Novel asthma polypeptide vaccine and preparation method thereof
A technology for vaccines and asthma, applied in the field of biomedicine, can solve the problems such as the absence of IL-13 polypeptide-related vaccines, and achieve the effects of breaking immune tolerance, small molecular weight, and convenient synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Example 1 Screening and Determination of Asthma IL-13 Short Peptide in the Present Invention
[0099] By consulting the library and detecting the binding affinity of IL-13 antigen short peptides to MHC, as well as the interaction between IL-13 antigen short peptides and IL-13 receptors, the best of the new asthma vaccines can be established. IL-13 antigen short peptide.
[0100] Specific method: From the Allele Frequency Net Database (AFND, http: / / www.allelefrequencies.net / ) database, the coverage of asthma-related screening ranges from 13.5% (DRB1*09:01) to 1% (DRB1*14:05) DR epitopes of human leukocytes, covering 95.9% of the Chinese population in total, obtained 20 epitopes. As a vaccine candidate antigen polypeptide, it needs to have high affinity with MHC, so 20 cases of Chinese HLA-DRB1 epitopes were predicted by six commonly used Immune Epitope Database (IEDB, http: / / www.iedb.org) method to filter. Among the 20 epitopes, the epitopes that can bind to MHC class...
Embodiment 3
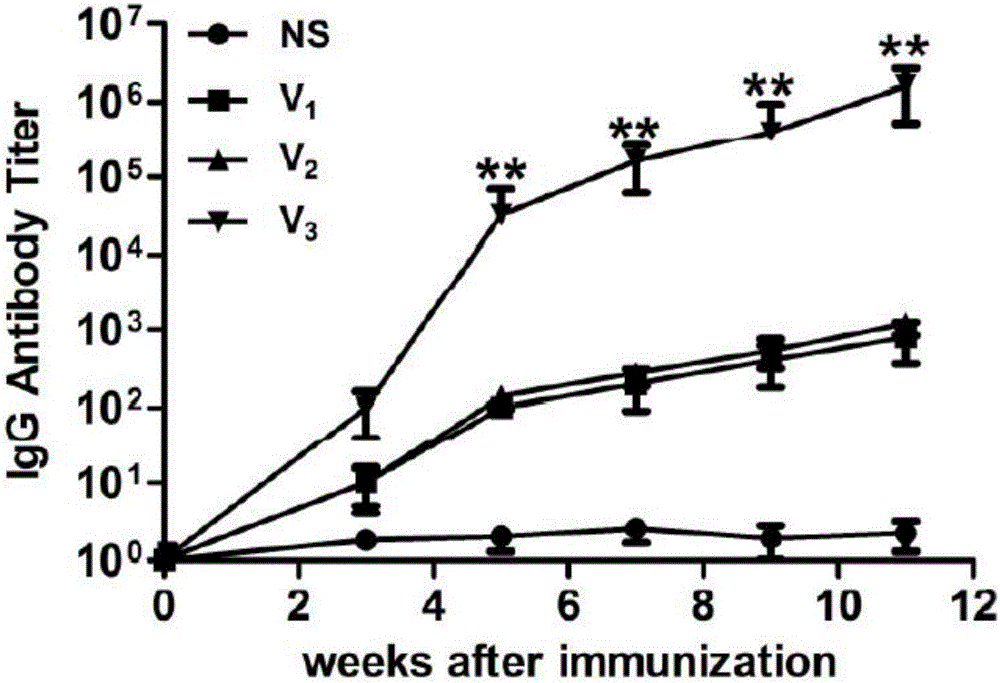
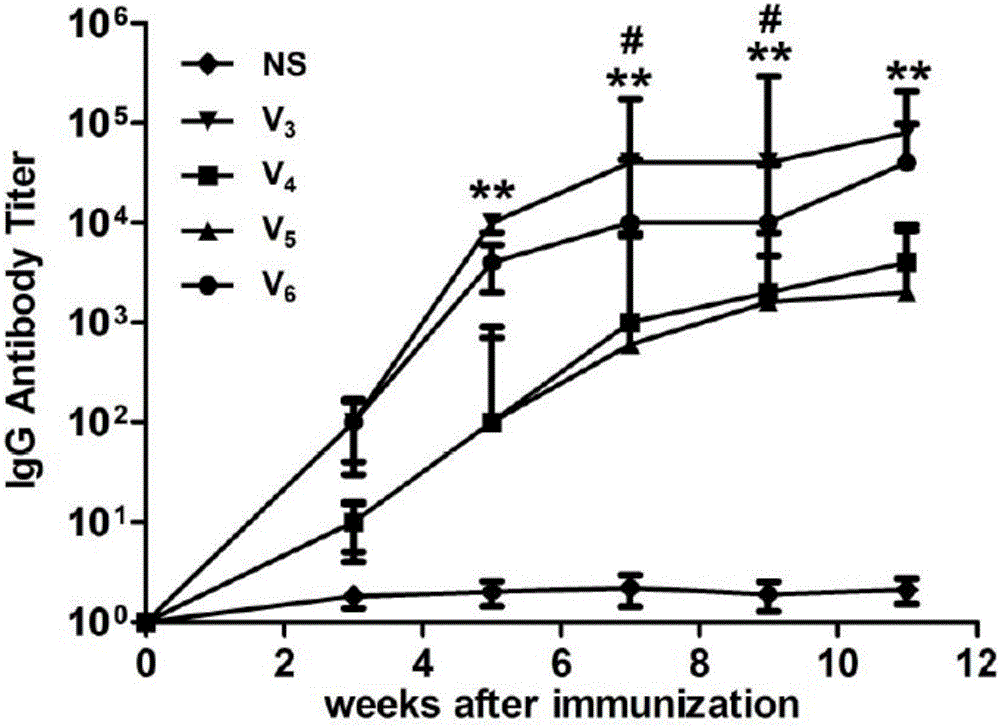
[0116] Example 3 Animal immunization experiments and antibody titer evaluation of four polypeptides constructed on the basis of E3
[0117] After determining E3 as the optimal epitope with the function of treating asthma among the three candidate IL-13 antigen short peptides, the present invention considers coupling different helper T cell epitope polypeptides to enhance IL-13 antigen short peptide Immunogenicity, so as to obtain the best candidate polypeptide for the treatment of asthma, to be developed as a vaccine. Because when determining the IL-13 antigen short peptide epitope, the coupled helper T cell DiTOX epitope peptide (AYNFVESIINLFQVVHNSYN). However, it is not sure that this epitope has the best effect of increasing the immunogenicity of short peptides of IL-13 antigen, so we screened three other epitope peptides: PADRE; TT8; TT9; respectively coupled with E3 to obtain TT8- E3(V4), TT9-E3(V5) and PADRE((V6) are used together with DiTOX-E3(V3) to evaluate the thera...
