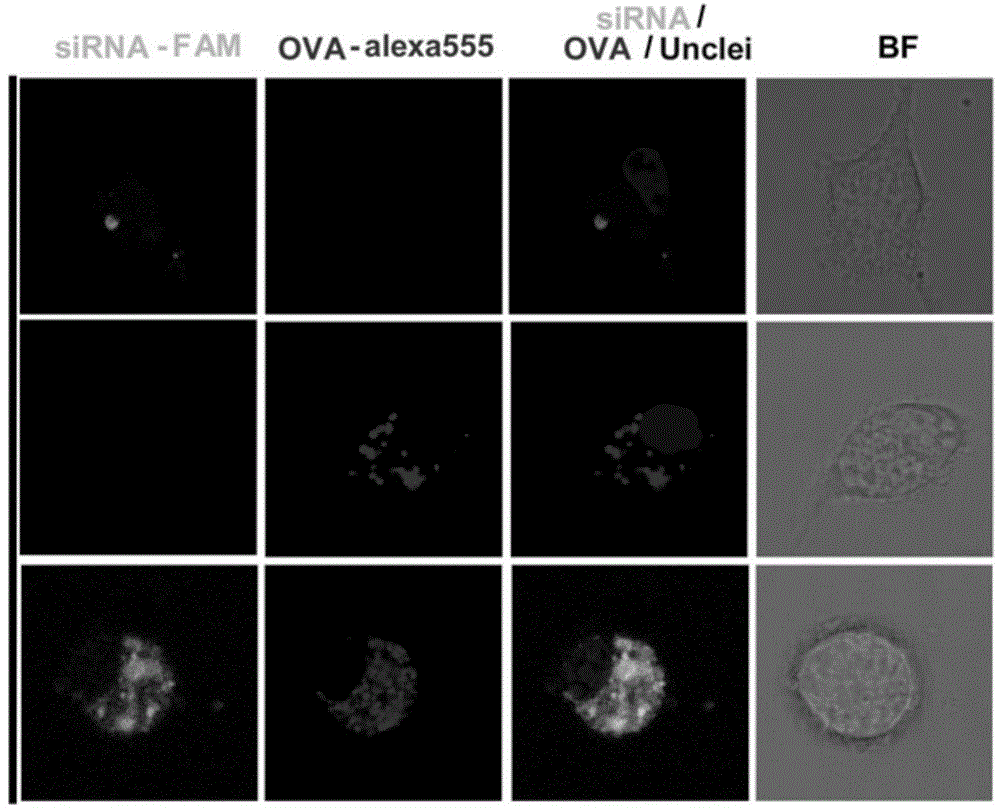
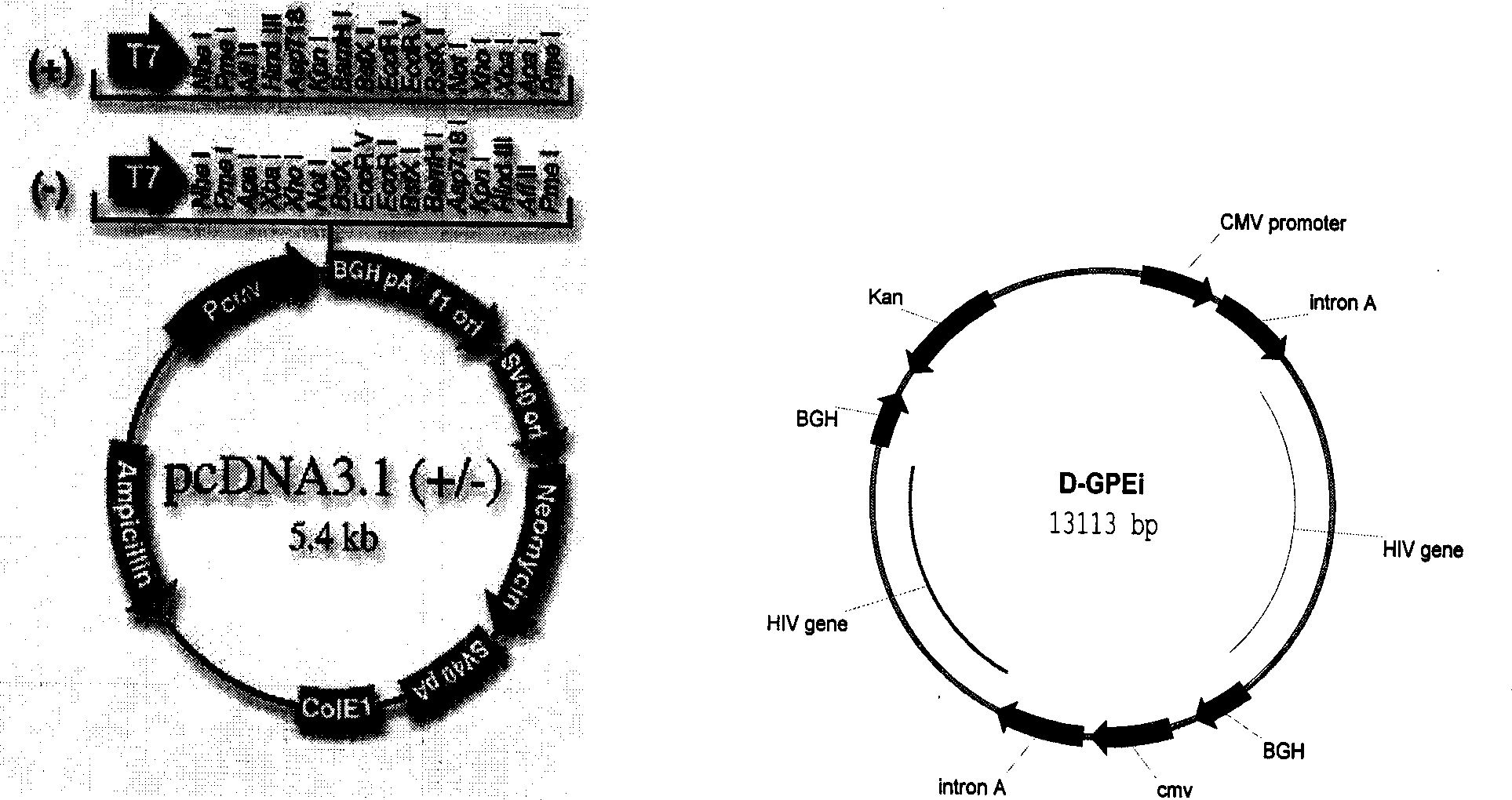
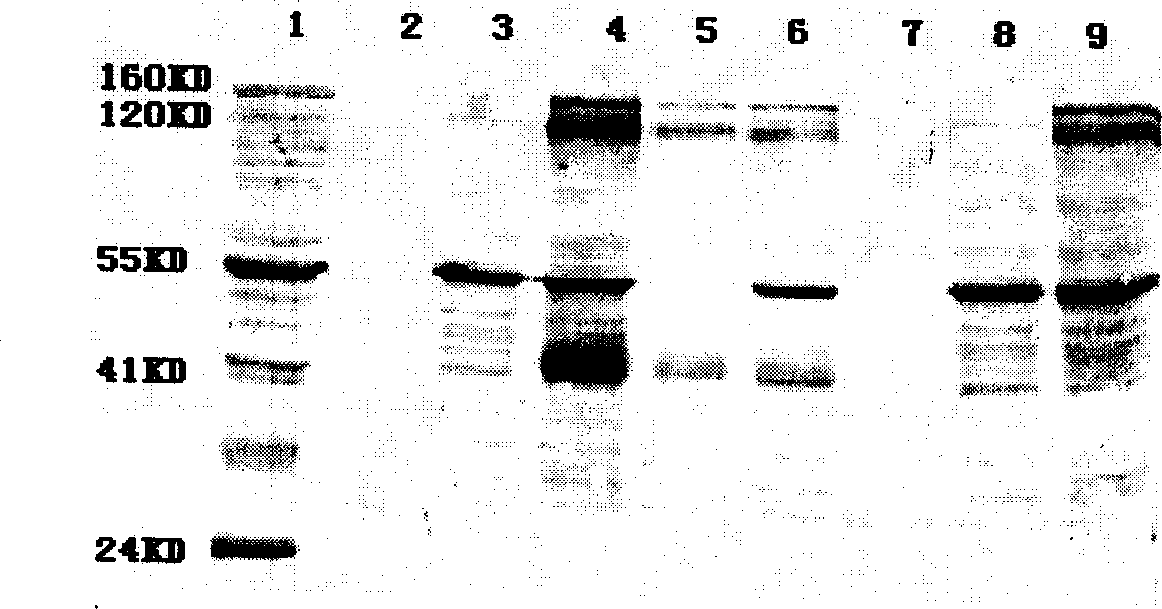
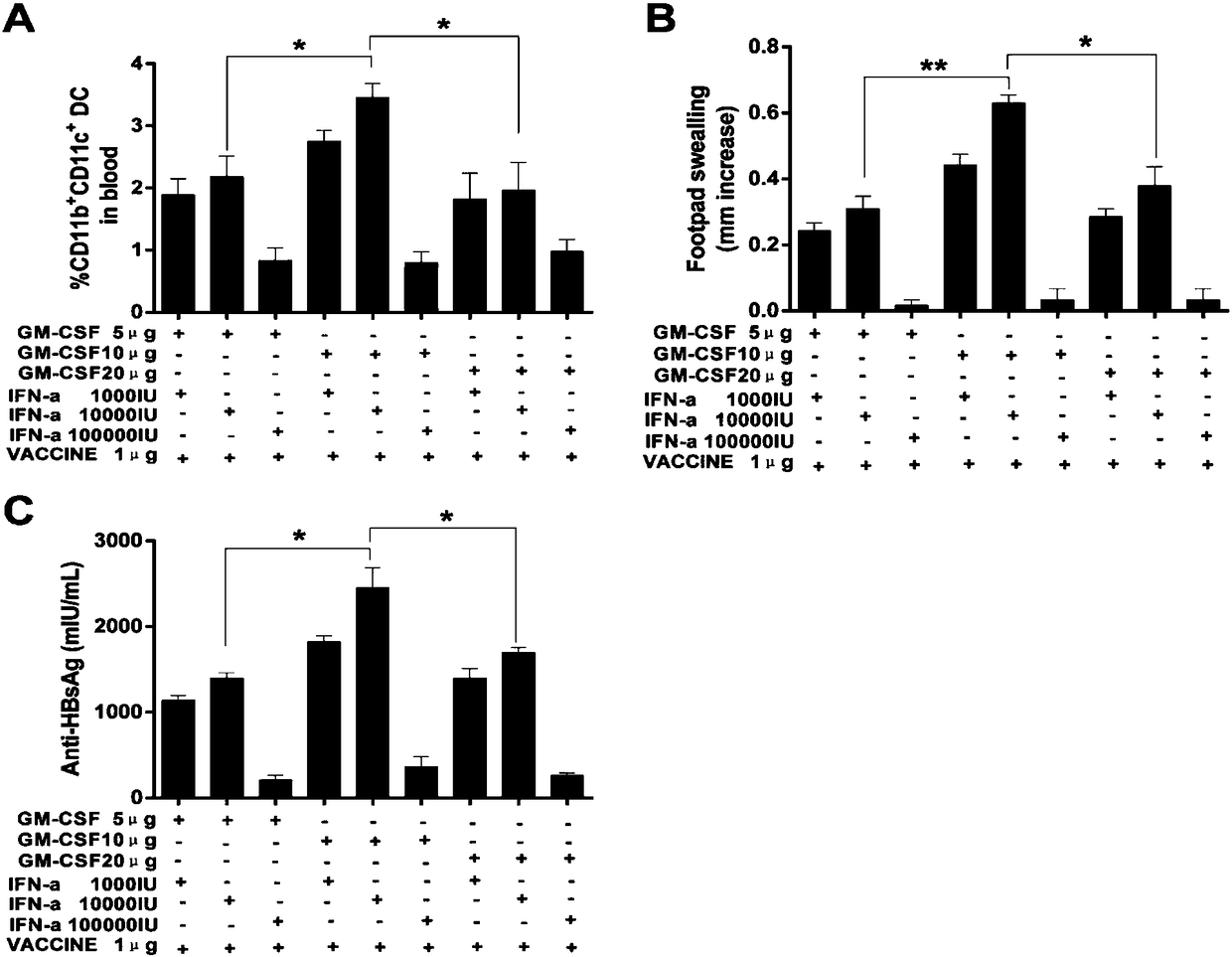
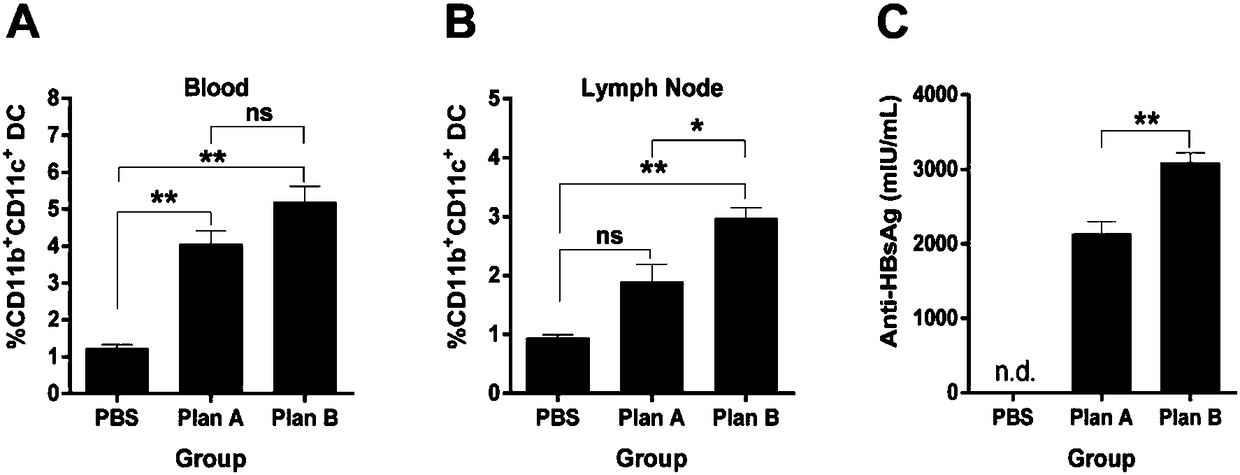
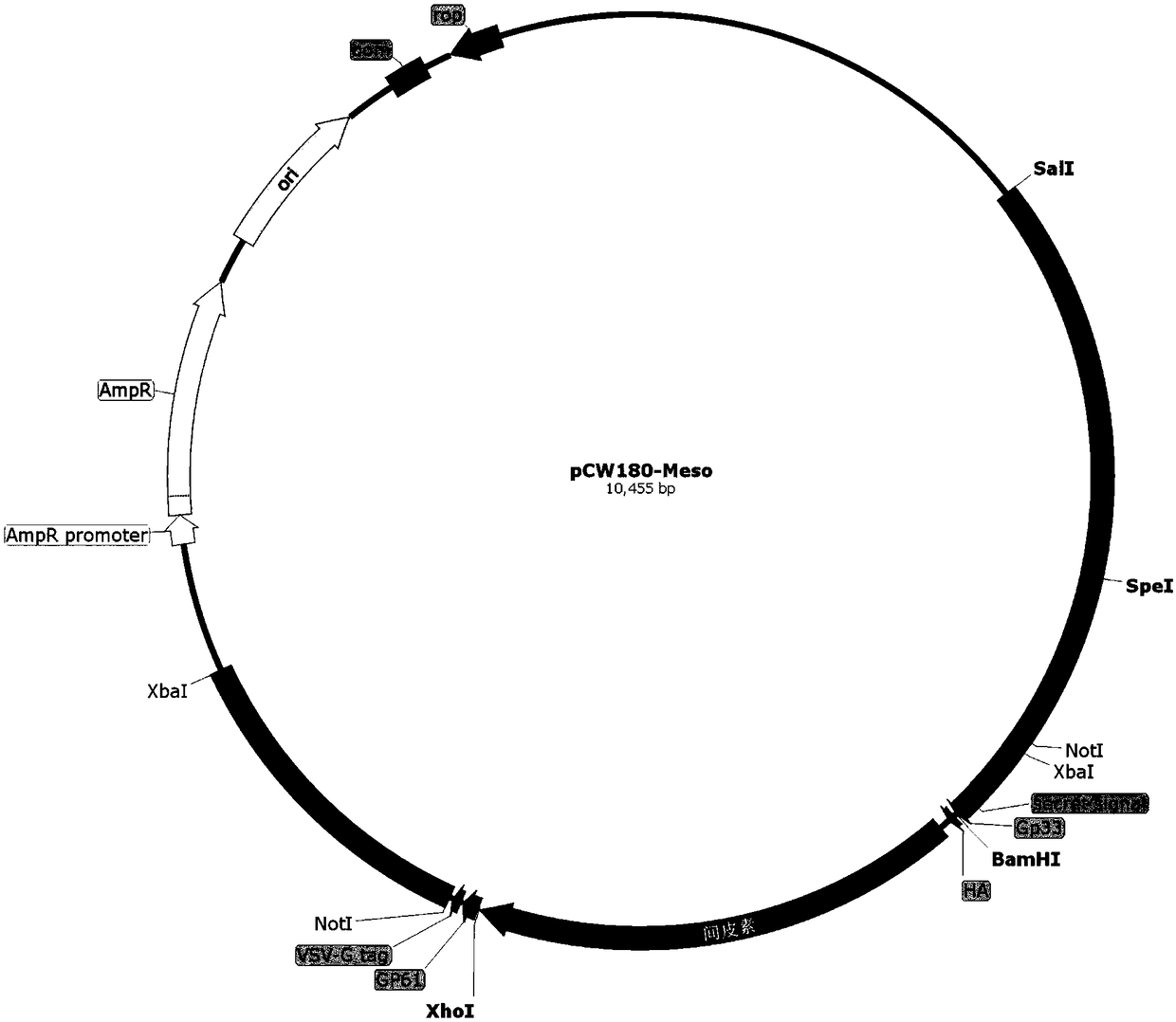
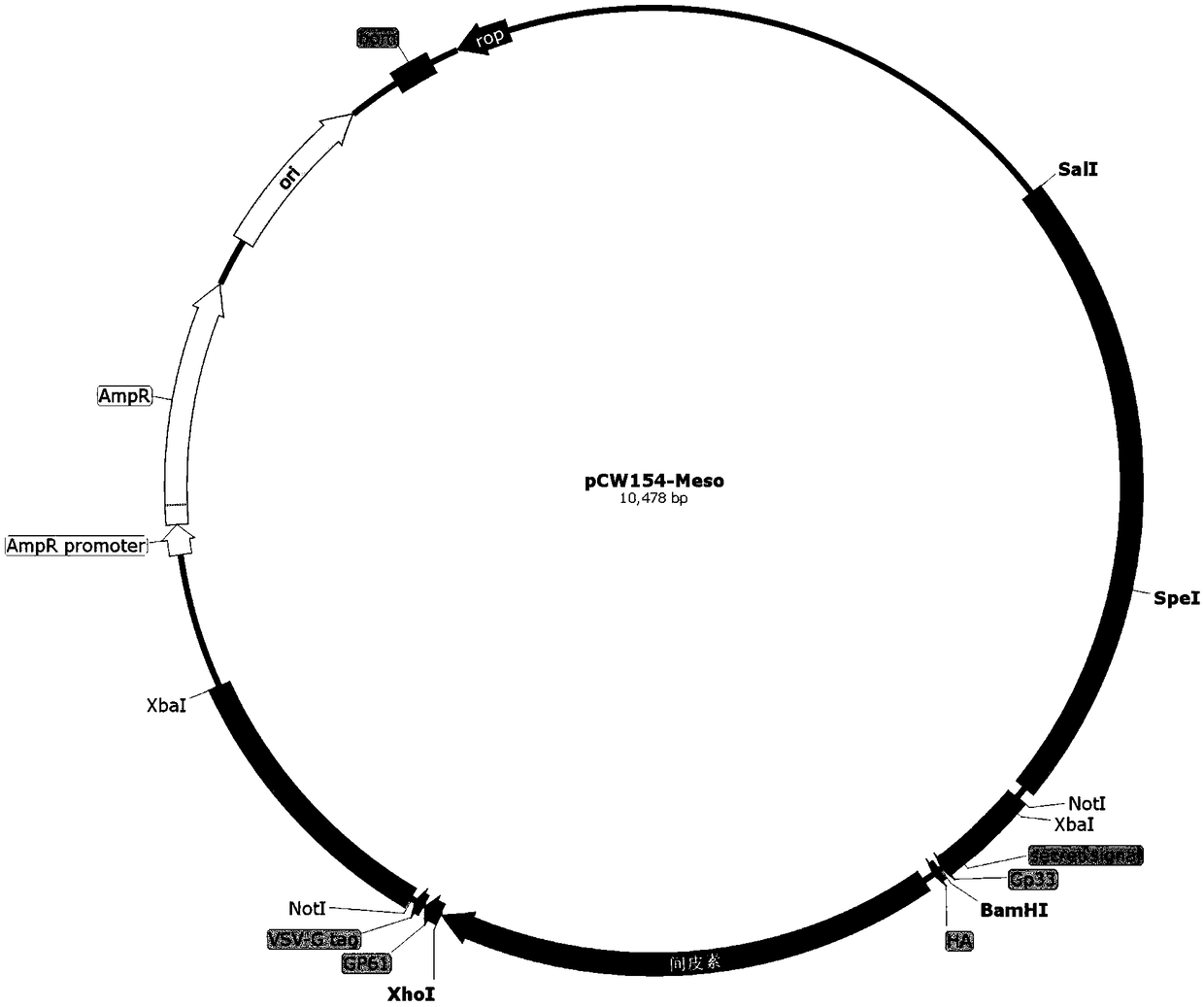
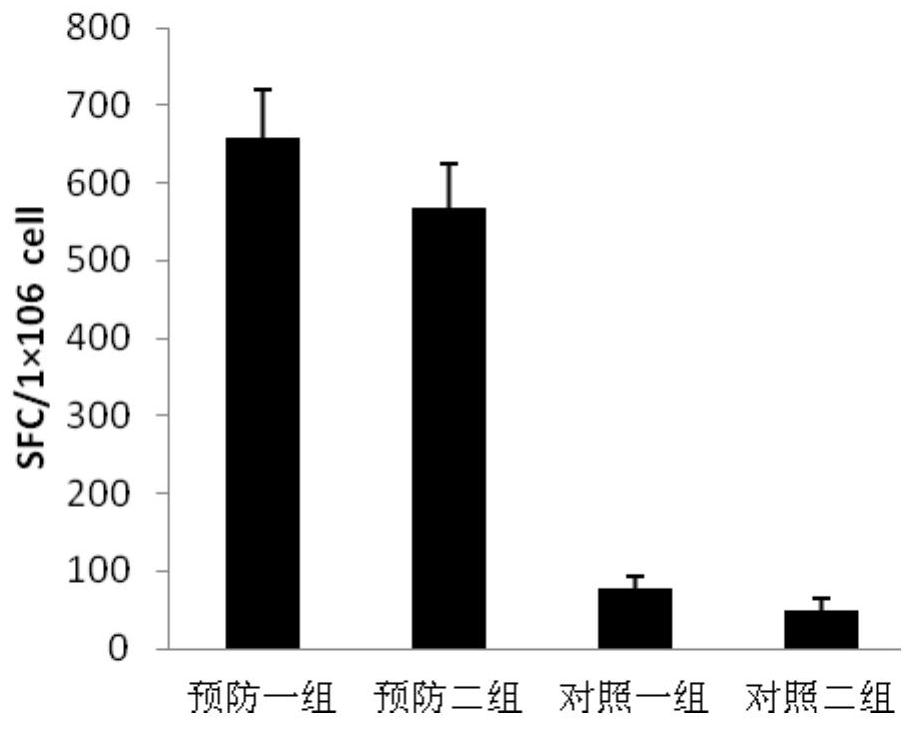
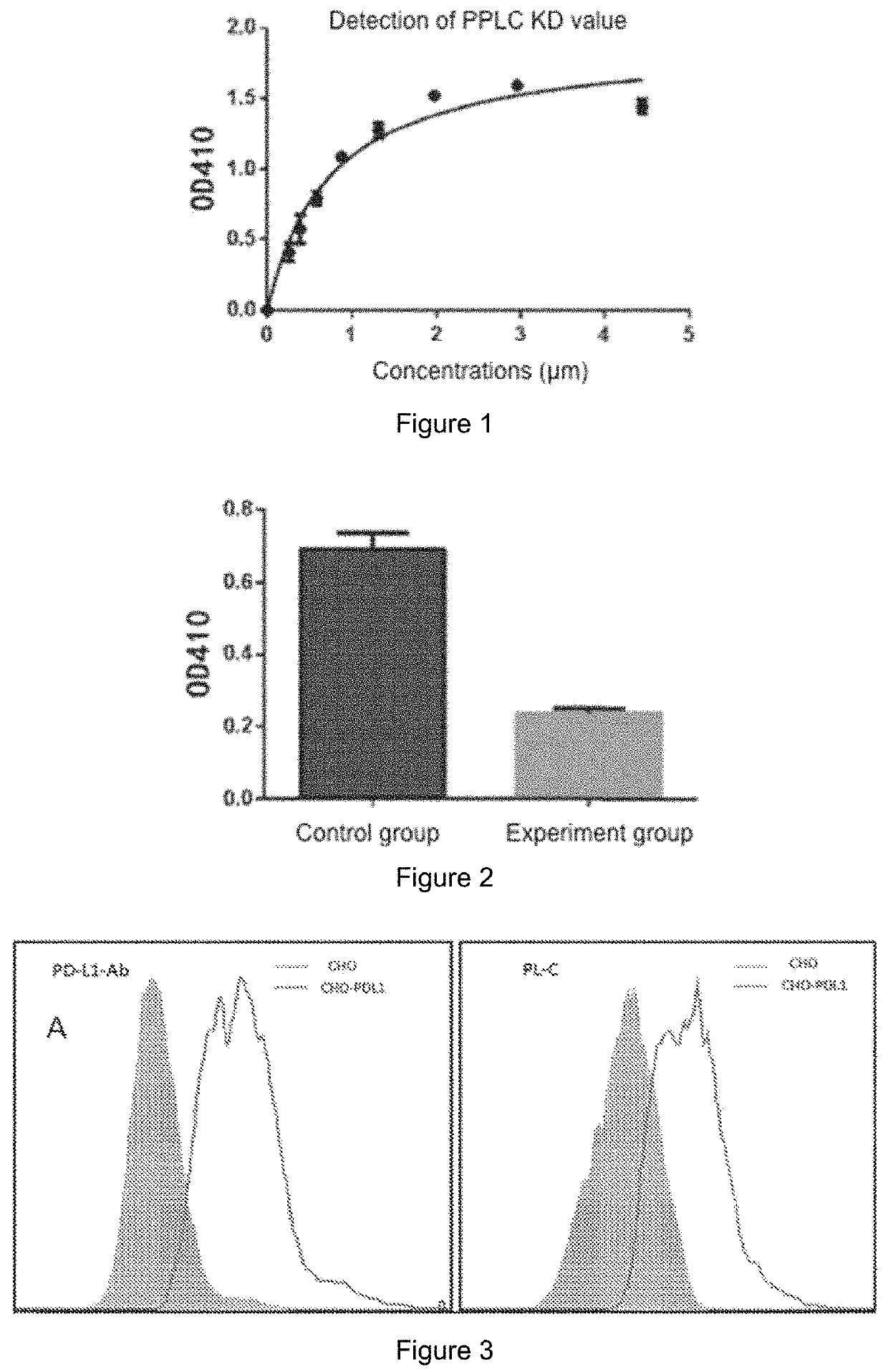
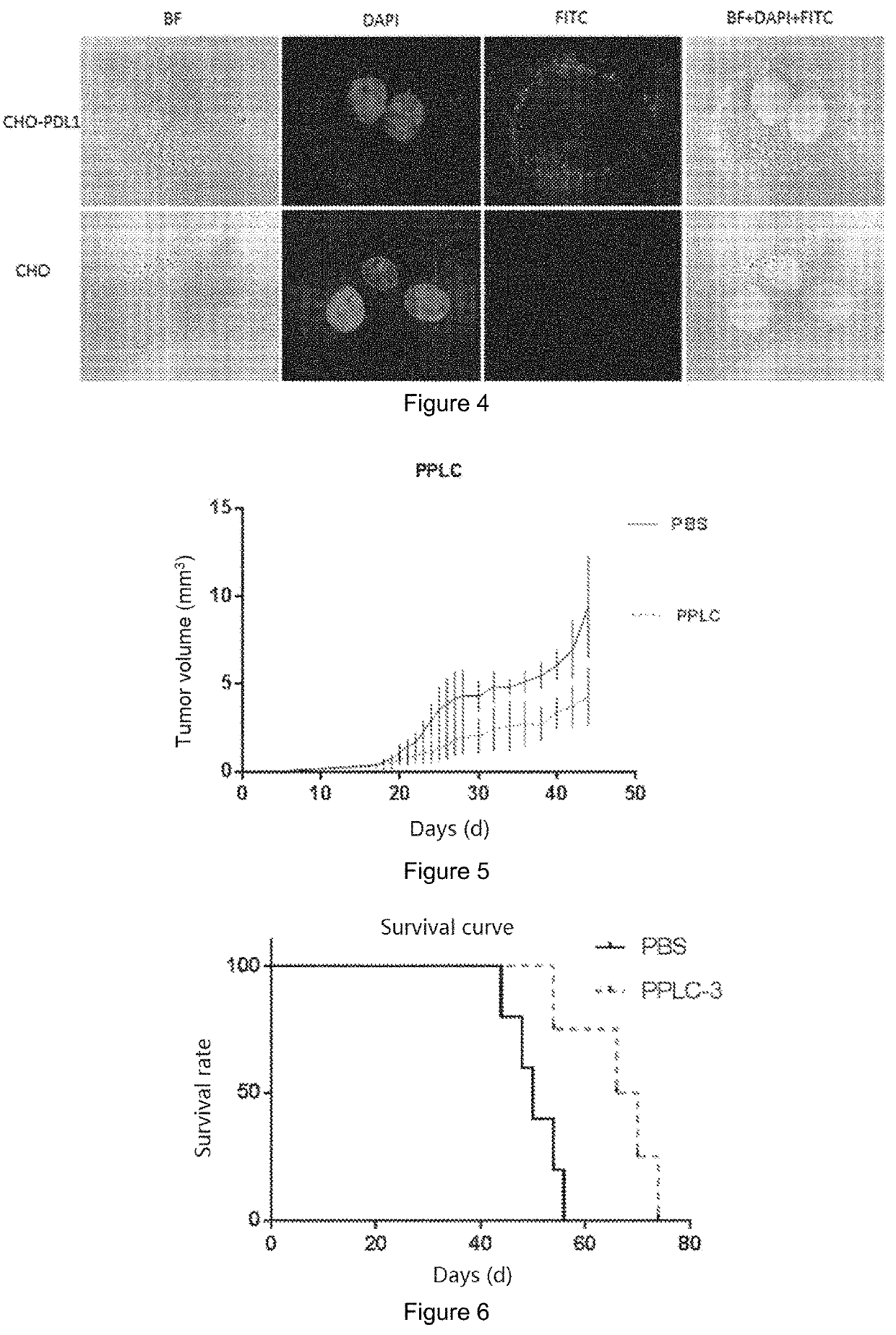
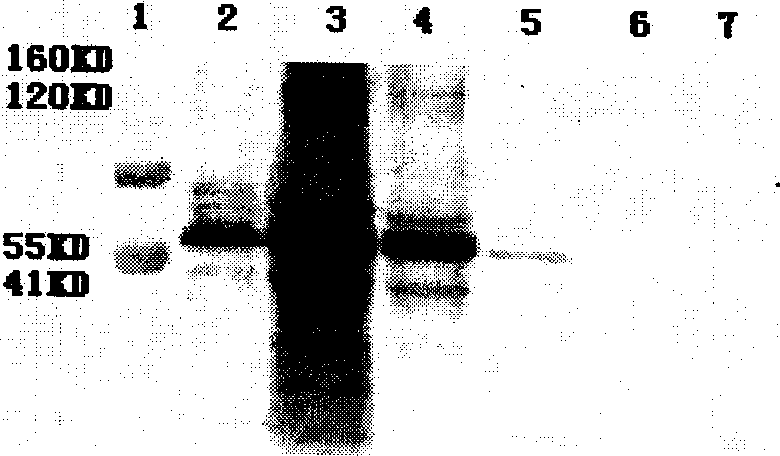Patents
Literature
46results about How to "Break immune tolerance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
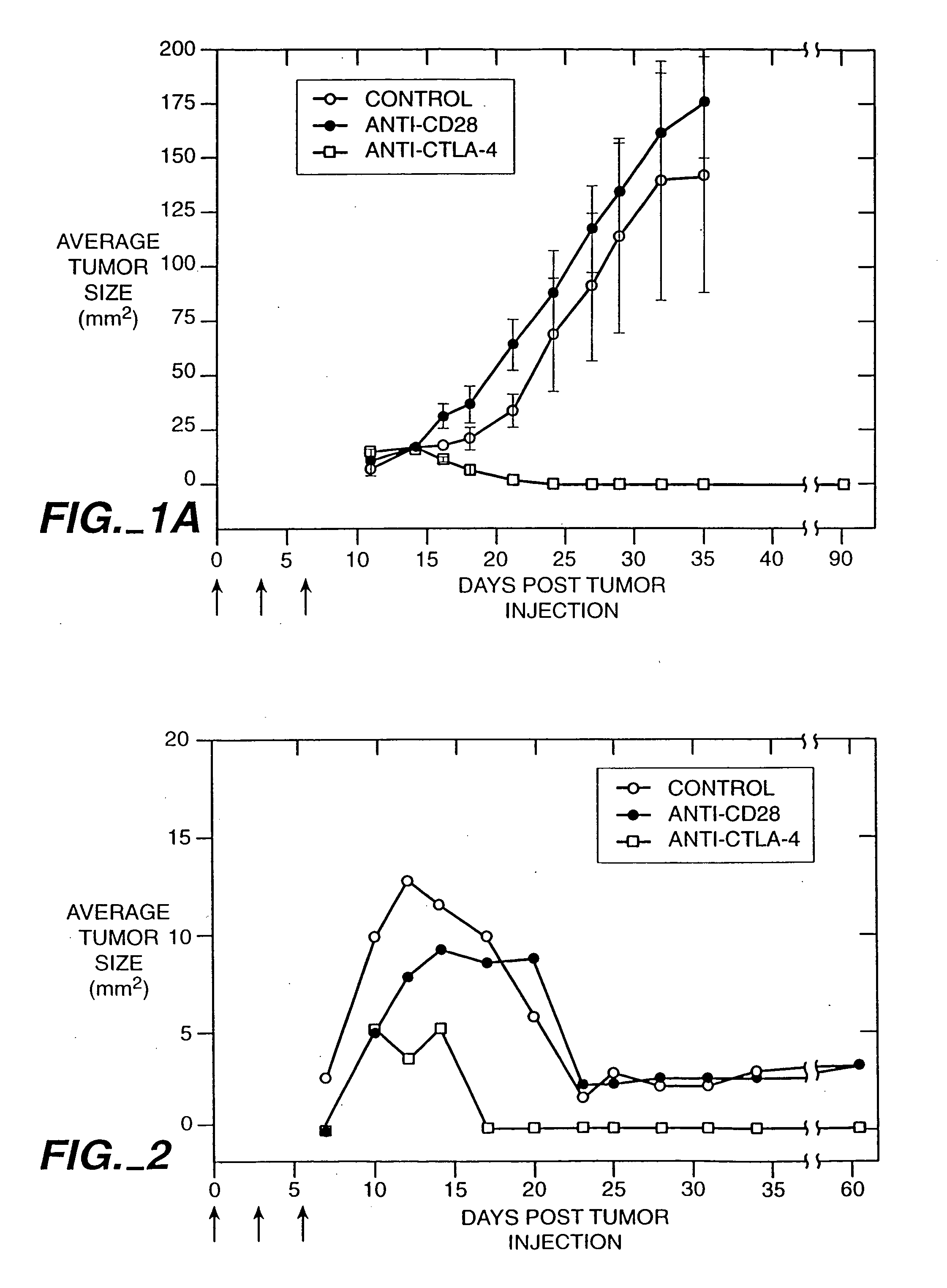
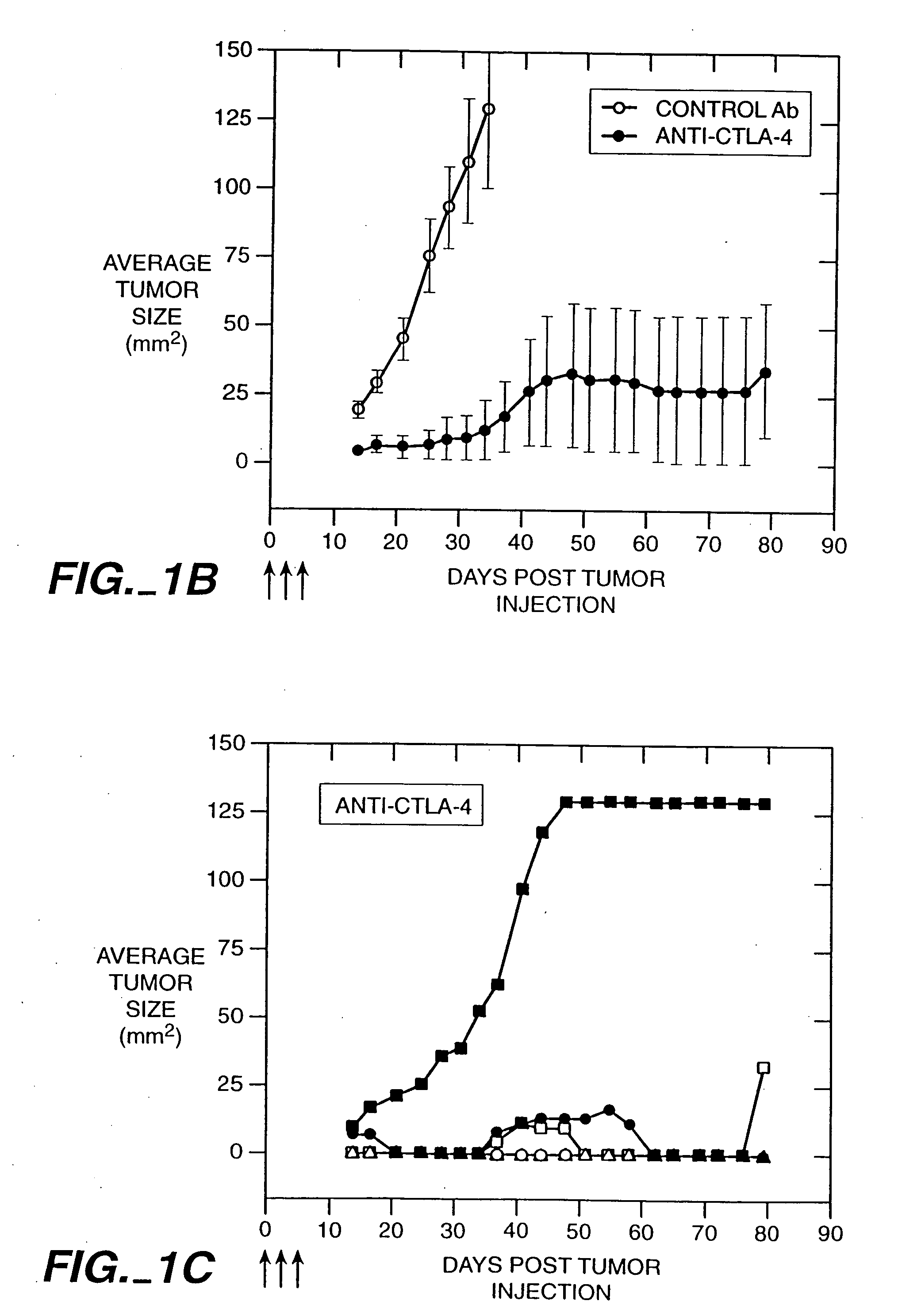
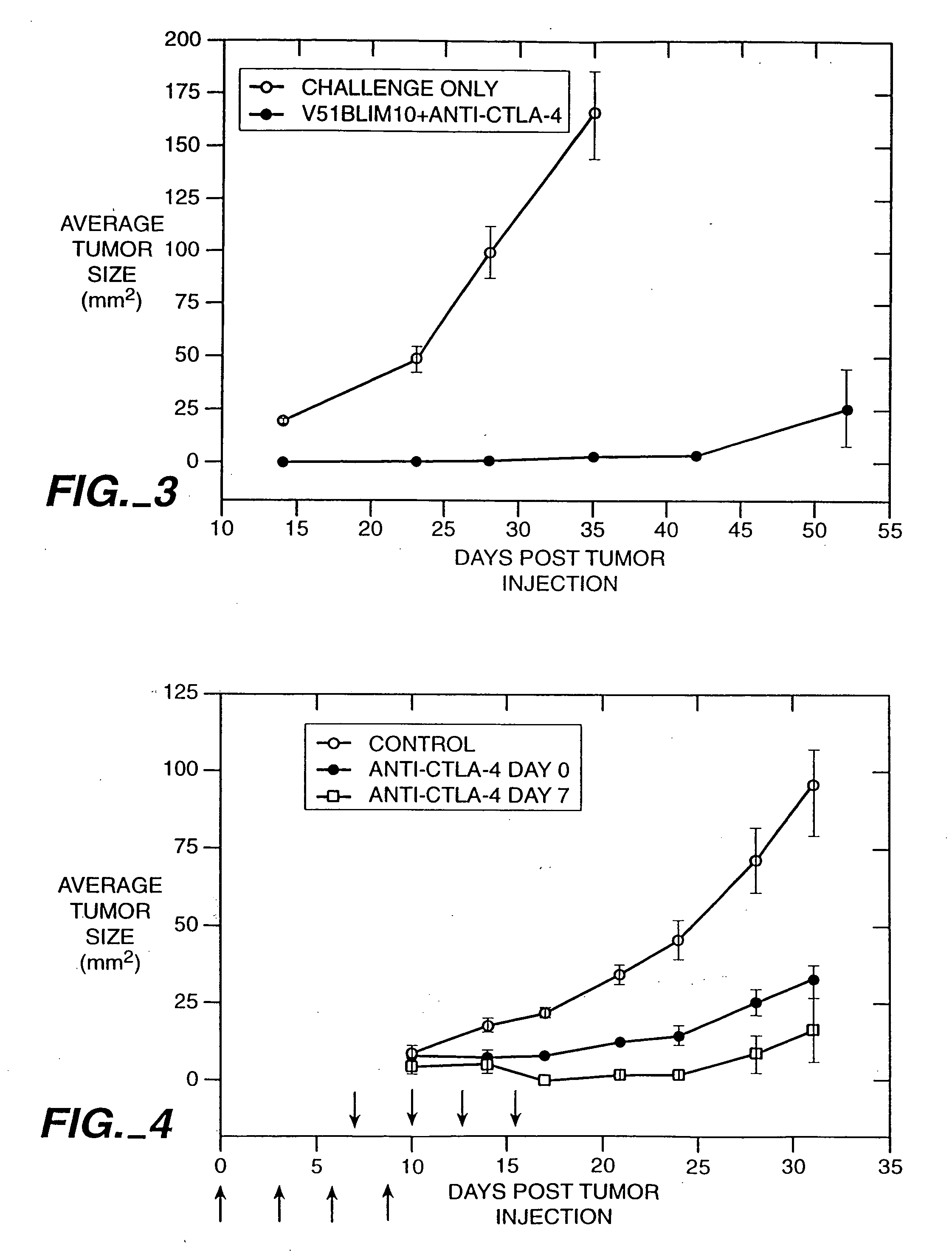
Stimulation of T cells against self antigens using CTLA-4 blocking agents
InactiveUS20060034844A1Enhanced T cell responseBreak immune tolerancePeptide/protein ingredientsSnake antigen ingredientsCTLA4 ProteinImmune tolerance
Stimulation of T cells to respond to self antigens is achieved through a blockade of CTLA-4 signaling. CTLA-4 blocking agents are combined with antigen preparations, either alone or with additional immune response stimulating agents, in costimulation