Immunopotentiator, immunotherapy pharmaceutical composition and its preparation and application
The technology of an immunotherapy drug and an immunopotentiator is applied in the field of immunotherapy pharmaceutical composition and its preparation, and immunopotentiator, which can solve the problems such as the toxic and side effects of adjuvants, and achieve the effects of less side effects, improved presentation efficiency, and convenient use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0199] Example 1, IFN-α / GM-CSF / VACCINE triggers humoral immunity and cellular immunity in wild-type mice
[0200] 1.1 Optimal dosage ratio of GM-CSF, IFN-α and VACCINE
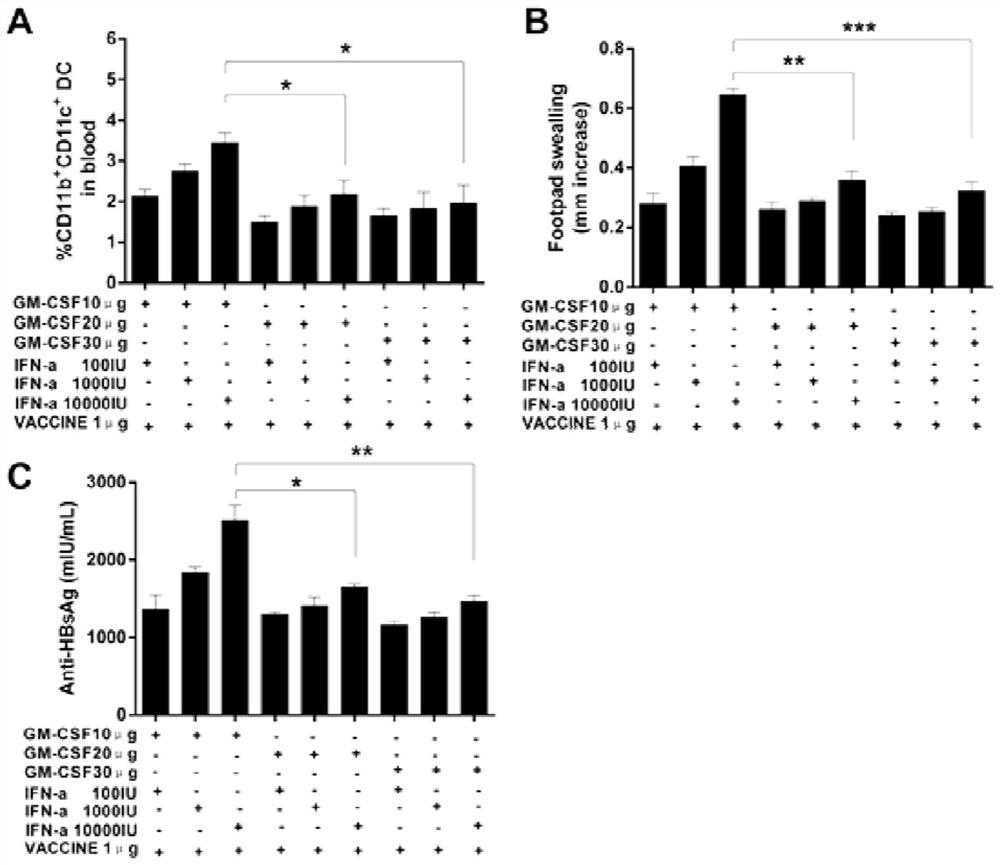
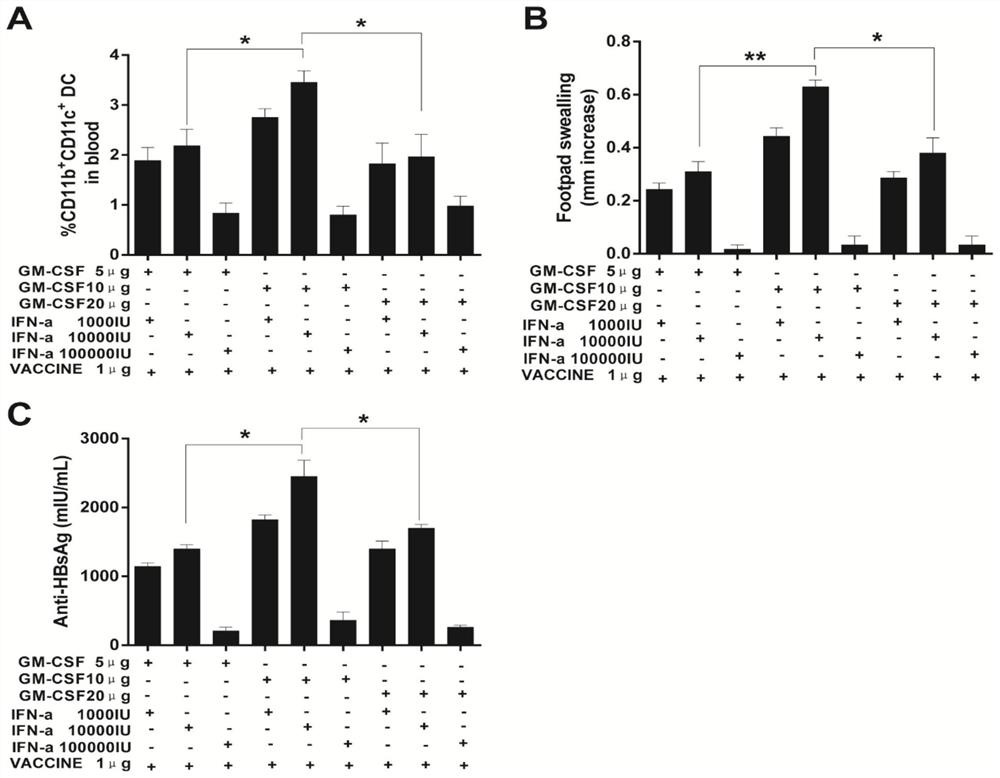
[0201] In order to verify that the mixture of GM-CSF, IFN-α and VACCINE achieves the same effect as the injection of GM-CSF (3×GM-CSF+VACCINE) 3 days before injection of hepatitis B vaccine, the effects of GM-CSF, IFN-α and VACCINE The optimal dosage ratio of the mixture was tested. We found that when 10μg GM-CSF and 10000IUIFN-α were mixed with 1μg VACCINE, the DTH response induced in normal mice was not different from that of 3×GM-CSF+VACCINE, but was significantly higher than other dose combinations. At the same time, we also saw that after 10μg GM-CSF, 10000IU IFN-α and 1μg VACCINE mixed immunization and 3×GM-CSF+VACCINE immunization, the anti-HBsAg produced by ordinary mice was the highest, and there was no significant statistical difference between the two difference, the anti-HBsAg produced by other d...
Embodiment 2
[0232] Example 2, IFN-α / GM-CSF / VACCINE breaks the immune tolerance of rAAV-1.3HBV mice
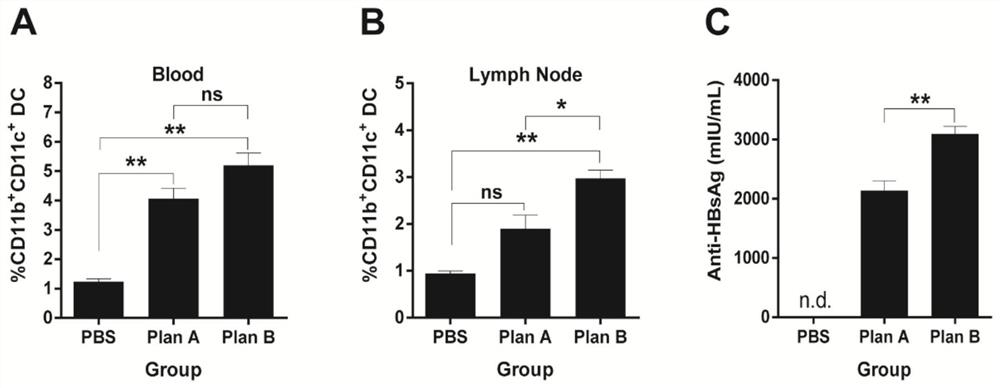
