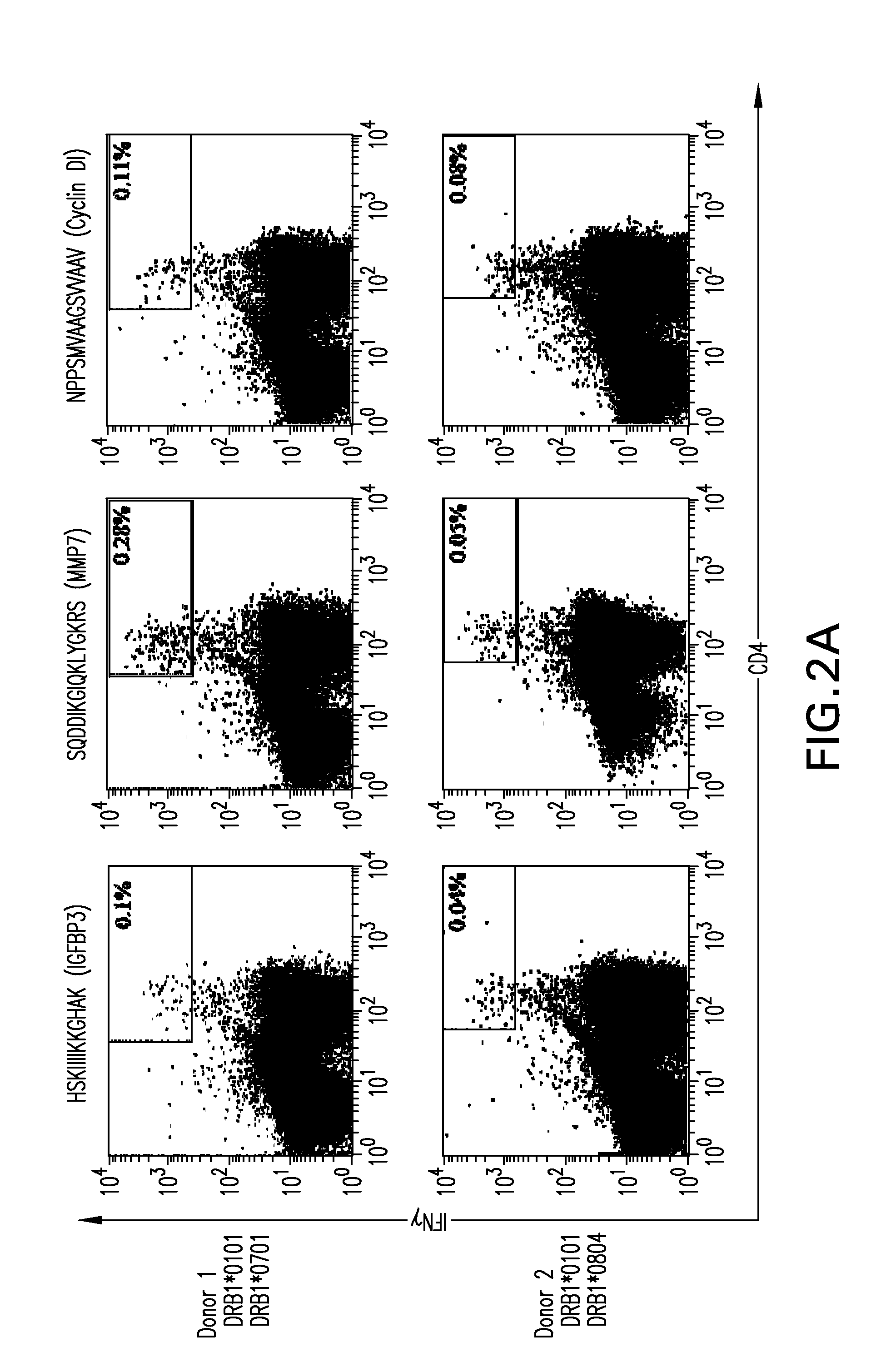
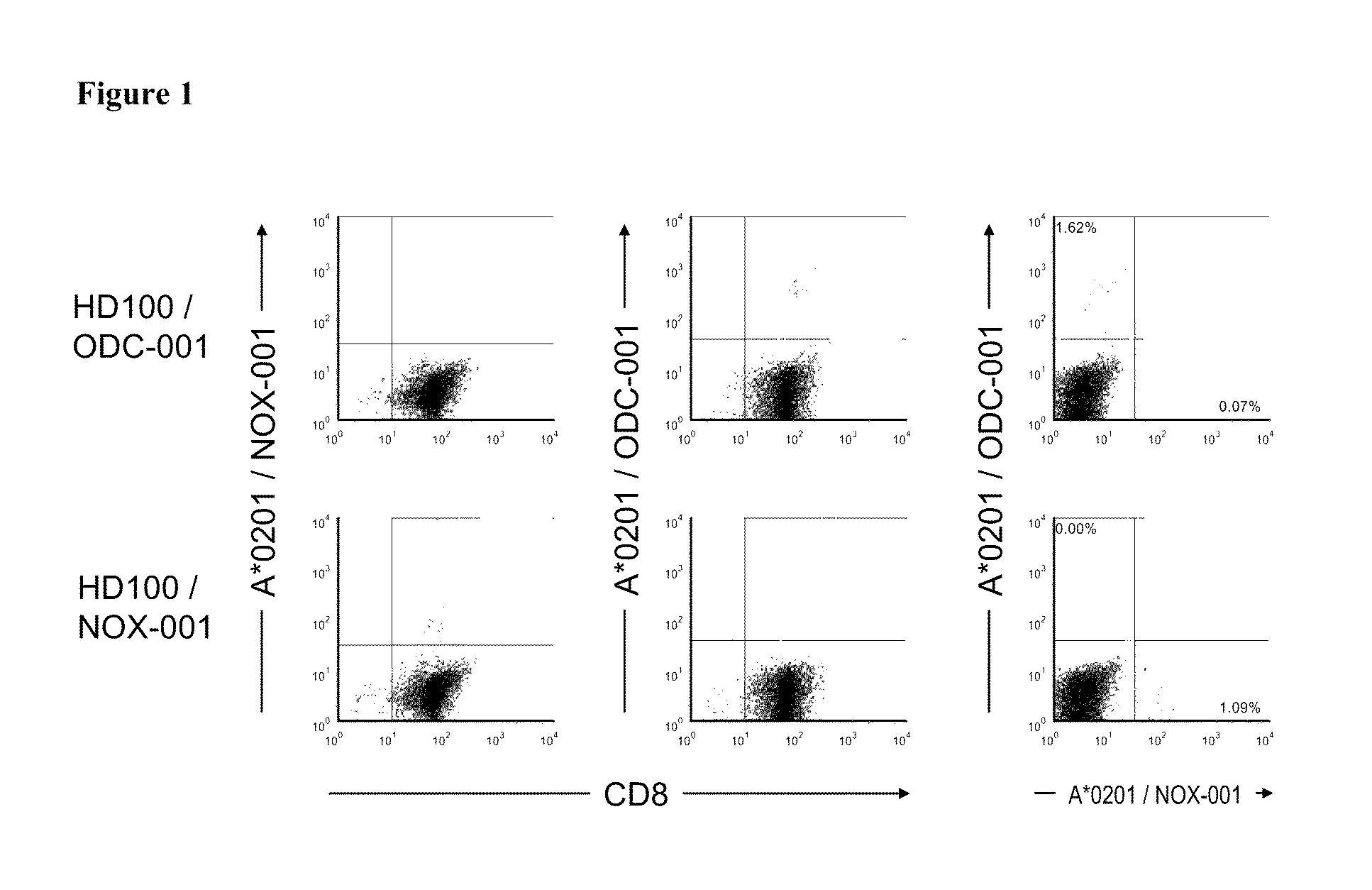
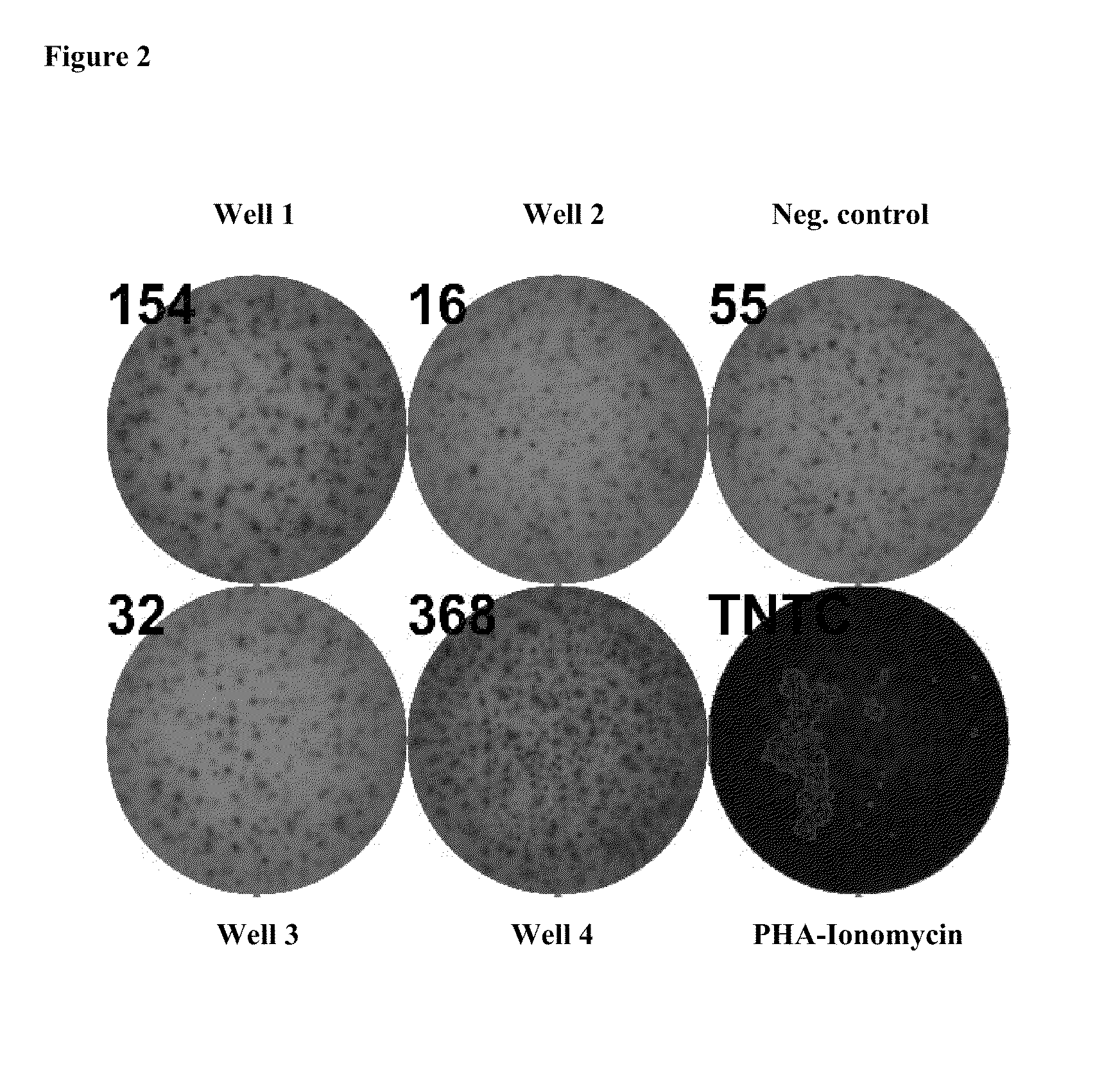
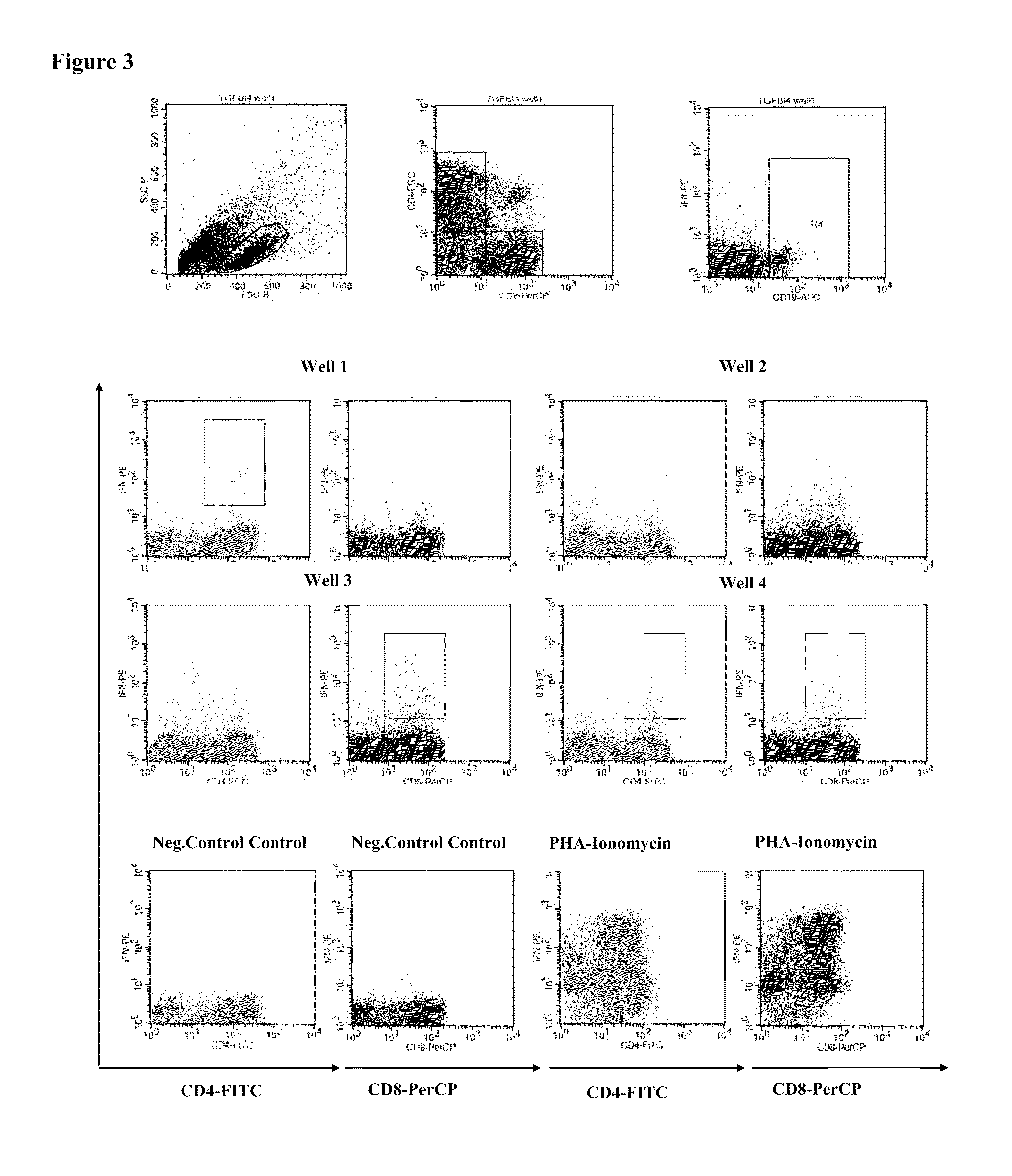
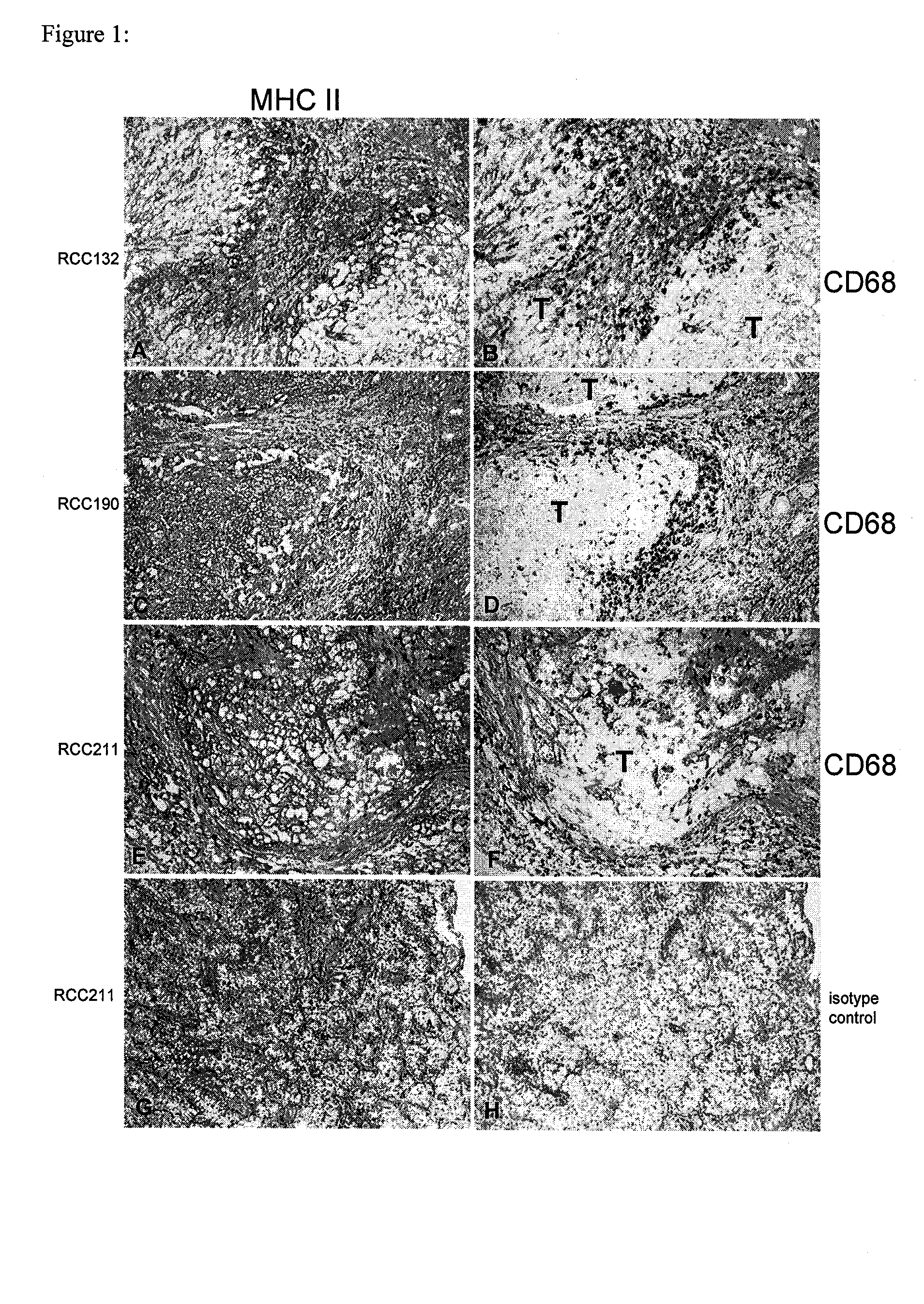
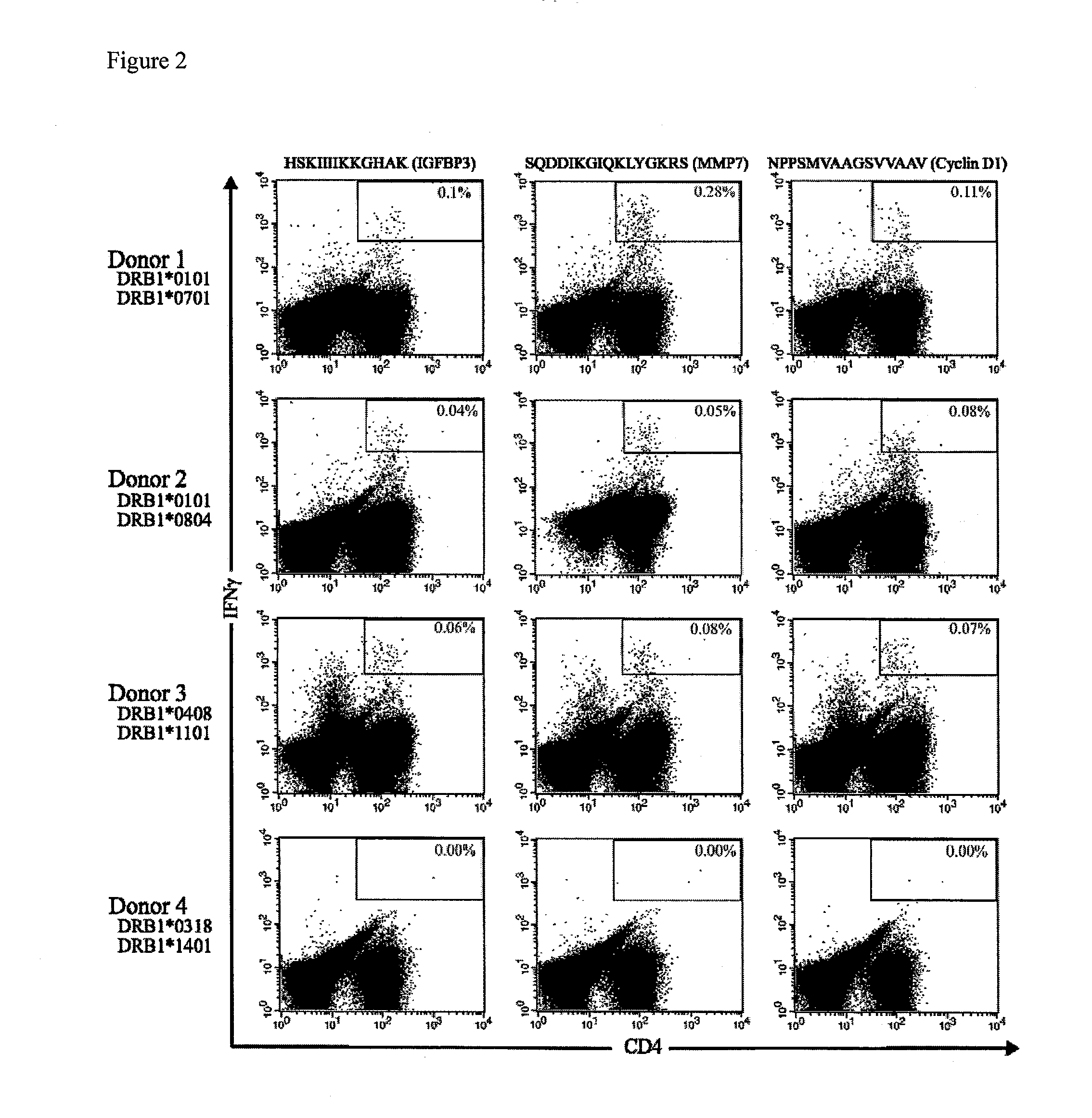
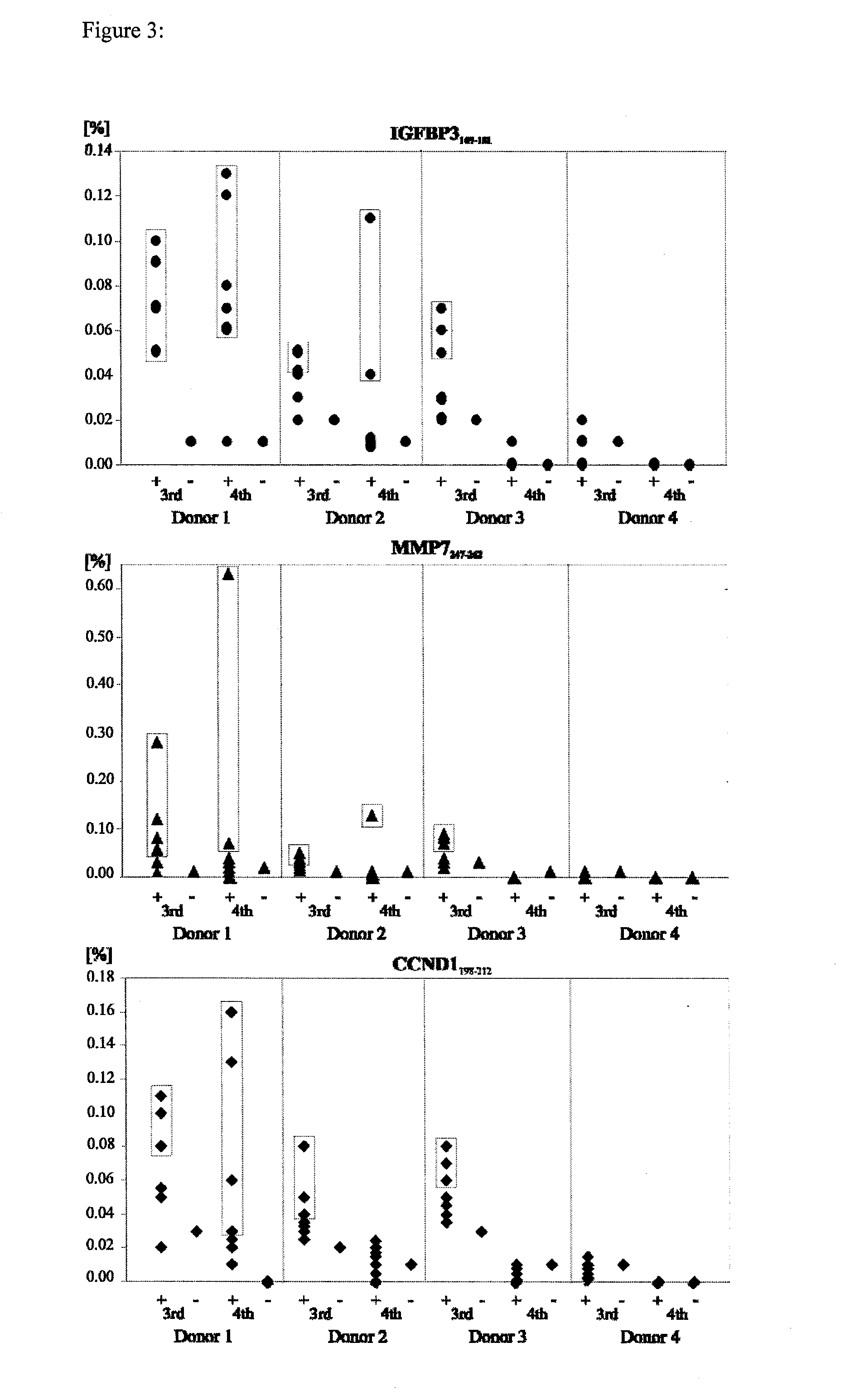
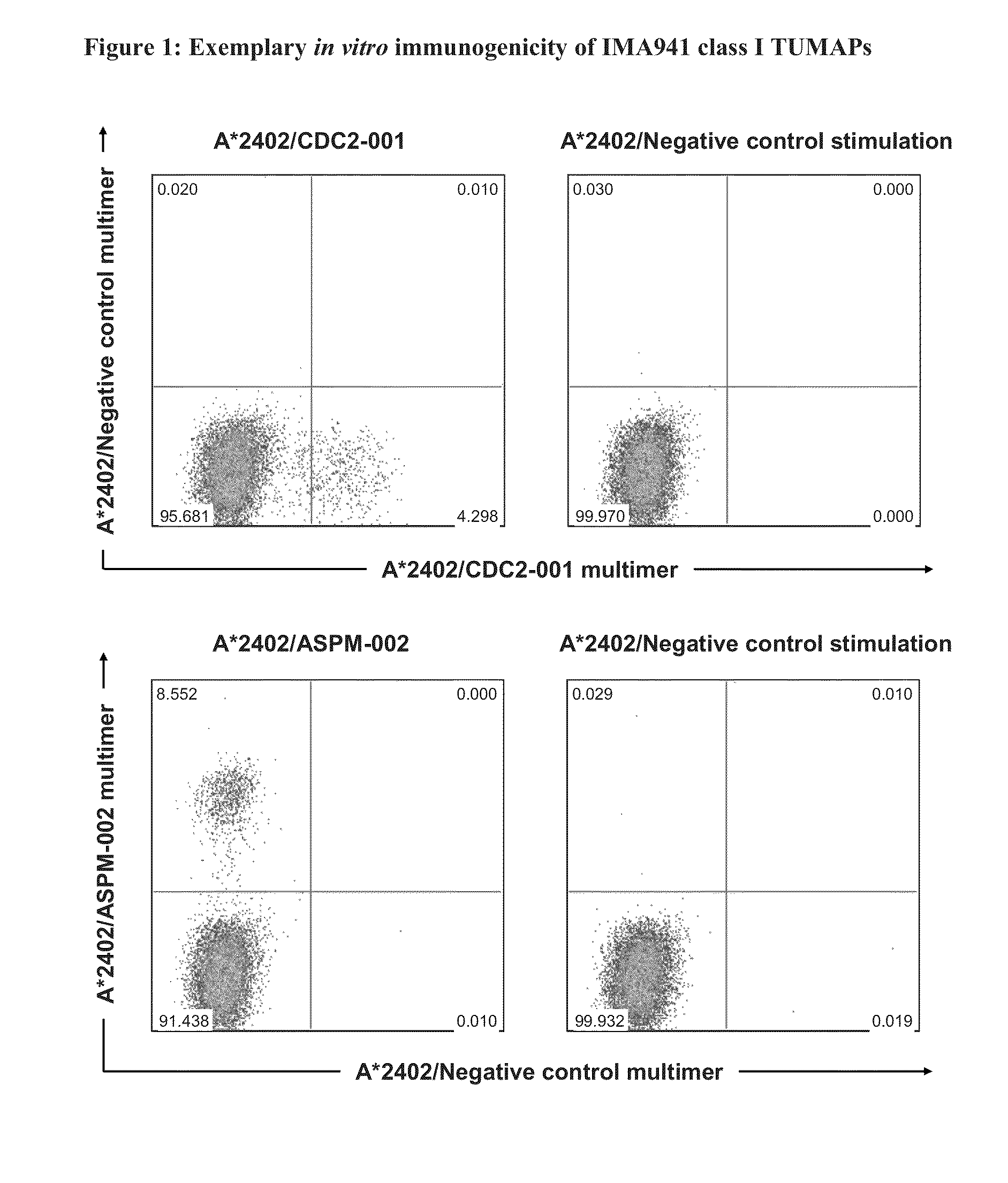
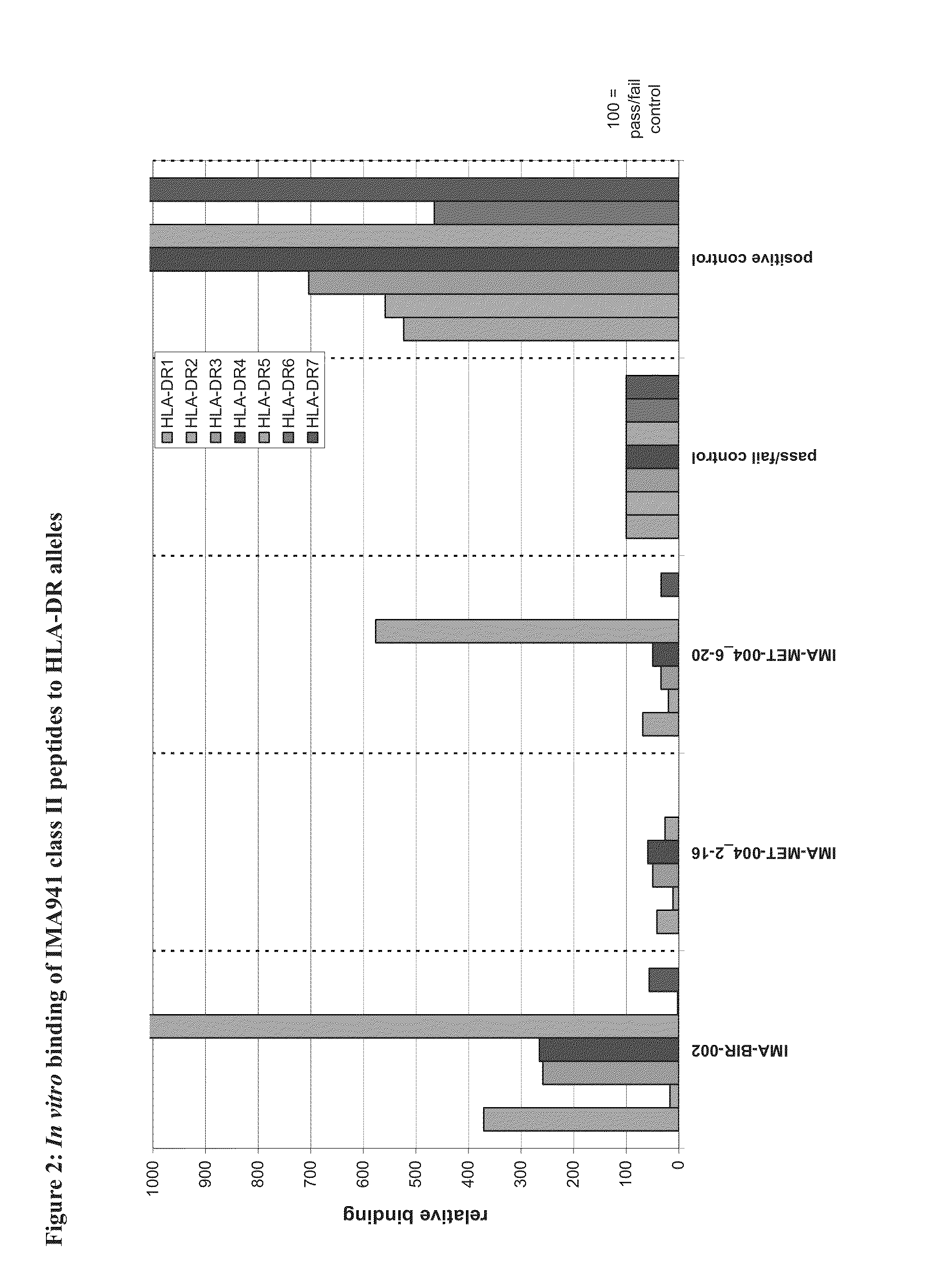
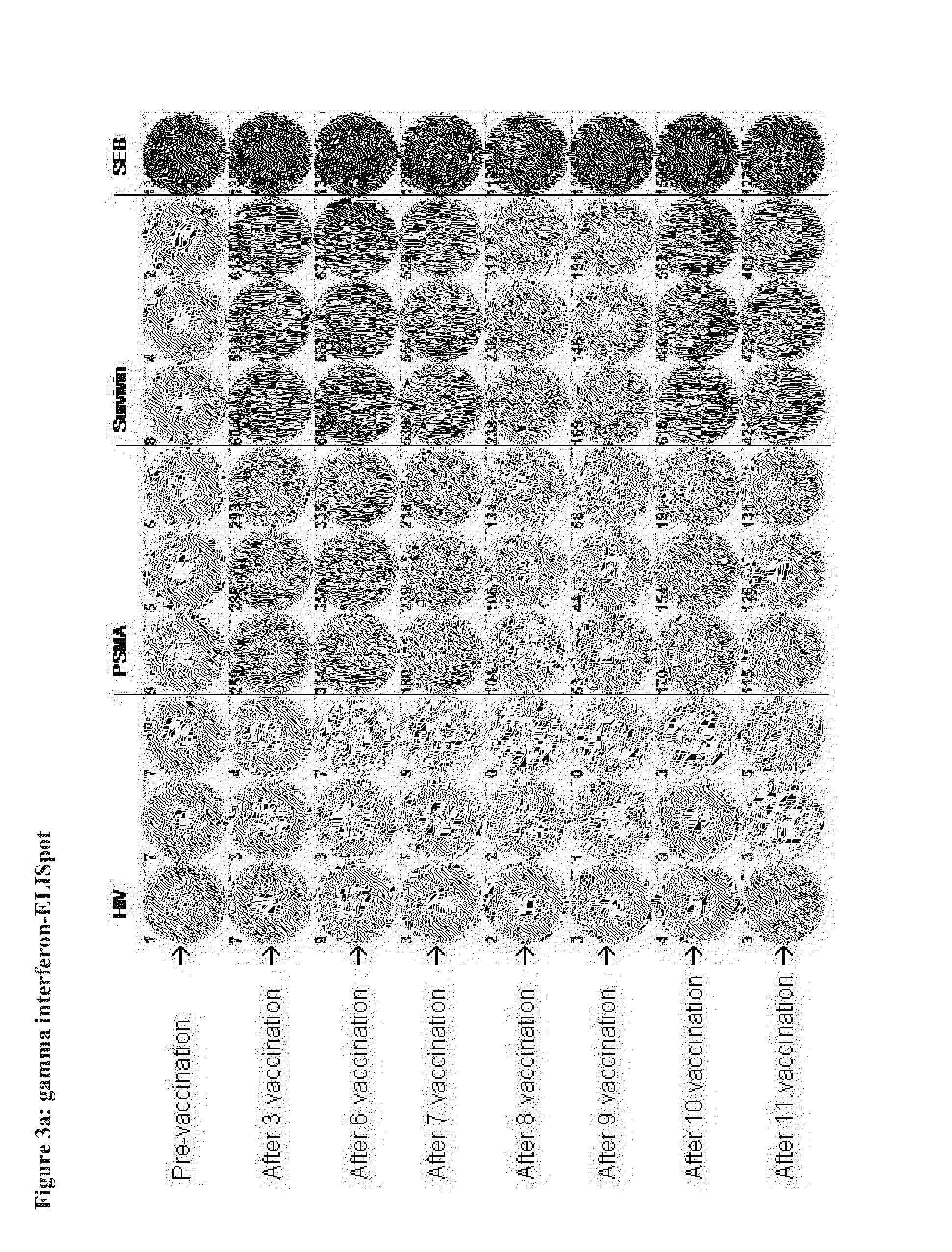
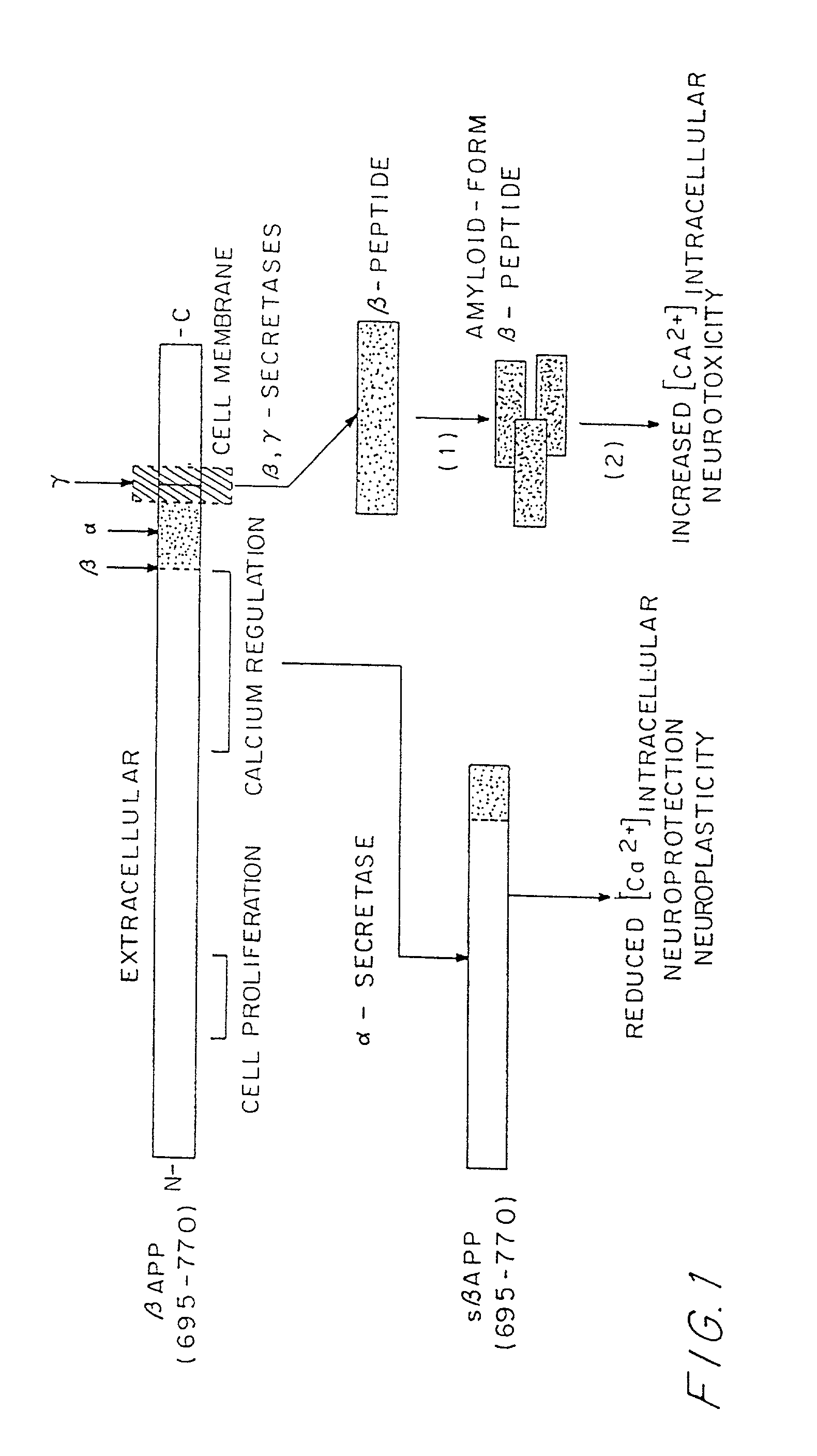
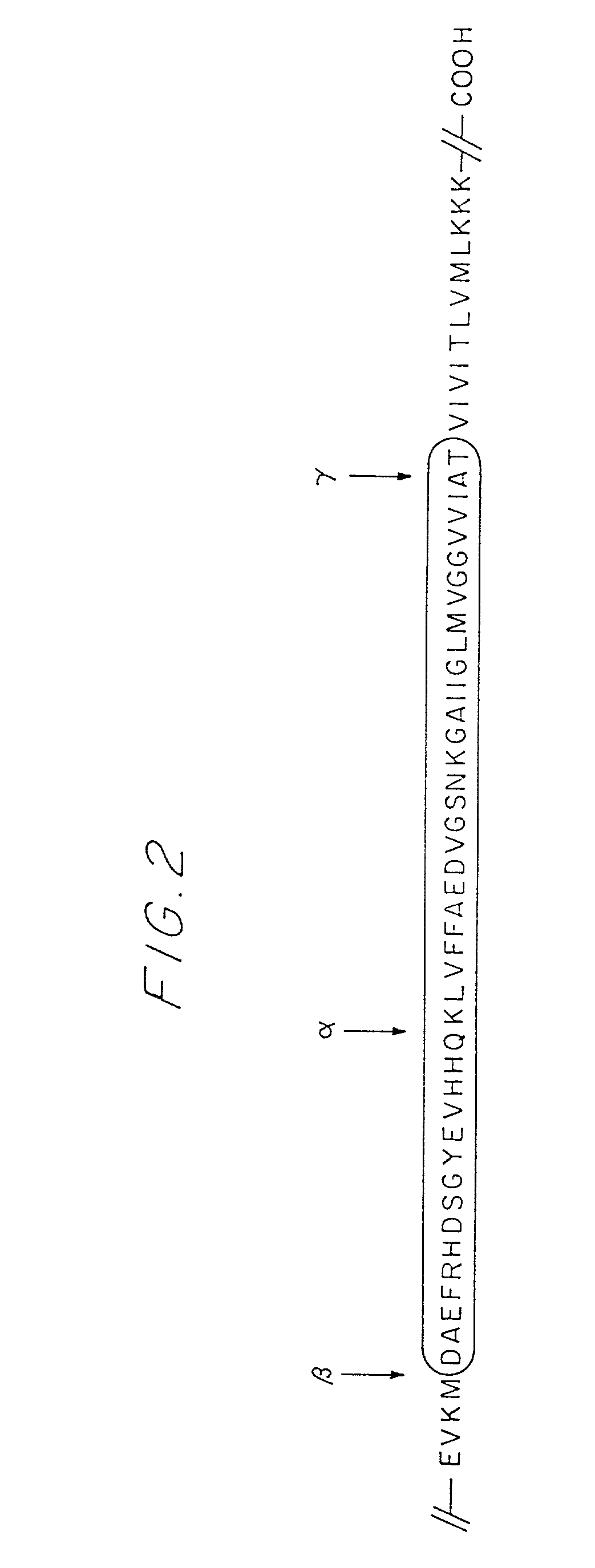
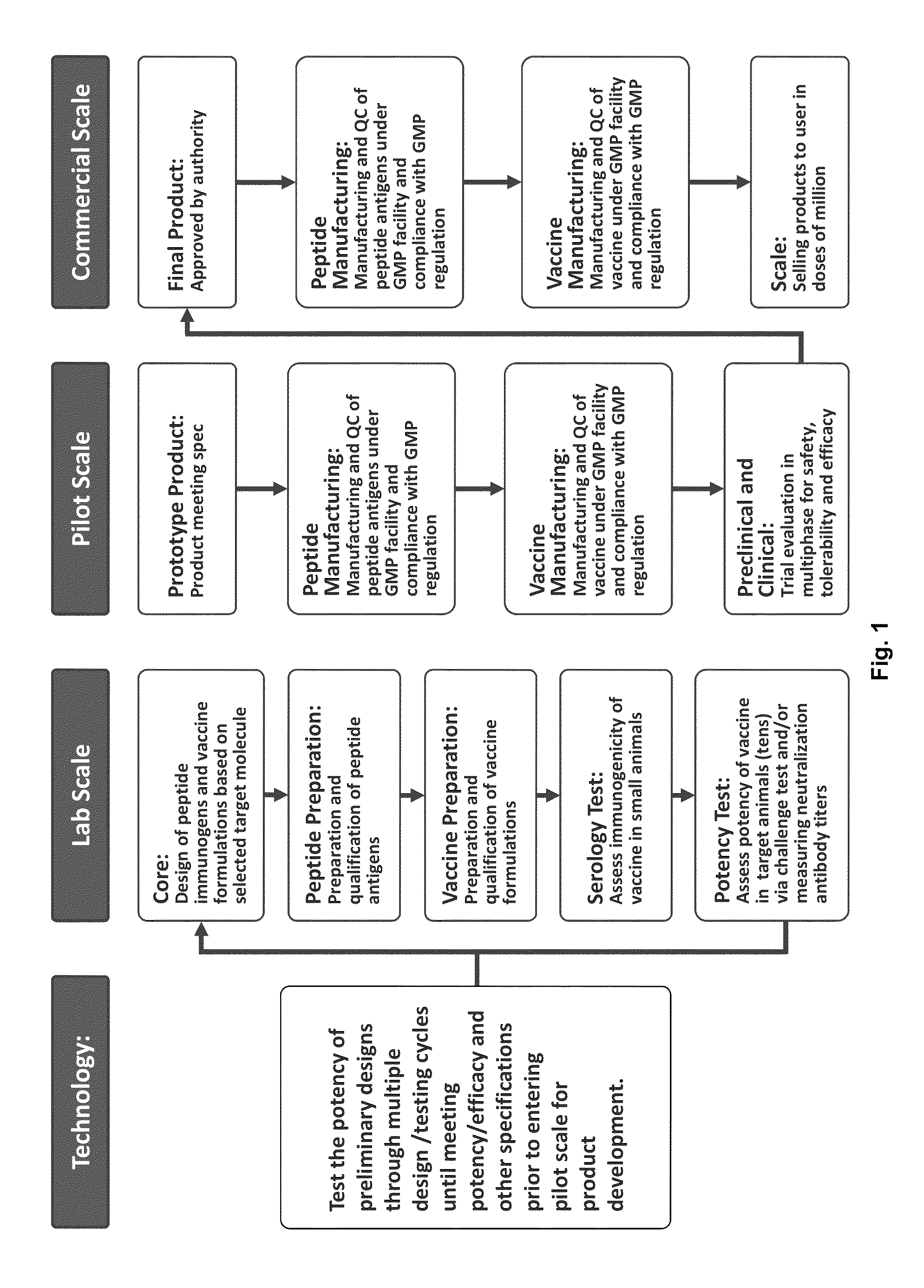
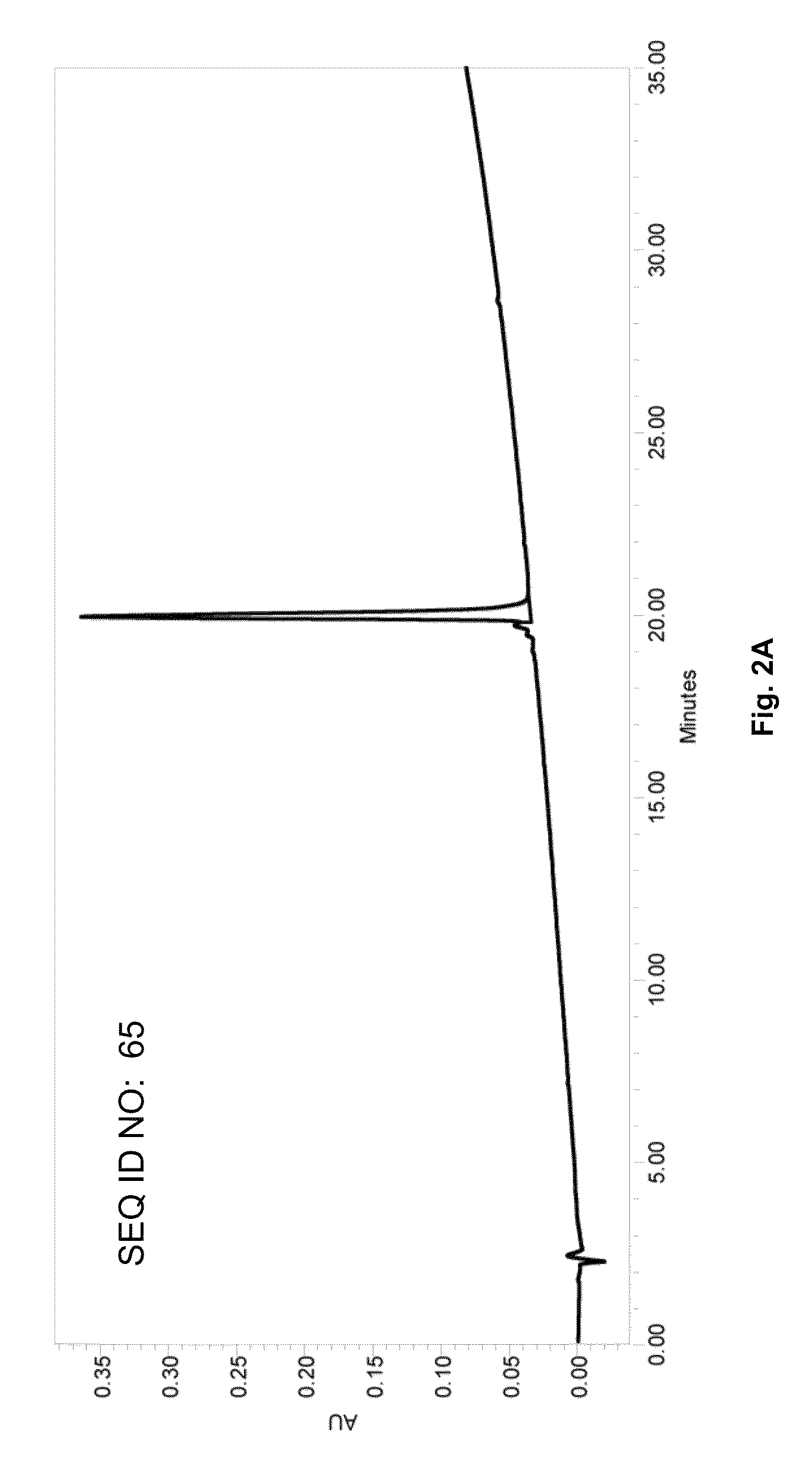
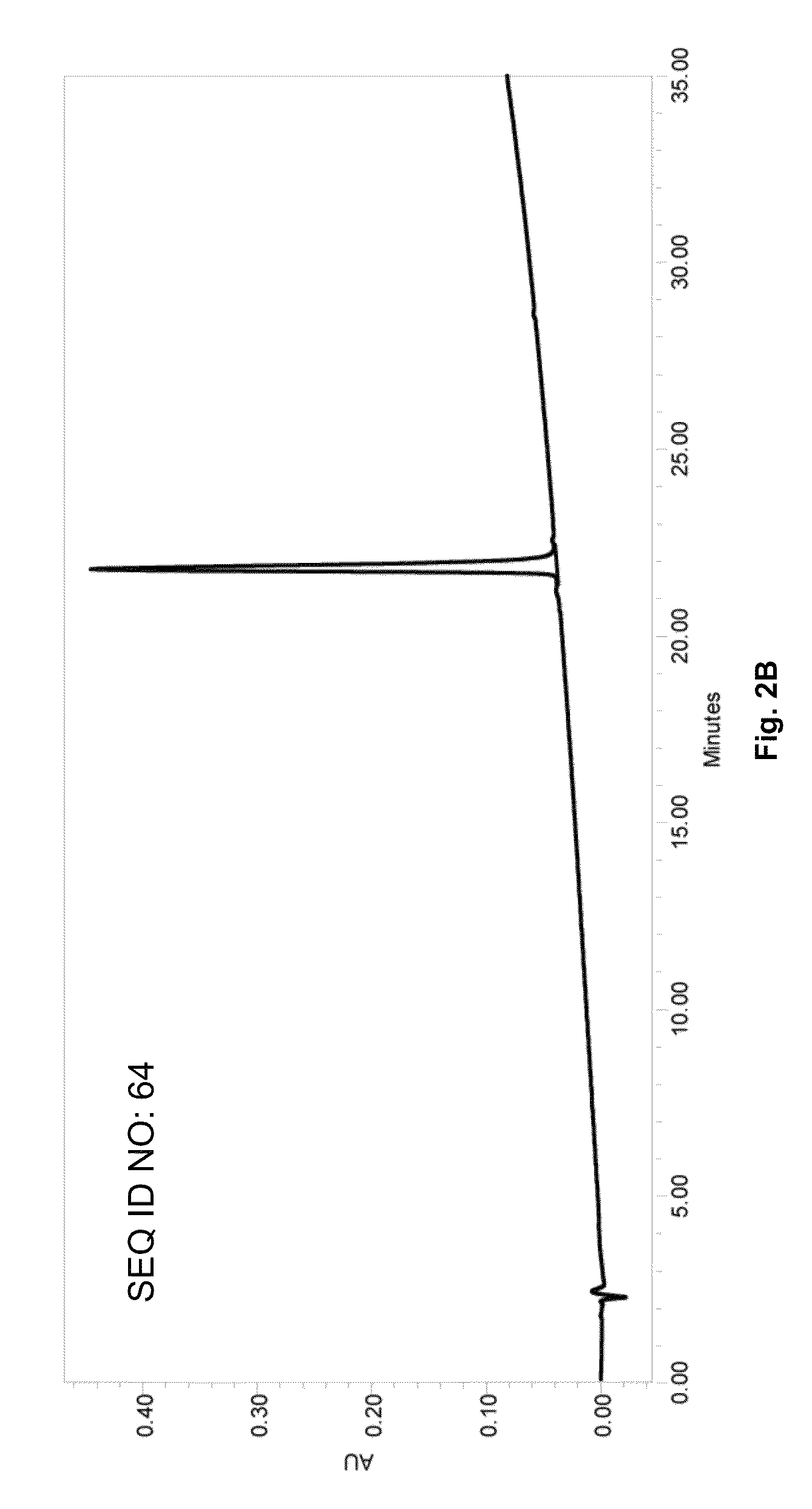
Patents
Literature
105 results about "T helper cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The T helper cells (Tₕ cells), also known as CD4⁺ cells, are a type of T cell that play an important role in the immune system, particularly in the adaptive immune system. They help the activity of other immune cells by releasing T cell cytokines. These cells help suppress or regulate immune responses. They are essential in B cell antibody class switching, in the activation and growth of cytotoxic T cells, and in maximizing bactericidal activity of phagocytes such as macrophages.
Tumour-associated peptides binding to human leukocyte antigen (HLA) class i or ii molecules and related Anti-cancer vaccine
InactiveUS20090274714A1Organic active ingredientsPeptide/protein ingredientsHla class iiAdditive ingredient
The present invention relates to immunotherapeutic methods, and molecules and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumour-associated T-helper cell peptide epitopes, alone or in combination with other tumour-associated peptides, that serve as active pharmaceutical ingredients of vaccine compositions which stimulate anti-tumour immune responses. In particular, the present invention relates to two novel peptide sequences derived from HLA class II molecules of human tumour cell lines, which can be used in vaccine compositions for eliciting anti-tumour immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Novel immunogenic epitope for immunotherapy
