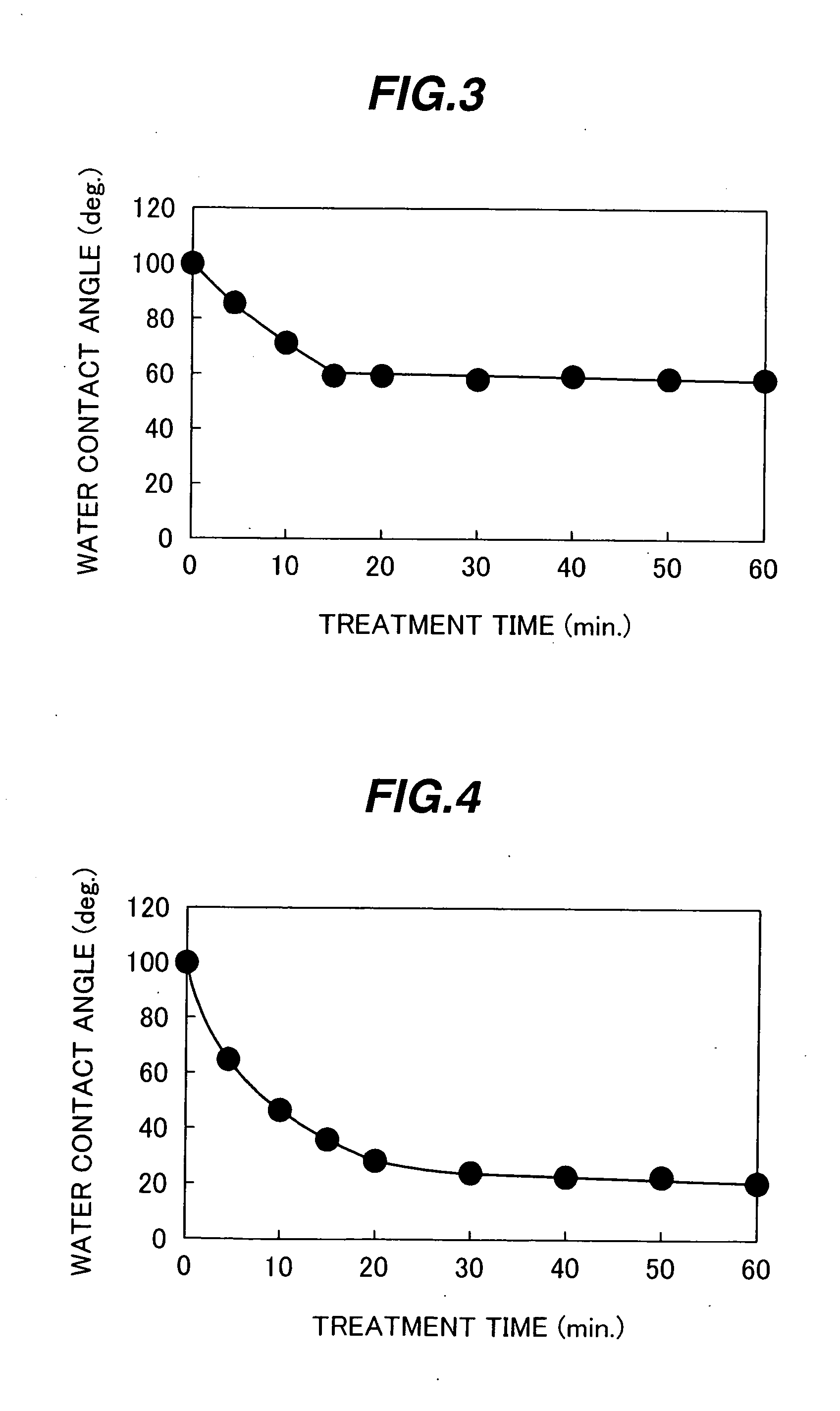
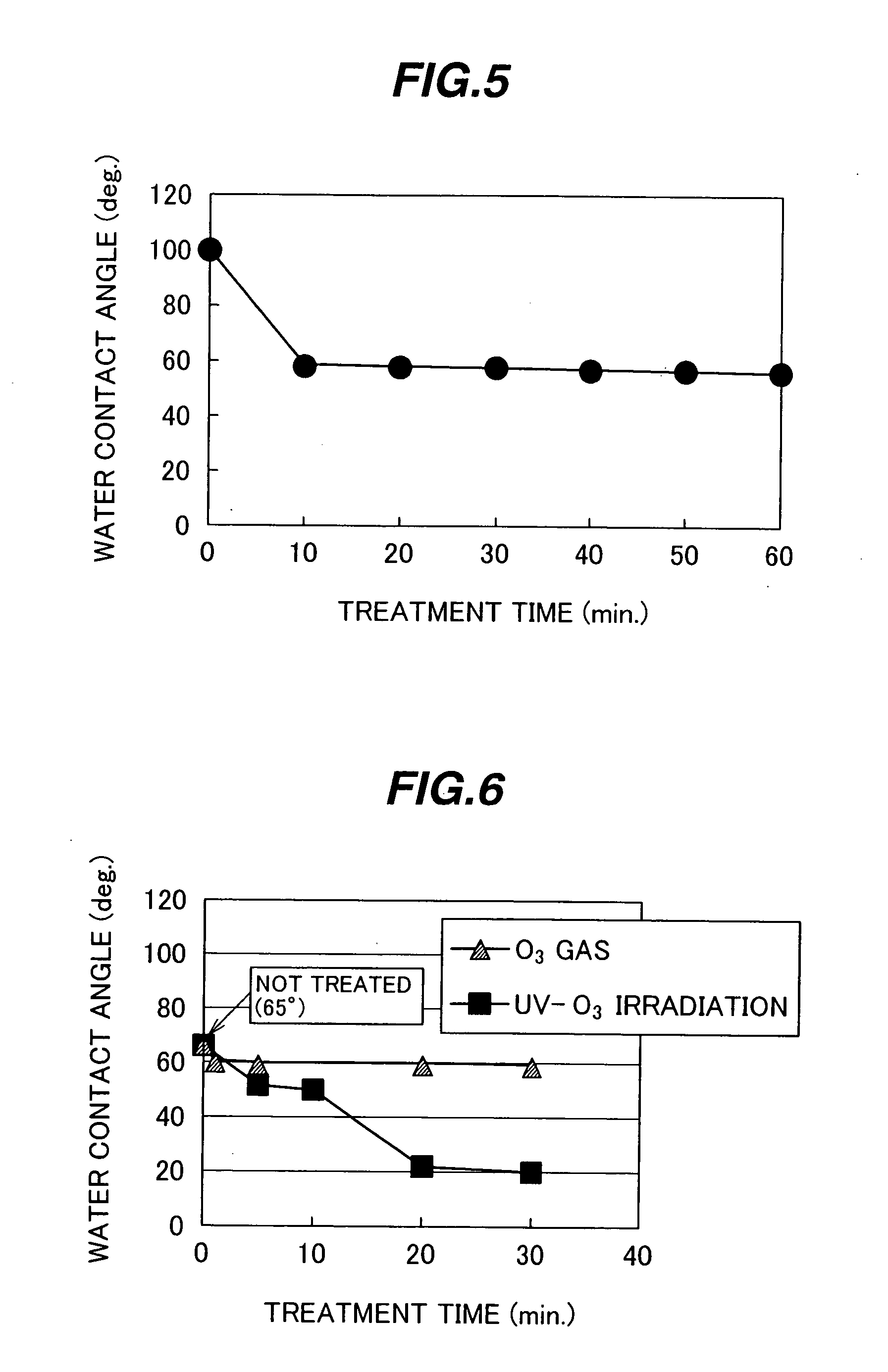
A highly reliable reaction container capable of restraining an initial detection failure (bubble attachment), and a biochemical and / or immunological automatic analyzer loaded with the reaction container. In a reaction container made of a synthetic resin and used for receiving a biological sample and a reagent, developing a biochemical and / or immunological reaction between the biological sample and the reagent, and measuring proceedings of the reaction and / or the state at a predetermined point in time by optical means, an inner wall surface of the reaction container in contact with the biological sample, the reagent, and a reaction product of the biological sample and the reagent has a critical surface tension of not smaller than 25.0 mN / m, or a contact angle between the inner wall surface of the reaction container and a solvent of a reaction solution is not larger than 60 degrees.
