MAGE-A3/HPV 16 peptide vaccines for head and neck cancer
a peptide vaccine and head and neck cancer technology, applied in the field of peptide vaccines for head and neck cancer, can solve the problems of limited efficacy of antigen-specific cytotoxic t cells, and high cost of maturation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of MAGE-A3 Antigenic Epitopes
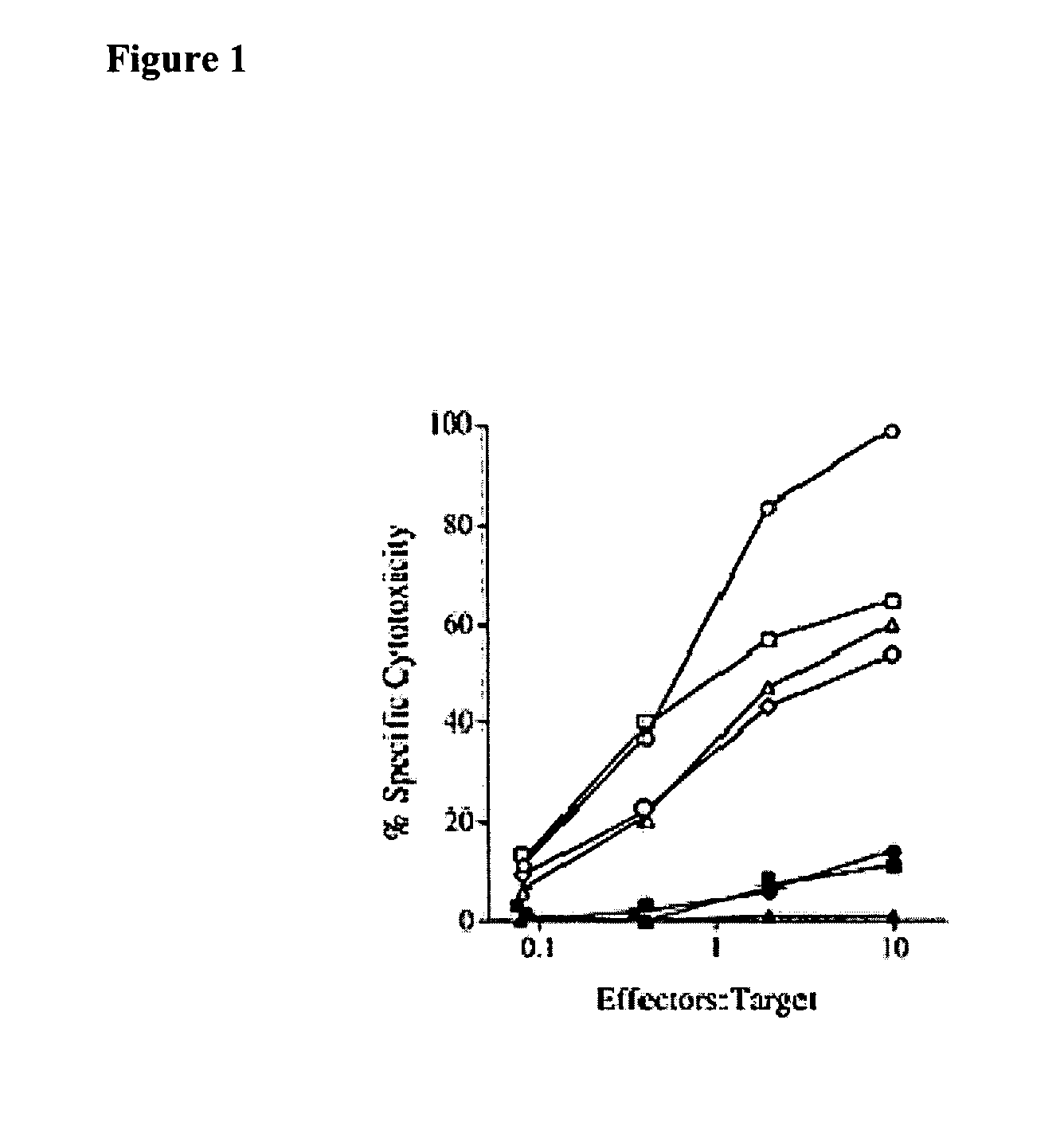
[0123] Using a predictive algorithm based on the presence of MHC binding motifs, an HLA-A1-binding peptide from MAGE-A3 that induced in vitro anti-tumor CTL responses with lymphocytes from normal individuals was identified (Celis et al. Induction of anti-tumor cytotoxic T lymphocytes in normal humans using primary cultures and synthetic peptide epitopes, PNAS USA 91(6):2105-2109 (1994)). In addition to the HLA-A1-restricted epitope, an HLA-A2 restricted CTL epitope, which is more frequently found in the general population than HLA-A1, was identified (Kawashima et al. The multi-epitope approach for immunotherapy for cancer: identification of several CTL epitopes from various tumor-associated antigens expressed on solid epithelial tumors. Human Immunology 59(1):1-14 (1998)). CTL-induced by peptide MAGE-A3112-120 (KVAELVHFLL; SEQ ID NO:2) were quite effective in recognizing tumor cells expressing MAGE-A3 antigen and HLA-A2 (FIG. 1).
