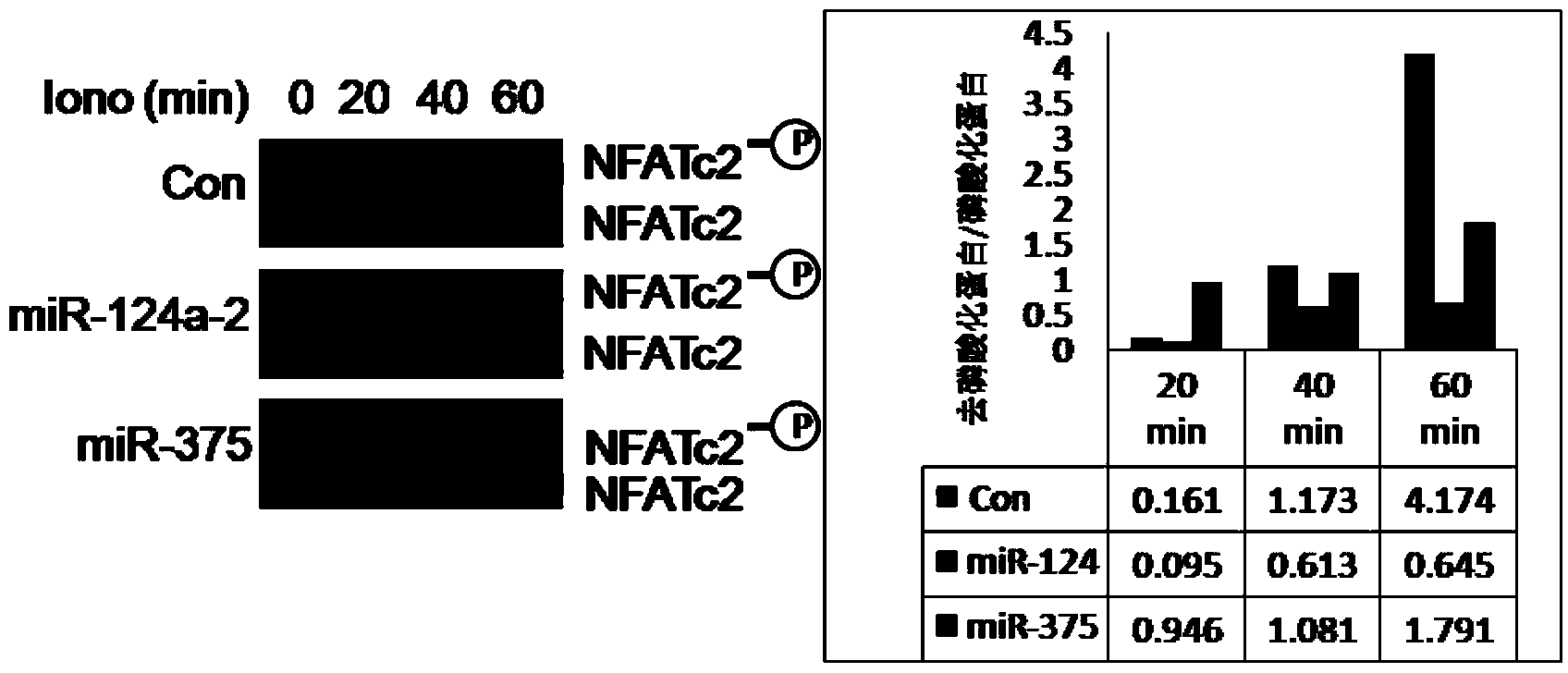
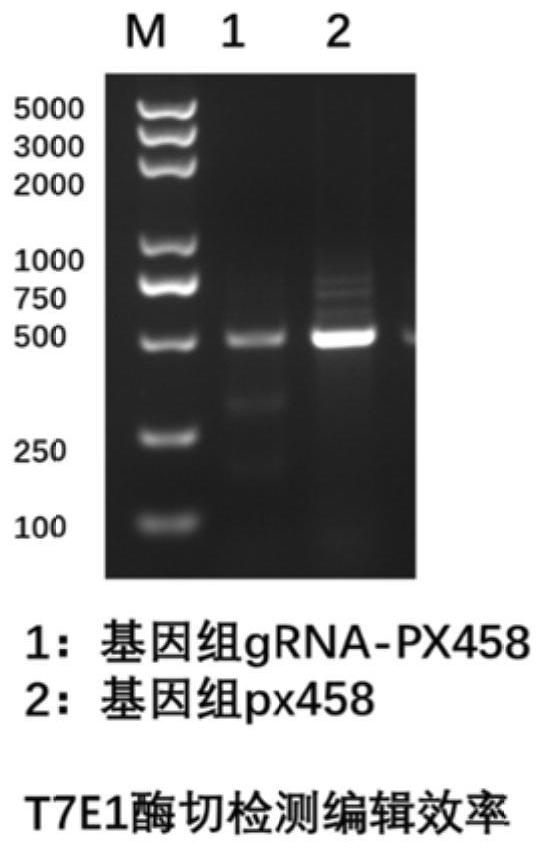
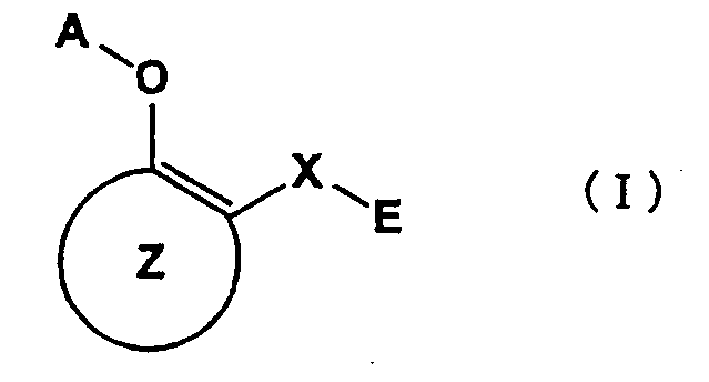Patents
Literature
67 results about "NFAT" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
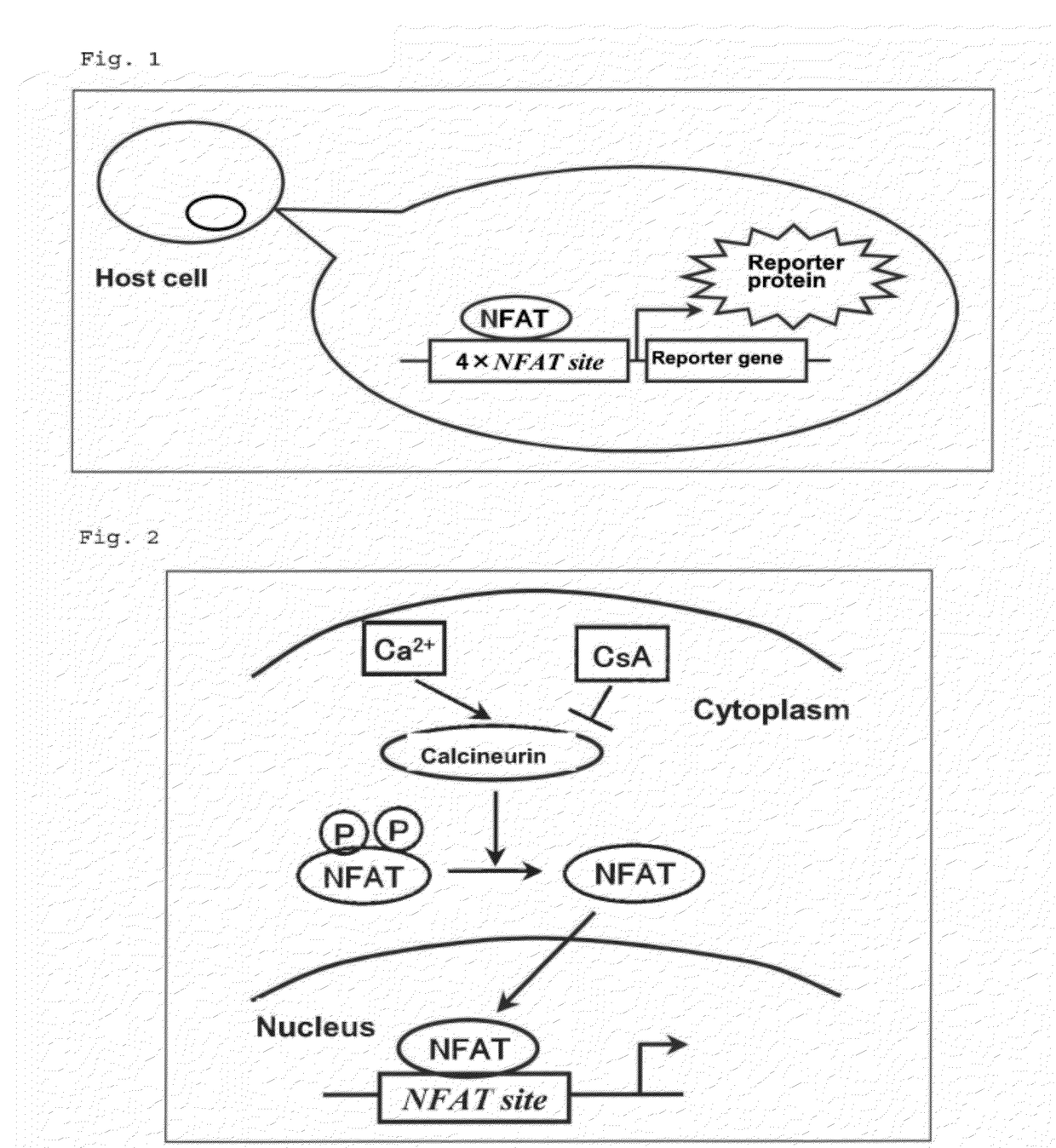
Nuclear factor of activated T-cells (NFAT) is a family of transcription factors shown to be important in immune response. One or more members of the NFAT family is expressed in most cells of the immune system. NFAT is also involved in the development of cardiac, skeletal muscle, and nervous systems. NFAT was first discovered as an activator for the transcription of interleukin-2 in T cells, as a regulator for T cell immune response, but has since been found to play an important role in regulating many other body systems. NFAT transcription factors are involved in many normal body processes as well as in development of several diseases, such as inflammatory bowel diseases and several types of cancer. NFAT is also being investigated as a drug target for several different disorders.
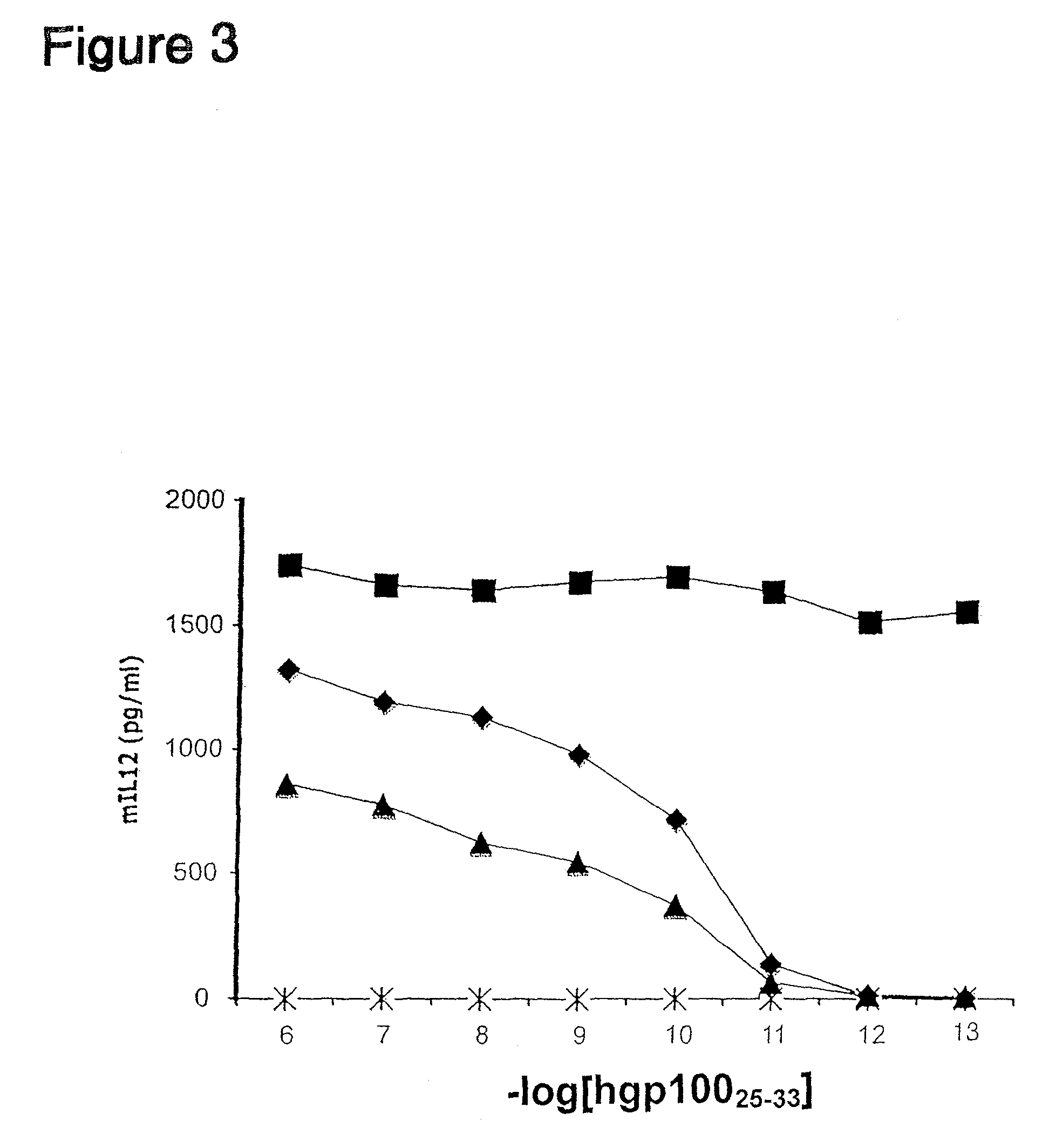
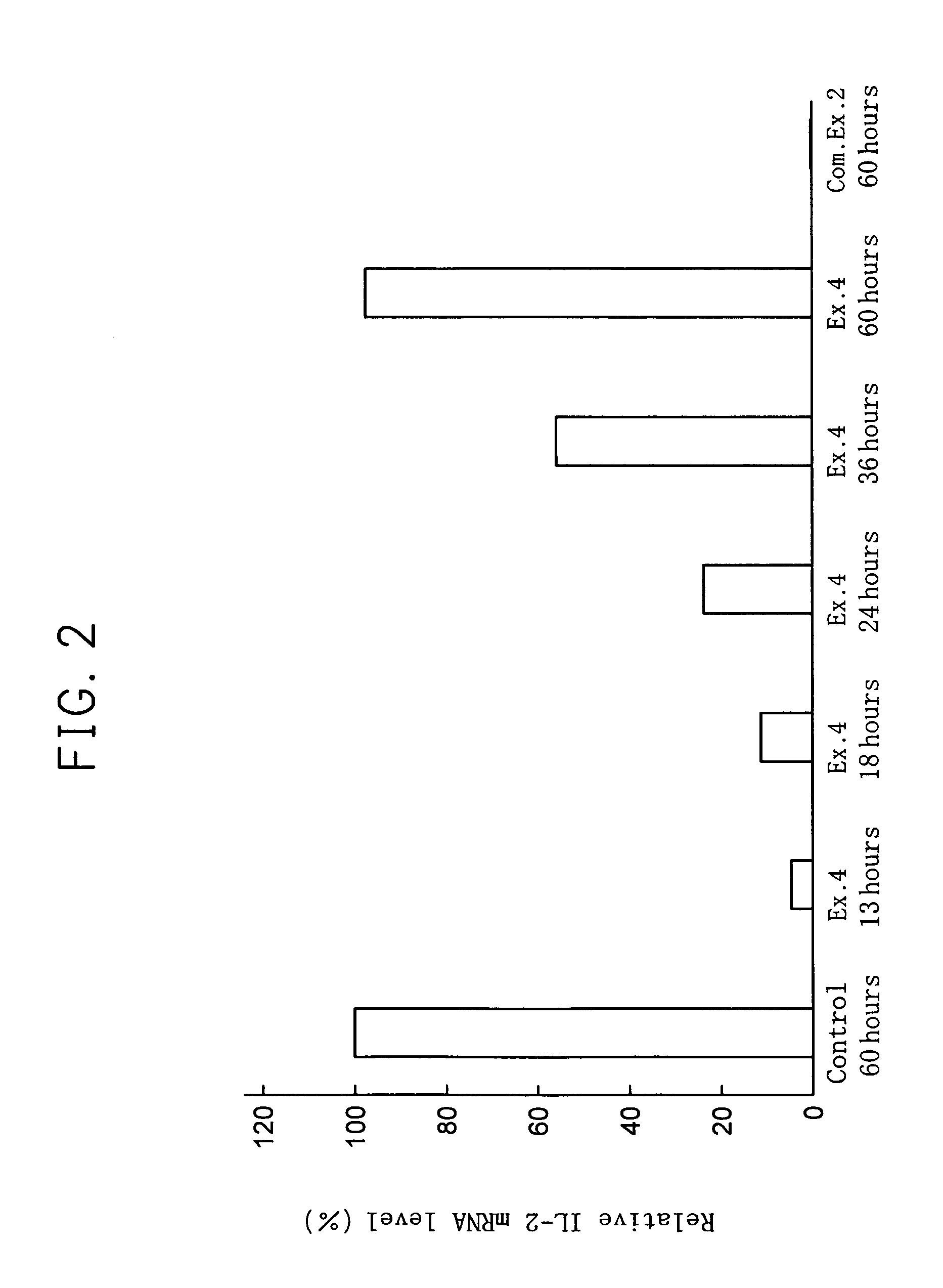
Inducible interleukin-12
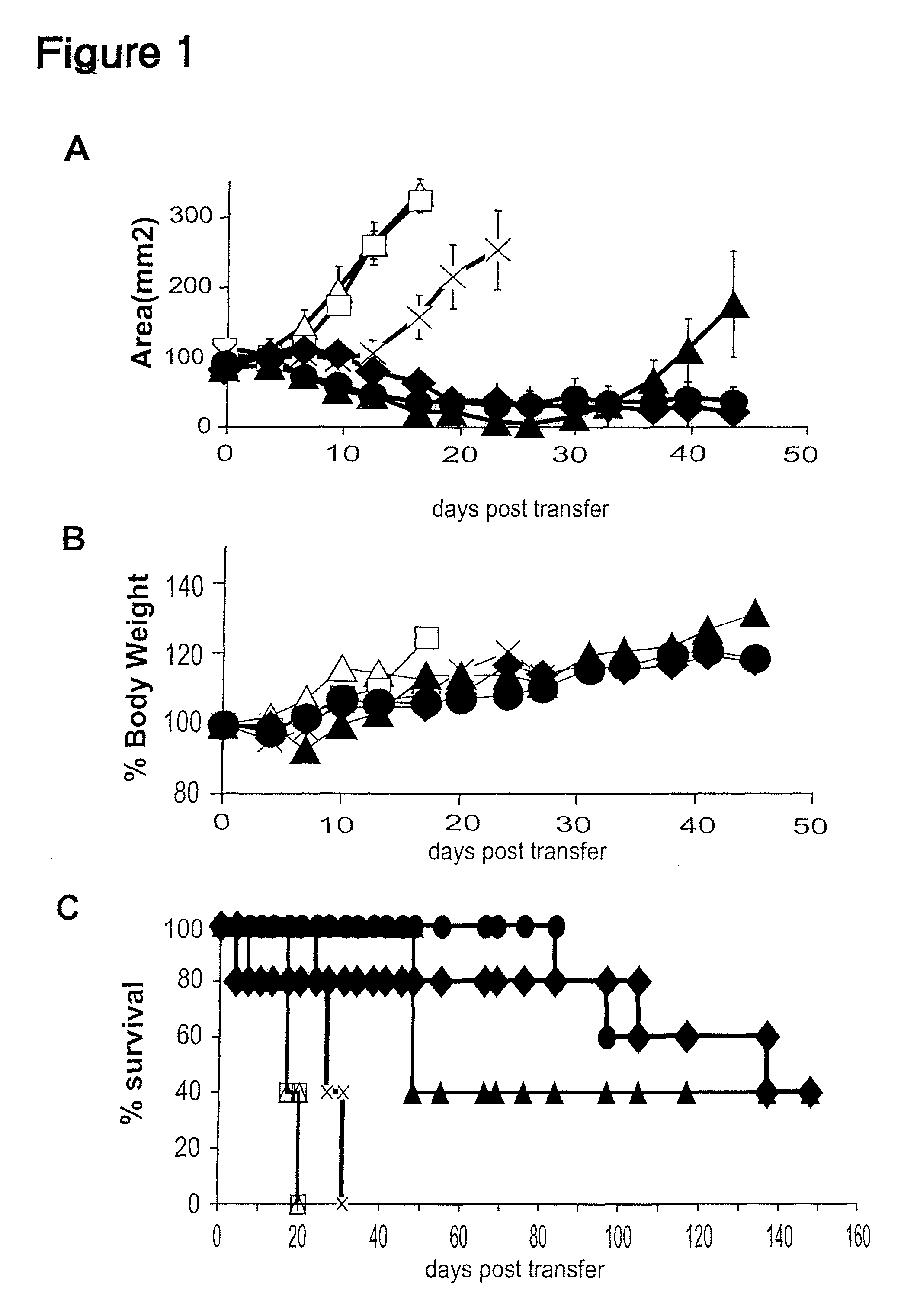
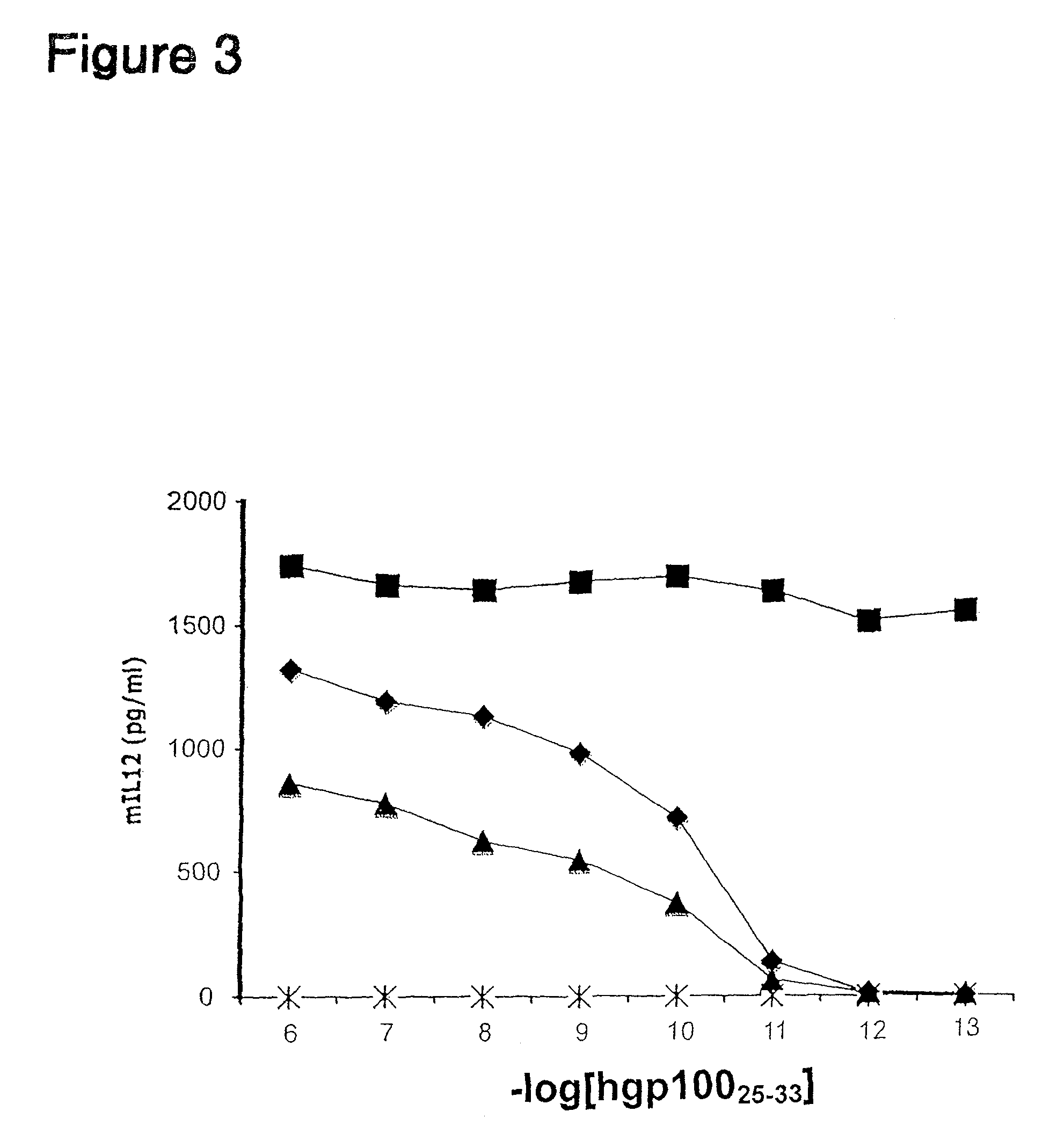
The invention provides an isolated or purified nucleic acid comprising a nucleotide sequence encoding a nuclear factor of activated T-cells (NFAT) promoter operatively associated with a nucleotide sequence encoding IL-12. The invention also provides a nucleic acid comprising a nucleotide sequence encoding a nuclear factor of activated T-cells (NFAT) promoter operatively associated with a nucleotide sequence encoding IL-12, wherein the NFAT promoter is located 3′ of the nucleotide sequence encoding IL-12. Also provided are related recombinant expression vectors, host cells, populations of cells, and pharmaceutical compositions. The invention further provides the use of the inventive nucleic acids or related materials in the treatment or prevention of cancer or an infectious disease in a mammal and in the induction of IL-12 expression in a mammal.
