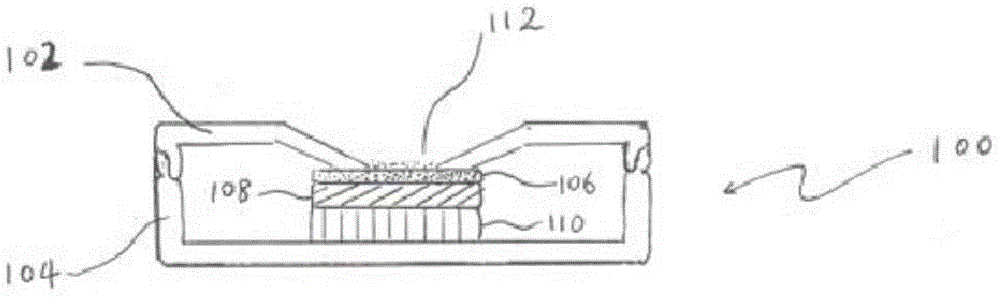
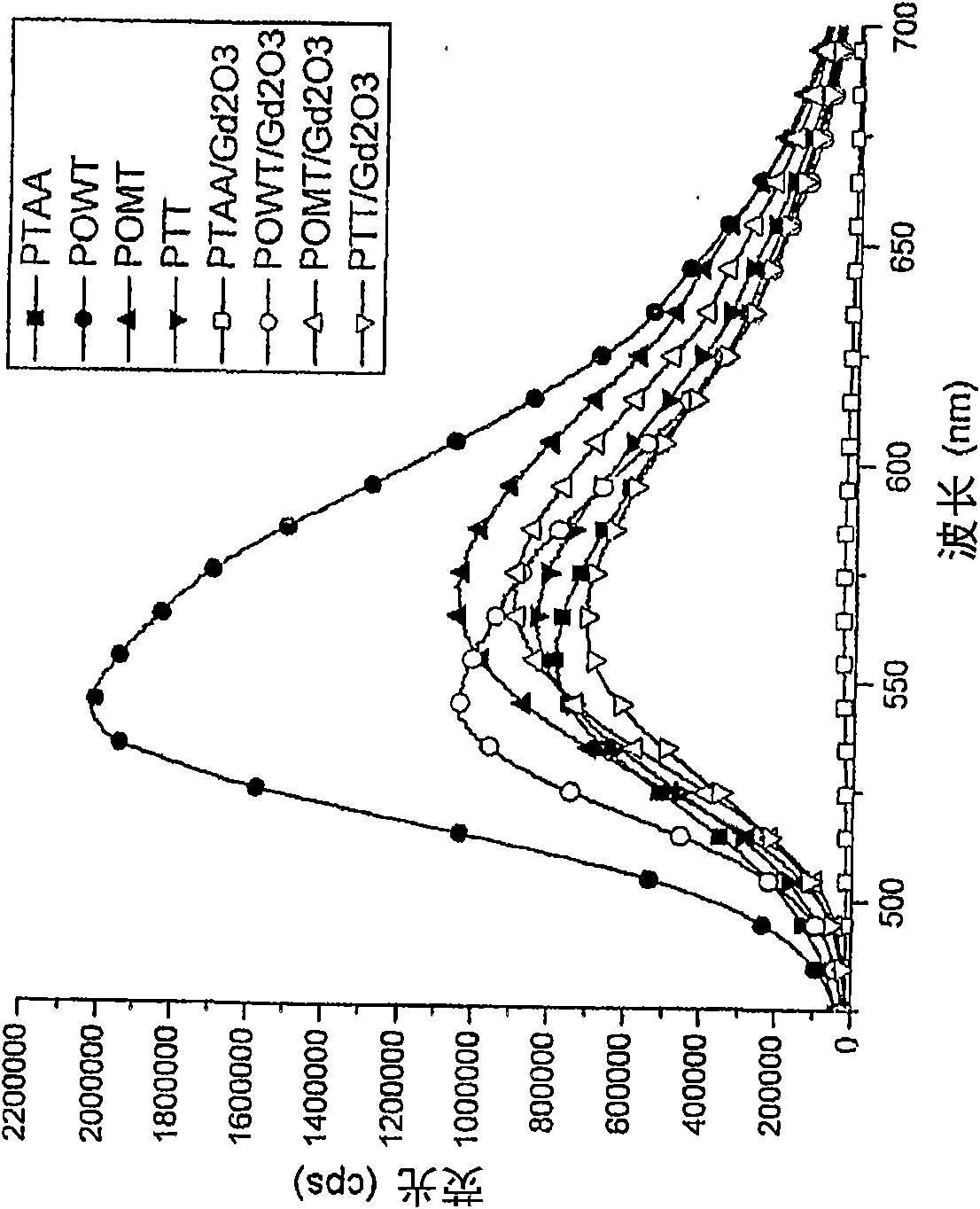
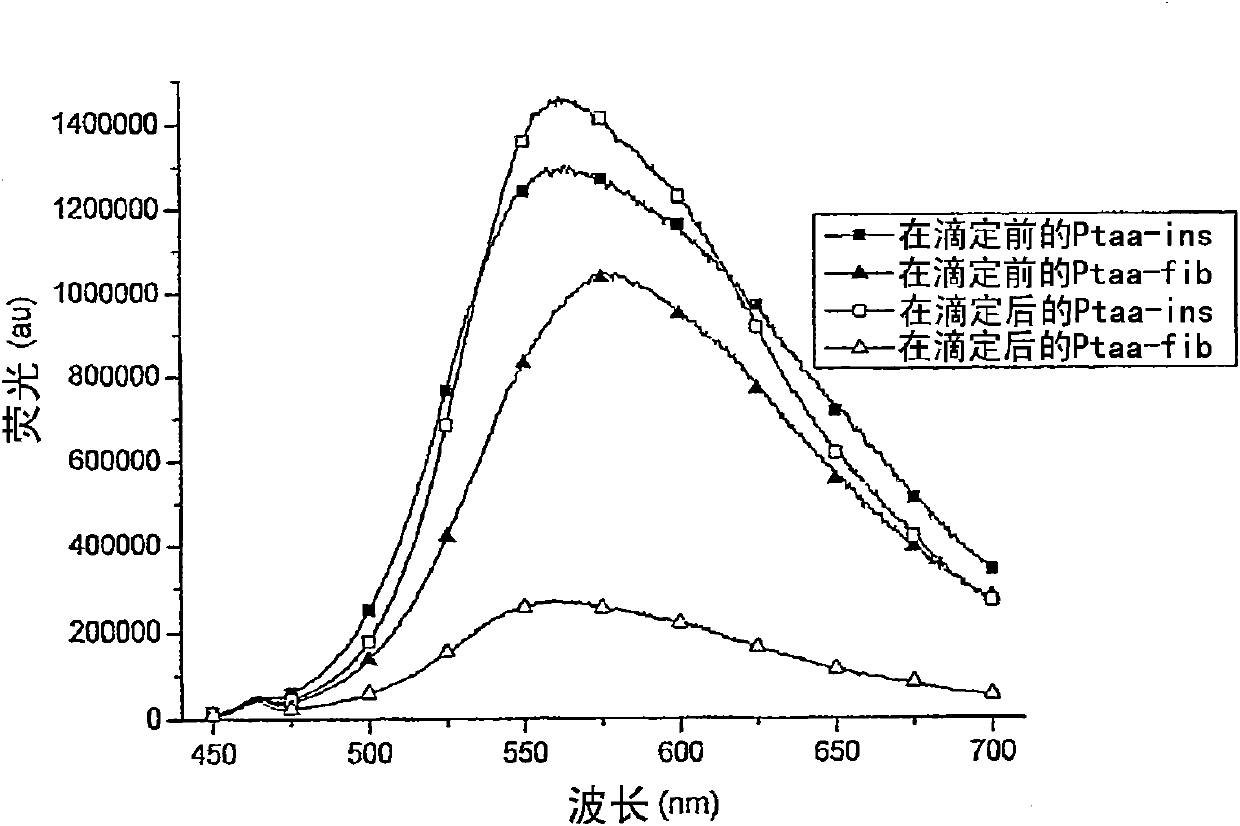
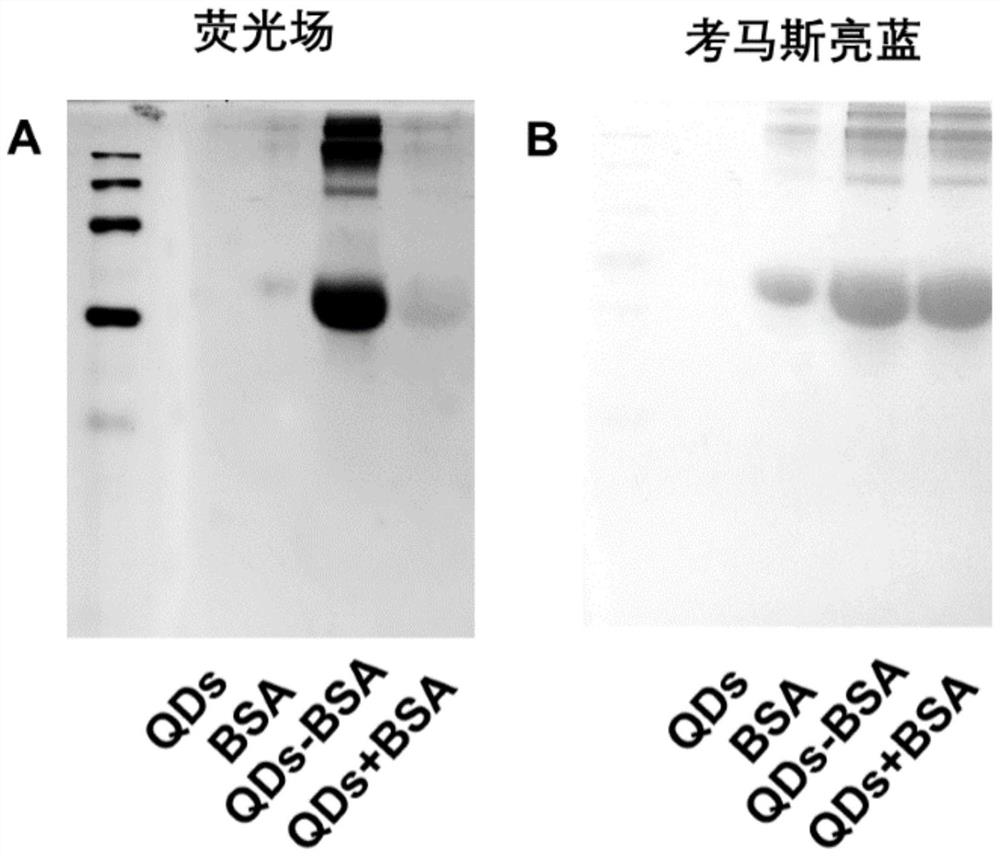
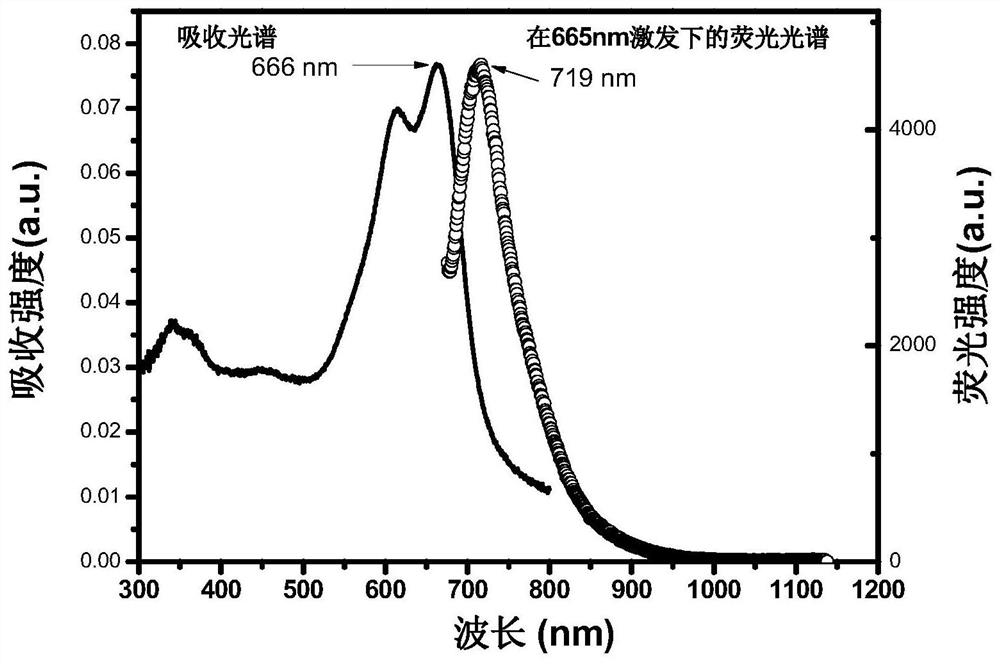
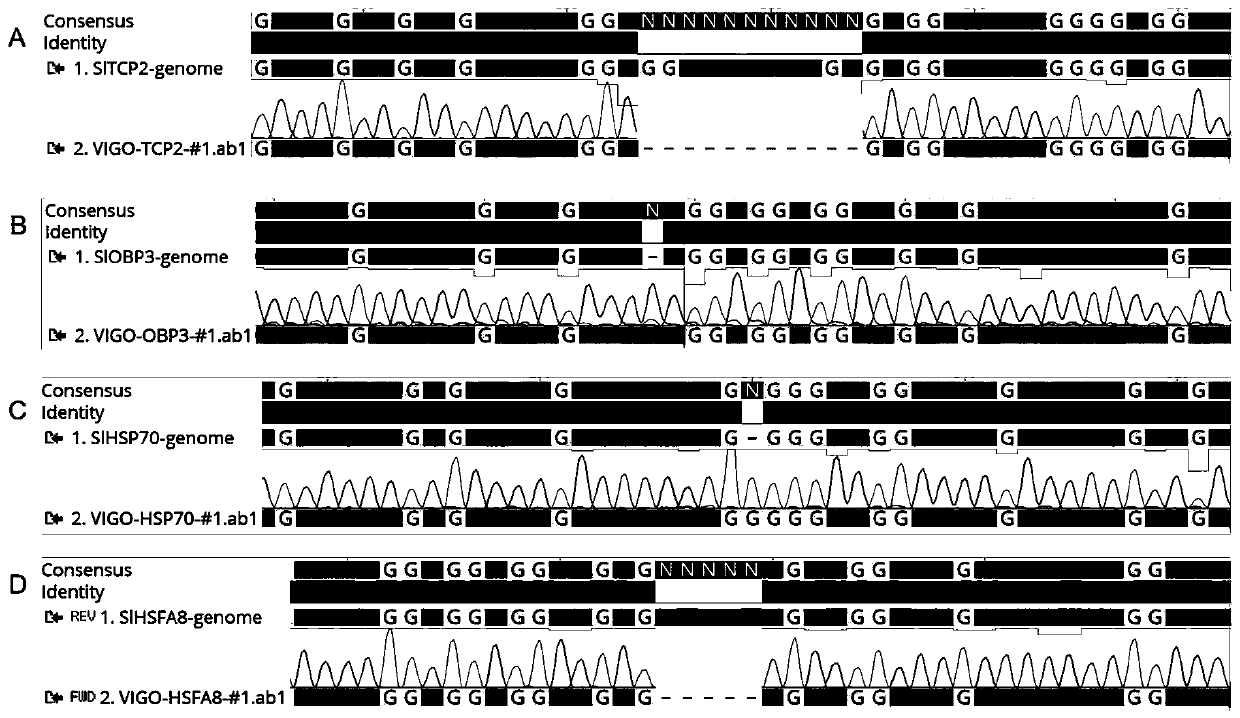
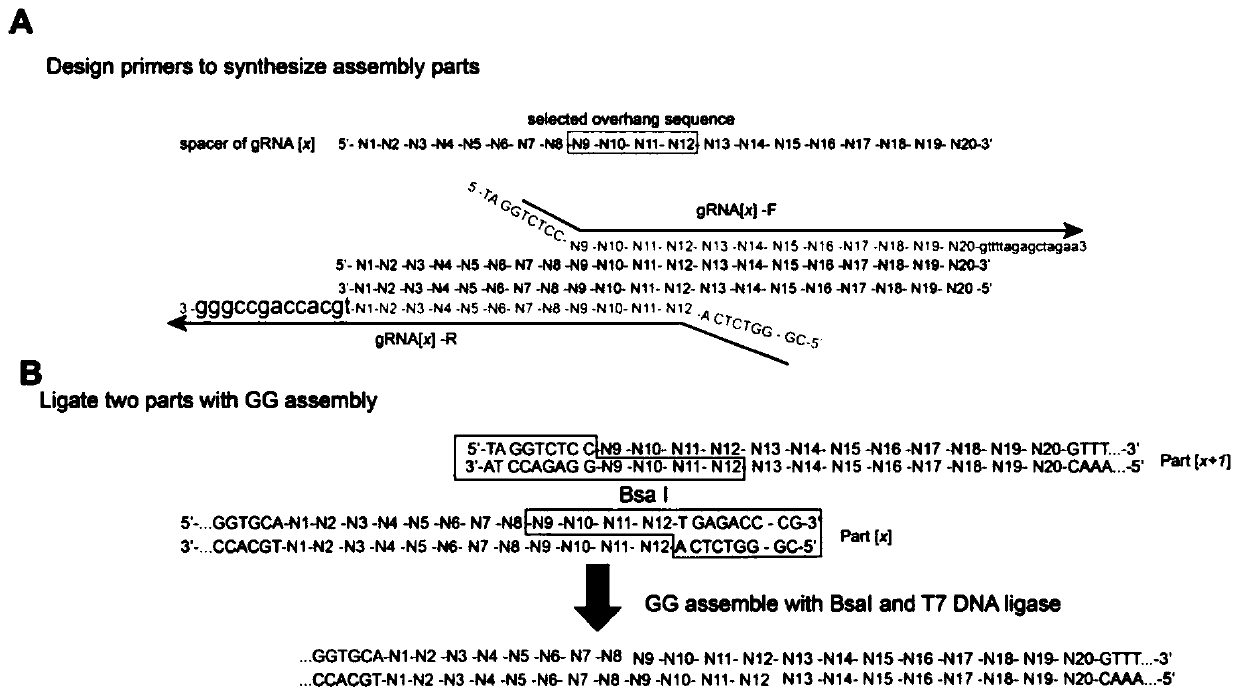
Patents
Literature
41 results about "Normal protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hmgi proteins in cancer and obesity
InactiveUS20030051260A1Peptide/protein ingredientsMicrobiological testing/measurementProtein regulationEmbryonic Stage
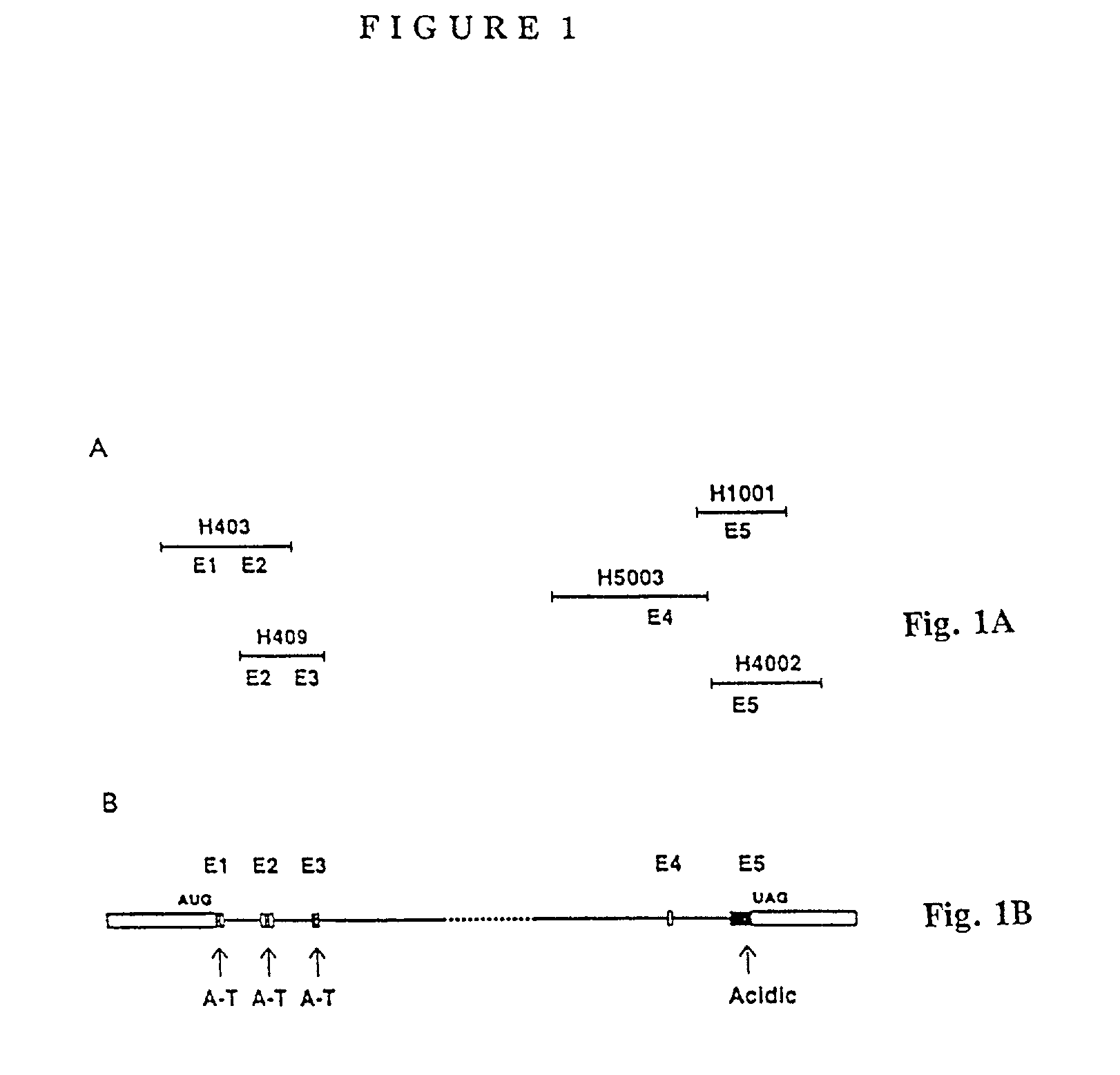
The present invention pertains to a method for treating obesity in a mammal which comprises reducing the biological activity of HMGI genes in the mammal. In another embodiment, the invention pertains to a method for treating a tumor in a patient by reducing the biological activity of normal HMGI genes which comprises administering to the patient a therapeutically effective amount of an inhibitor compound active against normal HMGI-C or HMGI(Y) genes. In another embodiment, the invention pertains to a method of producing a transgenic non-human mammal, the germ cells and somatic cells of which contain an inactivated HMGI gene sequence introduced into the mammal at an embryonic stage. In another embodiment, the invention pertains to a method for screening candidate compounds capable of inhibiting the biological activity of normal HMGI proteins. In another embodiment, the invention pertains to a method for screening candidate compounds capable of inhibiting the biological activity of normal HMGI genes. In another embodiment, the invention pertains to a method for detecting normal HMGI proteins as a diagnostic marker for a tumor using a probe. that recognizes normal HMGI