strategies to break immune tolerance and stimulate an enhanced T-cell response against self antigens. This enhanced response is useful for the treatment of non-immunogenic and poorly-immunogenic tumors, as well as other medical conditions requiring selective tissue ablation.
Owner:RGT UNIV OF CALIFORNIA
Preparation of specific tumor killing cell
ActiveCN102526716ABreak immune tolerancePowerful killing functionMammal material medical ingredientsBlood/immune system cellsCell immunityT lymphocyte
The invention relates to an antitumor cell immunotherapy technology, in particular to preparation of a specific tumor killing cell. The preparation method disclosed by the invention comprises the following steps of: 1, sampling a single prokaryotic cell from peripheral blood; 2, separating a DC (Dendritic Cell) from a T cell; 3, maturing the DC and preparing a DC vaccine; 4, preparing a CIK (Cytokine Induced Killer); 5, preparing a CTL (cytotoxic T lymphocyte); and 6, preparing a specific DC-CIK-CTL cell preparation.
Owner:玥特农生物科技河北有限责任公司
NY-ESO-1 tumour antigen mimic epitope and use thereof
InactiveCN101381402AImproving immunogenicityStrong specificityPeptidesAntibody medical ingredientsCtl epitopePredictive methods
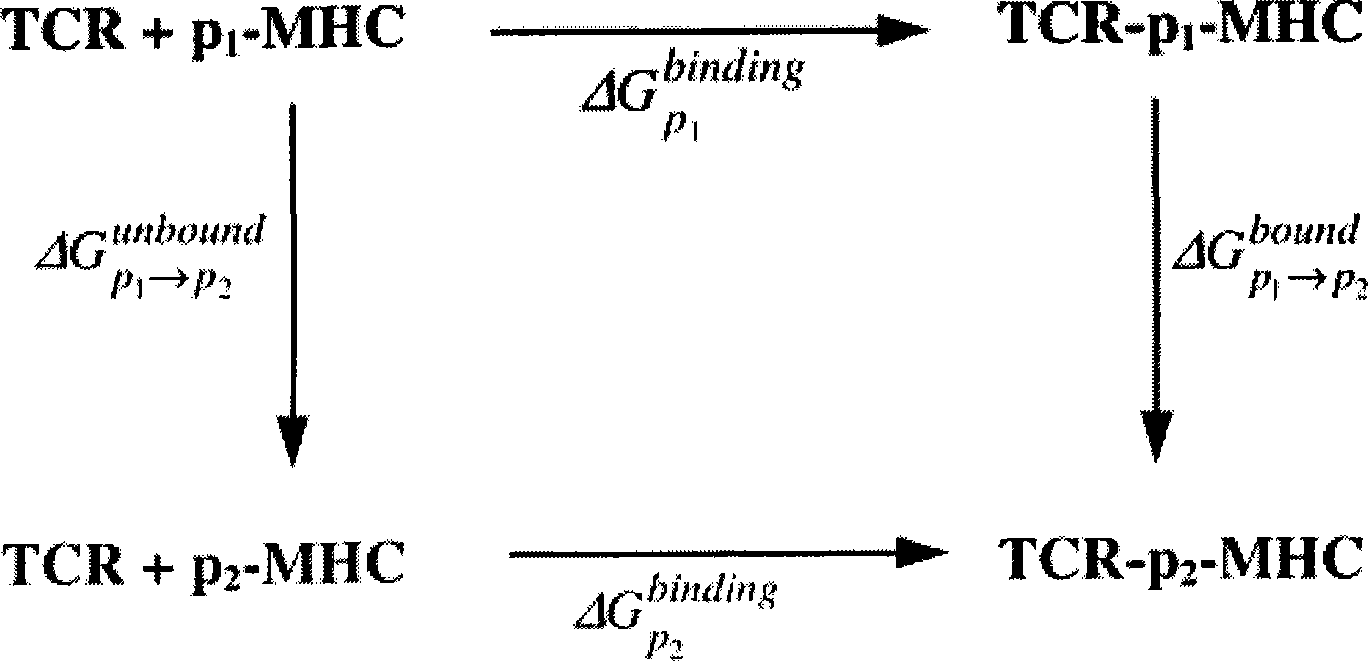
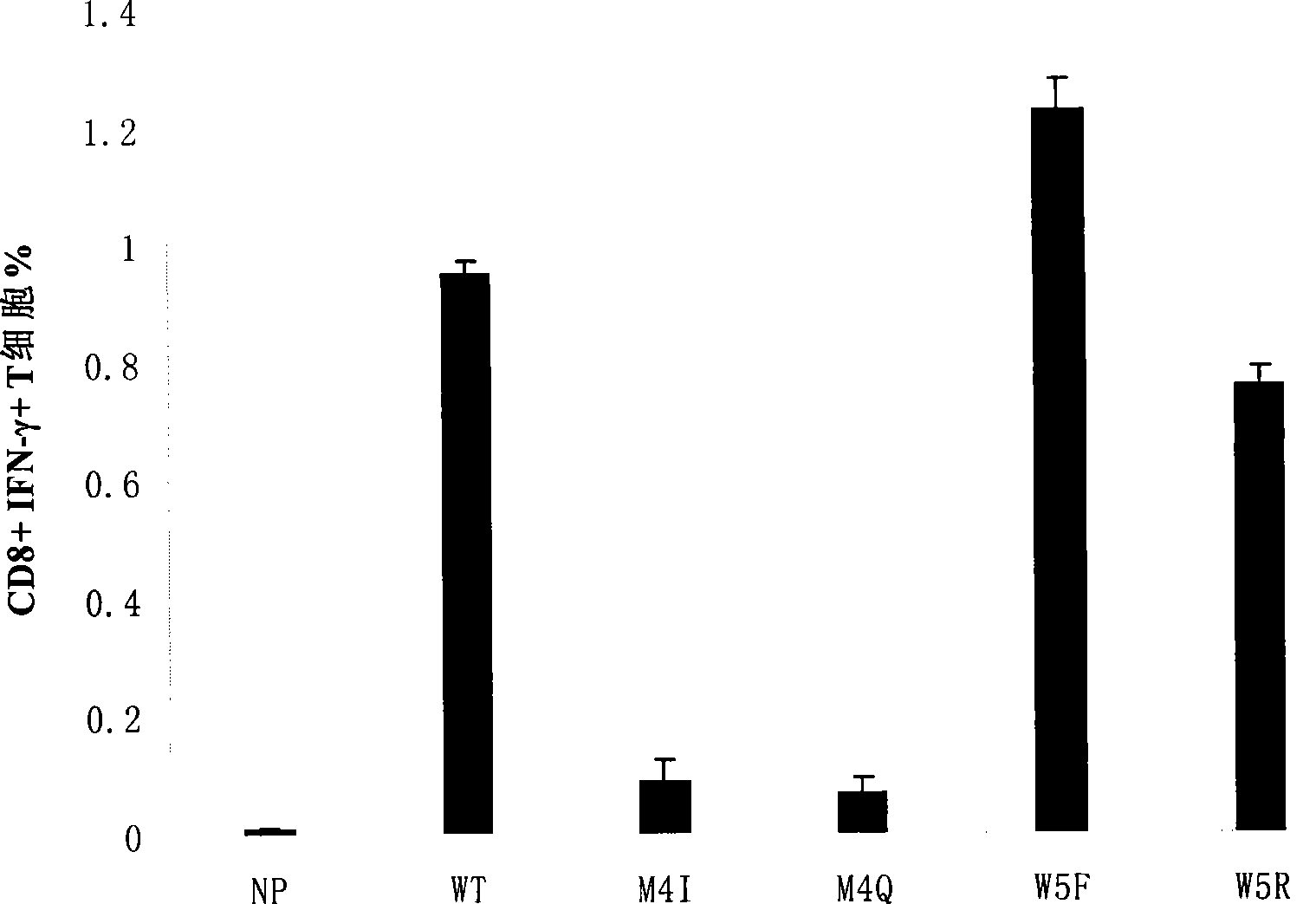
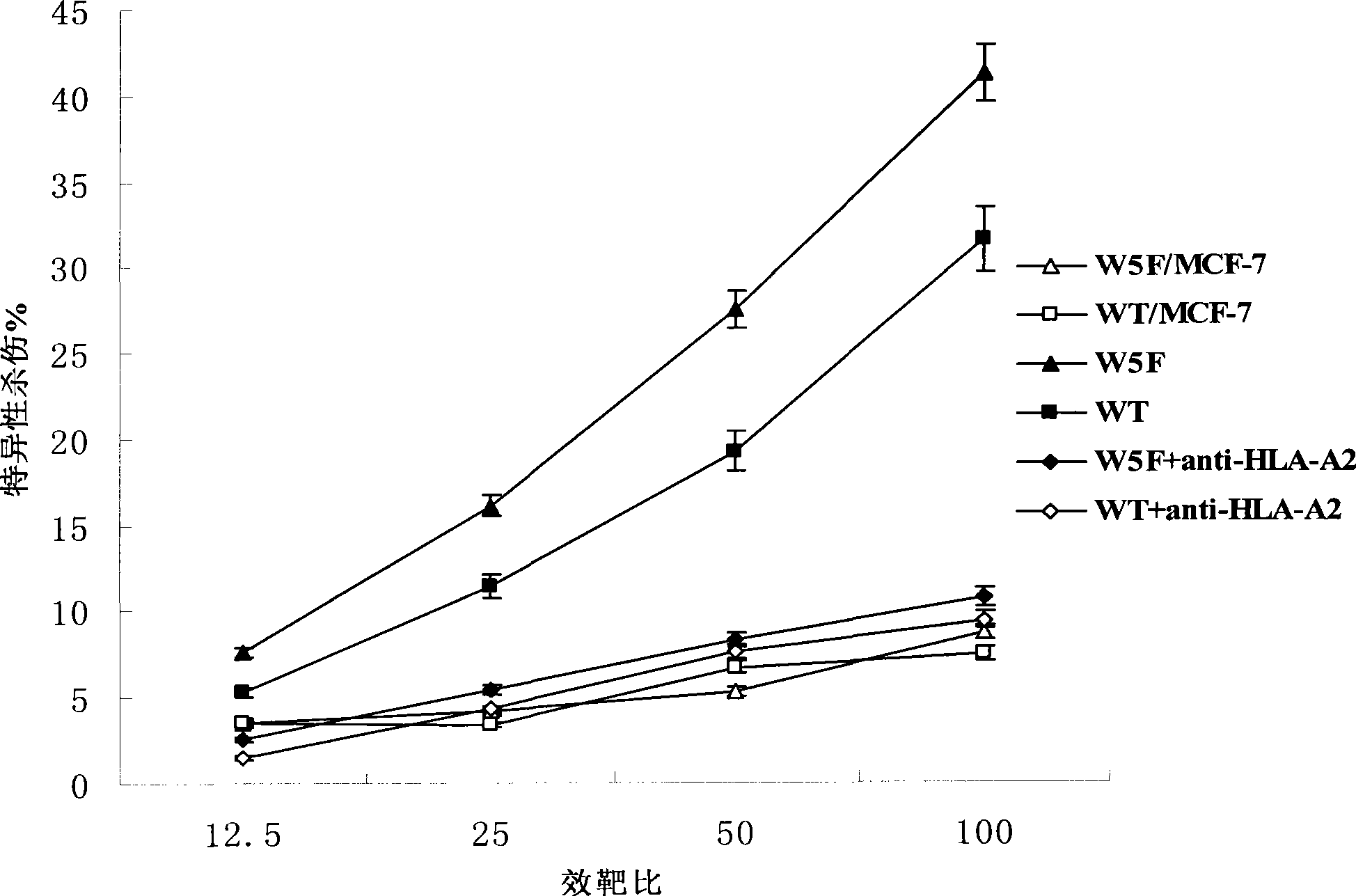
The invention discloses epitope for an NY-ESO-1 tumor antigen. The amino acid sequence of the mimic epitope is Ser-Leu-Leu- Met-Phe-Ile-Thr-Trp-Cys, namely SLLMFITWC; the mimic epitope can raise a CTL immunity response, can undergo a cross reaction with a natural epitope, has the characteristics of strong immunogenicity, immune tolerance breaking, strong specificity, safety, low cost, and easy synthesis and storage, and can be used to prepare tumor-therapeutic polypeptide vaccine. The invention also discloses a method for predicting the mimic epitope, which comprises steps of the establishment of a structural model of a TCR-pMHC complex, the analysis of TCR binding sites of the epitope, and the analysis of amino acid replacement of the TCR binding sites of the epitope. The method is also applicable to the computer-aided modification of CTL epitopes of other antigens, and can provide a useful tool for the design and research of therapeutic polypeptide vaccine.
