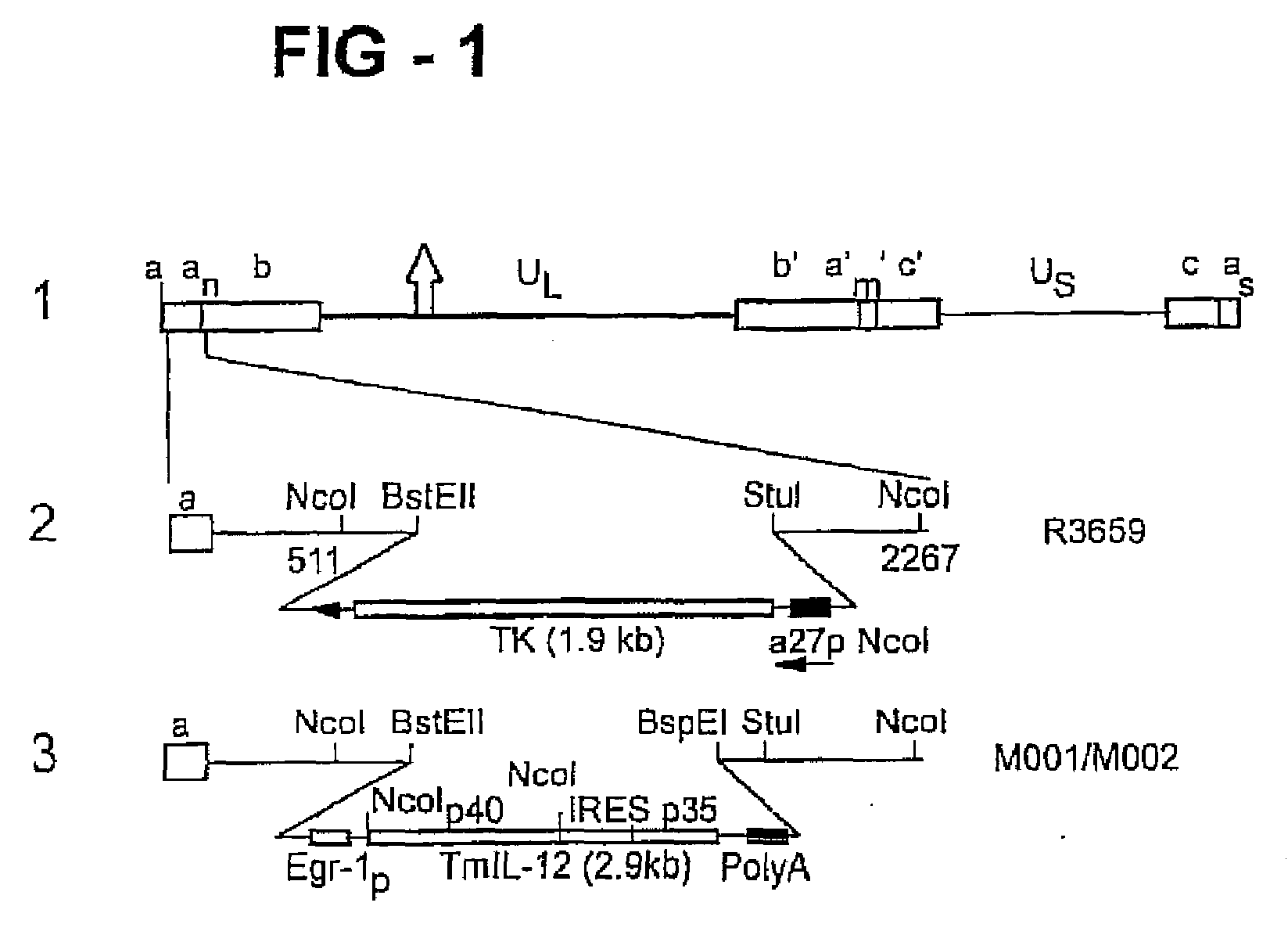
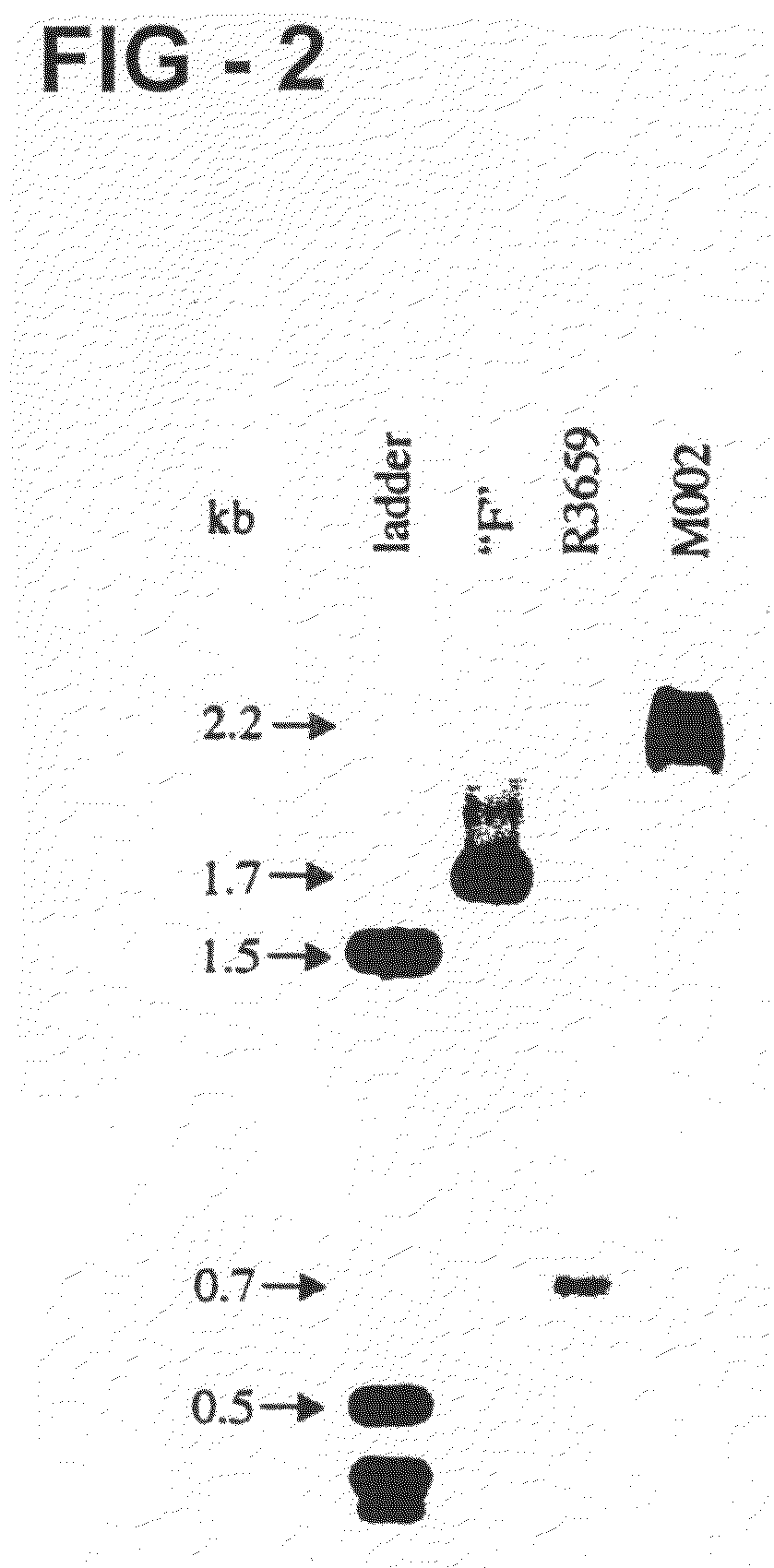
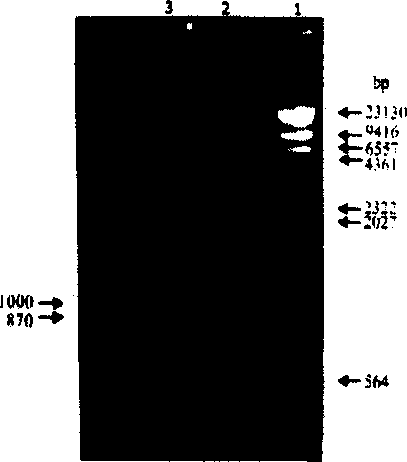
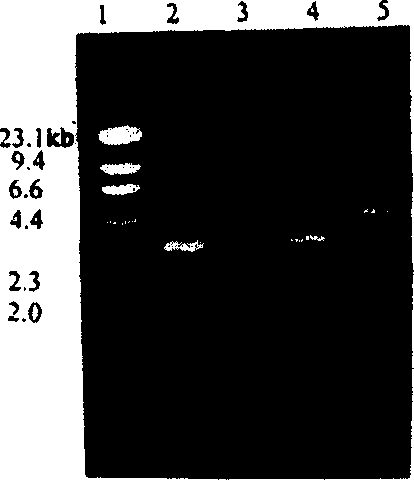
Patents
Literature
169 results about "Interleukin 12" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Interleukin 12 (IL-12) is an interleukin that is naturally produced by dendritic cells, macrophages, neutrophils, and human B-lymphoblastoid cells (NC-37) in response to antigenic stimulation.
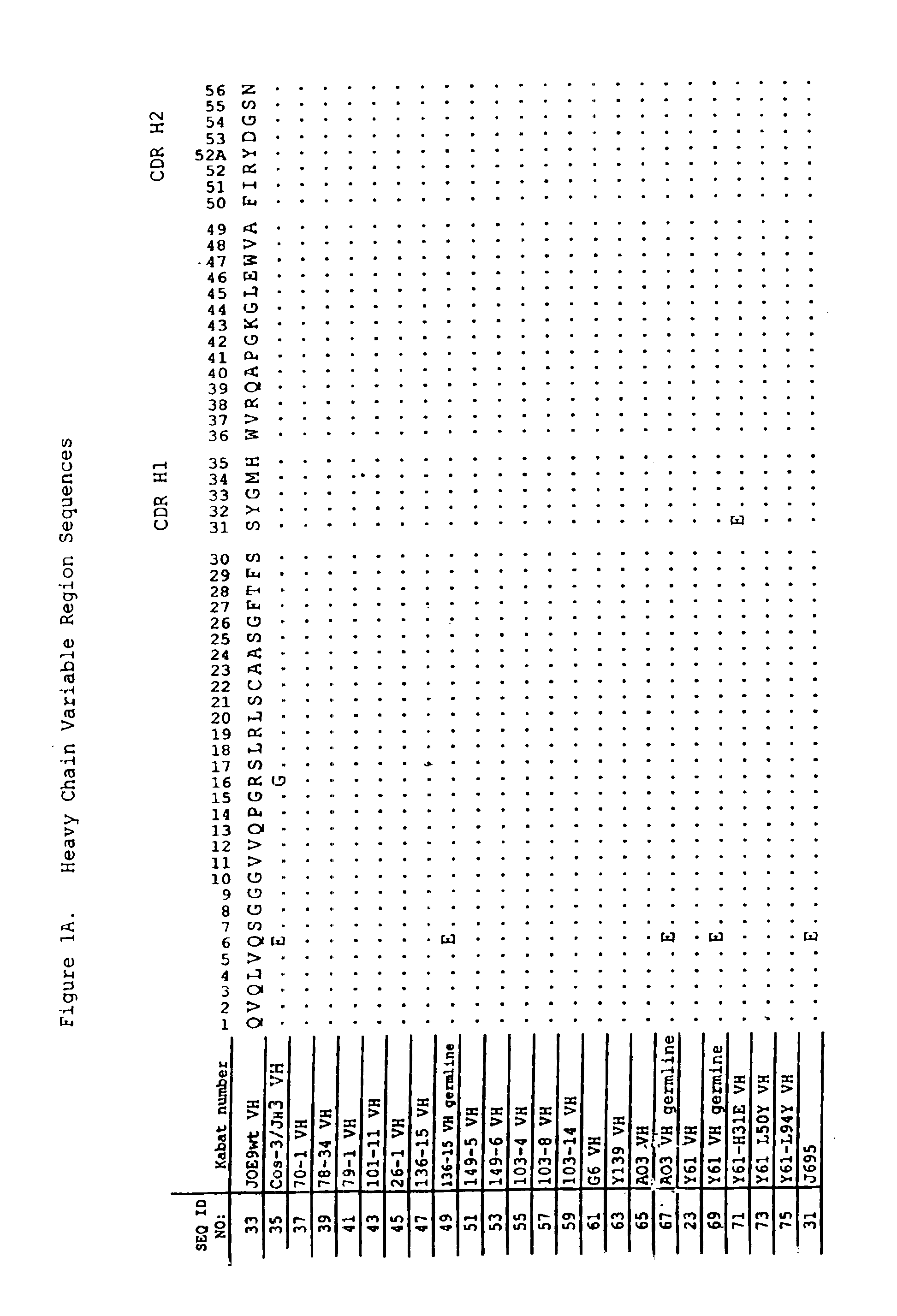
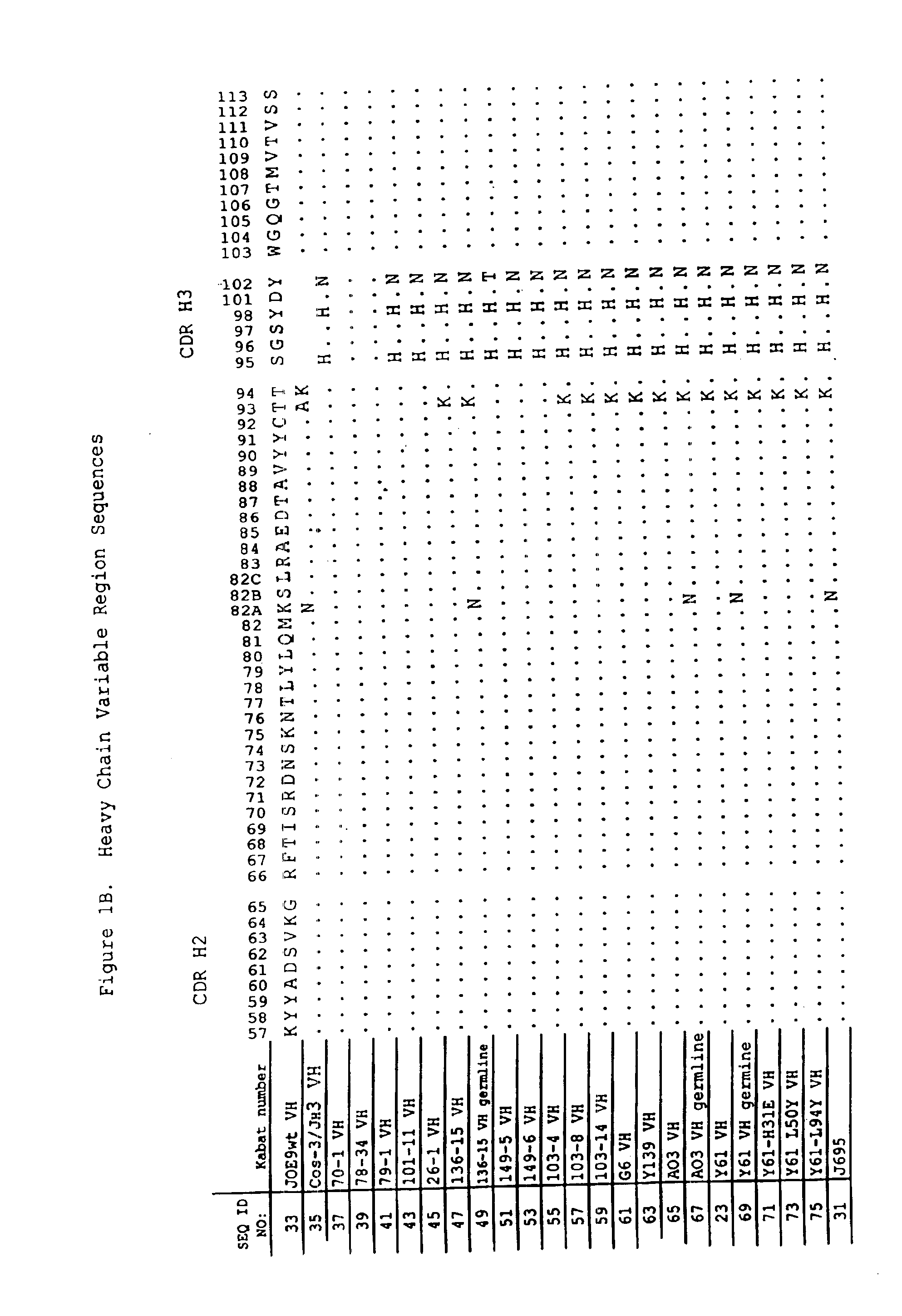
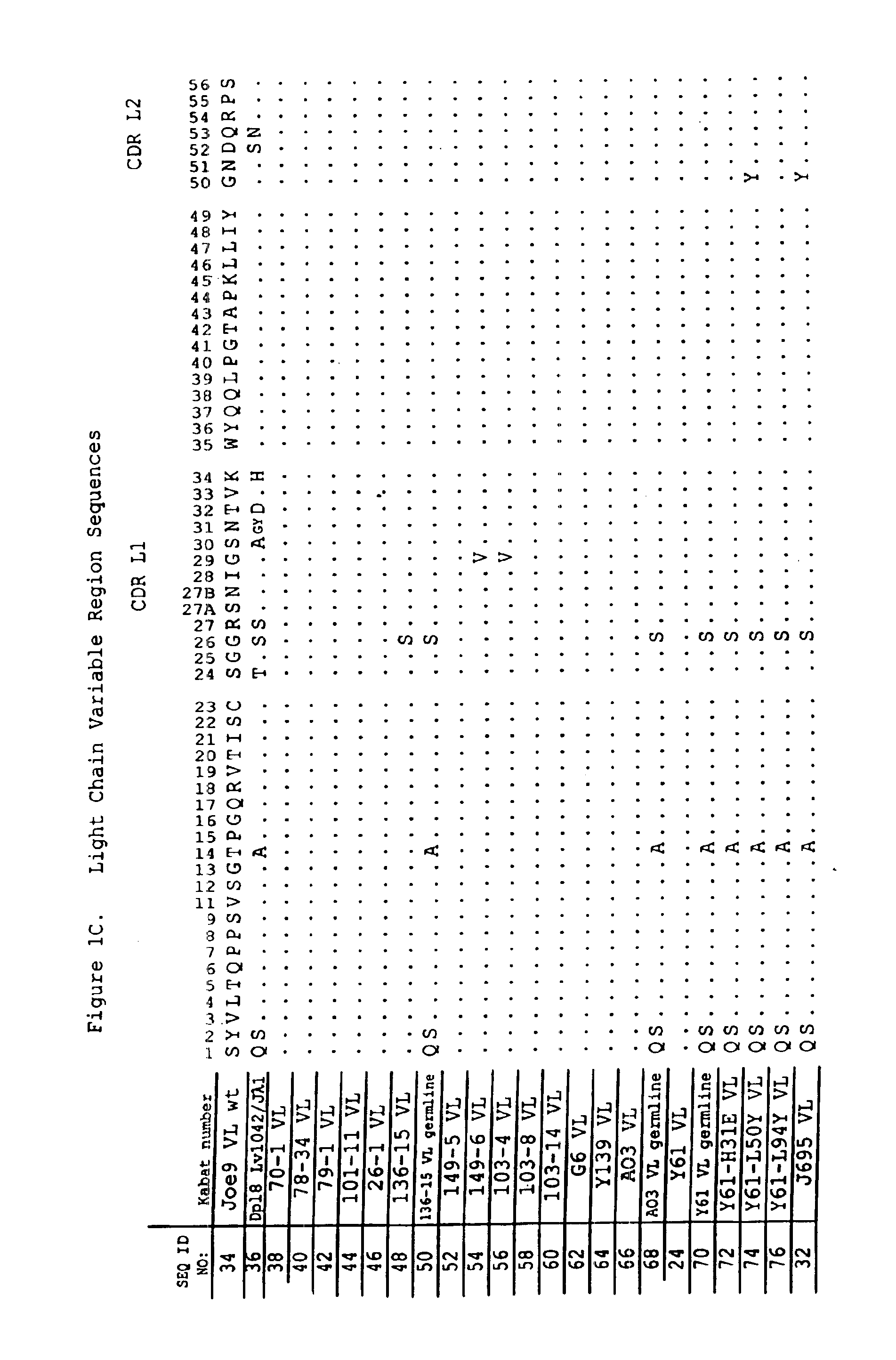
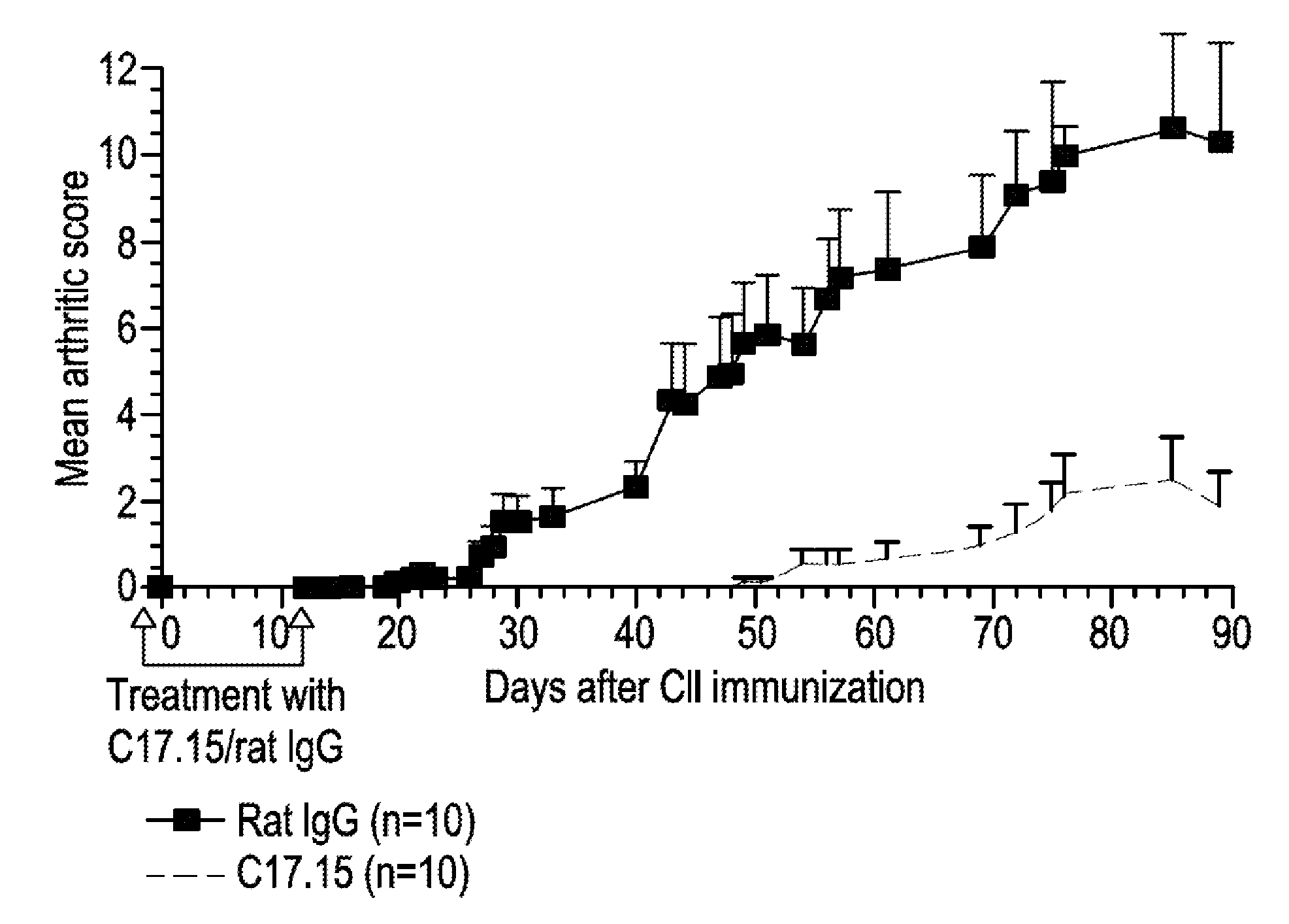
Human antibodies that bind human IL-12 and methods for producing
InactiveUS6914128B1Avoid interferencePreservationNervous disorderPeptide/protein ingredientsAntigen bindingIn vivo
Human antibodies, preferably recombinant human antibodies, that specifically bind to human interleukin-12 (hIL-12) are disclosed. Preferred antibodies have high affinity for hIL-12 and neutralize hIL-12 activity in vitro and in vivo. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. The antibodies, or antibody portions, of the invention are useful for detecting hIL-12 and for inhibiting hIL-12 activity, e.g., in a human subject suffering from a disorder in which hIL-12 activity is detrimental. Nucleic acids, vectors and host cells for expressing the recombinant human antibodies of the invention, and methods of synthesizing the recombinant human antibodies, are also encompassed by the invention.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Human antibodies that bind human il-12 and methods for producing
InactiveUS20110123544A1Avoid interferencePreservationAntibacterial agentsSenses disorderAntigen bindingIn vivo
Human antibodies, preferably recombinant human antibodies, that specifically bind to human interleukin-12 (hIL-12) are disclosed. Preferred antibodies have high affinity for hIL-12 and neutralize hIL-12 activity in vitro and in vivo. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. The antibodies, or antibody portions, of the invention are useful for detecting hIL-12 and for inhibiting hIL-12 activity, e.g., in a human subject suffering from a disorder in which hIL-12 activity is detrimental. Nucleic acids, vectors and host cells for expressing the recombinant human antibodies of the invention, and methods of synthesizing the recombinant human antibodies, are also encompassed by the invention.
Owner:ABBOTT GMBH & CO KG
Interleukin-12p40 variants with improved stability
ActiveUS20070154453A1Improve stabilityResistant to digestionPeptide/protein ingredientsTissue cultureProteinase activityIl 12p40
Modified interleukin-12 (IL-12) p40 polypeptides are disclosed. The modified polypeptides have alterations in the IL-12p40 subunit to eliminate the protease site between positions Lys260 and Arg261. The modified IL-12p40 polypeptides according to the invention have improved stability compared to wild-type mature human IL-12p40 polypeptides.
