Patents
Literature
163 results about "Leucocyte antigens" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Artificial antigen presenting cells and methods of use thereof
InactiveUS20020131960A1Palliating their conditionReduce riskBiocideCompound screeningEpitopeAccessory molecule
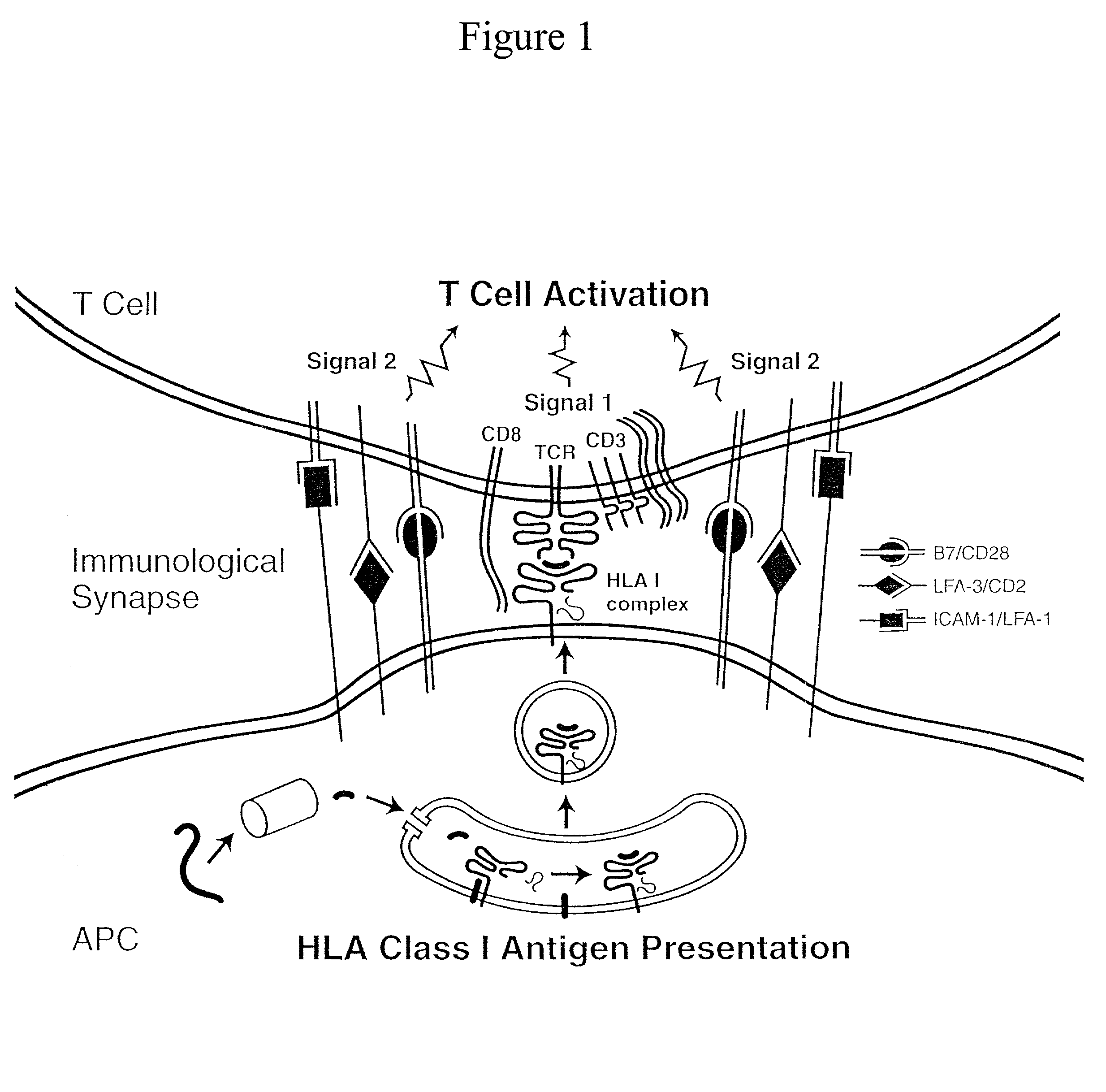
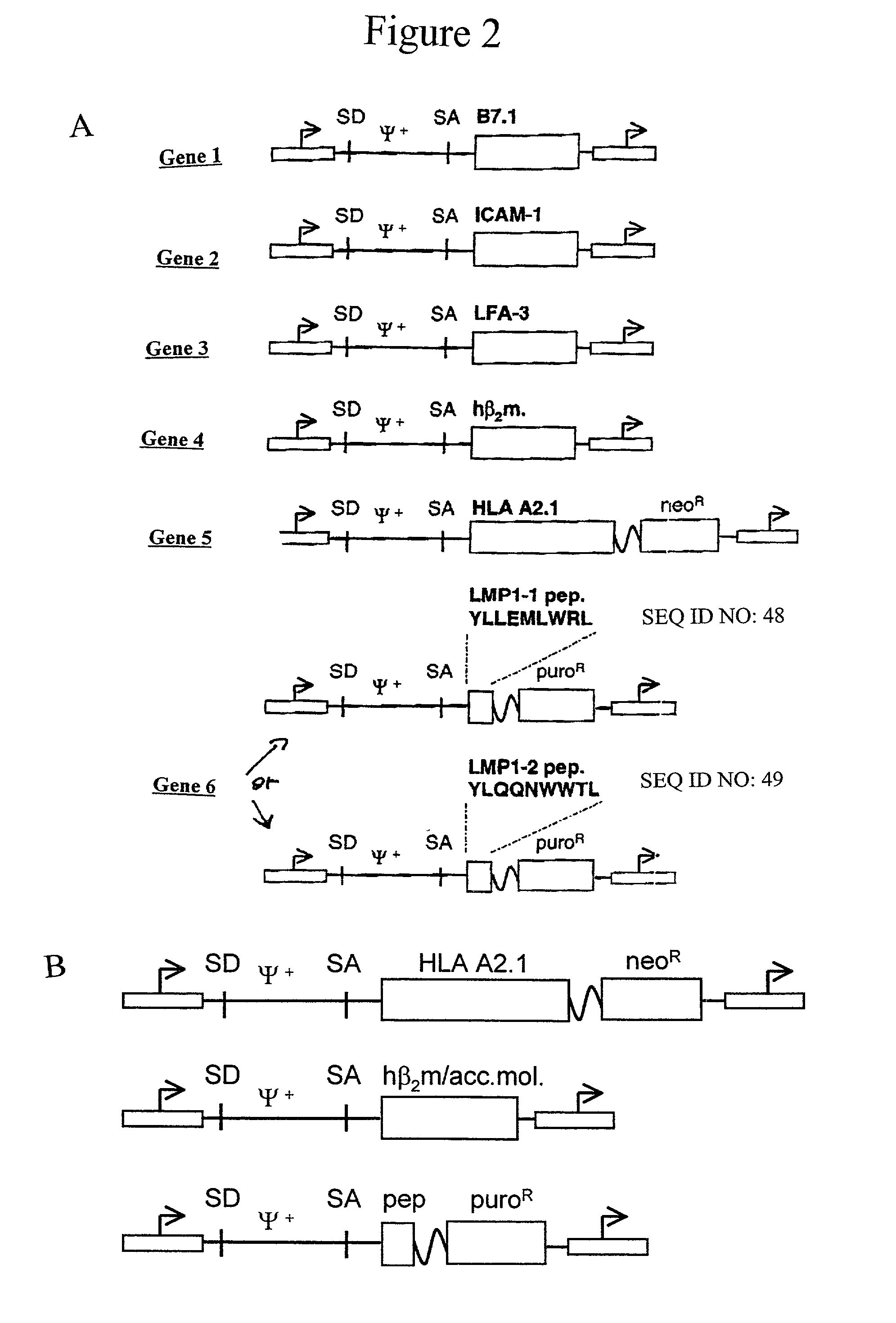
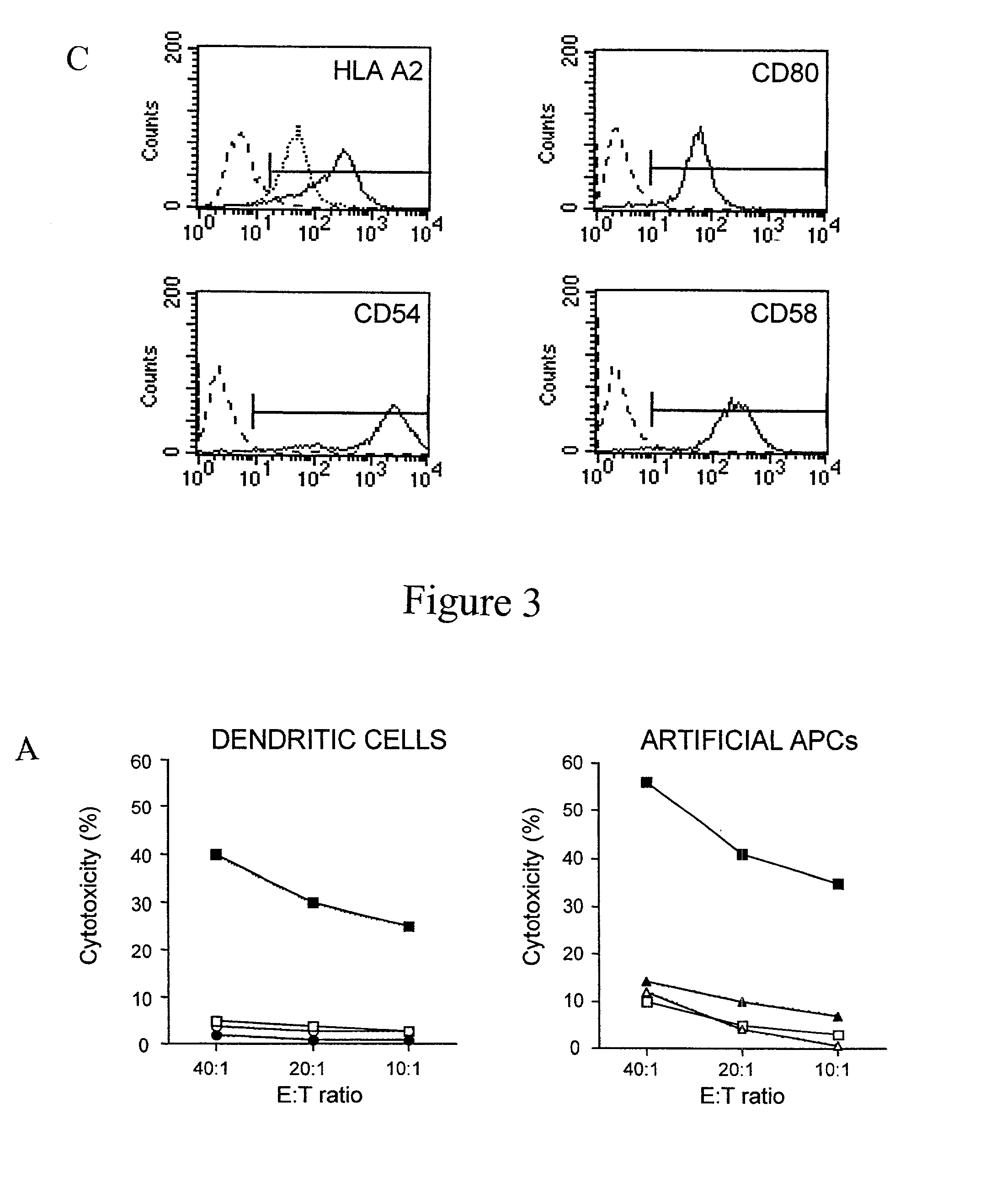
The invention provides an artificial antigen presenting cell (AAPC) comprising a eukaryotic cell expressing an antigen presenting complex comprising a human leukocyte antigen (HLA) molecule of a single type, at least one exogenous accessory molecule and at least one exogenous T cell-specific epitope. Methods of use for activation of T lymphocytes are also provided.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Tumour-associated peptides binding to human leukocyte antigen (HLA) class i or ii molecules and related Anti-cancer vaccine
InactiveUS20090274714A1Organic active ingredientsPeptide/protein ingredientsHla class iiAdditive ingredient
The present invention relates to immunotherapeutic methods, and molecules and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumour-associated T-helper cell peptide epitopes, alone or in combination with other tumour-associated peptides, that serve as active pharmaceutical ingredients of vaccine compositions which stimulate anti-tumour immune responses. In particular, the present invention relates to two novel peptide sequences derived from HLA class II molecules of human tumour cell lines, which can be used in vaccine compositions for eliciting anti-tumour immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Tumor-associated Peptides Binding Promiscuously to Human Leukocyte Antigen (HLA) Class II Molecules
InactiveUS20080206216A1Organic active ingredientsPeptide/protein ingredientsHla class iiAdditive ingredient
The present invention relates to immunotherapeutic methods, and molecules and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumour-associated T-helper cell peptide epitopes, alone or in combination with other tumour-associated peptides, that serve as active pharmaceutical ingredients of vaccine compositions which stimulate anti-tumour immune responses. In particular, the present invention relates to 49 novel peptide sequences derived from HLA class II molecules of human tumour cell lines which can be used in vaccine compositions for eliciting anti-tumour immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
