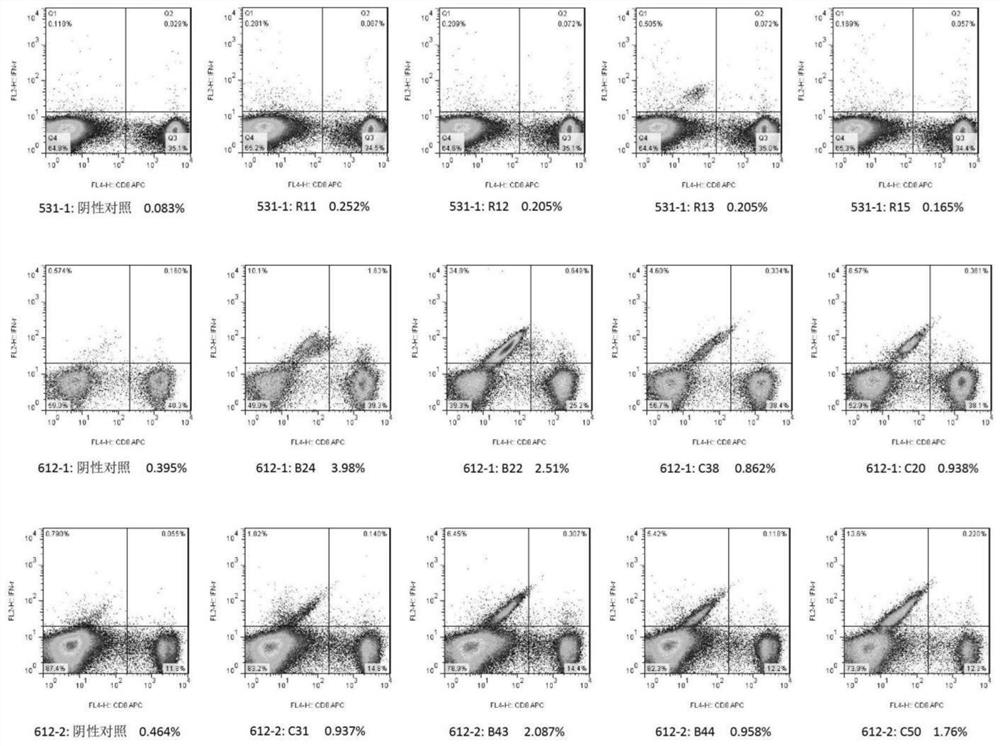
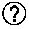Patents
Literature
404 results about "Peptide vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A peptide vaccine is any peptide which serves to immunize an organism against a pathogen. Peptide vaccine are often synthetic and mimic naturally occurring proteins from pathogens. In addition to infectious pathogens, peptide vaccines can be utilized as therapeutic cancer vaccines, where peptides from tumor associated antigens are used to induce effective anti-tumor T cell response. Synthetic long peptides (SLP) have shown promising successful results.
Method for differentially quantifying naturally processed hla-restricted peptides for cancer, autoimmune and infectious diseases immunotherapy development
ActiveUS20130096016A1Efficient use ofBiological material analysisLibrary member identificationDiseaseAntigen
The invention relates to a method for quantitatively identifying relevant HLA-bound peptide antigens from primary tissue specimens on a large scale without labeling approaches. This method can not only be used for the development of peptide vaccines, but is also highly valuable for a molecularly defined immunomonitoring and the identification of new antigens for any immunotherapeutic strategy in which HLA-restricted antigenic determinants function as targets, such as a variety of subunit vaccines or adoptive T-cell transfer approaches in cancer, or infectious and autoimmune diseases.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Formyl methionyl peptide vaccine adjuvant
InactiveUS6017537AConvenient easy to formulateStimulate immune responseBiocideNanotechPeptide vaccinePeptide
The present invention relates to immunological adjuvants comprised of the N-formyl methionyl peptide fMLP. FMLP, when used as an adjuvant in accordance with the present invention, provides for an immune response to suboptimal doses of recombinant antigens.
Owner:VIROGENETICS
Method for Differentially Quantifying Naturally Processed HLA-Restricted Peptides for Cancer, Autoimmune and Infectious Diseases Immunotherapy Development
The invention relates to a method for quantitatively identifying relevant HLA-bound peptide antigens from primary tissue specimens on a large scale without labeling approaches. This method can not only be used for the development of peptide vaccines, but is also highly valuable for a molecularly defined immunomonitoring and the identification of new antigens for any immunotherapeutic strategy in which HLA-restricted antigenic determinants function as targets, such as a variety of subunit vaccines or adoptive T-cell transfer approaches in cancer, or infectious and autoimmune diseases.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Peptide vaccines against group A streptococci
Owner:HEALTH & HUMAN SERVICES DEPT OF THE GOVERNMENT OF THE UNITED STATES OF AS REPRESENTED BY THE SEC
Antigen specific multi epitope vaccines
ActiveUS20100074925A1Strong and comprehensive responseEffective immune responseTumor rejection antigen precursorsSugar derivativesMHC class IProtein target
The present invention relates to cancer vaccines composed of the signal peptide domain of tumor associated antigens or proteins. The peptide vaccines of the invention are characterized by having multiple MHC class I and class II epitopes which are highly abundant in the population. Therefore, these vaccines are likely to induce a strong, comprehensive immune response against the target proteins in the majority of the vaccinated population, and thereby induce an immune reaction against tumors expressing such target proteins. Specifically, the invention relates to peptide vaccines composed of the signal peptide domain of Mucin (MUC1), BAGE-1 or ARMET, and their use for the treatment of cancers which express Mucin (MUC1), BAGE-1 or ARMET.
Owner:VAXIL BIOTHERAPEUTICS
Enhancing Class I Antigen Presentation With Synthetic Sequences
InactiveUS20080206270A1Enhance antigen presentationInduces antitumor and antiviral CTLTumor rejection antigen precursorsCell receptors/surface-antigens/surface-determinantsCancer cellT lymphocyte
The invention relates generally to the treatment and prevention of human cancer and viral diseases. More specifically, this invention relates to development of a new generation of vaccines that rely on eliciting cellular immune responses, specifically induction of cytotoxic T lymphocytes (CTL), against cancer cells and virus-infected cells via administration of a vaccine comprising a fusion peptide or a modified peptide. Such a fusion peptide is composed of an insertion signal sequence and a peptide derived from a tumor antigen or a viral antigen, which improves antigen presentation and induces CTL with higher efficiency against cancer cells and virus-infected cells. An exemplary antigen utilized in the invention is HER2 / neu. The peptides peptide vaccines of the invention are derived from the antigens PRAME, OFA / iLRP, STEAP and SURVIVIN.
Owner:RGT UNIV OF CALIFORNIA
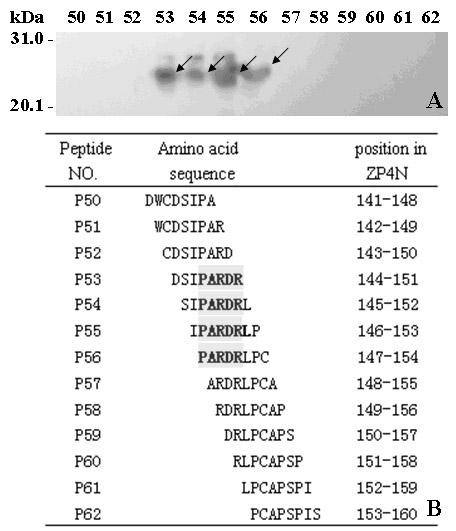
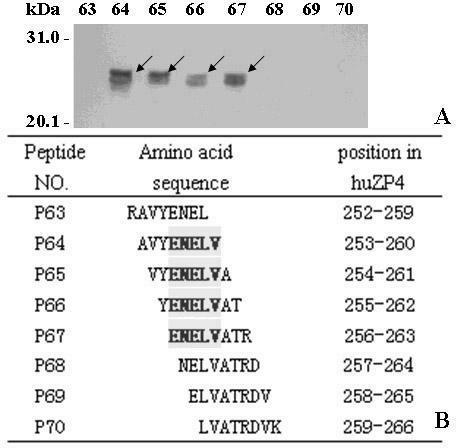
Epitope minimal motif peptides of human zona pellucida protein-4 and extended short peptides and application thereof
The invention belongs to the technical field of biomedicine and biological detection, in particular to two linear epitope minimal motif peptides capable of being identified by human zona pellucida protein-4 (ZP4) monoclonal antibodies MA-1662 and MA-1671 on human ZP4 and extended eight peptides and application thereof. The invention provides two candidate epitopes which can be used for developing human immune birth control recombinant multi-epitope vaccines, and the amino acid sequences of the candidate epitopes are shown as SEQ ID No. 1 and marked as huZP4147-151 or shown as SEQ ID No.2 and marked as huZP4256-260; and the invention also provides candidate epitopes which can be used for developing novel high-specificity high-sensitivity recombinant multi-epitope detection antigens for diagnosing serum ZP antibodies of ZP autoimmune infertile women, and the amino acid sequences of the candidate epitopes are shown as SEQ ID No.3-SEQ ID No.6.
