Patents
Literature
1055 results about "T-cell Antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
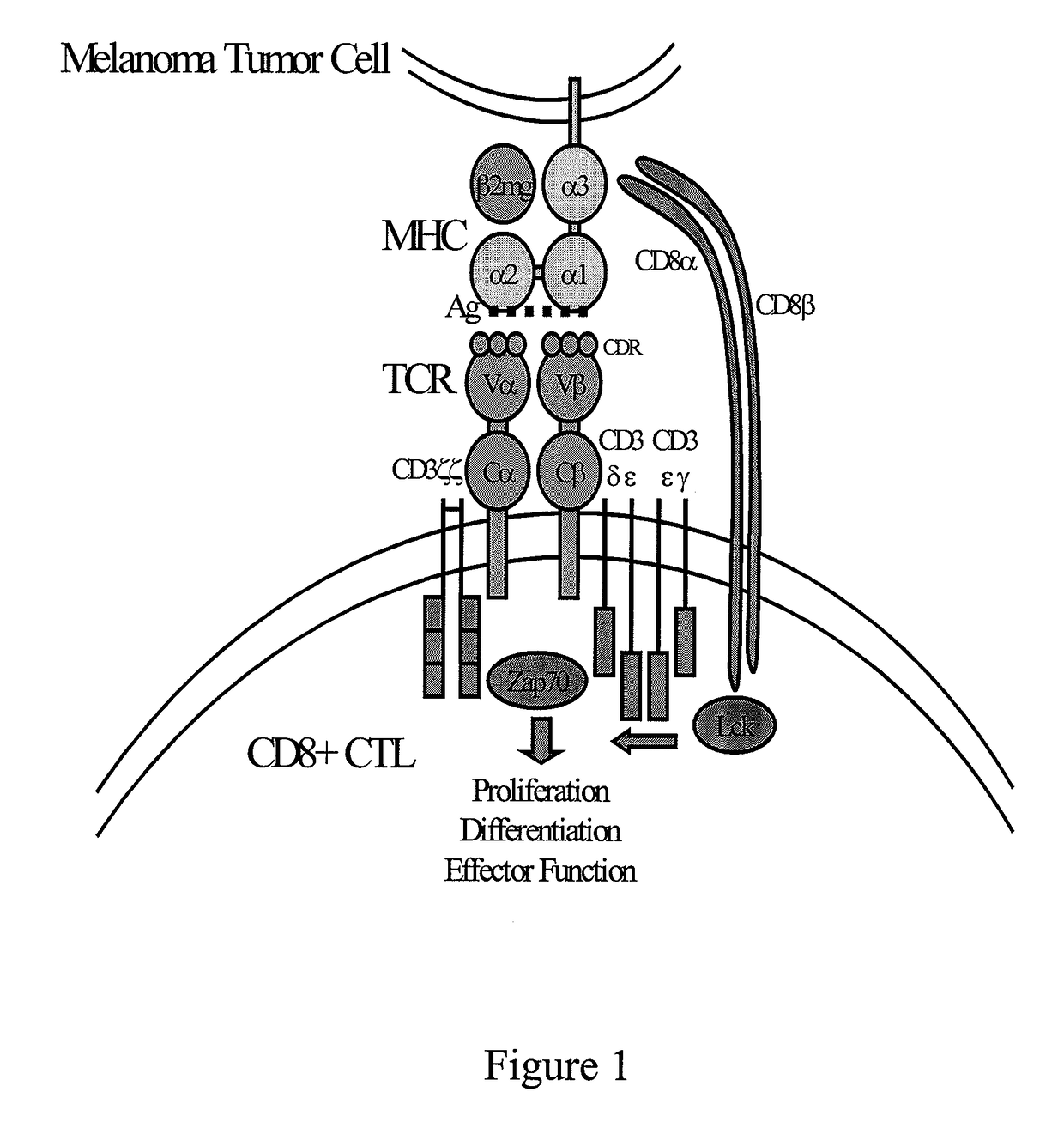
Antigen presentation stimulates T cells to become either "cytotoxic" CD8+ cells or "helper" CD4+ cells. The T-cell receptor, or TCR, is a molecule found on the surface of T cells, or T lymphocytes, that is responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules.
Methods for selectively stimulating proliferation of T cells
InactiveUS6905681B1Increase the number ofVirusesPeptide/protein ingredientsAccessory moleculeExogenous growth
Owner:GENETICS INST INC +2
Methods for selectively stimulating proliferation of T cells
InactiveUS6887466B2Expanding population of cellIncrease the number ofVirusesPeptide/protein ingredientsAccessory moleculeExogenous growth
Methods for inducing a population of T cells to proliferate by activating the population of T cells and stimulating an accessory molecule on the surface of the T cells with a ligand which binds the accessory molecule are described. T cell proliferation occurs in the absence of exogenous growth factors or accessory cells. T cell activation is accomplished by stimulating the T cell receptor (TCR) / CD3 complex or the CD2 surface protein. To induce proliferation of an activated population T cells, an accessory molecule on the surface of the T cells, such as CD28, is stimulated with a ligand which binds the accessory molecule. The T cell population expanded by the method of the invention can be genetically transduced and used for immunotherapy or can be used in methods of diagnosis.
Owner:GENETICS INST INC +2
Methods for selectively stimulating proliferation of T cells
InactiveUS7175843B2Increase the number ofBiocideCell receptors/surface-antigens/surface-determinantsAccessory moleculeExogenous growth
Owner:GENETICS INST LLC +2
Methods of treating HIV infected subjects
InactiveUS6905680B2Expanding population of cellIncrease the number ofVirusesPeptide/protein ingredientsAccessory moleculeExogenous growth
Methods for inducing a population of T cells to proliferate by activating the population of T cells and stimulating an accessory molecule on the surface of the T cells with a ligand which binds the accessory molecule are described. T cell proliferation occurs in the absence of exogenous growth factors or accessory cells. T cell activation is accomplished by stimulating the T cell receptor (TCR) / CD3 complex or the CD2 surface protein. To induce proliferation of an activated population T cells, an accessory molecule on the surface of the T cells, such as CD28, is stimulated with a ligand which binds the accessory molecule. The T cell population expanded by the method of the invention can be genetically transduced and used for immunotherapy or can be used in methods of diagnosis.
Owner:GENETICS INST INC +2
Methods for selectively stimulating proliferation of T cells
InactiveUS7144575B2Increase the number ofBiocidePeptide/protein ingredientsAccessory moleculeExogenous growth
Methods for inducing a population of T cells to proliferate by activating the population of T cells and stimulating an accessory molecule on the surface of the T cells with a ligand which binds the accessory molecule are described. T cell proliferation occurs in the absence of exogenous growth factors or accessory cells. T cell activation is accomplished by stimulating the T cell receptor (TCR) / CD3 complex or the CD2 surface protein. To induce proliferation of an activated population T cells, an accessory molecule on the surface of the T cells, such as CD28, is stimulated with a ligand which binds the accessory molecule. The T cell population expanded by the method of the invention can be genetically transduced and used for immunotherapy or can be used in methods of diagnosis.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY +2
Nucleic acids encoding chimeric T cell receptors
ActiveUS7446190B2Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsCytotoxicityBiological activation
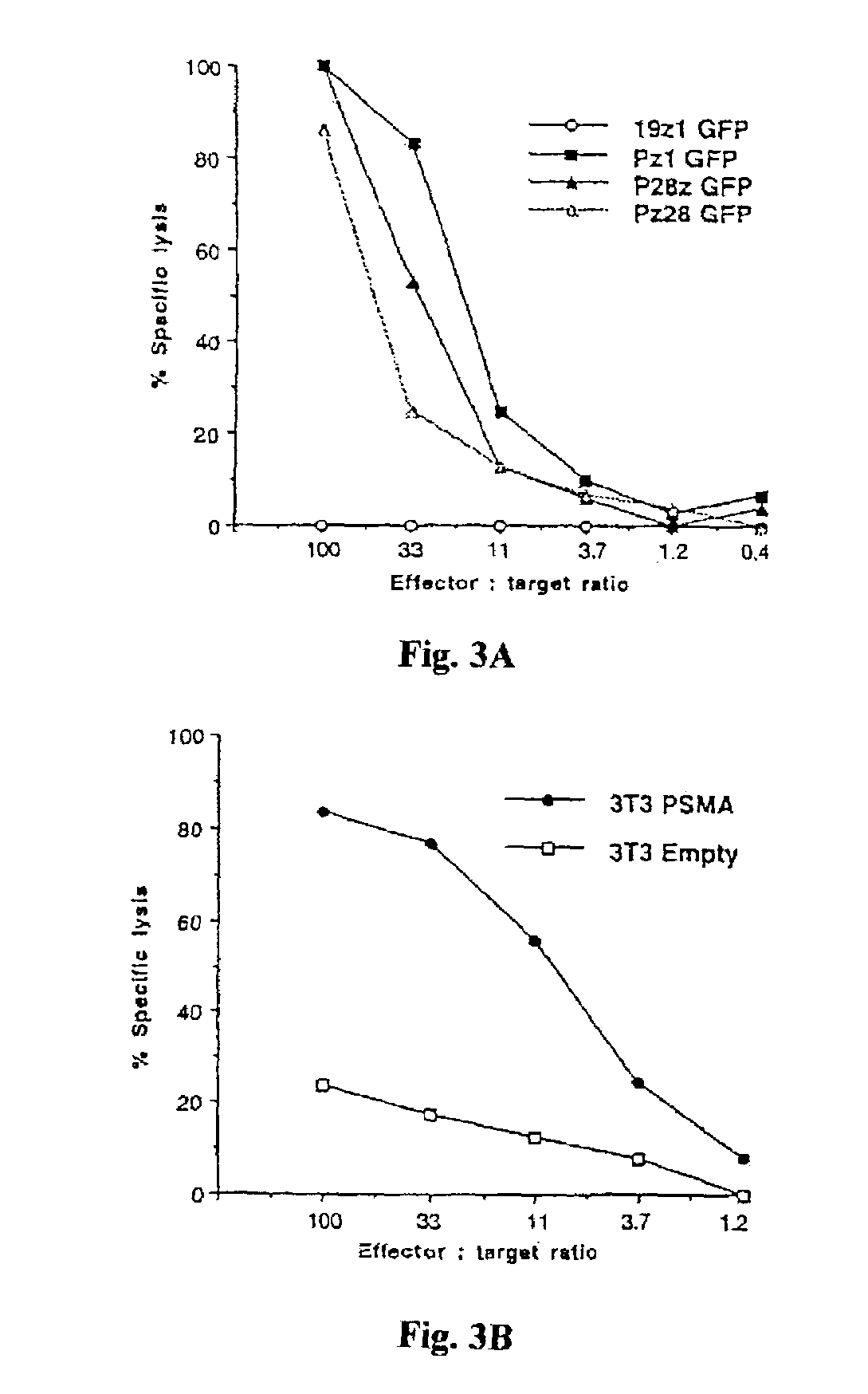
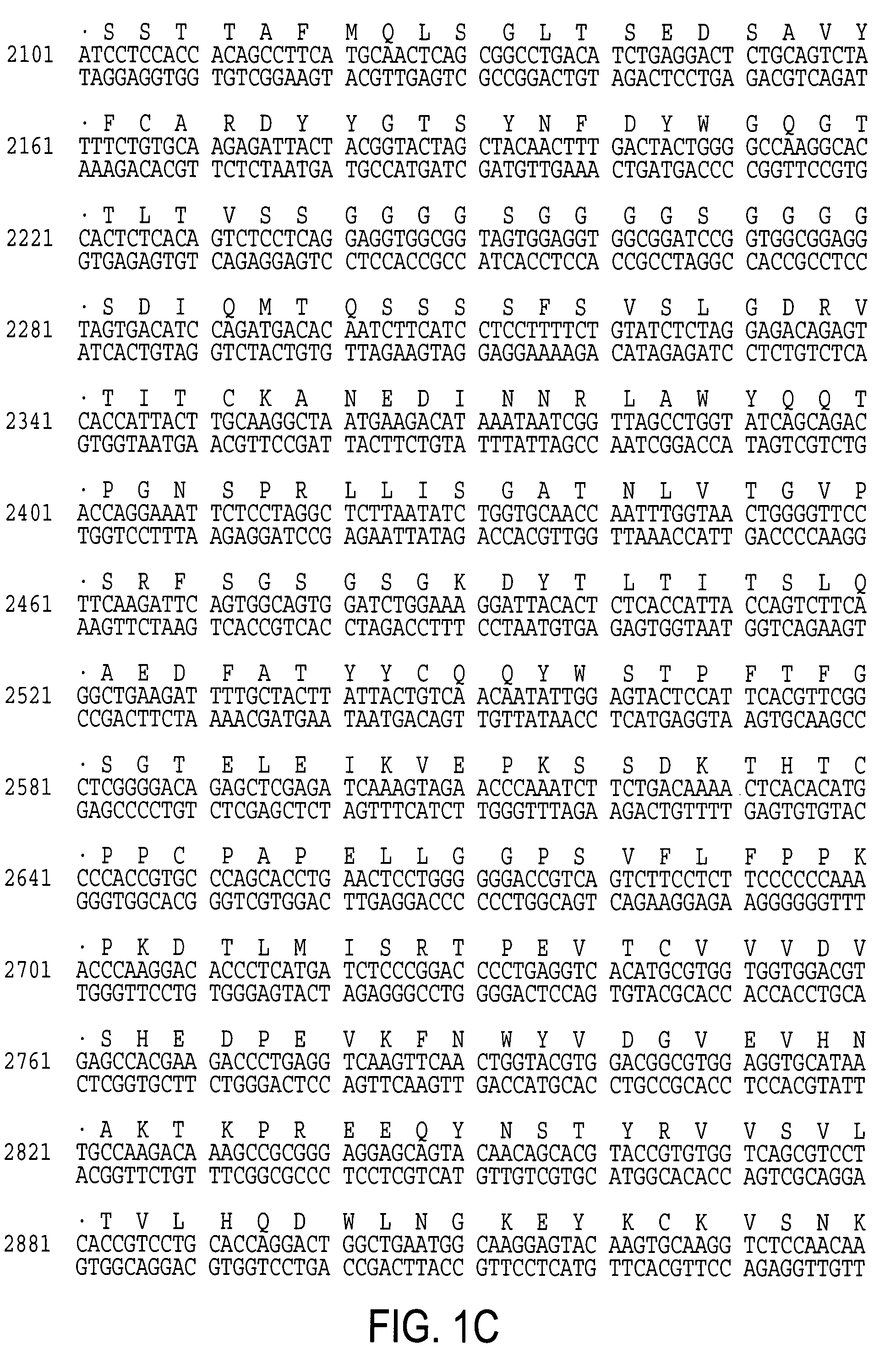
Chimeric T cell receptors (TCR) are provided that combine, in a single chimeric species, the intracellular domain of CD3 ζ-chain, a signaling region from a costimulatory protein such as CD28, and a binding element that specifically interacts with a selected target. When expressed, for example in T-lymphocytes from the individual to be treated for a condition associated with the selected target, a T cell immune response is stimulated in the individual to the target cells. The chimeric TCR's are able to provide both the activation and the co-stimulation signals from a single molecule to more effectively direct T-lymphocyte cytotoxicity against the selected target and T-lymphocyte proliferation.
Owner:SLOAN KETTERING INST FOR CANCER RES
CE7-specific redirected immune cells
Genetically engineered, CE7-specific redirected immune cells expressing a cell surface protein having an extracellular domain comprising a receptor which is specific for CE7, an intracellular signaling domain, and a transmembrane domain, and methods of use for such cells for cellular immunotherapy of CE7+ neuroblastoma are disclosed. In one embodiment, the immune cell is a T cell and the cell surface protein is a single chain FvFc:ζ receptor where Fv designates the VH and VL chains of a single chain monoclonal antibody to CE7 linked by peptide, Fc represents a hinge —CH2—CH3 region of a human IgG1, and ζ represents the intracellular signaling domain of the zeta chain of human CD3. DNA constructs encoding a chimeric T-cell receptor and a method of making a redirected T cell expressing a chimeric T cell receptor by electroporation using naked DNA encoding the receptor are also disclosed.