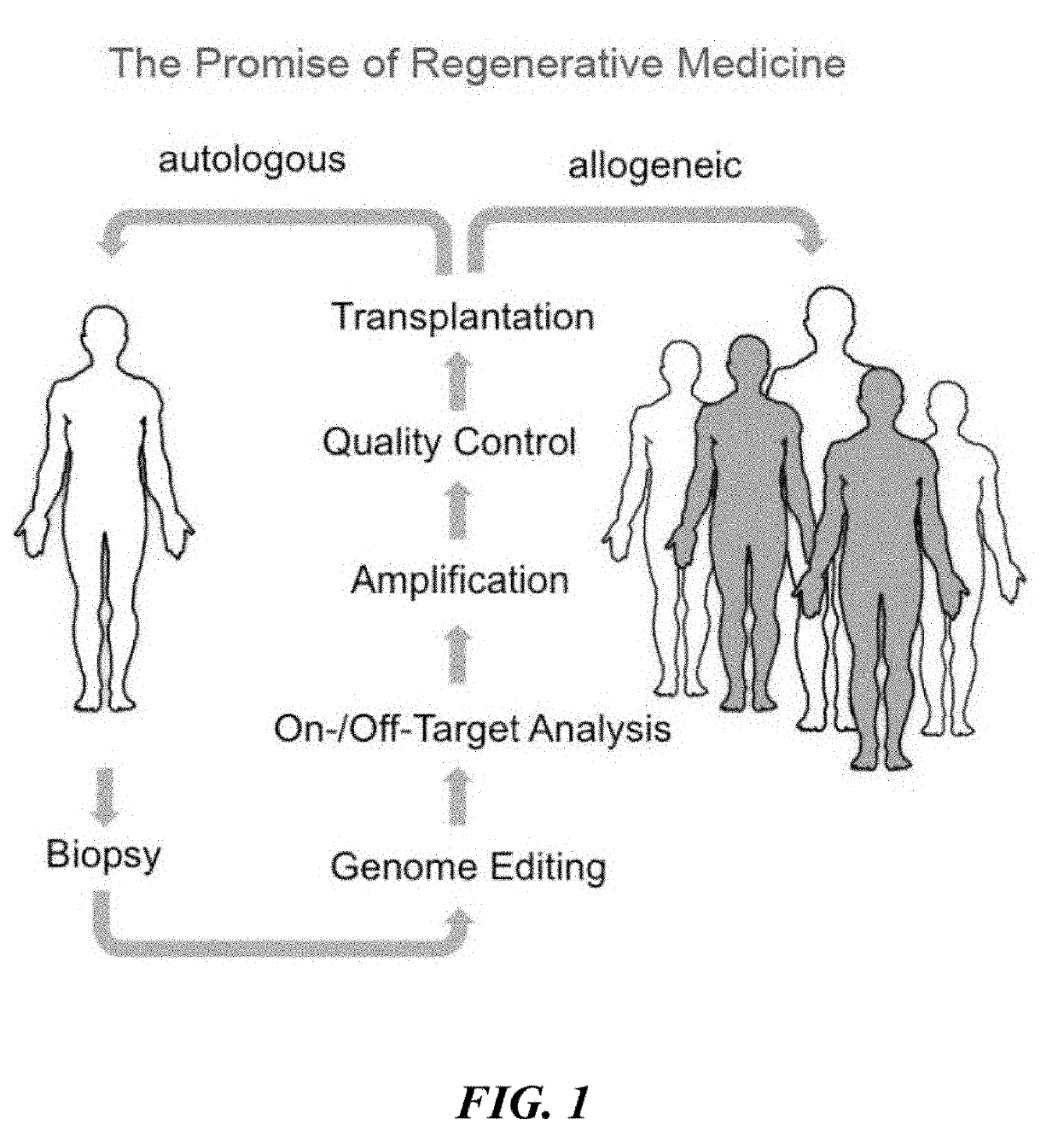
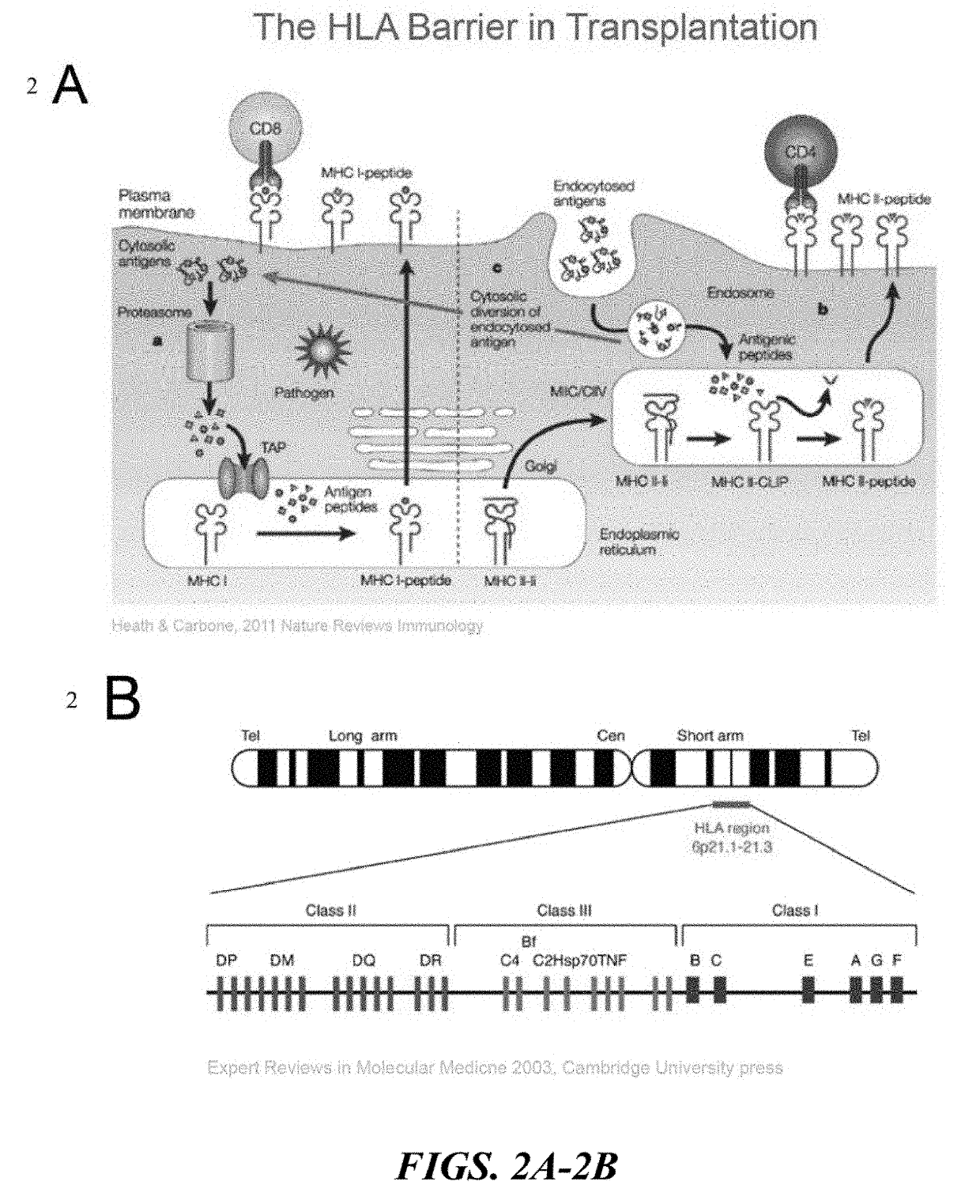
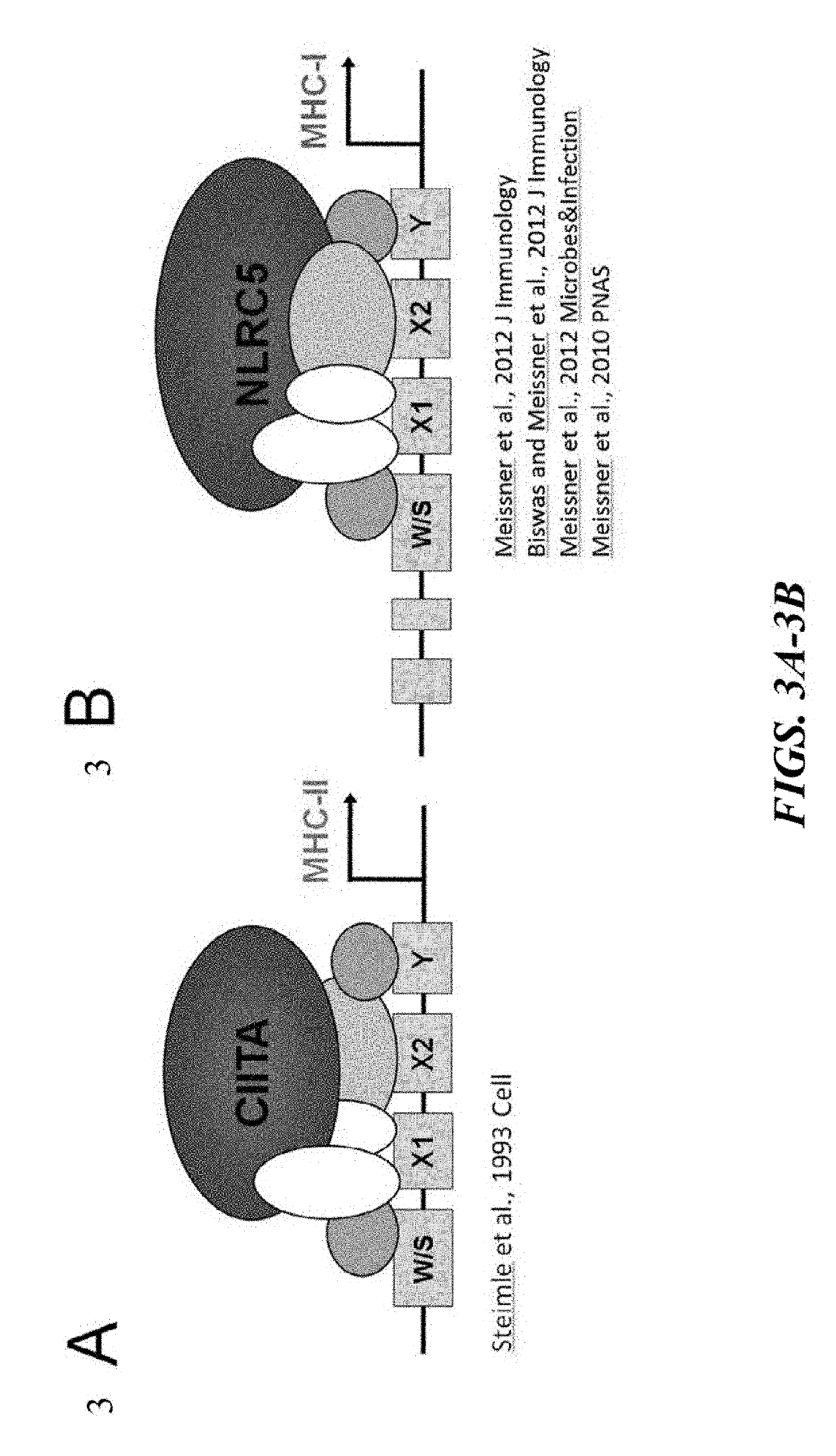
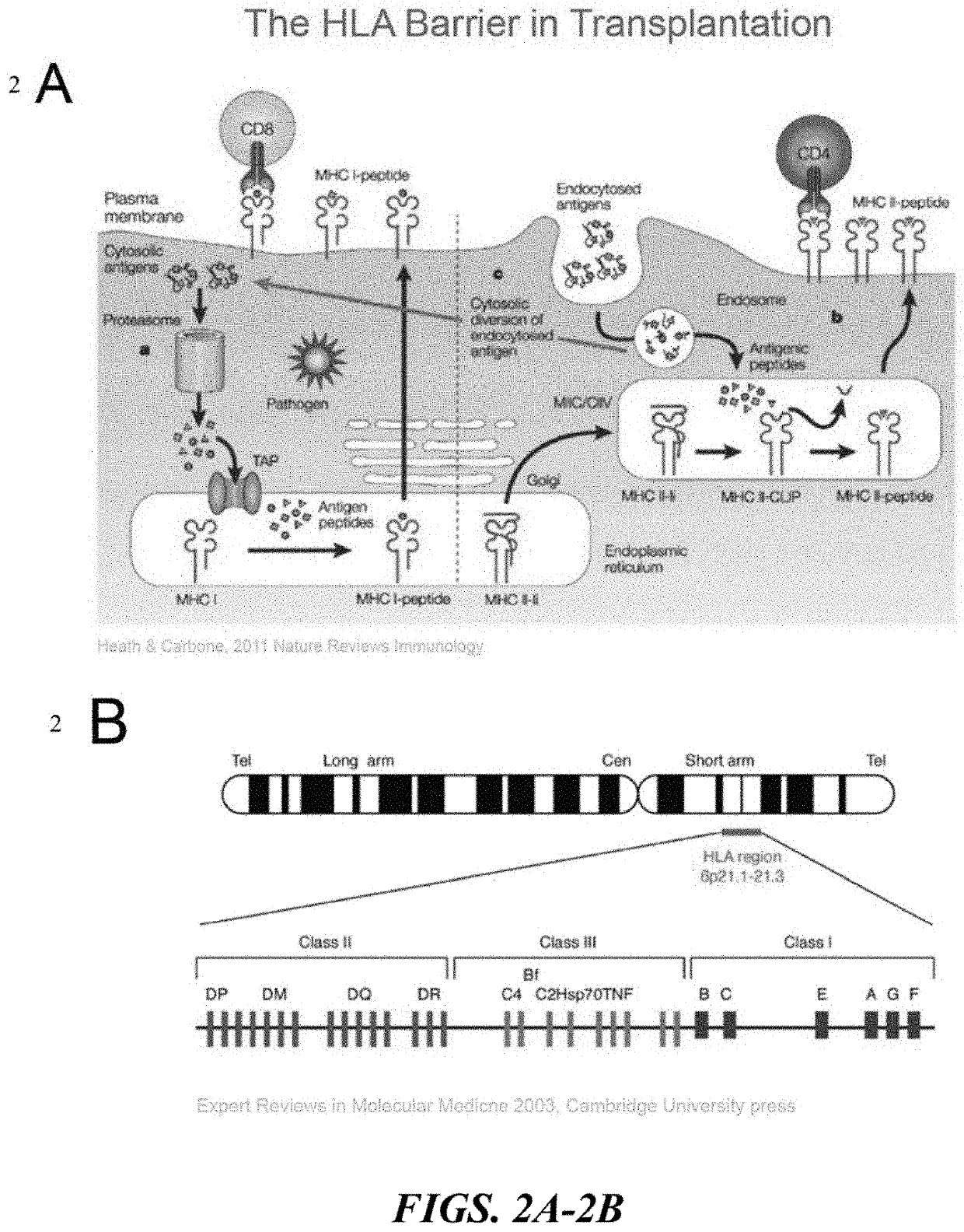
HLA Class II Deficient Cells, HLA Class I Deficient Cells Capable of Expressing HLA Class II Proteins, and Uses Thereof
The invention provides isolated primate cells preferably human cells that comprise a genetically engineered disruption in a human leukocyte antigen (HLA) class II-related gene, which results in deficiency in MHC class II expression and function. This invention also provides isolated cells further comprising a genetically engineered disruption in a beta-2 microglobulin (B2M) gene, which results in HLA class I / class II deficiency. Also provided are the method of using the cells for transplantation and treating a disease condition.
Owner:UNIV OF WASHINGTON +1
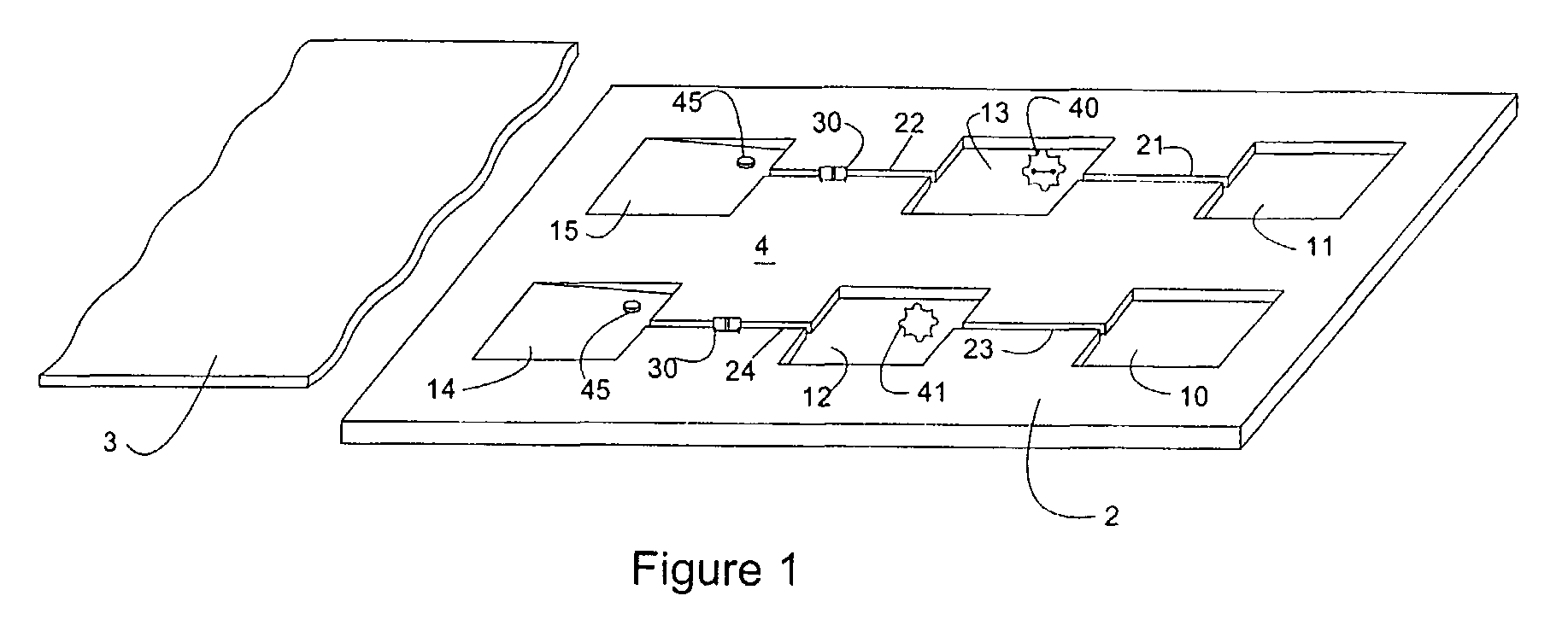
Microfluidic device and leucocyte antigen mediated microfluidic assay
ActiveUS9023641B2Bioreactor/fermenter combinationsBiological substance pretreatmentsCellular antigensWhite blood cell
Owner:ADVANCED ANIMAL DIAGNOSTICS
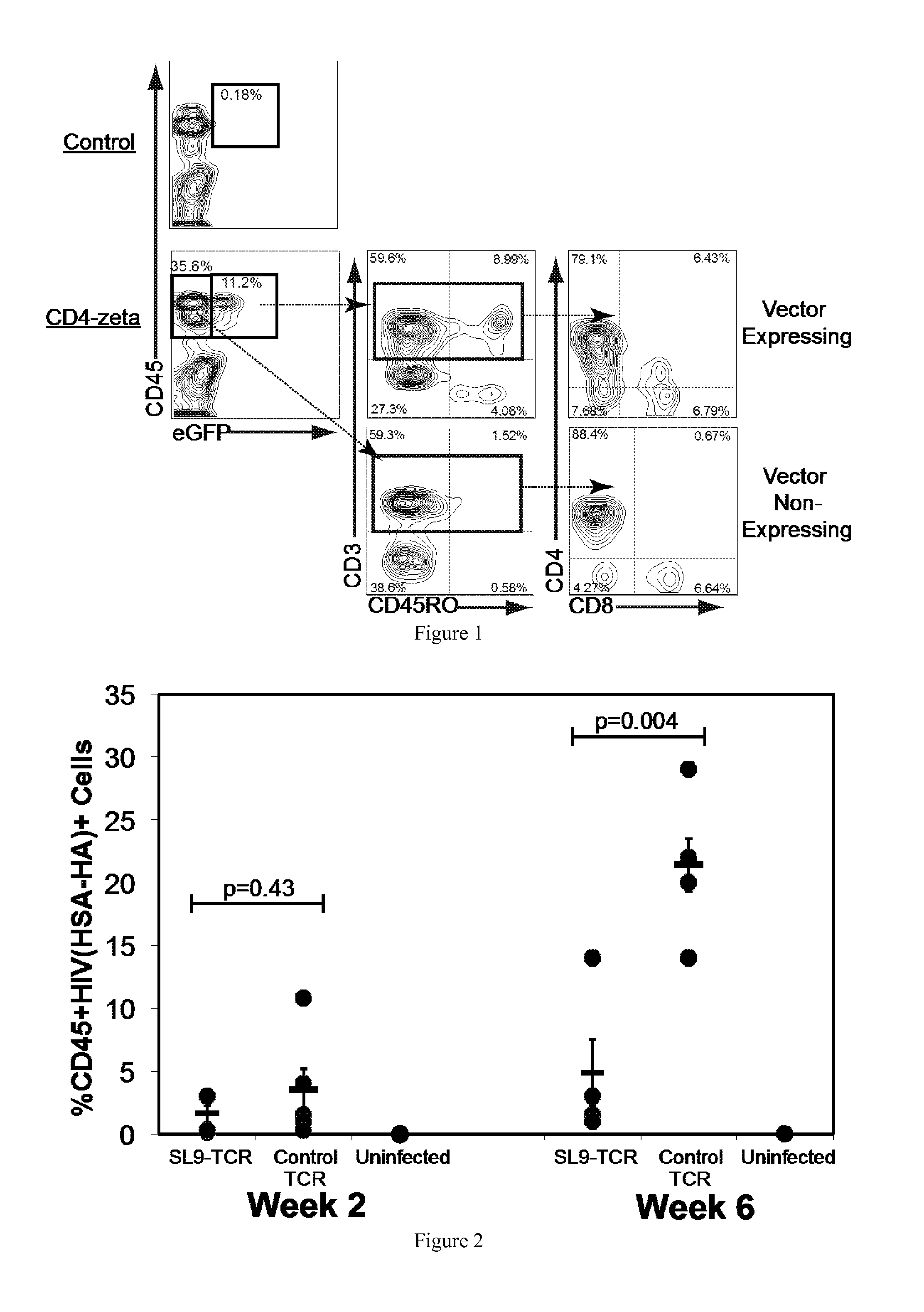
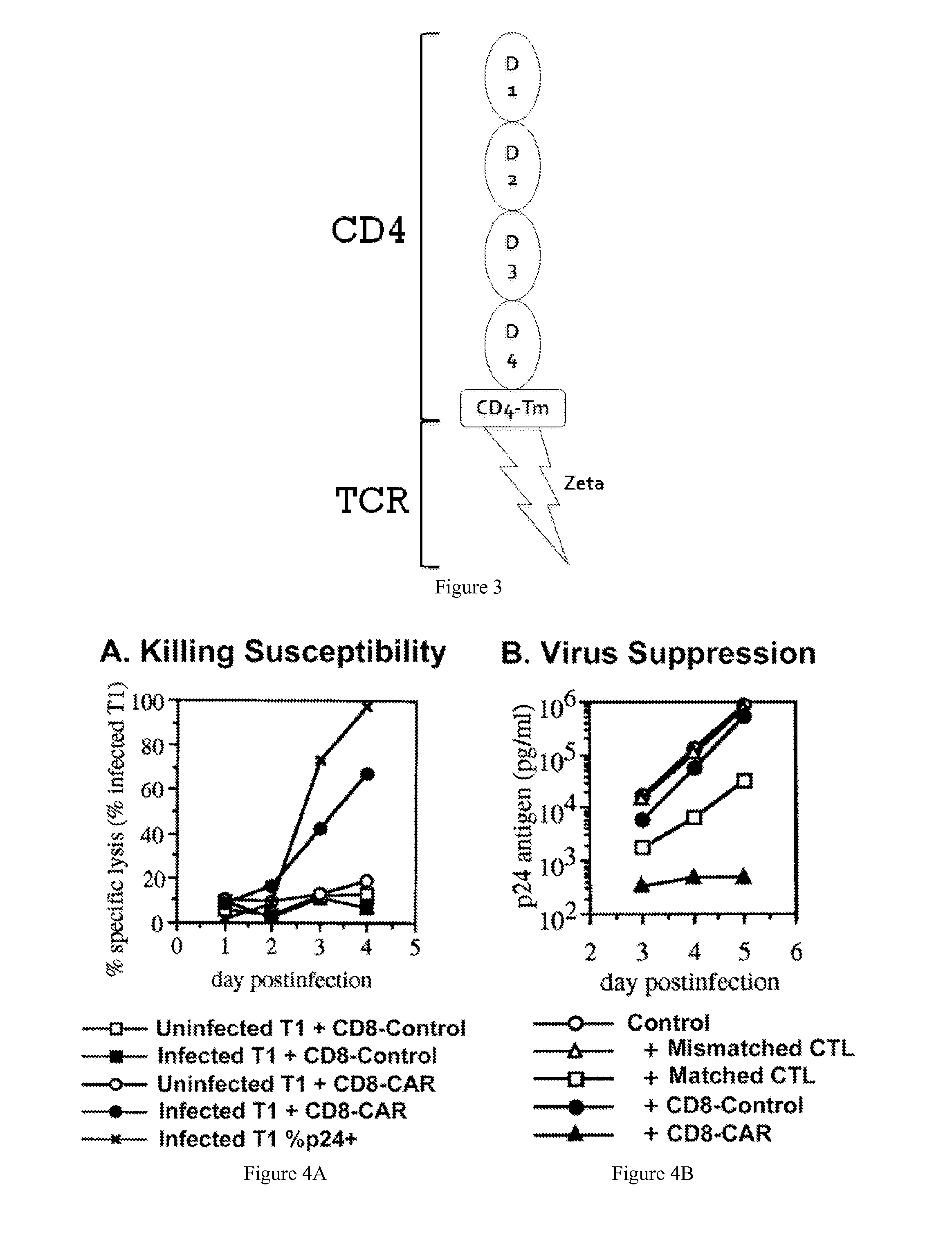
Engineering Antiviral T Cell Immunity through Stem Cells and Chimeric Antigen Receptors
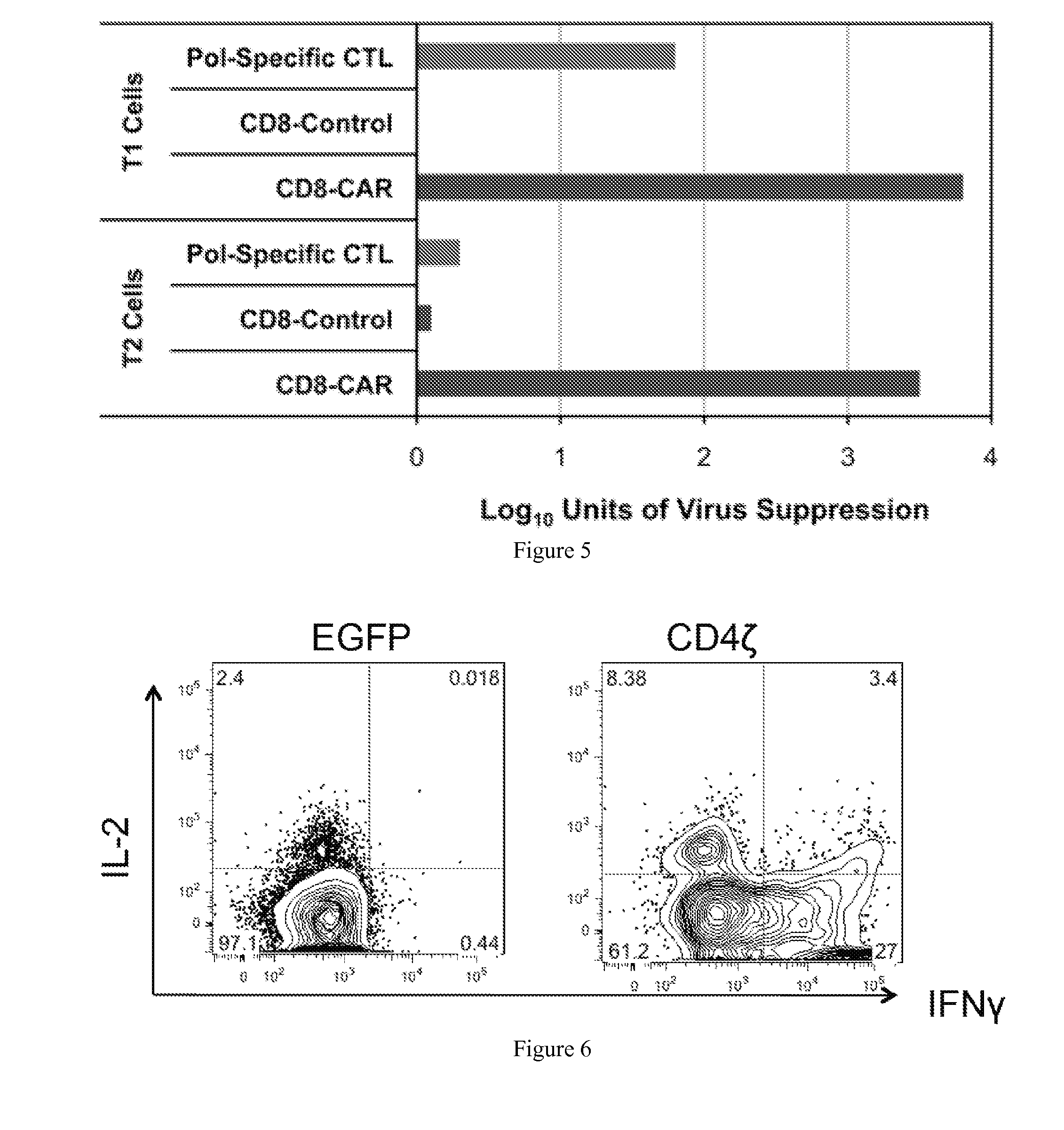
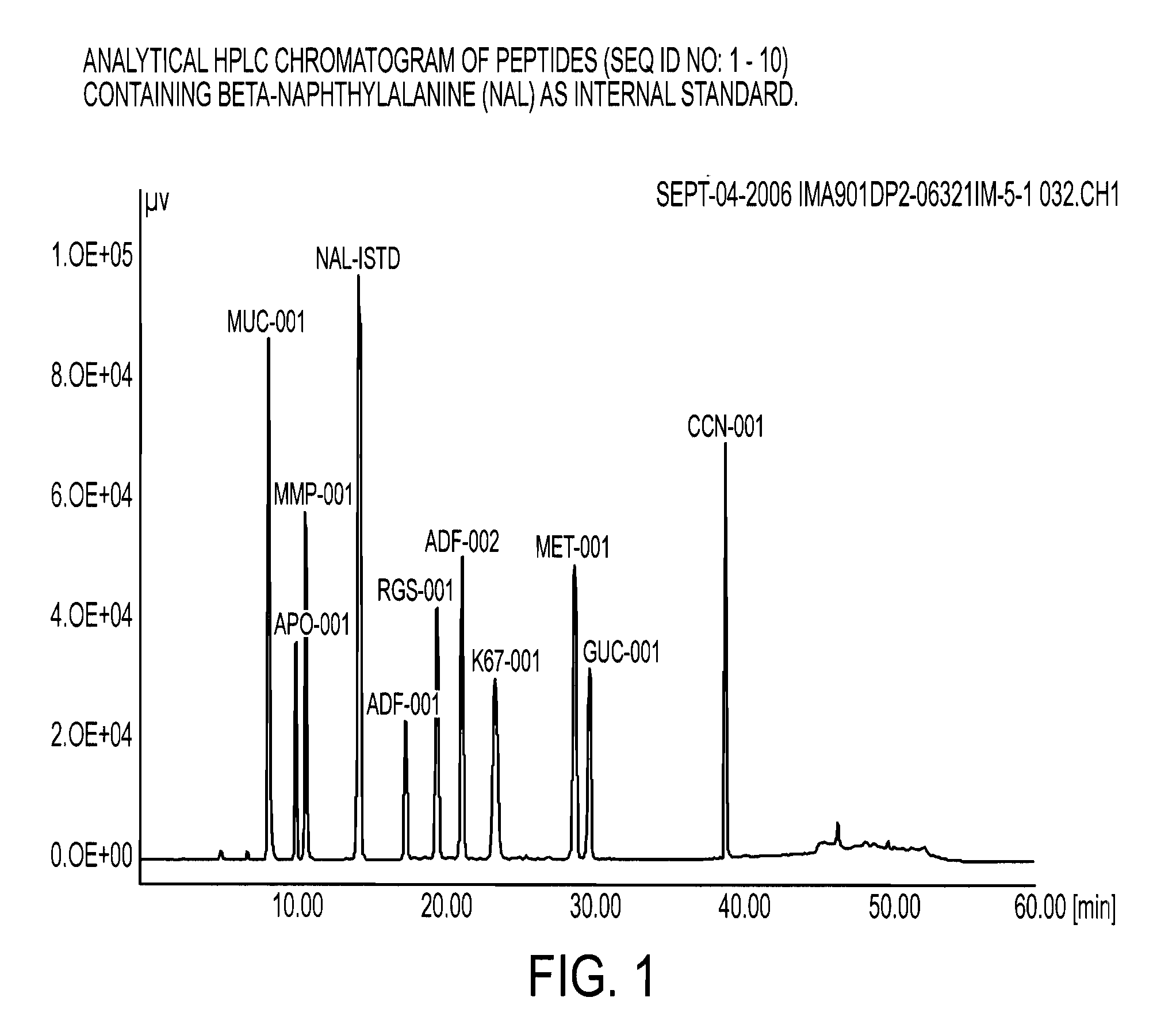
The HIV-specific cytotoxic T lymphocyte (CTL) response is a critical component in controlling HIV replication and is an important part of the ultimate failure to eradicate the virus. Disclosed herein are methods for genetically enhancing the HIV-specific CTL response to allow long-term viral suppression or viral clearance. Human hematopoietic stem cells (HSCs) were genetically modified such that they differentiate into mature CTLs that will kill HIV infected cells. As disclosed herein, the functional effector cells are not human leukocyte antigen (HLA)-restricted. As disclosed herein, stem cells are transduced with non HLA-restricted chimeric antigen receptors (CARs) that allow the recognition of HIV or HIV infected cells when expressed by a CTL.