Owner:SHANGHAI INST OF PLANNED PARENTHOOD RES +1
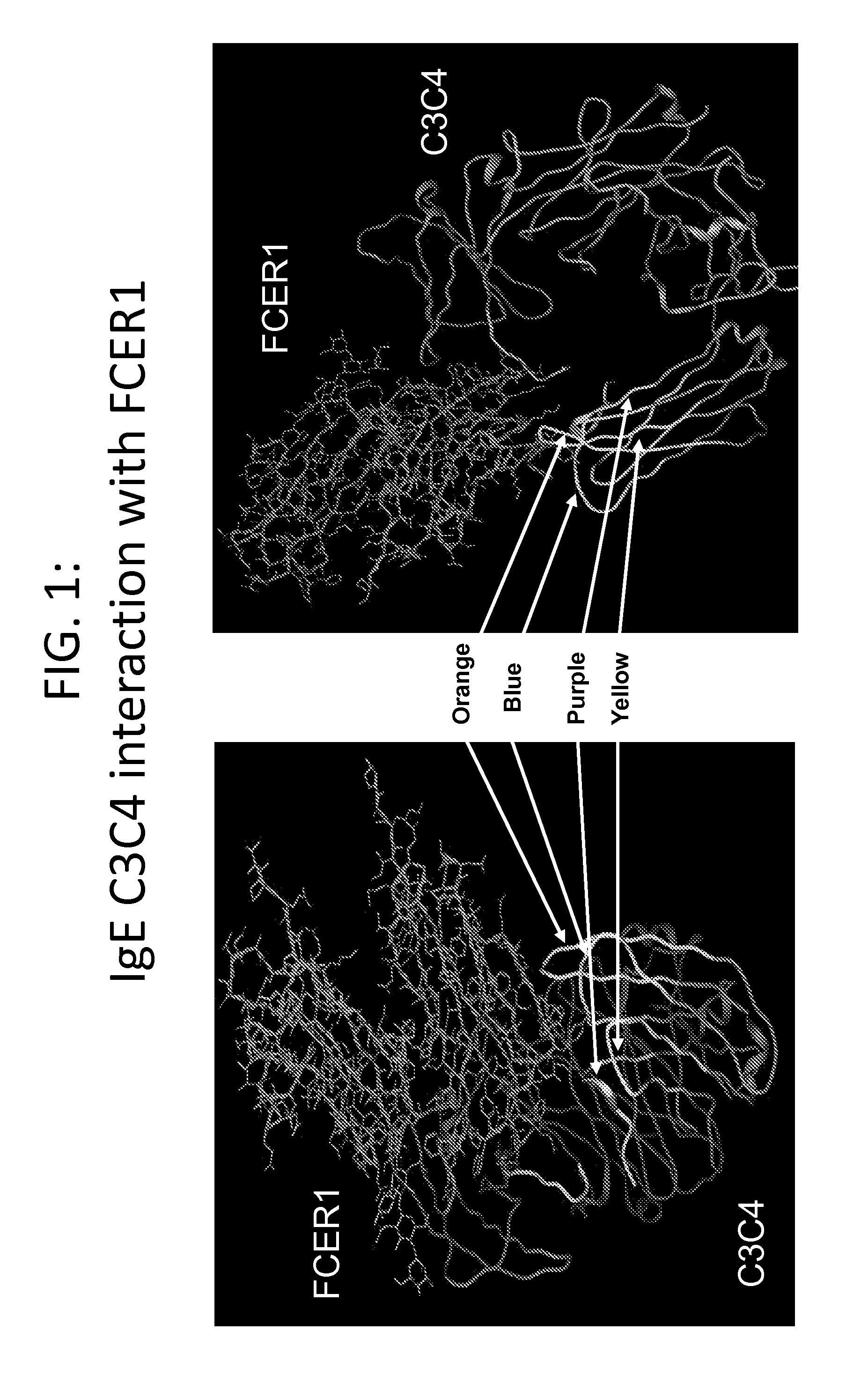
IgE CH3 Peptide Vaccine
InactiveUS20110300163A1Reducing treatmentPreventing and alleviatingPeptide/protein ingredientsVertebrate antigen ingredientsPeptide vaccineImmunogenicity
The present invention relates to the provision of novel immunogens comprising an antigenic IgE peptide preferably linked to an immunogenic carrier, compositions comprising the immunogens, and methods for the prevention, treatment or alleviation of IgE-mediated disorders. The invention further relates to methods for production of these medicaments, immunogenic compositions and pharmaceutical compositing thereof and their use in medicine.
Owner:PFIZER VACCINES
Epitope screening method capable of exciting anti-mycobacterium tuberculosis protective immunological reaction of body and uses
ActiveCN101289496AHelp predictHelp determineAntibacterial agentsPeptide preparation methodsScreening methodPeptide vaccine
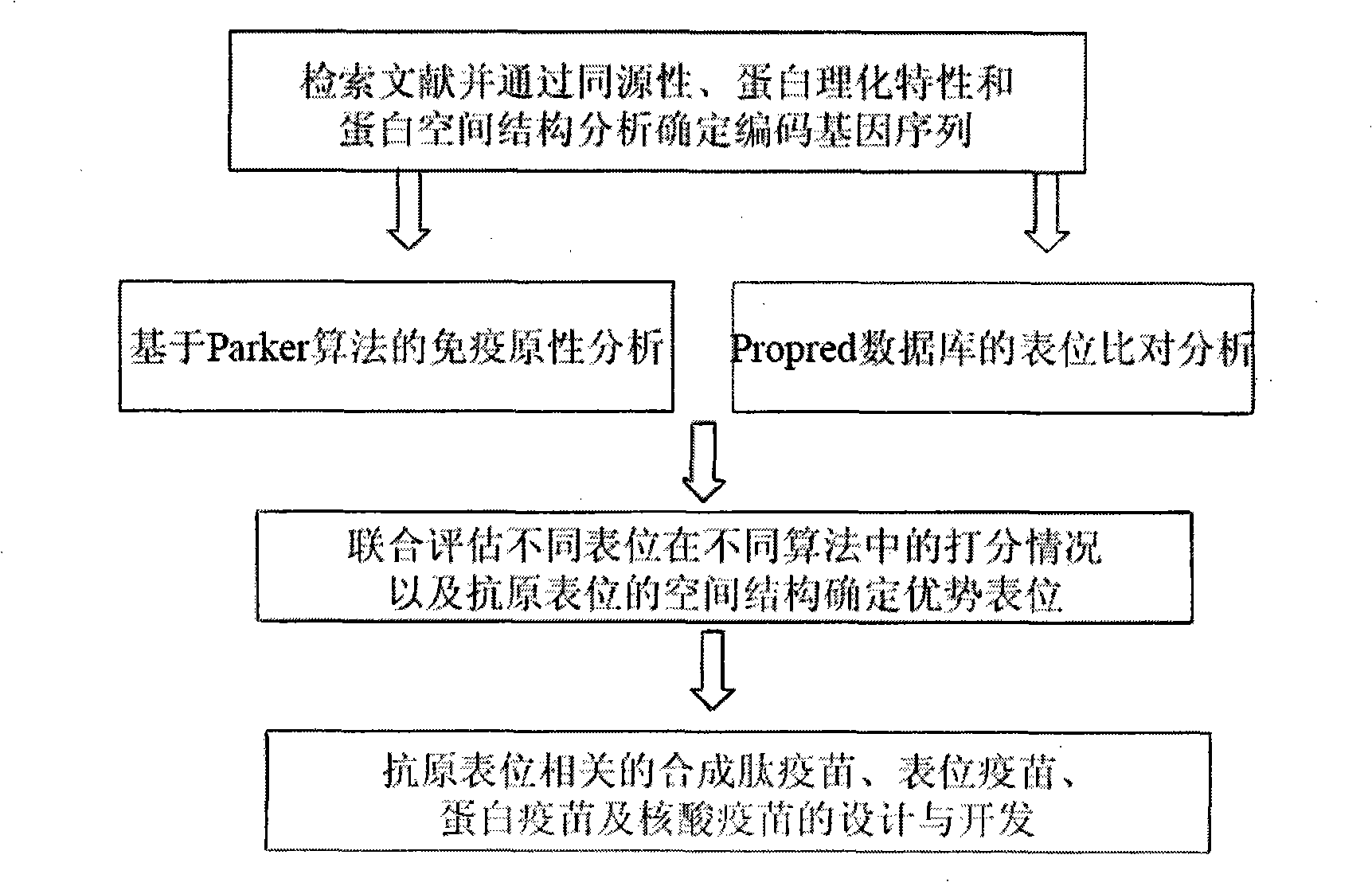
The invention relates to a selection method for epitope which can stimulate the protective immune response of the body anti-mycobacterium tuberculosis and the function thereof, in particular to the molecule mimic peptide of the epitope with vaccine development prospect from the mycobacterium tuberculosis and the coding DNA thereof. The selection method of epitope which can stimulate the protective immune response of the body anti-mycobacterium tuberculosis and the function thereof provides T lymphocyte epitope contained in one important gene Ag85B in the research of mycobacterium tuberculosis and the method of deducting or selecting the epitope, which is beneficial to further developing novel multivalent and poly epitope tuberculosis vaccine and prevent and control the happening and development of tuberculosis; the method makes a foundation of the future development of synthesizing peptide vaccine epitope vaccine and dna vaccine by using epitope and provides molecule mimic peptide of epitope which can stimulate the protective immune response of the body anti-mycobacterium tuberculosis and the peptide has the amino acid sequence of FVRSSNLKFQDAYNA(SEQ ID NO:1).