proteins, which comprises the steps of (a) contacting normal HMGI proteins from a sample from a patient with a probe which binds to HMGI proteins; and (b) analyzing for normal HMGI proteins by detecting levels of the probe bound to the normal HMGI proteins, wherein the presence of normal HMGI proteins in the sample is positive for a tumor. In another embodiment, the invention pertains to a method for detecting antibodies to normal HMGI proteins using a probe that recognizes antibodies to HMGI normal proteins, which comprises the steps of (a) treating a sample from a patient with a probe which binds to antibodies to normal HMGI proteins; and (b) analyzing for antibodies to HMGI proteins by detecting levels of the probe bound to the antibodies to HMGI proteins, wherein the presence of antibodies to normal HMGI proteins in the sample is positive for a tumor. In another embodiment, the invention pertains to HMGI genes and proteins for use as a starting point to isolate downstream target genes regulated by the HMGI genes and proteins.
Owner:MEDICINE & DENTISTRY OF NEW YORK UNIV OF
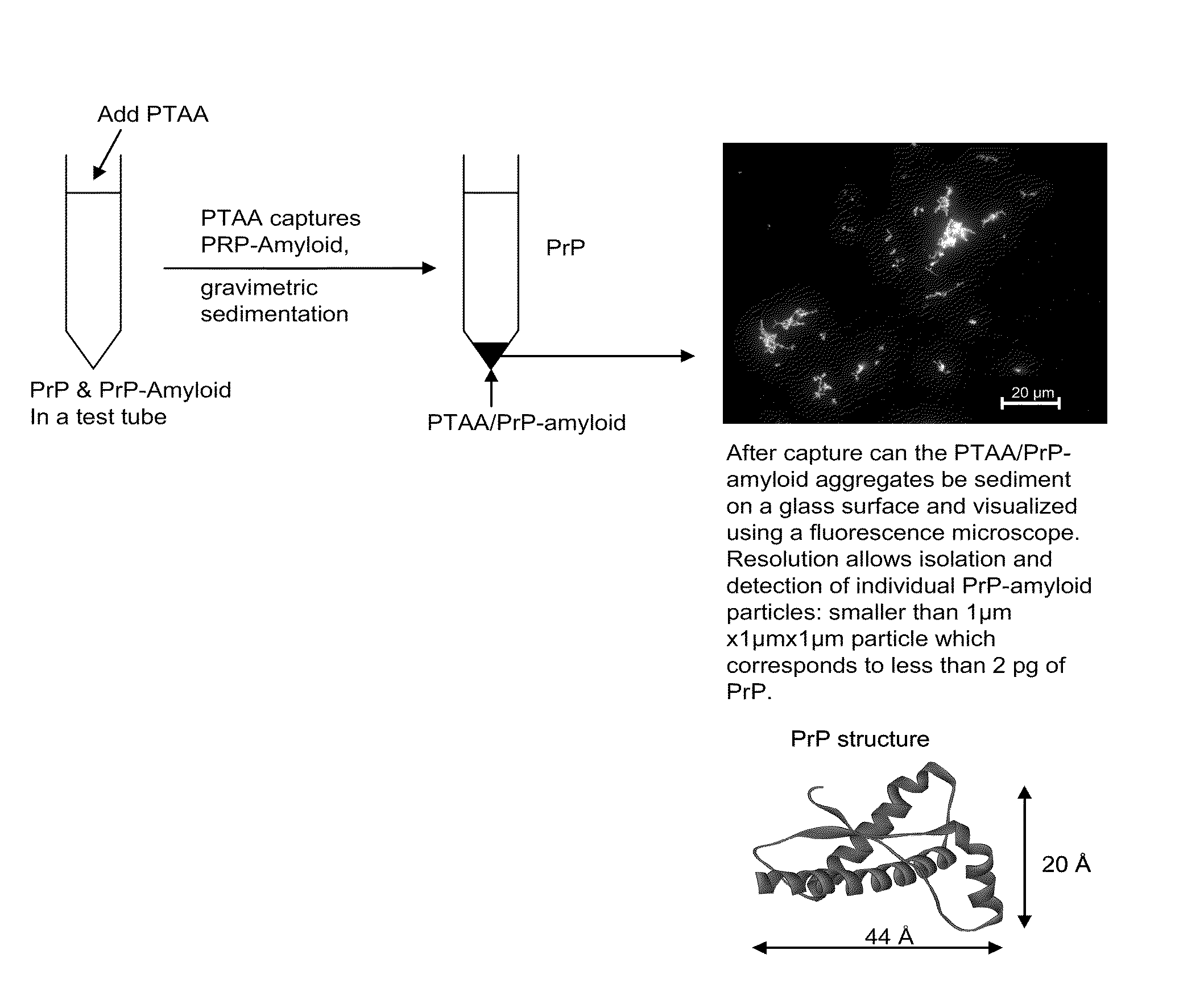
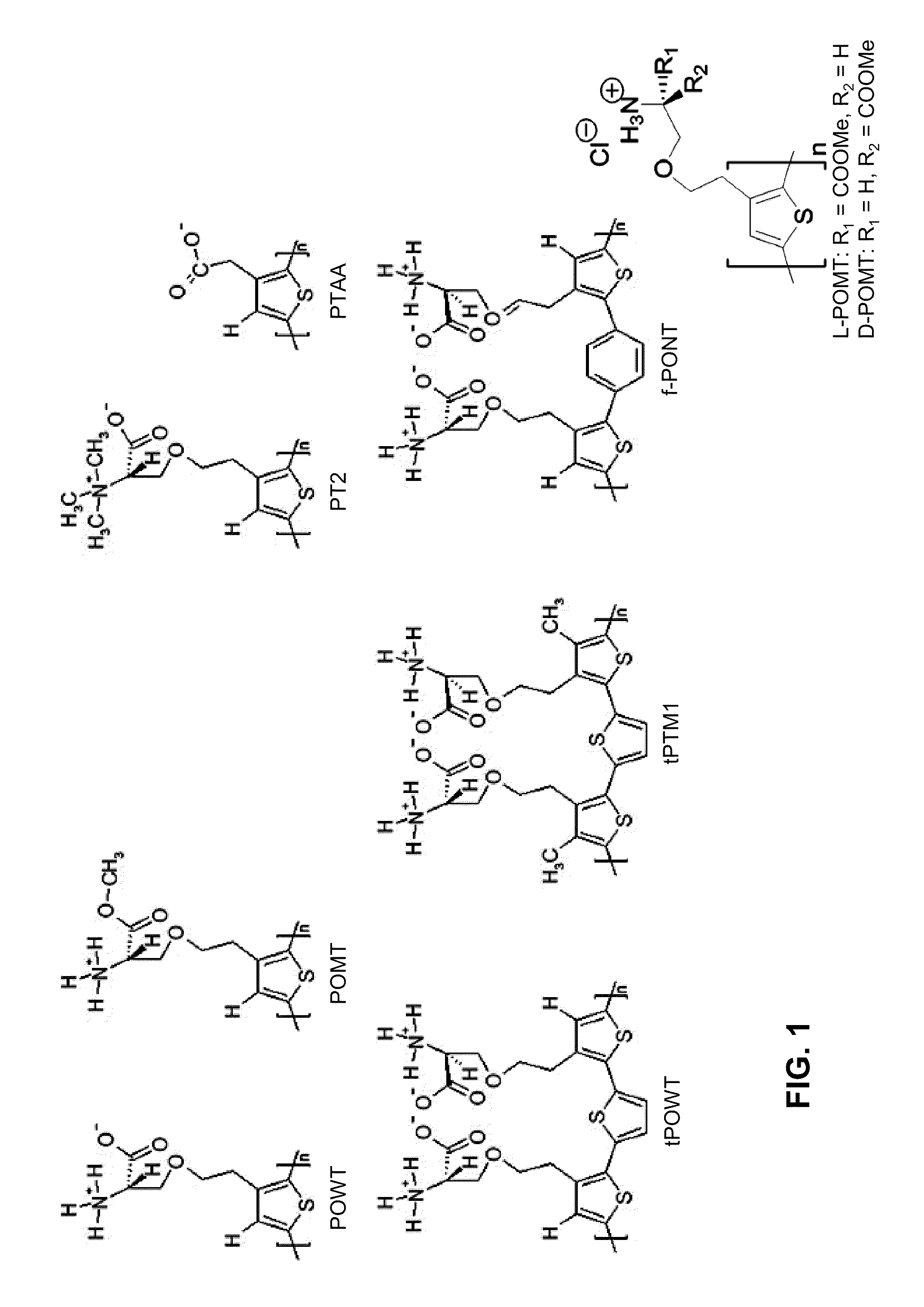
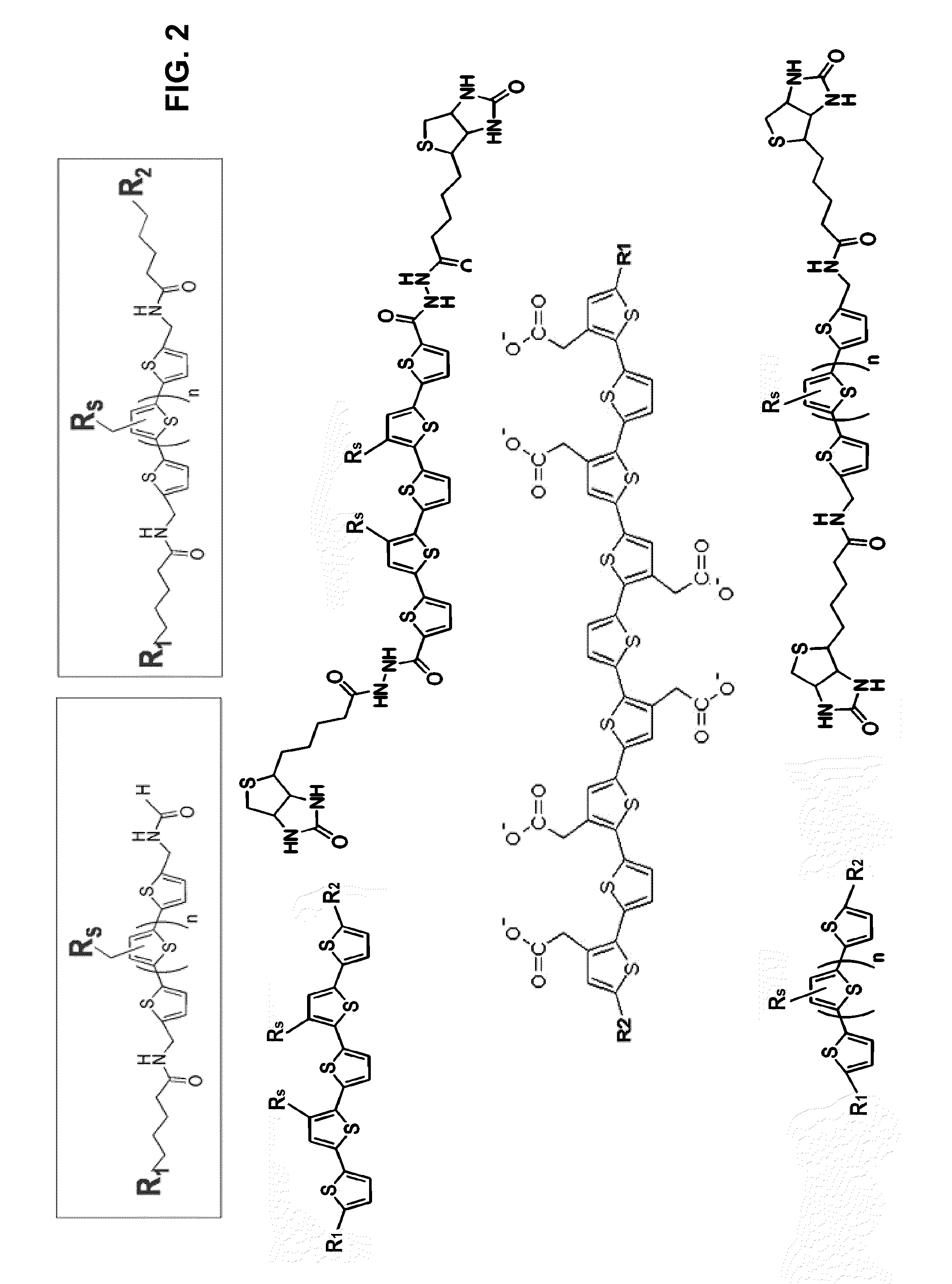
Binding of pathological forms of proteins using conjugated polyelectrolytes
InactiveUS20100310462A1High selectivityEasy to captureNervous disorderMetabolism disorderNormal proteinConjugated Polyelectrolytes
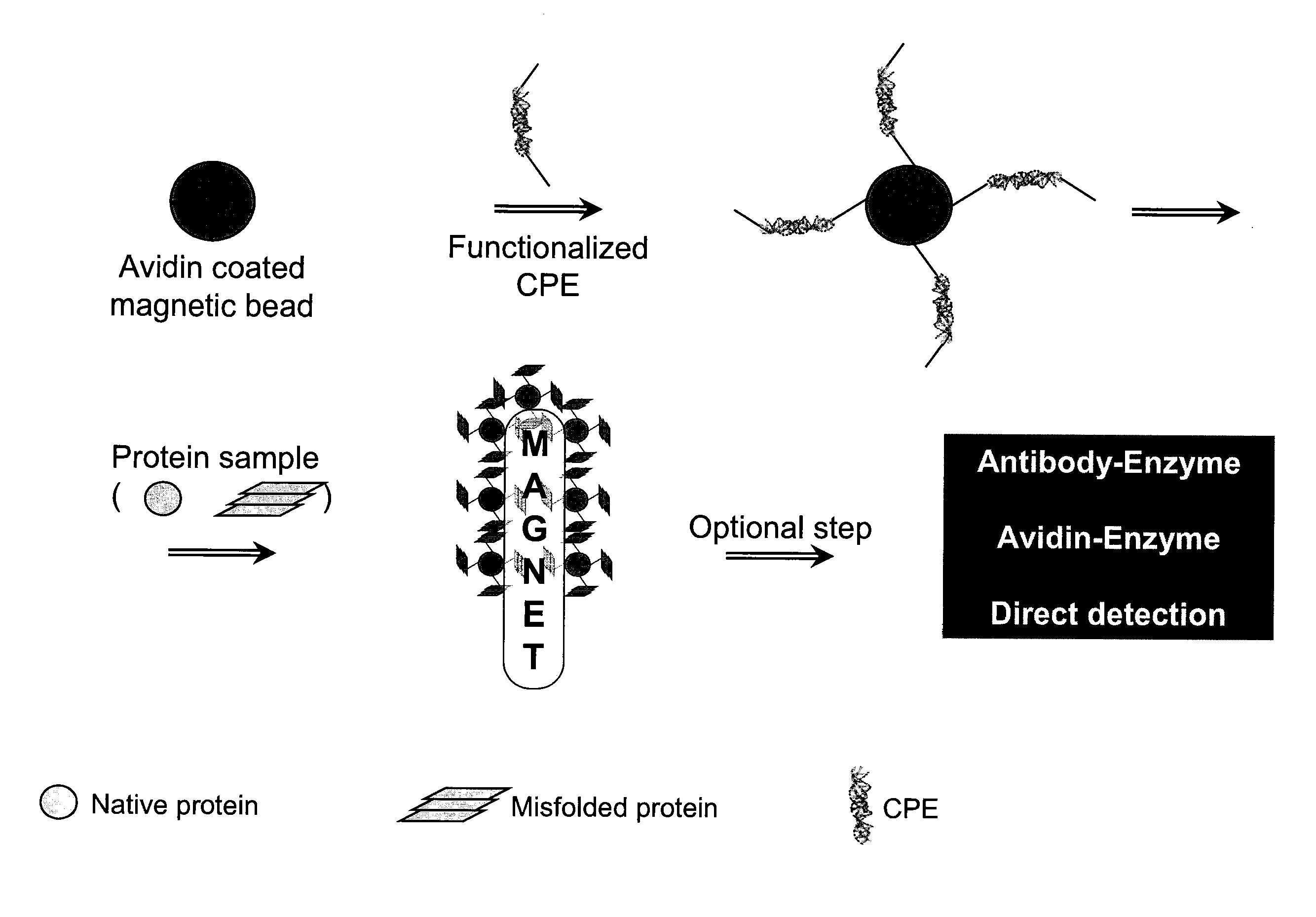
A method for separation of an aggregated misfolded protein from an environment including a non-aggregating normal form of the protein includes contacting both the misfolded and normal protein with a conjugated polyelectrolyte (CPE) and separating the CPE / protein complex from the other constituents of the sample.