Owner:RGT UNIV OF CALIFORNIA
Novel formulations of tumour-associated peptides binding to human leukocyte antigen (HLA) class i or class ii molecules for vaccine
ActiveUS20100158929A1Good adjuvant effectIncrease heightPeptide/protein ingredientsMetabolism disorderKidney cancerGlioblastoma
The present invention relates to novel formulations of tumour-associated peptides binding to human leukocyte antigen (HLA) class I or II molecules as vaccines for the use in immunotherapeutic methods. In particular, the present invention relates to formulations for the immunotherapy of cancer, in particular renal and brain cancer, in particular glioma, especially glioblastoma cancer. The present invention furthermore relates to vaccine compositions for eliciting anti-tumour immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
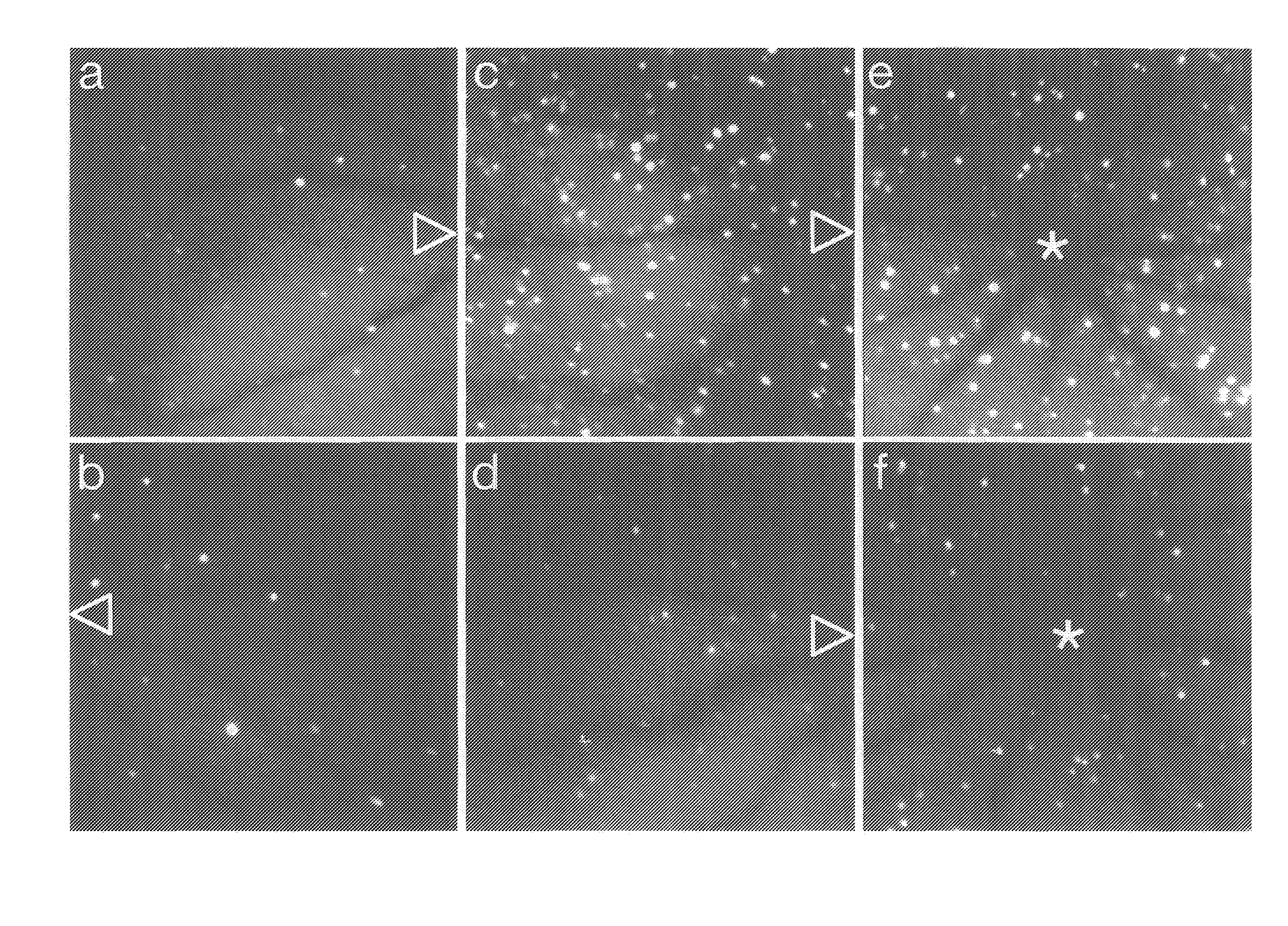
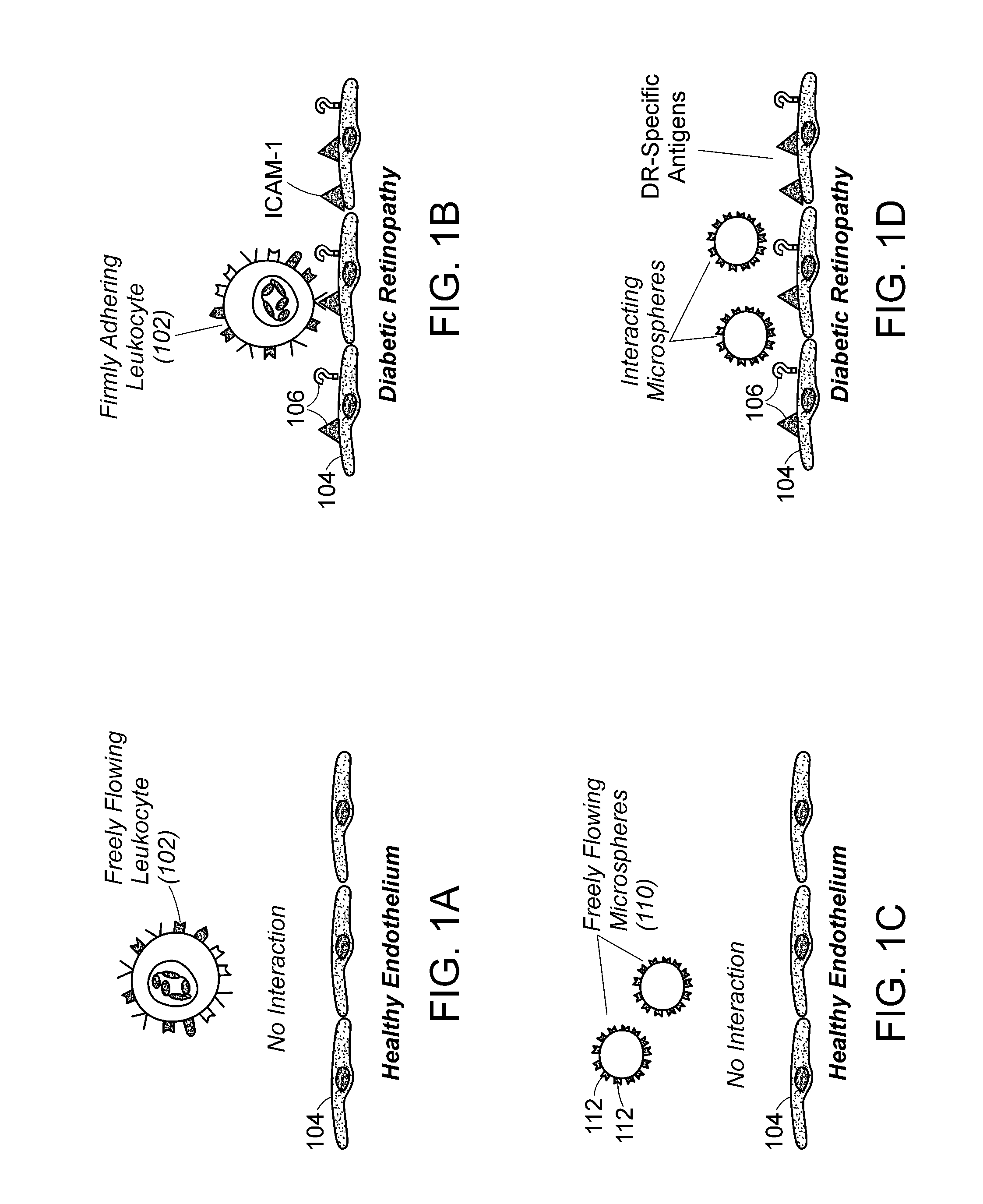
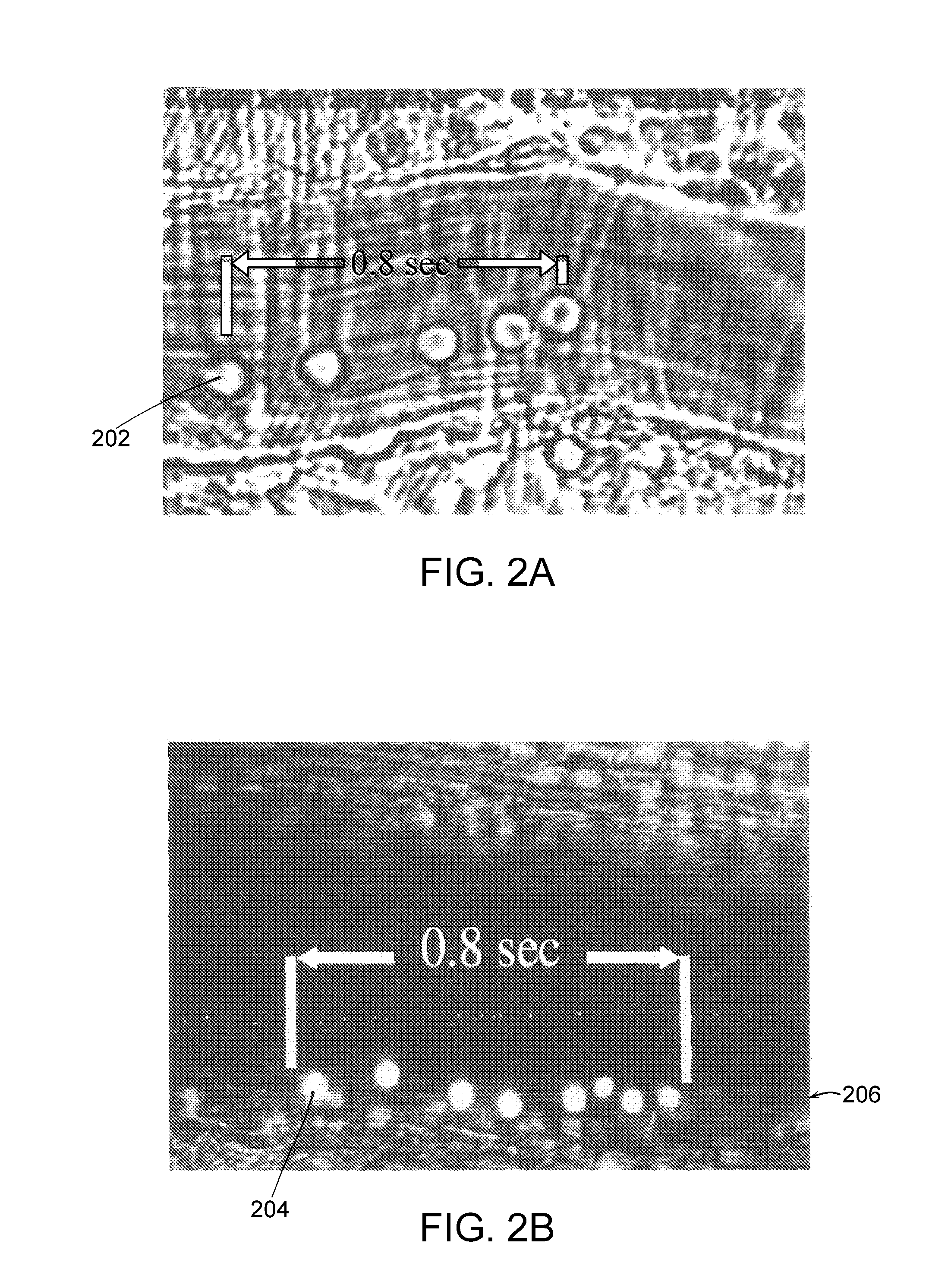
Molecular imaging methods for diagnosis and evaluation of ocular and systemic diseases
InactiveUS20080305046A1Provide informationNon-invasive and minimally-invasive detectionUltrasonic/sonic/infrasonic diagnosticsPowder deliveryDiabetic retinopathyWhole body
This invention relates generally to minimally-invasive, in vivo methods of detecting one or more ligands on an intraluminal surface of a blood vessel using microparticles coated with one or more ligand binding partners. More particularly, in certain embodiments, the invention relates to minimally-invasive, in vivo methods of detecting endothelial and leukocyte antigens that are predictive of diabetic retinopathy (DR) and / or other conditions using protein-conjugated microparticles detectable by a non-invasive detection system, for example, a scanning laser opthalmoscope. In other embodiments, the invention relates to targeted delivery of drugs or other substances to specific regions of an intraluminal surface of a blood vessel using drug-containing microparticles coated with one or more ligand binding partners.
