Immunogene therapeutic drug for chronic hepatitis B and preparation method for immunogene therapeutic drug
A chronic hepatitis B, gene therapy technology, applied in gene therapy, drug combination, pharmaceutical formula, etc., can solve the problems of long treatment course, inability to suppress drug-resistant virus, and low sustained response rate, so as to enhance immune response and realize The effect of effective continuous control, increased safety and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
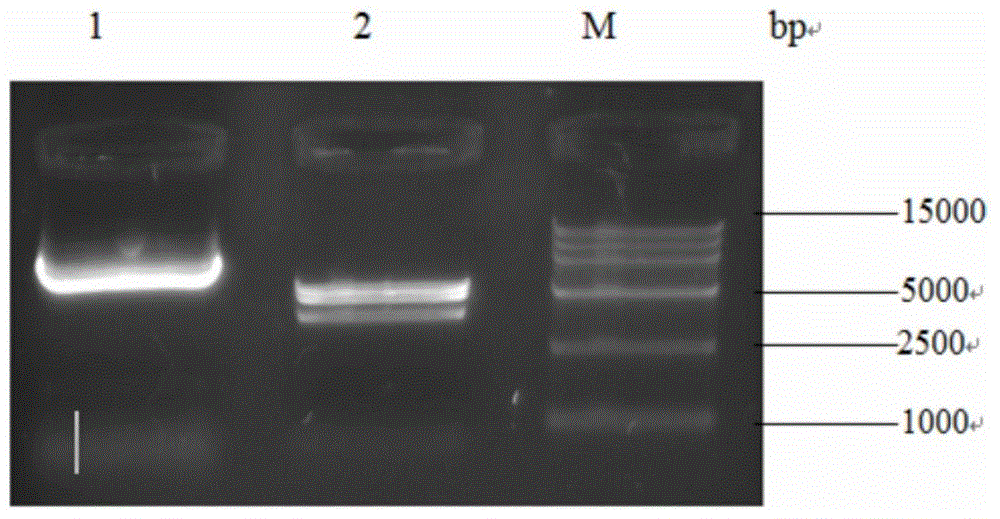
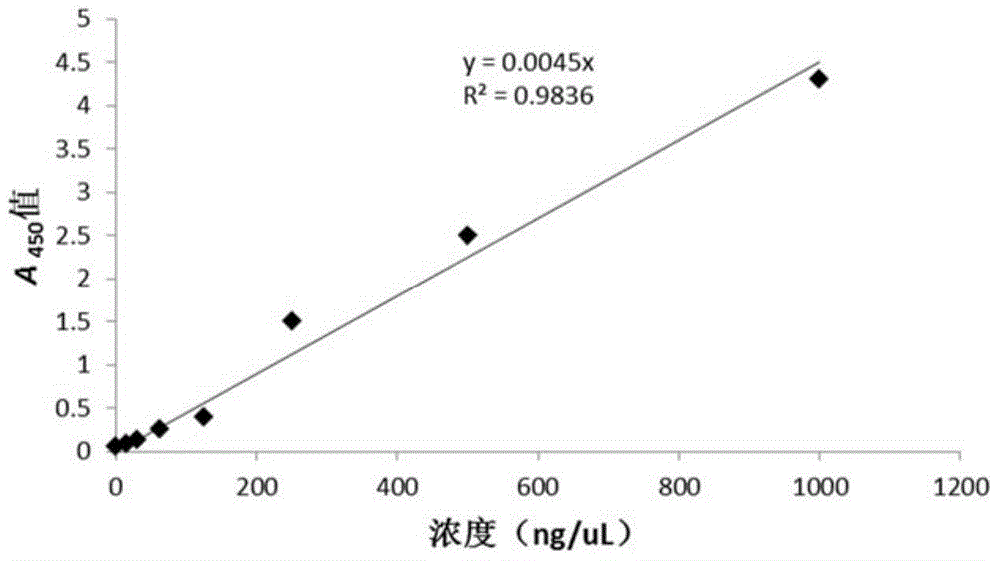
[0062] Example 1. Construction and expression of antibody targeting interferon (dsFvα) and interleukin-12 fusion expression vector pVAX-HBVE
[0063] 1. Experimental materials and instruments
[0064] 1. Bacterial strains and cell lines
[0065] E.coli.trans5α competent cells were purchased from Quanshijin Company; HEK293T cells were purchased from Beijing Union Medical College Cell Bank.
