Recombinant antibody-like T cell antigen receptor, T cell antigen receptor conjugate, bi-specific molecule and application
A technology of bispecific molecules and cell antigens, applied in the field of biomedicine, can solve problems such as cumbersome processes, unsatisfactory affinity, and application limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1G4113 mammalian cell expression plasmid construction.
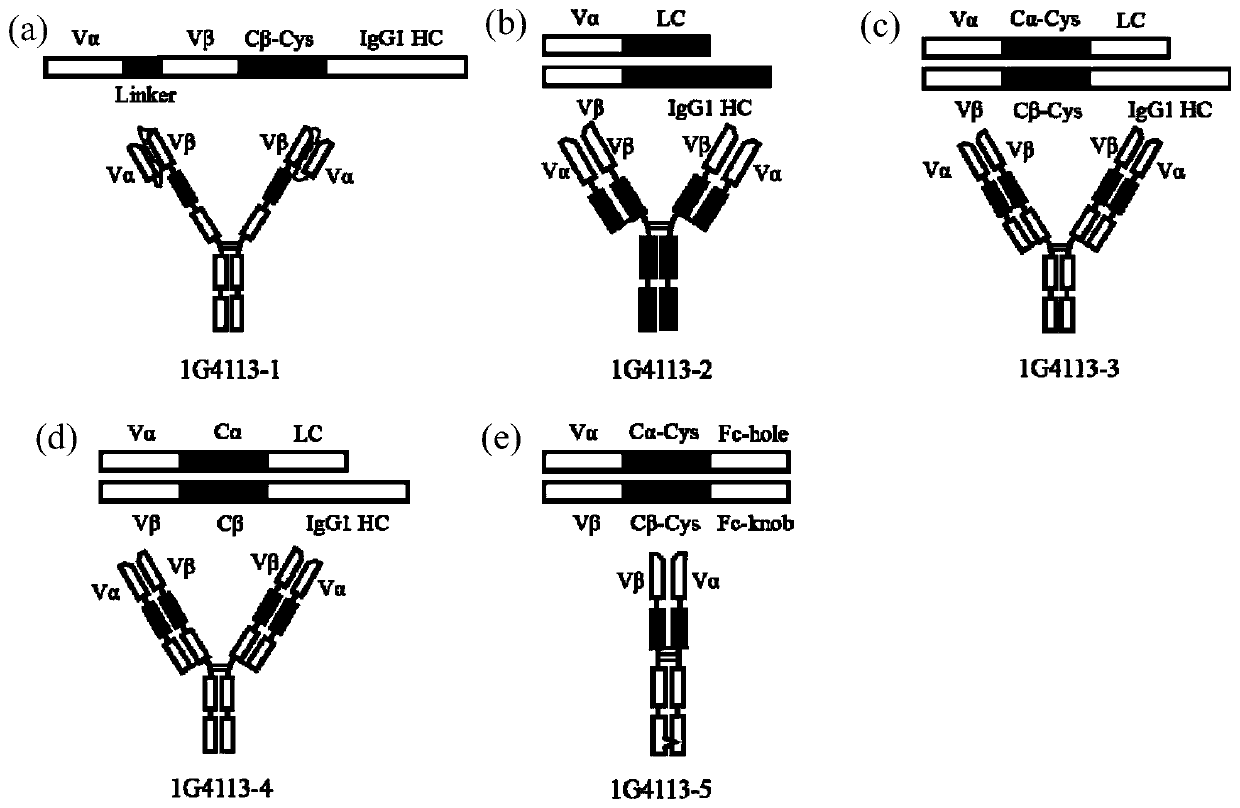
[0048] TCR selection 1G4113 (affinity-matured clone of TCR (named 1G4) targeting the complex of SLLMWITQC polypeptide and HLA-A*0201), from patent: US20110014169A1. A total of 5 different truncated and combined 1G4113 and IgG1 antibody constant regions were designed (the gene sequence of the heavy chain constant region and knob-into-hole mutation is shown in SEQ ID No.14, and the gene sequence of the light chain constant region is shown in SEQ ID Shown in No.15) Fusion expression form ( figure 1 ), and introduce GGGGSLPETGG polypeptide sequence (G 4 S-LPETGG) is used for subsequent catalytic coupling of sortase A to construct 9 human kidney epithelial cell (293F) expression plasmids.