Owner:BIOCHROMIX
Phosphorodiamidate morpholino oligomers (PMOS) and their use in suppression of mutant huntingtin expression and attenuation of neurotoxicity
InactiveUS20160017327A1Improve neurotoxicityDecrease HTT protein expressionOrganic active ingredientsSugar derivativesHuntingtons choreaADAMTS Proteins
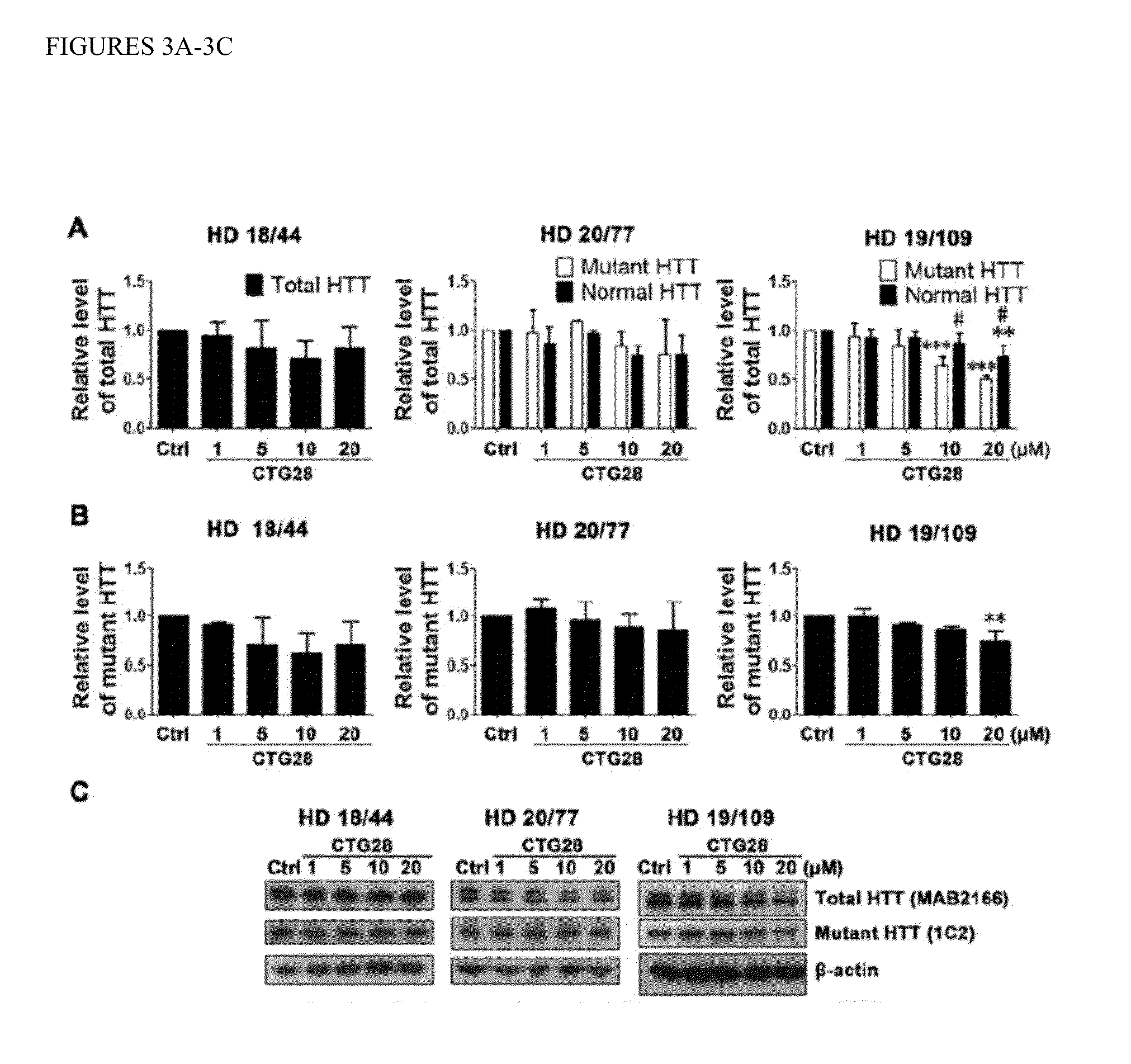
The present invention provides antisense phosphorodiamidate morpholino oligomers which are useful for the suppression or inhibition of the HTT gene involved in Huntington's disease. The oligomers can selectively suppress mutant forms of the HTT protein while allowing the normal protein to be expressed in sufficient quantity to retain its function in the cell. Methods for treatment of Huntington's disease are also provided.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
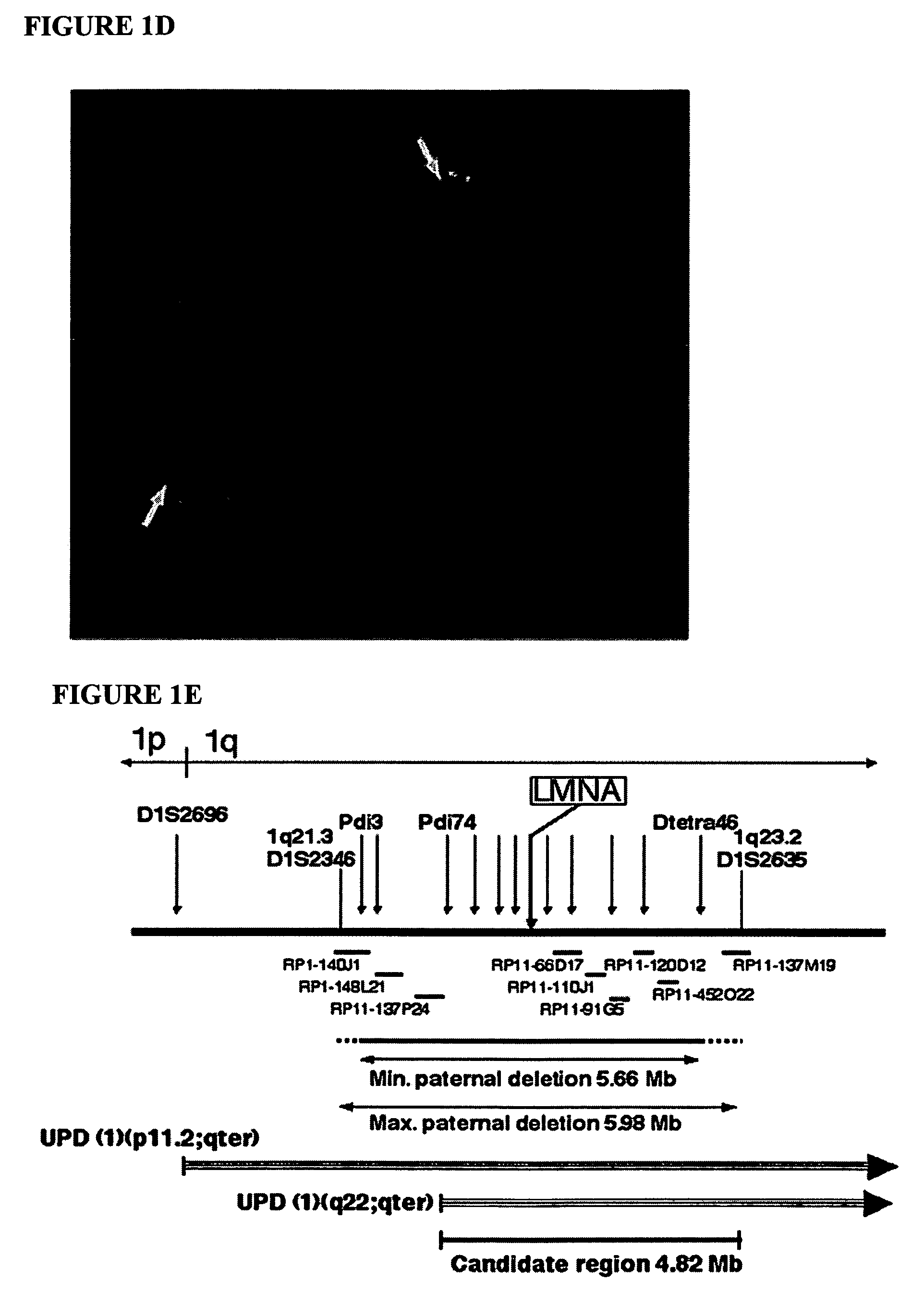
LMNA gene and its involvement in hutchinson-gilford progeria syndrome (HGPS) and arteriosclerosis
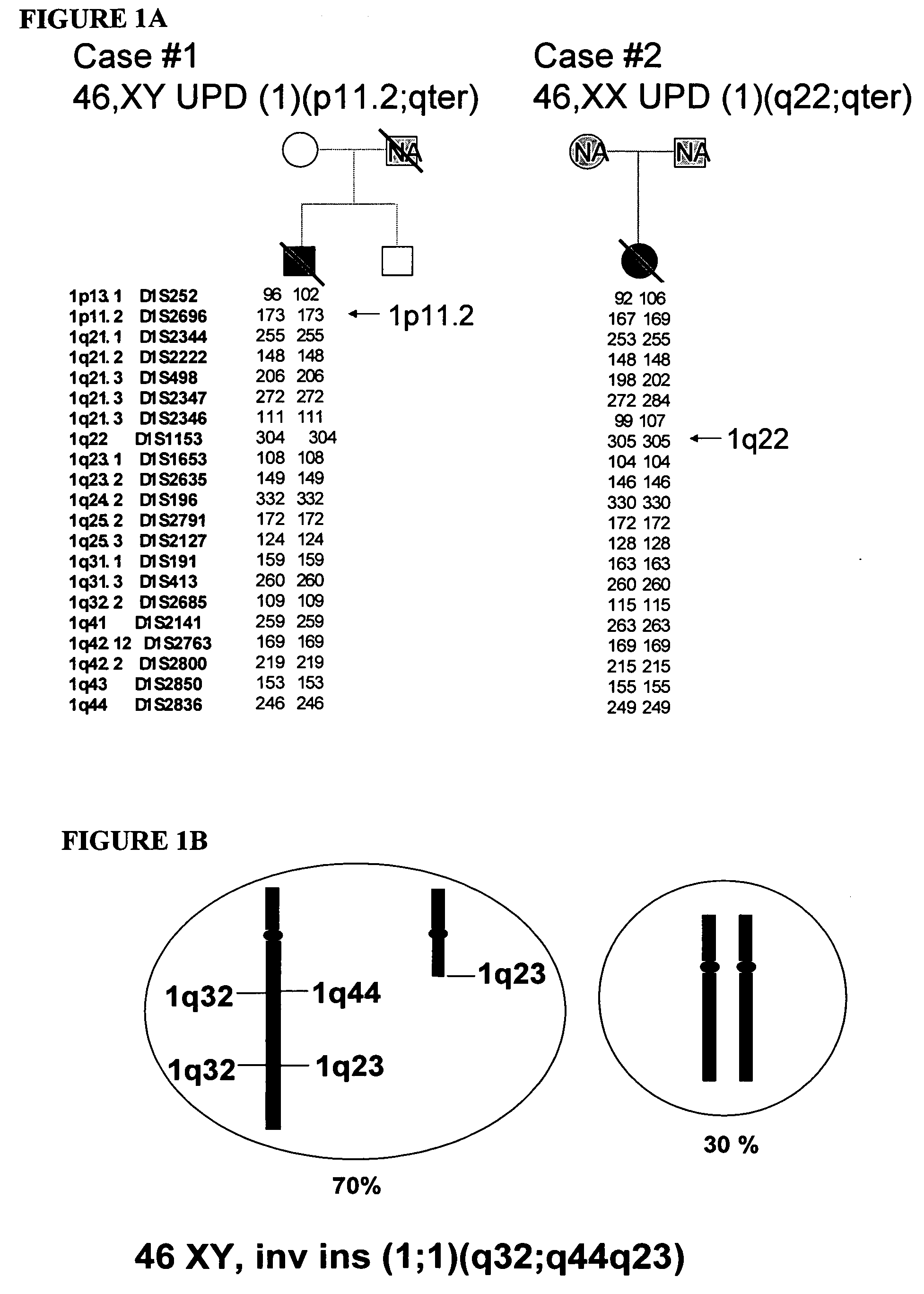
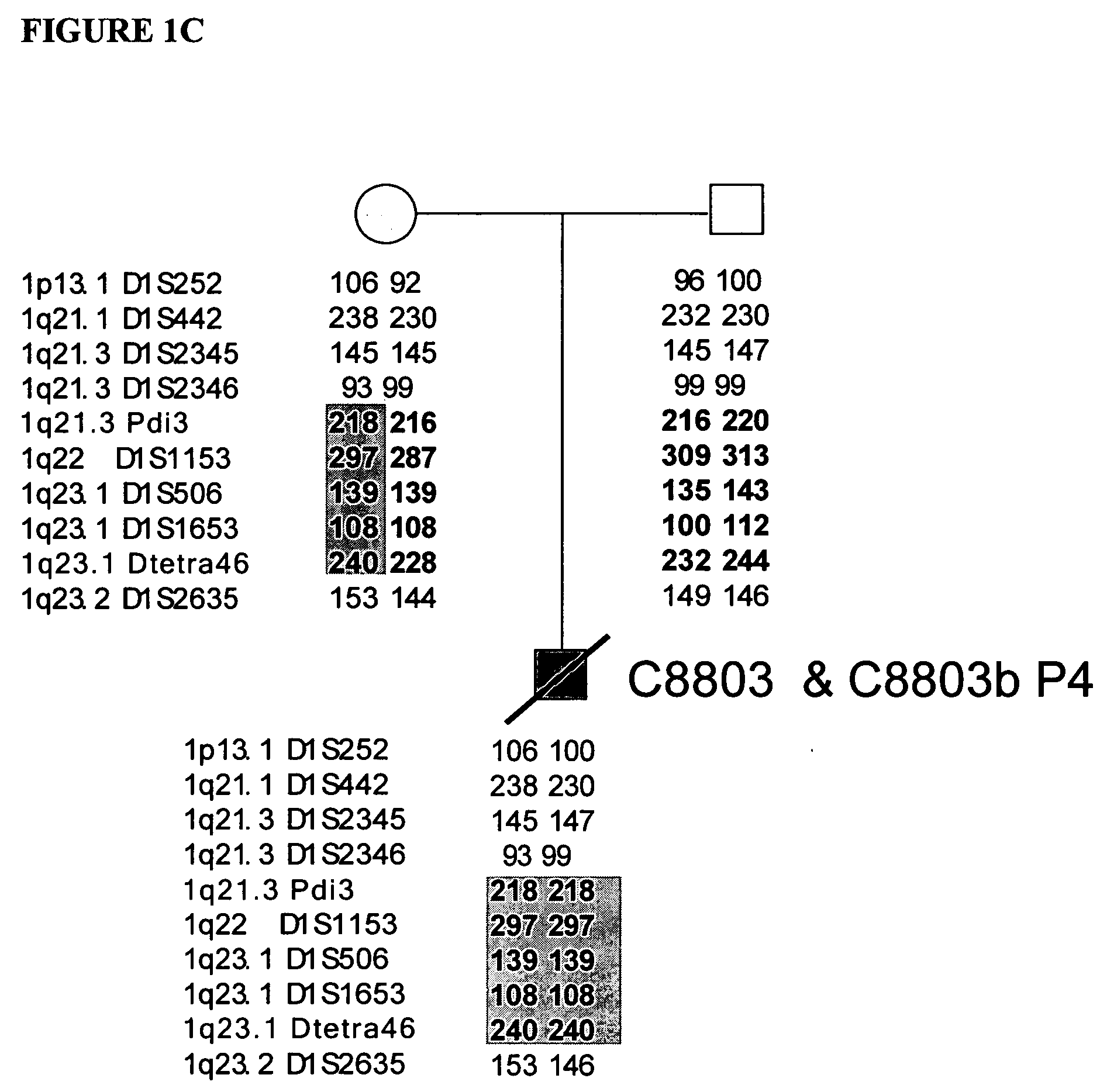
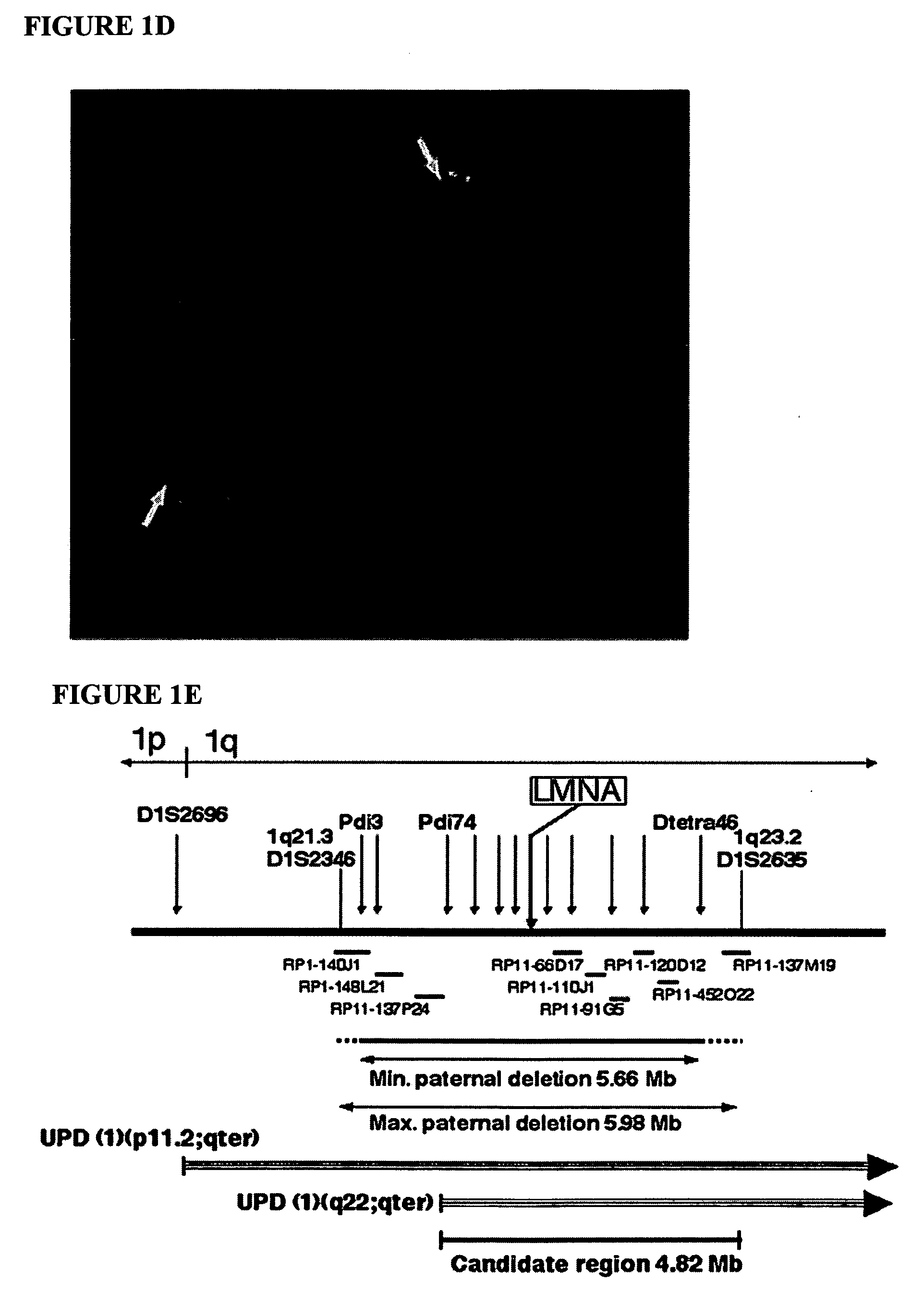
Disclosed herein are point mutations in the LMNA gene that cause HGPS. These mutations activate a cryptic splice site within the LMNA gene, which leads to deletion of part of exon 11 and generation of a mutant Lamin A protein product that is 50 amino acids shorter than the normal protein. In addition to the novel Lamin A variant protein and nucleic acids encoding this variant, methods of using these molecules in detecting biological conditions associated with a LMNA mutation in a subject (e.g., HGPS, arteriosclerosis, and other age-related diseases), methods of treating such conditions, methods of selecting