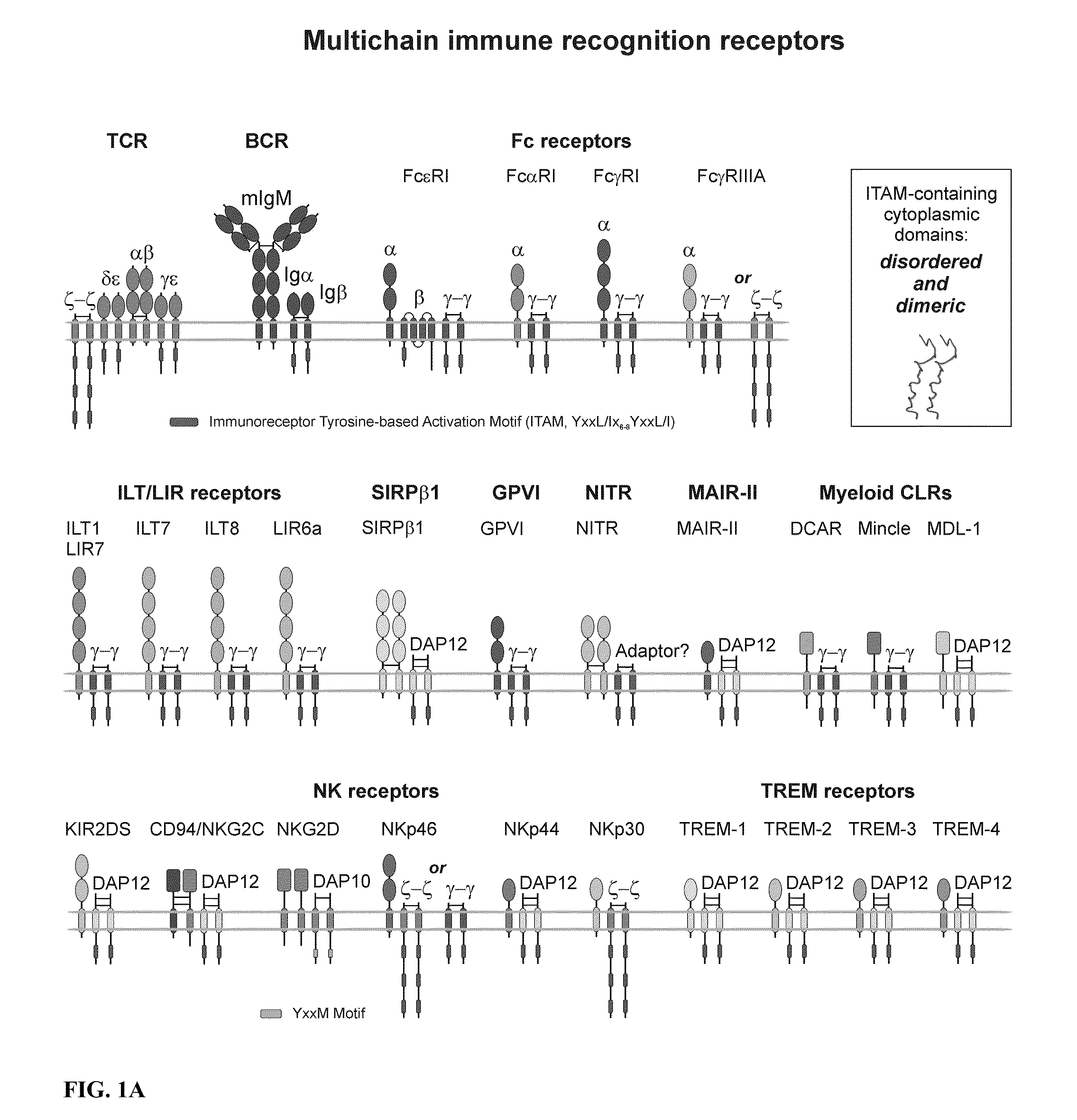
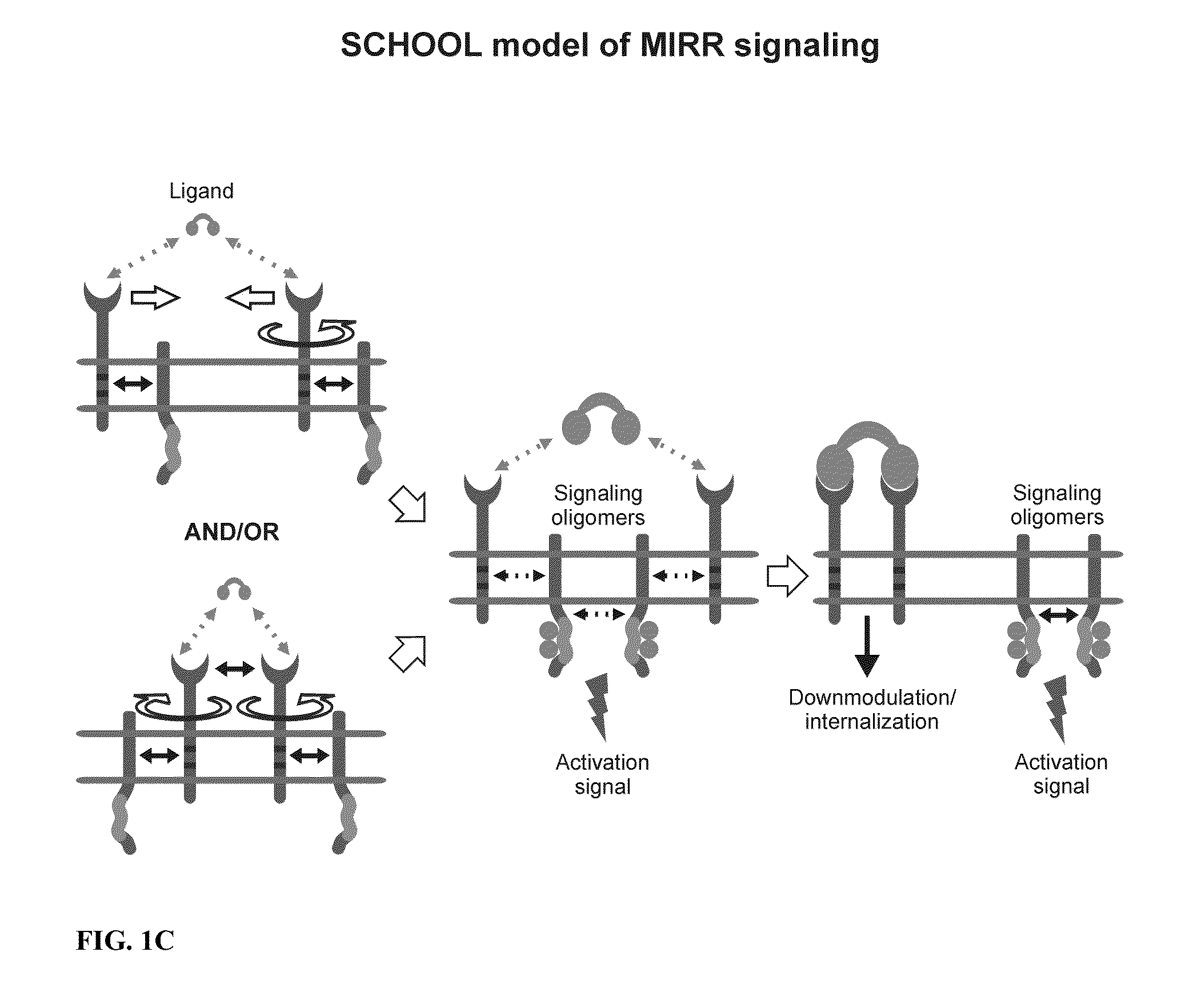
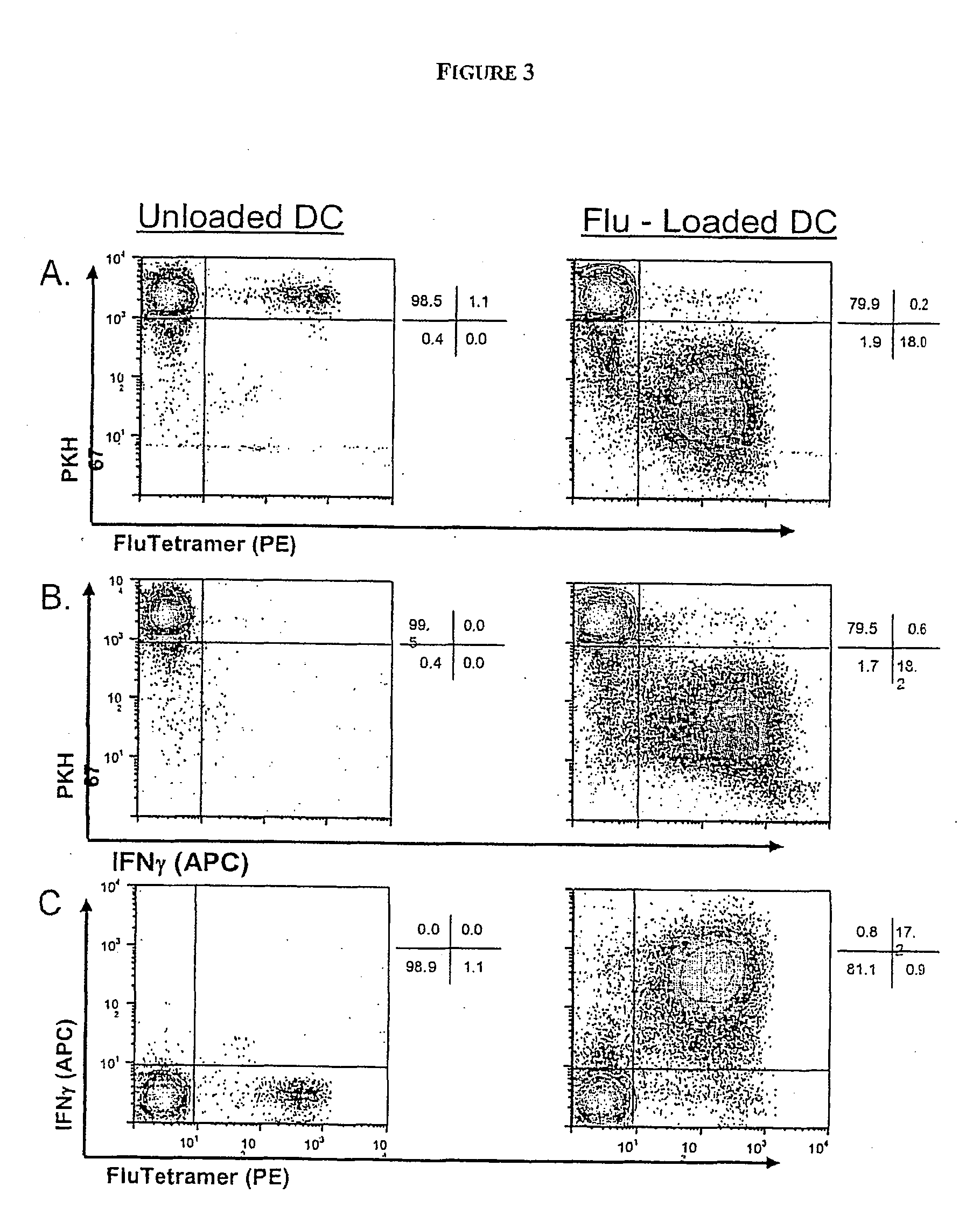
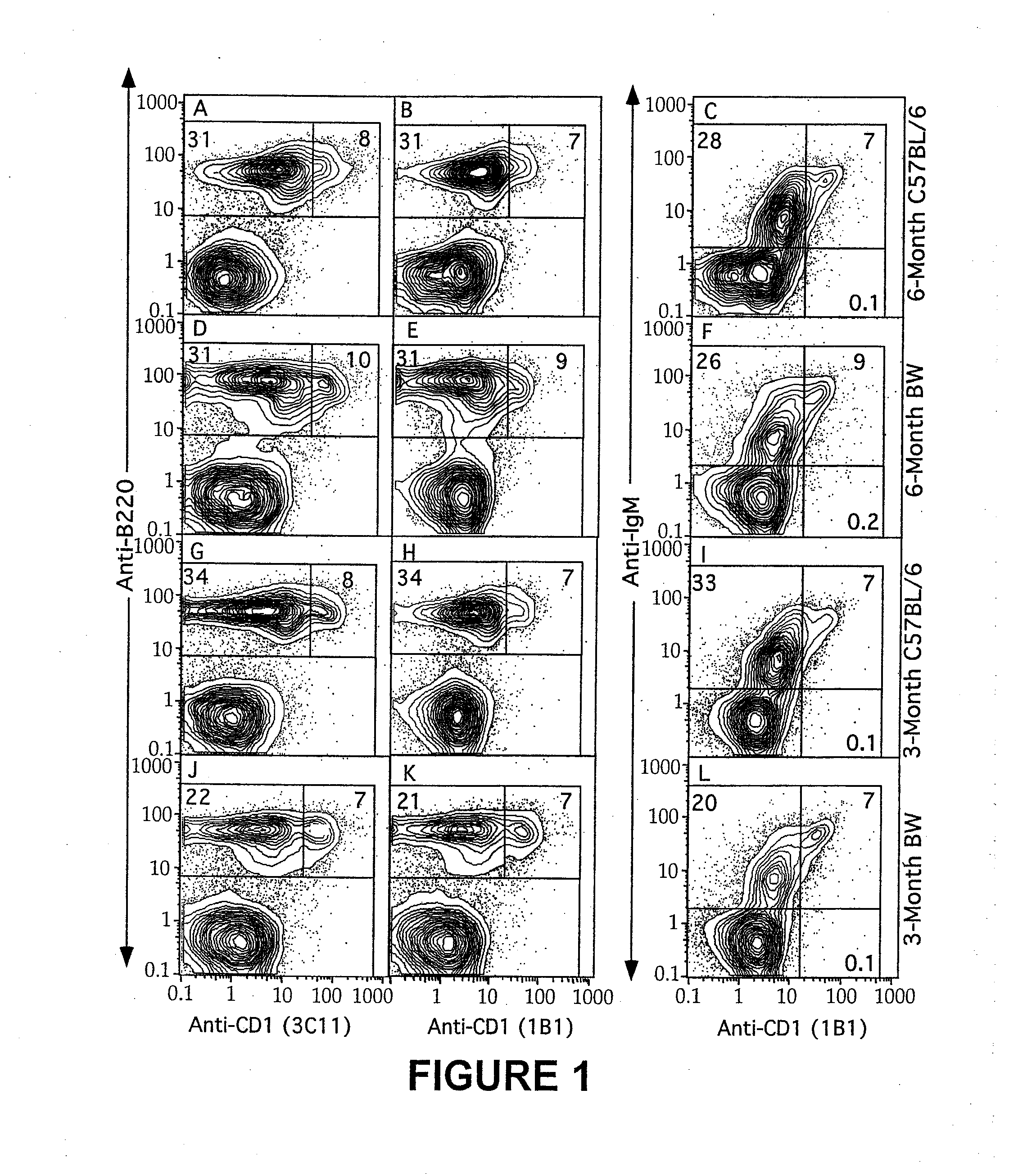
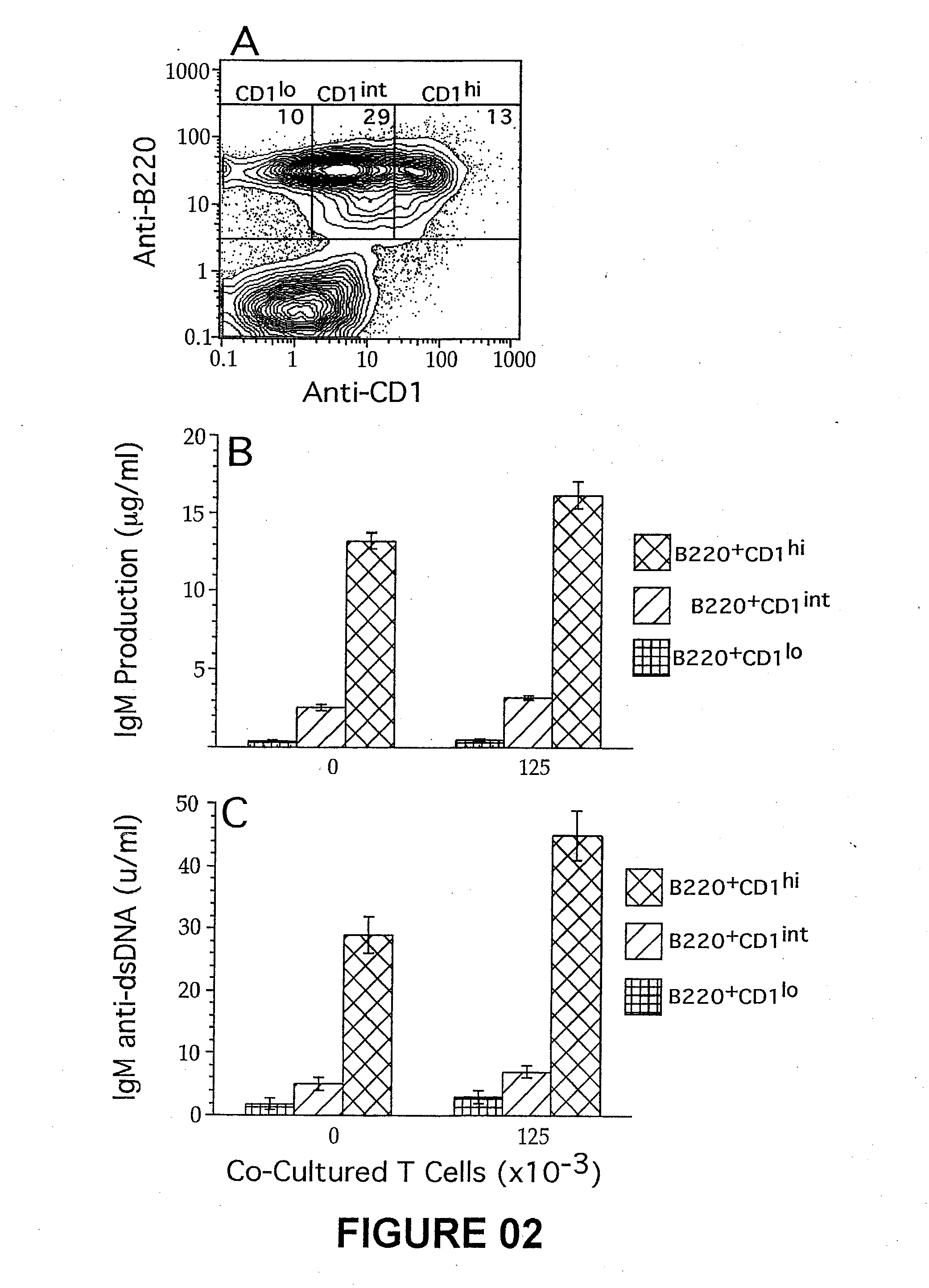
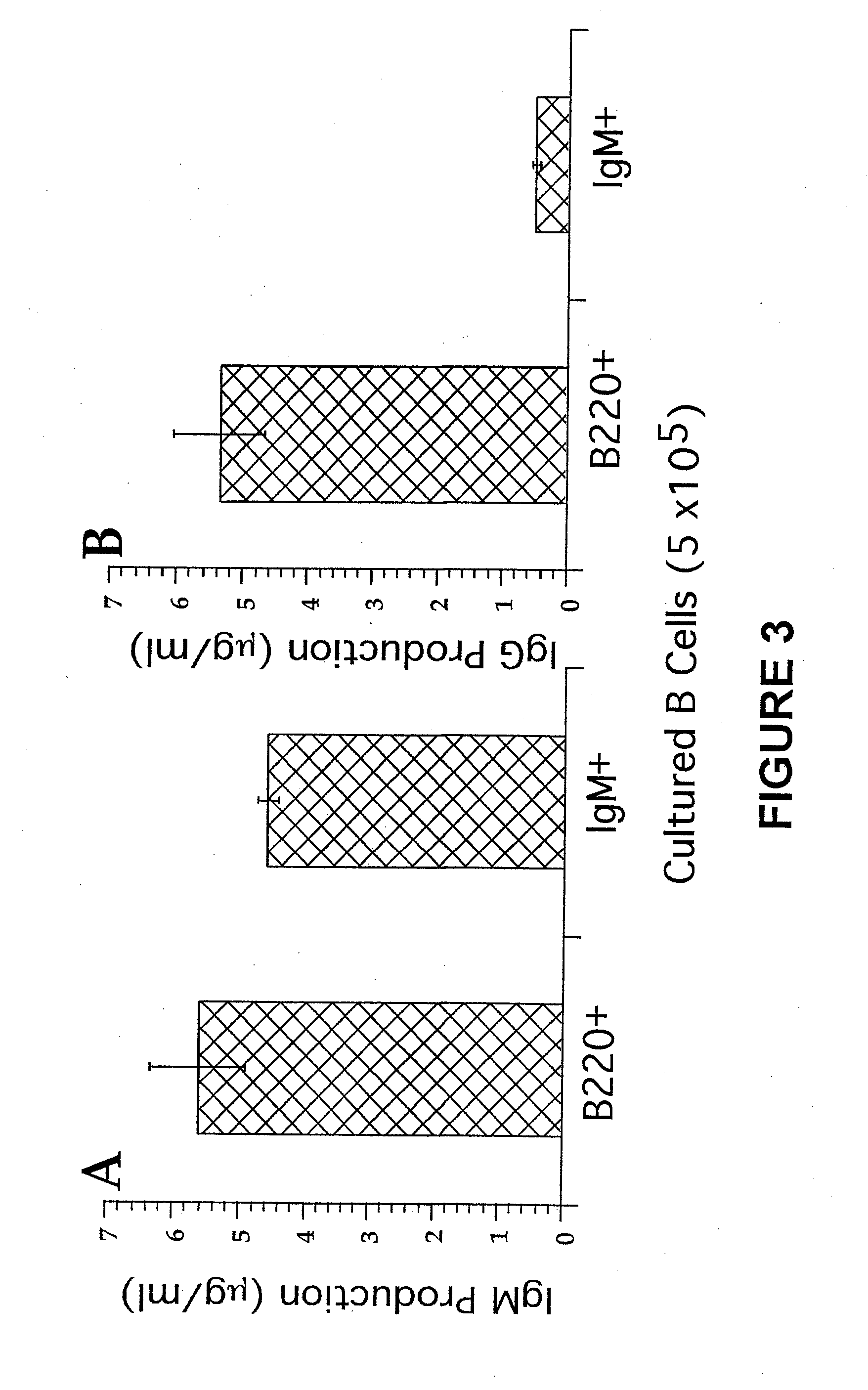
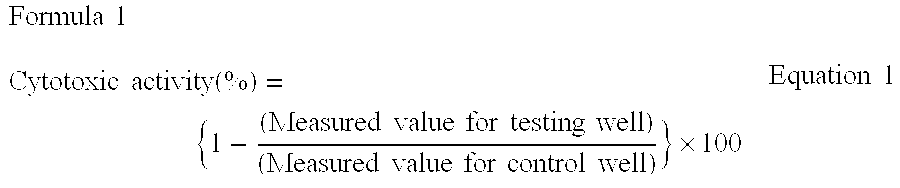Patents
Literature
47 results about "T-Cell Antigen Receptors" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
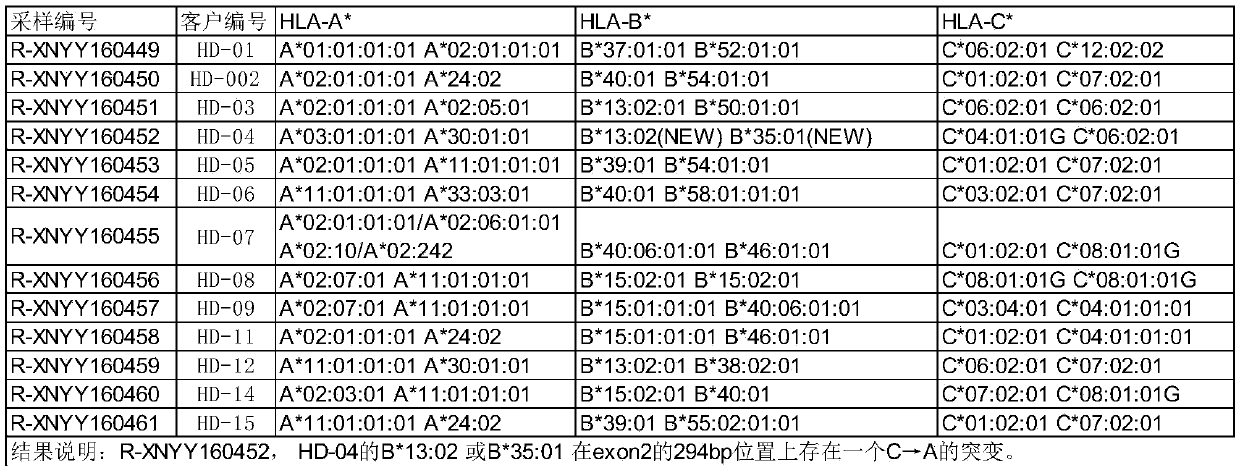
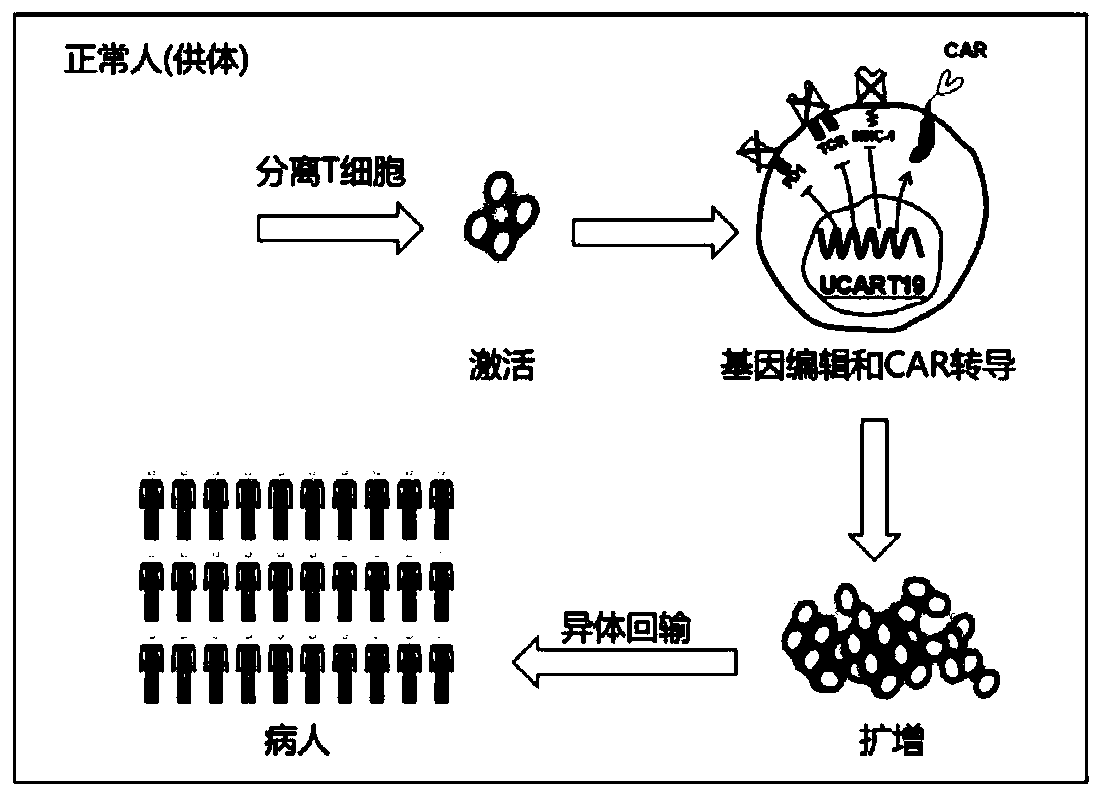
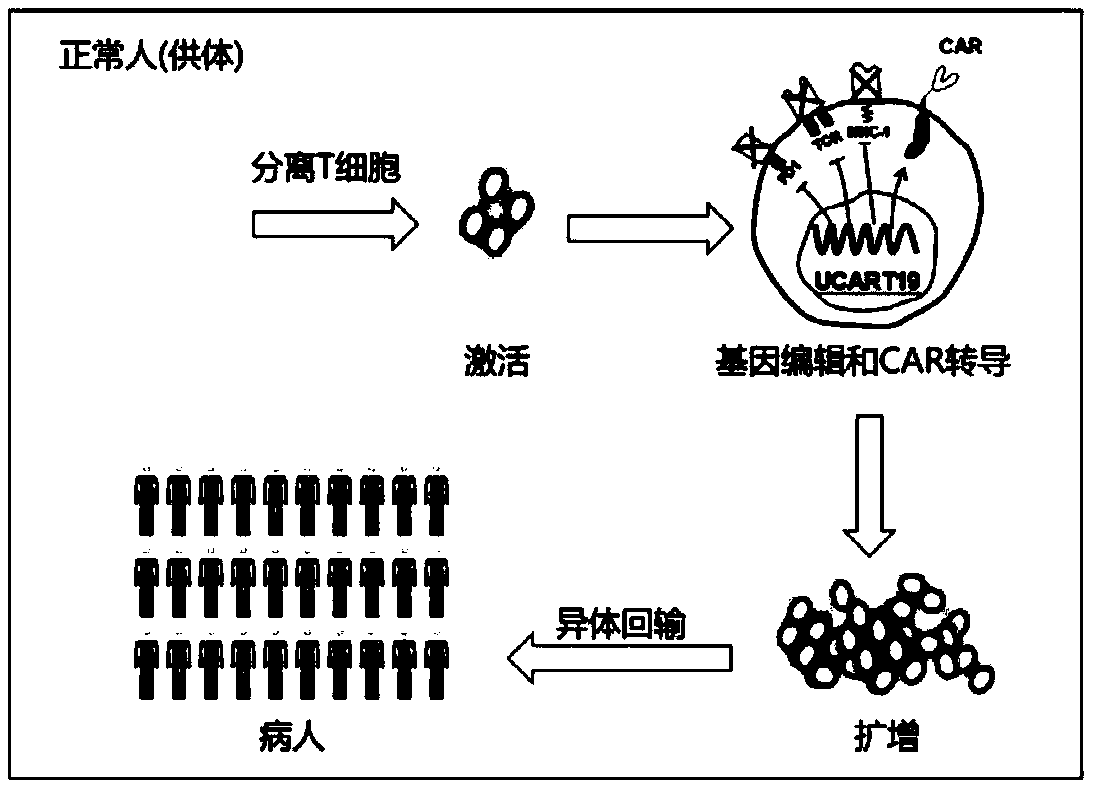
Universal CAR-T cell and preparation method and application thereof
ActiveCN107723275AImprove expression rateImprove effectiveness and safetyMammal material medical ingredientsNucleic acid vectorAbnormal tissue growthDisease
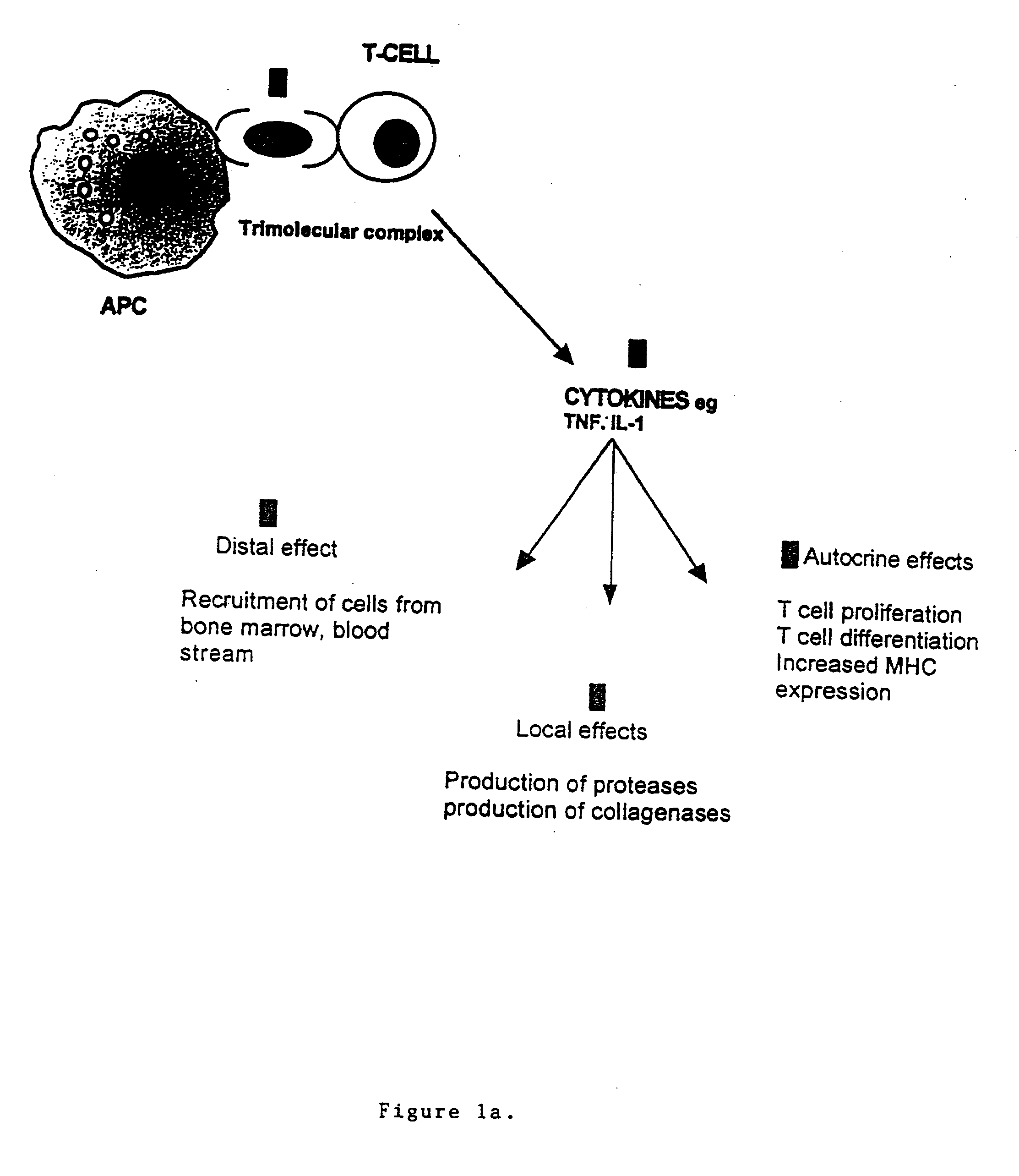
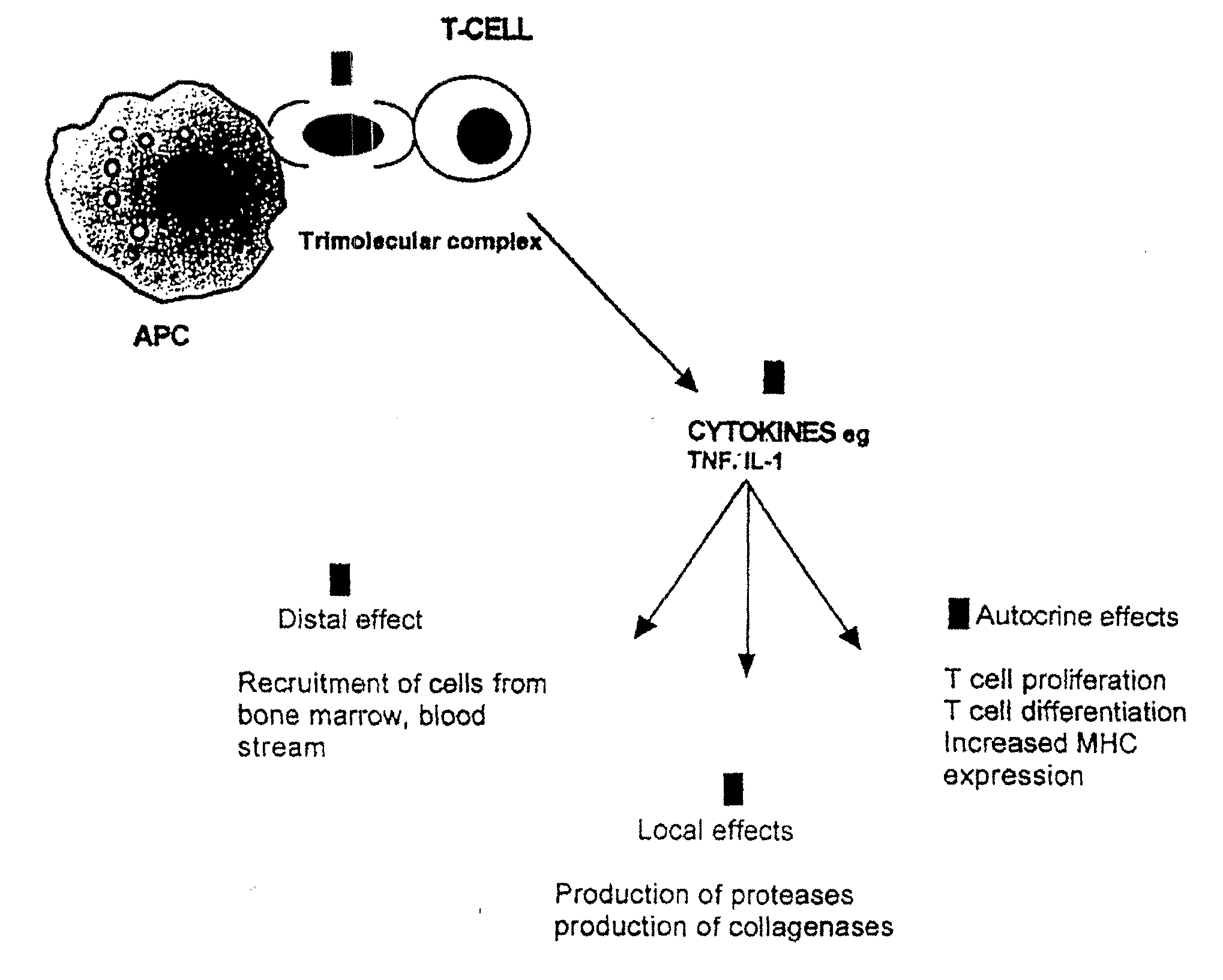
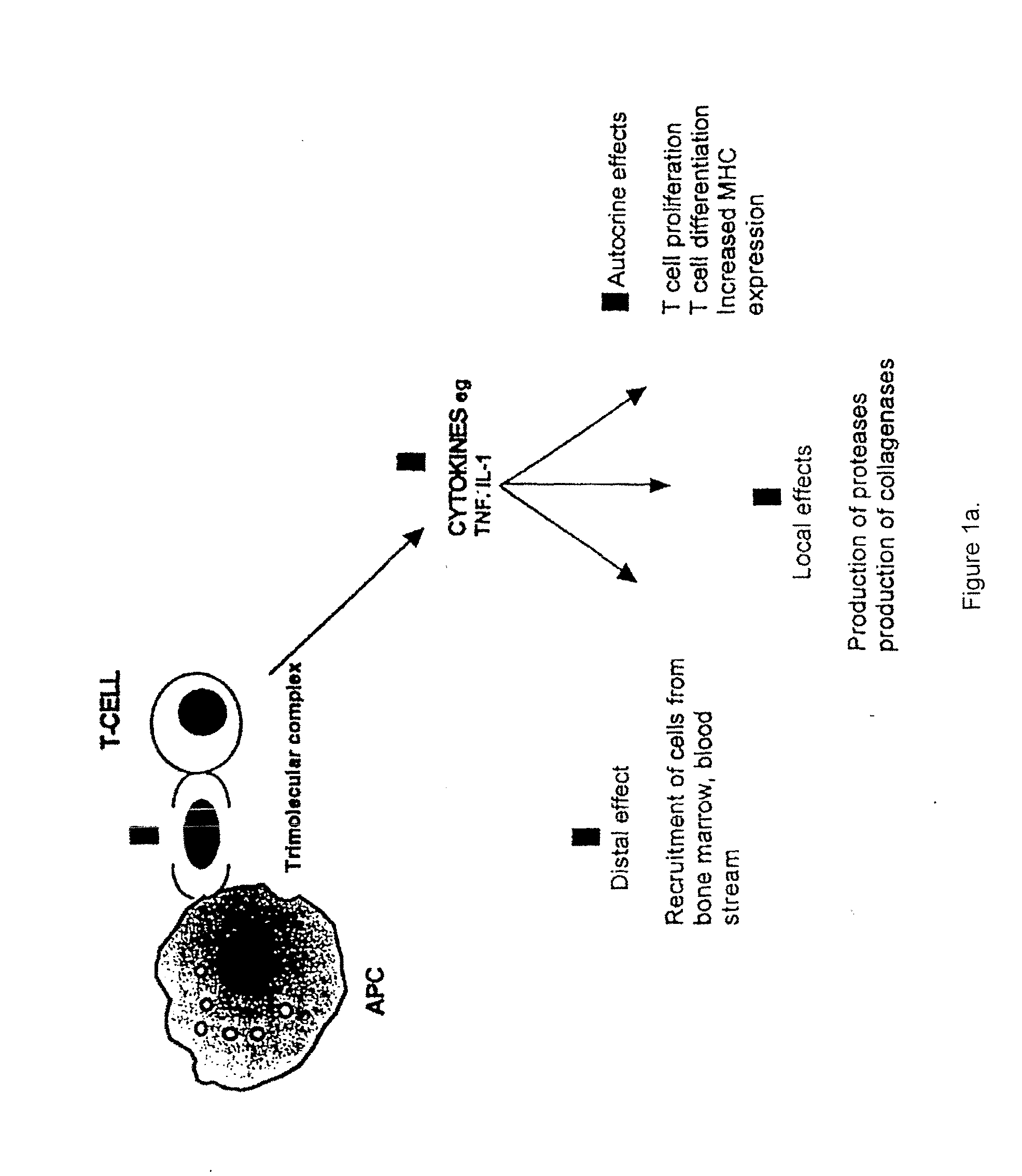
The invention belongs to the field of immunotherapy and relates to a universal CAR-T cell and a preparation method and application thereof. In the universal CAR-T cell, the functions of a T cell antigen receptor (TCR) and major histocompatibility complexes (MHC I and MHC II) in the T cell are inhibited while multi-gene knockout is performed; a gene encoding the TCR includes TRAC and / or TRBC; genesencoding the major histocompatibility complexes include HLA-A, B2MH and CIITA. The universal CAR-T cell can target relevant markers of specific tumors and inactivate the functions of the TCR and theMHC on the cell surface, can reduce immunological rejection caused by allogeneic cell therapy and safely and effectively remove tumor cells in the diseased human body, is not affected by the disease or a treatment mode of a patient in use and can be prepared at any time, treatment can be provided at the optimum time, and treatment effectiveness is ensured.
Owner:CHONGQING PRECISION BIOTECH CO LTD
T-Cell Receptor Antibodies And Methods of Use Thereof