ActiveUS20090136528A1Organic active ingredientsPeptide/protein ingredientsAdditive ingredientWilms' tumor
The present invention relates to peptides, nucleic acids and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumour-associated T-helper cell peptide epitopes, alone or in combination with other tumour-associated peptides that serve as active pharmaceutical ingredients of vaccine compositions which stimulate anti-tumour immune responses. The present invention relates to novel peptide sequences and their variants derived from HLA class I and class II molecules of human tumour cells which can be used in vaccine compositions for eliciting anti-tumour immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Antigen specific T cell therapy
Provided are methods for generating immune cells of the desired type and specificity in a host. The methods may be used to treat a disease or disorder, such as a tumor in a patient. Target cells, preferably hematopoietic stem cells such as primary bone marrow cells are transfected with a polynucleotide encoding a T cell receptor with the desired specificity. The transfected cells are then transferred to the host where they develop into mature, functional immune cells. The source of the T cell receptor can determine the stem cell's fate. Thus transfecting stem cells with TCRs from cytotoxic cells will lead to the generation of cytotoxic T cells in the host, while TCRs from helper cells will produce helper cells. Both arms of T cell immunity can be generated simultaneously in a host. Additionally, the immune response to the desired antigen can be further stimulated by immunizing the host with the antigen.
Owner:CALIFORNIA INST OF TECH
Tumor-associated Peptides Binding Promiscuously to Human Leukocyte Antigen (HLA) Class II Molecules