Owner:CITY OF HOPE
CD19-specific chimeric T cell receptor
InactiveUS7446179B2Peptide/protein ingredientsAntibody mimetics/scaffoldsIntracellular signallingTransmembrane domain
The present invention relates to a genetically engineered, CD19-specific chimeric T cell receptor and to immune cells expressing the chimeric receptor The present invention also relates to the use of such cells for cellular immunotherapy of CD9+ malignancies and for abrogating any untoward B cell function. The chimeric receptor is a single chain scFvFc:ζ receptor where scFvFc designates the extracellular domain, scFv designates the VH and VL chains of a single chain monoclonal antibody to CD19, Fc represents at least part of a constant region of an IgG1, and ζ represents the intracellular signaling domain of the zeta chain of human CD3. The extracellular domain scFvFc and the intracellular domain ζ are linked by a transmembrane domain such as the transmembrane domain of CD4. In one aspect, the chimeric receptor comprises amino acids 23-634 of SEQ I DNO:2. The present invention further relates to a method of making a redirected T cell expressing a chimeric T cell receptor by electroporation using naked DNA encoding the receptor.
Owner:CITY OF HOPE
Human Antibodies to PD-1
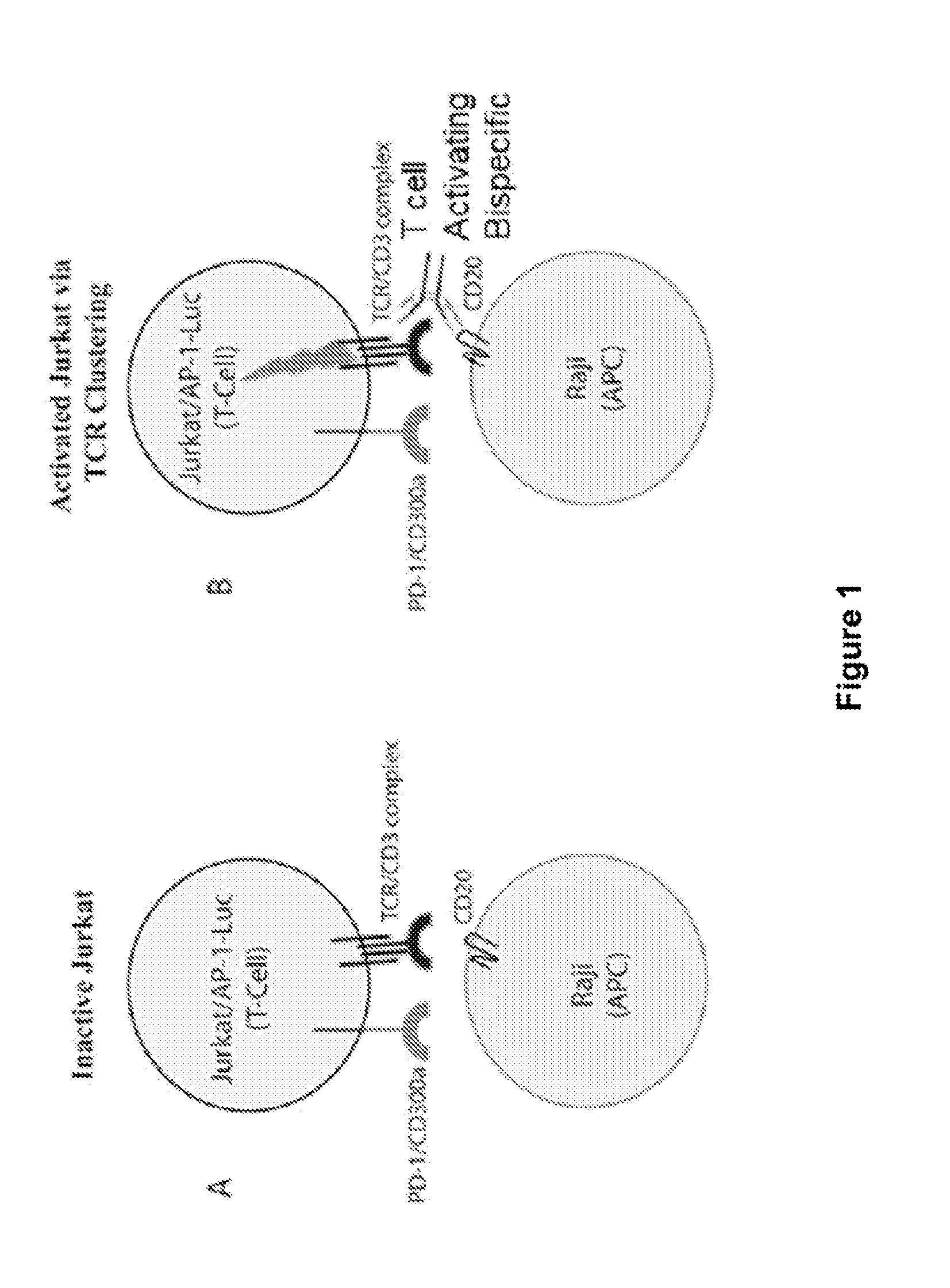
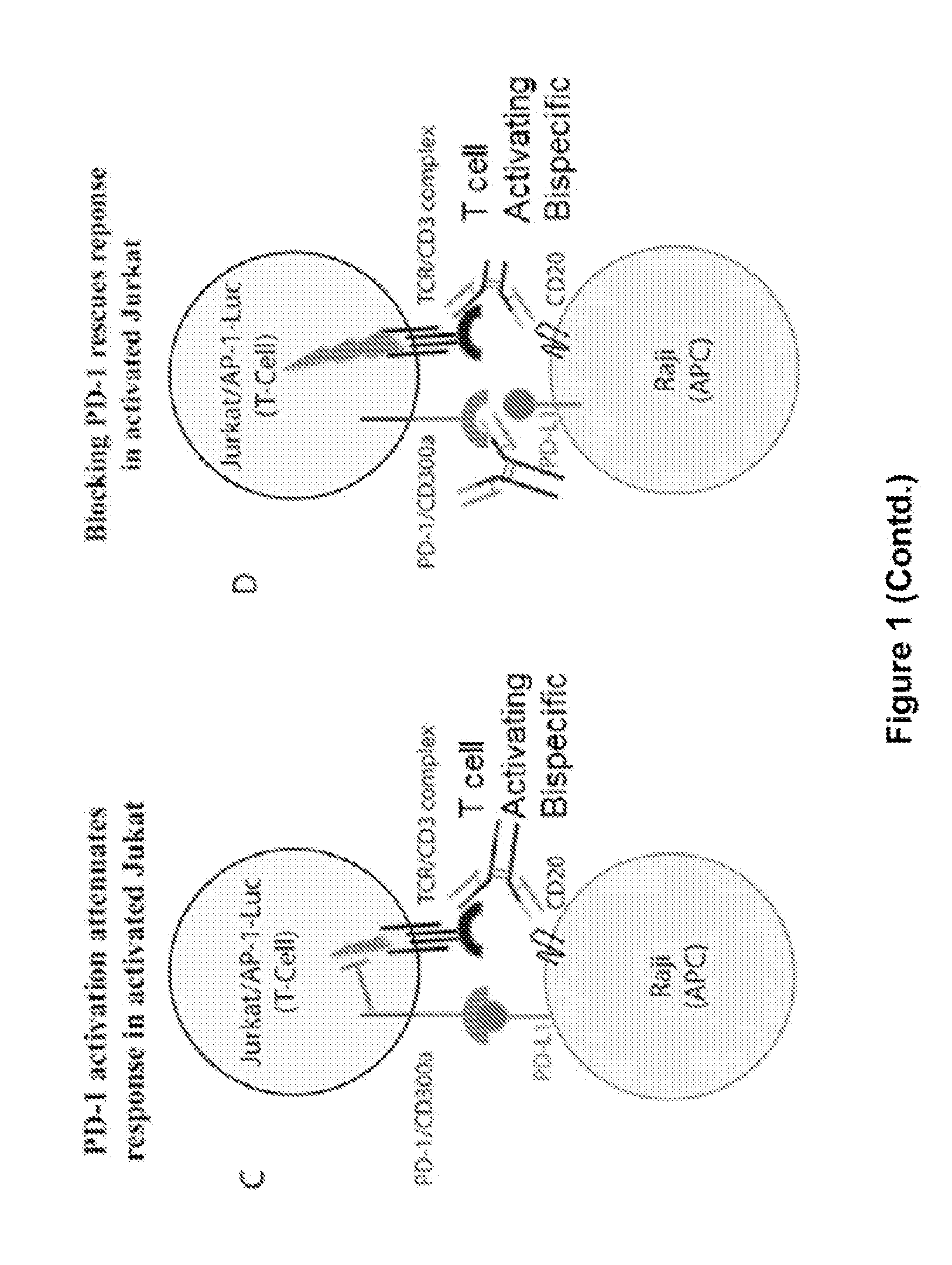
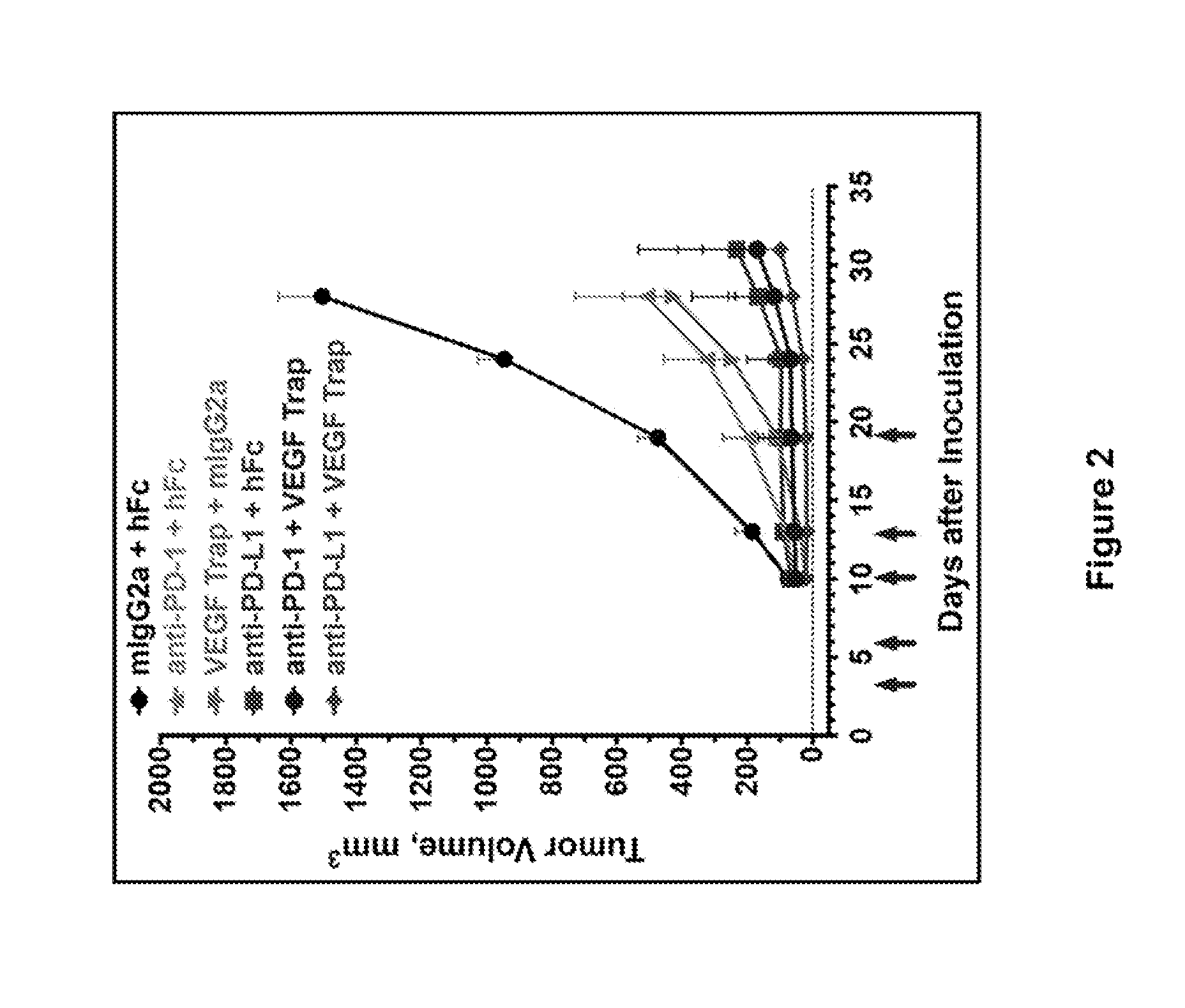
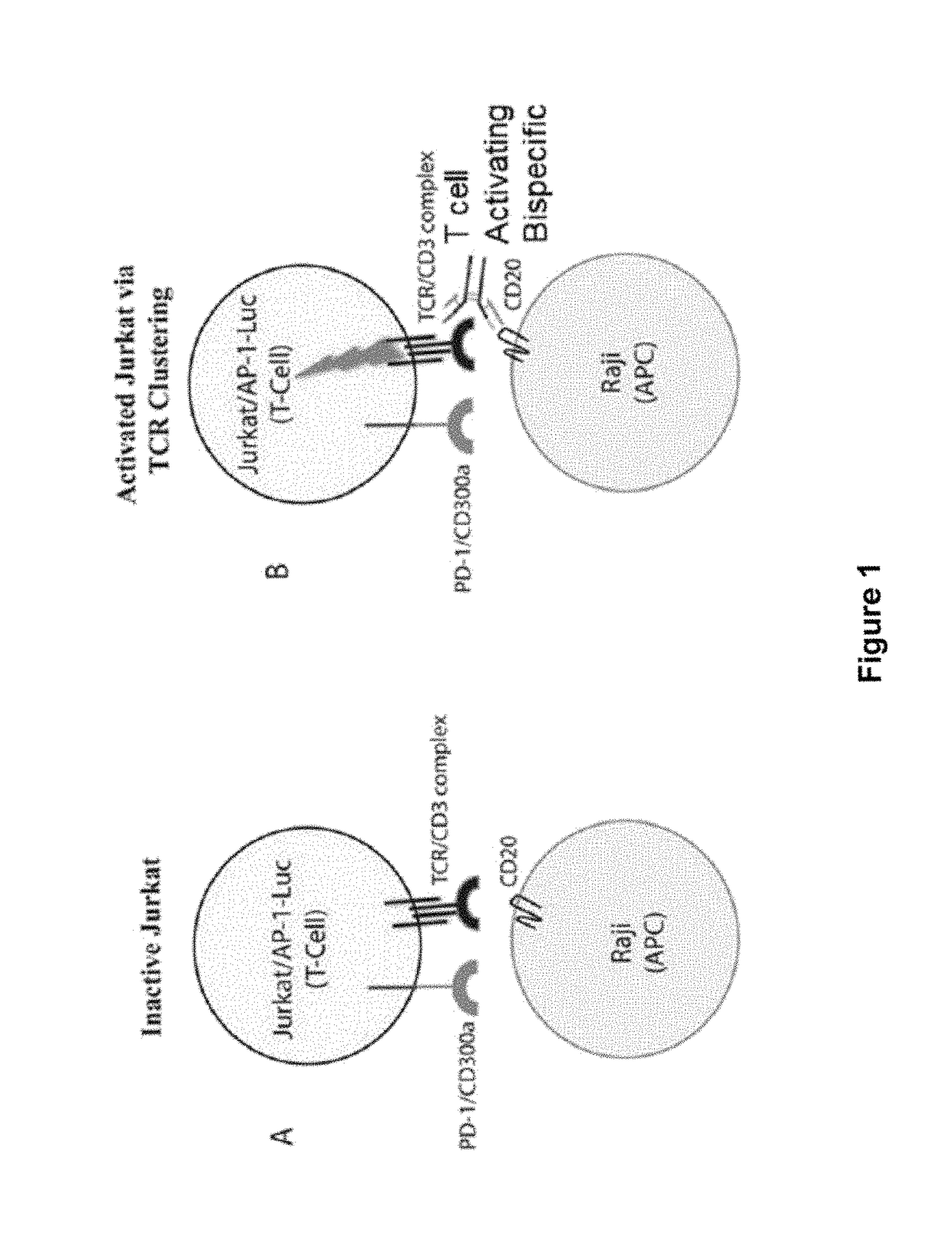
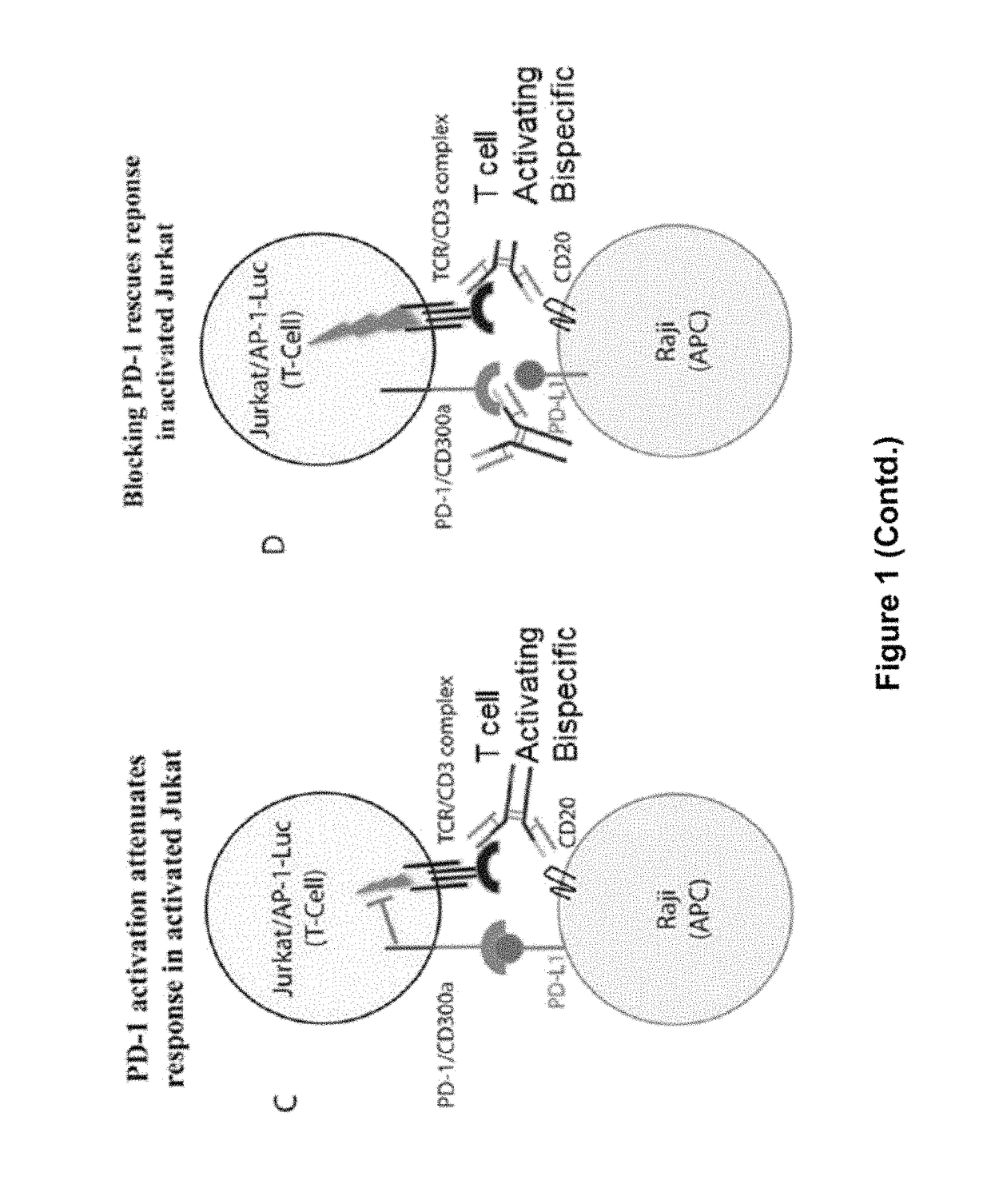
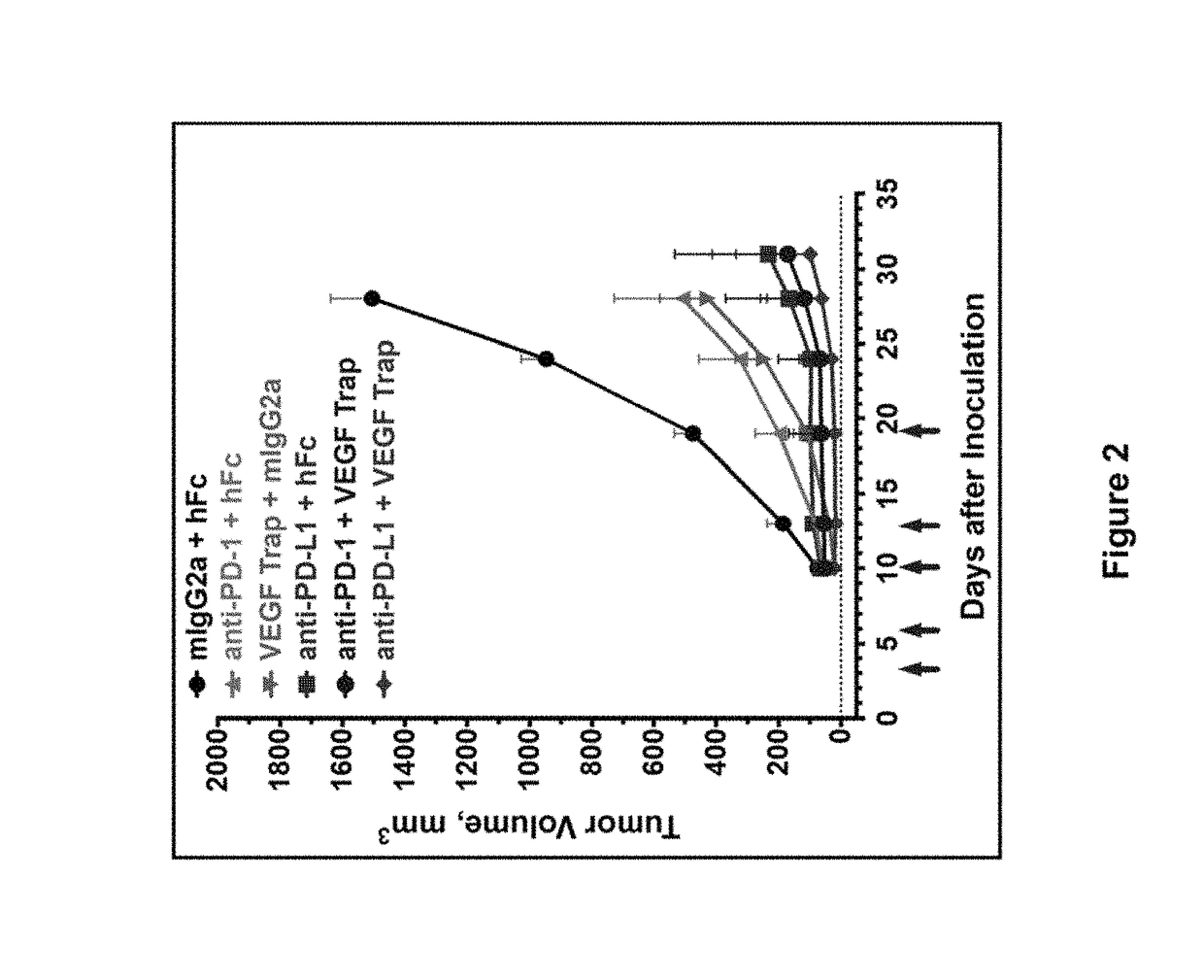
ActiveUS20150203579A1Rescue T-cell signalingInhibit tumor growthNervous disorderAntipyreticFc(alpha) receptorDisease
The present invention provides antibodies that bind to the T-cell co-inhibitor programmed death-1 (PD-1) protein, and methods of use. In various embodiments of the invention, the antibodies are fully human antibodies that bind to PD-1. In certain embodiments, the present invention provides multi-specific antigen-binding molecules comprising a first binding specificity that binds to PD-1 and a second binding specificity that binds to an autoimmune tissue antigen, another