InactiveUS20120034221A1Good curative effectAvoid managementFungiBacteriaDiseaseT-Cell Antigen Receptors
The present invention is directed to the production and use of monoclonal antibodies, or antigen binding fragments thereof, that specifically bind the T cell antigen receptor (TCR) and their use for immunomodulation. In preferred embodiments, the antibody or antigen binding fragment of the invention specifically binds the constant region of the α chain of the TCR, or otherwise specifically binds the α chain regardless of TCR clonal origin (i.e., is pan specific for TCR). The antibodies of the invention may be used, for example, in immunosuppressive therapies for transplant maintenance and the treatment of autoimmune diseases, and / or as targeting molecules for use in the treatment of T-cell malignancies.
Owner:MACROGENICS INC
Monoclonal antibodies reactive with defined regions of the T cell antigen receptor
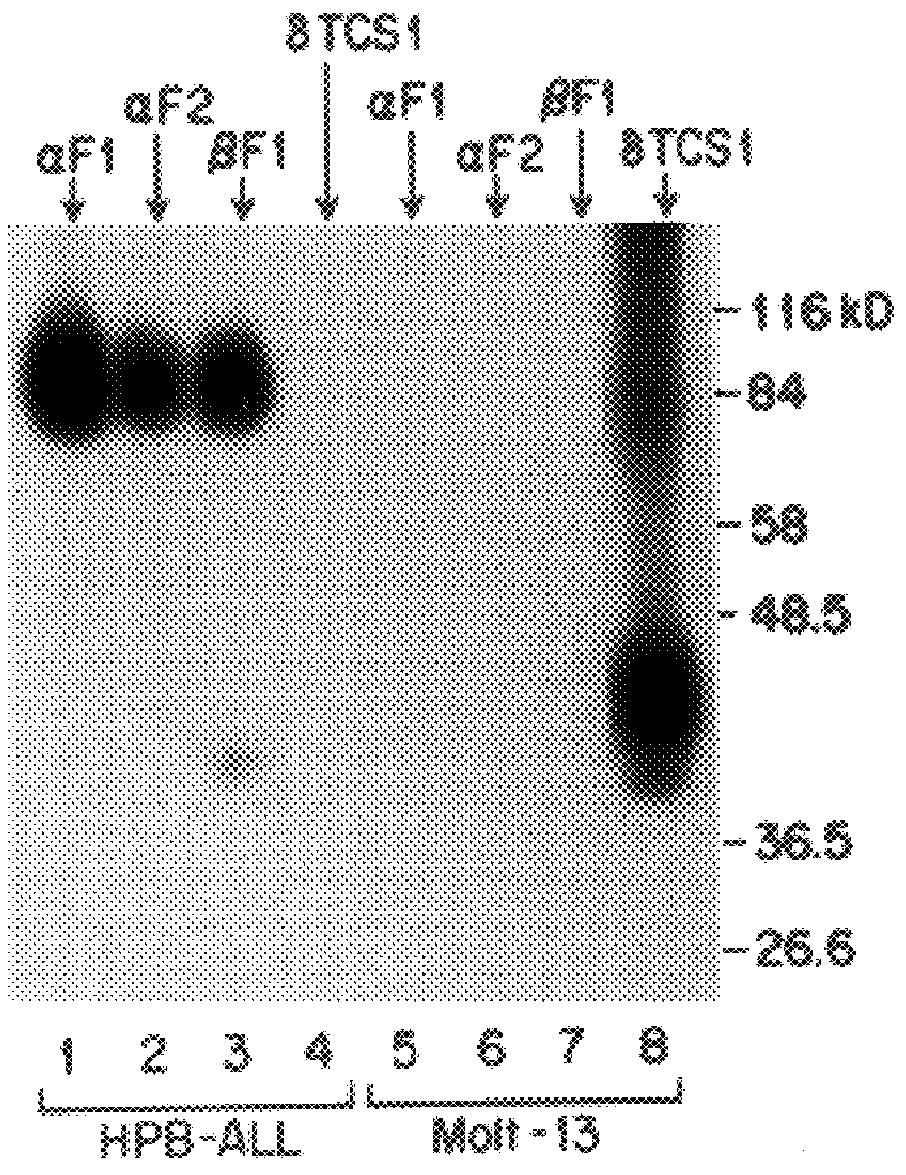
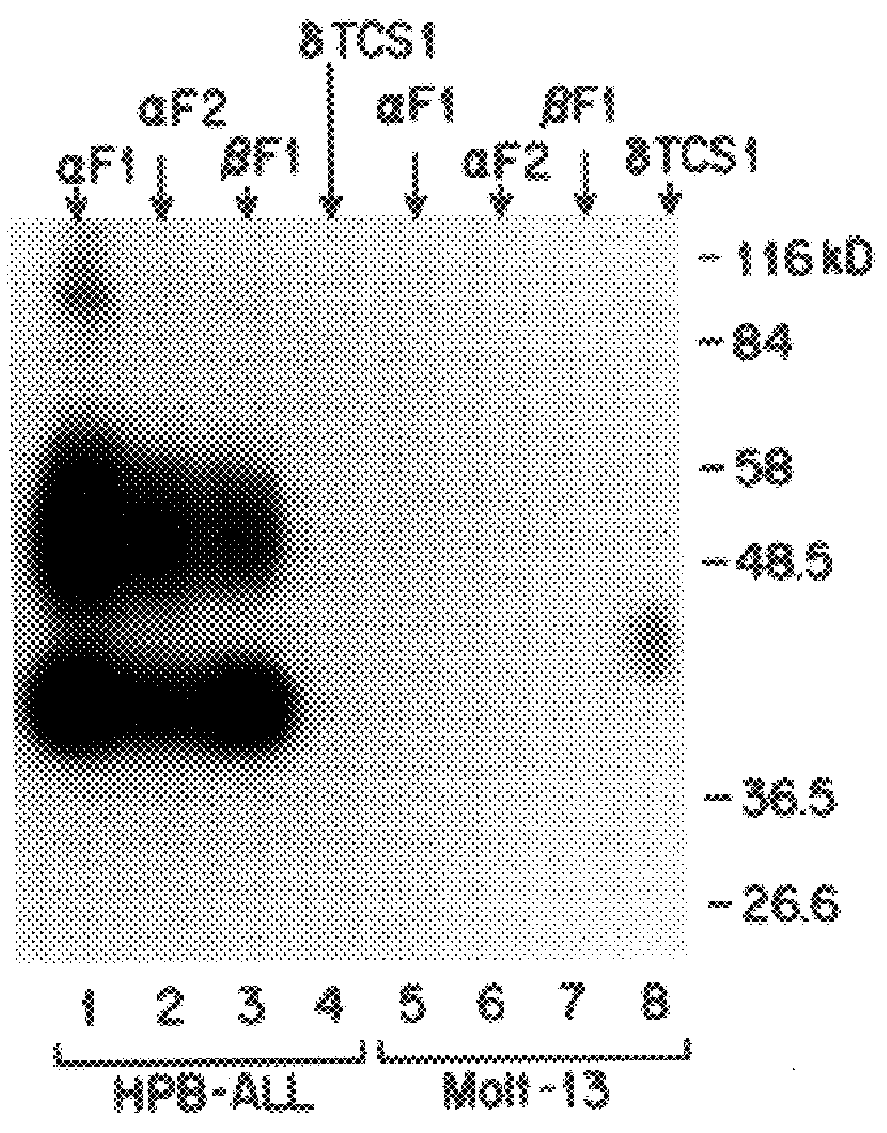
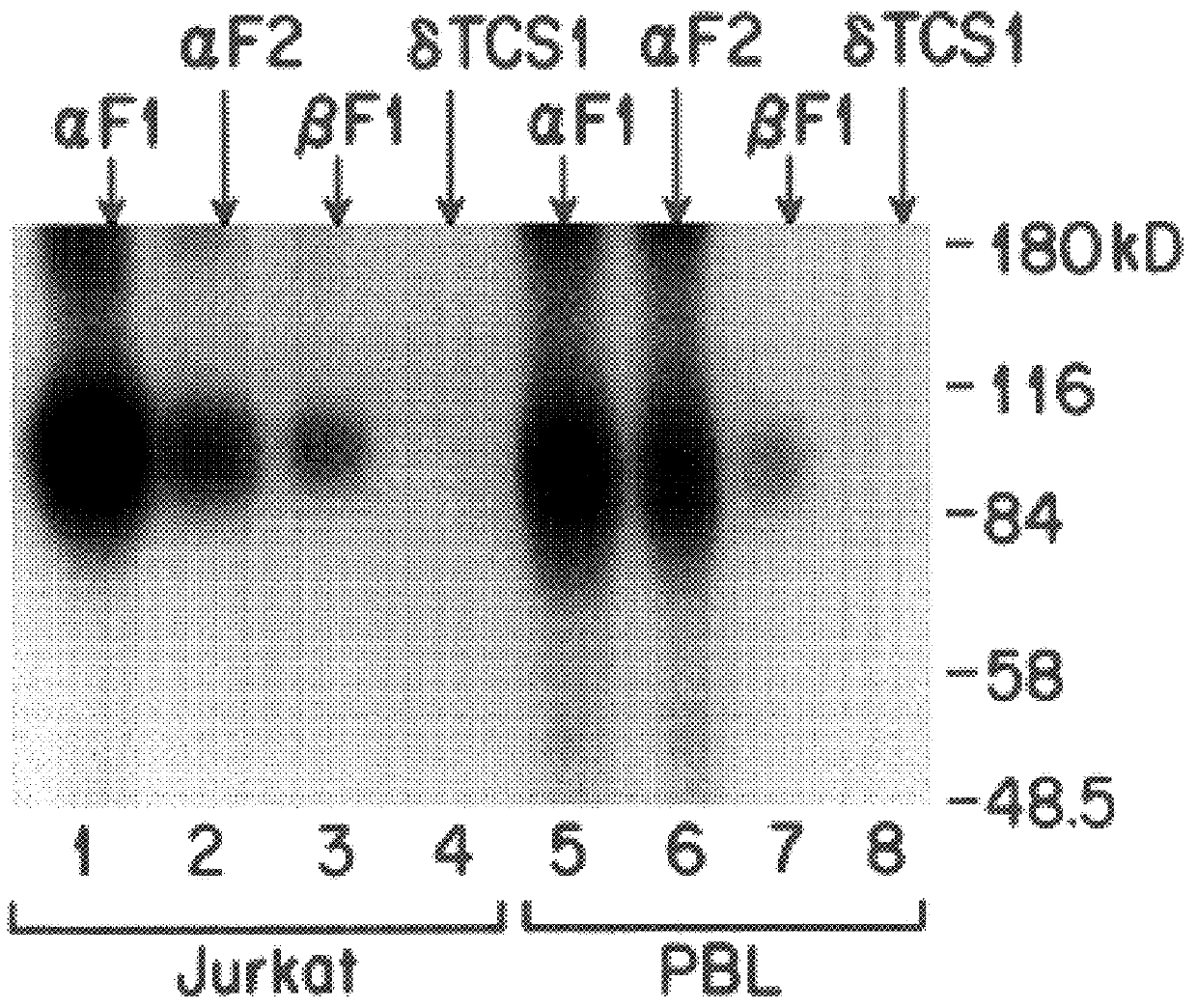
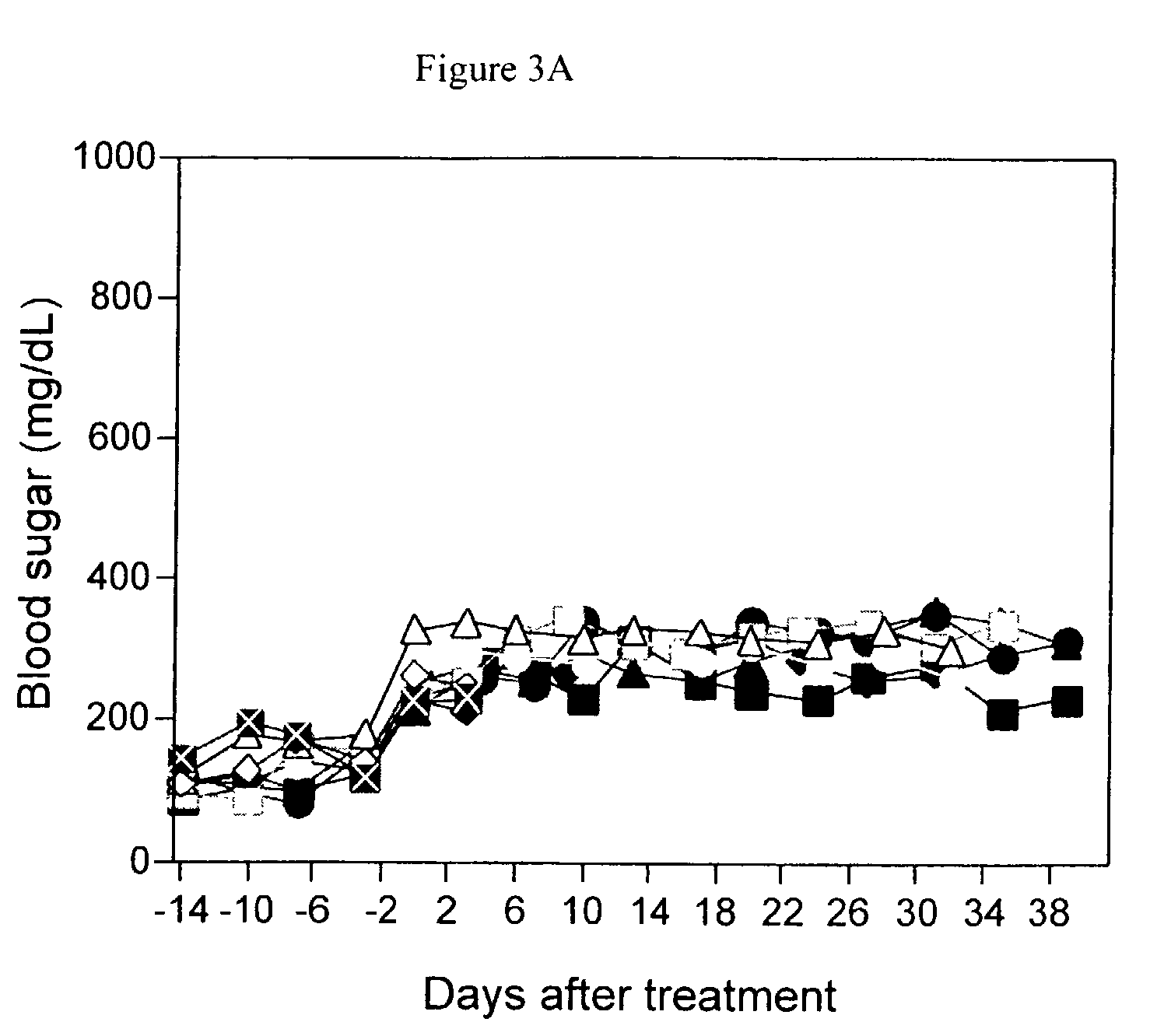
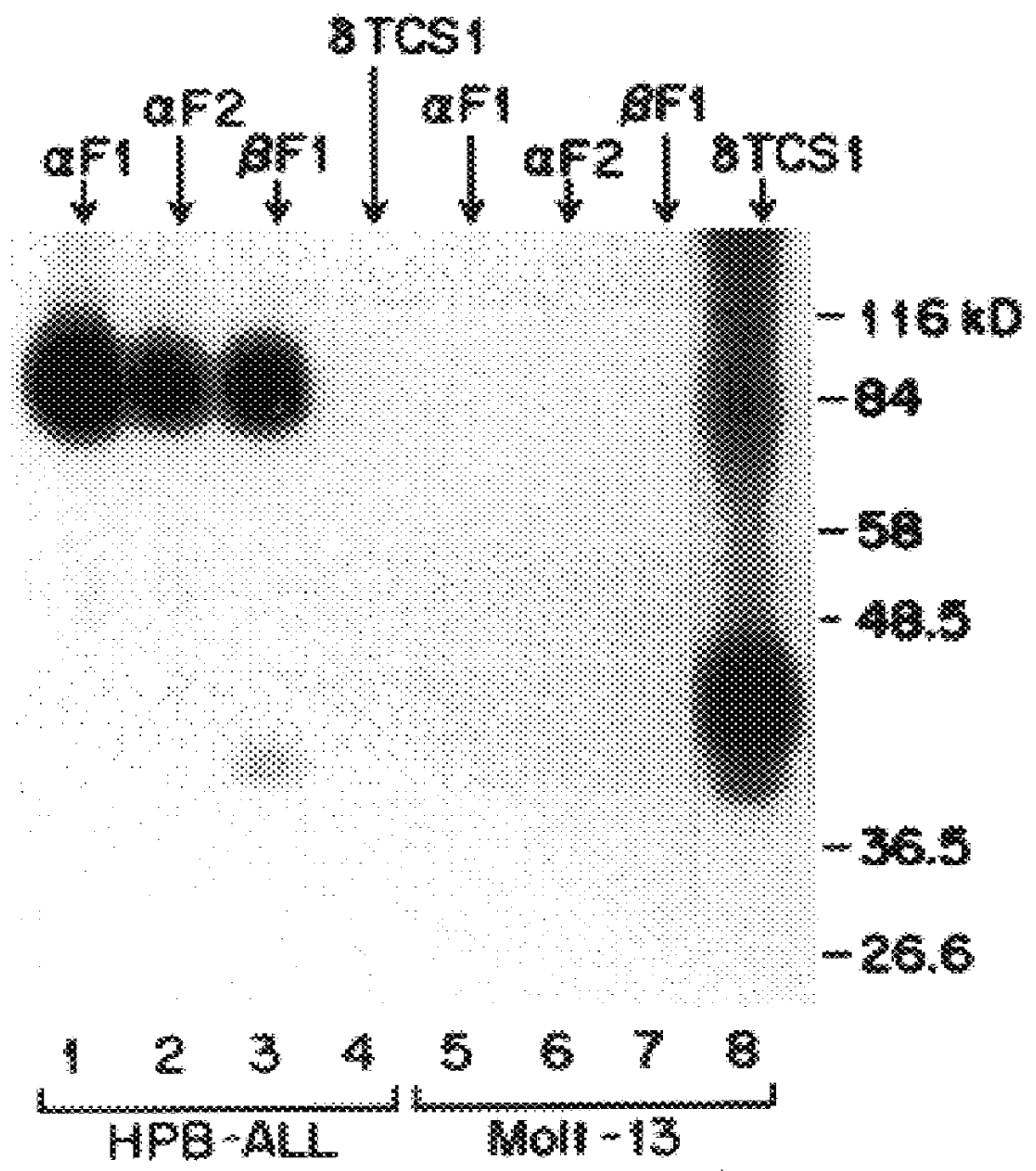
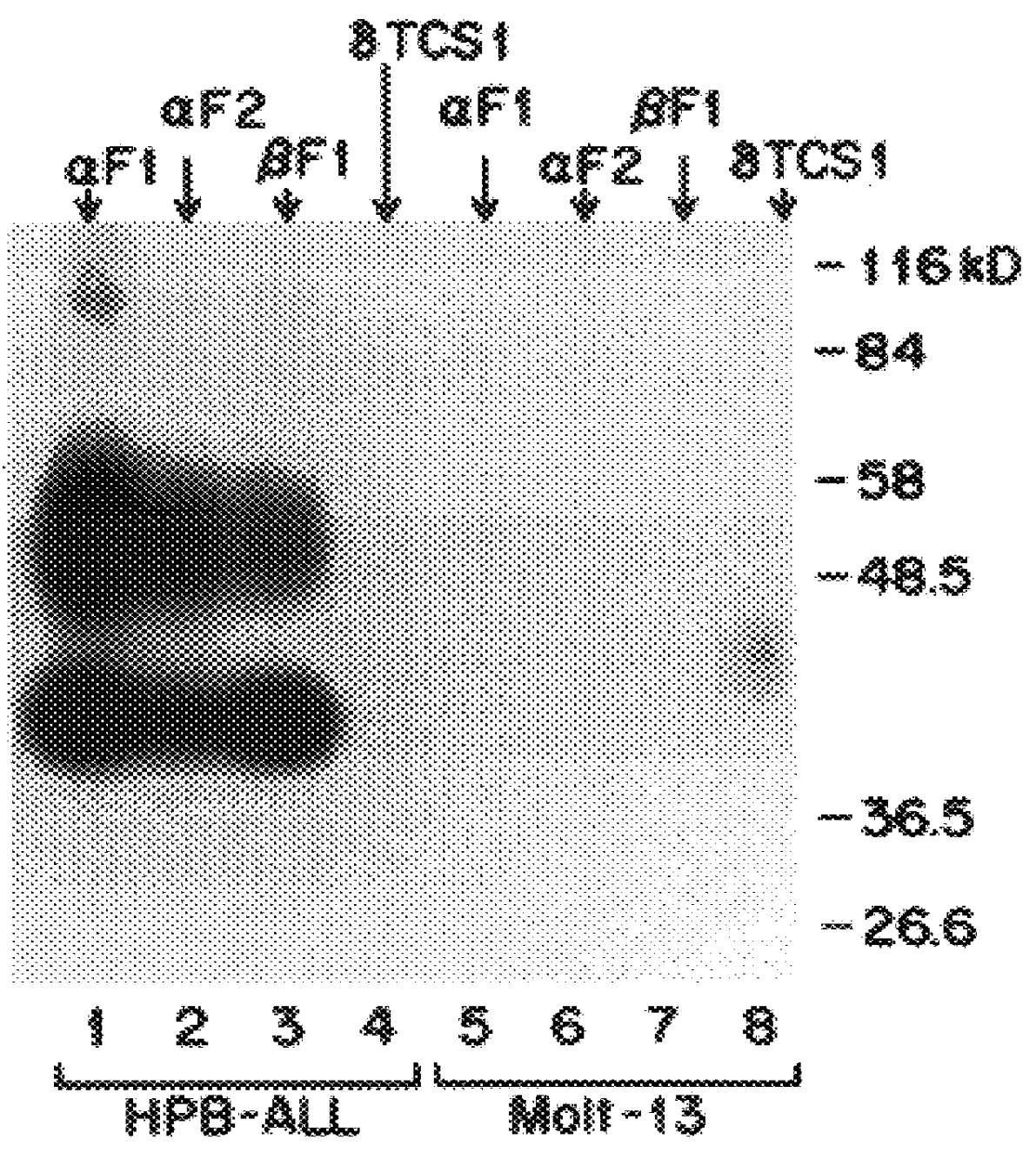
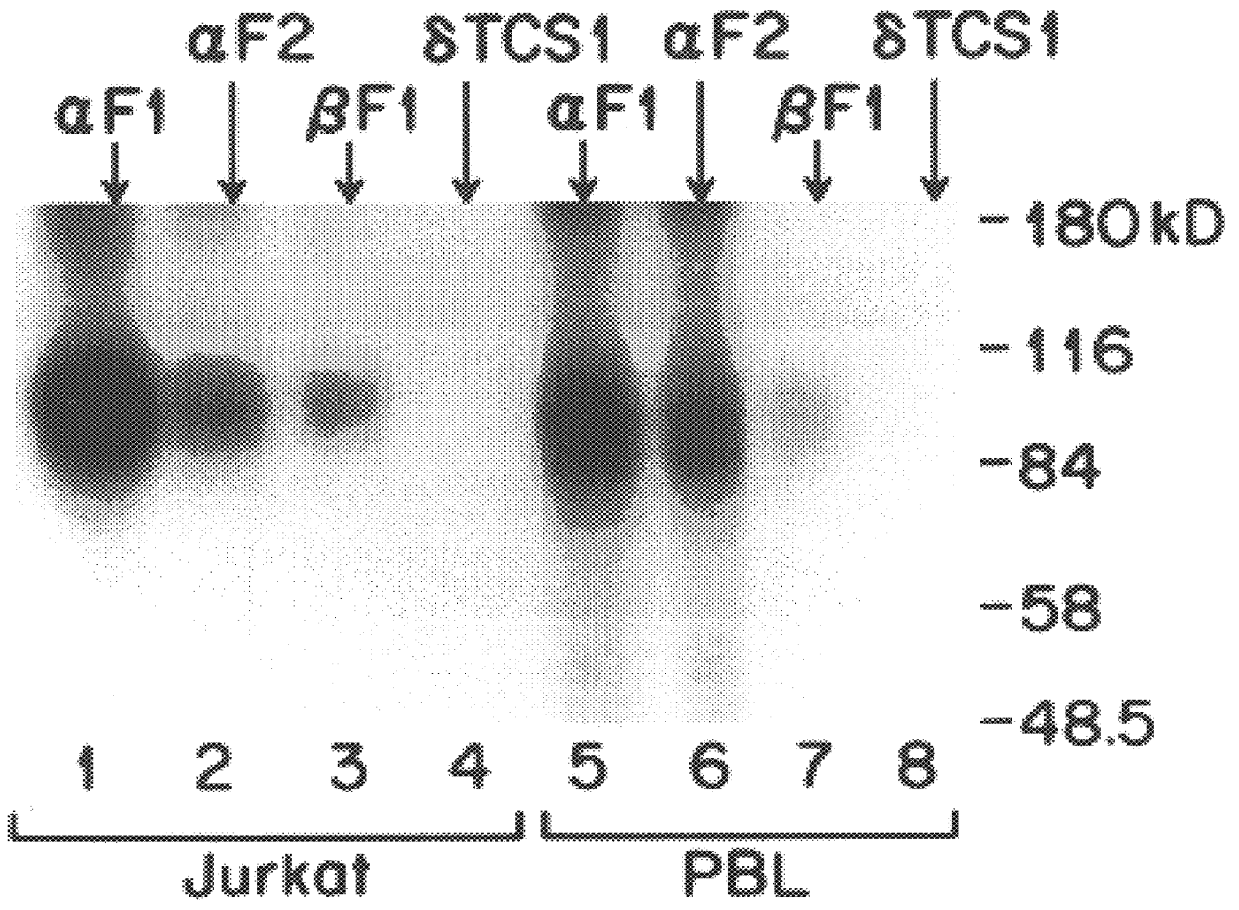
The present invention relates to monoclonal antibodies which recognize defined regions of the T-cell receptor (TCR). In a specific embodiment, the invention provides monoclonal antibodies which are reactive with a constant region of the alpha chain of the TCR. In particular embodiments, the invention relates to two monoclonal antibodies, termed alphaF1 and alphaF2, which react with two different epitopes on the framework region of the alpha monomer of the TCR molecule. In another specific embodiment, the invention is directed to monoclonal antibodies reactive with a variable region of the beta chain of the TCR. In particular, the invention provides two monoclonal antibodies, termed