Tuberculosis medicament resistance related tuberculosis-resisting cytotoxic T lymphocyte (CTL) epitope peptide derived from refflux protein and application thereof
A technology for efflux proteins and epitope peptides, which is applied in the direction of peptides, antibacterial drugs, bacterial antigen components, etc., and can solve the problem of few reports on membrane proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The anti-tuberculosis CTL epitope peptide derived from the tuberculosis drug resistance-related efflux protein of the present invention is a nonapeptide, and the amino acid sequence of the nonapeptide is:
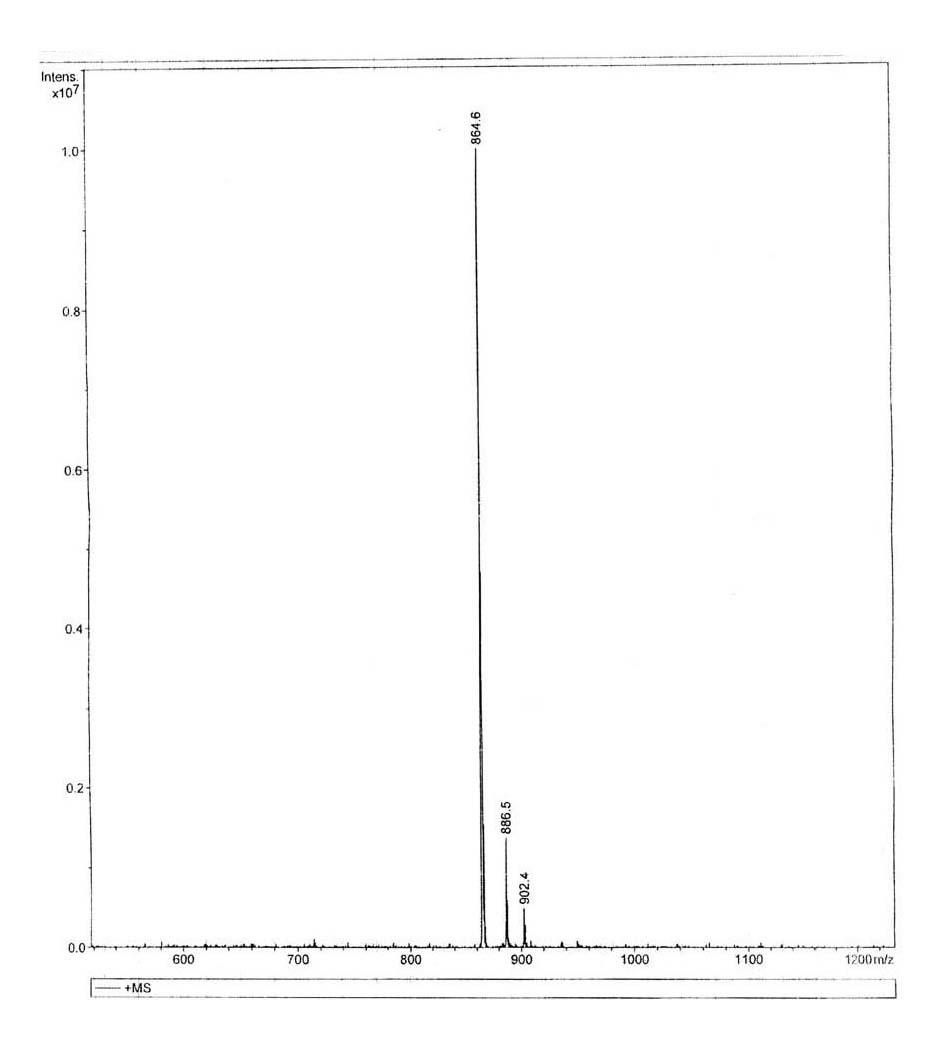
[0020] P5: Tyr-Leu-Gly-Gly-Thr-Thr-Gly-Pro-Val (YLGGTTGPV) molecular weight: 864.6 (theoretical value: 863.44)