Owner:MERCK PATENT GMBH
Heterodimeric fusion proteins useful for targeted immune therapy and general immune stimulation
InactiveUS20050137384A1Extended half-lifeAltering pharmacology and biodistributionPeptide/protein ingredientsAntibody mimetics/scaffoldsWhite blood cellStepwise approach
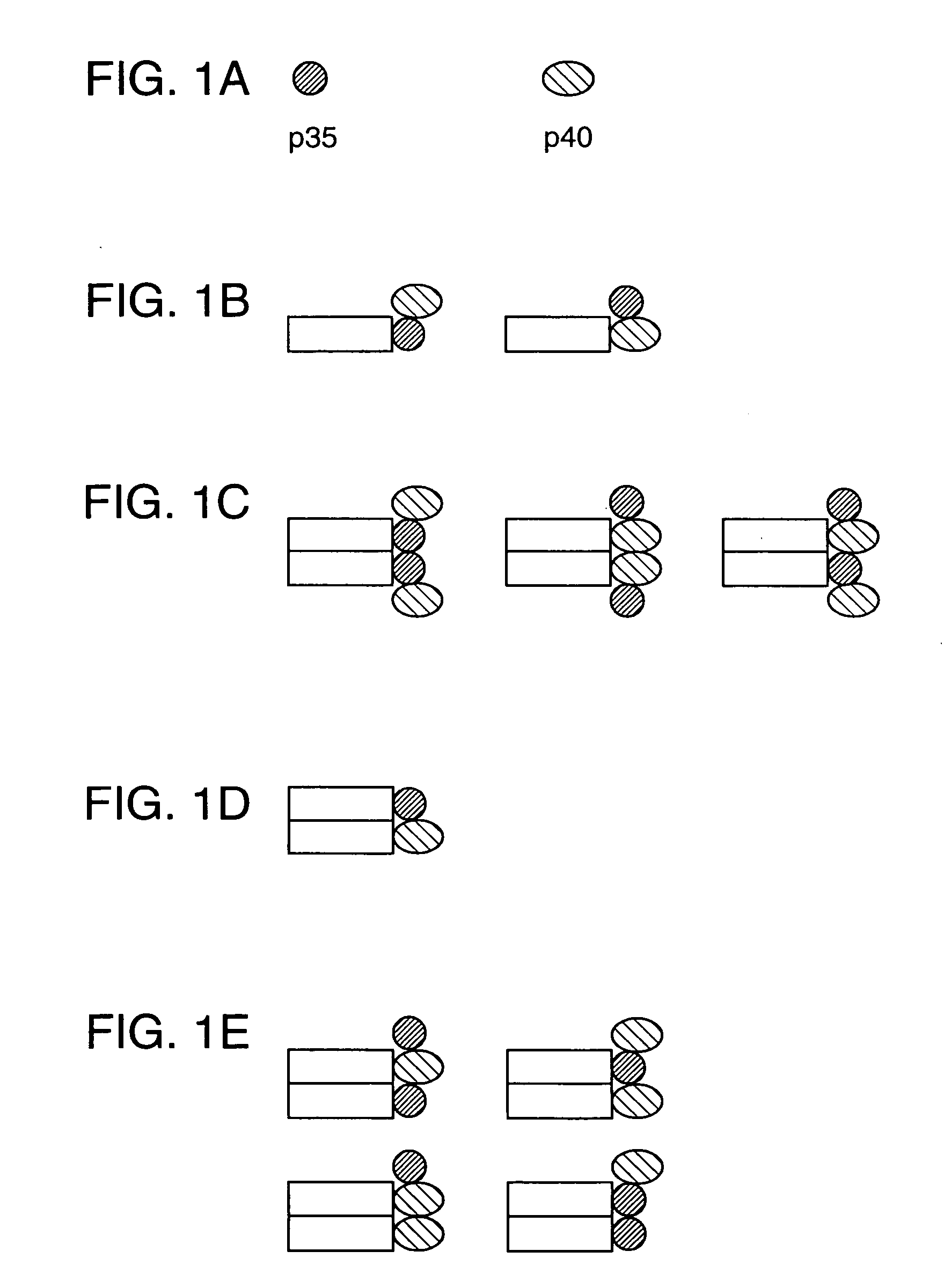
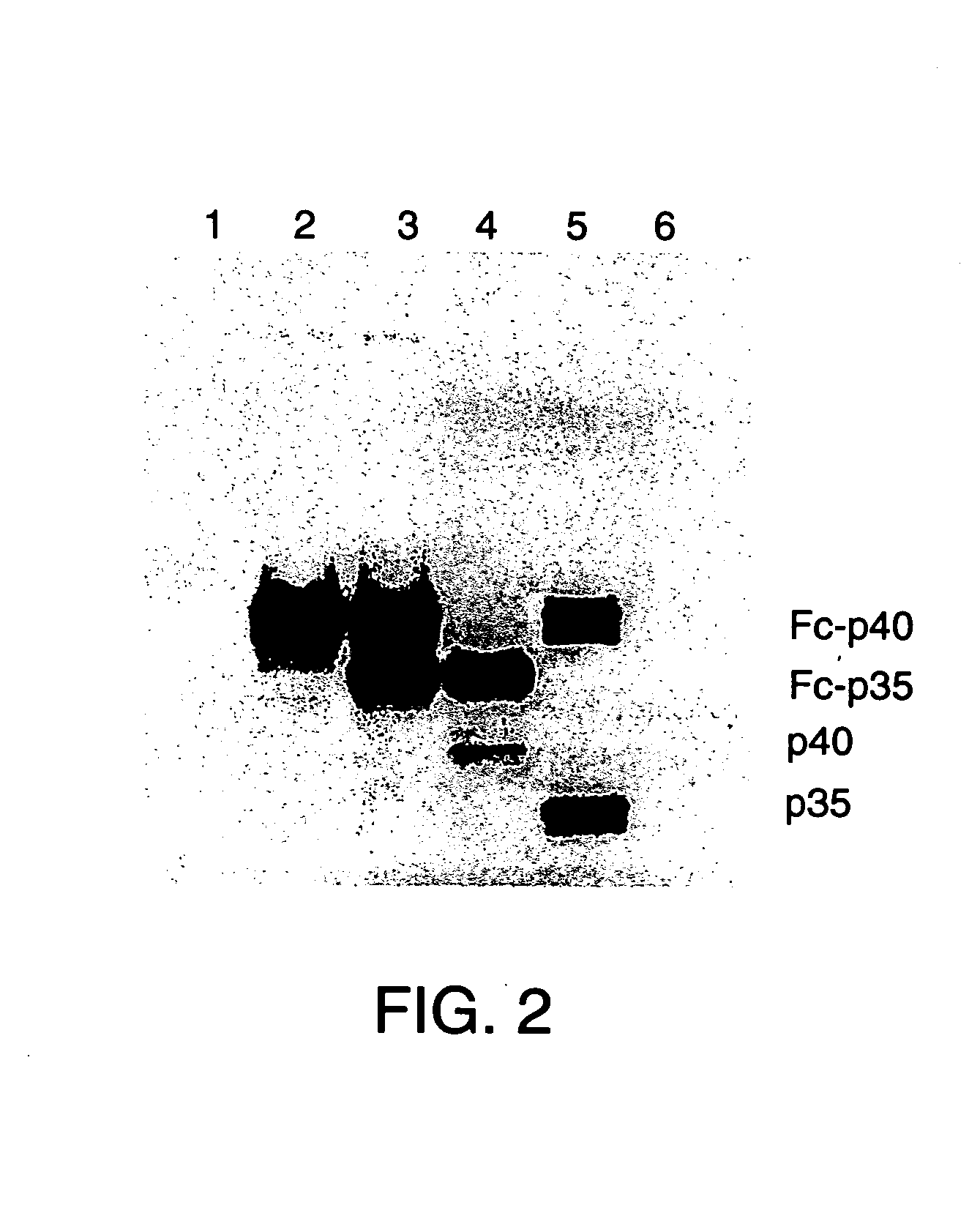
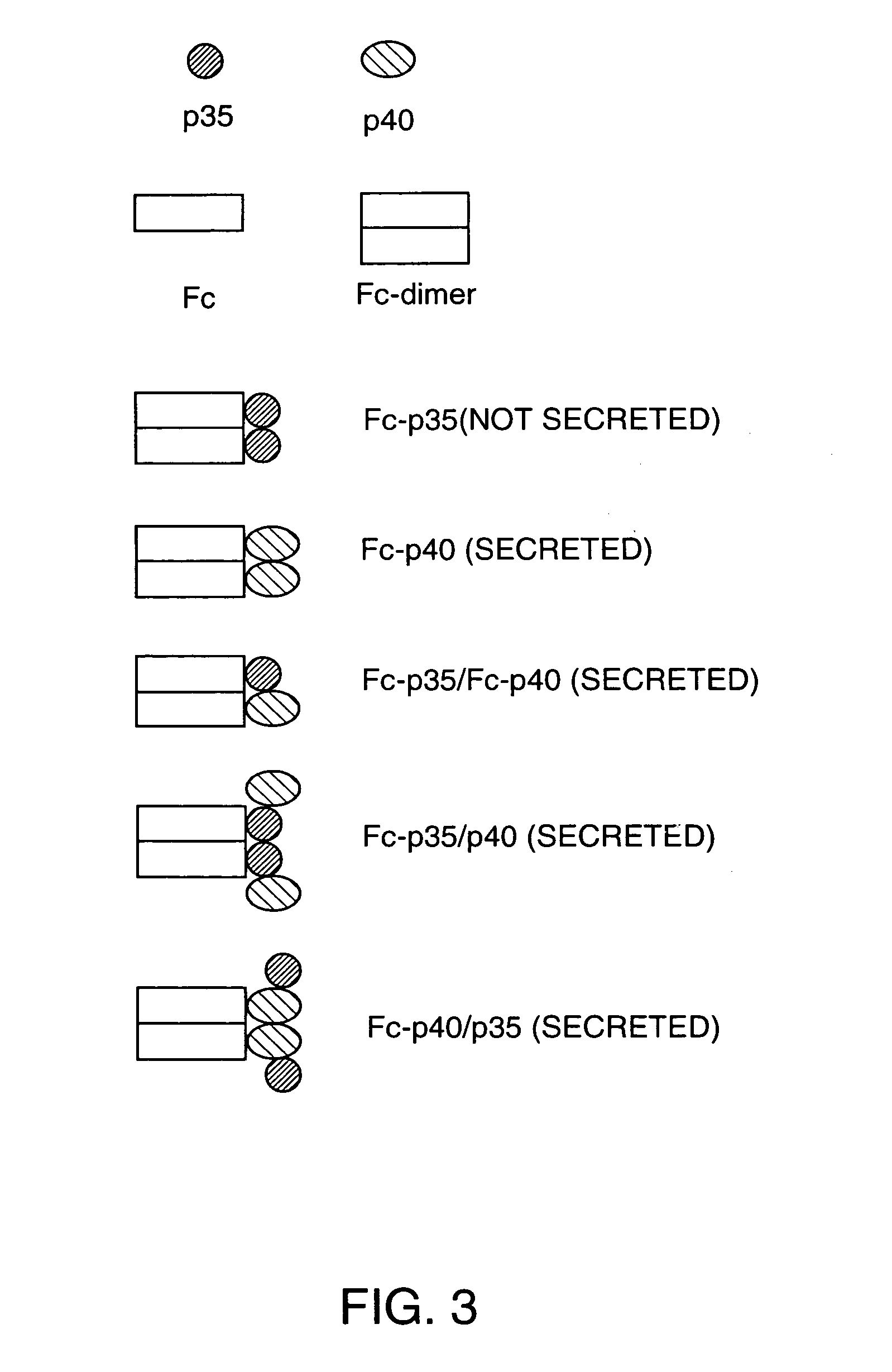
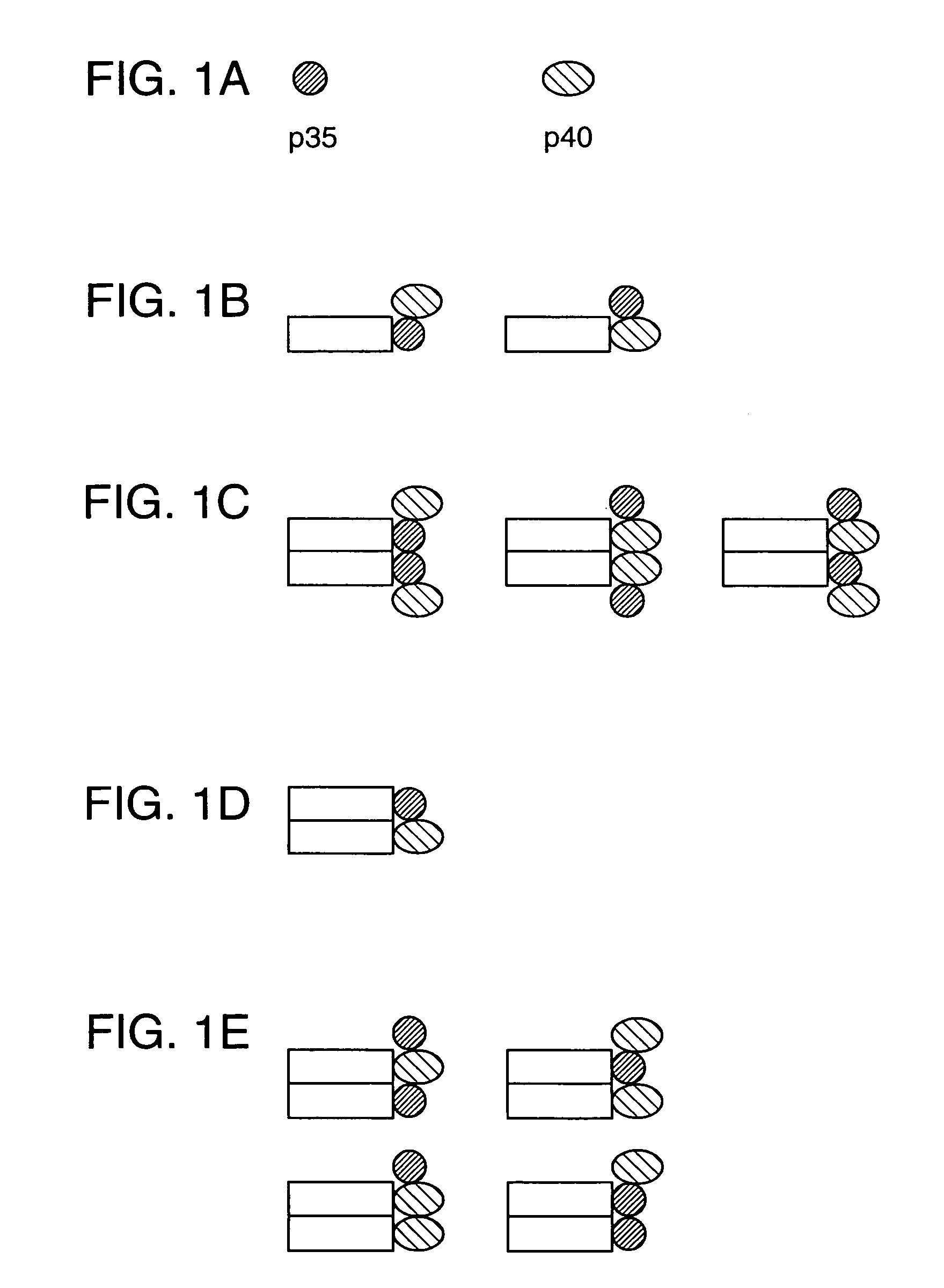
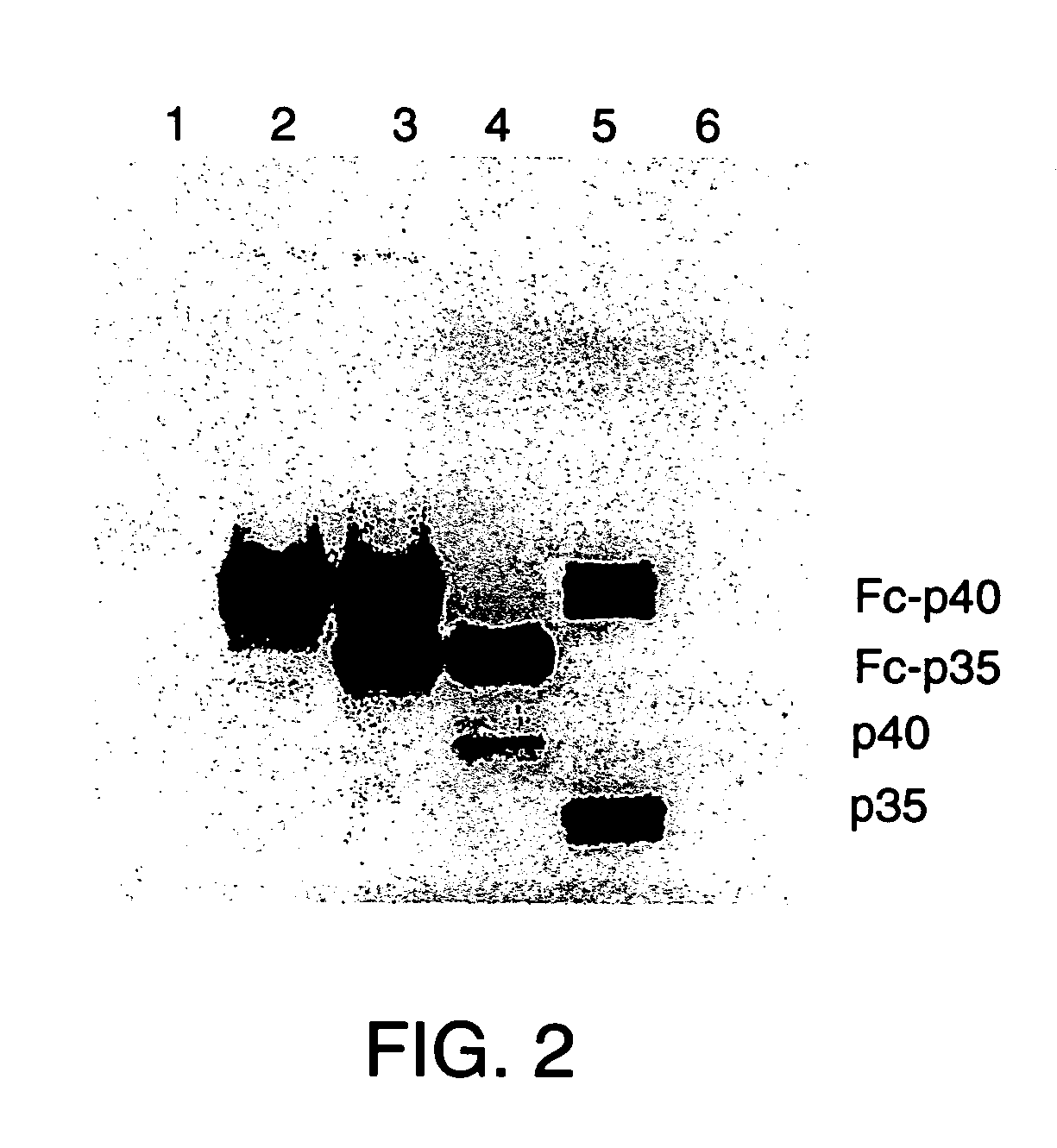
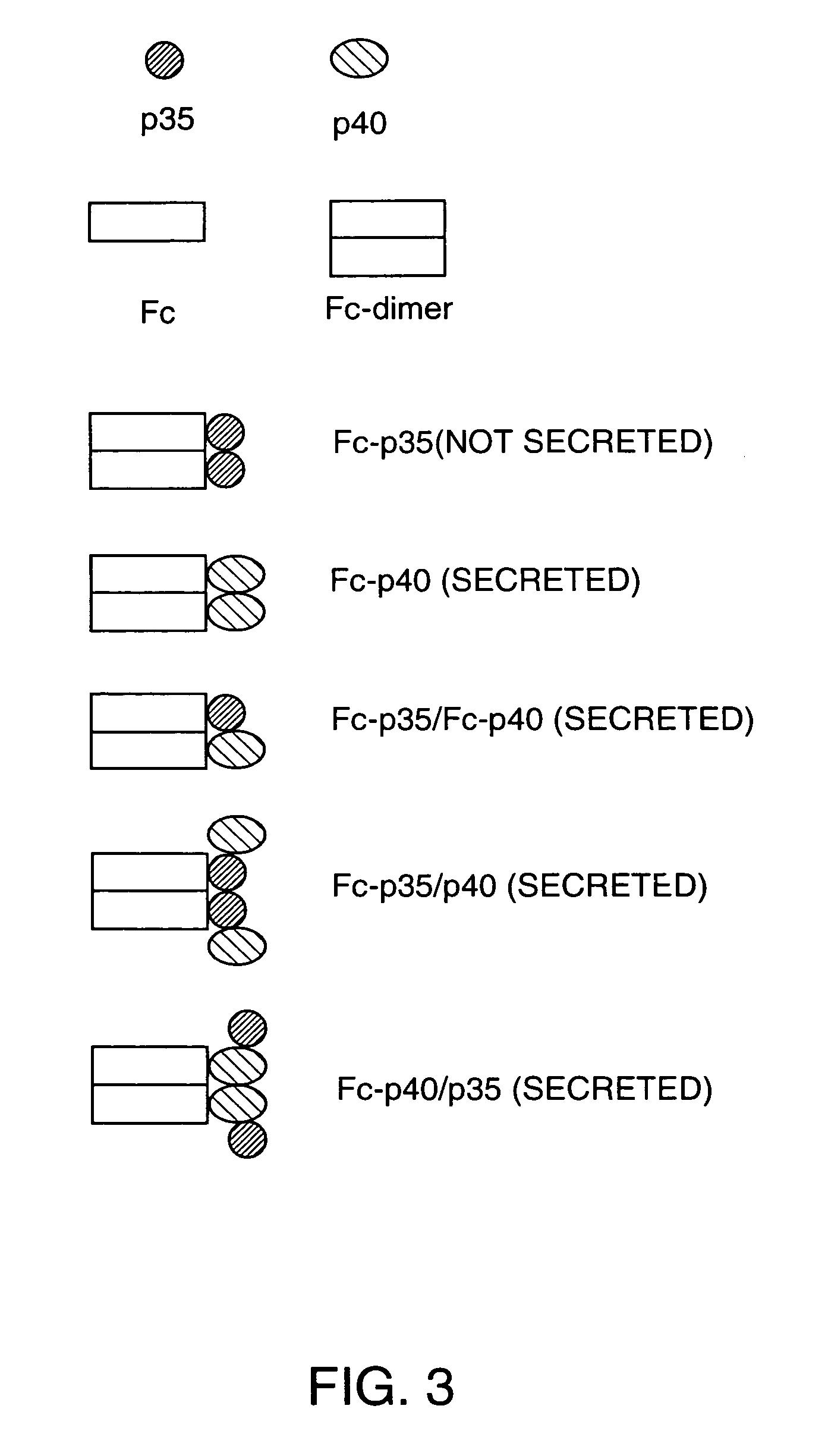
Disclosed are methods for producing fusion proteins with the heterodimeric cytokine, interleukin-12. In order to insure that the proper ratio of fused and non-fused subunits are obtained in the fusion protein, a specific stepwise approach to genetic engineering is used. This consists of first expressing the non-fused p40 IL-12 subunit in a production cell line, followed by or simultaneously expressing in the same cell, a second recombinant fusion protein consisting of the fused polypeptide linked by a peptide bond to the p35 subunit of IL-12. Molecules containing the p35 fusion protein cannot be secreted from the transfected mammalian cell without first complexing in a one to one ratio with the p40 subunit, thus ensuring the production of active heterodimeric fusion proteins.
Owner:MERCK PATENT GMBH
Heterodimeric fusion proteins useful for targeted immune therapy and general immune stimulation
InactiveUS7226998B2Altering pharmacology and biodistributionIncreasing its circulating half-life and its affinityPeptide/protein ingredientsAntibody mimetics/scaffoldsStepwise approachImmune therapy
Disclosed are methods for producing fusion proteins with the heterodimeric cytokine, interleukin-12. In order to insure that the proper ratio of fused and non-fused subunits are obtained in the fusion protein, a specific stepwise approach to genetic engineering is used. This consists of first expressing the non-fused p40 IL-12 subunit in a production cell line, followed by or simultaneously expressing in the same cell, a second recombinant fusion protein consisting of the fused polypeptide linked by a peptide bond to the p35 subunit of IL-12. Molecules containing the p35 fusion protein cannot be secreted from the transfected mammalian cell without first complexing in a one to one ratio with the p40 subunit, thus ensuring the production of active heterodimeric fusion proteins.
Owner:MERCK PATENT GMBH
Inducible interleukin-12