W112 and 2D1, which react with beta chain variable regions Vbeta5.3 and Vbeta8.1, respectively. In another specific embodiment, the invention is directed to monoclonal antibodies reactive with a variable region of the delta chain of the TCR. In particular, the invention provides monoclonal antibody deltaTCS1, isotype IgG2a. The monoclonal antibodies of the invention have value in diagnosis and therapy and are useful tools for study of the immune system.
Owner:ASTRAZENECA AB
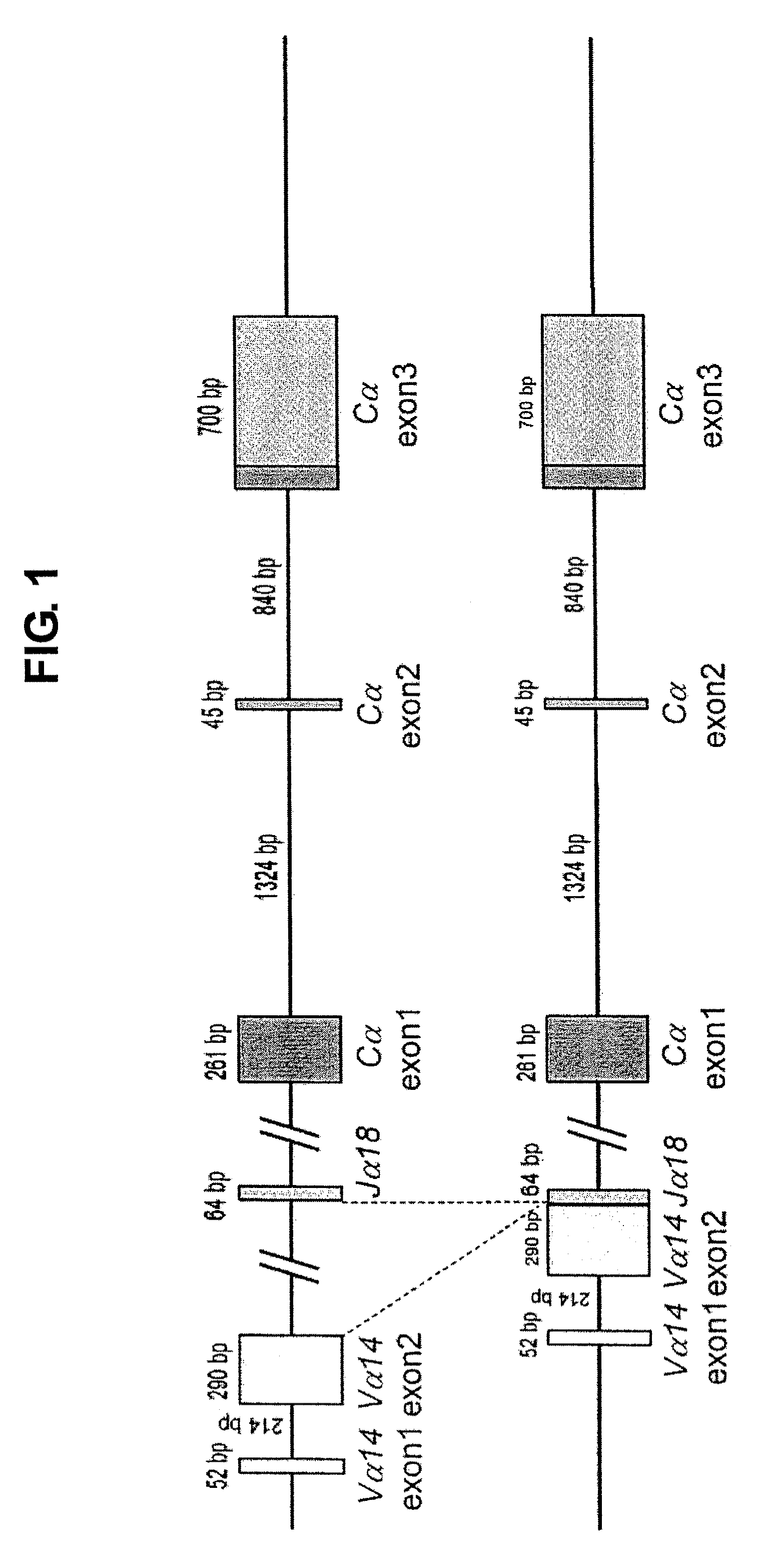
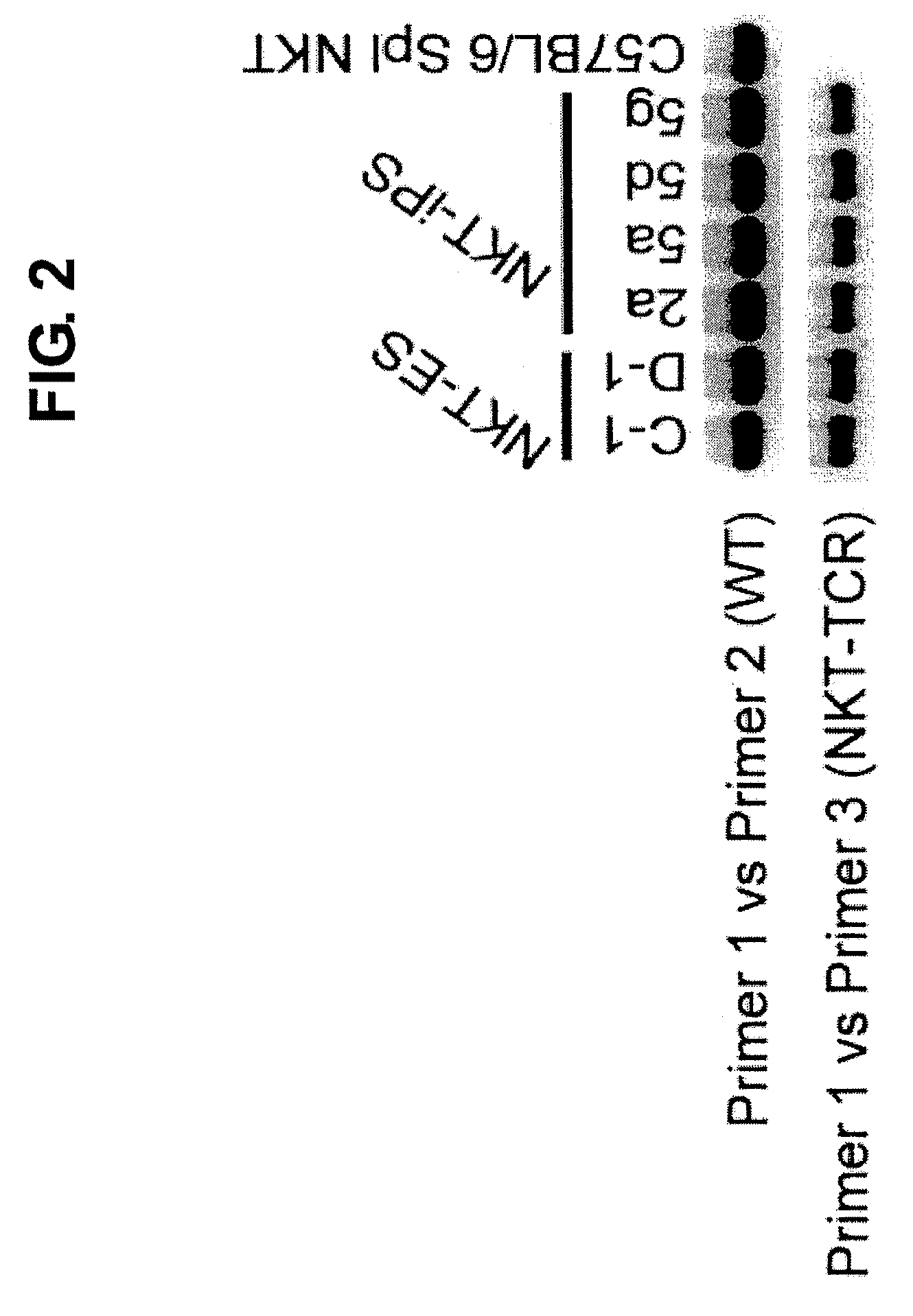
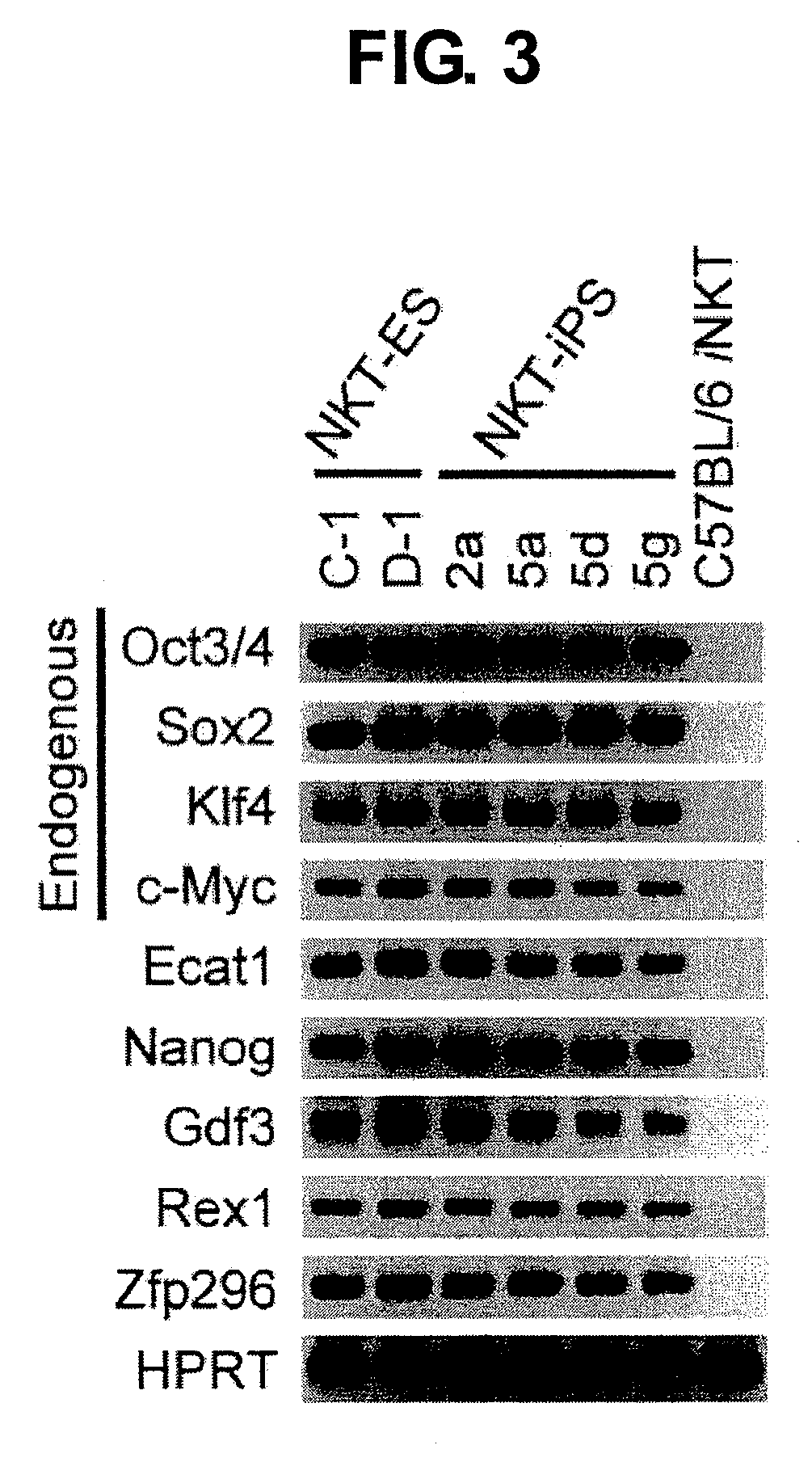
NKT CELL-DERIVED iPS CELLS AND NKT CELLS DERIVED THEREFROM
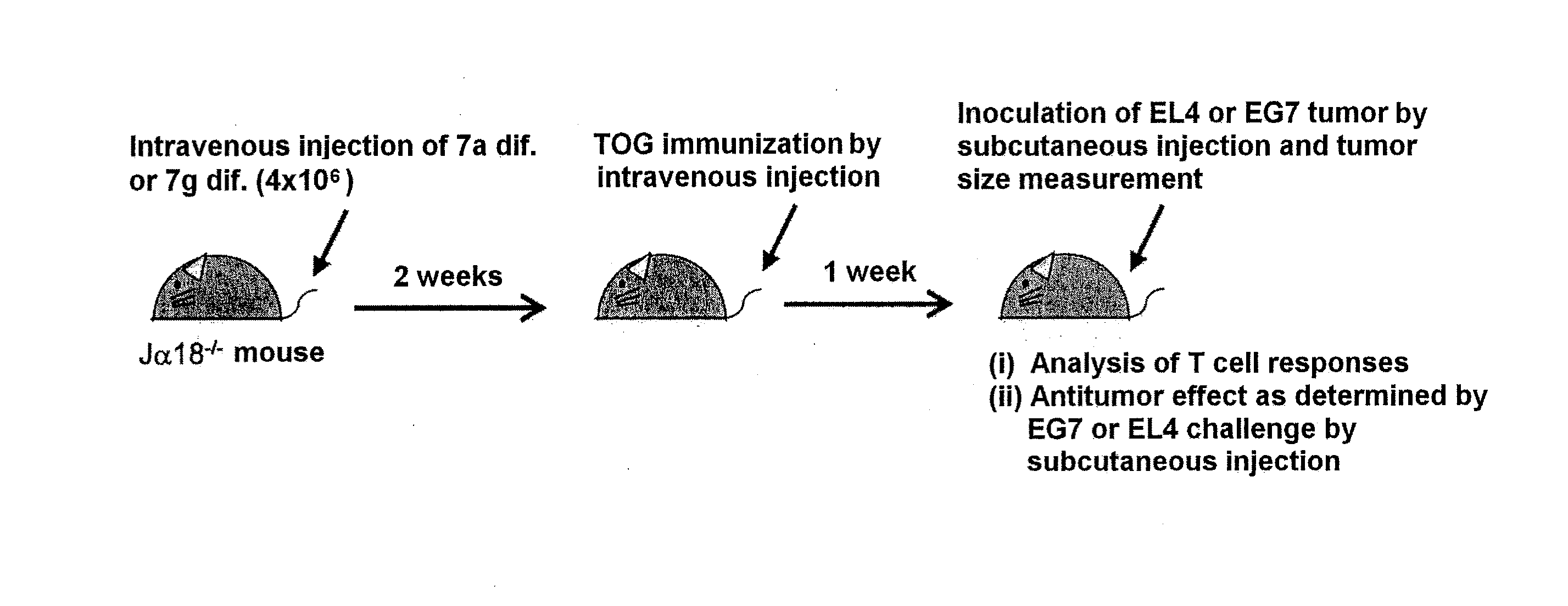
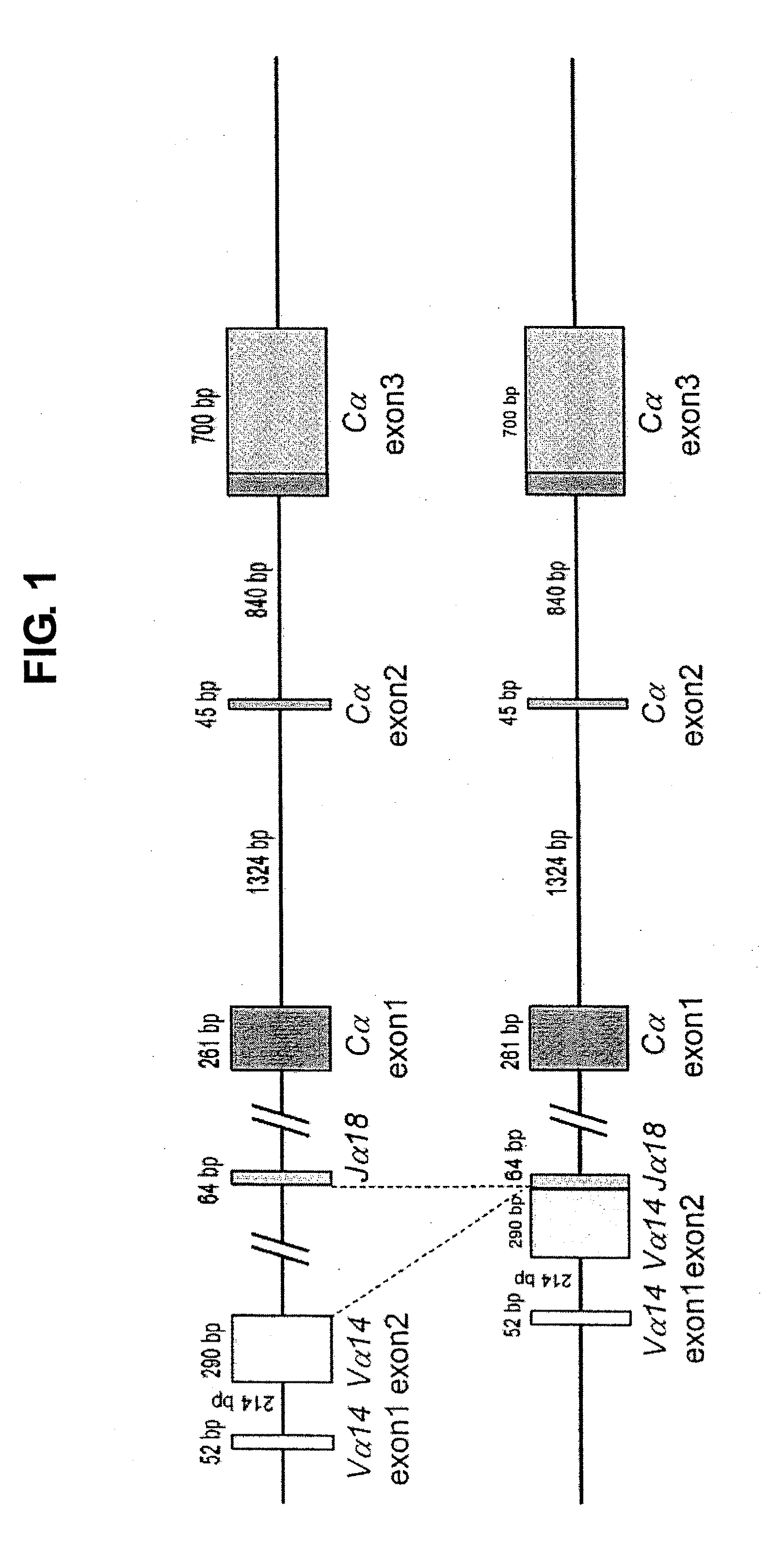
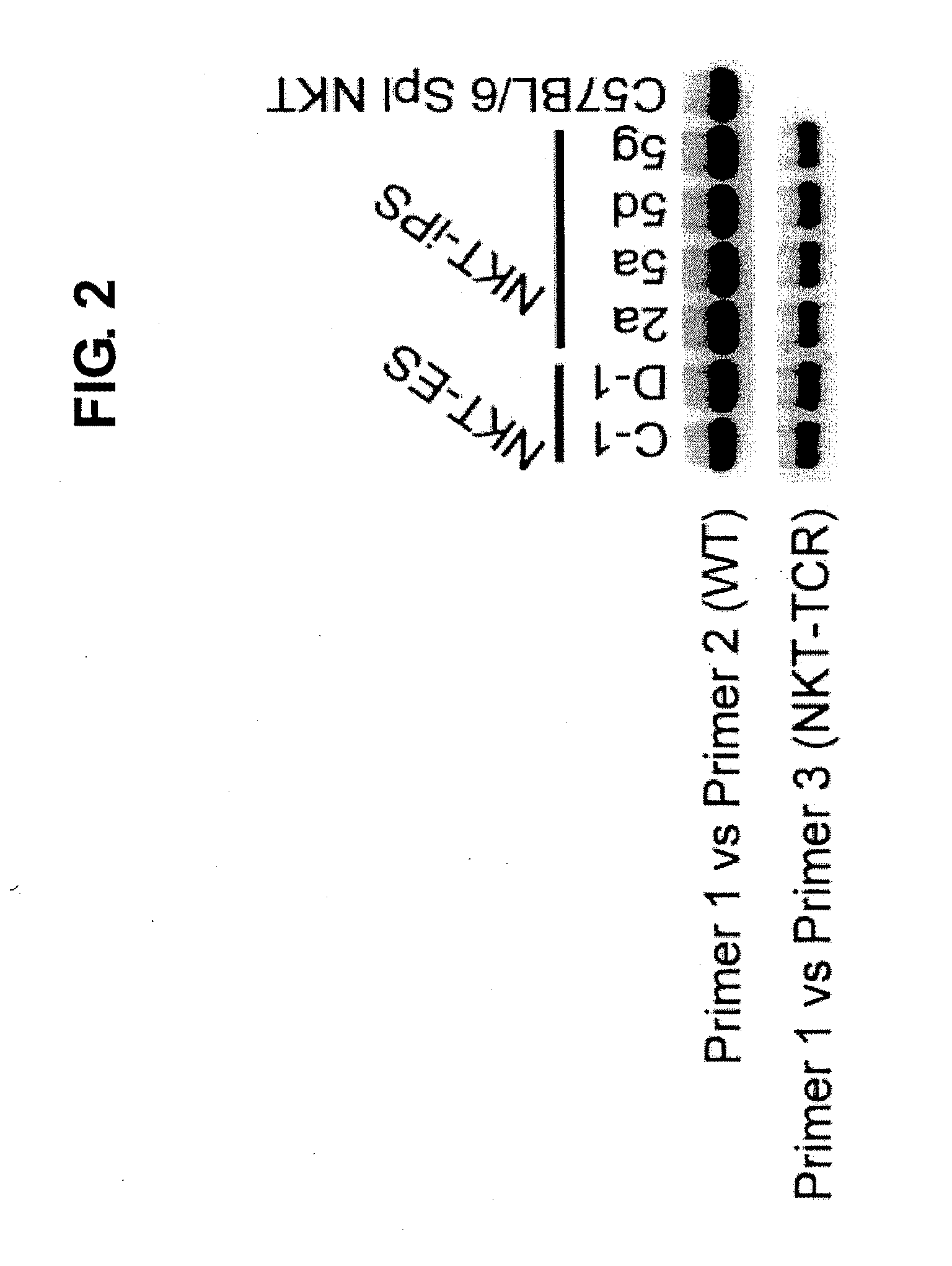
Provided are an iPS cell derived from a somatic cell such as an NKT cell, having the α-chain region of the T cell antigen receptor gene rearranged to uniform Vα-Jα in an NKT cell receptor-specific way, NKT cells differentiated from the iPS cell, a method of creating the same, and an immune cell therapy agent prepared using cells differentiated from the iPS cell. Also provided are an iPS cell having TCRα rearranged to NKT-TCR (NKT-iPS cell), obtained by contacting a somatic cell, such as an NKT cell, having the α-chain region of the T cell antigen receptor gene rearranged to uniform Vα-Jα in an NKT cell receptor-specific way, with nuclear reprogramming factors, isolated NKT cells obtained by differentiating the iPS cell ex vivo (iPS-NKT cell), a method of generating CD4 / CD8-double positive NKT cells (DP-NKT cells) and mature NKT cells from NKT-iPS cells by altering the combination of feeder cells and / or cytokines, a method of expanding the iPS-NKT cells, and an NKT cell cytotherapy agent comprising NKT cells activated with α-galactosyl ceramide (α-GalCer), or iPS-NKT cells, and α-GalCer in combination.