Owner:UNITED STATES OF AMERICA
Method for determining biology activity of PD-1 pathway inhibitor
ActiveCN104673897AMicrobiological testing/measurementForeign genetic material cellsEffector cellPD-L1
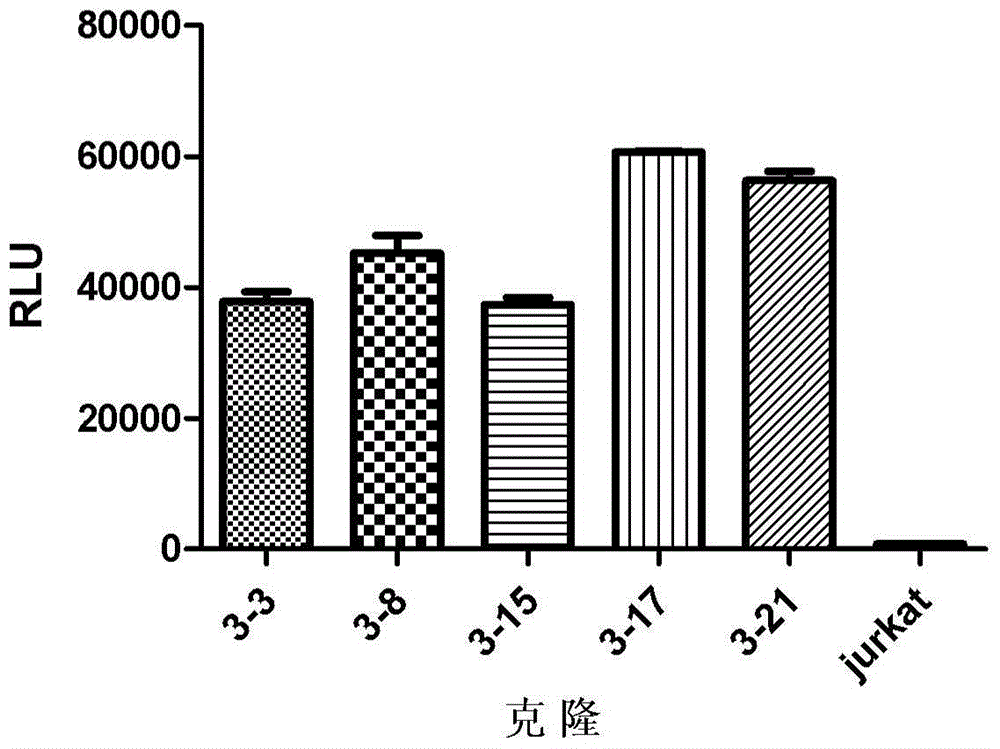
The invention discloses a method for testing biology activity of a PD-1 pathway inhibitor. The method comprises the steps: contacting target cells expressing alpha CD3TM and PD-L1 and effector cells expressing NFAT report genes and PD-1 with the PD-1 pathway inhibitor and testing signals of the NFAT report genes to determine the biology activity of the PD-1 pathway inhibitor.
Owner:JIANGSU SIMCERE PHARMA
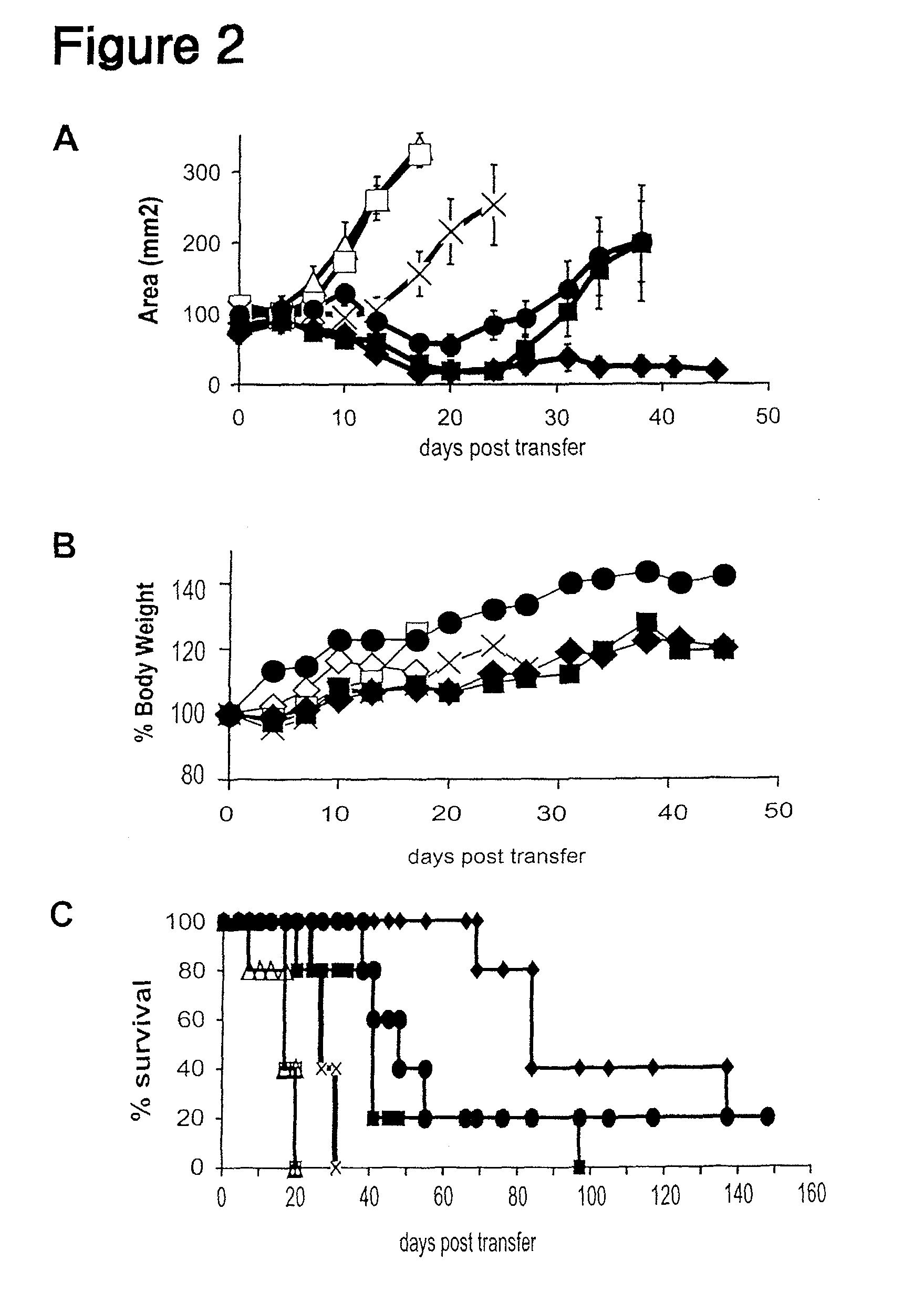
Inducible interleukin-12
ActiveUS20120071859A1Treat and prevent cancerOrganic active ingredientsFungiNucleotideWhite blood cell
The invention provides an isolated or purified nucleic acid comprising a nucleotide sequence encoding a nuclear factor of activated T-cells (NFAT) promoter operatively associated with a nucleotide sequence encoding IL-12. The invention also provides a nucleic acid comprising a nucleotide sequence encoding a nuclear factor of activated T-cells (NFAT) promoter operatively associated with a nucleotide sequence encoding IL-12, wherein the NFAT promoter is located 3′ of the nucleotide sequence encoding IL-12. Also provided are related recombinant expression vectors, host cells, populations of cells, and pharmaceutical compositions. The invention further provides the use of the inventive nucleic acids or related materials in the treatment or prevention of cancer or an infectious disease in a mammal and in the induction of IL-12 expression in a mammal.
Owner:UNITED STATES OF AMERICA
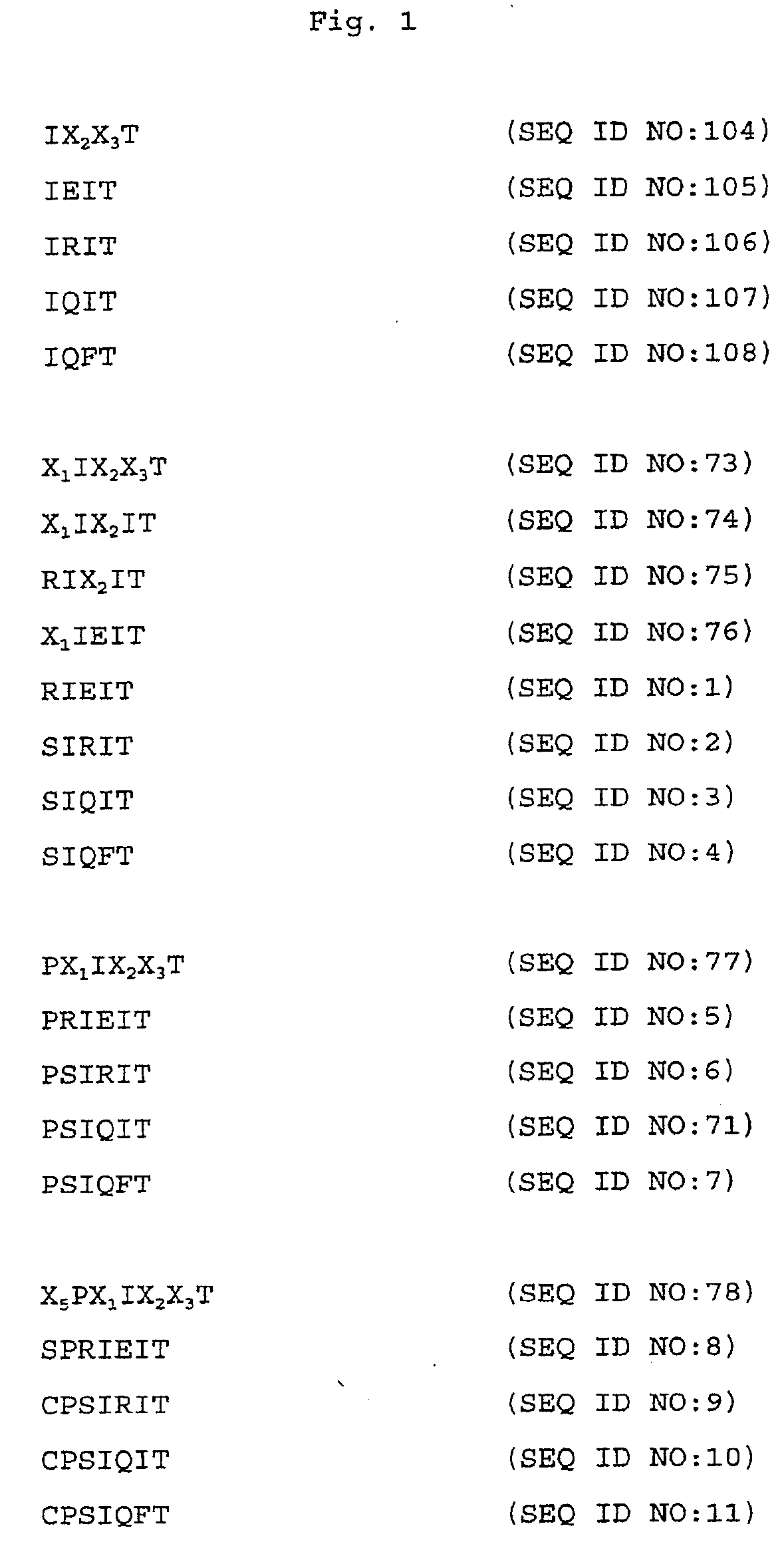
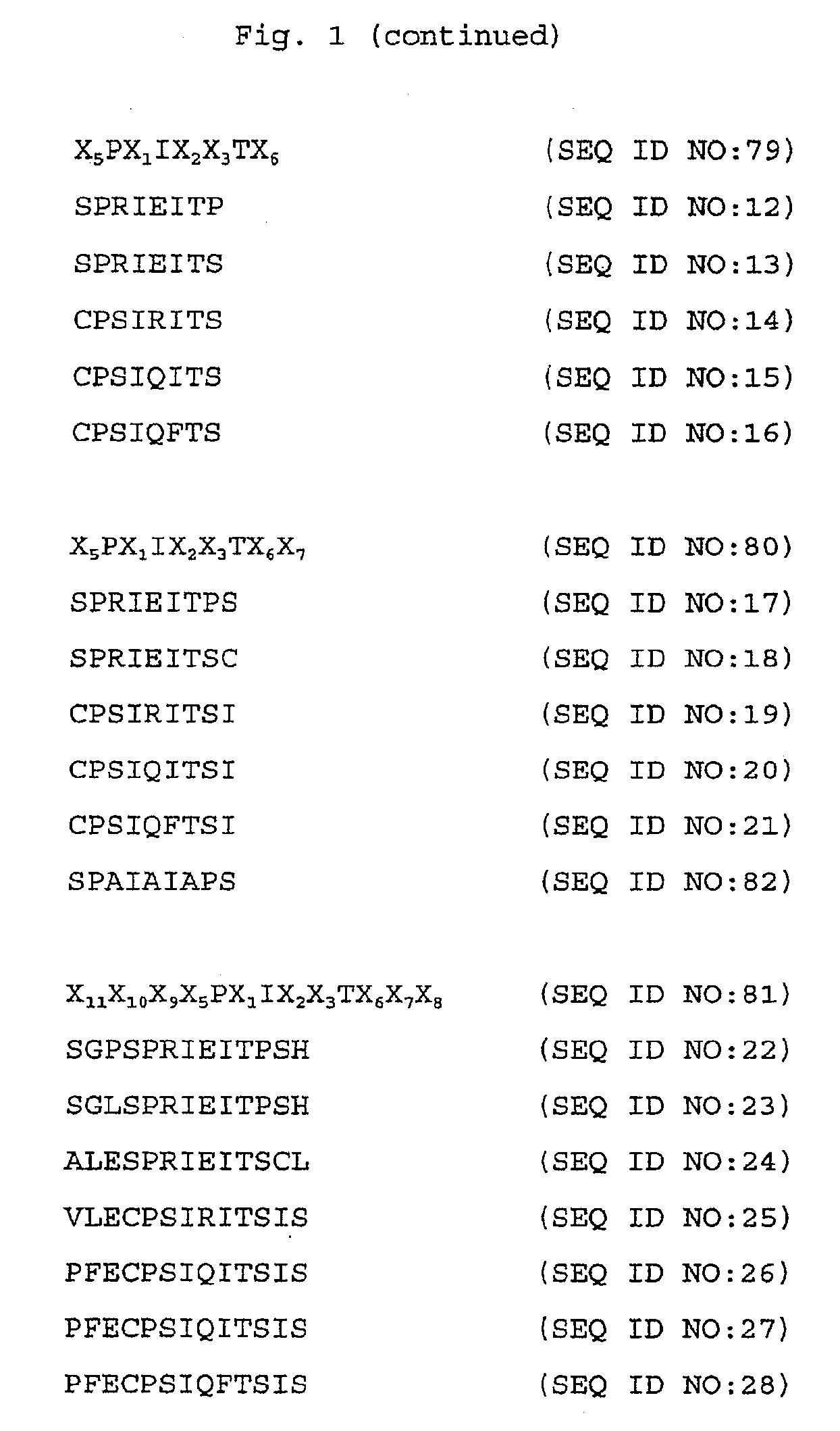
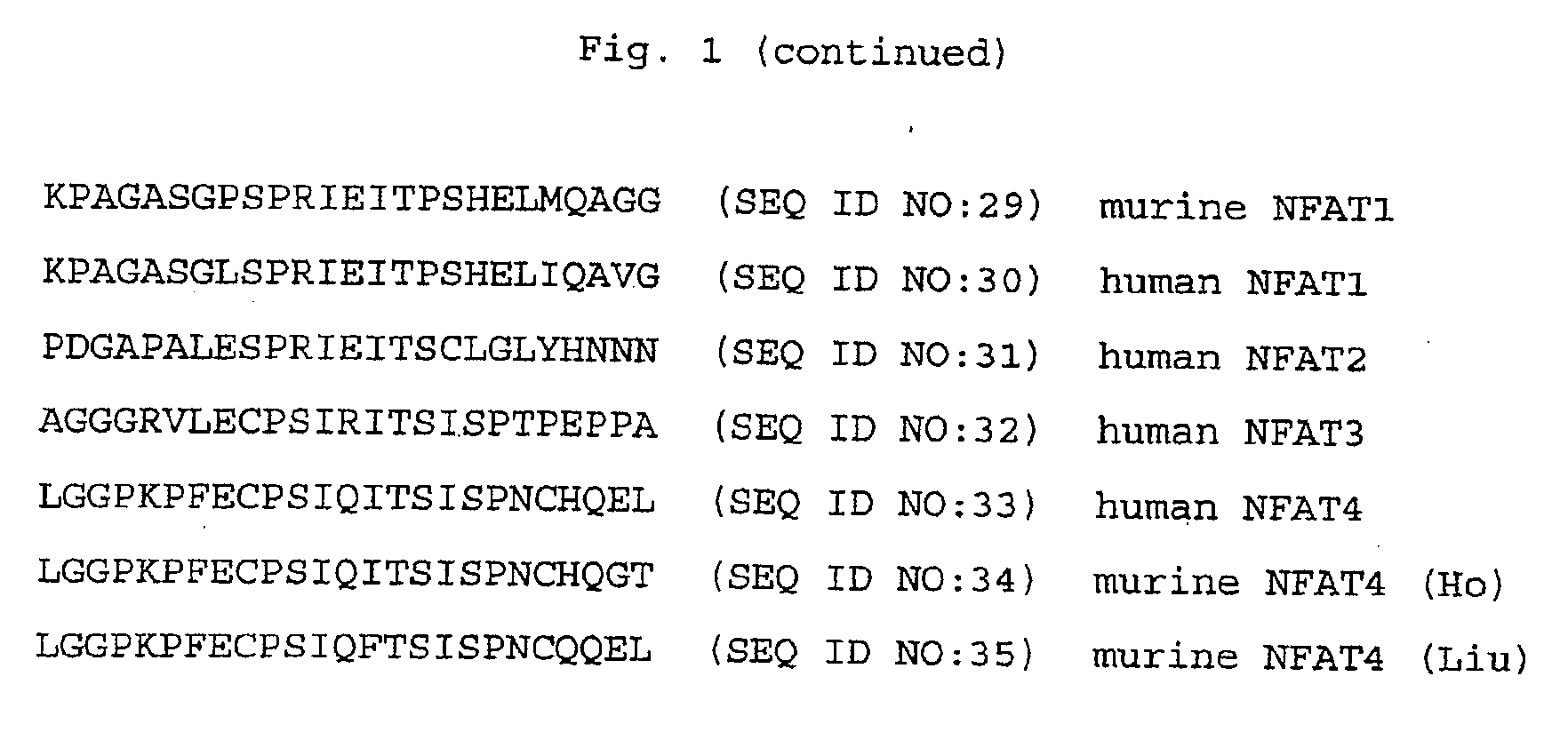
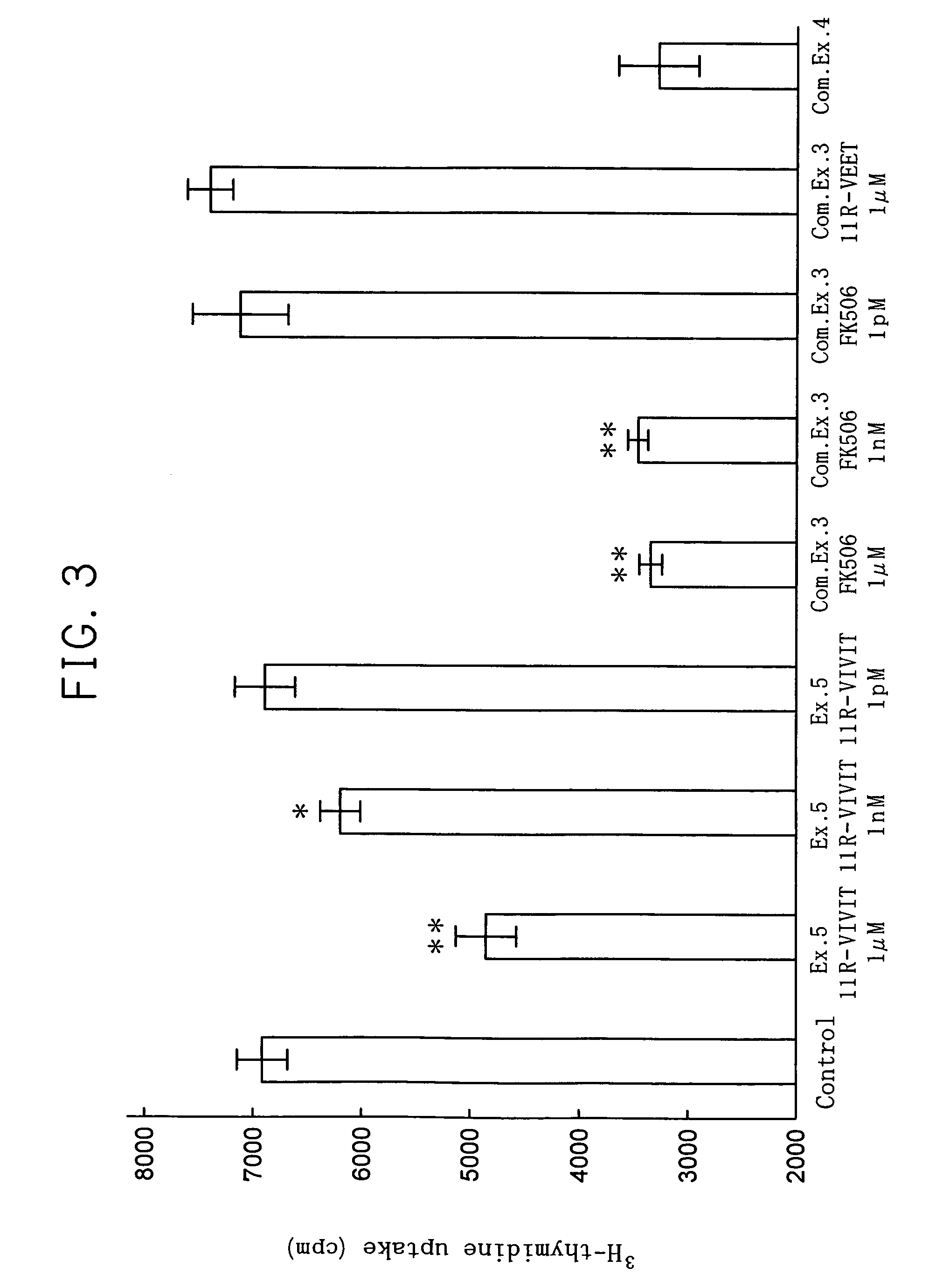
Specific inhibitors of nfat activation by calcineurin and their use in treating immune-related diseases
InactiveUS20090186422A1Low toxicityTripeptide ingredientsDepsipeptidesNucleotideBiological activation
Isolated peptide fragments of the conserved regulatory domain of NFAT protein capable of inhibiting protein-protein interaction between calcineurin and NFAT, or a biologically active analog thereof are described. Isolated polynucleotides and gene therapy vectors encoding such peptide fragments are also described. In addition, methods for treating immune-related diseases or conditions and methods for high throughput screening of candidate agents are described. Pharmaceutical compositions are also provided.