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
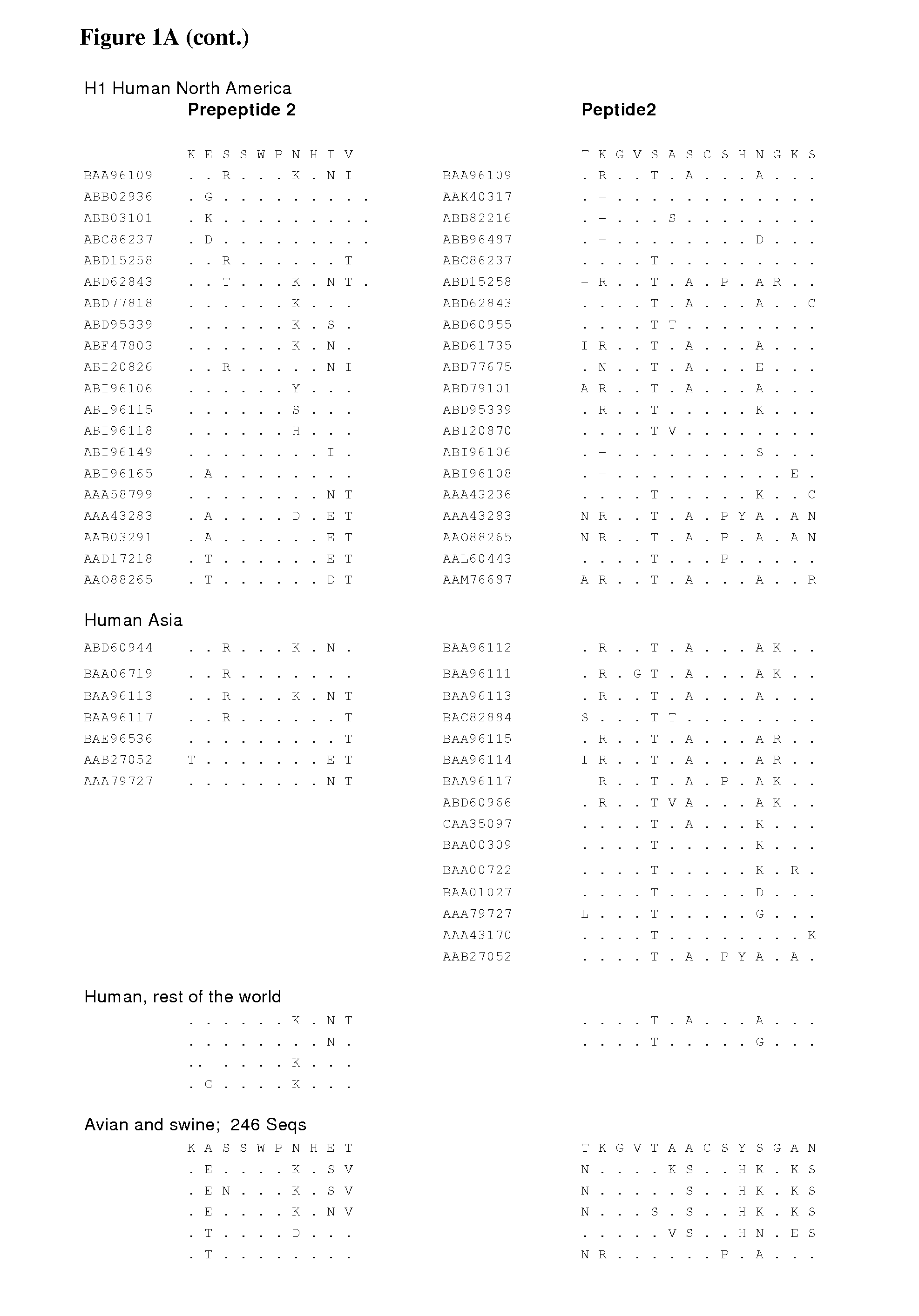
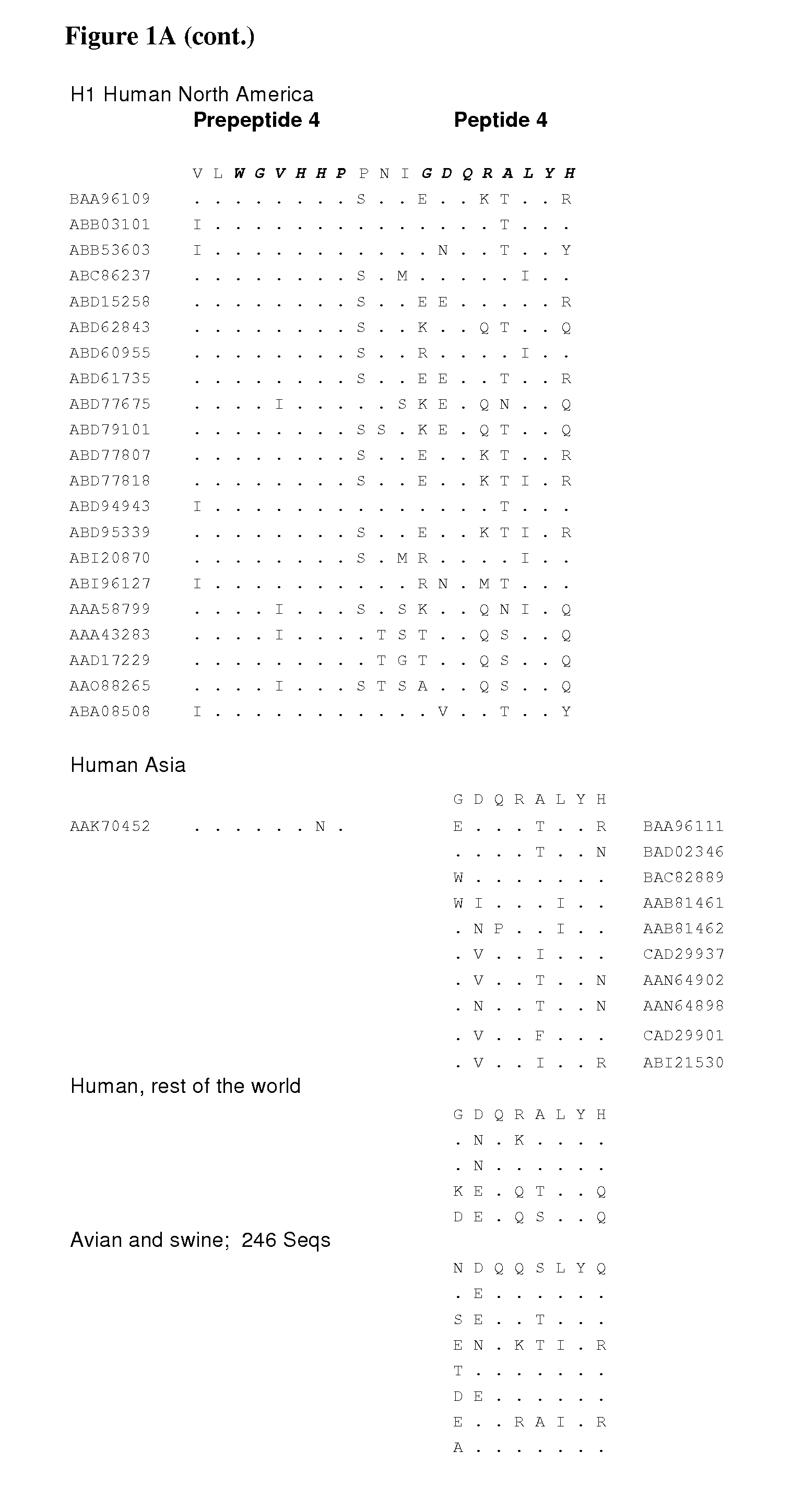
Peptide vaccine for influenza virus
The invention provides peptide epitopes for use in the prevention and / or treatment of influenza or for the development of such treatment or vaccine against influenza. The invention also relates to a method for evaluating the potential of a chemical entity, such as an antibody, to bind to a peptide epitope derived from the divalent sialoside binding site of hemagglutinin protein of influenza virus, and to conjugates containing one or more such peptide epitopes. The peptide epitopes of the invention are cyclic peptides comprising a 7-mer peptide derived from H1, H3 or H5 hemagglutinin of influenza virus. The 7-mer peptide has a sequence corresponding to the loop sequence at positions 220-226 of X31-hemagglutinin.
Owner:GLYKOS FINLAND
O type foot-and-mouth disease virus variant as well as coding gene and application thereof
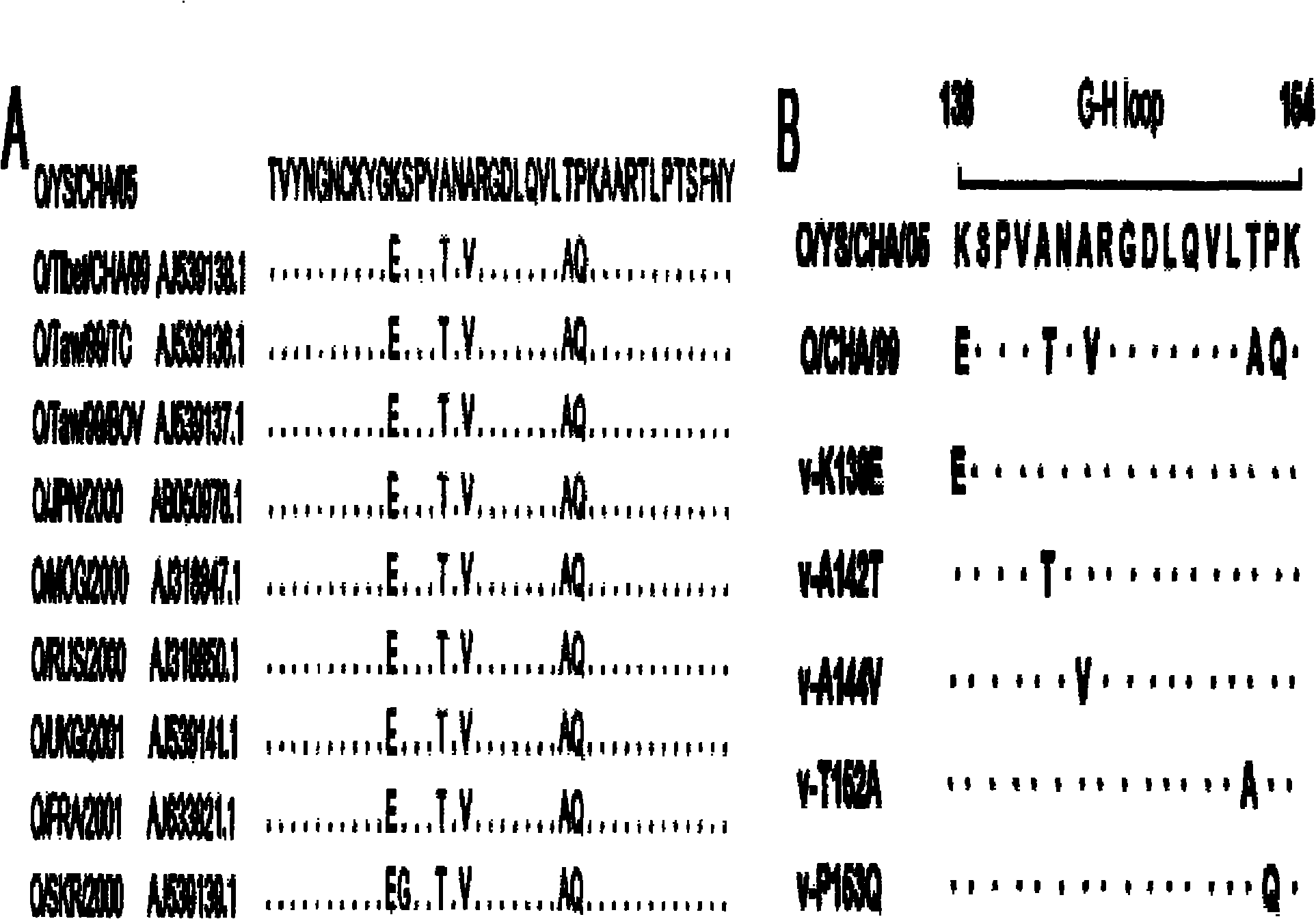
The invention discloses an O type foot-and-mouth disease virus variant as well as a coding gene and application thereof. In the invention, an O type foot-and-mouth disease virus pan-Asia strain O / YS / CHA / 05 is firstly separated out, the nucleotide sequence of the O type foot-and-mouth disease virus pan-Asia strain O / YS / CHA / 05 SEQ ID NO: 1, and the amino acid sequence is SEQ ID NO: 2. In comparison with a VP1 amino acid sequence, the virus strain has 7 variable sites, five of which are centralized in a G-H ring. Mutation of the sites ensures that the virus variant has the capability of escaping from host immunity so as to have the superiority for becoming a popular virus strain. Therefore, the variant can be employed to prepare an inactivated vaccine for prevention and treatment of the variant and relevant strains, dominant antigen epitope of the variant can be employed to prepare a synthetic peptide vaccine, and the variant can be employed to develop novel O type foot-and-mouth disease virus vaccines such as VLP vaccine and the like. Therefore, the invention has important value in controlling the popularity of O / YS / CHA / 05 and relevant variable strains.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Peptide vaccines for cancers expressing mphosph1 or depdc1 polypeptides
The present invention provides peptides having an amino acid sequence as set forth in SEQ ID NO: 7, 8, 9, 10, 11, 12, 192, 195, 197, 209, 225, 226, 228, 230, 240, 241, 243, 244, 249, 253, 254 or 255, as well as peptides having the above-mentioned amino acid sequences in which 1, 2, or several amino acids are substituted, deleted, or added, wherein the peptides possess cytotoxic T cell inducibility. The present invention also provides drugs for treating or preventing a disease associated with the over-expression of MPHOSPH1 and / or DEPDC1, e.g. cancers, containing these peptides as an active ingredient. The peptides of the present invention can also be used as vaccines.