The invention provides an isolated or purified nucleic acid comprising a nucleotide sequence encoding a nuclear factor of activated T-cells (NFAT) promoter operatively associated with a nucleotide sequence encoding IL-12. The invention also provides a nucleic acid comprising a nucleotide sequence encoding a nuclear factor of activated T-cells (NFAT) promoter operatively associated with a nucleotide sequence encoding IL-12, wherein the NFAT promoter is located 3′ of the nucleotide sequence encoding IL-12. Also provided are related recombinant expression vectors, host cells, populations of cells, and pharmaceutical compositions. The invention further provides the use of the inventive nucleic acids or related materials in the treatment or prevention of cancer or an infectious disease in a mammal and in the induction of IL-12 expression in a mammal.
Owner:UNITED STATES OF AMERICA
Genetic adjuvants for immunotherapy
The present invention pertains to methods and pharmaceutical compositions for modulating an immune response. The method of the present invention involves administration of an effective amount of nucleic acid molecules encoding interleukin-12 (IL-12), interferon-gamma (IFN-γ), or a combination thereof, to a patient in need of such treatment. The pharmaceutical compositions of the invention contain nucleic acid molecules encoding IL-12 and / or IFN-γ and an operably-linked promoter sequence. In another aspect, the present invention concerns expression vectors containing a nucleotide sequence encoding IL-12 and IFN-γ, and an operably-linked promoter sequence. In another aspect, the present invention concerns cells generally modified with a nucleotide sequence encoding IL-12 and IFN-γ.
Owner:SOUTH FLORIDA UNIVESITY OF
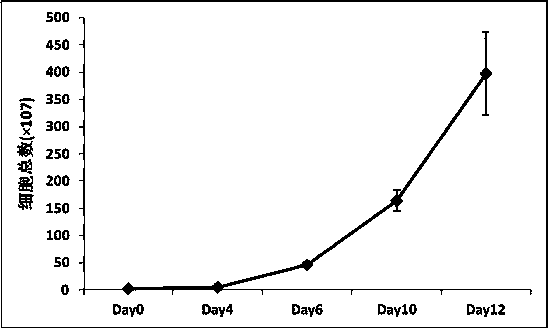
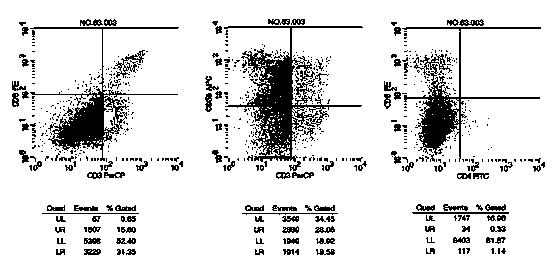
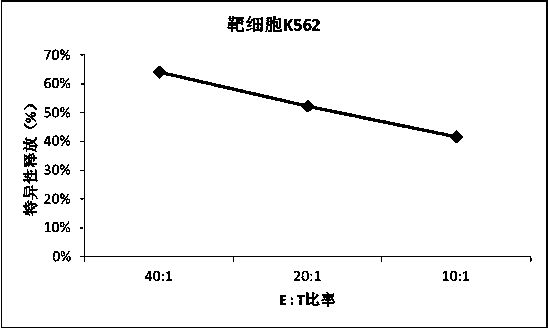
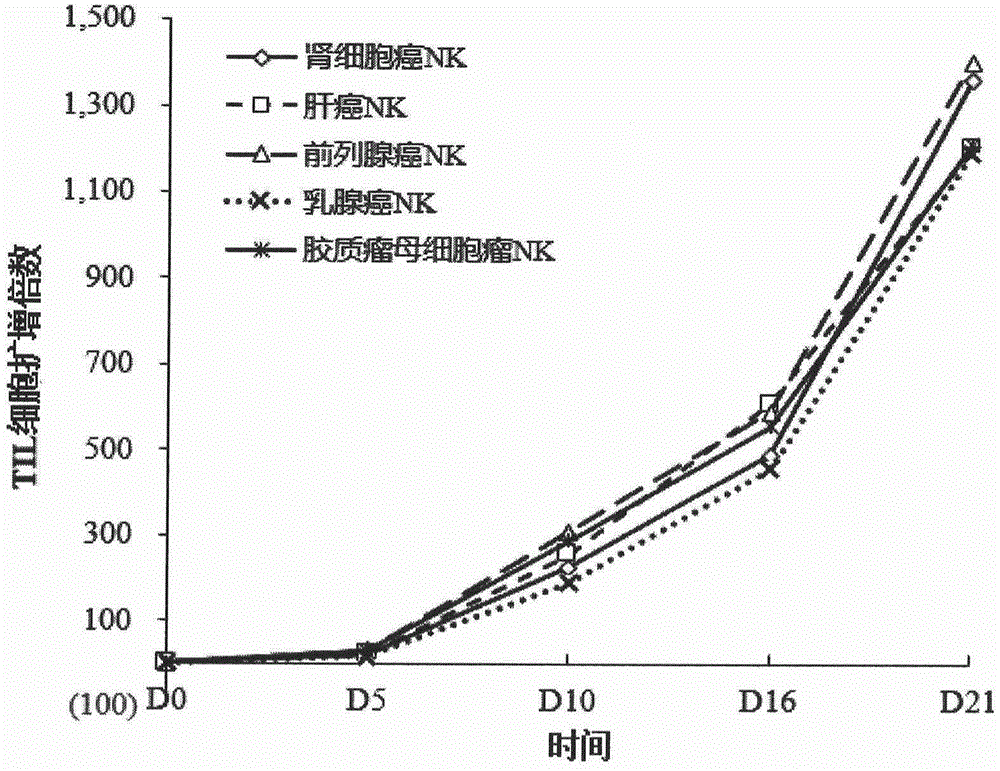
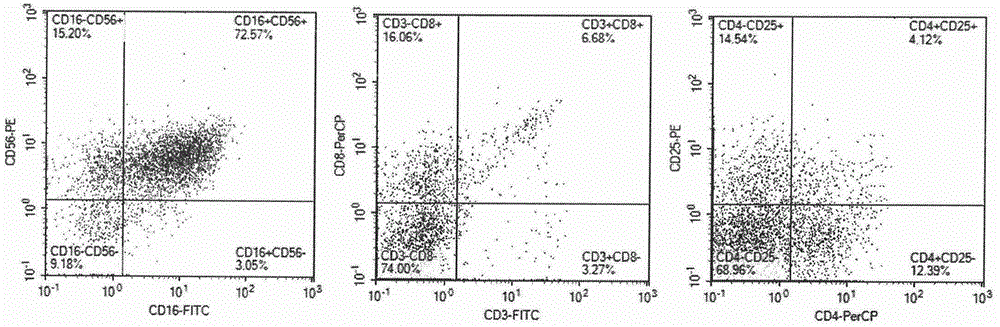
Culture medium efficiently amplifying autologous NK cells and cultural method
The invention relates to a culture medium efficiently amplifying autologous NK cells and a culture method. The culture medium internally contains interleukin 2(IL-2), interleukin 15(IL-15), interleukin 7(IL-7), interleukin 12(IL-12), tumor necrosis factor alpha (TNFalpha) and CD3-antibody, can efficiently amplify and activate NK cells so as to obtain a large quantity of highly active immune cells with majority of NK cells to be transferred back to human bodies, and has remarkable curative effect in anti-tumor, anti-virus infection and immune adjustment related diseases. The culture medium efficiently amplifying autologous NK cells comprises the culture medium for cultivating cells and added factors added into the culture medium for cultivating cells, is used for cultivating and amplifying a large quantity of autologous activated NK cells, and is characterized in that the added factor comprises interleukin 2(IL-2), interleukin 15(IL-15), interleukin 7(IL-7), interleukin 12(IL-12), tumor necrosis factor alpha (TNF alpha) and CD3-antibody.