Owner:IMMUNE DISEASE INST INC
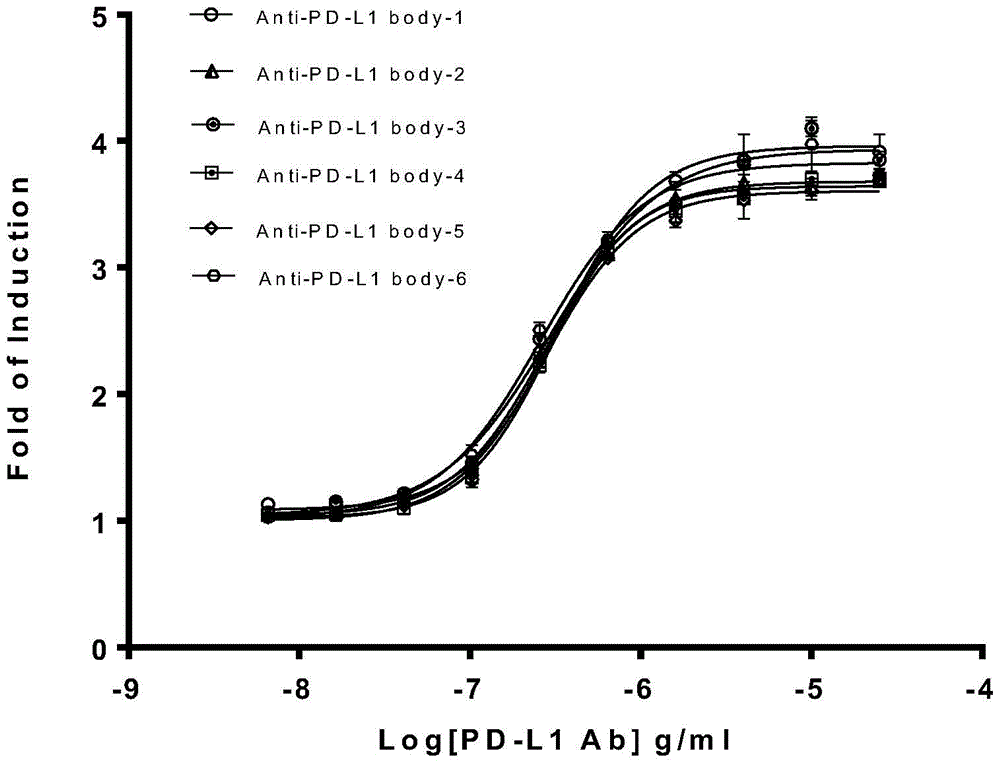
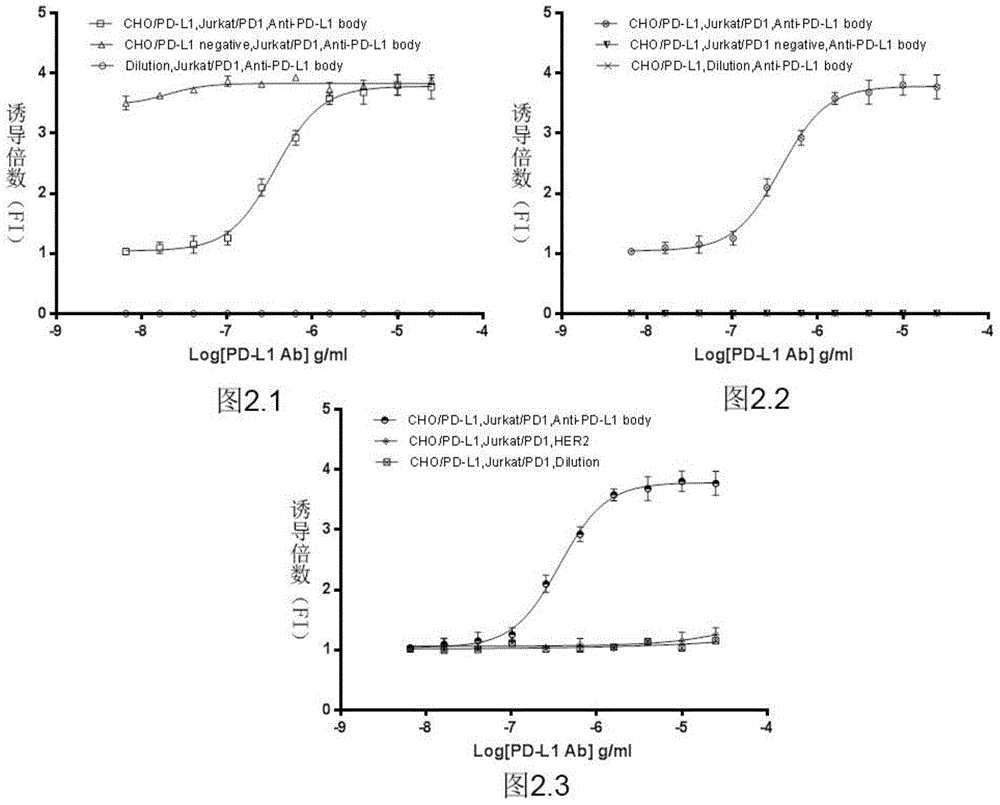
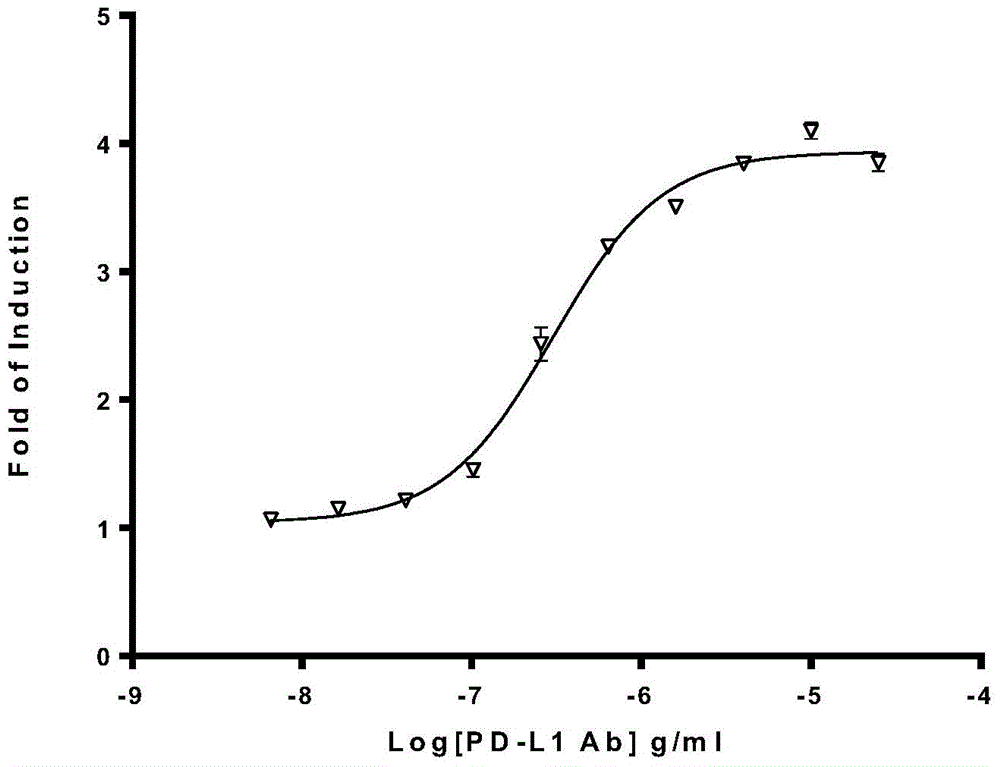
Biological activity determination method for anti-PD-L1 monoclonal antibody
The invention relates to the field of activity detection of biological medicine, in particular to a biological activity determination method for an anti-PD-L1 monoclonal antibody. According to the method, a PD1 / PD-L1 interruption detection system is used, binding of PD1 and PD-L1 protein is interrupted when the anti-PD-L1 monoclonal antibody is bound with PD-L1 expressed by target cells, a transcription factor NFAT is activated through signal transduction, expression of luciferase is started, the expression quantity of luciferase is positively correlated to the biological activity of the anti-PD-L1 monoclonal antibody, the chemiluminescence value of the anti-PD-L1 monoclonal antibody is determined after a cell lysis solution and a luciferase substrate are added, a dose-effect curve is drawn, and accordingly the biological activity of the anti-PD-L1 monoclonal antibody is determined. The method is easy to implement, strong in specificity and high in durability and has great significance on quality control and clinical application of the anti-PD-L1 monoclonal antibody.
Owner:兆科(广州)肿瘤药物有限公司
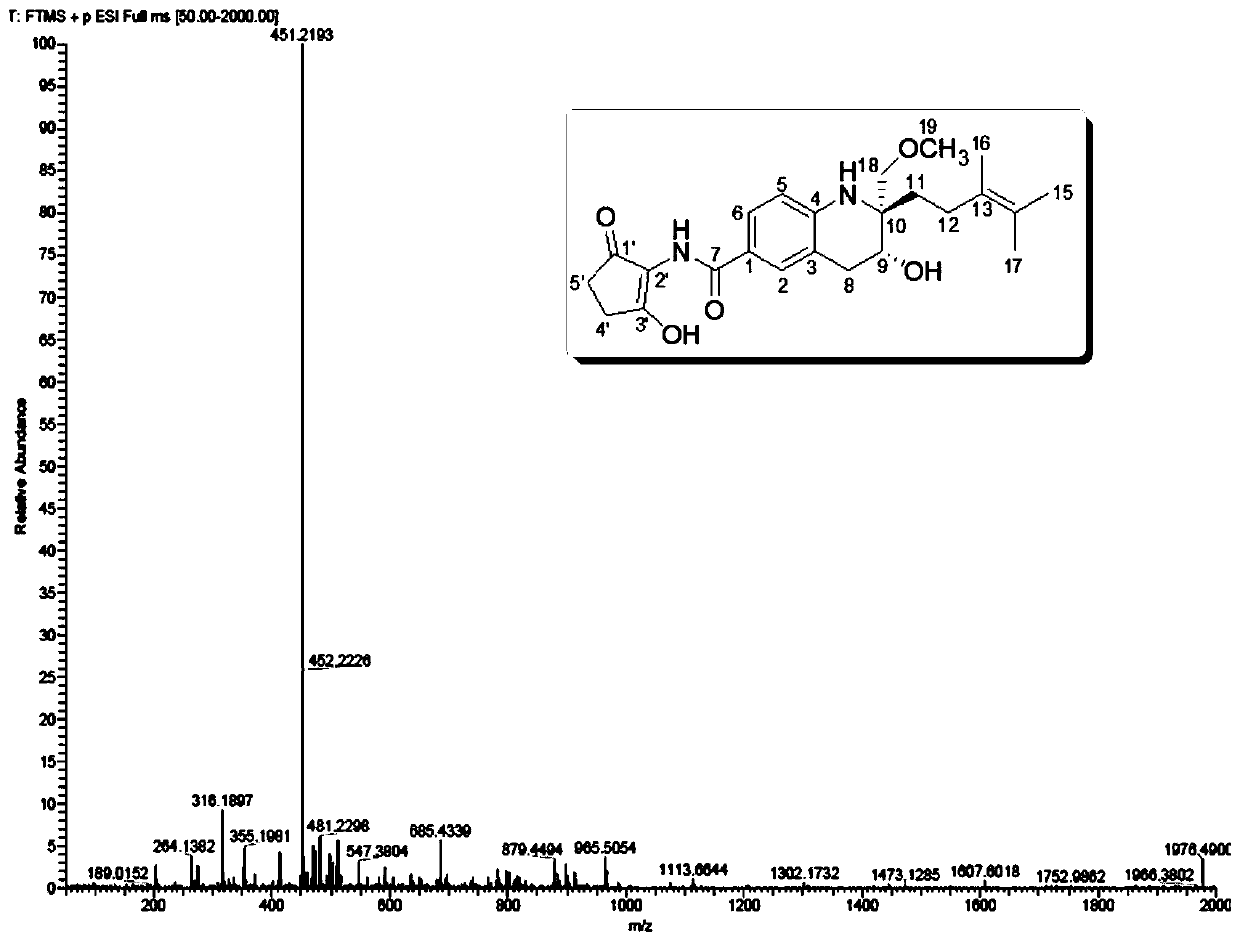
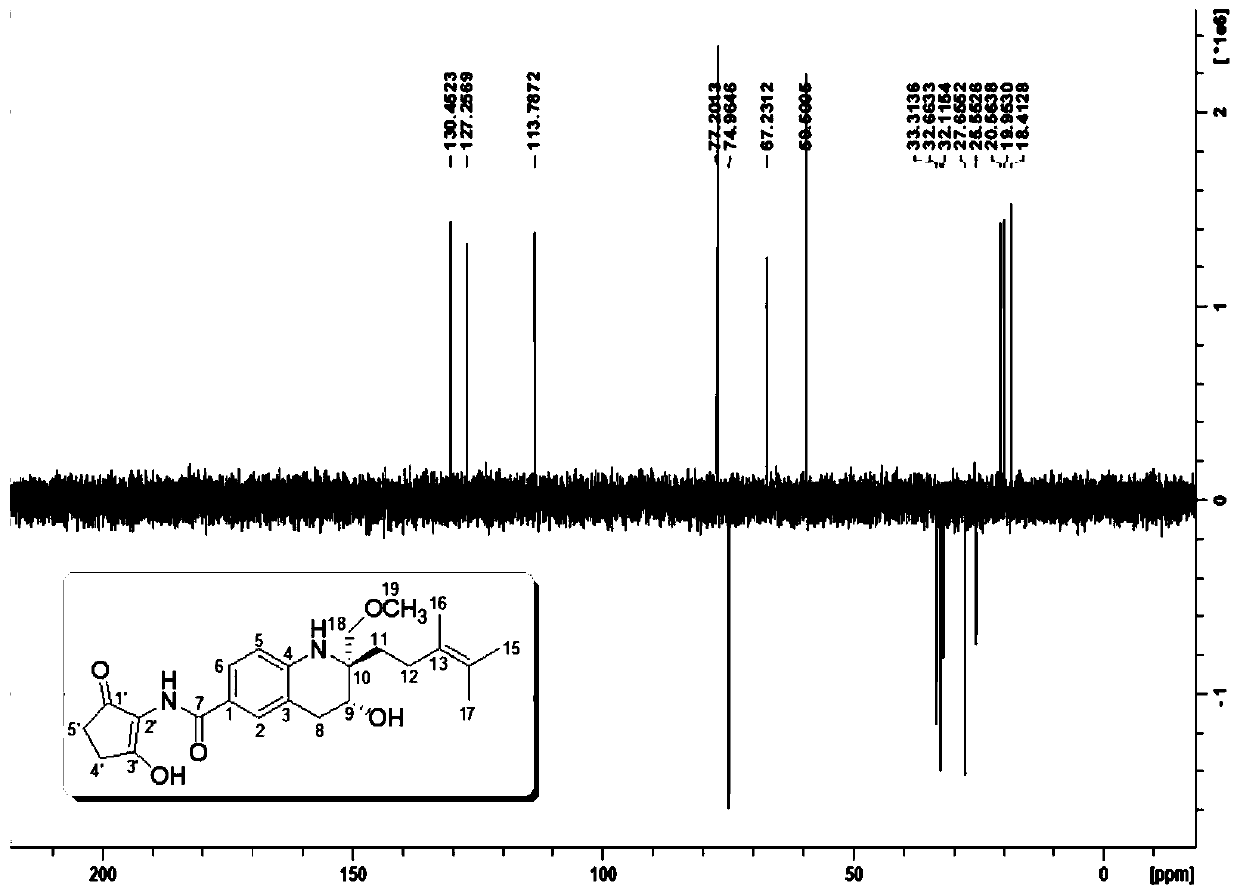
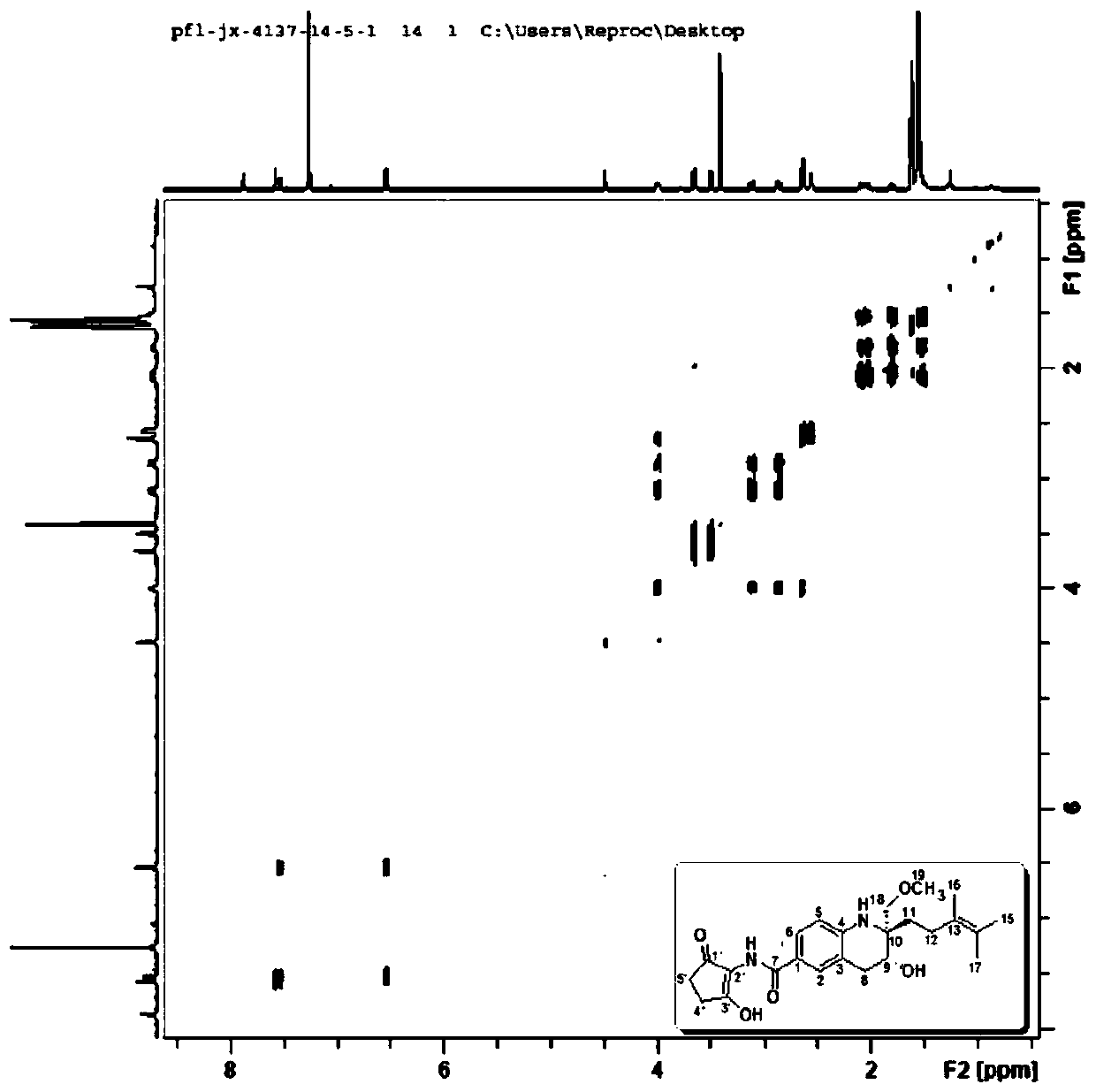
Tetrahydroquinoline alkaloid Malaysiensin with immunosuppression activity and production method and application of tetrahydroquinoline alkaloid Malaysiensin with immunosuppression activity
ActiveCN110724096APrevent proliferationOrganic chemistryImmunological disordersIMMUNE SUPPRESSANTSAutoimmune condition
The invention provides a tetrahydroquinoline alkaloid Malaysiensin with immunosuppression activity and a production method and application of the tetrahydroquinoline alkaloid Malaysiensin with the immunosuppression activity. The compound is obtained by conducting SFM solid medium fermentation on Streptomyces sp.DSM 4137 and separating and purifying a fermentation product. The compound can significantly inhibit ConA-induced proliferation of T cells, an immunosuppression effect of the compound is equivalent to that of an immunosuppressor cyclosporin A which is clinically and widely used, and action mechanism studies show that Malaysiensin exerts the immunosuppression activity for a targeted NFAT signal path, is an efficient immunosuppressor, and can be used for producing immunosuppressive medicines for organ transplantation and autoimmune diseases.
Owner:HAINAN UNIVERSITY
Regulators of nfat
Disclosed are methods of identifying an agent that modulates an NFAT regulator protein. One such method comprises contacting at least one test agent with a recombinant cell comprising at least one NFAT regulator protein or fragment or derivative thereof, assessing the effect of the test agent on an activity, interaction, expression, or binding to the NFAT regulator protein or fragment or derivative thereof, and identifying the test agent that has an effect on an activity, interaction, expression, or binding to the NFAT regulator protein or fragment or derivative thereof, whereby the identified test agent is characterized as an agent that modulates an NFAT regulator protein. Methods of identifying an agent that modulates intracellular calcium, methods to screen for an agent that modulates NFAT regulator function, methods to diagnose unexplained immunodeficiency in a subject, and methods for identifying an agent for treating or preventing a disease or disorder associated with a NFAT regulator protein or calcium signaling are also disclosed.