[0049] 1G4113-1: Vα-(G 4 S) 3 -ECDβ(with C-terminal cysteine)-IgG1HC-G 4 S-LPETGG (gene sequence shown in SEQ ID No.1), the fusion protein forms a homodimer.
[0050] 1G4113-2: Vα-GS-LC (gene sequence shown in SEQ ID No.2), Vβ...
Embodiment 2
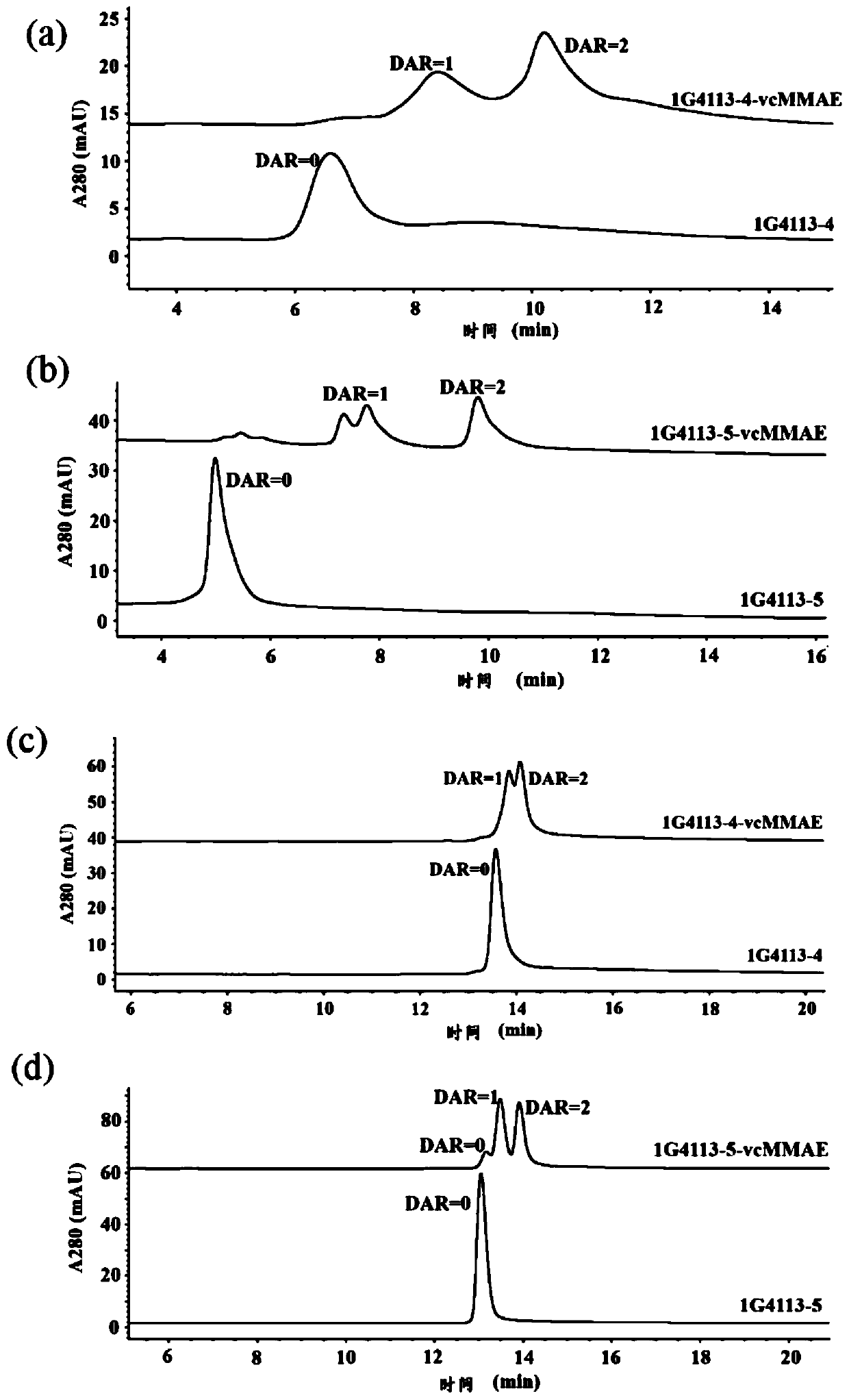
[0061] Expression and purification of 1G4113.