Owner:ONCOTHERAPY SCI INC
MAGE-A3/HPV 16 peptide vaccines for head and neck cancer
InactiveUS20060222656A1Tumor rejection antigen precursorsPeptide/protein ingredientsSquamous CarcinomasHead and neck
The present invention relates to Trojan antigens, and immunogenic compositions comprising the Trojan antigens. The present invention also relates to methods of generating an immune response in a subject using the Trojan antigens or immunogenic compositions. The present invention further relates to methods of treating squamous cell carcinoma of the head and neck (SCCHN) using the Trojan antigens and immunogenic compositions of the present invention.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES +1
Peptide vaccine for prevention and immunotherapy of dementia of the alzheimer's type
ActiveUS20140271690A1Reduce manufacturing costQuality improvementSsRNA viruses negative-senseNervous disorderPeptide vaccineImmunotherapy
The present disclosure is directed to individual Aβ peptide immunogen constructs, peptide compositions comprising these Aβ peptide immunogen constructs and mixtures thereof, pharmaceutical compositions including vaccine formulations comprising these Aβ peptide immunogen constructs, with the individual Aβ peptide immunogen constructs having the N-terminus of the Aβ peptide as the B cell (B) epitopes linked through spacer residue(s) to heterologous T helper cell (Th) epitopes derived from pathogen proteins that act together to stimulate the generation of highly specific antibodies directed against the N-terminus of the Aβ peptide offering protective immune responses to patients at risk for, or with, Alzheimer's Disease.
Owner:UNITED NEUROSCIENCE LIMITED
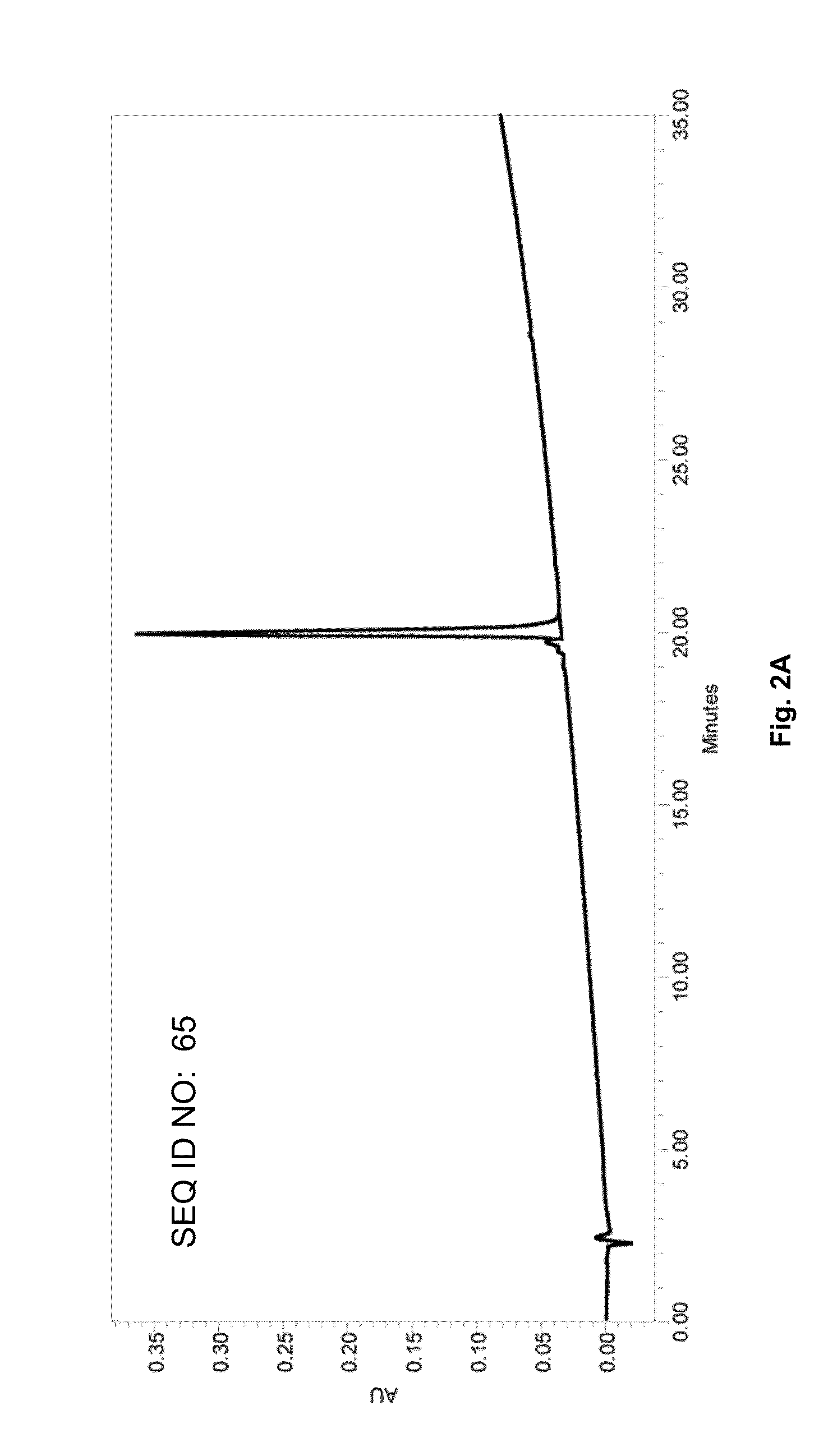
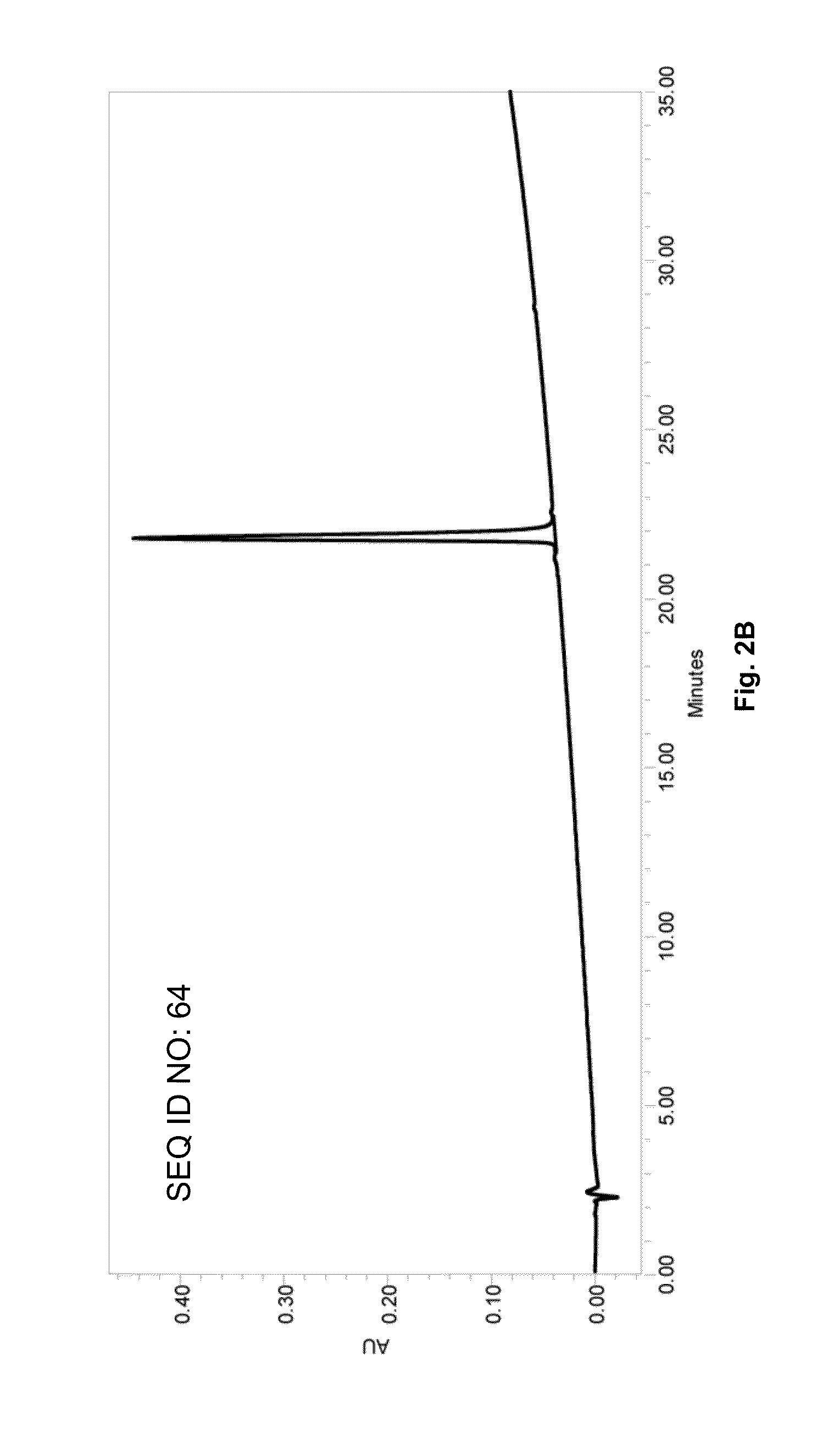
SARS-CoV-2 lymphocyte antigen epitope peptides and application thereof
InactiveCN112961223ASsRNA viruses positive-senseViral antigen ingredientsAntigen epitopeLymphocyte antigen
The invention discloses lymphocyte antigen epitope peptides and application thereof, belongs to the fields of medical immunology and infectious diseases, and particularly relates to 164 thymus dependent lymphocyte antigen epitope peptides of five proteins of SARS-CoV-2 and application thereof. The antigen epitope peptides can be presented by HLA-A molecules to stimulate activation, proliferation and differentiation of SARS-CoV-2 specific thymus dependent lymphocytes, so that the immune effect of resisting SARS-CoV-2 infection is exerted. The antigen peptides can be used for preparing mixed polypeptide vaccines of SARS-CoV-2, recombinant protein vaccines connected with a plurality of epitope peptides in series and DNA and RNA vaccines, can be used for preparing a detection kit for detecting SARS-CoV-2 specific thymus dependent lymphocytes, can also be used for preparing effector thymus dependent lymphocytes or drugs for treating SARS-CoV-2 infection, and have a potential application value in the prevention, treatment and diagnosis of SARS-CoV-2 infection and COVID-19.
Owner:SOUTHEAST UNIV +2
Porcine circovirus II-type (PCV2) epitope peptide vaccine and preparation method thereof
ActiveCN103536912AStrong specificityEasy to saveViral antigen ingredientsAntiviralsDiseaseCircovirus
The invention relates to a porcine circovirus II-type (PCV2) epitope peptide vaccine and a preparation method of the vaccine. The vaccine contains three b cell epitopes, the lysine of the vaccine is four-branch peptide with the same core matrix structure epitope monomer and is connected with a general T ancillary cell (Th) epitope in series, and the vaccine has the molecular weight of 13kDa; the epitope peptide is named PCV CP98-156-228; after being vaccinated, a mice has stronger immune response, and a high potency virus neutralizing antibody can be generated. The epitope peptide vaccine has the advantages of being low in price, safe, high in specificity and easy to store and apply, and plays an important role in preventing and controlling the porcine circovirus disease. The PCV2 epitope peptide vaccine is prepared by four steps including predicting and screening epitope peptide, designing the epitope peptide vaccine, synthesizing, purifying and authenticating the epitope peptide as well as preparing the epitope peptide vaccine; the method is easy in raw material obtaining, lower in cost and easy to control, and has better operability, thus being suitable for being popularized and applied.
Owner:CHONGQING UNIV OF TECH +2