Owner:CHILDRENS MEDICAL CENT CORP
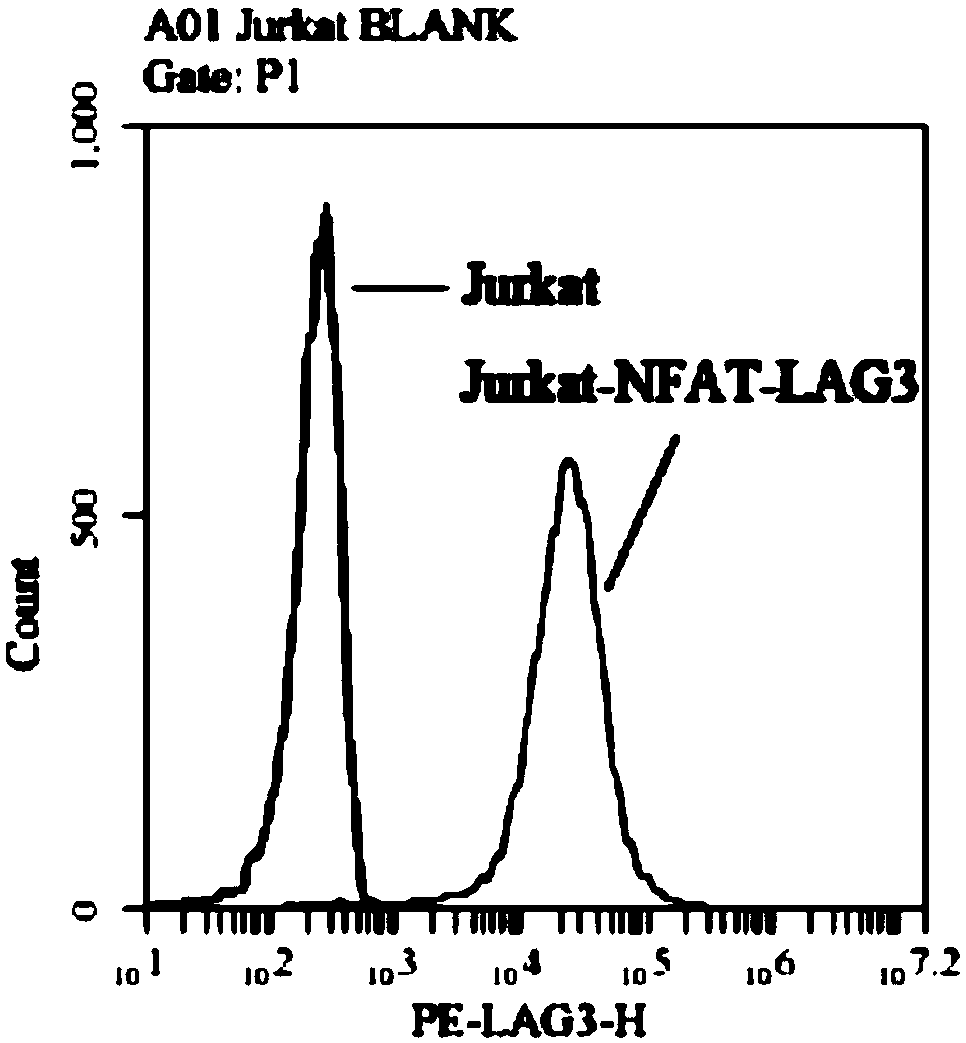
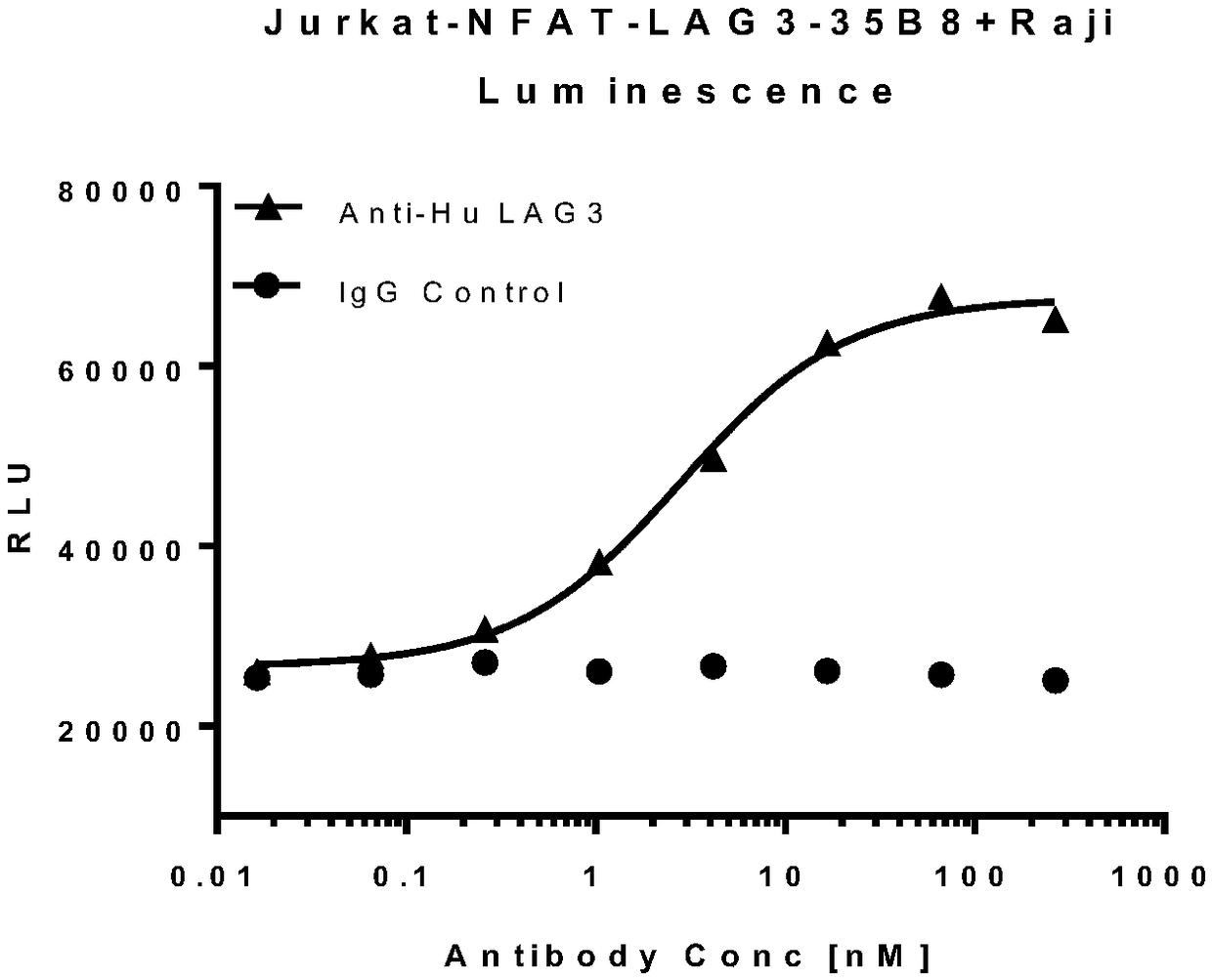
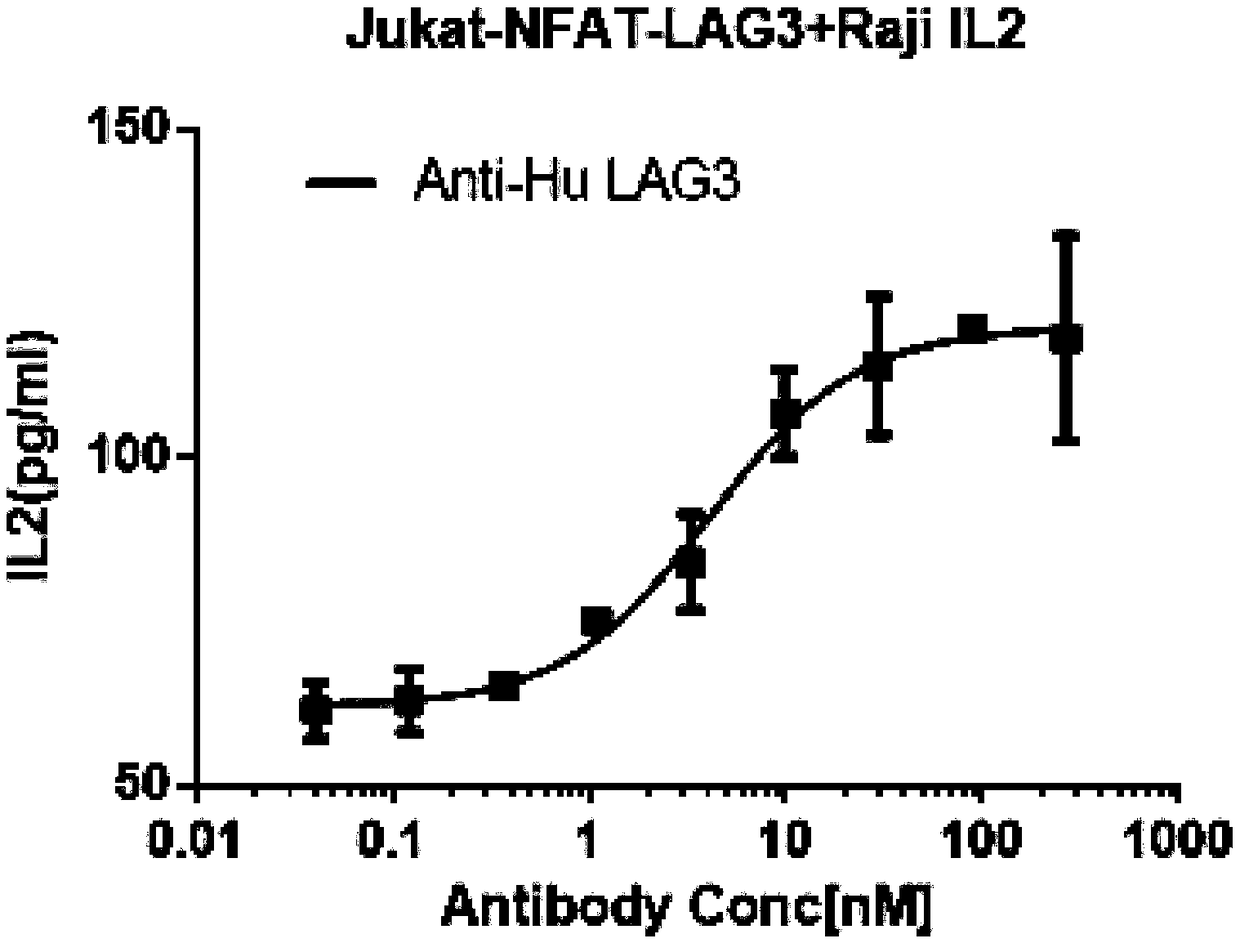
Method for determining biological activity of LAG3 (Lymphocyte Activation Gene-3) protein binding molecule
ActiveCN108949904AReduce luminescent signalKeep activeMicrobiological testing/measurementBiological testingLymphocyte activationDrug biological activity
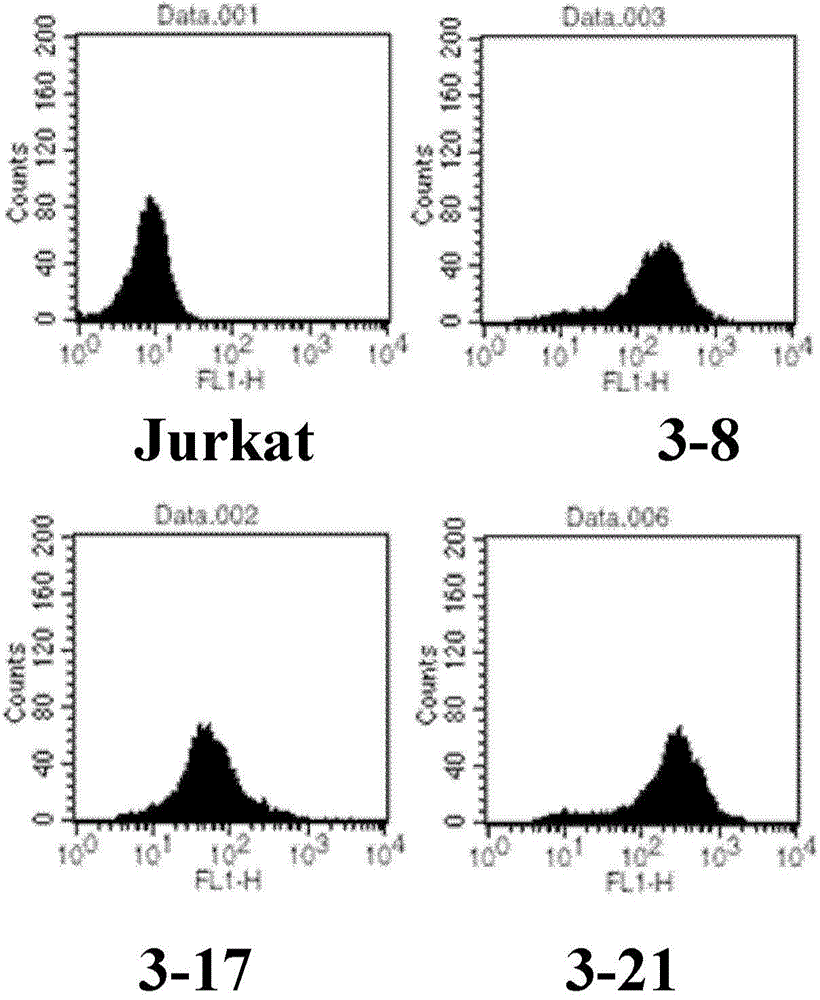
The invention discloses a method for determining the biological activity of an LAG3 (Lymphocyte Activation Gene-3) protein binding molecule. The method comprises the step of detecting the activity ofan anti-human LAG3 antibody by a co-expression NFAT (Nuclear Factor of Activated T cells) reporting element and Jurkat cells of human LAG3 protein under the condition of coexistence of SED and Raji cells. The method provided by the invention can be used for rapidly and accurately detecting the activity of the molecule bound with human LAG3 protein.
Owner:NANJING LEADS BIOLABS CO LTD
Method and reagent for regulation and control of germ layer differentiation of embryonic stem cell and early embryo
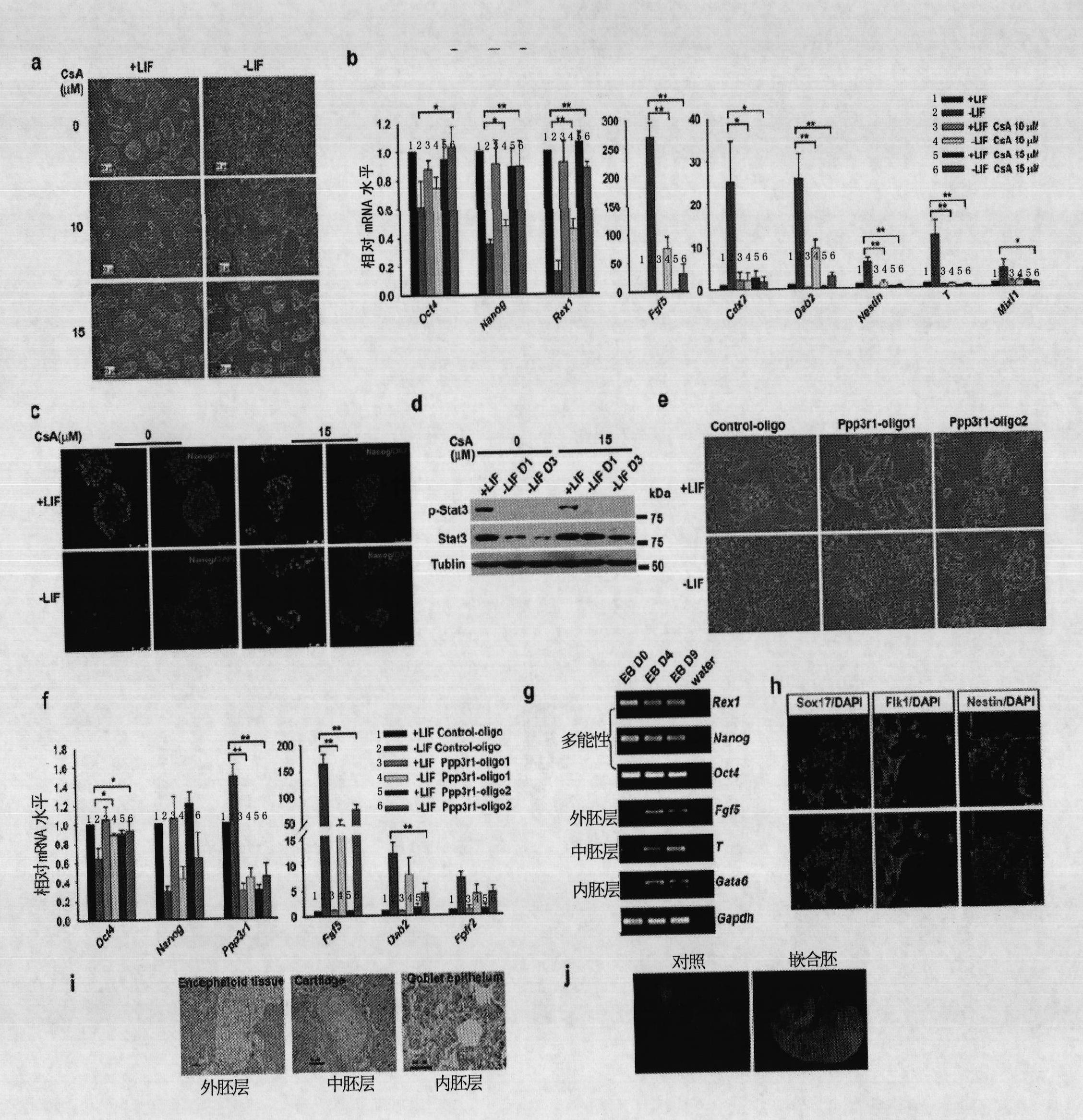
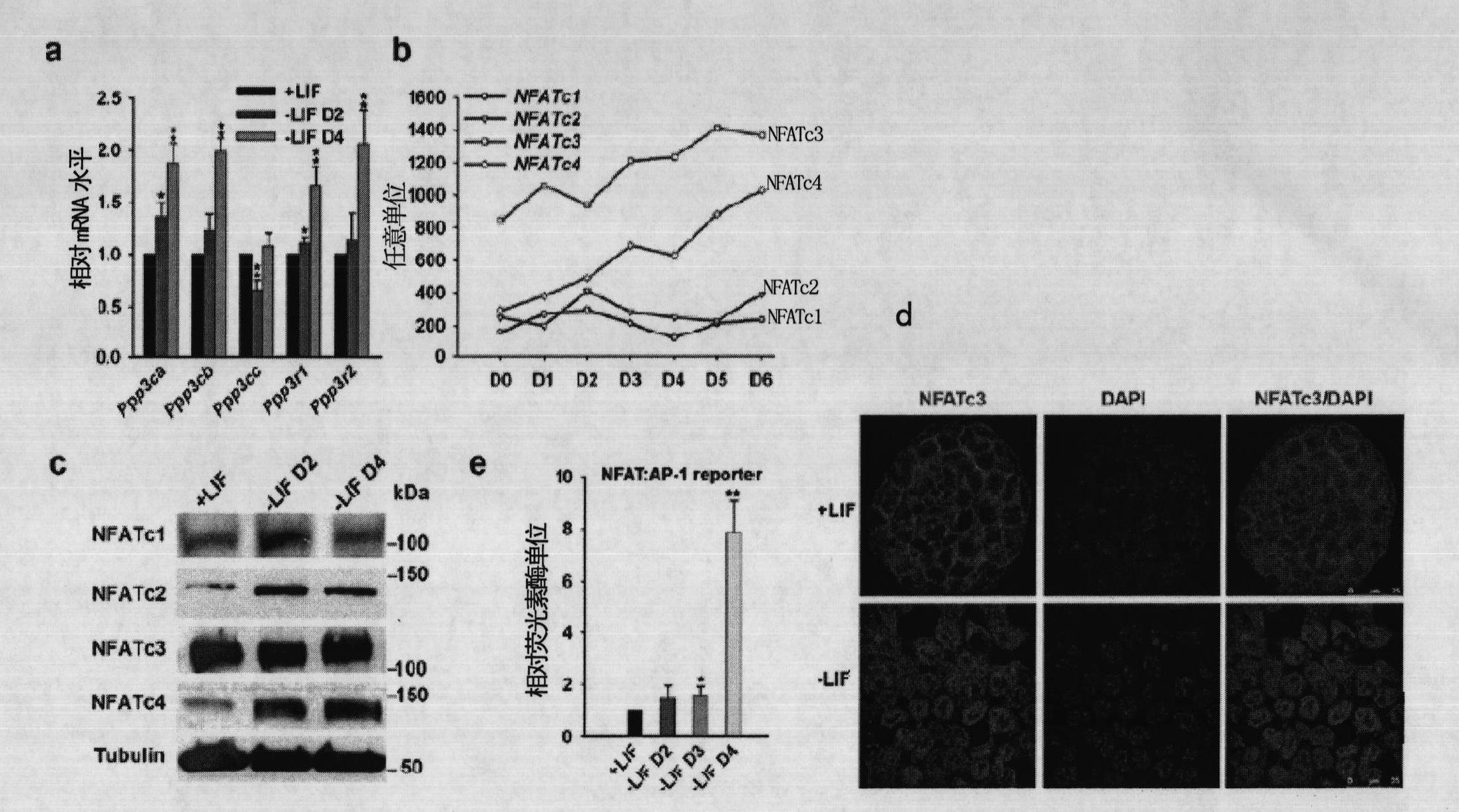
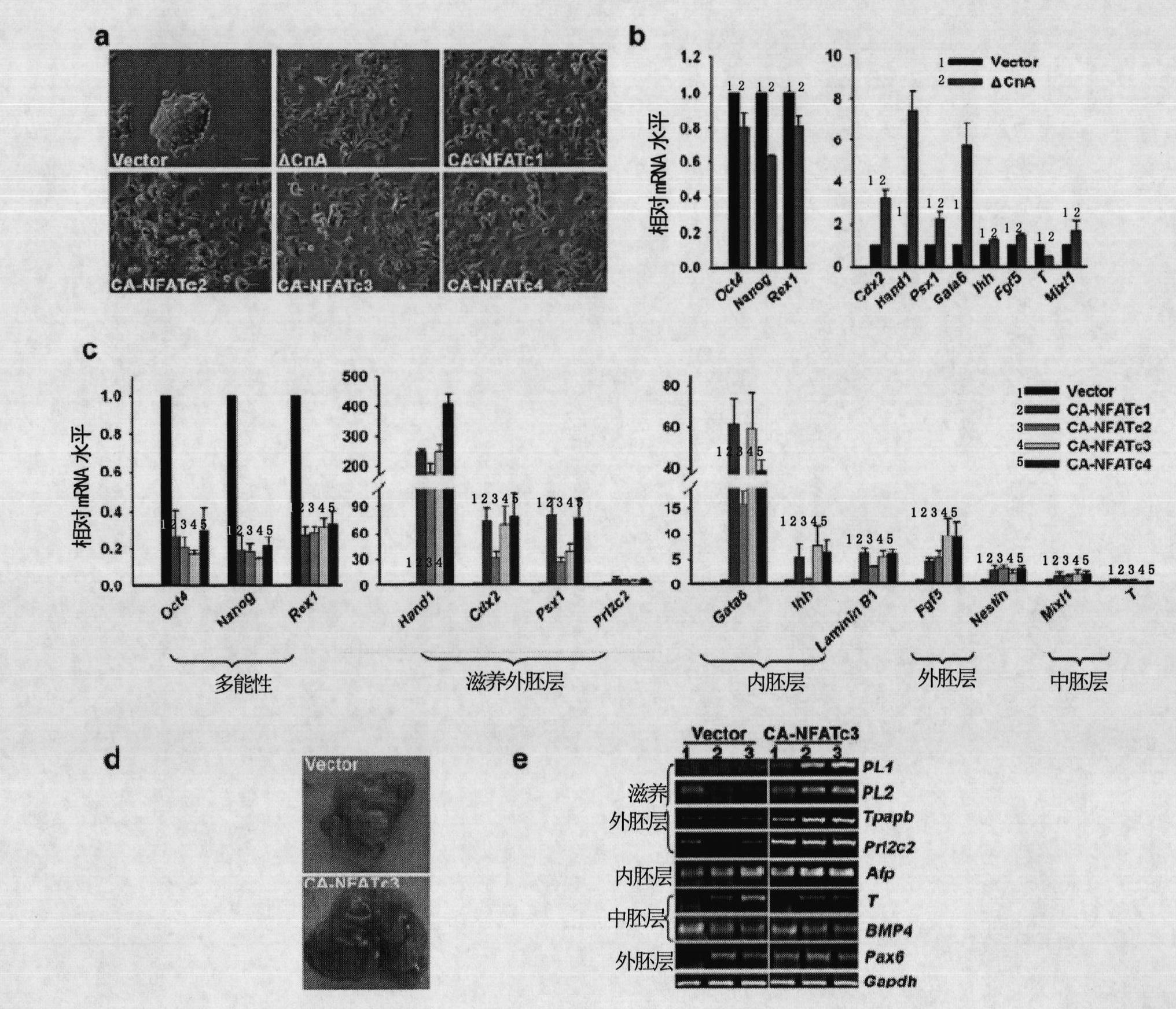
The invention relates to a method and a reagent for regulation and control of germ layer differentiation of an embryonic stem cell and an early embryo. The invention first reveals that activation of a Calcinuerin-NFAT signal path is closely related embryonic stem (ES) cell differentiation or self-renewal, wherein ES cell germ layer differentiation can be initiated through activation of the Calcinuerin-NFAT signal path, and ES cell self-renewal can be maintained for a long time through inhibition of the Calcinuerin-NFAT signal path.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
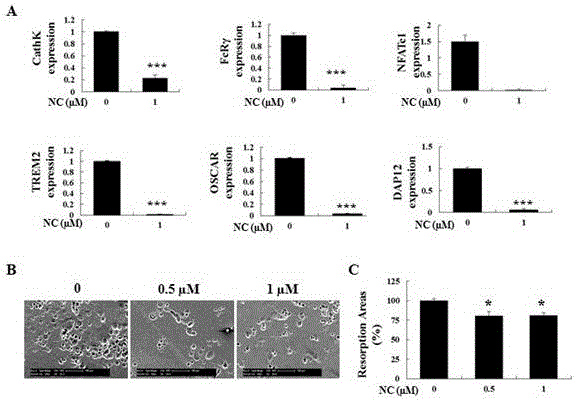
Application of nitidine chloride in preparing medicines for resisting osteoporosis and bone loss diseases
ActiveCN106389432AInhibition formationAvoid lossOrganic active ingredientsSkeletal disorderNitidine chlorideDisease
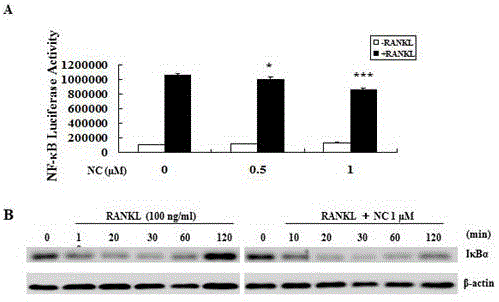
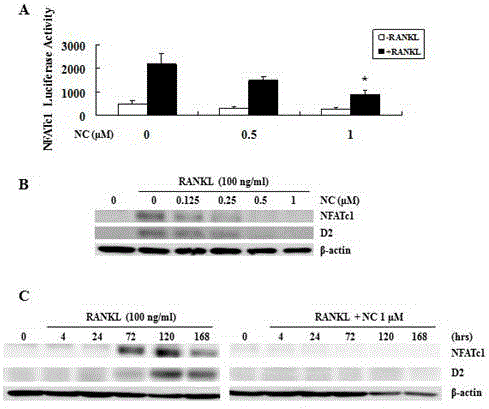
The invention discloses an application of nitidine chloride in resisting osteoporosis and in preventing and treating bone loss diseases caused by osteoclasts; the inventor, through researches, discovers that the nitidine chloride can inhibit the formation of the osteoclasts and a bone absorption function in a dose-dependent mode for the first time; it discovers that the nitidine chloride, by intervening RANKL-activated NF-kB and NFAT signaling pathways, can develop an effect of inhibiting the osteoclasts; and by establishing an ovariectomized osteoporosis mouse model, it further provides the effect of the nitidine chloride on the osteoclasts. The nitidine chloride can be used as an ideal medicine for preventing and treating the bone loss diseases caused by the osteoclasts in the future.