Owner:康思葆(北京)生物技术有限公司
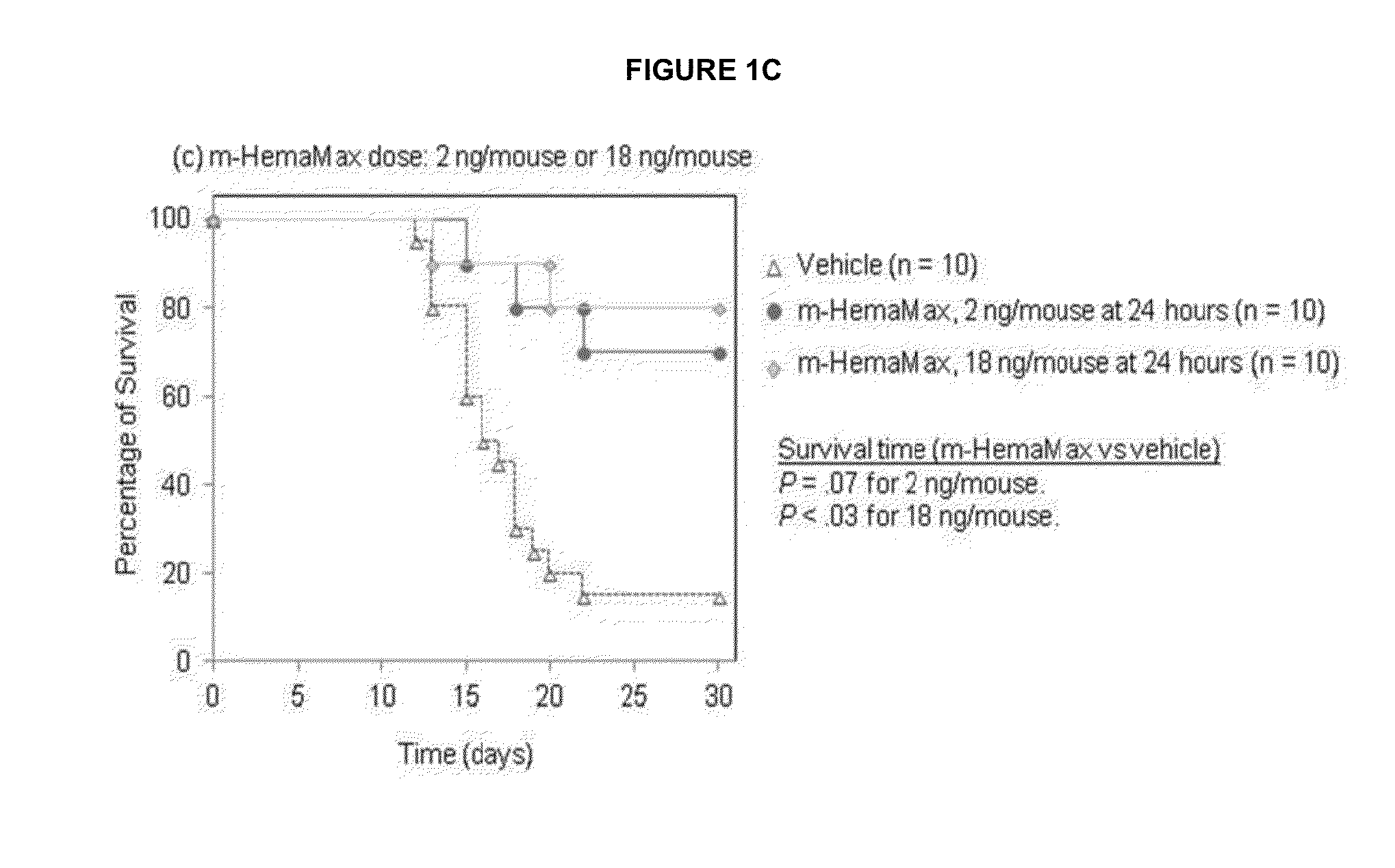
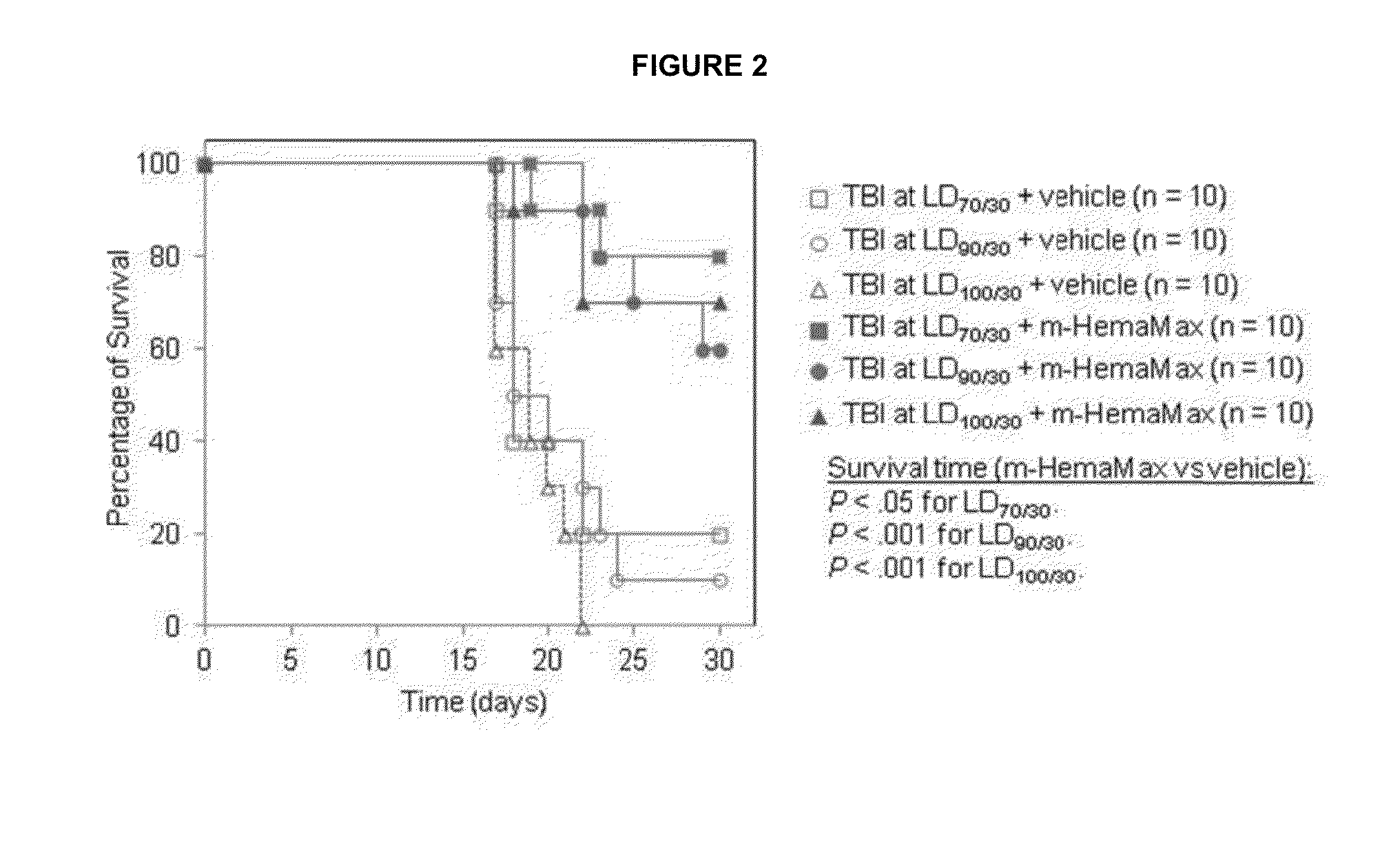
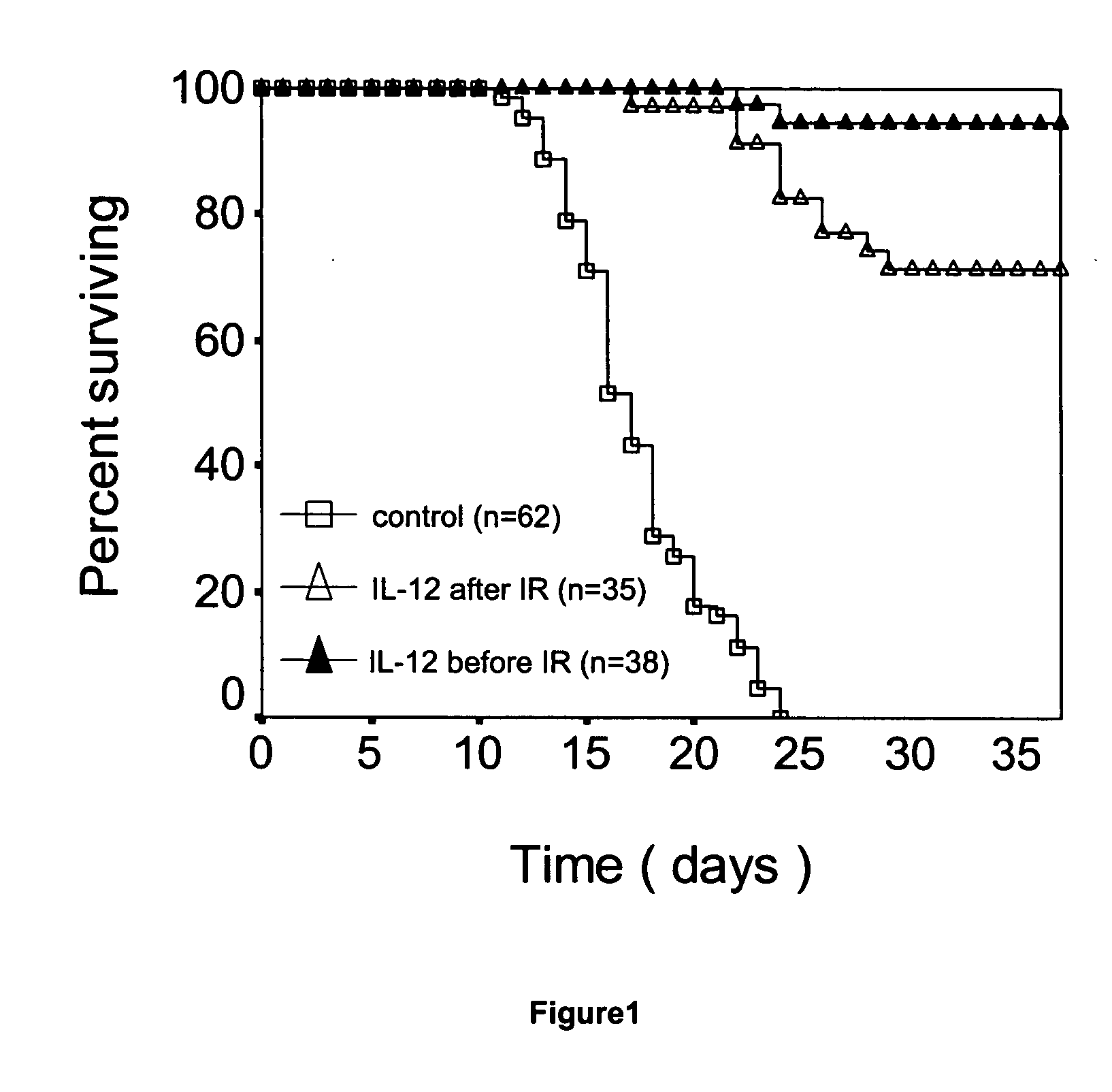
Il-12 for radiation protection and radiation-induced toxicity mitigation
ActiveUS20130243722A1Increase opportunitiesIncrease of effect of exposureNervous disorderPeptide/protein ingredientsMedicineRadiation induced toxicity
Aspects and embodiments of the instant disclosure provide therapeutic methods and compositions comprising interleukin 12 (IL-12) useful for treating radiation-induced damage in a subject. In particular, the instant disclosure provides methods and compositions for radiation protection and / or radiation toxicity mitigation for the treatment of acute radiation syndrome and radiation induced toxicity associated with the treatment of cutaneous T-cell lymphoma.
Owner:NEUMEDICINES INC
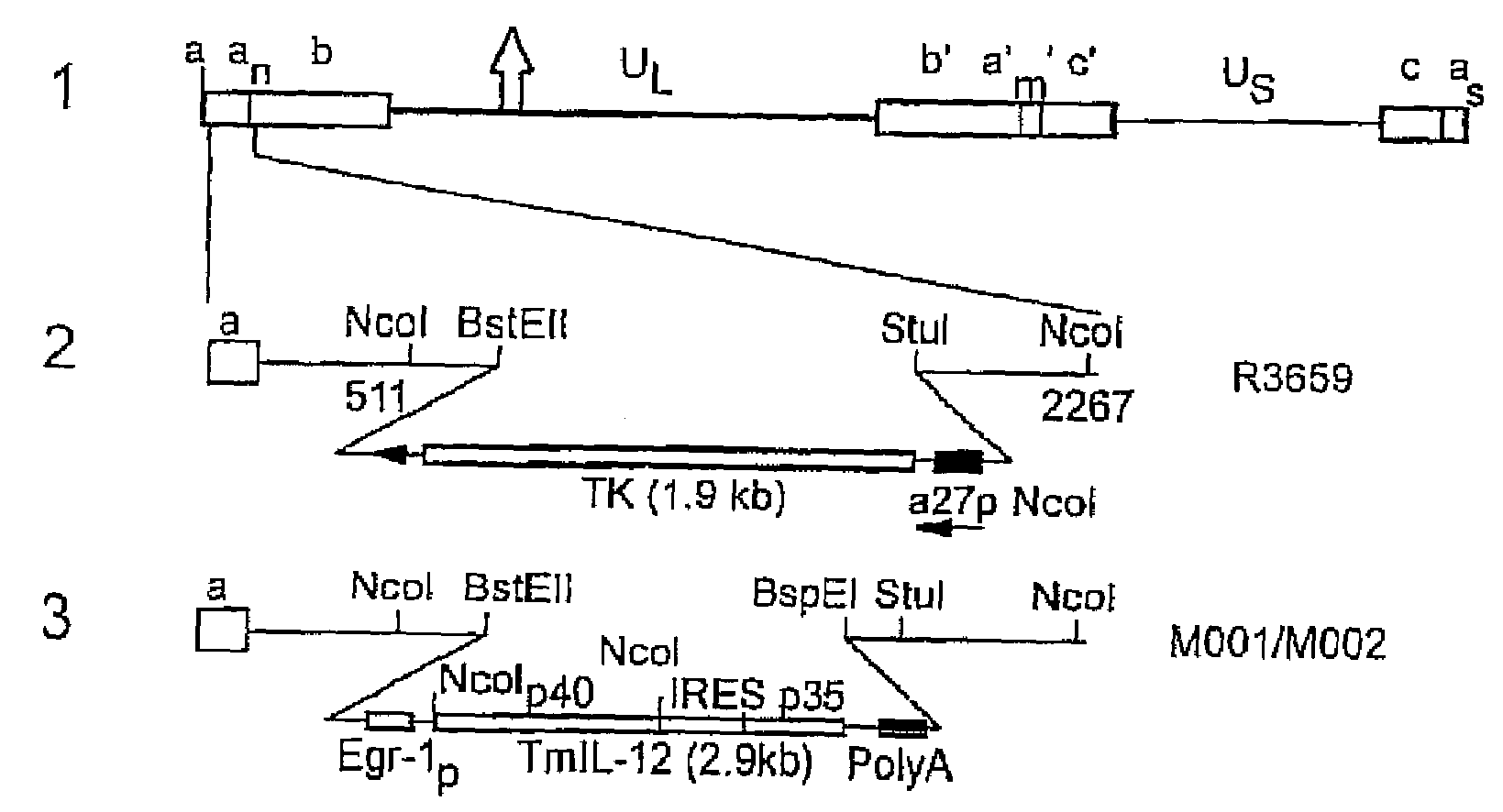
Herpes simplex virus expressing foreign genes and method for treating cancers therewith
An anti-cancer pharmaceutical composition includes a herpes simplex virus (HSV) vector into which a nucleic acid sequence encoding for an anti-cancer agent selected from interleukin-12, GM-CSF, and CD has been inserted. A method of treatment of a patient suffering from cancer includes administering to the patient the anti-tumor pharmaceutical composition including a HSV vector having a nucleic acid sequence encoding for an anti-cancer agent selected from interleukin-12, GM-CSF, and CD inserted therein.
Owner:UAB RES FOUND
Inducible interleukin-12
ActiveUS20120071859A1Treat and prevent cancerOrganic active ingredientsFungiNucleotideWhite blood cell
The invention provides an isolated or purified nucleic acid comprising a nucleotide sequence encoding a nuclear factor of activated T-cells (NFAT) promoter operatively associated with a nucleotide sequence encoding IL-12. The invention also provides a nucleic acid comprising a nucleotide sequence encoding a nuclear factor of activated T-cells (NFAT) promoter operatively associated with a nucleotide sequence encoding IL-12, wherein the NFAT promoter is located 3′ of the nucleotide sequence encoding IL-12. Also provided are related recombinant expression vectors, host cells, populations of cells, and pharmaceutical compositions. The invention further provides the use of the inventive nucleic acids or related materials in the treatment or prevention of cancer or an infectious disease in a mammal and in the induction of IL-12 expression in a mammal.
Owner:UNITED STATES OF AMERICA
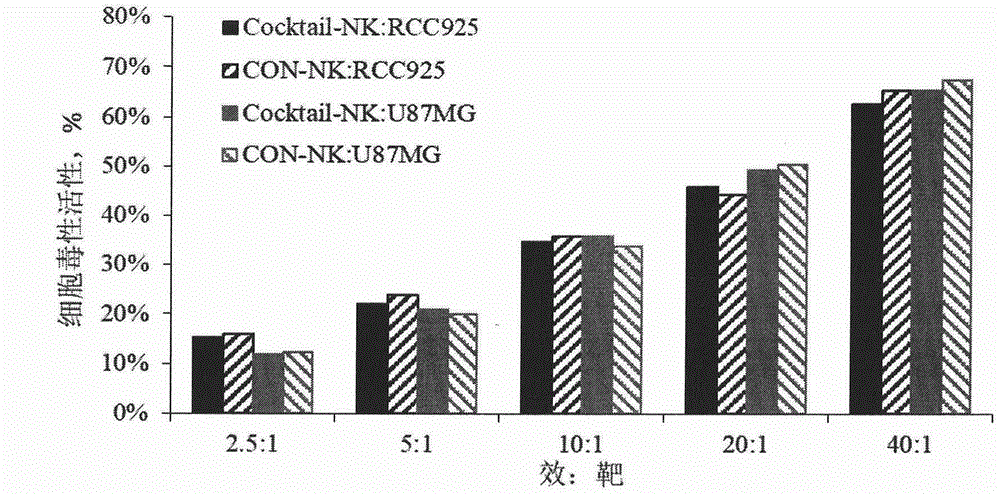
Method for preparing autologous natural killer cell in cocktail culture and and kit product
InactiveCN104789527AMammal material medical ingredientsBlood/immune system cellsPeripheral blood mononuclear cellRecombinant Human Interleukin-15
The invention discloses a method for preparing autologous natural killer cell in cocktail culture and a kit product. The method is characterized in that proliferation of natural killer cells of peripheral blood mononuclear cells from a cancer patient is activated under the combined action of a recombinant human interleukin 15, a recombinant human interleukin 18, a recombinant human interleukin 21, a recombinant human interleukin 12, a recombinant human interleukin 7 and a recombinant human MHC-1 chain related molecule A, and the natural killer cell killing potential is reinforced. The invention also discloses a kit containing the autologous natural killer cell in cocktail culture prepared by the method. The kit can be used for clinically acquiring plenty of natural killer cells and performing antitumor and antivirus treatment.