Owner:RIKEN
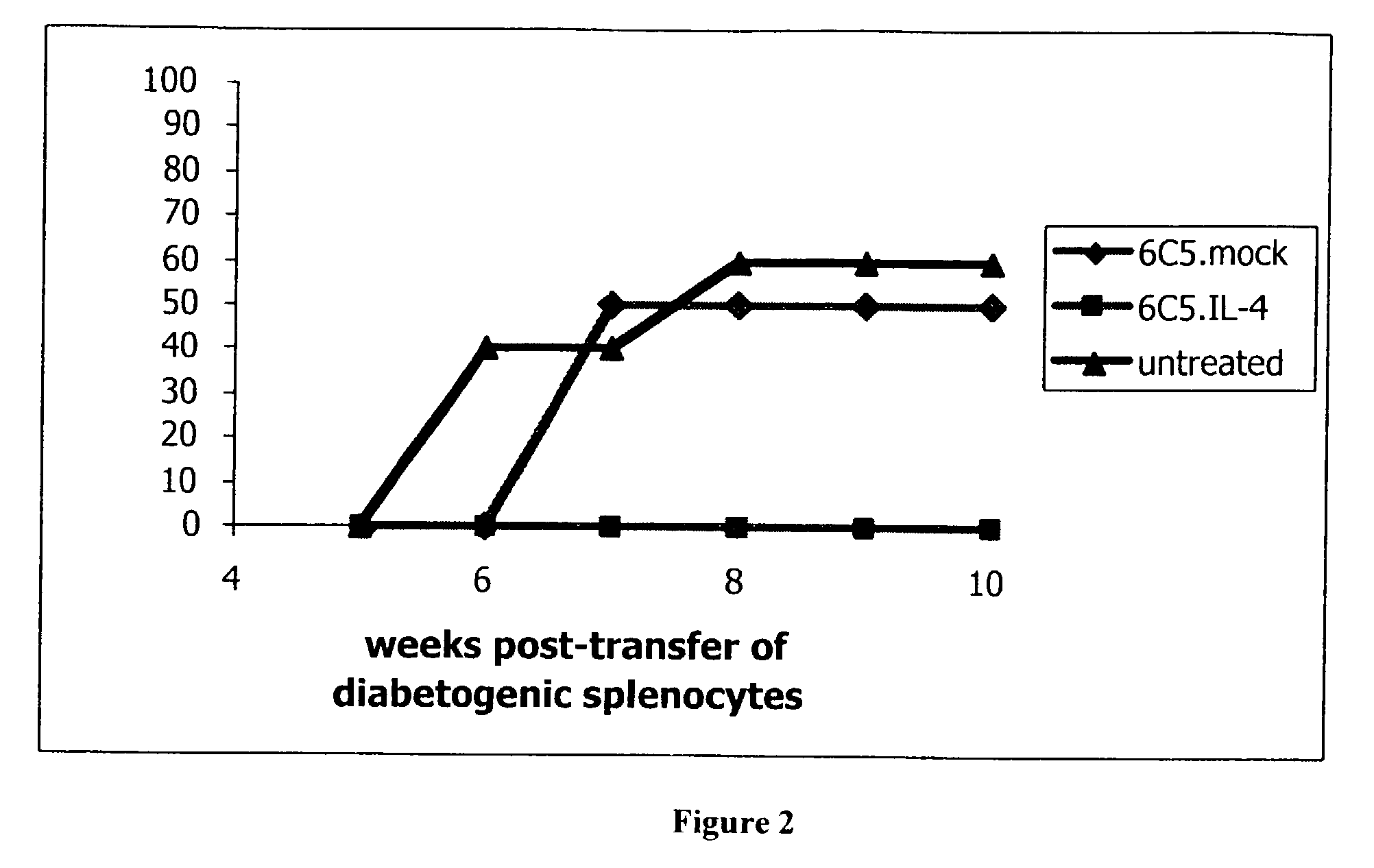
Gene therapy for the prevention of autoimmune disease
Autoimmune disease is treated by the delivery of a suppressive agent to the site of disease. Delivery is accomplished by introducing an expression vector encoding the suppressive agent into cells targeted for such sites, and administering the genetically modified cells to the patient. Suppressive agents of particular interest include IL-4; and anti-CD3 antibodies, particularly single chain anti-CD3 antibodies. Cells of interest for delivery include T cells and T cell hybridomas, where the T cell antigen receptor recognizes epitopes associated with the autoimmune disease. Alternatively, dendritic cells are used as delivery vectors.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Monoclonal antibodies reactive with defined regions of the T cell antigen receptor
The present invention relates to monoclonal antibodies which recognize defined regions of the T-cell receptor (TCR). In a specific embodiment, the invention provides monoclonal antibodies which are reactive with a constant region of the alpha chain of the TCR. In particular embodiments, the invention relates to two monoclonal antibodies, termed alpha F1 and alpha F2, which react with two different epitopes on the framework region of the alpha monomer of the TCR molecule. In another specific embodiment, the invention is directed to monoclonal antibodies reactive with a variable region of the beta chain of the TCR. In particular, the invention provides two monoclonal antibodies, termed W112 and 2D1, which react with beta chain variable regions V beta 5.3 and V beta 8.1, respectively. In another specific embodiment, the invention is directed to monoclonal antibodies reactive with a variable region of the delta chain of the TCR. In particular, the invention provides monoclonal antibody delta TCS1, isotype IgG2a. The monoclonal antibodies of the invention have value in diagnosis and therapy and are useful tools for study of the immune system.
Owner:ASTRAZENECA AB
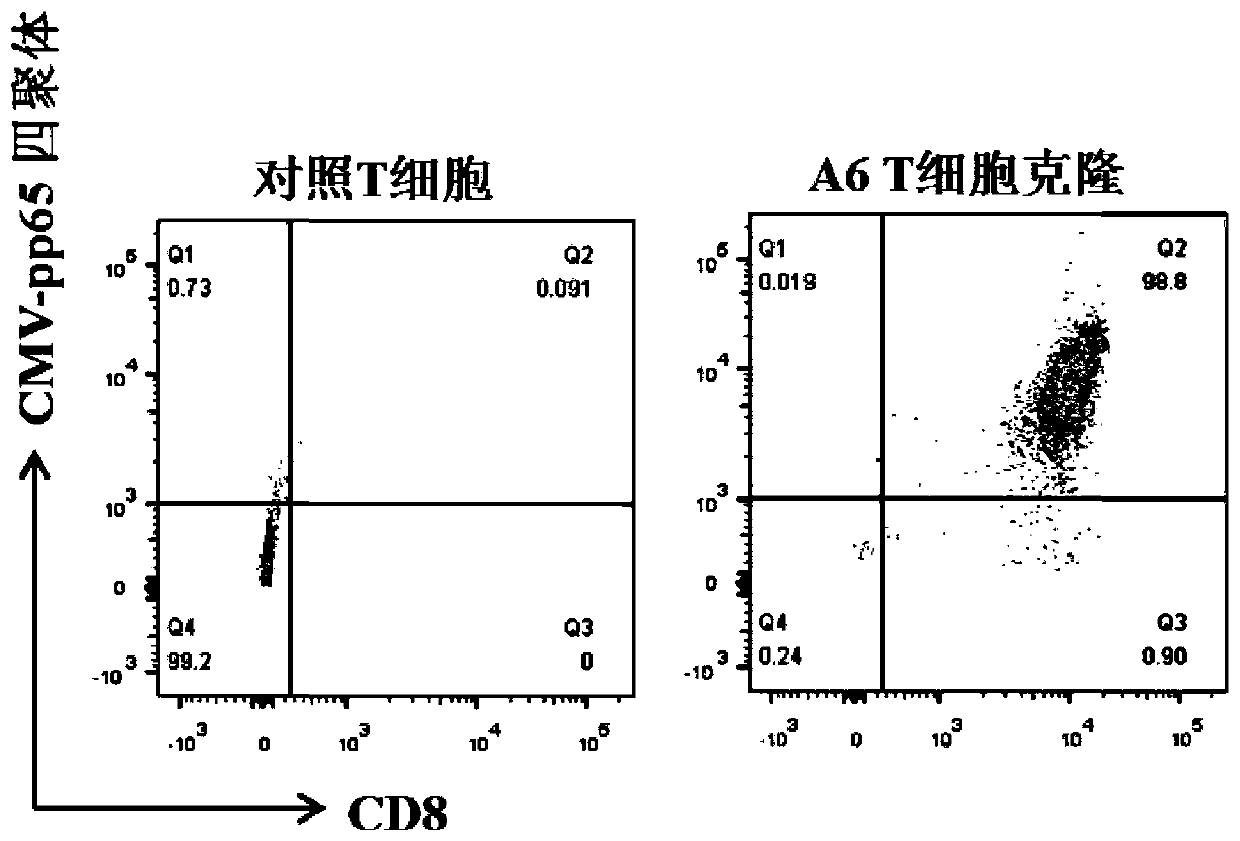
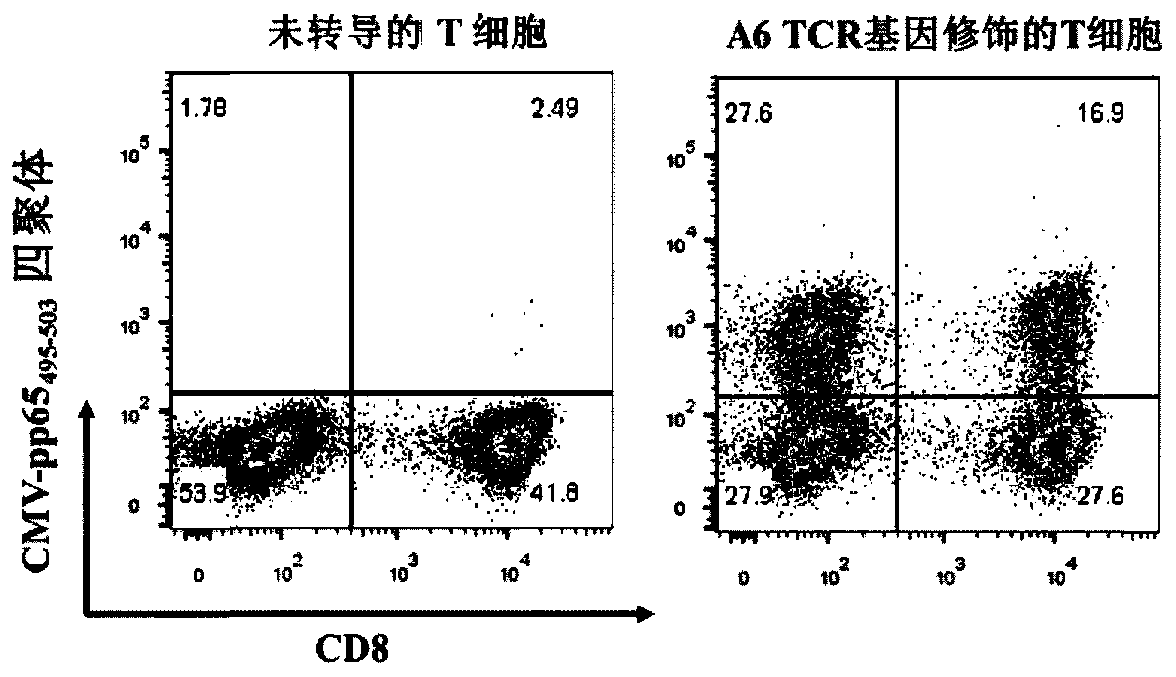
TCR for identifying human cytomegalovirus pp65 antigen
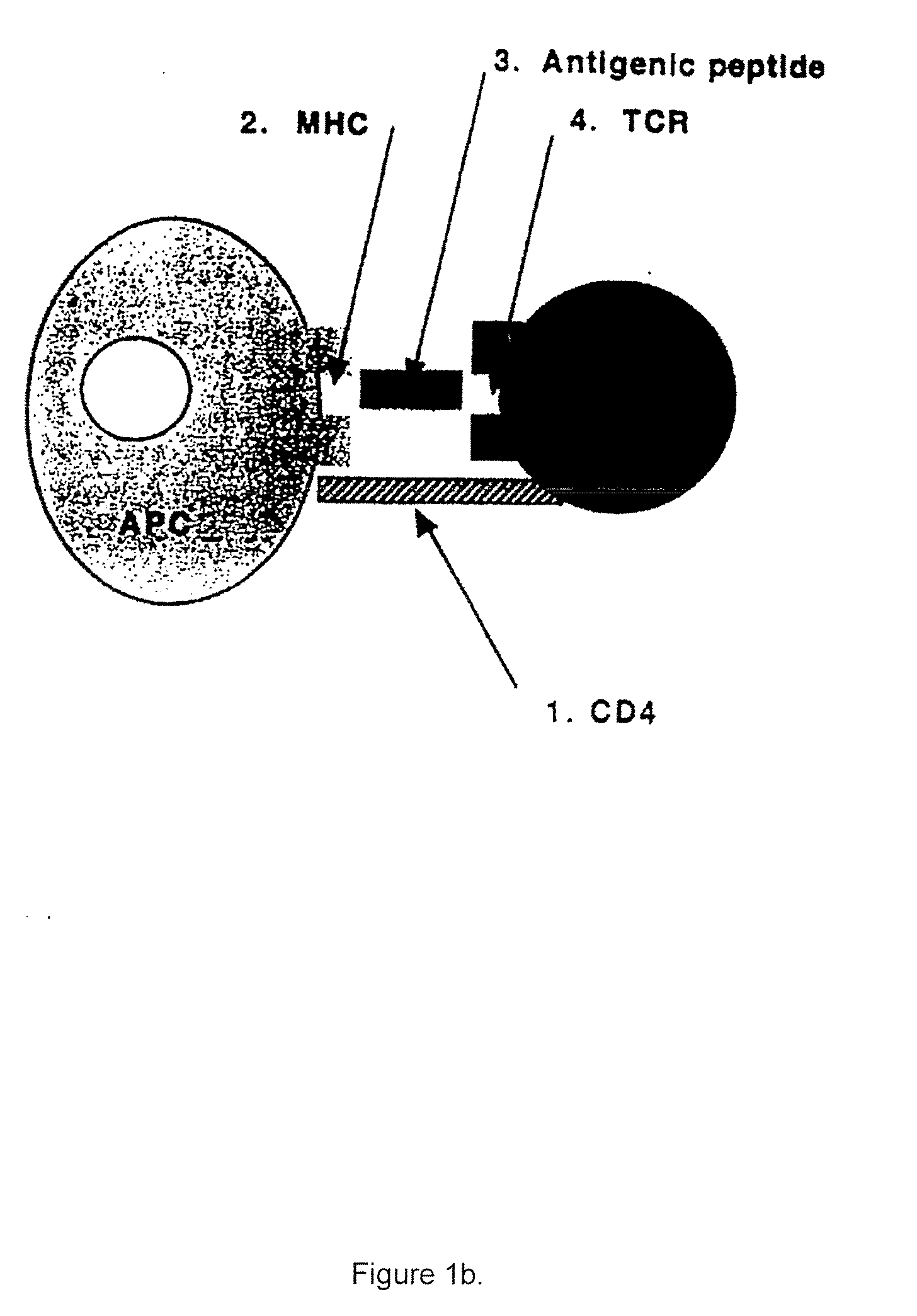
The invention relates to the technical field of T cell antigen receptors, in particular to TCR capable of identifying and combining with an HLA-A2-CMV-pp65495-503 antigen complex, a nucleotide sequence encoding the TCR or nucleic acid molecules of a complementary sequence of the nucleotide sequence, a carrier containing the nucleic acid molecules, cells transducing the nucleic acid molecules or carrier, a drug composition containing the TCR, the nucleic acid molecules, the carrier or the cells which are taken as active ingredients and application of the TCR, nucleic acid molecules, carrier, cells and drug composition which are used for preparing drugs for treating tumors or virus infection respectively.
