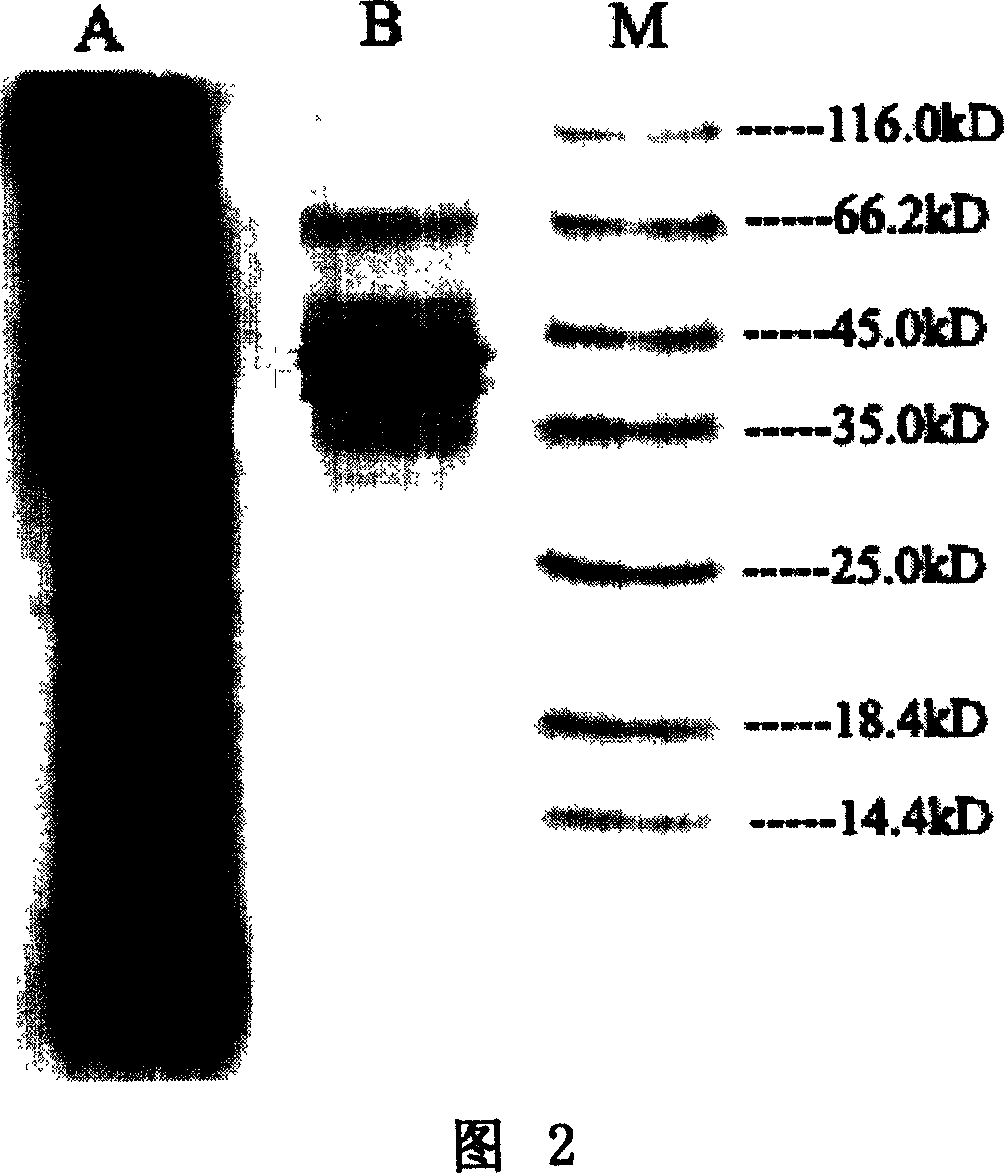Method of purifying and combining human interleukins 12
A technology for interleukin and cell culture, applied in the field of purification of recombinant human interleukin 12, can solve the problems of difficult industrial application, complicated process, large pore size, etc., achieve high recovery rate and purity, low cost of separation and purification, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Purification of embodiment 1 rhIL-12
[0026] 1. Sample filtration
[0027] The cell culture supernatant was filtered with a 0.45 μm microporous membrane to remove impurities, and the filtrate was diluted 4-fold with 10 mM PB, pH=6.0 for later use.