T-cell co-inhibitor, an Fc receptor, or a T-cell receptor. In some embodiments, the antibodies of the invention are useful for inhibiting or neutralizing PD-1 activity, thus providing a means of treating a disease or disorder such as cancer or a chronic viral infection. In other embodiments, the antibodies are useful for enhancing or stimulating PD-1 activity, thus providing a means of treating, for example, an autoimmune disease or disorder.
Owner:REGENERON PHARM INC
Method for the generation of antigen-specific lymphocytes
InactiveUS20070116690A1Function increaseEnhancing function of T cellBiocideVirusesAutoimmune conditionAutoimmune disease
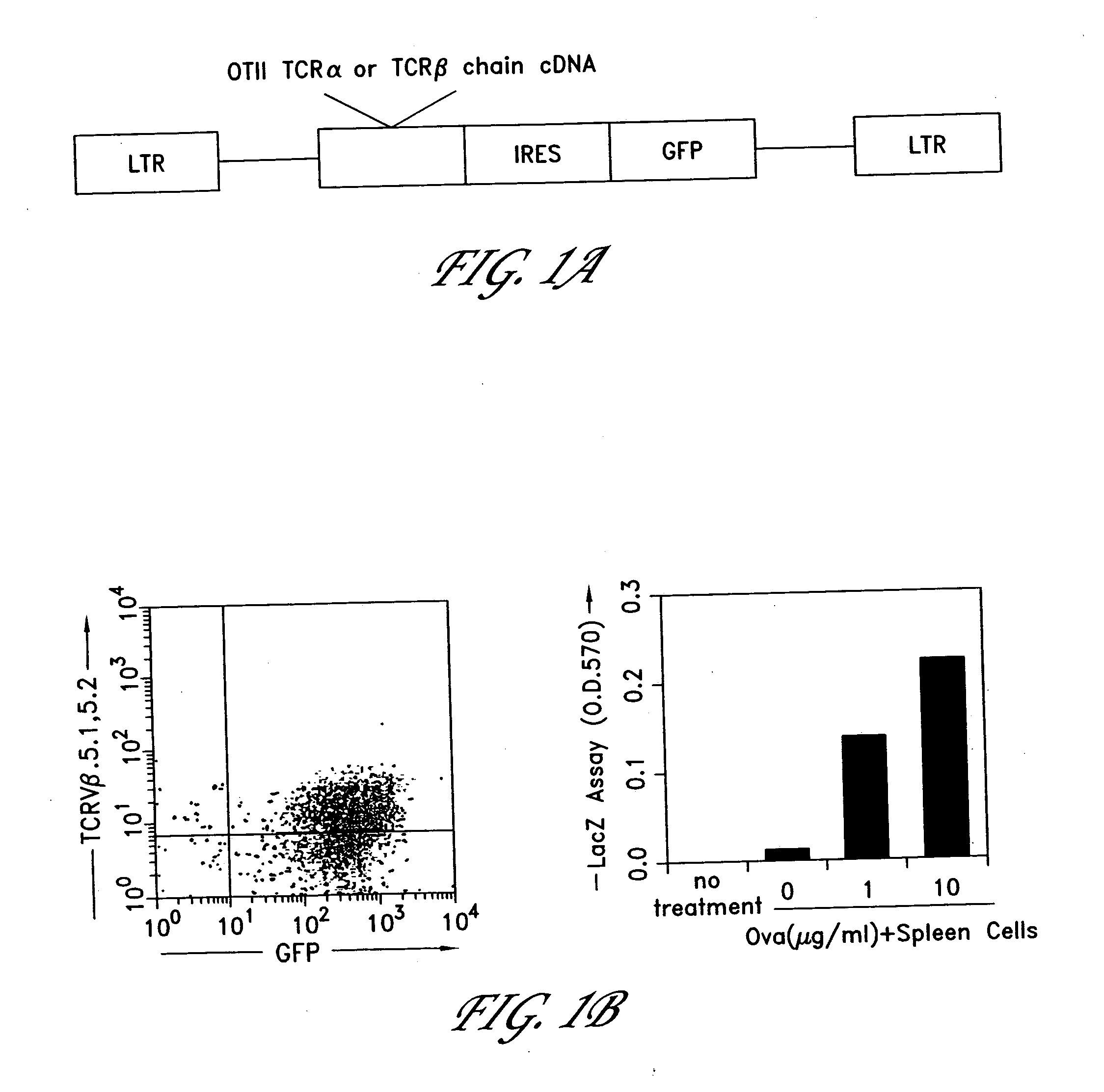
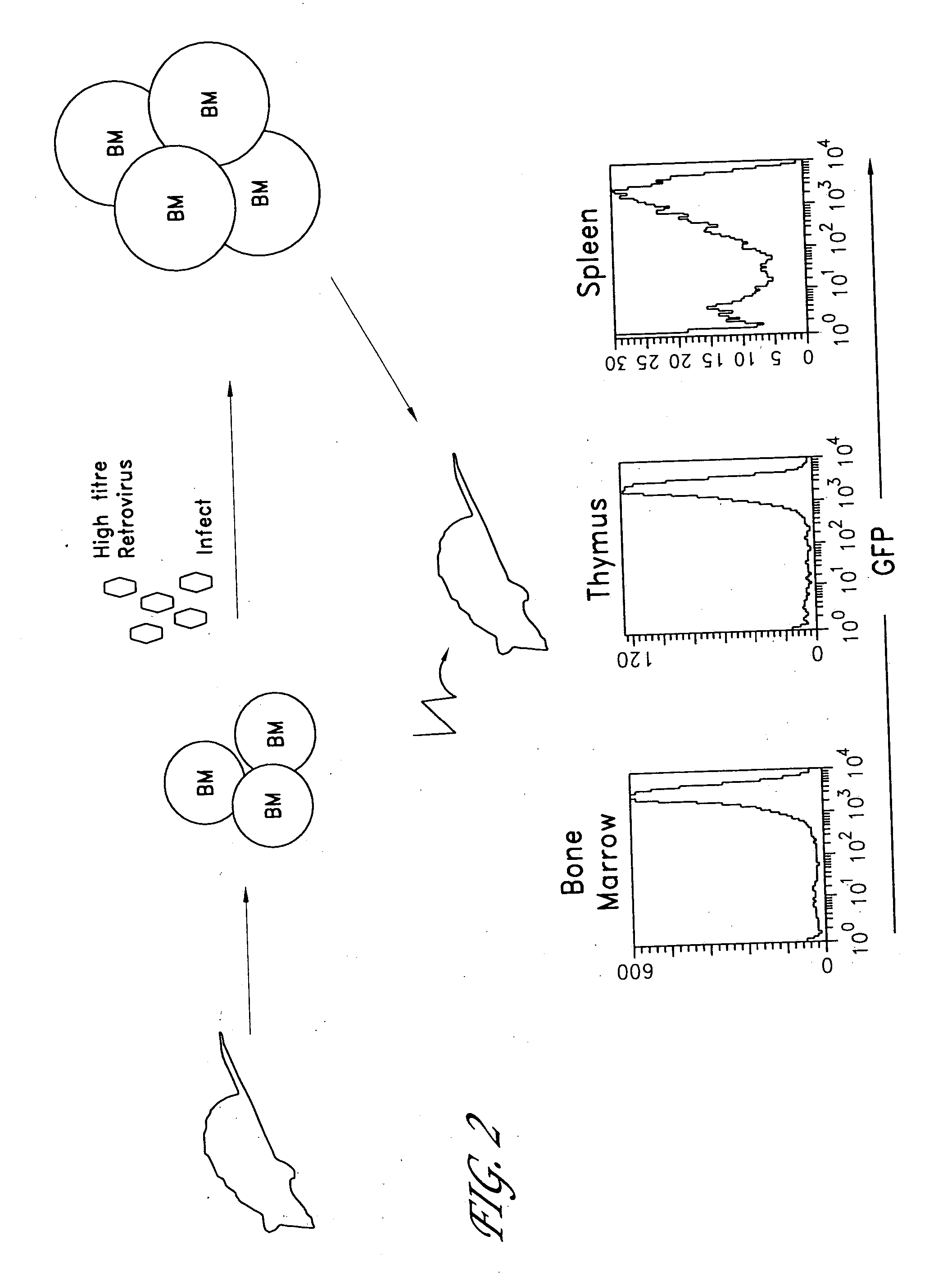
The invention provides systems and methods for the generation of lymphocytes having a unique antigen specificity. In a preferred embodiment, the invention provides methods of virally infecting cells from bone marrow with one or more viral vectors that encode antigen-specific antibodies for the production of, for example B cells and T cells. In some embodiments, the viral vectors include an IRES or 2A element to promote separation of, for example, the α subunit and β subunit of a T cell receptor (TCR) or heavy and light chains of a B-cell antibody. The resulting lymphocytes, express the particular antibody that was introduced in the case of B cells and TCR in the case of T cells. The lymphocytes generated can be used for a variety of therapeutic purposes including the treatment of various cancers and the generation of a desired immune response to viruses and other pathogens. The resulting cells develop normally and respond to antigen both in vitro and in vivo. We also show that it is possible to modify the function of lymphocytes by using stem cells from different genetic backgrounds. Thus our system constitutes a powerful tool to generate desired lymphocyte populations both for research and therapy. Future applications of this technology may include treatments for infectious diseases, such as HIV / AIDS, cancer therapy, allergy, and autoimmune disease.