Owner:MASSACHUSETTS EYE & EAR INFARY
Tumor-associated Peptides Binding Promiscuously to Human Leukocyte Antigen (HLA) Class II Molecules
The present invention relates to immunotherapeutic methods, and molecules and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumour-associated T-helper cell peptide epitopes, alone or in combination with other tumour-associated peptides, that serve as active pharmaceutical ingredients of vaccine compositions which stimulate anti-tumour immune responses. In particular, the present invention relates to 49 novel peptide sequences derived from HLA class II molecules of human tumour cell lines which can be used in vaccine compositions for eliciting anti-tumour immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
HLA high-resolution gene sequencing kit
InactiveCN101892317AAvoid problems that cannot be effectively typedHigh resolutionMicrobiological testing/measurementDNA/RNA fragmentationHLA-BExon
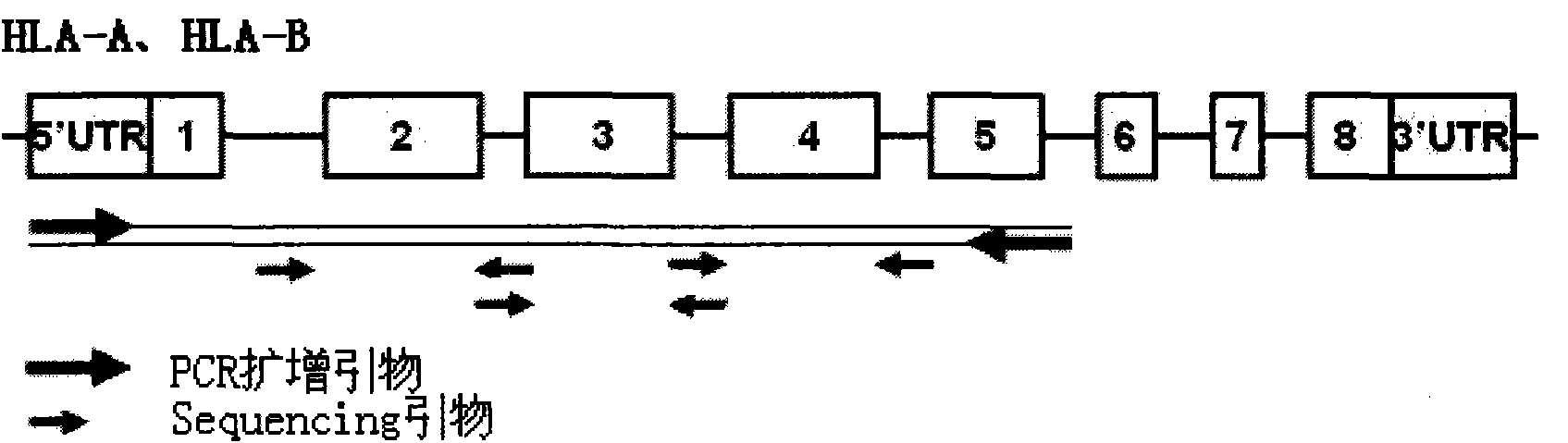
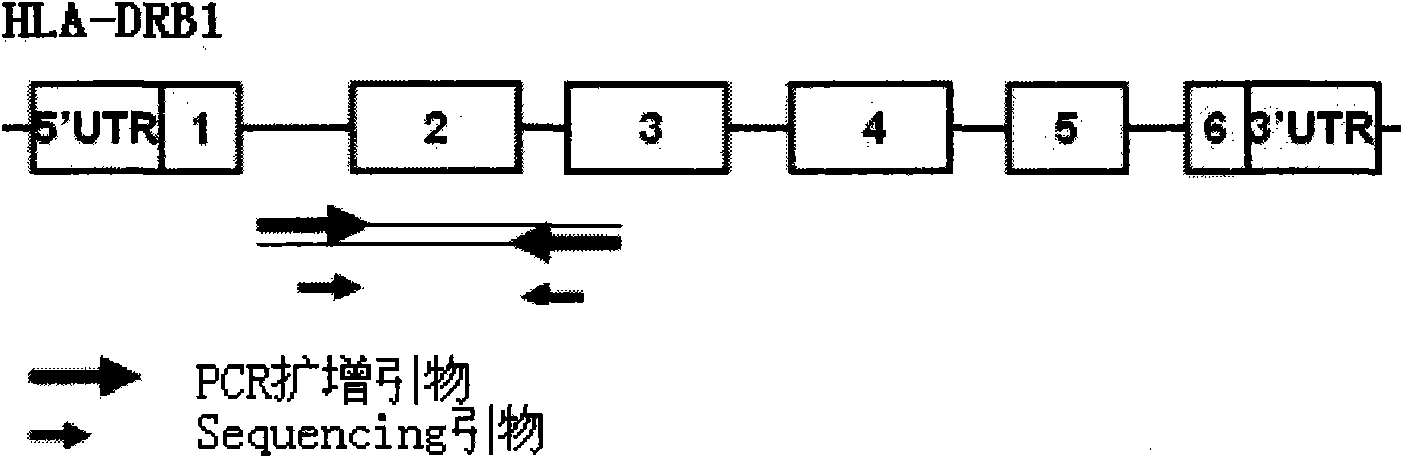
The invention discloses a parting method of leucocyte antigen gene of human being, comprising the following steps of: (1) extracting genome DNA to be tested by a regular technology, and amplifying a destination gene fragment to be analyzed by using PCR amplification primer: 2,3,4 exon of HLA-A, 2,3,4 exon of HLA-B and exon on the locus 2 of HLA-DRB; and (2) amplifying the PCR output obtained in the step (1) by using sequencing primer, amplifying the exon, sequencing the amplified exon and comparing the sequencing result with the standard sequence in a database to determine the gene parting result. As the 2,3,4 exon of HLA-A, 2,3,4 exon of HLA-B and exon on the locus 2 of HLA-DRB are effectively amplified as a result of optimized combination of the HLA gene sequencing kit and the test condition, and the corresponding exon is sequenced, the invention solves the problem that effective parting can not be performed when certain allelic gene nucleotide is located outside an amplification area during further parting, thereby improving the parting resolution and accuracy of the HLA gene.
Owner:SUZHOU UNIV +1
Tumor-associated peptides binding to human leukocyte antigen (HLA) class II molecules
ActiveUS20100040590A1Organic active ingredientsPeptide/protein ingredientsHla class iiAdditive ingredient
The present invention relates to immunotherapeutic methods, and molecules and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumour-associated T-helper cell peptide epitopes, alone or in combination with other tumour-associated peptides, that serve as active pharmaceutical ingredients of vaccine compositions which stimulate anti-tumour immune responses. In particular, the present invention relates to 49 novel peptide sequences derived from HLA class II molecules of human tumour cell lines which can be used in vaccine compositions for eliciting anti-tumour immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Universal donor stem cells and related methods
ActiveUS20190309259A1Reduce and eliminate and activityReduce and eliminate surface expressionGenetically modified cellsStable introduction of DNAHLA-BImmunogenicity
Disclosed herein are universal donor stem cells and related methods of their use and production. The universal donor stem cells disclosed herein are useful for overcoming the immune rejection in cell-based transplantation therapies. In certain embodiments, the universal donor stem cells disclosed herein do not express one or more MHC-I and MHC-II human leukocyte antigens. Similarly, in certain embodiments, the universal donor stem cells disclosed herein do not express one or more human leukocyte antigens (e.g., HLA-A, HLA-B and / or HLA-C) corresponding to MHC-I and MHC-II human leukocyte antigens, thereby rendering such cells hypoimmunogenic.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
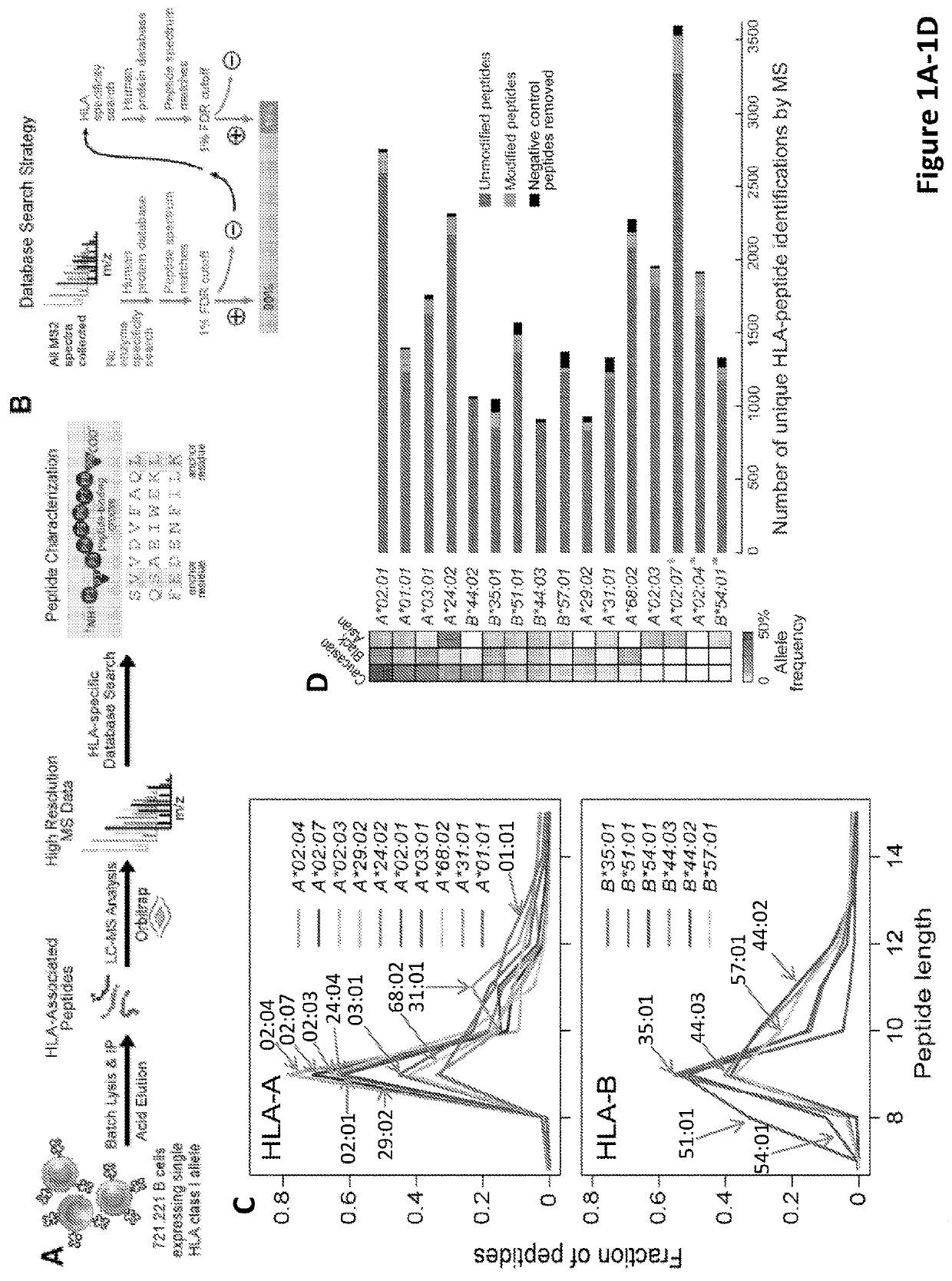
Improved HLA epitope prediction
PendingUS20190346442A1Microbiological testing/measurementMass spectrometric analysisT cellHuman health
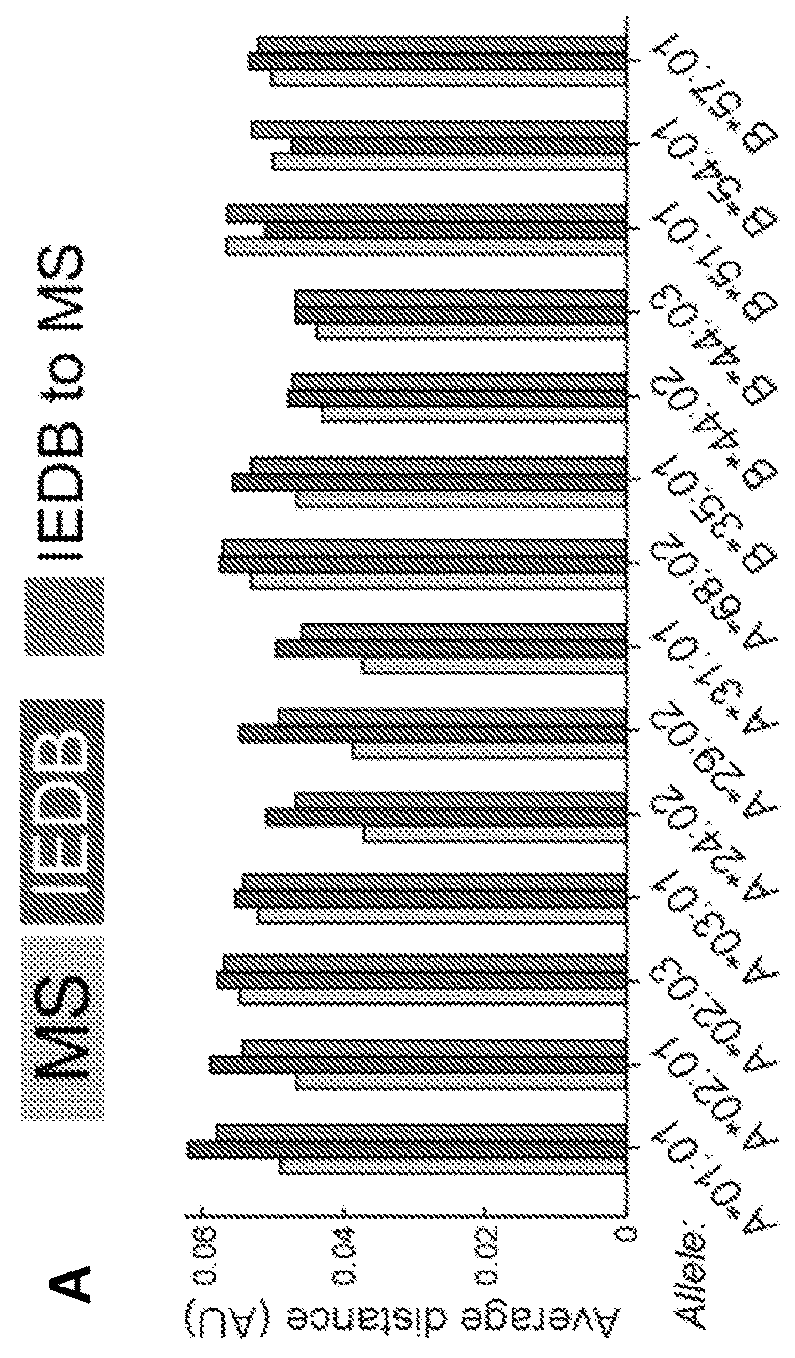
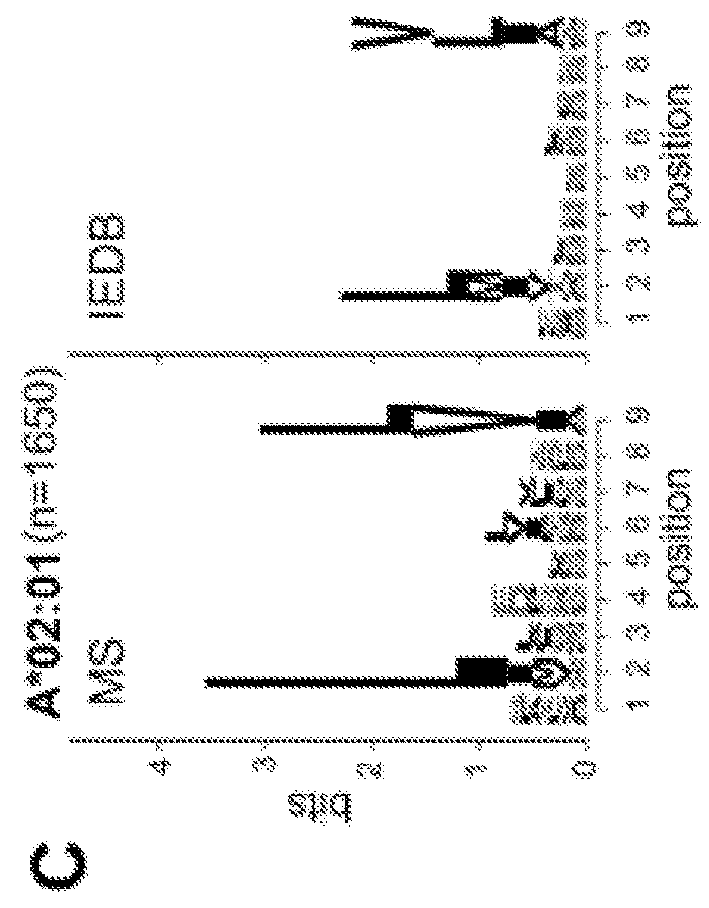
Adaptive immune responses rely on the ability of cytotoxic T cells to identify and eliminate cells displaying disease-specific antigens on human leukocyte antigen (HLA) class I molecules. Investigations into antigen processing and display have immense implications in human health, disease and therapy. To extend understanding of the rules governing antigen processing and presentation, immunopurified peptides from B cells, each expressing a single HLA class I allele, were profiled using accurate mass, high-resolution liquid chromatography-mass spectrometry (LC-MS / MS). A resource dataset containing thousands of peptides bound to 28 distinct class I HLA-A, -B, and -C alleles was generated by implementing a novel allele-specific database search strategy. Applicants discovered new binding motifs, established the role of gene expression in peptide presentation and improved prediction of HLA-peptide binding by using these data to train machine-learning models. These streamlined experimental and analytic workflows enable direct identification and analysis of endogenously processed and presented antigens.
