Compound-type nano-vaccine and preparation method thereof
A nano-vaccine and composite technology, applied in the field of biomedicine, can solve the problems of complex preparation, nano-carriers that cannot simultaneously load siRNA, cannot be degraded and metabolized, and achieve simple and easy preparation methods, improved anti-tumor effects, and biocompatibility good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
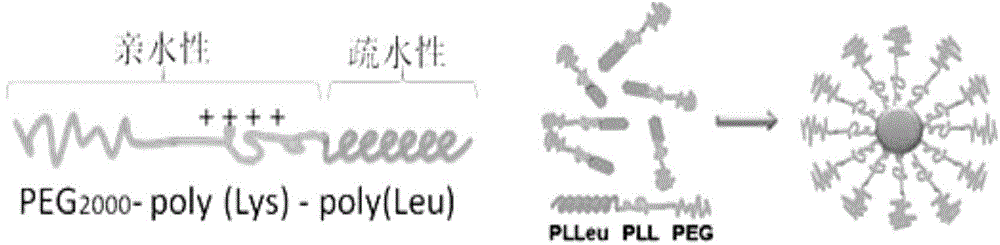
[0037] This example is the preparation process of the polyethylene glycol monomethyl ether-polylysine-polyleucine triblock polymer carrier used in the composite nano-vaccine disclosed in the present invention.
[0038] The preparation method comprises the following steps:
[0039] (1) After evacuating the polymerization tube, fill it with nitrogen for protection, and take 1g of CH with a molecular weight of 2000 3 O-PEG-NH 2 , dissolved in 20mL N, N-dimethylformamide (DMF) and added to the polymerization tube;
[0040] (2) According to the lysine cyclic anhydride monomer and CH 3 O-PEG-NH 2 Add Lys-NCA monomer in a ratio of 10:1, and react at constant temperature for 24 hours under the protection of nitrogen;
[0041] Then, according to the leucine cyclic anhydride monomer and CH 3 O-PEG-NH 2 Add the Leu-NCA monomer with a molar ratio of 10:1, and continue the reaction at constant temperature for 24 hours under the protection of nitrogen;
[0042] After the reaction, ad...
Embodiment 2
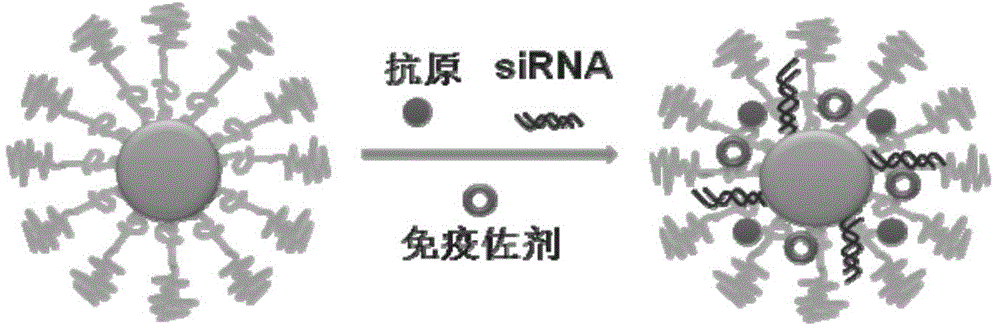
[0047] This example is the process of using the PEG-PLys-PLeu triblock copolymer prepared in Example 1, tumor antigen (OVA), TLR3 agonist (PIC), and synthetic STAT3 siRNA to prepare a composite nano-vaccine, and the obtained polymer The structure of micelles in aqueous solution and the structure of composite nanovaccine are as follows figure 1 and figure 2 shown.
[0048] (1) Weigh 1 mg of the PEG-PLys-PLeu triblock copolymer prepared in Example 1 and dissolve it in 1 ml of ultrapure water to form a uniform and transparent polymer nanomicelle aqueous solution ( figure 1 );
[0049] (2) Mix and shake tumor antigen (OVA), TLR3 agonist (PIC), synthetic STAT3 siRNA (5′-GGAAAUUUAACAUUCUGGGCACGAA-3′, SEQ ID NO.1) and polypeptide nanomicelle aqueous solution (OVA: PIC: siRNA: NPPEG -PLL 30 -PLLeu 40 =16:4:1:79, w / w) for 1 hour, and let stand at room temperature for 30 minutes to obtain a composite nano-vaccine (see vaccine structure figure 2 ).
