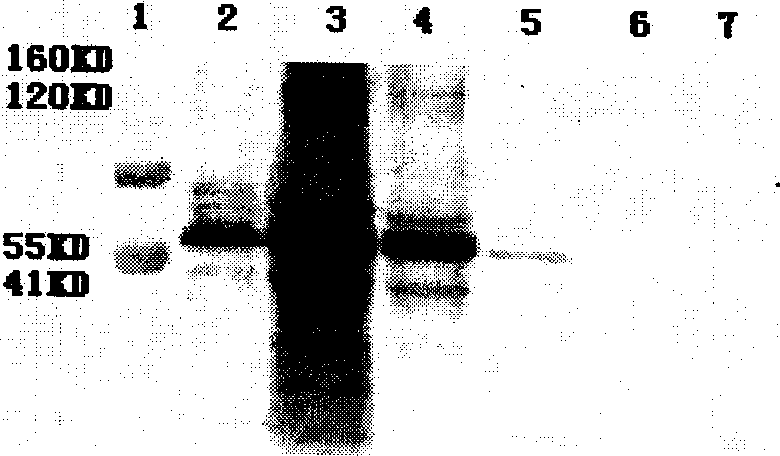HIV-1 virus-like particle and its prepn and use
An HIV-1, virus-like technology, applied in biochemical equipment and methods, viruses/phages, viral antigen components, etc., can solve the problems of low immune response, difficulty in continuous production of VLP, rapid decline in expression, etc., to achieve strong immunity. Originality, good safety, the effect of improving the possibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1. Selection of plasmids and resistance selection markers
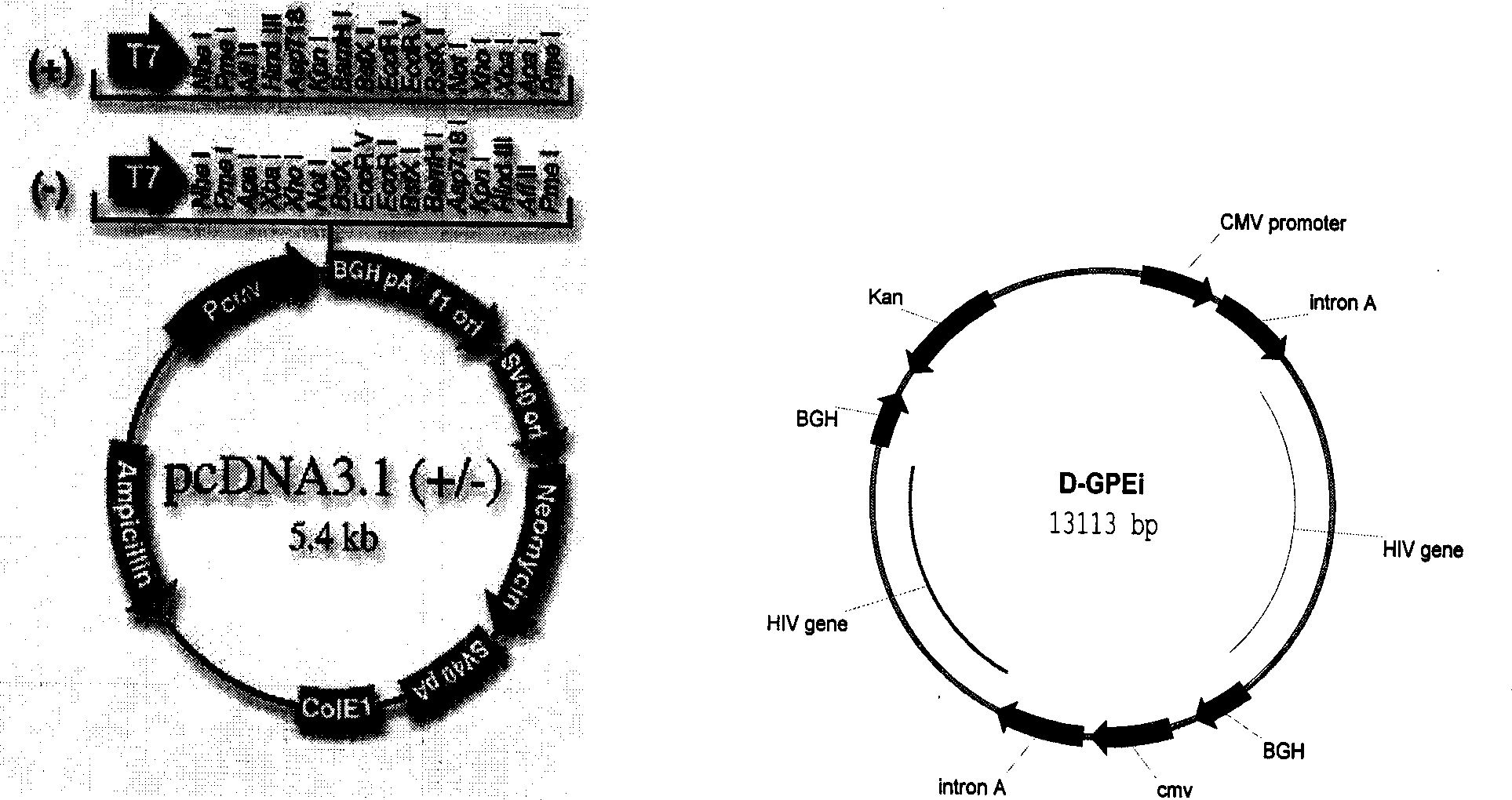
[0040] D-GPEi plasmid: We chose the eukaryotic cell expression plasmid D-GPEi, which was constructed by our laboratory, and cloned the main structural protein genes Gag, Pol and Env of the epidemic strain (regional) HIV-1 in China into a self-developed construction The new-generation expression vector pVR vector (see Patent Publication No. CN1631441A for specific construction methods). The vector is composed of CMV promoter, kanamycin resistance gene, prokaryotic cell high copy factor, intron A and other conventional components. It meets the safety standards of the US FDA for human clinical trials, and has the ability to stably and efficiently express foreign genes Features. The antigen genes contained in the constructed plasmid are GagPol and Env of the modified HIV-1 epidemic strain in China. The gene expression products are the same as the original gene, but a large number of expression inhibitors i...
