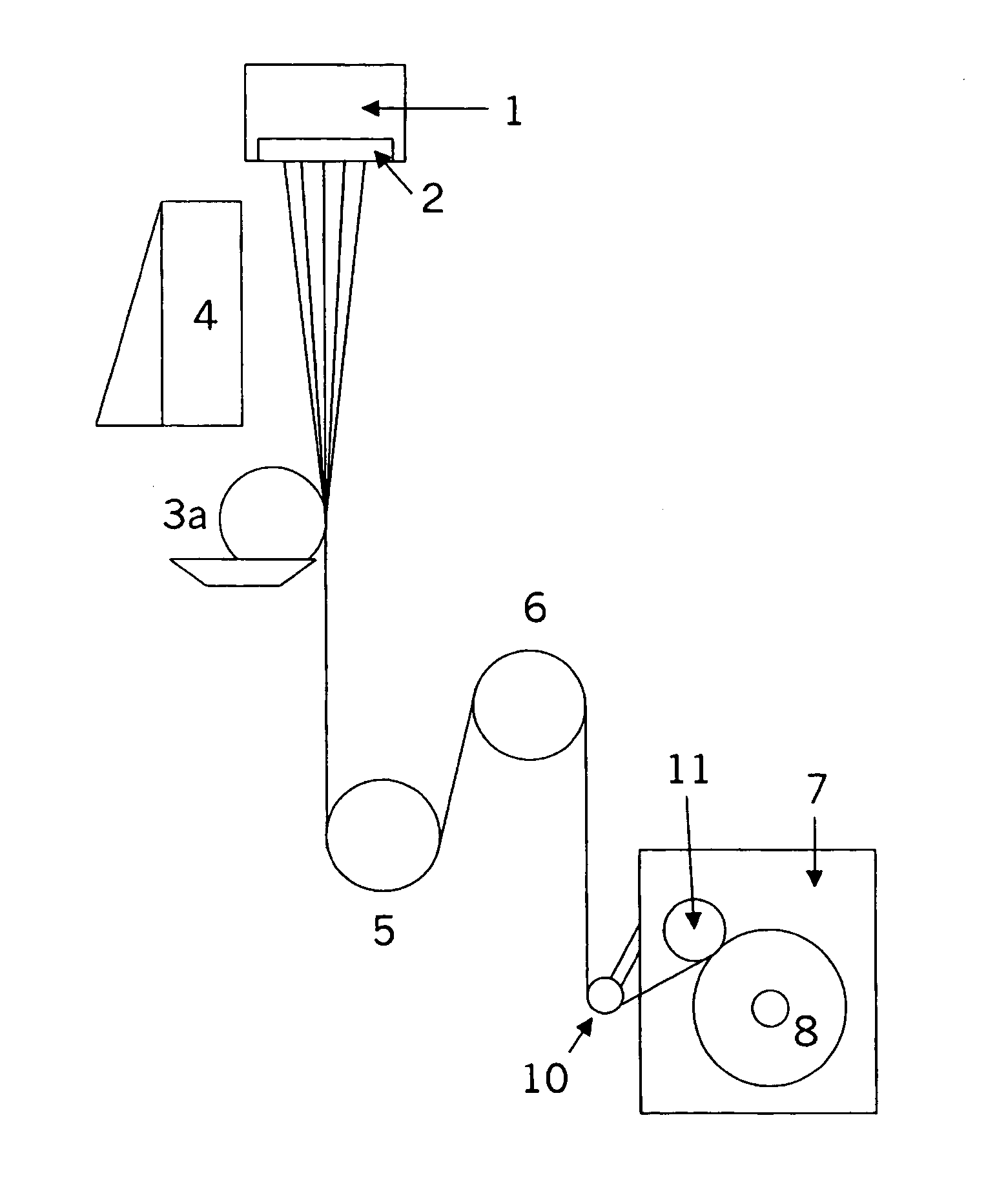
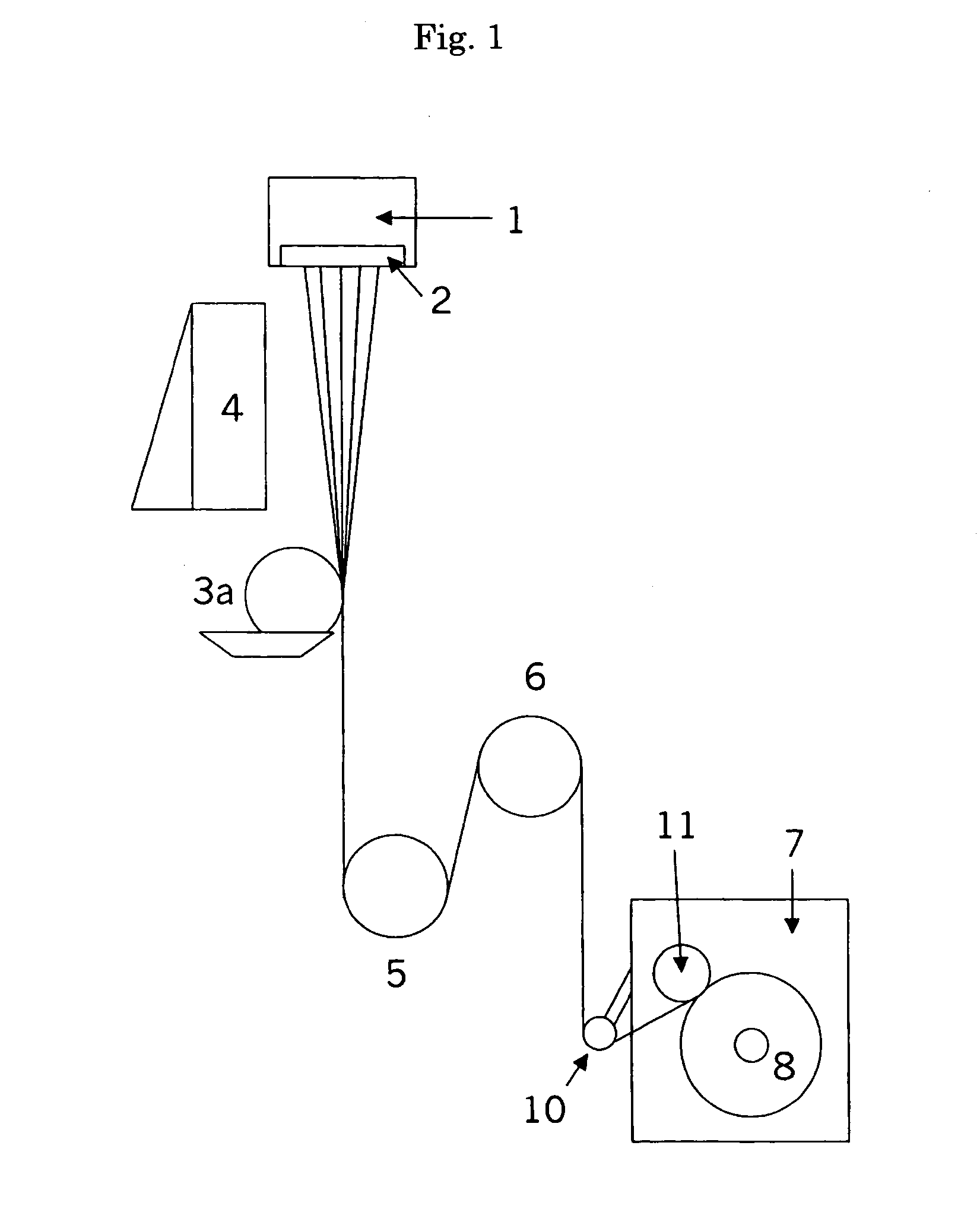
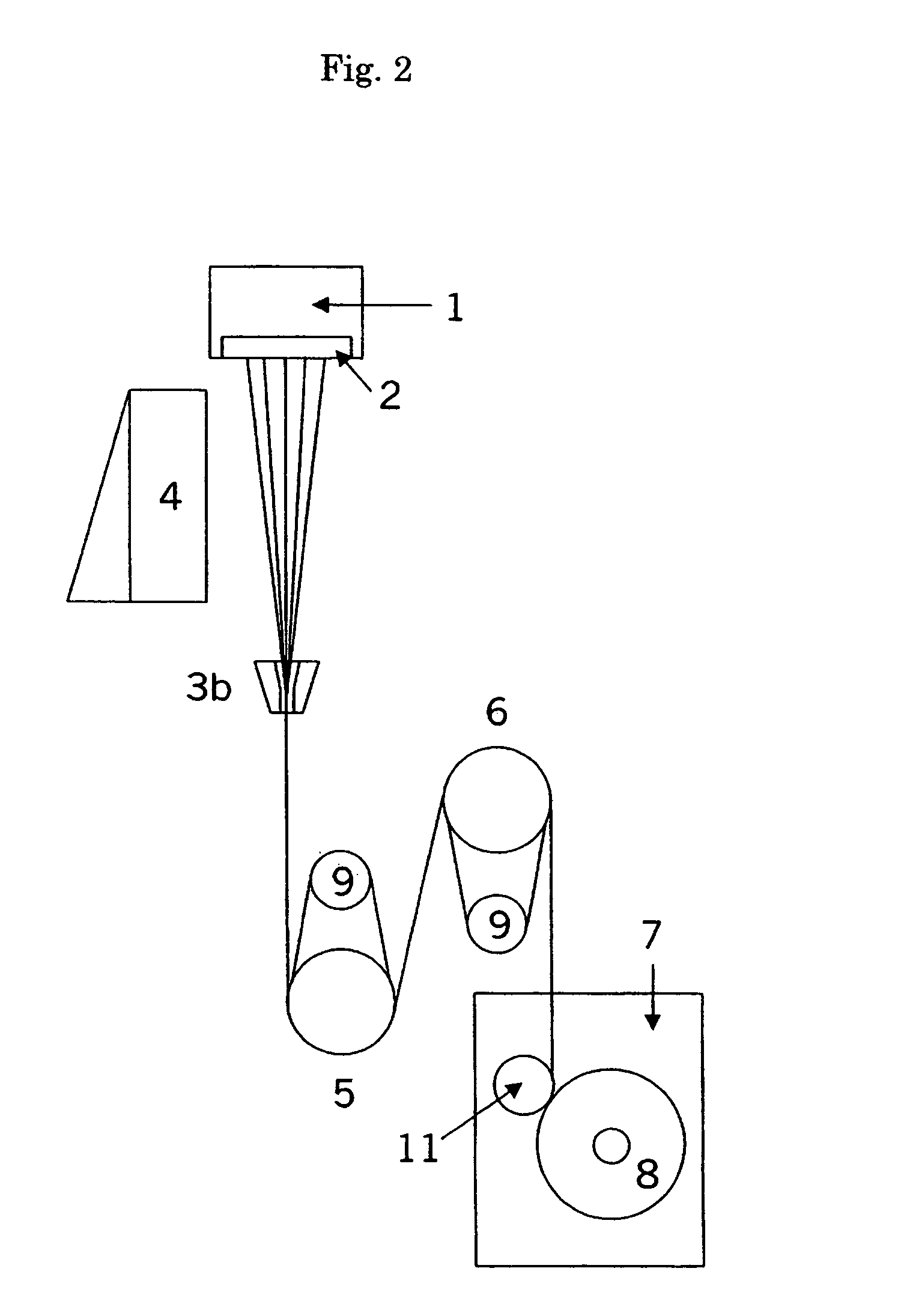
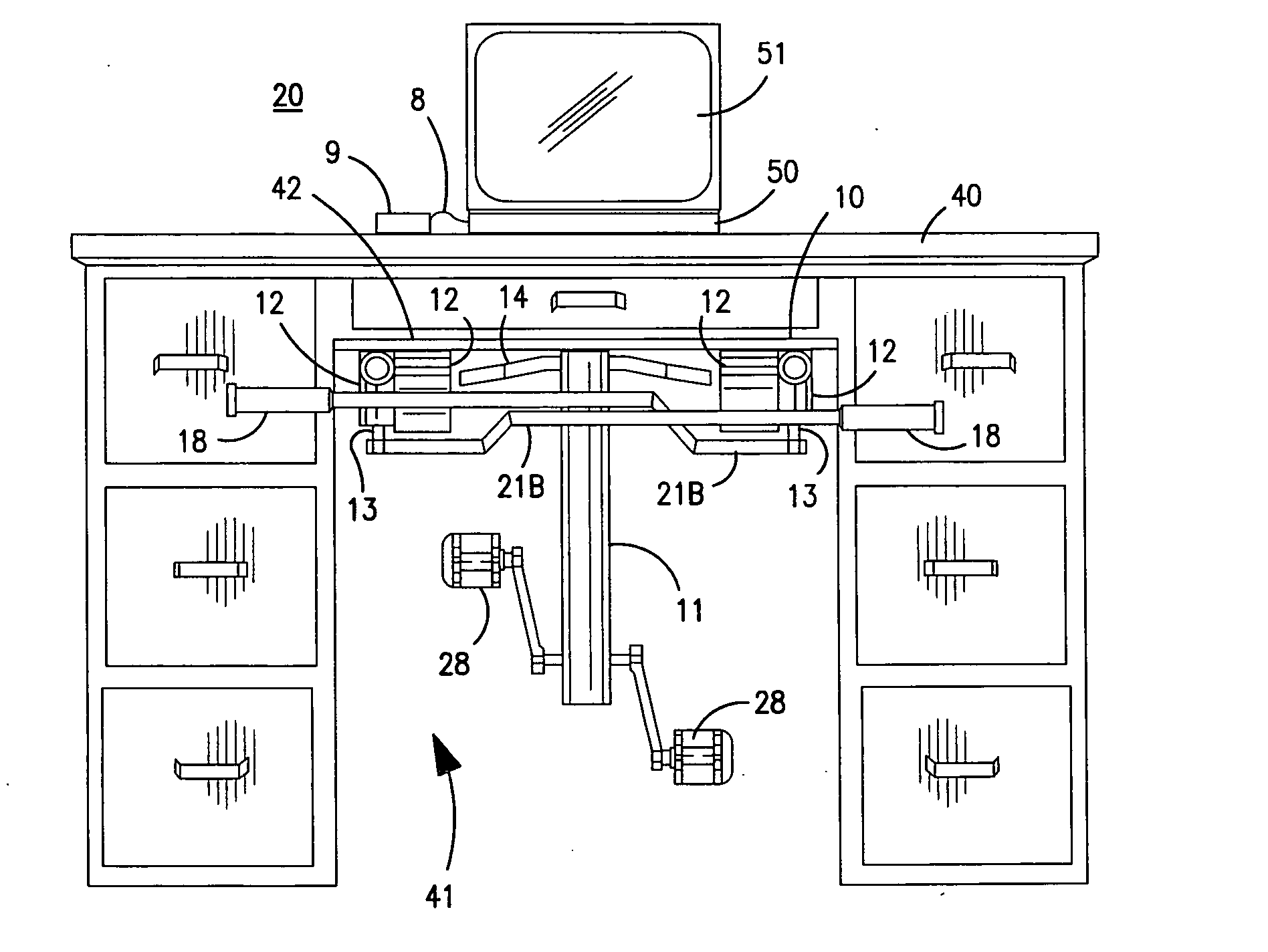
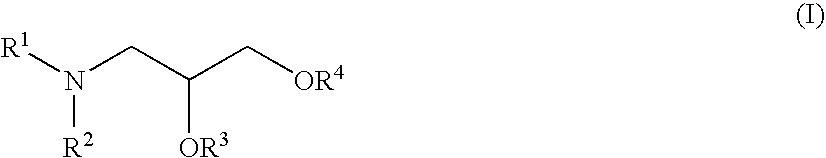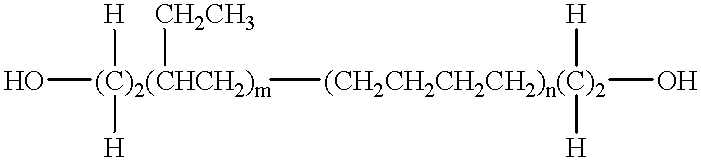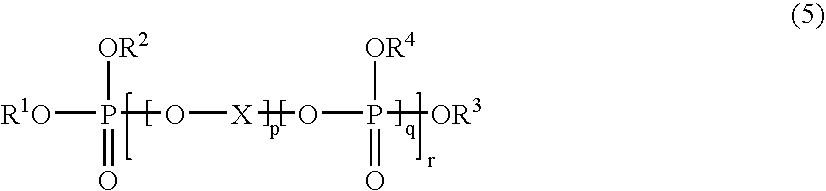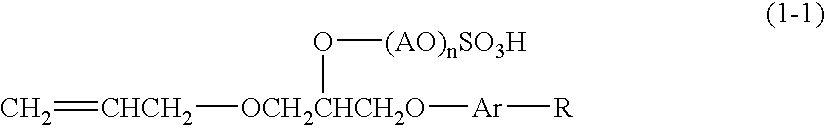Patents
Literature
687results about How to "Good fluidity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
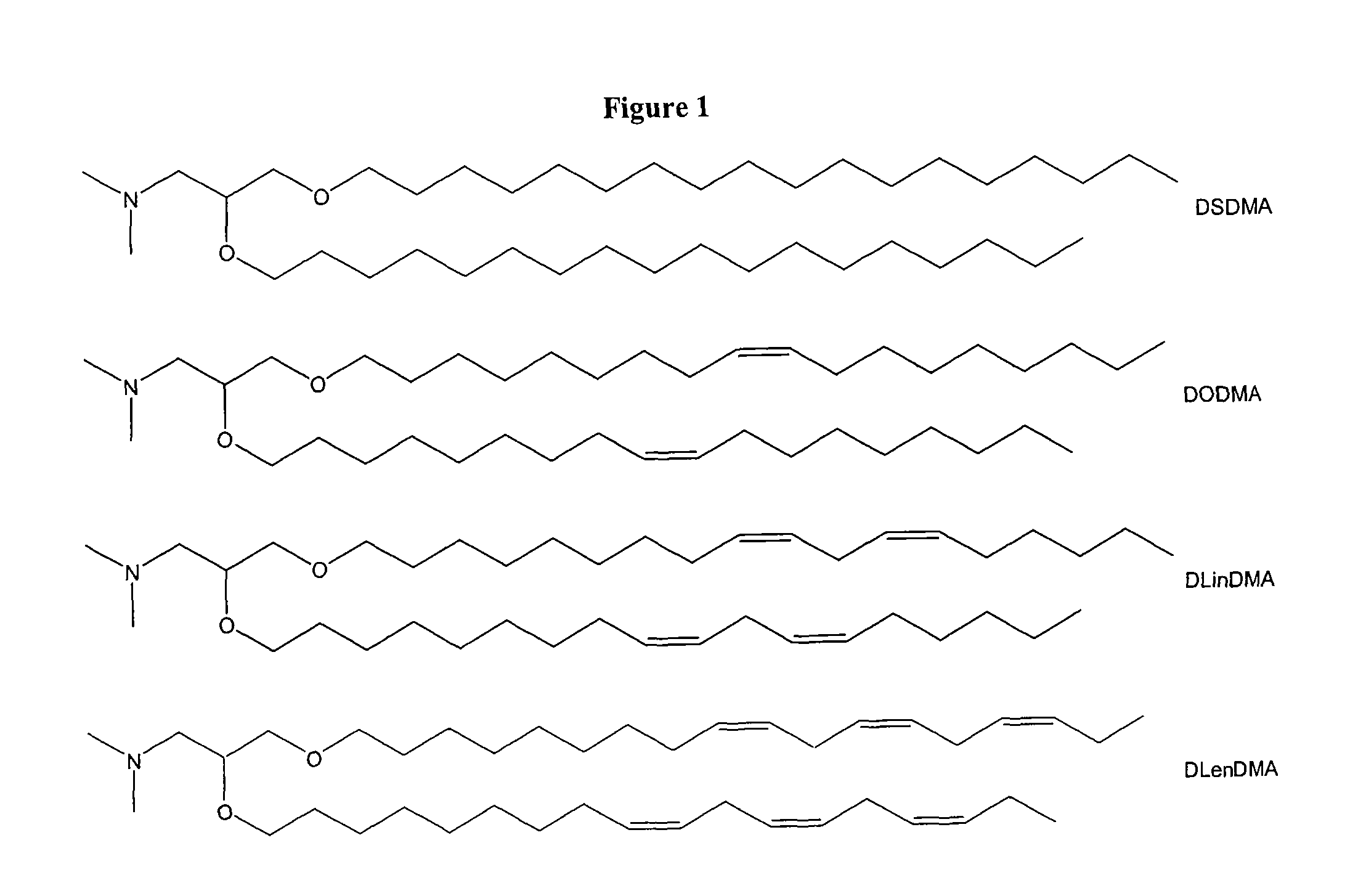
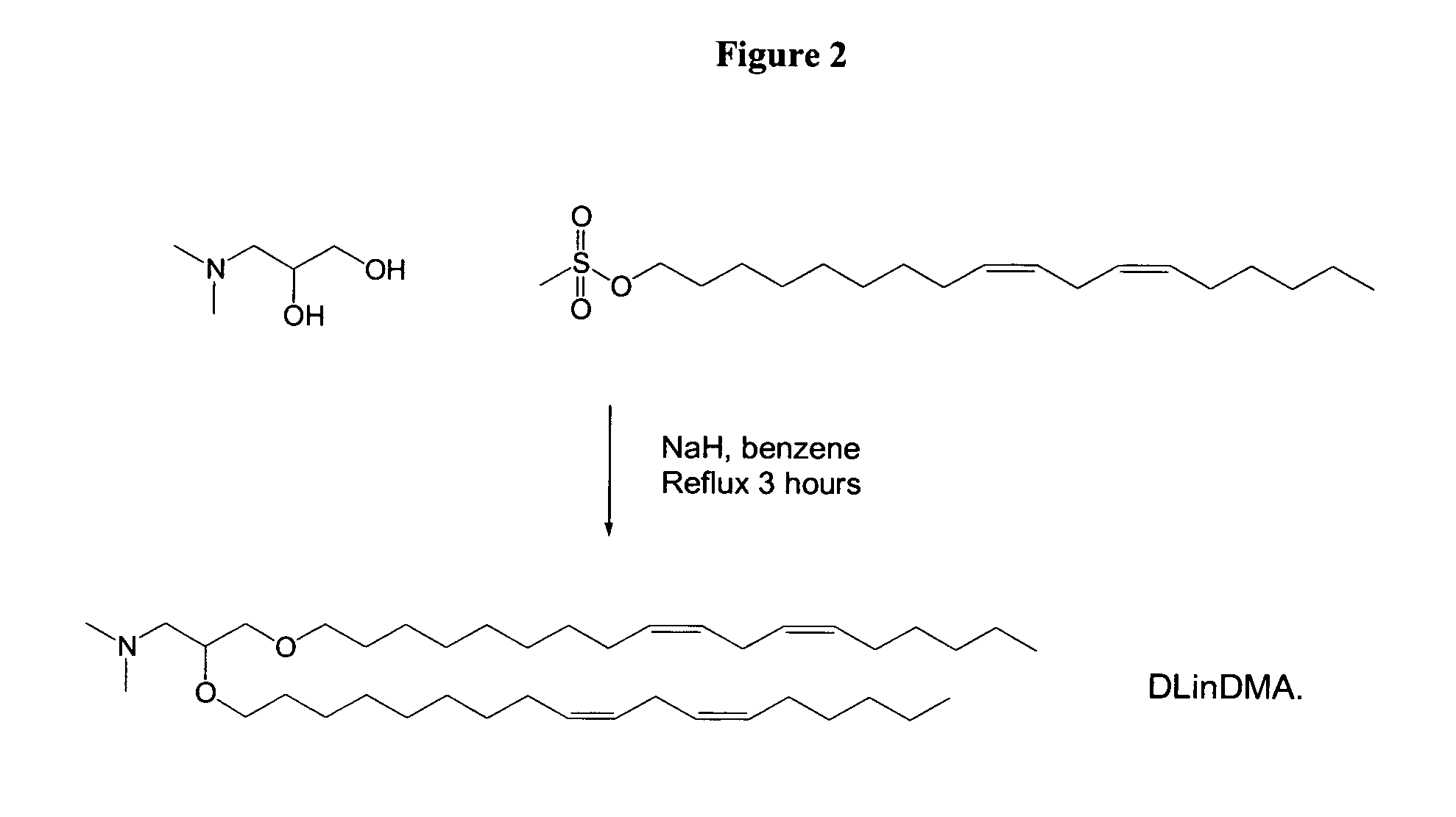
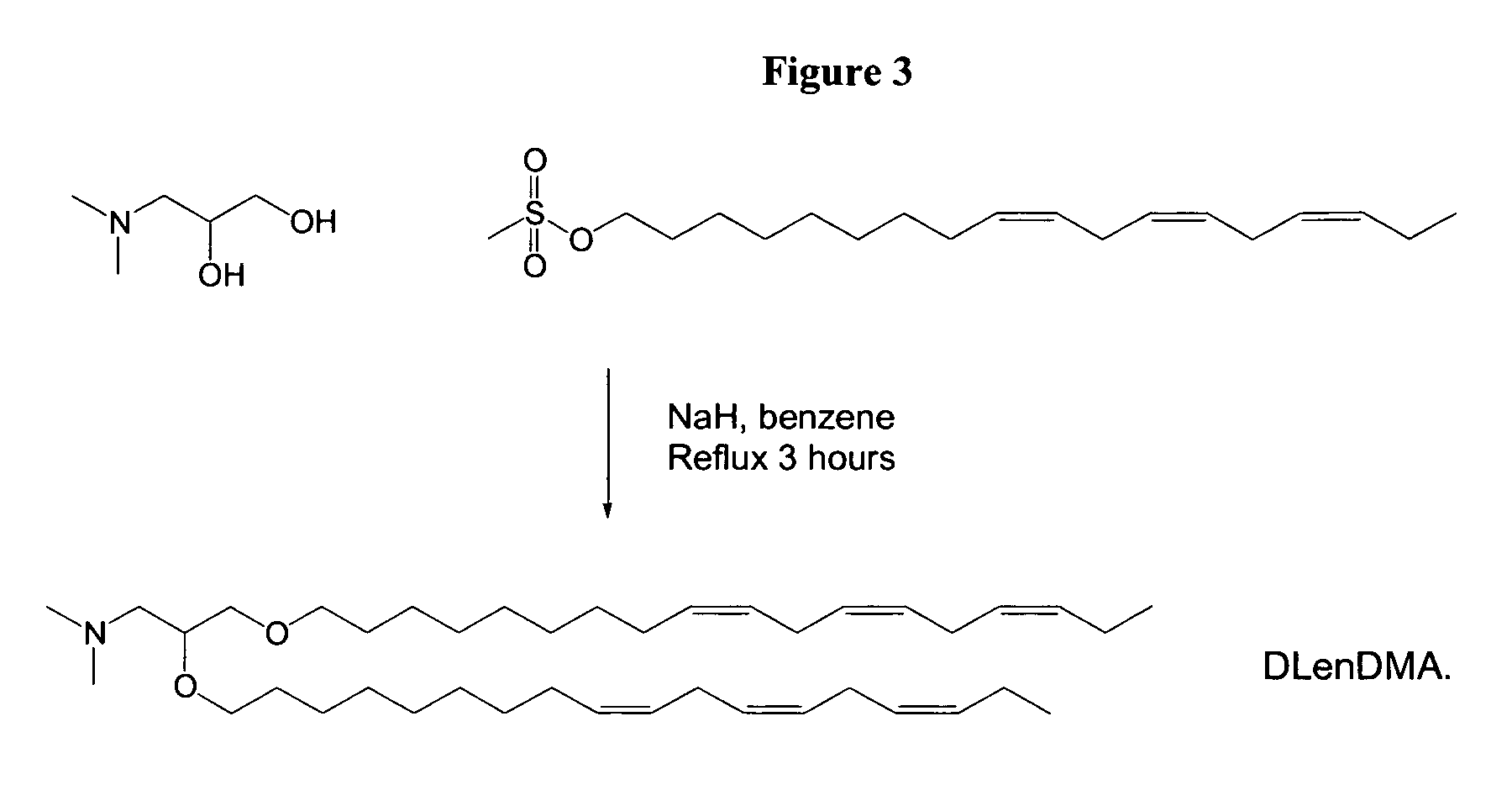
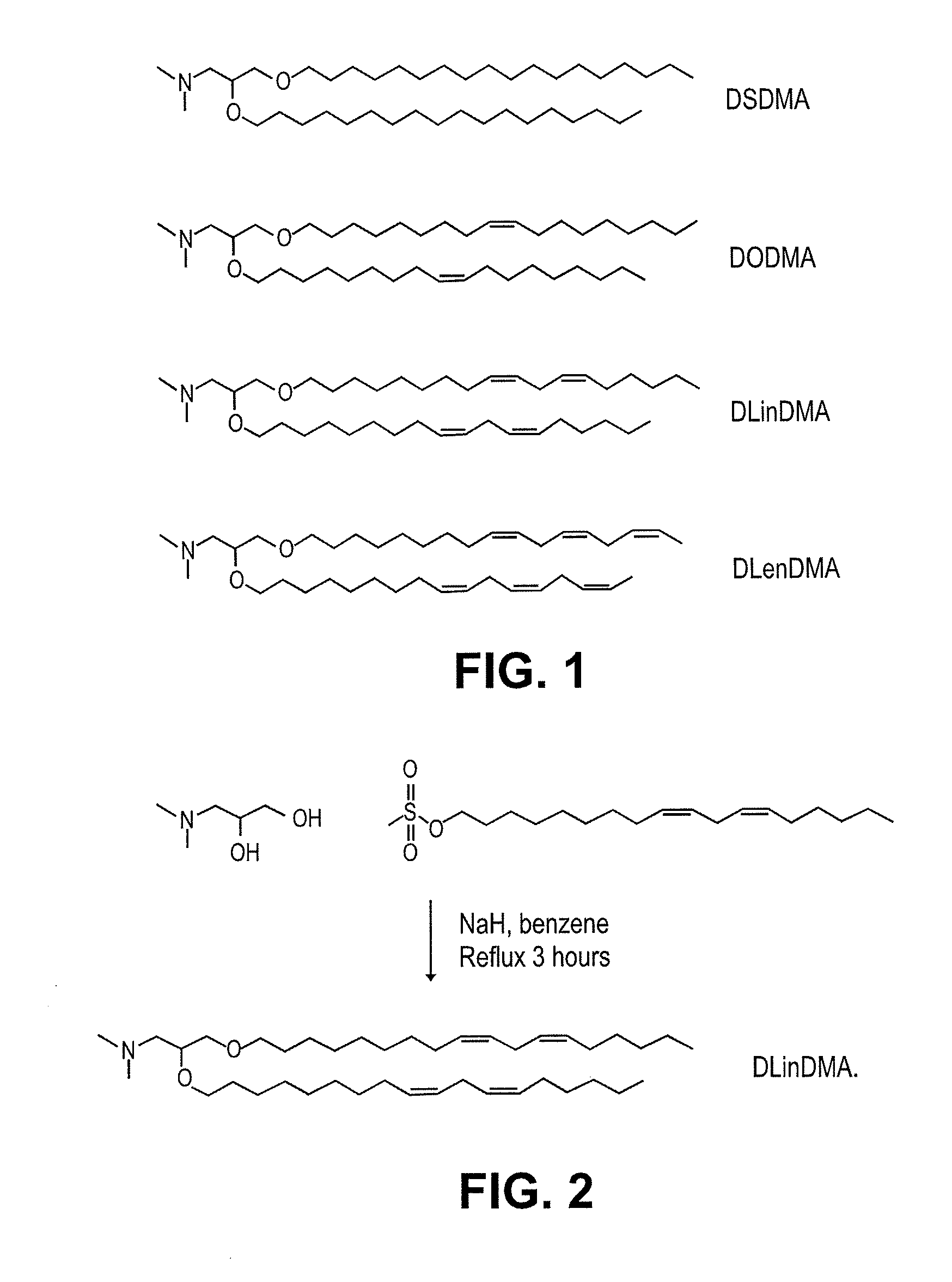
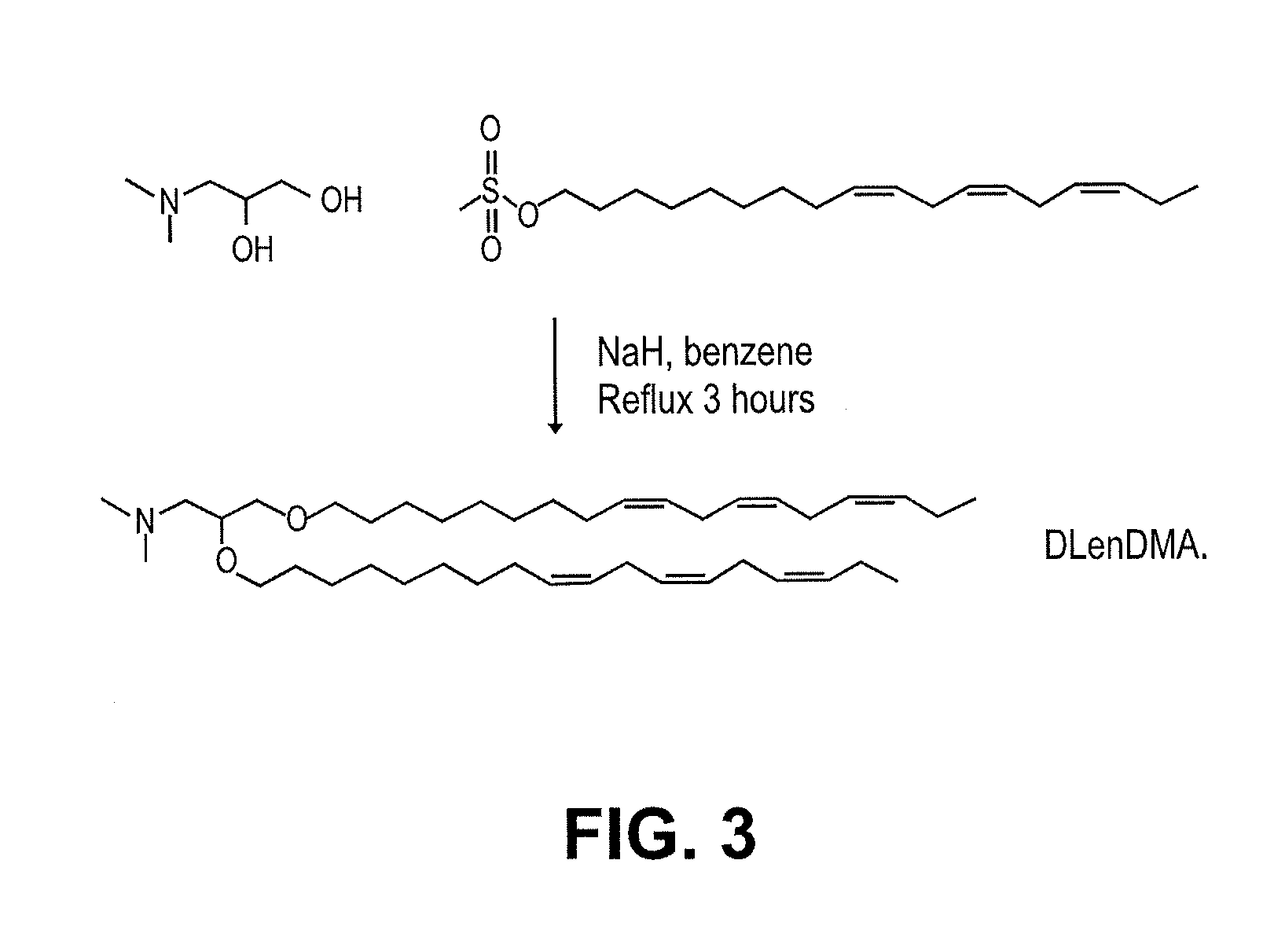
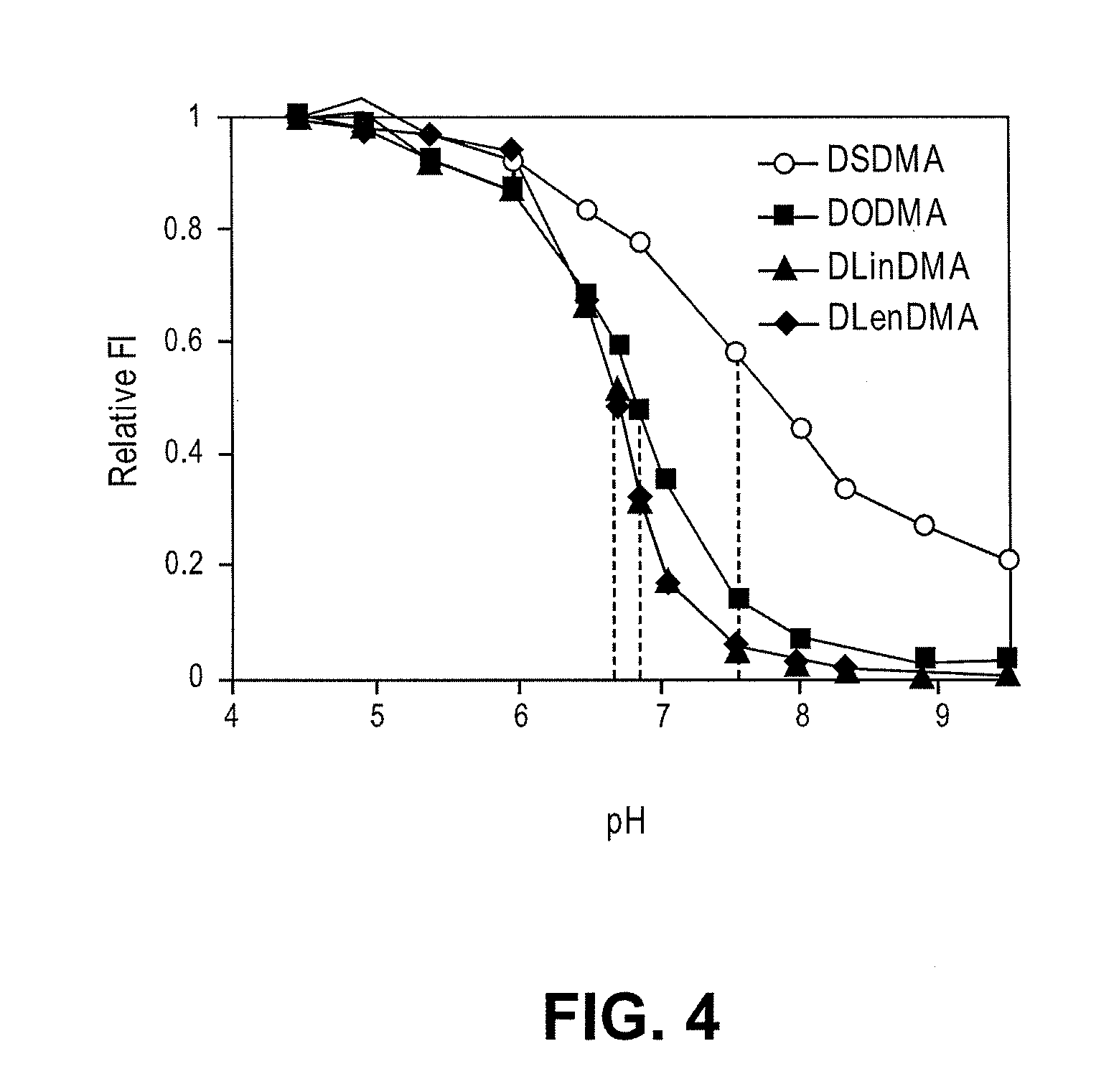
Cationic lipids and methods of use
ActiveUS20060083780A1Increase flexibilityImprove propertiesUltrasonic/sonic/infrasonic diagnosticsNervous disorderLipid formationLipid particle
The present invention provides compositions comprising cationic lipids, liposomes and nucleic acid-lipid particles comprising the cationic lipids, and methods of using such compositions, liposomes, and nucleic acid-lipid particles.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Cationic lipids and methods of use
ActiveUS7745651B2Increase flexibilityGood fluidityUltrasonic/sonic/infrasonic diagnosticsBiocideLipid particleLiposome
The present invention provides compositions comprising cationic lipids, liposomes and nucleic acid-lipid particles comprising the cationic lipids, and methods of using such compositions, liposomes, and nucleic acid-lipid particles.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Therapeutic delivery systems
InactiveUS6443898B1Low costSuitable for useUltrasonic/sonic/infrasonic diagnosticsPowder deliveryMicrosphereLiposome