Owner:ARMY MEDICAL UNIV
Immunogene therapeutic drug for chronic hepatitis B and preparation method for immunogene therapeutic drug
InactiveCN104940953AReduced viral copyImprove securityGenetic material ingredientsDigestive systemHBsAgChronic hepatitis
The invention relates to an immunogene therapeutic drug for chronic hepatitis B and a preparation method for the immunogene therapeutic drug. The active component of the drug is a replication-competent recombinant vector pSVK-dSFValpha-IRES-hIL-12 (for short, pSVK-HBVE), which is constructed by taking a pSVK carrier as a starting carrier and carries a fusion gene dSFValpha-IRES-hIL12. An antibody targeting interferon alpha and human IL-12 are co-expressed in the replication-competent recombinant vector pSVK to construct a humanized HBsAg dsFv antibody, human interferon-alpha and interleukin-12 fusion expression vector pSVK-dSFValpha-IRES-hIL-12, the fusion expression vector is efficiently expressed in an eukaryotic cell and in vitro, the combined application of human IL-12 and human IFN-alpha has an important synergistic effect during the tumor control process, and a safe and efficient immunogene therapeutic drug is provided for gene therapy of chronic hepatitis B.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Compound-type nano-vaccine and preparation method thereof
InactiveCN104645349AImprove Uptake PresentationImprove immune efficiencyGenetic material ingredientsMacromolecular non-active ingredientsDendritic cellBiocompatibility Testing
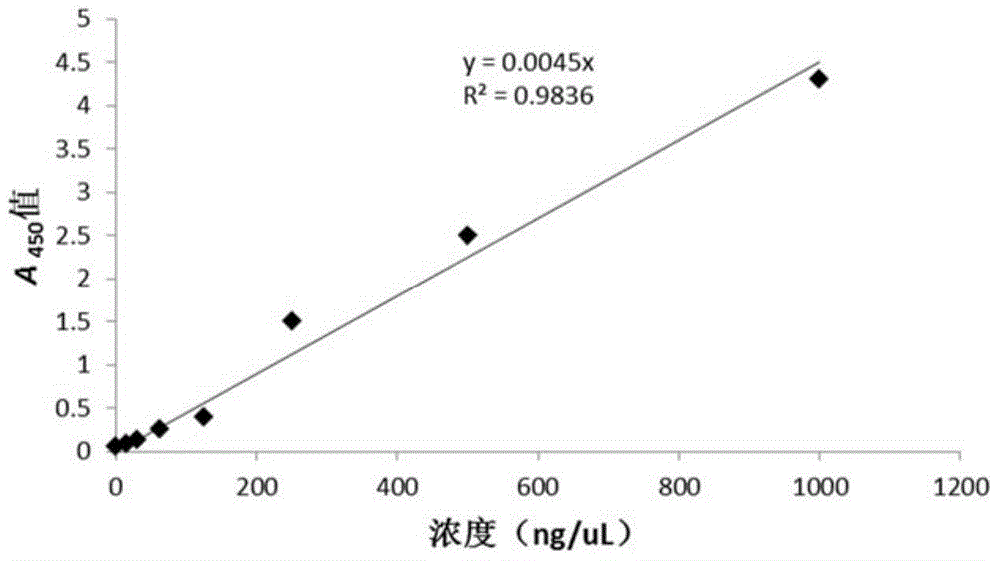
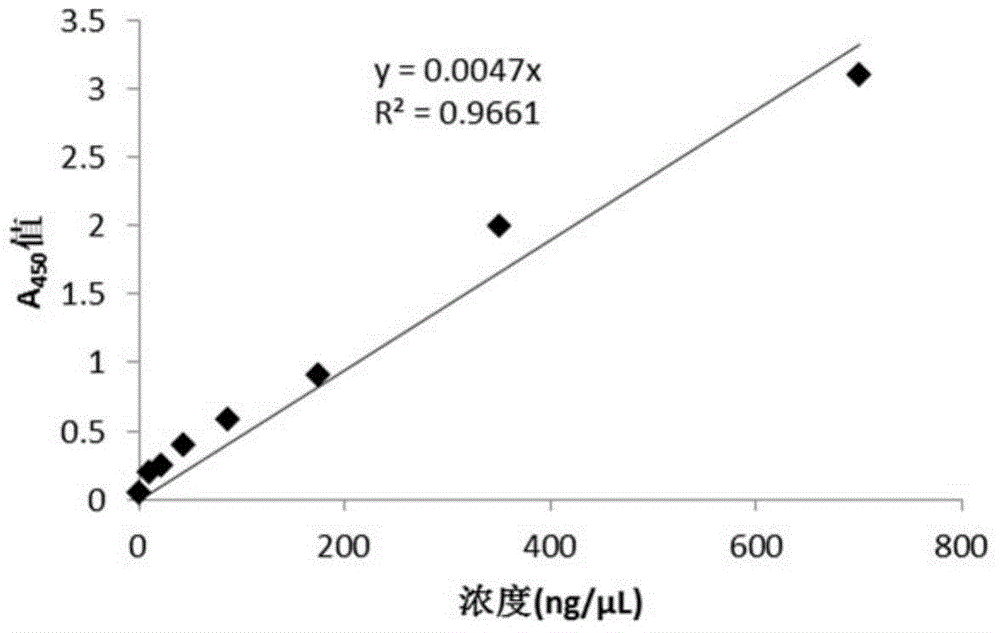
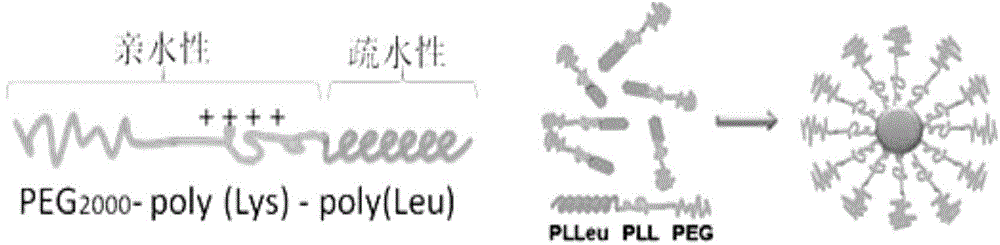
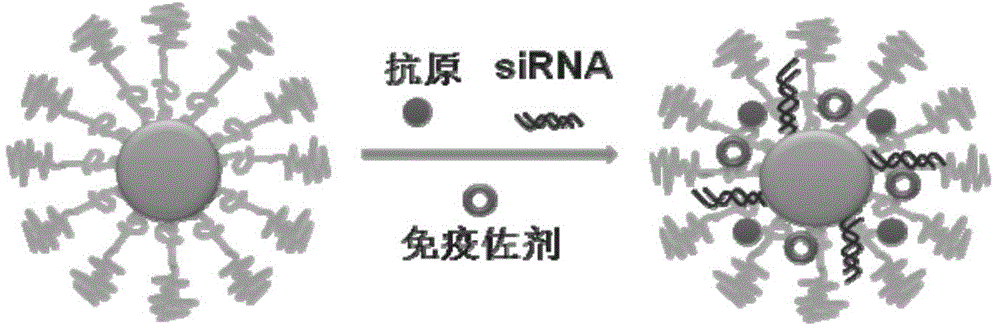
The invention discloses a compound-type nano-vaccine taking an amphiphilic three-block polymer of polyethylene glycol derivative-poly lysine-poly leucine as a nano-carrier bearing a tumor antigen, an immunological adjuvant and siRNA (si Ribonucleic Acid), and a preparation method of the compound-type nano-vaccine. According to the nano-vaccine, taking and presenting of the antigen by antigen-presenting cells can be improved by a nano-micelle bearing the antigen; siRNA is efficiently delivered to TADCs (Tumor-associated Dendritic Cells) to block immunosuppression signals of TADCs, and TADCs are induced and activated by collaboration with the immunological adjuvant, so that a tumor resisting effect of a tumor vaccine is improved; the nano-vaccine has the advantages that the adopted carrier of the nano-vaccine is good in biocompatibility and low in toxicity, and is degradable in a biological body; degradation products are nontoxic and harmless, and can be absorbed or metabolized; and the preparation method of the nano-vaccine is simple and convenient and feasible, good in stability and convenient for popularization.
Owner:SHENZHEN INST OF ADVANCED TECH
Method for preparing tumor-specific DC vaccine by applying CD34+ cells of umbilical cord blood
ActiveCN103405759ABreak immune toleranceBlood/immune system cellsAntibody medical ingredientsAntigenDc vaccine
The invention discloses a method for preparing a tumor-specific DC vaccine by applying CD34+ cells in umbilical cord blood. The method comprises (1) a step of preparing autologous tumor-related holoantigen; (2) a step of obtaining the umbilical cord blood; (3) a step of obtaining mononuclear cells derived from the umbilical cord blood; (4) a step of purifying CD34+ cells in the mononuclear cells derived from the umbilical cord blood; (5) performing induction culture for a precursor DC; (6) a step of performing amplification and culture of an immature DC; and (7) a step of preparing the DC vaccine.
Owner:玥特农生物科技河北有限责任公司
HIV-1 virus-like particle and its prepn and use
InactiveCN1772892AImprove securityReasonable assemblyViral antigen ingredientsViruses/bacteriophagesVirus-like particleCell Membrane Proteins
The present invention relates to one kind of and its preparation and use, and belongs to the field of biotechnology. The HIV-1 virus-like particle contains complete core protein gag, encoding enzyme protein pol and outer membrane protein env of HIV-1, and is nonreplication type. The present invention is superior in that stably expression cell line is established through cotransfection and monoclonal cell screening. The cell line can secrete HIV-1 virus-like particle stably and continuously, and the HIV-1 virus-like particle contains no virus nucleic acid and has high safety. The constituted VLP has reasonable assembling form and high similarity with natural virus in structure, and may be used as HIV-1 treating vaccine.
Owner:JILIN UNIV +1
Medicine for treating and/or preventing cancer and application
PendingCN111298111AImprove anti-tumor effectHigh killing efficiencyAntibody ingredientsImmunological disordersCancer preventionAntiendomysial antibodies
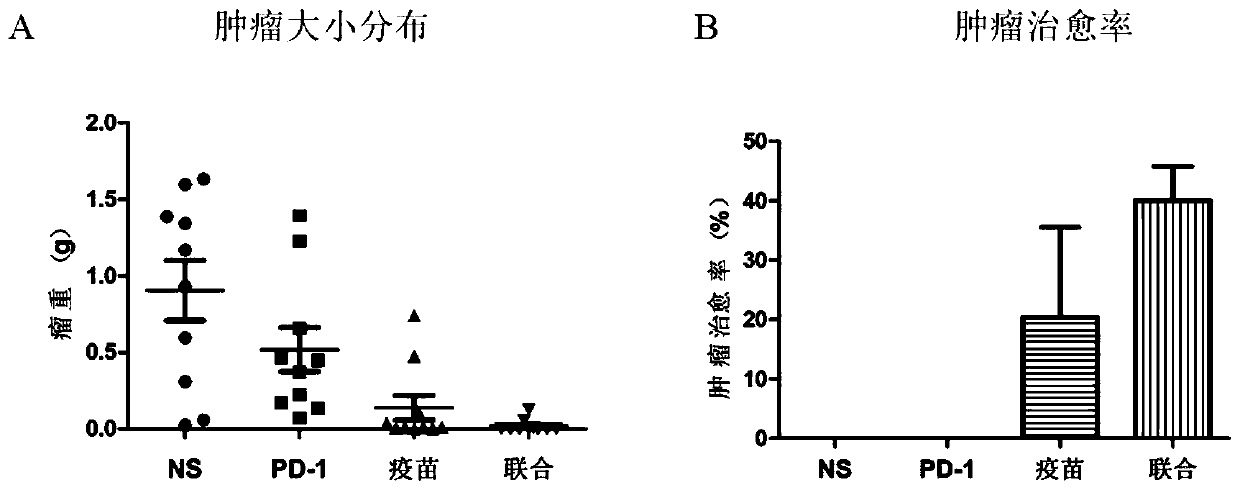
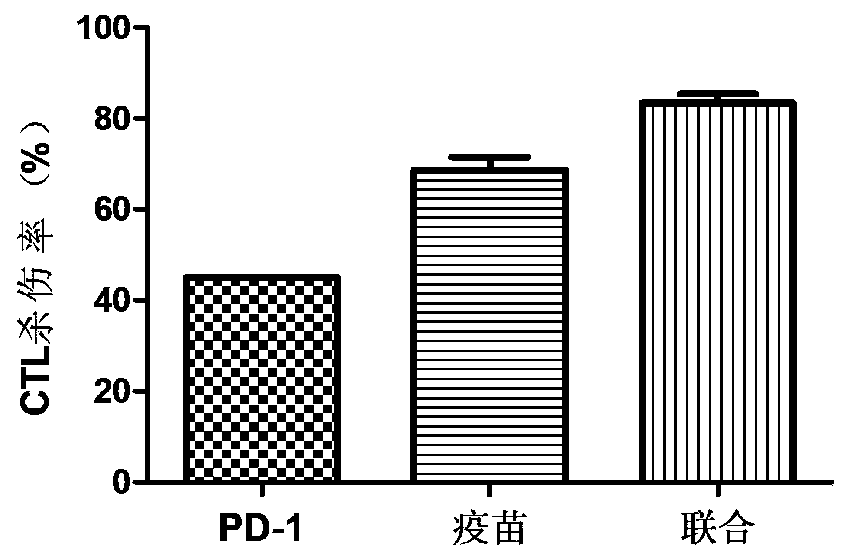
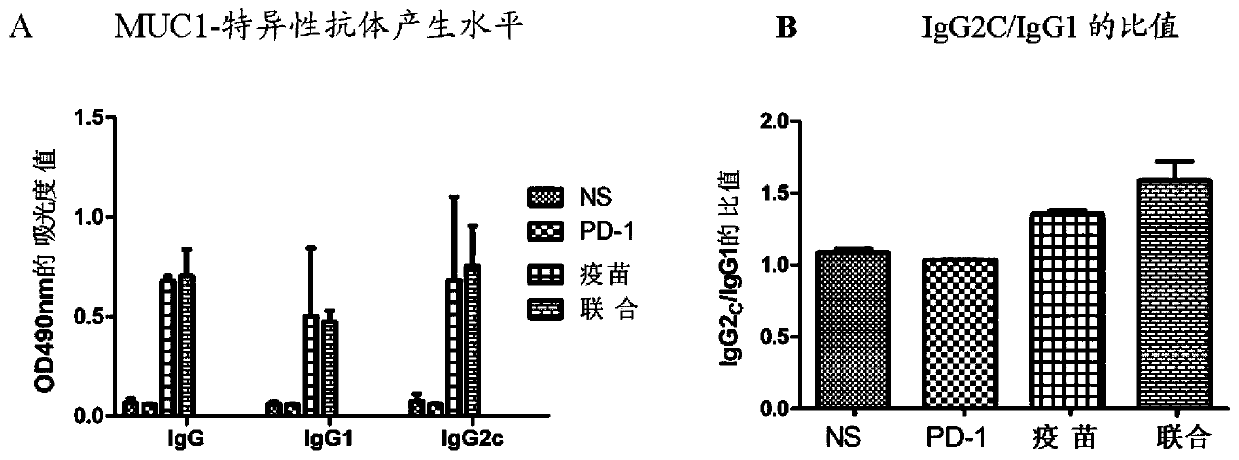
The invention relates to a medicine for treating and / or preventing cancer and an application. The medicine of the invention includes a recombinant MUC1-MBP fusion protein vaccine and a PD-1 antibody.The medicine is expected to break an immune tolerance state of a tumor microenvironment in vivo and enhance the MUC1 specific anti-tumor immune response, and is expected to completely clear tumors tobring gospel to the majority of cancer patients.