Owner:JIANGSU JIESHENG BIOSCI CO LTD
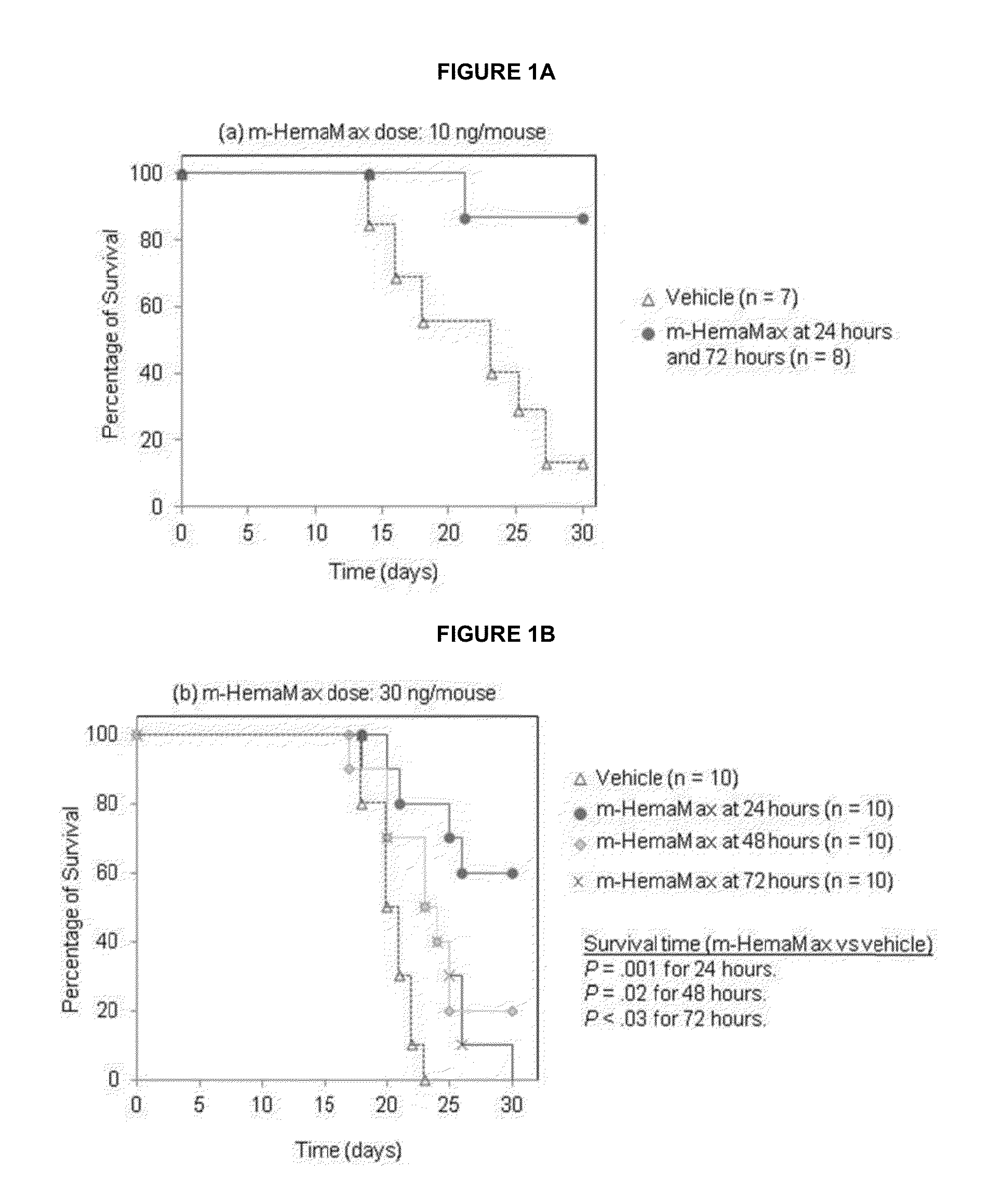
Uses of IL-12 in hematopoiesis
ActiveUS20050136034A1Reduces hematopoietic toxicityFacilitating eradicationOrganic active ingredientsPeptide/protein ingredientsWhite blood cellIn vivo
Embodiments of the present invention are directed to uses of Interleukin-12 (IL-12) in enhancing or stimulating hematopoiesis to yield hematopoietic recovery in a mammal in need. Particular embodiments of the invention are directed to uses of IL-12 as an adjuvant therapy to alleviate the hematopoietic toxicities associated with one or more treatment regimens used to combat a disease state. Other embodiments include uses of IL-12 to ameliorate various hematopoietic deficiencies. Still other embodiments are directed to uses of IL-12 for in-vivo proliferation of hematopoietic repopulating cell, hematopoietic progenitor cells and hematopoietic stem cells. Other disclosed embodiments are directed to uses of Il-12 for bone marrow preservation or recovery.
Owner:UNIV OF SOUTHERN CALIFORNIA
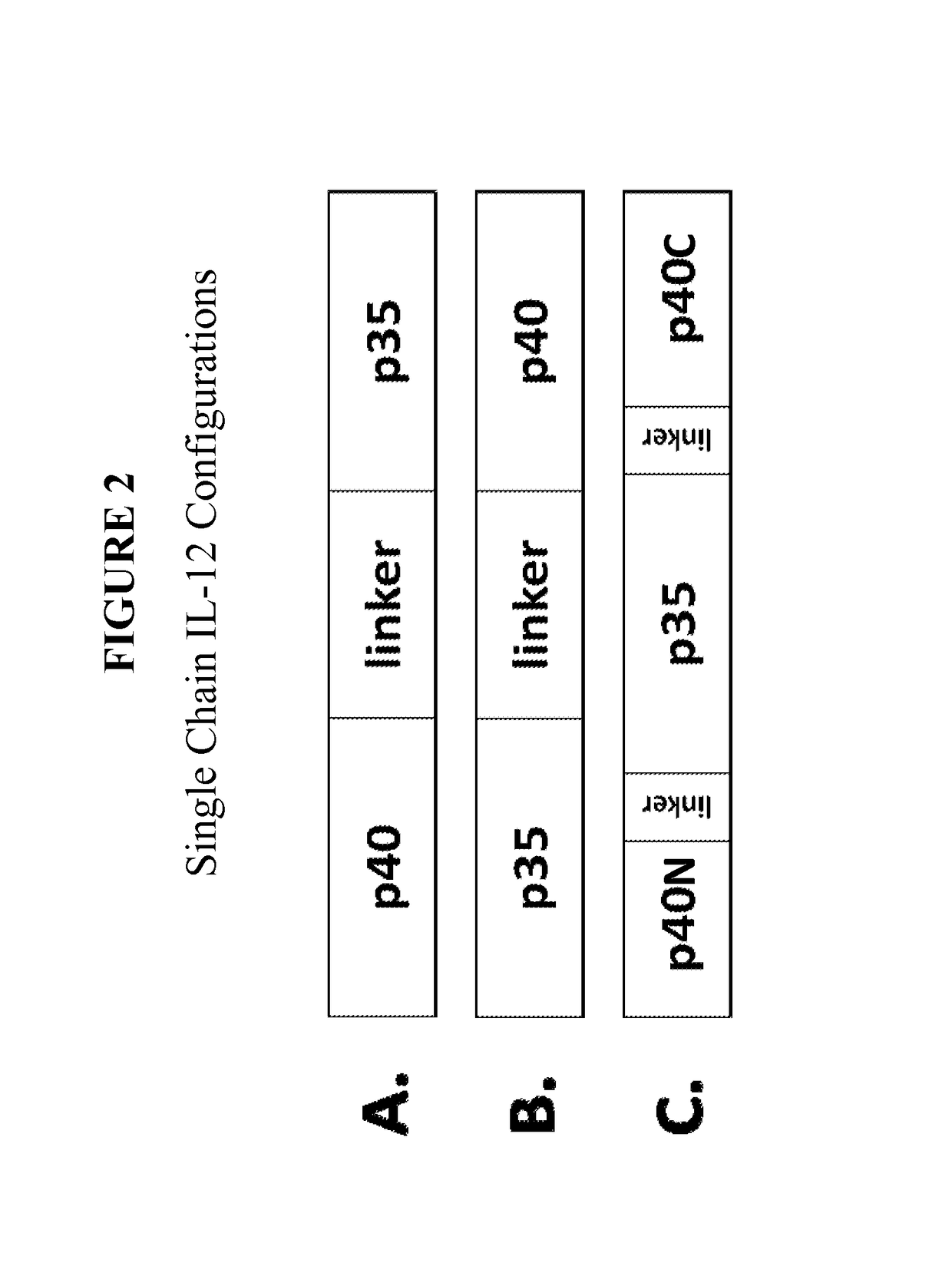
Improved therapeutic control of heterodimeric and single chain forms of interleukin-12
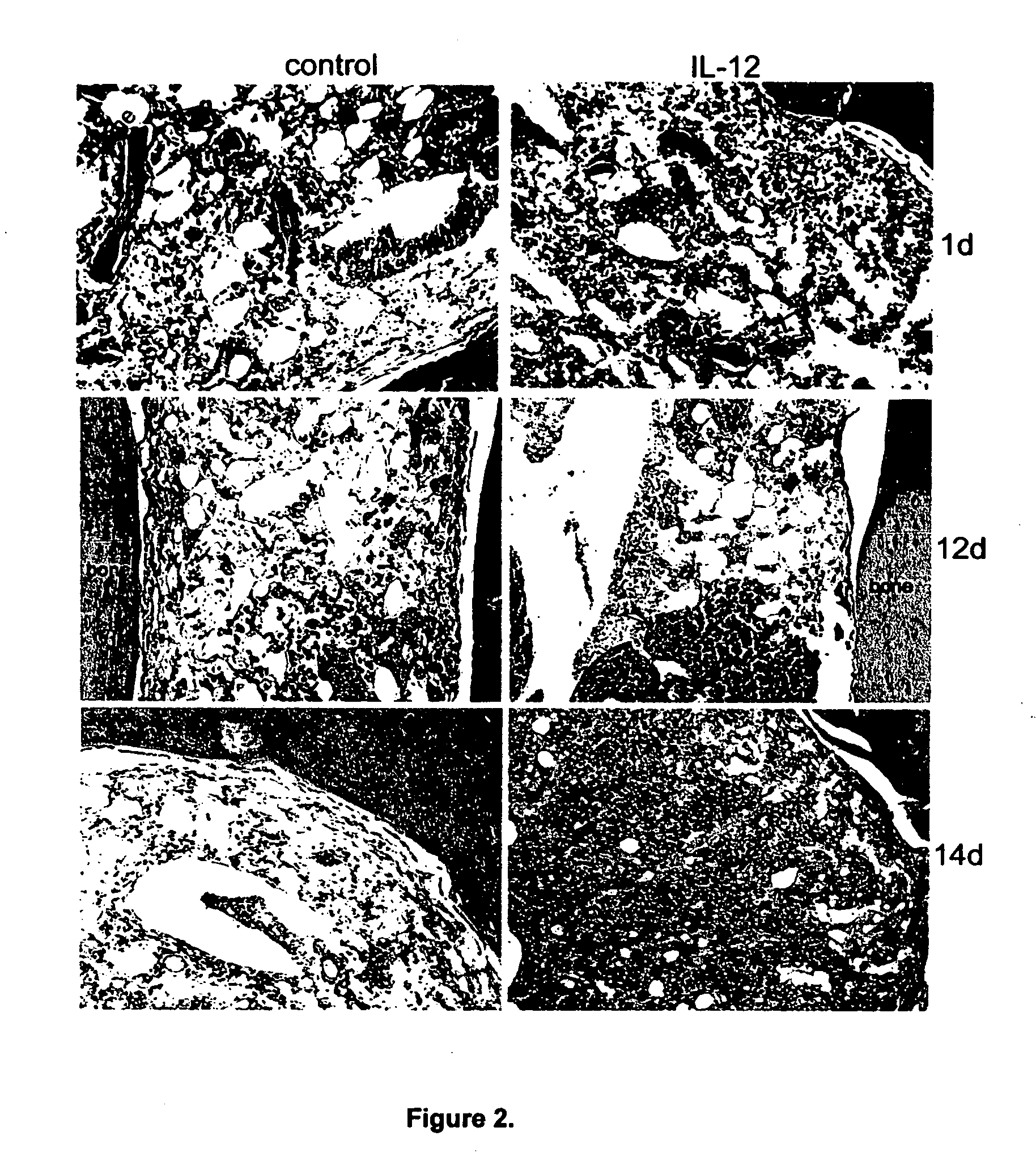
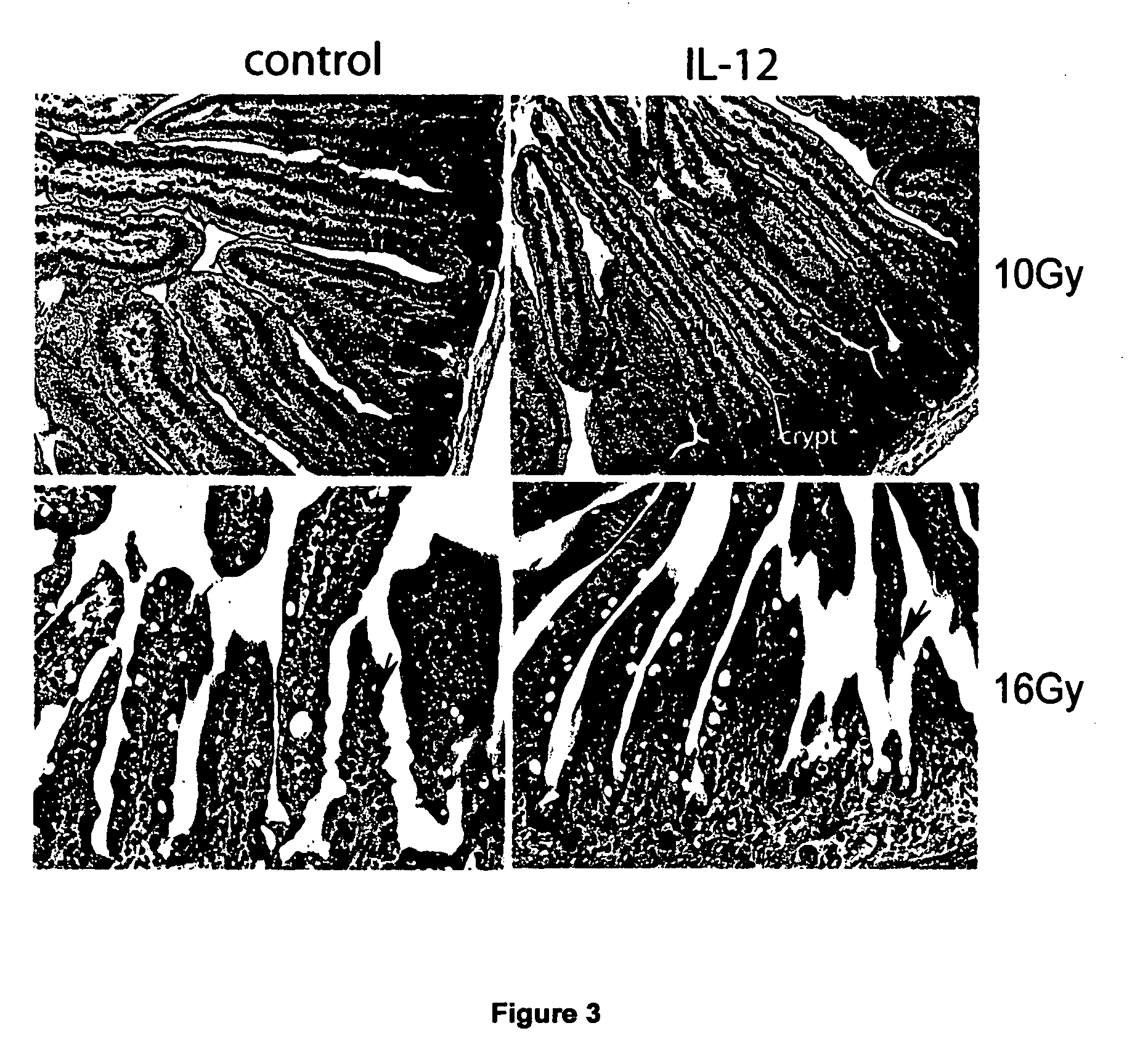
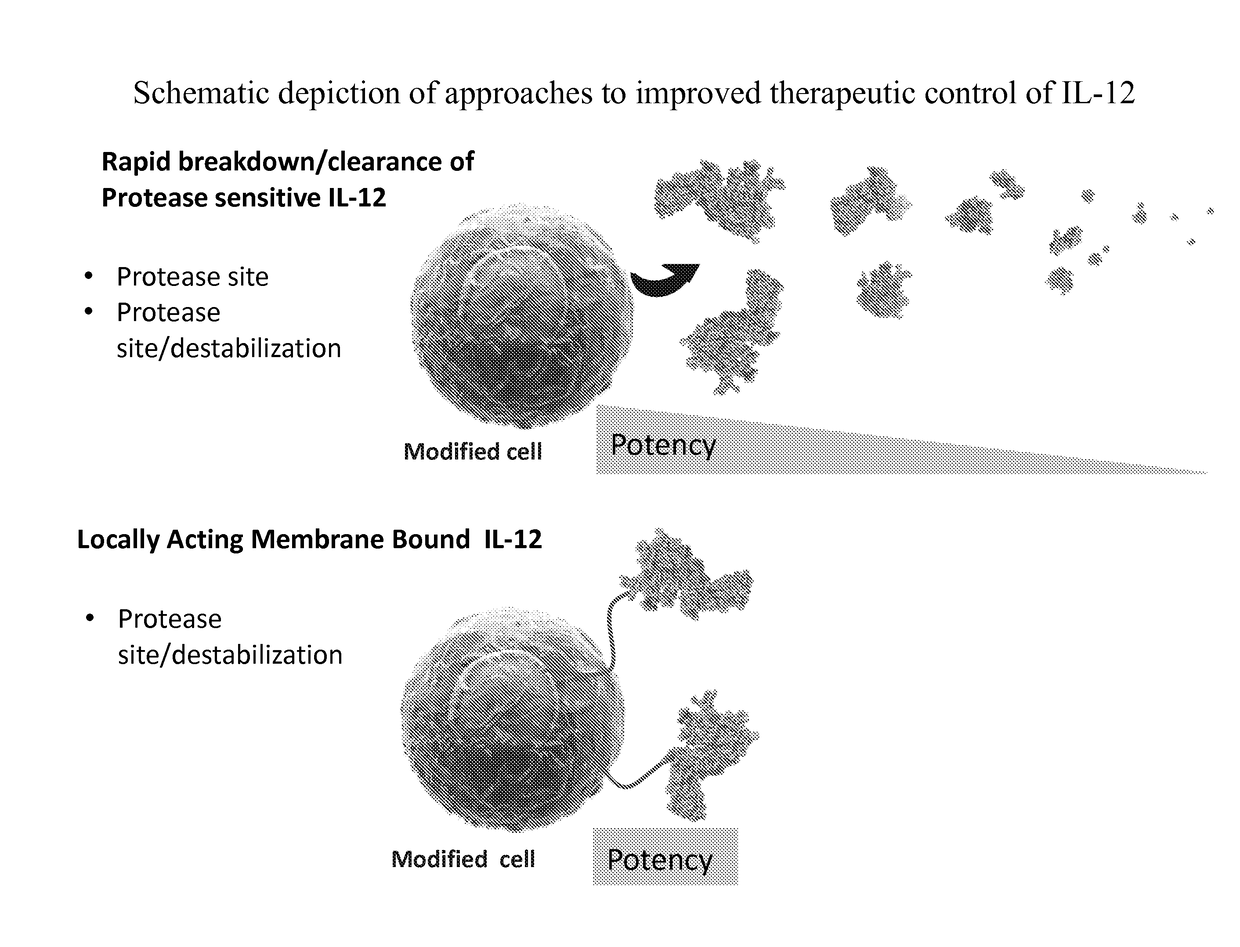
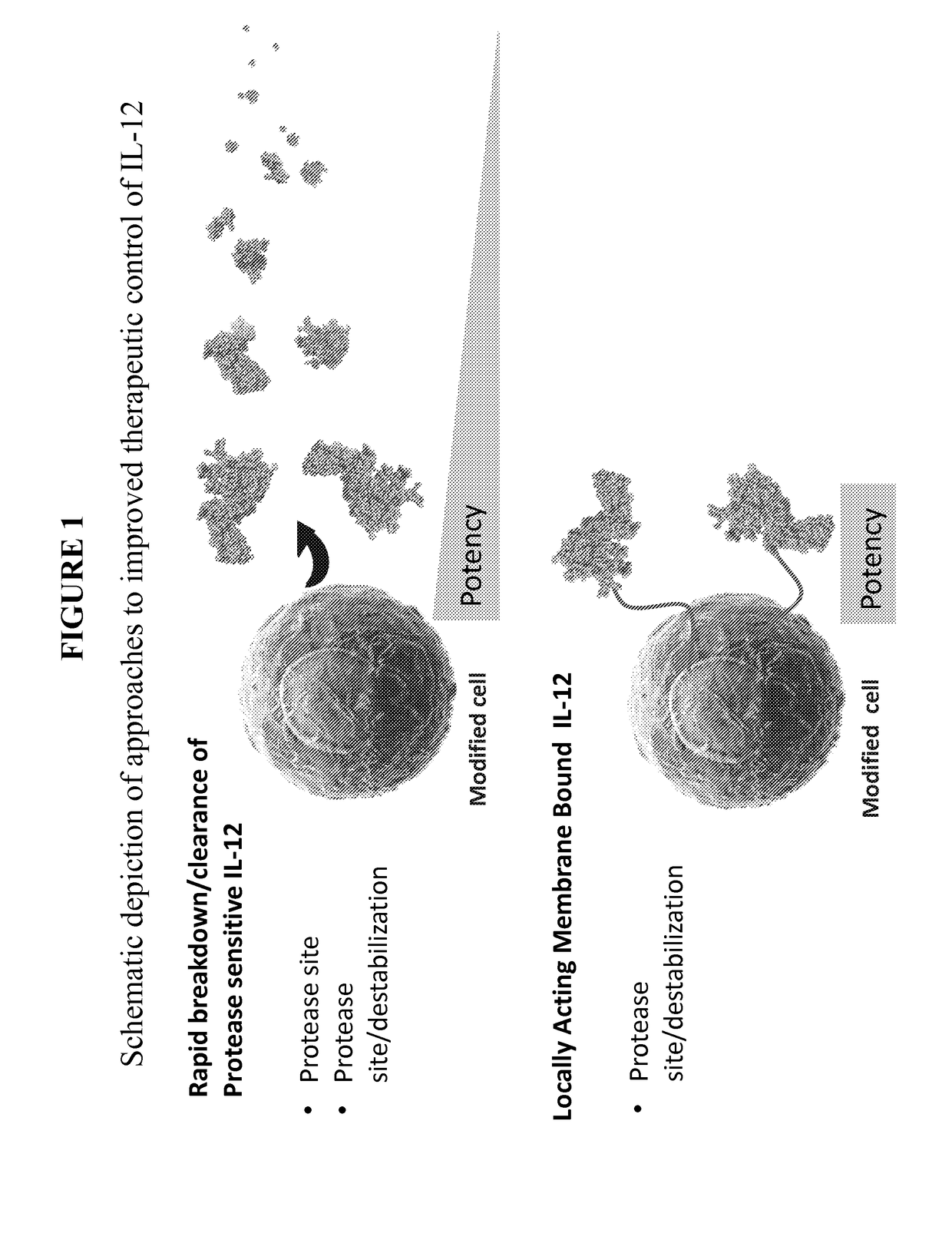
InactiveUS20170291934A1Shortened in vivo half-lifeEnhanced localization of biological effectPeptide/protein ingredientsBiological testingWhite blood cellHalf-life
The present invention relates to modified forms of IL-12. These modified forms of IL-12 may be engineered to have a shortened in vivo half-life compared and / or enhanced localization of biological effects compared to that of corresponding non-modified form of IL-12. Short half-life and membrane bound forms of IL-12 may provide greater therapeutic control for in vivo therapeutic delivery, in particular when used in combination with ligand inducible delivery of IL-12. Modified forms of IL-12 engineered to have shortened in vivo half-life and / or enhanced localization of biological effects include heterodimeric p35 / p40, single chain and membrane bound forms of IL-12 wherein a naturally occurring IL-12 amino acid sequence is genetically modified to enhance susceptibility of the IL-12 molecule to in vivo proteolytic degradation.