Owner:HITACHI LTD
Electrochemical immune sensor for phosphating protein
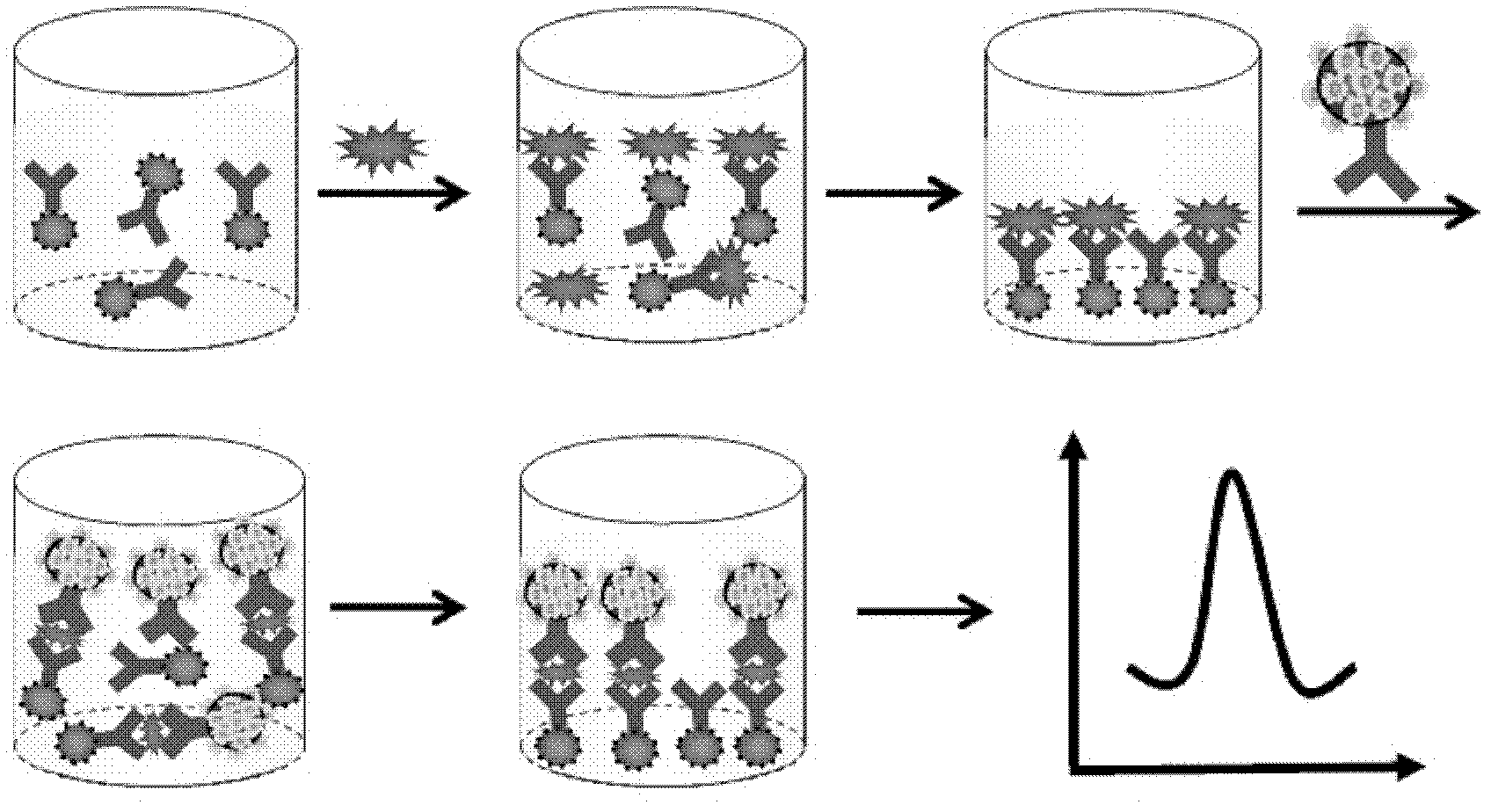
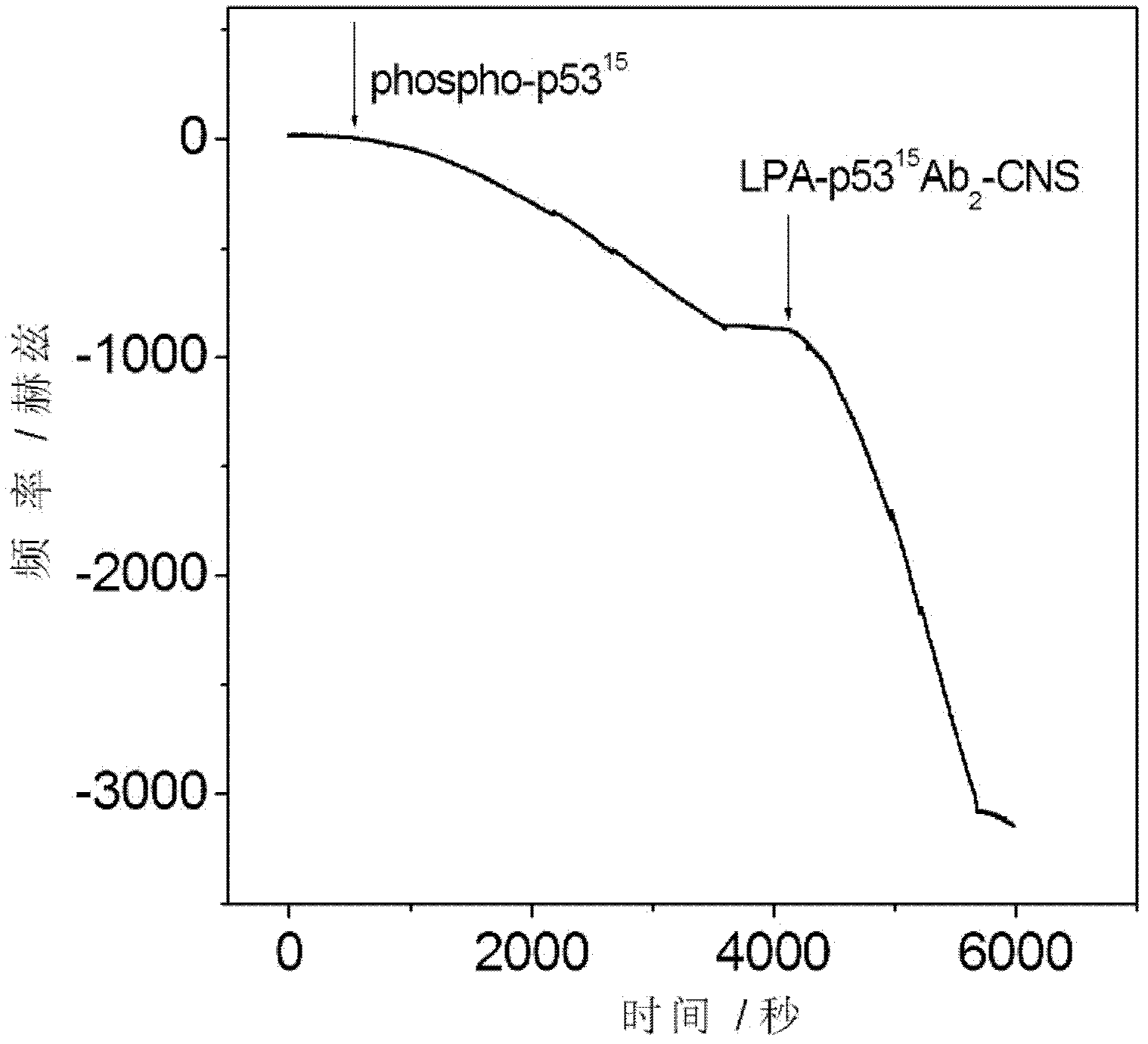
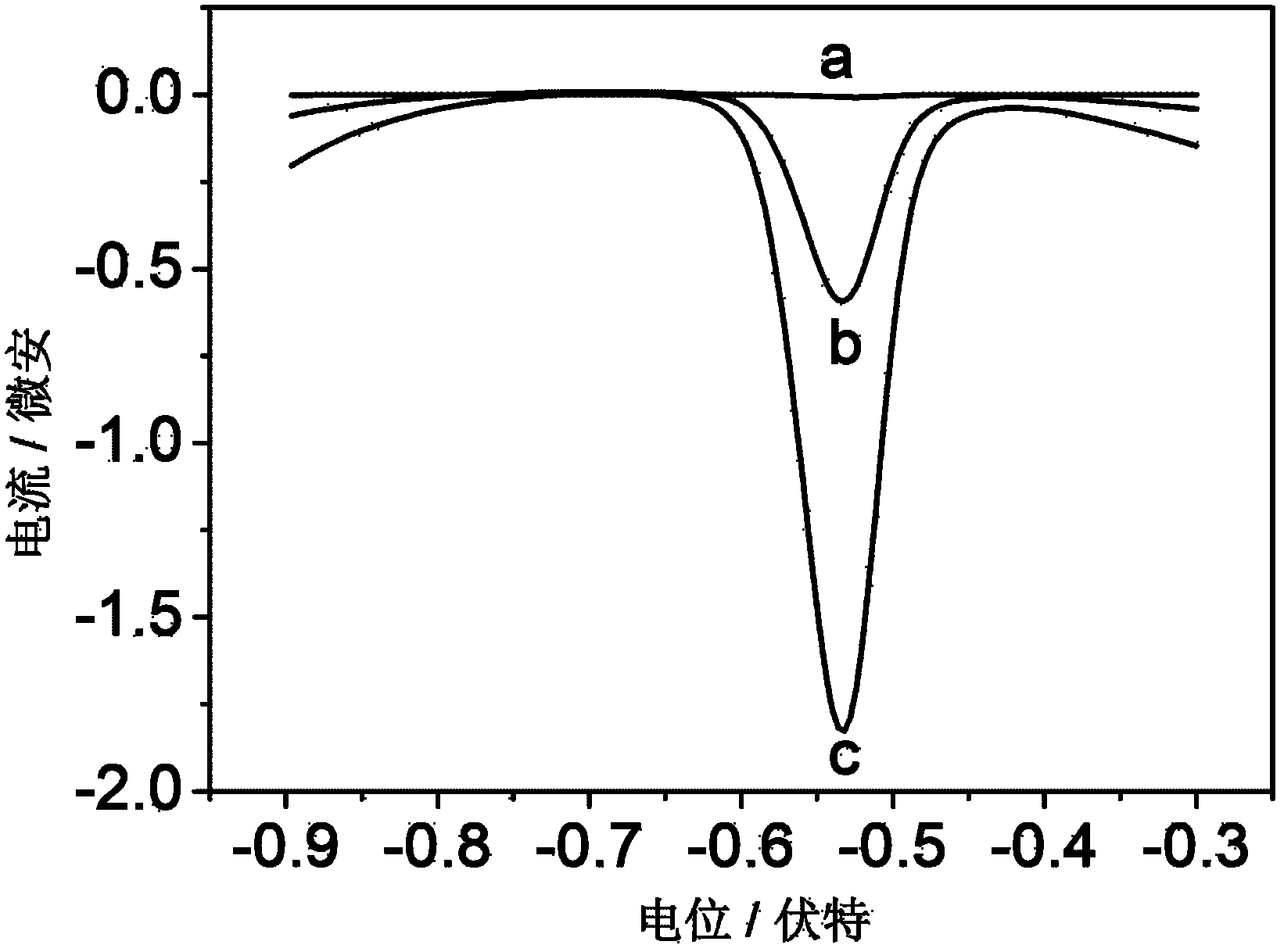
InactiveCN102253221AIncrease fixed amountGood biocompatibilityBiological testingMaterial electrochemical variablesEpoxySignalling molecules
The invention relates to an electrochemical immune sensor for phosphating protein. In the electrochemical immune sensor, a magnetic nanometer-antibody composition MPs-p53<15>Ab1 is used as an adsorbent to separate the phosphating protein, a carbon nanometer sphere CNS is used as a carrier, lead phosphate-apoferritin (LPA) and a phosphating protein antibody p5315Ab2 are modified on the surface of the CNS, and thus, LPA-p53<15>Ab2-CNS nanometer complexing agent is prepared for detecting signal amplification. According to the sandwich immunoassay principle, the content of the phosphating protein to be detected is in proportion to the captured LPA-p5315Ab2-CNS, and the content of lead ions encapsulated in LPA is detected according