treatments, methods of screening for compounds that influence Lamin A activity, and methods of influencing the expression of LMNA or LMNA variants are also described. Oligonucleotides and other compounds for use in examples of the described methods are also provided, as are protein-specific binding agents, such as antibodies, that bind specifically to at least one epitope of a Lamin A variant protein preferentially compared to wildtype Lamin A, and methods of using such antibodies in diagnosis, treatment, and screening. Also provided are kits for carrying out the methods described herein.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +2
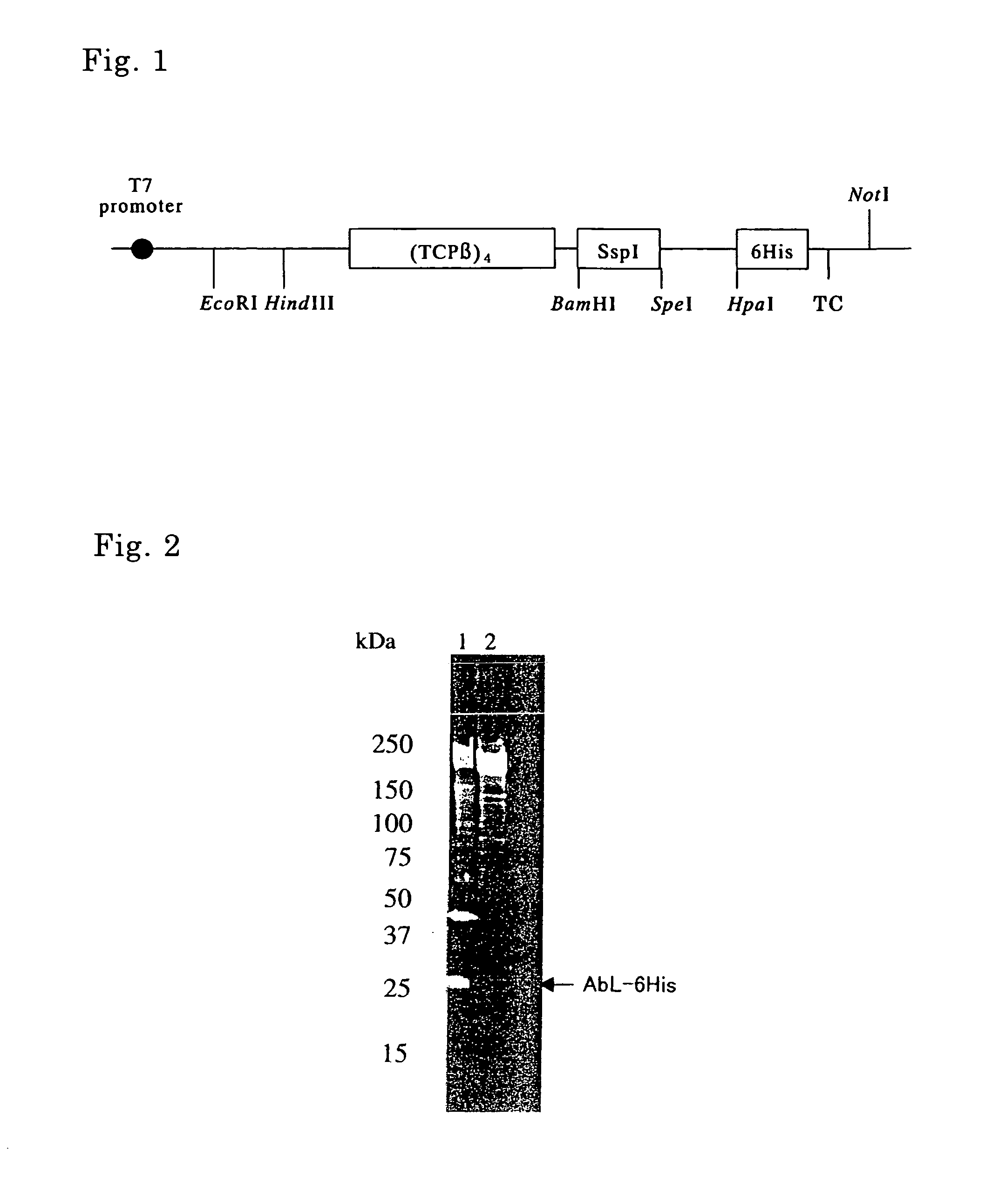
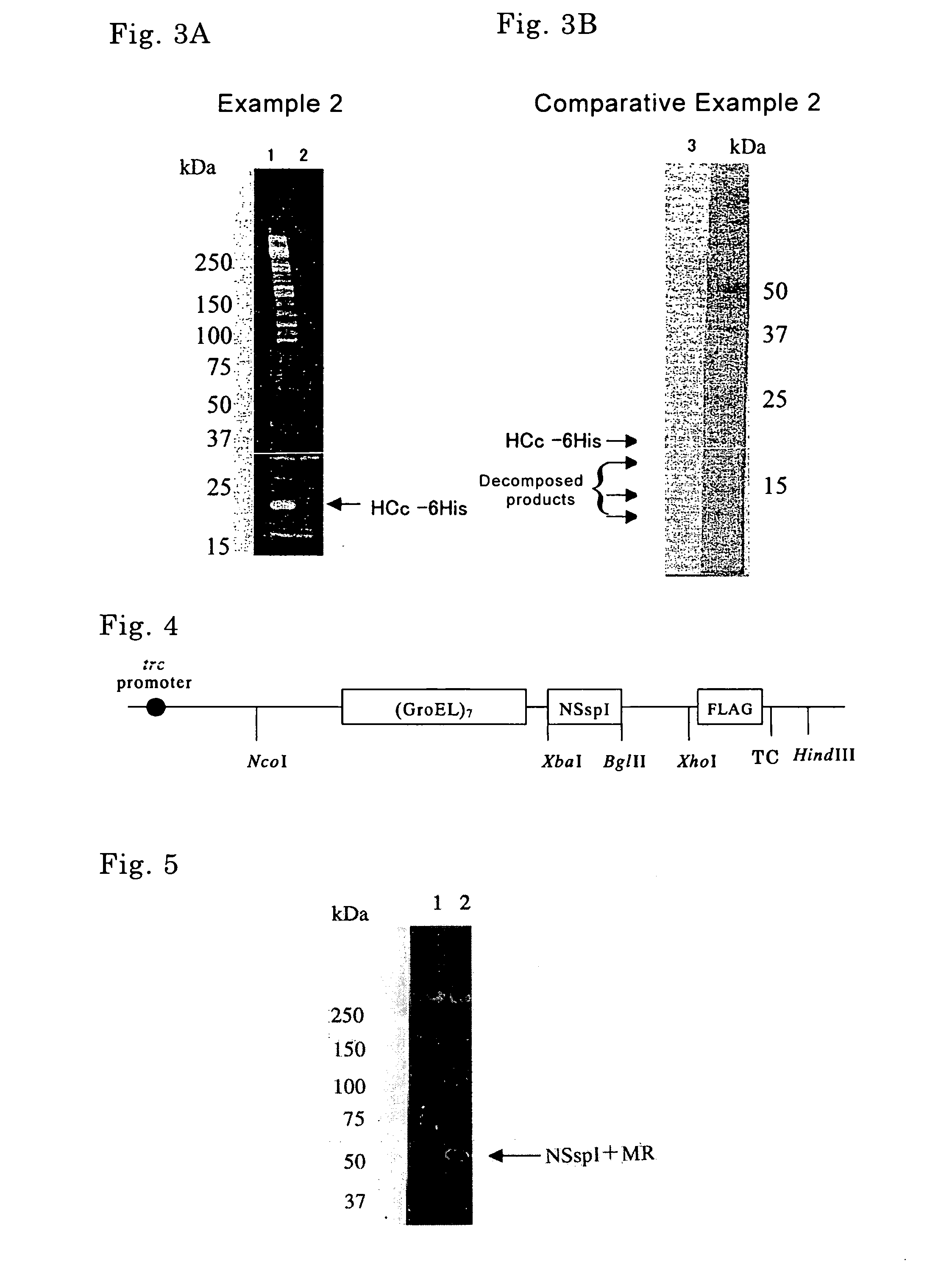
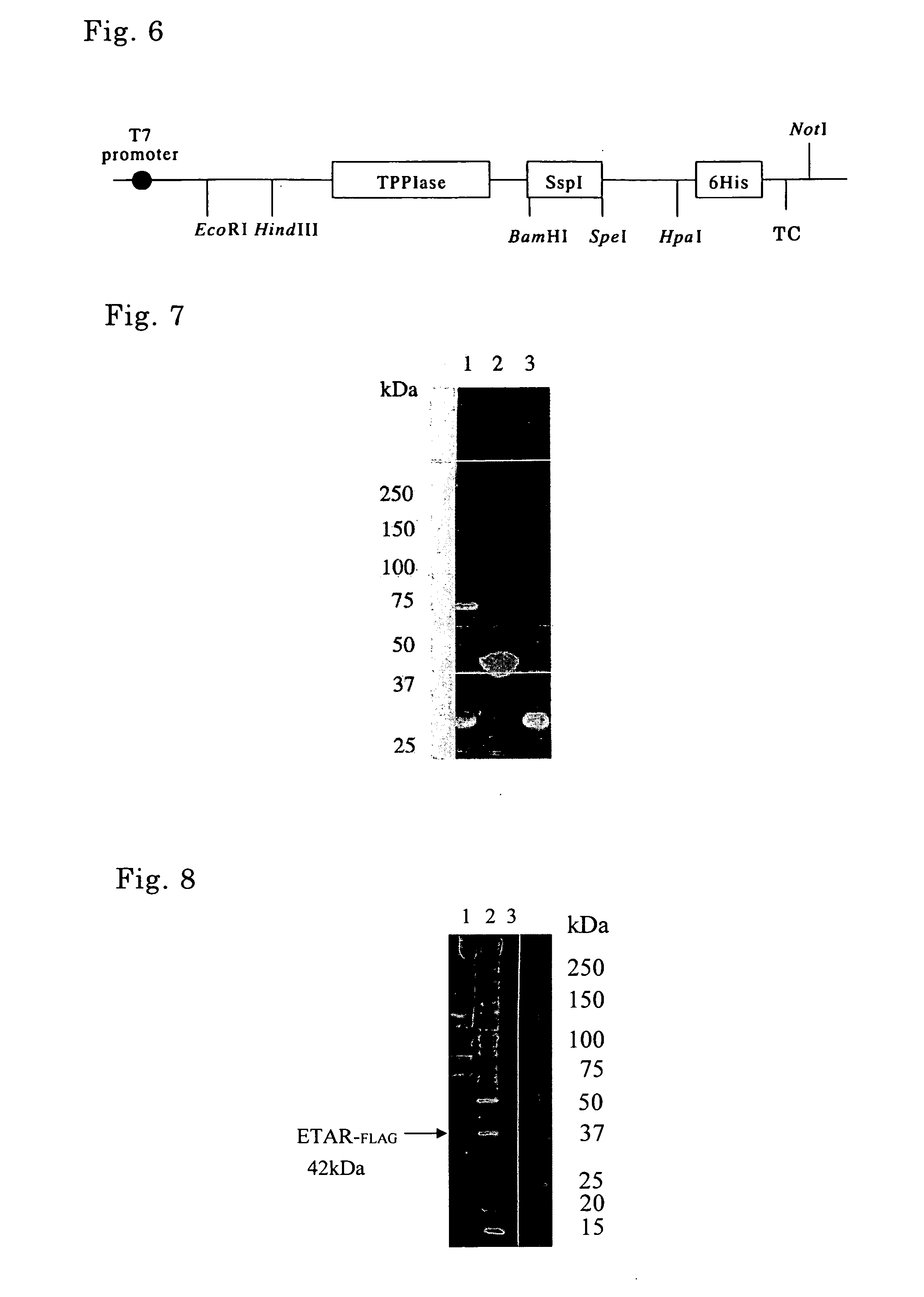
Method of producing target protein, fusion protein and gene thereof, protein consisting of partial sequence of intein and gene thereof, expression vector, and transformant
InactiveUS20070092937A1Easy to introduceEfficient productionFungiBacteriaNormal proteinProtein target
It is intended to provide techniques whereby a target protein is efficiently obtained as a normal protein even in the case of a protein which can be hardly expressed by usual recombinant DNA techniques. A fusion protein composed of a molecular chaperone, an intervening protein having an activity of cleaving a peptide bond, and a target protein is prepared, from which fusion protein the target protein is cleaved by the action of the intervening protein having the activity of cleaving a peptide bond. Examples of the intervening protein having the activity of cleaving a peptide bond include an intein and a protein consisting of a partial sequence of the intein.