Owner:深圳市因诺转化医学研究院 +1
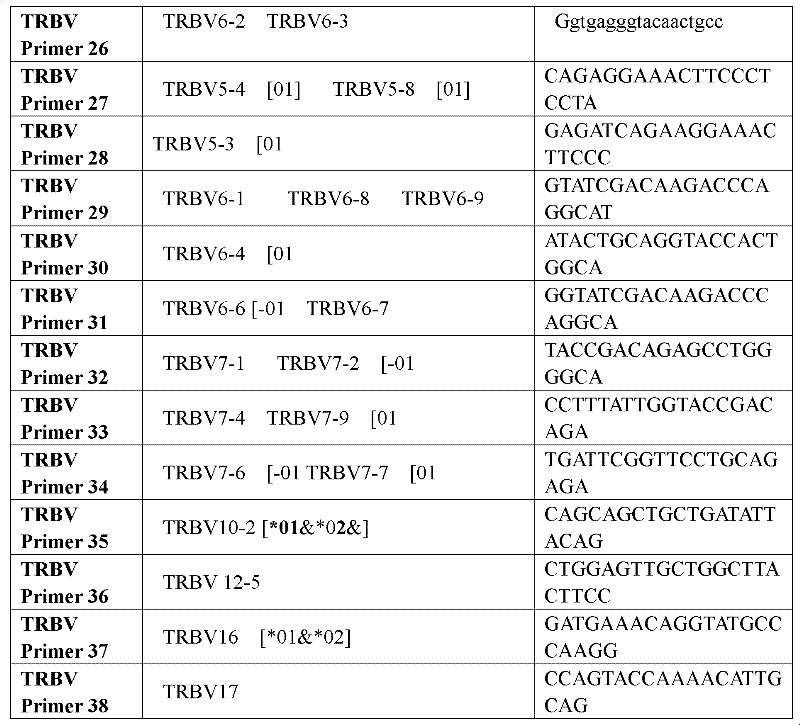
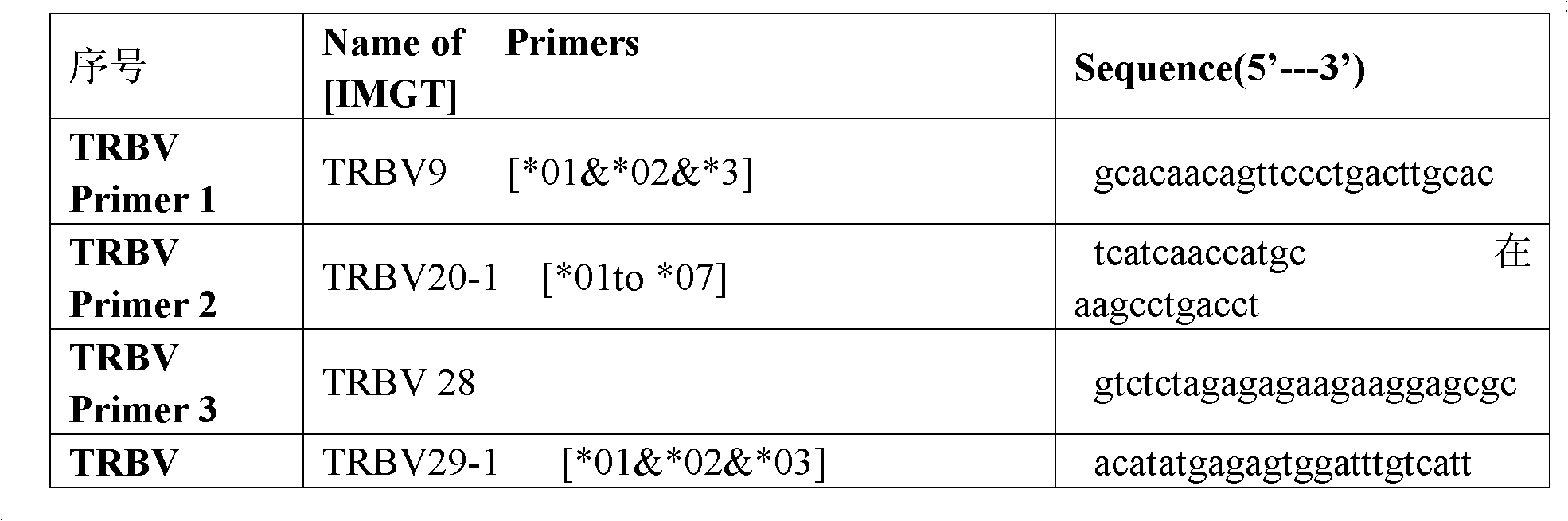
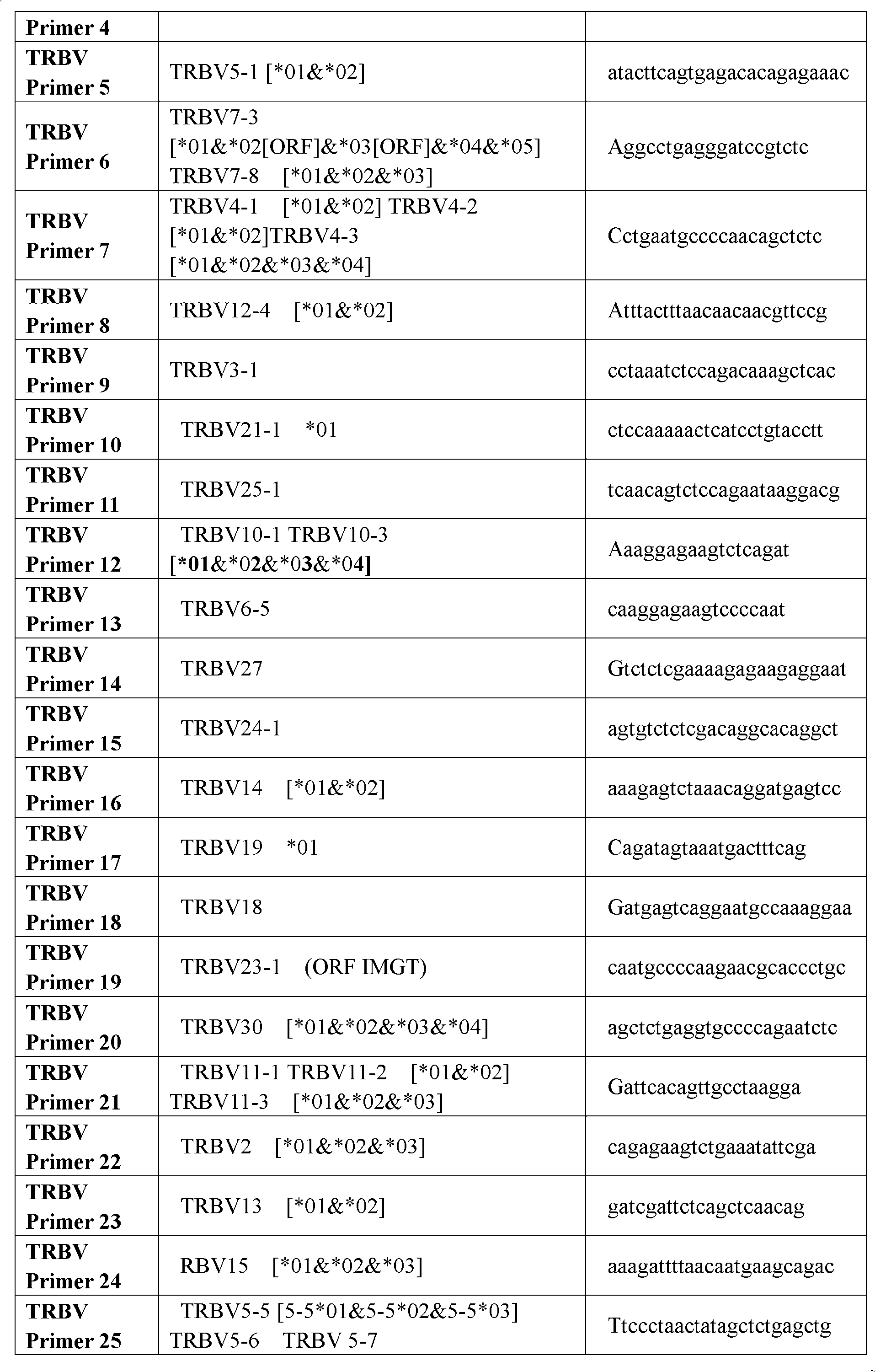
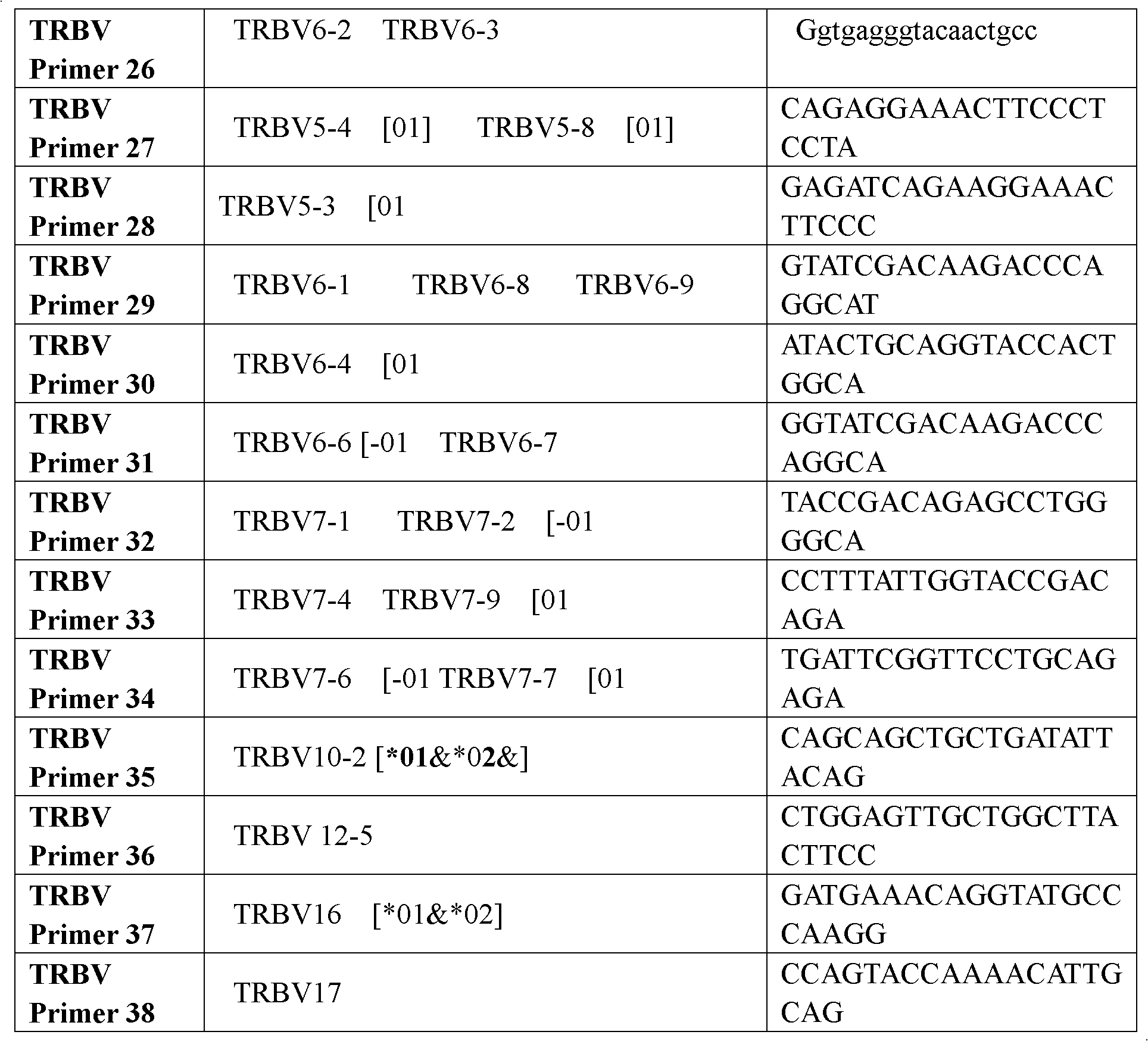
Method for simultaneously sequencing multi-sample CDR3 (complementary determining region 3) receptor library with high flux
InactiveCN102443624AHigh-throughput sequencing cost reductionMicrobiological testing/measurementComplementarity determining regionHigh flux
The invention discloses a method for simultaneously sequencing a multi-sample CDR3 (complementary determining region 3) receptor library with a high flux, comprising the following steps of: only designing one set of V-gene family upstream primers in the variable region of a TCR (T cell antigen receptor) or BCR (B cell antigen receptor) chain according to the homology of the V-gene family, then designing C or J gene characteristic downstream primers of the TCR or BCR chain as many as samples and suitable for being paired with the upstream primers, amplifying each sample by one characteristic downstream primer and the same set of upstream primers to obtain a CDR3 region of the TCR or BCR, then mixing all the amplified samples to be a to-be-sequenced sample library, finally sequencing, wherein the characteristic downstream primers are designed by different ATCG basic group compositions. The CDR3 sequences of multiple samples can be simultaneously sequenced by operating one high-flux sequencing program via the method for simultaneously sequencing a multi-sample CDR3 receptor library with a high flux disclosed by the invention, so that the high-flux sequencing cost is greatly decreased. Thus, a high-flux sequencing technology has a wide application value in a sequencing research of a CDR3 receptor library.
Owner:ZUNYI MEDICAL UNIVERSITY
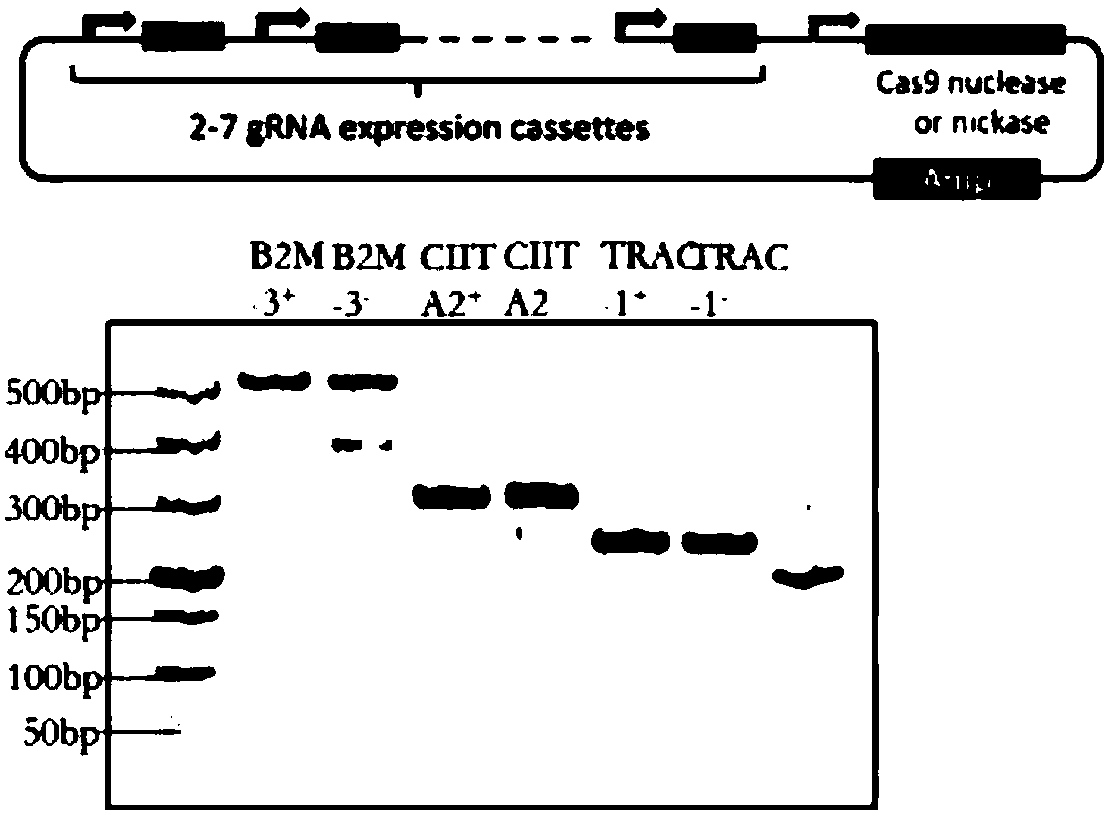
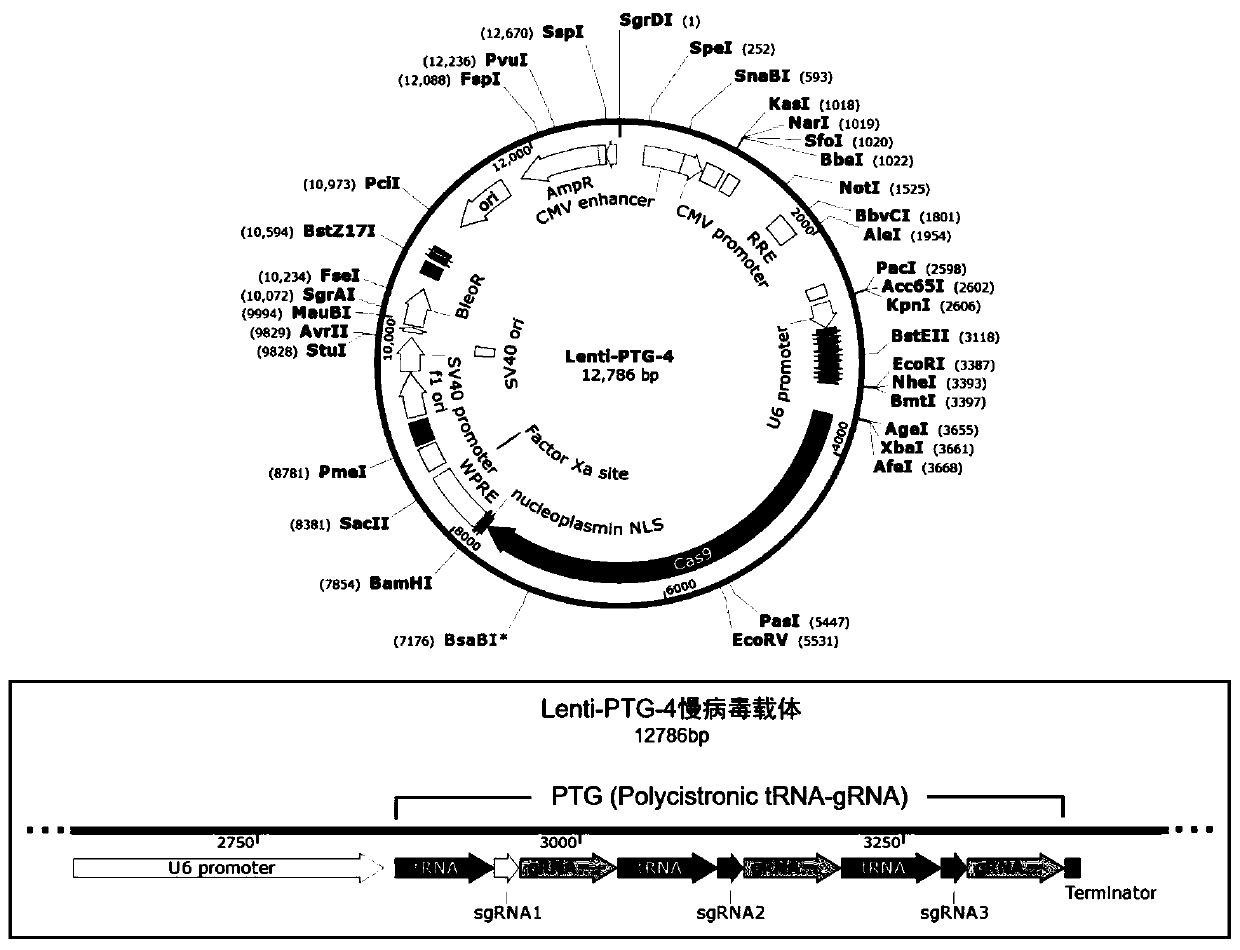
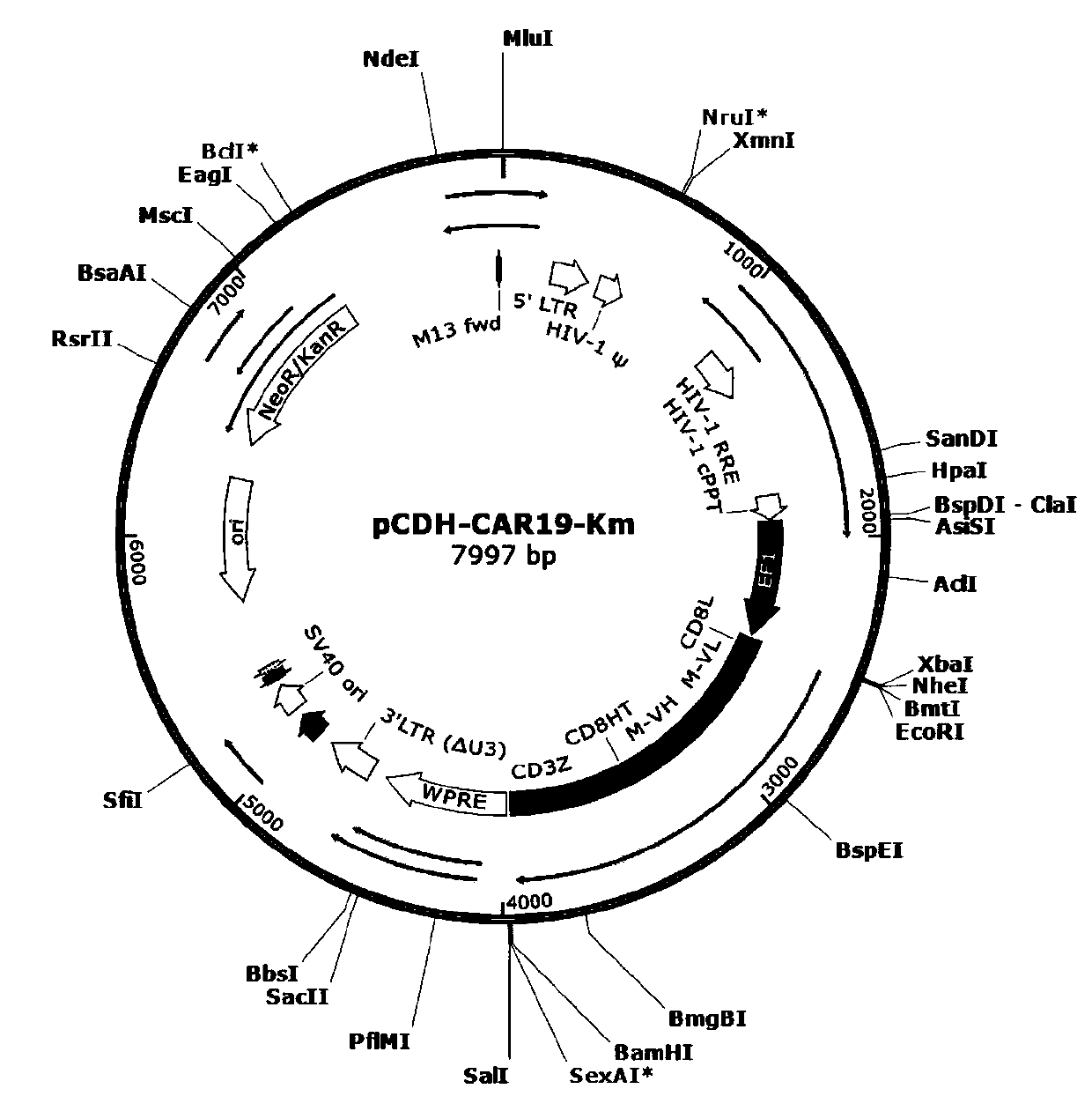
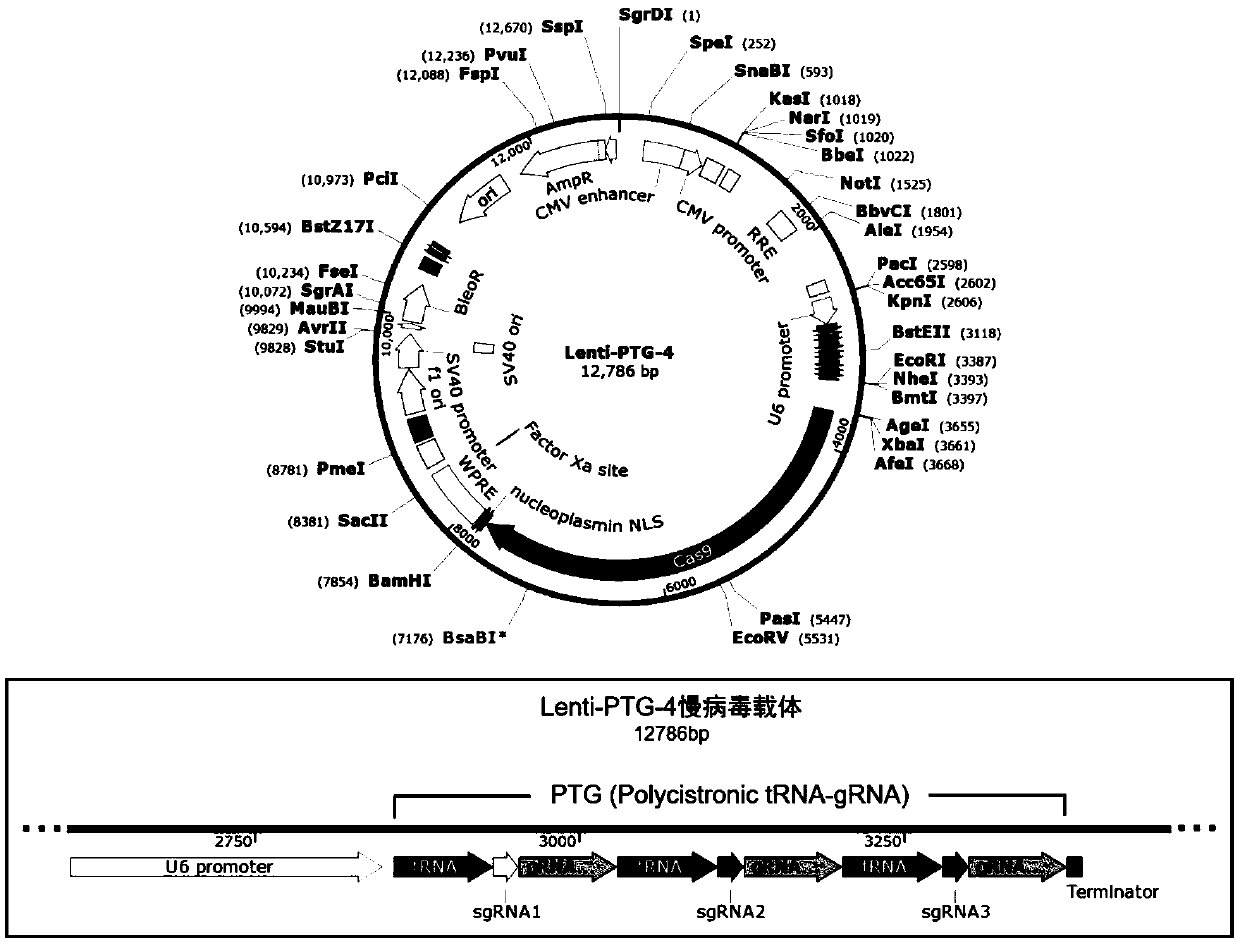
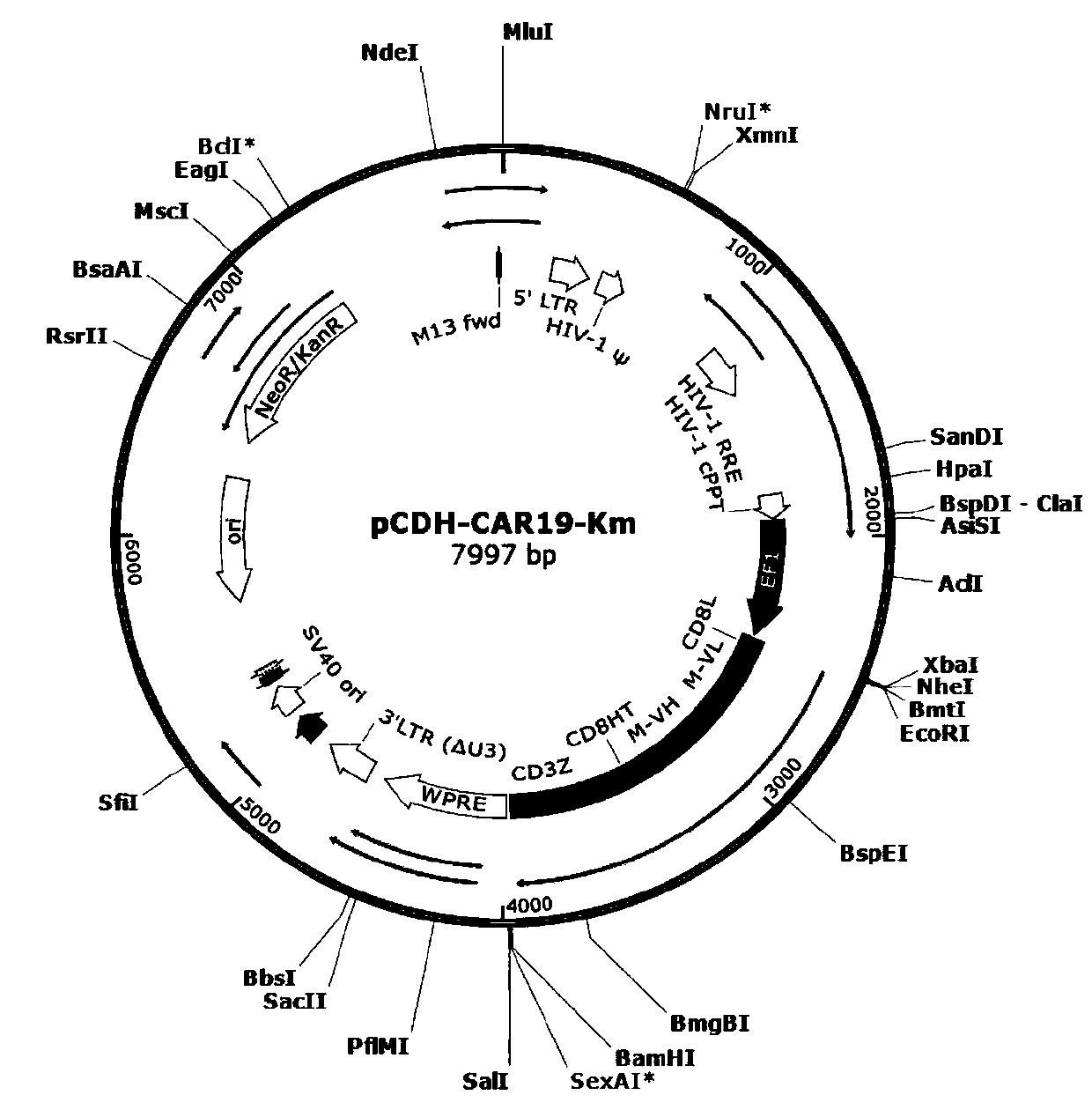
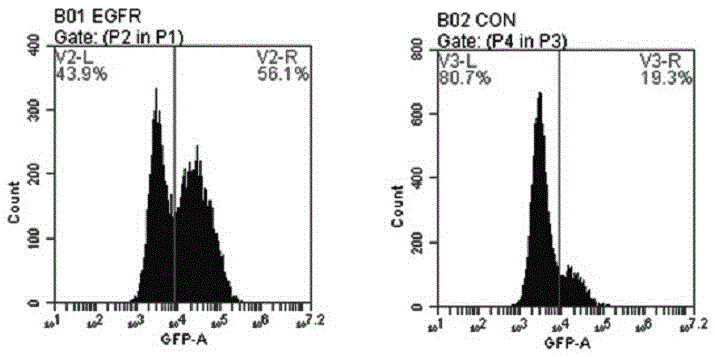
General type CAR-T cell and preparation method and use thereof
ActiveCN109722437AEfficient knockoutDoes not affect expressionStable introduction of DNAMammal material medical ingredientsT-Cell Antigen ReceptorsMicrobiology
The invention provides sgRNA or PTG (Polycistronic tRNA-gRNA), through combination with a CRISPR-Cas9 technique, a T cell antigen receptor (TCR) can be knocked out, or one or more of genes relevant tomajor histocompatibility complex (MHCI) and immunosuppression molecules can be knocked out. The invention also provides a general type CAR-T cell which can express chimeric antigen receptor (CAR) butcannot express TCR, and a preparation method of the general type CAR-T cell. The general type CAR-T cell prepared by the preparation method is suitable for heterogeneity antigen, has generality and has higher killability at the same time.