Therapeutic delivery systems comprising gaseous precursor-filled microspheres comprising a therapeutic are described. Methods for employing such microspheres in therapeutic delivery applications are also provided. Therapeutic delivery systems comprising gaseous precursor-filled liposomes having encapsulated therein a contrast agent or drug are preferred. Methods of and apparatus for preparing such liposomes and methods for employing such liposomes in therapeutic delivery applications are also disclosed.
Owner:CEREVAST MEDICAL
High flow engineering thermoplastic compositions and products made therefrom
InactiveUS20040108623A1Improve moldability and flowabilityGood balance of impact and heat resistanceOrganic dyesThermoplasticAcrylate
High flow engineering thermoplastic compositions made from a thermoplastic host polymer and a low molecular weight flow modifier polymer, and products made therefrom. The flow modifier polymer is made by polymerizing at least one vinyl aromatic monomer and at least one (meth)acrylate monomer. The high flow engineering thermoplastics provide improved flowability and processability without sacrificing impact strength or heat resistance
Owner:JOHNSON POLYMER INC
Therapeutic delivery systems
InactiveUS7083572B2Low costSuitable for useUltrasonic/sonic/infrasonic diagnosticsEchographic/ultrasound-imaging preparationsMedicineMicrosphere
Targeted therapeutic delivery systems comprising gas- or gaseous precursor-filled lipid microspheres comprising a therapeutic are described. Methods for employing such microspheres in therapeutic delivery applications are also provided.Targeted therapeutic delivery systems comprising gas- or gaseous precursor-filled liposomes having a drug encapsulated therein are preferred. Methods of and apparatus for preparing such liposomes and methods for employing such liposomes in therapeutic delivery applications are also disclosed.