Owner:PRECIGEN INC
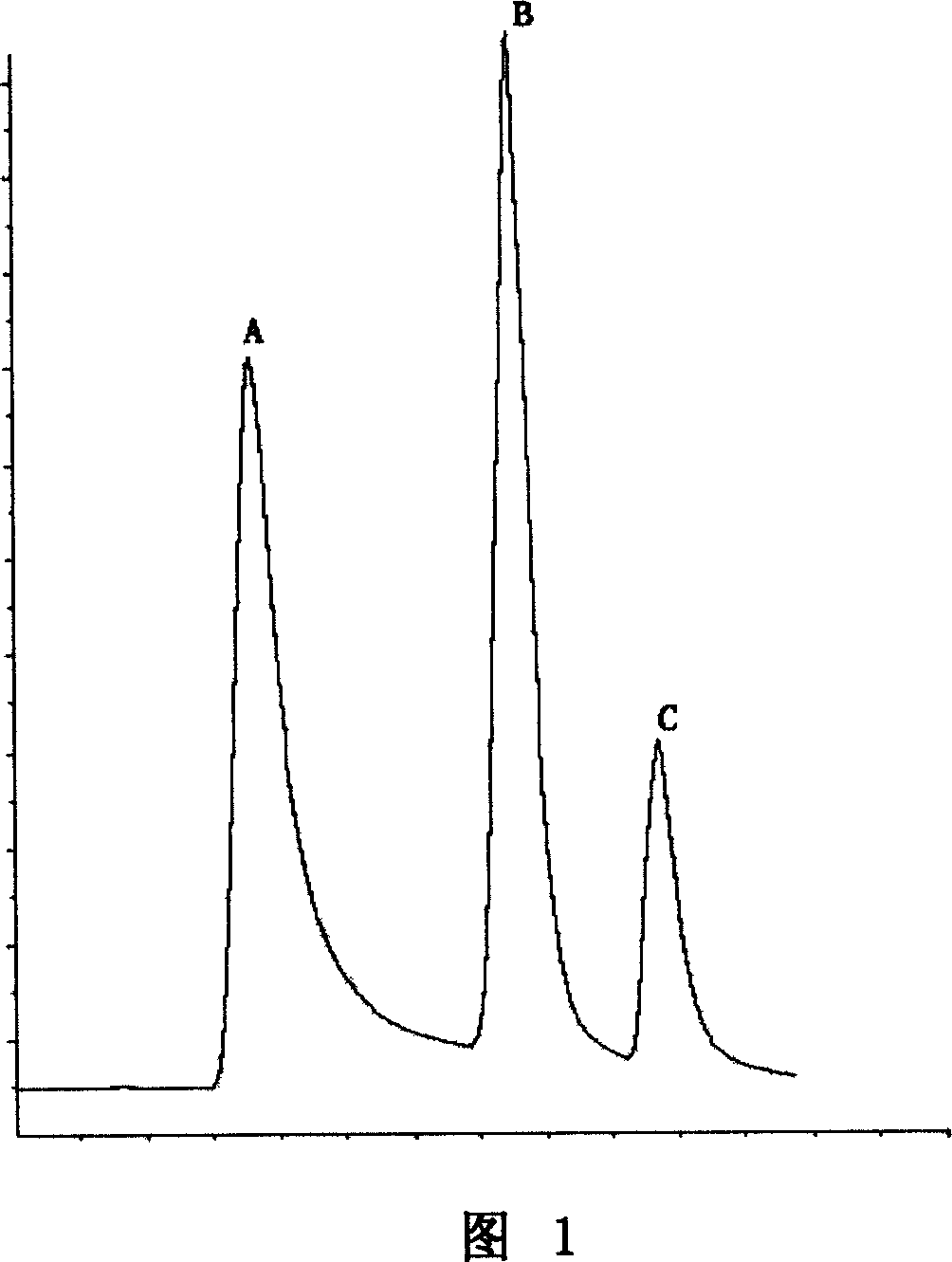
Method of purifying and combining human interleukins 12
ActiveCN101033254AReduce the cost of separation and purificationSimple and fast operationInterleukinsMolecular sieveAnion-exchange chromatography
This invention discloses a method to purify rhIL-12, belonging to the protein purification technology, which technical points are that the cell culture supernatant of rhIL-12 is conducted filtering, cation or anion exchange chromatography, precipitation of ammonium sulfate, anion or cation exchange chromatography and molecular sieve chromatography, and during the precipitation of ammonium sulfate, pH is far away from the isoelectric point of rhIL-12. This method uses ammonium sulfate precipitation to remove most of hybridproteins, making the future separation simple and effective.
Owner:广州市茵良强生物科技有限公司
Genetic adjuvants for immunotherapy
Owner:UNIV OF SOUTH FLORIDA
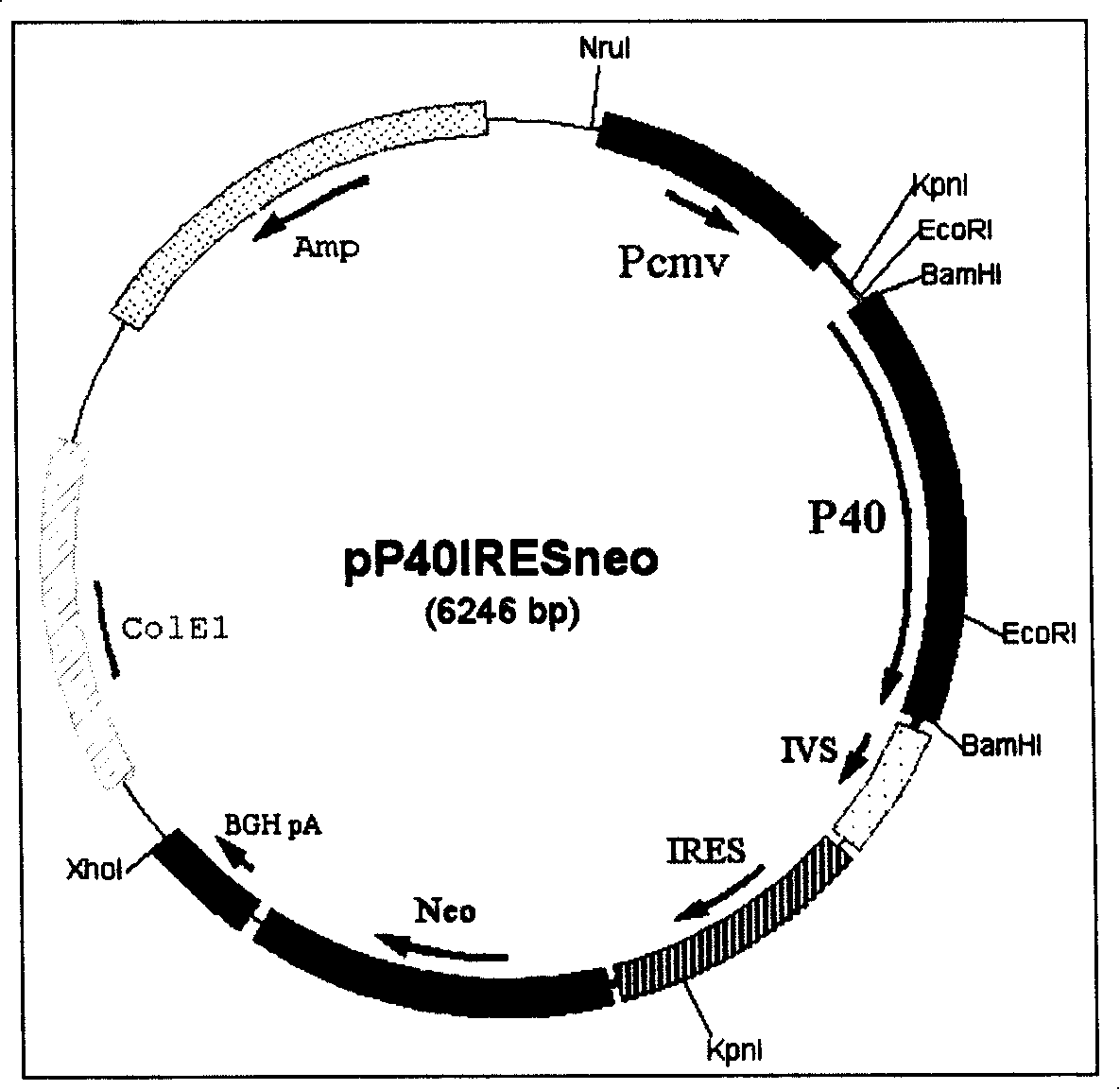
IL-12 expression carrier as well as eukaryotic cell strain expressed thereby and uses thereof
ActiveCN101200730APeptide/protein ingredientsFermentationTranscriptional Regulatory ElementsCell strain
The present invention relates to a novel method of using mammalian cell efficient expression to recombine human interleukin 12 (rhIL-12). The present invention transcripts two p35 subunits and one p40 subunit of rhIL-12 to be constructed inside a same carrier; each subunit is processed for expression level regulation by a transcription regulating element, and the efficient expression of rhIL-12 is realized by introducing the regulating elements of Intron and SV40Enhancer which can improve the expression level.
Owner:广州市茵良强生物科技有限公司
Immunogene therapeutic drug for chronic hepatitis B and preparation method for immunogene therapeutic drug
InactiveCN104940953AReduced viral copyImprove securityGenetic material ingredientsDigestive systemHBsAgChronic hepatitis
The invention relates to an immunogene therapeutic drug for chronic hepatitis B and a preparation method for the immunogene therapeutic drug. The active component of the drug is a replication-competent recombinant vector pSVK-dSFValpha-IRES-hIL-12 (for short, pSVK-HBVE), which is constructed by taking a pSVK carrier as a starting carrier and carries a fusion gene dSFValpha-IRES-hIL12. An antibody targeting interferon alpha and human IL-12 are co-expressed in the replication-competent recombinant vector pSVK to construct a humanized HBsAg dsFv antibody, human interferon-alpha and interleukin-12 fusion expression vector pSVK-dSFValpha-IRES-hIL-12, the fusion expression vector is efficiently expressed in an eukaryotic cell and in vitro, the combined application of human IL-12 and human IFN-alpha has an important synergistic effect during the tumor control process, and a safe and efficient immunogene therapeutic drug is provided for gene therapy of chronic hepatitis B.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Il-12/p40 binding proteins
The present invention encompasses IL-12p40 binding proteins, particularly antibodies that bind human interleukin-12 (hIL-12) and / or human IL-23 (hIL-23). Specifically, the invention relates to antibodies that are chimeric, CDR grafted and humanized antibodies. Preferred antibodies have high affinity for hIL-12 and / or hIL-23 and neutralize h IL-12 and / or hIL-23 activity in vitro and in vivo. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. Method of making and method of using the antibodies of the invention are also provided. The antibodies, or antibody portions, of the invention are useful for detecting hIL-12 and / or hIL-23 and for inhibiting hIL-12 and / or hIL-23 activity, e.g., in a human subject suffering from a disorder in which hIL-12 and / or hIL-23 activity is detrimental.
Owner:ABBVIE INC
Compositions and methods for immunotherapy of cancer and infectious diseases
The present invention provides compositions and methods for the prevention and treatment of primary and metastatic neoplastic diseases and infectious diseases, for stimulating an immune response in a subject, and for use as an alternative to interleukin-12 (IL-12) treatment. In particular, the present invention provides Apicomlexa-related proteins (ARPs) that have immune stimulatory activity and thus have uses in the treatment and prevention of cancer and infectious diseases and in immune modulation. Compositions comprising an ARP are provided. Methods of use of an ARP for the prevention and / or treatment of cancer and / or infectious diseases, for use as an alternative to interleukin-12 (IL-12) treatment, and for eliciting an immune response in a subject, are also provided.
Owner:MICHIGAN STATE UNIV
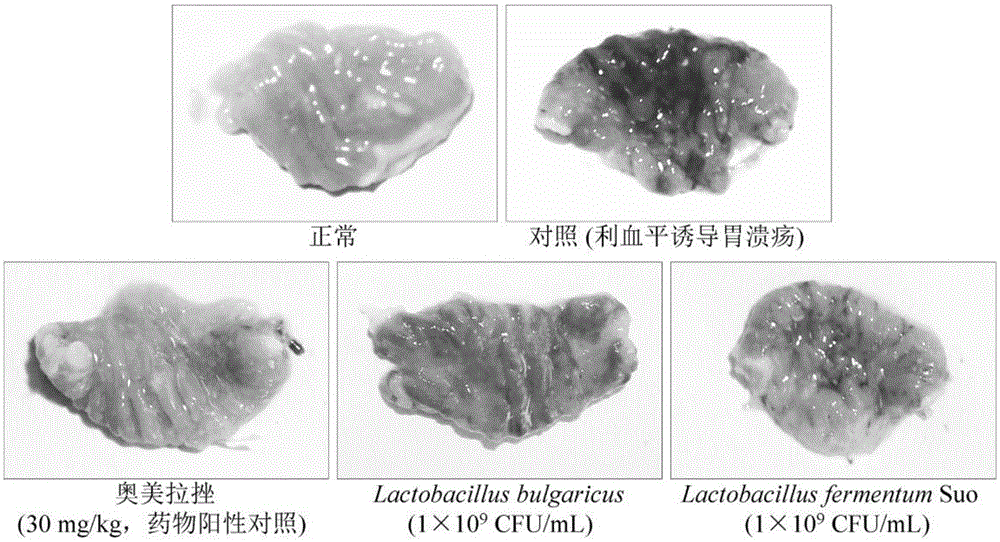
Lactobacillus fermentum strain suo capable of preventing gastric ulcers and application of lactobacillus fermentum strain suo
The invention discloses lactobacillus fermentum strain suo capable of preventing gastric ulcers and application of the lactobacillus fermentum strain suo. A bacterial strain of which the preservation number CCTCC NO is M2013511 has strong acid resistance, and the survival rate in artificial gastric juice of which the pH value is 3.0 for three hours reaches 92.46 plus or minus 4.06%, and the bacterial strain can slowly grow in cholate of which the concentration is 1.0%, and the growth efficiency reaches 17.36 plus or minus 1.19% of cholate-free cultivation; the hydrophobicity of lactobacillus fermentum strain suo cells also reaches 68.44 plus or minus 2.48%, and the lactobacillus fermentum strain suo cells can normally grow in human intestines. By adopting the lactobacillus fermentum strain suo, the areas and degrees of the gastric ulcers can be reduced; motilin (MOT) and P substances (SP) are reduced by different degrees; somatostatin (SS) and vasoactive intestinal peptides (VIP) are increased, and tumor necrosis factors-alpha (TNF-alpha), interleukin-6 (IL-6), interleukin-12 (IL-12) and interferon-gamma (IFN-gamma) can be lowered; the lactobacillus fermentum strain suo has a good gastric ulcer inhibiting effect, and the effect similar to omeprazole is achieved.
Owner:江苏新申奥生物科技有限公司
Interleukin-12p40 variants with improved stability
ActiveUS7872107B2Improve stabilityResistant to digestionPeptide/protein ingredientsImmunoglobulinsWild typeBiochemistry
Modified interleukin-12 (IL-12) p40 polypeptides are disclosed. The modified polypeptides have alterations in the IL-12p40 subunit to eliminate the protease site between positions Lys260 and Arg261. The modified IL-12p40 polypeptides according to the invention have improved stability compared to wild-type mature human IL-12p40 polypeptides.