Owner:SEKISUI CHEM CO LTD
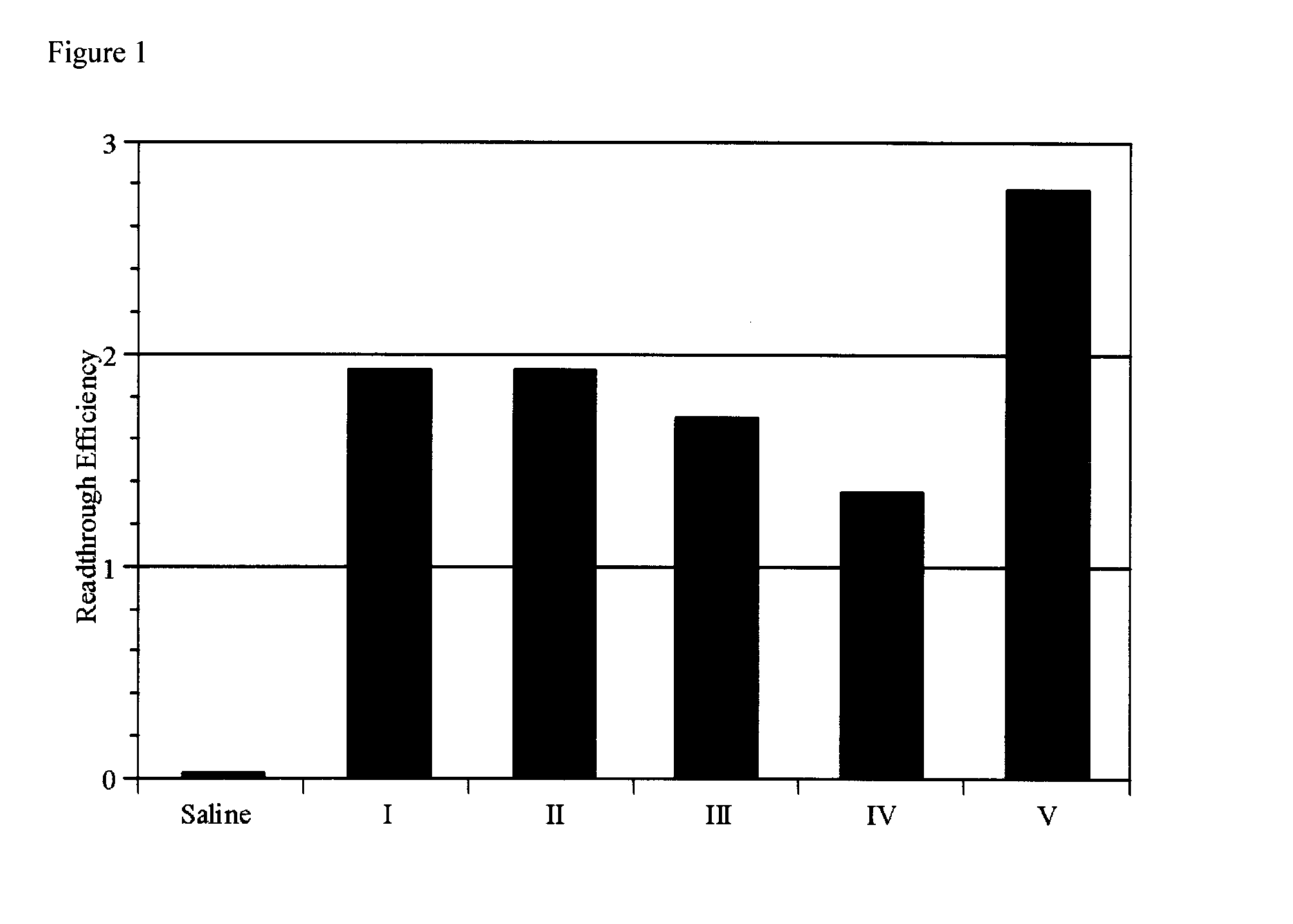
Method Of Treating Genetic Disease Caused By Nonsense Mutation
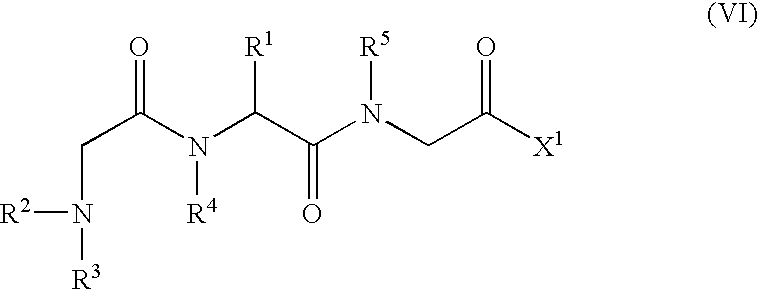
An object of the present invention is to provide compounds having read-through activity for use in treatment methods of genetic diseases caused by nonsense mutation, to provide pharmaceutical compositions comprising the compound, and to provide a treatment method of genetic diseases caused by nonsense mutation comprising administering the compound.The present invention can provide a method of producing wild type normal protein in a living body of a mammal from a gene with a premature termination codon being generated by a mutation, wherein the method comprises administering a compound expressed by the following formula (VI):(wherein R1, R2, R3, R4, R5 and X1 in the formula are as defined in description) or the like to the mammal.
Owner:THE UNIV OF TOKYO +1
LMNA gene and its involvement in Hutchinson-Gilford Progeria Syndrome (HGPS) and arteriosclerosis
Disclosed herein are point mutations in the LMNA gene that cause HGPS. These mutations activate a cryptic splice site within the LMNA gene, which leads to deletion of part of exon 11 and generation of a mutant Lamin A protein product that is 50 amino acids shorter than the normal protein. In addition to the novel Lamin A variant protein and nucleic acids encoding this variant, methods of using these molecules in detecting biological conditions associated with a LMNA mutation in a subject (e.g., HGPS, arteriosclerosis, and other age-related diseases), methods of treating such conditions, methods of selecting treatments, methods of screening for compounds that influence Lamin A activity, and methods of influencing the expression of LMNA or LMNA variants are also described. Oligonucleotides and other compounds for use in examples of the described methods are also provided, as are protein-specific binding agents, such as antibodies, that bind specifically to at least one epitope of a Lamin A variant protein preferentially compared to wildtype Lamin A, and methods of using such antibodies in diagnosis, treatment, and screening. Also provided are kits for carrying out the methods described herein.
Owner:UNITED STATES OF AMERICA +2
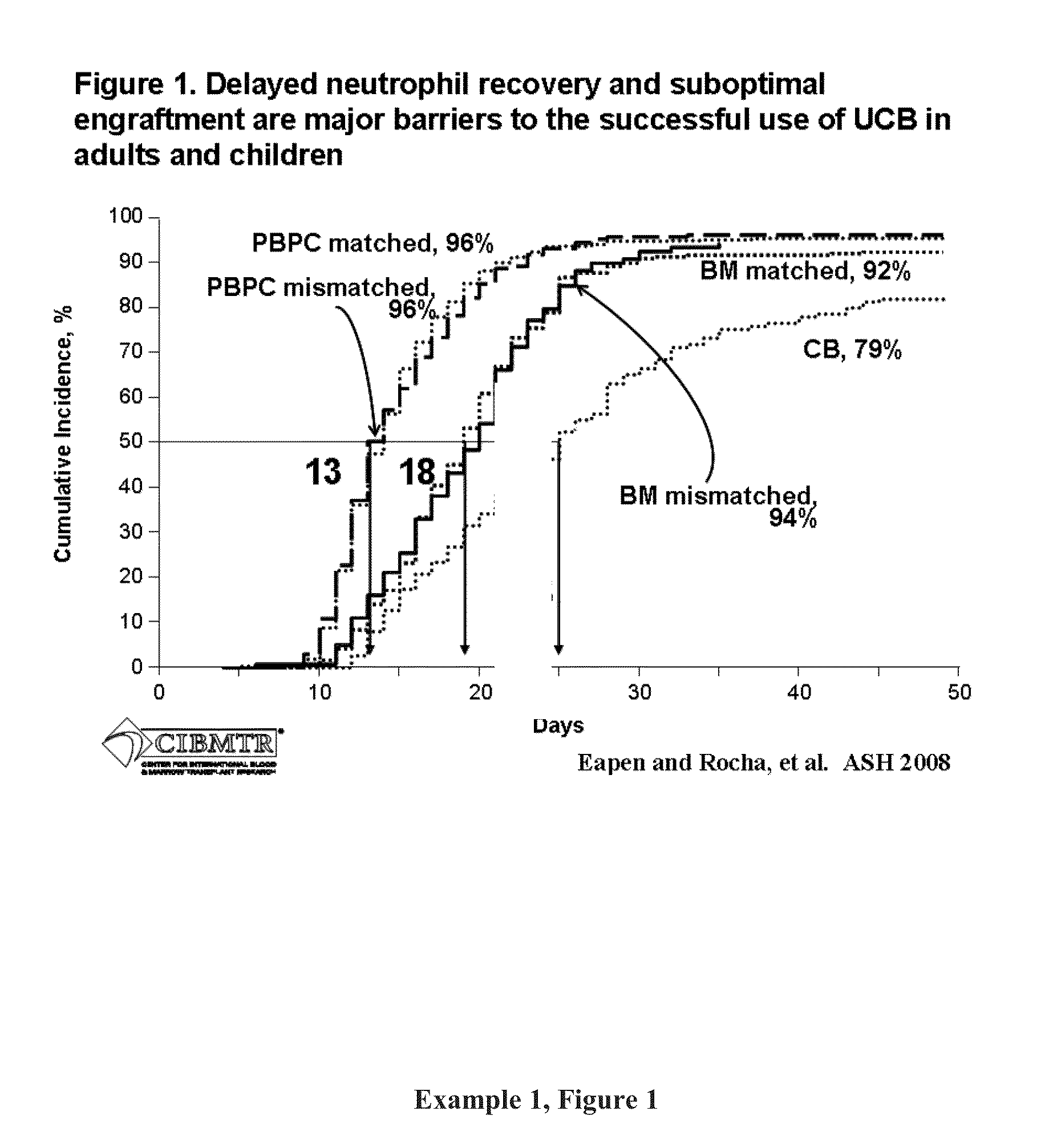
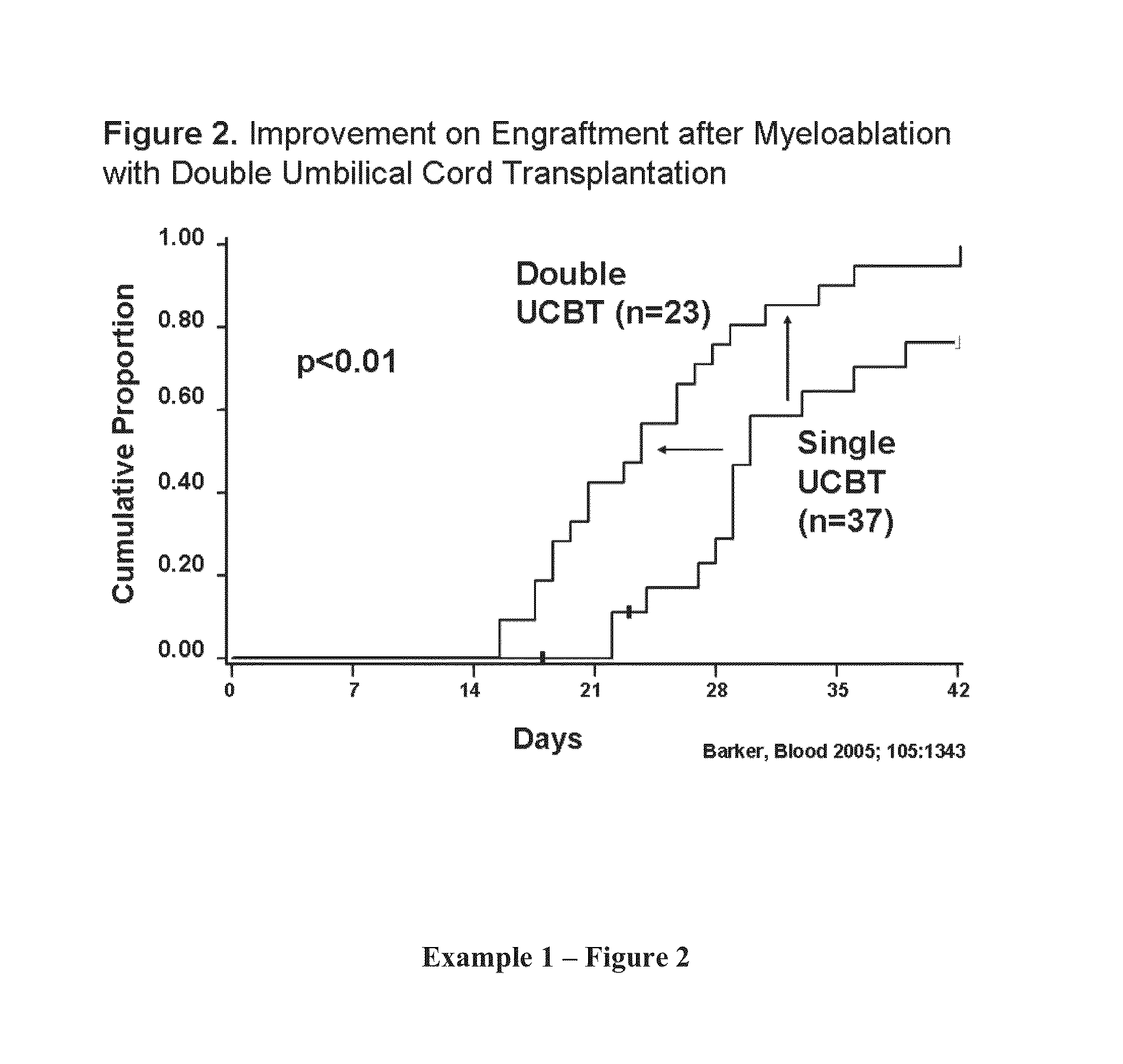
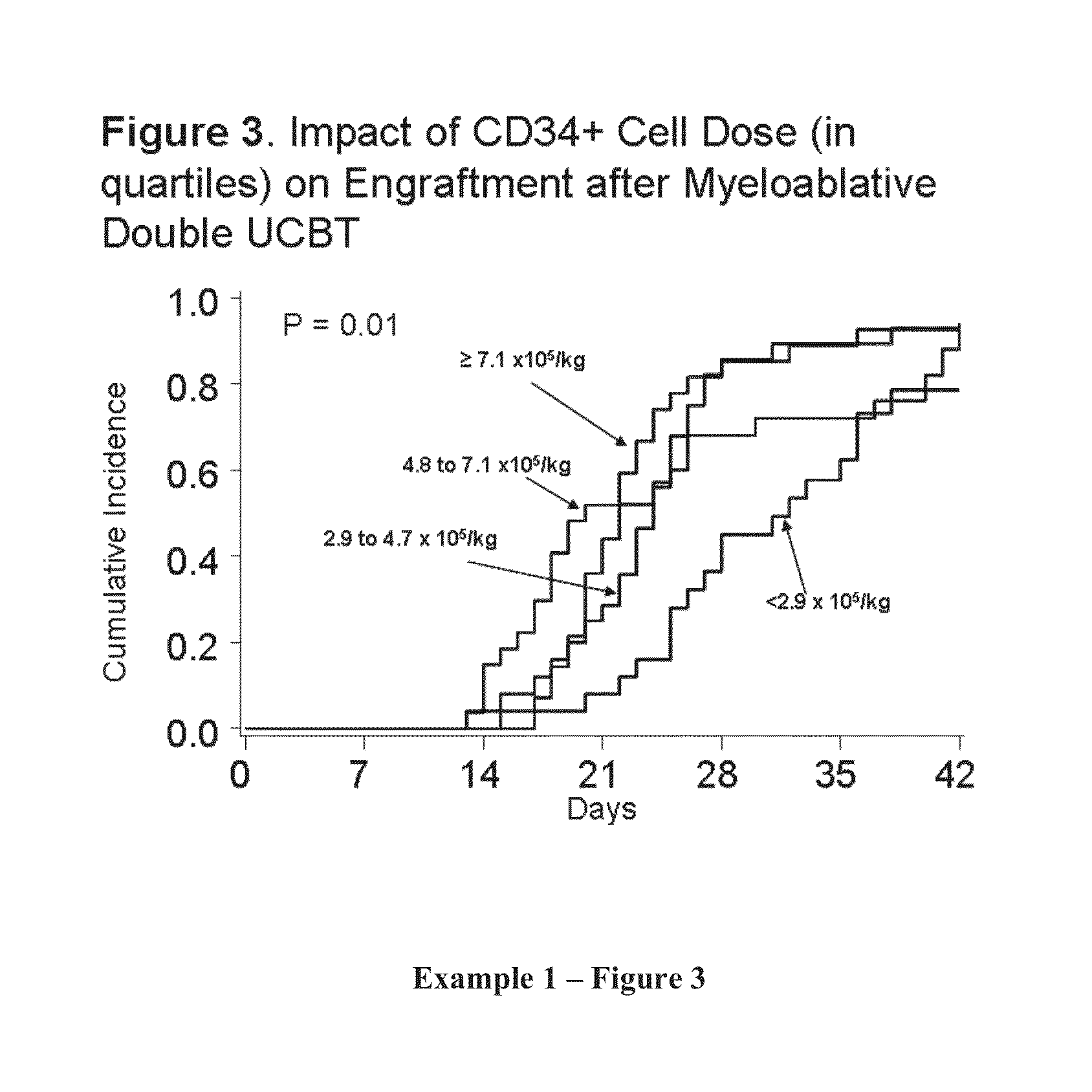
Compositions and methods for cxcr4 signaling and umbilical cord blood stem cell engraftment
ActiveUS20130236425A1Improve responsivenessEnhancing homingBiocideMammal material medical ingredientsProgenitorNormal protein
The present invention provides for enhancing engraftment by co-infusing at least two partially HLA matched umbilical cord blood (“UCB”) units. The invention further provides for positive C3a mediated priming on responsiveness to doses of SDF-1 and C3a induced incorporation of CXCR4 in membranes in HSC and progenitors. The invention further provides for enhancing the homing of UCB HSC and progenitors via the SDF-1 / CXCR4 pathway and that C3a and LL-37 are useful for this method. It is also disclosed herein that fragments of C3a (e.g., des-Arg) are effective in the methods of the invention, including enhancing homing of HSPCs to BM. The invention further encompasses the disclosure herein of NFAT1 regulation post-transcriptionally by both mir-184 and IFN-γ. The present invention further provides for measuring and using differences between UCB and adult CD4+ / 45RA+ T-cells as a means of defining strategies to enhance optimal allogeneic stem cell transplantation outcomes. The present invention further provides methods for maintaining IL-2 production in the absence of NFAT1 normal protein levels.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND +1
In-vitro diagnostic device and kit
The invention provides an infiltration detection device for predicting or detecting preeclampsia. The device comprises an enclosed shell, which is formed by an upper cover and a bottom groove, wherein the upper cover and bottom groove clamp and fix each other. In the enclosed shell, the upper cover is provided with a sample feed hole. A sample to be detected and other reagents such as coloring agent, eluent, and the like can be added into the shell through the sample feed hole. In the shell, from top to bottom, at least an infiltration layer and a water absorption layer are laminated. The infiltration layer comprises a micro-porous membrane, normal proteins and other micromolecular compounds can penetrate the micro-porous membrane, but protein aggregate which is mistakenly folded cannot penetrate the micro-porous membrane. The micro-porous membrane can be made of cellulose such as cellulose nitrate or cellulose acetate. Preferably, the micro-porous membrane in the infiltration layer comprises a labeling reagent, which can be specifically bound with mistakenly-folded protein aggregate, mistakenly-folded protein aggregate, or a mixture of the labeling reagent and mistakenly-folded protein aggregate.