Owner:BRISTOL MYERS SQUIBB MEDICAL IMAGING
Bioactive Complexes Compositions and Methods of Use Thereof
InactiveUS20070085059A1Enhanced soluble complexesGood fluidityCosmetic preparationsNervous disorderDiseaseCholesterol blood
A bioactive complex composition having enhanced oxidative stability, emulsion stability, mineral rich transparent beverages and a wide range of functional health benefits. The composition may include as a base composition individual ingredients or a synergistic blend of mineral salts, Omega-3 rich oils, phospholipids, chitosan, and alpha-casein, beta-casein, kappa-casein or protein fragments, glycopeptides, phosphopeptides. The composition may optionally be further utilized for the prevention of hypercholesterolemia, bone (and teeth) mineral loss, treatment of mental health diseases, heart health, additional nutritional supplementation, and treatment of additional medical conditions.
Owner:TEXAS A&M UNIVERSITY
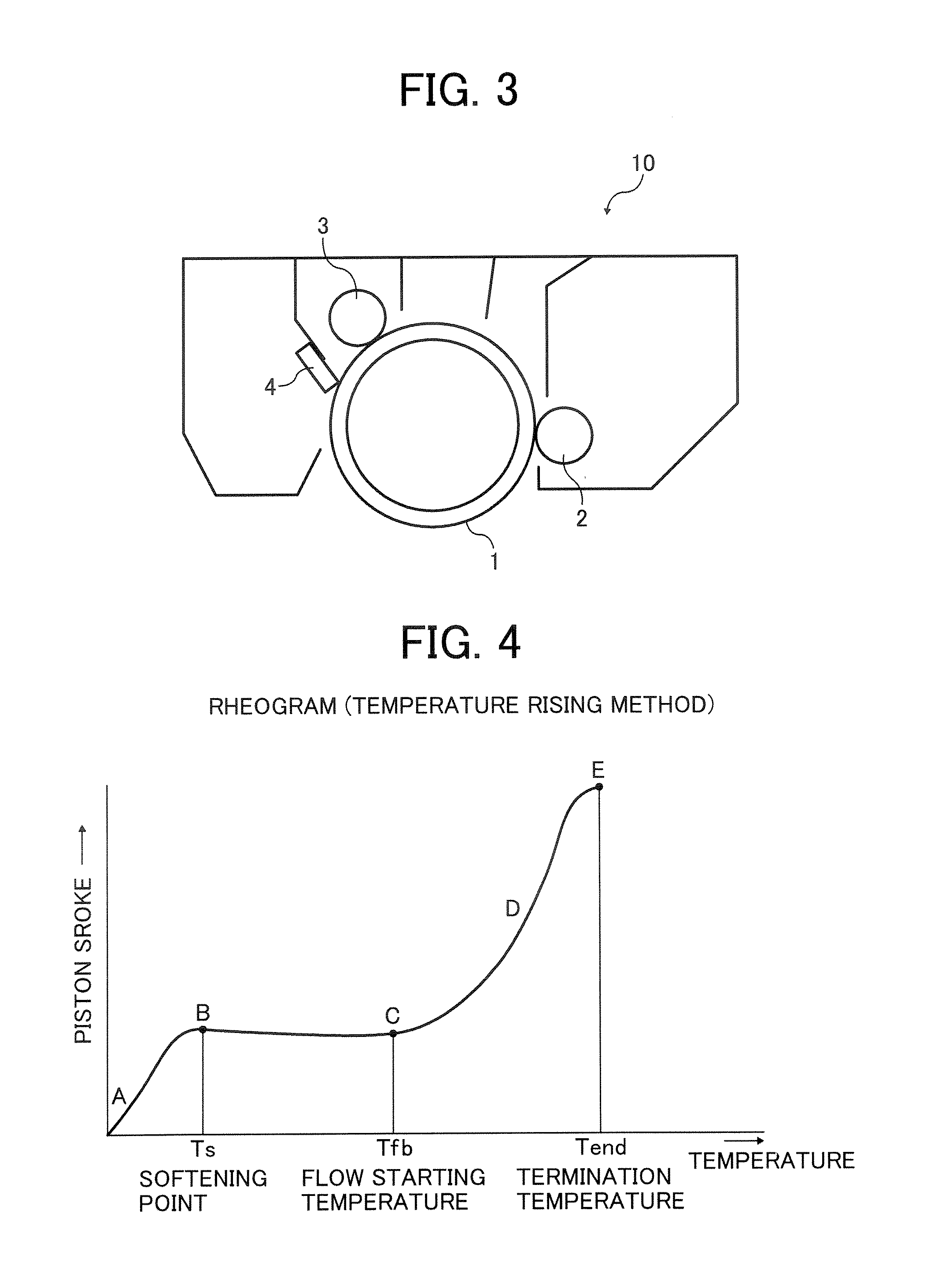
Toner and developer, and image forming method and apparatus using the developer
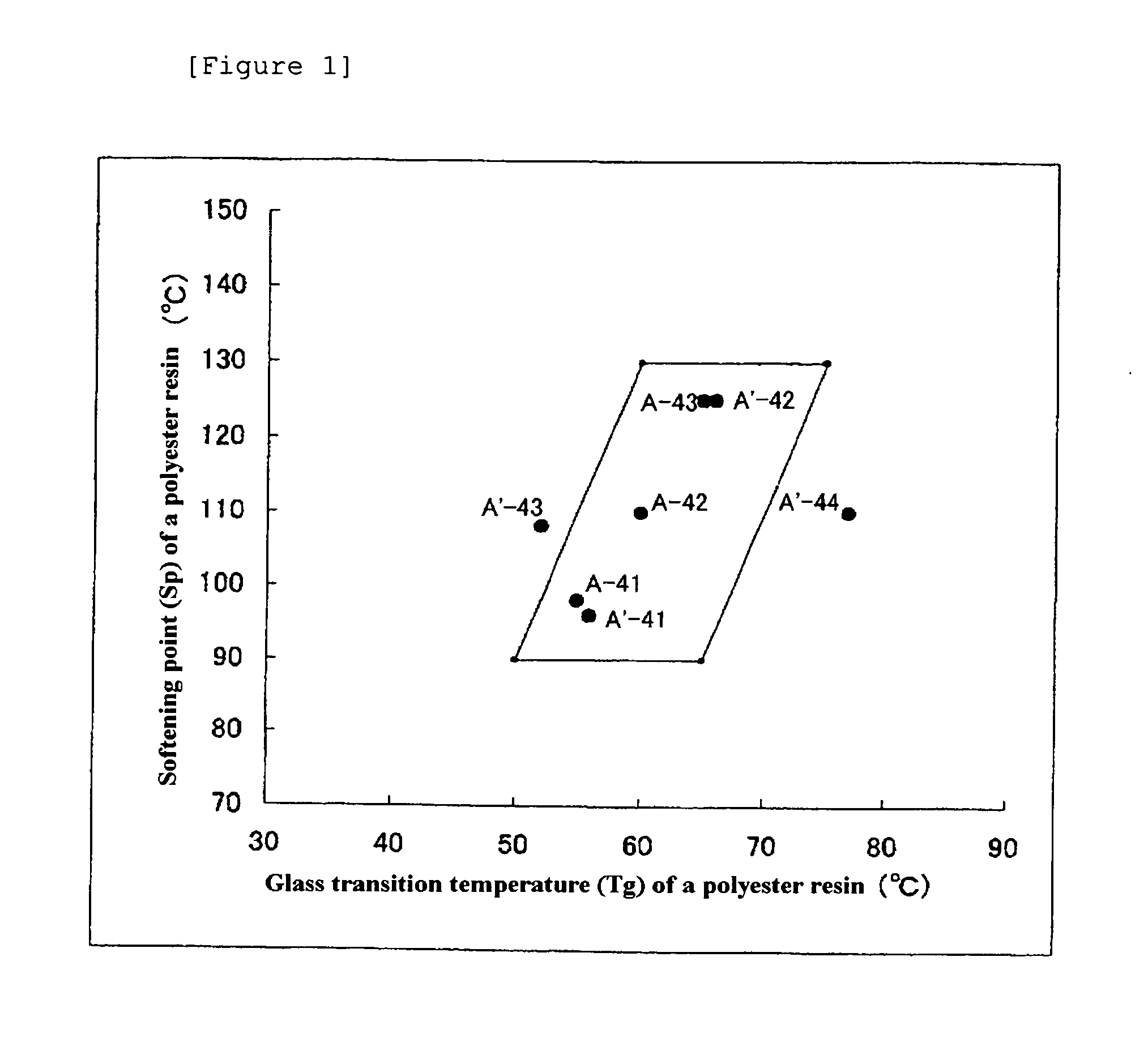
A toner is provided that contains a particulate toner material (mother toner) having an average circularity of from 0.93 to 0.99, and including a modified polyester resin and a colorant; and an external additive in an amount of from 0.3 to 5.0 parts by weight per 100 parts by weight of the mother toner, wherein the toner has a melting viscosity of from 70 to 140 Pa·s at 160° C., a weight-average particle diameter of from 3 to 7 μm, a ratio thereof to a number-average particle diameter of from 1.91 to 1.25, wherein particles satisfy at least one of (I) and (II): (I) particles having a diameter of 4 μm or less in an amount less than 10% by number; or (II) particles having a diameter of 8 μm or more in an amount less than 2% by volume, along with a one or two component developer containing the toner, a cartridge containing the toner, an image forming method using the toner and an image forming apparatus using the toner.
Owner:RICOH KK
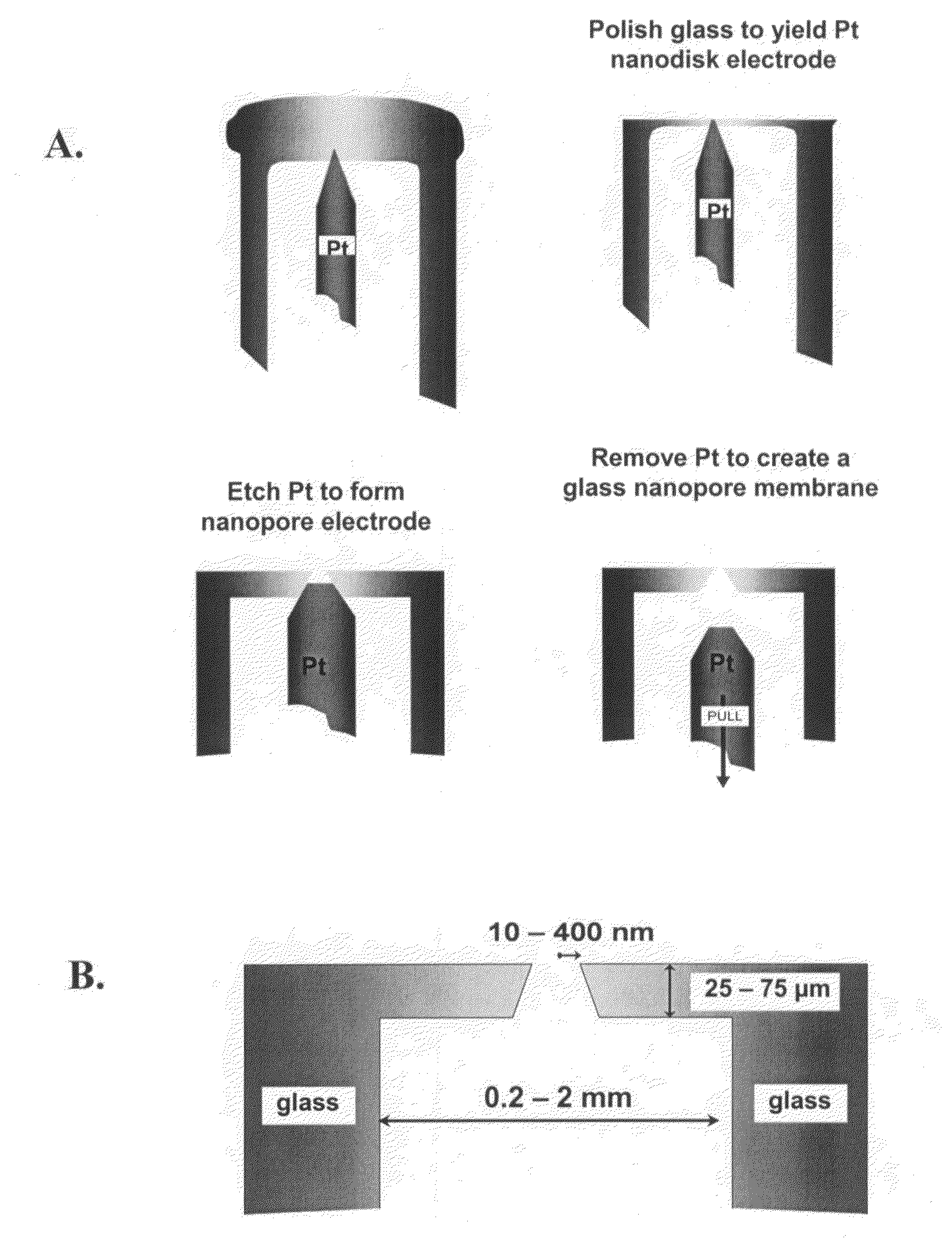
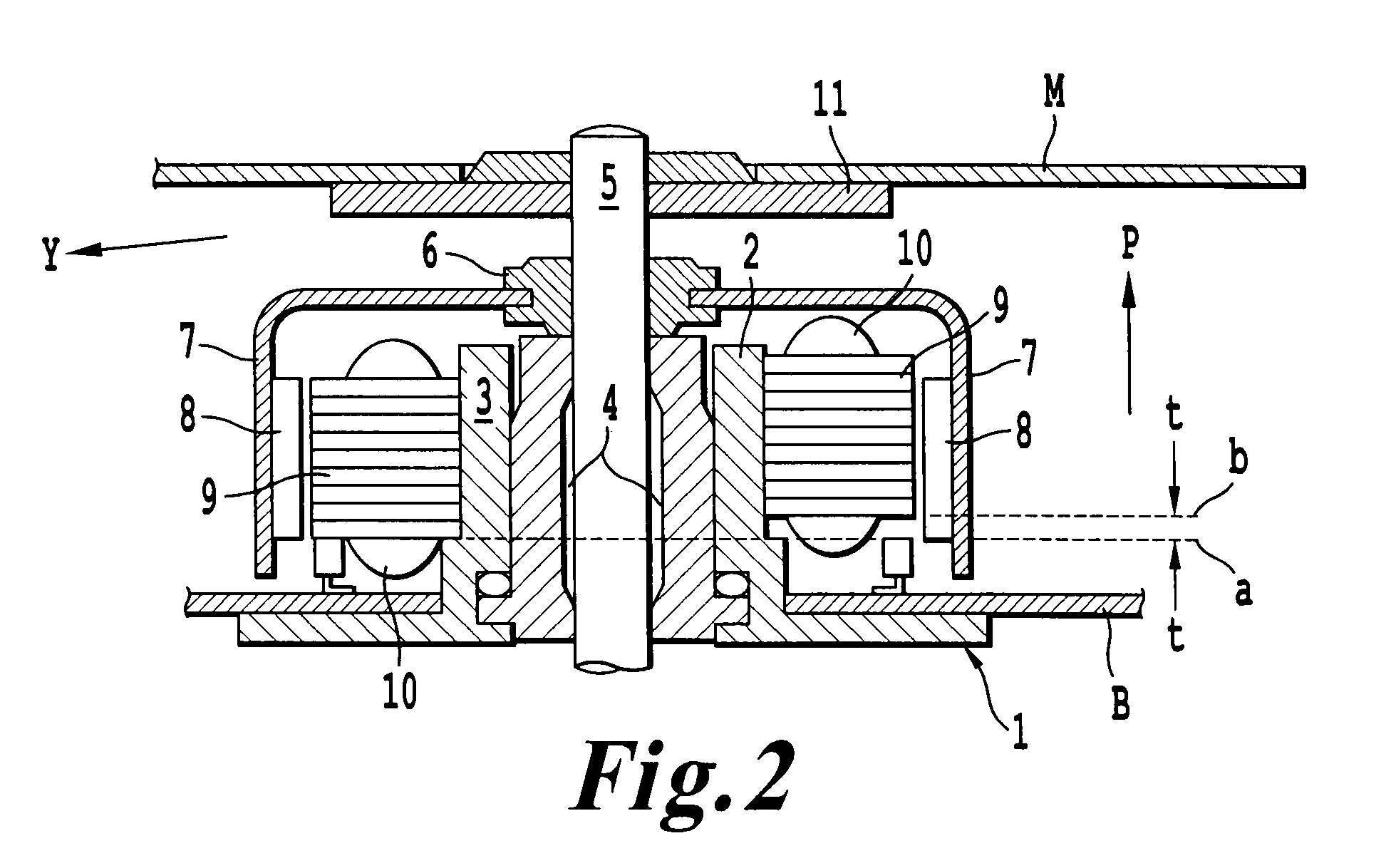
Nanopore platforms for ion channel recordings and single molecule detection and analysis
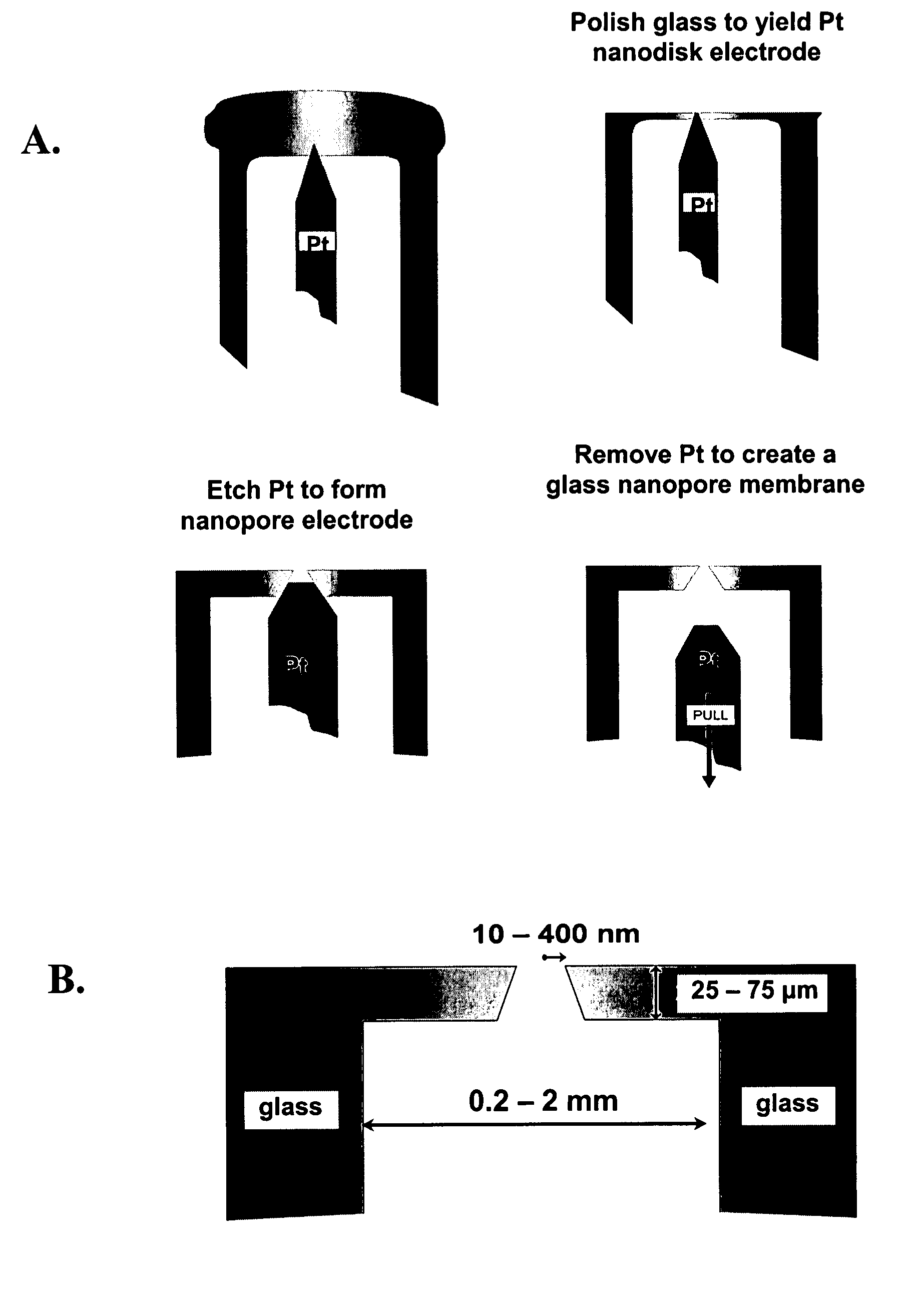
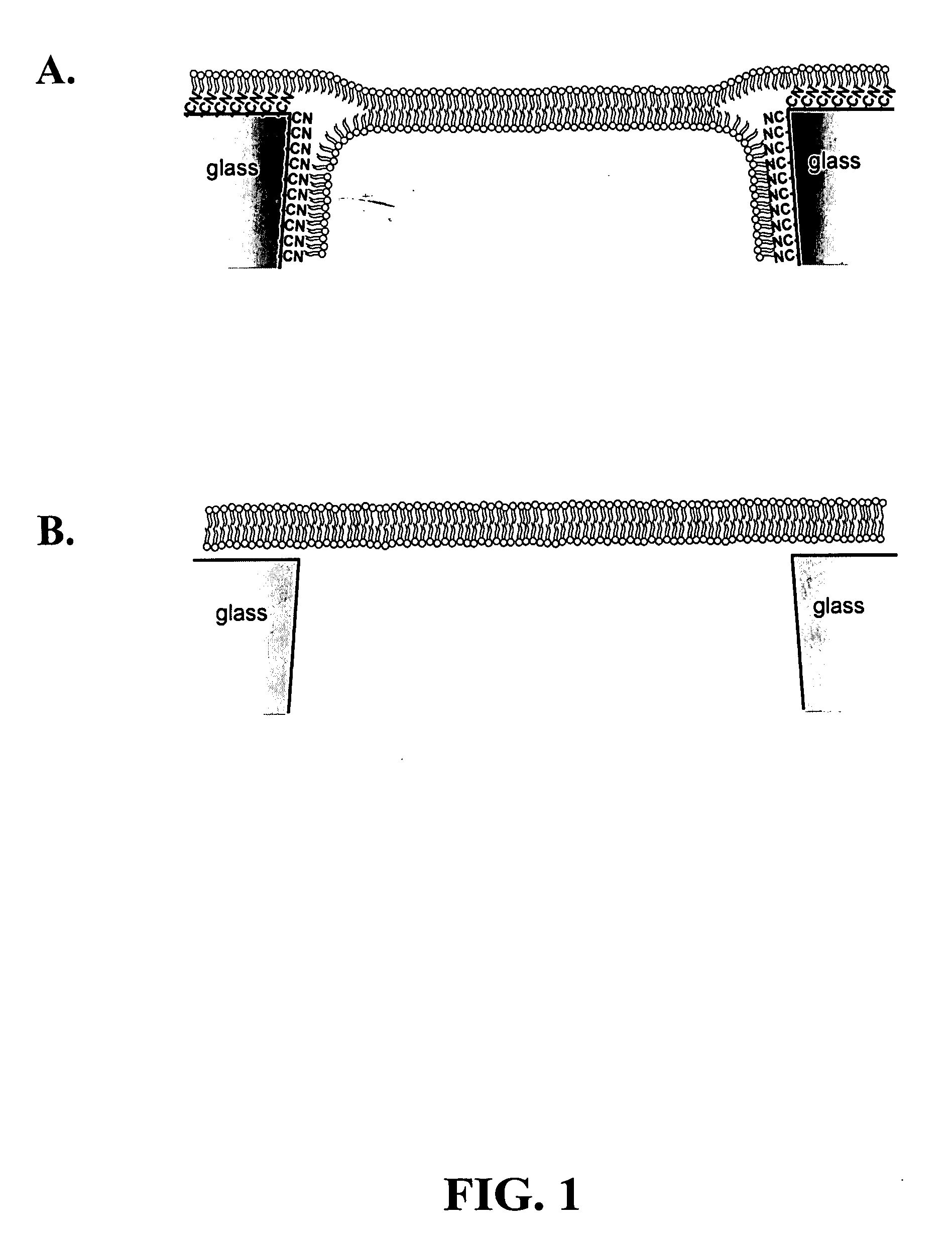
ActiveUS20080218184A1Increased electrical conductivityDetermining of stabilitySpark gapsCell seperators/membranes/diaphragms/spacersIonChemistry
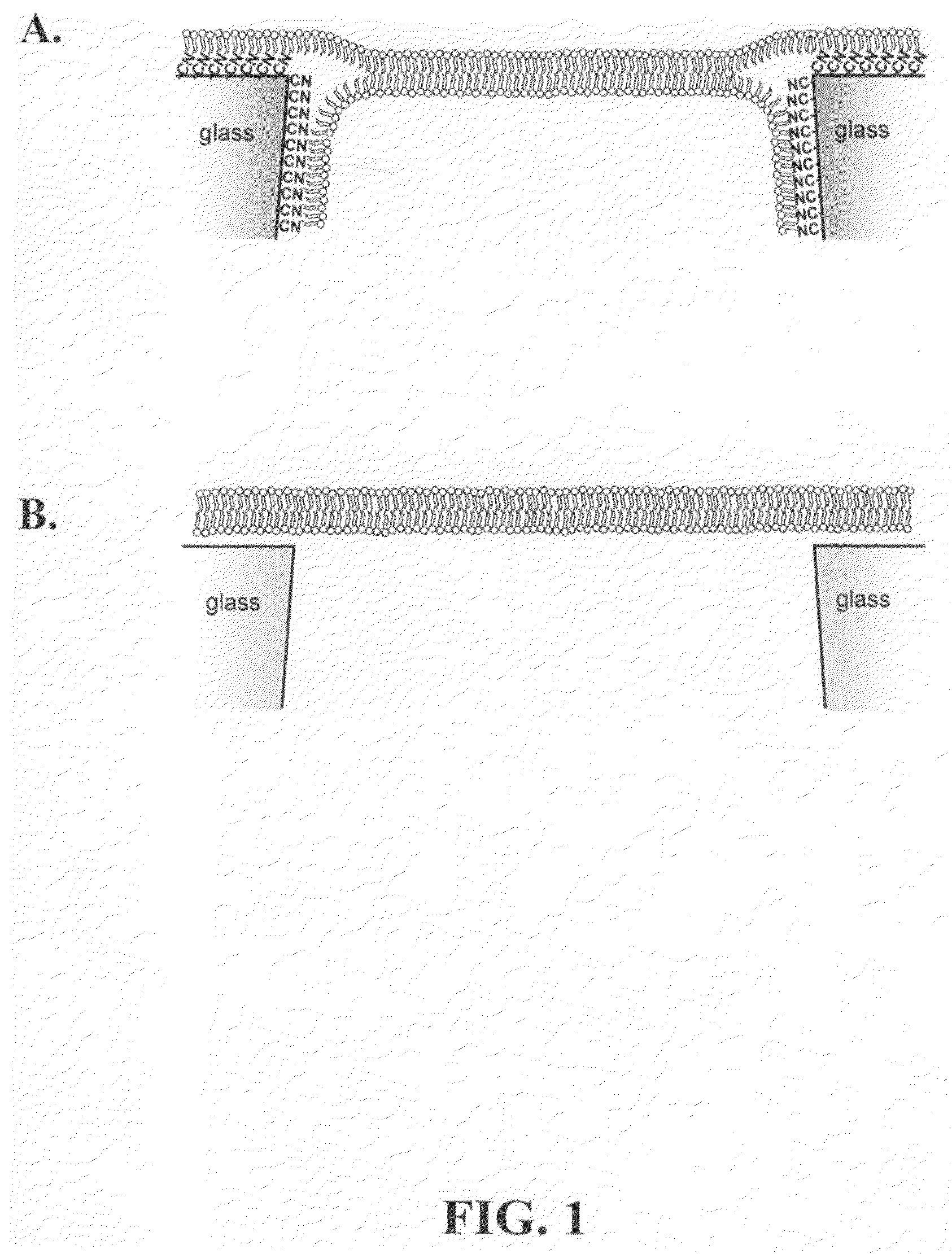