Owner:CALIFORNIA INST OF TECH
T cell receptor-deficient t cell compositions
The invention is directed to modified T cells, methods of making and using isolated, modified T cells, and methods of using these isolated, modified T cells to address diseases and disorders. In one embodiment, this invention broadly relates to TCR-deficient T cells, isolated populations thereof, and compositions comprising the same. In another embodiment of the invention, these TCR-deficient T cells are designed to express a functional non-TCR receptor. The invention also pertains to methods of making said TCR-deficient T cells, and methods of reducing or ameliorating, or preventing or treating, diseases and disorders using said TCR-deficient T cells, populations thereof, or compositions comprising the same.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Chimeric T cell receotors
ActiveUS20040043401A1Minimize the numberSmall doseAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsLymphocyte proliferationCytotoxicity
Chimeric T cell receptors (TCR) are provided that combine, in a single chimeric species, the intracellular domain of CD3 zeta-chain, a signaling region from a costimulatory protein such as CD28, and a binding element that specifically interacts with a selected target. When expressed, for example in T-lymphocytes from the individual to be treated for a condition associated with the selected target, a T cell immune response is stimulated in the individual to the target cells. The chimeric TCR's are able to provide both the activation and the co-stimulation signals from a single molecule to more effectively direct T-lymphocyte cytotoxicity against the selected target and T-lymphocyte proliferation.
Owner:SLOAN KETTERING INST FOR CANCER RES
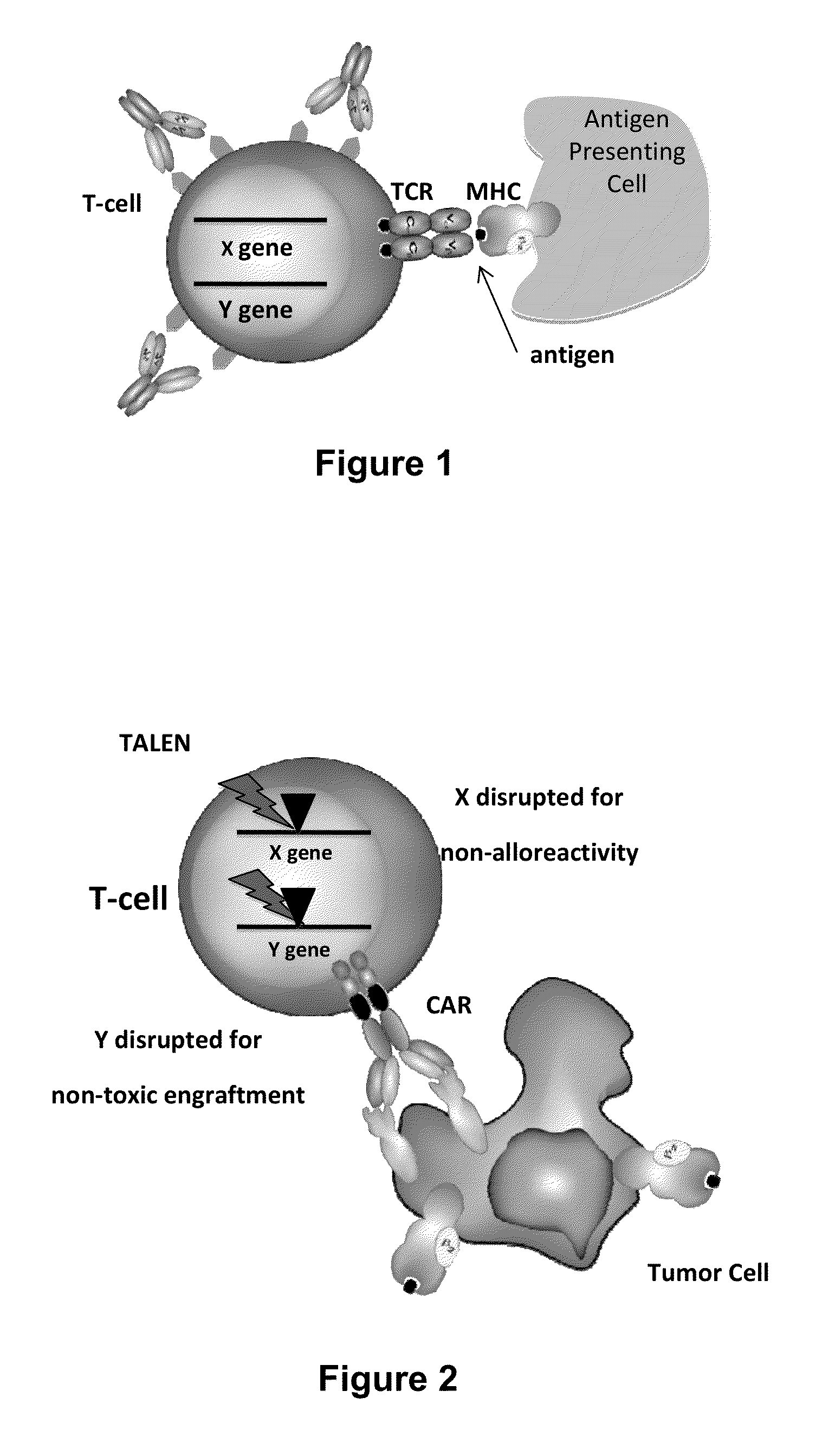
Methods for engineering allogeneic and immunosuppressive resistant t cell for immunotherapy
ActiveUS20130315884A1Precise positioningPeptide/protein ingredientsAntibody mimetics/scaffoldsImmunosuppressive drugPrimary cell
Methods for developing engineered T-cells for immunotherapy that are both non-alloreactive and resistant to immunosuppressive drugs. The present invention relates to methods for modifying T-cells by inactivating both genes encoding target for an immunosuppressive agent and T-cell receptor, in particular genes encoding CD52 and TCR. This method involves the use of specific rare cutting endonucleases, in particular TALE-nucleases (TAL effector endonuclease) and polynucleotides encoding such polypeptides, to precisely target a selection of key genes in T-cells, which are available from donors or from culture of primary cells. The invention opens the way to standard and affordable adoptive immunotherapy strategies for treating cancer and viral infections.
Owner:CELLECTIS SA
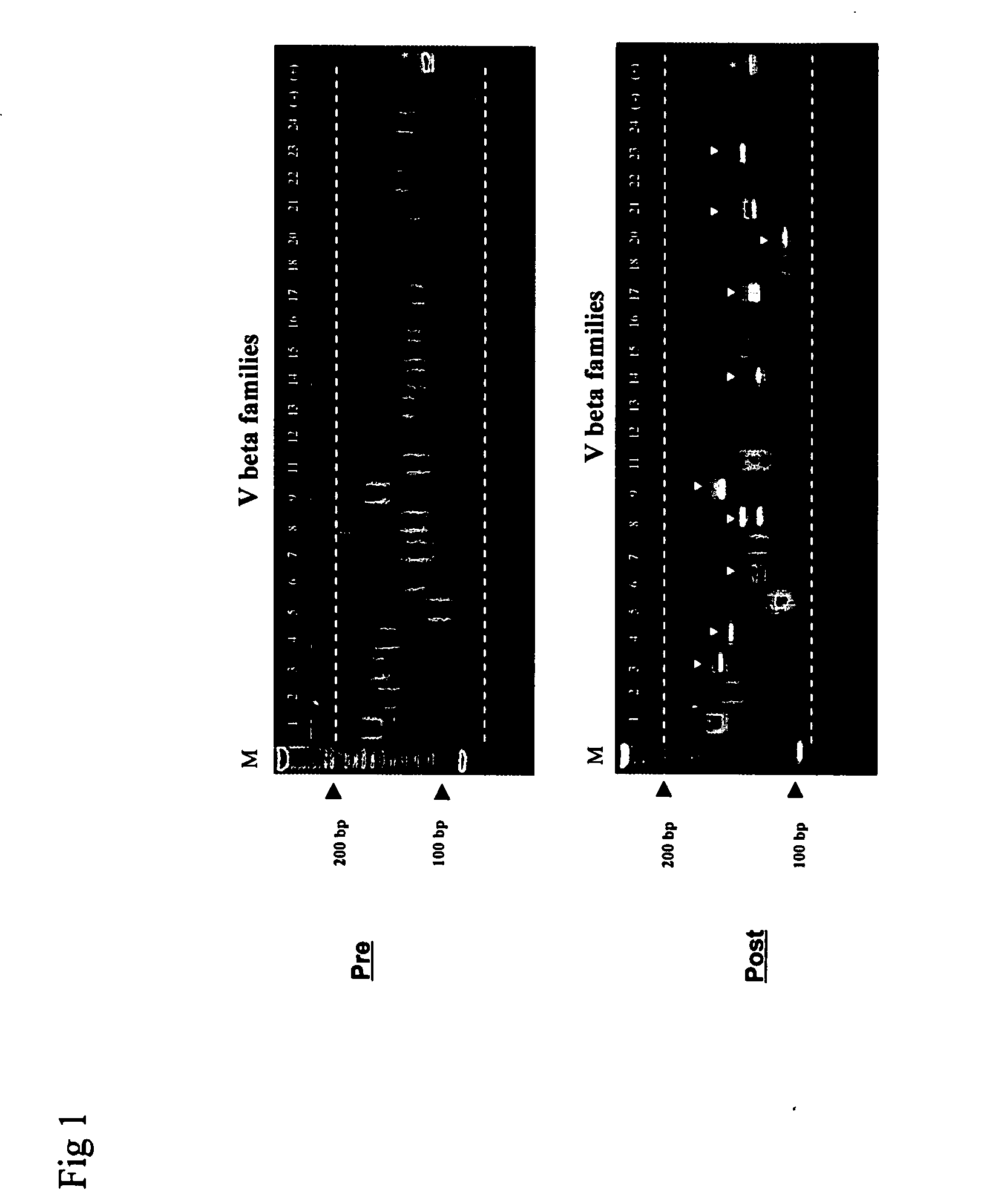
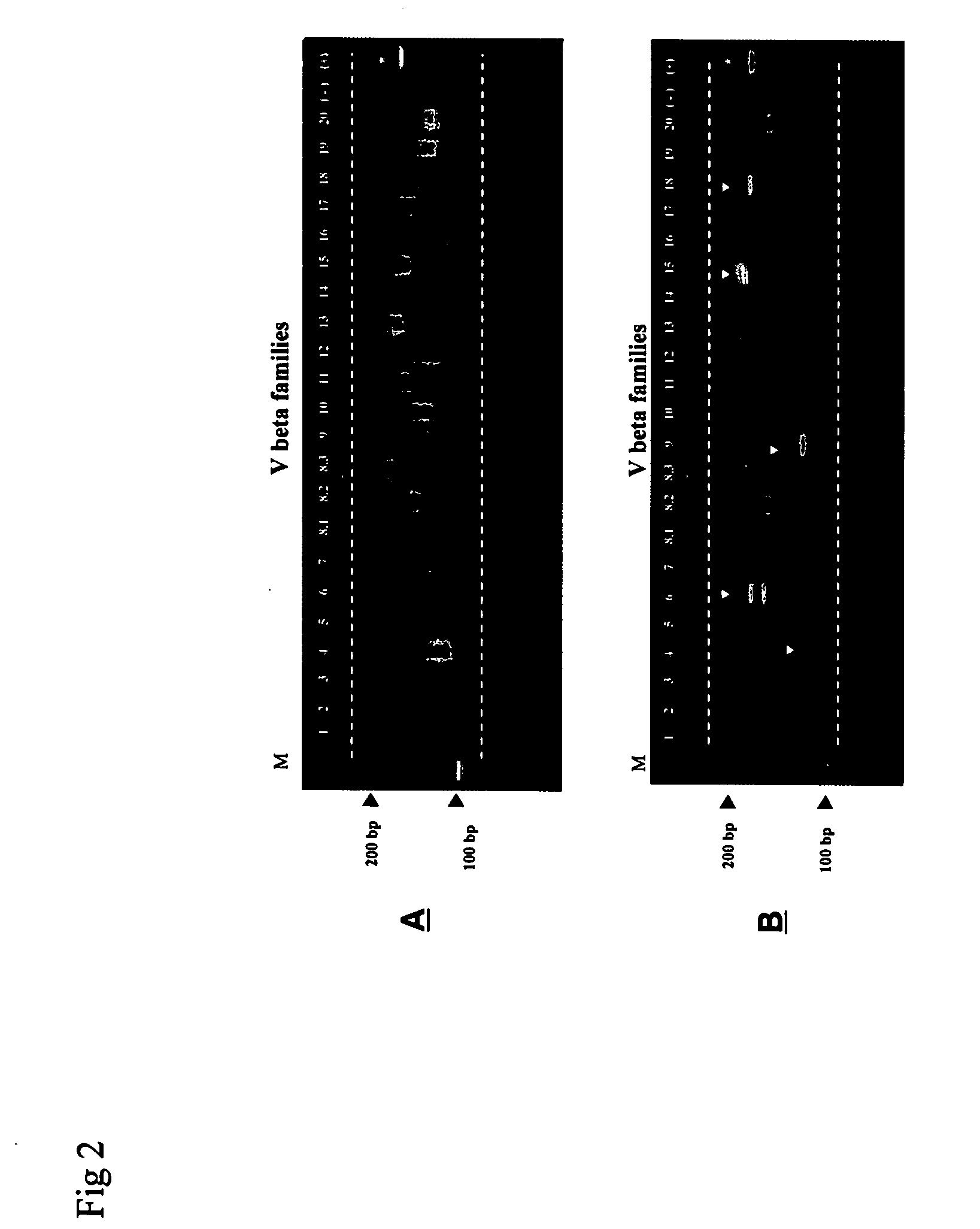
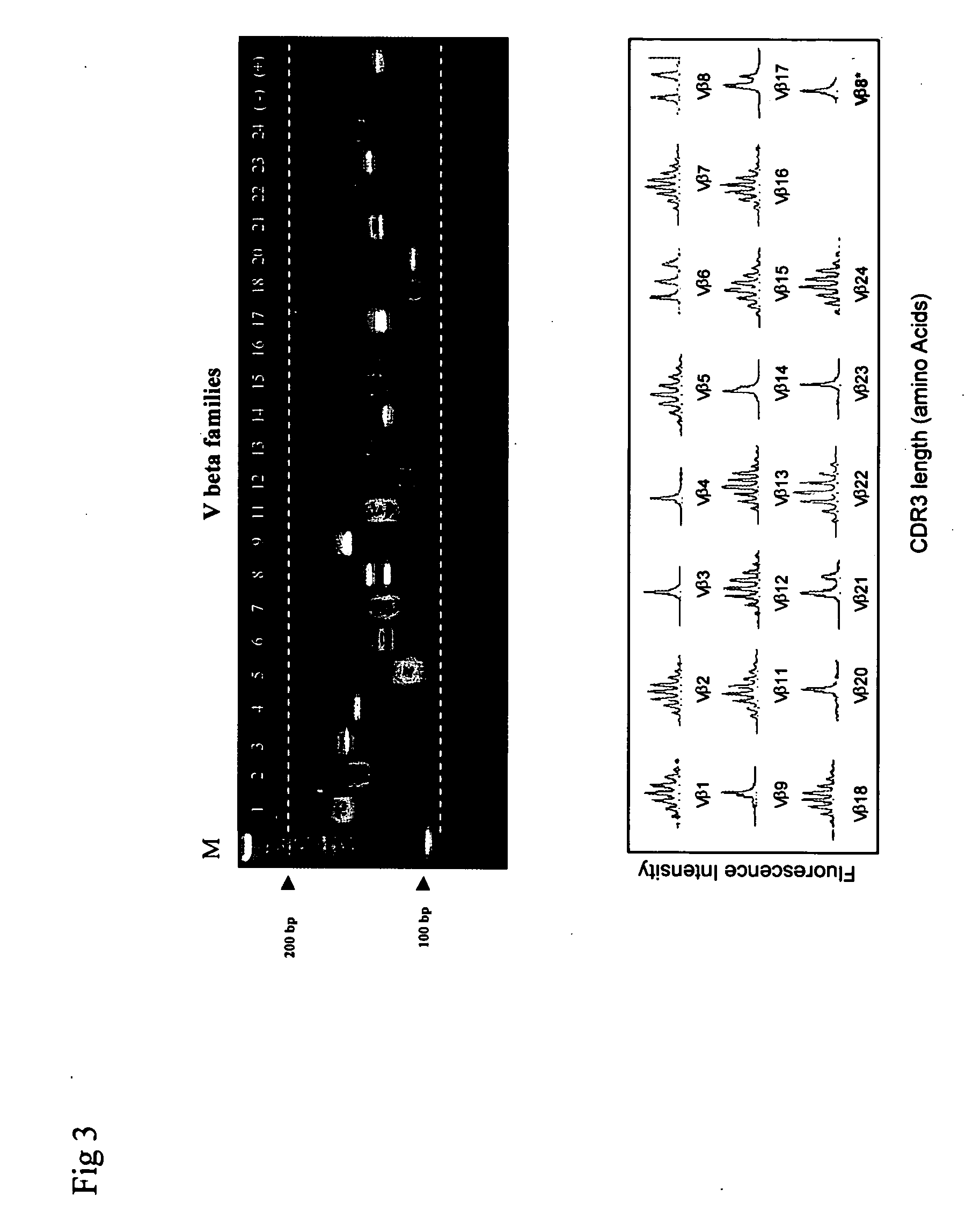
Method for detection and quantification of T-cell receptor Vbeta repertoire
ActiveUS20070117134A1Enhances PCR reaction sensitivityRapid determinationAnalysis using chemical indicatorsSugar derivativesVaccinationAntigen stimulation
The invention is a method for detecting and measuring T-cell receptor (TCR) repertoires from mammalian lymphocytes. The method is based on the use of the multiple sets of unique primers to amplify 22 regions of the TCR Vβ region and thereby detect clonal expansions related to antigen stimulation of the immune system. Kits containing sets of primers and specialized analytical statistical software for use in determining clonal expansion in humans and mice are disclosed. The reliability, efficiency and short assay time in using the method is well suited to monitoring immune response to vaccination and therapeutic treatments for immune disorders.