[0233] In Example 1, we found that IFN-α / GM-CSF / VACCINE combined immunization and 3×GM-CSF+VACCINE can stimulate humoral immunity and cellular immunity in wild-type mice, especially can significantly improve HBV-specific CTL killing ability of CD8+ effector T cells. The key problem that chronic hepatitis B is difficult to treat is the immune tolerance caused by HBV, and the body cannot complete the clearance of HBV by itself. In previous experiments, we found that 3×GM-CSF+VACCINE successfully broke the immune tolerance of HBV-sAg transgenic mice, promoted the clearance of HBsAg and the production of anti-HBsAg. In this experiment, we established a hepatitis B model in mice infected with adenovirus and recombinant hepatitis B virus (rAAV8-1.3HBV), and used this model to evaluate the effectiveness of the optimized IFN-α / GM-CSF / VACCINE combined immunization regimen. immune effect.
[0234...
Embodiment 3
[0262] In order to verify that GM-CSF, IFNα-2b and tumor antigen polypeptide or protein vaccine are mixed to achieve an immune activation response, first we use prostate cancer antigen epitope peptide (PAP, its sequence is: CMSAMTNLAALFPPEG, as shown in SEQID NO.1 ), using the polypeptide and carrier protein (KLH) coupling method for coupling. After purification, mix 50 μg of the conjugated vaccine with 10 μg GM-CSF and 10,000 IU IFNα-2b and supplemented with aluminum adjuvant, and subcutaneously immunize common BALB / C mice, with an interval of 2 weeks between each time, a total of 2 times , while the coupled antigen peptide 50 μg mixed aluminum adjuvant group was used as the control, and the immunization procedures were consistent. Two weeks after immunization, the serum of the mice was collected for ELISA to detect the level of humoral immunity. Afterwards, the paws were stimulated with 10 μg of polypeptide-conjugated BSA, and the swelling size of the paws was measured for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com