Chemical modification of a glass and fused silica nanopore surfaces results in surface properties that are ideal for localized bilayer formation over a nanopore and subsequent ion channel recording. With no surface modification, one may form a bilayer supported on the glass capillary extending across the nanopore orifice. Changing the surface properties from that of bare glass to a moderately hydrophobic surface produces a lipid monolayer above the glass and spontaneously yields a bilayer across the nanopore orifice, effectively corralling a single protein ion channel in the lipid bilayer region spanning nanopore orifice. The bilayer structure over the modified nanopore is such that current can only flow through the protein ion channel. The protein ion channel is able to diffuse in the bilayer above the pore opening, but cannot leave this area to enter the lipid monolayer. The bilayer formed across the nanopore orifice exhibits high electrical breakdown voltage, is stable to mechanical vibrations, and is long lived. Resistance through the protein channel can be measured electrically and is exploited for stochastic single-molecule detection. Protein ion channels can be inserted and removed from the bilayer by adjusting transmembrane pressure, and adapter molecules can be electrostatically trapped in the ion channel by applying high transmembrane voltages.
Owner:UNIV OF UTAH RES FOUND
Cationic lipids and methods of use
InactiveUS20110262527A1Increase flexibilityGood fluidityOrganic active ingredientsNervous disorderLipid particleLiposome
The present invention provides compositions comprising cationic lipids, liposomes and nucleic acid-lipid particles comprising the cationic lipids, and methods of using such compositions, liposomes, and nucleic acid-lipid particles.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
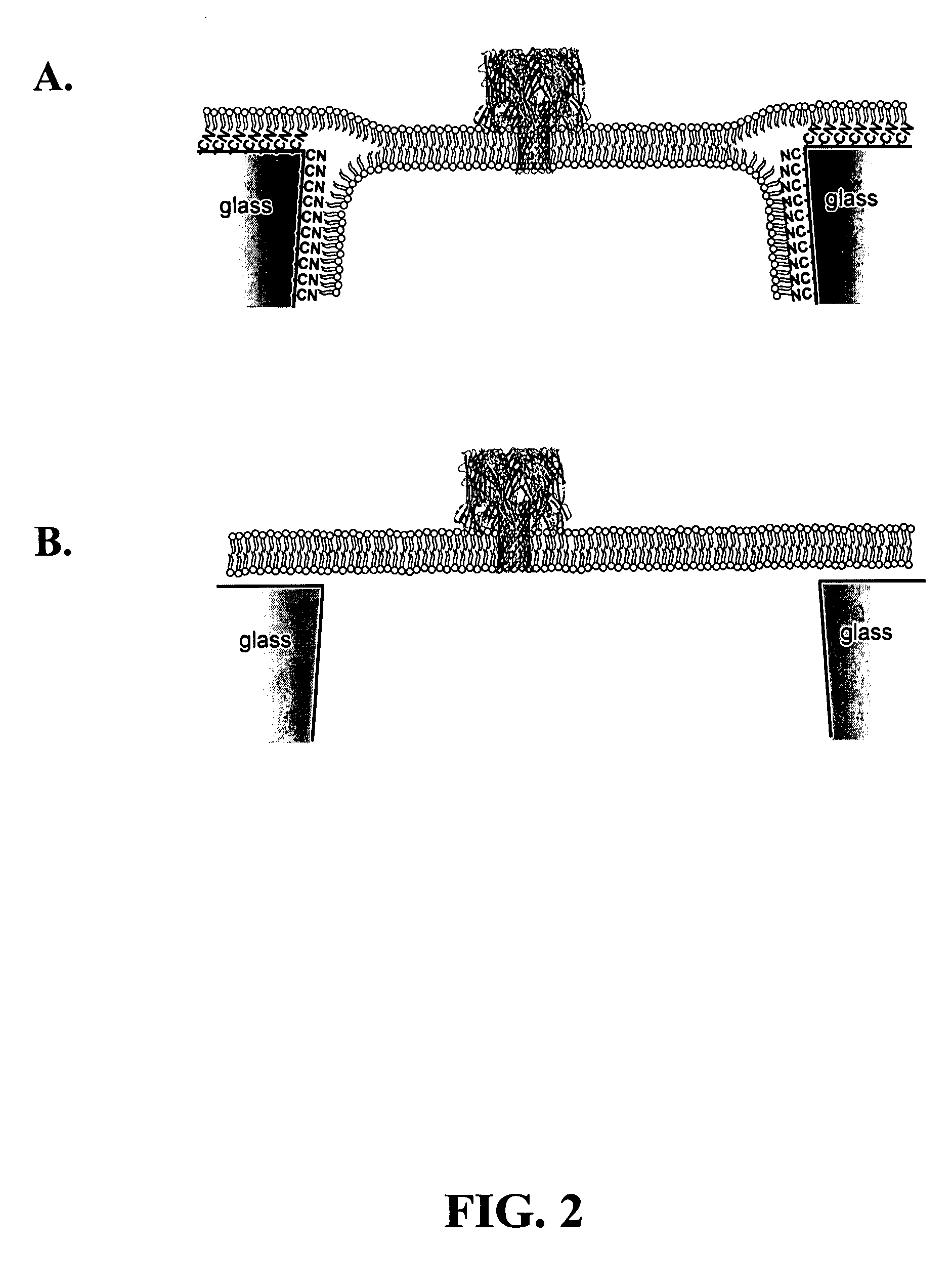
Nanopore platforms for ion channel recordings and single molecule detection and analysis
ActiveUS7777505B2Determining of stabilityGood fluiditySpark gapsResistance/reactance/impedenceLipid formationSpontaneous generation
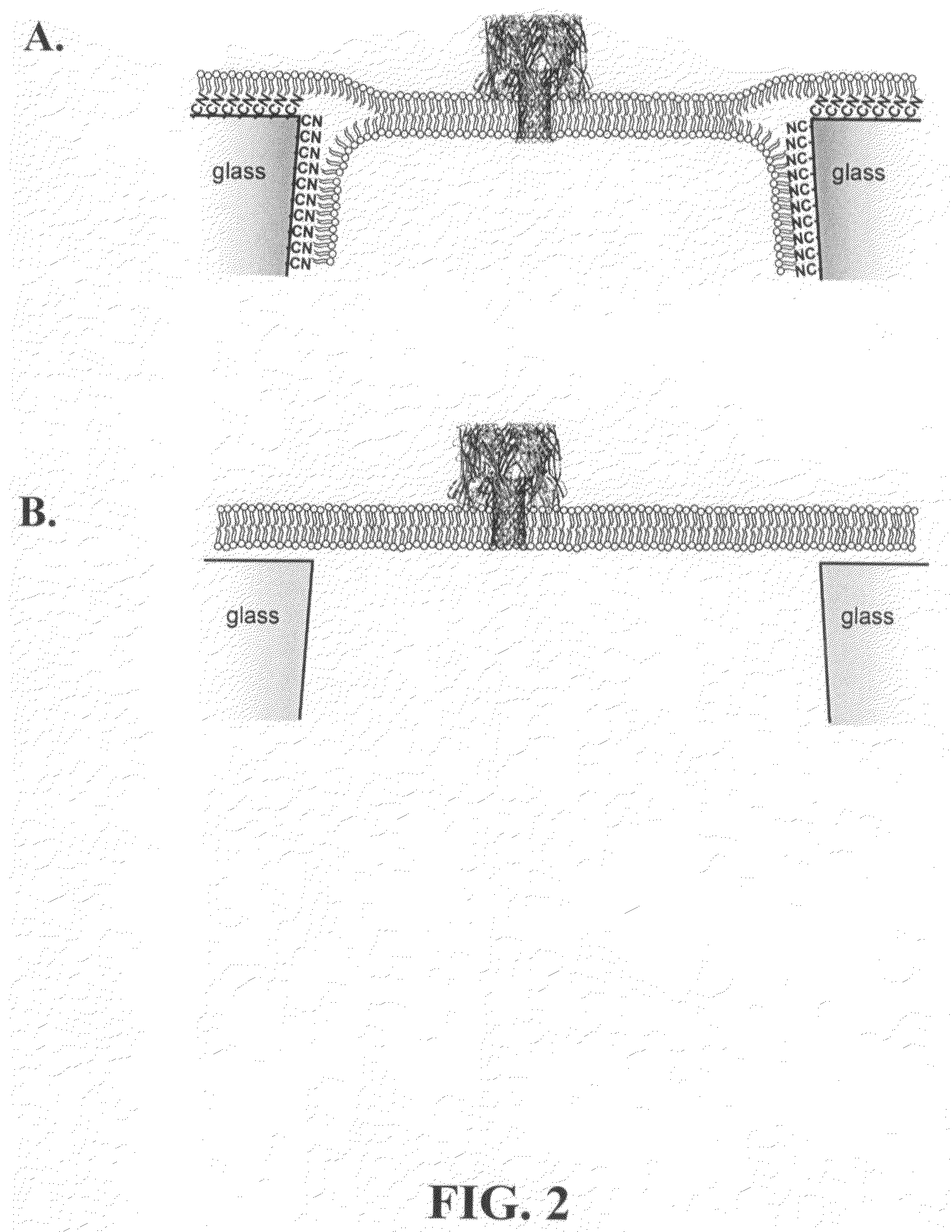
A nanopore device includes a membrane having a nanopore extending there through forming a channel from a first side of the membrane to a second side of the membrane. The surface of the channel and first side of the membrane are modified with a hydrophobic coating. A first lipid monolayer is deposited on the first side of the membrane, and a second lipid monolayer is deposited on the second side of the membrane, wherein the hydrophobic coating causes spontaneous generation of a lipid bilayer across the nanopore orifice. Sensing entities, such as a protein ion channel, can be inserted and removed from the bilayer by adjusting transmembrane pressure, and adapter molecules can be electrostatically trapped in the ion channel by applying high transmembrane voltages, while resistance or current flow through the sensing entity can be measured electrically.