Embodiment 3
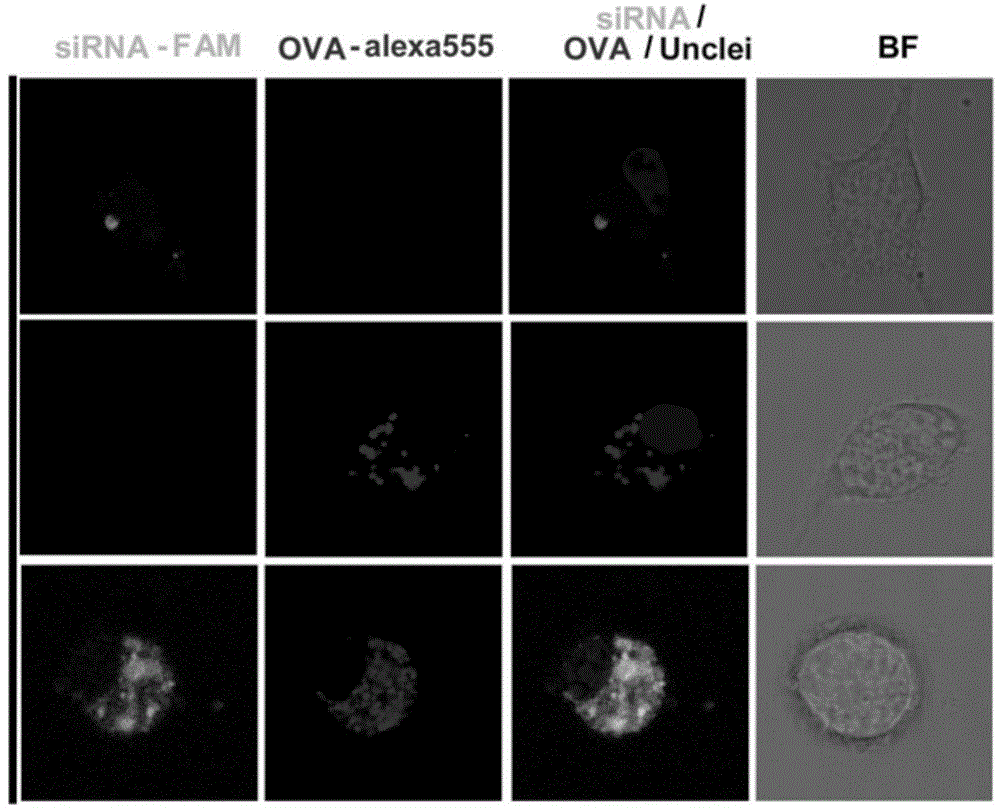
[0051] This example is a comparative experiment of TADC on the uptake of OVA and siRNA in the case of free state or siRNA-FAM wrapped in polypeptide nanomicelles. The experimental results are as follows image 3 shown.
[0052] 1. Experimental steps:
[0053] 1. Add PEG-PLL 30 -PLLeu 40 Polypeptide nanomicelle aqueous solution (50 μg / ml) was mixed with TLR3 agonist (PIC), tumor antigen (OVA-Alexa555) and FAM-labeled STAT3 siRNA (SEQ ID NO.1), and left to stand at room temperature for 30 minutes. Obtain a compound vaccine encapsulated by polypeptide nanomicelles (OVA: PIC: siRNA: NPPEG-PLL 30 -PLLeu 40 =5:5:1:50, w / w), namely NP / siRNA / OVA;
[0054] 2. Incubate free siRNA-FAM, free OVA-Alexa555 or NP / siRNA / OVA with TADCs (tumour-associated dendritic cells, tumor-associated dendritic cells) for 2 hours. The uptake of siRNA and OVA by TADC was detected by confocal microscopy.
[0055] 2. Experimental results:
[0056] Compared with free OVA-Alexa555 and free FAM-siRNA, pol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com