Owner:刘倩 +3
Application of micromolecule RNA used as immunosuppressor
InactiveCN103505745AInhibitory activityLow toxicityGenetic material ingredientsImmunological disordersDiseaseAutoimmune disease
The invention discloses application of micromolecule RNA (Ribose Nucleic Acid) used as an immunosuppressor, and particularly relates to application of the micromolecule RNA which has a base sequence of SEQ ID NO:1 and is used as the immunosuppressor to preparation of a medicament for treating disease caused by abnormal activation of an NFAT (Nuclear Factor Activated T Cell) signal pathway. The micromolecule RNA in the invention uses NFAT as a target, inhibits the NFAT signal pathway by inhibiting a key factor in the NFAT signal pathway, can obviously inhibit activity of NFAT and can be used as the novel immunosuppressor for treating organ transplantation rejection reaction and autoimmune diseases. The micromolecule RNA in the invention is an endogenous RNA of a human body and has low toxicity for the human body.
Owner:SHENZHEN UNIV
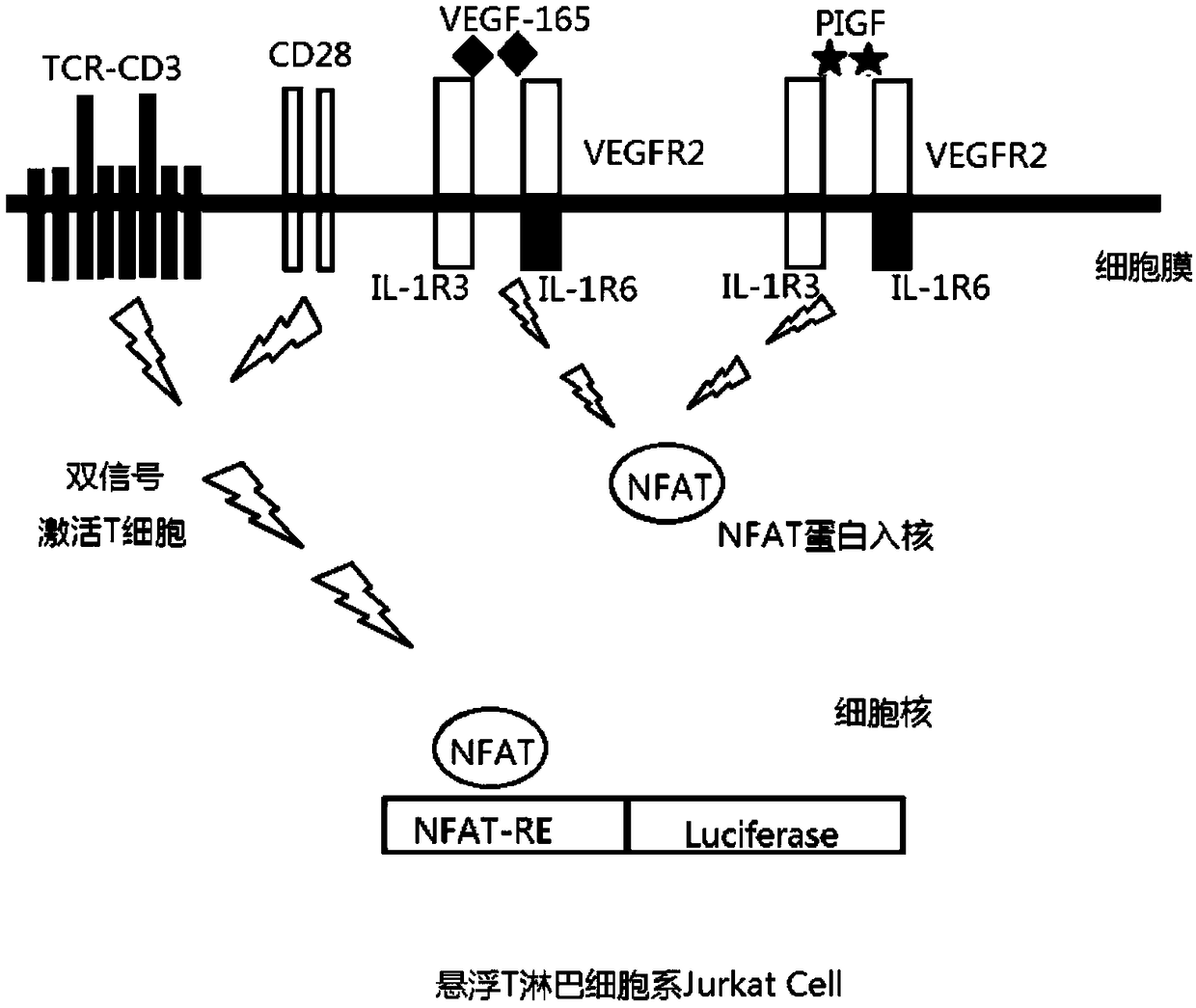
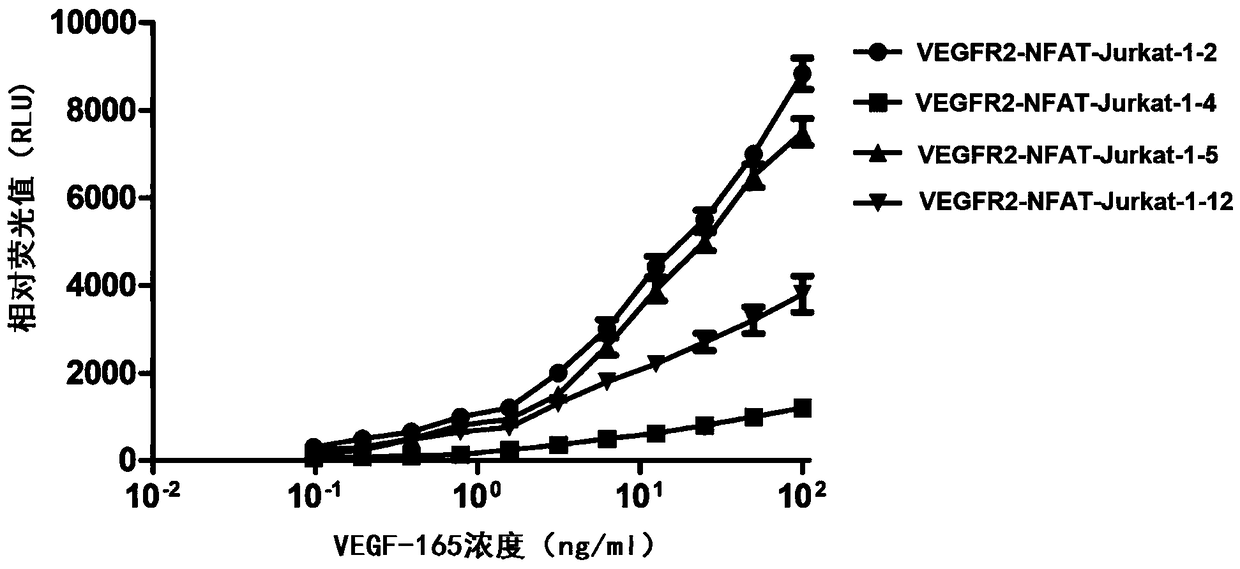
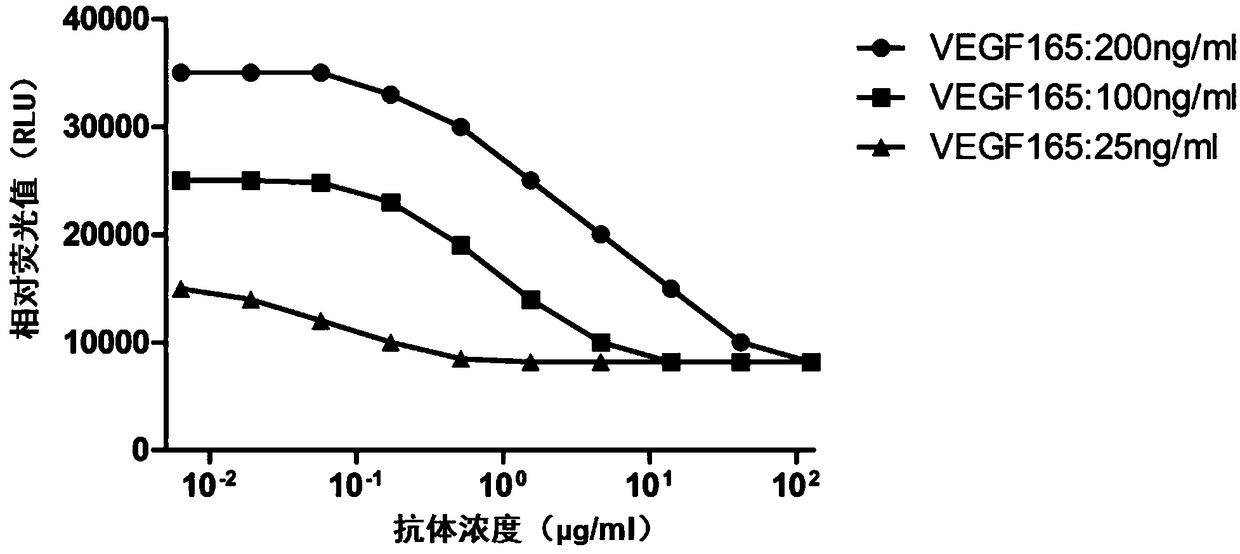
Method for measuring activity of anti-VEGF (Vascular Endothelial Growth Factor) antibody, and application thereof
ActiveCN108918892AEasy to operateShorten experiment timeBiological material analysisBiological testingAnti vegf antibodyEffector cell
The invention discloses a method for measuring the activity of an anti-VEGF (Vascular Endothelial Growth Factor) antibody. An effector cell which transfects a VEGF receptor, IL-1R3, IL-1R6 and a reporter gene is taken as a detection material, anti-CD3 and anti-CD28 antibodies activate Jurkat cells, a VEGF ligand is added and is combined with a VEGFR (Vascular Endothelial Growth Factor Receptor) onthe effector cell, so that IL-1R3 and IL-1R6 in the cell mutually act to activate a downstream NFAT (Nuclear Factor of Activated T) signal path, and therefore, the reporter gene can be expressed. After the anti-VEGF antibody is added, the anti-VEGF antibody can be competitively combined with the VEGF ligand so as to block the combination of the VEGF ligand and the receptor VEGFR thereof so as toreduce a downstream reporter gene expression quantity. The method has the advantages of convenient operation step, high detection sensitivity and good accuracy, and experiment time is greatly shortened.
Owner:BIO THERA SOLUTIONS LTD
Method of evaluating immunosuppression
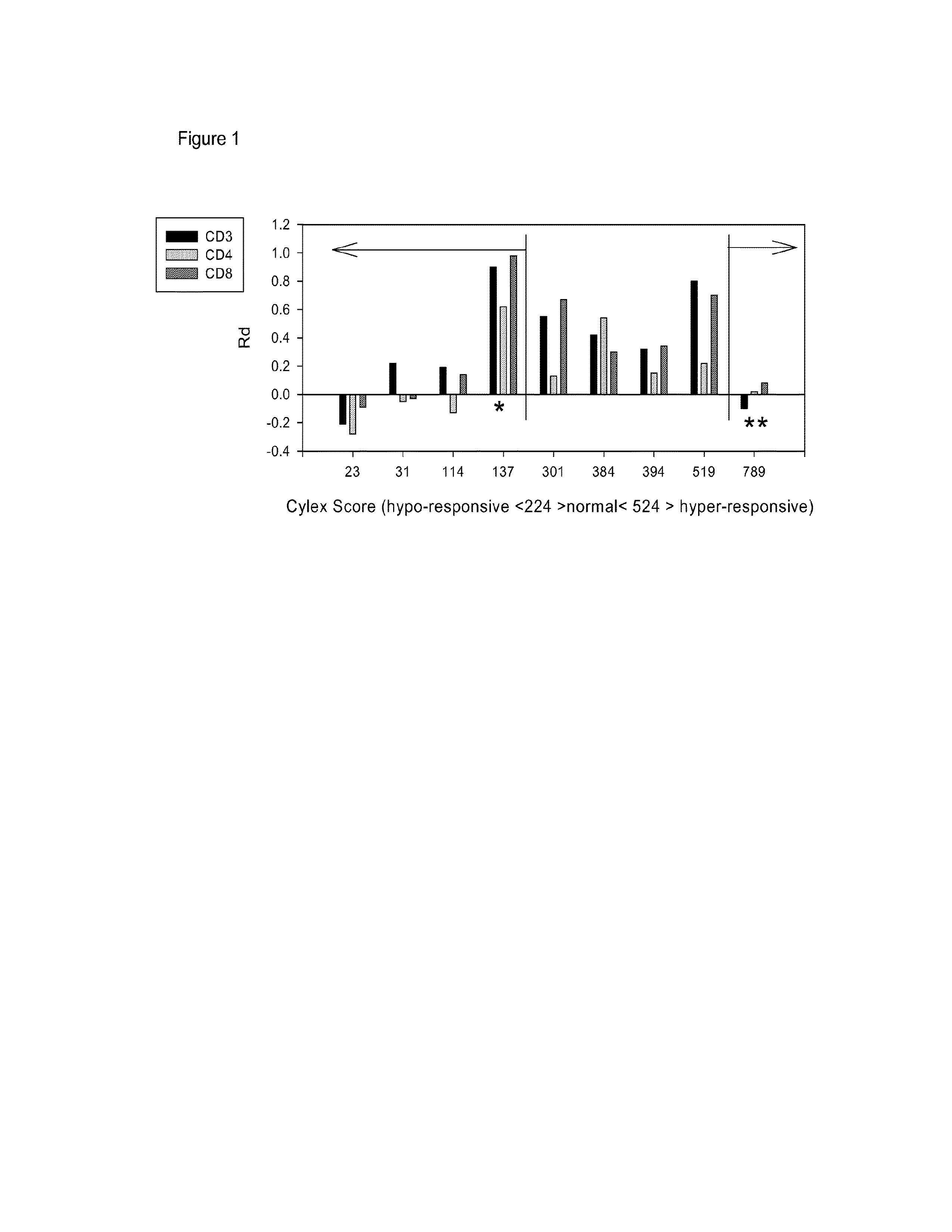
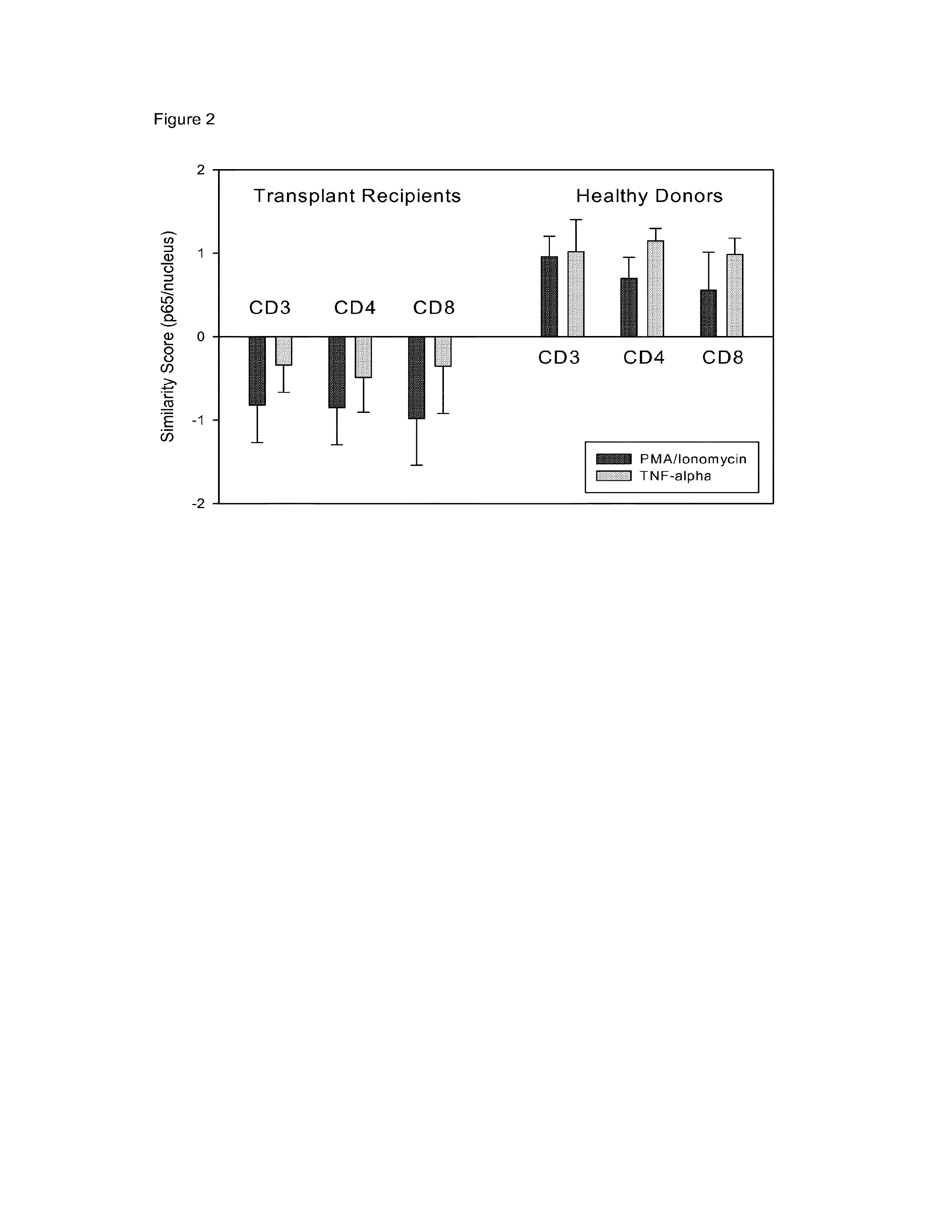
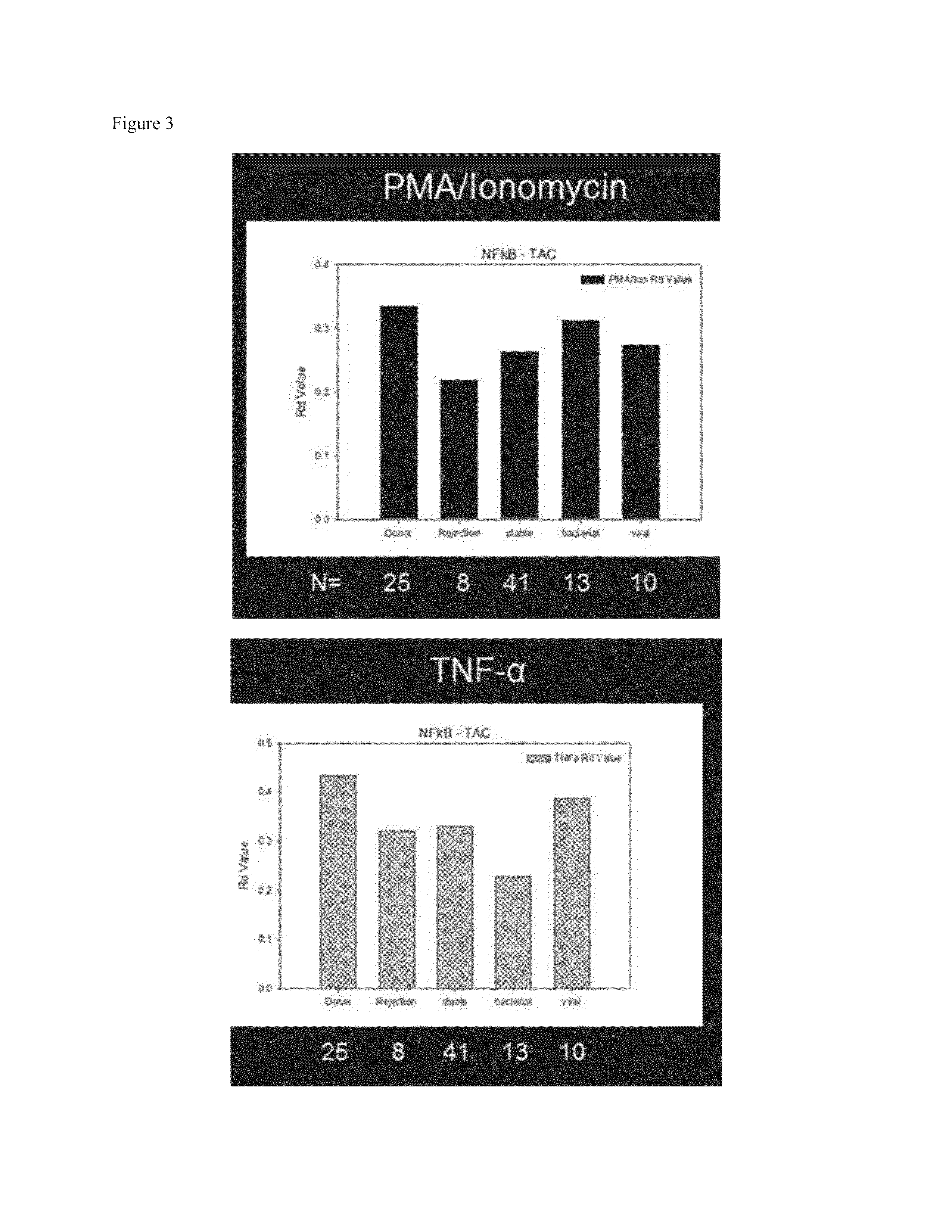
InactiveUS20130183686A1Small similarity scoreLess nuclear translocationDisease diagnosisBiological testingActivation cellsHematological test
Provided is a method for determining immunosuppression in an individual. The method entails testing blood cells for nuclear NFkB and / or nuclear NFAT. The blood cells can be from a sample of blood from an individual. The cells can be contacted with an activating agent to obtain activated cells, and the amount of nuclear NFkB and / or NFAT can be compared to a control. An amount of nuclear NFkB and / or NFAT that is higher than the control is considered to be indicative of insufficient immunosuppression in the individual. An amount of nuclear NFkB and / or NFAT that is lower than the control is considered to be indicative of excessive immunosuppression in the individual. An amount of nuclear NFkB and / or NFAT that is the same as the control is considered to be indicative of an appropriate amount of immunosuppression in the individual.
