Lipid and Nitrous Oxide Combination as Adjuvant for the Enhancement of the Efficacy of Vaccines
a technology of nitrous oxide and vaccine, which is applied in the direction of antibacterial agents, antibody medical ingredients, immunological disorders, etc., can solve the problems of inability to induce an appropriate, protective, immune response, and sustainably deliver vaccines to everyone at risk, so as to enhance the action of antigens, enhance the immune response, and enhance the effect of specific neutralizing antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation 1
Preparation of FAA-1 for the Parenteral Rabies and Nasal Diphtheria Toxoid (DT)-Vaccines
[0127]Step 1: The buffer solution applicable to the specific antigen is saturated with nitrous oxide at ambient pressure using a pressure vessel and sparger. In the case of rabies the buffer used was phosphate buffered saline (PBS), in the case of the DT for nasal administration, distilled water was used.[0128]Step 2: The following group of fatty acids was heated to 70° C.: 21% oleic acid, 34% linolenic acid, and 28% linoleic acid. These fatty acids were modified by esterification with an ethylene group of the carboxy terminal. The pegylated, hydrogenated fatty acid, ricinoleic acid (also known by the INCI name as PEG-n-Hydrogenated Castor Oil), was heated to 80° C. and mixed with the first group of fatty acids at 70° C. The ratio of the first group of fatty acids to the latter fatty acid was 3:1.