InactiveUS20080206216A1Organic active ingredientsPeptide/protein ingredientsHla class iiAdditive ingredient
The present invention relates to immunotherapeutic methods, and molecules and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumour-associated T-helper cell peptide epitopes, alone or in combination with other tumour-associated peptides, that serve as active pharmaceutical ingredients of vaccine compositions which stimulate anti-tumour immune responses. In particular, the present invention relates to 49 novel peptide sequences derived from HLA class II molecules of human tumour cell lines which can be used in vaccine compositions for eliciting anti-tumour immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Use of parasitic biological agents for prevention and control of allergic and other IgE-mediated disorders
InactiveUS20070087020A1Reduce and eliminate and ameliorate inappropriate immune responseStimulate immune responseProtozoa antigen ingredientsLeech/worm material medical ingredientsDiseaseInterleukin 10
The present invention describes using, on a repetitive basis, a non-human colonizing helminth compound, in an amount sufficient to establish a transitory parasitic helminth infection and or to simulate in a parasitic helminth infection, thereby having immunosuppressive effect against benign antigens and or stimulating a regulatory immune response characterized by the production of T helper cells 2 (Th2), T regulatory helper cells (TREG) and certain cytokines, including, but not limited to interleukin 10 (IL-10), as a therapy or prophylaxis of allergy and other IgE-mediated disorders, which are marked by an inappropriate IgE immune response including, but not limited to an aberrant and or enhanced IgE antibody production to benign antigens. The invention presents using helminth compound by administering it in a frequency and amount sufficient to eliminate or ameliorate the inappropriate immune response in an asthmatic and or allergic individual.