Concurrent chemotherapy and immunotherapy
InactiveUS20090220551A1Peptide/protein ingredientsAntibody mimetics/scaffoldsGlioblastomaConcurrent chemotherapy
The concurrent administration of chemotherapy and immunotherapy has been considered a contraindication because of the concern that the induced lymphopenia would ablate therapeutic efficacy of immunotherapy. Temozolomide has been shown to be an effective chemotherapeutic for patients with malignant gliomas and to deprive patients with glioblastoma (GBM) patients of this agent in order to treat with immunotherapy is controversial. Despite conventional dogma, we demonstrate that both chemotherapy and immunotherapy can be delivered concurrently without negating the effects of immunotherapy, in fact, the temozolomide induced lymphopenia may actually be synergistic with a peptide vaccine.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Synthetic peptide vaccine for treating porcine reproductive and respiratory syndrome and preparation method thereof
InactiveCN101845083AImprove securityImprove efficiencyViral antigen ingredientsVirus peptidesMedicinePorcine reproductive and respiratory syndrome virus
The invention provides a synthetic peptide vaccine for treating porcine reproductive and respiratory syndrome and a preparation method thereof, in particular relates to polypeptide of the synthetic peptide vaccine for treating the porcine reproductive and respiratory syndrome and a vaccine which contains the polypeptide and a method for preparing the polypeptide and the vaccine. The amino acid sequence of the polypeptide is an amino acid sequence shown in SEQ ID No.1, SEQ ID No.2, SEQ ID No.3 or SEQ ID No.4. The vaccine prepared from the polypeptide can effectively cope with the antigenic variation of a porcine reproductive and respiratory syndrome virus, ensures biological safety, is easy for large-scale synthesis and has good application prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
O-type aftosa synthetic peptide vaccine
ActiveCN101659695AImprove protectionFree from attackAntiviralsPeptide preparation methodsChemical synthesisPeptide vaccine
The invention provides O-type aftosa synthetic peptide vaccine, and in particular relates to polypeptide or polypeptide polymer thereof used in the vaccine as well as the vaccine containing the polypeptide or the polypeptide polymer thereof and a preparation method thereof. The polypeptide has amino acid sequences shown in SEQ ID No.1, SEQ ID No.2 and SEQ ID No.3. The O-type aftosa synthetic peptide vaccine carries out chemical synthesis of potential antigen site peptide segments by carrying out sequencing of domestic aftosa epidemic strains to study the variation of the main antigen sites ofaftosa and combining with computer assistant to carry out antigen site analytical prediction. Candidate polypeptide antigens are screened out by carrying out large numbers of animal experiments and aftosa virus antigen sites are optimized according to the screening result; and T cell epitope and B cell epitope are effectively combined to improve the immune effects of the polypeptide antigens. TheO-type aftosa synthetic peptide vaccine can effectively cope with the antigen variation of aftosa virus and has ideal biosafety and easy large-scale synthesis, thereby having a good application prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
Synthetic peptide vaccine for O-type foot and mouth disease of swine and preparation method thereof
InactiveCN102580076AImproving immunogenicityImprove stabilityAntiviralsAntibody medical ingredientsPeptide vaccineEngineered genetic
The invention discloses synthetic peptide vaccine for O-type foot and mouth disease of swine and the preparation method thereof. In the invention, synthetic peptide genes are expressed by selecting an eukaryotic expression system, then an expression product is emulsified into vaccine, since the application of genetic engineering technology, the synthetic peptide for O-type foot and mouth disease of swine can be efficiently and stably expressed, the production cost is low, the amplification is easy, and the toxic side effect and worries about biosafety can be avoided. Therefore, the synthetic peptide vaccine for the O-type foot and mouth disease of swine is suitable for large scale industrial production, and is classified as novel synthetic peptide vaccine for O-type foot and mouth disease of swine, is used for clinically preventing, controlling and curing foot and mouth disease.