[0124] B...
example 2
Identification of a Promiscuous T Helper Epitope from MAGE-A3
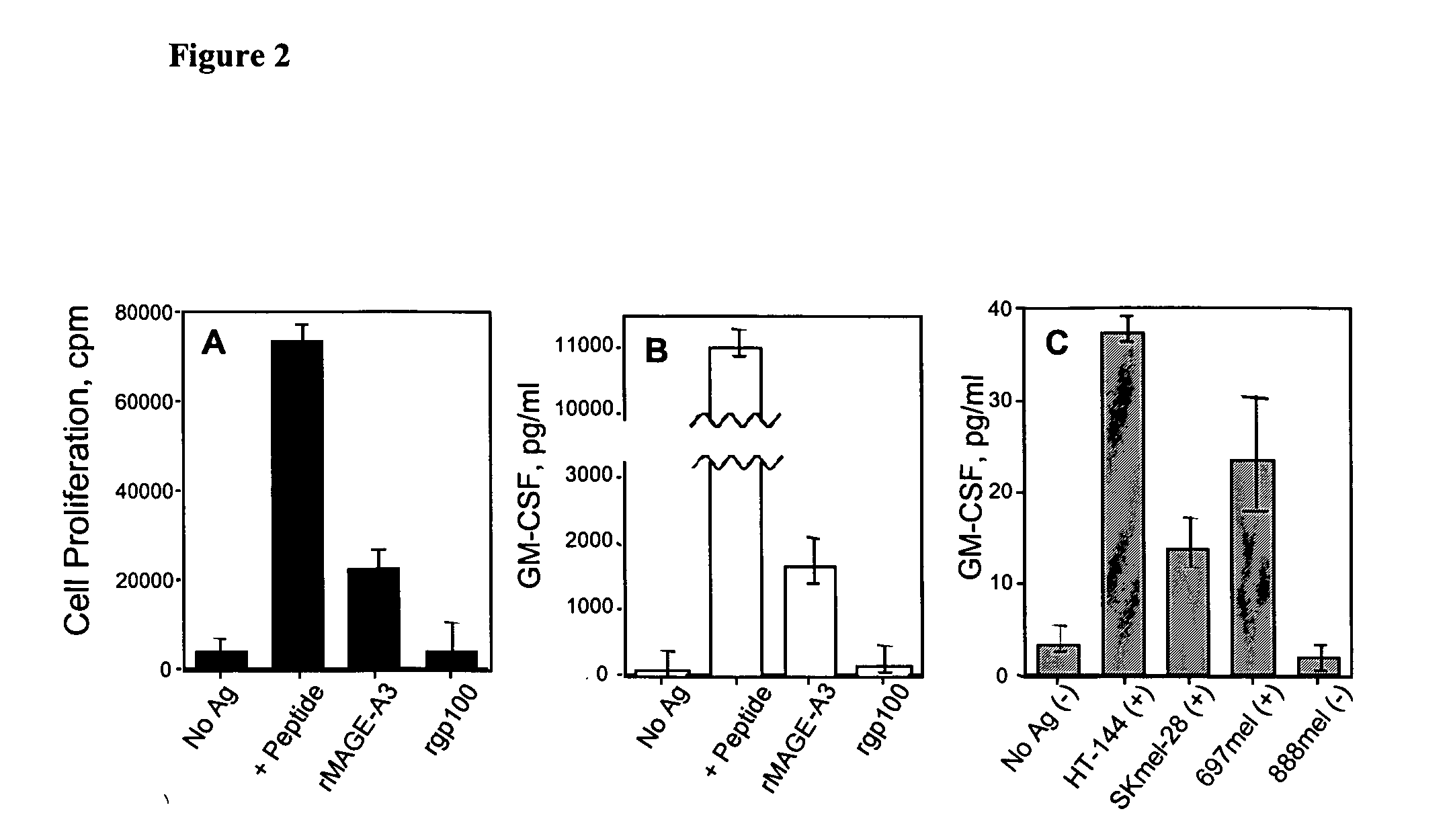
[0126] A promiscuous T helper epitope that is presented to T cells by HLA-DR4 and HLA-DR7, two of the most frequently found MHC class II alleles, was identified. Peptide MAGE-A3149-160 (VIFSKASSSLQL; SEQ ID NO:5) was found to stimulate T helper lymphocytes that recognized recombinant MAGE-A3 protein or cell lysates from tumors expressing MAGE-A3 antigen (Kobayashi et al. Tumor-reactive T helper lymphocytes recognize a promiscuous MAGE-A3 epitope presented by various major histocompatibility complex class II alleles. Cancer Research 61(12):4773-8 (2001)). As shown in FIG. 2, HLA-DR4-restricted HTL clone 8G9 recognizes naturally processed MAGE-A3 antigen. A: Proliferative T-cell response induced by MAGE-A3146-160 (+Peptide), recombinant MAGE-A3 protein (rMAGE-A3) or recombinant gp100 (rgp100). B: Tissue culture supernatants from experiment described in panel A, were collected after 48 hr and the concentration of GM-CSF was ...