Owner:DAVIDOVICH DONNA
Regulators of NFAT
Disclosed are methods of identifying an agent that modulates an NFAT regulator protein. One such method comprises contacting at least one test agent with a recombinant cell comprising at least one NFAT regulator protein or fragment or derivative thereof, assessing the effect of the test agent on an activity, interaction, expression, or binding to the NFAT regulator protein or fragment or derivative thereof, and identifying the test agent that has an effect on an activity, interaction, expression, or binding to the NFAT regulator protein or fragment or derivative thereof, whereby the identified test agent is characterized as an agent that modulates an NFAT regulator protein. Methods of identifying an agent that modulates intracellular calcium, methods to screen for an agent that modulates NFAT regulator function, methods to diagnose unexplained immunodeficiency in a subject, and methods for identifying an agent for treating or preventing a disease or disorder associated with a NFAT regulator protein or calcium signaling are also disclosed.
Owner:CHILDRENS MEDICAL CENT CORP
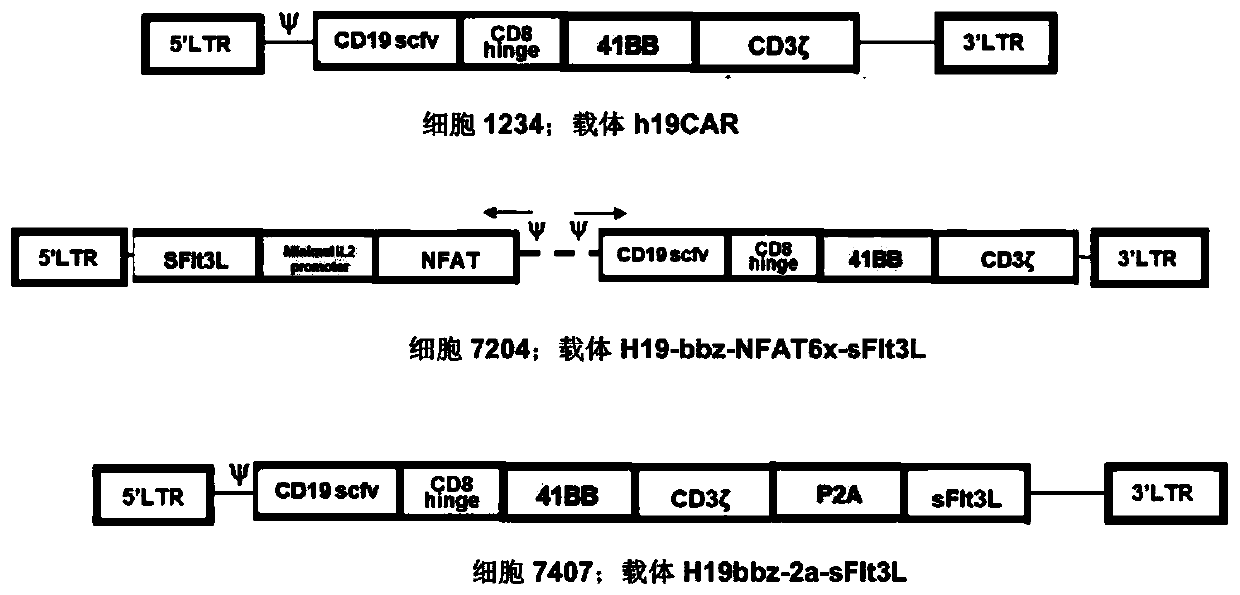
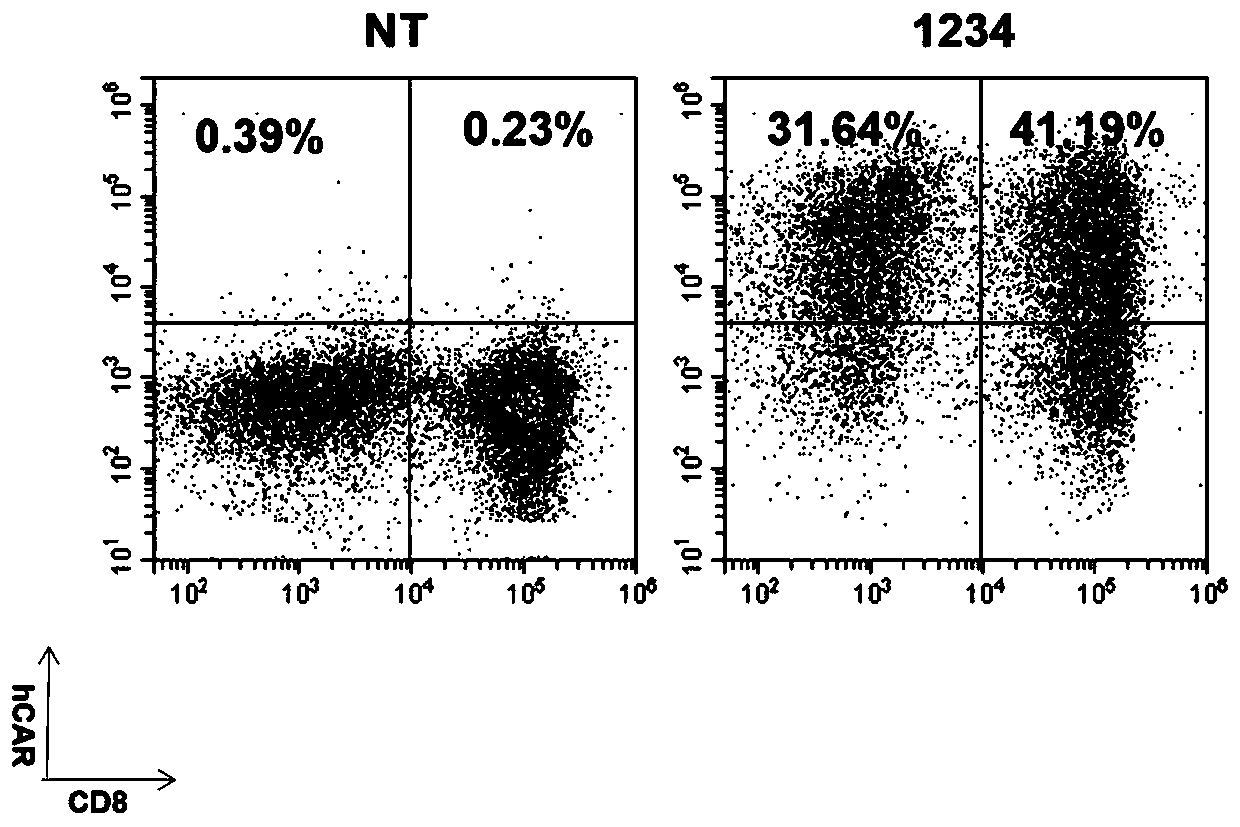
Inducible Fms-like tyrosine kinase 3 ligand (FLT3L) modified cells and application thereof
Owner:上海煦顼技术有限公司
Small molecule screen for inhibitors of nfat: ap-1: DNA interactions
The invention provides a screening assay for selecting inhibitors of NFAT:Transcription factor interactions. The invention also provides compositions and methods for inhibiting immune response in a subject.
Owner:CHILDRENS MEDICAL CENT CORP
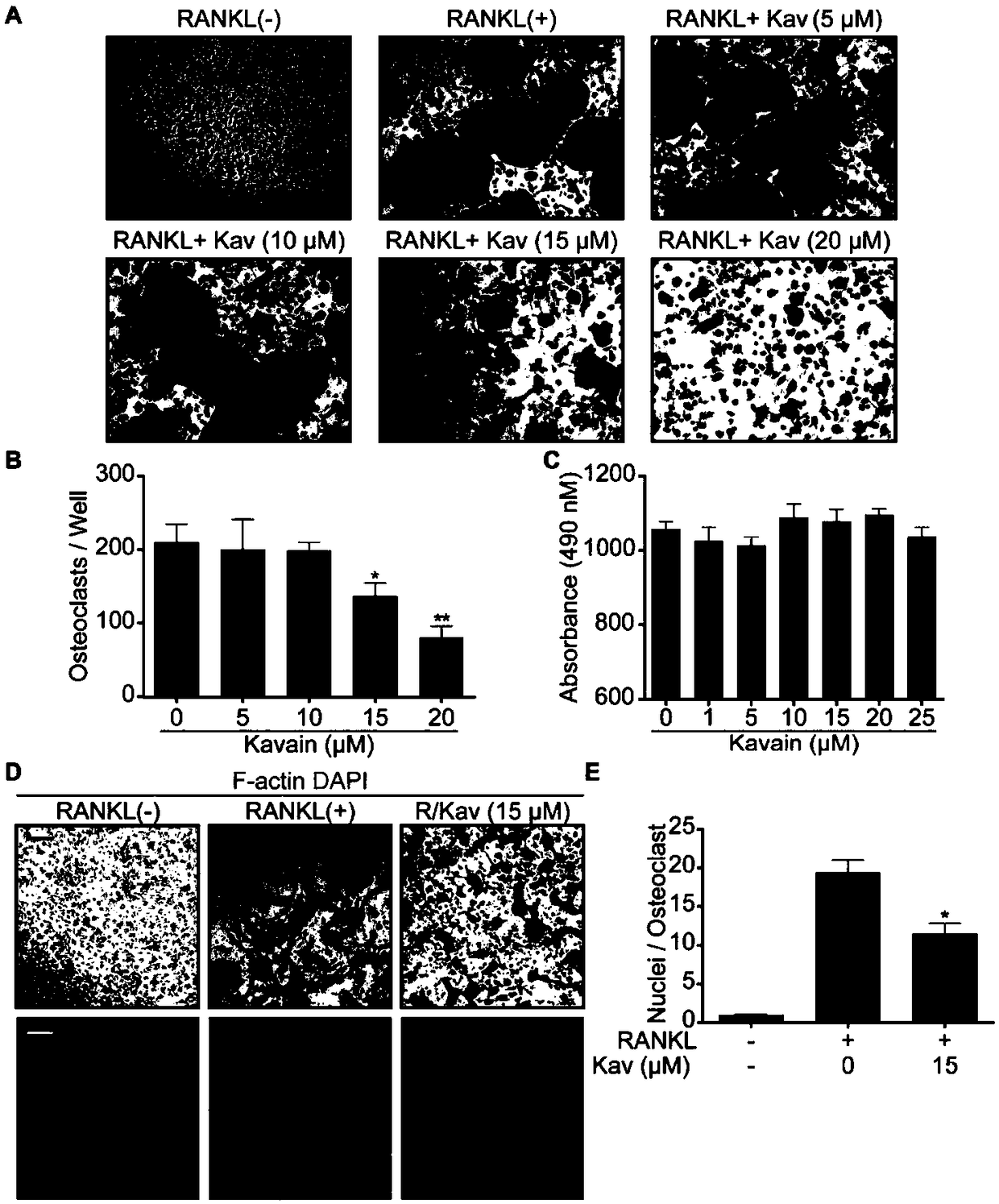
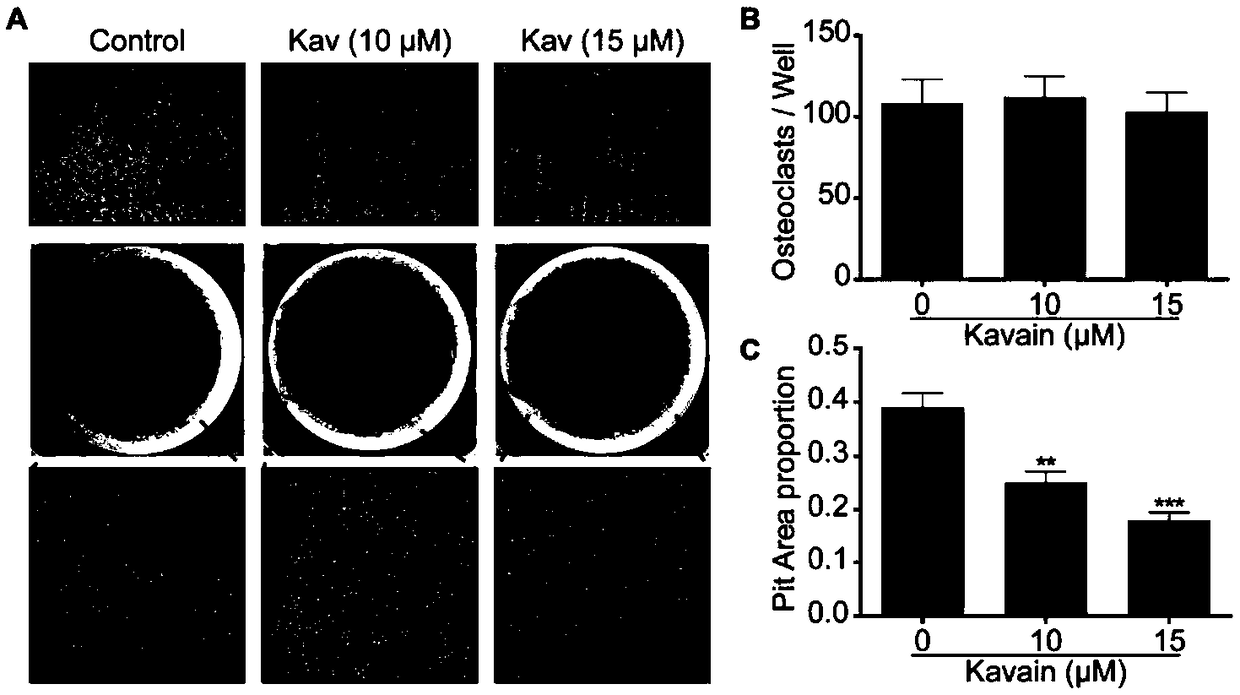
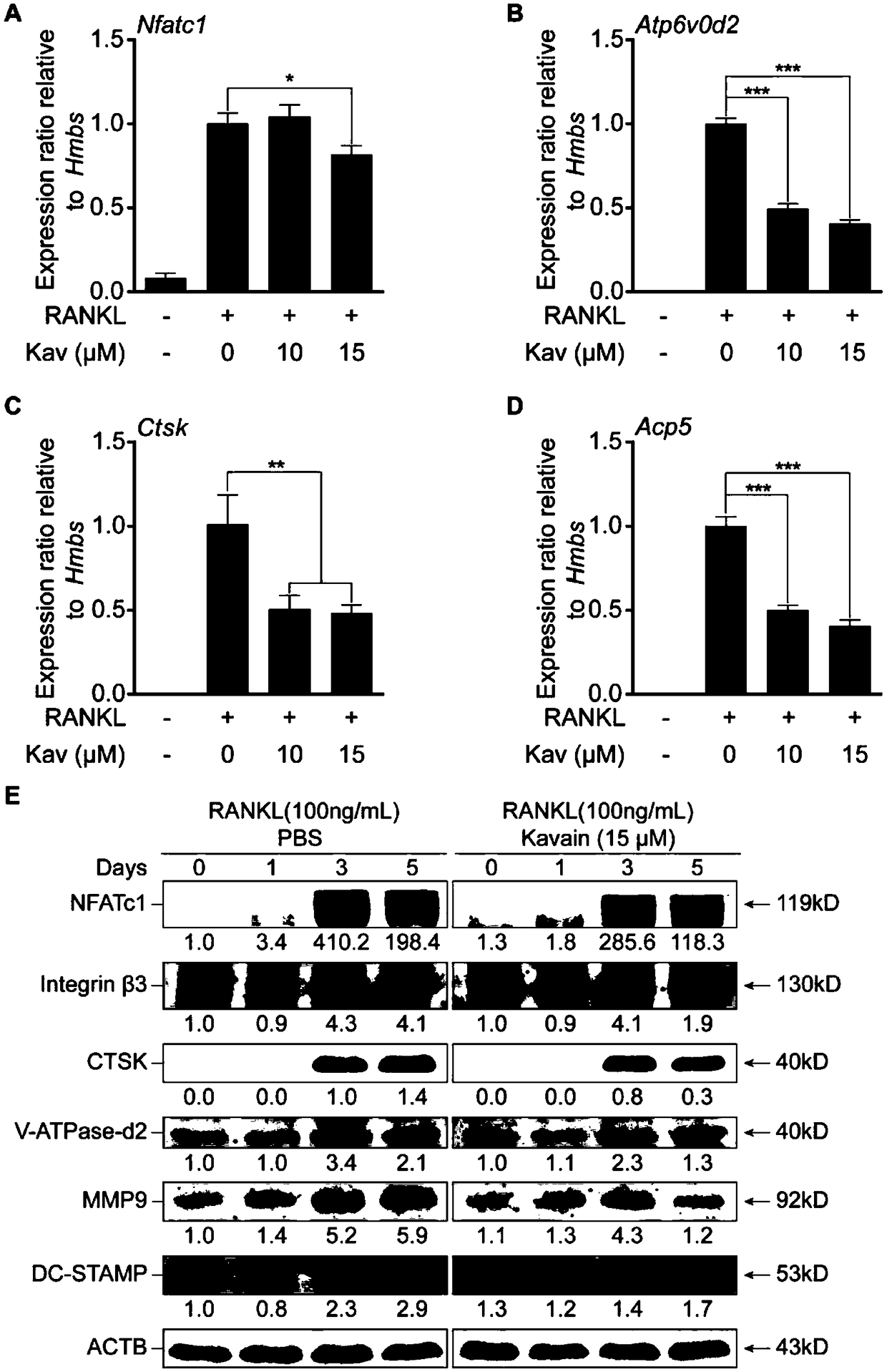
Application of kavain in preparing drug for treating osteoporosis
InactiveCN108685904AInhibit bone resorptionInhibition formationOrganic active ingredientsSkeletal disorderData displayDisease
The invention discloses application of kavain in preparing drug for treating osteoporosis. Impact of kavain on osteoclast differentiation, osteoclast absorption and osteoblast differentiaon is evaluated through tartrate-resistant acid phosphatase dyeing, immunofluorescence, reverse transcription-polymerase chain reaction and immunoblotting analysis. Results show that kavain inhibits RANKL inducedosteoclast by blocking NFAT and MAPK passages mediated by calcium while NF-kB is unaffected; kavain also inhibits bone resorption o osteoclast and has no obvious impact on differentiation and proliferation of osteoblast. In addition, kavain can inhibit formation of osteoclast to bone loss of ovariectomized mice. Data display that osteoclast of kavain which is a natural compound has specific effect, which shows that kavain has potential value in treating osteoporosis diseases.
Owner:GUANGXI MEDICAL UNIVERSITY
Transmembrane nfat inhibitory peptide
InactiveUS7160863B2Short timePolypeptide with localisation/targeting motifPeptide/protein ingredientsDiseaseSide effect
Owner:JAPAN SCI & TECH AGENCY 97 +2
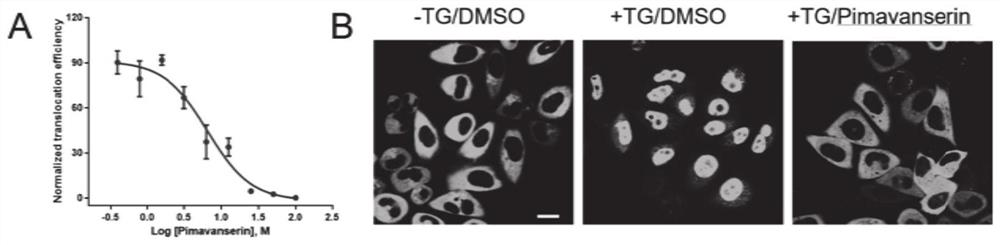
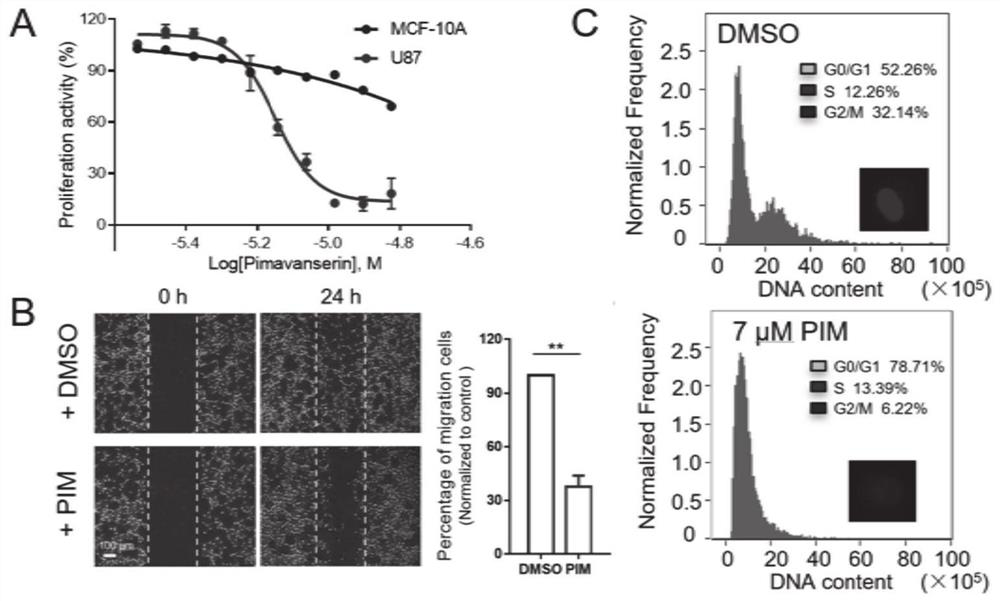
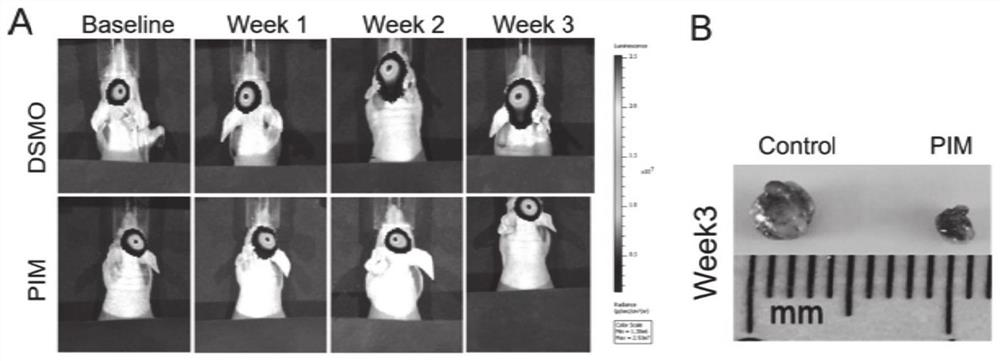
Application of pimavanserin tartrate in preparation of drug for treating glioma
ActiveCN112494490AGuaranteed efficient growthGrowth inhibitionOrganic active ingredientsAntineoplastic agentsOncologyCell migration
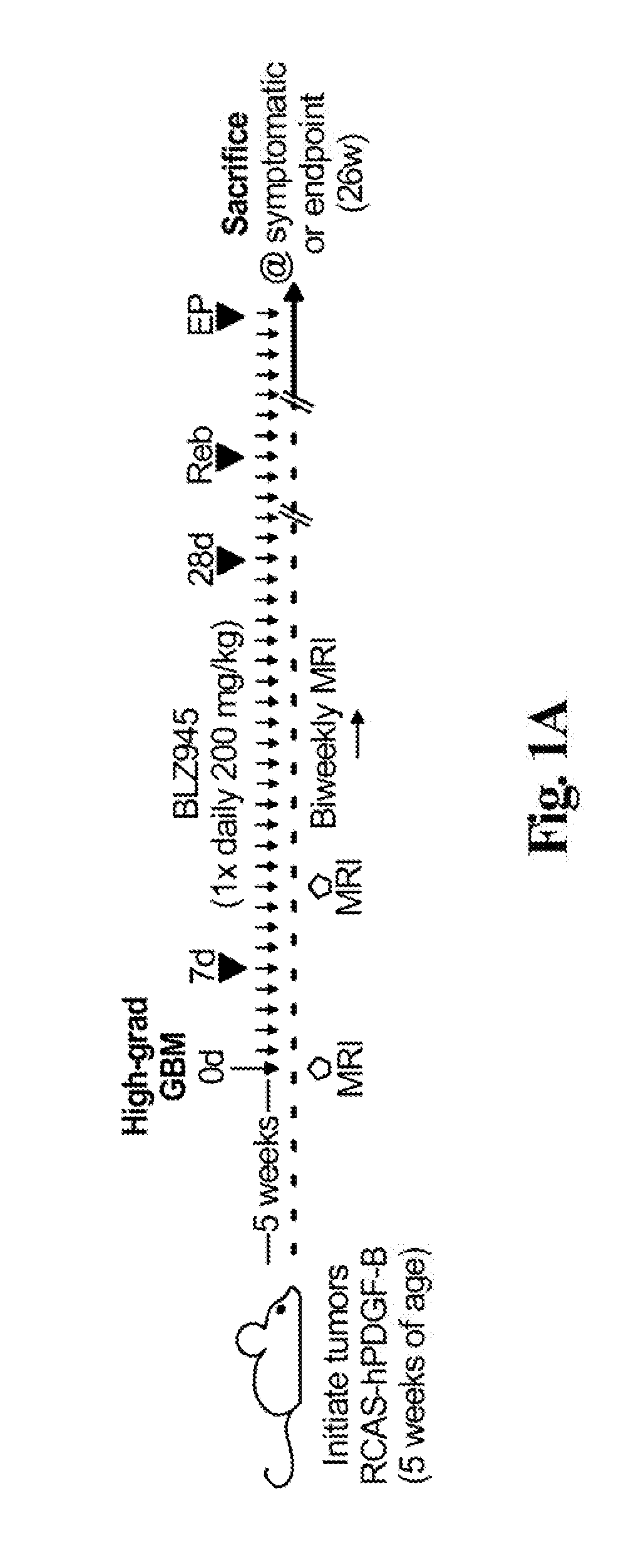
The invention relates to the technical field of medicines, in particular to application of pimavanserin tartrate in preparation of a drug for treating glioma, the glioma is polymorphic glioblastoma, and the application for treating glioma at least has any one or more of the following uses: (a) inhibiting the proliferation of glioma cells; (b) inhibiting the migration of glioma cells; (c) arrestingthe tumor cell cycle at G1 / S phase; and (d) inhibiting the growth of intracranial tumor cells in situ. The pimavanserin tartrate is found to be an effective NFAT signal pathway inhibitor, can inhibitE2F and MYC signal pathways and ATR and AuroraA / B signal pathways in tumor cells at the same time, has high anti-GBM activity in vivo, can effectively penetrate through a blood brain barrier (BBB) toenter the brain, can effectively inhibit growth of intracranial tumors in situ through oral administration, and is convenient to use. The invention provides a novel therapeutic drug for treating GBMtumors, which has important clinical significance.