[0129]Step 3: The buffer solution was heated to 70° C. and mixed with the fatty acid mix to a final c...
preparation 2
Preparation of FAA-2 for Parenteral Hepatitis 6 Vaccine
[0131]To the fatty acids contained in FAA-1 above was added[0132]1. dl-a-Tocopherol as anti-oxidant[0133]2. additional ethylated fatty acids DHA (decahexonoic acid) and EPA (eicosapentaenoic acid). The preferable amount of the two fatty acids for this invention was 0.2%.[0134]3. Entrapment of the Hepatitis B peptide occurred by mixing for 30 minutes in a Vibramix at ambient temperature.
[0135]Stable particles of fairly homogeneous sizes ranging from 20 nm to 50 μm can be manufactured with ease on a large scale. The size and shape of the particles can be reproducibly controlled. The use of FAA-1 and FAA-2 in animal studies as it pertains to this invention is described below. The following antigens considered to be representative and hence demonstrative albeit not exhaustive of the range of vaccines to which the invention relates, were used in the cell and animal studies to confirm the invention:
A toxoid as antigen (diphtheria)
An i...
example 1
Determination of the Capacity of an FAA-1 / DT Vaccine to Induce a Systemic Immune Response after Oral and Nasal Administration Respectively
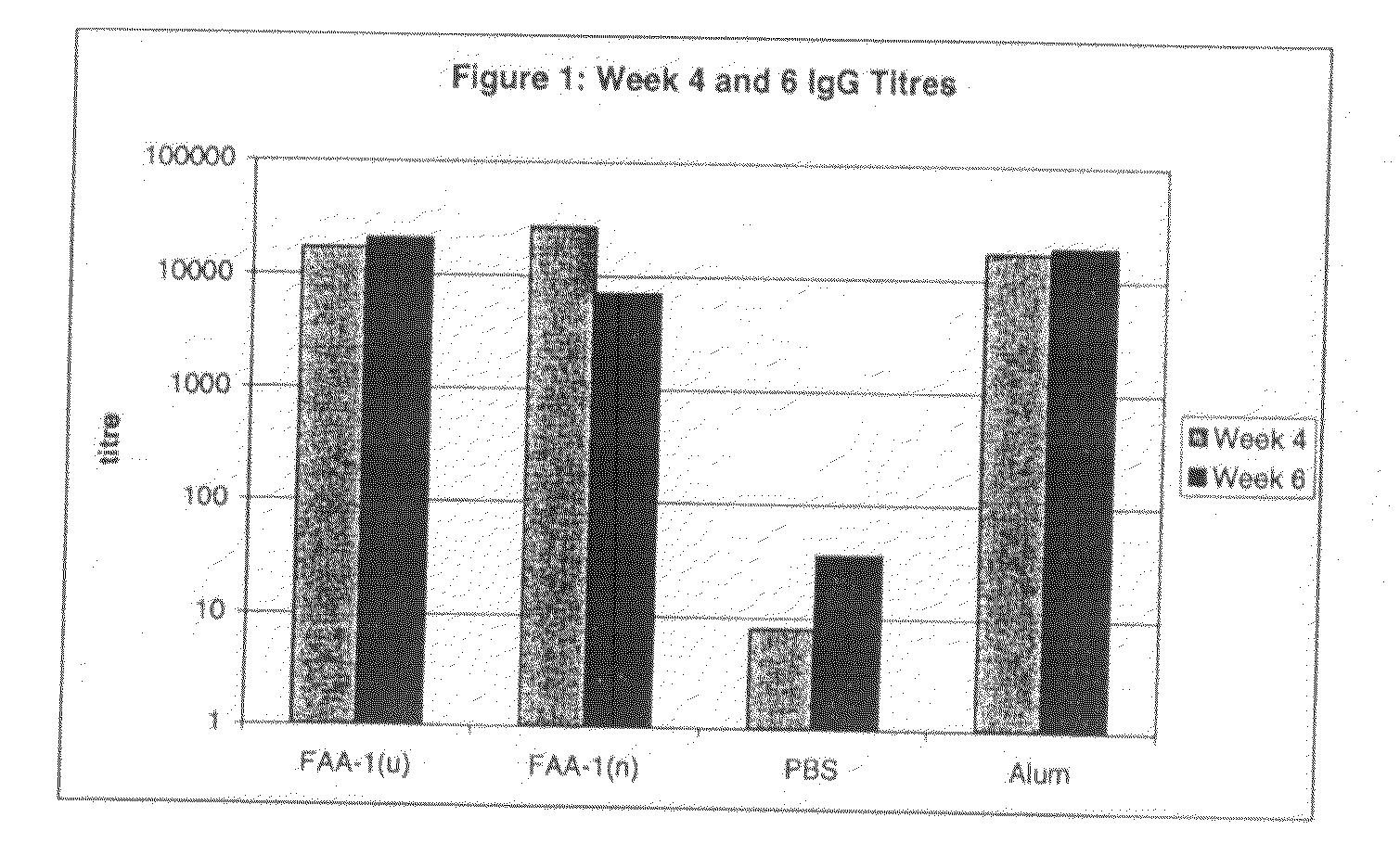
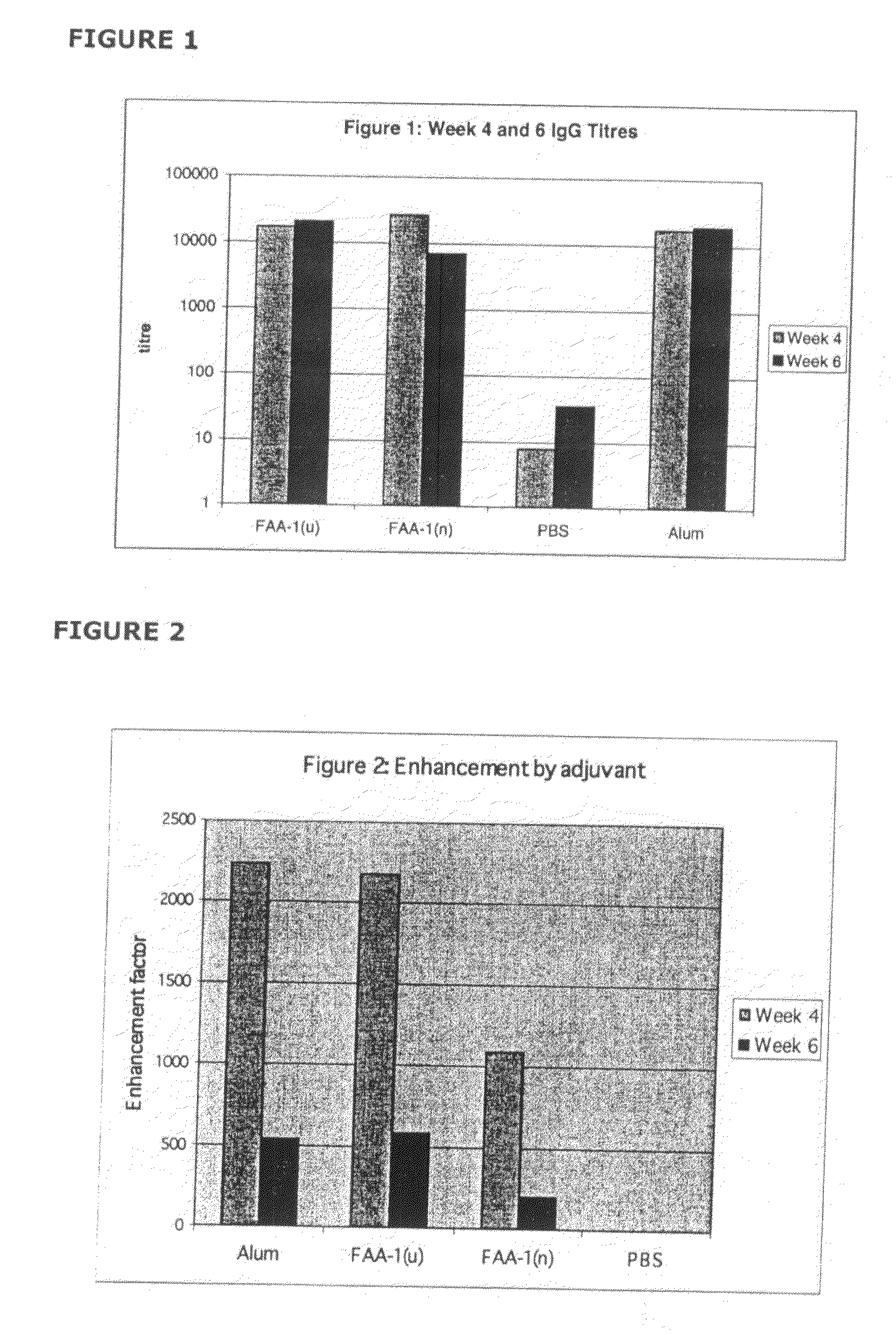
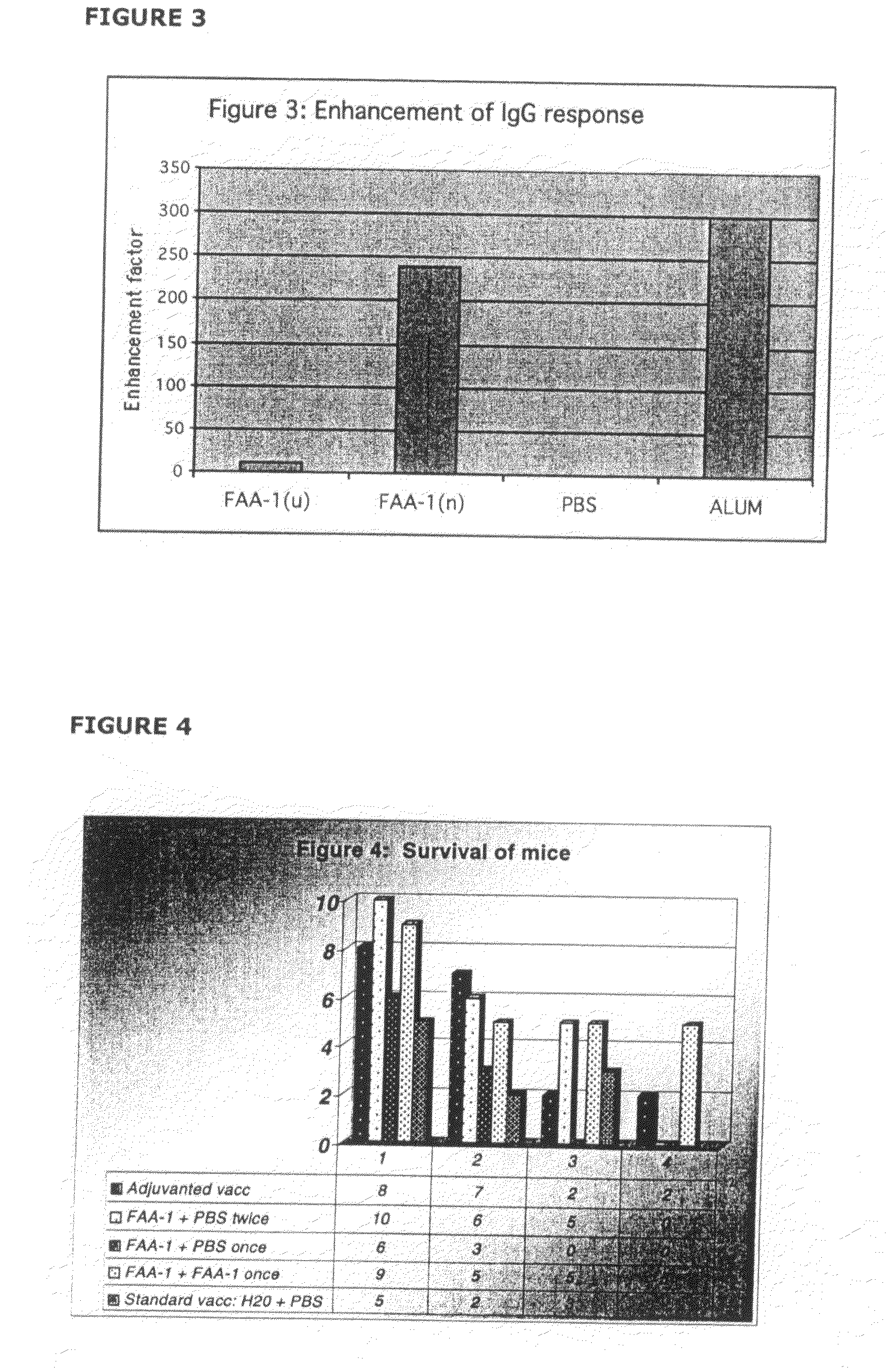
[0137]This example pertains to the enhancement of the immune response to the diphtheria toxoid specifically in a nasally and orally administered vaccine in animals in comparison to the currently used gold standard—an aluminium hydroxide (alum)-based parenteral vaccine.
1. Objective of the Study:
[0138]The primary objective of this study was to assess the efficacy of FAA-derived formulations of the present invention in enhancing the systemic immune response after oral and nasal administration of the model antigen DT when compared with antigen administered in
a) PBS saline
b) Alum by parenteral route.
[0139]Desai et al16 showed that chitosan particle uptake by M-cells is dependent on the size of the particles as well as the hydrophobic / hydrophilic character of the particles. It has been established that particles with sizes in the nanometer range are mor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Acidity | aaaaa | aaaaa |
| Content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com