Owner:GENIFARM LAB INC
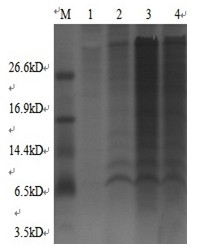
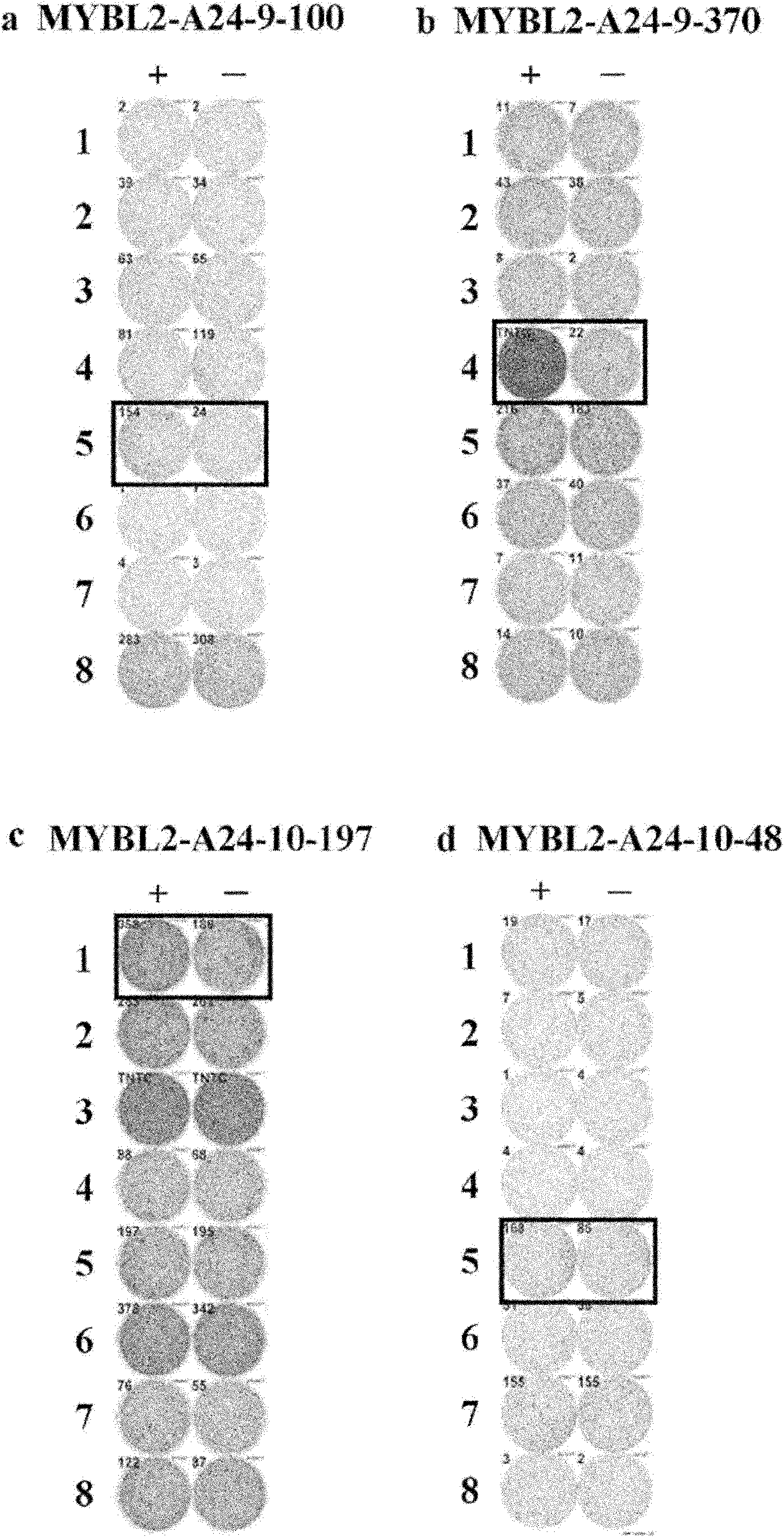
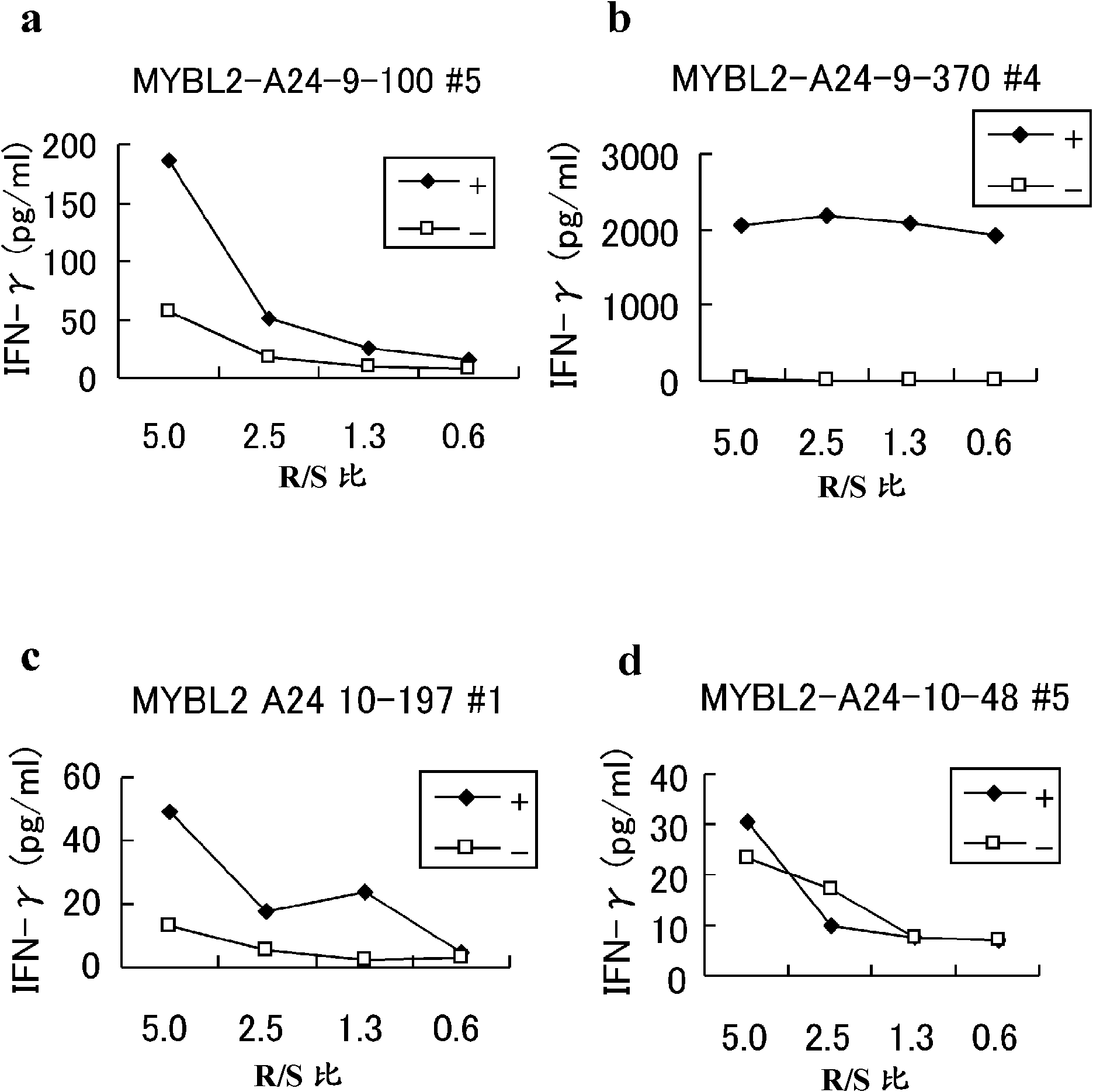
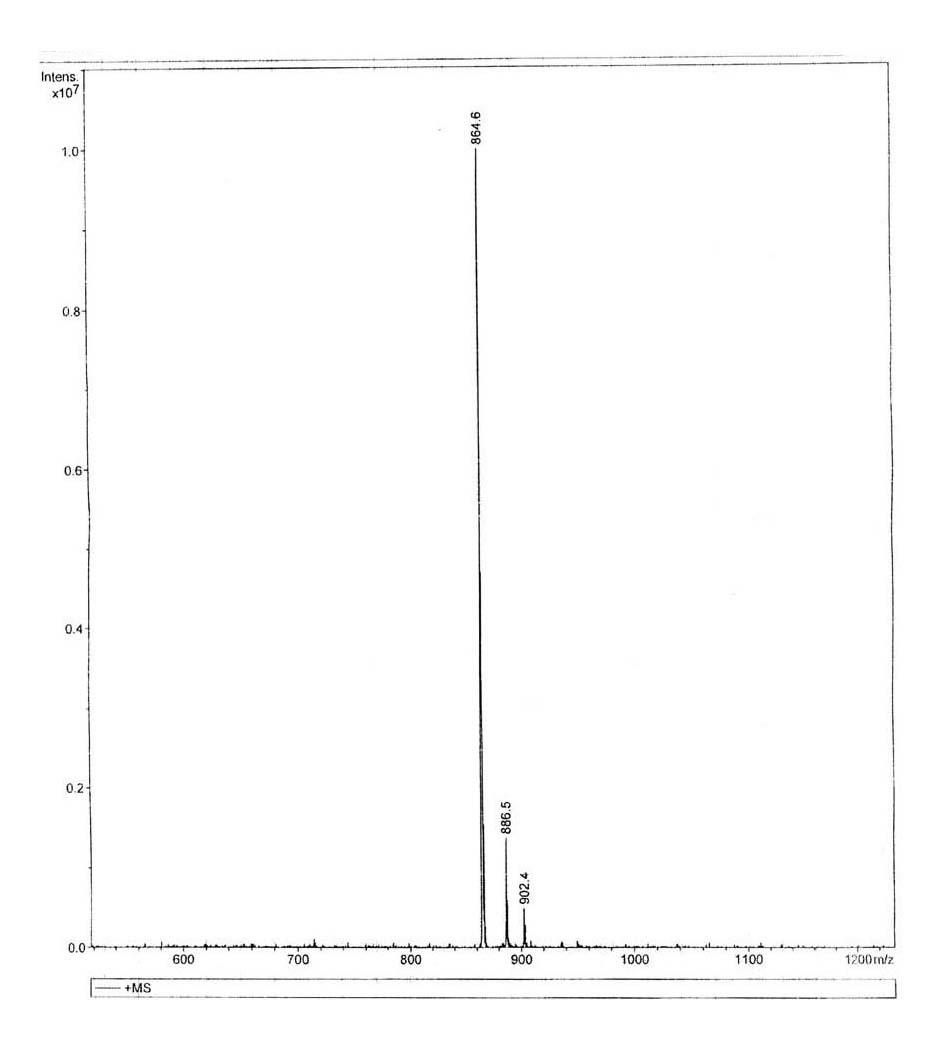
MYBL2 epitope peptides and vaccines containing the same
InactiveCN102119170APeptide/protein ingredientsVaccination/ovulation diagnosticsTesticular stromal tumorHLA-A24
Peptide vaccines against cancer are described herein. In particular, the present invention describes epitope peptides derived from MYBL2 that elicit CTLs. The present invention also provides established CTLs that specifically recognize HLA-A24 positive target cells pulsed with the peptides. Antigen-presenting cells and exosomes that present any of the peptides, as well as methods for inducing antigen-presenting cells are also provided. The present invention further provides pharmaceutical agents containing the MYBL2 polypeptides or polynucleotides encoding thereof, as well as exosomes and antigen-presenting cells as active ingredients. Furthermore, the present invention provides methods for treating and / or prophylaxis of (i.e., preventing) cancers (tumors), and / or prevention of postoperative recurrence thereof, as well as methods for inducing CTLs, methods for inducing anti-tumor immunity, using the MYBL2 polypeptides, polynucleotides encoding the polypeptides, exosomes or antigen-presenting cells presenting the polypeptides, or the pharmaceutical agents of the present invention. The cancers to be targeted include, but are not limited to, testicular tumor, pancreatic cancer, bladder cancer, non-small cell lung cancer, small cell lung cancer and esophageal cancer.