Owner:SHUWEN BIOTECH CO LTD
Method for binding pathologic protein by using conjugated polyelectrolyte
InactiveCN101948505APeptide preparation methodsBiological testingNormal proteinConjugated Polyelectrolytes
The invention relates to a method for separating aggregated misfolded protein from an environment comprising protein in a non-aggregated normal form, which comprises the following steps: using the conjugated polyelectrolyte (CPE) to contact with the misfolded protein and normal protein and separating a CPE / protein complex from other components of a sample.
Owner:BIOCHROMIX
Fluorescent protein marker preparation method and application
InactiveCN103923939AGuaranteed accuracyBatch-to-batch repeatability assuranceMaterial analysis by electric/magnetic meansVector-based foreign material introductionProtein markersNormal protein
The invention discloses a fluorescent protein marker preparation method and application. Carriers containing different fusion tags are selected according to the size of needed protein markers, primer sequences are determined according to the design of plasmids and insertion sequences, and the length is controlled to be about 20 bp; enzyme cutting sites are determined according to the different plasmids and different GFP gene segment sequences; the constructed plasmids are normally converted respectively, the plasmids are induced according to a method recommended by a manufacturer to express GFP recombinant protein in different sizes and be purified, then the 28 KD-80 KD fluorescent protein markers can be obtained, SDS-PAGE is adopted to detect the molecular weight and the purity of protein, and normal protein is quantified for standby application. According to the fluorescent protein marker preparation method and application, the expression carriers containing GFP open frames and the fusion tags in different sizes are constructed through recombinant, the carriers are induced to conduct expression and be purified respectively, the green fluorescent protein markers are prepared after the GFP protein in different sizes are selected to be mixed according to the needs, the inter-batch repeatability can be completely guaranteed and the experiment accuracy can be guaranteed.
Owner:ZHENGZHOU IMMUNO BIOTECH
Method and kit for detecting susceptibility of ankylosing spondylitis
The invention discloses a method for detecting susceptibility of ankylosing spondylitis, which comprises the step of detecting whether transporter antigenic peptides 1 (TPA1), transcripts and / or protein of an individual exist mutation or not compared with normal TPA1, normal transcripts and / or normal protein; and if the mutation exists, the possibility that the individual suffers from the ankylosing spondylitis is larger than the possibility that general group suffers form the ankylosing spondylitis. The invention also discloses a corresponding detecting kit.
Owner:CHINESE NAT HUMAN GENOME CENT AT SHANGHAI
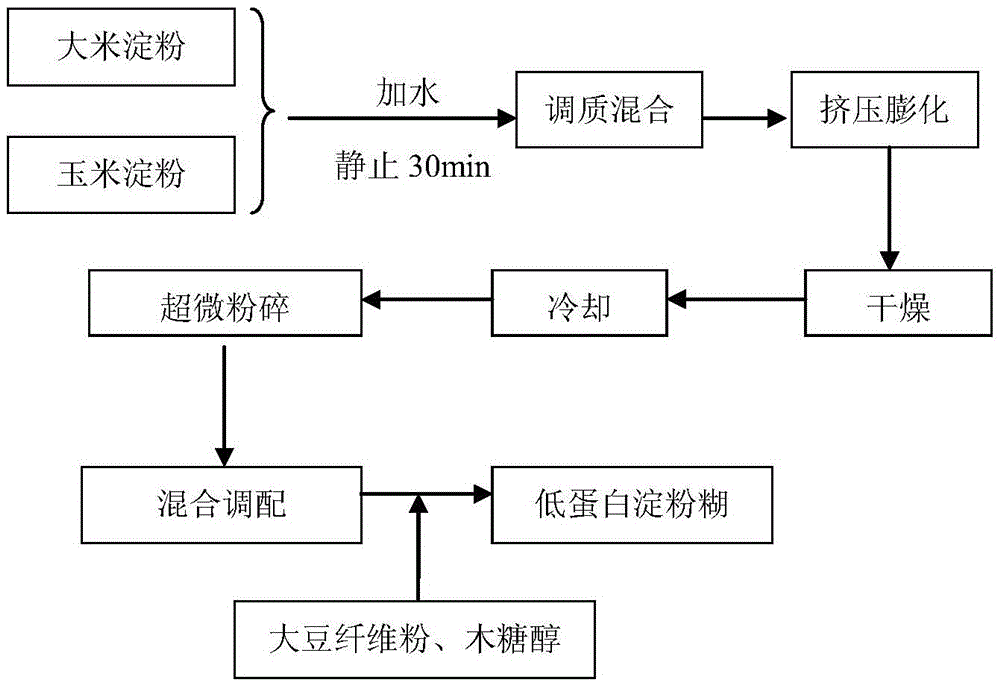
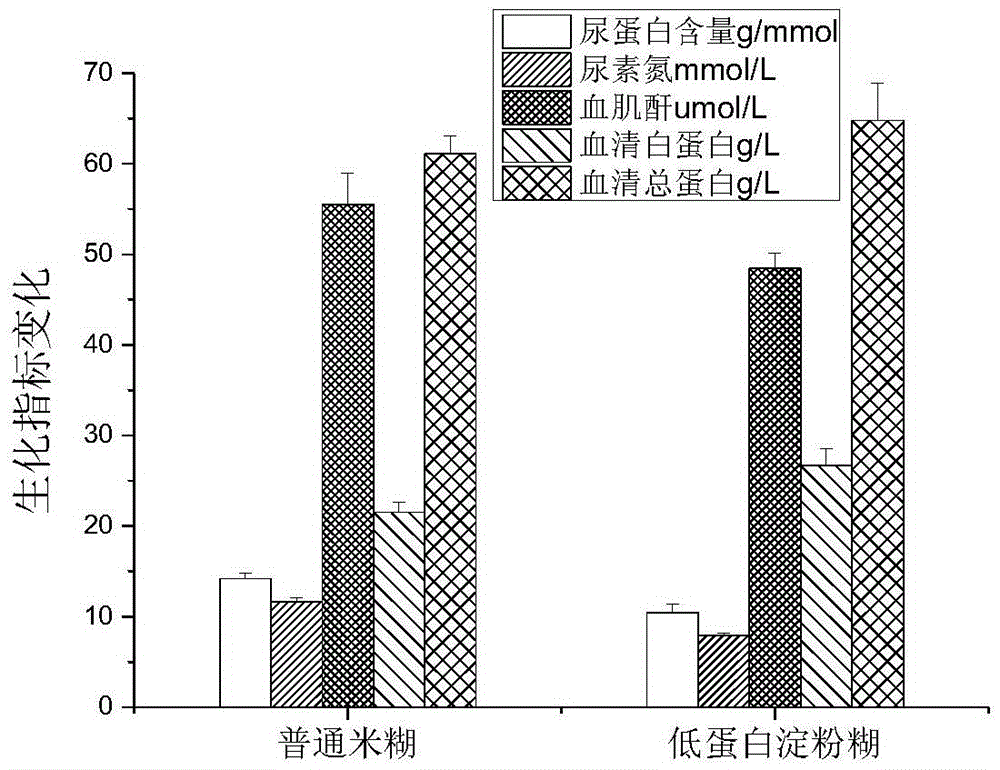
Low-protein starch paste easy in brewing and preparation method thereof
InactiveCN105559049AGreat tasteImprove absorption and utilizationFood ingredient functionsFiberProtein intake
The invention relates to low-protein starch paste easy in brewing. The low-protein starch paste is mixed and prepared from, by weight percentage, 35-50% of rice starch, 30-45% of corn starch, 8-13% of soybean fiber powder and 4-7% of xylitol, wherein the rice starch and corn starch are the main raw materials, the soybean fiber powder, the xylitol and the like are auxiliary materials, and the auxiliary materials are added after the main raw materials are processed. A preparation method of the low-protein starch paste includes the steps of mixing and regulating, extruding, drying, superfine grinding, mixing and blending, and the like. The low-protein starch paste has the advantages that an extruding generator in multi-gradient physical-field generators is fully utilized and combined with the superfine grinding technology, the prepared low-protein starch paste can satisfy the protein intake requirement of a patient with kidney disease, and the low-protein starch paste is good in taste and edibility, high in digestion rate and utilization rate, and the like as compared with other low-protein food. In addition, compared with paste products with normal protein contents, the low-protein starch paste has the advantages that after the patient with the kidney disease eats the low-protein starch paste for a certain period of time, the urinary protein, serum creatinine and urea nitrogen contents of the patient are in a decreasing trend, the total serum protein and serum albumin contents of the patient are in an increasing trend, and the state of the kidney disease can be relieved.
Owner:SHENYANG NORMAL UNIV
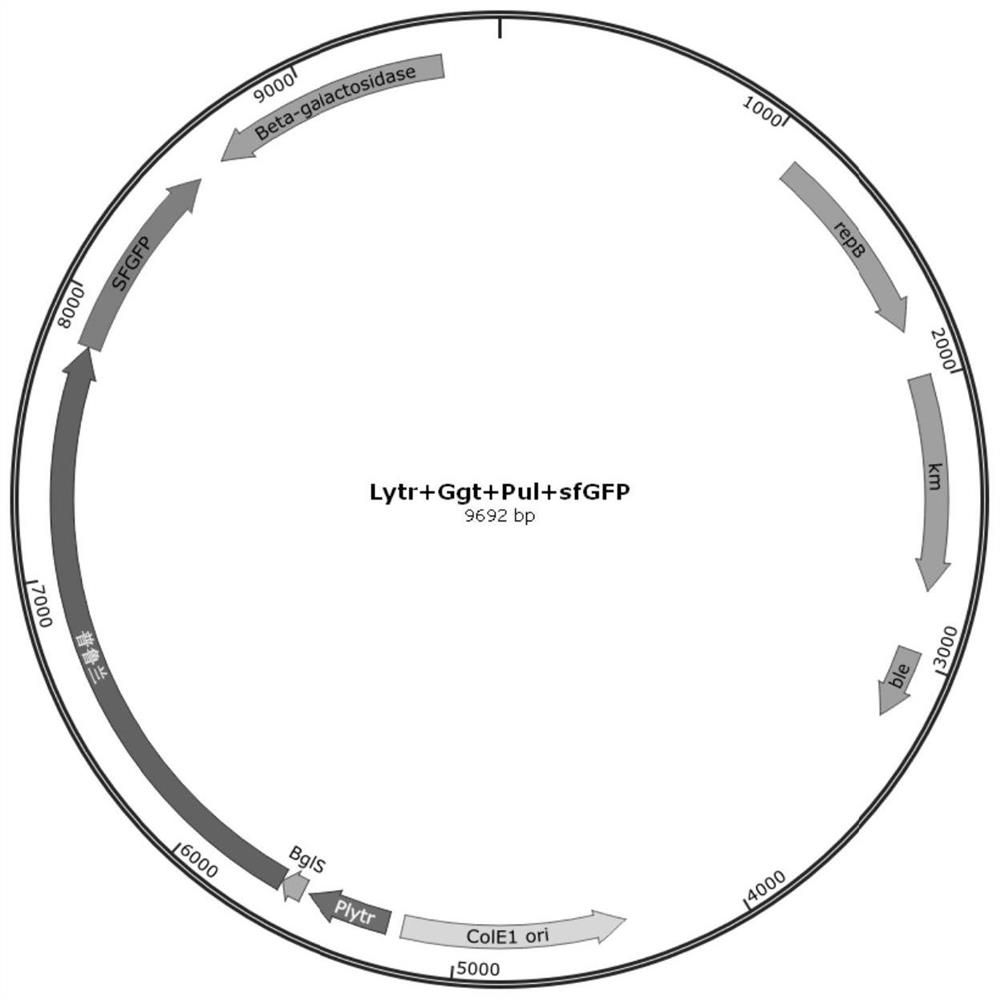
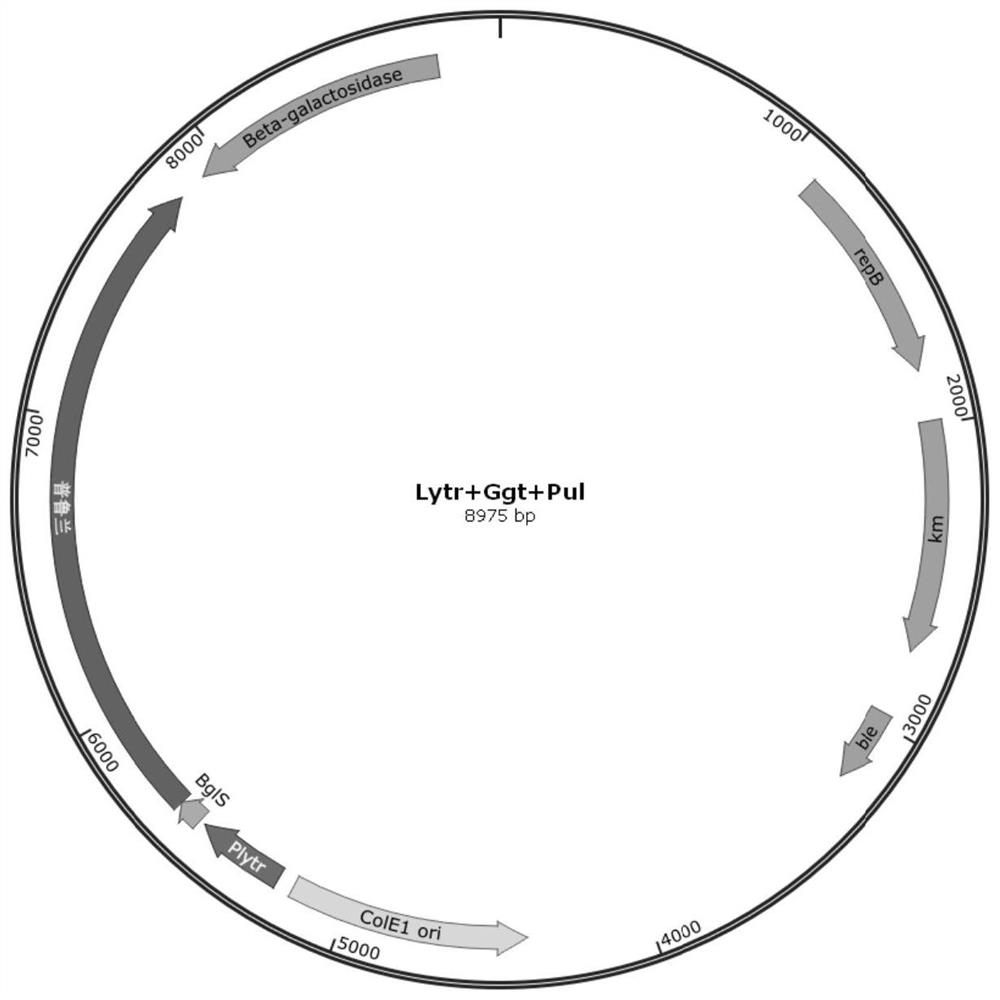
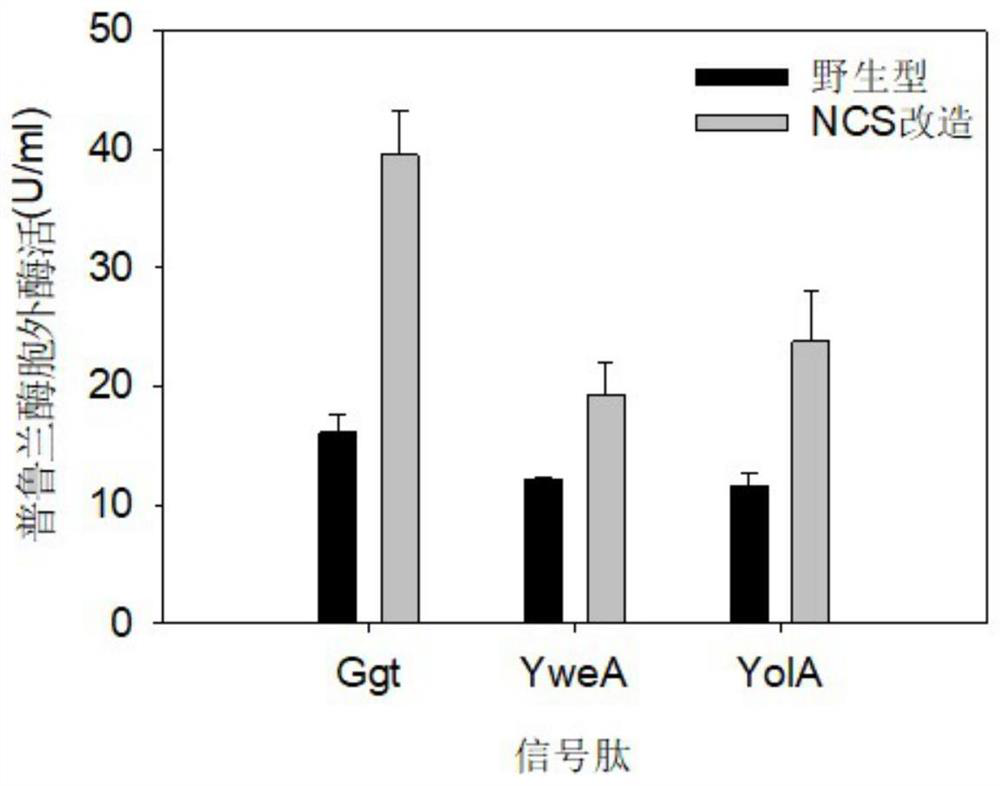
Signal peptide for promoting protein extracellular expression
PendingCN111850008AIncrease extracellular enzyme activityPolypeptide with localisation/targeting motifBacteriaGeneticsExtracellular proteins
The invention discloses a signal peptide for promoting protein extracellular expression, and belongs to the technical field of gene engineering and enzyme engineering. According to the invention, bacillus subtilis is used as a host, and the method comprises the following steps: performing synonymous mutation on endogenous signal peptides Ggt, YweA and YolA of bacillus subtilis, selecting monoclones with high and low fluorescence values through high-throughput screening, and after sequencing identification and signal peptide mutation, inoculating variants into a shake flask for fermentation verification, so that a synonymous mutation sequence capable of remarkably improving the extracellular protein quantity is obtained, and the protein expression quantity can be increased while normal protein translation is guaranteed. The synonymous mutation sequences of the Ggt signal peptide, the YweA signal peptide and the YolA signal peptide respectively enable the extracellular enzyme activity tobe improved by 1.45 times, 0.59 times and 1.04 times compared with that of a wild type, so that a new strategy is provided for extracellular high-efficiency expression of enzymes.