Owner:SHANGHAI CELLULAR BIOPHARMACEUTICAL GROUP LTD
Method for linking sequences of interest
ActiveUS7749697B2Efficient methodAntibacterial agentsSugar derivativesNucleotideNucleotide sequencing
Multiplex overlap-extension RT-PCR provides an efficient method of linking two or more nucleotide sequences encoding for domains or subunits of a heteromeric protein, in a single reaction. Especially, the linkage of variable region encoding sequences from e.g. immunoglobulins, T cell receptors or B cell receptors is eased with the method of the present invention. This allows for a more efficient way of generating libraries of variable region encoding sequences. The capability to perform the multiplex overlap-extension RT-PCR using template derived from an isolated single cell enables the generation of cognate pair libraries in a high-throughput format.
Owner:LES LAB SERVIER
Sequence analysis of complex amplicons
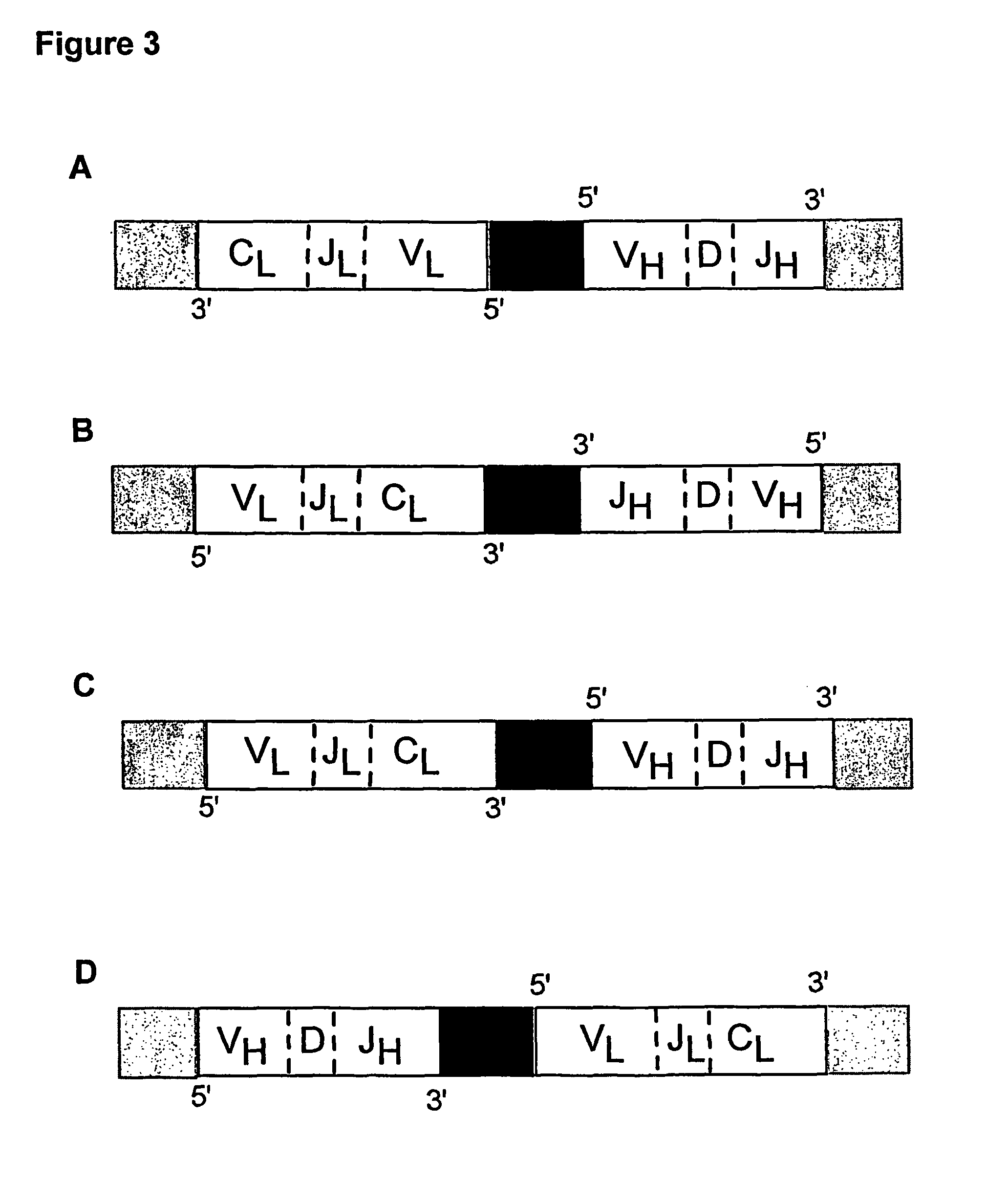
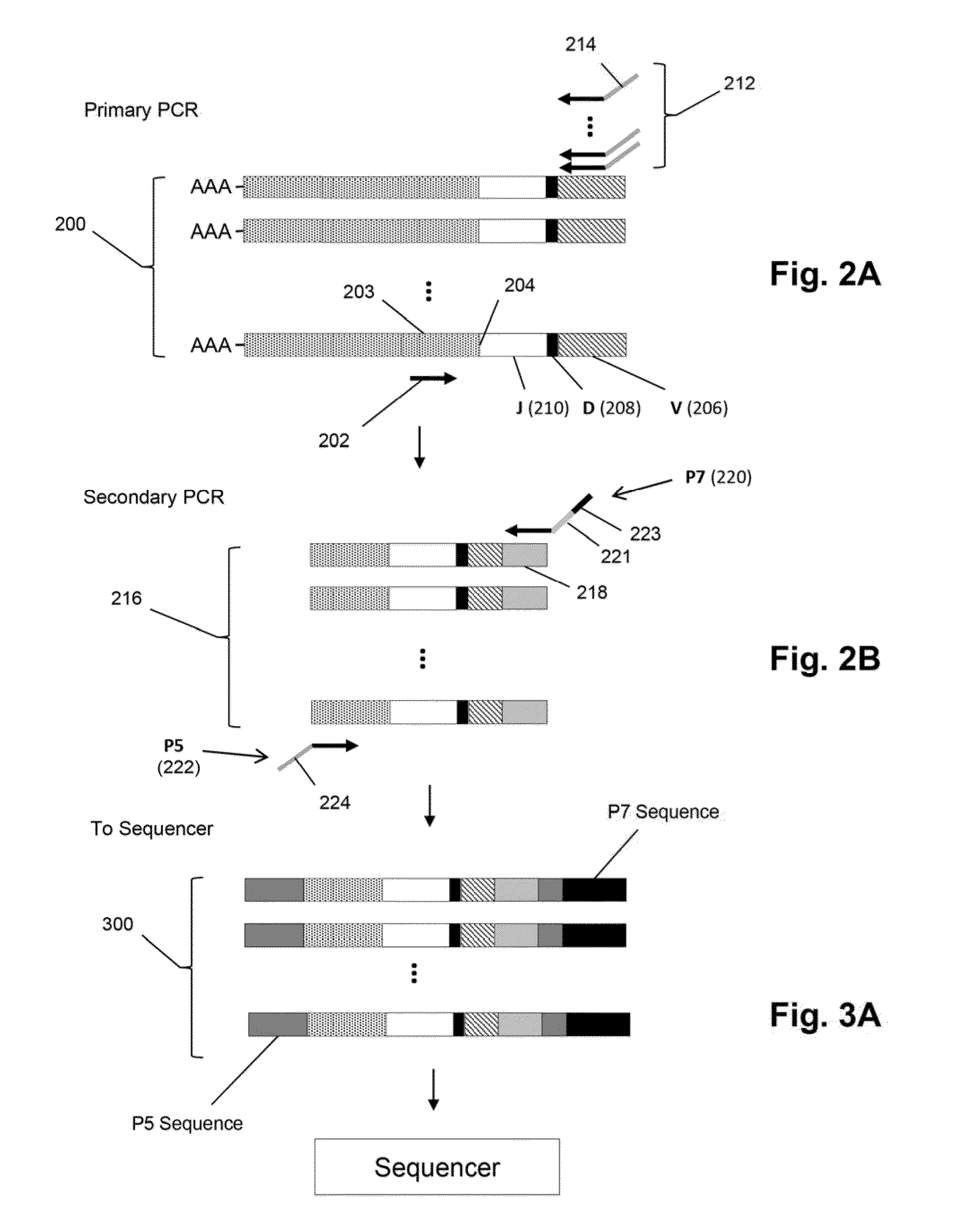
ActiveUS20140315725A1Microbiological testing/measurementLibrary member identificationSequence analysisB-cell receptor
The invention is directed to methods of generating sequence profiles of populations of nucleic acids, whose member nucleic acids contain regions of high variability, such as populations of nucleic acids encoding T cell receptors or B cell receptors. In one aspect, the invention provides pluralities of sets of primers for generating nested sets of templates from nucleic acids in such populations, thereby insuring the production of at least one template from which sequence reads are generated, despite such variability, or despite limited lengths or quality of sequence reads. In another aspect, members of such populations are bidirectionally sequenced so that further sequence information is obtained by analyzing overlapping sequence reads in the zones of highest variability.