Owner:GUANGZHOU BIO GENE TECH CO LTD
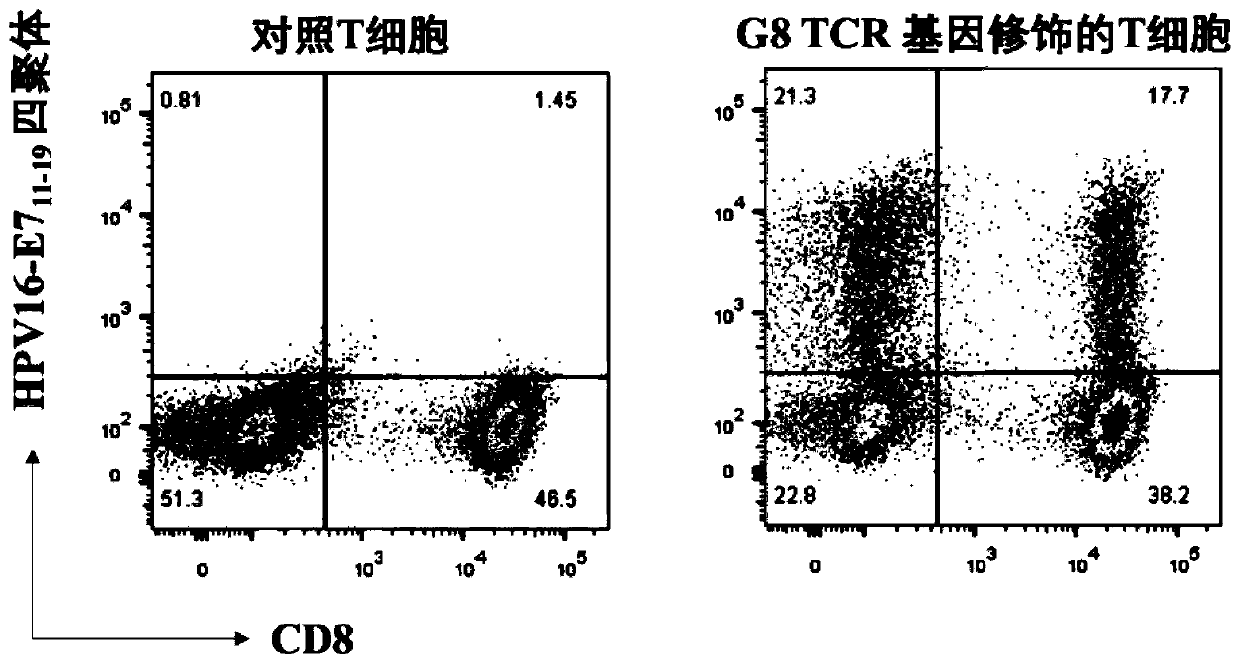
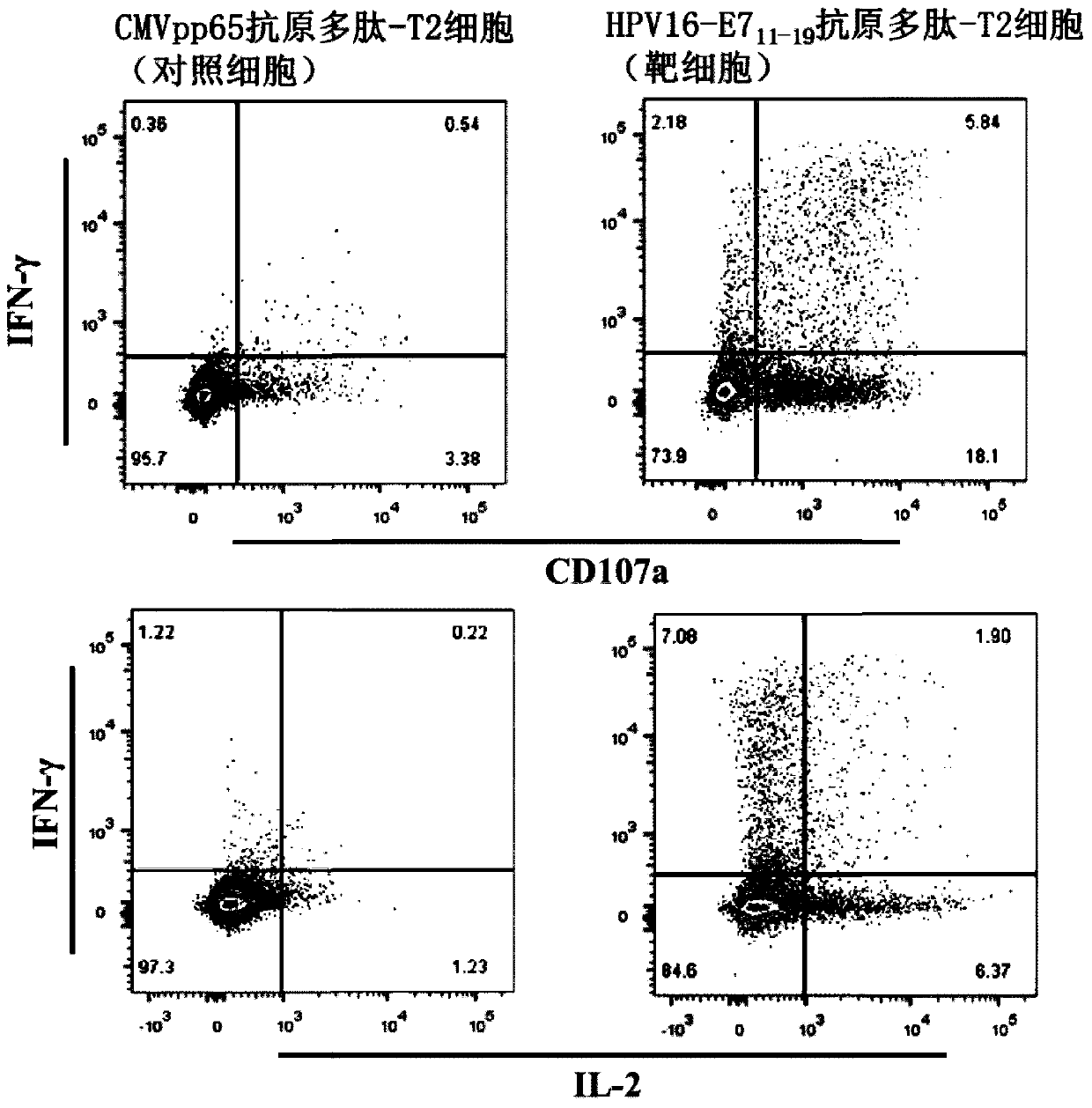
TCR for identifying human papilloma virus HPV16-E7 antigen
ActiveCN110357952AImmunoglobulin superfamilyMammal material medical ingredientsAntigenT-Cell Antigen Receptors
The invention relates to the technical field of T cell antigen receptors, in particular to TCR capable of specifically identifying and combining with a human papilloma virus antigen HPV16-E7, a nucleotide sequence encoding the TCR or nucleic acid molecules of a complementary sequence of the nucleotide sequence, a carrier containing the nucleic acid molecules, cells transducing the nucleic acid molecules or carrier, a drug composition containing the TCR, the nucleic acid molecules, the carrier or the cells which are taken as active ingredients and application of the TCR, nucleic acid molecules,carrier, cells and drug composition which are used for preparing drugs for treating tumors or virus infection respectively.
Owner:深圳市因诺转化医学研究院 +1
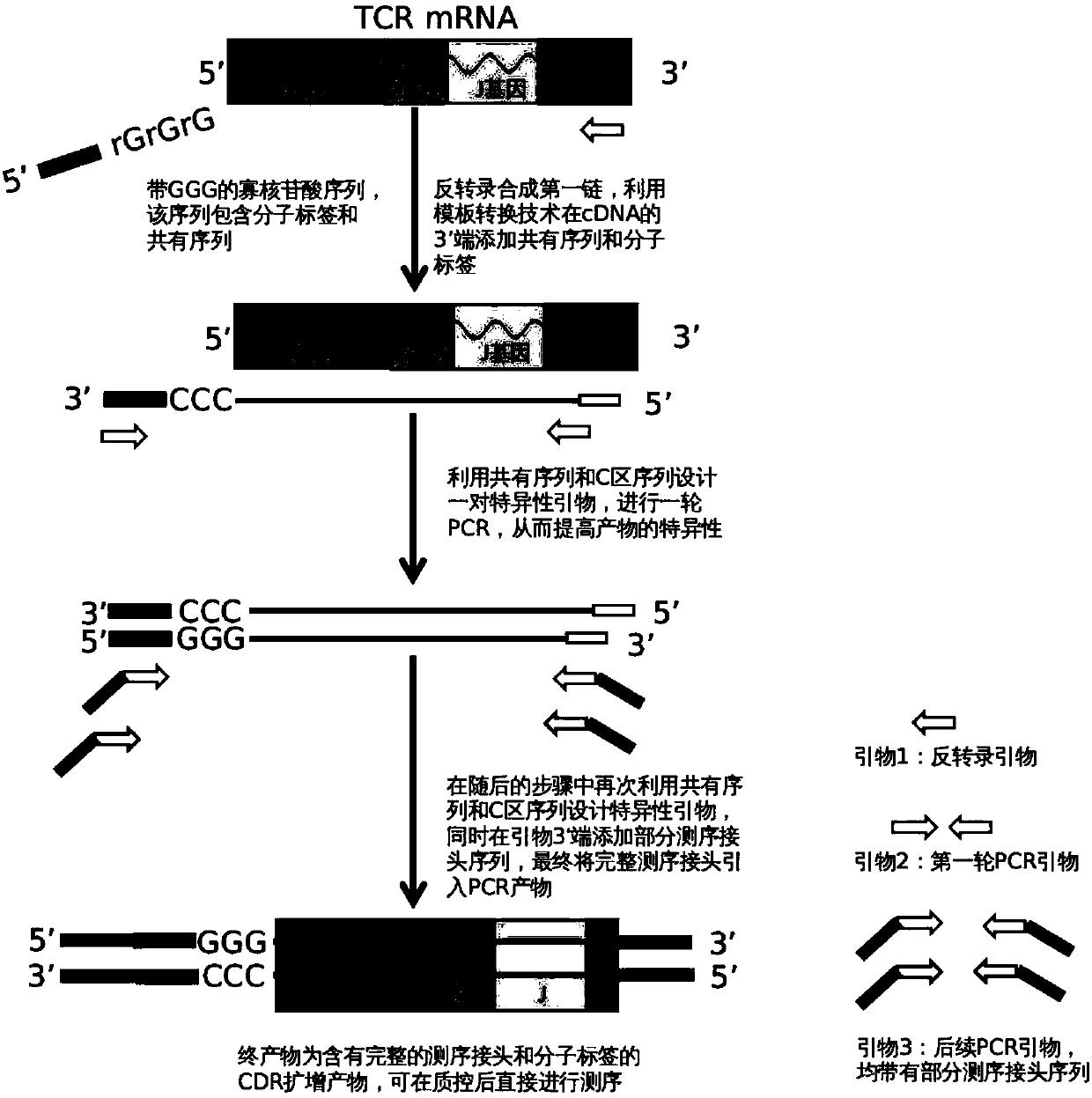
Construction method of T cell antigen receptor diversity sequencing library and kit
ActiveCN107779495AImplement the buildCorrect mistakesMicrobiological testing/measurementLibrary creationSequence designT-Cell Antigen Receptors
The invention relates to a construction method of a T cell antigen receptor diversity sequencing library and a kit. The construction method provided by the invention comprises the following steps: amplifying regions V, D and J of a TRB gene expressed by a human or mouse T lymphocyte by utilizing a designed specific primer specific to a consensus sequence of a region C of the human or mouse TRB gene, synchronously introducing a next generation sequencing joint sequence into an amplified product, and directly sequencing the regions V, D and J of the TRB gene.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
New application of acyl coenzyme A:cholesterol acyltransferase ACAT1 inhibitor
ActiveCN106955353AInhibitory activityEnhance antigen-specific immune responseAntibody ingredientsAntineoplastic agentsEffect functionImmunocompetence
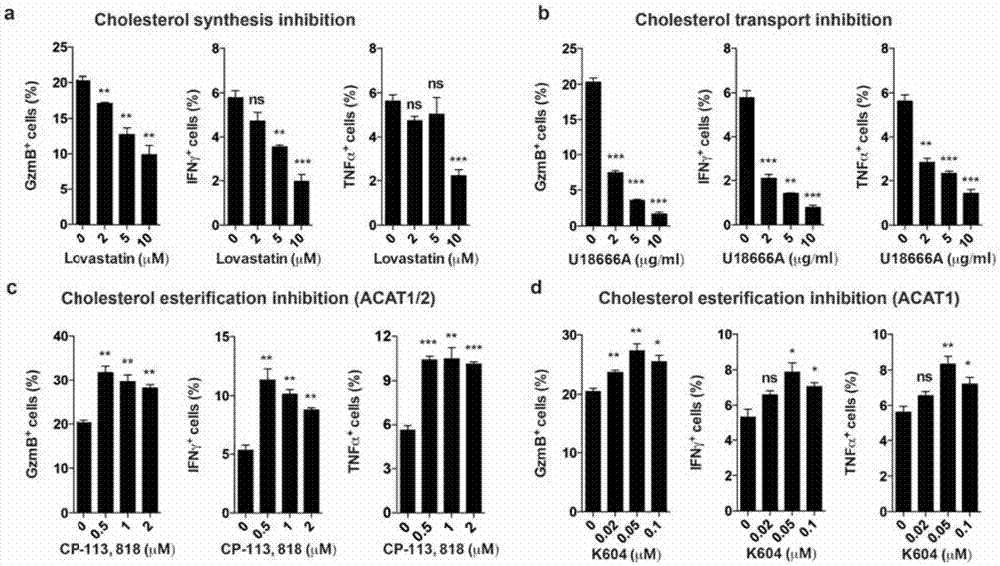
The invention provides a new application of an acyl coenzyme A:cholesterol acyltransferase ACAT1 inhibitor. The invention discloses the acyl coenzyme A:cholesterol acyltransferase ACAT1 inhibitor. The acyl coenzyme A:cholesterol acyltransferase ACAT1 inhibitor disclosed by the invention has the advantages that the immune response capacity of CD8 T cells can be enhanced; the killing activity of the CD8 T cells for tumors can be enhanced; the effect function of the CD8 T cells can be enhanced; the multiplication of the CD8 T cells can be promoted; the apoptosis of the CD8 T cells can be reduced; the clustering of T-cell antigen receptors (TCR) on plasma membranes of the CD8 T cells can be promoted; the directional release of cytotoxic particles from the CD8 T cells can be promoted; and the formation of immunological synapses of the CD8 T cells can be promoted. The acyl coenzyme A:cholesterol acyltransferase ACAT1 inhibitor can be used for immunotherapy of the tumors or improving the immunocompetence to the tumors.
Owner:CENT FOR EXCELLENCE IN MOLECULAR CELL SCI CHINESE ACAD OF SCI
Generating a mature NKT cell from a reprogrammed somatic cell with a T-cell antigen receptor α-chain region rearranged to uniform Va-Ja in a NKT-cell specific way
Provided are an iPS cell derived from a somatic cell such as an NKT cell, having the α-chain region of the T cell antigen receptor gene rearranged to uniform Vα-Jα in an NKT cell receptor-specific way, NKT cells differentiated from the iPS cell, a method of creating the same, and an immune cell therapy agent prepared using cells differentiated from the iPS cell. Also provided are an iPS cell having TCRα rearranged to NKT-TCR (NKT-iPS cell), obtained by contacting a somatic cell, such as an NKT cell, having the α-chain region of the T cell antigen receptor gene rearranged to uniform Vα-Jα in an NKT cell receptor-specific way, with nuclear reprogramming factors, isolated NKT cells obtained by differentiating the iPS cell ex vivo (iPS-NKT cell), a method of generating CD4 / CD8-double positive NKT cells (DP-NKT cells) and mature NKT cells from NKT-iPS cells by altering the combination of feeder cells and / or cytokines, a method of expanding the iPS-NKT cells, and an NKT cell cytotherapy agent comprising NKT cells activated with α-galactosyl ceramide (α-GalCer), or iPS-NKT cells, and α-GalCer in combination.
Owner:RIKEN
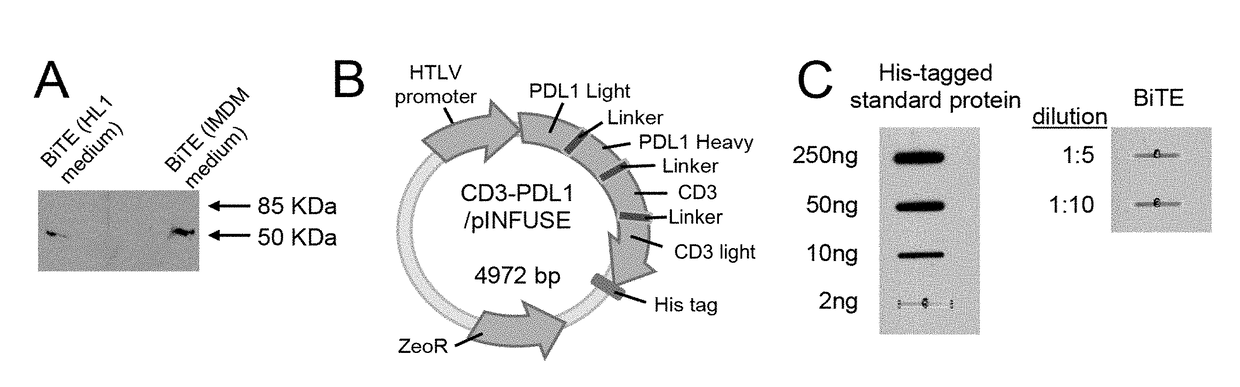
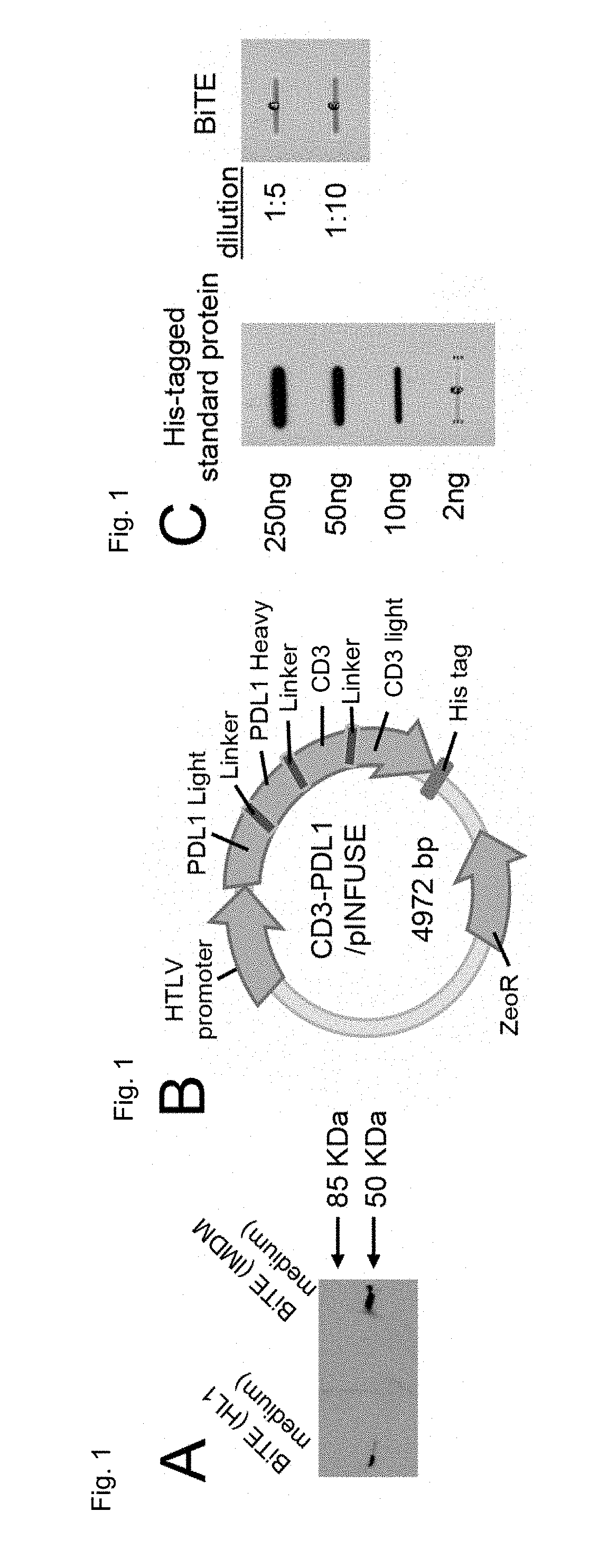
Recombinant bi-specific polypeptide for coordinately activating tumor-reactive t-cells and neutralizing immune suppression
PendingUS20180291114A1Reduce capacityReducing growth and killingHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsCancer cellCellular antigens
The present invention relates generally to the field of generating fusion proteins to be used in cancer therapy, and more specifically, a bispecific T-Cell engager recombinant polypeptide comprising an antibody, fragment thereof, or single chain variable fragment that binds to CD3 of a T-cell antigen receptor and an antibody, fragment thereof, or single chain variable fragment that binds to Programmed Death Ligand 1 (PDL1) on a cancerous tumor cell to counteract the immune tolerance of cancer cells.
Owner:UNIV OF MARYLAND BALTIMORE COUNTY
Inhibition of TCR Signaling with Peptide Variants
ActiveUS20130039948A1Current is limitedIn-vivo radioactive preparationsVirus peptidesADAMTS ProteinsT cell
The present invention provides compositions comprising peptides derived from amino acid sequences (or from combinations thereof) of fusion and other protein regions of various viruses, including but not limited to, severe acute respiratory syndrome coronavirus, herpesvirus saimiri, human herpesvirus 6, Lassa virus, lymphocytic choriomeningitis virus, Mopeia virus, Tacaribe virus, Friend murine leukemia virus; human T lymphotropic virus type 1; herpesvirus ateles; Marburg virus; Sudan Ebola virus; Zaire Ebola virus, and comprising L- and / or D-amino acids and combinations thereof, which affect T cells by acting on the T cell antigen receptor (TCR). More specifically, the peptides act on the TCRαβ-CD3δε-CD3γε-ζζ signaling complex. Yet more specifically, the peptides act on the TCRα / CD3δε / ζζ signaling module of TCR. The present invention further relates to the prevention and therapy of various T cell-related disease states involving the use of these compositions. Specifically, the compositions are useful in the treatment and / or prevention of a disease or condition where T cells are involved or recruited. The compositions of the present invention also are useful in the production of medical devices comprising peptide matrices (for example, medical implants and implantable devices).
Owner:SIGNABLOK
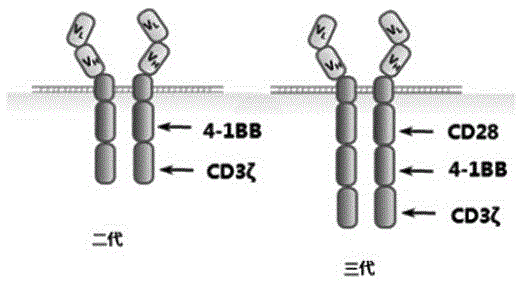
General CAR-T cell with enhanced function and preparation method and application thereof
ActiveCN109652378AHigh activityFunction increaseImmunoglobulin superfamilyGenetically modified cellsT-Cell Antigen ReceptorsT cell
The invention provides a CAR-T cell and a preparation method. The CAR-T cell restrains a T cell antigen receptor (TCR), a major histocompatibility complex (MHCI) and an immunosuppression molecule related gene. The CAR-T cell expresses a chimeric antigen receptor (CAR), does not express the TCR, MHCI and an immunosuppression molecule PD1, is suitable for a heterogenous receptor, has generality, andmeanwhile has higher killability.
Owner:GUANGZHOU BIO GENE TECH CO LTD
Novel human t-cell population
InactiveUS20090297490A1Reduce rejectionBiocideArtificial cell constructsT-Cell Antigen ReceptorsStromal cell
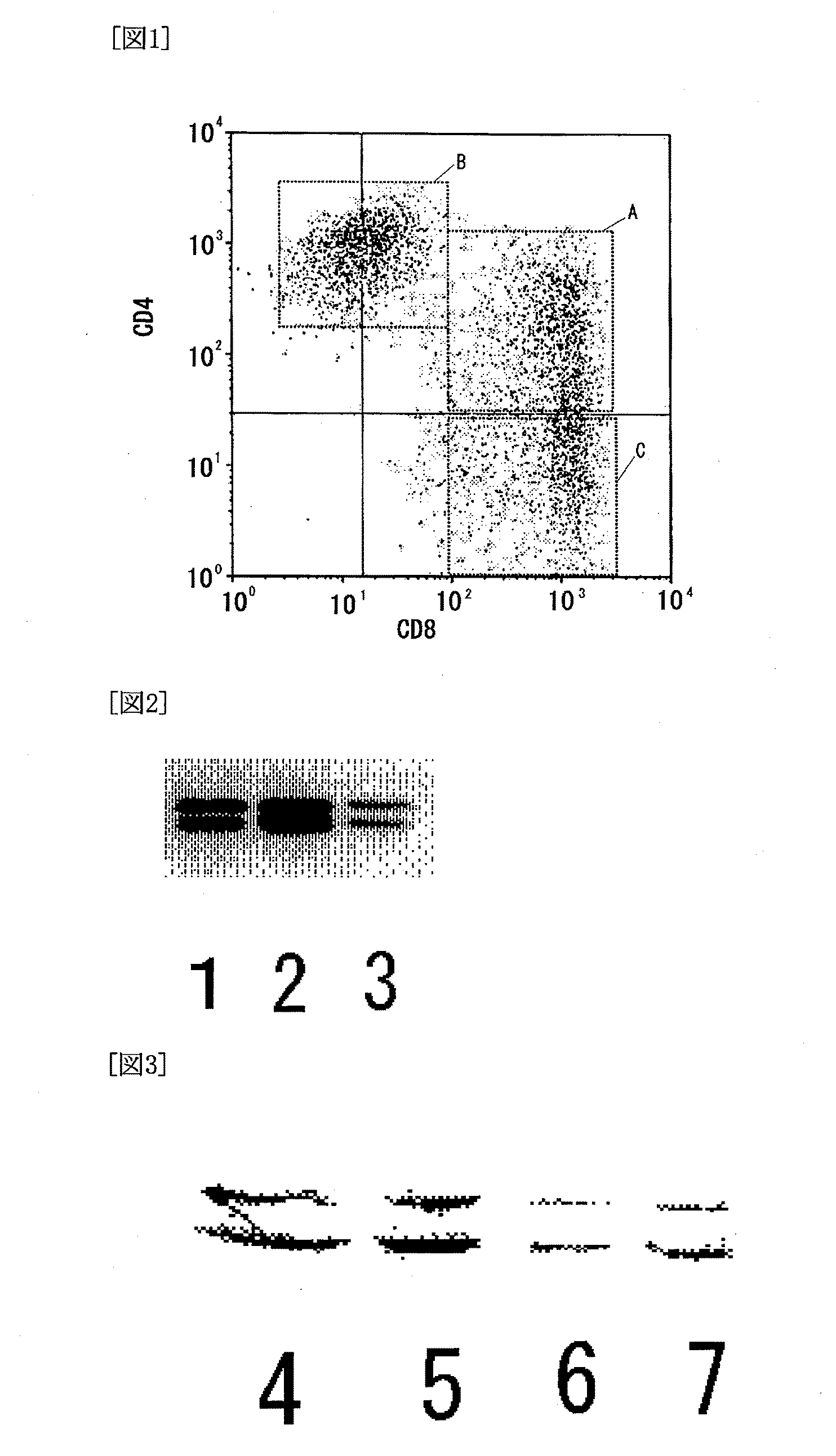
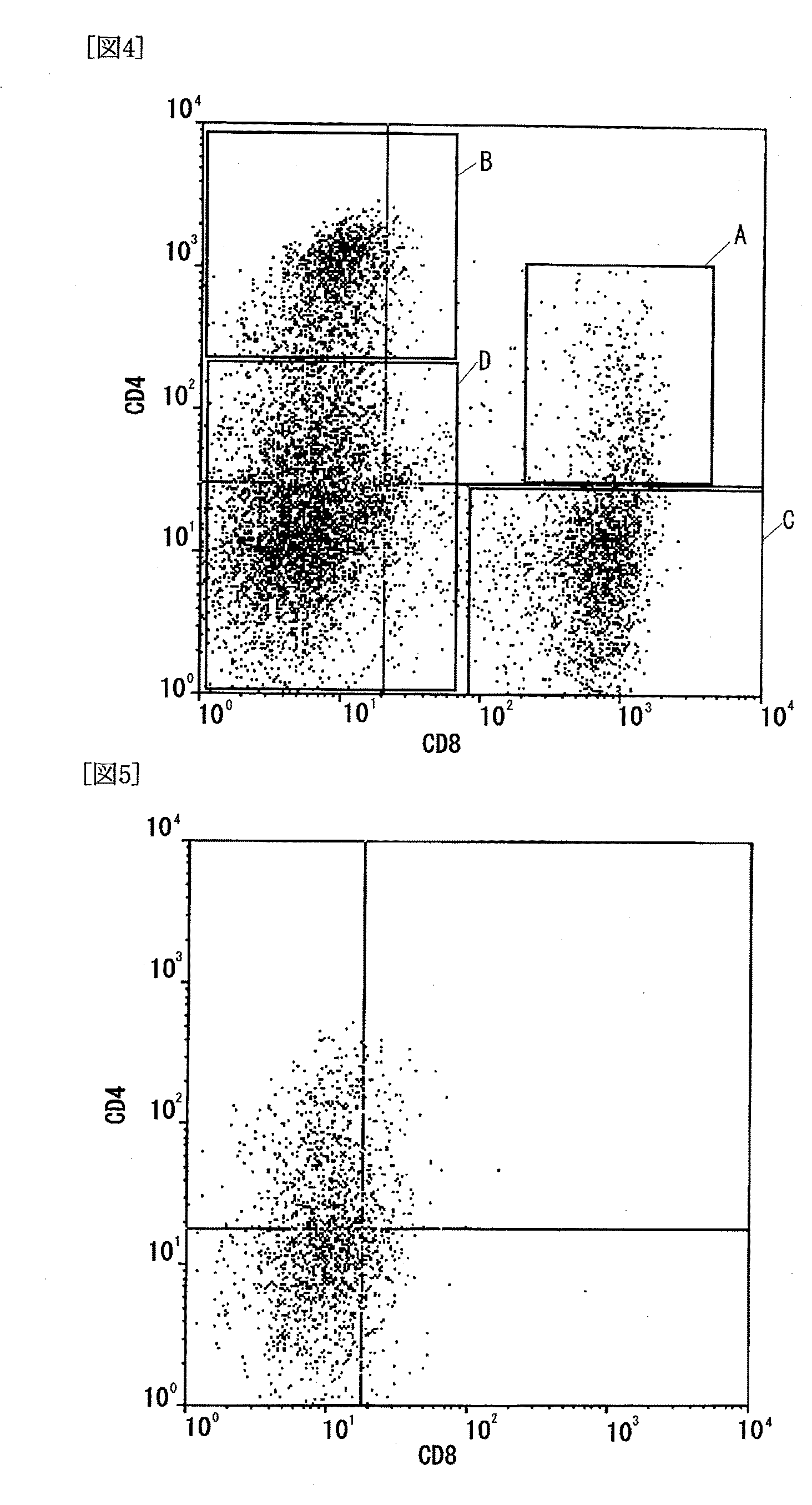
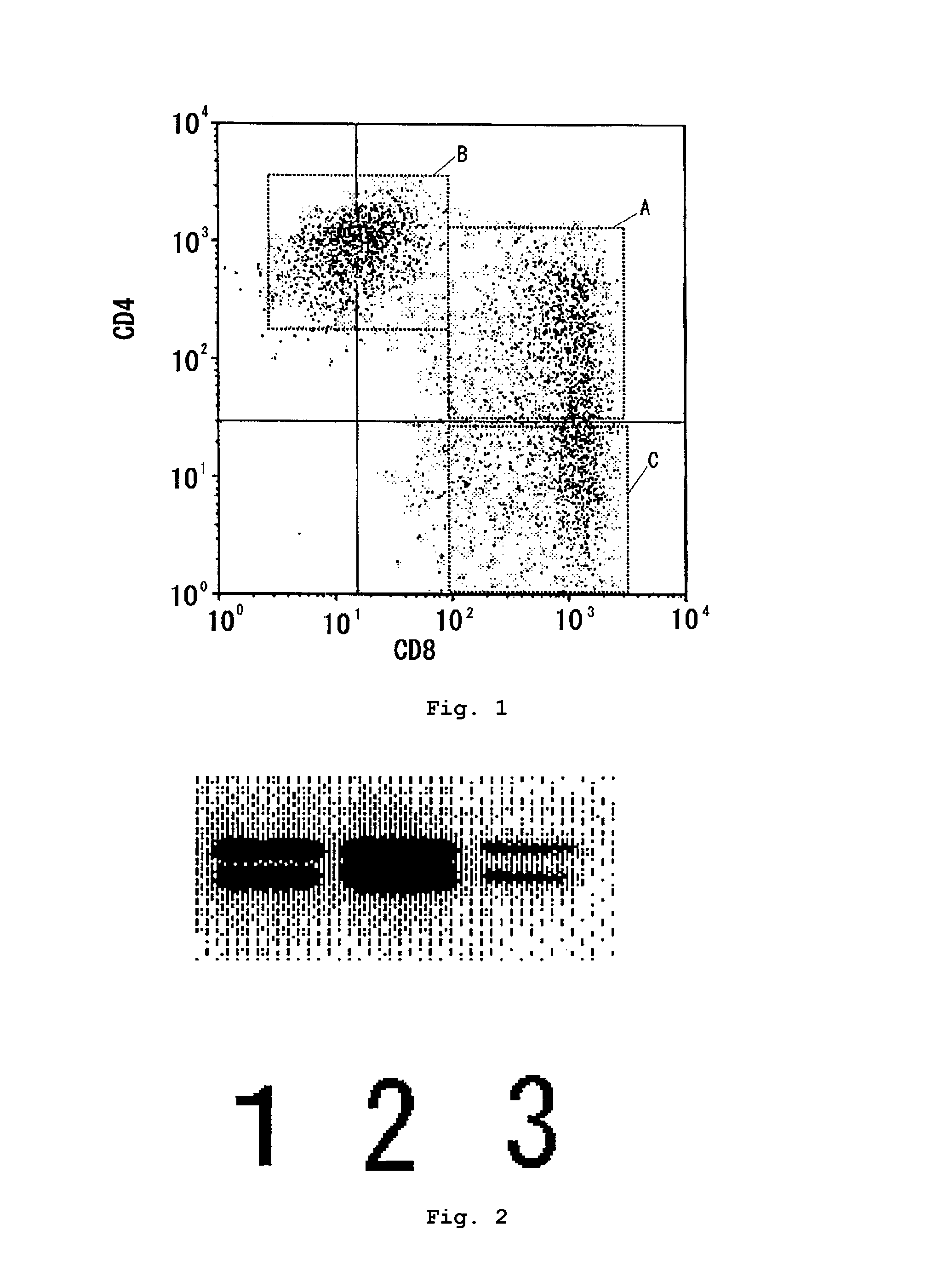
The present invention has objects to provide a novel human T-cell population having both cytotoxic and immunosuppressive activities, and to a method for preparing the same. The above objects are attained by providing a human T-cell population which is obtainable by coculturing mononuclear cells, collected from human blood, with stroma cells and which has the following features:(1) being positive for CD3, CD25, CD28 and T-cell antigen receptor αβ;(2) essentially consisting of three groups of a CD4 positive and CD8 positive (CD4+CD8−) T-cell group, a CD4 positive and CD8 dimly positive (CD4+CD8dim) T-cell group, and a CD4 negative and CD8 positive (CD4−CD8+) T-cell group;(3) exerting a cytotoxic activity against the cocultured stroma cells; and(4) exerting an immunosuppressive activity against activated T cells.