Owner:UNIV OF UTAH RES FOUND
Process for producing saturated aliphatic hydrocarbon compound, and lubricant composition
InactiveUS20080146469A1Low-temperature fluidityImprove Oxidation StabilityHydrocarbon by hydrogenationCatalystsAlpha-olefinCoordination complex
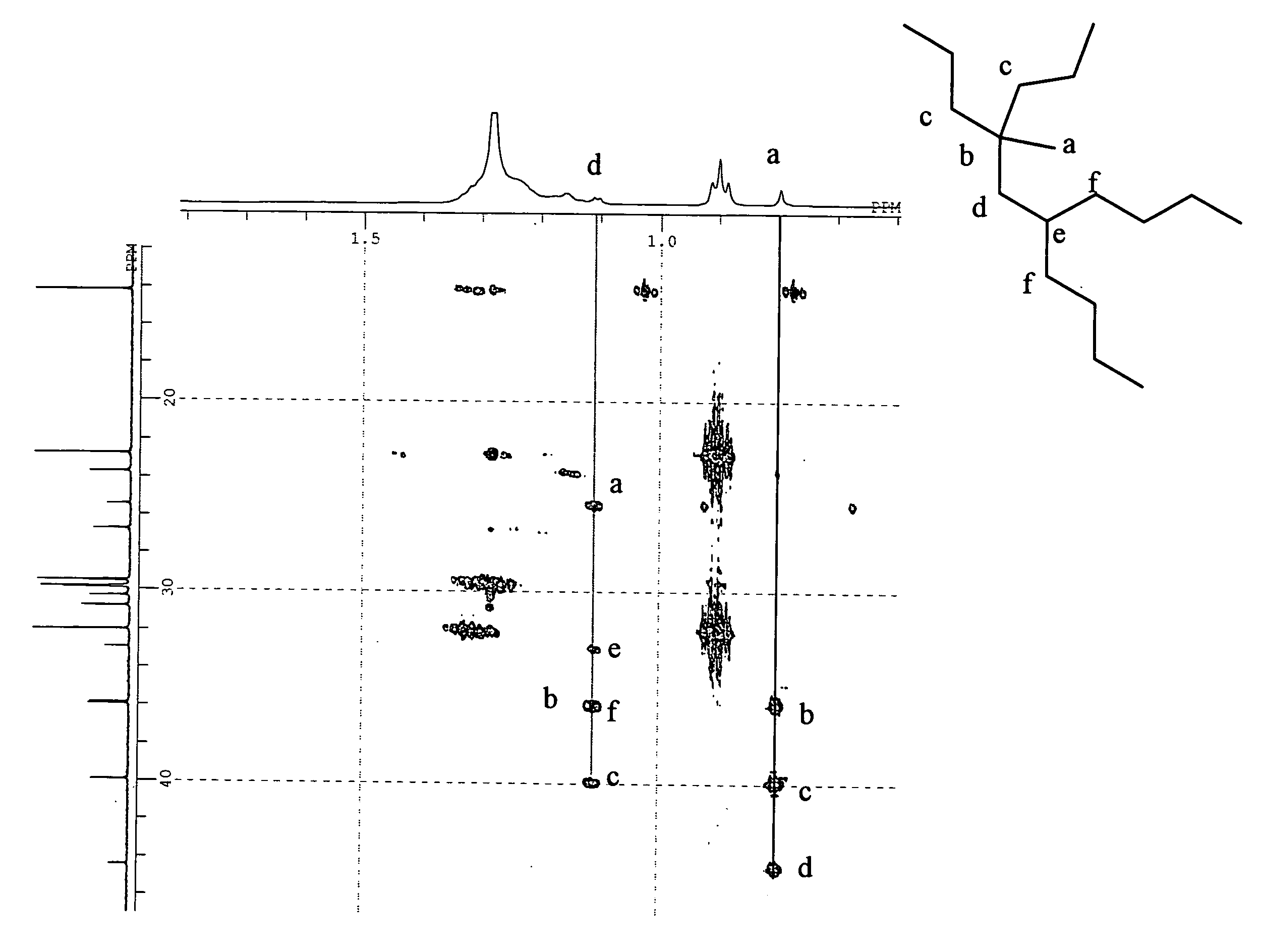
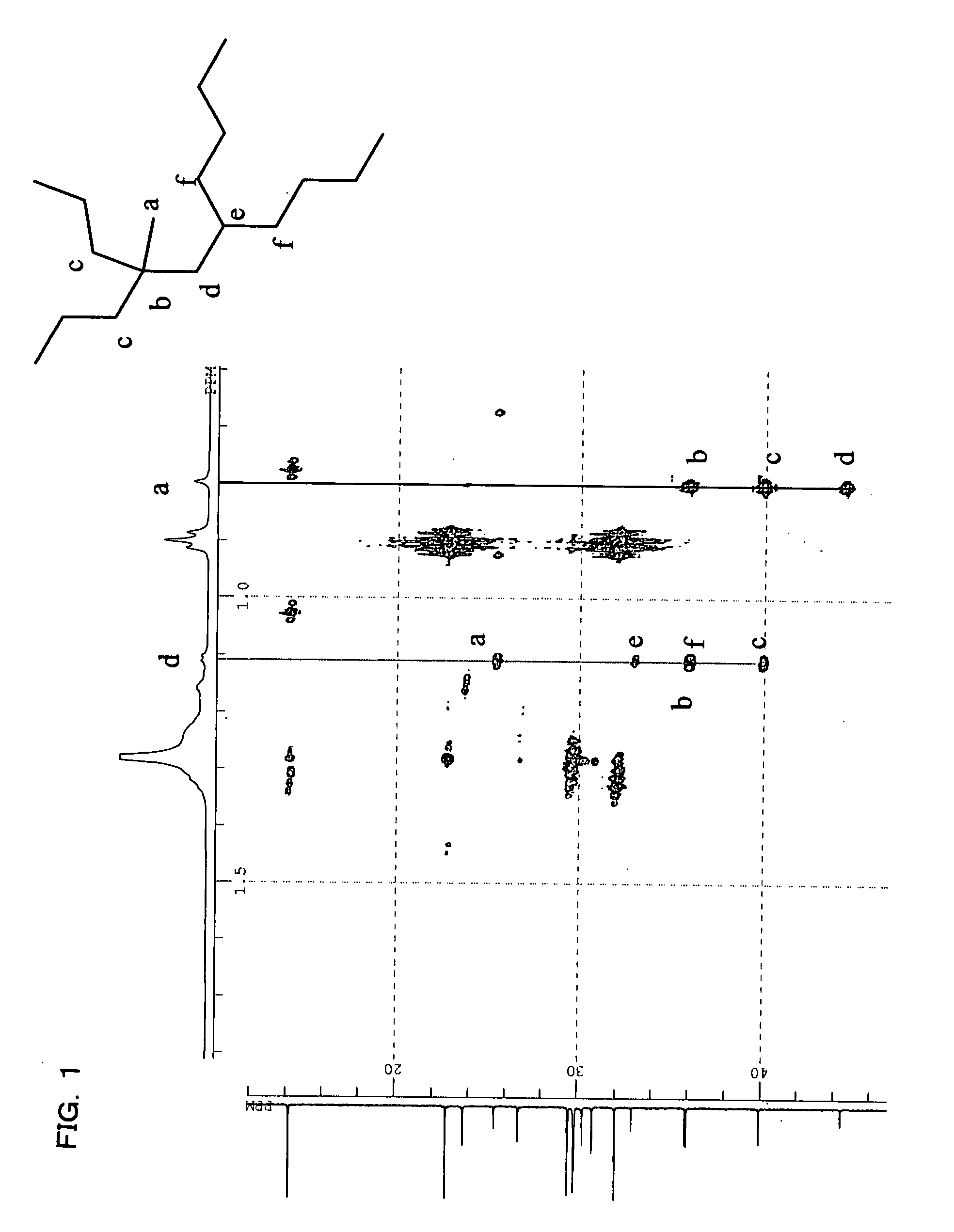
The present invention provides a process for producing a saturated aliphatic hydrocarbon prepared using an α-olefin as a raw material and represented by the general formula (1), including the steps of: (I) producing a vinylidene olefin by dimerizing the α-olefin in the presence of a metallocene complex catalyst; (II) further dimerizing the vinylidene olefin in the presence of an acid catalyst; and (III) hydrogenating the obtained dimer. Further, there are provided a lubricant composition containing the saturated aliphatic hydrocarbon compound produced by the above process, a bearing oil consisting of the lubricant composition, and making use of the same, a bearing and gyral equipment. The saturated aliphatic hydrocarbon compounds produced by the process of the present invention have low-temperature fluidity, exhibiting low evaporativity, and excellent in thermal stability and oxidation stability. Thus, the saturated aliphatic hydrocarbon compounds are suitable for use as, for example, a base oil of lubricant composition for hydraulic pressure, turbine, working machine, bearing, gear, metal-working, etc.
Owner:IDEMITSU KOSAN CO LTD
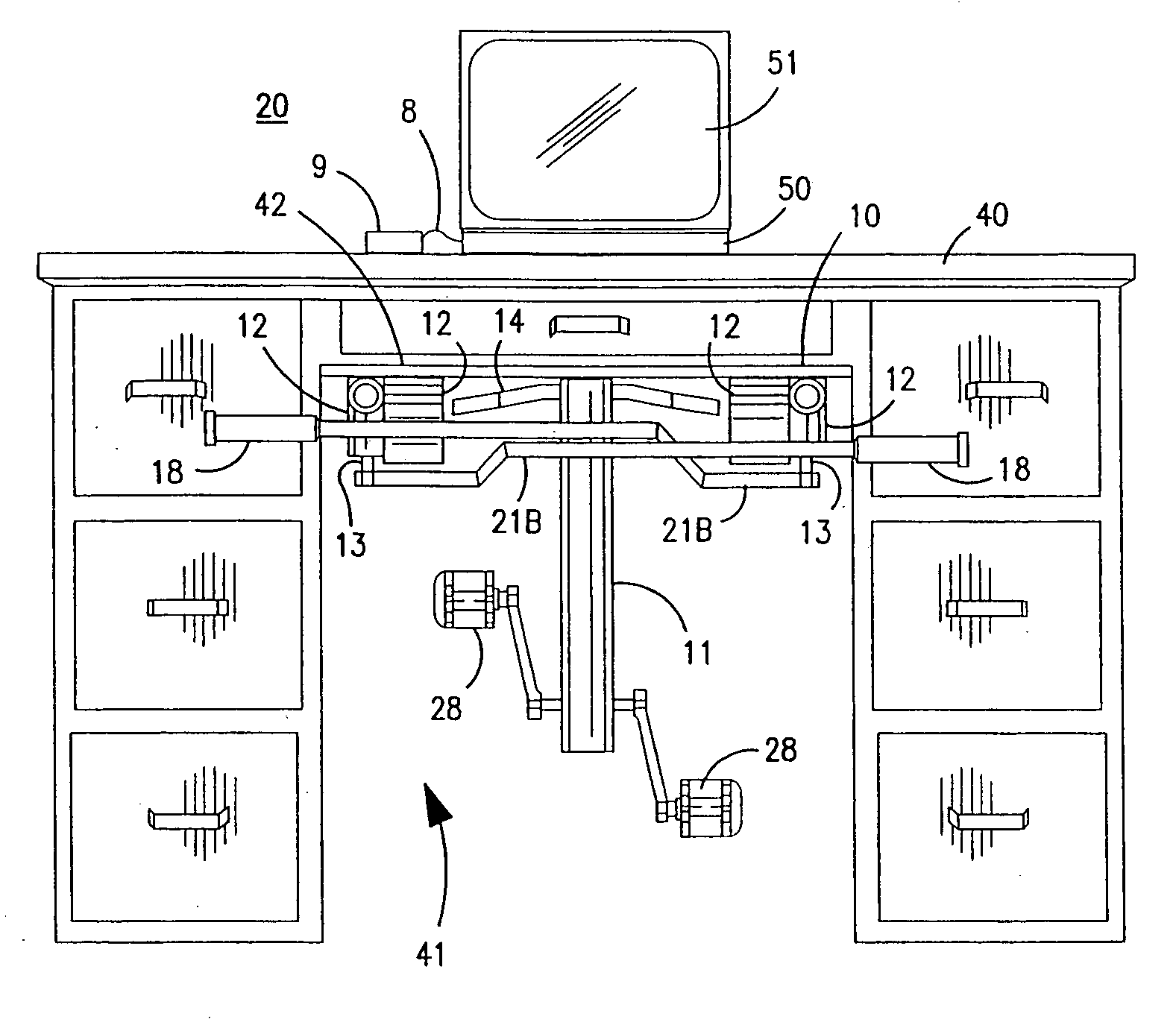
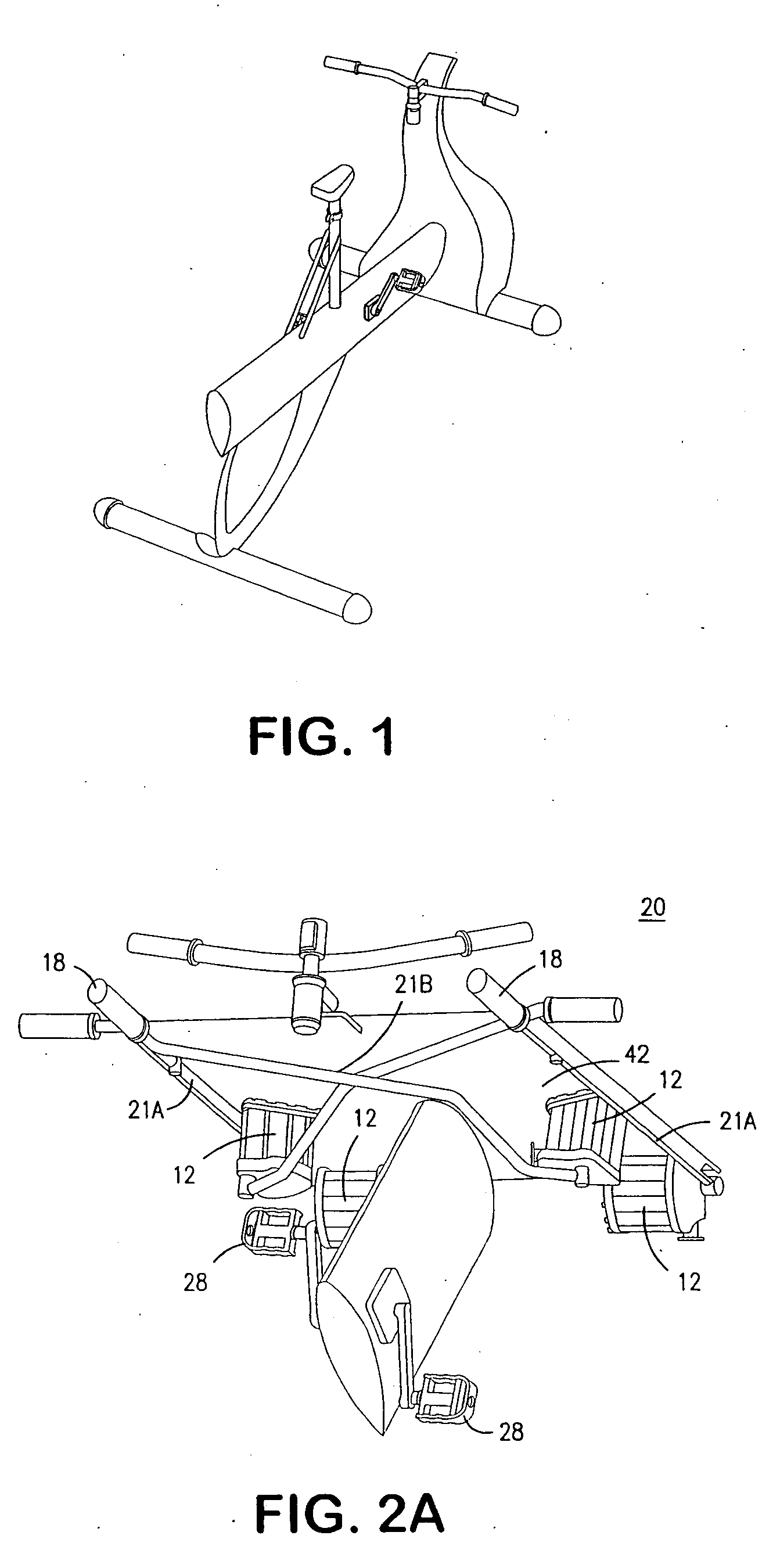
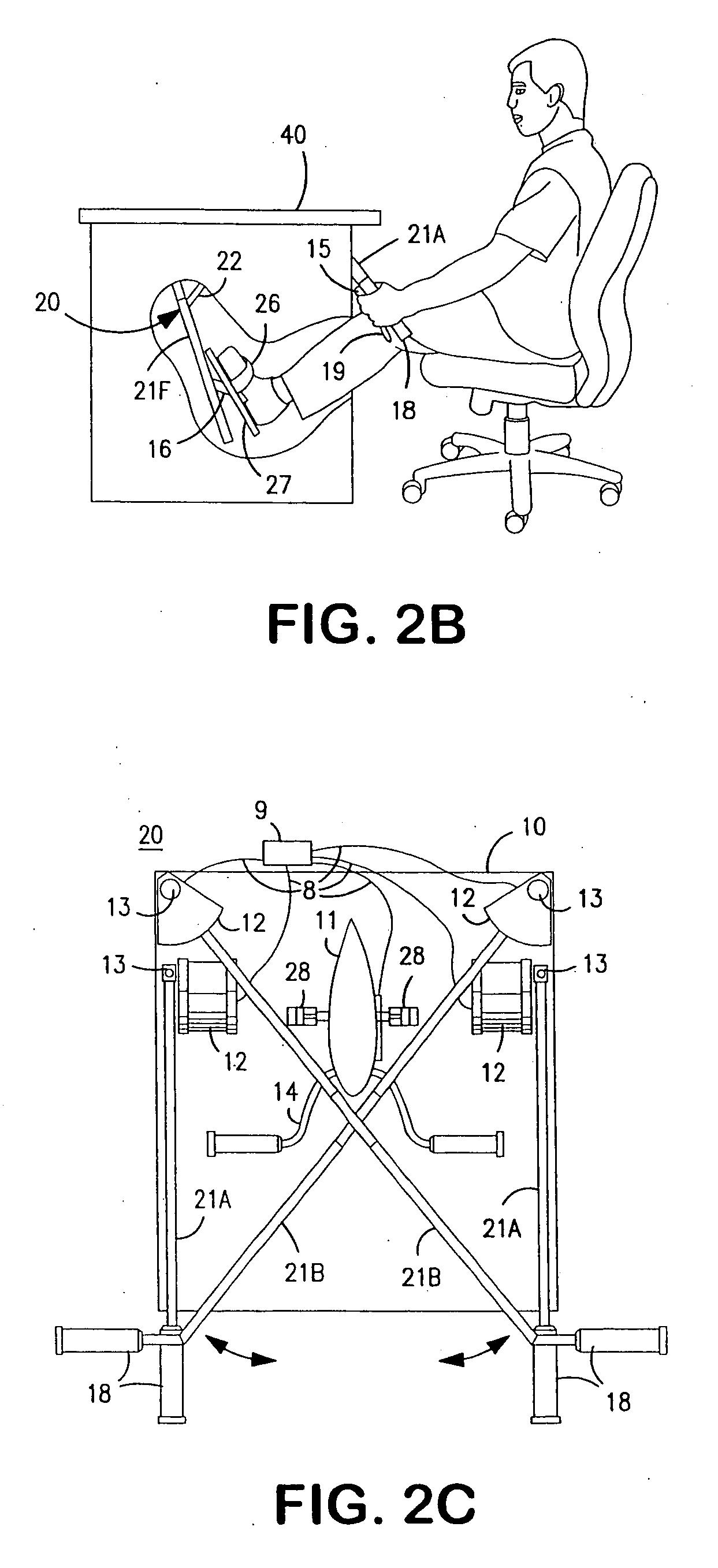
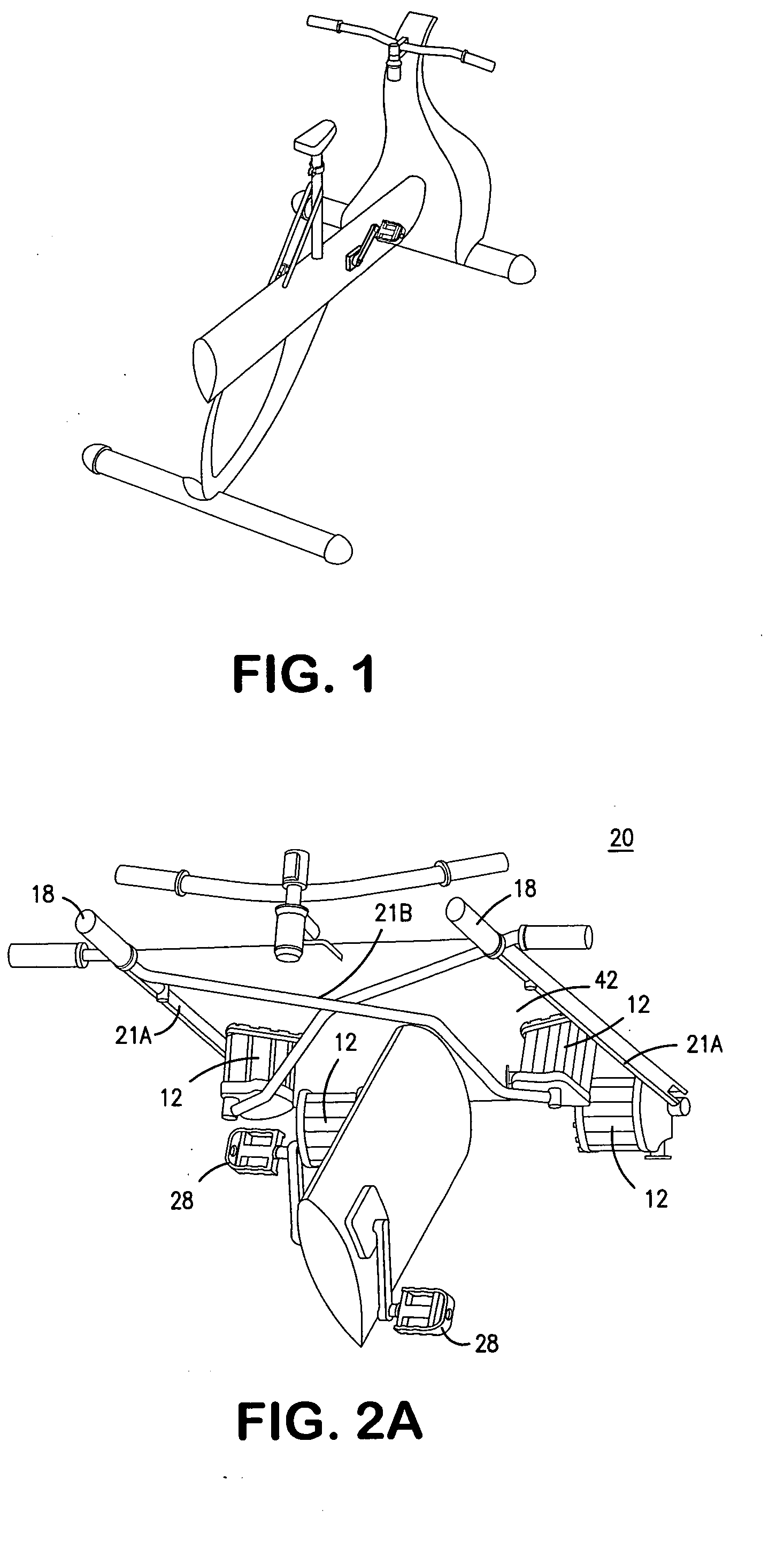
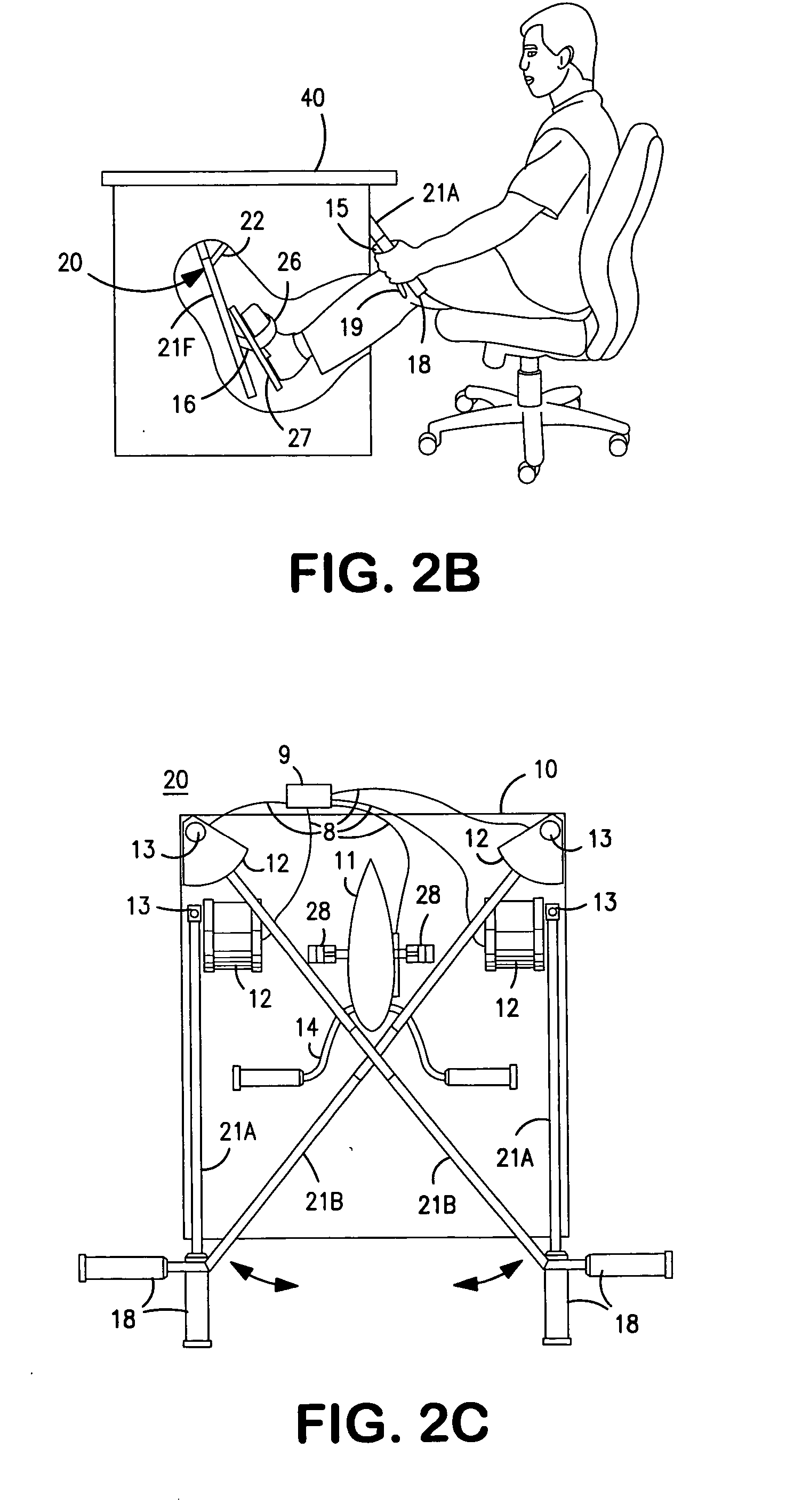
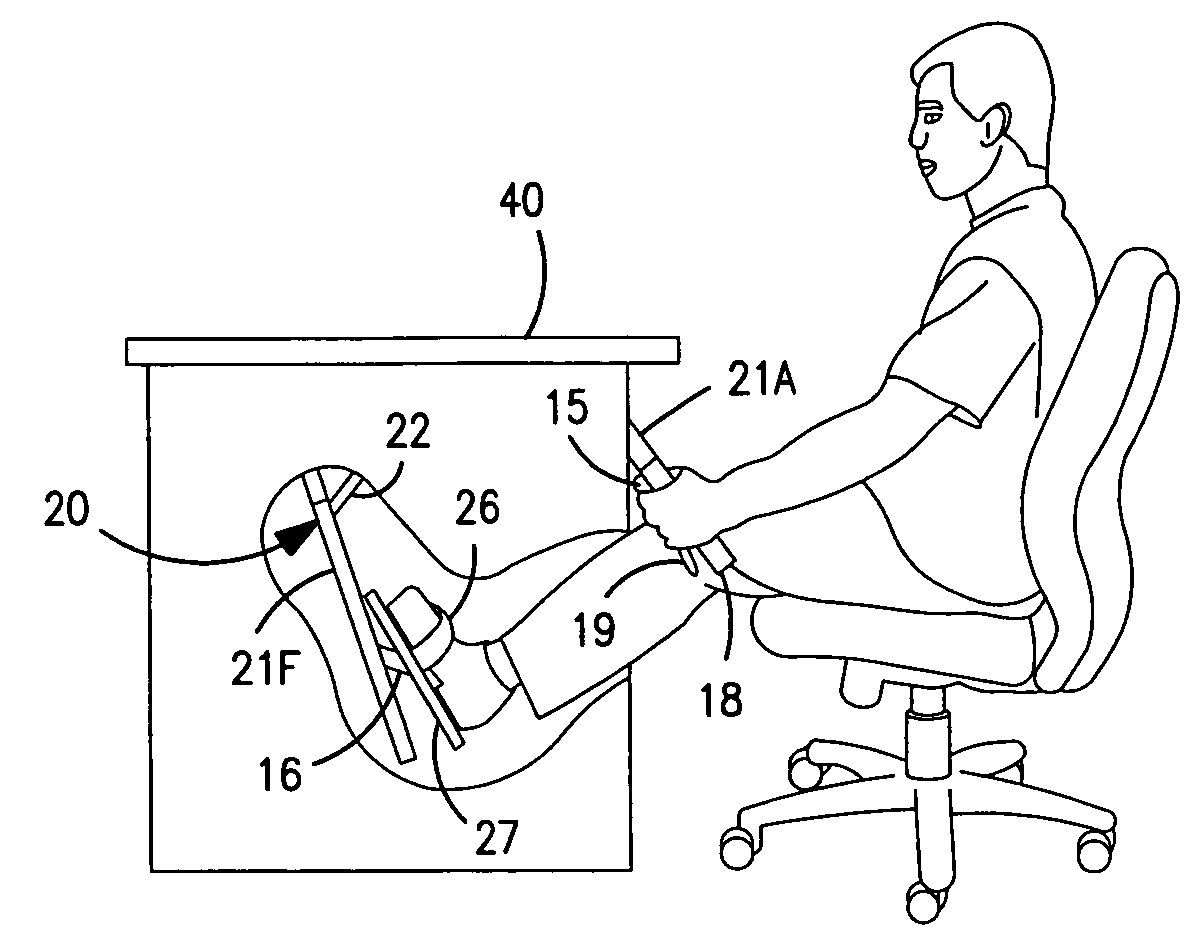
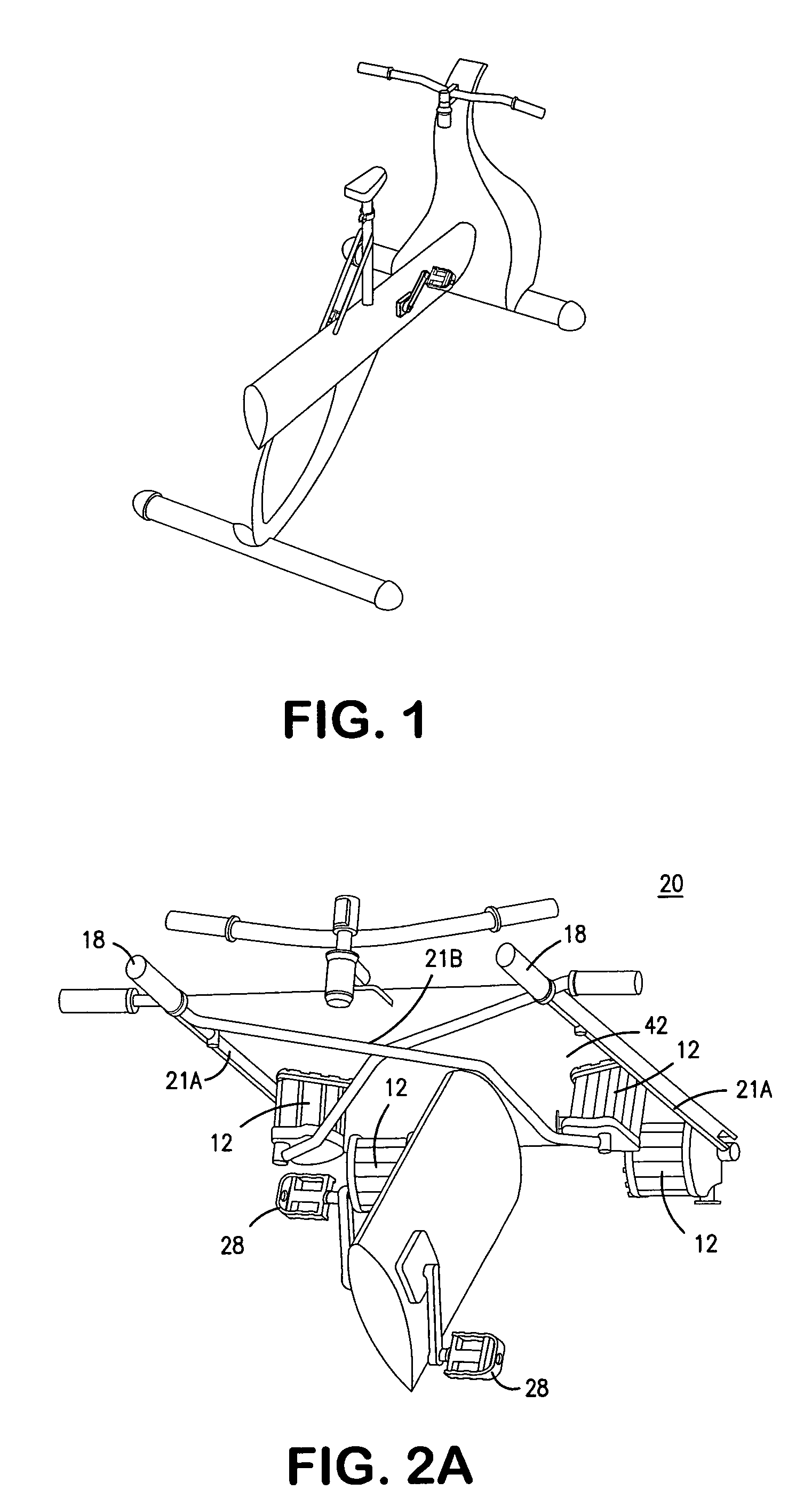
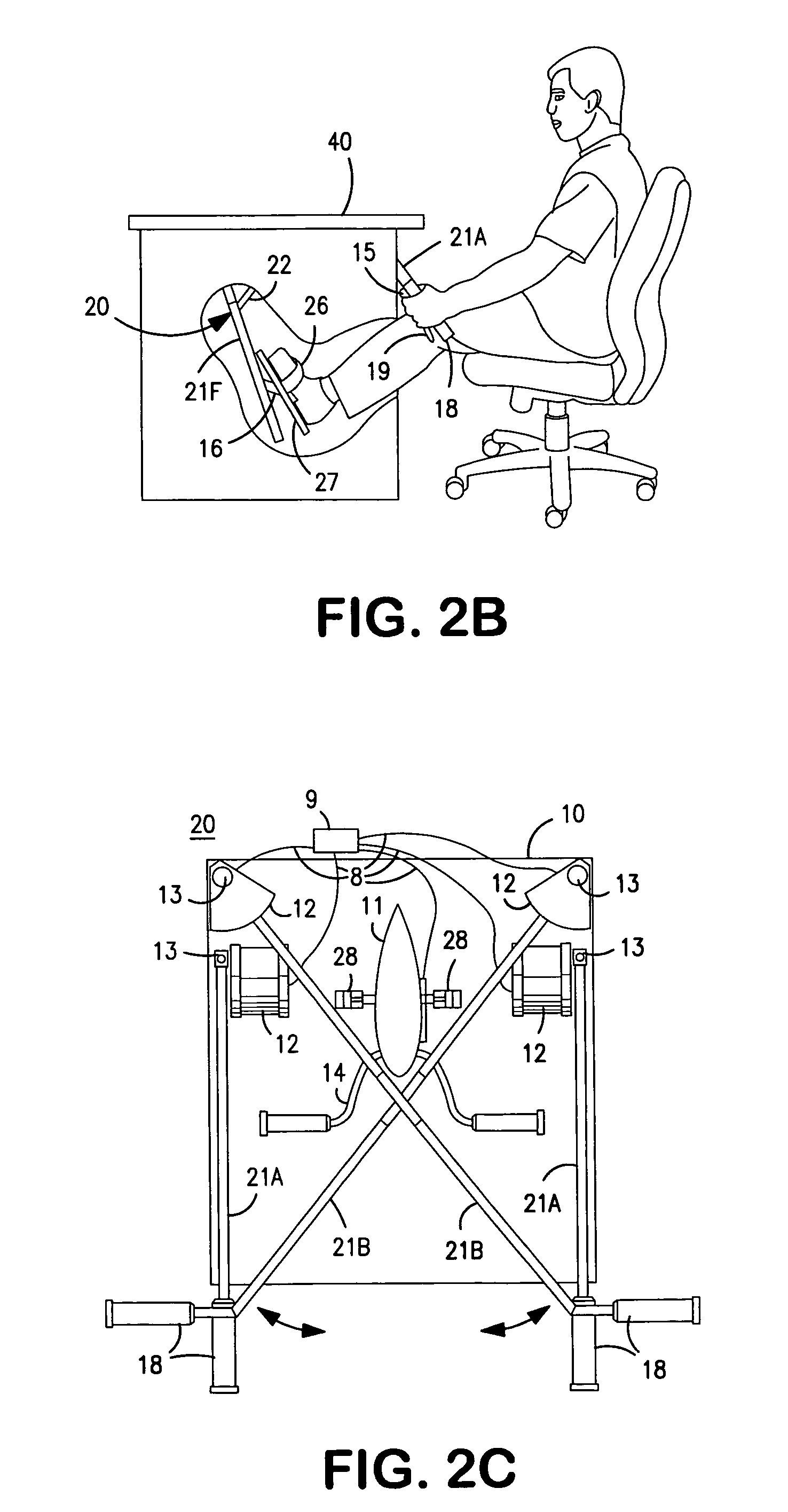
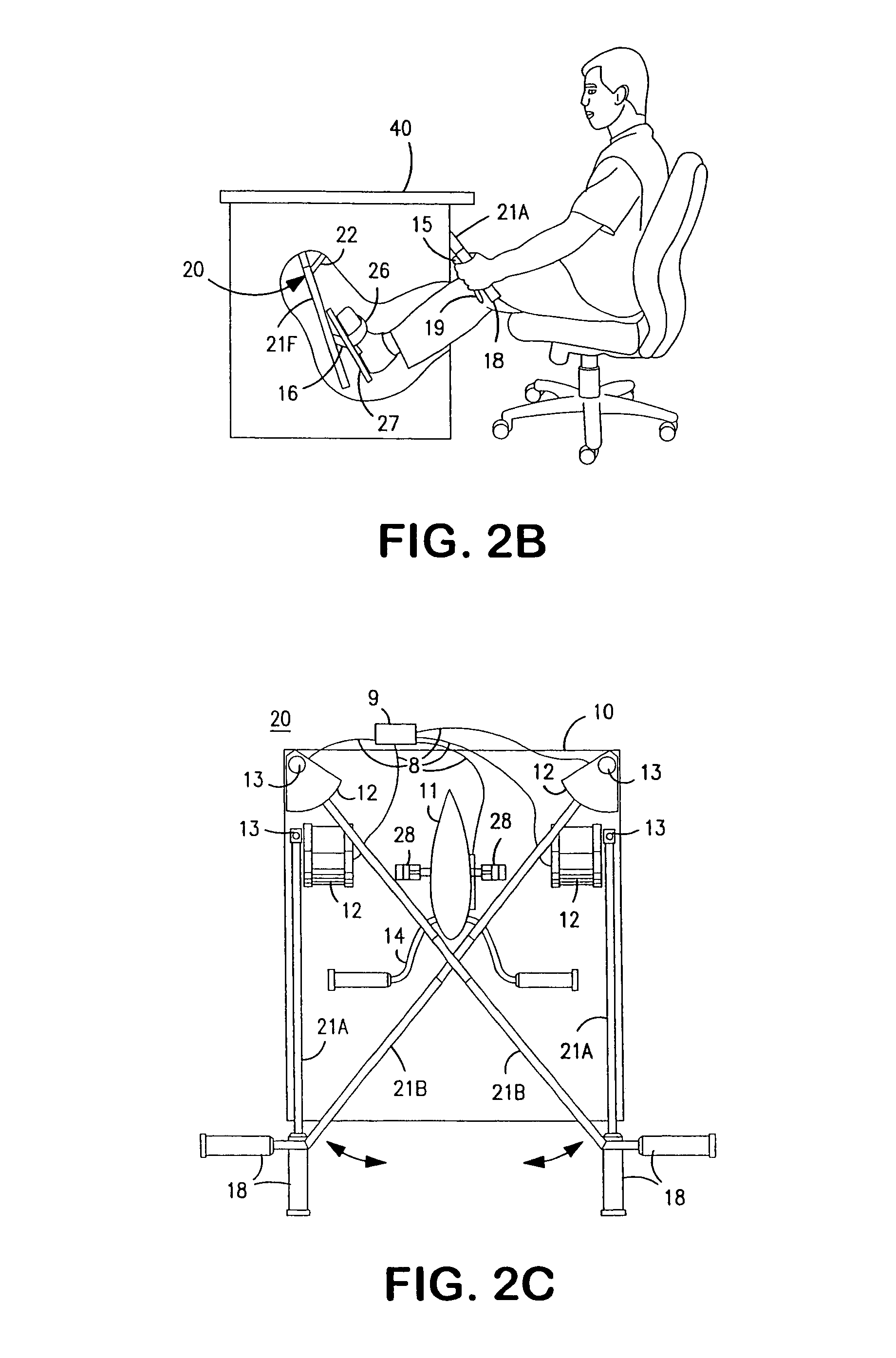
Interactive computer simulation enhanced exercise machine
InactiveUS20070117680A1Improve sports experienceEfficient executionIndoor gamesSpace saving gamesExercise machineVisual perception
A computer simulation enhanced exercise device is provided which engages the user by directly relating the users exercise motion in real time to a visual simulation or interactive game. The exercise device may comprise any variety of machines including, stationary bikes, rowing machines, treadmills, stepper, elliptical gliders or under desk exercise. These exercise devices are configured with sensors to measure physical movements as the user exercises and are