Owner:ADAPTIVE BIOTECH
Car+ t cells genetically modified to eliminate expression of t-cell receptor and/or HLA
ActiveUS20140349402A1Suitable for storageReduce/eliminate T cellsGenetically modified cellsMammal material medical ingredientsAntigen receptorsAutoimmunity
The present invention concerns methods and compositions for immunotherapy employing a modified T cell comprising disrupted T cell receptor and / or HLA and comprising a chimeric antigen receptor. In certain embodiments, the compositions are employed allogeneically as universal reagents for “off-the-shelf treatment of medical conditions such as cancer, autoimmunity, and infection. In particular embodiments, the T cell receptor-negative and / or HLA-negative T cells are generated using zinc finger nucleases, for example.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
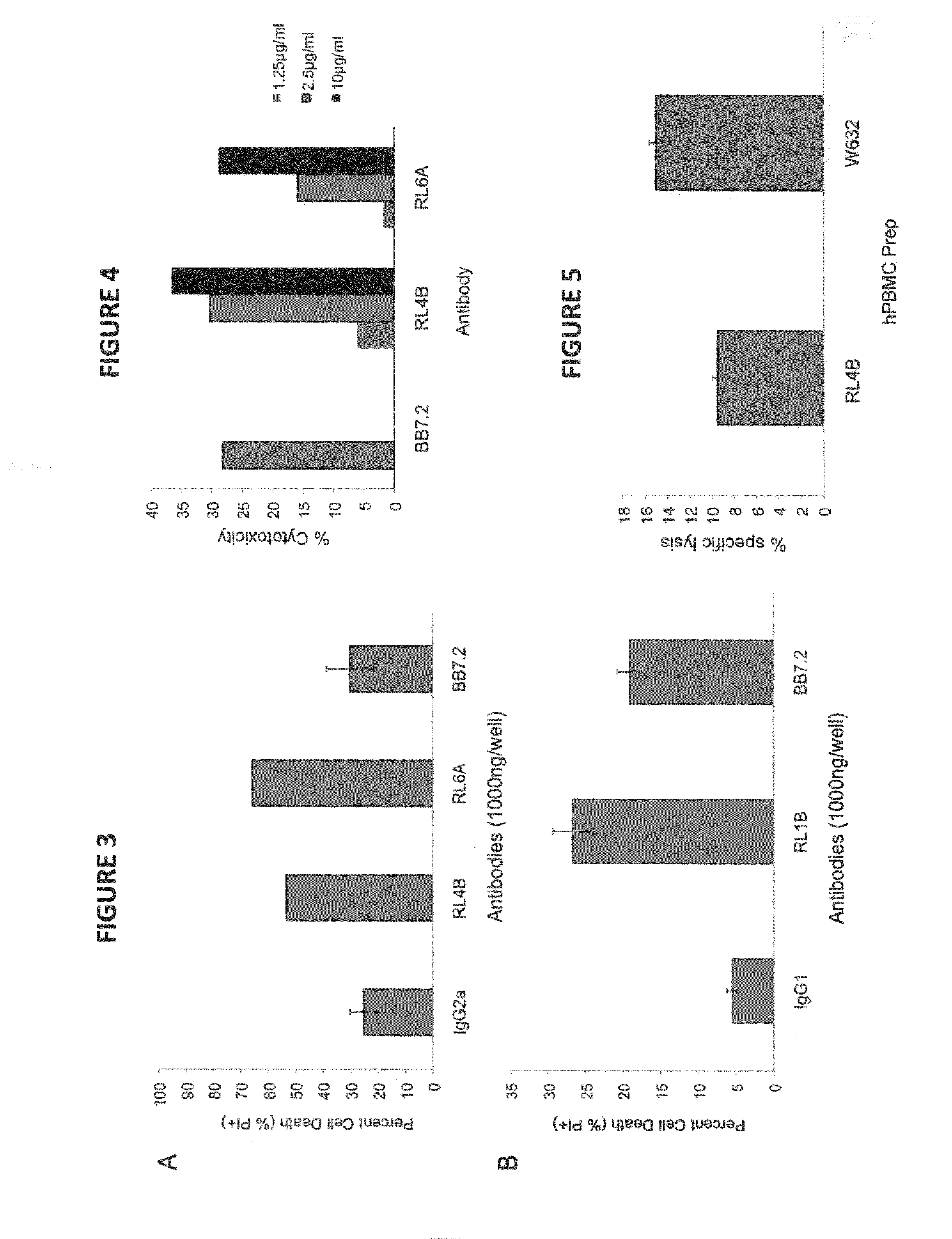
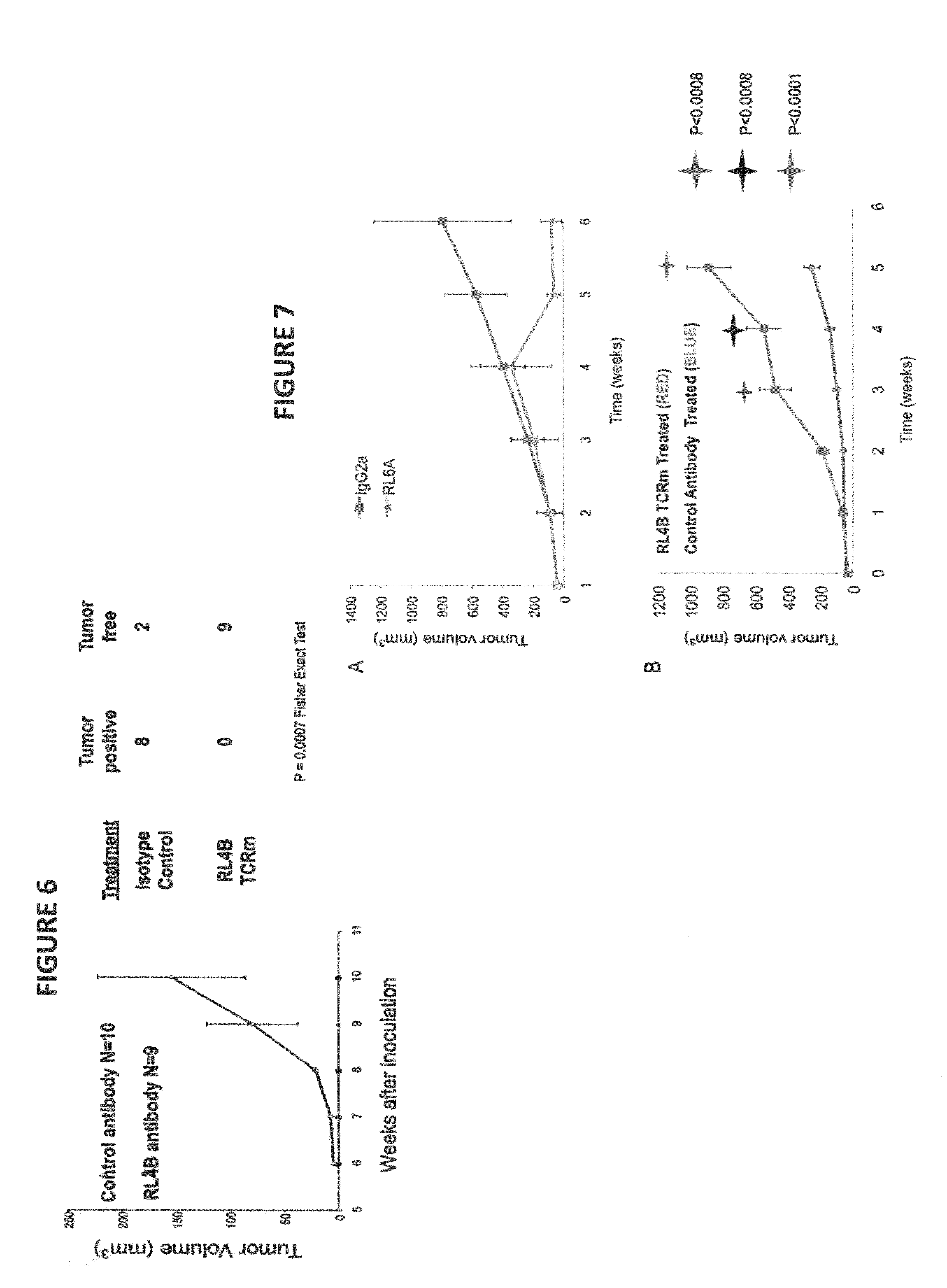
Antibodies as T cell receptor mimics, methods of production and uses thereof
The presently disclosed and claimed invention relates to a methodology of producing and utilizing antibodies that recognize peptides associated with a tumorigenic or disease state, wherein the peptides are displayed in the context of HLA molecules. These antibodies may be utilized in therapeutic methods of mediating cell lysis.
Owner:TEXAS TECH UNIV SYST
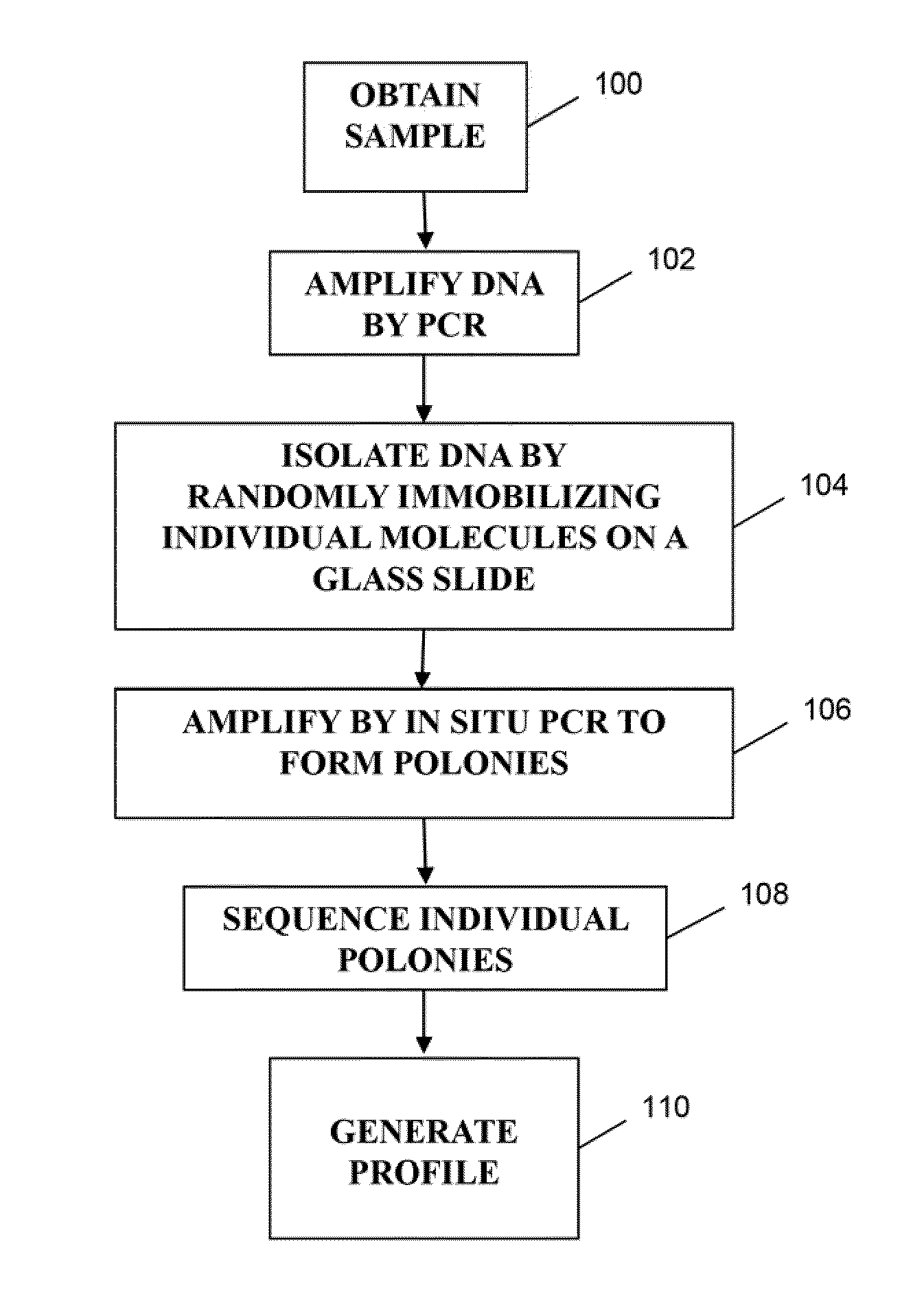
Methods of monitoring conditions by sequence analysis
ActiveUS20110207135A1Low quality scoreMicrobiological testing/measurementICT adaptationSequence analysisGenetics
The invention is directed to methods of generating sequence profiles of populations of nucleic acids, whose member nucleic acids contain regions of high variability, such as populations of nucleic acids encoding T cell receptors or B cell receptors. In one aspect, the invention provides pluralities of sets of primers for generating nested sets of templates from nucleic acids in such populations, thereby insuring the production of at least one template from which sequence reads are generated, despite such variability, or dispite limited lenghs or quality of sequence reads. In another aspect, members of such populations are bidirectionally sequenced so that further sequence information is obtained by analyzing overlapping sequence reads in the zones of highest variability.
Owner:ADAPTIVE BIOTECH
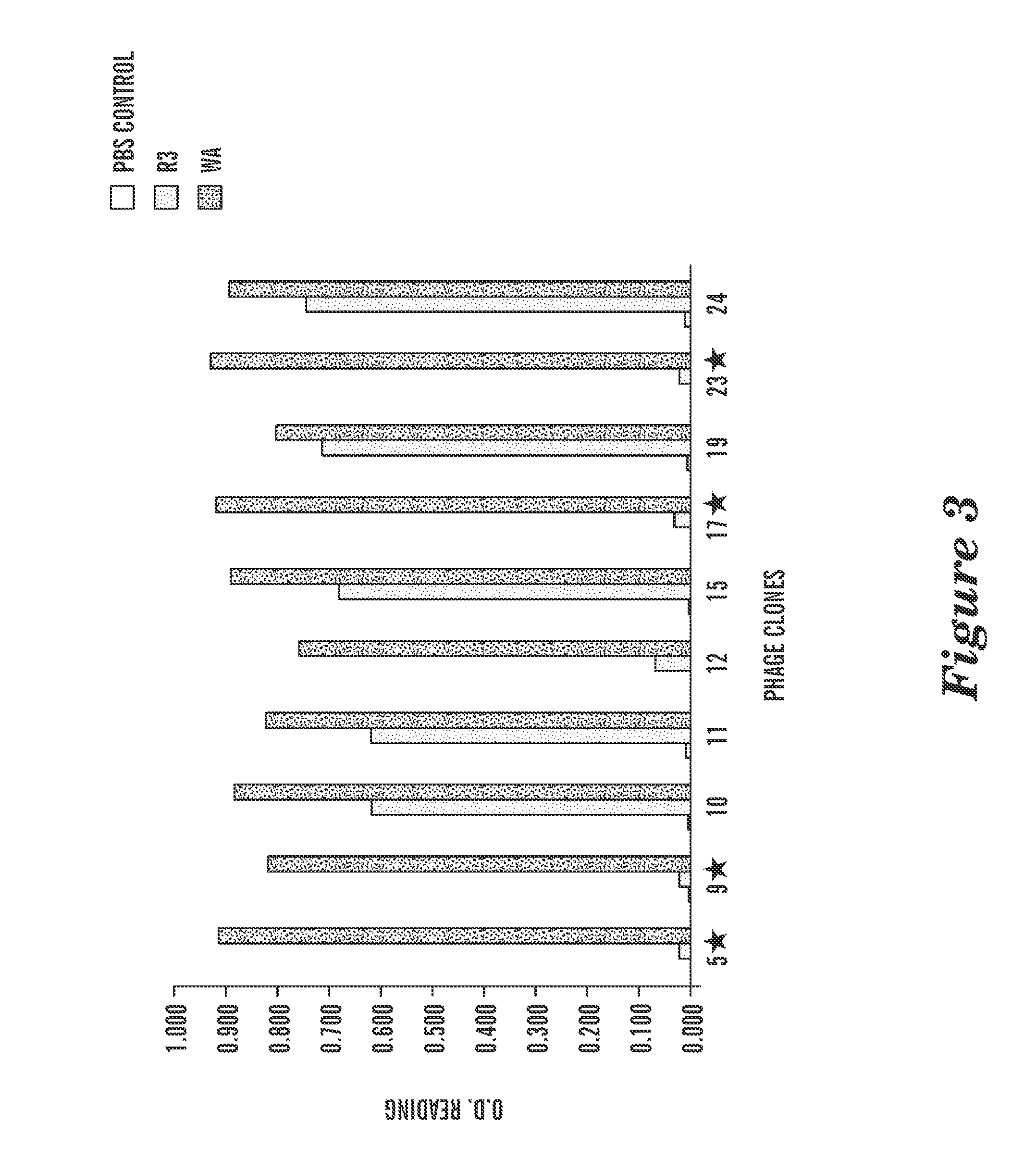
High affinity T cell receptor and use thereof
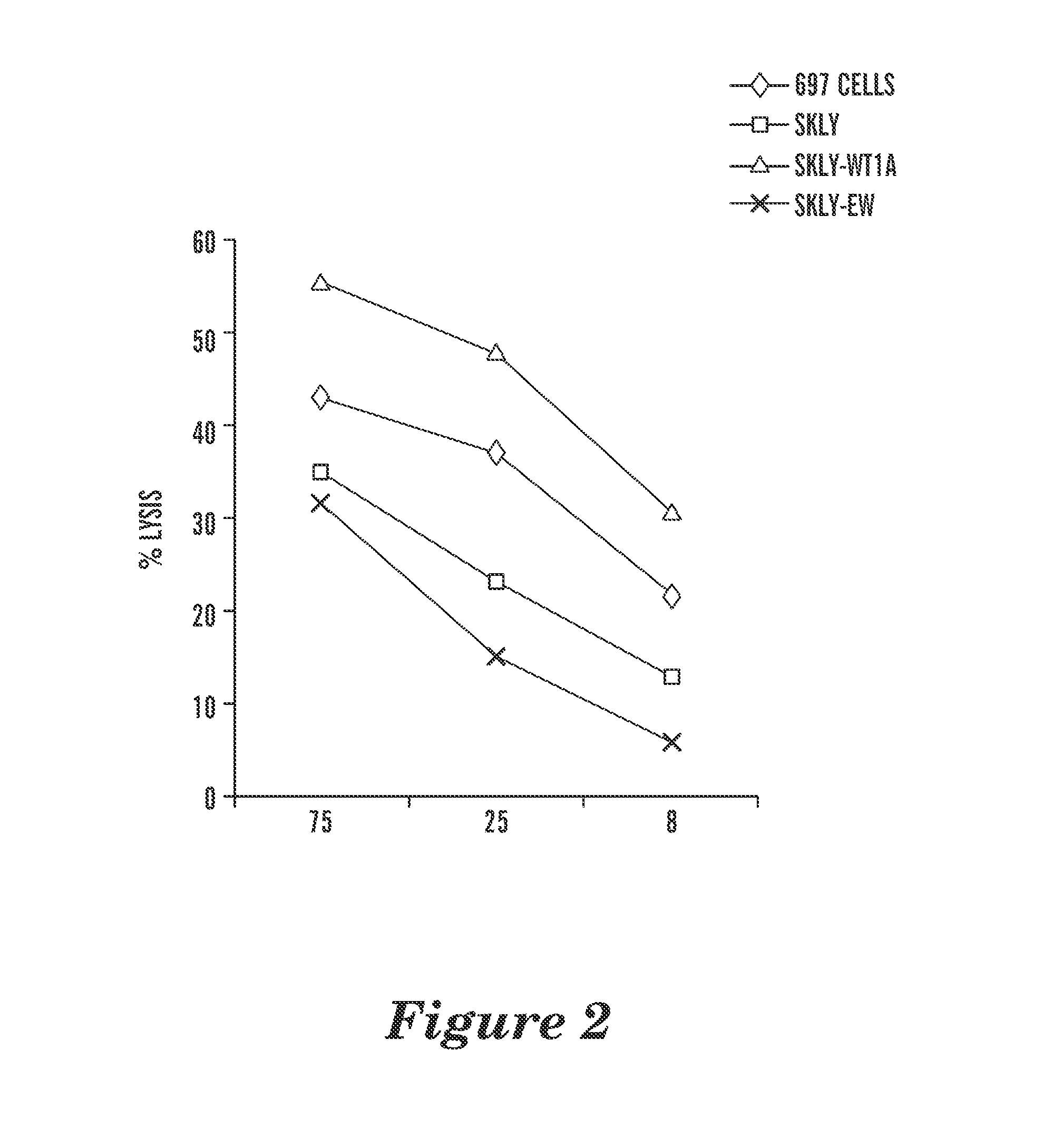
ActiveUS8697854B2High affinityEffective treatmentBiocidePeptide/protein ingredientsDiseaseWilms' tumor
The present invention is directed to a high affinity T cell receptor (TCR) against a tumor-associated antigen, an isolated nucleic acid molecule encoding same, a T cell expressing said TCR, and a pharmaceutical composition for use in the treatment of diseases involving malignant cells expressing said tumor-associated antigen.