[0066] 2. Enzymes and reagents
[0067] DNA restriction endonucleases were purchased from New England Biolabs (NEB) biological company; plasmid recovery kit and gel recovery kit were purchased from Beijing Kangwei Century Company; fetal bovine serum for experiment was purchased from Hyclone Company; MEM medium was purchased from Hyclone Company; PCR enzymes and dNTPs were purchased from TaKaRa Company; human IFN-αELISA detection kits and human IL-12ELISA detection kits were purchased from Beijing Xinbosheng Co., Ltd.; kanamycin and ampicillin were purchased from Biotech Biotech Co., Ltd....
Embodiment 2
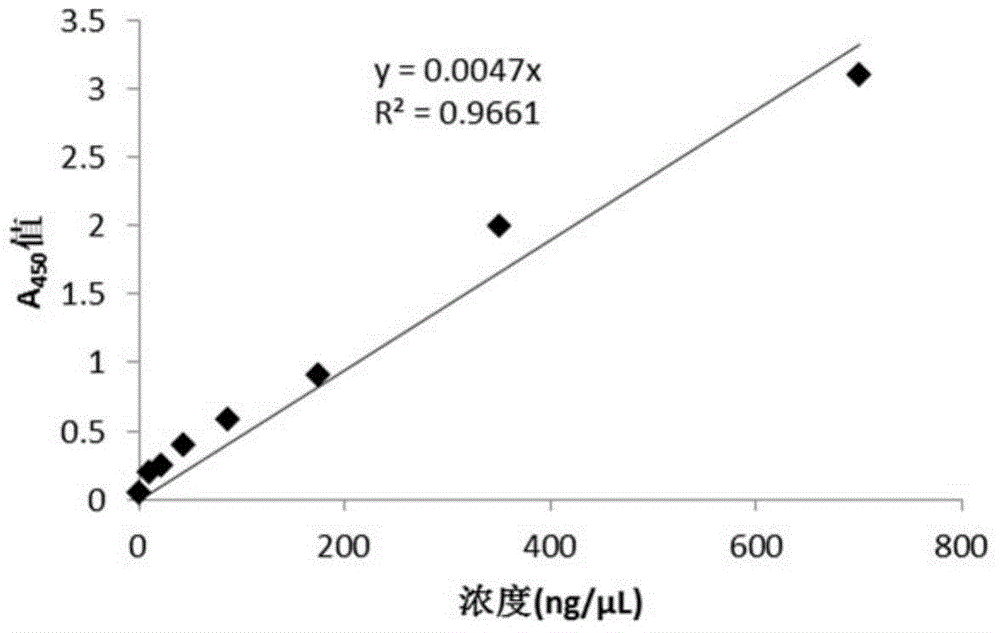
[0147] Example 2. Construction, expression and preliminary functional verification of replicable vector pSVK-HBVE
[0148] 1. Experimental materials and instruments
[0149] 1. Bacterial strains, cell lines and experimental animals
[0150] E.coli.DH5α competent cells were purchased from Beijing Quanshijin Company; HEK293T cells were purchased from Beijing Union Medical College Cell Bank. Female transgenic mice (6-8 weeks old, with 1.3 copies of HBV whole genome transferred), weighing about 20 g, were purchased from Guangzhou Air Force General Hospital.
[0151] 2. Enzymes and reagents
[0152] DNA restriction endonucleases were purchased from New England Biolabs (NEB) biological company; plasmid recovery kit and gel recovery kit were purchased from Beijing Kangwei Century Company; fetal bovine serum for experiment was purchased from Hyclone Company; MEM medium was purchased from Hyclone Company; enzymes and dNTPs used in PCR were purchased from TaKaRa Company; human IFN-αE...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com