Enhanced bivalent DC (dendritic cell) vaccine of human papillomavirus HPV-16/18
A technology of human papillomavirus and HPV-16, applied in the field of biology and new medicine, can solve the problems of large amount of infected tissue, difficulty in determining the optimal stimulation dose, and induction of autoimmune reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Induction of imDC
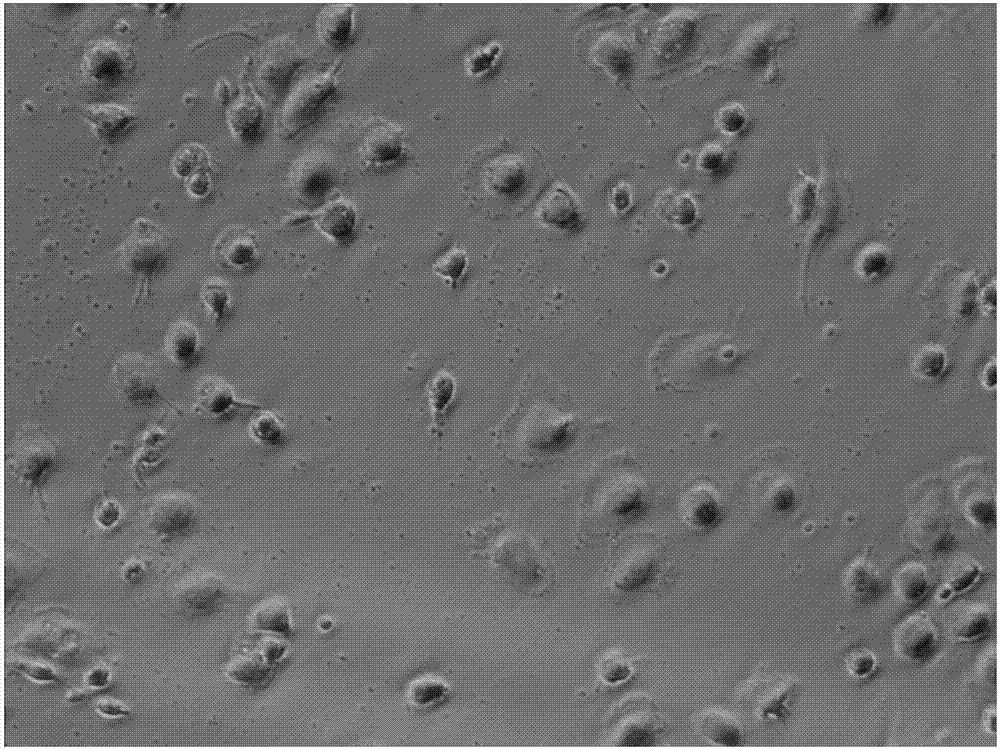
[0032] (1) Collect 50 ml of umbilical cord blood from healthy puerperas, use lymphocyte separation medium to separate mononuclear cells, and culture medium (purchased from TaKaRa Company, GT-T551), 37 ° C, 5% CO 2After culturing under the condition for 2-3 hours, discard the suspended dead cells, keep the adherent cells, and add cytokines rhIL-4 (final concentration: 50ng / ml), rhGM-GSF (final concentration: 100ng / ml) to induce single Differentiate nuclear cells into DCs, replace the new medium every 48 hours, obtain imDCs on the fifth day of culture, observe the cell morphology of DCs before maturation through an inverted microscope, detect the expression of cell surface molecular markers CD83+ and CD86+ by flow cytometry, and comprehensively identify the induced imDC.
