Protein vaccine aiming at tumor necrosis factor alpha and applications of protein vaccine
A vaccine and application technology, applied in the field of biomedicine, can solve the problem of high cost, achieve the effect of low therapeutic dose, improve immune effect, and inhibit inflammatory response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 Obtaining of TNF-α mutein of the present invention
[0035] Using computer-aided design, combined with previous studies of the present invention, it is found that the active center of hTNF-α includes four domains of 29-36AA, 84-91AA, 117-119AA and 143-148AA. Among them, amino acid residues such as Glu23, Leu29, Arg32, Ala33, Asn34, Leu75, Ala84, Tyr87, Asp143 may be important amino acids involved in the biological activity of hTNF-ɑ.
[0036] The present invention designs mutant proteins for the following sites, and according to the sequence homology comparison of human and mouse TNF-α proteins, designs corresponding mouse mutant proteins for preclinical research (specific mutation sites and mutation methods As shown in Table 1). On this basis, the present invention synthesizes the gene sequence encoding the above-mentioned mutant protein through conventional nucleic acid synthesis technology, and uses this as a template to carry out PCR amplification thro...
Embodiment 2
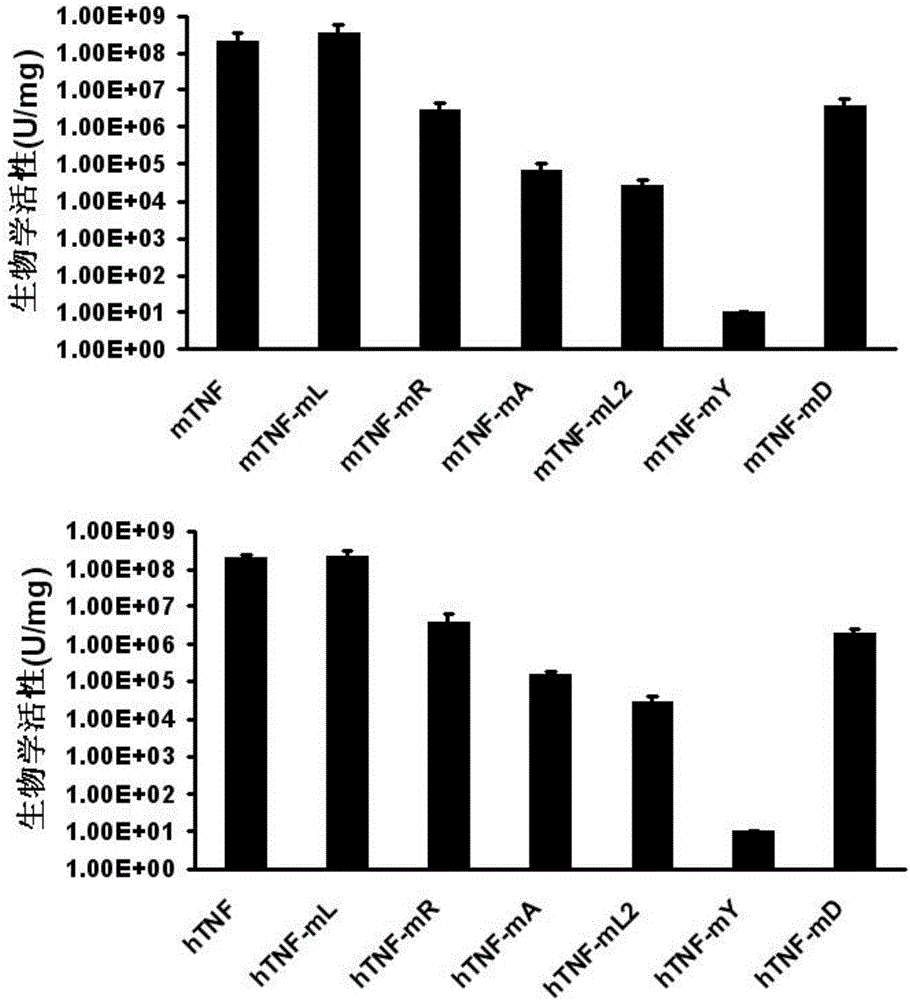
[0046] Example 2: Identification of Biological Activity of TNF-α Mutants
[0047] The identification of the biological activity of TNF-α mutants is determined by the activity test of L929 cells. L929 cells are mouse fibroblasts sensitive to TNF-α activity. This method is a standard method for measuring the biological activity of TNF-α protein. The brief description is as follows : L929 in logarithmic growth phase at 1.5×10 4 per well in a 96-well plate. Design 10 dose gradients, each dose gradient has 2 duplicate wells, the actual dilution concentration of TNF-α mutant or standard control substance is 1.0×10 -6 mg / ml. Each protein was diluted in complete medium PRMI 1640 containing 3% FBS and 0.7 μg / ml actinomycin D. After culturing at 37° C. for 24 hours, the number of surviving cells was detected by MTT method, and a curve was drawn to determine the biological activity of each mutant protein at the level of 50% effective concentration (IC50).