Owner:MILESTONE RES
Methods and compositions to enhance vaccine efficacy by reprogramming regulatory t cells
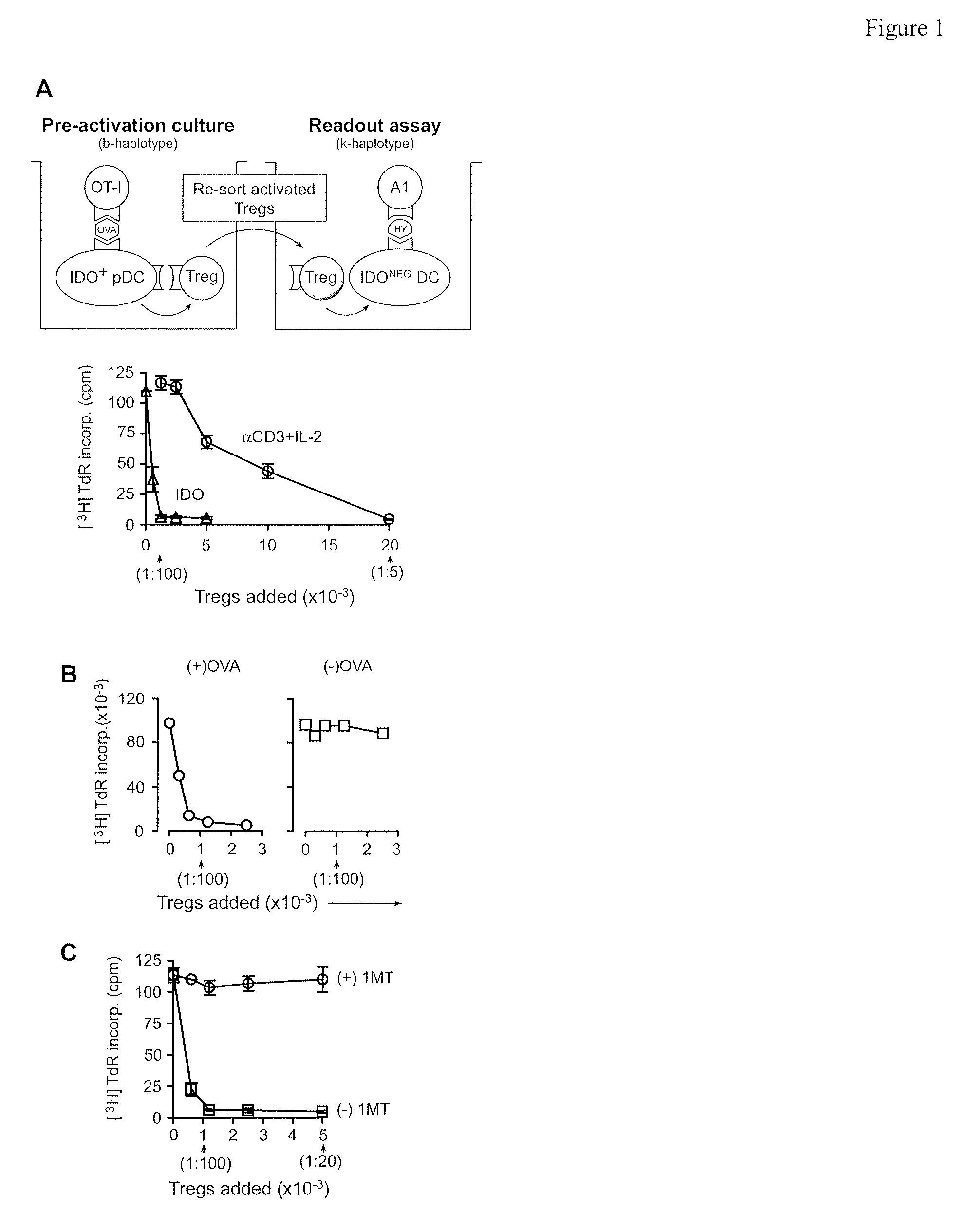
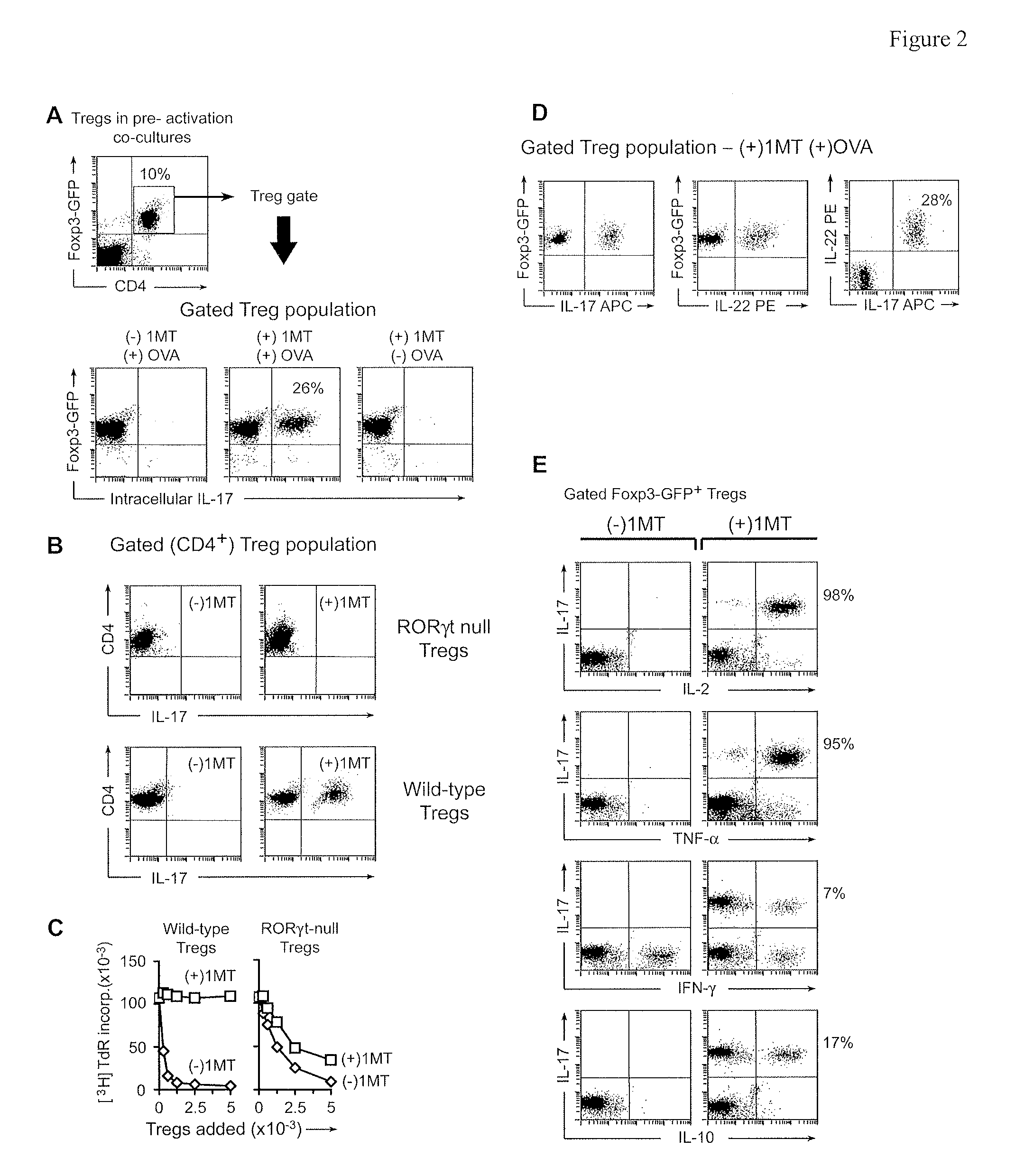
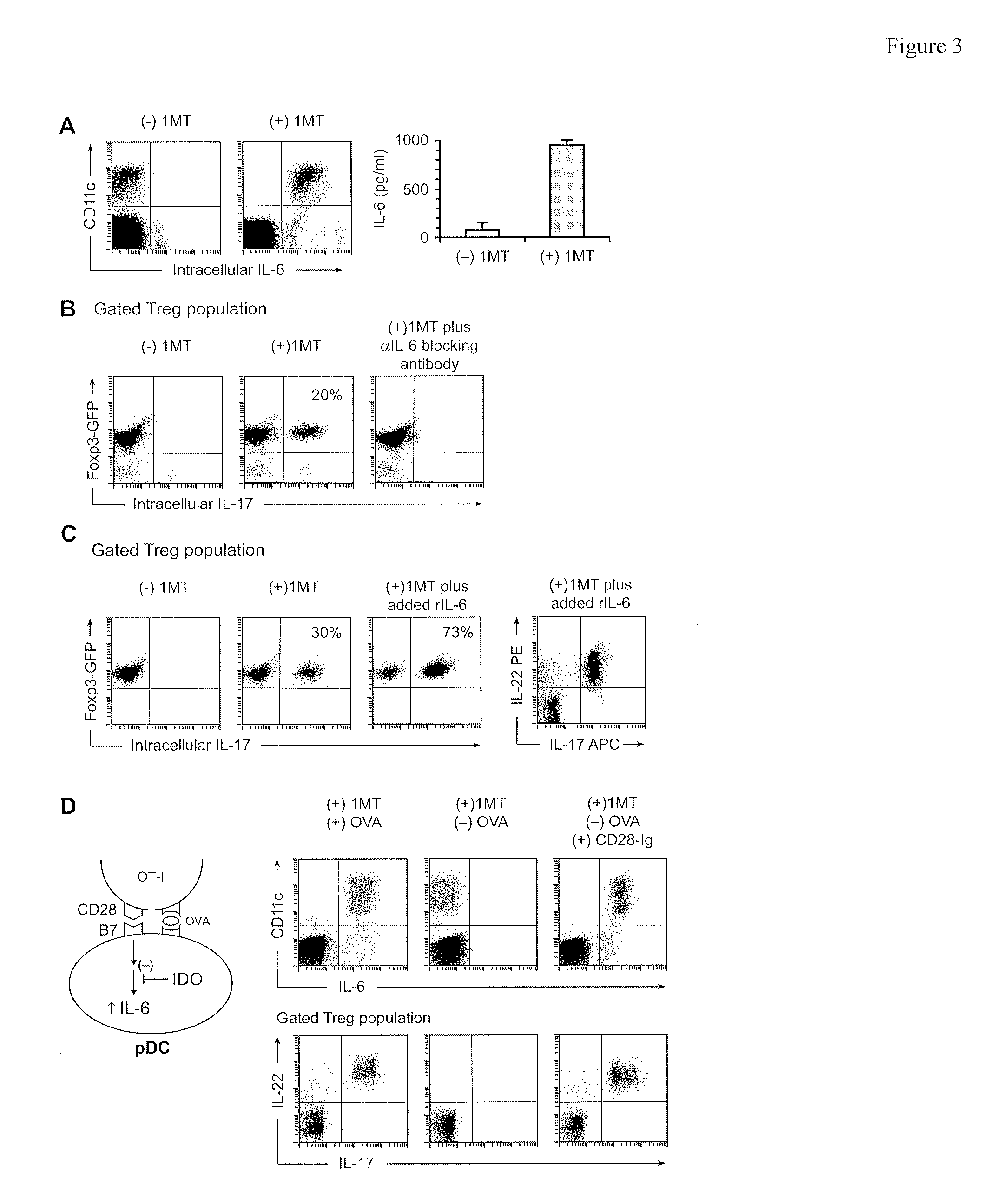
The immunoregulatory enzyme indoleamine 2,3-dioxygenase (IDO) is expressed by a subset of murine plasmacytoid DCs (pDCs) in tumor-draining LNs, where it can potently activate Foxp3 regulatory T cells (Tregs). We now show that IDO functions as a molecular switch in tumor-draining LNs, maintaining Tregs in their normal suppressive phenotype when IDO was active, but allowing inflammation-induced conversion of Tregs to a polyfunctional T-helper phenotype similar to proinflammatory TH17 cells when IDO was blocked. In vitro, conversion of Tregs to the TH17-like phenotype was driven by antigen-activated effector T cells, and required IL-6 produced by activated pDCs. IDO regulated this conversion by dominantly suppressing production of IL-6 in pDCs, in a GCN2-kinase dependent fashion. In vivo, using a model of established B16 melanoma, the combination of an IDO-inhibitor drug plus anti-tumor vaccine caused upregulation of IL-6 in pDCs and in situ conversion of a majority of Tregs to the TH17 phenotype, with marked enhancement of CD8+ T cell activation and anti-tumor efficacy. Thus, Tregs in tumor-draining LNs can be actively re-programmed in vitro and in vivo into T-helper cells, without the need for physical depletion, and IDO serves as a key regulator of this critical conversion.
Owner:GEORGIA HEALTH SCI UNIV RES INST
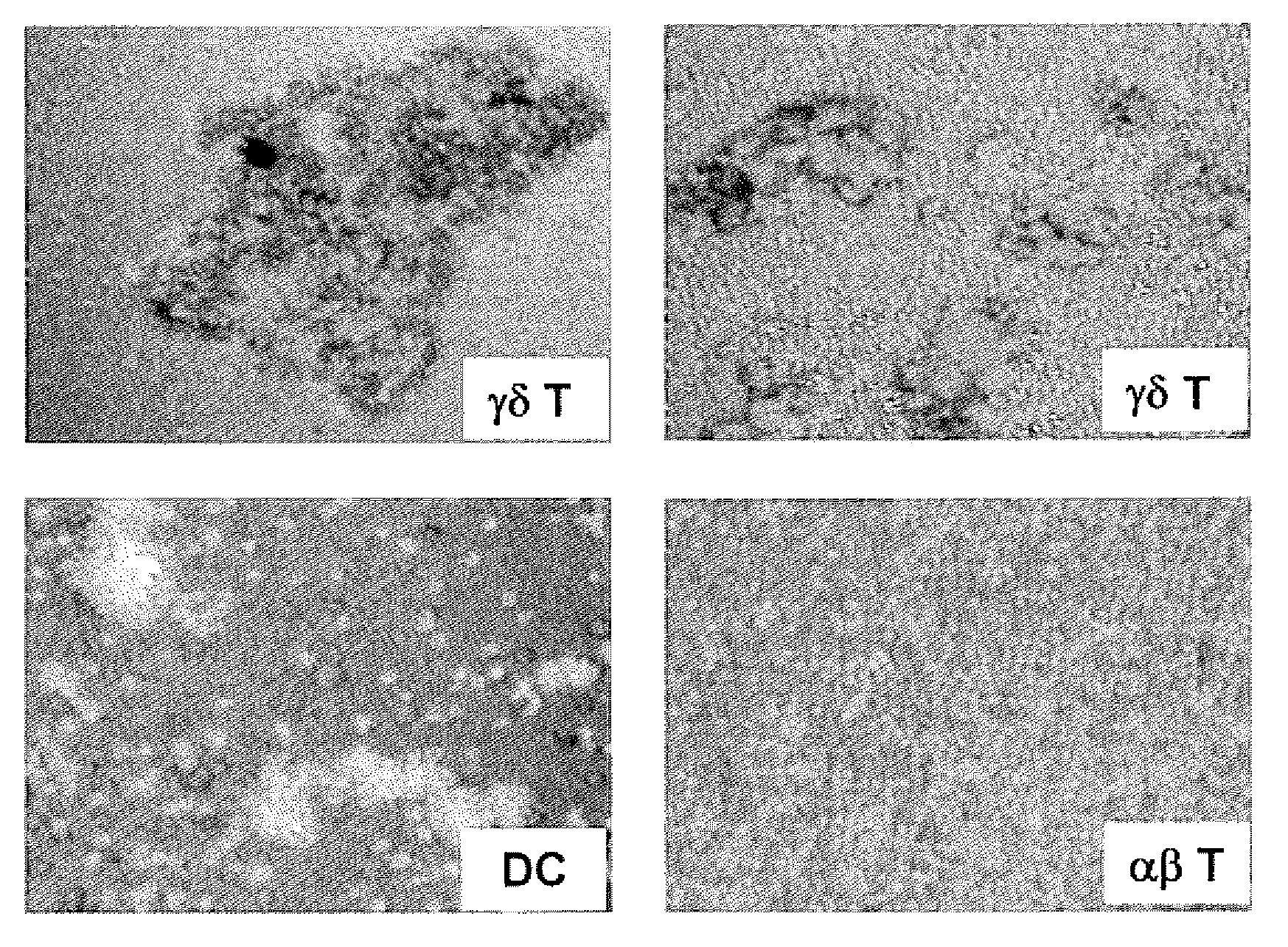
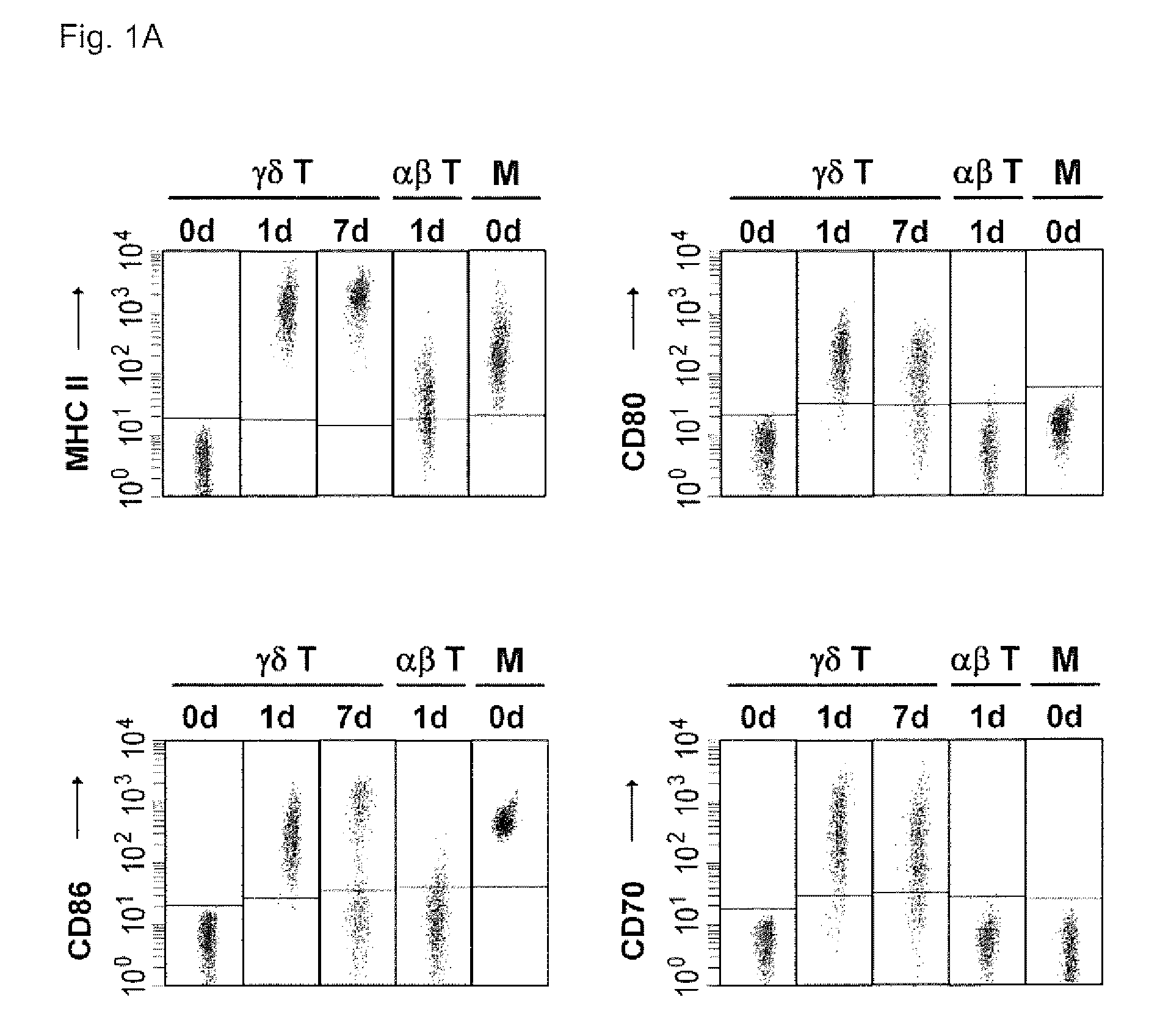
Preparation of antigen-presenting human gamma delta t cells and use in immunotherapy
InactiveCN101031641AGood effectEffective antigen uptakeTissue cultureVaccinationEpidermal Dendritic Cells
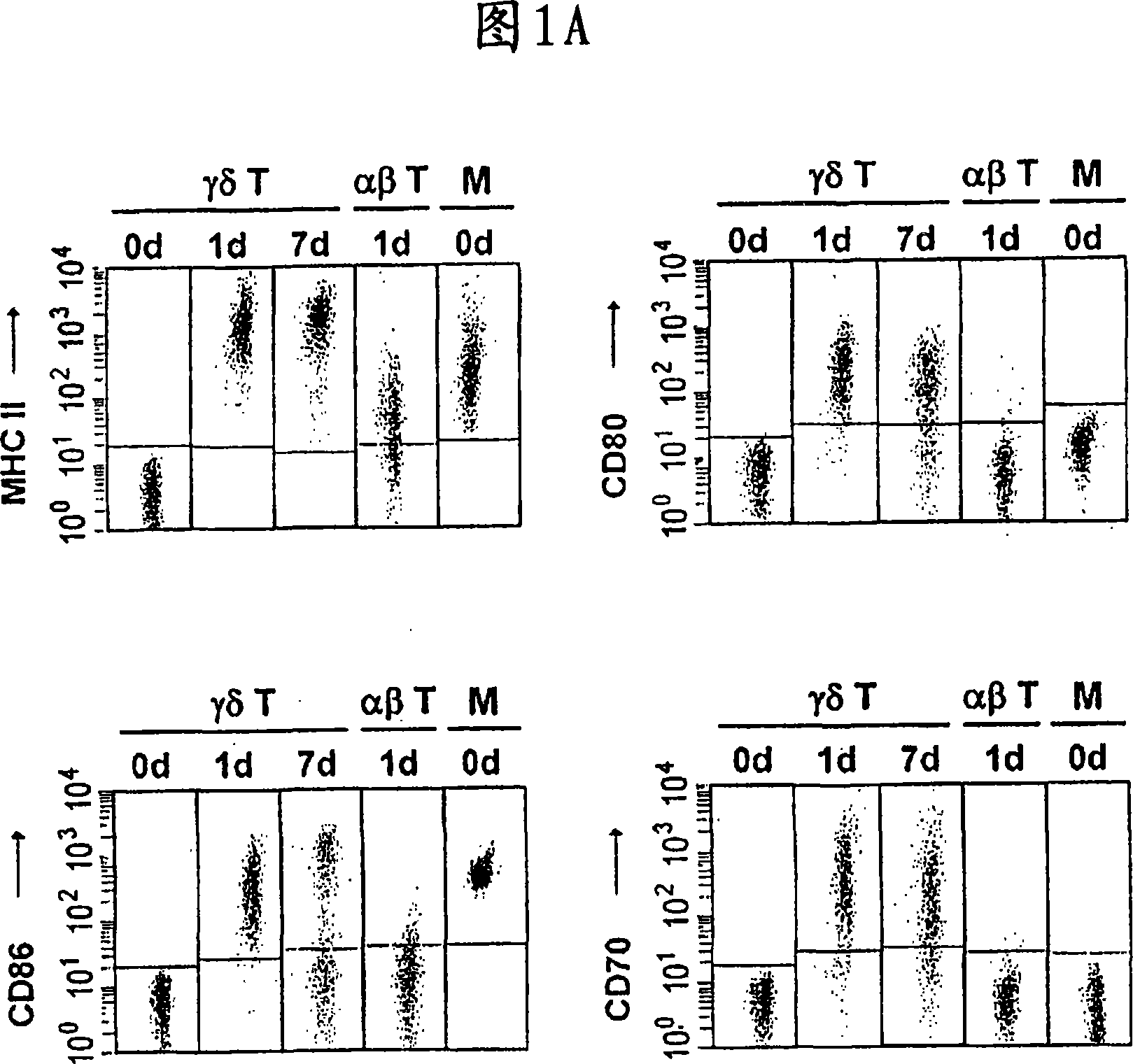
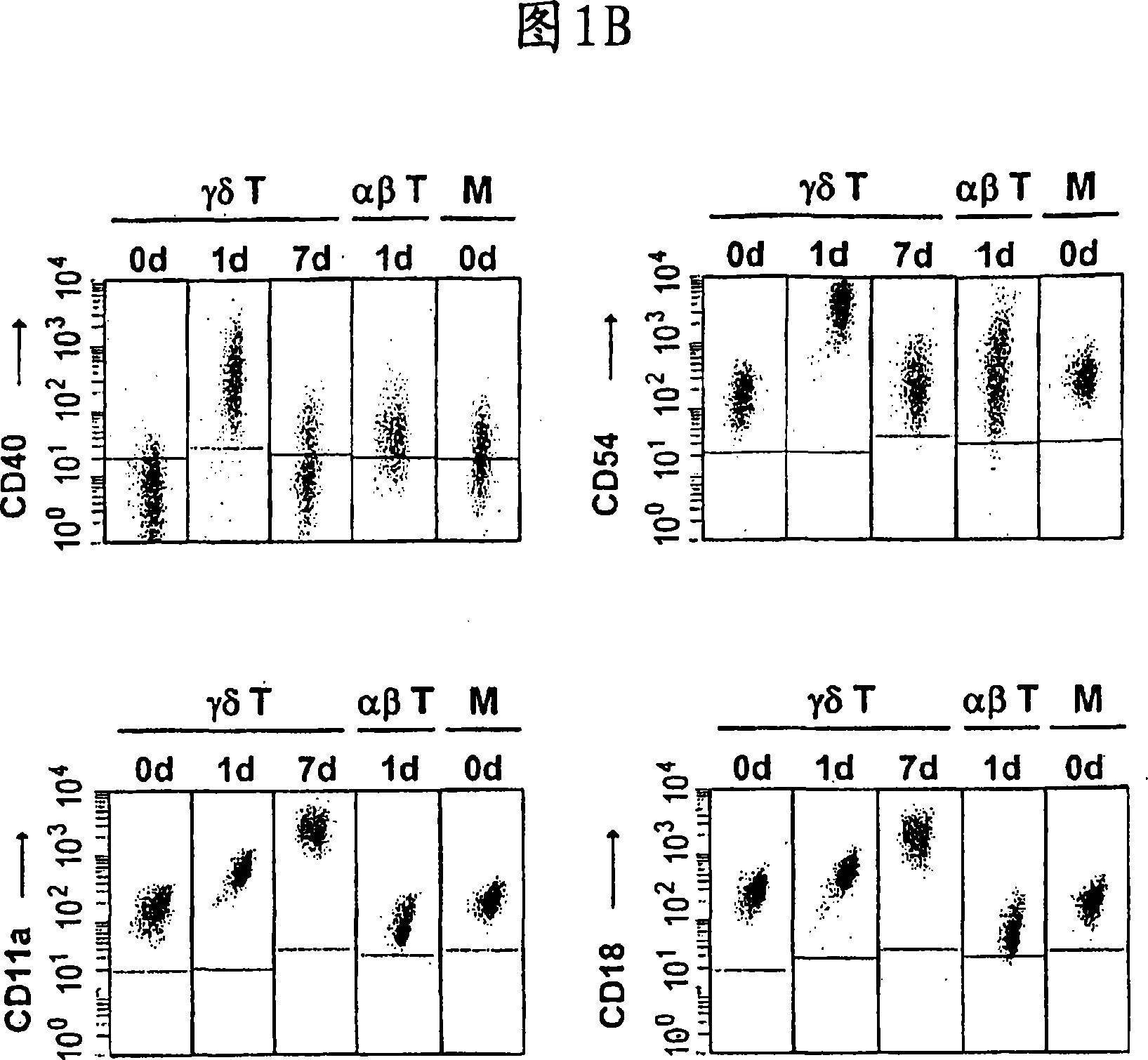
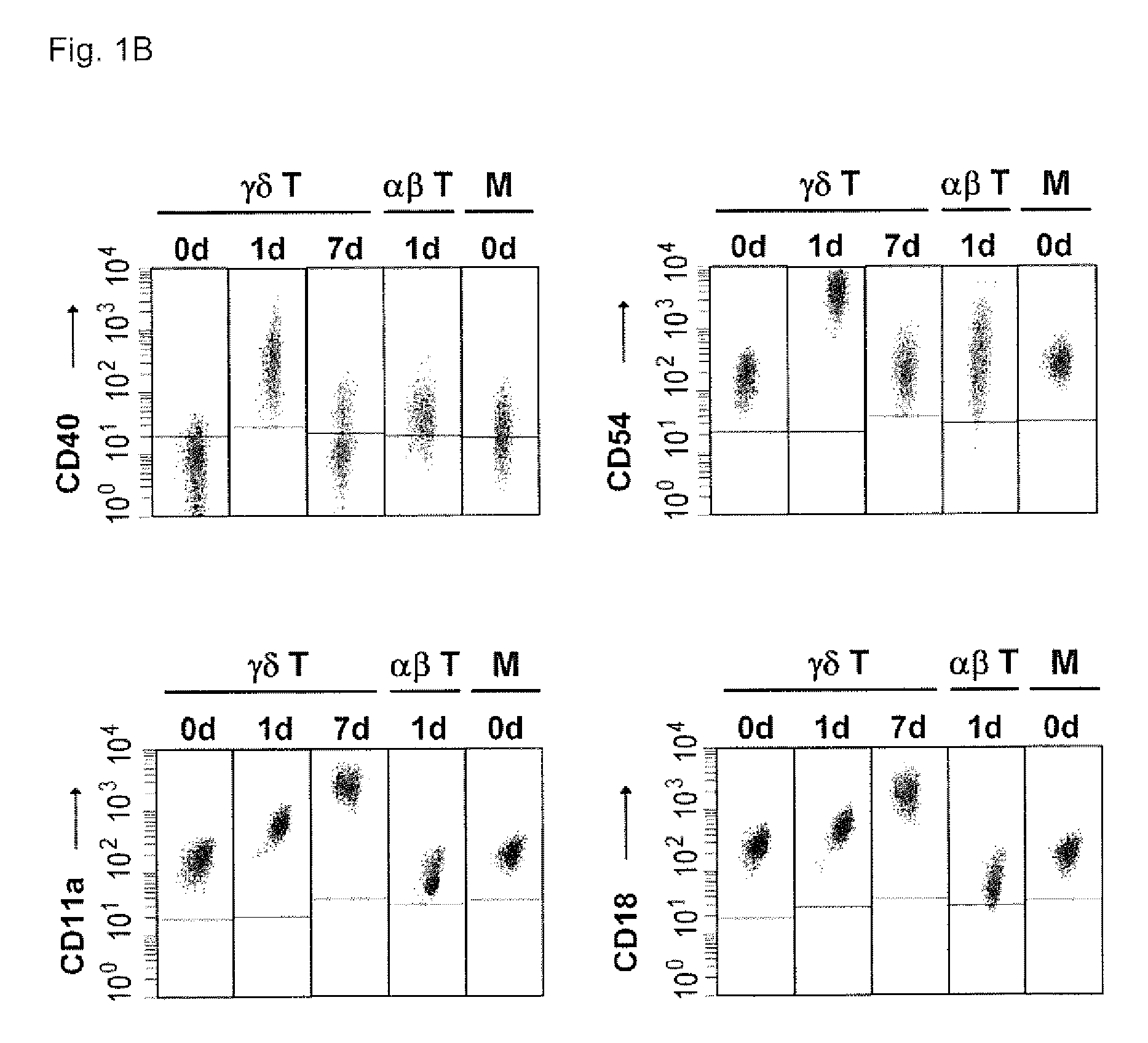
The invention relates to a method for the preparation of efficient antigen-presenting human Gamma Delta T cells, to the Gamma Delta T cells prepared by such a method, and to their use in immunotherapy, vaccination, vaccine development and diagnostics. Similar to dendritic cells (DCs) in potency and efficacy, these human Gamma Delta T cells process antigens and present antigenic peptides to Alpha Beta T cells and induce antigen-specific responses (proliferation and differentiation) in nave Alpha Beta T cells. Gamma Delta T cells are easily purified from peripheral blood, acquire''maturation'' status (expression of essential adhesion, co-stimulatory and major histocompatibility complex molecules) within 1 day of in vitro culture under stimulation and induce strong primary and secondary T helper cell and cytotoxic T cell responses. The Gamma Delta T cells may be used in a method of treatment of tumors or chronic or recurrent infectious diseases, in identification of novel tumor or pathogen-derived antigens, and in the diagnosis of the immune competence of a patient.