Owner:MAX DELBRUECK CENT FUER MOLEKULARE MEDIZIN
T cell receptor-like antibodies specific for a wti peptide presented by hla-a2
The present invention provides antigen binding proteins that specifically bind to Wilms' tumor protein (WT1), including humanized, chimeric and fully human antibodies against WT1, antibody fragments, chimeric antigen receptors (CARs), fusion proteins, and conjugates thereof. The antigen binding proteins and antibodies bind to HLA-A0201-restricted WT1 peptide. Such antibodies, fragments, fusion proteins and conjugates thereof are useful for the treatment of WT1 associated cancers, including for example, breast cancer, ovarian cancer, prostate cancer, chronic myelocytic leukemia, multiple myeloma, acute lymphoblastic leukemia (ALL), acute myeloid / myelogenous leukemia (AML) and myelodysplastic syndrome (MDS). In more particular embodiments, the anti-WT1 / A antibodies may comprise one or more framework region amino acid substitutions designed to improve protein stability, antibody binding and / or expression levels.
Owner:EUREKA THERAPEUTICS INC +1
Single chain recombinant T cell receptors
InactiveUS7569664B2Improve stabilityUseful purposeCompound screeningApoptosis detectionExtracellularC-terminus
A single chain T cell receptor (scTCR) comprising an a segment constituted by a TCR α chain variable region sequence fused to the N terminus of a TCR α chain constant region extracellular sequence, a β segment constituted by a TCR β chain variable region fused to the N terminus of a TCR β chain constant region extracellular sequence, and a linker sequence linking the C terminus of the α segment to the N terminus of the β segment, or vice versa, the constant region extracellular sequences of the α and β segments being linked by a disulfide bond, the length of the linker sequence and the position of the disulfide bond being such that the variable region sequences of the α and β segments are mutually orientated substantially as in native αβ T cell receptors. Complexes of two or more such scTCRs, and use of the scTCRs in therapy and in various screening applications are also disclosed.
Owner:IMMUNOCORE LTD +1
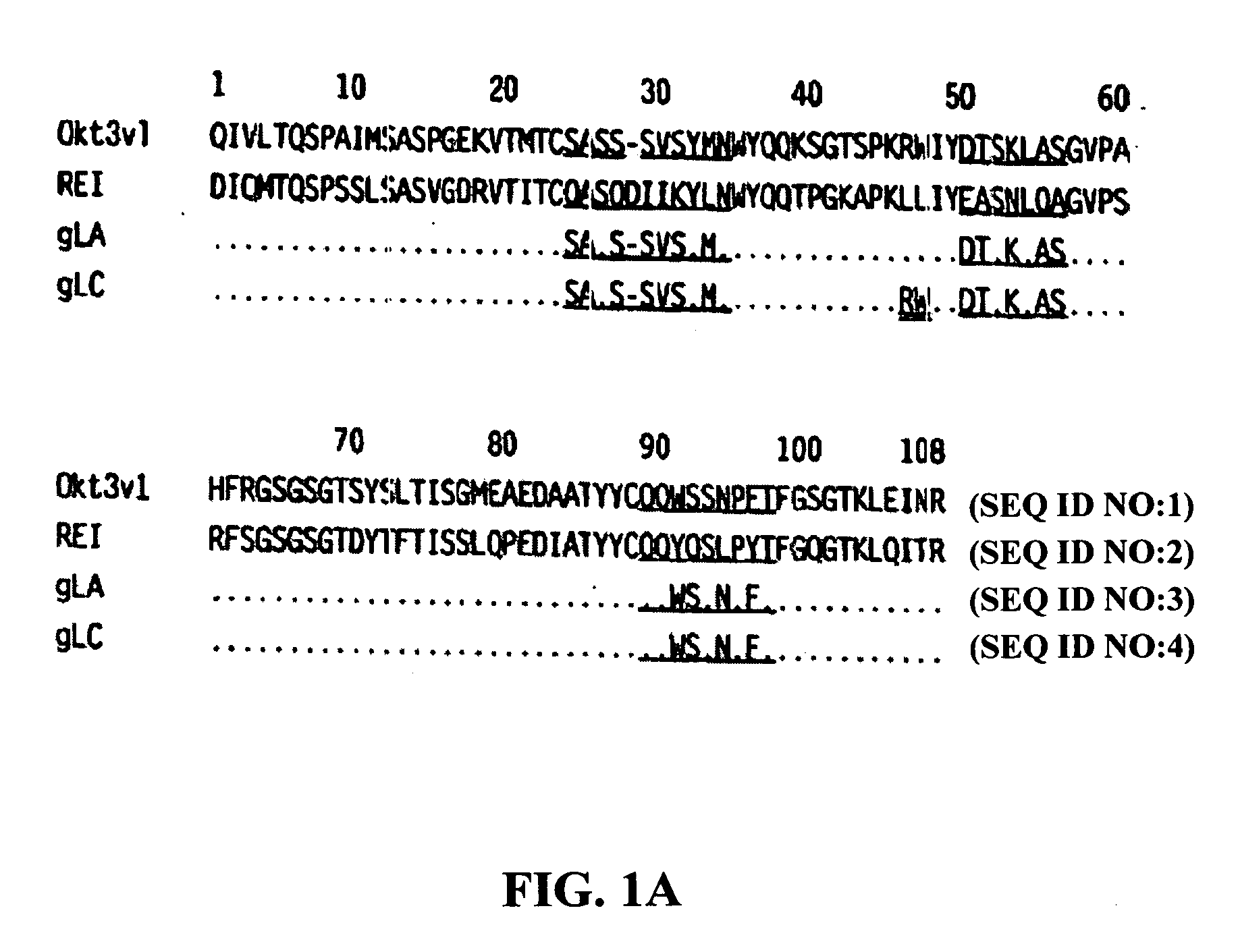
Methods for the treatment of autoimmune disorders using immunosuppressive monoclonal antibodies with reduced toxicity
ActiveUS20070077246A1Treating and preventing and ameliorating symptomSlow and reduce damagePeptide/protein ingredientsAntipyreticAutoimmune conditionAutoimmune disease
The present invention provides methods of treating, preventing or ameliorating the symptoms of T cell-mediated immunological diseases, particularly autoimmune diseases, through the use of anti-CD3 antibodies. In particular, the methods of the invention provide for administration of antibodies that specifically bind the epsilon subunit within the human CD3 complex. Such antibodies modulate the T cell receptor / alloantigen interaction and, thus, regulate the T cell mediated cytotoxicity associated with autoimmune disorders. Additionally, the invention provides for modification of the anti-CD3 antibodies such that they exhibit reduced or eliminated effector function and T cell activation as compared to non-modified anti-CD3 antibodies.
Owner:PROVENTION BIO INC
Antibodies as T cell receptor mimics, methods of production and uses thereof
InactiveUS20060034850A1Aid in stabilizationAnimal cellsImmunoglobulin superfamilyHla moleculesDisease cause
The present invention relates to a methodology of producing antibodies that recognize peptides associated with a tumorigenic or disease state, wherein the peptides are displayed in the context of HLA molecules. These antibodies will mimic the specificity of a T cell receptor (TCR) but will have higher binding affinity such that the molecules may be used as therapeutic, diagnostic and research reagents. The method of producing a T-cell receptor mimic of the present invention includes identifying a peptide of interest, wherein the peptide of interest is capable of being presented by an MHC molecule. Then, an immunogen comprising at least one peptide / MHC complex is formed, wherein the peptide of the peptide / MHC complex is the peptide of interest. An effective amount of the immunogen is then administered to a host for eliciting an immune response, and serum collected from the host is assayed to determine if desired antibodies that recognize a three-dimensional presentation of the peptide in the binding groove of the MHC molecule are being produced. The desired antibodies can differentiate the peptide / MHC complex from the MHC molecule alone, the peptide alone, and a complex of MHC and irrelevant peptide. Finally, the desired antibodies are isolated.
Owner:TEXAS TECH UNIV SYST
Antibodies as T cell receptor mimics, methods of production and uses thereof
InactiveUS20090226474A1Less timeEfficient at generating a specific responseImmunoglobulin superfamilyImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseSerum ige
