Application of recombinant attenuated Listeria in preparation of therapeutic vaccine for cervical cancer
A therapeutic vaccine and Listeria technology are applied in the application field of recombinant attenuated Listeria in the preparation of cervical cancer therapeutic vaccines, and can solve problems such as defects and low immunogenicity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
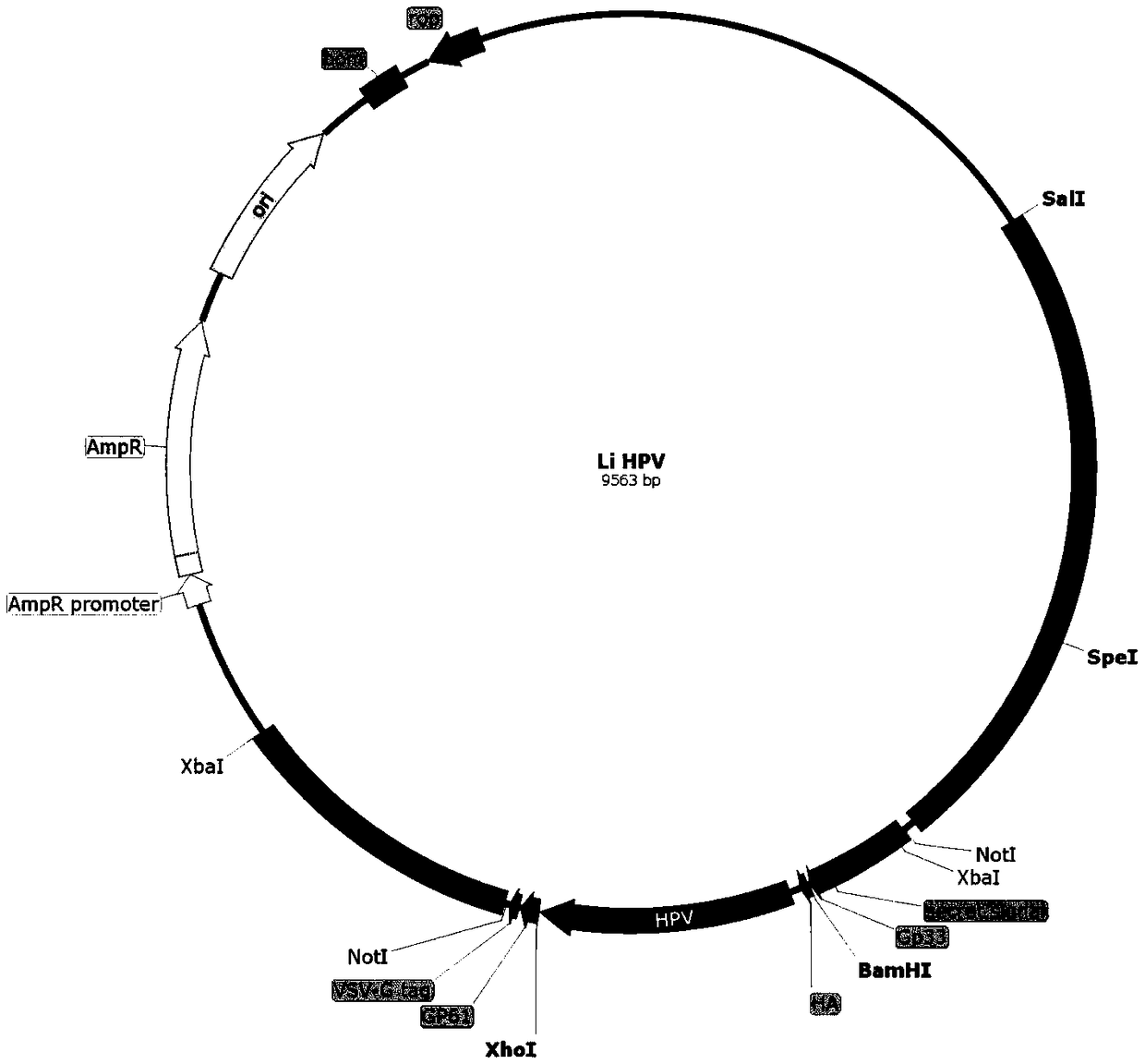
[0100] Example 1. Preparation of vaccine bacteria LiΔactAplcB-E6E7
[0101] (1) HPV16 E6E7 gene fragment synthesis
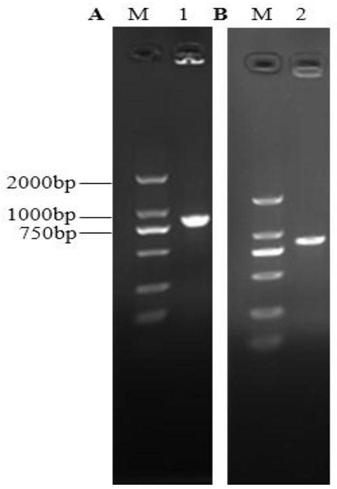
[0102] Query HPV 16 E6 protein and E7 protein genes from NCBI, use online software to analyze and predict their dominant T cell antigen epitopes, select and combine them into fusion antigen epitope peptide genes, and then optimize according to Listeria codons, And a HindⅢ restriction site was added upstream, and an XhoI restriction site was added downstream, and finally the DNA sequence was directly obtained by synthesis (obtained in the form of the cloning plasmid pUC57-E6E7 provided by the company), and the fragment length was 866bp (the sequence is shown in the sequence table sequence 1 shown).