example 3
Identification and Selection of CTL and T Helper Epitopes from HPV 16 E7
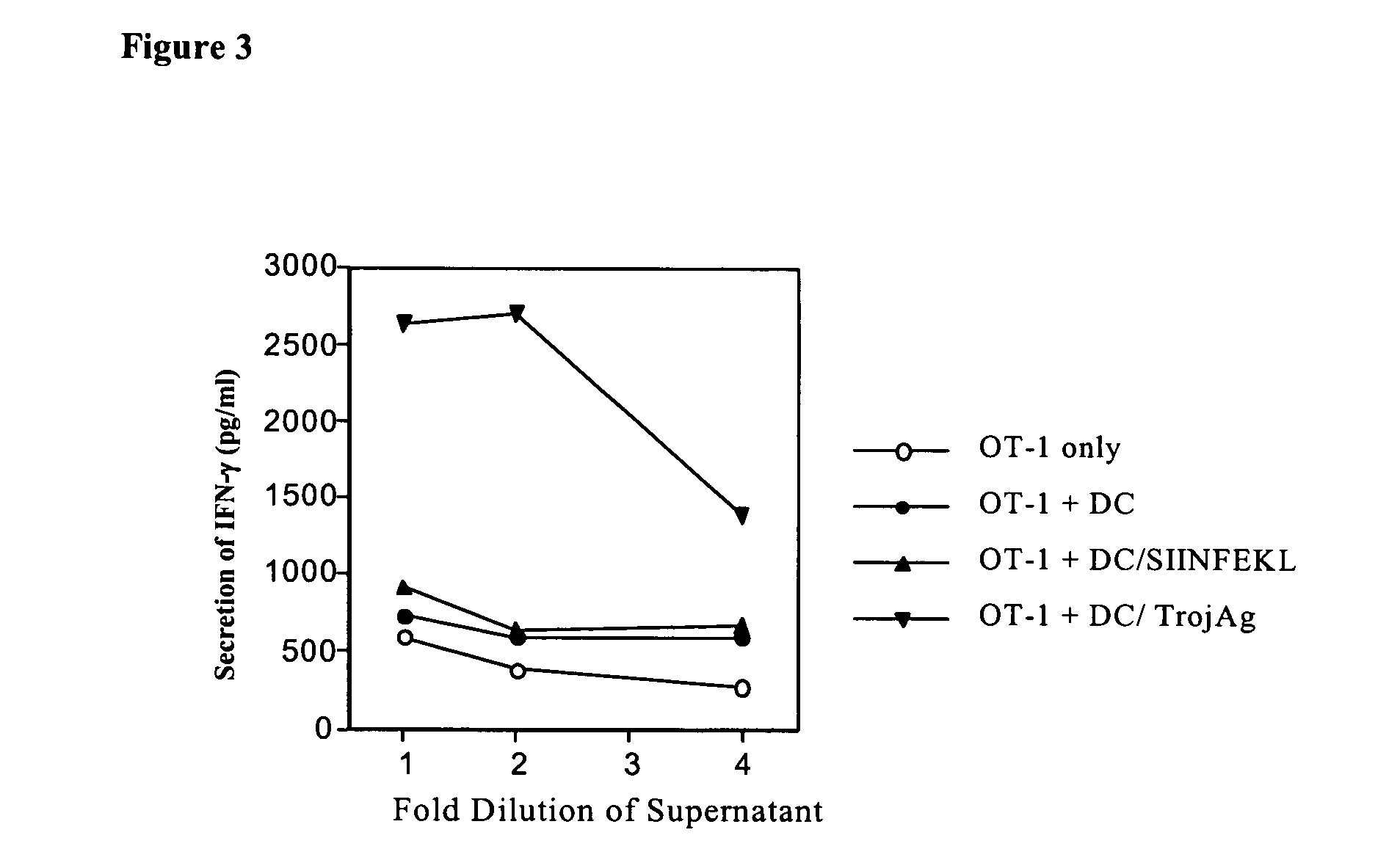
[0127] Two HLA-A2-restricted CTL epitopes and one T helper epitope were selected. The identification and description of these epitopes have been published (de Jong et al. Frequent detection of human papillomavirus 16 E2-specific T-helper immunity in healthy subjects. Cancer Research 62(2):472-479 (2002); Kast et al. Role of HLA-A motifs in identification of potential CTL epitopes in human papillomavirus type 16 E6 and E7 proteins. Journal of Immunology 152(8):3904-3912 (1994)). Furthermore, the HLA-A2-restricted CTL epitopes were shown to induce CTL responses (Jager et al. Monitoring CD8 T cell responses to NY-ESO-1: correlation of humoral and cellular immune responses. PNAS USA 97(9):4760-4765 (2000); den Haan et al. Identification of a graft versus host disease-associated human minor histocompatibility antigen. Science 268(5216):1476-1480 (1995); Bennouna et al. Application of IL-5 ELISPOT assays to quantific...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com