Owner:JILIN COFCO BIOCHEM +1
Method and kit for detecting susceptibility of ankylosing spondylitis
The invention discloses a method for detecting susceptibility of ankylosing spondylitis, which comprises the step of detecting whether transporter antigenic peptides 1 (TPA1), transcripts and / or protein of an individual exist mutation or not compared with normal TPA1, normal transcripts and / or normal protein; and if the mutation exists, the possibility that the individual suffers from the ankylosing spondylitis is larger than the possibility that general group suffers form the ankylosing spondylitis. The invention also discloses a corresponding detecting kit.
Owner:上海市生物医药技术研究院
Binding of pathological forms of proteins using conjugated polyelectrolytes
InactiveUS20100310461A1Easy to captureHigh selectivityEnergy modified materialsNanomedicineConjugated PolyelectrolytesDisease
A method for treatment of a disease caused by aggregation of misfolded proteins including subjecting a body fluid of a patient to a separation of an aggregated misfolded protein which includes contacting both the misfolded and normal protein with a conjugated polyelectrolyte (CPE) and separating the CPE / protein complex from the other constituents of the sample.
Owner:BIOCHROMIX PHARMA
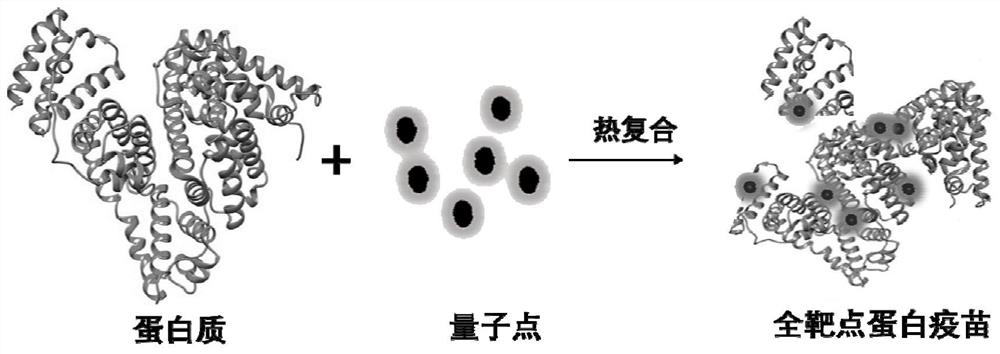
Quantum dot modified protein vaccine as well as preparation method and application thereof
PendingCN113456809AExcellent immune activation propertiesEasy to preparePeptide preparation methodsCancer antigen ingredientsCancer cellImmunogenicity
The invention discloses a quantum dot modified protein vaccine as well as a preparation method and application thereof, which relates to the field of nano materials and biomedicine. Raw materials of the quantum dot modified protein vaccine comprise quantum dot particles and antigen protein, the surfaces of the quantum dot particles comprise biocompatible functional groups, and the antigen protein and the functional groups act to enable the protein to be wound on the surfaces of the quantum dot particles under the action of non-covalent bonds. The antigen protein is modified through the quantum dots, the spatial conformation of the antigen protein can be changed, the immunogenicity of normal protein is remarkably enhanced due to conformation modification, and meanwhile the function of immunosuppressive protein on cancer cells is deregulated. And the specific recognition probability can be enhanced. According to the quantum dot modified protein vaccine, the immunogenicity of protein can be greatly improved, so that the quantum dot modified protein vaccine has better immune activation characteristics, the preparation method is simple, and the operation conditions are mild. The obtained quantum dot modified protein vaccine can be applied to personalized immunotherapy of cancers.
Owner:UNIVERSITY OF MACAU
Virus vector capable of realizing polygene editing of plants as well as construction method and application of virus vector
ActiveCN111334527APrecise Targeted KnockoutLow mRNA expressionMicrobiological testing/measurementFermentationBiotechnologyGolden Gate Cloning
The invention provides a virus vector capable of realizing polygene editing of plants as well as a construction method and application of the virus vector, and relates to the technical field of gene editing of the plants. According to the virus vector, tRNA and sgRNA are sequentially linked to form unit sequences, and the unit sequences are linked with vectors pCE2; a target sequence of a target gene is inserted between the tRNA and sgRNA of each unit sequence by virtue of a ''golden-gate'' cloning method, the multiple unit sequences are linked and arrayed in series to form a series-wound sequence, the series-wound sequence is inserted into a TRV2 virus vector, after plants capable of overexpressing Cas9 proteins are infected by TRV, and a tRNA-sgRNA expression sequence unit assembled withdifferent unit sequences is transcribed, thereby finishing a polygene editing process, so that editing of multiple genes or combined editing of different genes according to targets can be realized. According to the construction method, the expression quantity of mRNA capable of being translated into normal protein sequences in the plants is lower, a gene silencing phenotype is relatively stable,and meanwhile, multiple genes can be precisely knocked out in a targeting manner.
Owner:JIANGSU UNIV OF SCI & TECH
Novel use of xCT protein and its encoding gene
InactiveCN1883700AHigh expressionNervous disorderPeptide/protein ingredientsNerve degenerationDisease
Disclosed is a novel use of xCT protein and gene coded by same in generation of nerve degeneration diseases. It is discovered for the first time that xCT protein and gene coded by same relates to generating mechanism of nerve degeneration diseases. A novel gene causing nerve degeneration diseases is discovered. Significance of the invention lies in that medicine improving expression quantity of Slc7a11 gene and xCT is screened by using Slc7a11 gene and xCT as targets, to improve expression quantity of Slc7a11 gene(normal gene) and xCT(normal protein), so to improve oxidation damage resistance ability of neurocyte, and as a result to prevent and slow down generation of nerve degeneration diseases. Animal models with the nerve degeneration disease of low expression quantity of Slc7a11 gene and xCT can be used to screen protein, nucleic acid, small organic molecule, and etc. which can improve expression quantity of Slc7a11 gene and xCT and use as candidate medicine.
Owner:INST OF GENETICS & DEVELOPMENTAL BIOLOGY CHINESE ACAD OF SCI
Binding of pathological forms of proteins using conjugated polyelectrolytes
InactiveCN101784559ANervous disorderPeptide preparation methodsNormal proteinProtein-protein complex
The invention relates to a method for separation of an aggregated misfolded protein from an environment comprising a non-aggregating normal form of the protein, comprising contacting both said misfolded and normal protein with a conjugated polyelectrolyte (CPE) and separating the CPE / protein complex from the other constituents of the sample.
Owner:BIOCHROMIX
Method and kit for detecting susceptibility of ankylosing spondylitis
The invention discloses a method for detecting susceptibility of ankylosing spondylitis, which comprises the step of detecting whether transporter antigenic peptides 1 (TPA1), transcripts and / or protein of an individual exist mutation or not compared with normal TPA1, normal transcripts and / or normal protein; and if the mutation exists, the possibility that the individual suffers from the ankylosing spondylitis is larger than the possibility that general group suffers form the ankylosing spondylitis. The invention also discloses a corresponding detecting kit.
Owner:上海市生物医药技术研究院
Functional biofood containing hericium erinaceus
InactiveCN106798316APromote digestion and absorptionImprove immunityFood ingredient functionsSide effectAdditive ingredient
The invention discloses a functional biofood containing hericium erinaceus. The biofood is characterized by comprising the following ingredients in parts by weight: 10-30 parts of hericium powder, 0.1-5 parts of selenium protein powder, 1-5 parts of fishmea, 0.1-0.5 part of low-sodium flavoring peptide salts, and 0.5-1 part of excipients. The product can be taken with water directly as an electuary, 20-50g daily, thus being capable of meeting the absorption of protein and selenium element; and the biofood can be added into food as a raw material of the biofood, such as cooked wheaten food, puffed food, jelly, lotus root starch, nutritional granules and the like. The product is rich in nutrients, fine and free from fishy smell in mouthfeeling, the synthesis and supplementation of in-vivo normal protein can be accelerated, and organic seleneium is easily absorbed and utilized by a human body and has no toxic or side effects to a human body, and has remarkable physiological health-care functions on the human body.
Owner:安徽米乐食品有限公司
Feed for meat pigeons in brooding period in winter
InactiveCN106071288AGuaranteed supplyPromote digestion and absorptionFood processingAnimal feeding stuffCalcium bicarbonateAnimal Foraging
The invention discloses feed for meat pigeons in the brooding period in winter. The feed is prepared from, by mass percentage, 5-15% of rice bran, 20-30% of barley, 5-10% of cottonseed cakes, 2-5% of shredded ginger, 8-12% of bran, 4-8% of red beans, 10-17% of fish meal, 0.1-0.5% of calcium bicarbonate, 8-12% of vegetable leaves, 5-10% of forage, 0.1-0.4% of salt and 0.1-0.5% of shellfish meal. Meat pigeons in the brooding period require a large amount of protein, particularly in winter, so the use amount of protein supplement feed should be increased to guarantee normal protein supply in the following development process. Functions of the digestive systems of meat pigeons in the brooding period are weak, so a proper amount of green forage is added according to a certain proportion, on one hand, digestion and absorption of the feed are benefited, and on the other hand, the resistance of the meat pigeons can be improved. The temperature is low in winter, and the functions of activating blood circulation and keeping warmth can be achieved by adding shredded ginger to the feed. Besides, diseases are reduced.
Owner:LIUZHOU DINGDIAN TECH CO LTD
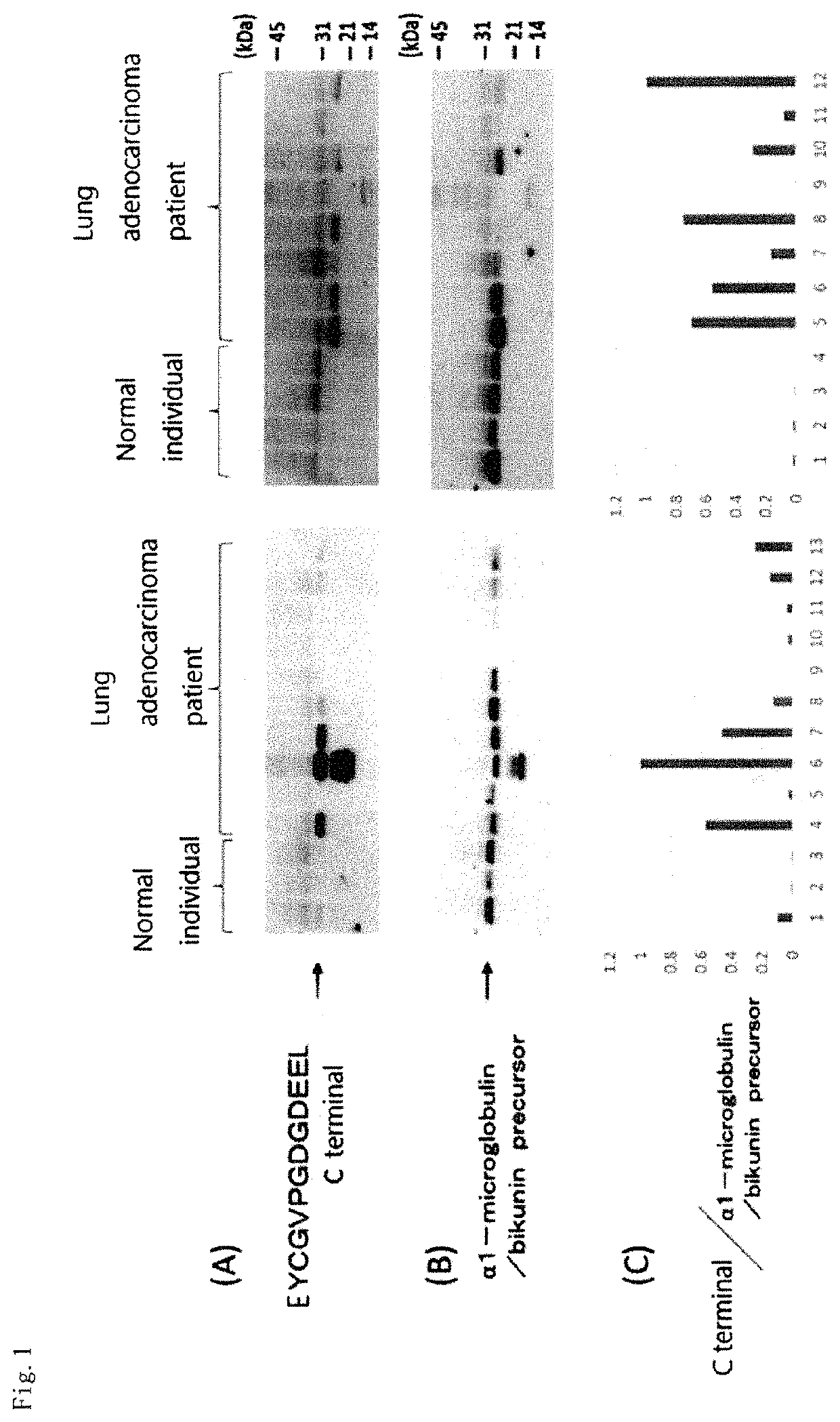
Adenocarcinoma detection method
There is provided a simple and minimally invasive adenocarcinoma detection method. The adenocarcinoma detection method of the present invention includes a step of detecting in vitro a presence or absence of an abnormal cleavage in a specific protein in a test subject-derived sample. The abnormal cleavage in the specific protein is, for example, a cleavage resulting in one or more breaks in a peptide bond in the specific protein and / or a cleavage resulting in a deletion of one or two more amino acid residues at one or more sites of the specific protein. The adenocarcinoma detection method of the present invention includes a step of detecting a presence or amount of a protein having the abnormal cleavage or a decrease in an amount of a normal protein.
Owner:UNIVERSITY OF MIYAZAKI +1
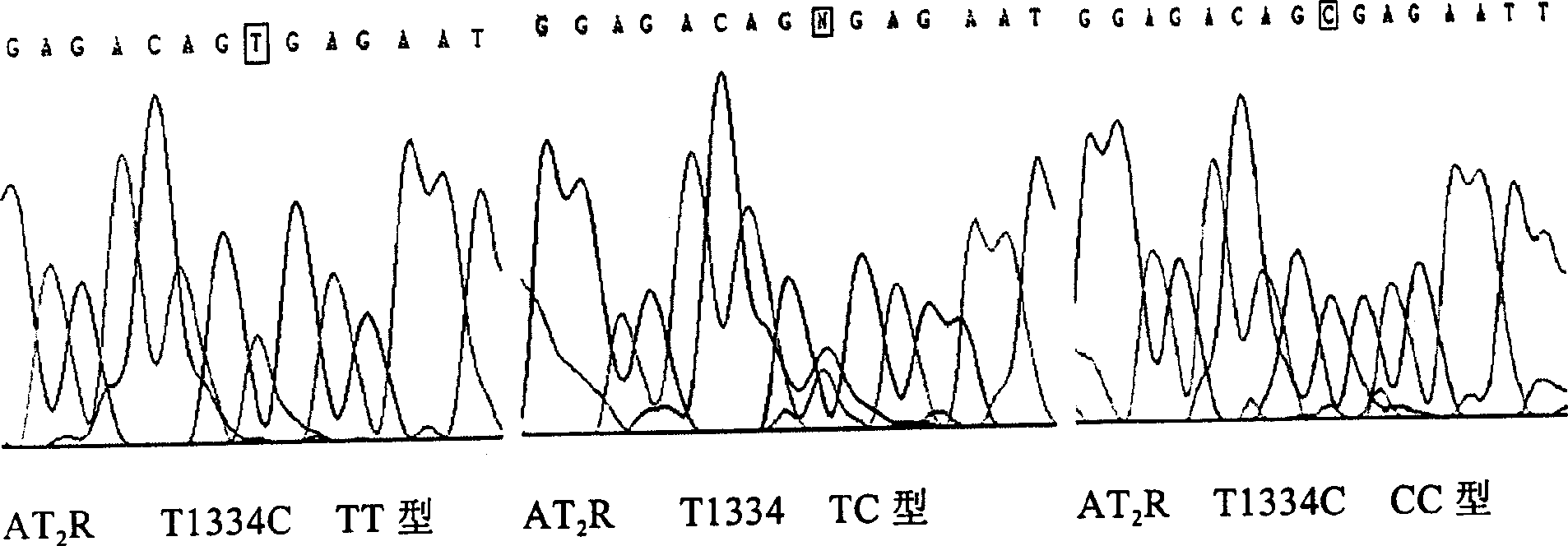
Angiotensin II-2 type receptor gene and its correlation of essential hypertension
The invention was involved in a method to detect primary hypertension susceptibility. The method contained two steps,namely assaying acceptor gene AT2R of individual angiotensin II2 and analysing variation between the protein and the normal protein. The variation showed that the individual had more chance to get primary hypertension than normal people. The invention provided corresponding detection kit.
Owner:SHANGHAI INST OF HYPERTENSION +1
Binding of pathological forms of proteins using conjugated polyelectrolytes
InactiveUS20140024813A1Easy to captureHigh selectivityNervous disorderMetabolism disorderNormal proteinConjugated Polyelectrolytes
A method for separation of an aggregated misfolded protein from an environment including a non-aggregating normal form of the protein includes contacting both the misfolded and normal protein with a conjugated polyelectrolyte (CPE) and separating the CPE / protein complex from the other constituents of the sample.
Owner:BIOCHROMIX
Sensors and methods for detecting diseases caused by a single point mutation
ActiveUS20090042210A1Rapid and accurate methodAccurate and Rapid DiagnosisImmunoglobulins against blood coagulation factorsBioreactor/fermenter combinationsProtein targetProtein insertion
A method for generating antibodies preferable to either a normal protein and a mutated form of the normal protein, respectively, where a mutation associated with the mutated form includes either a single point mutation or a small number of point mutations where the method includes creating first and second antigenic peptides of a predetermined length corresponding respectively to common regions of the normal target protein and the mutated form, where the common regions are identical to one another except for the point mutation of the mutated form, obtaining first and second antibodies by multiplying the first and second antigenic peptides via hybridoma methods, and identifying the respective affinities of the first and second antibodies for the normal target protein and the mutated form. Also included are methods of using the first and second antibodies to detect and quantify respective amounts of a normal target protein and a mutated form of the target protein. Also included are immunological sensors the include the first and second antibodies for determining the presence and quantity of normal target proteins and mutant forms of the normal target proteins.