to a dissolving volt-ampere method to measure the concentration of the phosphating protein in a sample. The method has the advantages of simplicity and convenience for operation and simple and efficient separation, and the advantage that an integral immunologic reaction is finished in an epoxy (EP) tube; a large number of LPA signal molecules are introduced by taking the CAN as the carrier, and each LPA molecule contains a large number of Pb<2+>, so the sensitivity of detection is improved; and the dissolved Pb<2+> is used as a detection signal without adding an enzyme substrate, so the electrochemical immune sensor is a reagent-free sensor. In the electrochemical immune sensor for the phosphating protein, the linear concentration range of the phosphating protein phosphor-p53<15> is from 0.02 to 20 ng mL<-1>, and the detection limit is 0.01 ng mL<-1>.
Owner:HUAZHONG NORMAL UNIV
Pharmaceutical composition for treatment and/or prophylaxis of cancer
ActiveUS9181348B2Treatment and/or prevention of cancerReduce usageNervous disorderDigestive systemCancer preventionCancer cell
An object of the present invention is to prepare an antibody that targets CAPRIN-1 specifically expressed on the surface of cancer cells and is superior in antitumor activity to conventional antibodies and to provide use of the antibody as a therapeutic and / or preventive agent for cancer. The present invention provides use of an antibody targeting an identified cancer antigenic protein specifically expressed on the surface of cancer cells as a therapeutic and / or preventive agent for cancer, specifically, a pharmaceutical composition for treatment and / or prevention of cancer, comprising as an active ingredient an antibody or a fragment thereof which has immunological reactivity with a CAPRIN-1 protein, the antibody or the fragment thereof comprising a heavy chain variable region comprising amino acid sequences represented by SEQ ID NOs: 5, 6, and 7 and a light chain variable region comprising amino acid sequences represented by SEQ ID NOs: 9, 10, and 11.
Owner:TORAY IND INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com