Owner:UNIV COLLEGE CARDIFF CONSULTANTS LTD
Composition of tumour-associated peptides and related Anti-cancer vaccine
The present invention relates to immunotherapeutic peptides and their use in immunotherapy, in particular the immunotherapy of cancer. The present invention discloses tumour-associated T-helper cell peptide epitopes, alone or in combination with other tumour-associated peptides that serve as active pharmaceutical ingredients of vaccine compositions which stimulate anti-tumour immune responses. In particular, the composition of the peptides of the present invention can be used in vaccine compositions for eliciting anti-tumour immune responses against colorectal cancer.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Tumor-associated Peptides Binding Promiscuously to Human Leukocyte Antigen (HLA) Class II Molecules
The present invention relates to immunotherapeutic methods, and molecules and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumour-associated T-helper cell peptide epitopes, alone or in combination with other tumour-associated peptides, that serve as active pharmaceutical ingredients of vaccine compositions which stimulate anti-tumour immune responses. In particular, the present invention relates to 49 novel peptide sequences derived from HLA class II molecules of human tumour cell lines which can be used in vaccine compositions for eliciting anti-tumour immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Composition of tumour-associated peptides and related anti-cancer vaccine
The present invention relates to immunotherapeutic peptides and their use in immunotherapy, in particular the immunotherapy of cancer. The present invention discloses tumor-associated T-helper cell peptide epitopes, alone or in combination with other tumor-associated peptides that serve as active pharmaceutical ingredients of vaccine compositions which stimulate anti-tumor immune responses. In particular, the composition of the peptides of the present invention can be used in vaccine compositions for eliciting anti-tumor immune responses against colorectal cancer.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Tumor-associated peptides binding to human leukocyte antigen (HLA) class II molecules
ActiveUS20100040590A1Organic active ingredientsPeptide/protein ingredientsHla class iiAdditive ingredient
The present invention relates to immunotherapeutic methods, and molecules and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumour-associated T-helper cell peptide epitopes, alone or in combination with other tumour-associated peptides, that serve as active pharmaceutical ingredients of vaccine compositions which stimulate anti-tumour immune responses. In particular, the present invention relates to 49 novel peptide sequences derived from HLA class II molecules of human tumour cell lines which can be used in vaccine compositions for eliciting anti-tumour immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Composition of tumor-associated peptides and related Anti-cancer vaccine for the treatment of gastric cancer and other cancers
ActiveUS20130115188A1Aid in diagnosisTumor rejection antigen precursorsPeptide/protein ingredientsEpitopeMISCELLANEOUS TUMORS
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Chimeric peptides as immunogens, antibodies thereto, and methods for immunization using chimeric peptides or antibodies
The invention provides a chimeric peptide or mixture of chimeric peptides that can be formulated as an immunizing composition and used in a method for immunization of a mammal against an internal peptide cleavage product derived from a precursor or mature protein, for which the peptide cleavage product and the precursor or mature protein are self molecules. The chimeric peptide or peptides have an end-specific B cell epitope from a naturally-occurring internal peptide cleavage product of a precursor or mature protein, as a free N- or C-terminus, fused with or without spacer residues to a T helper cell epitope derived from a living source different from that of the internal peptide cleavage product.
Owner:COLLATERAL AGENTS
Method of inducing anergic T helper cells
InactiveUS7144728B1Reduce riskLower Level RequirementsMicrobiological testing/measurementGenetically modified cellsDiseaseAutoimmune disease
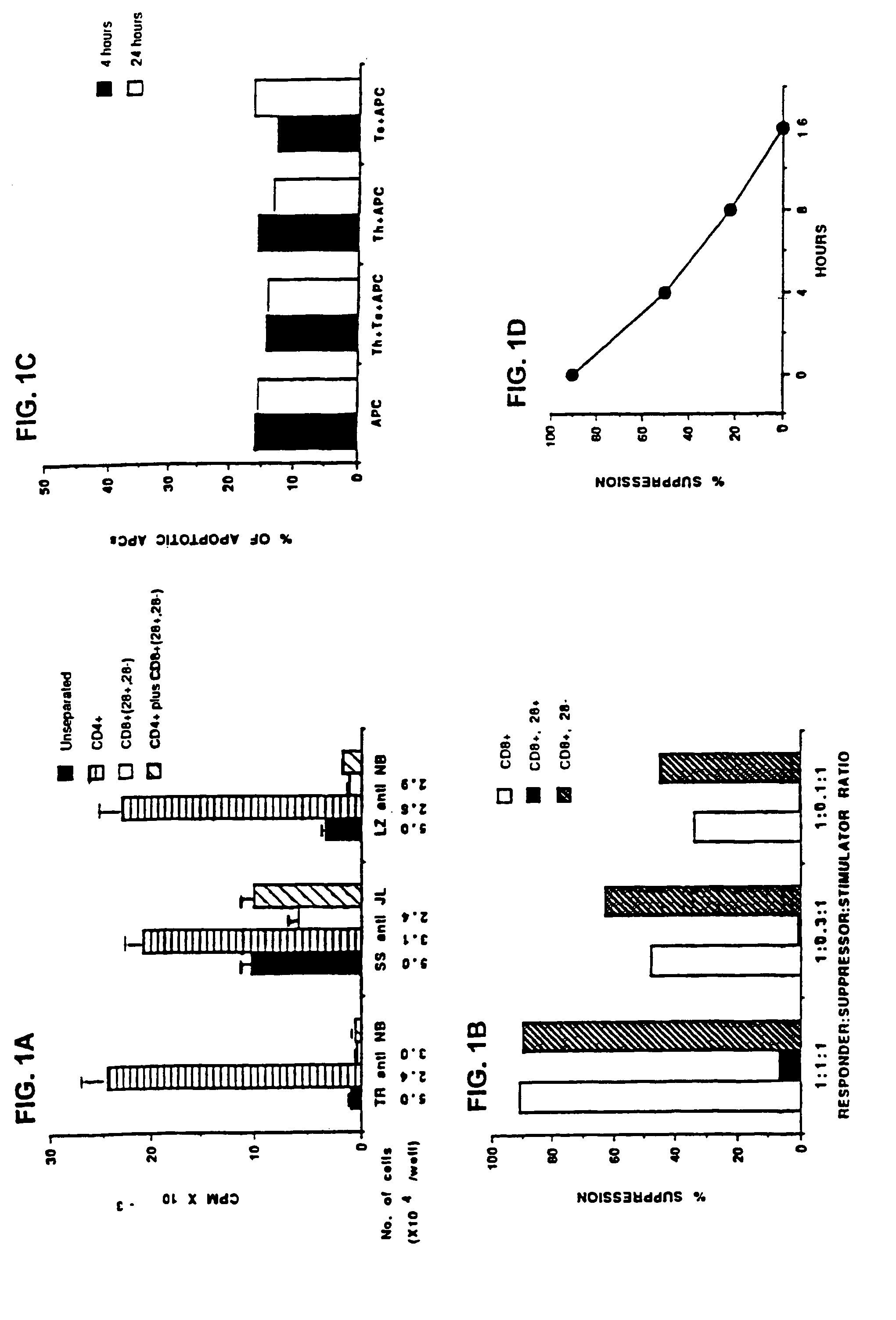
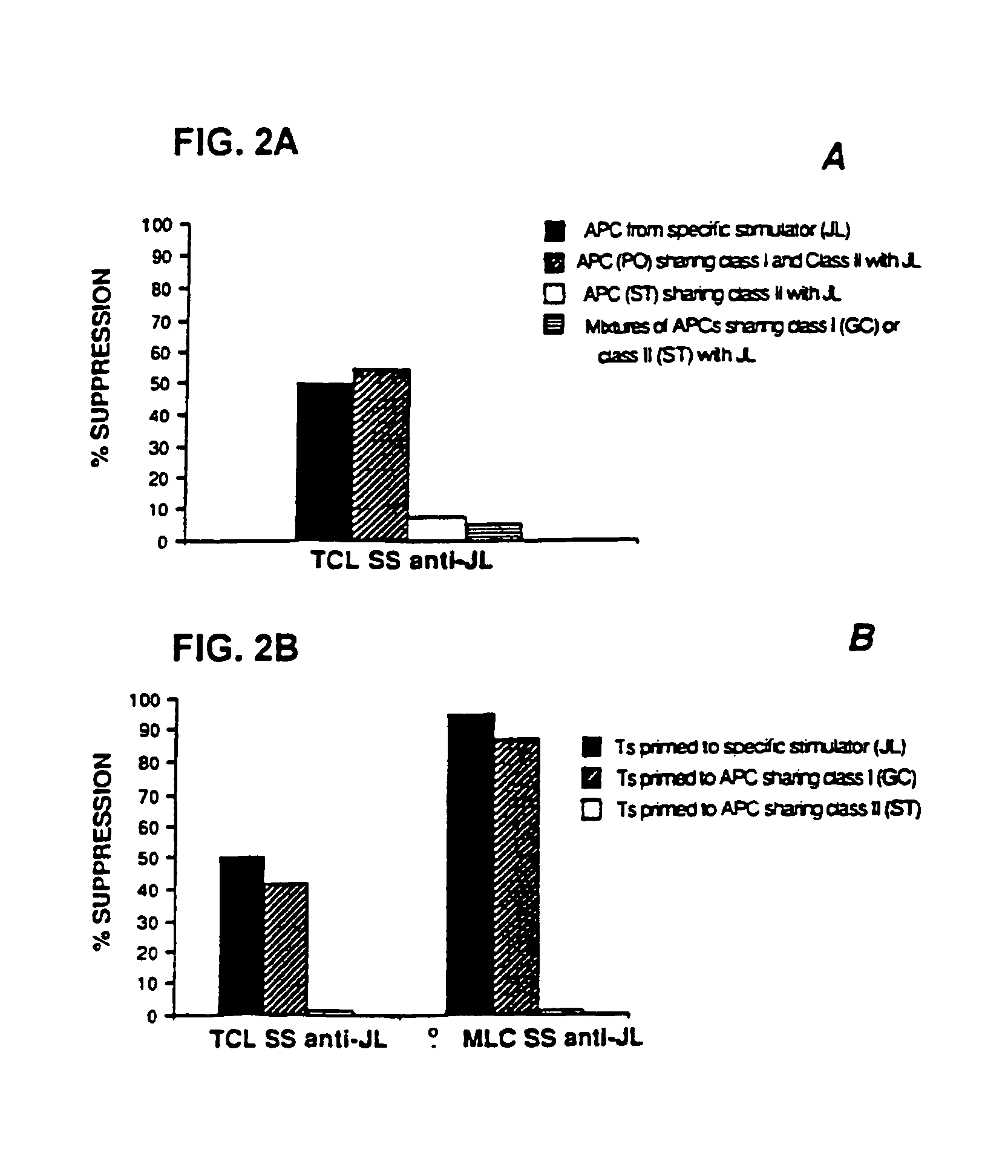
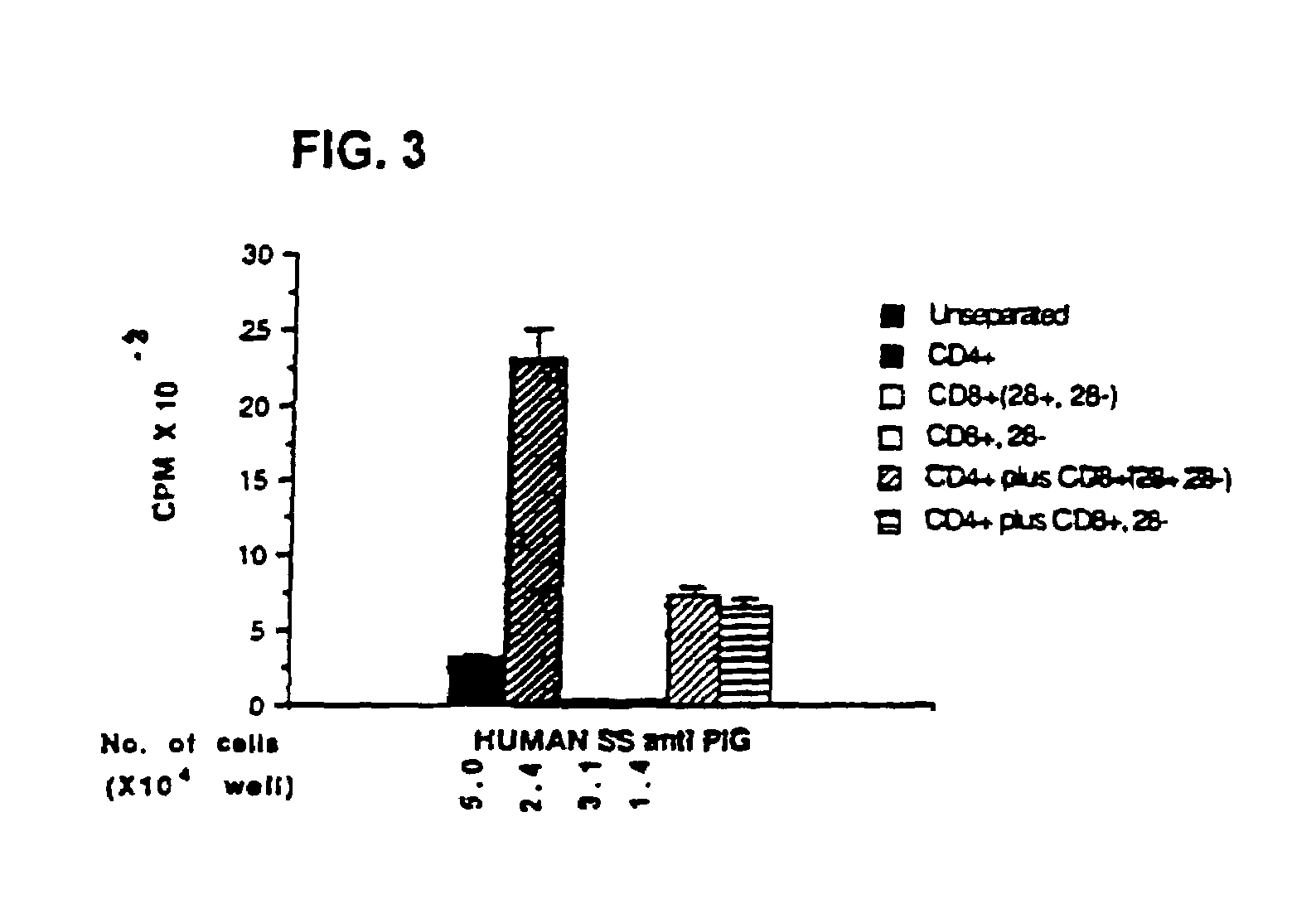
This invention also provides a method of generating antigen specific human suppressor CD8+CD28− T cells. This invention further provides a method of generating allopeptide antigen specific human suppressor CD8+CD28− T cells. Methods of tent for reduction of risk of rejection of allografts and xenografts and autoimmune diseases using the human suppressor CD8+CD28− T cells so produced are also provided, as are methods of preventing rejection and autoimmune diseases, and vaccines comprising the produced suppressor T cells. Methods of diagnosis to determine whether a level of immuno-suppressant therapy requires a reduction are provided.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
HIV vaccine composition
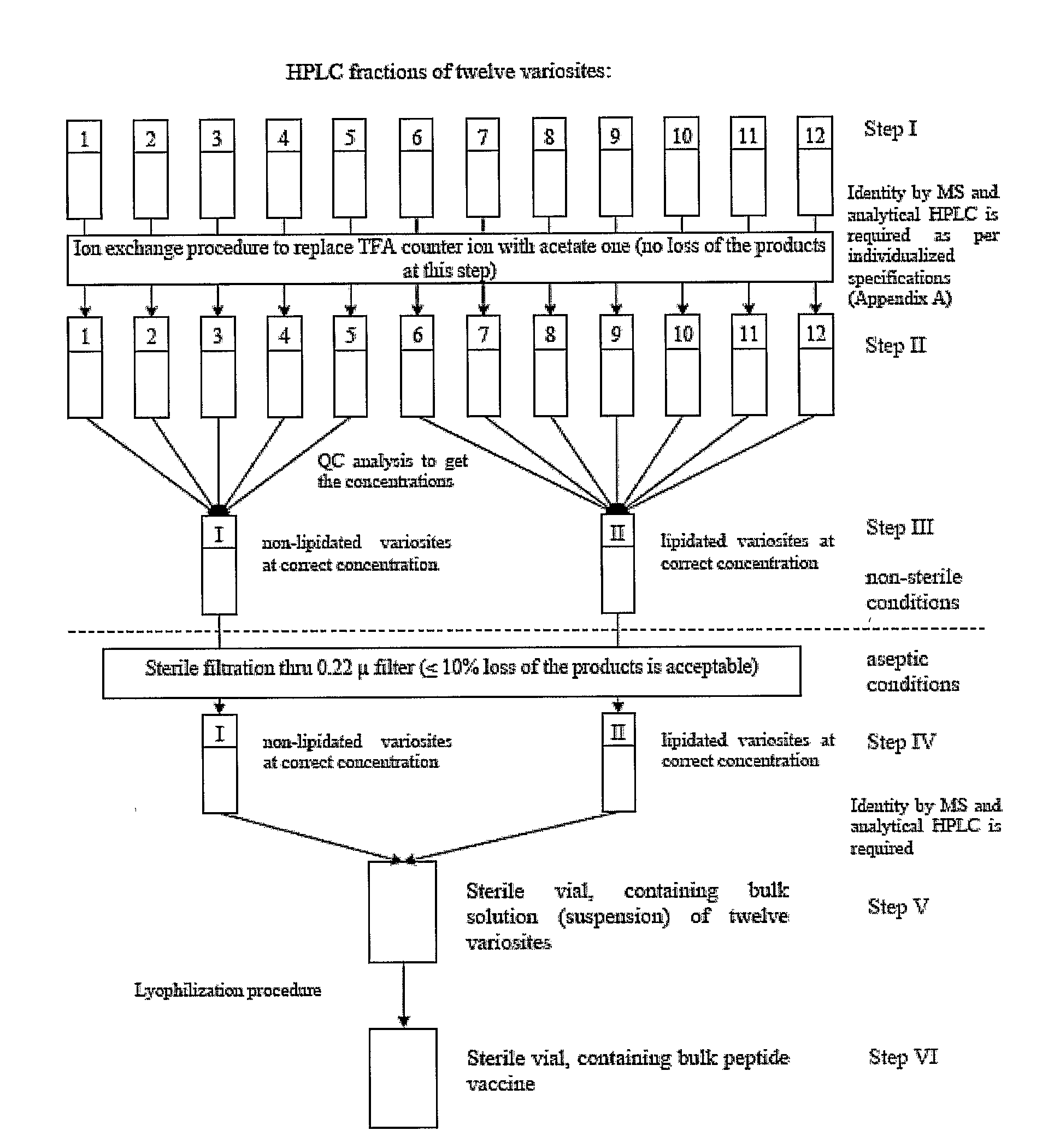
An anti-HIV vaccine composition is disclosed. The vaccine comprises an combination of immunogenic peptide mixtures, which mixtures may be prepared in a single synthesis. The composition collectively represents the in vivo variability seen in immunogenic epitopes from highly variable regions of HIV. Immunization with the vaccine elicits broadly reactive immunity (CTL and T helper cell responses) against the divergent strains of HIV upon which it is based. The vaccine may be formulated to target regionally distinct variability based on an HIV clade predominant in a geographical region.