coupled to computer hardware with modeling and virtualization software to create the system. These sensor measurements are then sent to a computer for use in the physics based modeling and real-time visual simulation. The computer simulation enhanced exercise device is further provided with features including manual and automatic adjustment of resistance levels, visualization of accurate caloric burn rates and correlation to everyday food items, and network connectivity providing for multiplayer network simulations and directed advertising.
Owner:CUBEX
Thermoplastic cellulose derivative composition and fiber comprising the same
InactiveUS6984631B2Good fluidityIncrease resistanceOrganic active ingredientsBiocidePolyesterSide chain
A thermoplastic cellulose derivative composition of the present invention contains, as a main component, a cellulose ester having an aliphatic polyester side chain having a repeat unit having 2 to 5 carbon atoms, wherein a rate of heating loss at 200° C. is 5 wt % or less, a melt viscosity at 200° C. and 1000 sec−1 is 50 to 300 Pa·sec, and a melt tension at the time of take-up at 200° C. and 100 m / min is 0.1 to 40 mN. The present invention can provide excellent fiber products by melt spinning of the composition.
Owner:TORAY IND INC
Interactive computer simulation enhanced exercise machine
InactiveUS20070093360A1Enhancement of exercise experienceReduce computational costInput/output for user-computer interactionIndoor gamesPhysical exerciseAutomatic tuning
A computer simulation enhanced exercise device is provided which engages the user by directly relating the users exercise motion in real time to a visual simulation or interactive game. The exercise device may comprise any variety of machines including, stationary bikes, rowing machines, treadmills, stepper, elliptical gliders or under desk exercise. These exercise devices are configured with sensors to measure physical movements as the user exercises and are coupled to computer hardware with modeling and virtualization software to create the system. These sensor measurements are then sent to a computer for use in the physics based modeling and real-time visual simulation. The computer simulation enhanced exercise device is further provided with features including manual and automatic adjustment of resistance levels, visualization of accurate caloric bum rates and correlation to everyday food items, and network connectivity providing for multiplayer network simulations and directed advertising.