Owner:ONCOTHERAPY SCI INC
Tuberculosis medicament resistance related tuberculosis-resisting cytotoxic T lymphocyte (CTL) epitope peptide derived from refflux protein and application thereof
The invention discloses a tuberculosis medicament resistance related tuberculosis-resisting cytotoxic T lymphocyte (CTL) epitope peptide derived from refflux protein, namely, nonapeptide. The amino acid sequence of the nonapeptide is as follows: P5: YLGGTTGPV, or P6: YIVGFCLLV, or P7: TLTWLFAFV, or P8: GLVAGLSAV, or P9: ALGMLIAGL, or P10: MLIAGLPCL, or P11: LLCAIFAEV, or P12: RLWPTVGCL. Accordingto the invention, the HLA-A*0201 restrictive CTL epitope of a tuberculosis medicament resistance related protein antigen is predicted and analyzed by applying SYFPEITHI, BIMAS and NetCTL1.2 databasesand using an immunoinformatics mean according to a primary structure of the antigen so that the epitope peptide is obtained by virtue of selection, and the identified nonapeptide is not reported in documents. The epitope peptide is identified through an in-vitro enzyme linked immunospot (ELISPOT) experiment; and according to the result, a theoretical basis is provided for developing tuberculosis vaccine based on the medicament resistance related protein antigen and more information is provided for designing a tuberculosis polyepitope peptide vaccine based on mixed T cell epitope.
Owner:ZHENGZHOU UNIV
Schweineseuche O-shaped synthetic peptide vaccine and preparation thereof
Owner:SHANGHAI SHEN LIAN BIOMEDICAL CORP
A kind of o/asia type I foot-and-mouth disease virus bivalent genetic engineering polypeptide vaccine and its preparation method and application
InactiveCN102274496AEffective controlNo pollution in the processBacteriaMicroorganism based processesInclusion bodiesAdjuvant
The invention relates to an O / Asia I type foot and mouth disease virus bivalent genetic engineering polypeptide vaccine, its preparation method and its purpose. The method comprises the following steps: selecting two serotypes of O type and Asia I type, taking B cell determinant 15 amino acid fragments of VP1 and T-cell helper of VP4, performing a series connection, cloning without containing carrier protein, constructing O / Asia I gene engineering bacteria. An antigen protein product can be obtained after passing through the processes of high density fermenting, cell disrupting, inclusion body renaturating, fusion protein separating, and is homogenized with an adjuvant to form the O / Asia I type foot and mouth disease virus bivalent genetic engineering polypeptide vaccine. The vaccine of the present invention contains 2<n-1> polypeptide connected in series which is coded by a nucleic acid sequence shown in SEQ ID, wherein, n is an integer of 1-5. The invention has the advantages of good security and high immune efficacy, and can be used once in half year for immunization; and is suitable for large scale production and convenient preservation and transportation; and is capable of effectively preventing and controlling two serotypes foot and mouth disease of O type and Asia I type which is useful in our country; foot and mouth disease virus non-structural protein 3A.B. will not generate, so that the infective animals can be differentiated easily.
Owner:吴晓琰 +2
Cattle food-and-mouth disease virus A type synthetic peptide and preparation and application thereof
ActiveCN103193869AImprove the efficacy of immune protectionGood protective effectVirus peptidesAntiviralsAntigenFoot mouth disease virus
The invention discloses a cattle food-and-mouth disease virus A type synthetic peptide. The cattle food-and-mouth disease virus A type synthetic peptide has the following amino acid sequence: acetyl-YDLDF EALKP HFKSL GQTIT PADKS PPS VYNGT CKYSA PATRR GDLGS LAARL AACLP ASFNY GAIRA T-amide. The cattle food-and-mouth disease virus A type synthetic peptide can be used as an effective novel vaccine for the A type food-and-mouth disease in production practice; simultaneously, the realization of the test provides a certain foundation for further perfecting the construction of a food-and-mouth disease virus A type synthetic peptide vaccine and the construction of other food-and-mouth disease subtype synthetic peptide vaccines; and the new ideal proposed in the research of the synthetic peptide vaccine and the construction of a new method of the synthetic peptide vaccine provide theoretical foundation and technical support for further perfecting the development and research of the multiple antigenic peptide, multivalent peptides and joint peptide vaccines in future.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Moringa seed micro-molecular peptide extracted from moringa seeds, and extraction method thereof
InactiveCN105238831AMeet the needs of useSafe large-scale preparation and useHydrolysed protein ingredientsAntiviralsAntibacterial activityPeptide vaccine
The invention relates to a moringa seed micro-molecular peptide extracted from moringa seeds, and an extraction method thereof. A fermentation, electrolytic and enzymatic hydrolysis comprehensive technology is adopted, and a fermentation agent comprises papain, alkaline proteinase and trypsin according to a mass ratio of 2:2:1. The proportion of moringa seed micro-molecular peptides with the molecular amount of 0-480 dalton in the moringa seed micro-molecular peptide disclosed in the invention is not lower than 87.5%. The moringa seed micro-molecular peptide can be used as a diagnosis peptide, a peptide vaccine, an antibiotic active peptide, a cytokine mimic peptide, an antiviral peptide or an antitumor peptide, and a peptide health product.
Owner:潘爱国
O-type foot and mouth disease virus antigen epitope molecular mimic peptide and application thereof
InactiveCN101597327AAntigenicNon-toxicAntiviralsPeptidesVirulent characteristicsMolecular Immunology
The invention relates to the molecular immunology field. Although the existing foot-and-mouth disease weak poison vaccines and inactivated vaccines and other routine vaccines have good immunogenicity, but the vaccines have some unsafe factors such as reversion of virulence, incomplete virus blanching, the escape of live-virus from a preparation factory and the like so that people are motivated to find a more safe and effective FMD vaccine. In the molecular mimic peptide invention, phage display techniques are used to sieve effective FMDV epitopes to provide an O-type foot and mouth disease virus antigen epitope molecular mimic peptide and the invention also provides an application thereof in preparing immunogen and swine foot-and-mouth disease epitope peptide vaccine. The FMDV epitope peptide vaccine using the molecular mimic peptide of the invention has the antigenicity of target molecule without toxicity, is very safe and has low mass production cost and good economic effect and social effect.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
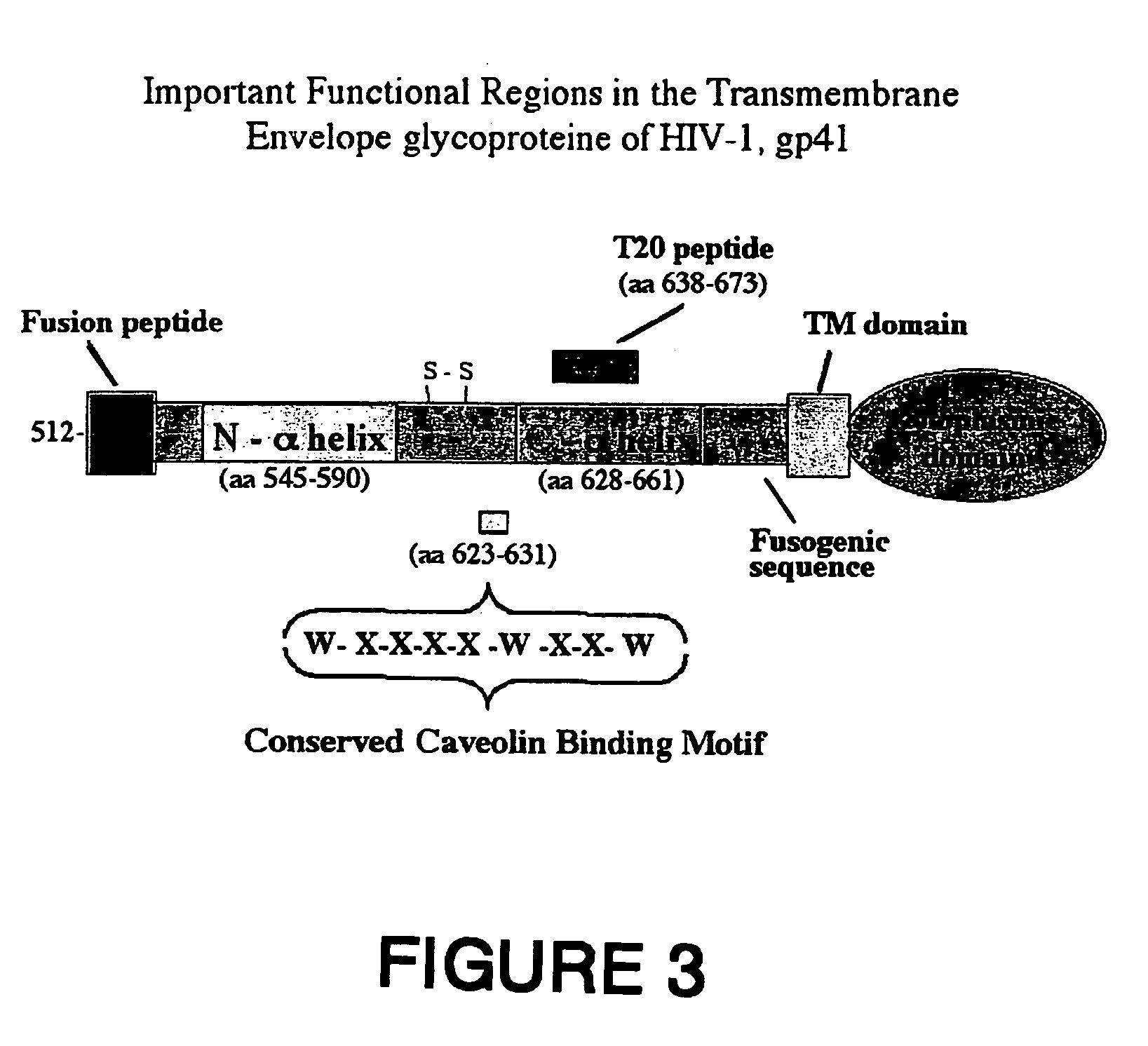
Synthetic peptide vaccines for HIV: the CBD epitope as an effective immunogen to elicit broadly neutralizing antibodies against HIV
Owner:INST PASTEUR +1
Vaccine for preventing and curing tumor
InactiveCN101920009AGenetic material ingredientsAntibody medical ingredientsReverse transcriptaseNucleotide
The invention belongs to vaccine and preparation thereof, in particular to a vaccine for preventing and curing tumor and preparation thereof. The vaccine is prepared by steps of: choosing a telomerase reverse transcriptase epitope sequence, serially connecting nucleotides the telomerase reverse transcriptase with multi-epitope, preparing the DNA vaccine of the telomerase reverse transcriptase with multi-epitope, and preparing the multi-epitope vaccine of the telomerase reverse transcriptase with multi-epitope. The invention overcomes the shortcomings that a single peptide vaccine only has a clinical effect in several tumor patients. After the vaccine has an immune body, the epitope specific CD4+T cells and the CD8+T cells can be activated. Under the assistance of the Th1 cell factor excreted from the CD4+T cells, the CD8+CTL can kill the tumor cell presenting TERT so as to prevent and cure tumor.