Owner:MERCK PATENT GMBH
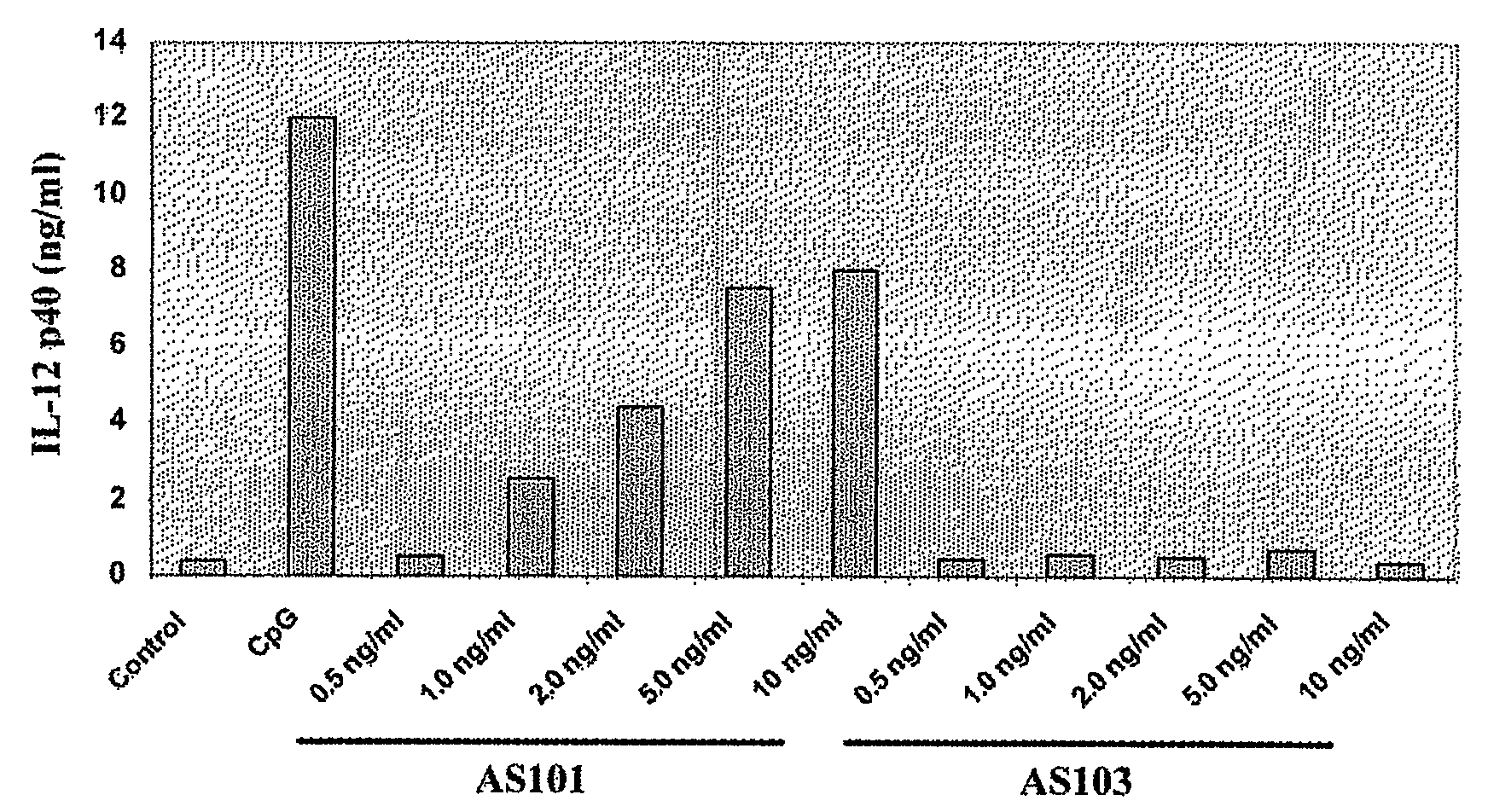
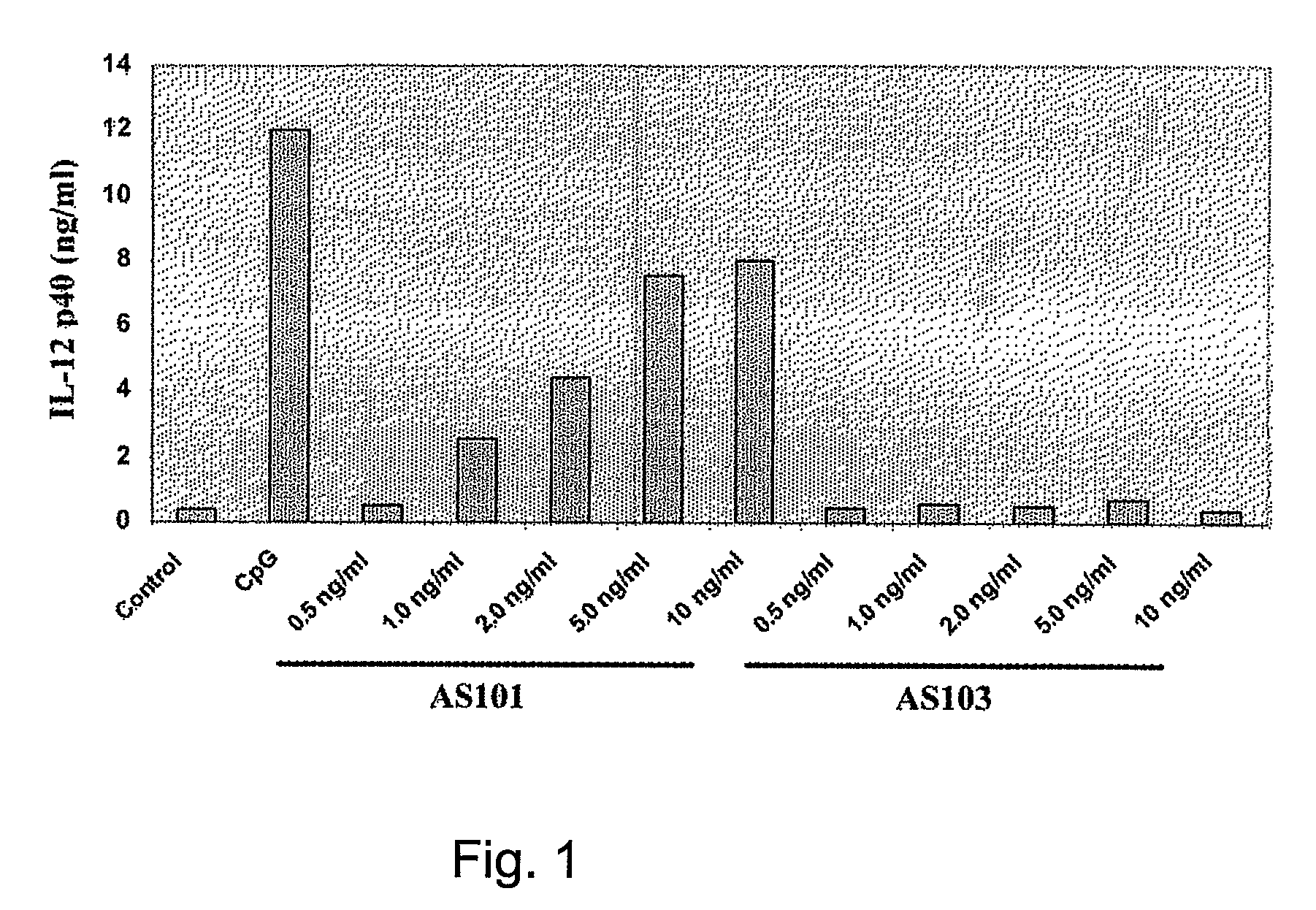
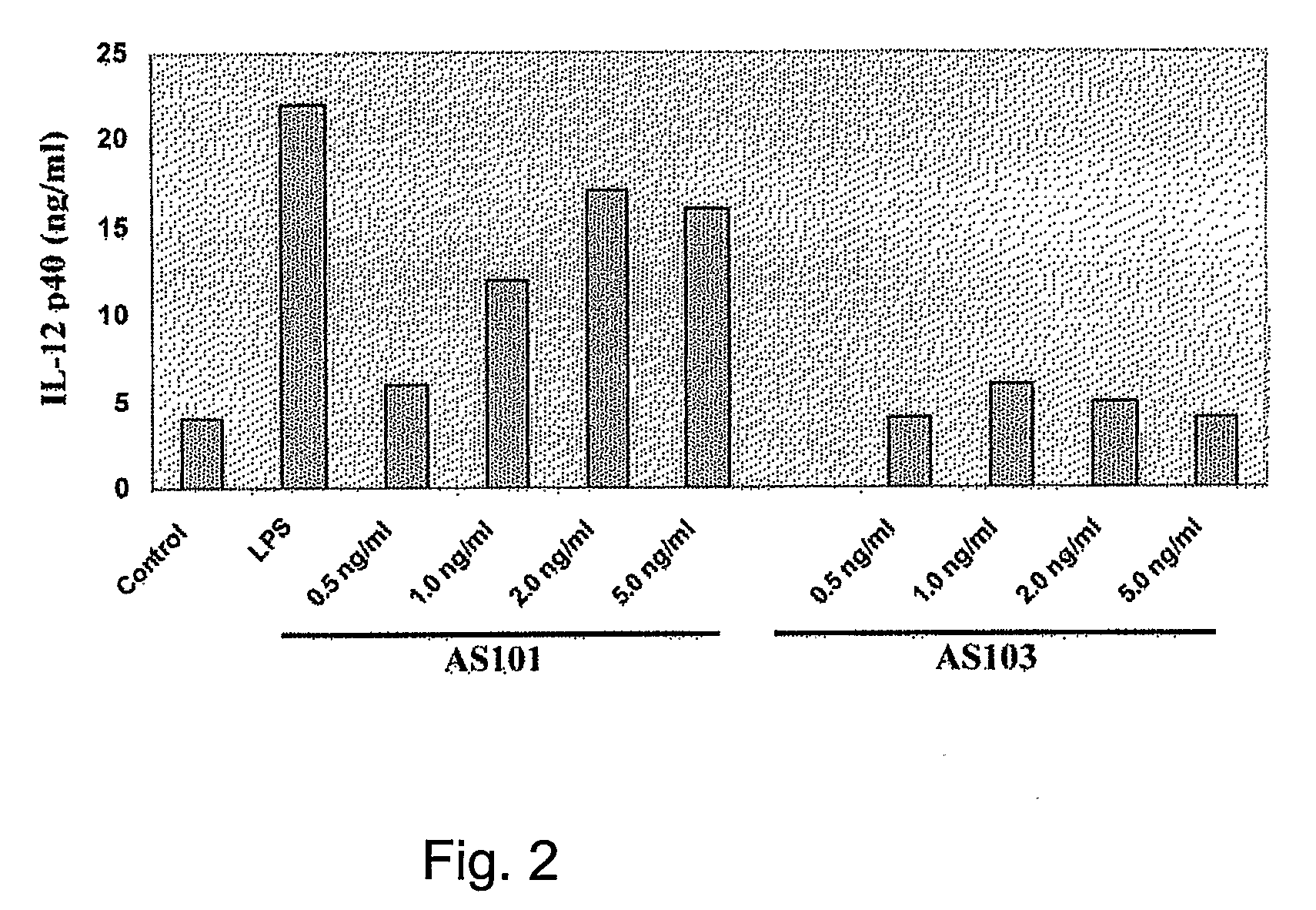
Use of Tellurium Compounds as Adjuvants
InactiveUS20080260770A1Enhance immune responseEffective adjuvanting amountOrganic non-active ingredientsCarrier-bound antigen/hapten ingredientsWhite blood cellTellurium compounds
Methods for enhancing the immune response of a subject to an immunoeffector, and methods of enhancing interleukin-12 production, which are effected by administering an amount of the immunoeffector and an effective adjuvanting amount of a tellurium-containing compound are provided. The enhanced immune response may be a cell-mediated or a humoral immune response. A pharmaceutical composition, which comprises the tellurium-containing compound, the immunoeffector and a pharmaceutically acceptable carrier is also provided. Use of a tellurium-containing compound as an adjuvant for immunization is also provided.
Owner:BIOMAS
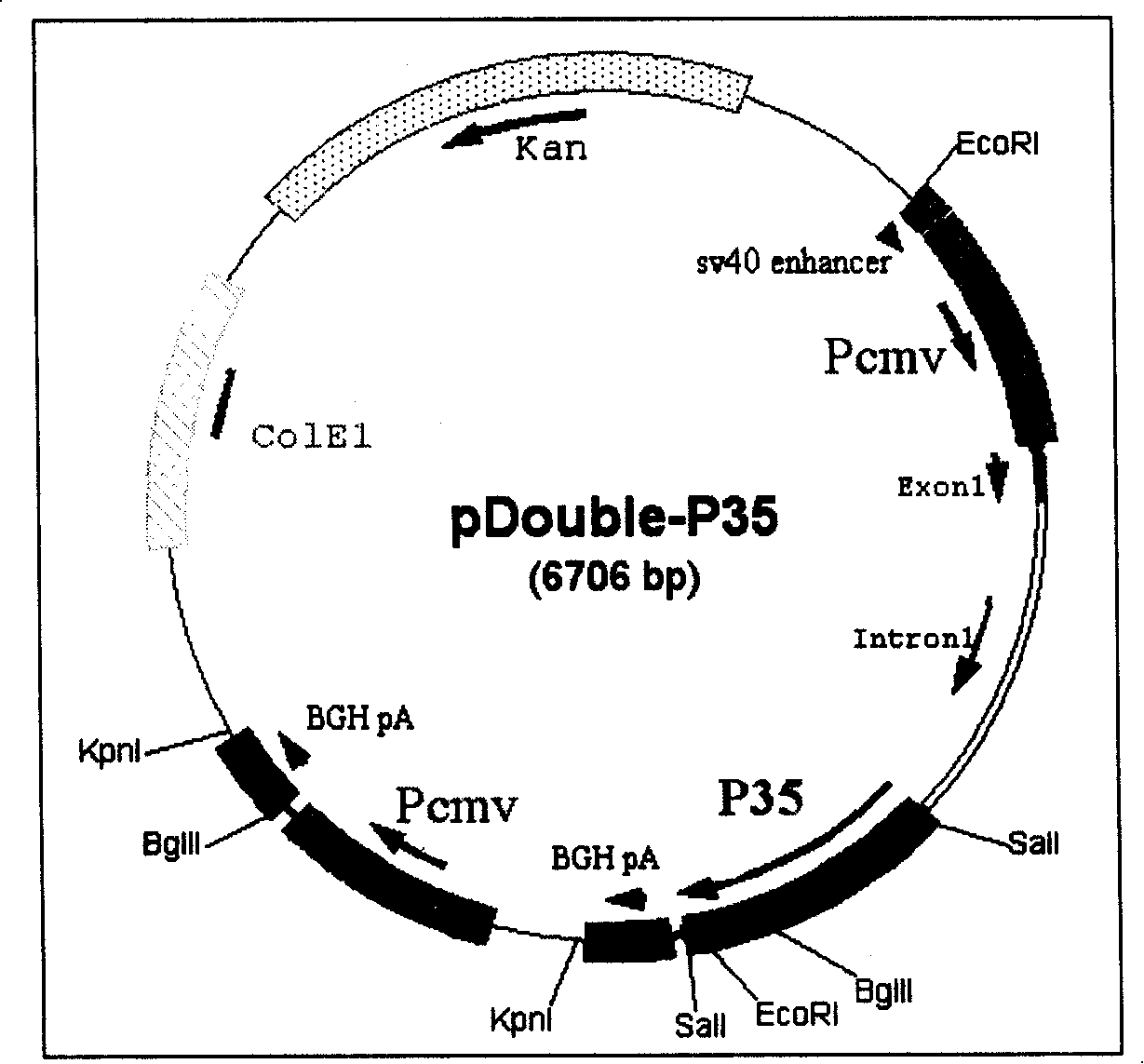
Construction and application of recombinant human interleukin 12 eukaryotic expression vector
InactiveCN101638657AFully extendedFully foldedGenetic material ingredientsAntibody medical ingredientsLaboratory mouseClinical trial
The invention provides a construction and an application of recombinant human interleukin 12 eukaryotic expression vector and relates to the development of recombinant human interleukin 12 (rhIL-12) eukaryotic expression vector, the screening of stable expression cell lines and the adjuvant function of nucleic acid vaccine gene and the therapeutical effect of tumor gene. Ten hydrophobic flexible amino acid joints are used to connect P40 and P35 to obtain fusion gene to construct rhIL-12 eukaryotic expression plasmid (pCDNA6-IL-12), then the expression plasmid is transferred to CHO cell and finally stable expression cell lines are screened. The expression product has good biological activity. Nucleic acid vaccine and pCDNA6-IL-12 are used for coimmune so as to significantly increase the immune effect. Strong antitumor effect can be exerted when the expression vector of the invention is directly injected inside the tumor of laboratory mouse tumor model or is first used to perform gene modification to tumor cells and then implanted in tumor. The expression vector of the invention can be used in the search of clinical trial stage after further perfecting.