[0048] The result is as f...
Embodiment 3
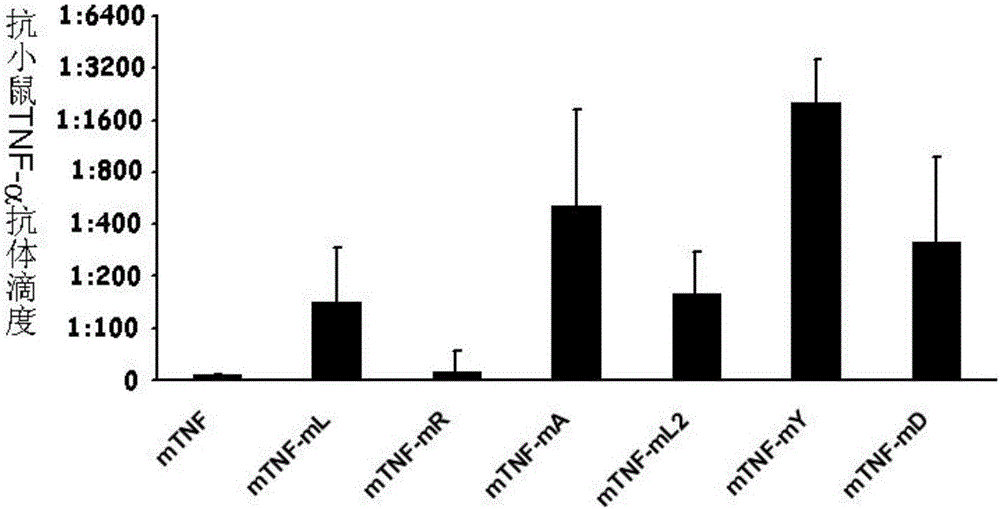
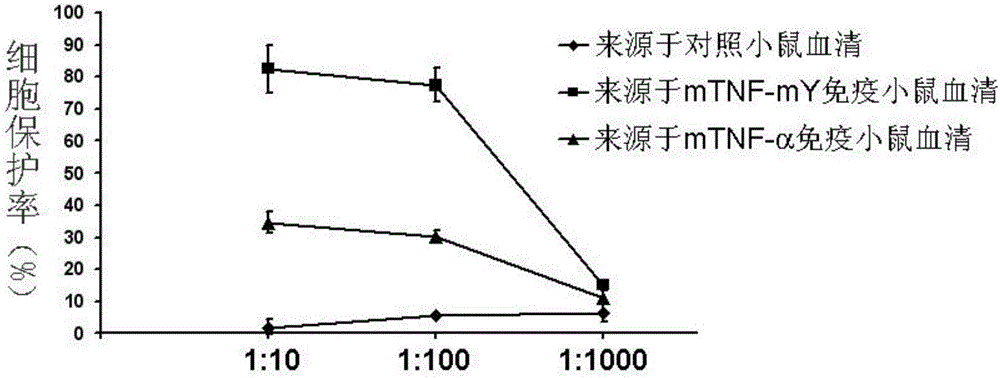
[0049] Example 3 Y86K breaks immune tolerance and produces neutralizing antibodies
[0050] Female Bab / c mice aged 6-8 weeks were randomly divided into 6 groups: wild-type protein immunization group, mutant protein immunization group (mTNF-mL, mTNF-mR, mTNF-mA, mTNF-mL2, mTNF-mY, mTNF-mD), 6 rats in each group. In this study, Al(OH) 3 as an adjuvant. Add 10μg protein to 50μl 50mM PB, add Al(OH) in equal volume 3 50μl (concentration: 10mg / ml), mix well. Mice were immunized subcutaneously on both sides of the groin, once every other week, a total of 6 times. Before the first immunization and 1 week after each immunization, blood was collected from the orbital canthus venous plexus of the mice, and the serum was separated. Anti-mouse TNF-α antibody titers in serum were detected by ELISA. The result is as figure 2 Shown, compared with wild-type protein, each mutant vaccine all can stimulate mice to produce higher antibody titer, wherein mTNF-mY (amino acid sequence is sho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com