Owner:CUBEX
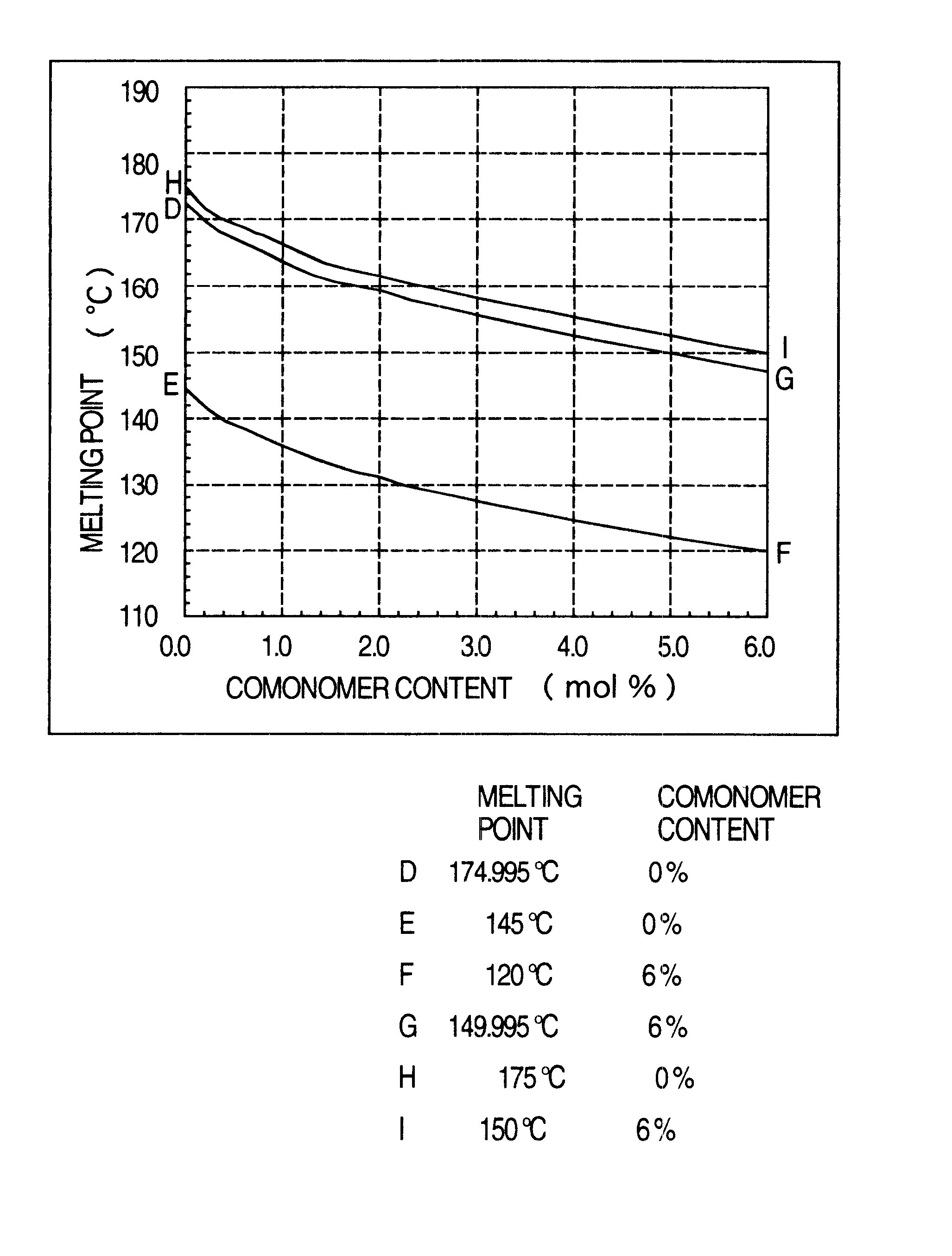
Low-molecular weight oxymethylene polymer and composition thereof
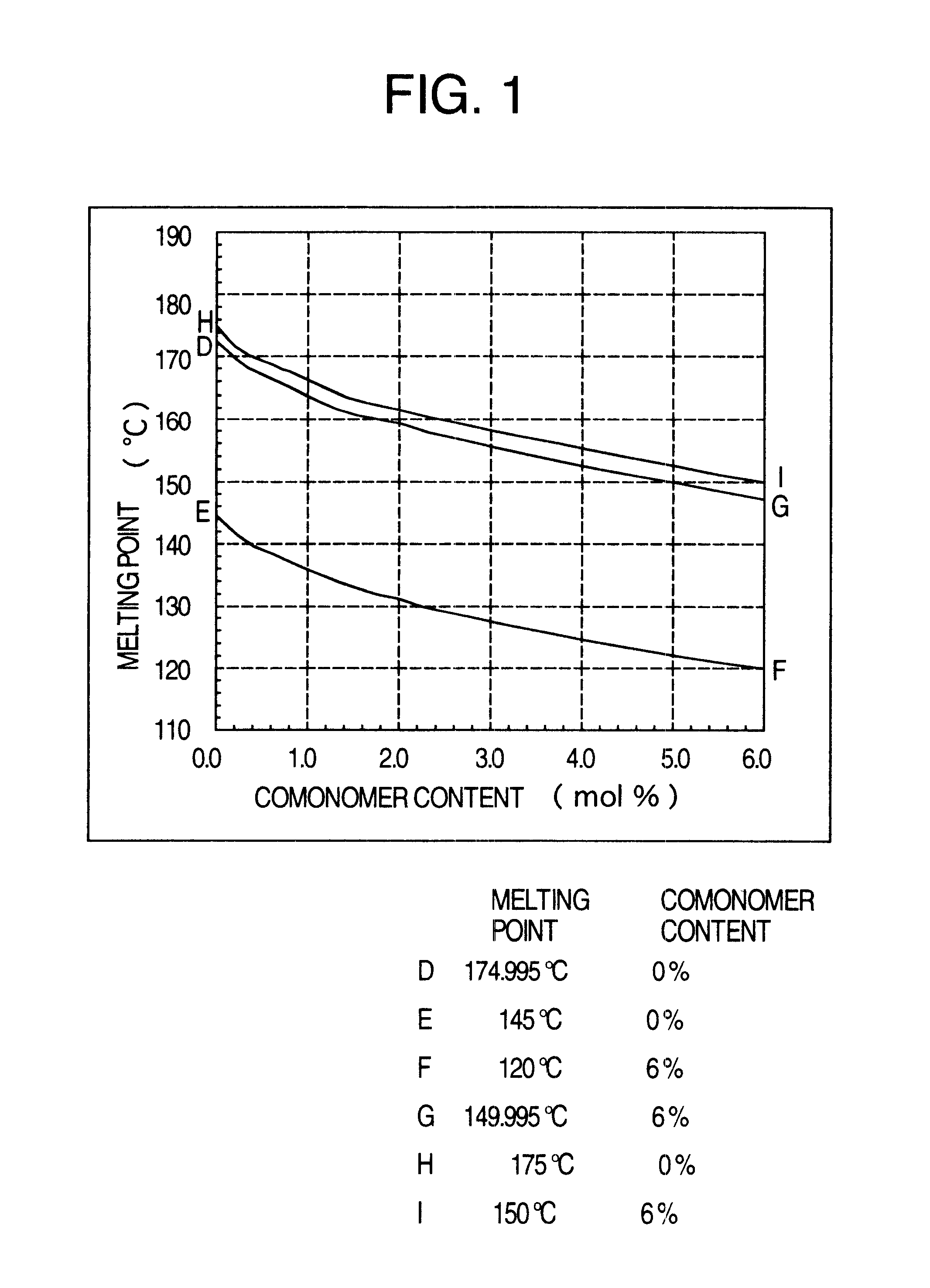
A low-molecular-weight oxymethylene polymer characterized by having a straight-chain molecular structure, a number-average molecular weight of 1,000 to 8,000 and a molecular weight distribution (Mw / Mn) of 1.0 to 3.0, and containing 0 to 30 mol % of a co-monomer.
Owner:ASAHI KASEI KK
Molding material, prepreg and fiber-reinforced composite material, and method for producing fiber-reinforced molding substrate
InactiveUS20100068518A1Increase productivitySatisfactory dispersionCoatingsYarnPolymer scienceFiber bundle
This invention relates to: a molding material comprising a bundle of continuous reinforcing fibers (A), a polyarylene sulfide prepolymer (B) comprising at least 50% by weight of cyclic polyarylene sulfide and having the weight average molecular weight of less than 10,000 or polyarylene sulfide (B′) having the weight average molecular weight of 10,000 or greater and the degree of dispersion of 2.5 or lower, and thermoplastic resin (C); a prepreg comprising a resin composition comprising the polyarylene sulfide prepolymer (B) impregnated into a reinforcing fiber; and a method for producing a fiber-reinforced molding substrate comprising step (I) of continuously feeding a bundle of continuous reinforcing fibers, step (II) of combining cyclic polyarylene sulfide with the reinforcing fiber bundle, step (III) of heating the composite obtained in step (II) to subject the cyclic polyarylene sulfide to ring-opening polymerization to convert into polyarylene sulfide, and step (IV) of cooling the composite obtained in step (III) and withdrawing the same.
Owner:TORAY IND INC
Thermoplastic resin composition and engineering plastic composition
InactiveUS20050159533A1Improve liquidityImprove melt fluiditySpecial tyresEngineering plasticTape recorder
Owner:MITSUBISHI CHEM CORP
Interactive computer simulation enhanced exercise machine
InactiveUS7497812B2Improve sports experienceLow costInput/output for user-computer interactionIndoor gamesPhysics basedVirtualization
A computer simulation enhanced exercise device is provided which engages the user by directly relating the users exercise motion in real time to a visual simulation or interactive game. The exercise device may comprise any variety of machines including, stationary bikes, rowing machines, treadmills, stepper, elliptical gliders or under desk exercise. These exercise devices are configured with sensors to measure physical movements as the user exercises and are coupled to computer hardware with modeling and virtualization software to create the system. These sensor measurements are then sent to a computer for use in the physics based modeling and real-time visual simulation. The computer simulation enhanced exercise device is further provided with features including manual and automatic adjustment of resistance levels, visualization of accurate caloric burn rates and correlation to everyday food items, and network connectivity providing for multiplayer network simulations and directed advertising.
Owner:CUBEX
Pigment dispersing agent and pigment composition containing the same
InactiveUS6123763APromote resultsGood dispersionPigmenting treatmentOrganic chemistryHuePigment composition
Owner:TOYO INK SC HOLD CO LTD
Fluidity-improving agent, aromatic polycarbonate resin composition, and shaped article thereof
ActiveUS20100029855A1Improve fluidityGood fluidityRecord information storageOptical recording/reproducingPolycarbonatePhenyl group
A large sized thin-walled shaped article having a complicated shape can be realized by improving fluidity at the time of shaping without deterioration of the intrinsic characteristic properties of an aromatic polycarbonate resin, namely, transparency, resistance to exfoliation of surface layer, thermal resistance, impact resistance, and chemical resistance. Specifically disclosed is a fluidity-improving agent containing 0.5 to 99.5 parts by mass of a polymer (A) which is obtained by polymerizing a monomer mixture (a) containing 0.5 to 99% by mass of (a1) an aromatic vinyl monomer, 0.5 to 99% by mass of (a2) a phenyl(meth)acrylate or a phenyl(meth)acrylate having a substituent in a phenyl group, and 0.5 to 5% by mass of (a3) a vinyl monomer having a functional group (X), and 0.5 to 99.5 parts by mass of a polymer (B) which is obtained by polymerizing a monomer mixture (b) comprising (b1) a phenyl (meth)acrylate or a phenyl (meth)acrylate having a substituent in a phenyl group with a compound having a functional group (Y) capable of reacting with the functional group (X), with the proviso that a total of the polymer (A) and the polymer (B) is 100 parts by mass.
Owner:MITSUBISHI CHEM CORP
Polyester Resin for Toner, Toner Composition and Resin Particle
ActiveUS20070281235A1Excellent low-temperatureExcellent grindabilityMixingDevelopersChemistryPolyester
[PROBLEMS] Disclosed is a heat-fusible electrostatic image developing toner which has an excellent balance between fixability at low temperatures and grindability and is excellent in glossiness after fixing. Also disclosed is a resin for toners. [MEANS FOR SOLVING PROBLEMS] A polyester resin for toners which is obtained by polycondensing a polyol component and a polycarboxylic acid component is characterized by containing 20-100 weight % of one or more polyester resins (A1) having a storage elastic modulus from 2.5×103 Pa to 5×106 Pa at 150° C. wherein the molar average cohesive energy of the polyol component is between 7.0×104 and 1.4×105 J.
Owner:SANYO CHEM IND LTD
Polycarbonate Resin Composition with Improved Scratch Resistance and Melt Flow Index
ActiveUS20100168272A1High fluidityImprove liquidityAntifouling/underwater paintsPaints with biocidesPolycarbonateChemistry
Owner:LOTTE ADVANCED MATERIALS CO LTD
Toner and method of manufacturing the same
InactiveUS20070218382A1Excellent low temperature fixabilitySmall sizeDevelopersMaterials scienceColoring agents
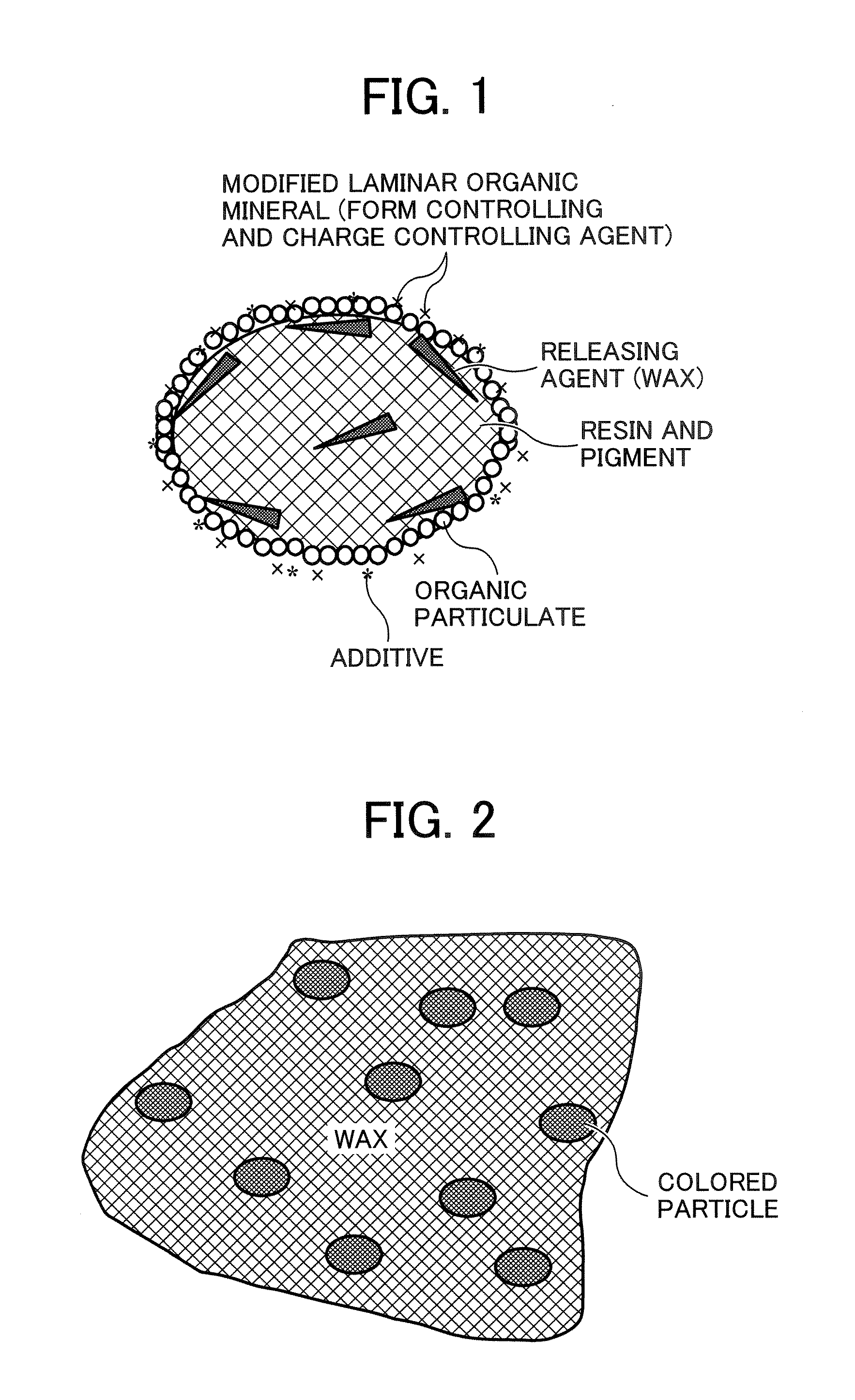
A toner containing a toner particle containing a binder resin, a colorant, a releasing agent and a laminar inorganic mineral in which part or all ions present between layers are modified by organic ions. The toner particle has a structure such that when the particle is heated at a temperature ranging from 65 to 90° C. the releasing agent is melted on the outside of the toner particle to form a particle having a sea-island structure.