Owner:VARIATION BIOTECHNOLOGIES INC
Tumor-associated Peptides Binding Promiscuously to Human Leukocyte Antigen (HLA) Class II Molecules
ActiveUS20080206218A1Organic active ingredientsPeptide/protein ingredientsHla class iiWhite blood cell
The present invention relates to immunotherapeutic methods, and molecules and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumour-associated T-helper cell peptide epitopes, alone or in combination with other tumour-associated peptides, that serve as active pharmaceutical ingredients of vaccine compositions which stimulate anti-tumour immune responses. In particular, the present invention relates to 49 novel peptide sequences derived from HLA class II molecules of human tumour cell lines which can be used in vaccine compositions for eliciting anti-tumour immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Composition of tumor-associated peptides and related anti-cancer vaccine for the treatment of gastric cancer and other cancers
ActiveUS9132177B2Tumor rejection antigen precursorsPeptide/protein ingredientsAbnormal tissue growthEpitope
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Peptide vaccine for prevention and immunotherapy of dementia of the alzheimer's type
ActiveUS20140271690A1Reduce manufacturing costQuality improvementSsRNA viruses negative-senseNervous disorderPeptide vaccineImmunotherapy
The present disclosure is directed to individual Aβ peptide immunogen constructs, peptide compositions comprising these Aβ peptide immunogen constructs and mixtures thereof, pharmaceutical compositions including vaccine formulations comprising these Aβ peptide immunogen constructs, with the individual Aβ peptide immunogen constructs having the N-terminus of the Aβ peptide as the B cell (B) epitopes linked through spacer residue(s) to heterologous T helper cell (Th) epitopes derived from pathogen proteins that act together to stimulate the generation of highly specific antibodies directed against the N-terminus of the Aβ peptide offering protective immune responses to patients at risk for, or with, Alzheimer's Disease.
Owner:UNITED NEUROSCIENCE LIMITED
Methods for treating diseases or conditions with peptide constructs
InactiveUS20070003542A1Enhance immune responseImprove biological activityPeptide/protein ingredientsMicrobiological testing/measurementAntigenAdjuvant
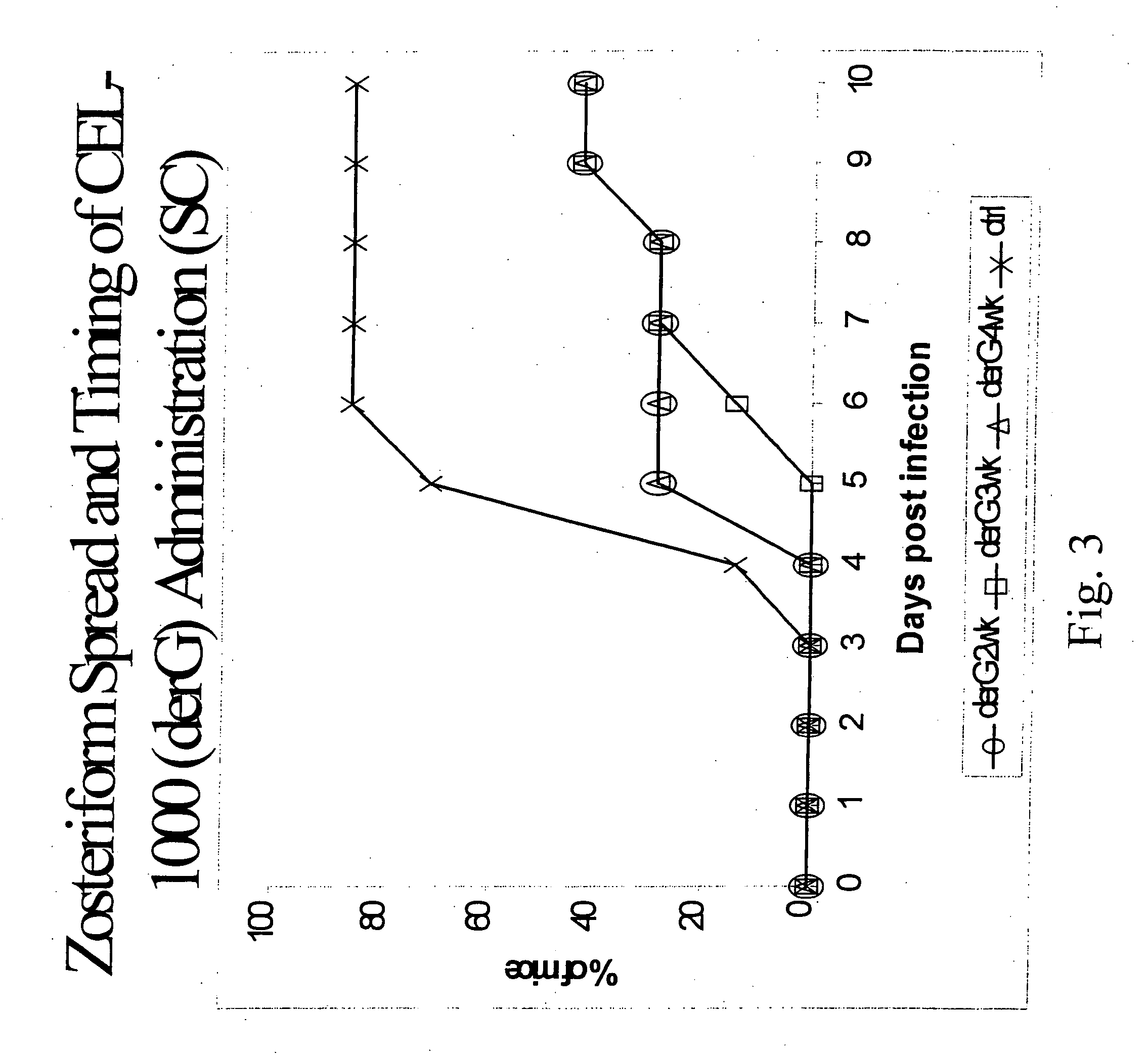
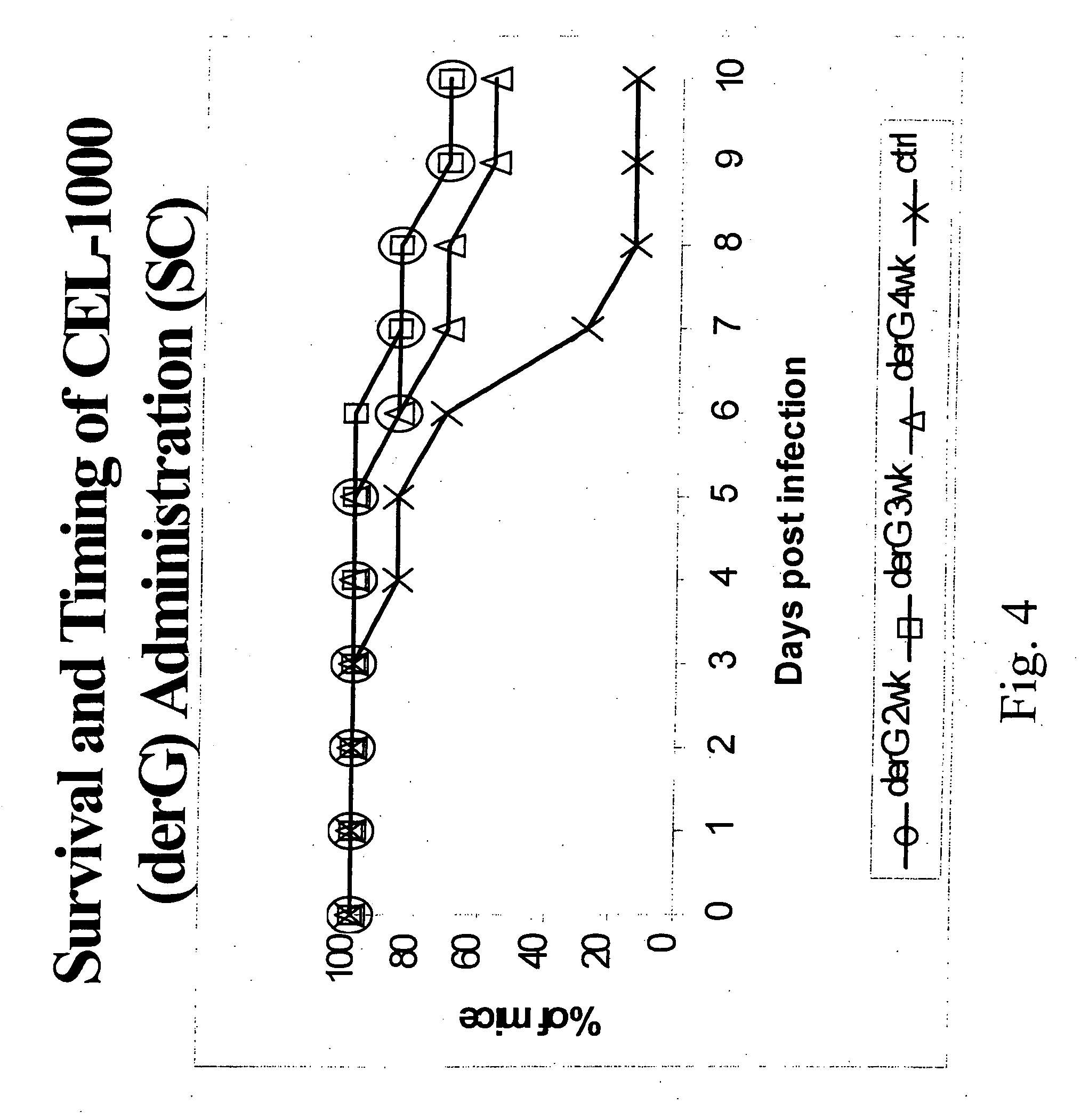
This invention relates to peptides directing a CD4 related T helper cell response wherein the peptides may be used as an adjuvant provided with an antigen or as an immunomodulatory agent without an antigen and compositions comprising modification of a fifteen-mer peptide sequence from the MHC IIβ chain at positions 135-149 known as Peptide G or a derivative of derG or other derivatives wherein the derivatives enhance the immune response of antigens and methods for treating cancer, autoimmune disease, transplant conditions, infectious conditions or allergies caused by foreign eukaryotic organisms, and infectious conditions or allergies caused by prokaryotic organisms or nonliving agents such as viruses, phages and prions with polypeptides.
Owner:CEL SCI CORP
Methods for inhibiting t helper cell differentiation
InactiveUS20100144737A1Inhibition of differentiationBiocideBlood/immune system cellsT helper cellCell biology
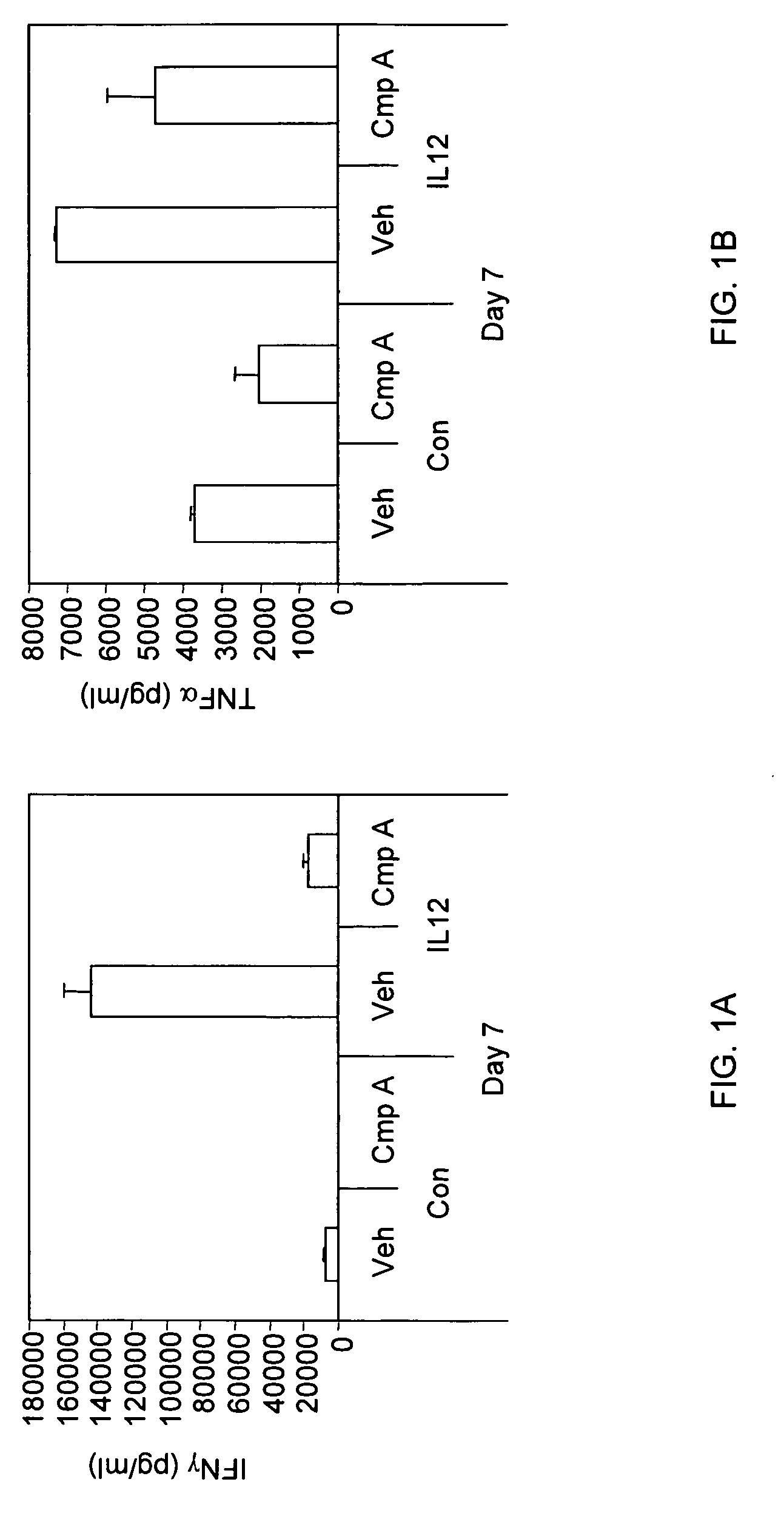
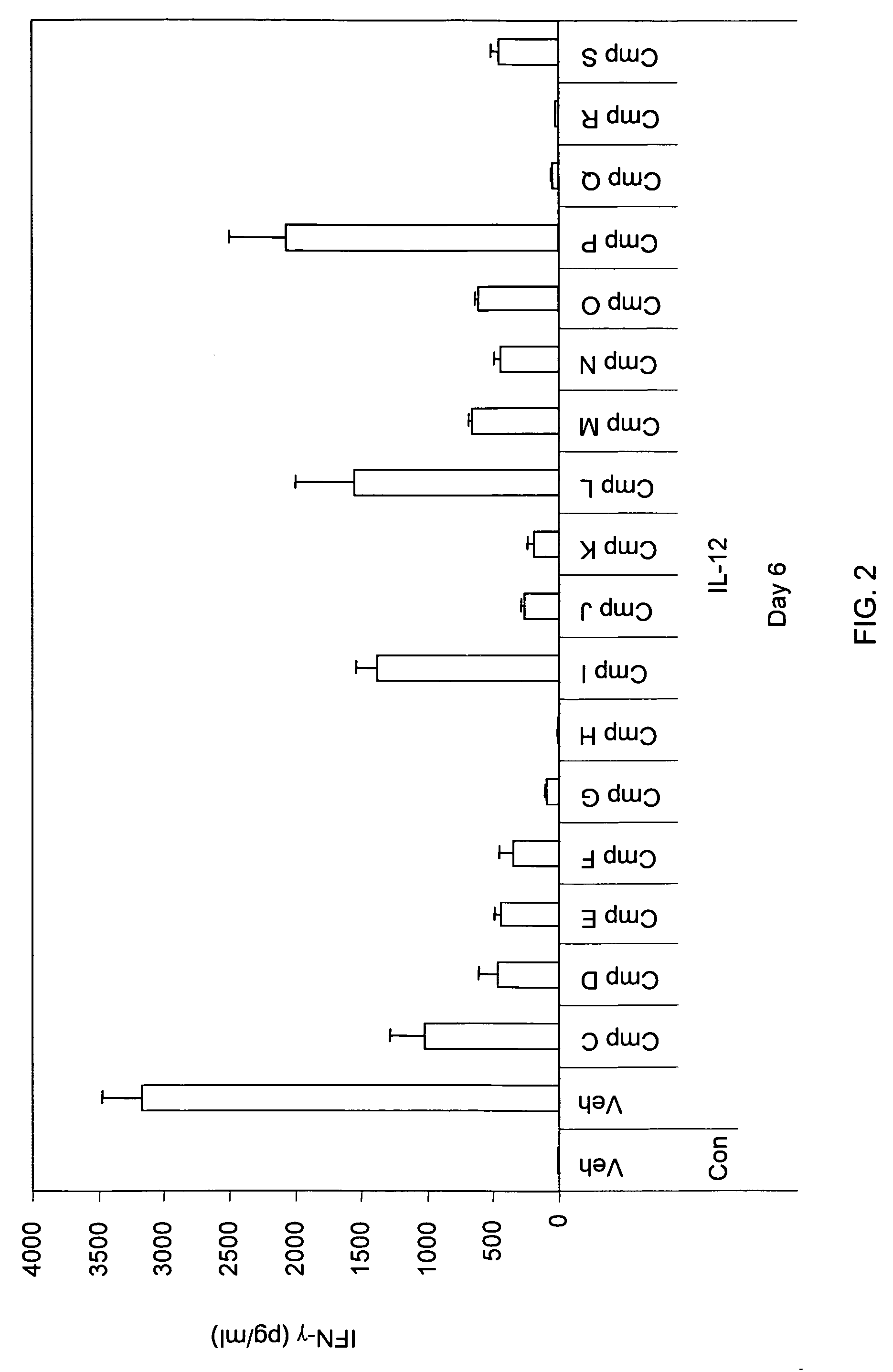
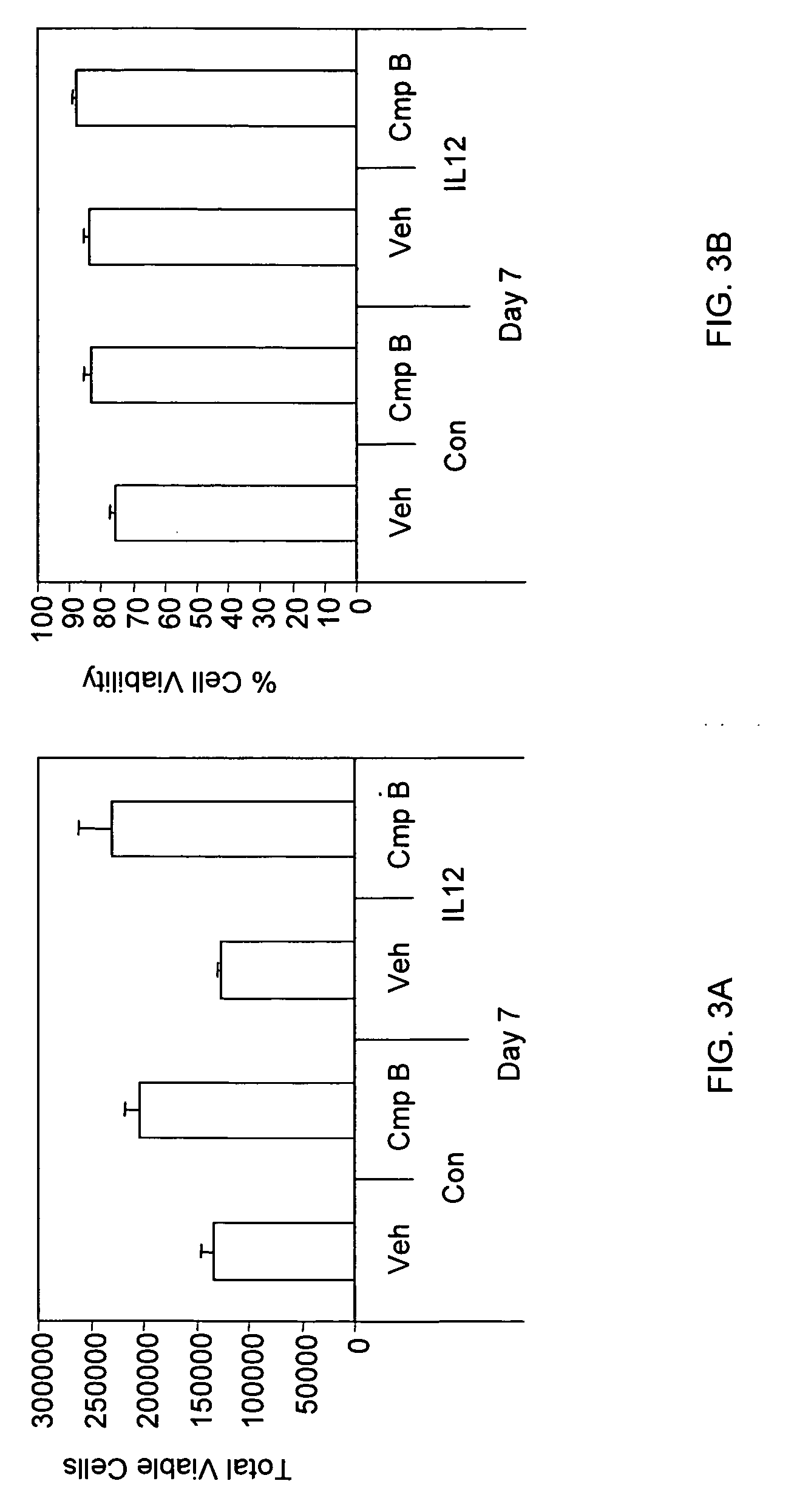
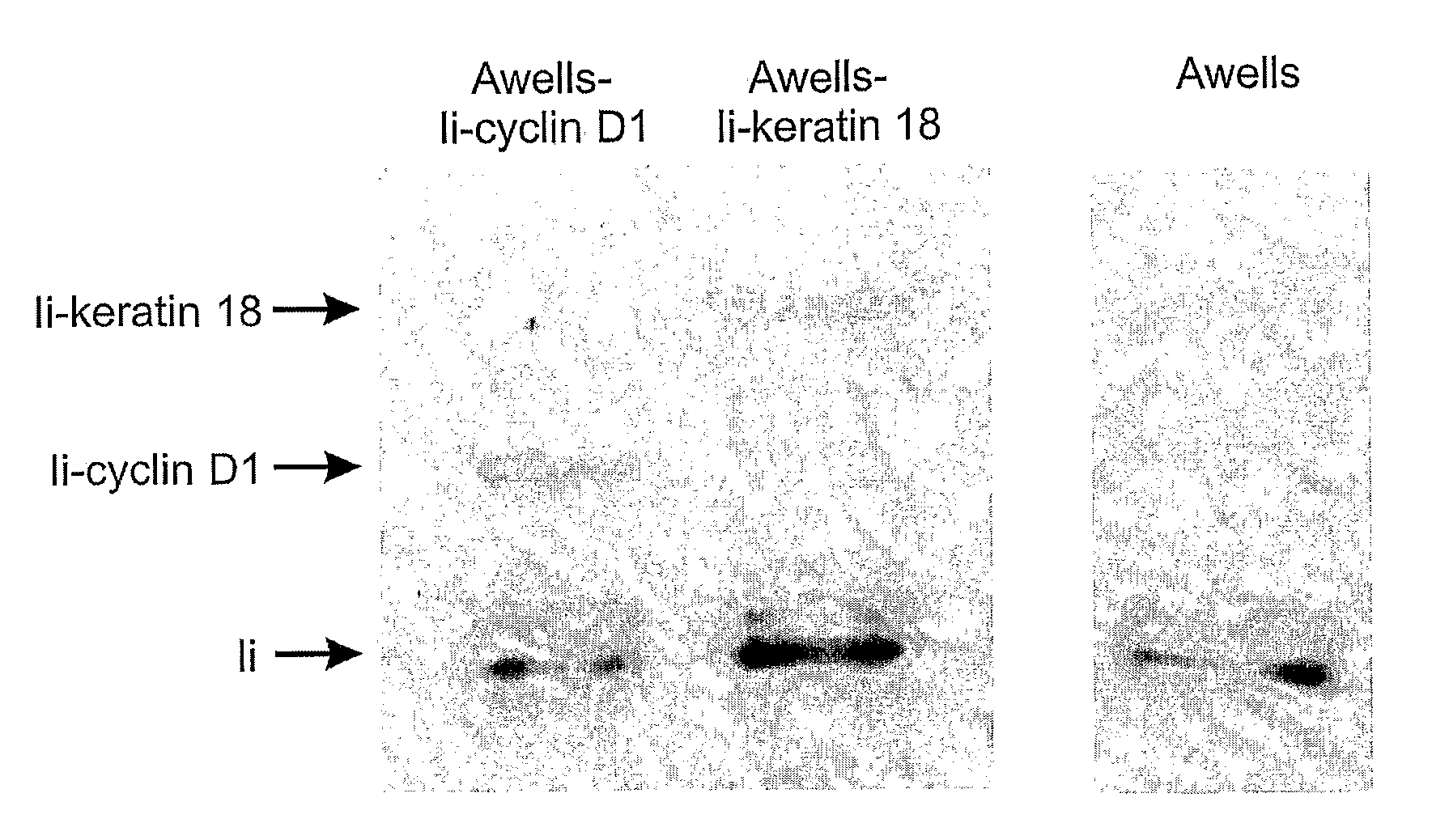
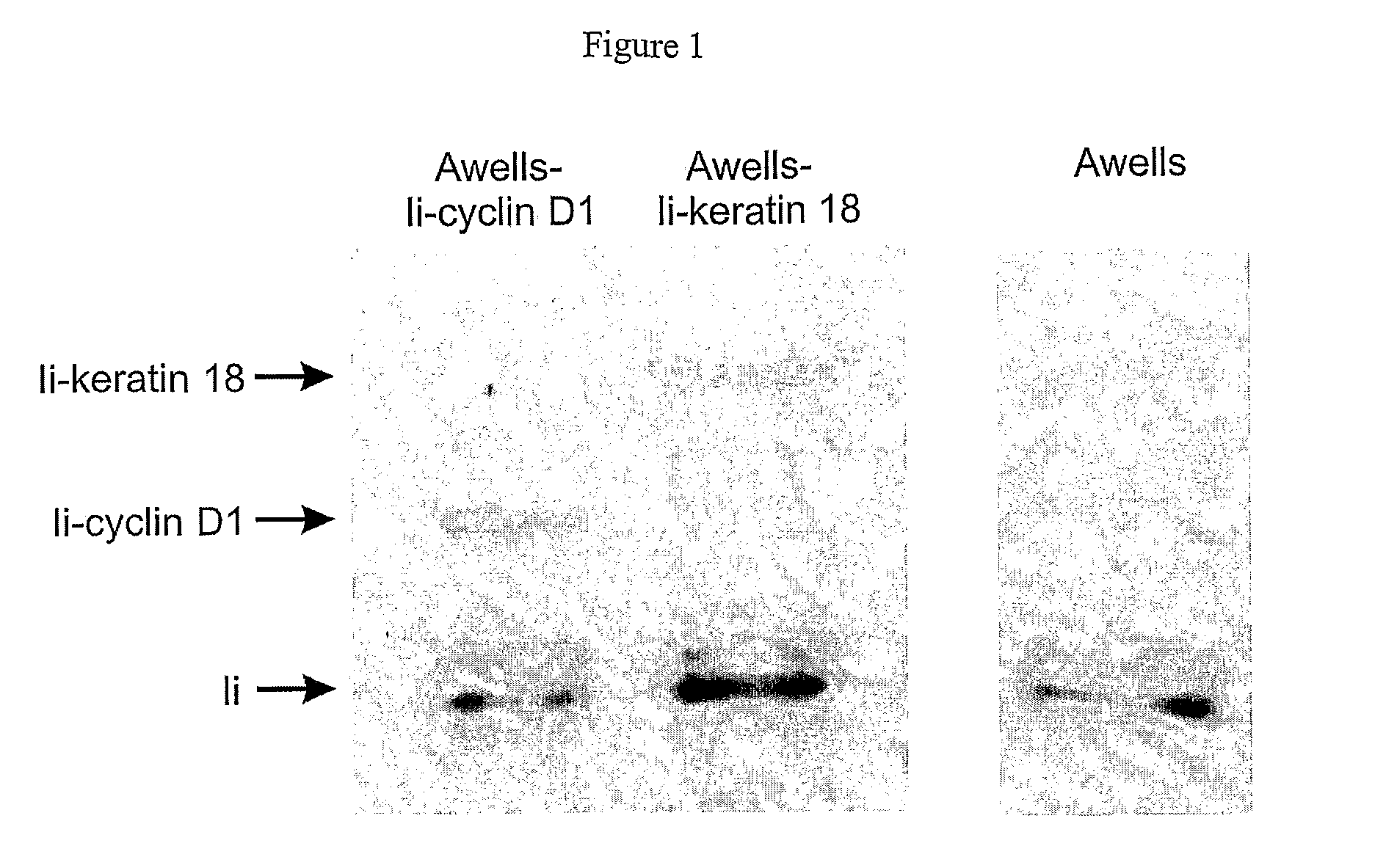
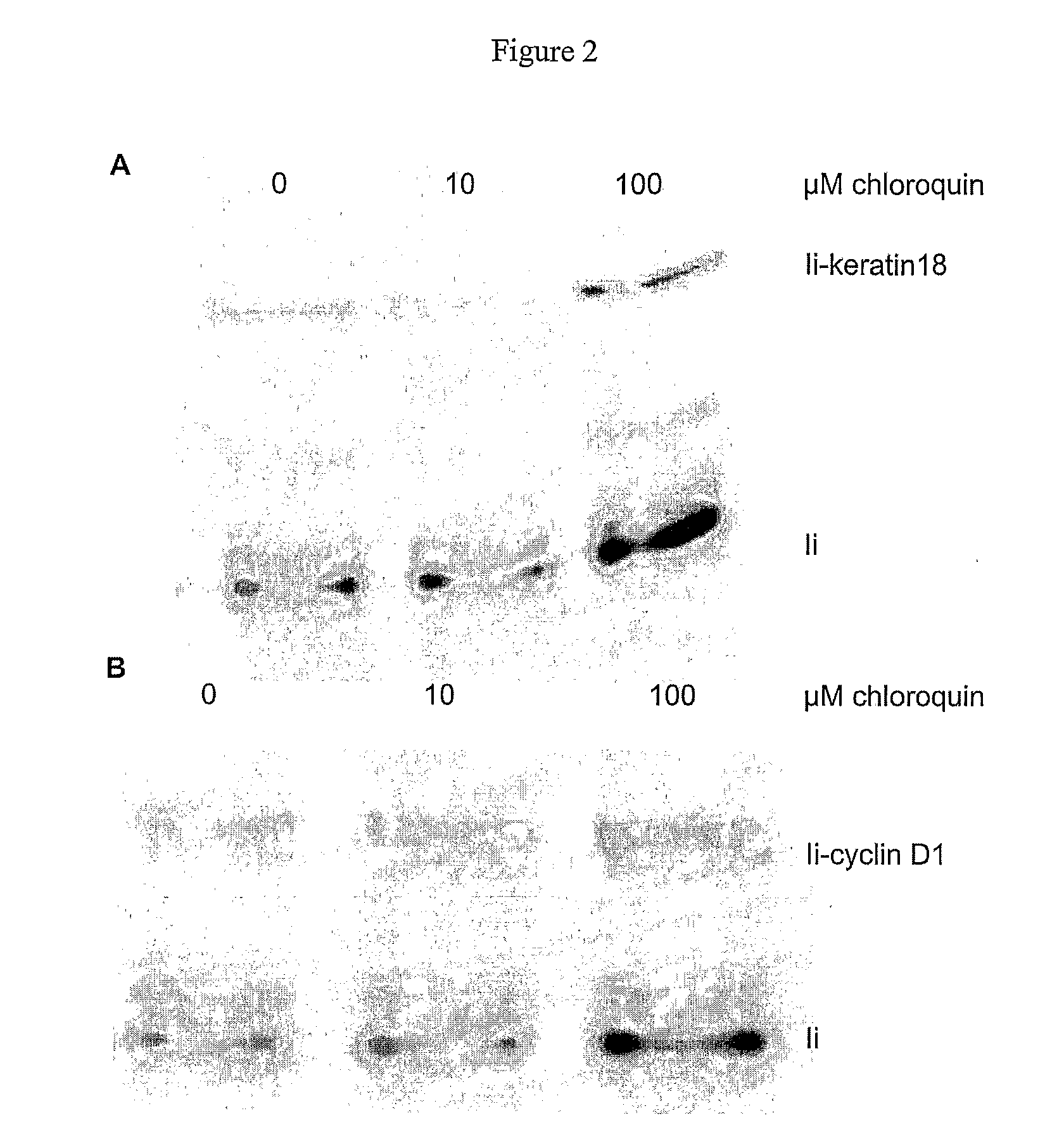
The present invention relates to methods and compounds useful for inhibiting T helper cell differentiation. Methods and compounds for decreasing IL-12 signaling in T helper cells are also provided.
Owner:FIBROGEN INC
Immunogenic T-Helper Epitopes From Human Tumour Antigens and Immunotherapeutic Methods Using Said Epitopes
InactiveUS20090317428A1Organic active ingredientsPeptide/protein ingredientsHla class iiAdditive ingredient
The present invention relates to immunotherapeutic methods, and molecules and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer, in particular renal cancer. The present invention furthermore relates to tumour-associated T-helper cell peptide epitopes, alone or in combination with other tumour-associated peptides, that serve as active pharmaceutical ingredients of vaccine compositions which stimulate anti-tumour immune responses. In particular, the present invention relates to 338 novel peptide sequences derived from HLA class II molecules of human tumour cell lines which can be used in vaccine compositions for eliciting anti-tumour immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Proteins for use in diagnosing and treating infection and disease
InactiveUS20100166789A1Nervous disorderPeptide/protein ingredientsAllergyObstructive Pulmonary Diseases
The present invention describes compositions of thymus derived peptides and uses therefore in diagnostic methods and for the treatment of diseases associated with reduced T helper cell counts, diseases such as infection, e.g., HIV infection and other viral infections, parasitic, and bacterial infection, AIDS, ARC, multiple sclerosis, chronic fatigue syndrome, rheumatoid arthritis, Alzheimer's disease, asthma, allergy, dermatitis, type 1 diabetes mellitus, colitis, inflammatory bowel disease / irritable bowel syndrome, Crohn's disease, Psoriasis, Chronic obstructive pulmonary disease, Systemic lupus erythematosus, transplant rejection and cancer.