Embodiment 4
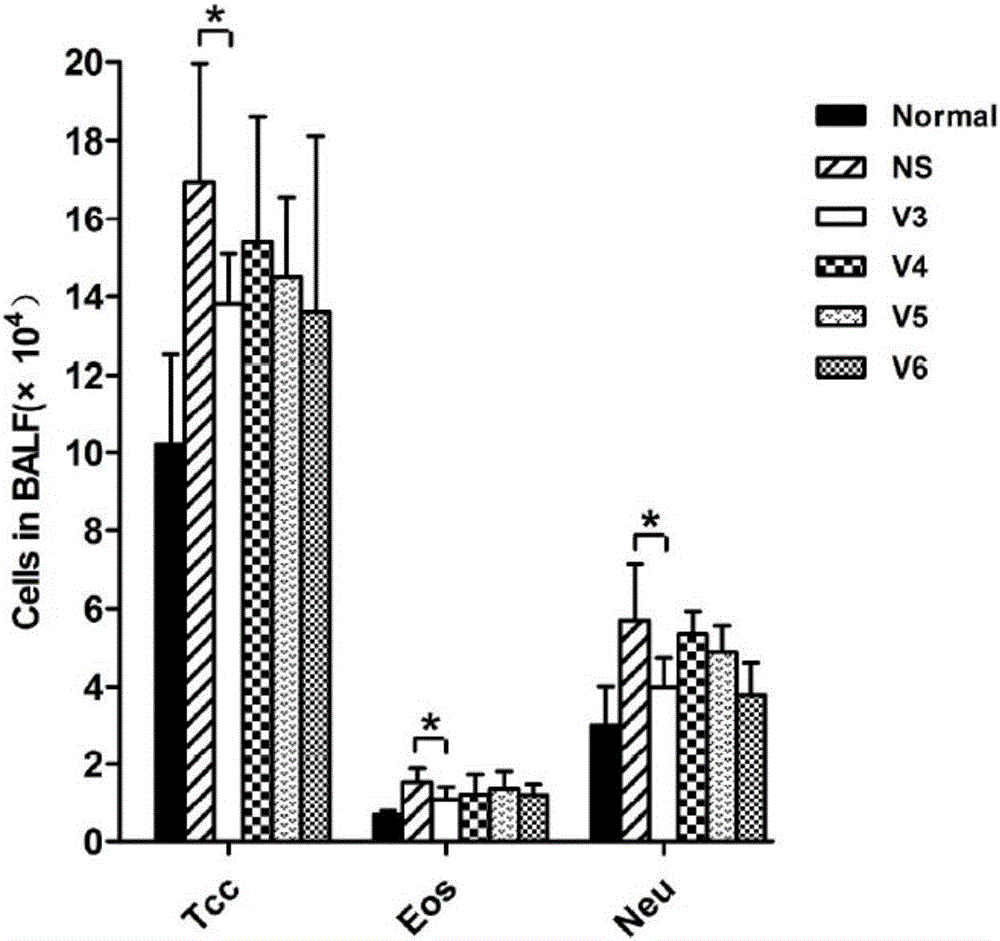
[0132] Example 4 The results of the alveolar lavage fluid (BALF) cell count and related cytokine detection results of the animal immunization experiment of four polypeptides constructed on the basis of E3:
[0133] See embodiment 3 for grouping experimental treatment.
[0134] BALF count detection method is as follows: when the mice are killed, use pre-cooled saline for alveolar lavage, collect about 1mL of alveolar lavage fluid; Resuspended in saline and sent for GLP detection to count eosinophils. The supernatant was detected using the relevant kit detection method.
[0135] IFN-γ: The detection kit is R&D Systems Mouse IFN-gamma (Catalog Number: VAL607)
[0136] IL-4: The detection kit is R&D Systems Mouse IL-4 (Catalog Number: VAL603)
[0137] IL-13: The detection kit is eBioscience Mouse IL-13 (Catalog Number: 88-7137)
[0138] At the same time, the eyeballs were picked to collect blood, and the supernatant was collected by centrifugation and stored at -20°C for the d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com