Owner:THE BROAD INST INC +4
Chimeric human leukocyte antigen and epitope-bearing molecules having immunosuppressant activity
InactiveUS20040234531A1Hybrid immunoglobulinsAntibody mimetics/scaffoldsAutoimmune responsesWhite blood cell
The present invention relates to chimeric molecules that comprise human major histocompatibility complex elements, linked to epitopes of interest, that have been joined together to form dimers or higher multimers. Such chimeric molecules may be used to treat or prevent diseases associated with autoimmunity, particularly diabetes. It is based, at least in part, on the discovery that a chimeric molecule comprising an IILA-DR element linked to an epitope of GAD65, an antigen associated with autoimmune diabetes, stimulated the secretion of the inhibitory cytokine IL-10 from CD4 T cells of Type I diabetic patients.
Owner:MT SINAI SCHOOL OF MEDICINE
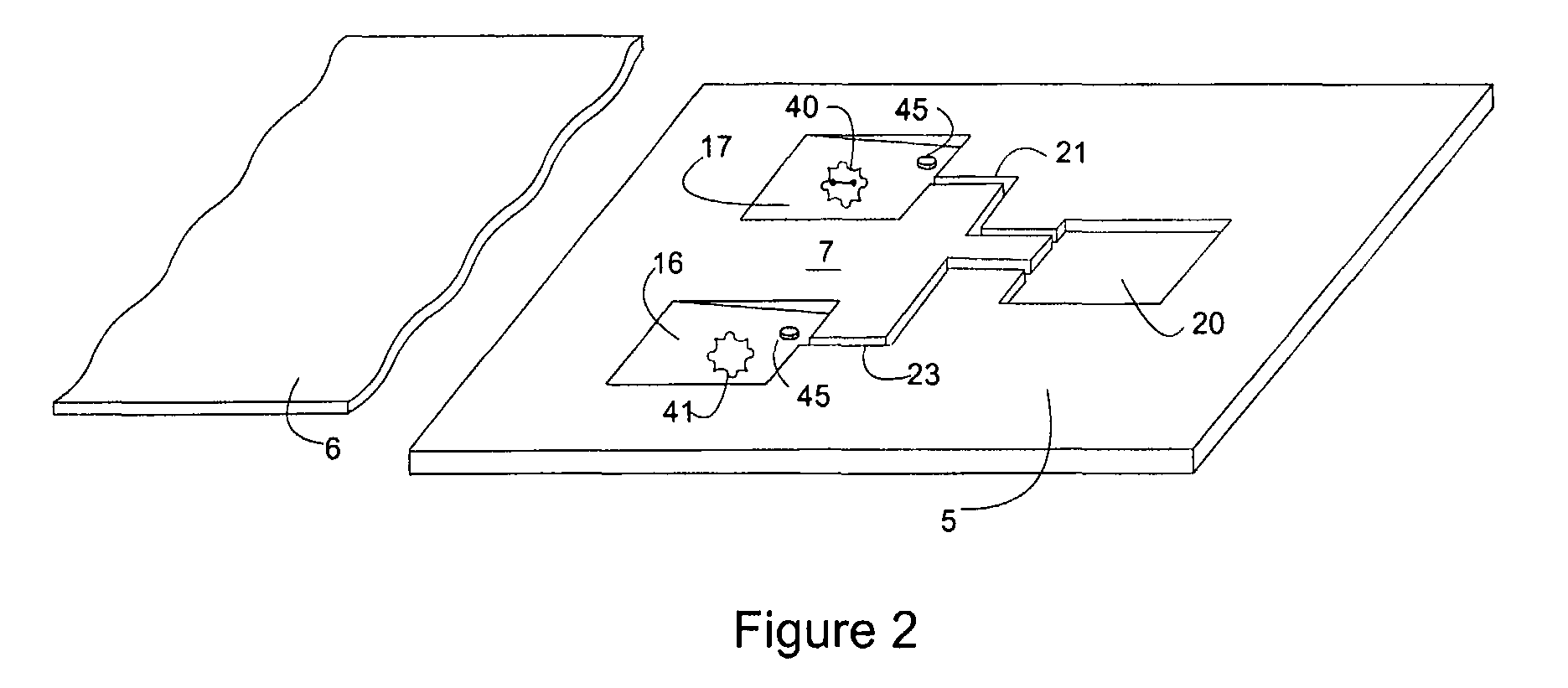
Microfluidic device and leucocyte antigen mediated microfluidic assay
ActiveUS20090011451A1Easy to doMicrobiological testing/measurementBiological material analysisCellular antigensWhite blood cell
Owner:ADVANCED ANIMAL DIAGNOSTICS
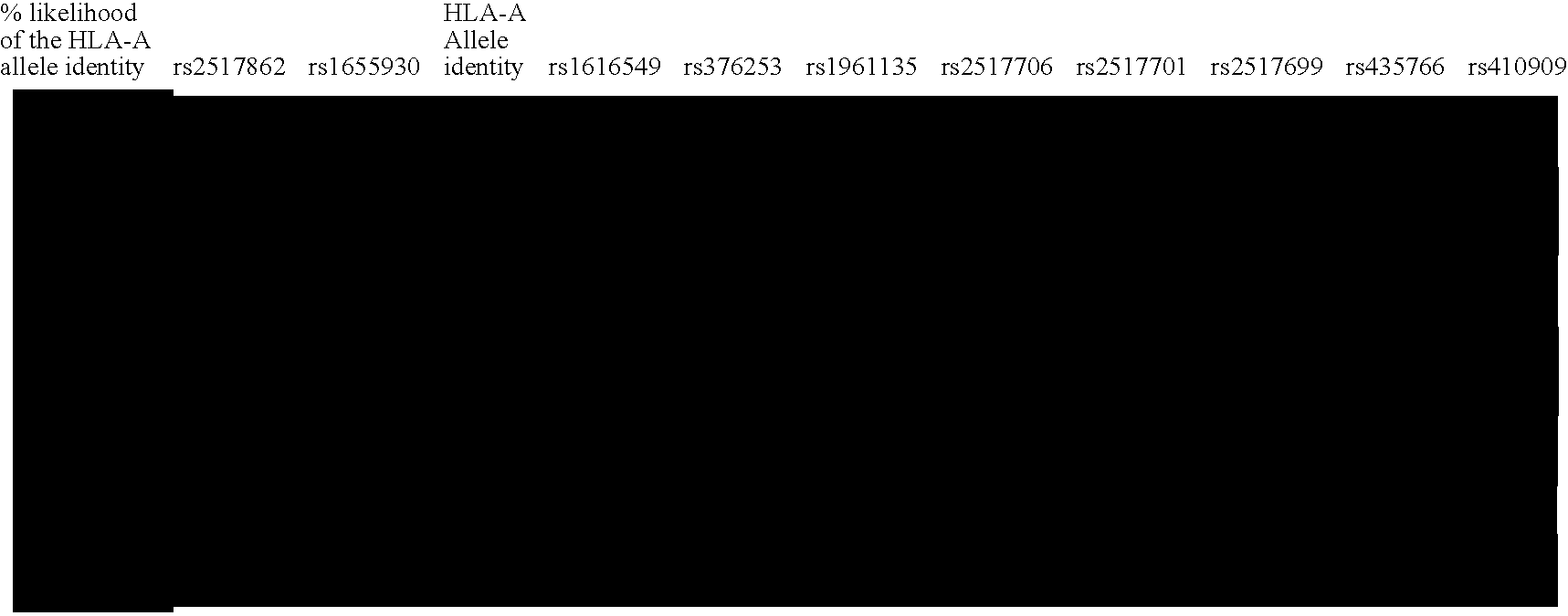
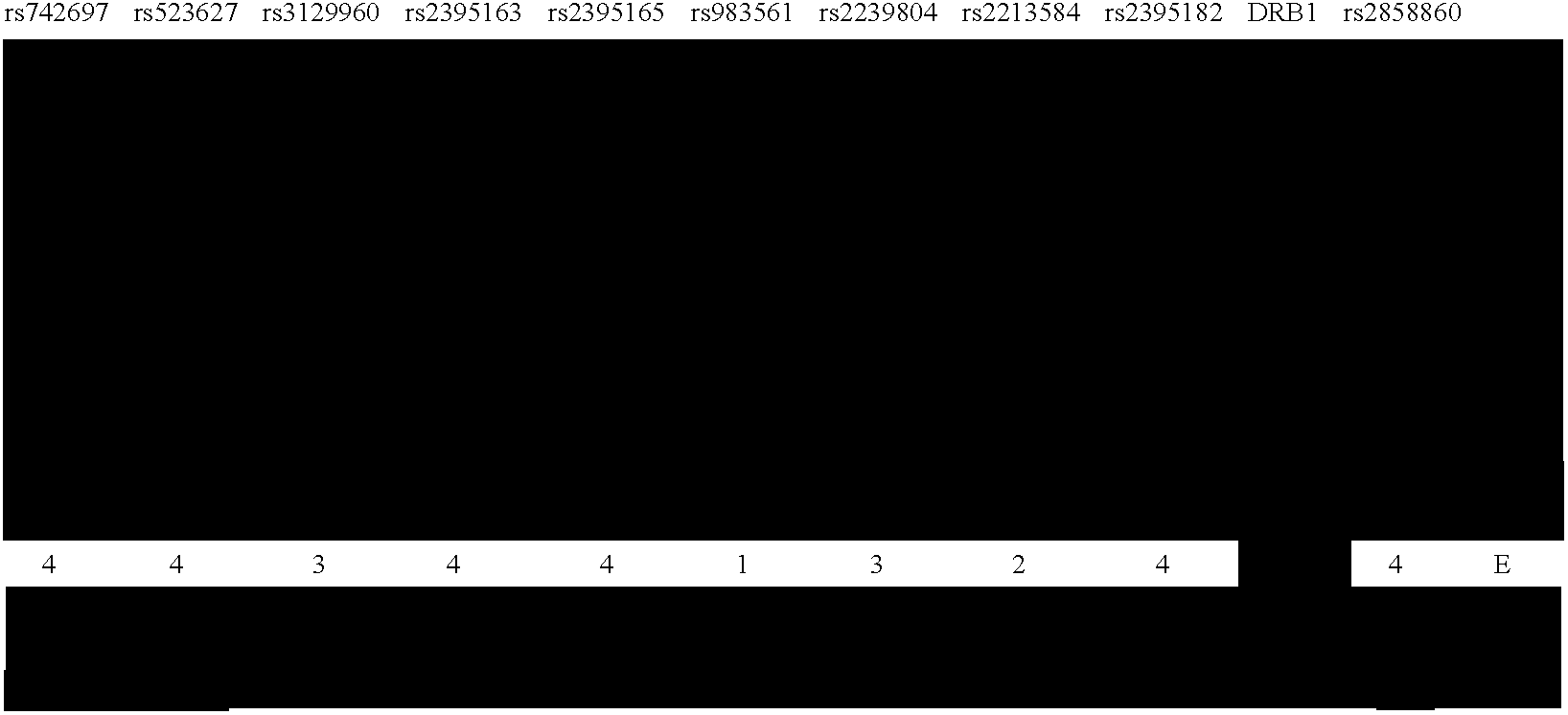
Methods of Human Leukocyte Antigen typing by neighboring single nucleotide polymorphism haplotypes
InactiveUS20050266410A1Quick and inexpensive genotypingAvoid pitfallMicrobiological testing/measurementBiological testingGeneticsGenotyping
The disclosure relates to novel approaches to mapping the MHC region and provides novel methods of genotyping the HLA loci. A haplotype map of the region and methods of using the map are also disclosed.