Owner:UNIV OF COLORADO THE REGENTS OF +1
Long peptides of 22-45 amino acid residues that induce and/or enhance antigen specific immune responses
InactiveUS7202034B2Efficient synthesisEfficient processingAntibacterial agentsPeptide/protein ingredientsCtl epitopeBacteroides
The invention is concerned with epitopes derived from human papilloma virus, and peptides having a size of about 22–45 amino acid residues comprising minimal T cell epitopes. The invention further provides clinically relevant approaches for immunizing subjects against (Myco)bacterially and / or virally infected cells or tumor cells, and in particular against HPV. The invention demonstrates that peptide sequences of 22–35 amino acid residues in length can induce both peptide-specific CD8+ cytolytic cells and CD4+ T-helper cells. Moreover, the invention demonstrates that vaccination with 22–35 residue long peptides results in a more vigorous CD8+ cytolytic T-cell response than vaccination with peptides of the exact minimal CTL epitope length. The invention further demonstrates that the intrinsic capacity of certain minimal CTL epitopes which instead of activating cytolytic effector cells tolerize these cytolytic cells, can be overcome by use of these 22–35 amino acid long peptides. The invention further provides clinically relevant approaches for vaccination and / or treatment of subjects against HPV. The invention also provides methods and uses suited to treat subjects suffering from progressive lesions and / or cervical cancer.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT
Immune responses to fusion proteins
InactiveUS6936464B1Improve aspectEnhance vaccine efficacyPolypeptide with localisation/targeting motifAntibody mimetics/scaffoldsFusion Protein ExpressionIn vivo
The invention relates to a nucleic acid construct for delivery into living cells in vivo for inducing an immune response in a patient to an idiotypic determinant present on a malignant B cell in the patient; the construct directing the expression of a fusion protein, said fusion protein comprising the idiotypic determinant and at least one T helper cell epitope from tetanus toxin. The invention further relates to a method of making the nucleic acid construct, a method of treating a patient, and to a composition comprising the nucleic acid construct.
Owner:CANCER RES TECH LTD
Artificial T helper cell epitopes as immune stimulators for synthetic peptide immunogens
InactiveCN1306439AAvoid infectionPromote growthAntibacterial agentsSsRNA viruses positive-senseB-Cell EpitopesImmune Stimulation
The present invention is directed to novel artificial T helper cell epitopes (Th epitopes) designed to provide optimum immunogenicity when used in peptide immunogens comprising B cell epitopes or peptide haptens, a target antigenic site of a target antigen for eliciting antibodies thereto. The artificial Th epitopes are covalently linked to the target antigenic site and optionally an immunostimulatory sequence to provide effective and safe peptide immunogens.
Owner:SHANGHAI SHEN LIAN BIOMEDICAL CORP +1
Anti-Human Immunodeficiency Virus Surrogate Target Agent Technology Filter Intended to Neutralize or Remove Human Immunodeficiency Virus Virions From Blood
InactiveUS20090281473A1Effectively averting AIDSHaemofiltrationMedical devicesImmunodeficiency virusHuman immunodeficiency virus disease
The Human Immunodeficiency Virus posses a significant threat to the world's population. Current strategies utilized to treat infectious agents have not been adequate to contain and eradicate this deadly viral infection. HIV seeks out its host, a T-Helper cell, by utilizing glycoprotein 120 probes to engage a CD4 cell-surface receptor located on the surface of a T-Helper cell. Developing blood filtering techniques that incorporate filter mediums that offer HIV virion's probes the opportunity to engage the cell-surface receptors they are seeking offers a means of neutralizing and removing HIV. Filtering the blood of a patient with filter mediums comprised of T-Helper cells, sheets of lipid bilayer or virus-like structures with each type of medium possessing cell-surface receptors intended to attract and engage HIV virions provides an effective strategy to prevent and treat AIDS.
Owner:SCHEIBER LANE BERNARD +1
Peptide vaccine for prevention and immunotherapy of dementia of the Alzheimer's type
ActiveUS9102752B2Increase response ratePromote generationSsRNA viruses negative-senseNervous disorderHeterologousPeptide vaccine
The present disclosure is directed to individual Aβ peptide immunogen constructs, peptide compositions comprising these Aβ peptide immunogen constructs and mixtures thereof, pharmaceutical compositions including vaccine formulations comprising these Aβ peptide immunogen constructs, with the individual Aβ peptide immunogen constructs having the N-terminus of the Aβ peptide as the B cell (B) epitopes linked through spacer residue(s) to heterologous T helper cell (Th) epitopes derived from pathogen proteins that act together to stimulate the generation of highly specific antibodies directed against the N-terminus of the Aβ peptide offering protective immune responses to patients at risk for, or with, Alzheimer's Disease.