Owner:RICOH KK
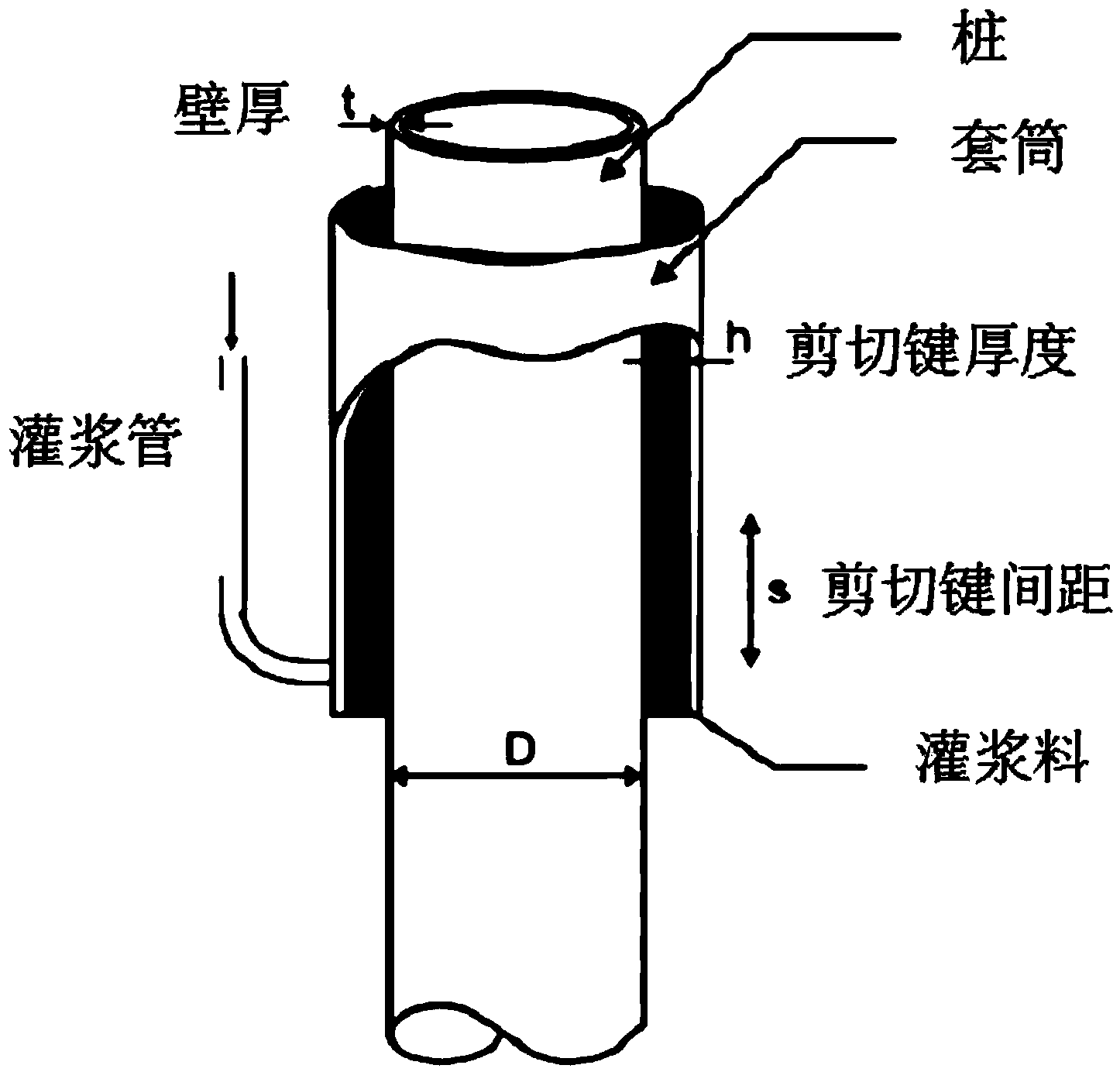
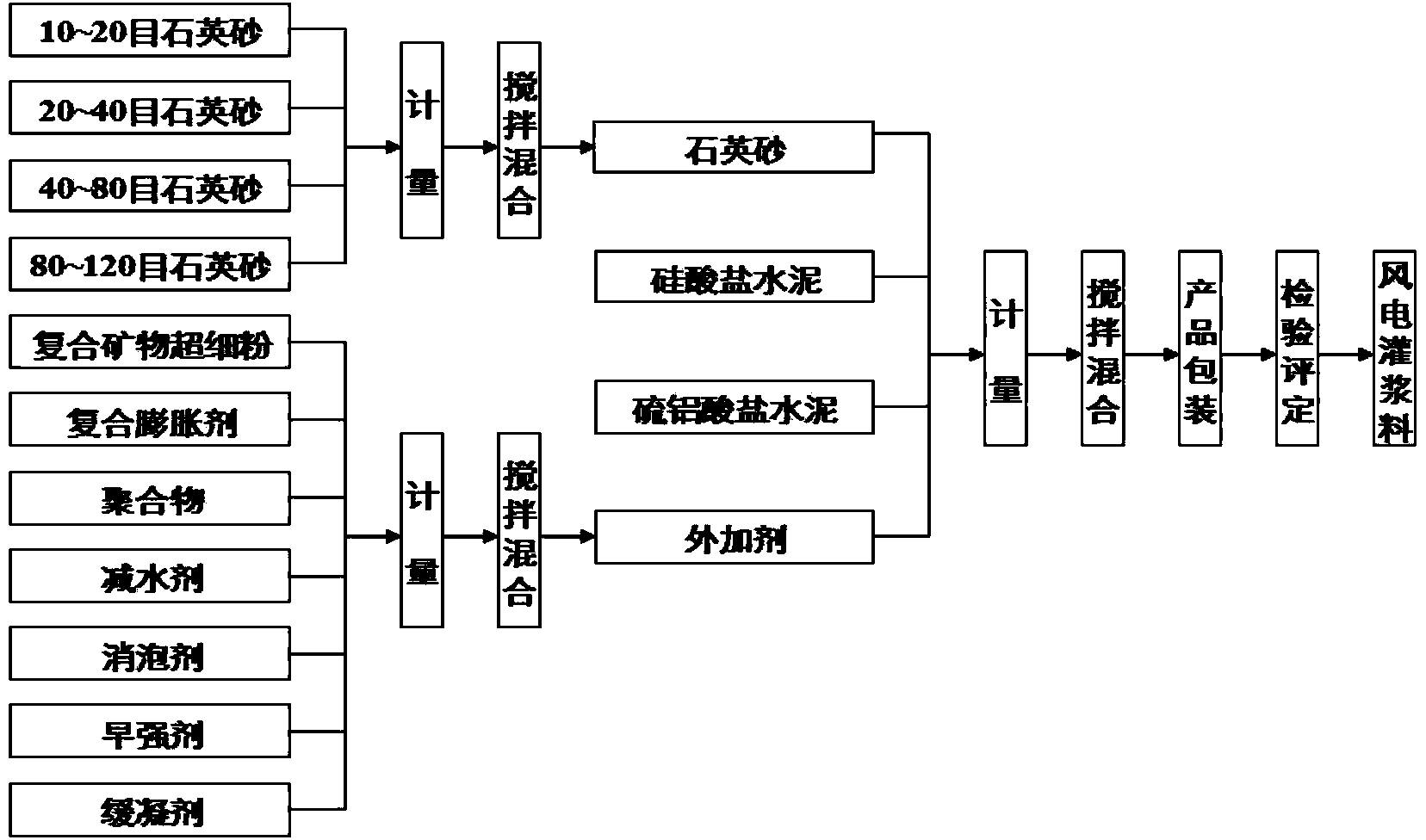
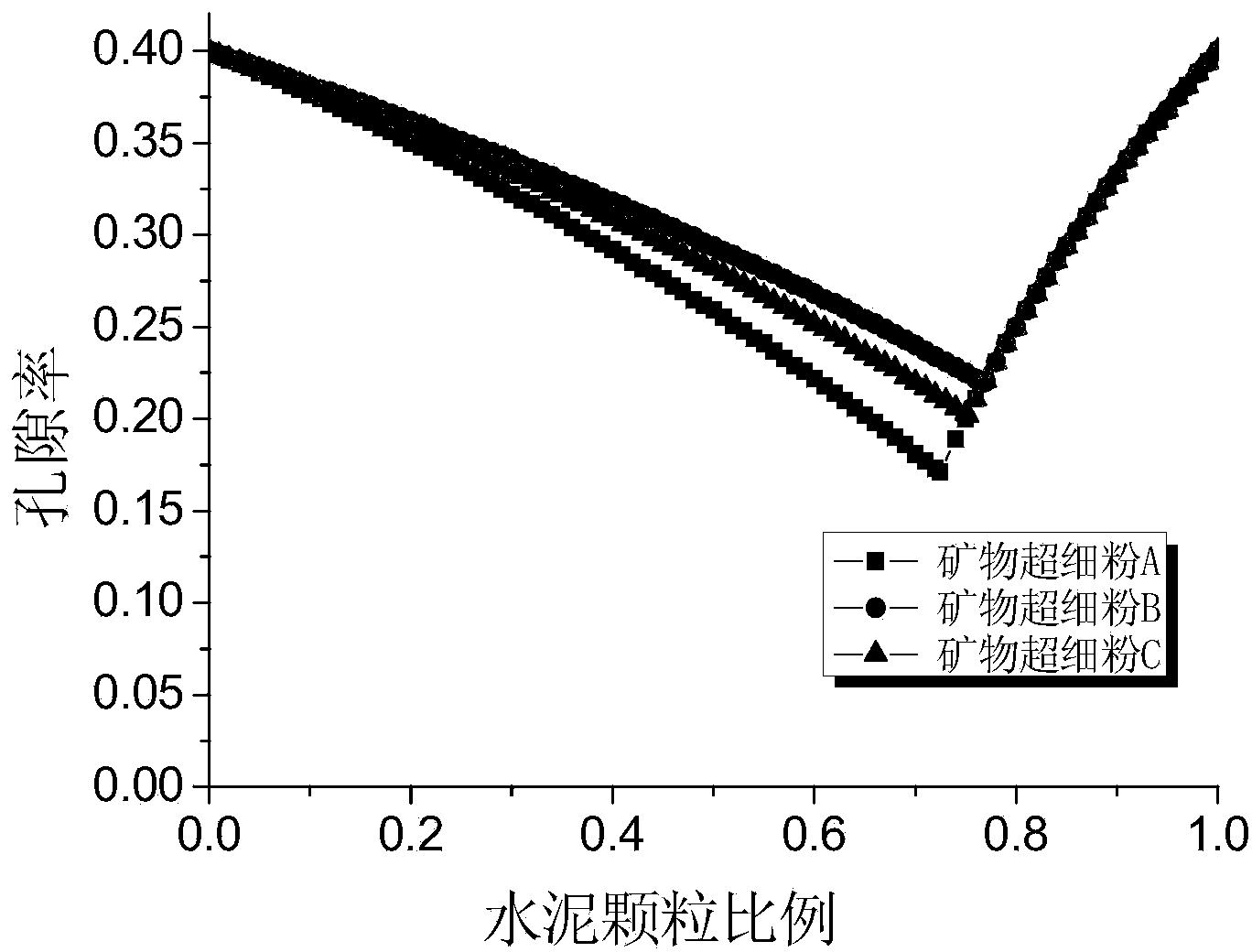
Offshore wind power duct rack grouting material and construction method thereof
ActiveCN104003681AMeet the index requirements for groutingImprove workabilityFoundation engineeringPortland cementOffshore wind power
The invention provides an offshore wind power duct rack grouting material which comprises a dry material and water, wherein the dry material comprises the following components in percentage by mass: 25.0-40.0 percent of Portland cement, 1.0-5.0 percent of sulphoaluminate cement, 45.0-55.0 percent of quartz sand and 5.0-15.0 percent of admixture; the addition amount of the water is proper. The invention further provides a construction method of the offshore wind power duct rack grouting material. According to the offshore wind power duct rack grouting material and the construction method thereof, the material has the characteristics of high liquidity, high pumpability, super-high early-strength, ultrahigh strength, high durability, good water dispersion resistance, zero shrinkage and high fatigue resistance, can be conveyed by a rubber pipeline by adopting a pumping and grouting construction mode, is suitable for underwater grouting and is also suitable for grouting connection between the offshore wind power duct rack and steel pipe pile foundation.
Owner:CCCC THIRD HARBOR ENG +2
Interactive computer simulation enhanced exercise machine
InactiveUS7497807B2Improve sports experienceLow costIndoor gamesSpace saving gamesVirtualizationPhysics based
A computer simulation enhanced exercise device is provided which engages the user by directly relating the users exercise motion in real time to a visual simulation or interactive game. The exercise device may comprise any variety of machines including, stationary bikes, rowing machines, treadmills, stepper, elliptical gliders or under desk exercise. These exercise devices are configured with sensors to measure physical movements as the user exercises and are coupled to computer hardware with modeling and virtualization software to create the system. These sensor measurements are then sent to a computer for use in the physics based modeling and real-time visual simulation. The computer simulation enhanced exercise device is further provided with features including manual and automatic adjustment of resistance levels, visualization of accurate caloric burn rates and correlation to everyday food items, and network connectivity providing for multiplayer network simulations and directed advertising.
Owner:CUBEX
Nanoemulsion compositions and methods of use thereof
InactiveUS20070085058A1Improve bioavailabilityEnhanced soluble complexesCosmetic preparationsToilet preparationsAdditive ingredientCholesterol blood
A nanoemulsion composition having enhanced oxidative stability, emulsion stability, and health benefits. The composition may include individual ingredients or a synergistic blend of non-reducing sugars, sugar polyols, medium-chain triglycerides, polysaccharides, polyphenols, phospholipids, chitosan, and alpha-casein, beta-casein, kappa-casein or protein fragments, glycopeptides, phosphopeptides. The composition may optionally be further utilized for the prevention of hypercholesterolemia or bone (and teeth) mineral loss.
Owner:PETTY BLAKE
Toner, and two component developer and image forming apparatus using the toner
ActiveUS20050186498A1Improve chargeabilityGood fluidityElectrographic process apparatusDevelopersEngineeringColoring agents
A toner is provided that contains a binder resin; a colorant; a release agent; and an external additive, wherein the toner has an average circularity of from 0.940 to 0.965 and a crater having a depth of from 0.02 to 0.1 μm, and wherein the crater has an amount of the external additive larger than an average amount thereof on the toner, along with a two-component developer containing the toner and an image forming apparatus using the toner.
Owner:RICOH KK
Processing net forming integrated method for amorphous alloy workpiece
InactiveCN101164722ASolve the problem of inability to prepare structurally complex partsRealize the integration of preparation and processing net shapeTest sampleMolten alloy
The present invention relates to a preparation processing method of amorphous alloy workpiece. Said method includes the following steps: heating and melting alloy mother material, then pouring the molten alloy mother material into a breakable mould, after the molten alloy is cooled, an amorphous alloy can be obtained, after the mould is broken, cleaning the test sample so as to obtain the invented required workpiece.
Owner:BEIHANG UNIV
No-shrinkage grouting filler with adjustable performance
The present invention belongs to the field of building material, and is especially one kind of no-shrinkage grouting filler with adjustable performance. The no-shrinkage grouting filler is hydraulic mixture comprising Portland cement and fly ash or silica fume as cementing material, quartzite, water reducing agent, expanding agent, water loss reducing agent, early settling preventing agent and early contract preventing agent. It has high flowability, no settling, no weeping, micro plastic expansion, no shrinkage, no cracking, adjustable performance, and no plastic settling and other excellent performance, and may be applied widely in installing large precise equipment, large area leveling, emergency repair engineering, etc.
Owner:INNER MONGOLIA UNIV OF SCI & TECH
Method and apparatus for manufacturing toner and toner manufactured by the apparatus and method
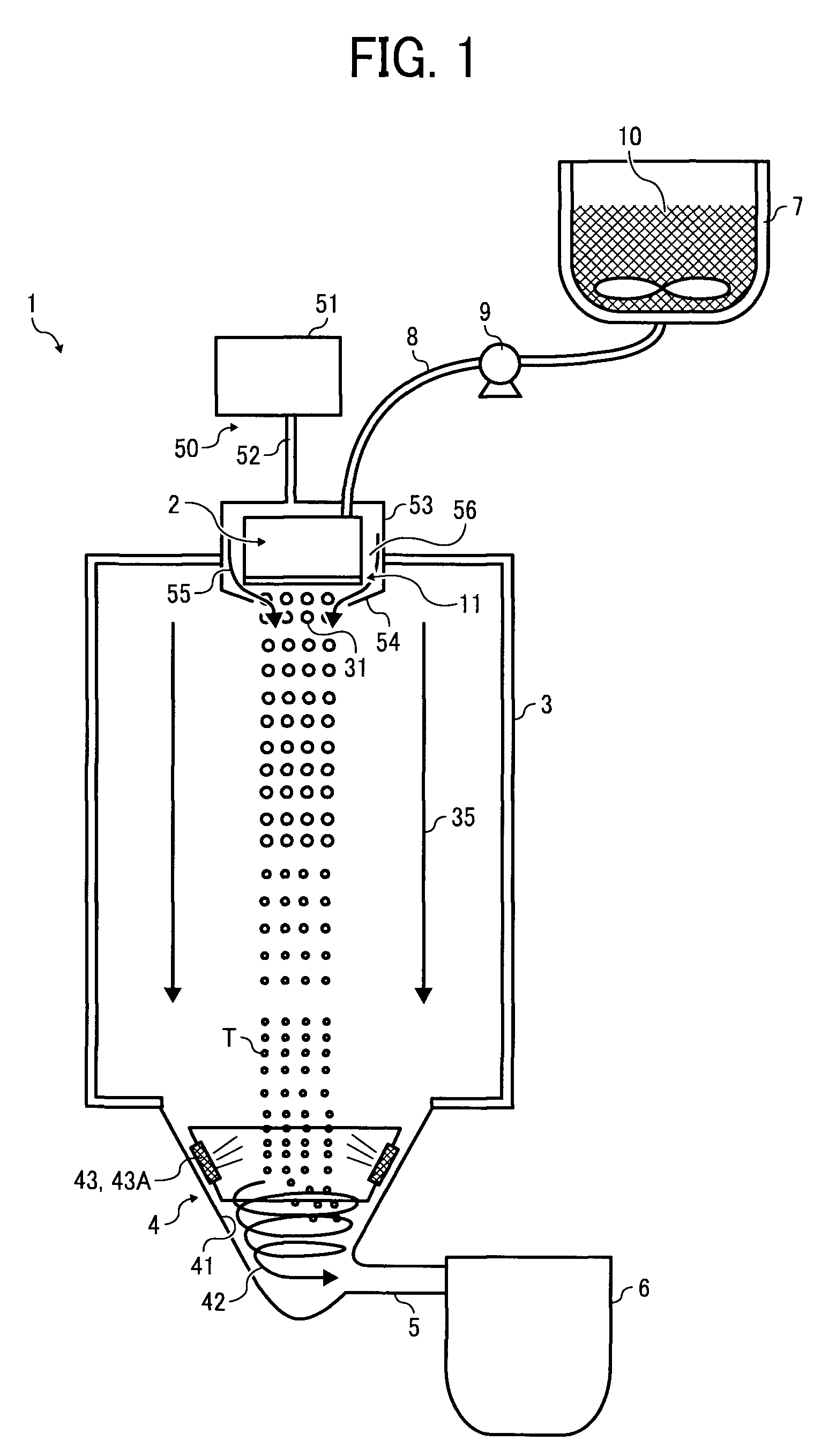
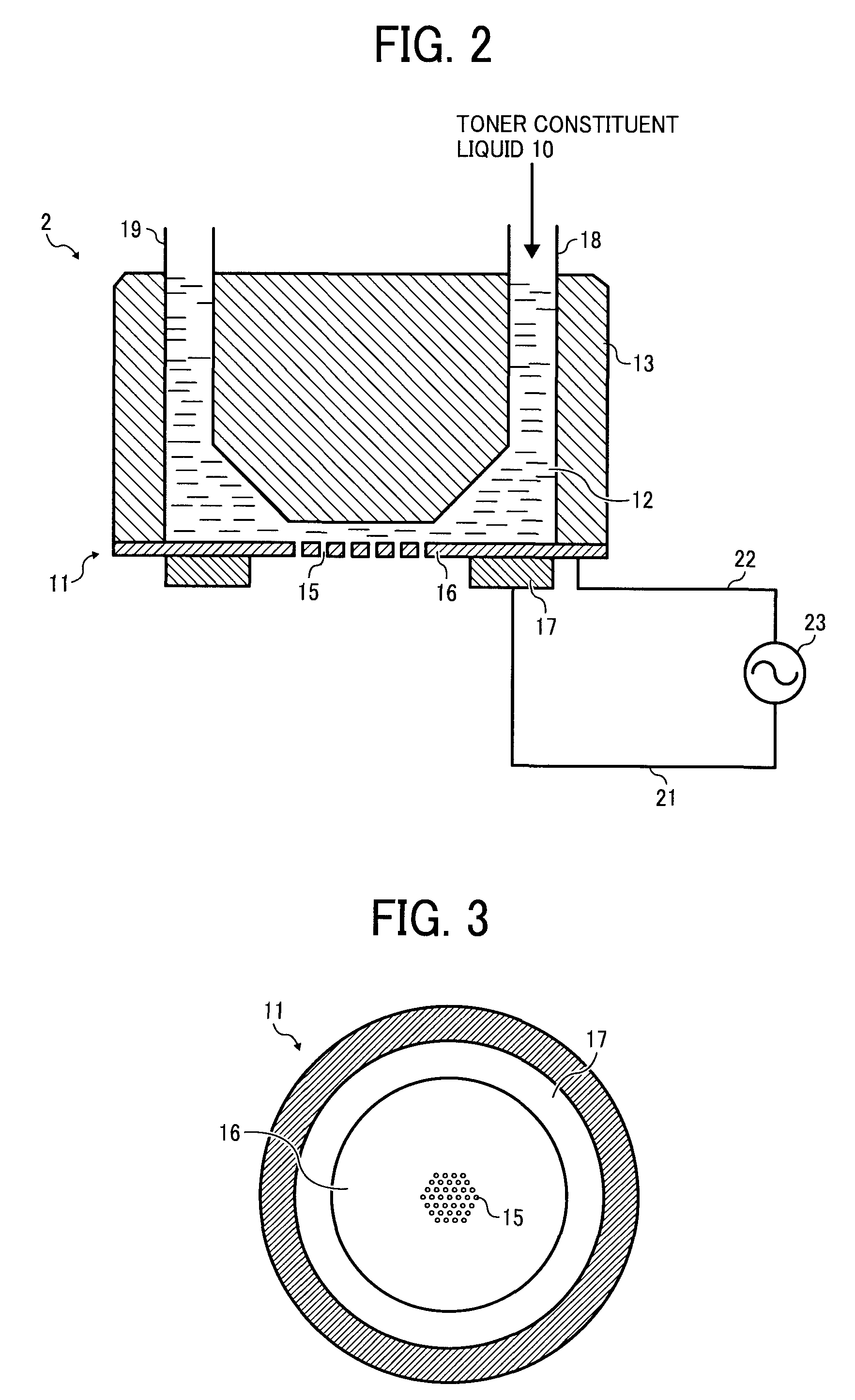
ActiveUS20080286680A1Efficiently and reliably manufacturingQuality improvementGranulation by liquid drop formationAuxillary shaping apparatusEngineeringColoring agents
A method for manufacturing a toner, including periodically forming liquid droplets of a toner constituent liquid comprising a resin and a colorant by vibrating a thin film having a plurality of holes provided on a retention part thereof to discharge the liquid droplets of the toner constituent liquid from the plurality of holes and forming toner particles by solidifying the liquid droplets discharged from the plurality of holes. In periodically forming the liquid droplets of the toner constituent liquid, a flow of gas in a direction of discharge of the liquid droplets is formed by a gas flow generation unit provided downstream from the thin film relative to the direction of discharge of the liquid droplets, including a tapered part forming an aperture corresponding to a hole formation area of the thin film.
Owner:RICOH KK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com