Tetrahydroquinoline alkaloid Malaysiensin with immunosuppression activity and production method and application of tetrahydroquinoline alkaloid Malaysiensin with immunosuppression activity
An immunosuppressive and tetrahydroquinoline technology, applied in the field of biomedicine, can solve problems such as expensive, induced tumors, and hyperlipidemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation of embodiment 1-compound
[0045] A kind of tetrahydroquinoline alkaloid with immunosuppressive activity, its preparation method comprises the following steps:
[0046] S1. Streptomyces sp.DSM 4137 was fermented and cultured; Streptomyces sp.DSM 4137 was deposited in the China Center for Type Culture Collection, date of deposit: August 1, 2018, deposit number: CCTCCNo: M 2018512; Streptomyces For the fermentation and cultivation method of Streptomyces sp.DSM 4137, refer to the patent previously applied by the inventor, publication number CN 109467579A, title of invention: a PKS type I polyketide compound with immunosuppressive activity and its preparation method and application.
[0047] S2. After the fermentation culture is completed, fully soak in methanol, filter to obtain methanol extract, concentrate under reduced pressure, recover methanol, and obtain 6.2g of strain fermentation product;
[0048] S3. Gradient elution is performed on the fermentati...
Embodiment 2
[0050] Embodiment 2-confirmation of compound structure
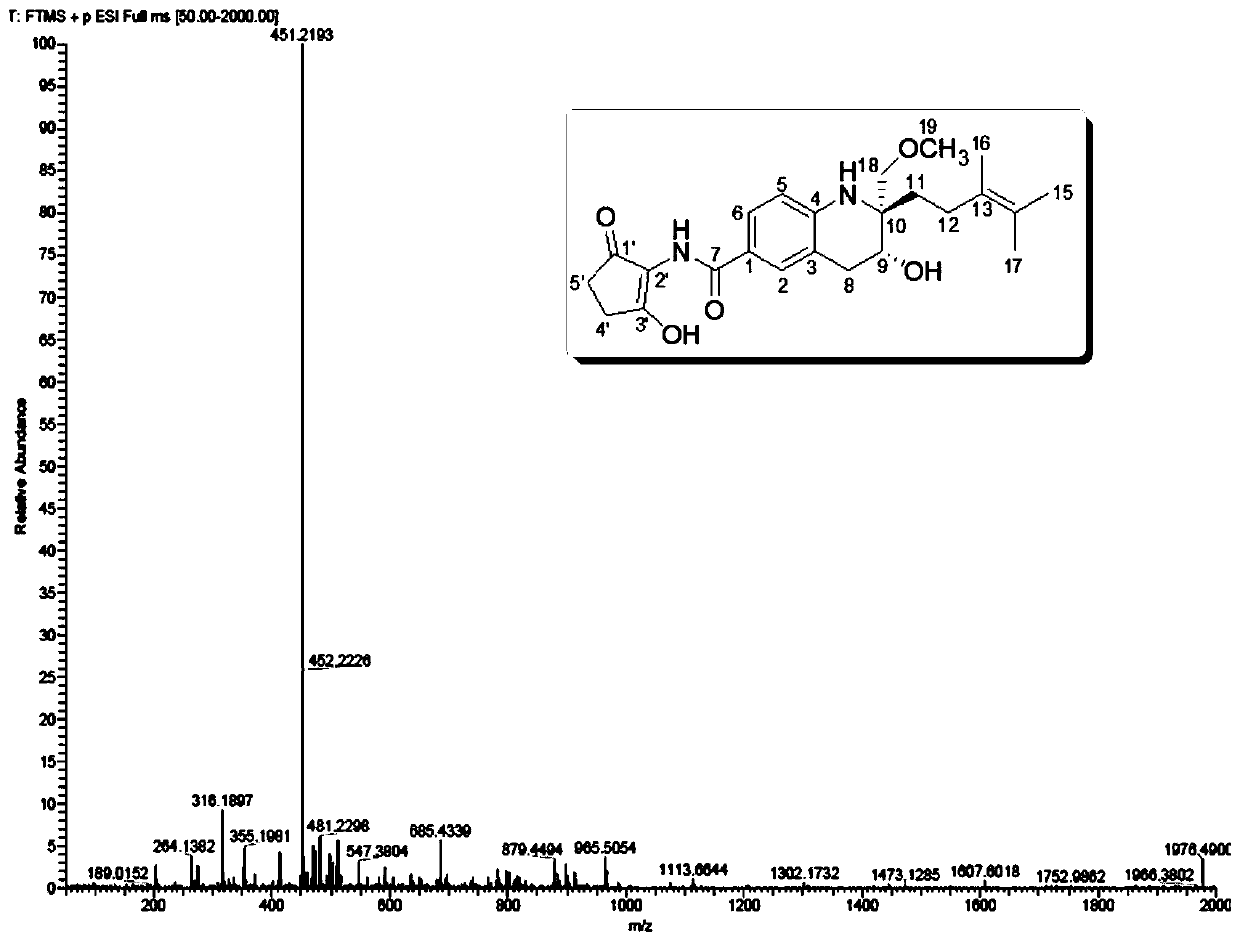
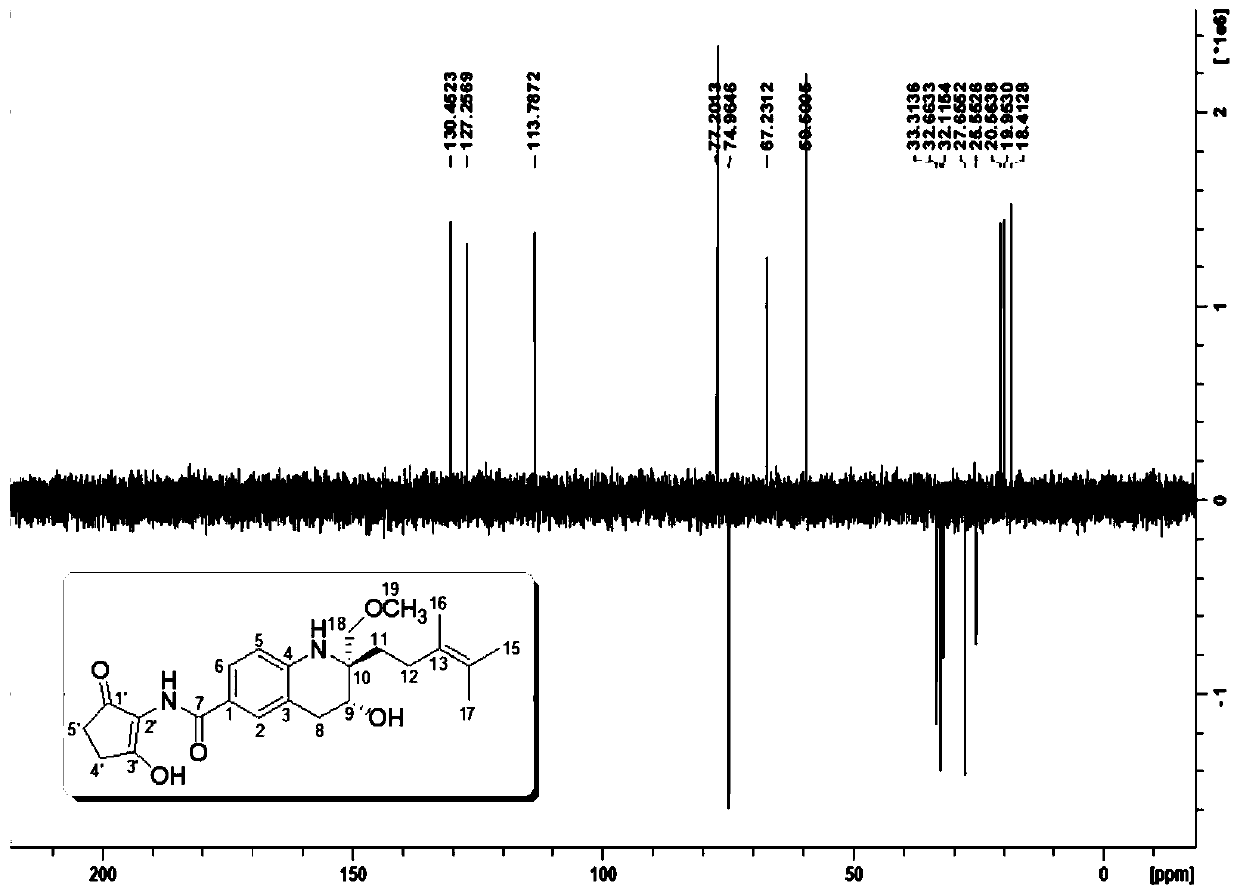
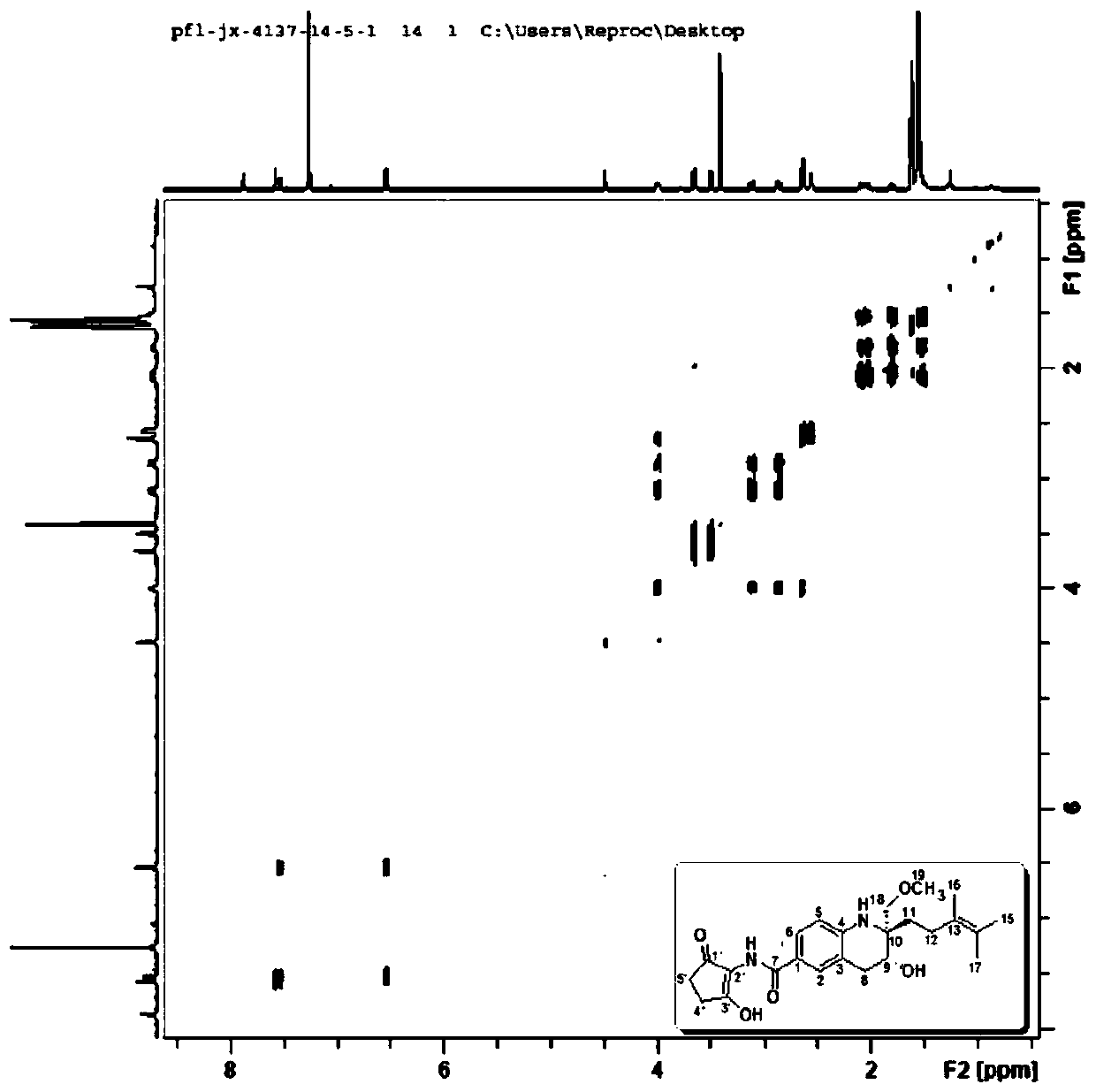
[0051] Using a variety of spectra (IR, UV, CD), spectrum ( 1 H NMR, 13 C NMR, DEPT, H-HCOSY, HMQC, HMBC, NOESY) and MS (ESI-MS, HRMS) and other structural identification techniques to determine the chemical structure of the obtained active ingredients and discover new structural active ingredients. see results Figure 1-Figure 3 .
[0052] Malaysiensin (compound 1), white solid, specific rotation [α] 25 D =+10 (c 0.001, MeOH), UV absorption UV(MeOH) λmax 202, 332nm, cationic HRESI-MS gives [M+Na] at m / z 451.2193 + peak( figure 1 ), so the molecular formula of this compound is C 24 h 32 N 2 o 5 , with an unsaturation of 10. The hydrogen-carbon spectrum of compound 1 ( figure 2 , image 3 , Table 1) show that 7 unsaturations originate from one amide carbonyl, one keto carbonyl and five carbon-carbon double bonds. Therefore, the molecule is presumed to be a tricyclic structure. DEPT spectrum ( Figure 4 ) s...
Embodiment 3
[0057] Example 3-Con A induced T cell proliferation experiment
[0058] 1. Experimental materials
[0059] 1.1 Experimental animals
[0060] Healthy Balb / c mice, female, weighing 18-20g / mouse, aged 6-8 weeks, were purchased from Hainan Medical College and kept at about 25°C and 50-80% relative humidity.
[0061] 1.2 Drugs and reagents
[0062] Concanavalin A (Sigma), CCK-8 (Biosharp), RPMI-1640 culture medium (HyClone), fetal bovine serum (Sijiqing), red blood cell lysate (Biosharp), trypan blue (Sigma)), etc.
[0063] 1.3 Main instruments
[0064] CO 2 Cell incubator (Galaxy R, British RS Biotech company), ultra-clean bench (SW-CJ-2FD, Suzhou Purification Equipment Co., Ltd.), inverted microscope (BX51-32P01, Japan Olympus), autoclave (HVE-50 , Japan Co., Ltd.), centrifuge (DM0412S, American SCILOGEX), microplate reader (ST-360, Shanghai Kehua Experimental System Co., Ltd.), 96-well cell culture plate (3599, American Costar), constant temperature water bath (DK- S28, Sh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com