Owner:HEBEI MEDICAL UNIVERSITY
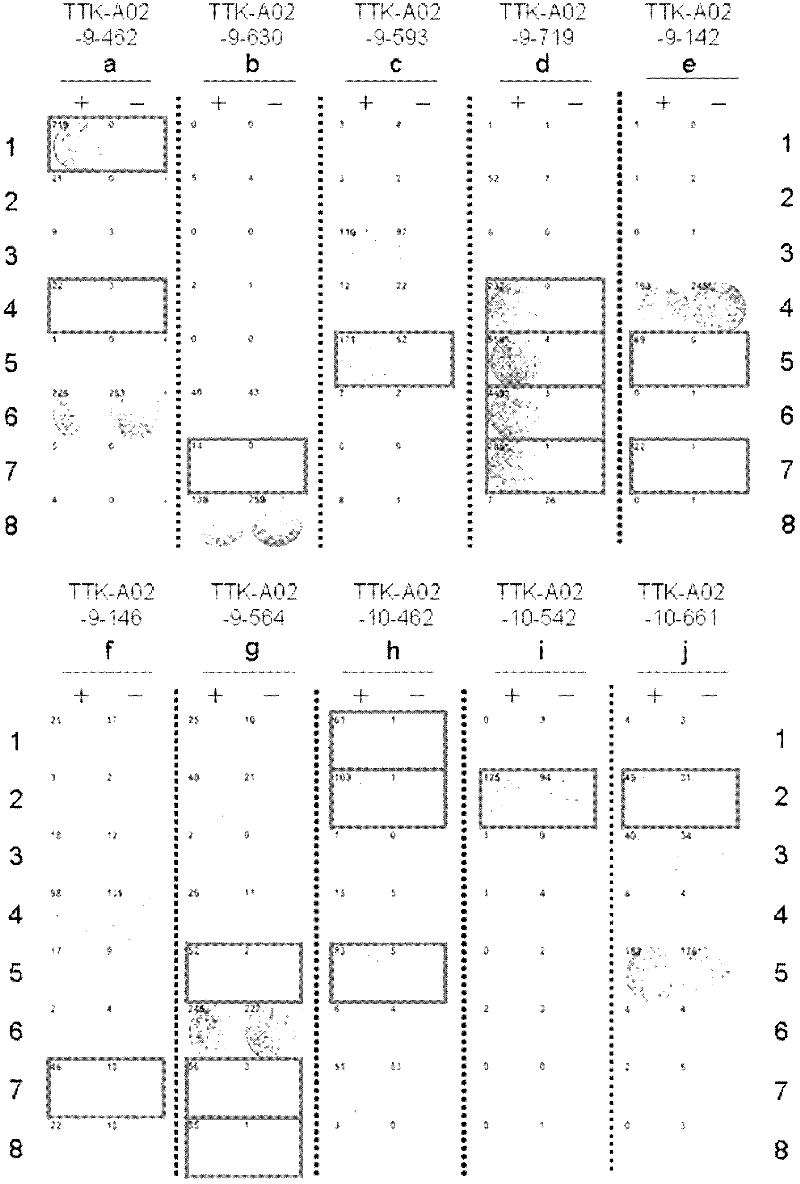
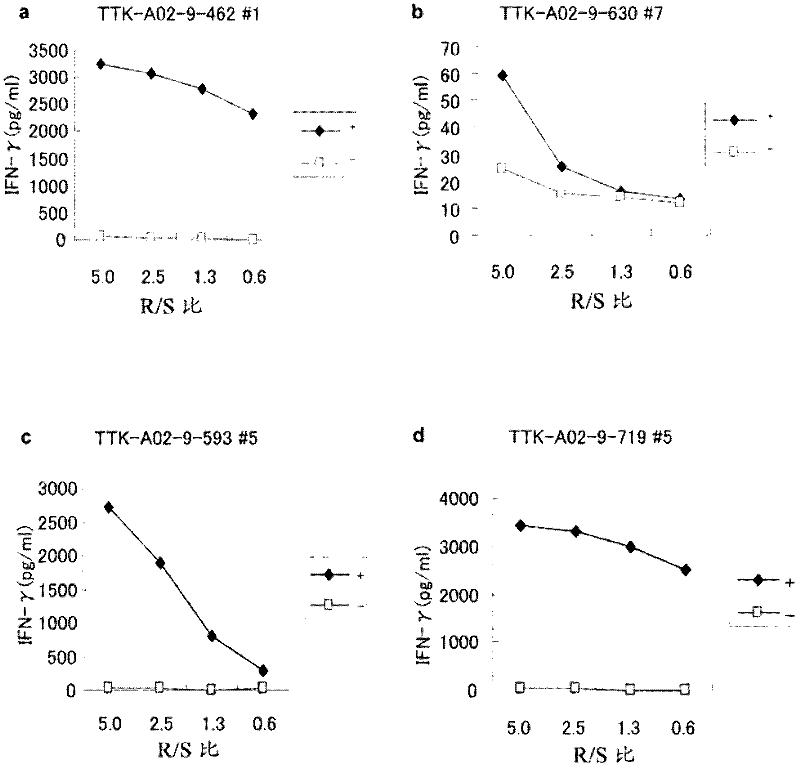
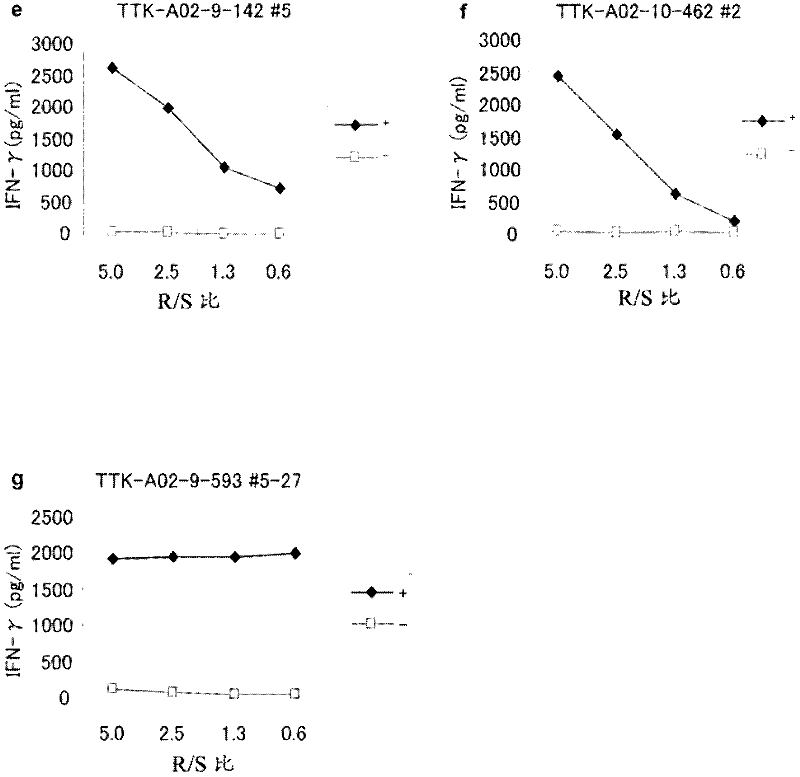
Ttk peptides and vaccines including the same
InactiveCN102459314ATumor rejection antigen precursorsPeptide/protein ingredientsNucleotidePeptide vaccine
Peptide vaccines against cancer are described herein. In particular, epitope peptides derived from the TTK gene that elicit CTLs are provided. Antigen-presenting cells and isolated CTLs that target such peptides, as well as methods for inducing the antigen-presenting cell, or CTL are also provided. The present invention further provides pharmaceutical compositions containing as active ingredients peptides derived from TTK or polynucleotides encoding the peptides. Furthermore, the present invention provides methods for the treatment and / or prophylaxis (i.e., prevention) of cancers (tumors), and / or the prevention of postoperative recurrence thereof, as well as methods for inducing CTLs, methods for inducing anti-tumor immunity, using the peptides derived from TTK, polynucleotides encoding the peptides, or antigen-presenting cells presenting the peptides, or the pharmaceutical compositions of the present invention.
Owner:ONCOTHERAPY SCI INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com