Owner:WHITEHEAD INST FOR BIOMEDICAL RES
Tumor-associated Peptides Binding Promiscuously to Human Leukocyte Antigen (HLA) Class II Molecules
ActiveUS20080206218A1Organic active ingredientsPeptide/protein ingredientsHla class iiWhite blood cell
The present invention relates to immunotherapeutic methods, and molecules and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumour-associated T-helper cell peptide epitopes, alone or in combination with other tumour-associated peptides, that serve as active pharmaceutical ingredients of vaccine compositions which stimulate anti-tumour immune responses. In particular, the present invention relates to 49 novel peptide sequences derived from HLA class II molecules of human tumour cell lines which can be used in vaccine compositions for eliciting anti-tumour immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Compositions and methods for detecting sla reactivity
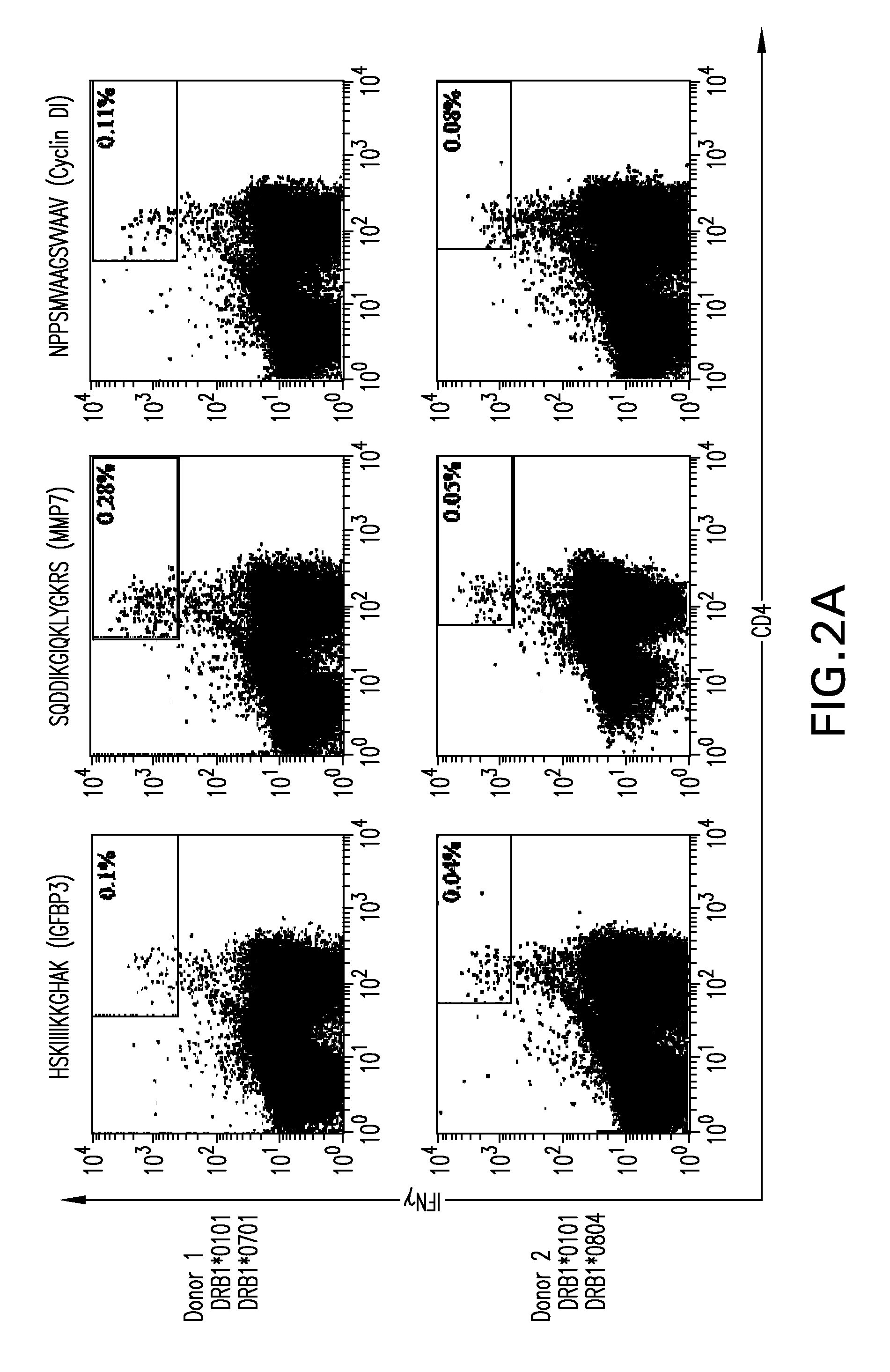
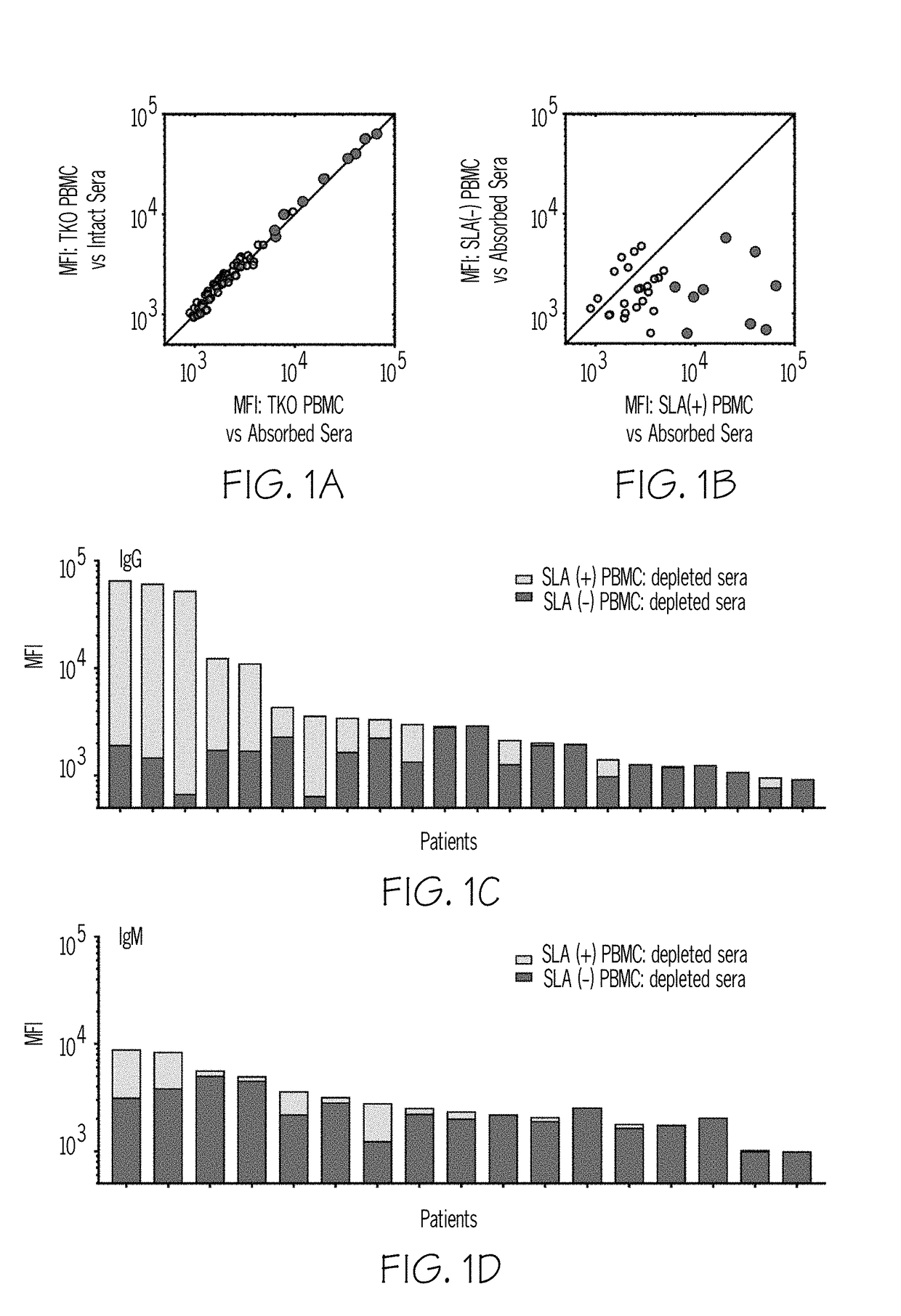
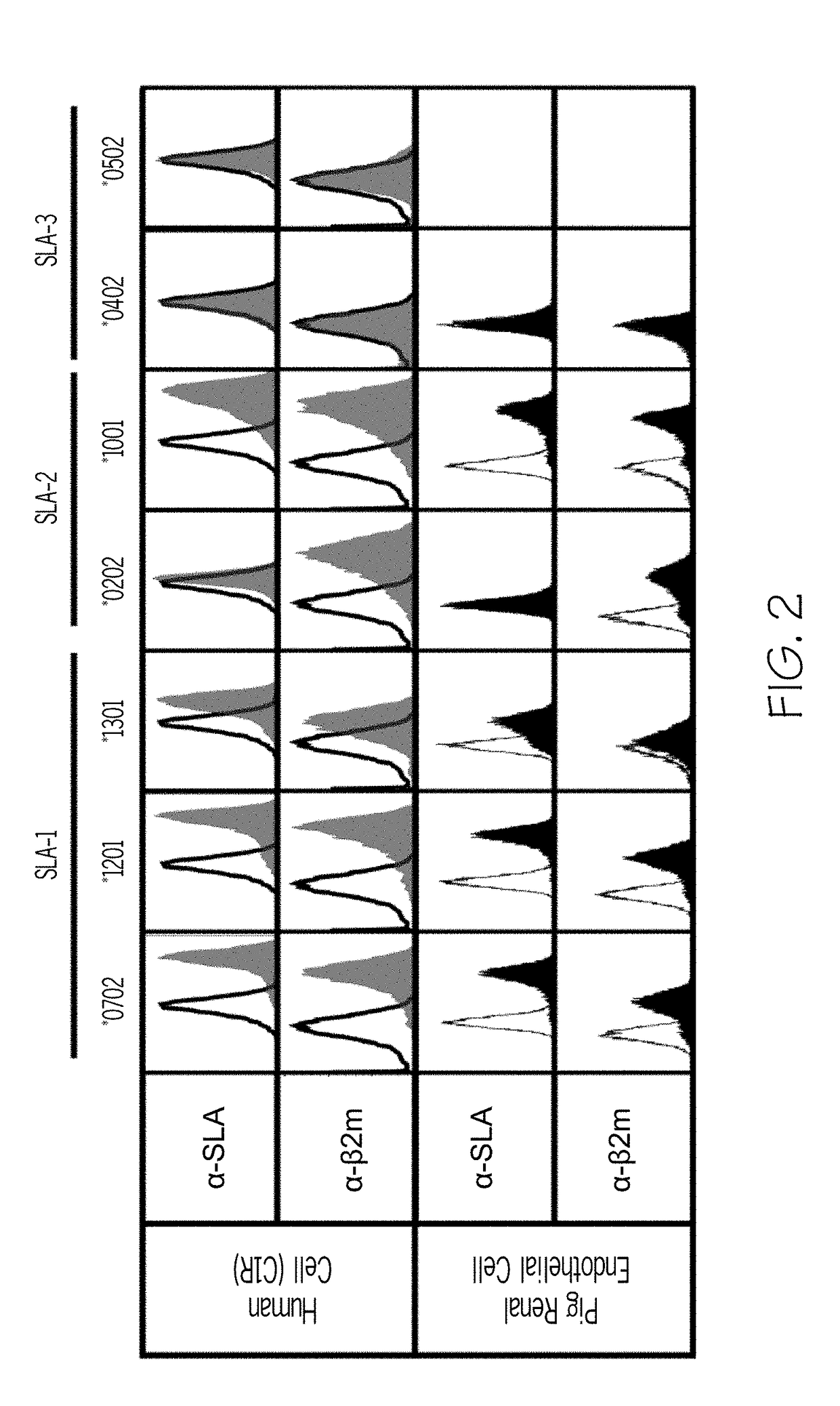
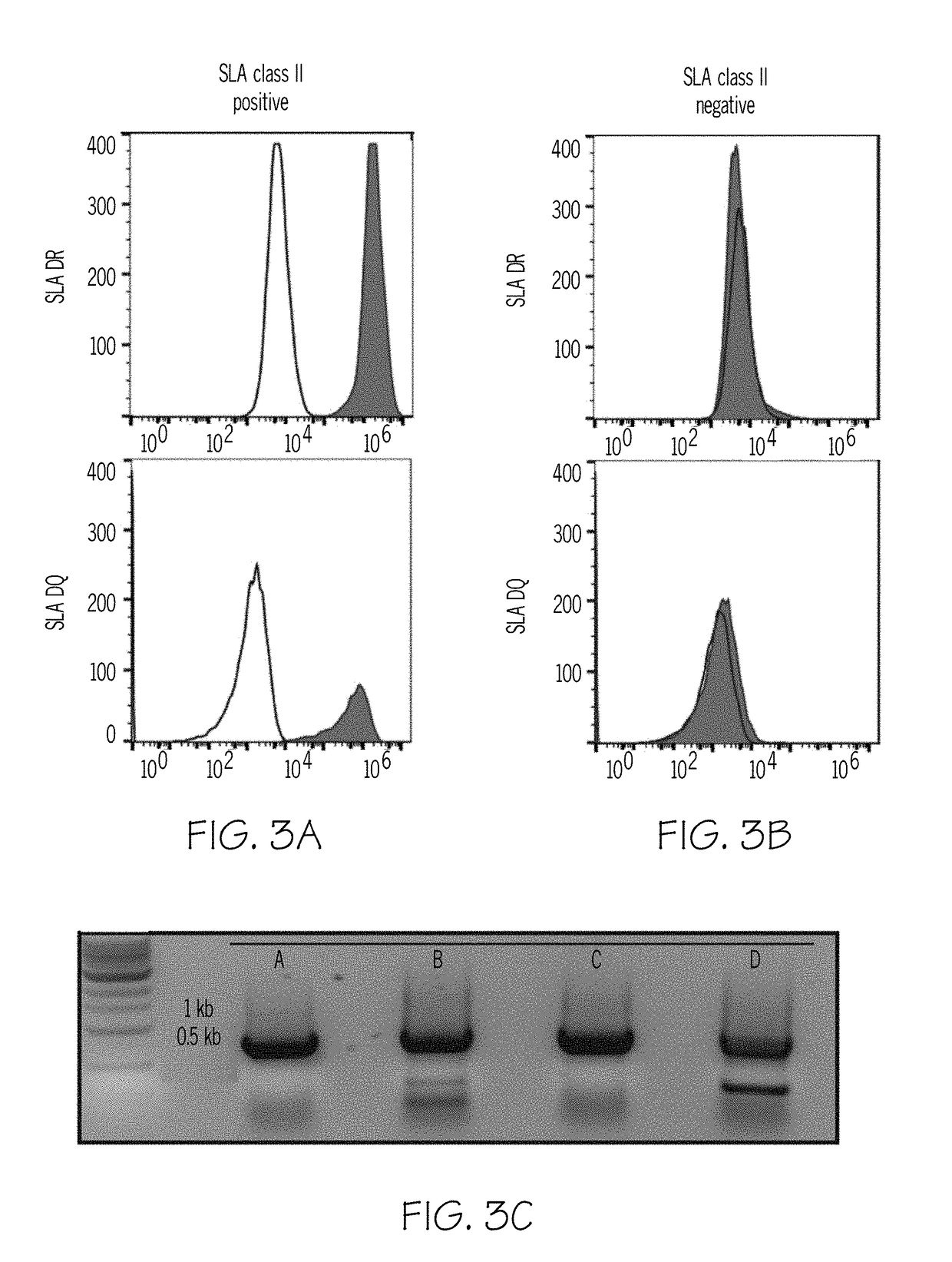
InactiveUS20190004063A1Reducing antibody bindingReduce the amount requiredTransferasesDepsipeptidesSerum igeClinical trial
In human-human transplants, the organ recipient's serum is tested against a broad array of HLA (human leukocyte antigens) alleles. The current clinical assay almost performs a virtual crossmatch which allows elimination of incompatible donor organs in advance, but the current assay presents fragments of HLA polypeptides that are not normally visible to antibodies. As a result, the assay yields false positives. Further the current clinical assay is optimized for human to human transplant, not swine to human transplant. Some HLA antibodies also bind swine leukocyte antigens (SLA). Rather than using beads to present antigens, the compositions and methods provide cellular presentation of potential antigens. The application provides a modified human C1R cell line with significantly reduced antigenicity to human sera. Multiple cell lines, each expressing a different SLA, were created. The modified C1R line may express HLAs or other potential antigens of interest. The application also provides modified HEK293T cells for antigen display.
Owner:INDIANA UNIV RES & TECH CORP
Human leukocyte antigen gene detection kit and application thereof
PendingCN106282328AContribute to the establishment of a method for screening drug eruptions caused by metronidazoleTo establish a method for screening metronidazole-induced drug eruptionMicrobiological testing/measurementMetaboliteWhite blood cell
The invention belongs to the fields of biomedicine and reagent detection, and relates to a human leukocyte antigen gene detection kit and application thereof in screening metronidazole-induced adverse drug reactions of skin. The kit contains a reagent for detecting human leukocyte antigen gene HLA-B*39:01 nucleic acid or protein, and amplification primers or a labeled probe of the human leukocyte antigen gene or a specific antibody containing the human leukocyte antigen. The HLA-B*39:01 gene can be used as a labeled gene for predicting metronidazole-induced epispasis. The detection kit can be used for further preparing or evaluating a metronidazole-induced epispasis screening kit, thereby providing valuable reference data for instructing clinical medication, and reducing the possibility of metronidazole-induced epispasis; or the detection kit can also be used for preparing or evaluating therapeutic drugs and especially targeted drugs for metronidazole-induced epispasis, thereby preventing the interactions between the drugs and metronidazole or metabolites thereof in disease development.