[0028] 2. Purification of rhIL-12 by cationic column chromatography
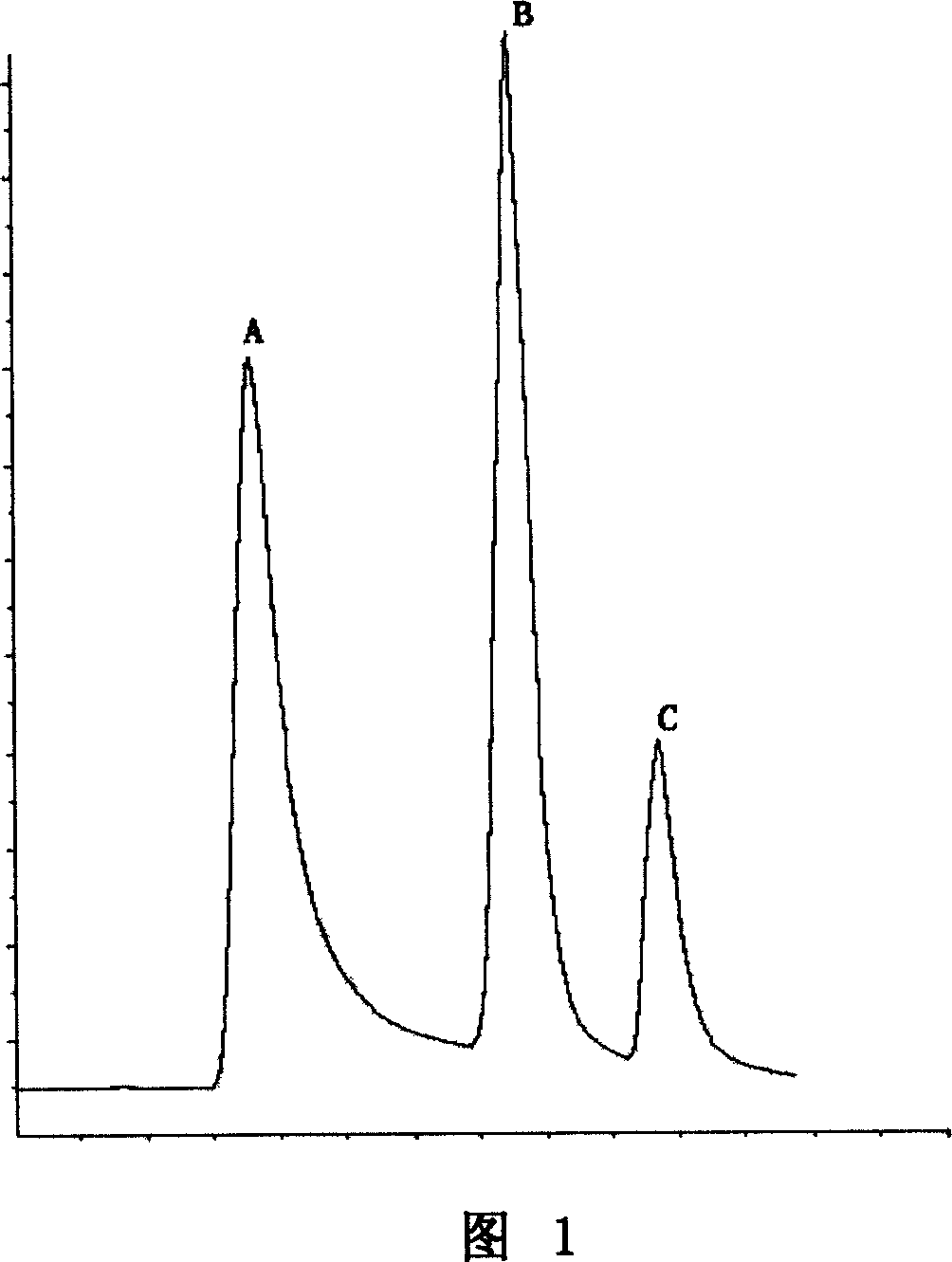
[0029] Select SP Sepharose High Performance cation exchange column, and equilibrate with 2 to 3 column volumes of equilibration buffer (20mM PB, pH6.0). After the column is fully equilibrated, load the dilution obtained in step 1. The equilibration buffer washes the column until A 280 Reach the baseline or stabilize to the vicinity of the baseline; wash the chromatographic column with eluent I (20mM PB, 0.30M NaCl, pH6.0), collect the elution peak I, and fully wash the chromatographic column until the baseline is stable; continue to use The eluent II (20mM PB, 0.60M NaCl, pH6.0) was used to wash the column, and the elution peak II was collected, and th...
Embodiment 2
[0038] Example 2 Purification of rhIL-12
[0039] 1. Sample filtration
[0040] The cell culture supernatant was filtered with a 0.45 μm microporous membrane to remove impurities, and the filtrate was diluted 4 times with 10 mM Tris-Cl, pH 8.0 for use.
[0041] 2. Purification of rhIL-12 by anion column chromatography
[0042] Choose DEAE Sepharose Fast Flow anion exchange column, equilibrate 2 to 3 column volumes with equilibration buffer (20mMTris-Cl, pH8.0), and load the rhIL-12-containing dilution obtained in step 1 after the chromatographic column is fully equilibrated. After loading the sample, wash the column with equilibration buffer until A 280 Reach the baseline or stabilize to the vicinity of the baseline; wash the chromatographic column with eluent I (20mM Tris-Cl, 0.15M NaCl, pH8.0), collect the elution peak I, and fully wash the chromatographic column until the baseline is stable; Continue to wash the chromatographic column with eluent II (20mM Tris-Cl, 0.28M ...