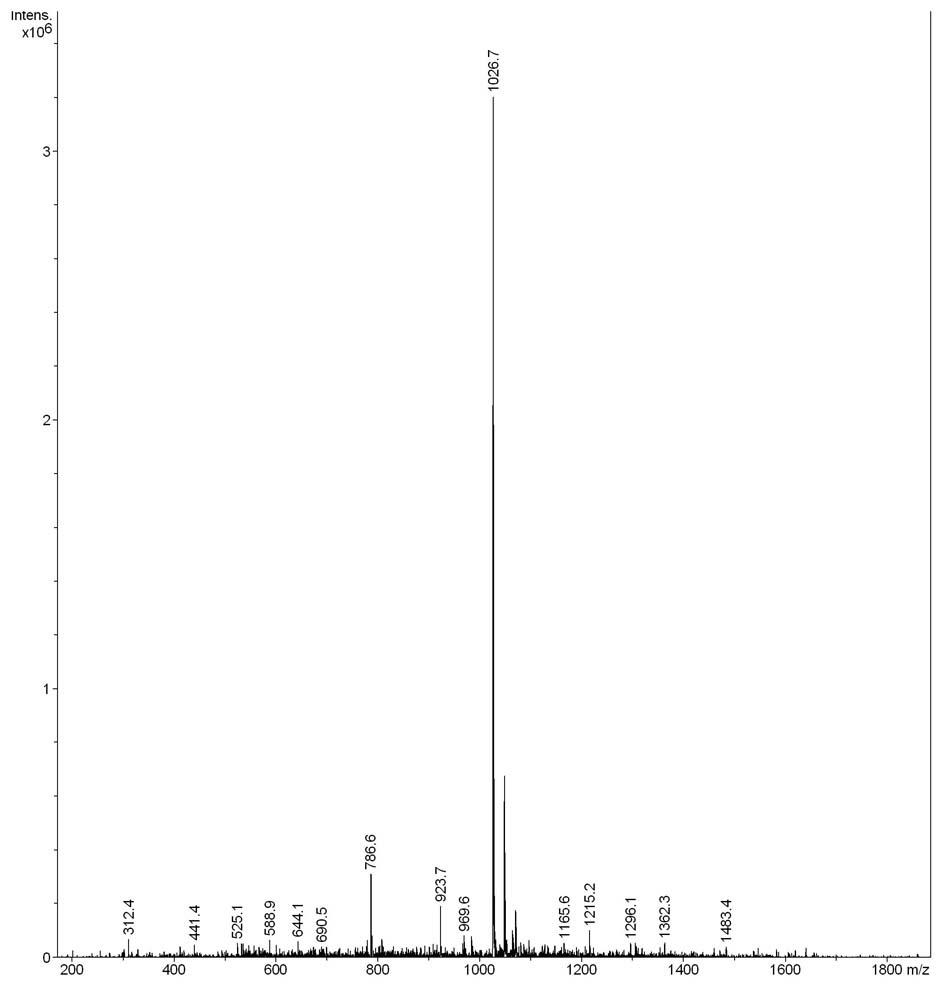
[0021] Or P6: Tyr-Ile-Val-Gly-Phe-Cys-Leu-Leu-Val (YIVGFCLLV) Molecular weight: 1026.7 (theoretical value: 1025.86)
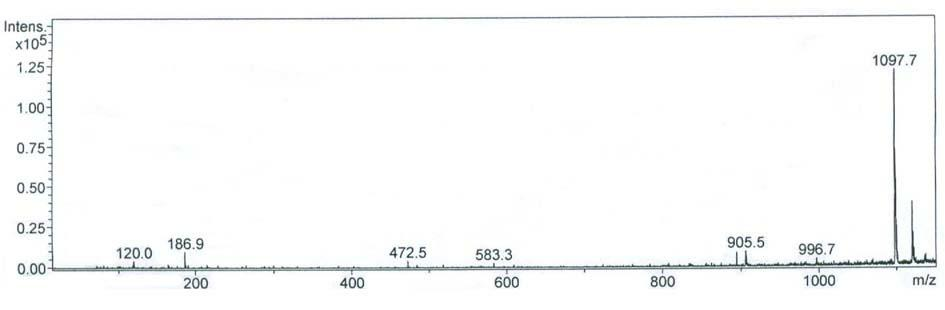
[0022] or P7: Thr-Leu-Thr-Trp-Leu-Phe-Ala-Phe-Val (TLTWLFAFV) molecular weight: 1097.7 (theoretical value: 1097.32)
[0023] or P8: Gly-Leu-Val-Ala-Gly-Leu-Ser-Ala-Val (GLVAGLSAV) molecular weight: 786.7 (theoretical value: 786.7)
[0024] Or P9: Ala-Leu-Gly-Met-Leu-Ile-Ala-Gly-Leu (ALGMLIAGL) molecular weight: 858.7 (theoretical value: 857.81)
[0025] or P10: Met-Leu-Ile-Ala-Gly-Leu-Pro-Cys-Leu (MLIAGLPCL) molecular weight: 930.6 (theoretical value: 930.24)
[0026] or P11: Leu-Leu-Cys-Ala-Ile-Phe-Ala-Glu-Val (LLCAIFAEV) molecular weig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com