Owner:长春康悦生物科技有限公司
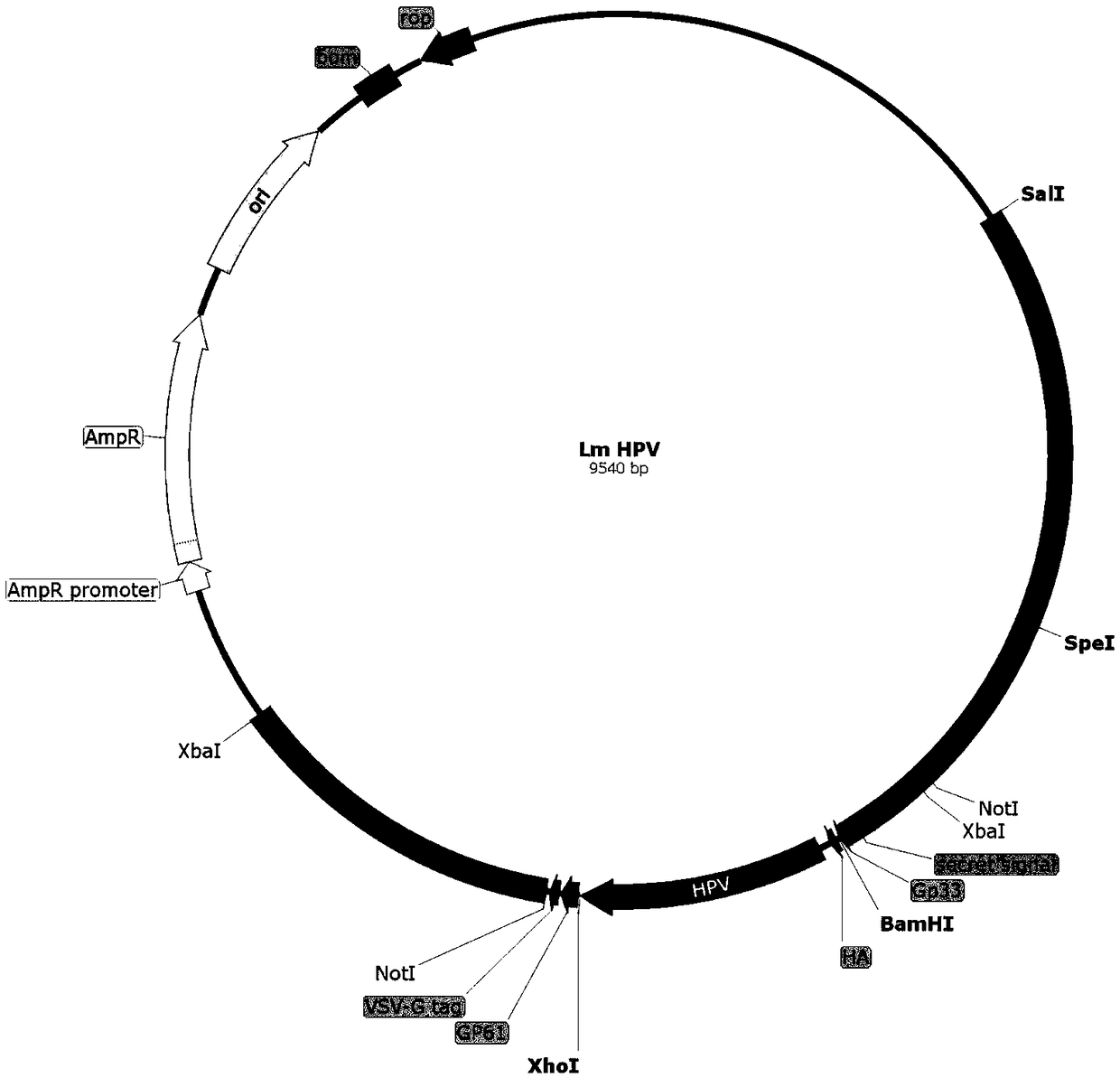
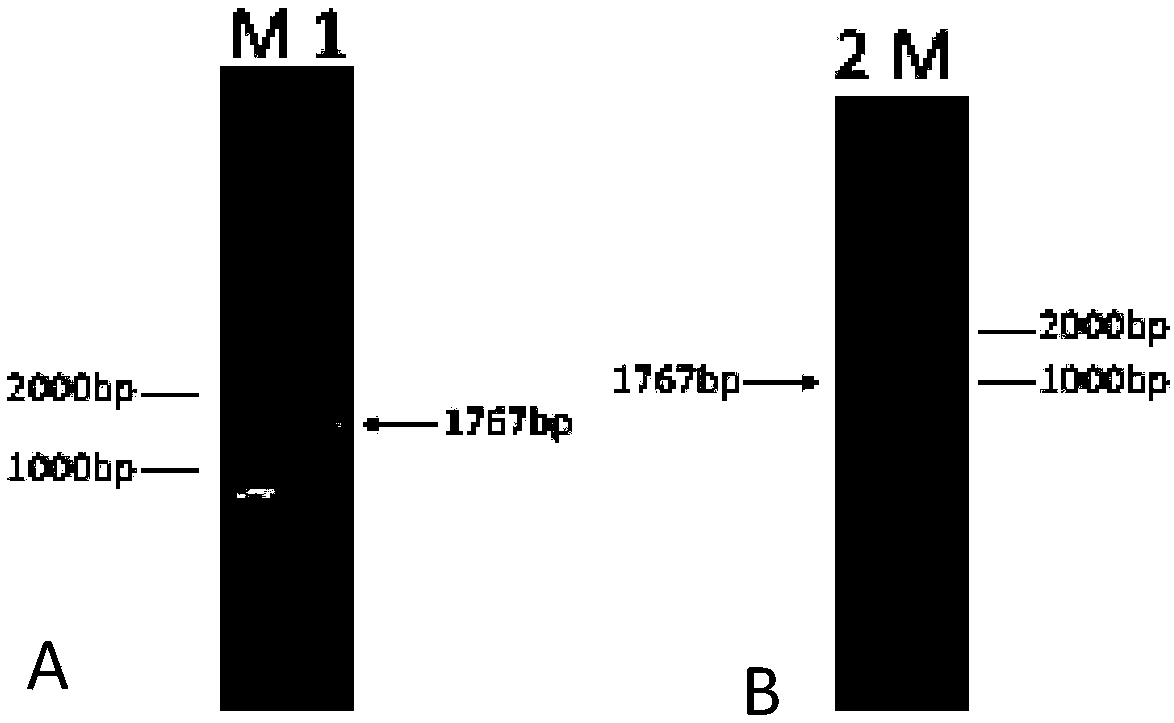
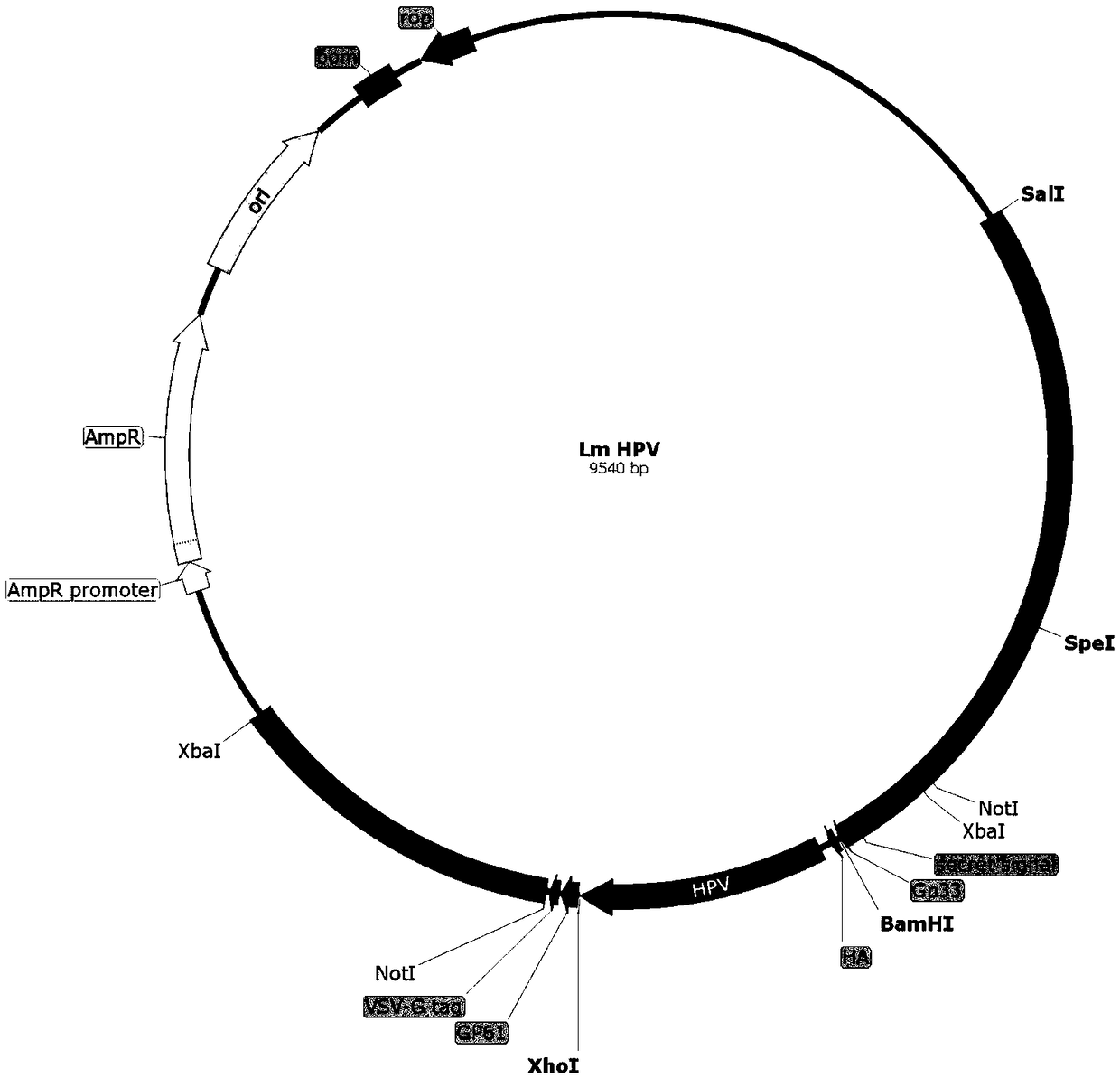
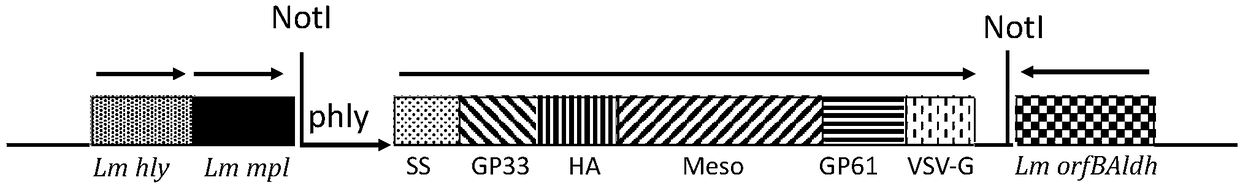
Cervical cancer therapeutic vaccine based on recombinant attenuating sheep listeria ivanovii
ActiveCN108939064ABreak immune toleranceMake up for the disadvantage of lower immunogenicityBacteriaViral antigen ingredientsAbnormal tissue growthListeria floridensis
The invention relates to a cervical cancer therapeutic vaccine based on recombinant attenuating sheep listeria ivanovii. By taking the attenuating sheep listeria ivanovii as a vector, specific immuneresponse in a tumor microenvironment is improved effectively and immune tolerance of a body is broken by means of a unique characteristic that the listeria ivanovii can grow in a main phagocyte and isa natural T cell immune activation adjuvant, so that continuous virus infection of the body is eliminated and the good therapeutic effect of eliminating the focus and inhibiting tumor development isachieved. The vaccine has the advantages that the defect that nucleic acid and protein polypeptide vaccines are relatively low in immunogenicity is made up, and the attenuating sheep listeria ivanoviialso has high safety while retaining the characteristic that an original strain can grow in cells to achieve antigen presentation.
Owner:南京颂悦生物科技有限公司
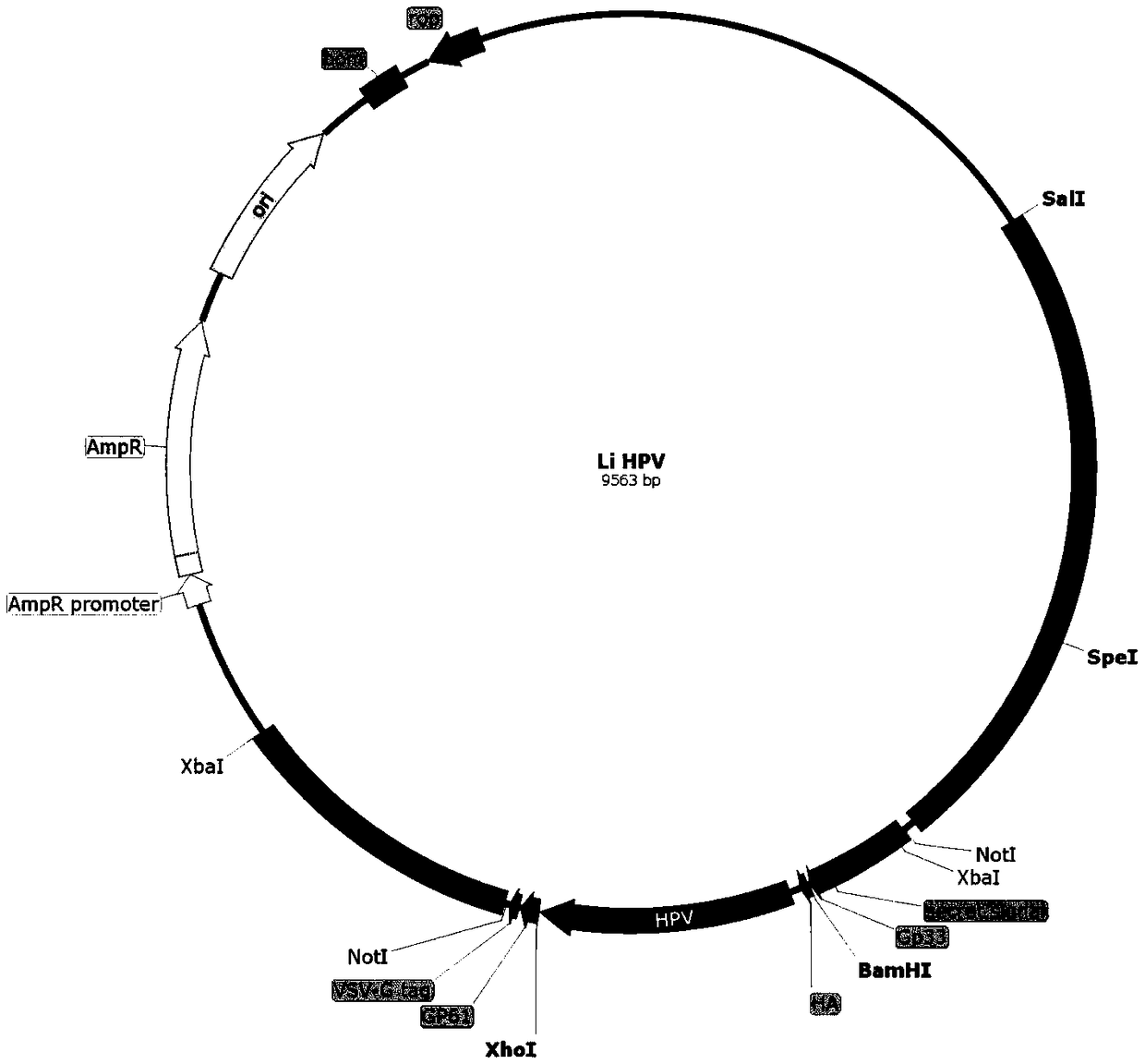
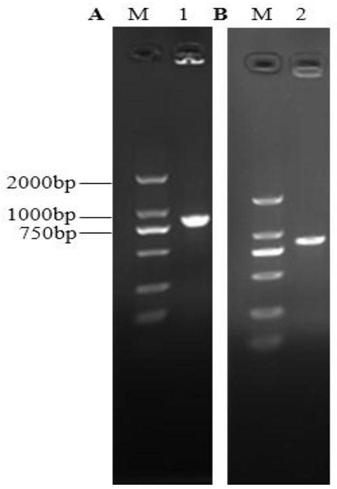
Cervical cancer therapeutic vaccine based on recombinant attenuated Listeria monocytogenes
ActiveCN108992665ABreak immune toleranceMake up for the disadvantage of lower immunogenicityBacteriaViral antigen ingredientsAdjuvantSpecific immunity
The invention relates to a cervical cancer therapeutic vaccine based on recombinant attenuated Listeria monocytogenes. Attenuated Listeria monocytogenes (hereinafter referred to as Lm) is used as a vector, and the unique ability of Listeria to grow in a host phagocytic cell is the characteristic of a natural T cell immune activation adjuvant, and is used to effectively improve the specific immuneresponse in a tumor microenvironment and break the immune tolerance of bodies, so persistent viral infection of the bodies is removed, and good therapeutic effects of clearing focus and restraining tumor development are achieved. The cervical cancer therapeutic vaccine makes up for the shortcoming of low immunogenicity of nucleic acid and protein peptide vaccines, and the attenuated Listeria reserves the intracellular growth and antigen presentation completion characteristics of an original strain, and has a high safety.