Owner:UNIV OF LOUISVILLE RES FOUND INC
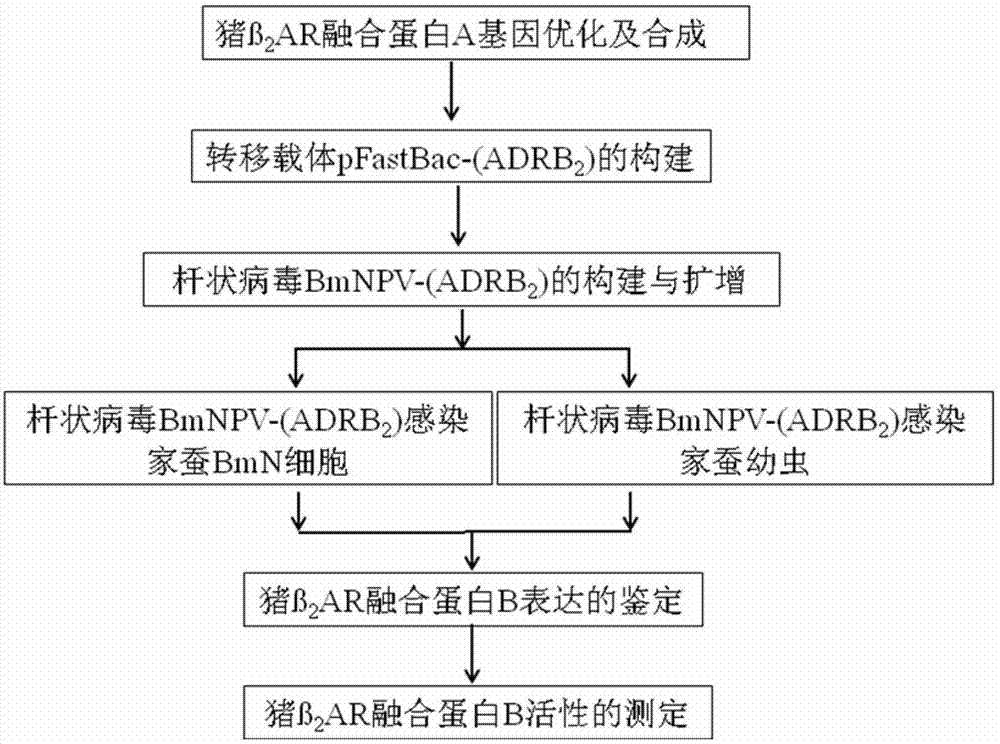
Porcine β2 adrenergic receptor fusion protein, coding gene and expression method thereof
ActiveCN103665150BReduce manufacturing costImprove expression efficiencyPolypeptide with localisation/targeting motifAntibody mimetics/scaffoldsProtein structureBiology
The invention provides a porcine beta2 epinephrine receptor fusion protein as well as an encoding gene thereof and a method for expressing the fusion protein by utilizing silkworm bioreactor. The method comprises the following steps of (1) colonizing a gene of an artificial synthesized encoded fusion protein A into a Bac-to-Bac baculoviru expression system carrier; (2) integrating the gene of the encoded fusion protein A on the transferring expression carrier obtained in the step (1) through the locus specificity transposition into baculovirus plasmid; (4) infecting the silkworm BmN cells with the baculovirus plasmid, and piercing inoculating the silkworm larva or pupae aged 1 to 5 by utilizing the obtained baculovirus; (5) expressing the fusion protein B through the infected silkworm BmN cell or the inoculated silkworm larva. The encoding gene is obtained by executing the codon optimization for the silkworm bioreactor, so that the protein expression efficiency is high, and the fusion protein is enabled to have a normal protein structure and biological activity.
Owner:江苏晶红生物医药科技股份有限公司
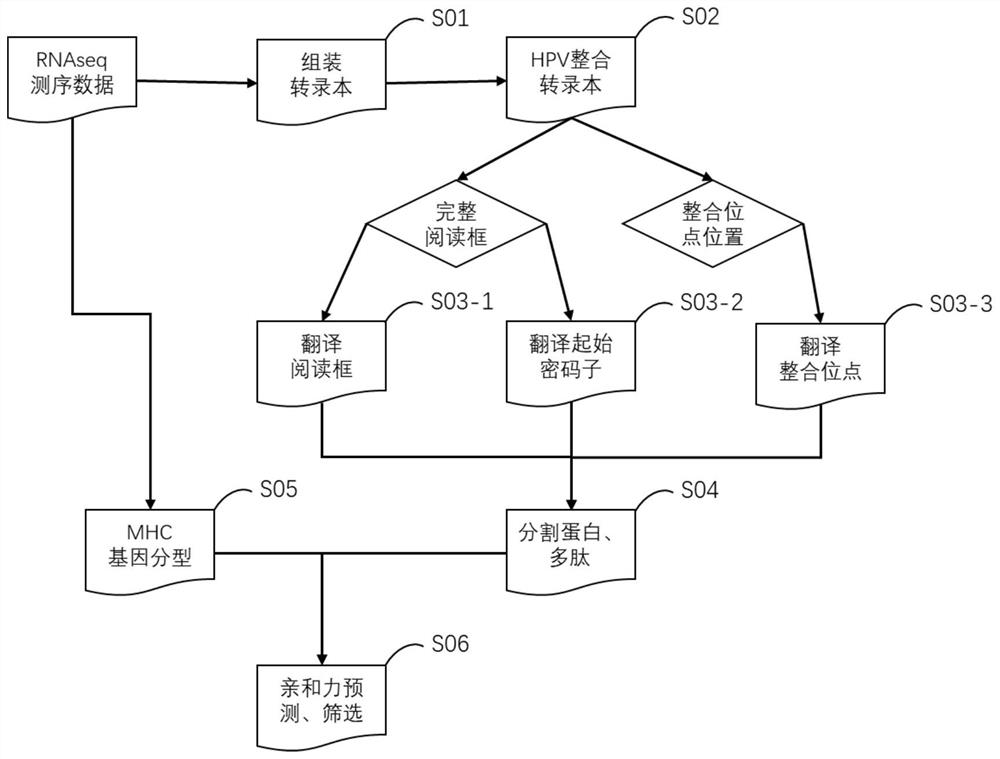
Tumor neoantigen prediction method based on HPV integration
PendingCN113362896AIgnoring the Importance of IntegrationMicrobiological testing/measurementSequence analysisHuman papillomavirusWhite blood cell
The invention discloses a tumor neoantigen prediction method based on HPV integration, and belongs to the technical field of bioinformatics. The method comprises the following steps: S01, assembling a tumor sample transcript; s02, screening a sample HPV (human papillomavirus) integrated transcript; s03, translating the HPV and integrating the transcript into polypeptide; s04, obtaining polypeptide short sequence fragments, and filtering polypeptides in human normal proteome; s05, performing genetic typing on the human leukocyte antigen of the sample; and S06, predicting the affinity of the peptide fragment, and screening new antigens. The new antigen obtained by the invention is a result of HPV integration and is closely related to a disease progression process. The new antigen obtained by the invention comes from RNA sequencing data, is a result of cell transcription, and has higher probability of translation to produce protein.
Owner:NEOCURA BIO-MEDICAL TECH CO LTD
Protein biomarkers for memory loss
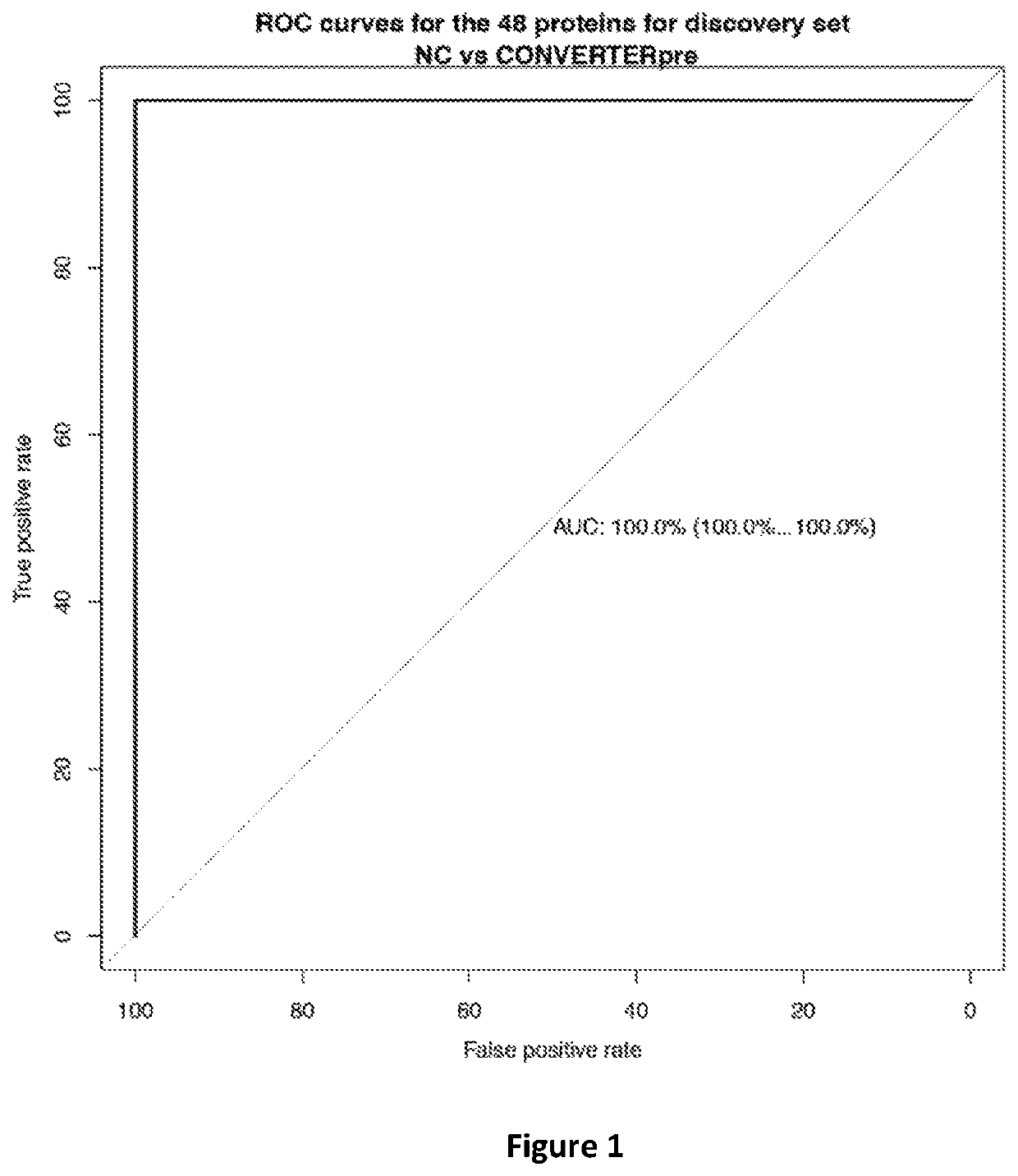
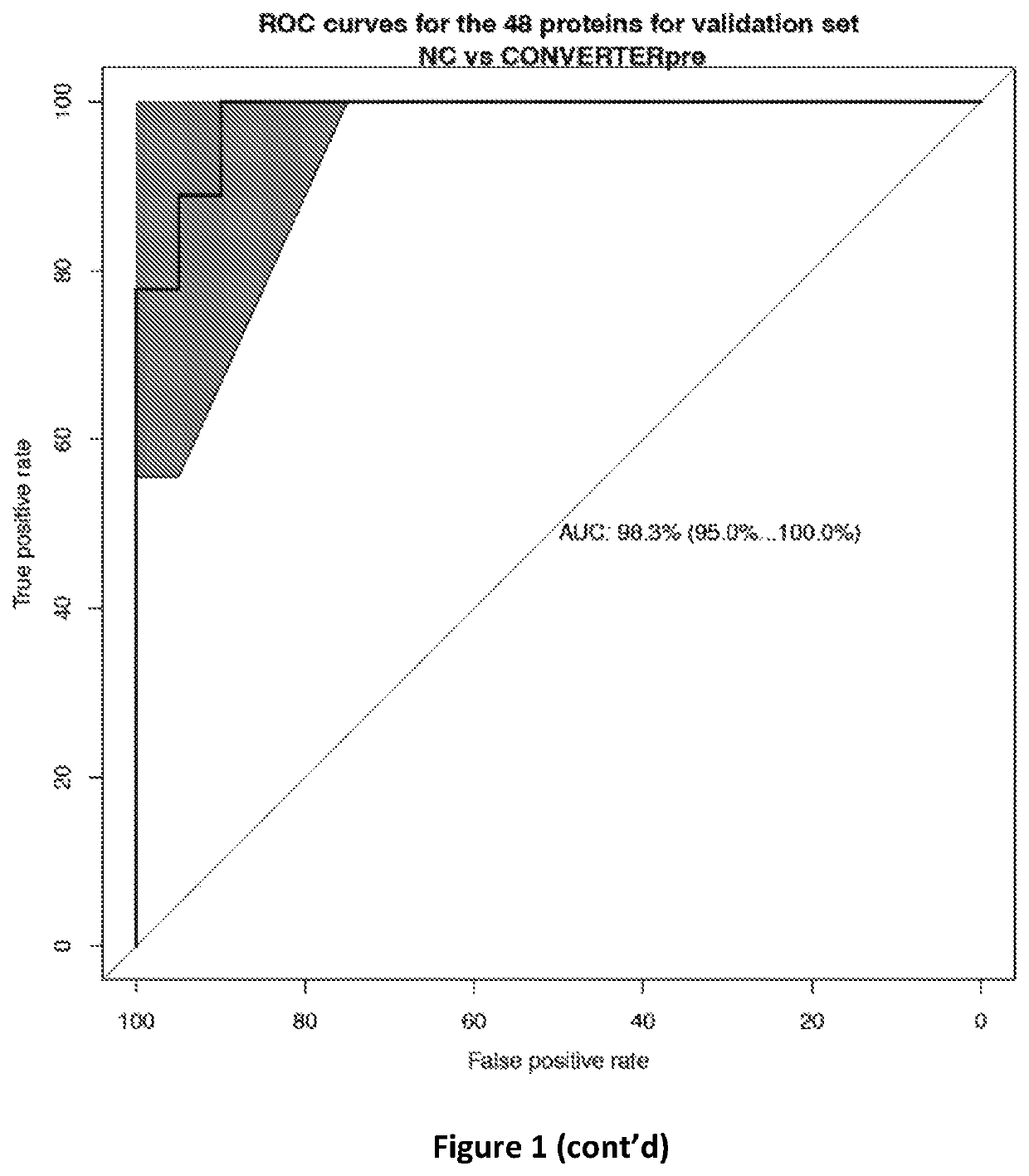
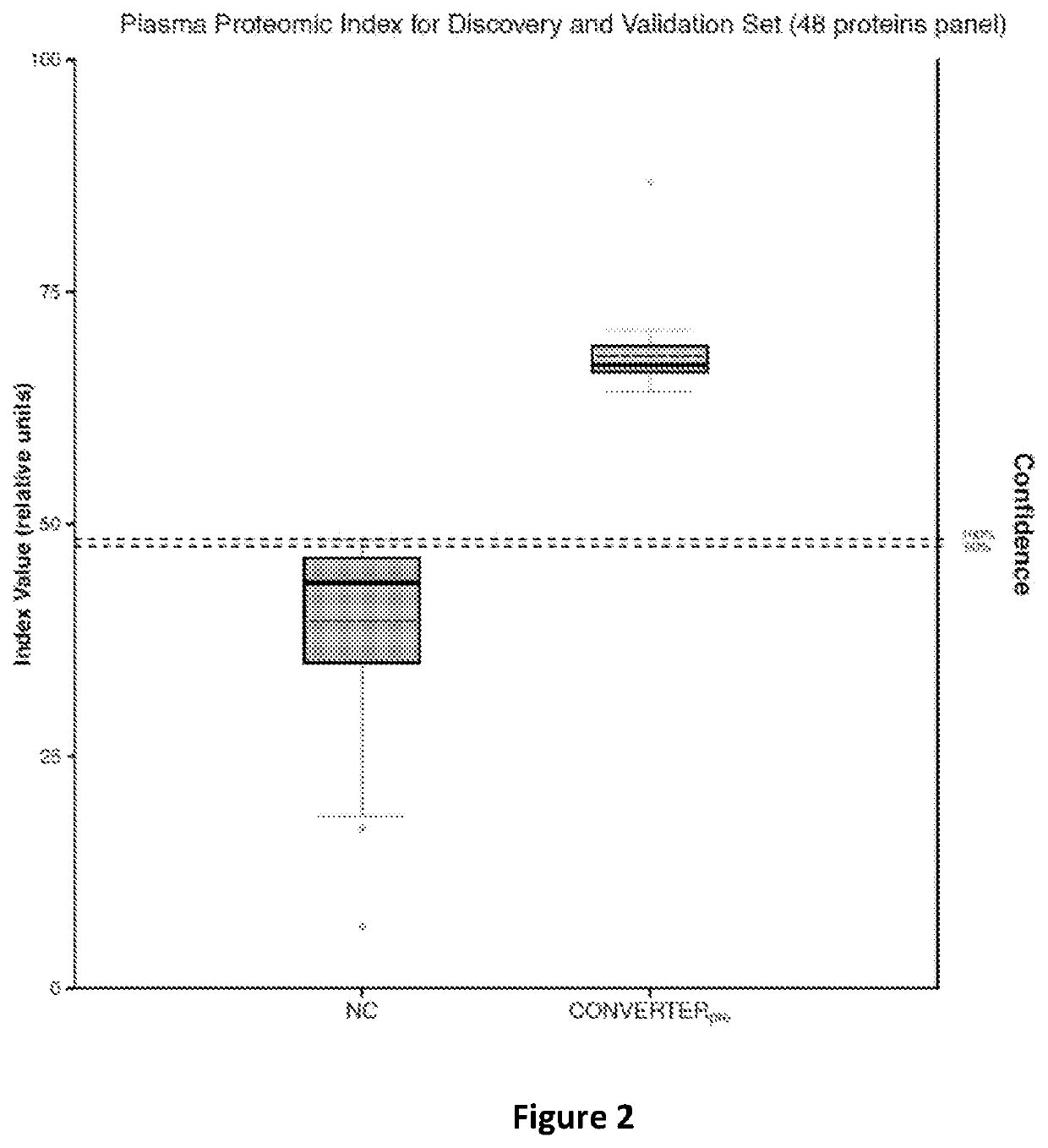
ActiveUS10900977B2Peptide/protein ingredientsHealth-index calculationProteomic ProfileMemory disorder
The present invention relates to methods of determining if a subject has an increased risk of suffering from memory impairment. The methods comprise analyzing at least one plasma sample from the subject to determine a value of the subject's proteomic profile and comparing the value of the subject's proteomic profile with the value of a normal proteomic profile. A change in the value of the subject's proteomic profile, over normal values is indicative that the subject has an increased risk of suffering from memory impairment compared to a normal individual.
Owner:UNIVERSITY OF ROCHESTER +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com