The present invention relates to a methodology of producing antibodies that recognize peptides associated with a tumorigenic or disease state, wherein the peptides are displayed in the context of HLA molecules. These antibodies will mimic the specificity of a T cell receptor (TCR) but will have higher binding affinity such that the molecules may be used as therapeutic, diagnostic and research reagents. The method of producing a T-cell receptor mimic of the present invention includes identifying a peptide of interest, wherein the peptide of interest is capable of being presented by an MHC molecule. Then, an immunogen comprising at least one peptide / MHC complex is formed, wherein the peptide of the peptide / MHC complex is the peptide of interest. An effective amount of the immunogen is then administered to a host for eliciting an immune response, and serum collected from the host is assayed to determine if desired antibodies that recognize a three-dimensional presentation of the peptide in the binding groove of the MHC molecule are being produced. The desired antibodies can differentiate the peptide / MHC complex from the MHC molecule alone, the peptide alone, and a complex of MHC and irrelevant peptide. Finally, the desired antibodies are isolated.
Owner:WEIDANZ JON A +2
Reducing the immunogenicity of fusion proteins
InactiveUS6992174B2Low immunogenicityHigh expressionPeptide/protein ingredientsAntibody mimetics/scaffoldsMHC class IIVaccine Immunogenicity
Disclosed are compositions and methods for producing fusion proteins with reduced immunogenicity. Fusion proteins of the invention include a junction region having an amino acid change that reduces the ability of a junctional epitope to bind to MHC Class II, thereby reducing its interaction with a T-cell receptor. Methods of the invention involve analyzing, changing, or modifying one or more amino acids in the junction region of a fusion protein in order to identify a T-cell epitope and reduce its ability to interact with a T cell receptor. Compositions and methods of the invention are useful in therapy.
Owner:MERCK PATENT GMBH
Cd123-specific chimeric antigen receptor redirected t cells and methods of their use
ActiveUS20140271582A1BiocideAntibody mimetics/scaffoldsCD19-specific chimeric antigen receptorT-Cell Specificity
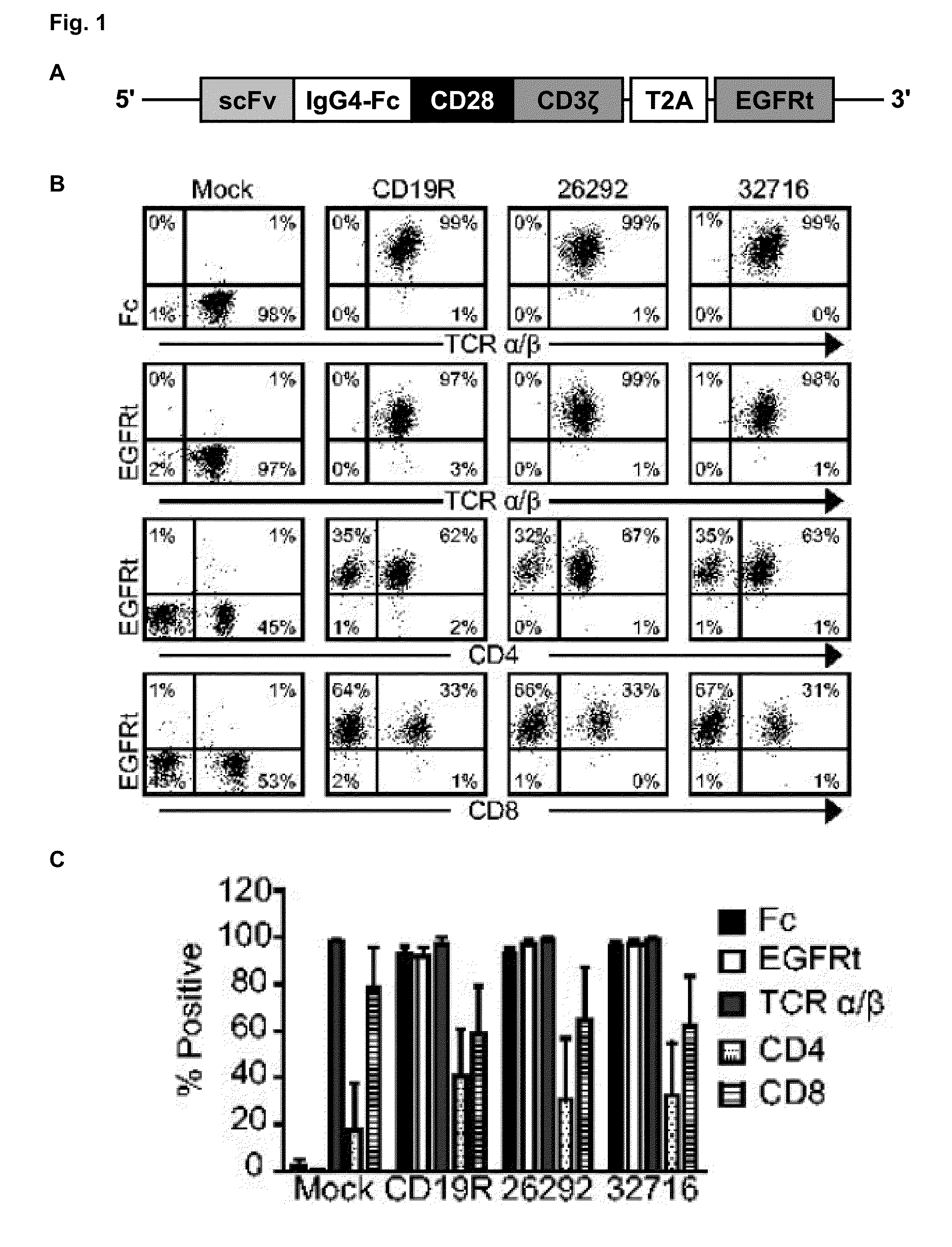
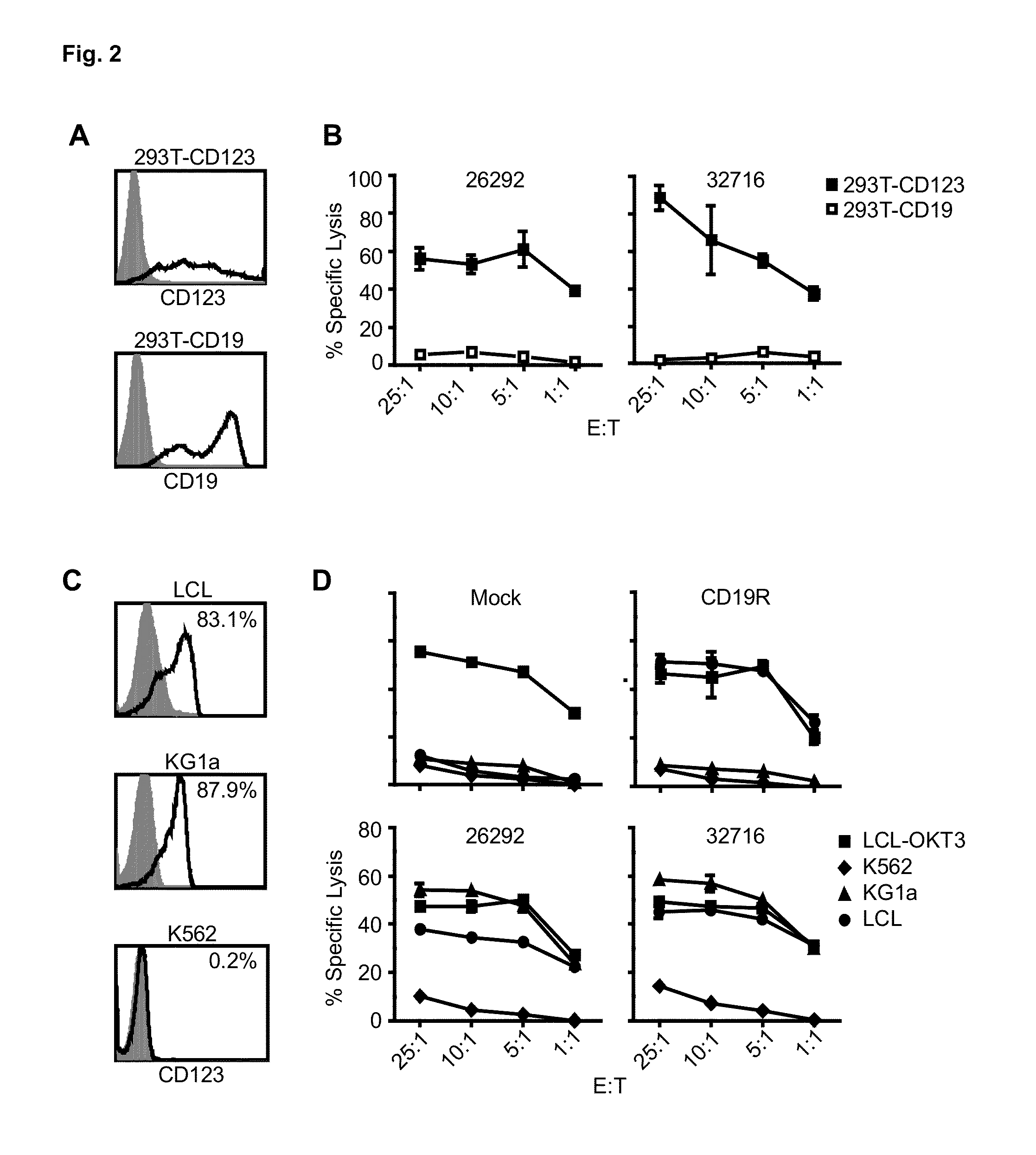
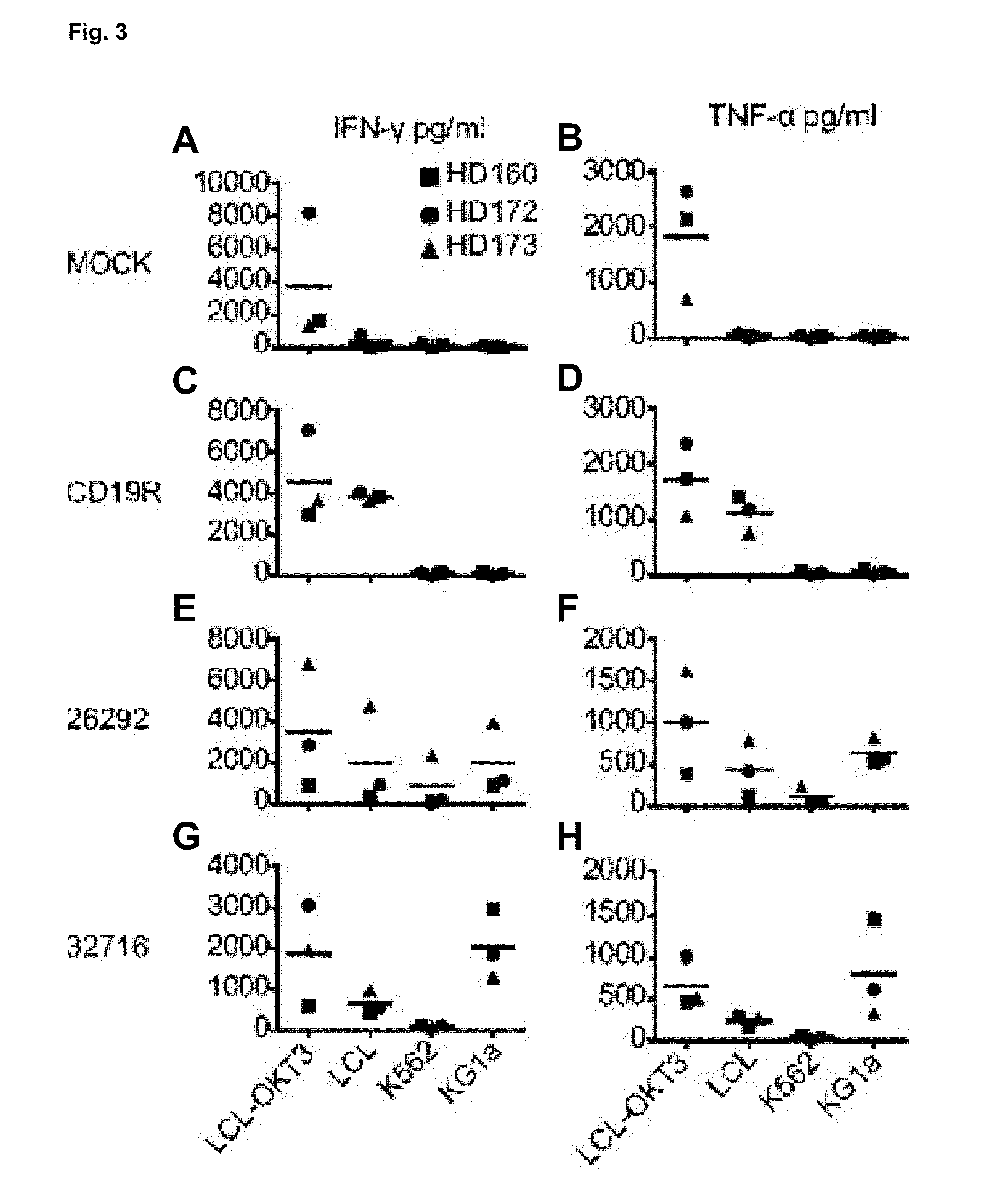
A family of chimeric antigen receptors (CARs) containing a CD123 specific scFv was developed to target different epitopes on CD123. In some embodiments, such a CD123 chimeric antigen receptor (CD123CAR) gene includes an anti-CD123 scFv region fused in frame to a modified IgG4 hinge region comprising an S228P substitution, an L235E substitution, and optionally an N297Q substitution; a costimulatory signaling domain; and a T cell receptor (TCR) zeta chain signaling domain. When expressed in healthy donor T cells (CD4 / CD8), the CD123CARs redirect T cell specificity and mediated potent effector activity against CD123+ cell lines as well as primary AML patient samples. Further, T cells obtained from patients with active AML can be modified to express CD123CAR genes and are able to lyse autologous AML blasts in vitro. Finally, a single dose of 5.0×106 CAR123 T cells results in significantly delayed leukemic progression in mice. These results suggest that CD123CAR-transduced T cells may be used as an immunotherapy for the treatment of high risk AML.
Owner:CITY OF HOPE
Human antibodies to PD-1
ActiveUS9987500B2Rescues T-cell signalingInhibit tumor growthNervous disorderAntipyreticFc(alpha) receptorDisease
The present invention provides antibodies that bind to the T-cell co-inhibitor programmed death-1 (PD-1) protein, and methods of use. In various embodiments of the invention, the antibodies are fully human antibodies that bind to PD-1. In certain embodiments, the present invention provides multi-specific antigen-binding molecules comprising a first binding specificity that binds to PD-1 and a second binding specificity that binds to an autoimmune tissue antigen, another T-cell co-inhibitor, an Fc receptor, or a T-cell receptor. In some embodiments, the antibodies of the invention are useful for inhibiting or neutralizing PD-1 activity, thus providing a means of treating a disease or disorder such as cancer or a chronic viral infection. In other embodiments, the antibodies are useful for enhancing or stimulating PD-1 activity, thus providing a means of treating, for example, an autoimmune disease or disorder.
Owner:REGENERON PHARM INC
Compositions comprising t cell receptors and methods of use thereof
InactiveUS20090053184A1High affinityEfficient killingOrganic active ingredientsBiocideMelanomaBiology
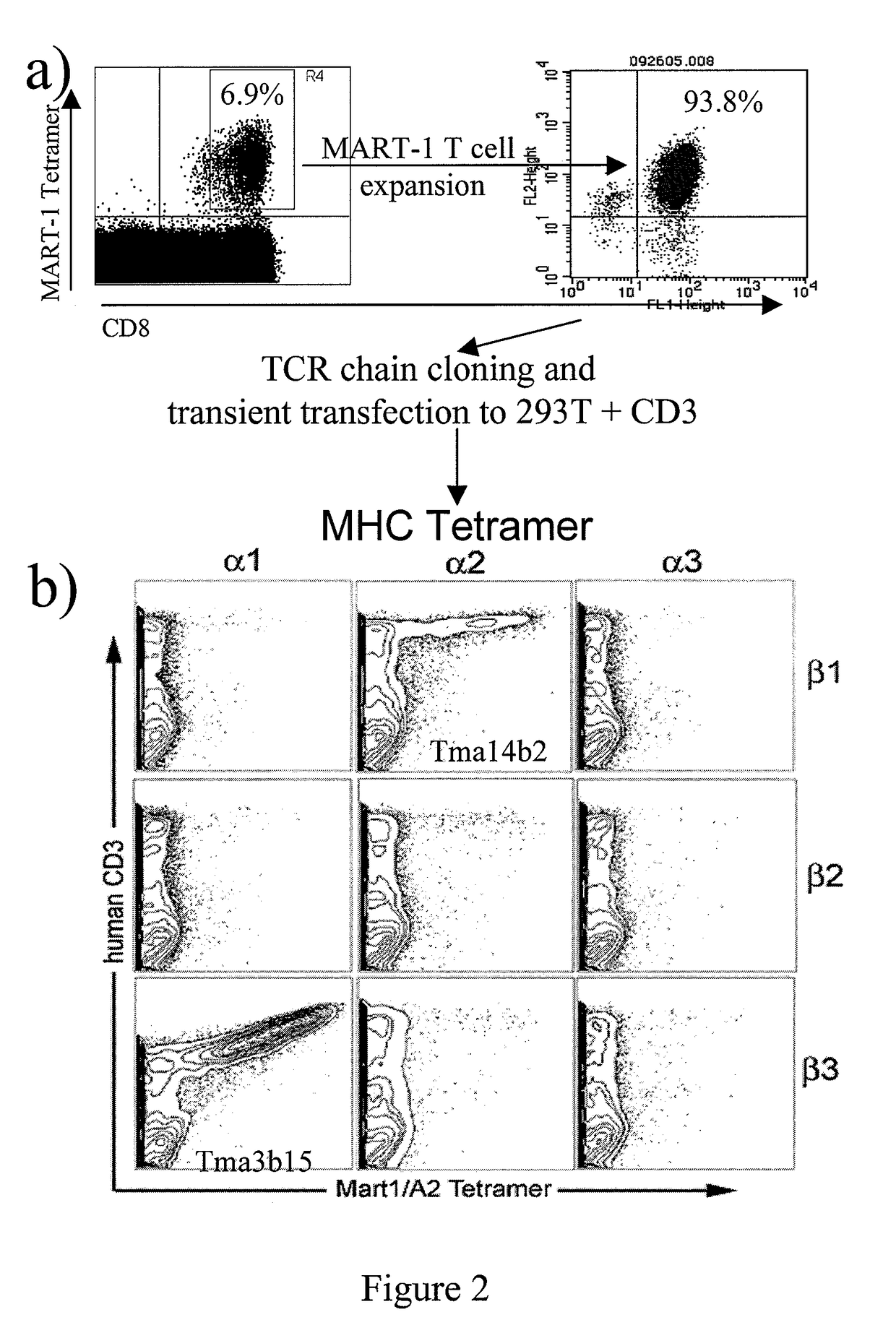
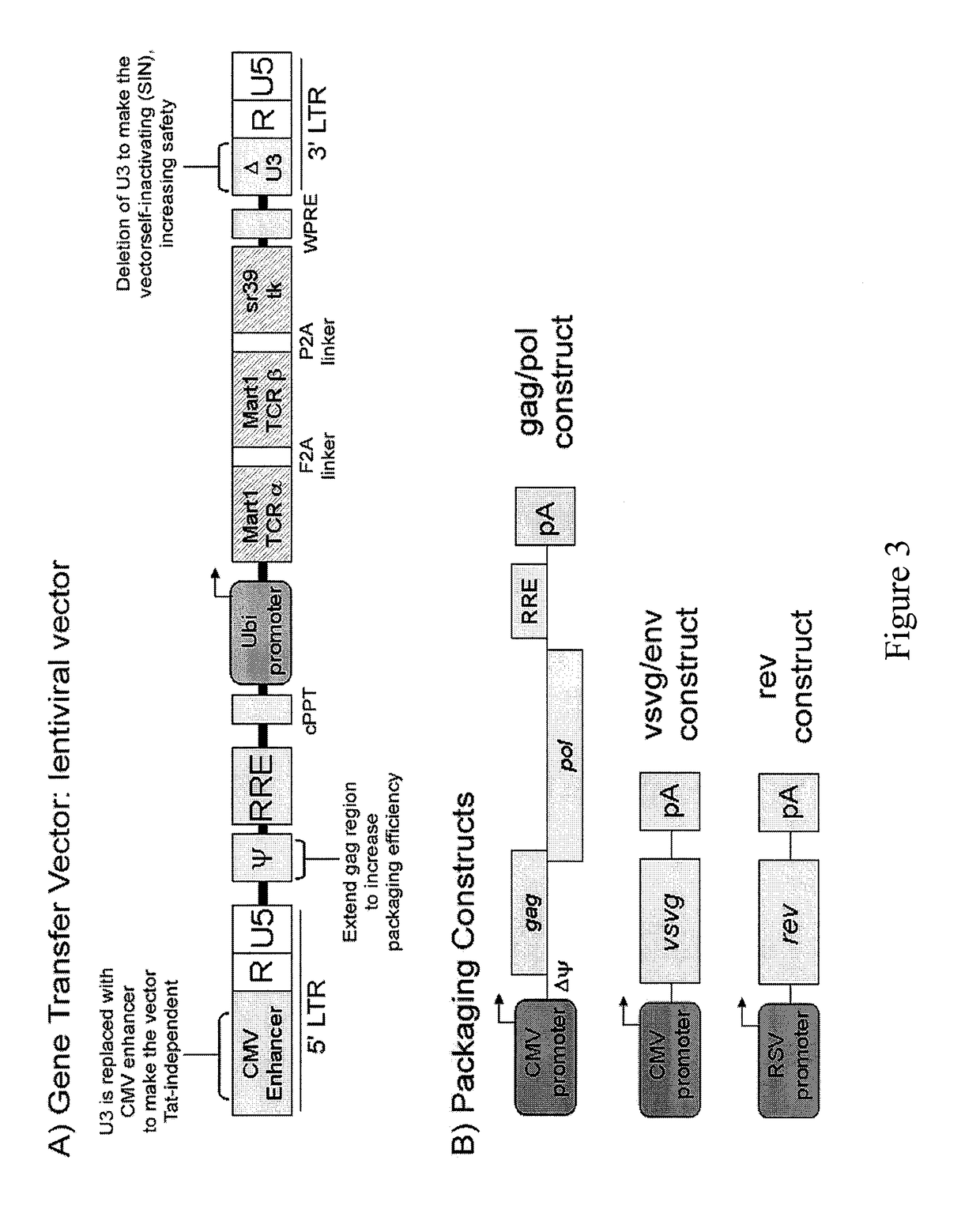
Nucleic acids encoding antitumor TCRs recognizing MART-1, NY-ESO-1, and melanoma gp100 peptides; vectors and cells comprising the same; and methods of using the foregoing.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com