Owner:SHANDONG NORMAL UNIV
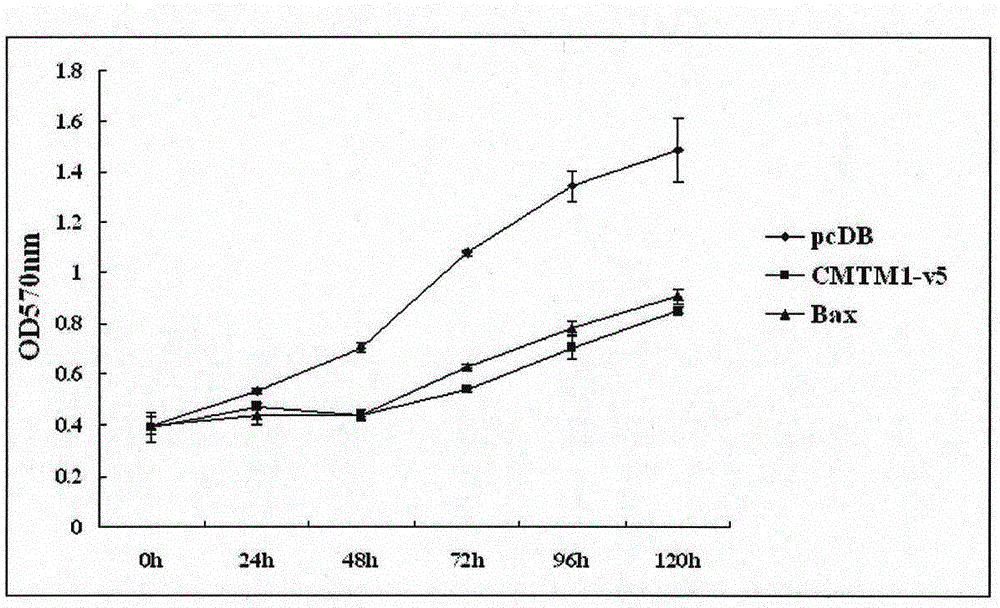
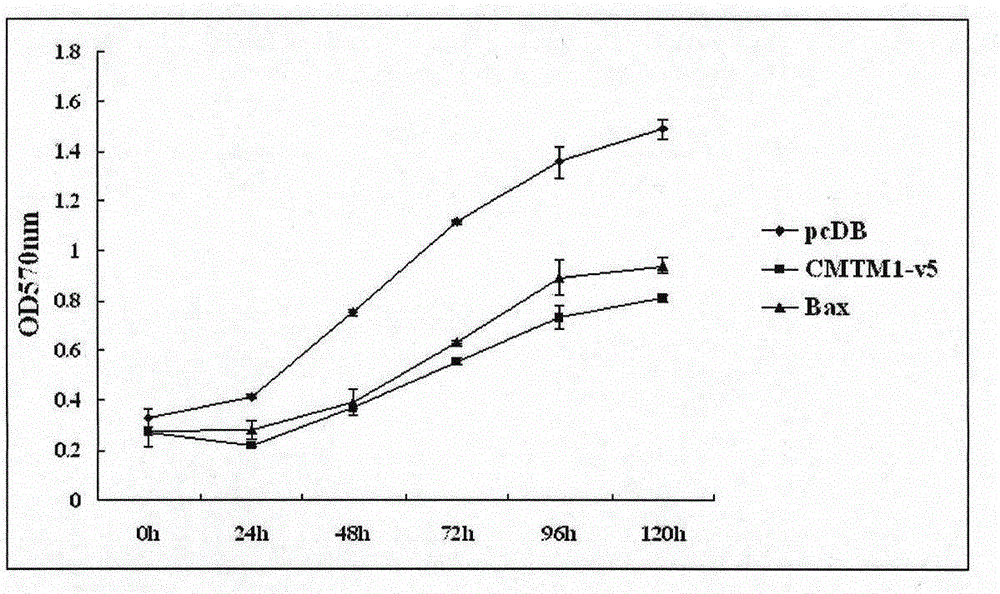
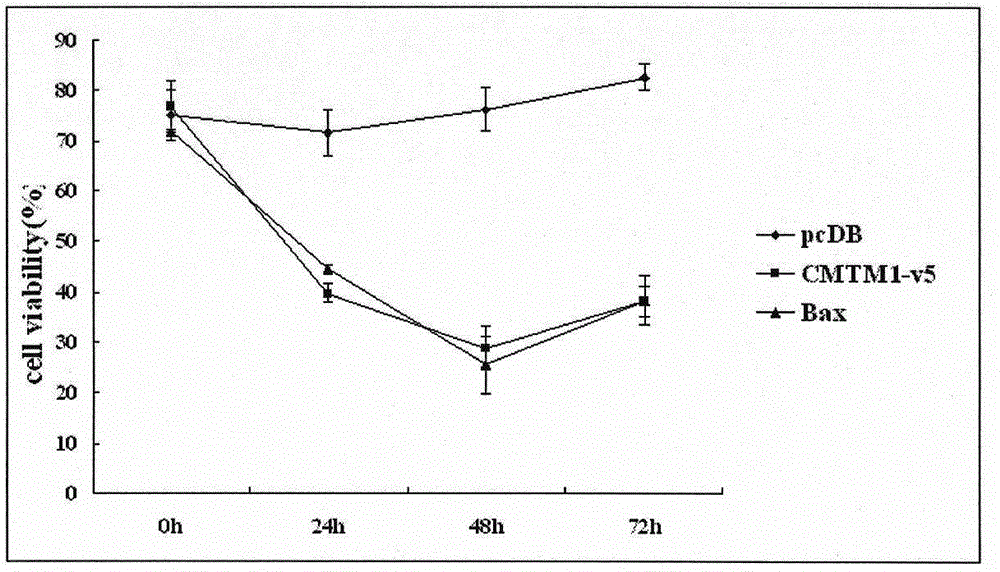
Use of CMTM1-V5 gene and its encoded protein
InactiveCN105879053AProlong lifePeptide/protein ingredientsGenetic material ingredientsAutoimmune conditionAutoimmune disease
The invention relates to a use of a CMTM1-V5 polypeptide or a CMTM1-V5 polypeptide encoding polynucleotide as a biological medicine in treatment on lymphocytic leukemia and other leukemia. The invention also relates to a use of the CMTM1-V5 polypeptide or the CMTM1-V5 polypeptide encoding polynucleotide in treatment on inflammation and autoimmune diseases through NFKB and NFAT activation inhibition. The invention also relates to a pharmaceutical composition for treating leukemia, inflammation and autoimmune diseases.
Owner:PEKING UNIV
Gene of restraining activation NF-kB and NFAT, and coded polypeptide
A human gene NFIF1 for suppressing the activation of NF-kB and NFAT, its cDNA sequence, the polypeptide coded by it, the process for testing its functions by dual-leuciferinase report gene method, and its application in preparing the medicines for preventing and treating inflammation, anaphylactia, autoimmunopathy, tumor etc are disclosed.
Owner:SINOGENOMAX +1
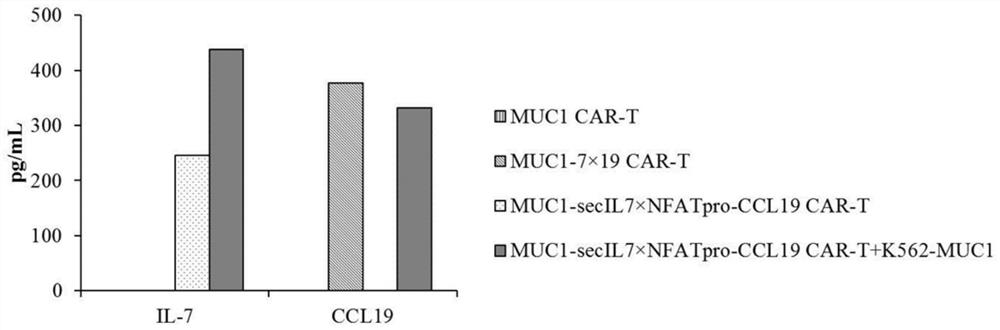
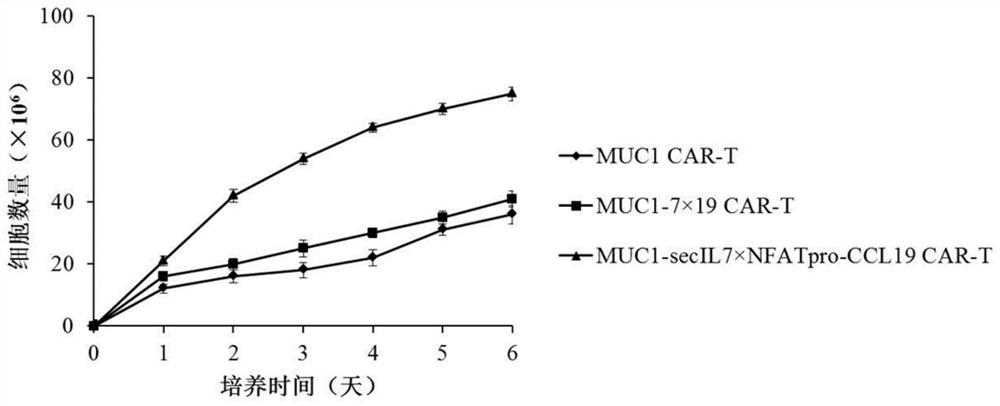
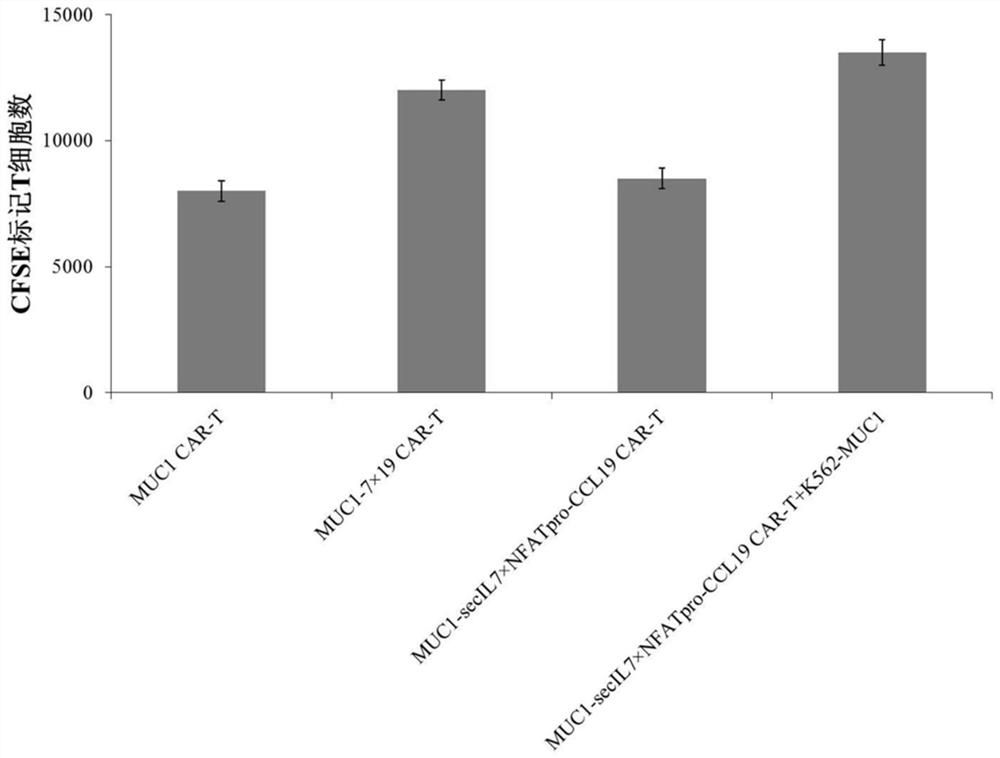
Chimeric antigen receptor T cells and application thereof
InactiveCN112175998AGive full play to the promotion effect of proliferationPromote recruitmentAntibody mimetics/scaffoldsImmunoglobulinsT cellSecretory protein
The invention provides chimeric antigen receptor T cells and application thereof. An expression vector system for preparing the chimeric antigen receptor T cells comprises a first expression vector containing a chimeric antigen receptor coding gene, a second expression vector containing a signal peptide IL-2 coding gene and an IL-7 coding gene and a third expression vector containing a NFAT regulating and controlling type promoter and a CCL19 coding gene; the NFAT regulating and controlling type promoter comprises a NFAT regulating and controlling sequence and a promoter sequence; and the NFATregulating and controlling sequence comprises a nucleic acid sequence shown in SEQ ID NO:1. According to the Chimeric antigen receptor T cells and the application thereof, the signal peptide IL-7 isreplaced with a signal peptide of a T-cell secretory protein IL-2, the expression of the T cells to CCL19 is regulated and controlled by employing the NFAT regulating and controlling type promoter, and the secretory expression of the IL-7 by CAR-T cells and the regulated and controlled type expression of CCL19 by NFAT are achieved.
Owner:汤朝阳
NFAT signal inhibitor and calcineurin inhibitor
To provide a drug, an external use composition, and a cosmetic composition, which exhibit an NFAT signal inhibitory action, a calcineurin inhibitory action, and a hair growth-promoting effect.The NFAT signal inhibitor contains, as an active ingredient, American angelica or an extract thereof.
Owner:KAO CORP
Cell strain as well as preparation method and application thereof
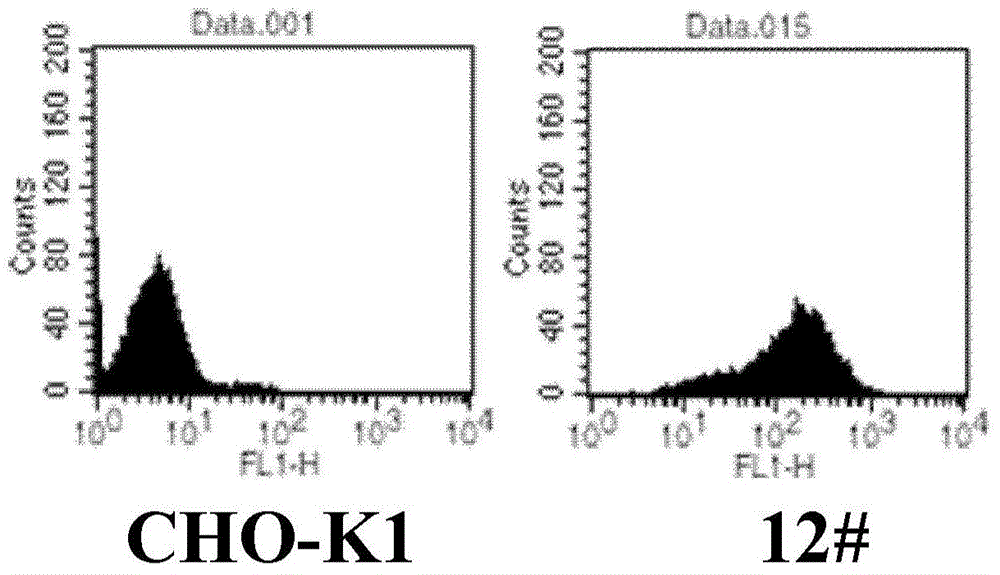
PendingCN112961832AImprove stabilityHigh sensitivityGenetically modified cellsMicrobiological testing/measurementGenome stabilityBiological studies
The invention relates to the technical field of biology, in particular to a cell strain as well as a preparation method and application thereof, a safe port site of a cell strain genome contains an NFAT element and a reporter gene regulated by the NFAT element, and the NFAT element and the reporter gene regulated by the NFAT element are NFAT-RE-Luciferase genes. The NFAT-RE-Luciferase gene in the cell strain is integrated in the genome in a specific site, and the existing cell strain is mainly obtained through a random integration technology, so that the cell strain has remarkable genome stability, and has higher transcriptional activity and higher fluorescence level compared with the existing random integration cell line, thereby the detection stability and sensitivity of the cell strain are improved. The cell strain provided by the invention also provides a good platform and solution for biological research or drug activity detection.
Owner:SHANGHAI JIAO TONG UNIV
Inhibitors against the activation of AP-1 and NFAT
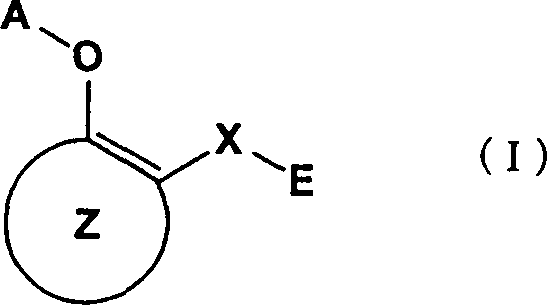
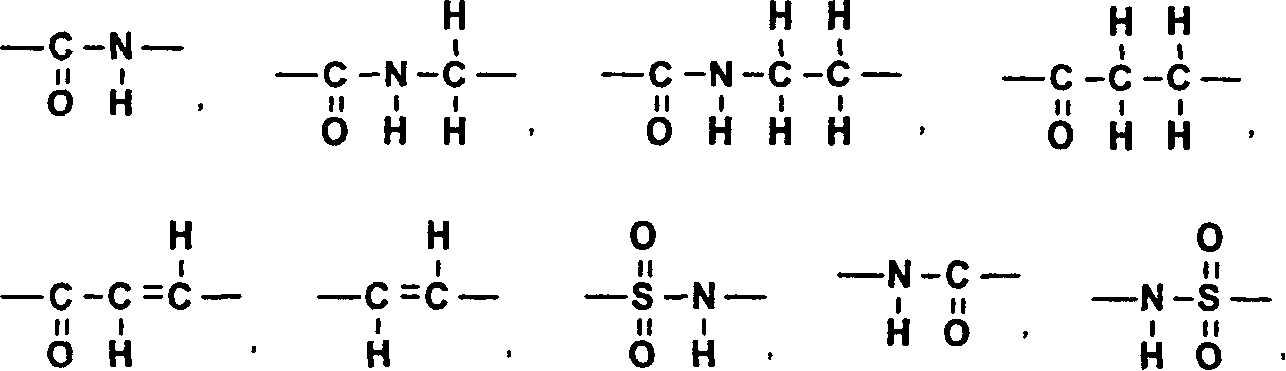
A medicament inhibiting the activation of AP-1 which comprises as an active ingredient a substance selected from the group consisting of a compound represented by the following general formula (I) and a pharmacologically acceptable salt thereof, and a hydrate thereof and a solvate thereof: wherein X represents a connecting group whose number of atoms in the main chain is 2 to 5 (said connecting group may be substituted), A represents hydrogen atom or acetyl group, E represents an aryl group which may be substituted or a hetero aryl group which may be substituted, ring Z represents an arene which may have one or more substituents in addition to the group represented by formula -O-A wherein A has the same meaning as that defined above and the group represented by formula -X-E wherein each of X and E has the same meaning as that defined above, or a heteroarene which may have one or more substituents in addition to the group represented by formula -O-A wherein A has the same meaning as that defined above and the group represented by formula -X-E wherein each of X and E has the same meaning as that defined above.