Owner:FUDAN UNIV +1
Hla-restricted epitopes encoded by somatically mutated genes
InactiveUS20180086832A1Raise the ratioPolypeptide with localisation/targeting motifAntibody mimetics/scaffoldsWild typeCancer research
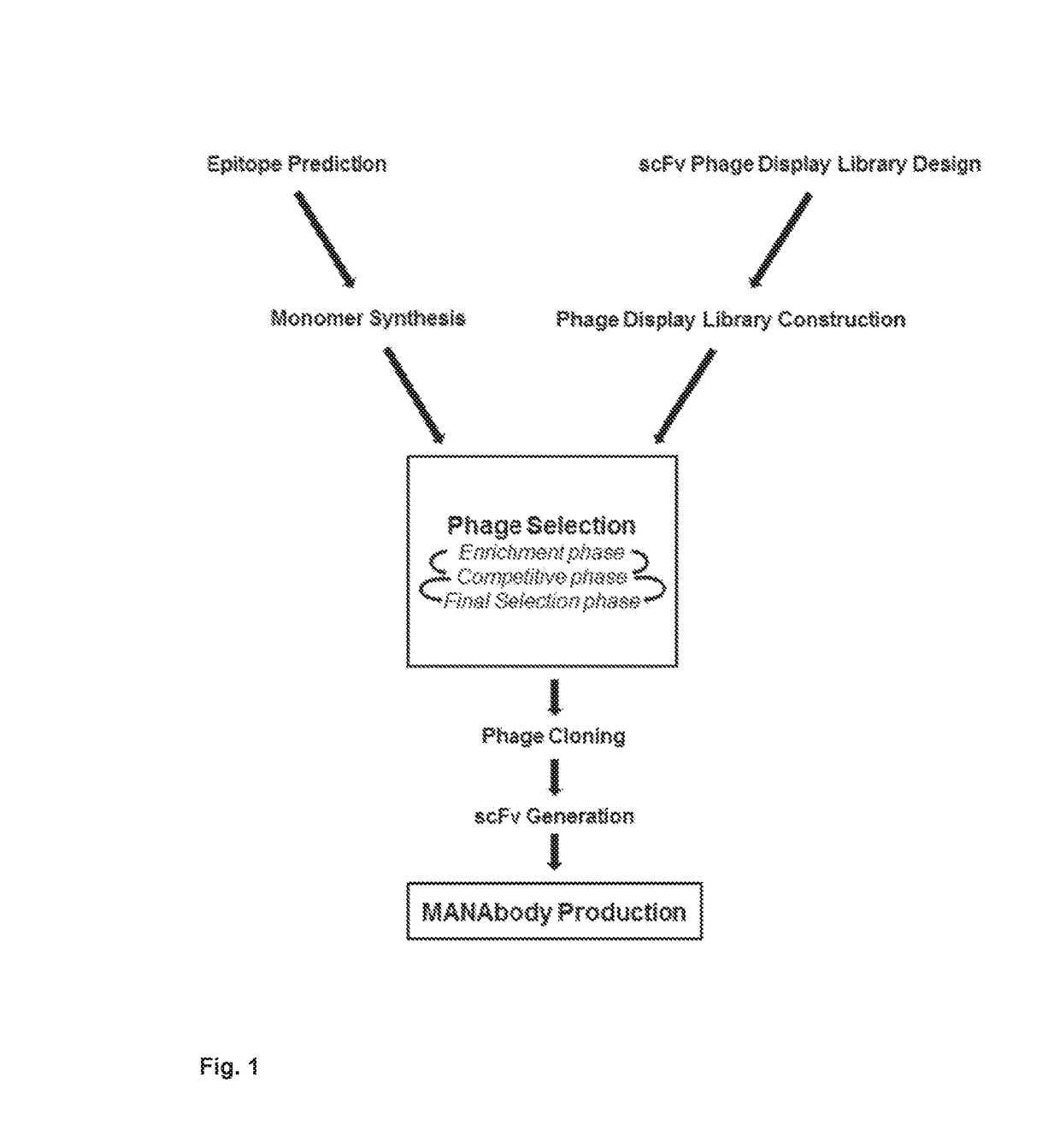
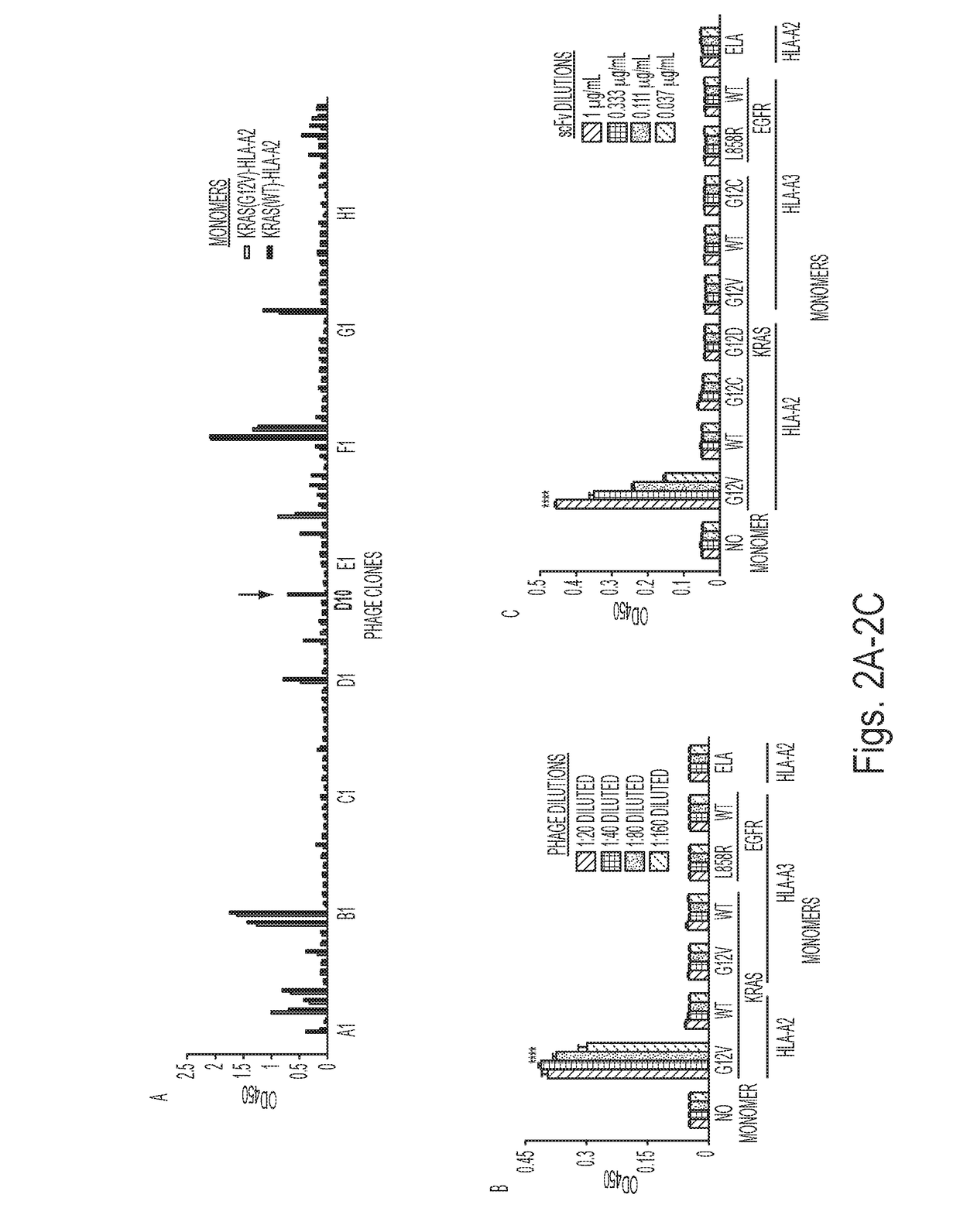
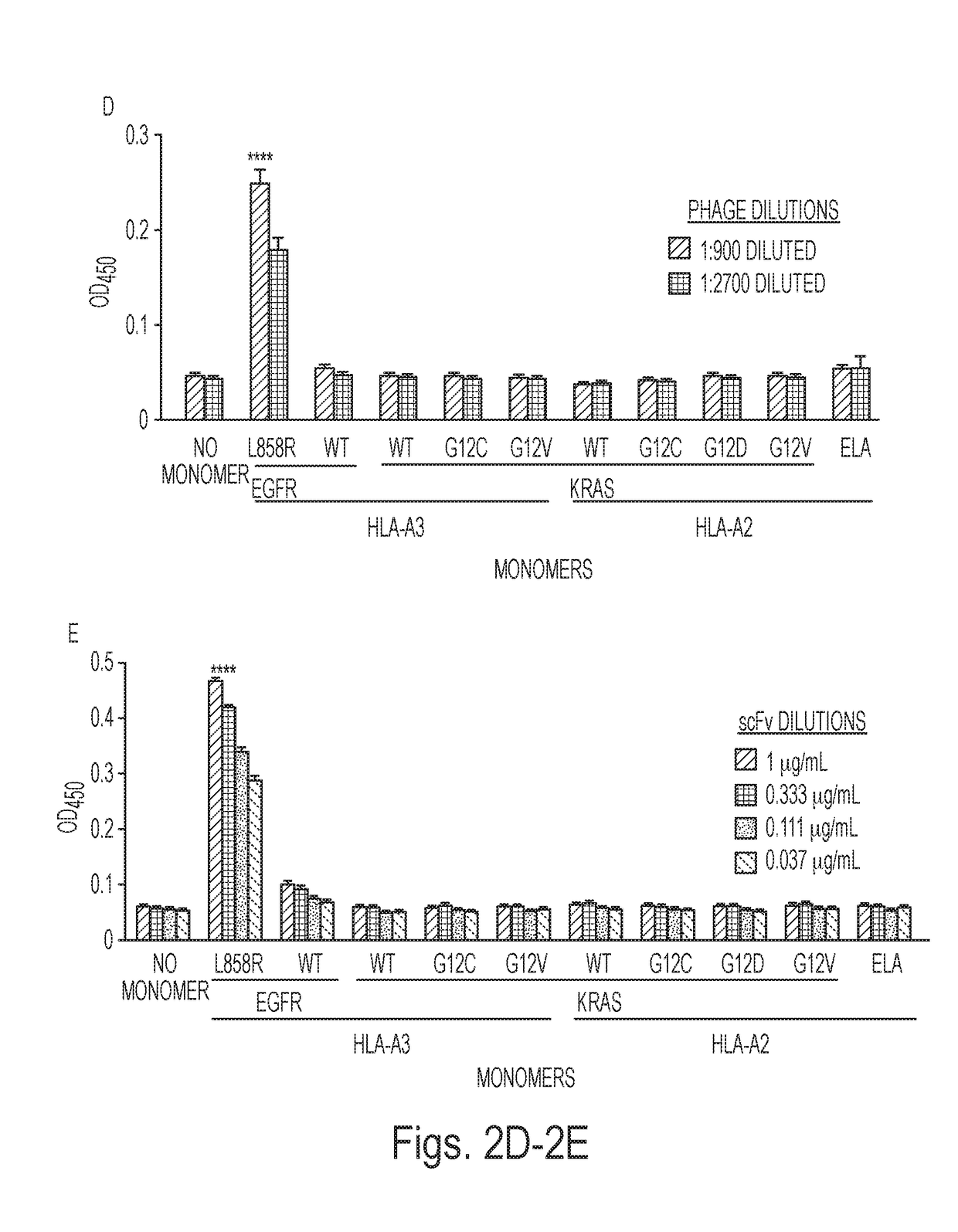
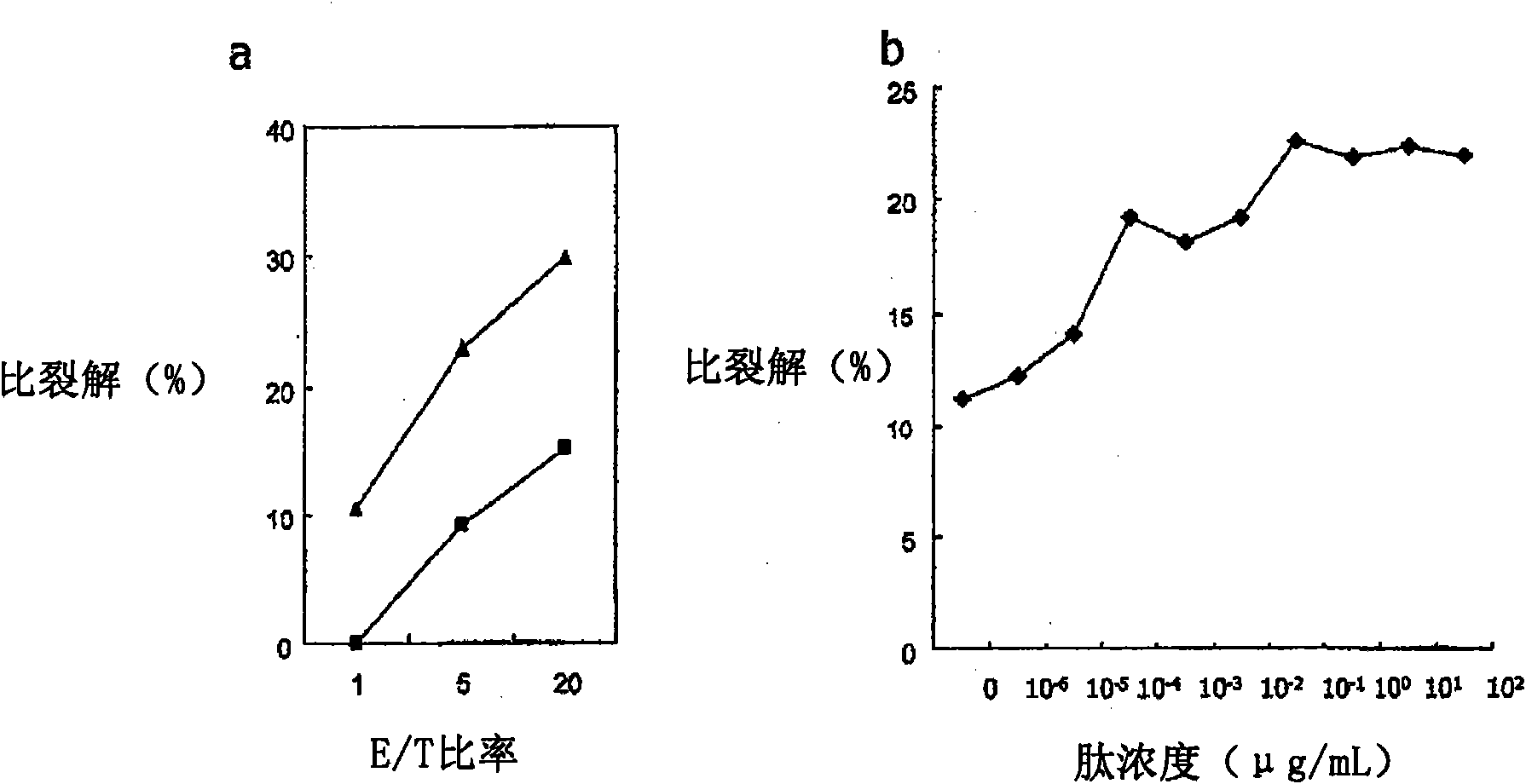
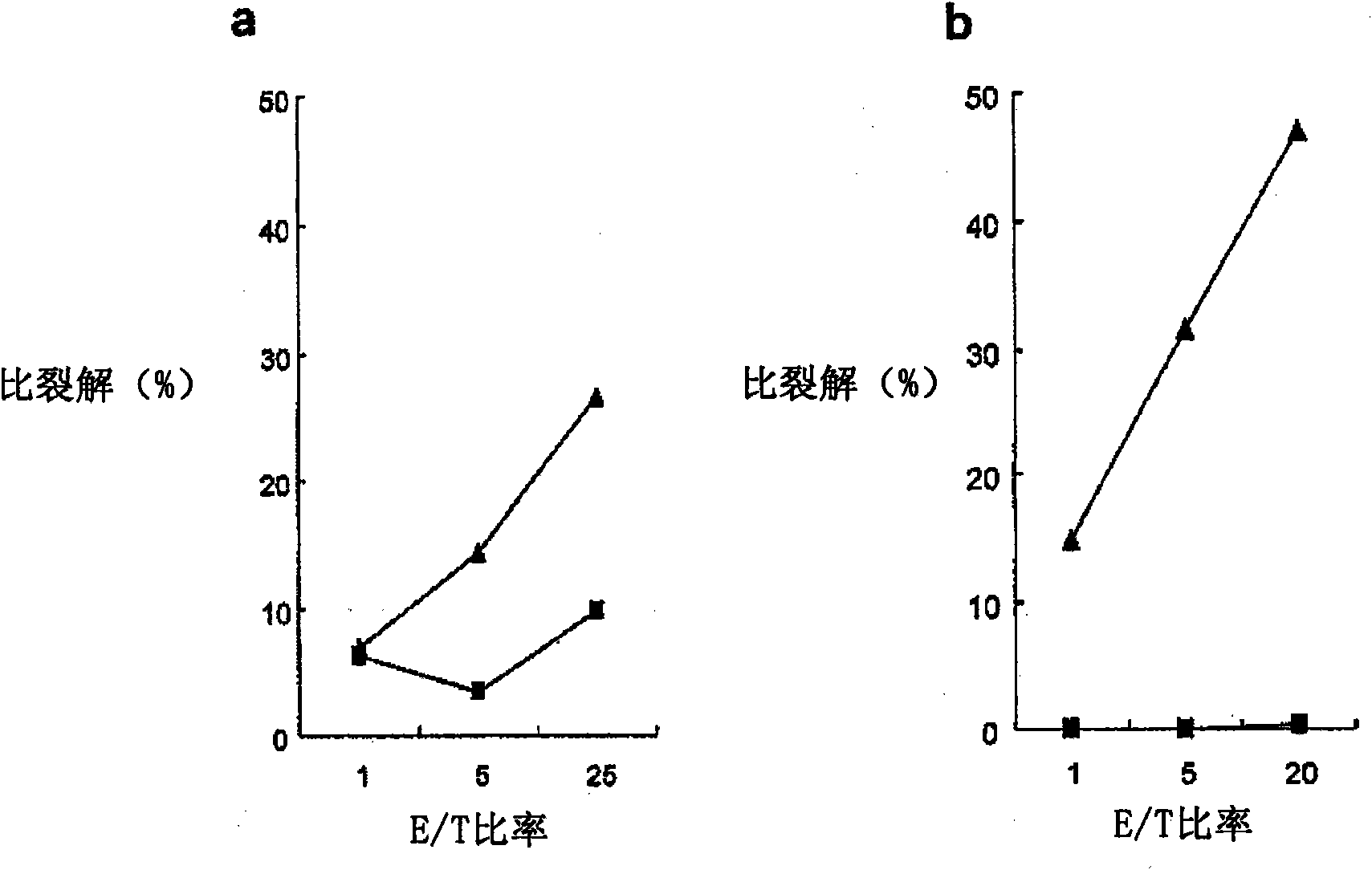
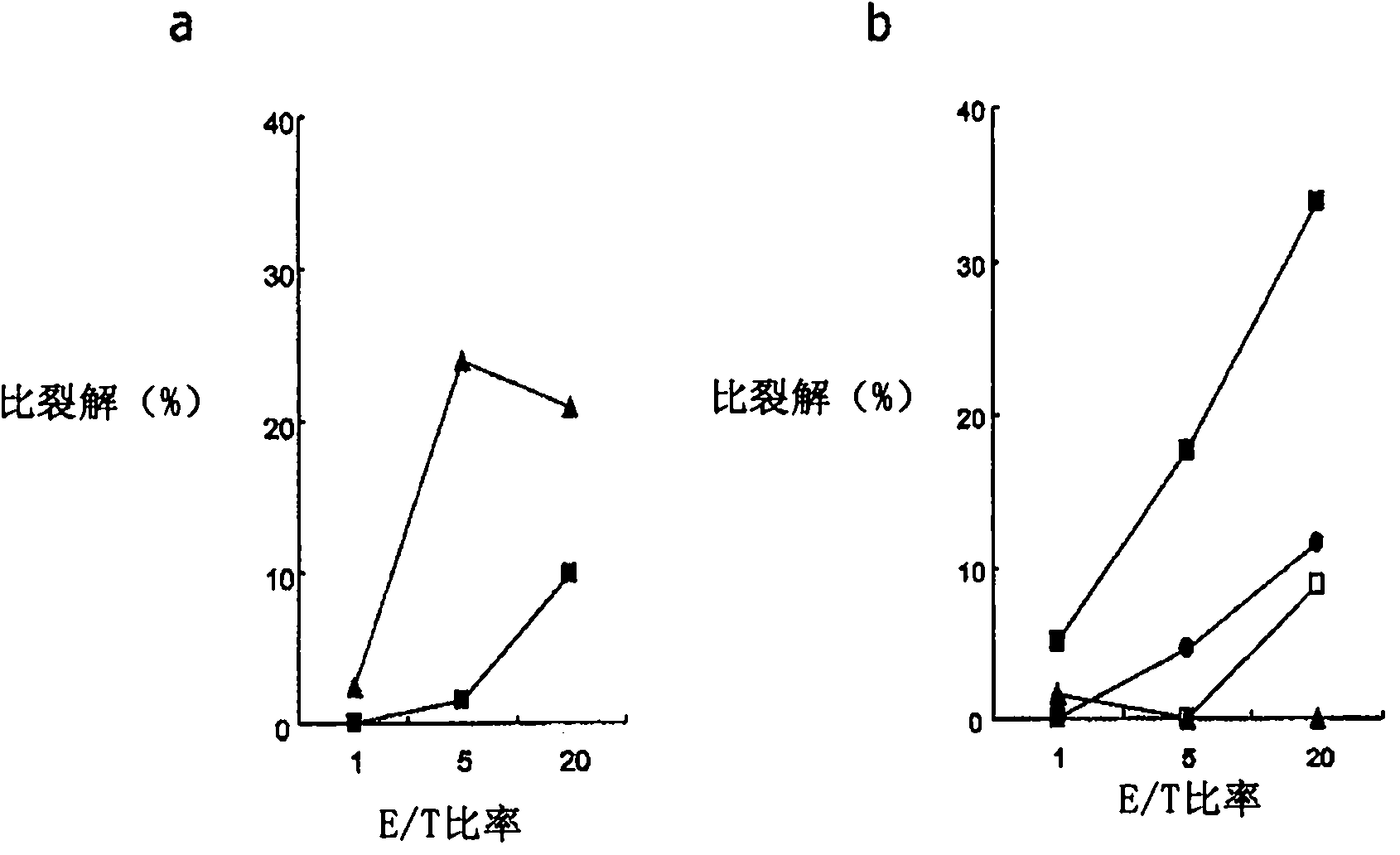
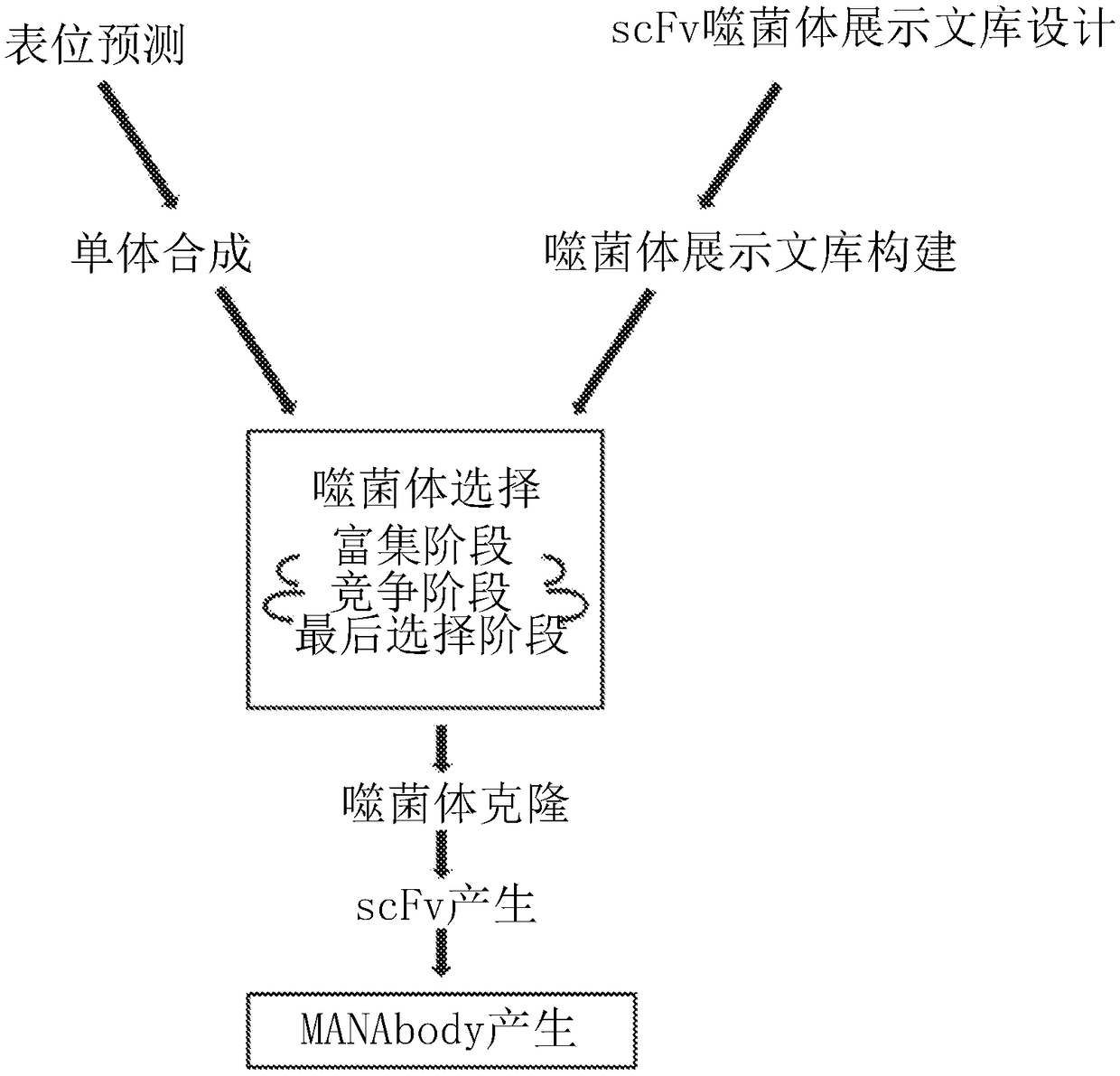
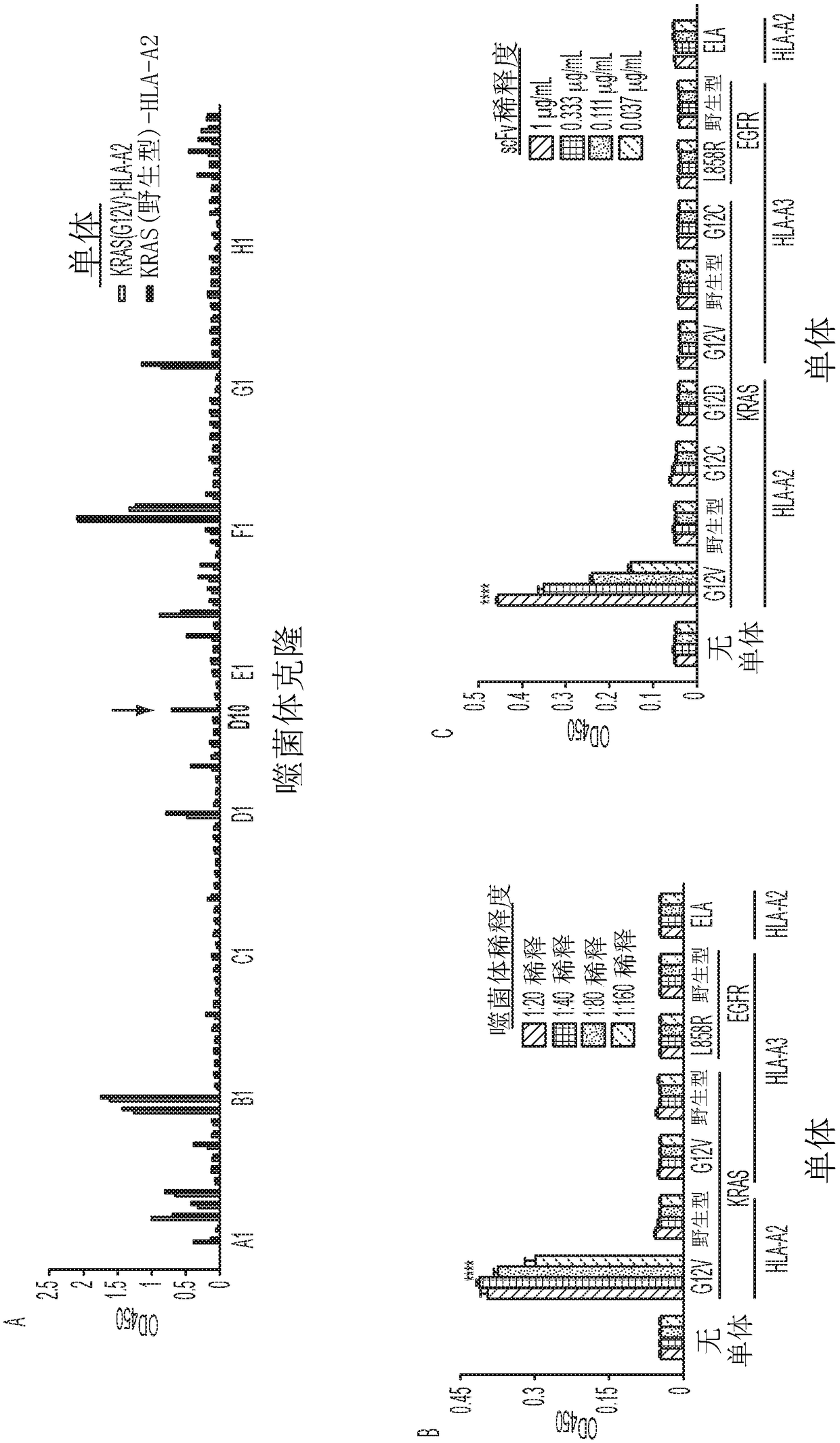
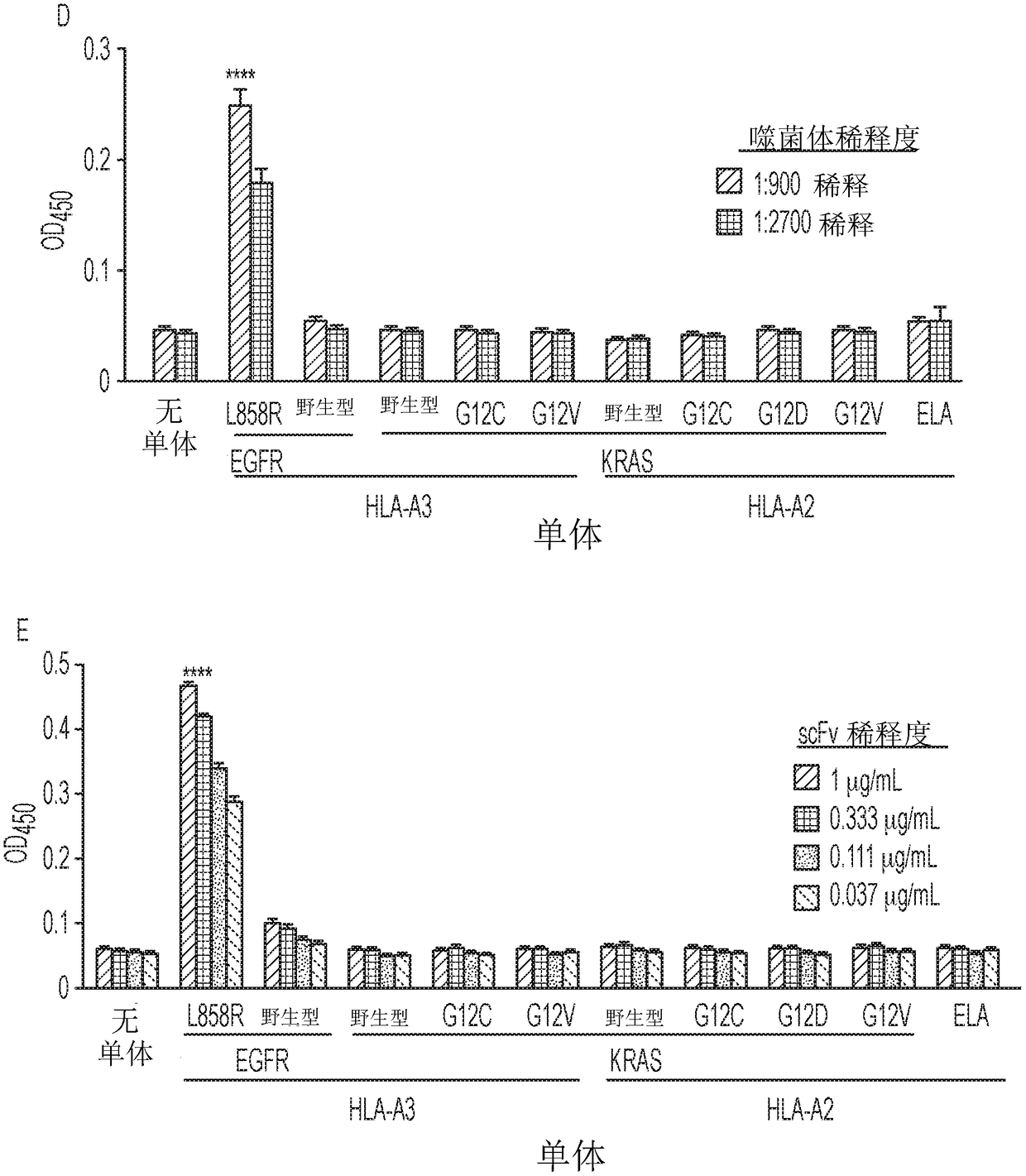
Mutant epitopes encoded by cancer genes are virtually always located in the interior of cells, making them invisible to conventional antibodies. We generated single chain variable fragments (scFvs) specific for mutant peptides presented on the cell surface by human leukocyte antigen (HLA) molecules. These scFvs can be converted to full-length antibodies, termed MANAbodies, targeting “Mutation Associated Neo-Antigens” bound to HLA. A phage display library representing a highly diverse array of single-chain variable fragment sequences was first designed and constructed. A competitive selection protocol was then used to identify clones specific for peptides bound to pre-defined HLA types. In this way, we obtained scFvs, including one specific for a peptide encoded by a common KRAS mutant and another by a common EGFR mutant. Molecules targeting MANA can be developed that specifically react with mutant peptide-HLA complexes even when these peptides differ by only one amino acid from the normal, wild-type form.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Kit and method for detecting human leucocyte antigen HLA-B*1502 genetype
InactiveCN103114130AEasy to operateAccurate operationMicrobiological testing/measurementHuman leucocyte antigenForward primer
The invention discloses a kit and a method for detecting human leucocyte antigen HLA-B*1502 genetype. The kit disclosed by the invention is characterized by comprising the following primer sequences: a forward primer FP: 5'-CGACGCCGCGAGTCCCAGG-3'; and a reverse primer RP: 5'-CGTCGTAGGCGGACTGGTCATA-3'. The kit further comprises the following probe sequence: a probe PR: 5'-Cy5-AACACACAGATCTCCAAGACCAACACAC-p-3'. The kit disclosed by the invention has good specificity and sensitivity for carrying out PCR (Polymerase Chain Reaction) detection of the human leucocyte antigen HLA-B*1502. The PCR detection method disclosed by the invention has the advantages of being simple for operation (closed pipe), rapid, accurate and the like and is applied to clinical molecular diagnosis.
Owner:瀚吉康生物科技(北京)有限公司
Cancer vaccine composition
ActiveCN101888852ATumor rejection antigen precursorsPeptide/protein ingredientsGene productFhit gene
Disclosed is a cancer vaccine composition for a human leukocyte antigen (HLA)-A*0206-positive subject, which is characterized by comprising a protein which is a gene product of a tumor suppressor gene WT1 or a partial peptide of the protein.
Owner:INT INST OF CANCER IMMUNOLOGY INC
Hla-restricted epitopes encoded by somatically mutated genes
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Universal donor stem cells and related methods
ActiveUS10968426B2Reduce and eliminate and activityReduce and eliminate expressionGenetically modified cellsStable introduction of DNAWhite blood cellImmunogenicity
Disclosed herein are universal donor stem cells and related methods of their use and production. The universal donor stem cells disclosed herein are useful for overcoming the immune rejection in cell-based transplantation therapies. In certain embodiments, the universal donor stem cells disclosed herein do not express one or more MHC-I and MHC-II human leukocyte antigens. Similarly, in certain embodiments, the universal donor stem cells disclosed herein do not express one or more human leukocyte antigens (e.g., HLA-A, HLA-B and / or HLA-C) corresponding to MHC-I and MHC-II human leukocyte antigens, thereby rendering such cells hypoimmunogenic.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Novel formulations of tumour-associated peptides binding to human leukocyte antigen (HLA) class i or class ii molecules for vaccines
ActiveUS20120107337A1Envelopes/bags making machineryPeptide/protein ingredientsKidney cancerWhite blood cell
The present invention relates to novel formulations of tumour-associated peptides binding to human leukocyte antigen (HLA) class I or II molecules as vaccines for the use in immunotherapeutic methods. In particular, the present invention relates to formulations for the immunotherapy of cancer, in particular renal and brain cancer, in particular glioma, especially glioblastoma cancer. The present invention furthermore relates to vaccine compositions for eliciting anti-tumour immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
SPA-antibody tripolymer, cell treating kit containing tripolymer, preparation method and application thereof
InactiveCN101799473ASimple methodLow costBlood/immune system cellsBiological testingProgenitorRed blood cell