Owner:HAYASHIBARA CO LTD
Human T-cell population
InactiveUS8883495B2Reduce rejectionBiocideMammal material medical ingredientsT-Cell Antigen ReceptorsStromal cell
The present invention has objects to provide a novel human T-cell population having both cytotoxic and immunosuppressive activities, and to a method for preparing the same. The above objects are attained by providing a human T-cell population which is obtainable by coculturing mononuclear cells, collected from human blood, with stroma cells and which has the following features:(1) being positive for CD3, CD25, CD28 and T-cell antigen receptor αβ;(2) essentially consisting of three groups of a CD4 positive and CD8 positive (CD4+CD8+) T-cell group, a CD4 positive and CD8 dimly positive (CD4+CD8dim) T-cell group, and a CD4 negative and CD8 positive (CD4−CD8+) T-cell group;(3) exerting a cytotoxic activity against the cocultured stroma cells; and(4) exerting an immunosuppressive activity against activated T cells.
Owner:HAYASHIBARA BIOCHEMICAL LAB INC
Method to measure a t cell response and its uses to qualify antigen-presenting cells
A method to characterize a T-cell response of a final population of T-lymphocytes resulting from the co-incubation of an initial population of T lymphocytes with a composition of antigen-presenting cells (APCs), said method comprising the steps of a) simultaneous measuring on a single cell basis at least two parameters: (i) proliferation of T lymphocytes and (ii) presence of a T cell antigen receptor on the surface of T lymphocytes and / or presence of at least one biological molecule produced by T lymphocytes, and the attribution of a positive or a negative value to each of these parameters, and b) classification of the final T-lymphocytes population into 2n different subsets of T lymphocytes, n being the number of parameters, each subset being characterized by a positive or a negative value respectively to each parameter and the determination of the proportion of T lymphocytes present in each subset with respect to the number of T lymphocytes in the final population, with said proportion being characteristic of the T-cell response. Use of the method for batch release assay and potency assay.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE +1
Methods for inhibition of polyclonal b cell activation and immunoglobulin class switching to pathogenic autoantibodies by blocking cd1-mediated interactions
InactiveUS20110038860A1Slow onsetReduces level of serum IgGBiocidePeptide/protein ingredientsAntigen receptorT-Cell Antigen Receptors
Pathogenic polyclonal B cell activation and immunoglobulin class switching to pathogenic autoantibodies is inhibited by binding molecules that specifically interfere with CD1 antigen, but do not activate signaling (blocking agents), or by molecules that bind to the T cell antigen receptor on T cells that recognize CD1. When CD1 mediated signaling is thus blocked, the T cell response is diminished, resulting in reduced polyclonal B cell activation and reduced immunoglobulin class switching to pathogenic autoantibodies.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Method for simultaneously sequencing multi-sample CDR3 (complementary determining region 3) receptor library with high flux
InactiveCN102443624BHigh-throughput sequencing cost reductionMicrobiological testing/measurementComplementarity determining regionHigh flux
The invention discloses a method for simultaneously sequencing a multi-sample CDR3 (complementary determining region 3) receptor library with a high flux, comprising the following steps of: only designing one set of V-gene family upstream primers in the variable region of a TCR (T cell antigen receptor) or BCR (B cell antigen receptor) chain according to the homology of the V-gene family, then designing C or J gene characteristic downstream primers of the TCR or BCR chain as many as samples and suitable for being paired with the upstream primers, amplifying each sample by one characteristic downstream primer and the same set of upstream primers to obtain a CDR3 region of the TCR or BCR, then mixing all the amplified samples to be a to-be-sequenced sample library, finally sequencing, wherein the characteristic downstream primers are designed by different ATCG basic group compositions. The CDR3 sequences of multiple samples can be simultaneously sequenced by operating one high-flux sequencing program via the method for simultaneously sequencing a multi-sample CDR3 receptor library with a high flux disclosed by the invention, so that the high-flux sequencing cost is greatly decreased. Thus, a high-flux sequencing technology has a wide application value in a sequencing research of a CDR3 receptor library.
Owner:ZUNYI MEDICAL UNIVERSITY
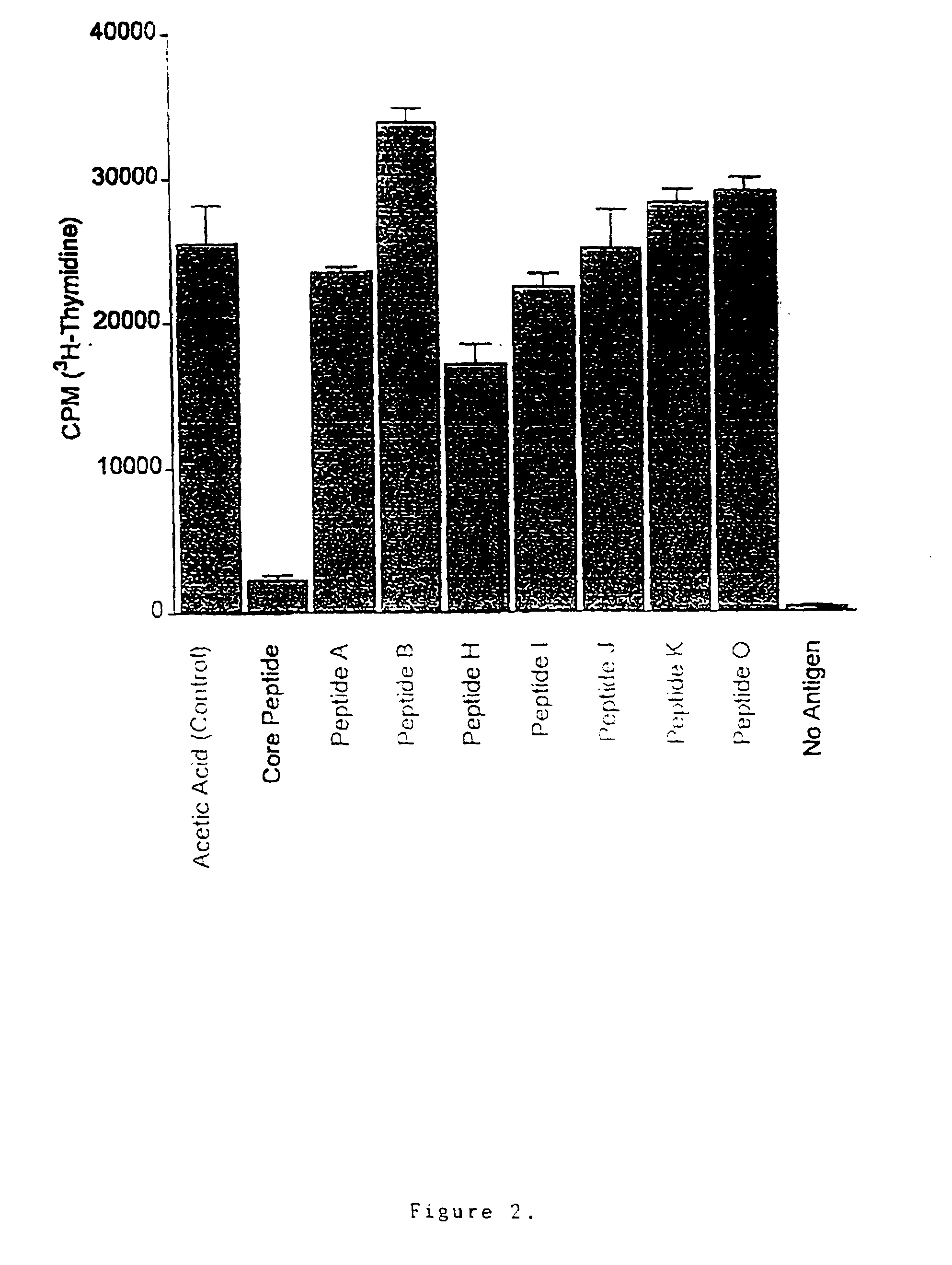
T cell antigen receptor peptides
InactiveUS20050070478A1Reduce inflammationFunctional disturbanceImmunoglobulin superfamilyPeptide/protein ingredientsDiseaseT-Cell Antigen Receptors
The present invention provides peptides which affect T-cells, presumably by action on the T-cell antigen receptor. The present invention further relates to the therapy of various inflammatory and autoimmune disease states involving the use of these peptides. Specifically, the peptides are useful in the treatment of disorders where T-cells are involved or recruited. In one aspect the peptides have the formula:- R1-A-B-A-R2 in which A is a hydrophobic amino acid or a hydrophobic peptide sequence comprising between 2 and 10 amino acids B is a charged amino acid R1 is NH2 and R2 is COOH In another aspect the peptides have the formula: R1-A-B-C-R2 in which A is a peptide sequence of between 0 and 5 amino acids; B is cysteine; C is a peptide sequence of between 2 to 10 amino acids; R1 is NH2; and R2 is COOH.
Owner:NOVOZYMES BIOPHARMA DK AS +1
Tuberculosis infection T cell immune tetramer detection method
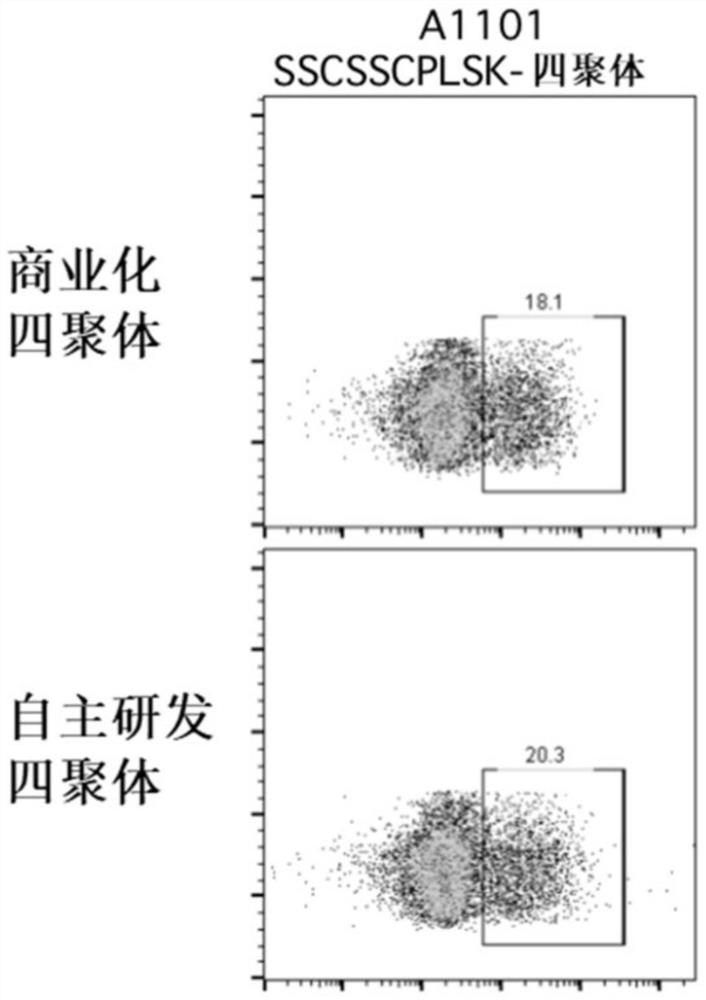
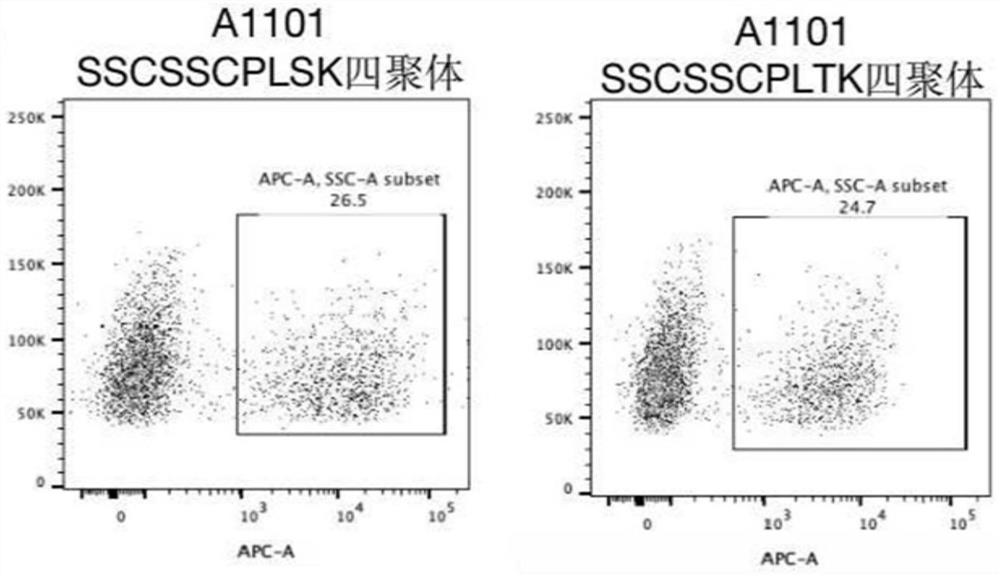
InactiveCN104422766AReduce non-specificityNo damageDisease diagnosisPeripheral blood mononuclear cellT-Cell Antigen Receptors
The invention discloses a tuberculosis infection T cell immune tetramer detection method, peripheral blood mononuclear cells are separated from a whole blood sample, the peripheral blood mononuclear cells are incubated together with mycobacterium tuberculosis specific antigen, when effector T lymphocytes exist, TCR (T cell antigen receptor) in the mycobacterium tuberculosis specific antigen and MHC (major histocompability complex)-polypeptide compound are combined, with the aid of biotin-streptavidin system, MHC-polypeptide compound forms a tetramer molecule, single MHC-polypeptide tetramer molecule is combined with a plurality of TCR on the T cell surface to improve the MHC-polypeptide compound and TCR affinity and stability; a biotin labeled antibody mark detection PE (poly ethylene) fluorescent dye is added, a flow cytometer is used to count specific T cells for auxiliary diagnosis of tuberculosis, and the tuberculosis infection T cell immune tetramer detection method has the advantages of high in detection sensitivity, accuracy and product specificity, easy and simple application, safety and reliablility.
Owner:SHANGHAI YINWEI IND CO LTD
T cell antigen receptor peptides
InactiveUS7192928B1Reduce inflammationFunctional disturbanceOrganic active ingredientsPeptide/protein ingredientsDiseaseT-Cell Antigen Receptors
The present invention provides peptides which affect T-cells, presumably by action on the T-cell antigen receptor. The present invention further relates to the therapy of various inflammatory and autoimmune disease states involving the use of these peptides. Specifically, the peptides are useful in the treatment of disorders where T-cells are involved or recruited. In one aspect the peptides have the formula: R1-A—B—A—R2 in which A is a hydrophobic amino acid or a hydrophobic peptide sequence comprising between 2 and 10 amino acids; B is a charged amino acid; R1 is NH2 and R2 is COOH. In another aspect the peptides have the formula: R1-A—B—C—R2 in which A is a peptide sequence of between 0 and 5 amino acids; B is cysteine; C is a peptide sequence of between 2 to 10 amino acids; R1 is NH2; and R2 is COOH.
Owner:NOVOZYMES BIOPHARMA DK AS +1
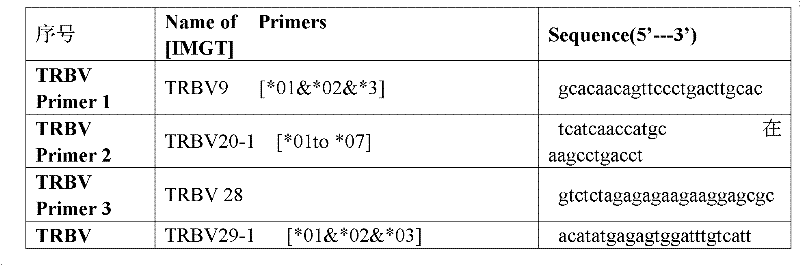
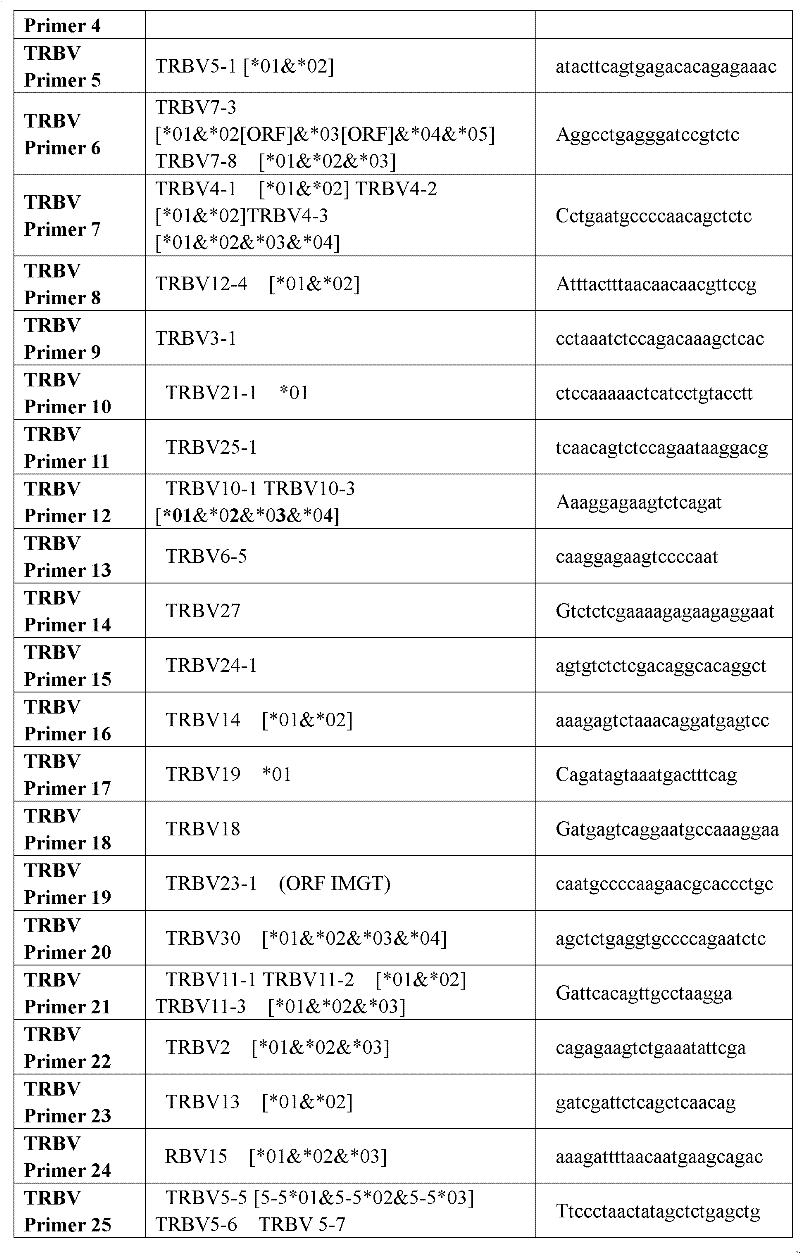
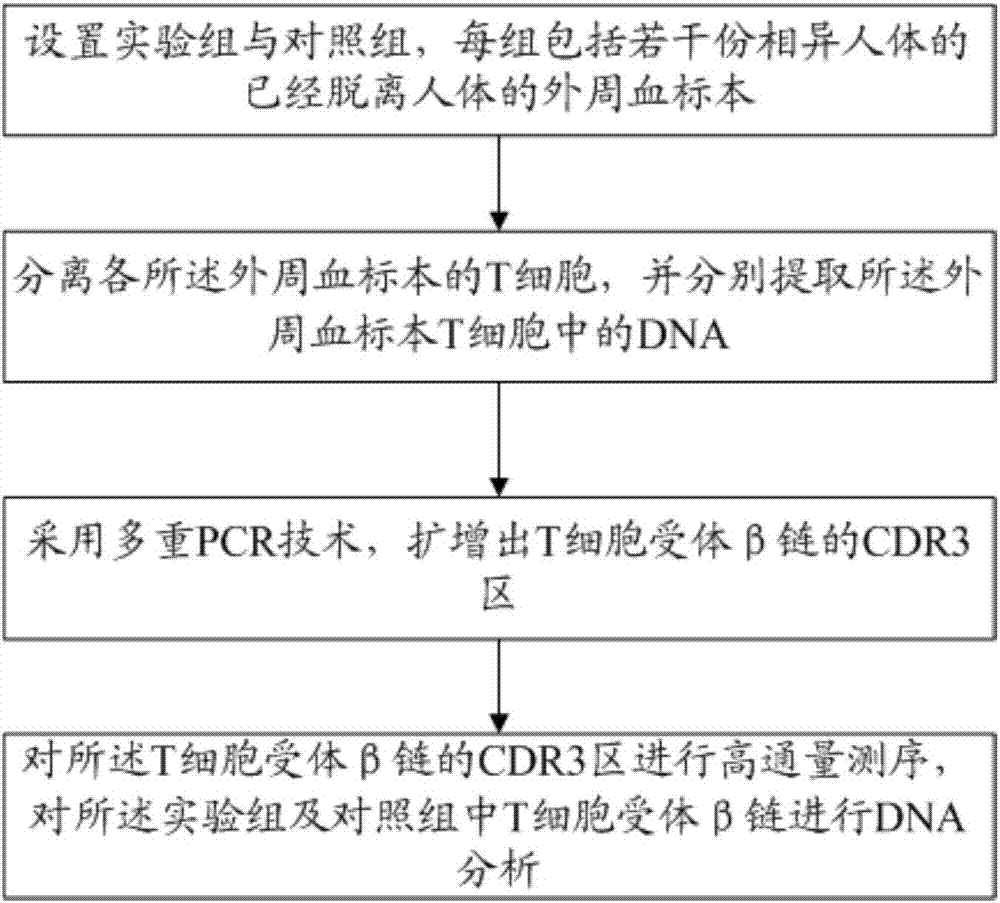
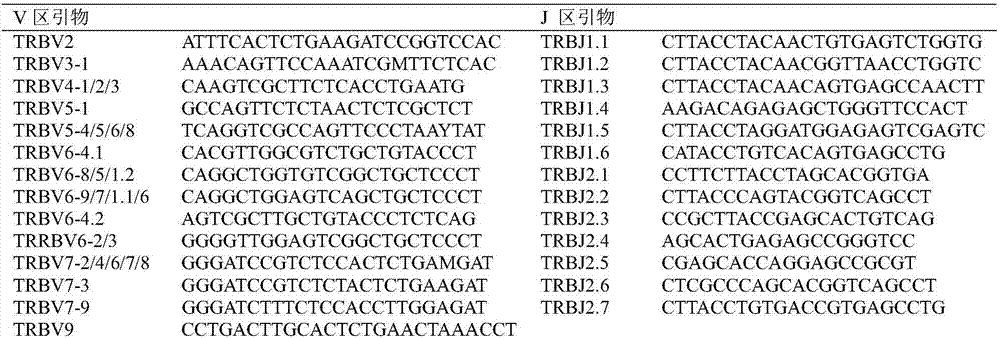
Method for treating complementary determining regions 3 (CDRs3) of T cell antigen receptor beta chains
InactiveCN107345240AImprove short termHigh activityMicrobiological testing/measurementImmunosuppressive drugComplementarity determining region
The invention relates to a method for treating complementary determining regions 3 (CDRs3) of T cell antigen receptor beta chains. The method comprises the following steps: setting an experimental group and a control group, wherein each group comprises a plurality of parts of peripheral blood samples which are dissimilar to a human body and get away from the human body; separating T cells of the peripheral blood samples, and respectively extracting deoxyribonucleic acid (DNA) in the T cells of the peripheral blood samples; adopting a multiplex polymerase chain reaction (PCR) technique to amplify the CDRs3 of the T cell antigen receptor beta chains; carrying out high-throughput sequencing on the CDRs3 of the T cell antigen receptor beta chains, and carrying out DNA analysis on the T cell antigen receptor beta chains in the experimental group and the control group. The method for treating the CDRs3 of the T cell antigen receptor beta chains is reasonable and feasible in design, and can be used for effectively acquiring information, taken as intermediate results, about renal transplant recipients at the perioperative period and further verifying, analyzing and researching the information so as to help to study the pathogenesis of a rejection reaction and guide the individual rational application of immunosuppressive drugs, thus being beneficial to increment of short-term and long-term survival rate of renal transplant recipients after transplantation.