Owner:南京颂悦生物科技有限公司
Novel asthma polypeptide vaccine and preparation method thereof
InactiveCN106749674ASmall molecular weightEasy to synthesizeCell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsNitrogenImmune tolerance
The invention belongs to the field of biological medicines, and concretely relates to a novel asthma polypeptide vaccine and a preparation method thereof. The invention aims to solve the technical problem so as to provide a new effective selection for asthma treatment in the field. The technical scheme provided by the invention for solving the technical problem is characterized by connecting DiTOX polypeptide or PADRE polypeptide on a nitrogen end of E3 polypeptide to obtain a new fusion polypeptide, wherein the polypeptide can be used as a main active constituent for preparing the asthma vaccine. The polypeptide vaccine provided by the invention can effectively induce specific humoral immunity, can break immune tolerance, and has a better application prospect when being used as the polypeptide vaccine for asthma treatment.
Owner:SICHUAN UNIV
Immune dominant HLA-A3 super-type restrictive CTL epitope of hepatitis B virus core antigen and identification method and application thereof
InactiveCN101979405AImproving immunogenicityStrong specificityMicrobiological testing/measurementDigestive systemHepatitis B virus core AntigenCtl epitope
The invention relates to an antigen epitope, in particular to an immune dominant HLA-A3 (human leukocyte antigen A3) super-type restrictive CTL (cytotoxic T lymphocyte) epitope of a hepatitis B virus core antigen (HBcAg) and an identification method for the super-type CTL epitope. The super-type CTL epitope consists of the following amino acid sequence: Leu-Leu-Asp-Thr-Ala-Ser-Ala-Leu-Tyr-Arg. The method is also suitable for identification of other antigen super-type CTL epitopes, and can provide a useful tool for design and research of therapeutic polypeptide vaccines. The invention also relates to application of the super-type CTL epitope in preparing hepatitis B therapeutic polypeptide vaccines, and provides a new strategy and a new method for efficient research of the hepatitis B therapeutic polypeptide vaccines. The method is expected to break through hepatitis B immune tolerance, reconstruct the immune function of cells and efficiently inhibit and clear hepatitis B viruses.
Owner:ARMY MEDICAL UNIV
Traditional Chinese medicine preparation for treating hepatitis B
InactiveCN101385845AAdjust immune functionImprove clinical symptomsAnthropod material medical ingredientsDigestive systemMonkshoodsDisease
The invention discloses a preparation of traditional Chinese medicines for treating chronic hepatitis B, which is prepared by the following components according to weight percentage: 6-12 percent of Chinese ephedra, 3-9 percent of monkshood, 3-6 percent of asarum, 6-9 percent of dried ginger, 12 percent of Chinese honeylocust spine, 15-30 percent of white backleaf mallotus root, 11-13 percent of Chinese atractylodes, 11-13 percent of radix bupleuri, 14-16 percent of bitter orange, 8-10 percent of peach seed, 8-10 percent of safflower, 29-31 percent of radix astragali, 14-16 percent of angelica, 12-18 percent of herba epimedii and 3-6 percent of scolopendra. In the invention, Chinese medicine treatment is adopted in the early period to positively cure the disease, adjust immunity function of HBV infection patients, excite the immunity of the organism of chronic HBV carriers, break the state of immune tolerance and adjust the immunity function of the patients who suffer from HBeAg masculine minor chronic hepatitis B, thus improving clinical symptoms and physical sign of the two patients, shortening the course of disease of the patients and increasing the negative turning rate of HBeAg and HBV-DNA thereof, therefore, clinical curative effect on treating patients infected by HBV with the traditional Chinese medicine is improved and the curative effect on hepatitis B is enhanced.
Owner:THE THIRD AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
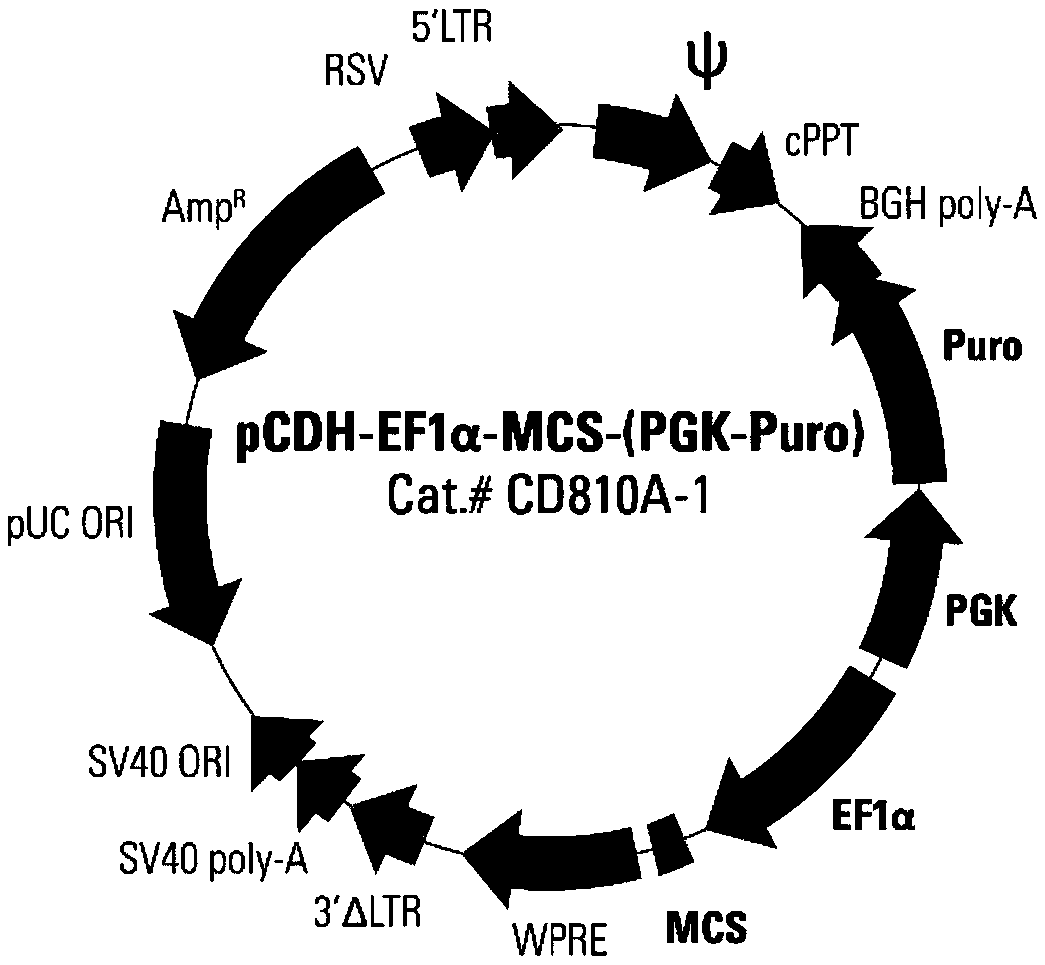
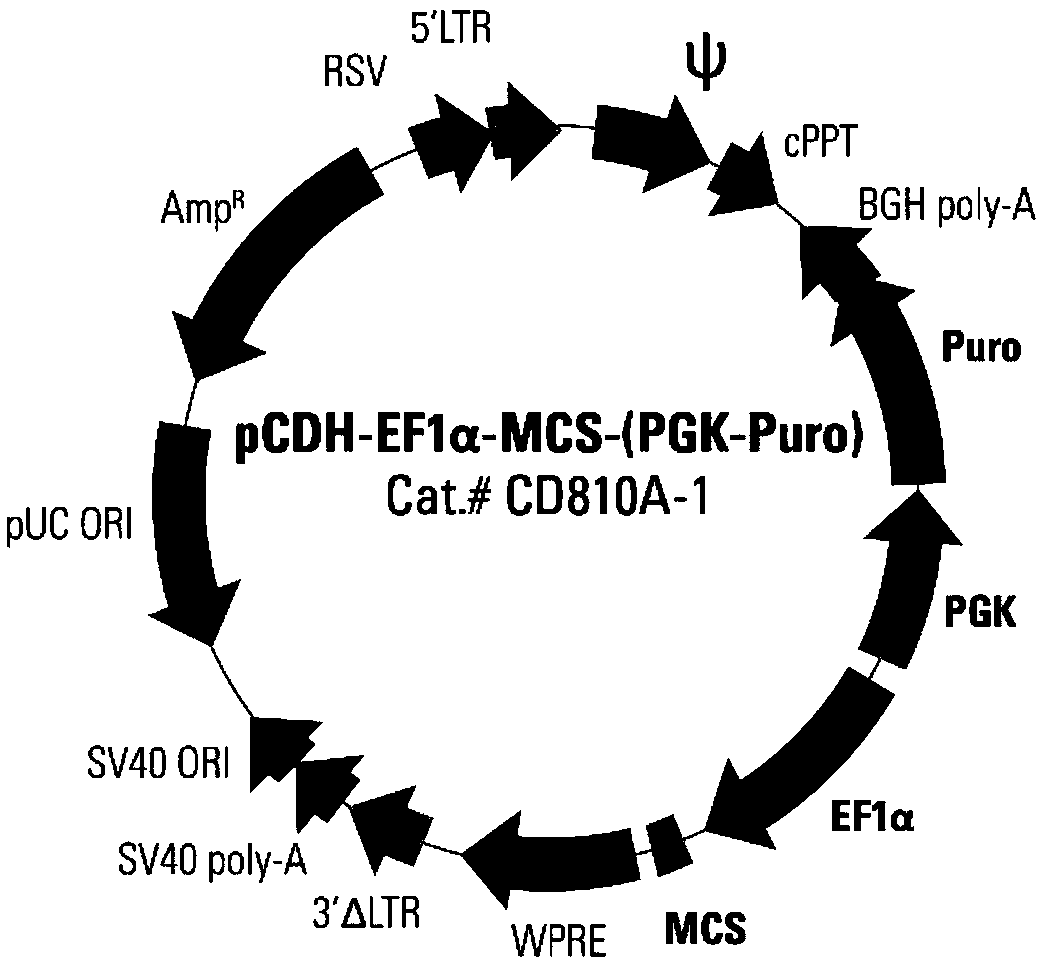
Double-target CAR-T therapy vector for colon cancer, and construction method and application of double-target CAR-T therapy vector
InactiveCN108641001AImprove proliferative abilityIncrease vitalityAntibody mimetics/scaffoldsMammal material medical ingredientsColon cancer cellCancer research
The invention relates to a double-target CAR-T therapy vector for colon cancer, and a construction method and application of the double-target CAR-T therapy vector. The double-target CAR-T therapy vector consists of two parts comprising a lentiviral expression vector pCDH-EF1aplpha-MCS-(PGK-Puro) and a CAR structure. The specific structure of the CAR is Igkappa-iRGD-CEAscFv-(G<4>S)<5>-Her-2scFv-CD8alpha-CD28-CD137-CD3zeta-T2A-CCL19. A T cell containing the therapy vector, namlely the CAR-T cell (Chimeric Antigen Receptor T-Cell), is obtained through the mode of virus infection, and the CAR-T cell can be used for targeted identification and killing of colon cancer cells by expressing the CAR structure.
Owner:SHANGHAI YIHAO BIOTECH CO LTD
Vector containing double-target chimeric antigen receptor gene, CAR-T cell and application of CAR-T cell
PendingCN111560075AExpand the scope of recognitionWide range of damagePolypeptide with localisation/targeting motifImmunoglobulin superfamilyCD3Molecular biology
The invention discloses a vector containing a double-target chimeric antigen receptor gene, a CAR-T cell and application of the CAR-T cell. The double-target chimeric antigen receptor is formed by sequentially splicing signal peptides, CD44 single-chain antibody ScFv, EpCAM single-chain antibody ScFv, strepII tag, CD8 molecular hinge region, CD28 molecular transmembrane region, intracellular structural domain of the CD28 molecular transmembrane region, intracellular costimulatory domain 4-1BB and CD3 molecular zeta chain from an N end to a C end. Meanwhile, a lentivirus expression recombinantplasmid pTK-881-CD44-EpCAM is constructed, lentivirus packaging is carried out, T cells are infected, and the double-target-point CAR-T cells capable of removing CD44 and EpCAM at the same time are obtained. Based on a CAR-T technology, gastric cancer stem cells are killed by only utilizing autoimmunity of a patient, so that a doctor is assisted in gastric cancer treatment.