Owner:张文卿
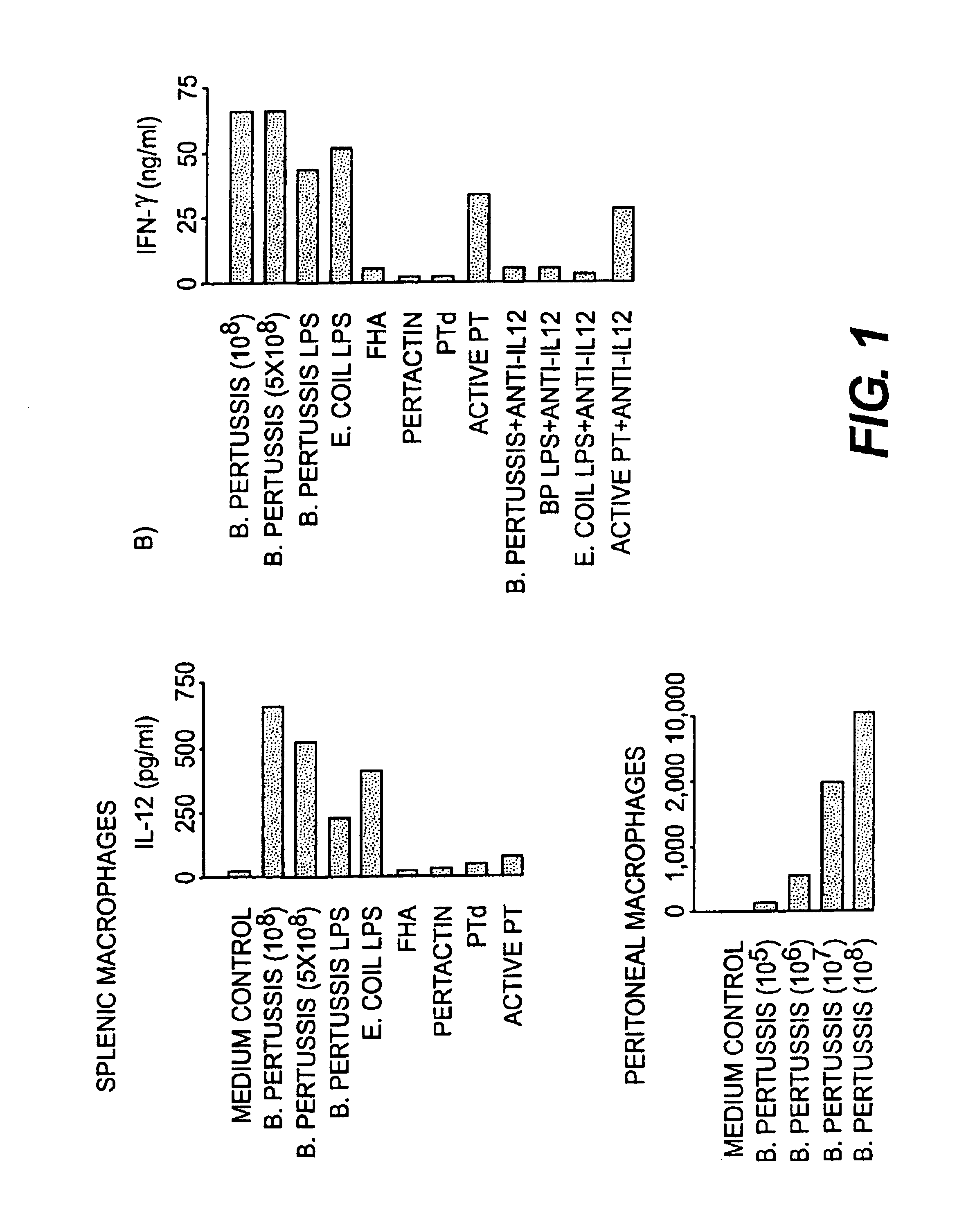
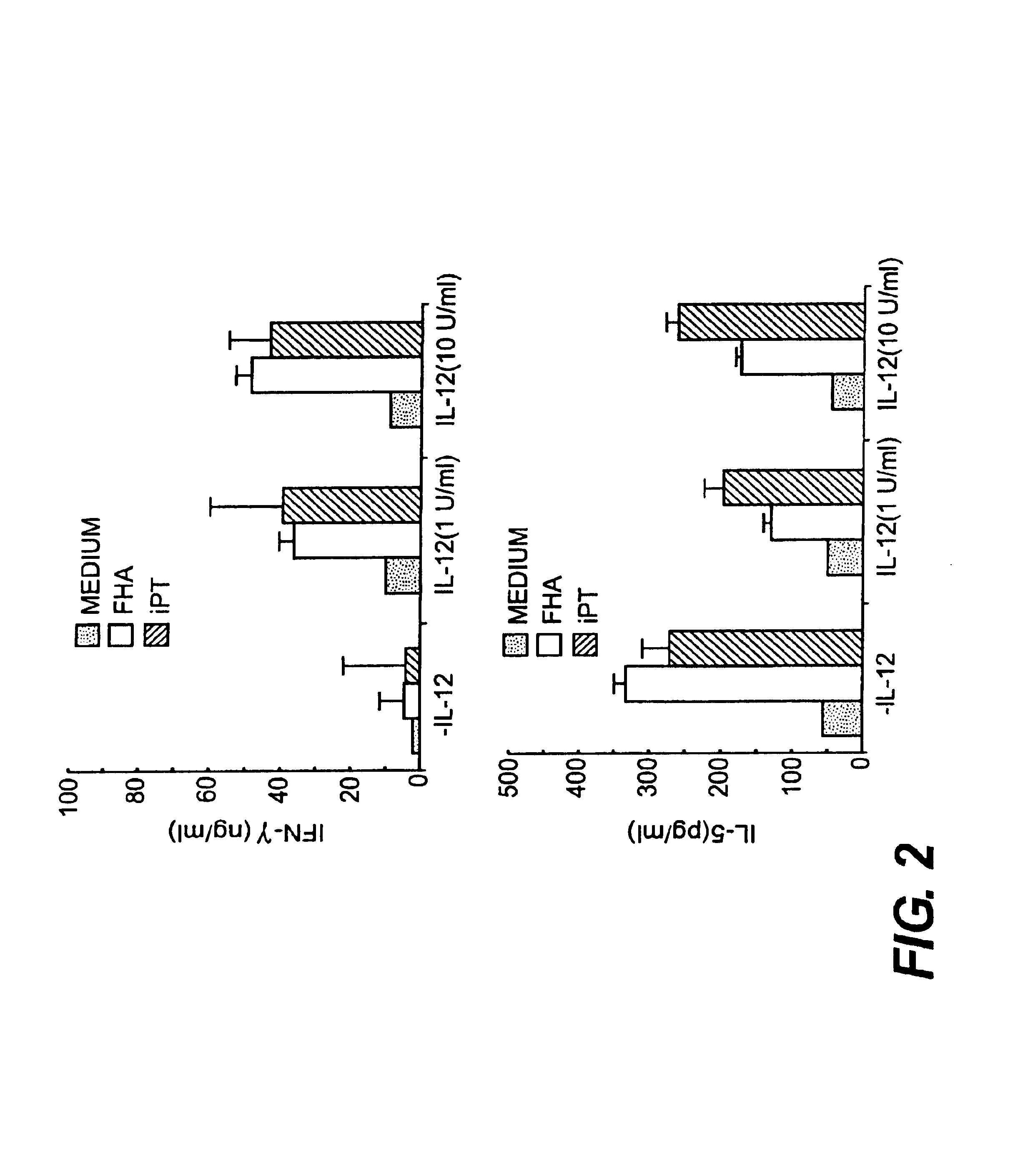
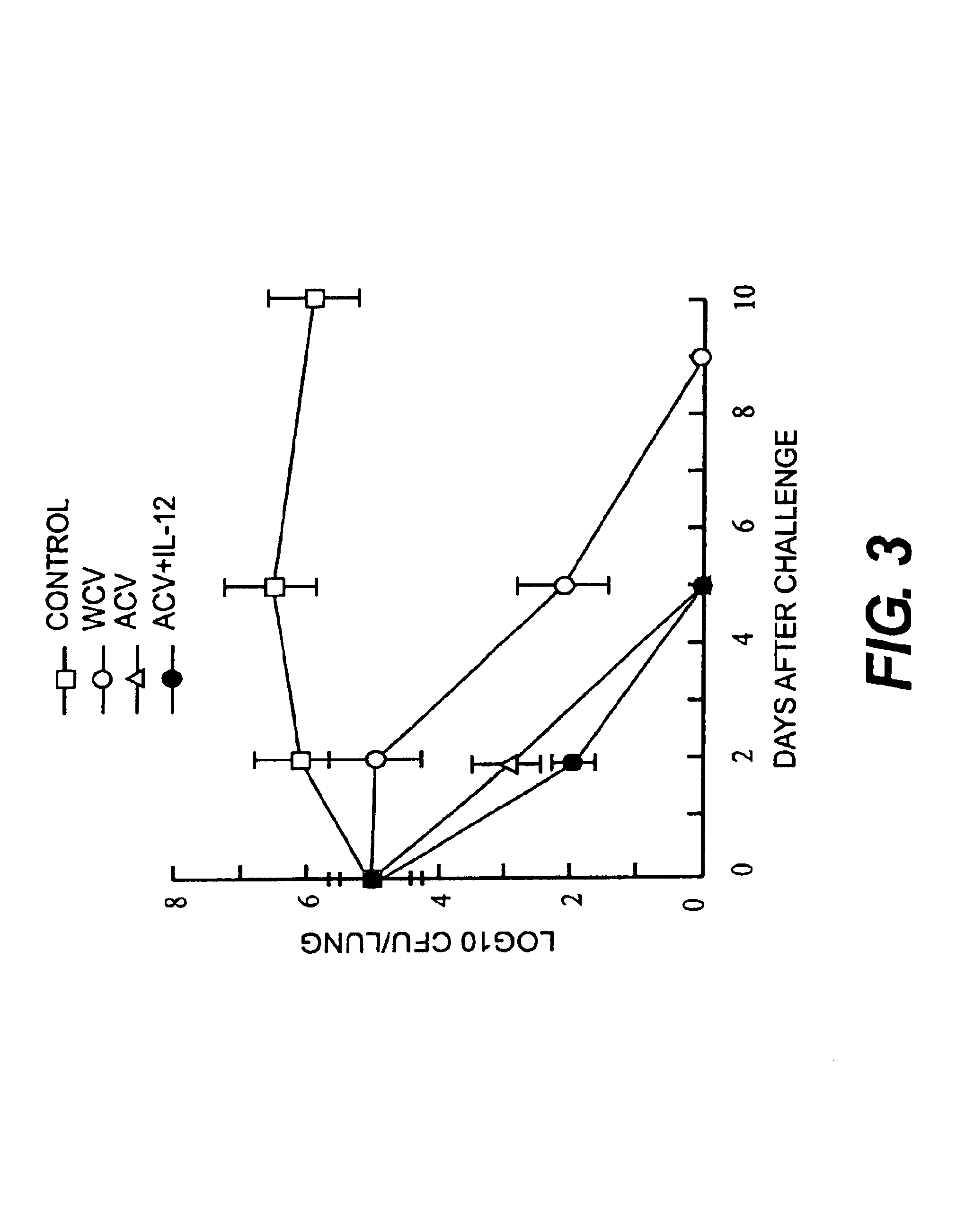
IL-12 as an adjuvant for Bordetella pertussis vaccines
This invention provides a composition of at least one Bordetella antigen and an effective adjuvant amount of interleukin-12 (IL-12), and uses thereof as a vaccine against Bordetella infection. Methods for using IL-12 as an adjuvant in combination with vaccines against Bordetella are also provided.
Owner:NATIONAL UNIVERSITY OF IRELAND
Herpes simplex virus expressing foreign genes and method for treating cancers therewith
An anti-cancer pharmaceutical composition includes a herpes simplex virus (HSV) vector into which a nucleic acid sequence encoding for an anti-cancer agent selected from interleukin-12, GM-CSF, and CD has been inserted. A method of treatment of a patient suffering from cancer includes administering to the patient the anti-tumor pharmaceutical composition including a HSV vector having a nucleic acid sequence encoding for an anti-cancer agent selected from interleukin-12, GM-CSF, and CD inserted therein.
Owner:UAB RES FOUND
Human interleukin-12 recombinant insect virus strain and its prepn
InactiveCN1357622AEfficient formationViruses/bacteriophagesFermentationWhite blood cellInfective disorder
The present invention discloses a human interleukin-12 recombinant insect virus strain, the recombinant Autographa california multiple nuclear polyhedrosis virus AcNPV-hIL12 virus strain, CCTCC No.2 V200108 and its preparation. The recombinant virus strain is inserted into human interleukin-12 expressing box, and the expressing box includes double promoter sequence, P35 subunit DNA encoding sequence of human interleukin-12 and P40 subunit DNA encoding sequence. P35 subunit gene locates in the downstream of Polyhederin and P40 subunit gene locates in the downstream of P10 promoter. The present invention can express human interleukin-12, and the cell factor is safe and reliable to human body and suitable for treating tumor bacteria, virus and various infections diseases.
Owner:WUHAN UNIV
Therapeutic hepatitis b vaccine and preparation method and use thereof
ActiveCN101361969AOvercome the defect of low antigenicityStrong specificityPeptide/protein ingredientsAntiviralsAdjuvantWhite blood cell
The invention discloses a curative hepatitis B vaccine and a preparation method and applications thereof. The key of the technical proposal is that: a plurality of CTL and HTL polypeptide epitopes originating from HBV envelope protein, core antigen and polymerase are selected to form a multiple antigenic peptide (MAP) system and recombinated human interleukin 12 (rhIL-12) is adopted as an adjuvant, so as to improve the immunogenicity and the response level, thus providing a biological preparation for curing chronic hepatitis B clinically.
Owner:广州市茵良强生物科技有限公司
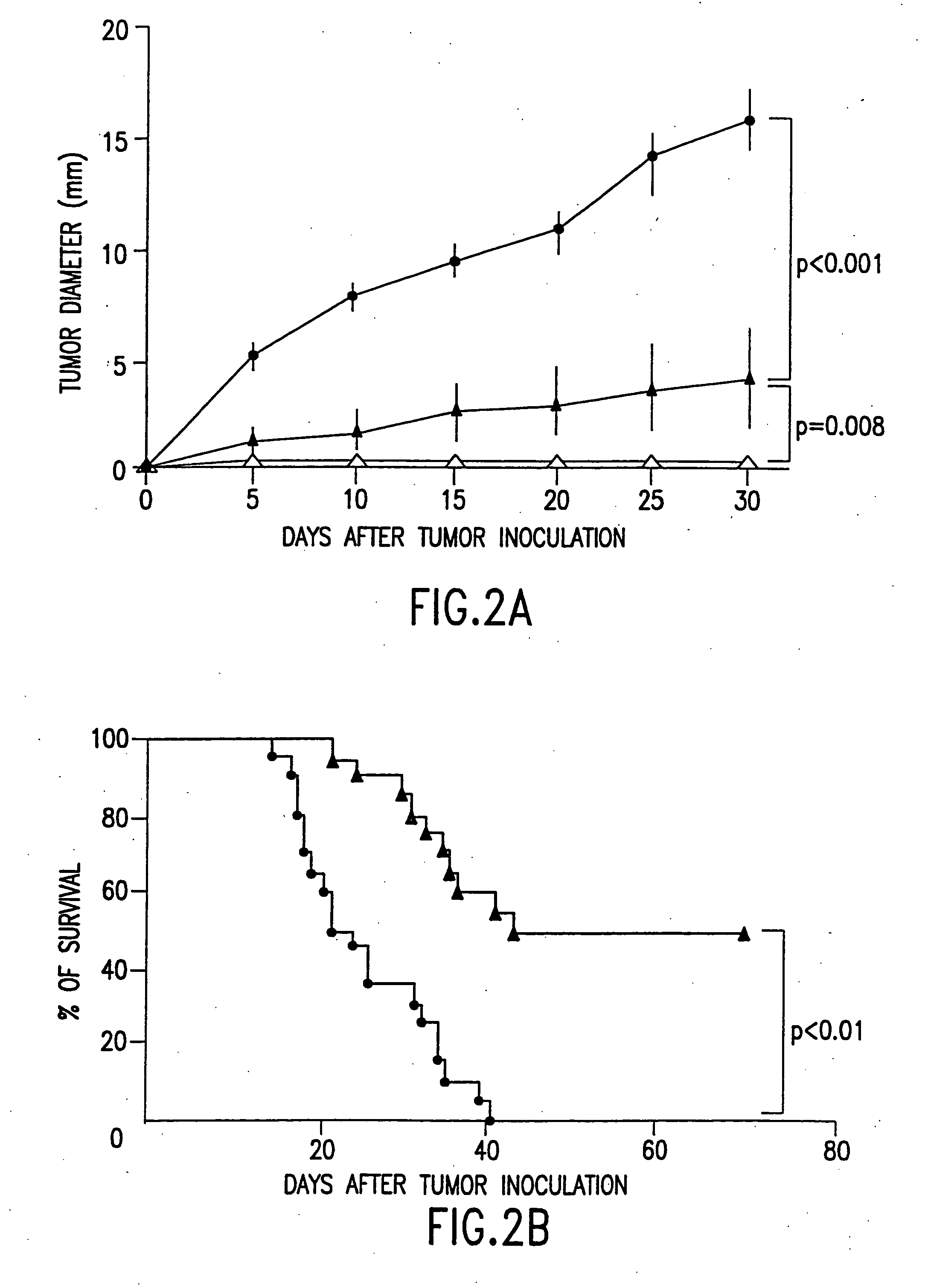
Combined immunotherapy of fusion cells and interleukin-12 for treatment of cancer
InactiveUS20050180951A1Increase stimulationBiocidePeptide/protein ingredientsWhite blood cellCancer research
The present invention relates to methods and treatment protocols for the immunotherapy of cancer by administering a therapeutically effective dose of fusion cells formed by fusion of autologous dendritic cells and autologous non-dendritic cells in combination with interleukin-12.
Owner:OHNO
Medium composition for culturing self-activated lymphocytes and method for culturing self-activated lymphocytes using same
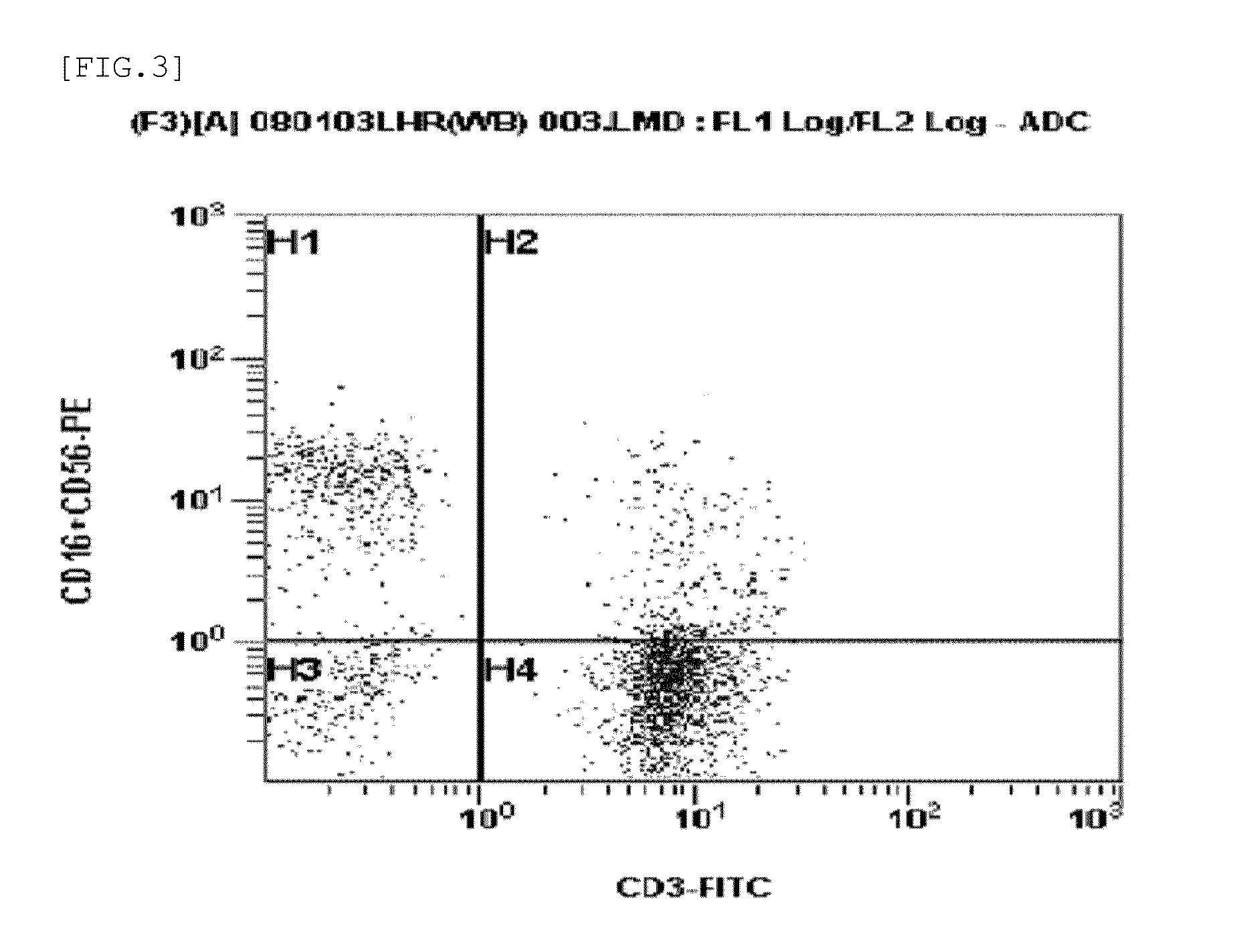
InactiveUS20130157364A1Raise the ratioLess side effectsCancer antigen ingredientsImmunoglobulinsNatural Killer Cell Inhibitory ReceptorsCD16
Disclosed is a medium composition for culturing self-activated lymphocytes, which contains anti-CD3 antibody and anti-CD16 antibody in addition to interleukin 2 (IL-2), interleukin 12 (IL-12) and interleukin 18 (IL-18) in a medium, and thus can efficiently proliferate and activate NK cells, T cells and NKT cells and, at the same time, can significantly increase the ratio of NK cells in lymphocytes so as to provide immunocytes having excellent effects on the treatment of various kinds of malignant tumors, and a method for culturing self-activated lymphocytes using the medium composition.
Owner:CELLTECH LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com