Owner:INST OF MEDICINAL MOLECULAR DESIGN
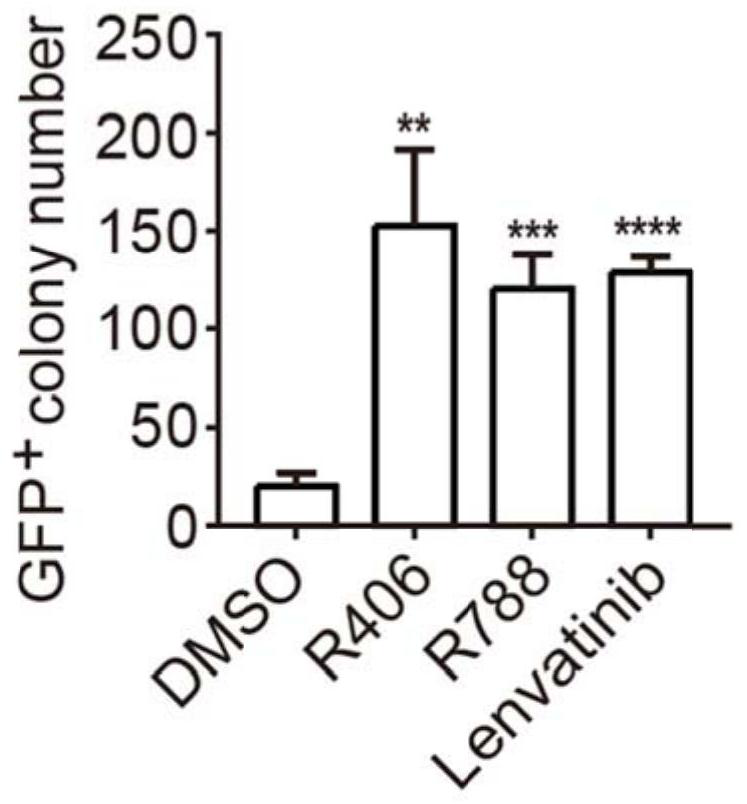
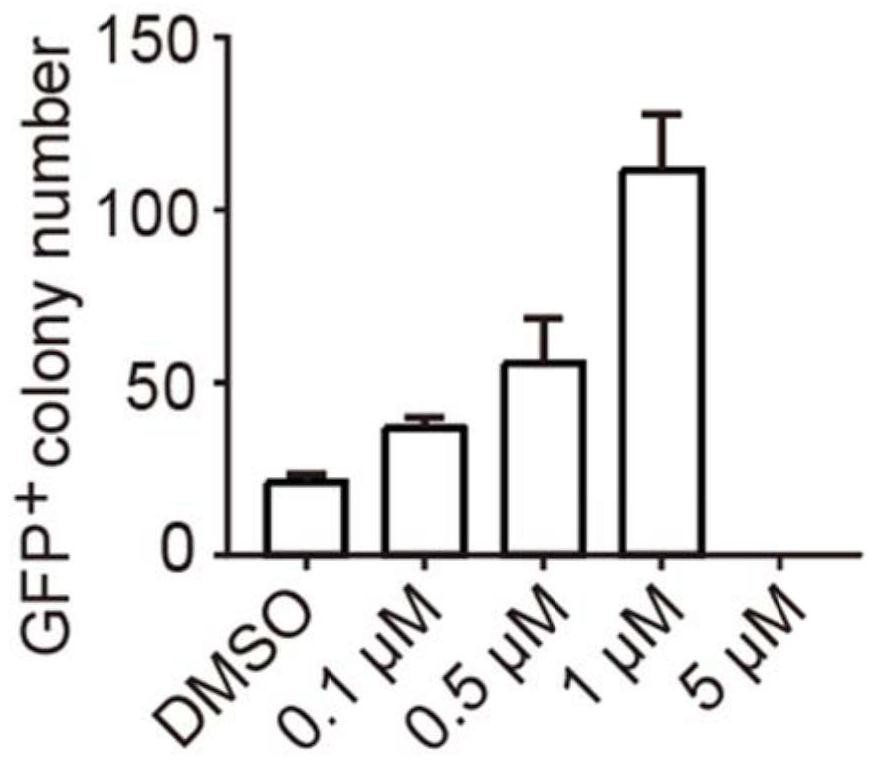
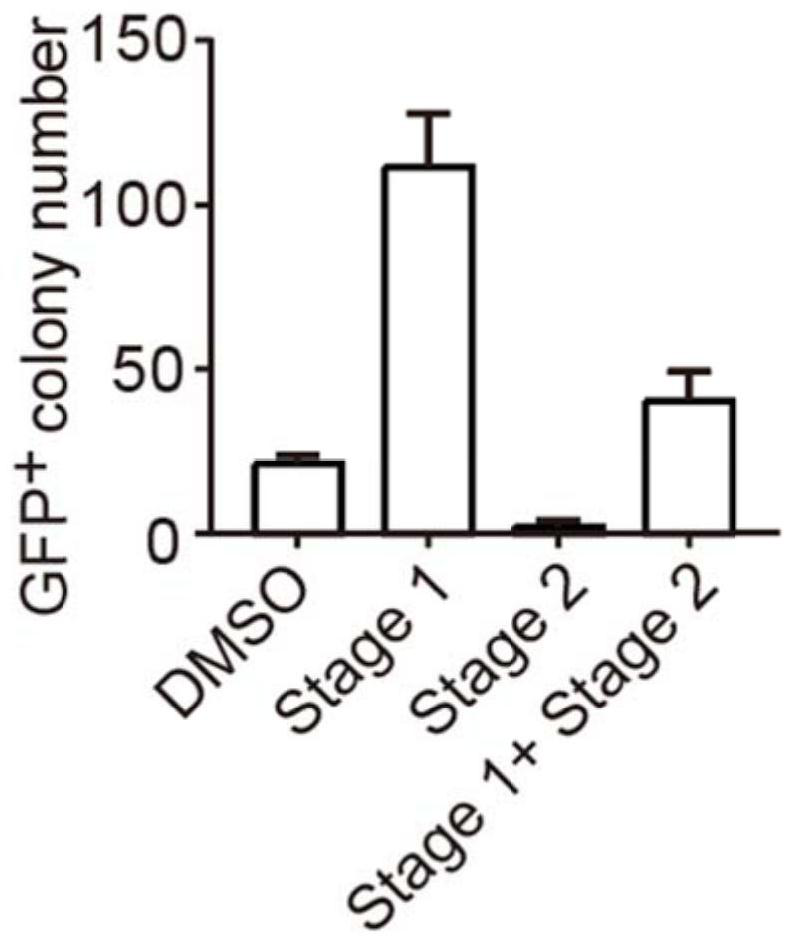
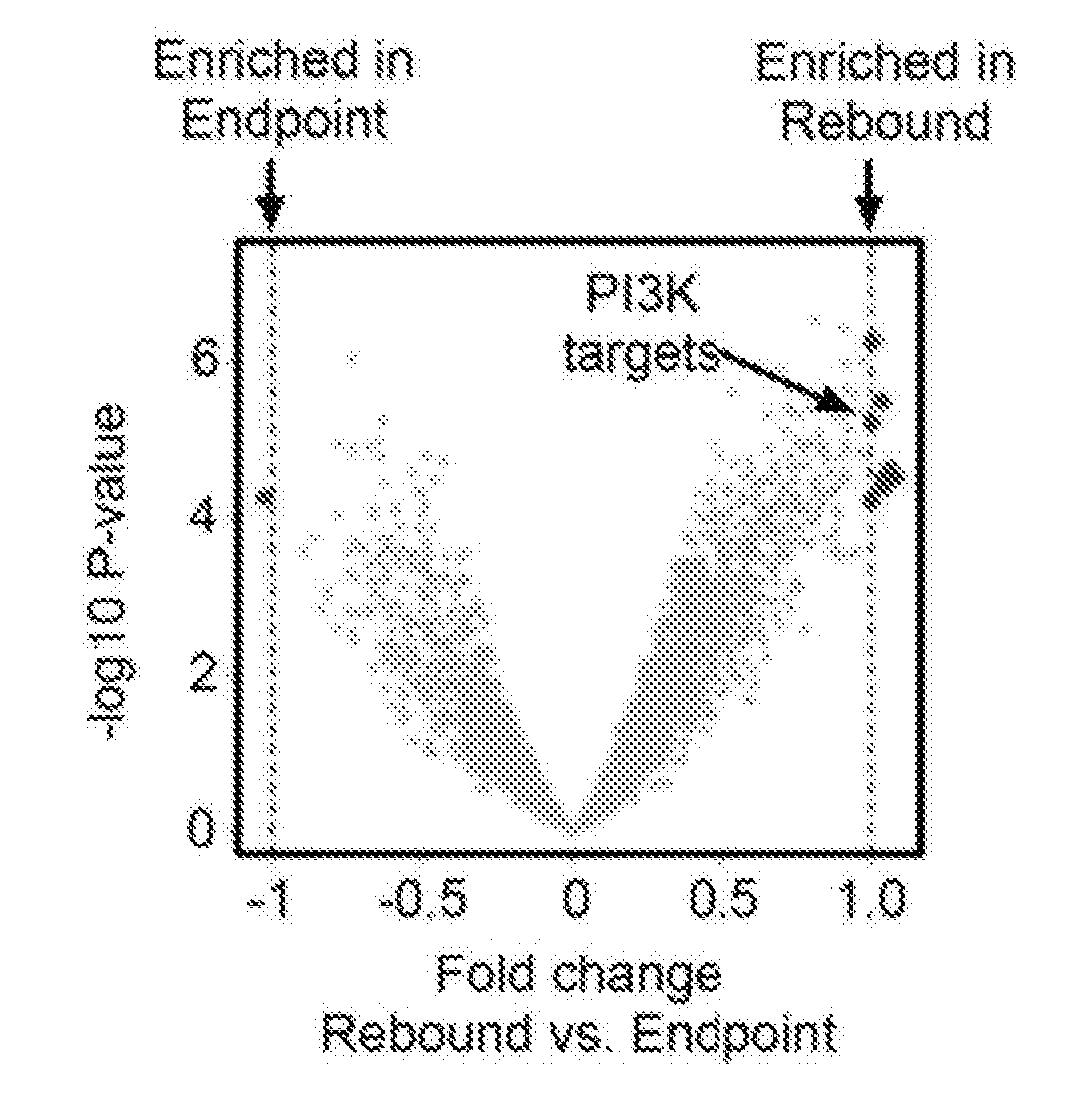
Application of R406 in promotion of somatic cell reprogramming, and reprogramming culture medium and method forpromotion of somatic cell reprogramming
ActiveCN113025562AImprove the efficiency of chemical reprogrammingWeaken direct bindingGenetically modified cellsArtificial cell constructsMetaboliteReprogramming
The invention discloses application of R406 in promotion of somatic cell reprogramming and a reprogramming culture medium and method thereof. The molecular formula of the R406 is shown in the specification, when the R406 is actually applied, the R406 can be prepared into a reprogramming culture medium for application, namely, the SYK inhibitor R406 is added into a basic culture medium to prepare the reprogramming culture medium. The R406 inhibits a Syk-Cn-NFAT signal channel and weakens direct binding of NFATc1 to glycine, serine and threonine metabolism related genes, so that expression of the genes is promoted, the content of metabolites in metabolism of glycine, serine and threonine and a downstream cysteine metabolic pathway is increased, and the intracellular H2S level is increased. H2S accumulated in cells regulates and controls mitochondrial oxidative phosphorylation activity and redox steady state, and finally promotes chemical reprogramming.
Owner:ZHEJIANG UNIV
Compositions and methods for treatment of glioma
In certain embodiments the present invention provides methods useful in the treatment of glioma, such as glioblastoma, such methods comprising administering to a subject in need thereof a CSF-IR inhibitor together with one or more additional active agents such as IGF-IR inhibitors, PI3K inhibitors, IL4 inhibitors, NFAT inhibitors, and / or Stat6 inhibitors. In certain embodiments the present invention provides pharmaceutical compositions comprising a CSF-IR inhibitor together with one or more of such additional active agents.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Nanoconstructs with pharmacological activity
Owner:UNIV DEGLI STUDI DI MILANO BICOCCA +1
Methods and substances for stimulating muscle regeneration
InactiveUS20110313027A1Increased fiber sizeAdd additional massHydrolasesMicrobiological testing/measurementDiseaseFiber
The invention is concerned with the field of muscle growth and regeneration. More particularly, the invention provides novel methods, substances and uses for facilitating the growth or regeneration of skeletal muscles. The methods, uses and substances of the invention are useful in the treatment or prevention of degenerative-regenerative muscular disorders or in the treatment of muscle injuries. The substances provided by the invention are capable of upregulating the calcineurin-NFAT-IL-4, advantageously by inhibiting the activity of the Ras or SERCA1b gene product. Introduction of the substances provided by the invention into only a small percentage of the fibers present in a regenerating muscle facilitates the regeneration of the whole muscle concerned.
Owner:UNIVERSITY OF SZEGED
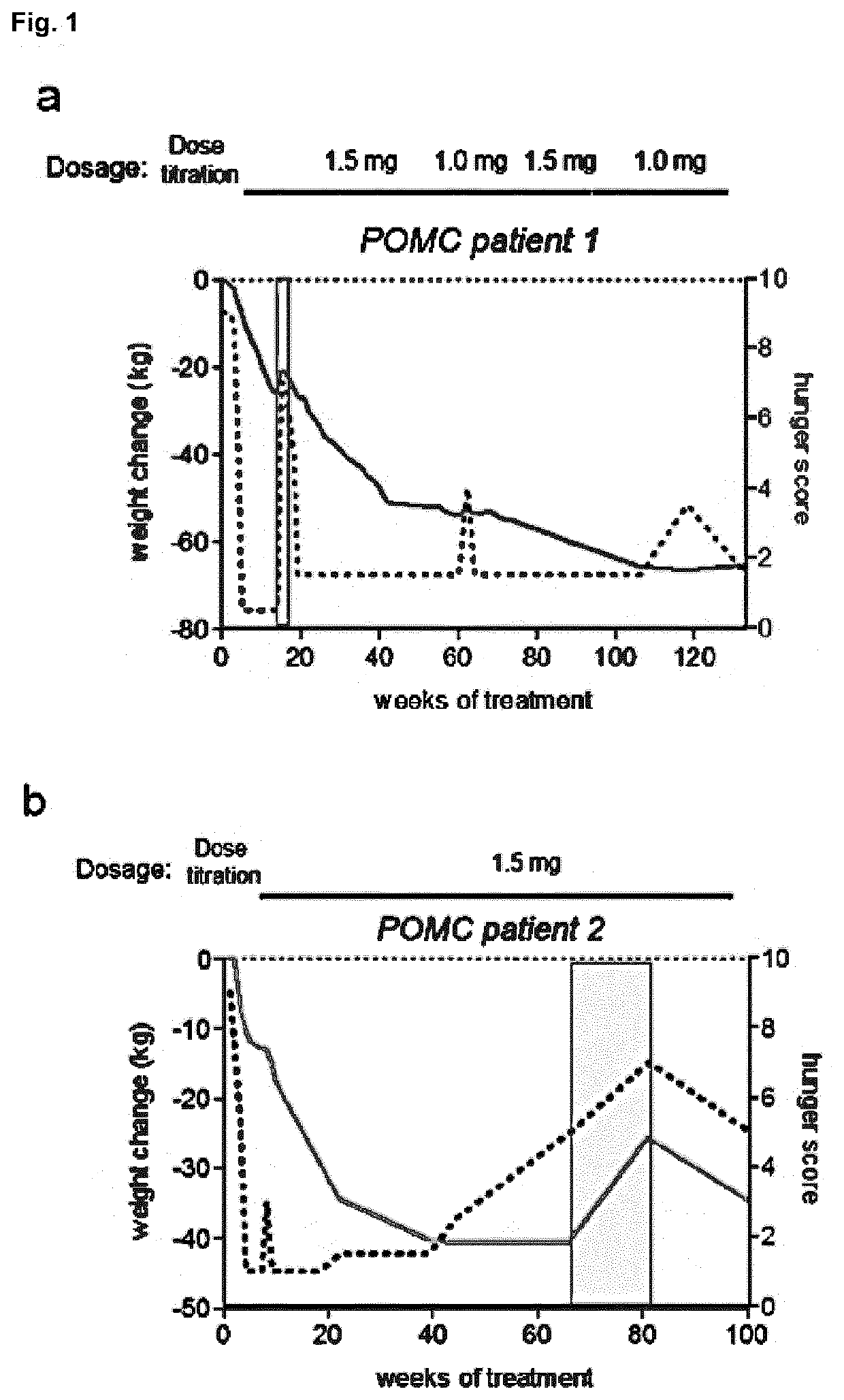
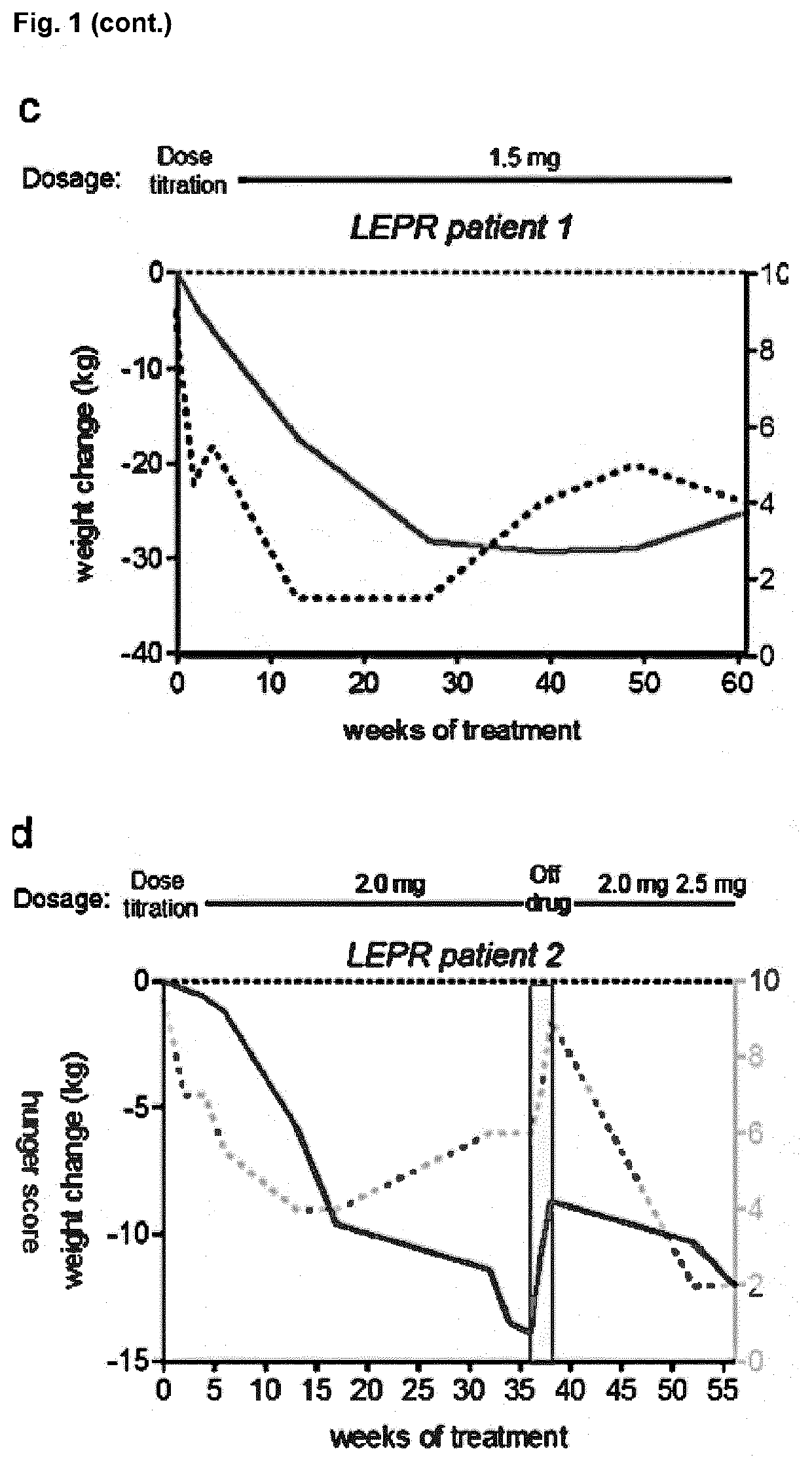
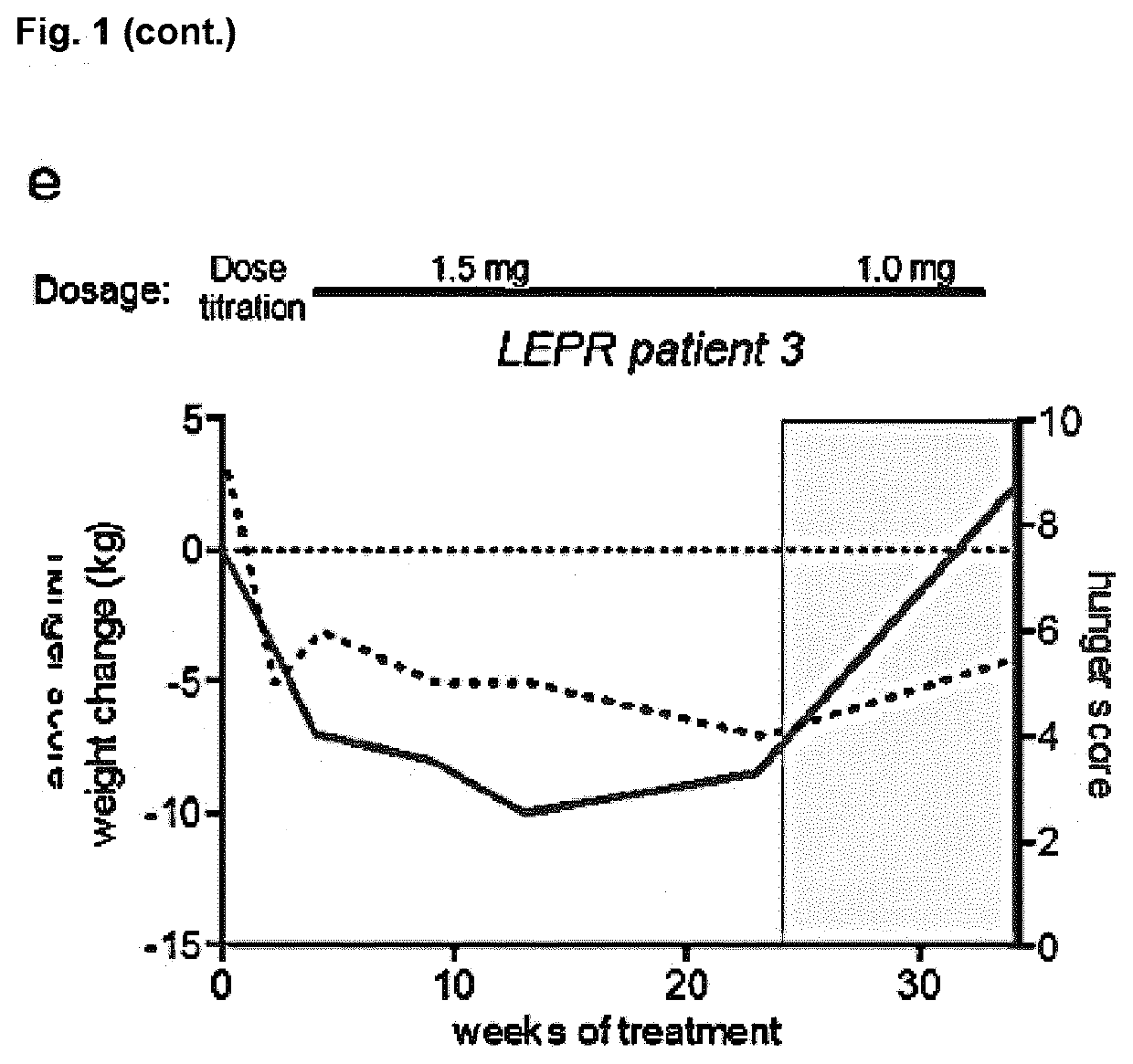
MC4R Agonist Efficacy in Subjects with MC4R Deficiencies and Impaired NFAT Signaling
PendingUS20210052551A1Reduced NFAT activationConvenient inductionOrganic active ingredientsMetabolism disorderActivation cellsEfficacy
The invention relates to a Melanocortin-4 receptor (MC4R) agonist that exhibits greater induction of NFAT signaling compared to α-MSH for use in the treatment and / or prevention of a medical condition associated with MC4R deficiency in a subject having an MC4R deficiency associated with impaired Nuclear factor of activated T-cells (NFAT) signaling. The present invention further relates to an in vitro method for the diagnosis, prognosis and / or assessment of likelihood of whether a subject with, or at risk of having and / or developing, a medical condition associated with MC4R deficiency, will respond to treatment with an MC4R agonist that exhibits greater induction of NFAT signaling compared to α-MSH, the method comprising (i) providing a sample from said subject, and (ii) determining whether the subject has an MC4R deficiency associated with impaired NFAT signaling by assessing said sample, (iii) wherein the presence of an MC4R deficiency associated with impaired NFAT signaling is indicative that treatment with an MC4R agonist that exhibits greater induction of NFAT signaling compared to α-MSH will be effective in said subject.
Owner:CHARITE UNIVS MEDIZIN BERLIN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com