Embodiment 2
[0033] Example 2: L1 sequence modification of HPV-16 and HPV-18 mature DC cells
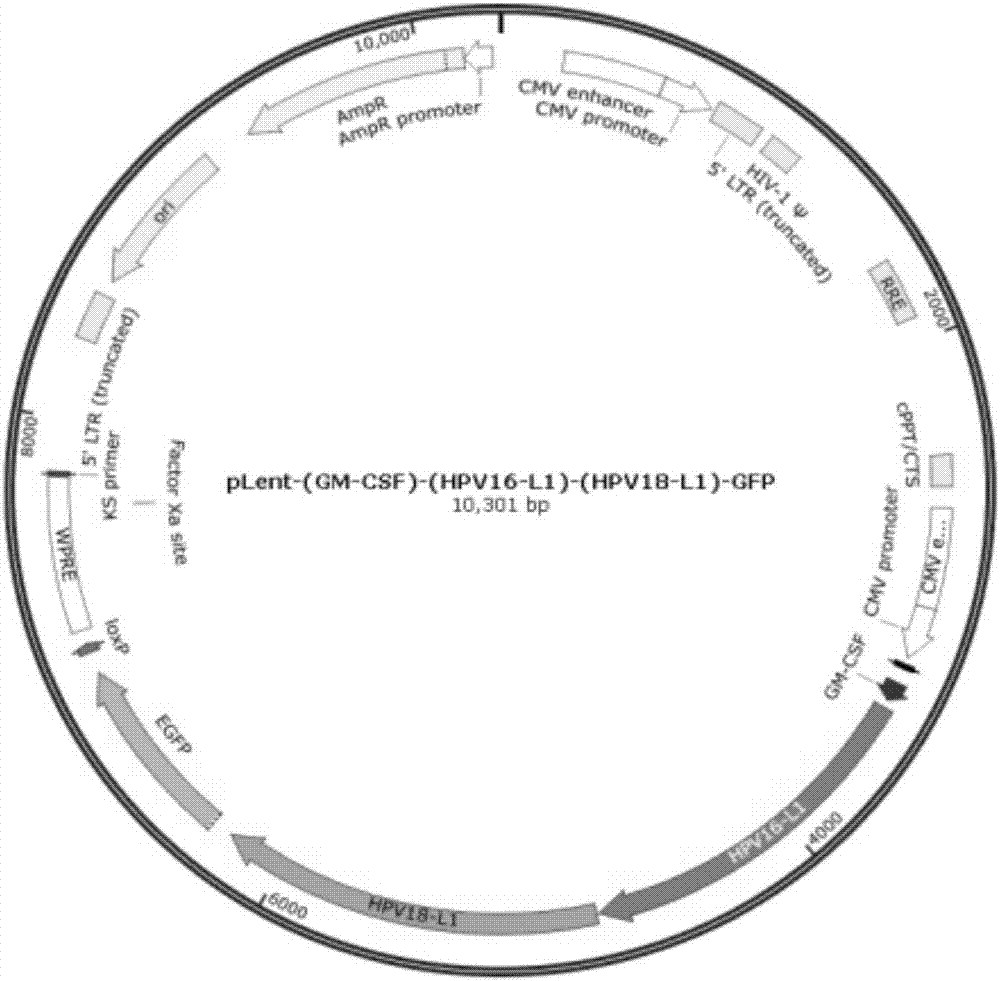
[0034] (1) Packaging and preparation of lentivirus: using gene cloning technology, PCR amplifies the full-length cDNA sequence of L1 gene of HPV-16 and HPV-18, and connects it into the lentiviral expression vector pLent-C-GFP to construct the lentivirus of L1 antigen. Viral expression vector, identified by PCR and nucleic acid sequencing. Perform virus titer determination.
[0035] (2) Infect imDC with the constructed L1 lentiviral vector, culture the infected imDC for 6 hours, add the maturation factor TNF-α, induce mature DC cells, observe the cell morphology of mature DC through an inverted microscope, and detect the cells by flow cytometry Expression of surface molecular markers CD83+ and CD86+ comprehensively identifies induced mDCs. The mDC can be directly injected as a vaccine against HPV16 and HPV18.
[0036] In order to verify the presence or absence of GM-CSF on the expression of HPV...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com