Owner:WUHAN UNIV OF SCI & TECH
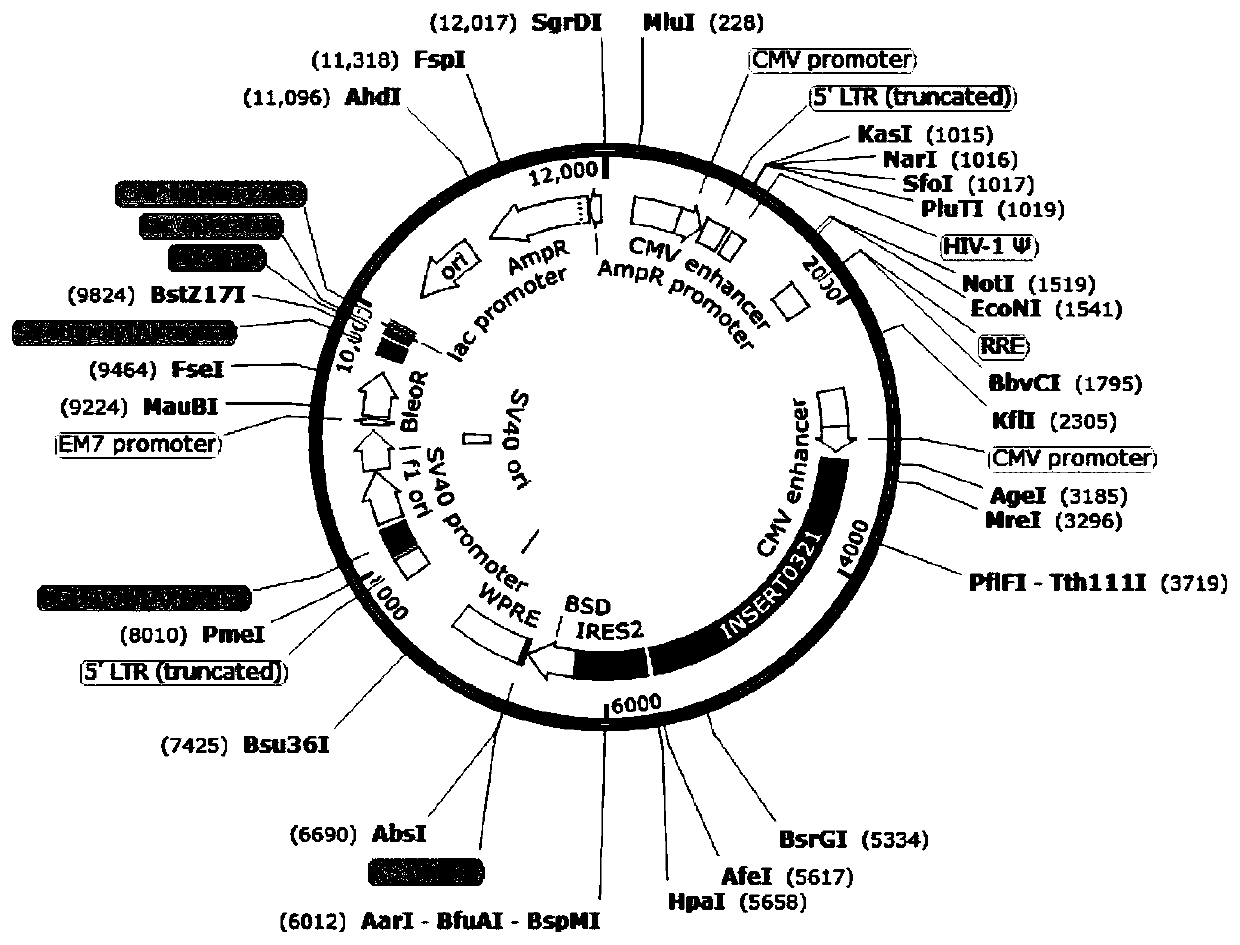
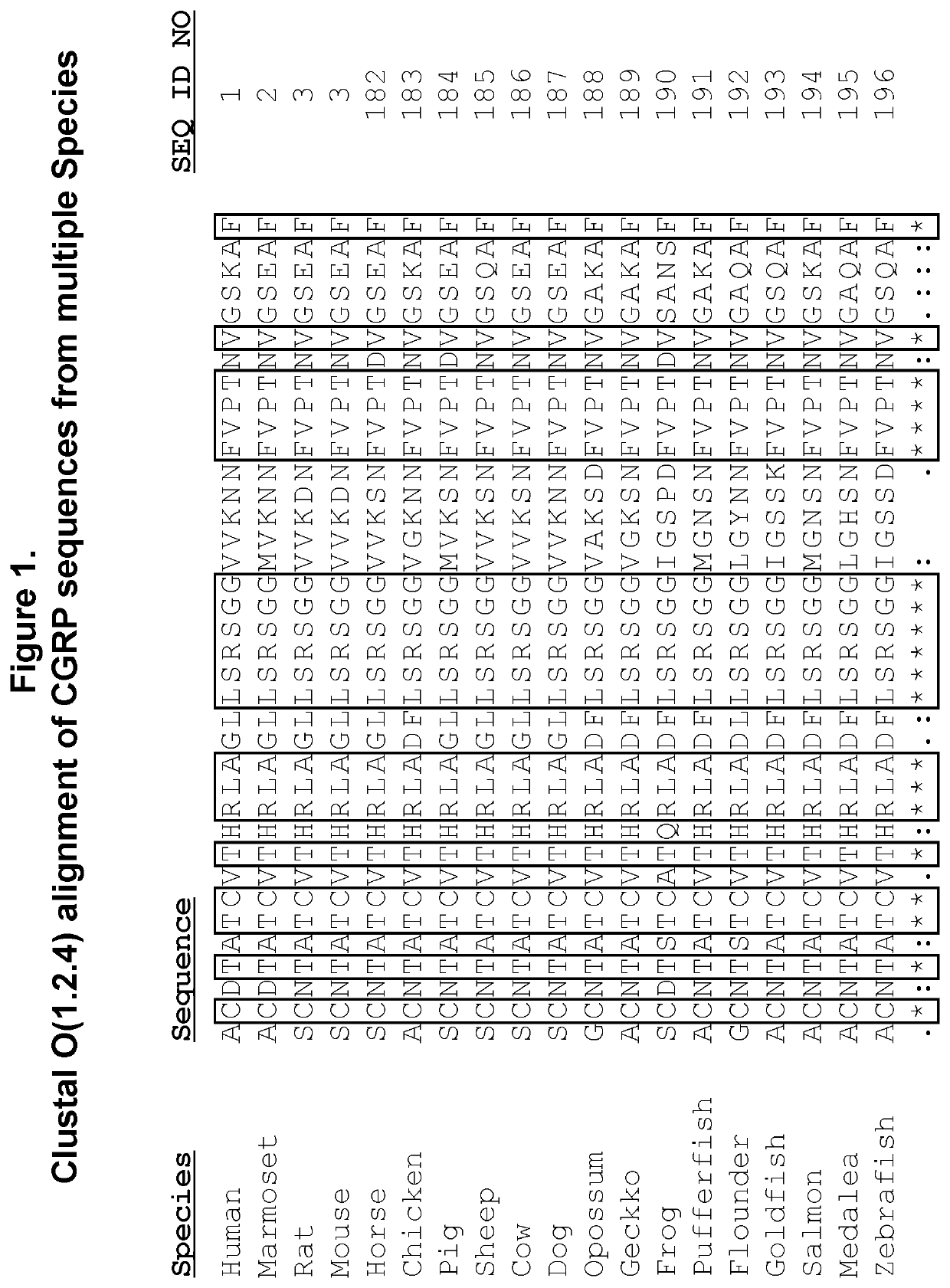
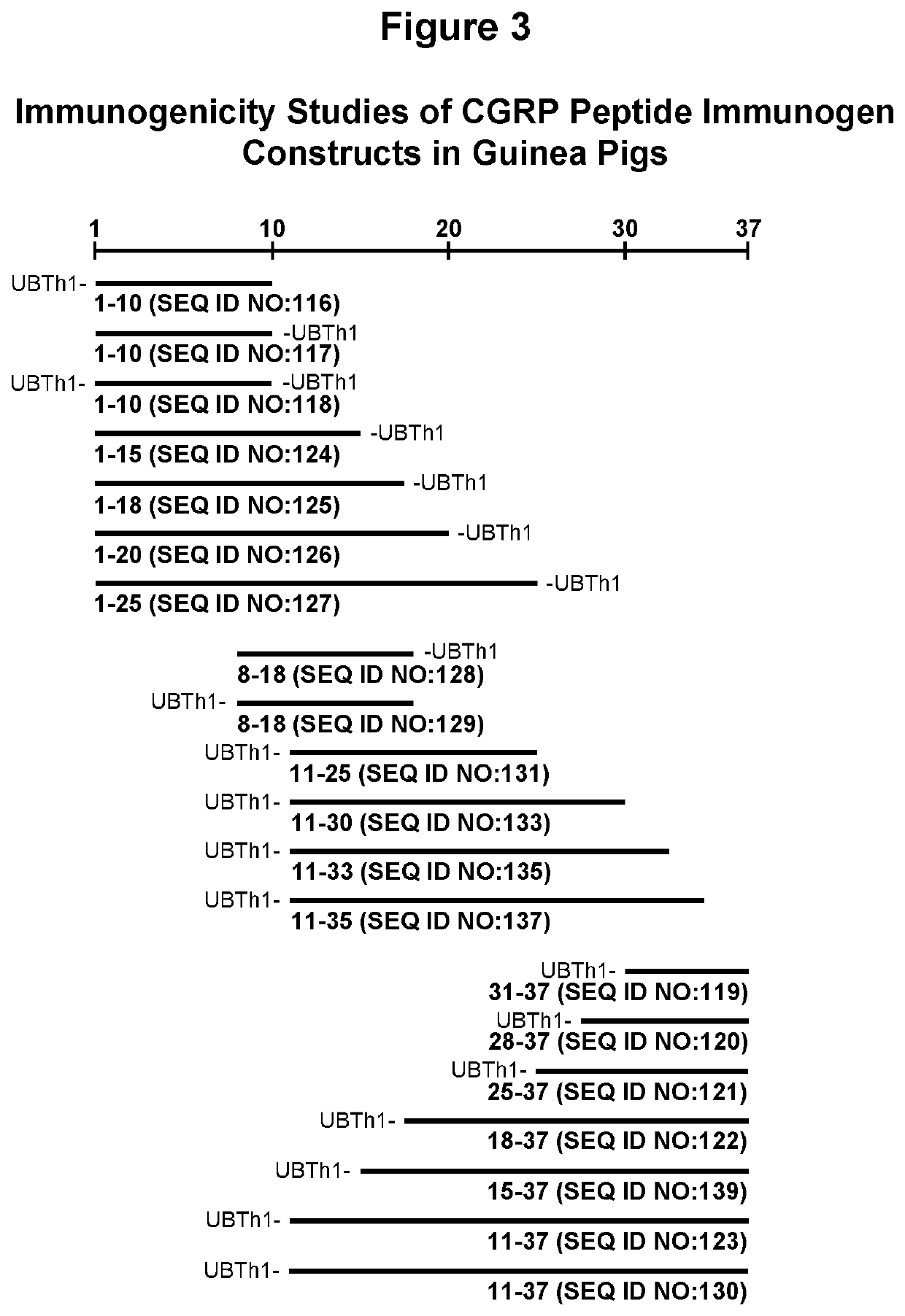
Peptide immunogens targeting calcitonin gene-related peptide (CGRP) and formulations thereof for prevention and treatment of migraine
PendingUS20220073582A1Prevention and treatment of migraineImproving immunogenicityOrganic active ingredientsNervous disorderHeterologousCGRP receptor
The present disclosure is directed to peptide immunogen constructs targeting portions of Calcitonin Gene-Related Peptide (CGRP), compositions containing the constructs, antibodies elicited by the constructs, and methods for making and using the constructs and compositions thereof. The disclosed peptide immunogen constructs have more than about 30 amino acids and contain (a) a B cell epitope having about more than about 7 contiguous amino acid residues from the CGRP receptor binding or activation regions of the full-length CGRP protein; (b) a heterologous Th epitope; and (c) an optional heterologous spacer. The disclosed CGRP peptide immunogen constructs stimulate the generation of highly specific antibodies directed CGRP for the prevention and / or treatment of migraine.
Owner:UNITED NEUROSCIENCE LIMITED
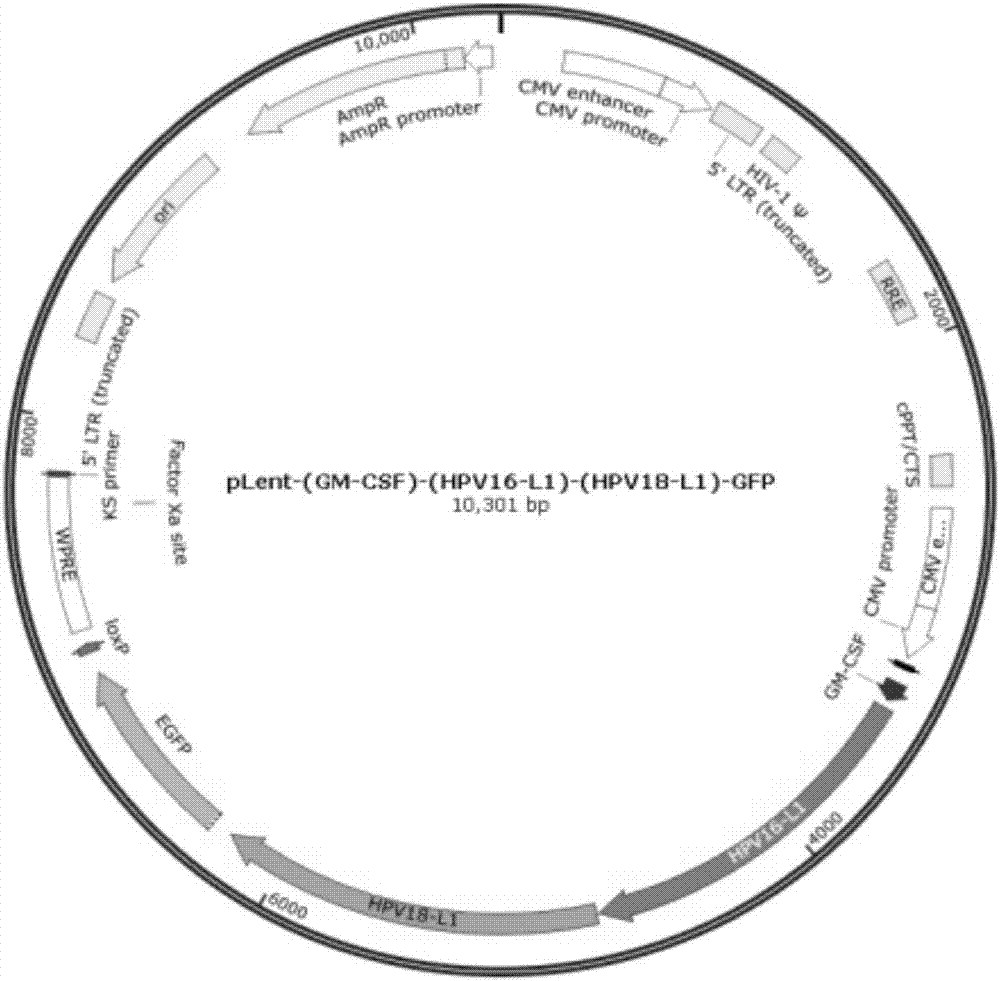
Enhanced bivalent DC (dendritic cell) vaccine of human papillomavirus HPV-16/18
InactiveCN106963945ABreak immune toleranceReduce intakePeptide/protein ingredientsViral antigen ingredientsHuman papillomavirusDc vaccine
The invention discloses an enhanced bivalent DC (dendritic cell) vaccine of human papillomavirus HPV-16 / 18, comprising genetically-modified dendritic cells, recombinant gene sequences in the genetically-modified dendritic cells are serially connected via granulocyte-macrophage stimulating factor and L1 transcription regions of human papillomavirus HPV-16 / 18, and the DC vaccine is composed of genetically-modified DC surface antigen, comprising granulocyte-macrophage stimulating factor expressed via an enhancement antigen, and L1 antigen proteins of human papillomavirus HPV-16 / 18. The DC vaccine is a prevention and treatment vaccine, capable of effectively preventing HPV-16 and HPV-18 infections and related diseases caused by HPV infections.
Owner:SHANDONG XINRUI BIOTECH CO LTD
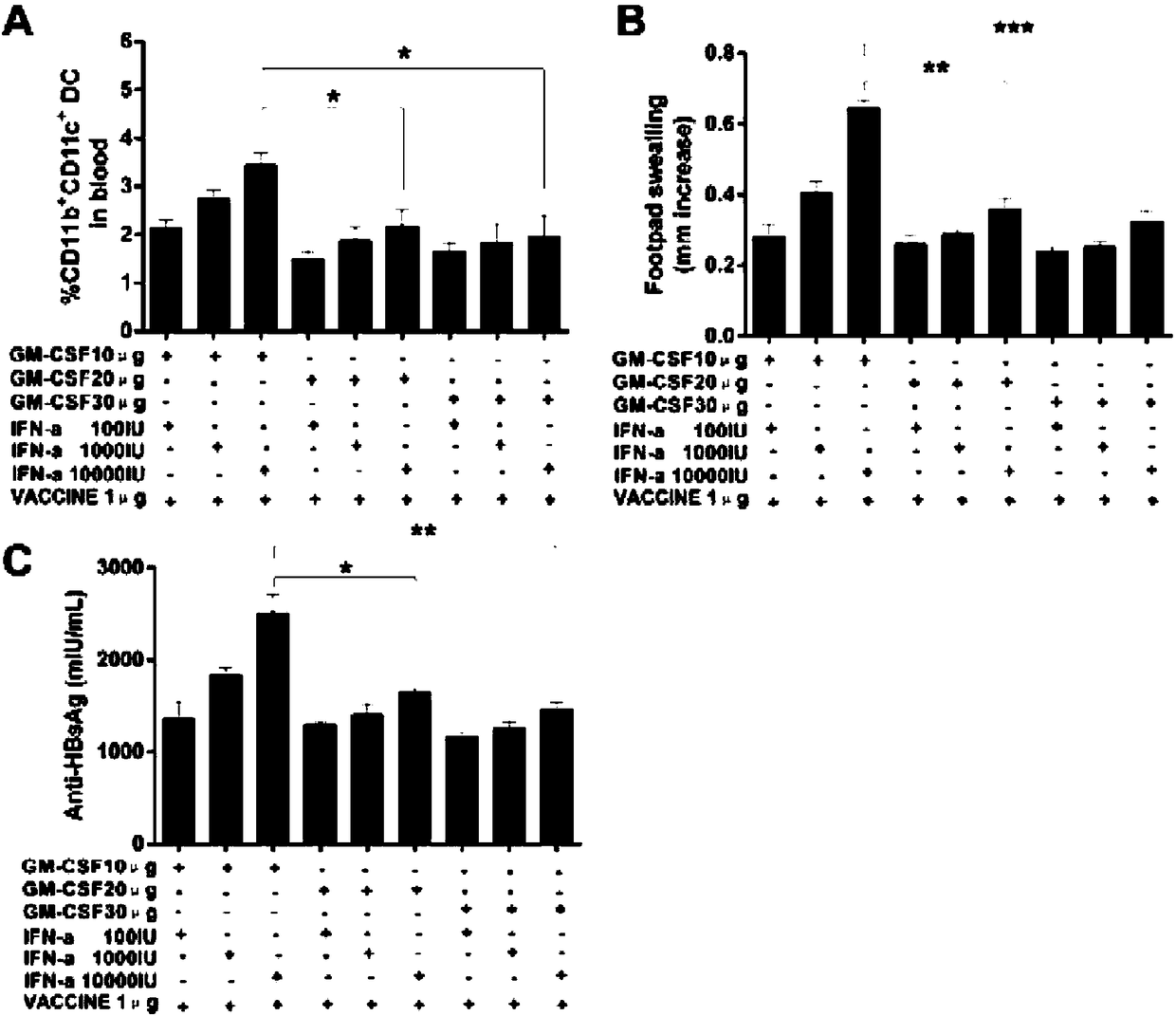
Immunoenhancer, immunotherapy medicine composition, preparation method of composition and application of immunoenhancer and composition
ActiveCN108567977AStrong immune responseImprove immunityVirus peptidesAntiviralsDiseaseGranular cell
The invention discloses an immunoenhancer, an immunotherapy medicine composition, a preparation method of the immunotherapy medicine composition and an application of the immunoenhancer and the immunotherapy medicine composition. The immunoenhancer at least comprises interferon and granular cell-macrophage colony stimulating factors, and the immunotherapy medicine composition at least comprises antigen and the immunoenhancer. The immunoenhancer can remarkably enhance the immunity of a body and improve the antigen presenting efficiency of the body, so that the body can establish effective immune activation and response. Strong antibody and cellular immune protection reaction and pathogen removing capacity can be generated, and the immunoenhancer can be applied to therapy of diseases and tumors caused by microorganisms such as viruses and bacteria.
Owner:FUDAN UNIV
Application of recombinant attenuated listeria in preparation of mesothelin high-expression cancer therapeutic vaccine
ActiveCN108714210ABreak immune toleranceMake up for the disadvantage of lower immunogenicityBacteriaMicroorganism based processesAbnormal tissue growthPhagocyte
The invention relates to application of recombinant attenuated listeria in the preparation of a mesothelin high-expression cancer therapeutic vaccine. By taking attenuated Listeria monocytogenes (Lm for short hereinafter) and attenuated Listeria ivanovii (Li for short hereinafter) as carriers, and special listeria can grow in phagocyte of a host and is a natural T-cell immune activation adjuvant,so that the specific immune response in a tumor microenvironment is effectively improved, the immune tolerance of a body is broken through, the continuous virus infection of the body is eliminated, and good treatment effects on the elimination of an infection focus and the containment of tumor development are achieved. The application has the advantages that the disadvantages that nucleic acid type vaccines and protein polypeptide type vaccines are relatively low in immunogenicity are remedied, the attenuated listeria preserves the properties that an original strain can grow in the cells and finish antigen presentation, and meanwhile, the attenuated listeria has relatively high safety.
Owner:苏州圣苏新药开发有限公司 +1
Application of recombinant attenuated Listeria in preparation of therapeutic vaccine for cervical cancer
ActiveCN109010819ABreak immune toleranceMake up for the disadvantage of lower immunogenicityAntibody mimetics/scaffoldsViral antigen ingredientsAdjuvantSpecific immunity
The invention relates to application of recombinant attenuated Listeria in preparation of a therapeutic vaccine for cervical cancer. Attenuated Listeria monocytogenes (hereinafter referred to as Lm) and attenuated Listeria ivanovii (hereinafter referred to as Li) are used as vectors, by use of the unique characteristic that the Listeria can grow in phagocytose cells in a host, and is a natural T cell immune activation adjuvant, specific immune response in tumor microenvironment can be effectively enhanced, body's immune tolerance can be broken, body's persistent viral infection can be eliminated, and good treatment results of clearing focuses and curbing tumor development can be achieved. The Listeria has the advantages that shortcomings of low immunogenicity of nucleic acid and protein peptide vaccines can be made up, the attenuated Listeria retains the characteristic that an attenuated original strain can grow intracellularly to complete antigen presentation, and meanwhile the attenuated Listeria has higher safety.