[0062] The above five kinds of plasmids containing different forms of 1G4113 (wherein 1G4113-1 is a plasmid, and 1G4113-2~5 include two plasmids for expressing two subunits respectively) were transfected into 293F cells for 4 days, 4000g, Centrifuge for 15 minutes, take the supernatant, filter it through a 0.45 μm filter membrane, and use Purification instrument and HiTrap Protein A HP prepacked column (purchased from GE, catalog number: 17-0403-01) for purification. Purification steps: HiTrap Protein A HP prepacked column was equilibrated with 100% Protein A loading buffer (50mM Tris-NaCl, 150mM NaCl, pH=7.4, filtered through a 0.45μm filter) and started to inject samples. After the sample injection, wash the prepacked column with 100% Protein A loading buffer until the unbound impurities are removed; finally use 100% Protein A elution buffer (50mM citric acid, pH=3.2, 0.45μm filter membrane filter) to collect the target prot...
Embodiment 3
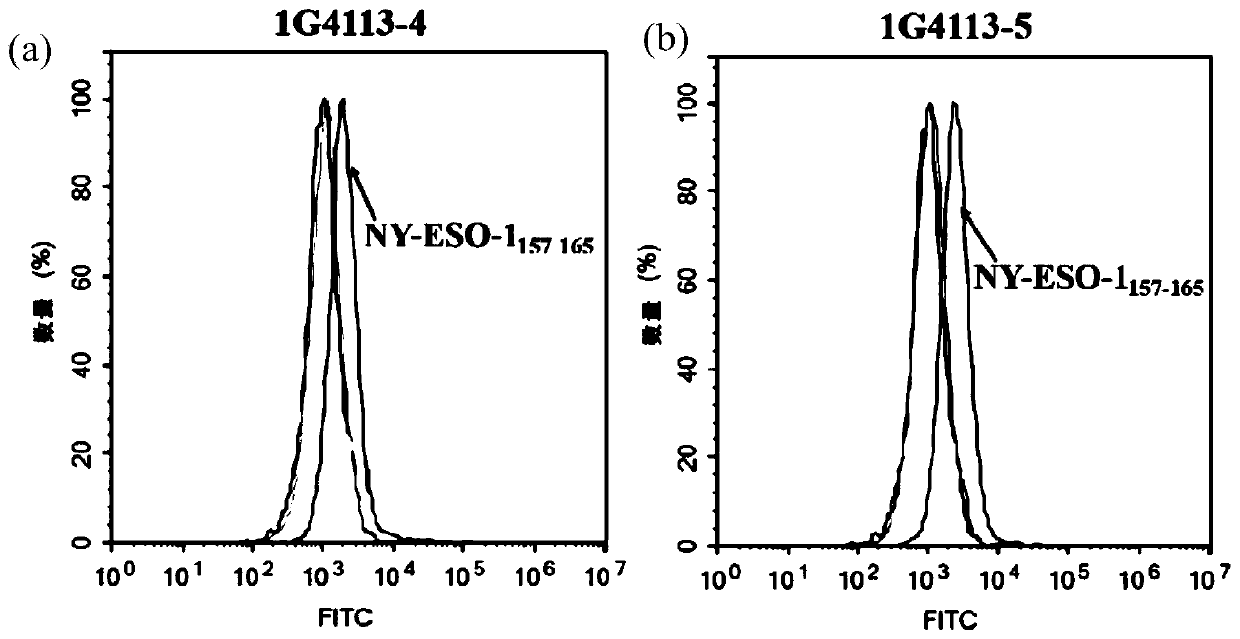
[0065] T2 cells validate the specificity and affinity of 1G4113.
[0066] T2 cells (purchased from ATCC and cultured in IMDM medium containing 20% serum) are TAP-deficient cell lines, and only empty HLA-A*0201 molecules exist on the cell surface. Form a specific pMHC complex for subsequent detection.
[0067] Collect the T2 cells in the logarithmic growth phase, centrifuge at 1000 rpm for 5 min, and discard the supernatant. Wash twice with serum-free RPMI-1640 medium. Resuspended in serum-free IMDM medium, according to 5 × 10 5 1 / well spread in 12-well plate, 1mL / well. Add peptide (synthesized by Hefei Guopeptide Biotechnology Co., Ltd., purity>95%) to a final concentration of 25 μg / mL, and β2m (purchased from Sigma, catalog number: M4890) to a final concentration of 5 μg / mL. After mixing well, place at 37°C, 5% CO 2 , and incubated in a constant temperature incubator with saturated humidity for 6h.
[0068] After the incubation, the cells were collected by centrifugat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com