Owner:UNITED NEUROSCIENCE LIMITED
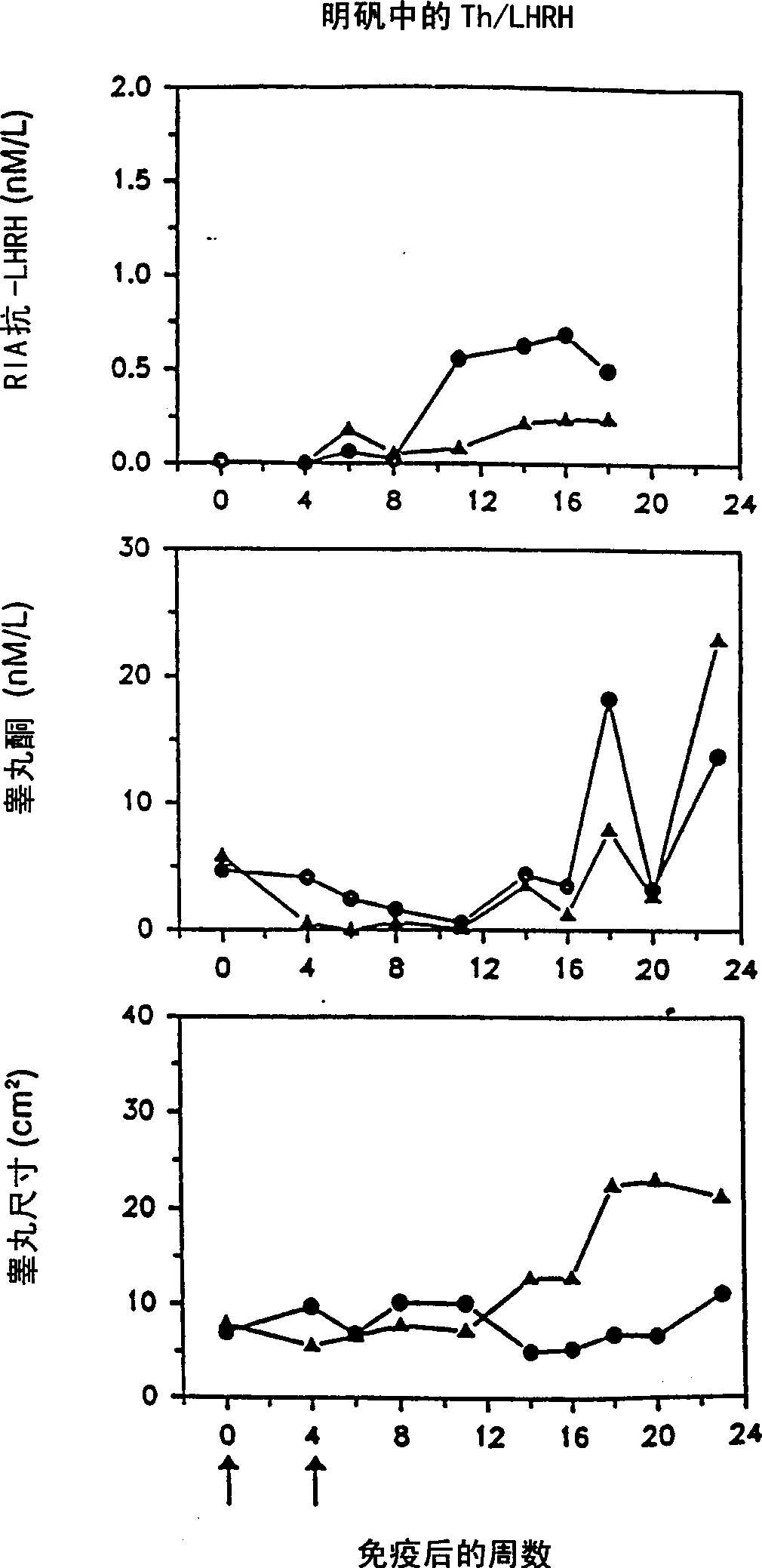
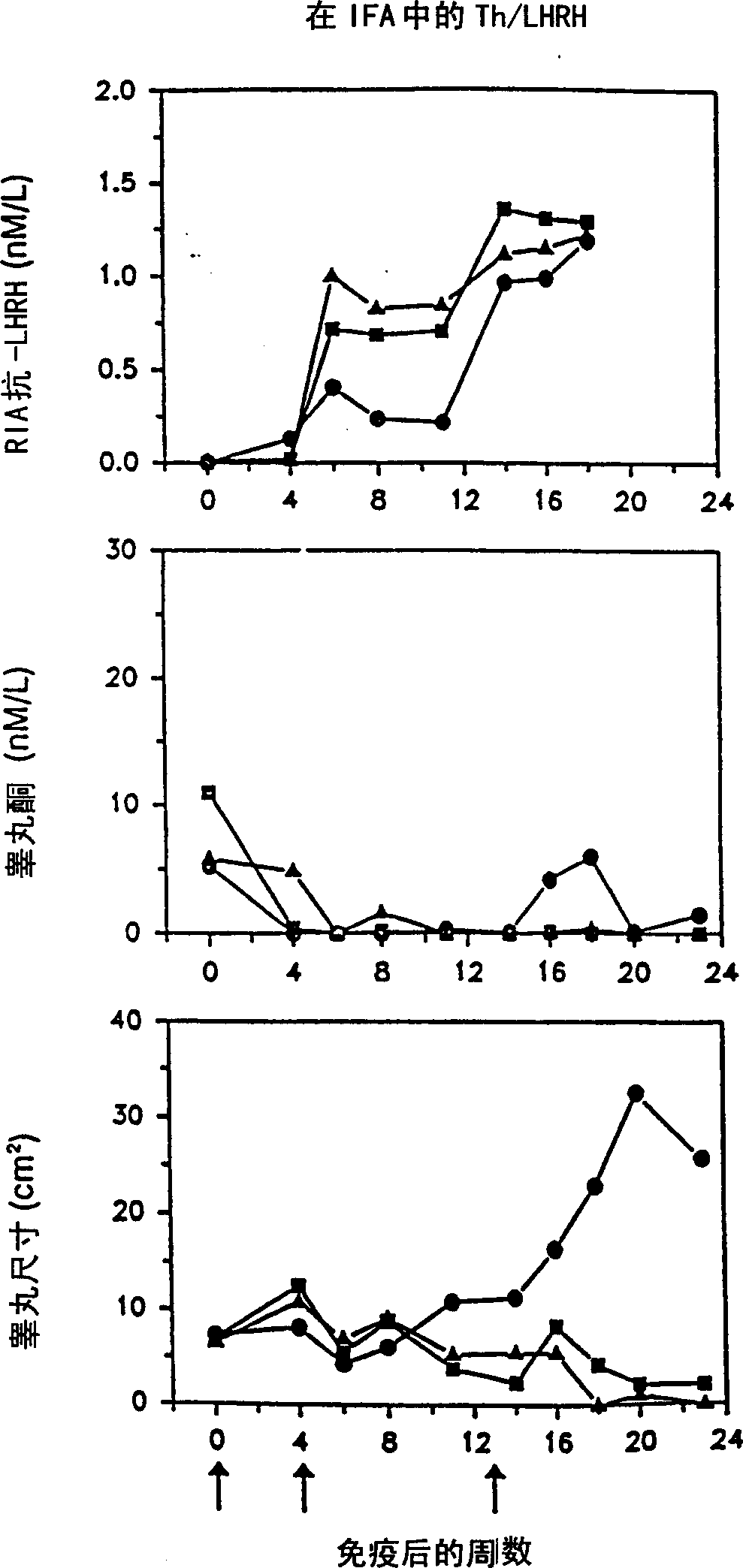
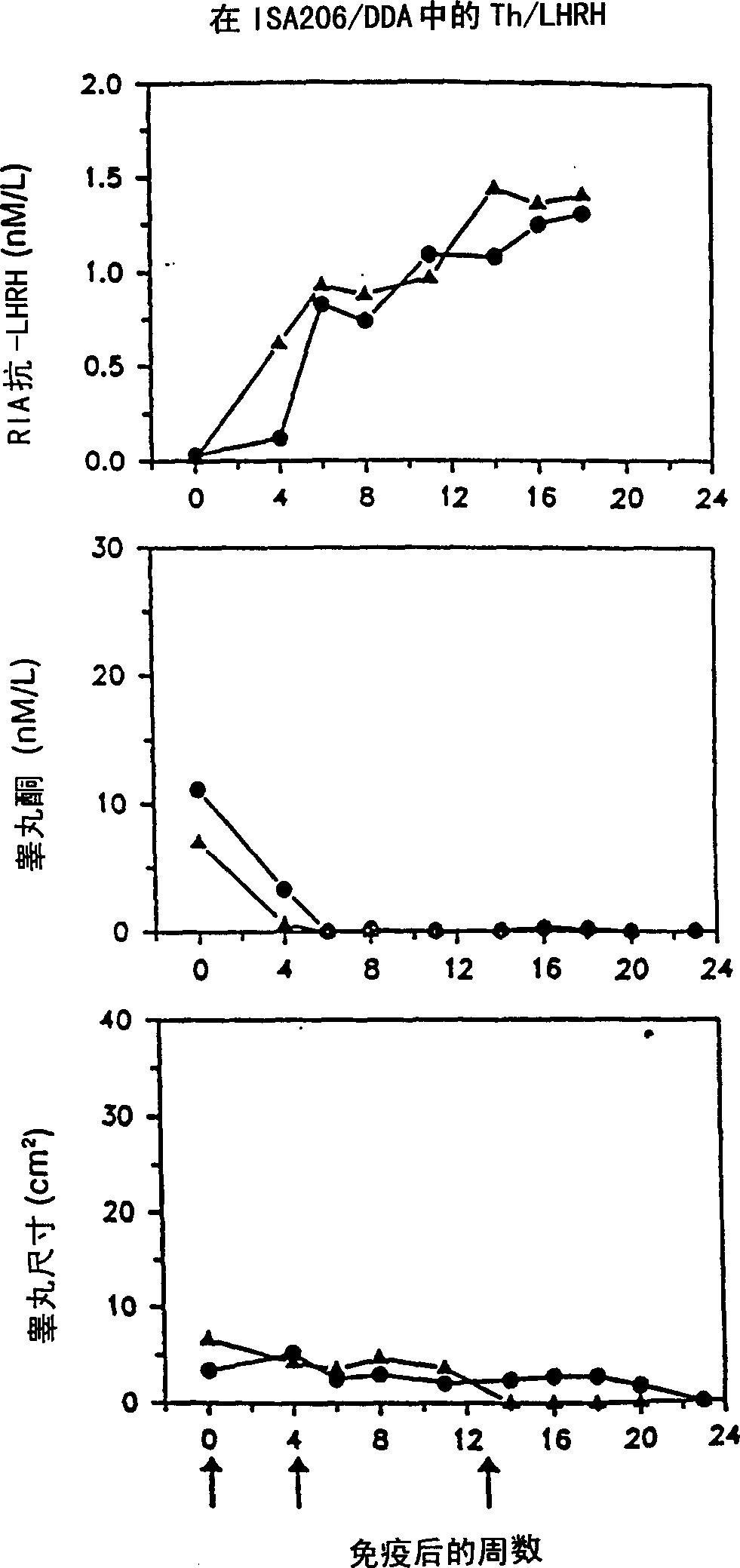
Artificial T helper cell epitopes as immune stimulators for synthetic peptide immunogens including immunogenic LHRH peptides
InactiveCN1315869ASomatostatinSsRNA viruses negative-sensePeptide/protein ingredientsImmunogenic peptideImmunogenicity
The present invention is directed to novel peptide immunogens for eliciting antibodies to LHRH comprising artificial T helper cell epitopes (Th epitopes) designed to provide optimum immunogenicity. The artificial Th epitopes are covalently linked to LHRH and optionally an immunostimulatory sequence.
Owner:UNITED BIOMEDICAL INC
Preparation Of Antigen-Presenting Human Gamma-Delta T Cells And Use In Immunotherapy
InactiveUS20090208517A1Improve purification effectEfficient workSnake antigen ingredientsDead animal preservationDendritic cellVaccination
The invention relates to a method for the preparation of efficient antigen-presenting human γδ T cells, to the γδ T cells prepared by such a method, and to their use in immunotherapy, vaccination, vaccine development and diagnostics. Similar to dendritic cells (DCs) in potency and efficacy, these human γδ T cells process antigens and present antigenic peptides to αβ T cells and induce antigen-specific responses (proliferation and differentiation) in naïve αβ T cells. γδ T cells are easily purified from peripheral blood, acquire “maturation” status (expression of essential adhesion, co-stimulatory and major histocompatibility complex molecules) within 1 day of in vitro culture under stimulation and induce strong primary and secondary T helper cell responses. The γδ T cells may be used in a method of treatment of tumors or chronic or recurrent infectious diseases, in identification of novel tumor or pathogen-derived antigens, and in the diagnosis of the immune competence of a patient.
Owner:MOSER BERNHARD +1
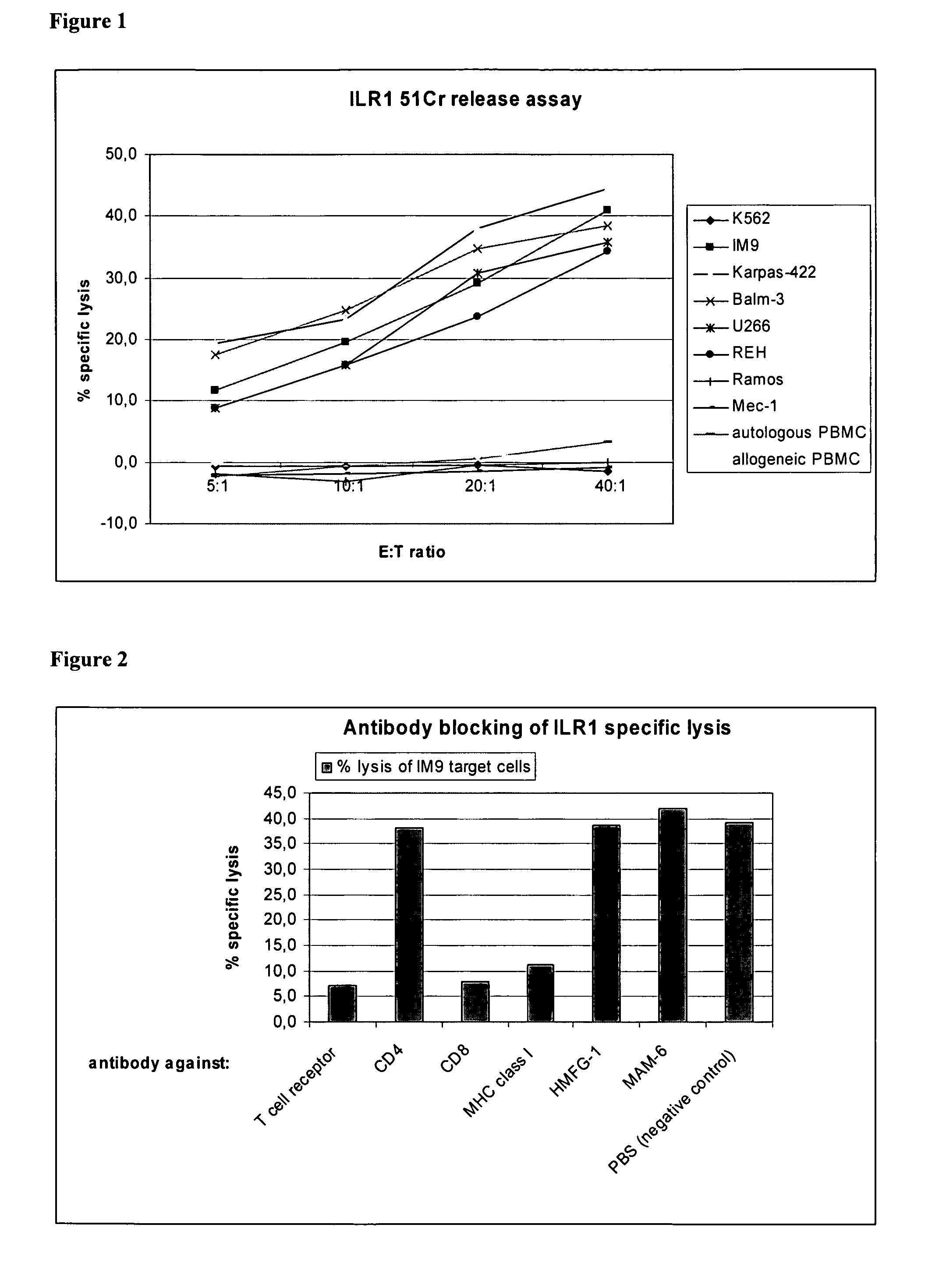
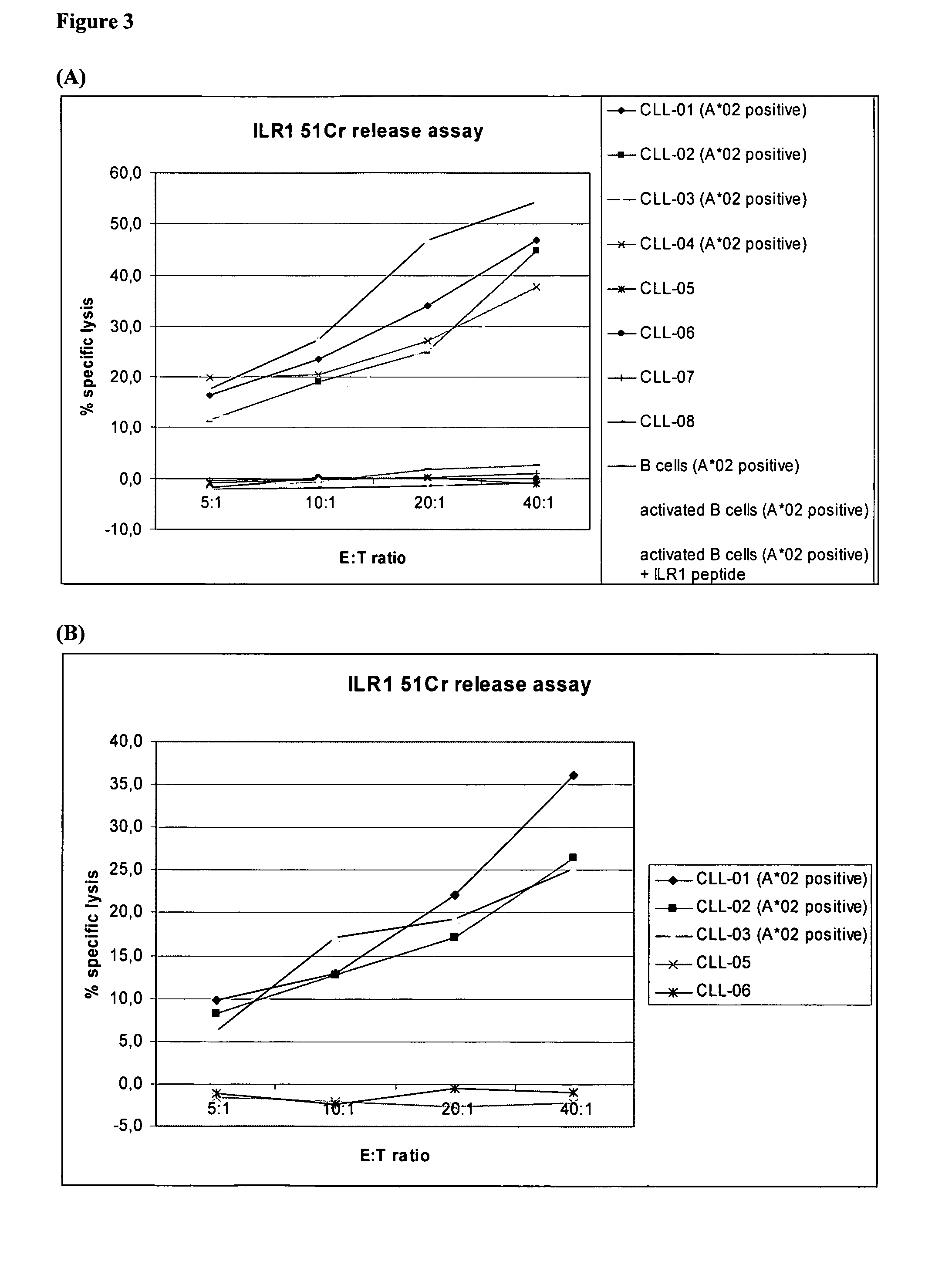
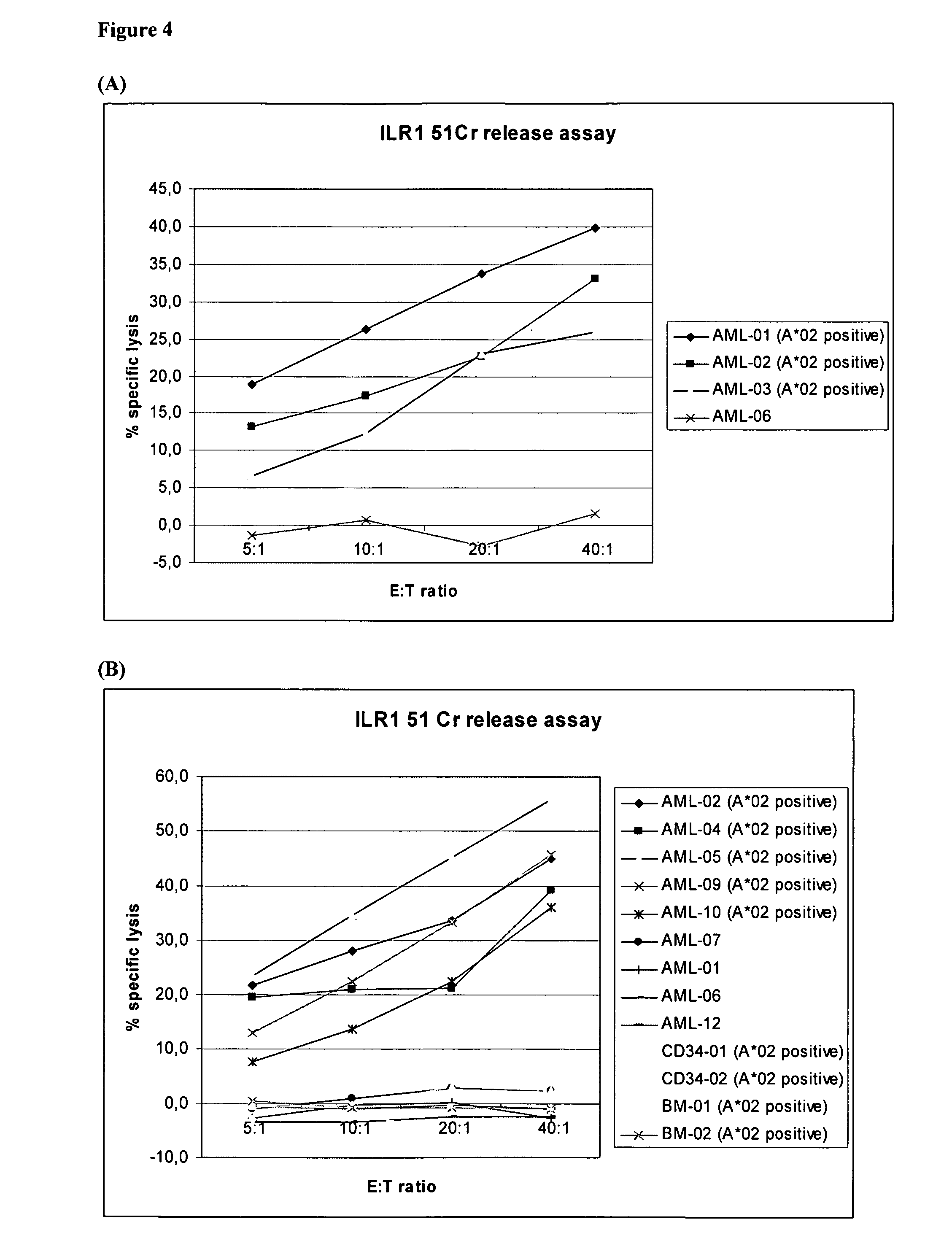
Identification of Hla-A2-Presented T-Cell Epitopes Derived from the Oncofoetal Antigen-Immature Laminin Receptor Protein and Uses Thereof
ActiveUS20090041794A1The relationship is accurateTumor rejection antigen precursorsPeptide/protein ingredientsImmunotherapeutic agentLaminin receptor protein
The present invention relates to the immunotherapB of cancer, in particular several tumor entities including hematological malignancies. The present invention relates to tumor-associated T-helper cell peptide epitopes, alone or in combination with other tumor-associated peptides, that serve as active pharmaceutical ingredients of vaccine compositions that stimulate anti-tumor immune responses. In particular, the present invention relates to two novel peptide sequences derived from HLA class I or II molecules of human oncofoetal antigen immature laminin receptor (OFA / iLR), which can be used in vaccine compositions for eliciting anti-tumor immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com