Embodiment 2
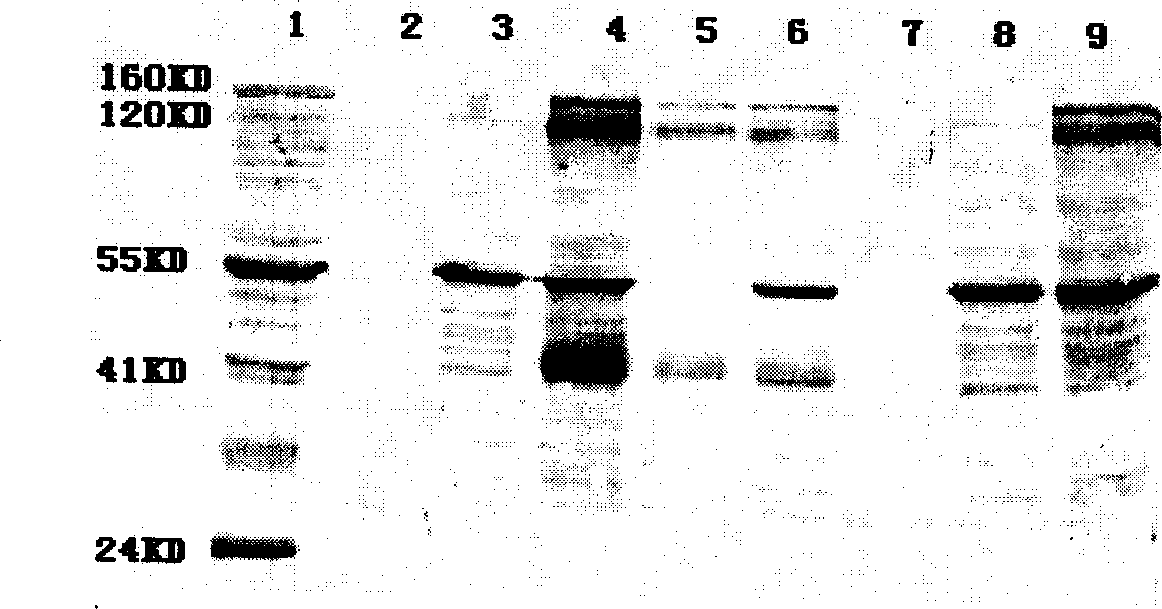
[0042] Embodiment 2. Construction of 293 stable cell lines
[0043] 1. Materials, reagents and instruments
[0044] 25cm 2 Cell culture flask, six-well plate, 96-well plate, CO2 incubator (Thermoforma), 293 cells, plasmid D-GPEi (constructed in our laboratory), vector pcDNA3.1 (purchased from Invitrogen), lipofectmine2000 (purchased from Invitrogen) , polyacrylamide gel electrophoresis (Bio-Rad), G418 (purchased from Dingguo, repackaged by gemview); HIV positive serum (collected from positive patients in Guangxi); HRP-IgG (Jackson ImmunoResearchlaboratories, INC).
[0045] Cell lysate: DTT 0.617g, SDS 0.8g, 1M Tris-HCl pH6.83.2ml, glycerin 4ml, bromofin 10.08g, water 12.8ml;
[0046] 5xTris-glycine electrophoresis buffer: 15.1g Tris base, 44g glycine, 5g SDS, add water to 1000ml;
[0047] Transfer buffer: dissolve 2.9g of glycine, 5.8g of Tris base, 0.37g of SDS, and 200ml of methanol in 800ml of water, and set the volume to 1000ml.
[0048] 2. Method steps
[0049] 2.1. ...
Embodiment 3
[0060] Construction of embodiment 3.Vero stable cell line
[0061] 1. Materials, reagents and instruments
[0062] 25cm 2 Cell culture flask, six-well plate, 96-well plate, CO2 incubator (Thermoforma), Vero cells, plasmid D-GPEi (constructed in our laboratory), vector pcDNA3.1 (purchased from Invitrogen), lipofectmine2000 (purchased from Invitrogen) , polyacrylamide gel electrophoresis instrument (Bio-Rad), G418 (purchased from Dingguo, gemview repackaged) HIV positive serum (collected from Guangxi positive patients); anti-human IgG (Jackson ImmunoResearchlaboratories, INC).
[0063] Cell lysate: DTT0.617g, SDS0.8g, 1M tris-HCl pH6.83.2ml, glycerin 4ml, bromphenol0.08g, H 2 O12.8ml;
[0064] 5xTris-glycine electrophoresis buffer: 15.1g Tris base, 44g glycine, 5g SDS, add water to 1000ml;
[0065] Transfer buffer: dissolve 2.9g of glycine, 5.8g of Tris base, 0.37g of SDS, and 200ml of methanol in 800ml of water, and set the volume to 1000ml.
[0066] 2. Method steps
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com