The invention relates to an SPA-antibody tripolymer, a cell treating kit containing the tripolymer, a preparation method and application thereof. The tripolymer comprises an anti erythrocyte antibody, an SPA and an antibody of a certain anti leukocyte antigen or other antigens. The tripolymer is combined with an erythrocyte through the anti erythrocyte antibody per se, the other antibody is combined with a corresponding leukocyte antigen (or other antigens), and then leukocyte (other cells, factors) and the erythrocyte are deposited through an erythrocyte sedimentation process or other methods of density gradient centrifugation and the like, thereby achieving the purpose of eliminating ingredients of corresponding cells and the like, connecting the erythrocyte with a corresponding antigen by the SPA, and being used as a mean for detecting a certain antigen. The invention has convenience and simpleness, and can be directly used for clinically separating and extracting stem cells / progenitor cells; and the collected and extracted stem cells / progenitor cells have no external markers. Meanwhile, the kit based on the tripolymer can be industrially produced so as to realize the popularization of the separation and purification technology of hematopoietic stem cells.
Owner:王信
Animal model with human immune system
InactiveUS20150282460A1Increase translatabilityCompounds screening/testingFermentationHuman cellImmunodeficiency
The present disclosure provides an immuno-deficient animal useful as an animal model for a human disease, wherein the animal comprises: (a) functional human immune cells; and (b) a human xenograft comprising a pathogenic human cell or tissue, wherein the human immune cells and the human xenograft meet at least one of the following criteria: i) the human xenograft expresses a threshold level of a therapeutic target for the human disease; ii) the human immune cells match with the human xenograft for at least one human leukocyte antigen (HLA) marker; and iii) the human xenograft cells expresses a desired level of the matched HLA marker. Also provides is a method of producing the animal model and use of the animal models.
Owner:CROWN BIOSCI INC TAICANG
Special primer and kit for detecting human leucocyte antigen-B27 (HLA-B27) gene
InactiveCN102337334AStrong specificityHigh sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceHuman leucocyte antigenWhite blood cell
The invention discloses a special primer for detecting a human leucocyte antigen-B27 (HLA-B27) gene. The special primer is characterized by comprising an upstream primer B27-1, a downstream primer B27-2 and a probe B27-probe which are used for detecting target genes, and an upstream primer Gapdh-1, a downstream primer Gapdh-2 and a probe Gapdh-probe which are used for detecting an internal control gene, namely glyceraldehyde-3-phosphate dehydrogenase (GAPDH), wherein the B27-1 is shown as GGGTCTCACACCCTCCAGAAT, the B27-2 is shown as CGGCGGTCCAGGAGCT, and the B27-probe is shown as FAM-TACCACCAGGACGCCTAC-TAMRA; and the Gapdh-1 is shown as CAGGTGGAGCGAGGCTAG, the Gapdh-2 is shown as CTACCCATGACTCAGCTTCTCC, and the Gapdh-probe is shown as HEX-ACCATGCCACAGCCACCACACCTC-BHQ1. The invention also provides a kit for detecting the HLA-B27 gene. A method for detecting the gene by using one pipe replaces the original method for detecting the gene by using two pipes, the amount of samples which can be detected by a machine at a time is increased, and the detection efficiency is improved to a great extent.
Owner:FUZHOU ADICON CLINICAL LAB INC
Detection kit for human leucocyte antigen genes
PendingCN104862379ABlocking interactionMicrobiological testing/measurementBiological testingMetaboliteHLA - Human leukocyte antigen gene
The invention belongs to the technical field of biological medicines and relates to a detection kit for human leucocyte antigen (HLA) genes--HLA-B*59: 01 genes, and the HLA-B*59: 01 genes can serve as marker genes for forecasting methazolamide caused SJS (Stevens-Johnson syndrome) and TEN (Toxic epidermal necrolysis). According to the detection kit, after DNA extracted from the peripheral blood of a patient, the PCR-SSO method is adopted to detect the HLA-B*59 :01 genes. The detection kit for HLA-B*59: 01 genes can be used for examining before application of methazolamide and screening targeted medicines for treating methazolamide caused SJS and TEN; and examination can guide clinical medication to reduce incidence of SJS and TEN caused by methazolamide, and the screening mainly acts on HLA-B*59: 01 molecules in a targeted manner, so that interaction between the HLA-B*59: 01 molecules and methazolamide or other metabolic products during attack.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV +1
Composition for Treating Baldness with Stem Cell Derived From Umbilical Cord Blood
InactiveUS20070298017A1Easy to produceSolve immune rejectionBiocideUnknown materialsTherapeutic TechniqueUmbilical Cord Blood Stem Cell
Provided is a therapeutic technique for treating baldness, using an umbilical cord blood-derived stem cell. Transplantation of a composition for treating baldness into a bald area of a patient can make great contributions to treatment for baldness, wherein the composition comprises stem cells isolated and cultured from umbilical cord blood in which 6 HLA (Human Leukocyte Antigen) are identical with a patient, or one or two HLA are not identical with the patient.
Owner:HAN HOON
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com