Embodiment 3
[0051] Example 3 Purification of rhIL-12
[0052] 1. Sample filtration
[0053] The cell culture supernatant was filtered with a 0.45 μm microporous membrane to remove impurities, and the filtrate was diluted 4 times with 10 mM PB, pH=6.5 for use.
[0054] 2. Purification of rhIL-12 by cationic column chromatography
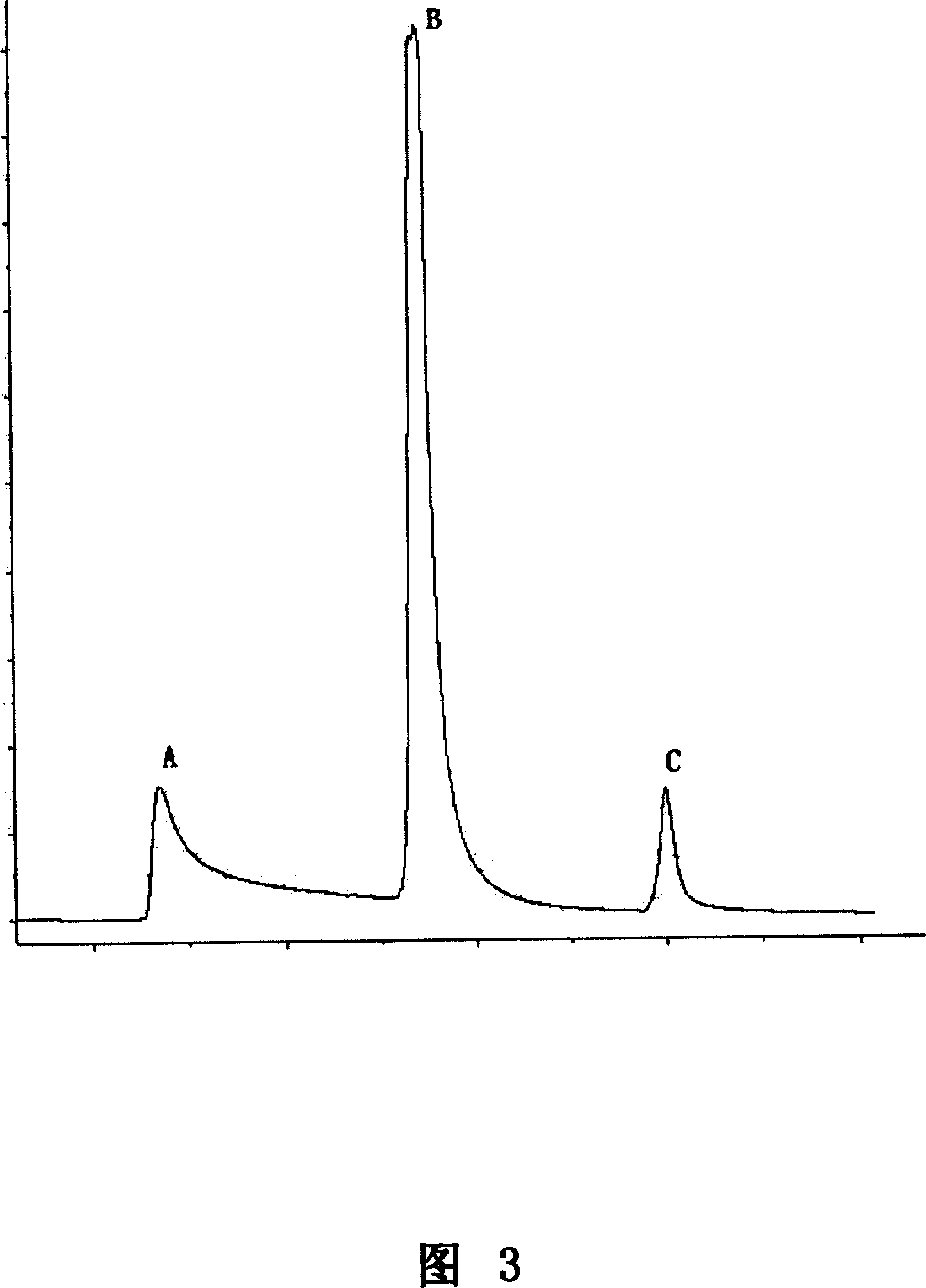
[0055] Select Capto S cation exchange column, and equilibrate 2 to 3 column volumes with equilibration buffer (20mM PB, pH6.5). The liquid washes the column until A 280 Reach the baseline or stabilize to the vicinity of the baseline; wash the chromatographic column with eluent I (20mM PB, 0.40M NaCl, pH6.5), collect the elution peak I, and fully wash the chromatographic column until the baseline is stable; continue to use Eluent II (20mM PB, 0.70MNaCl, pH6.5) washes the chromatographic column, collects the elution peak II, and fully washes the chromatographic column until the baseline is stable; , pH6.5) to wash the chromatographic column, collect the elution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com