Owner:眭维国 +2
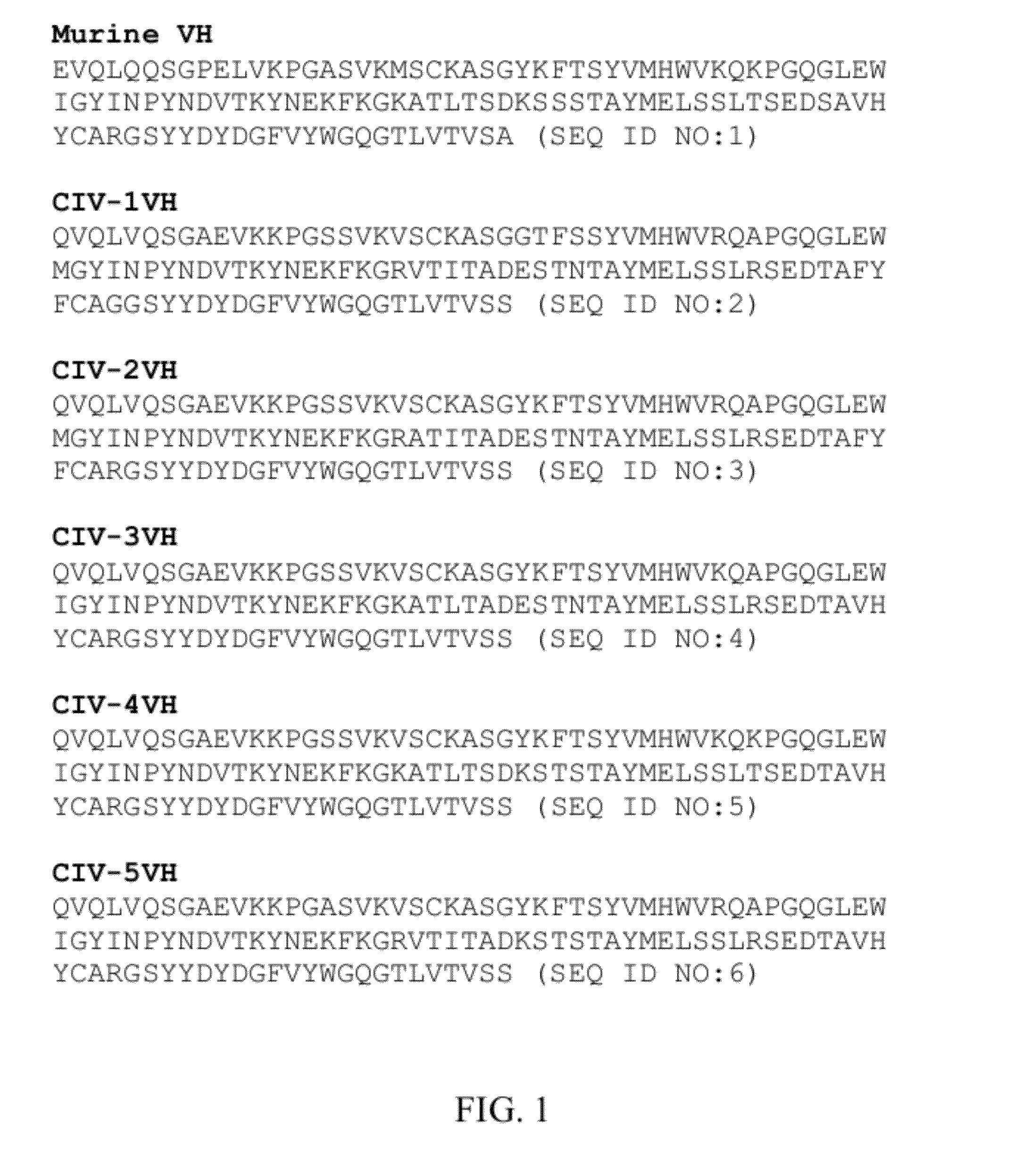
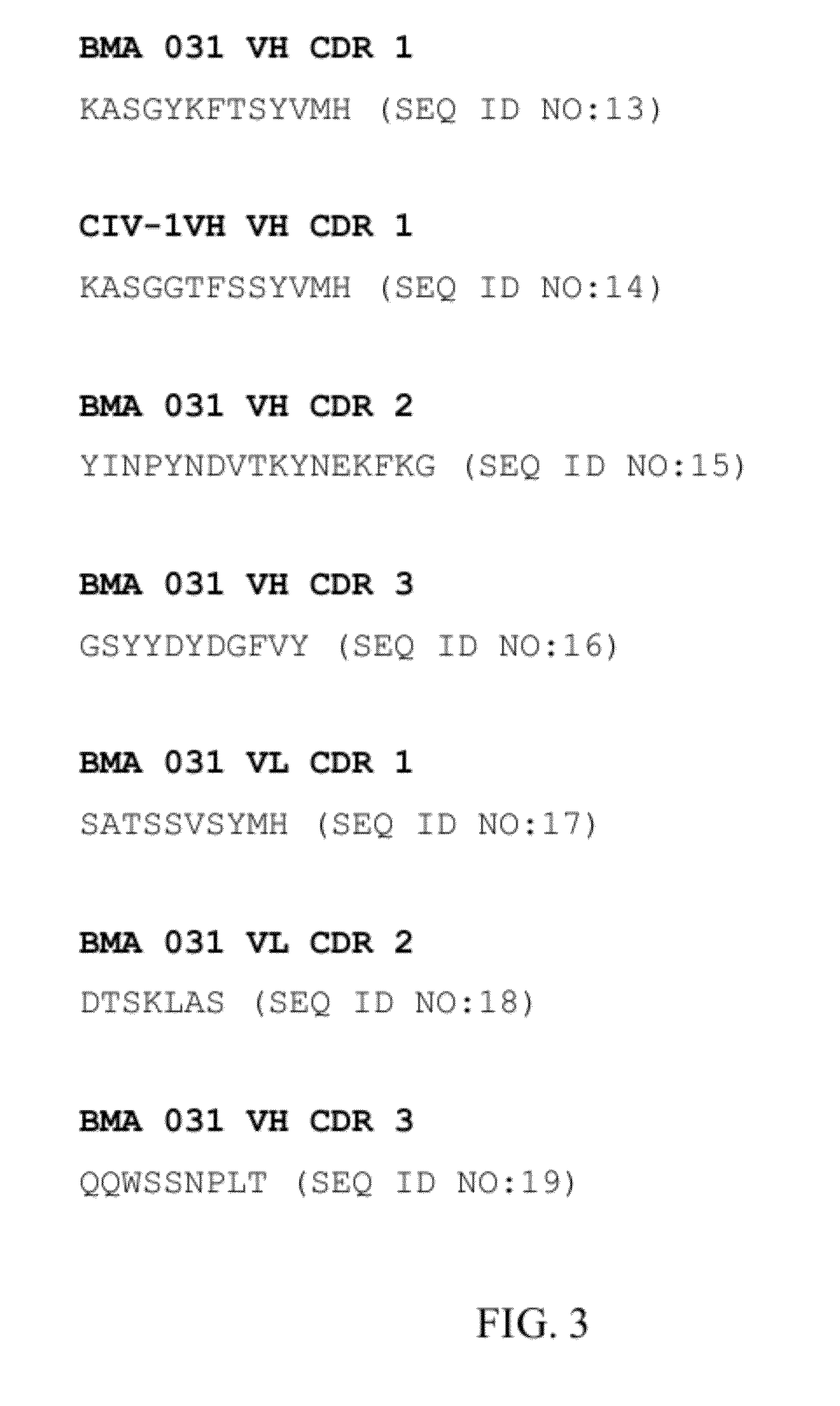
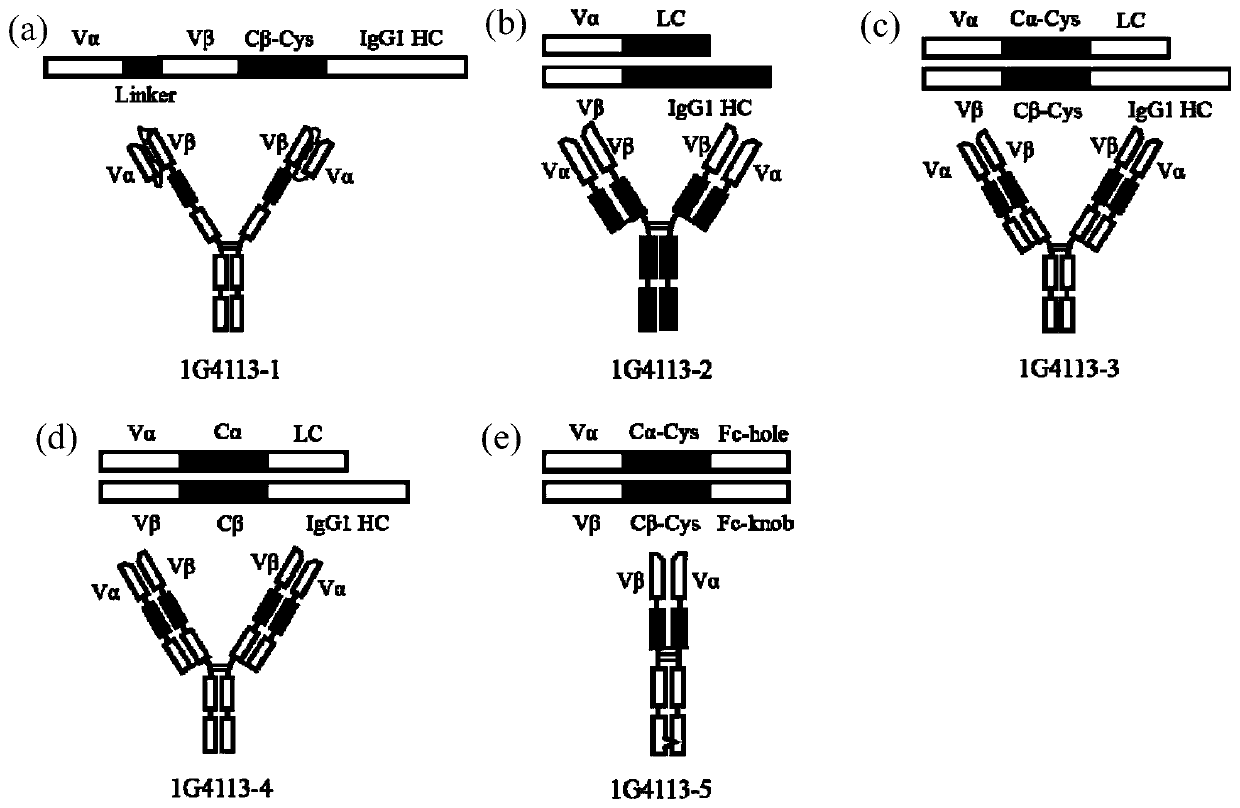
Recombinant antibody-like T cell antigen receptor, T cell antigen receptor conjugate, bi-specific molecule and application
ActiveCN110317275AHigh purityImmunoglobulin superfamilyAntibody mimetics/scaffoldsAntigen receptorT-Cell Antigen Receptors
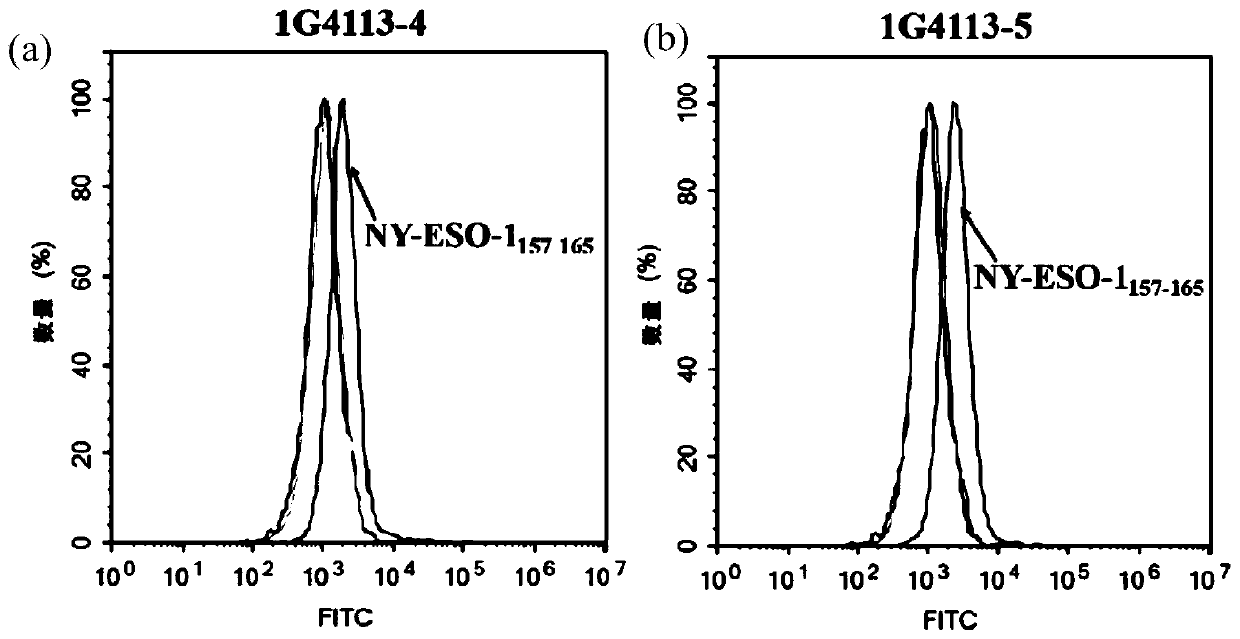
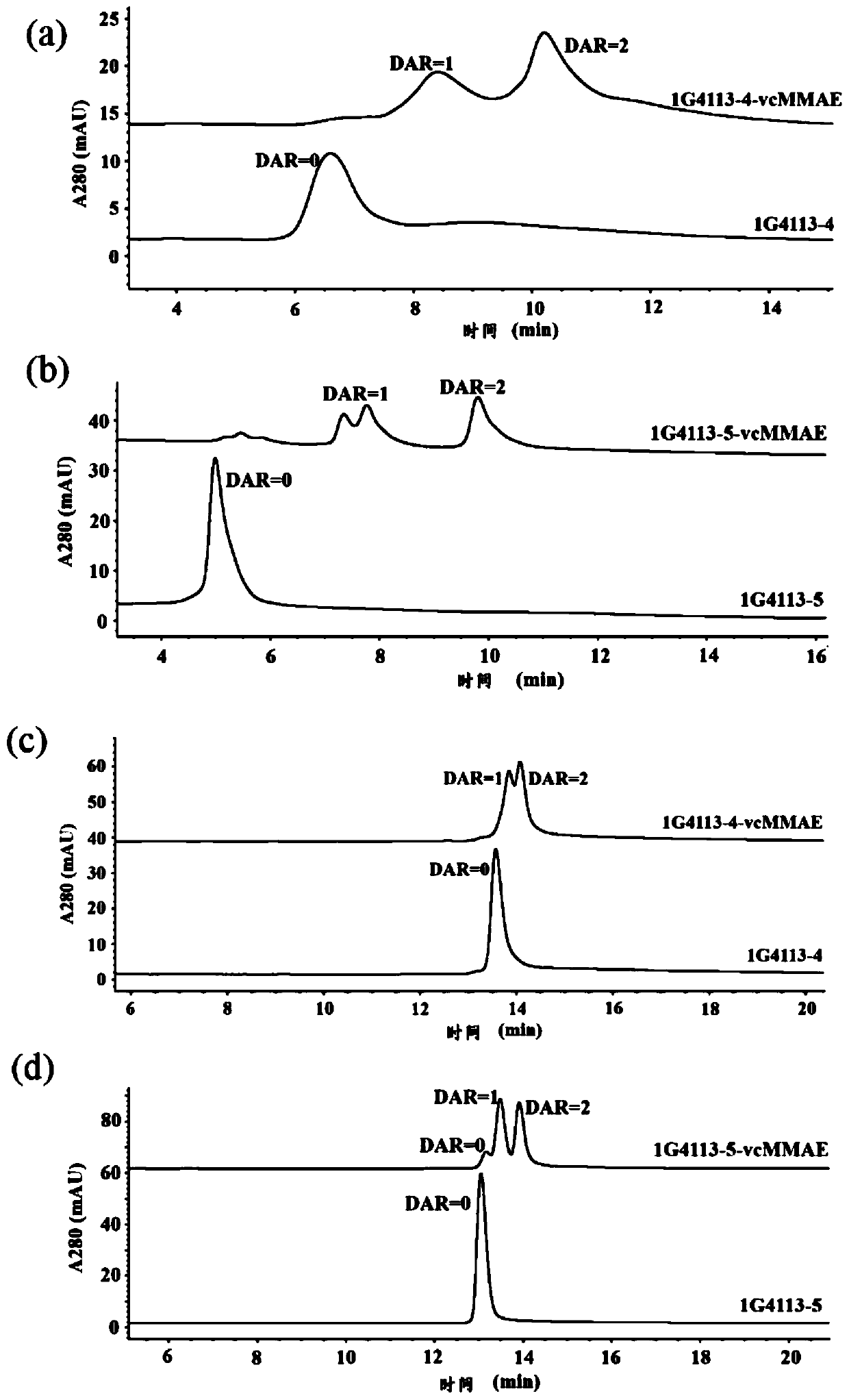
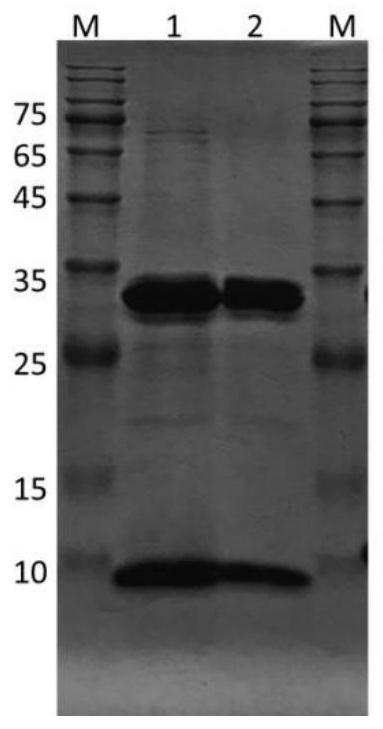
The invention discloses a recombinant antibody-like T cell antigen receptor, a T cell antigen receptor conjugate, a bi-specific molecule and application. By designing various IgG1 constant region and1G4113 fusion expression forms, the fusion protein form that soluble expression can be conducted in mammalian cells is obtained through screening, and the high-purity recombinant antibody-like T cellantigen receptor can be obtained simply by one-step purification. The recombinant antibody-like T cell antigen receptor is coupled with MMAE micromolecules to obtain the T cell antigen receptor conjugate, and the T cell antigen receptor conjugate can be used for being prepared into an anti-tumor drug. The recombinant antibody-like T cell antigen receptor is coupled with fluorescent dye molecules so as to be used for in-vivo positioning of the drug or tumor imaging. The recombinant antibody-like T cell antigen receptor is coupled with an anti-CD3 antibody to obtain the soluble expression bi-specific molecule, and the bi-specific molecule can be used for being prepared into the anti-tumor drug.
Owner:ZHEJIANG UNIV
T cell antigen receptor, polymer complex thereof, and preparation methods and applications of T cell antigen receptor and polymer complex thereof
PendingCN113621071AAntiviralsMammal material medical ingredientsCellular antigensT-Cell Antigen Receptors
The invention provides a T cell antigen receptor, an immune cell for expressing the T cell antigen receptor (TCR), and preparation methods and applications of the T cell antigen receptor and the immune cell. The TCR provided by the invention can specifically recognize a corresponding pMHC complex and activate TCR-T cells, so that high-level cell factors IFN[gamma], IL2 and TNF[alpha] are generated, target cells are remarkably killed, and the service life of a tumor-loaded mouse is prolonged.
Owner:CHINA IMMUNOTECH BEIJING BIOTECH CO LTD +1
T cell antigen receptor gene and application thereof
ActiveCN105821064APolypeptide with localisation/targeting motifImmunoglobulin superfamilyT-Cell Antigen ReceptorsFunctional activity
Owner:河北格尔泰生物科技有限公司
T cell antigen receptor peptides
InactiveUS20100267651A1Reduce biological activityHigh activityAntipyreticAnalgesicsAutoimmune conditionCysteine thiolate
The present invention provides peptides which affect T-cells, presumably by action on the T-cell antigen receptor. The present invention further relates to the therapy of various inflammatory and autoimmune disease states involving the use of these peptides. Specifically, the peptides are useful in the treatment of disorders where T-cells are involved or recruited. In one aspect the peptides have the formula:R1-A-B-A-R2 in whichA is a hydrophobic amino acid or a hydrophobic peptide sequence comprising between 2 and 10 amino acidsB is a charged amino acidR1 is NH2 andR2 is COOHIn another aspect the peptides have the formula:R1-A-B-C—R2 in whichA is a peptide sequence of between 0 and 5 amino acids;B is cysteine;C is a peptide sequence of between 2 to 10 amino acids;R1 is NH2; andR2 is COOH.
Owner:NOVOZYMES BIOPHARMA DK AS
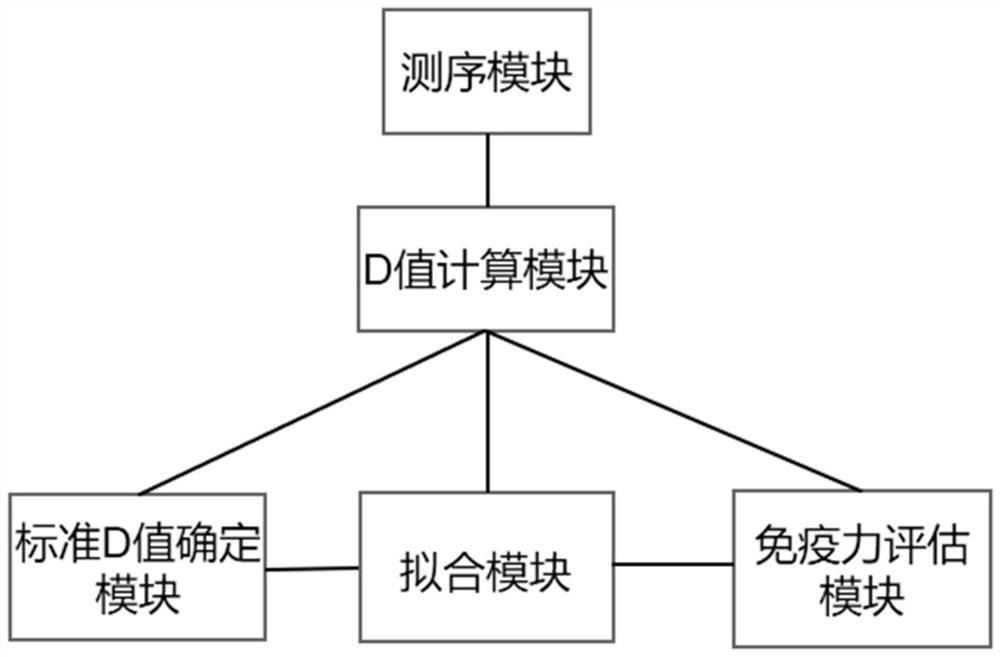
Method, device and equipment for evaluating immunity level and storage medium
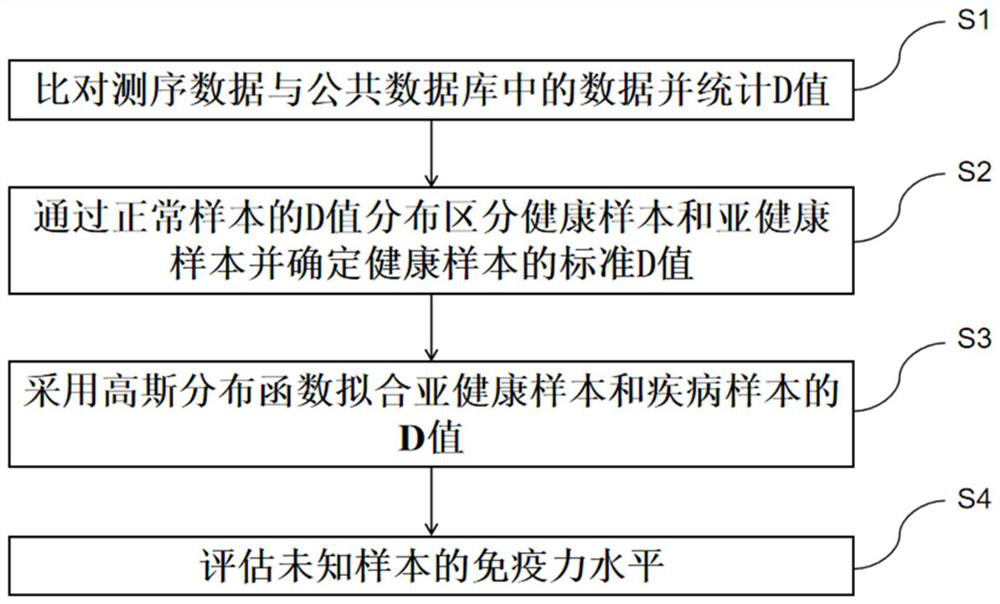
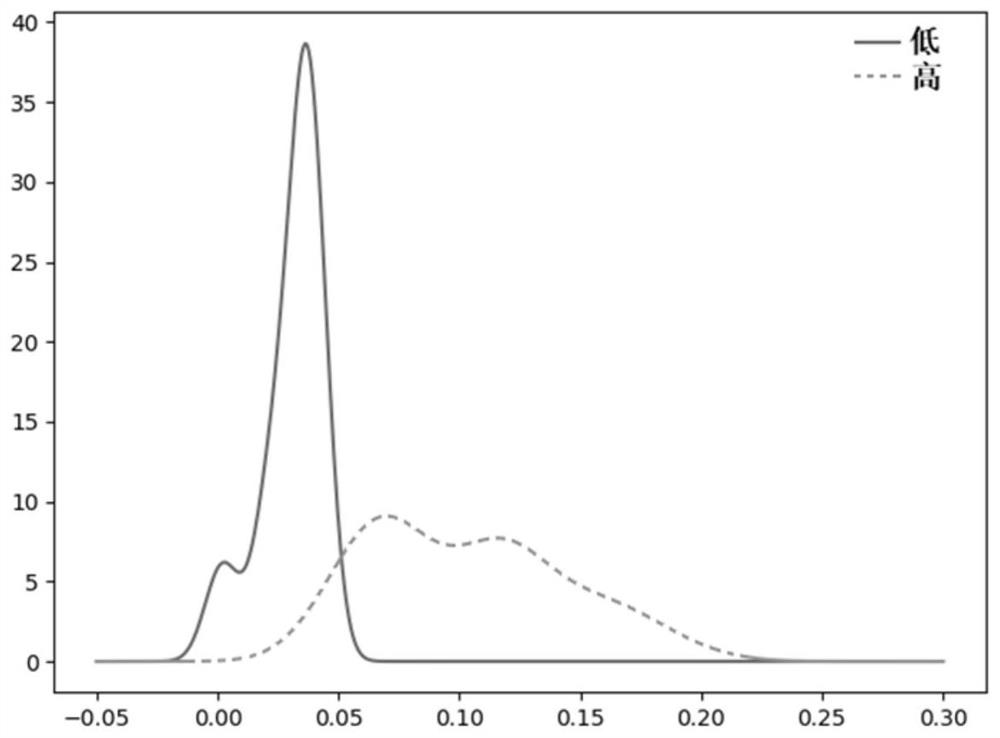
PendingCN113241177AEasy to classifyAccurate classificationHealth-index calculationMedical automated diagnosisDiseaseCellular antigens
The invention provides a method, device and equipment for evaluating the immunity level and a storage medium. The method comprises the following steps: (1) collecting sequencing data of a B cell antigen receptor variable region and / or a T cell antigen receptor variable region of a lymphocyte sample, comparing the sequencing data with data in a public database, and calculating a D value; (2) distinguishing healthy samples and sub-healthy samples according to the distribution diagram of the D values, fitting the D values of the healthy samples by adopting multiple Gaussian distribution, and determining a standard D value; (3) fitting the D values of the sub-health sample and the disease sample by adopting a Gaussian distribution function to obtain a Gaussian distribution function A and a Gaussian distribution function B; (4) evaluating the immunity level of the unknown sample according to the standard D value, the Gaussian distribution function A and the Gaussian distribution function B. According to the method, the sequencing result is analyzed and subjected to multiple Gaussian distribution fitting, and the effect of accurately, comprehensively and efficiently evaluating the immunity of the sample is achieved.
Owner:SHANGHAI BIOTECAN PHARMA +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com