Owner:南京颂悦生物科技有限公司
Protein vaccine aiming at tumor necrosis factor alpha and applications of protein vaccine
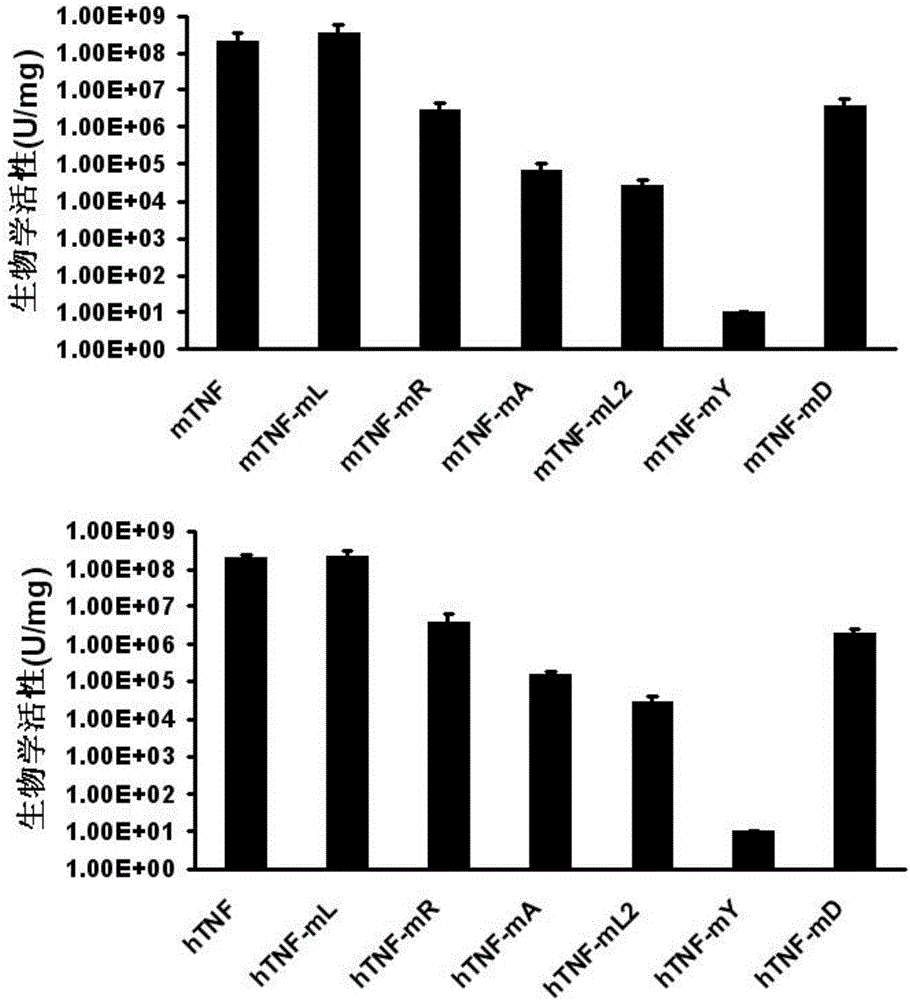
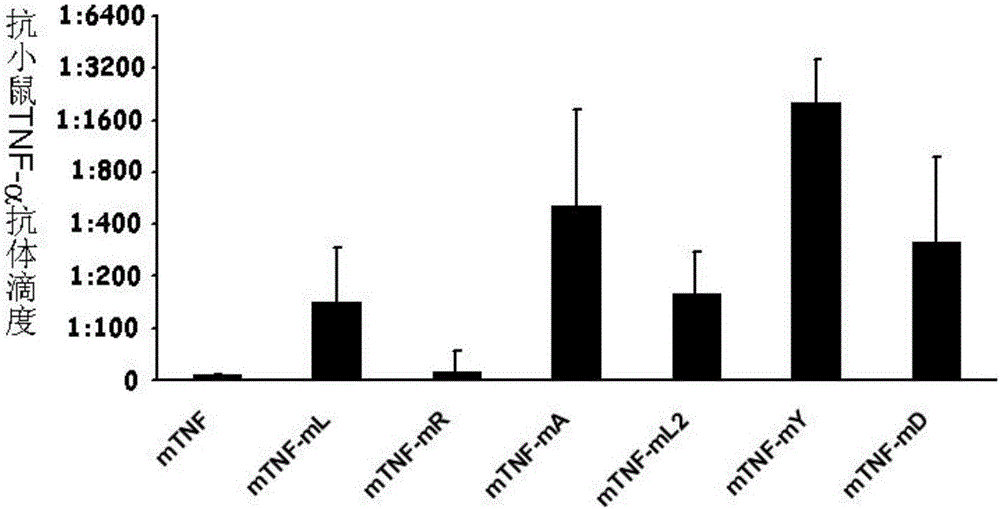
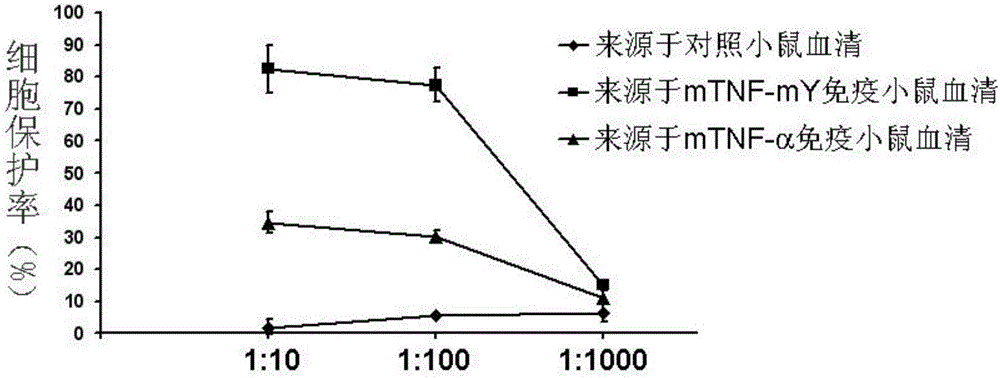
ActiveCN106540252AInhibit biological activityInhibit the inflammatory responseAntipyreticAntibody mimetics/scaffoldsImmunologic disordersMutated protein
The invention belongs to the field of biological medicines, and relates to a mutant protein vaccine aiming at tumor necrosis factor alpha (TNF-alpha) and applications of the mutant protein vaccine. The invention aims at providing the protein vaccine which is good in property and takes TNF-alpha as the target spot. According to the scheme, by immunizing the TNF-alpha mutant protein vaccine, the biological activity of the TNF-alpha mutant protein vaccine is inhibited, the cross immunologic reaction is generated in vivo, the biological functions of TNF-alpha are neutralized, and the autoimmune diseases and inflammatory diseases are inhibited. The protein vaccine aiming at TNF-alpha can be used for treating the autoimmune diseases and the inflammatory diseases and preventing the relapse of the autoimmune diseases and the inflammatory diseases.
Owner:SICHUAN UNIV
Dual-target CAR-T therapeutic vector for breast cancer and construction method and application thereof
PendingCN108864288AImprove proliferative abilityIncrease vitalityAntibody mimetics/scaffoldsNucleic acid vectorCD137CD28
The invention provides a dual-target CAR-T therapeutic vector for breast cancer and a construction method and application thereof. The dual-target CAR-T therapeutic vector consists of a lentiviral expression vector pCDH-EF1 alpha-MCS-(PGK-Puro) and a CAR structure. The CAR structure is specifically Igkappa-iRGD-mesothelin scFv-(G4S)5-CD133scFv-CD8 alpha-CD28-CD137-CD3 zeta-2A-CCL19. A virus infection mode is adopted to obtain a T cell containing the therapeutic vector, namely a CAR-T cell (Chimeric Antigen Receptor T-Cell), and breast cancer cells can be recognized and killed in a targeted mode through expression of the CAR structure.
Owner:SHANGHAI YIHAO BIOTECH CO LTD
Mesothelin high-expression cancer treatment vaccine based on recombinant attenuated listeria monocytogenes
InactiveCN108704126ABreak immune toleranceMake up for the disadvantage of lower immunogenicityBacteriaBacteria peptidesTreatment effectAdjuvant
The invention relates to a mesothelin high-expression cancer treatment vaccine based on recombinant attenuated listeria monocytogenes. The attenuated listeria monocytogenes (Lm) is used as a carier, the characteristics that the Lm can specifically grow in a host phagocyte and is a natural T cell immune activated reagent are utilized, specific immune response in a tumor microenvironment can be effectively improved, the immune tolerance of a body can be broken, persistent viral infection of the body can be cleaned, and an excellent treatment effect for cleaning the focus and inhibiting tumor development can be achieved. The mesothelin high-expression cancer treatment vaccine has the advantages of overcoming the defect of relatively low immunogenicity of nucleotide and protein polypeptide vaccines, the attenuated listeria remained with original strains can grow in cells, so that higher safety is achieved while the characteristic of antigen presentation can be completed.
Owner:南京颂悦生物科技有限公司
Method for preparing tumor-specific DC vaccine by applying mononuclear cells in umbilical cord blood
ActiveCN103405758ABreak immune toleranceBlood/immune system cellsAntibody medical ingredientsAntigenDc vaccine
The invention discloses a method for preparing a tumor-specific DC vaccine by applying mononuclear cells of umbilical cord blood. The method comprises (1) a step of preparing autologous tumor-related holoantigen; (2) a step of obtaining the umbilical cord blood; (3) a step of obtaining the mononuclear cells derived from the umbilical cord blood; (4) a step of performing induction culture for a precursor DC of the mononuclear cells derived from the umbilical cord blood; (5) a step of performing amplification and culture of an immature DC; and (6) a step of preparing the DC vaccine.
Owner:玥特农生物科技河北有限责任公司
TEM-1 targeted gene vaccine and construction and application thereof
InactiveCN111773381ANot strict immune toleranceBreak immune toleranceLectin superfamilyAntibody mimetics/scaffoldsAntigenAdjuvant
The invention relates to the technical field of biology, in particular to a TEM-1 targeted gene vaccine and construction and application thereof. According to the gene vaccine, a flagellin (SF) is used as an adjuvant, rTEM-1 is used as an antigen, and a rTEM-1-SF fusion gene is obtained, and is constructed in eukaryotic expression plasmids pcDNA3.1. The rTEM-1 / flagellin fusion gene vaccine can restrain formation of tumor vessels of breast cancer tumor-bearing mice, the size of the tumor and transfer of the tumor, can restrain the transfer of breast cancer cells to lung tissue and liver tissue, and has the effects of preventing and treating the breast cancer.
Owner:贵阳市第二人民医院
Immunopotentiator, immunotherapy pharmaceutical composition and its preparation and application
ActiveCN108567977BStrong immune responseImprove immunityVirus peptidesAntiviralsDiseaseImmunocompetence
The invention discloses an immune enhancer, which at least includes interferon and granulocyte-macrophage colony-stimulating factor, and an immunotherapy pharmaceutical composition, which at least includes an antigen and the above-mentioned immune enhancer. The invention also discloses the preparation method of the immunotherapy pharmaceutical composition, the application of the above immune enhancer and immunotherapy pharmaceutical composition. The immunopotentiator provided by the invention can significantly improve the immune ability of the body, improve the antigen presentation efficiency of the body, and enable the body to establish effective immune activation and response; produce stronger antibodies and cellular immune protection responses and the ability to remove pathogens, can It is used in the treatment of diseases and tumors caused by microorganisms such as viruses and bacteria.
Owner:FUDAN UNIV
Peptide having high affinity for pd-l1 protein and use thereof
ActiveUS20210230286A1Reduced growth rateProlong survival timePeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsHuman tumorImmunity
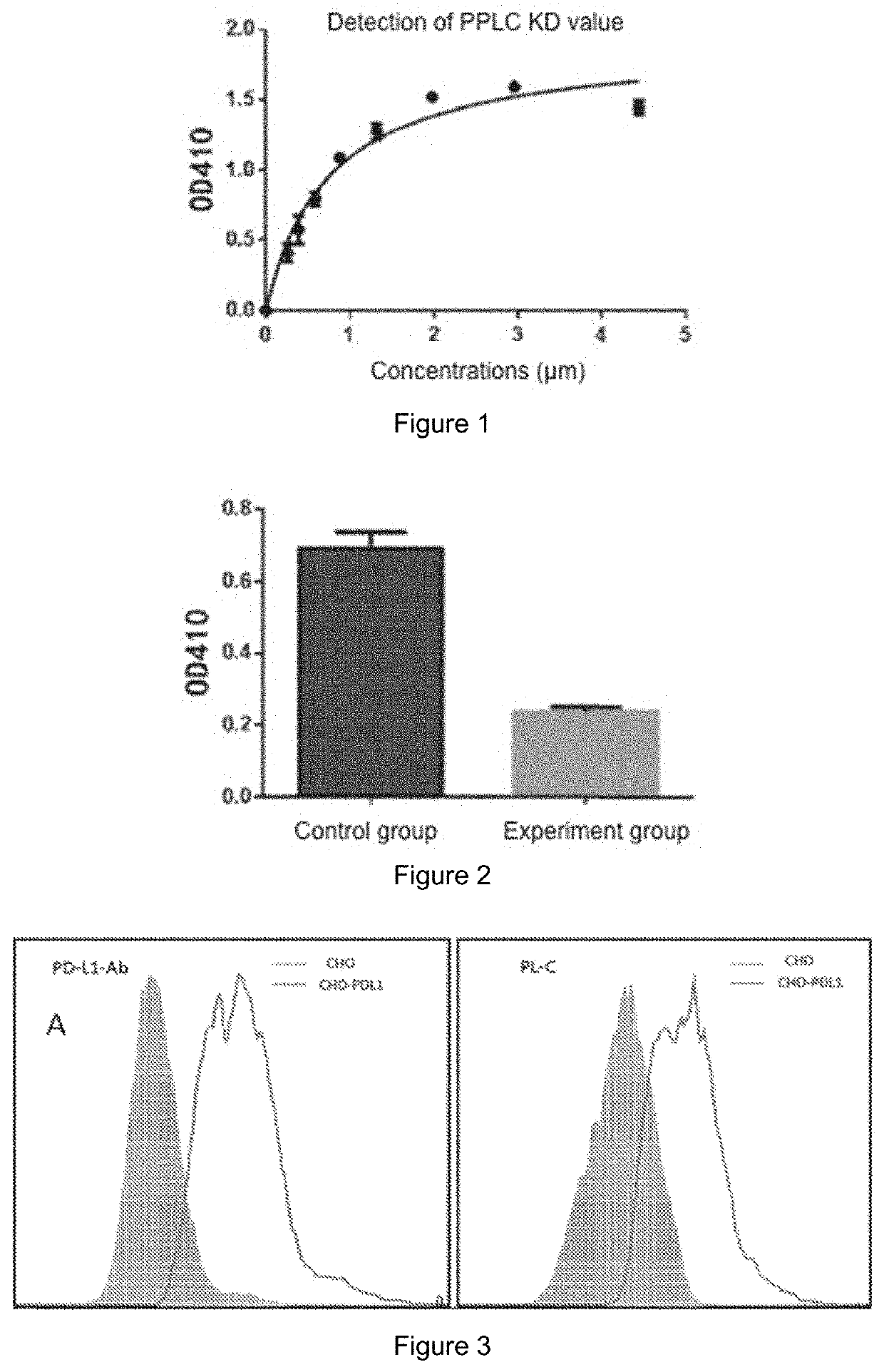
The present invention provides a peptide having high binding affinity for PD-L1 protein and use thereof. The peptide has an amino acid sequence of SEQ. ID. NO. 1, SEQ. ID. NO. 3 or SEQ. ID. NO. 4, or the peptide is a tandem or branched peptide with a single repeat or multiple repeats of SEQ. ID. NO. 1, SEQ. ID. NO. 3 and SEQ. ID. NO. 4 and has an amino acid sequence of SEQ. ID. NO. 5. The peptide can bind to human PD-L1 with high affinity, competitively block the affinity of PD-1 / PD-L1 protein, block the negative regulatory tolerance pathway of human tumors, activate immunity and increase the lethality of T cells against tumor cells.
Owner:ZHU NAISHUO +1
Method for preparing tumor-specific DC vaccine by applying CD34+ cells of umbilical cord blood
ActiveCN103405759BBreak immune toleranceBlood/immune system cellsAntibody medical ingredientsAntigenDc vaccine
Owner:玥特农生物科技河北有限责任公司
Therapeutic DC compound vaccine against herpes simplex virus and preparation method thereof
ActiveCN109701008BTreatment Prevention and TreatmentBreak immune toleranceAntiviralsAntibody medical ingredientsDendritic cellSpecific immunity
The invention discloses a therapeutic DC compound vaccine against herpes simplex virus and a preparation method thereof, comprising three kinds of dendritic cells loaded with HSV-2 virus-specific immune antigen gene fragments modified, and the three kinds of HSV-2 virus-specific immune antigen gene fragments loaded with dendritic cells The gene fragments of sexual immune antigens were gC+US10, DC‑UL47, and gD+ICP4, respectively. The present invention uses the granulocyte-macrophage cluster factor receptor signal peptide to guide the expression of the antigen, which can improve the expression efficiency of the antigen, enhance its immune effect, and generate a stronger anti-HSV specific immune response.
Owner:上海兴瑞一达生物科技有限公司
Peptide having high affinity for PD-L1 protein and use thereof
ActiveUS11377501B2High affinityBlock affinityPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsHuman tumorImmunity
The present invention provides a peptide having high binding affinity for PD-L1 protein and use thereof. The peptide has an amino acid sequence of SEQ ID NO: 1, SEQ ID NO: 3 or SEQ ID NO: 4, or the peptide is a tandem or branched peptide with a single repeat or multiple repeats of SEQ ID NO: 1, SEQ ID NO: 3 and SEQ ID NO: 4 and has an amino acid sequence of SEQ ID NO: 5. The peptide can bind to human PD-L1 with high affinity, competitively block the affinity of PD-1 / PD-L1 protein, block the negative regulatory tolerance pathway of human tumors, activate immunity and increase the lethality of T cells against tumor cells.
Owner:ZHU NAISHUO +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com