[0103] (2) Construction of targeting plasmid
[0104] ① Construction of intermediate plasmid pCW203-E6E7
[0105] Plasmids pUC57-E6E7 and pCW203 were extracted according to the instructions of the plasmids, and eluted with 30 μL Elution Buffer.
[0106] pUC57-E6E...
Embodiment 2
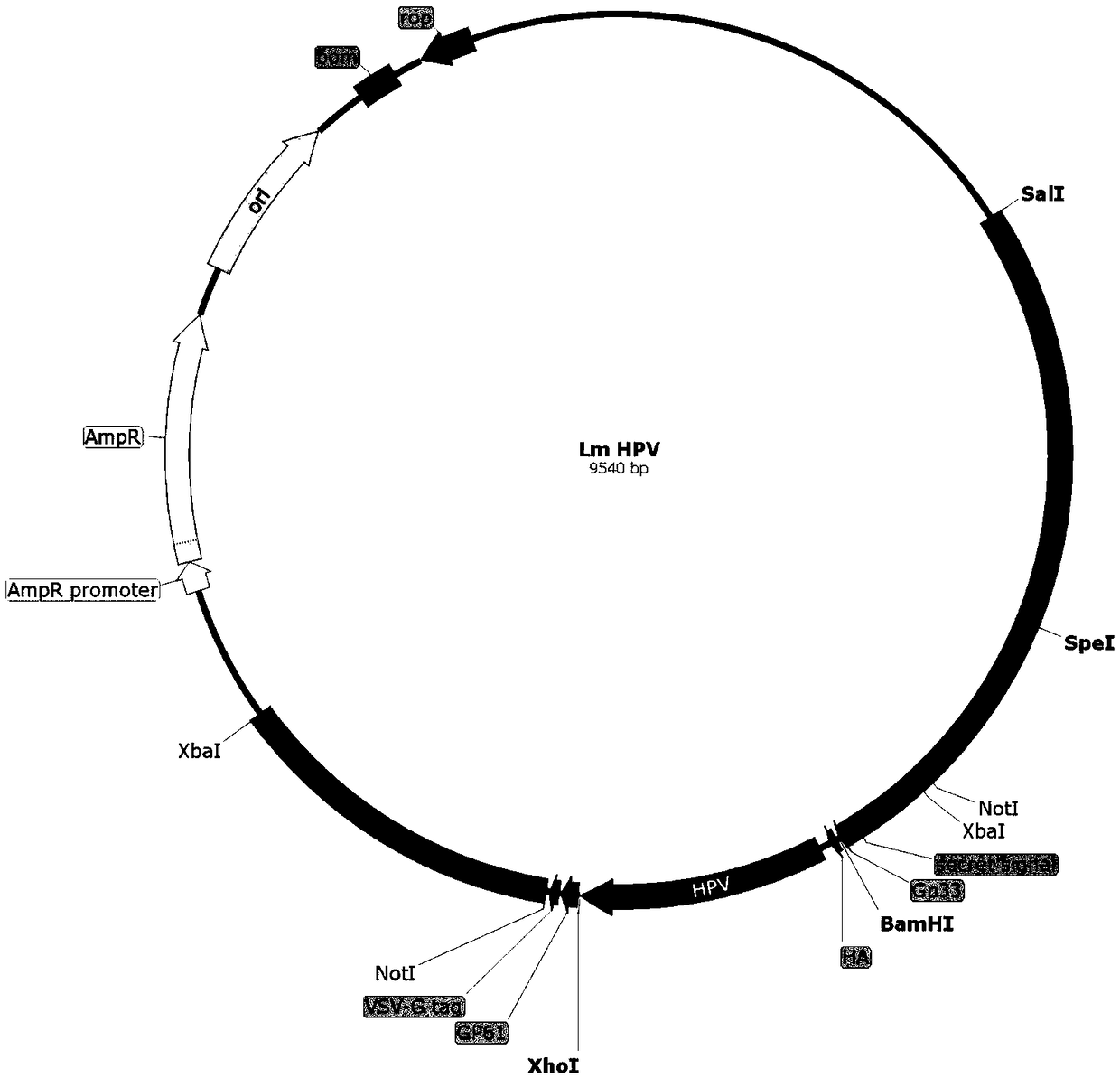
[0130] Embodiment 2: the preparation of vaccine bacterium LmΔactAplcB-E6E7
[0131](1) HPV16 E6E7 gene fragment synthesis
[0132] Same as Example 1(1)
[0133] (2) Construction of targeting plasmid
[0134] ①The construction of the intermediate plasmid pCW203-E6E7 is the same as in Example 1(2)①
[0135] ②Construction of targeting plasmid
[0136] Plasmid pCW180 was extracted and eluted with 30 μL Elution Buffer. Mix plasmid pCW180 and intermediate plasmid pCW203-E6E7 with restriction endonucleases BamHI and XhoI respectively according to the system of (2) ① (replace HindIII enzyme with BamHI enzyme), carry out enzyme digestion and dephosphorylation, and gel recovery after electrophoresis The small fragment after digestion of pCW203-E6E7 is the insert fragment (931bp) and the backbone of the pCW180 vector (long fragment after digestion, about 8621bp in length). Mix according to the system of (2)①, connect and transform into Escherichia coli DH5α, smear on LA plate, perfo...
Embodiment 3
[0150] Example 3: Evaluation of the therapeutic effect of vaccine strains on cervical cancer
[0151] (1) TC-1 tumor cell culture and establishment of cervical cancer model
[0152] Use RPMI1640 Medium containing 10% fetal bovine serum, 1% penicillin, and streptomycin at 37°C in 5% CO 2 Cultivate TC-1 cells in an incubator until the density reaches about 80%, rinse the cells with Hank’s solution for 2 to 3 times, digest with trypsin and resuspend with PBS for counting, and prepare the cells to a concentration of 1×10 6 100 μl of cell suspension was subcutaneously injected into the right abdomen of 6-8 week-old C57BL / 6 female mice to establish a mouse HPV-infected tumor model, and a macroscopic tumor was formed in about 7 days. Tumor volume according to the formula 1 / 2×(a×b 2 ) calculation (a, b are the longest diameter and shortest diameter of the tumor, respectively). Statistical analysis of data was performed using SPSS software. The t-test was used to compare the means ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com