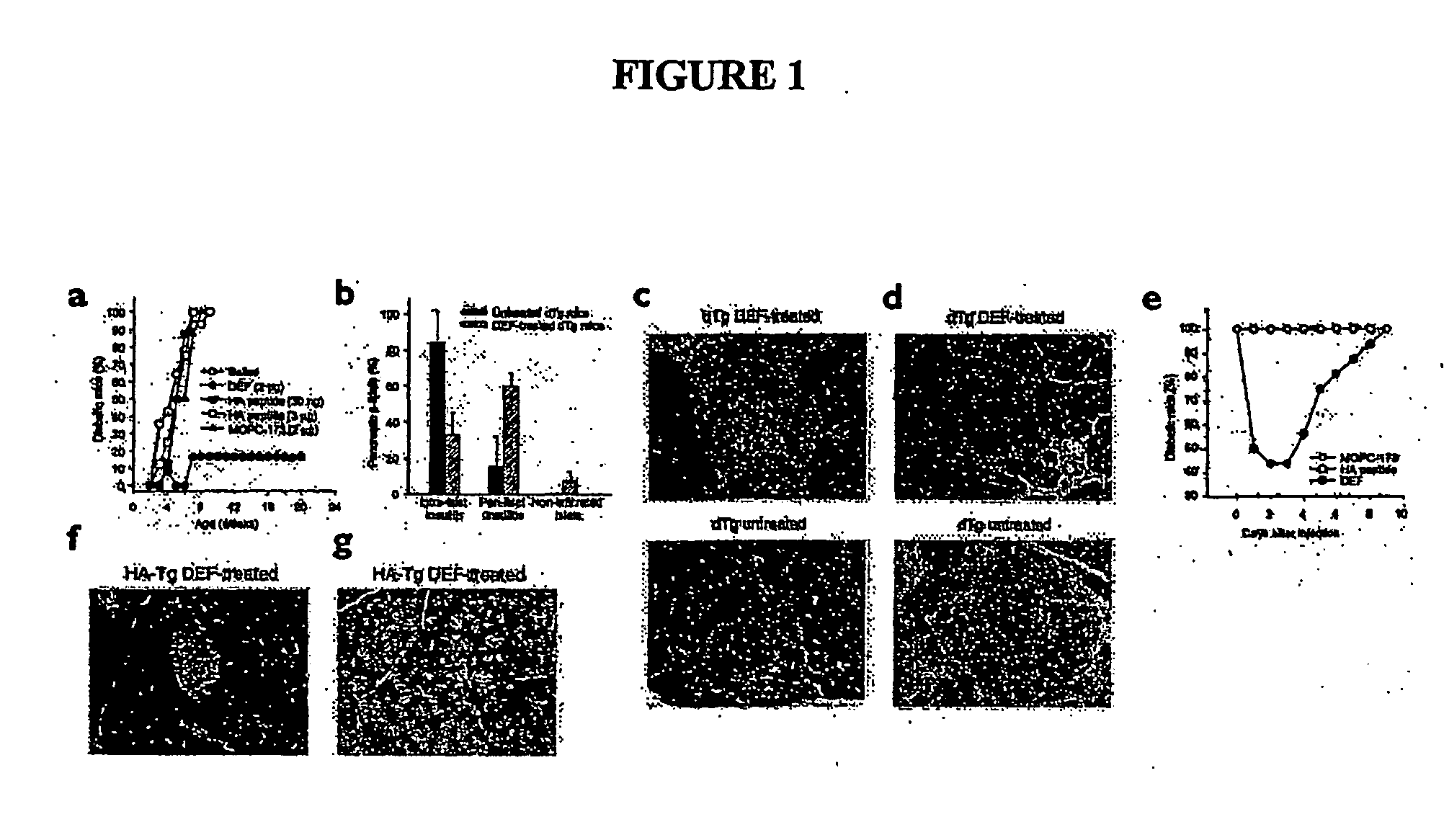
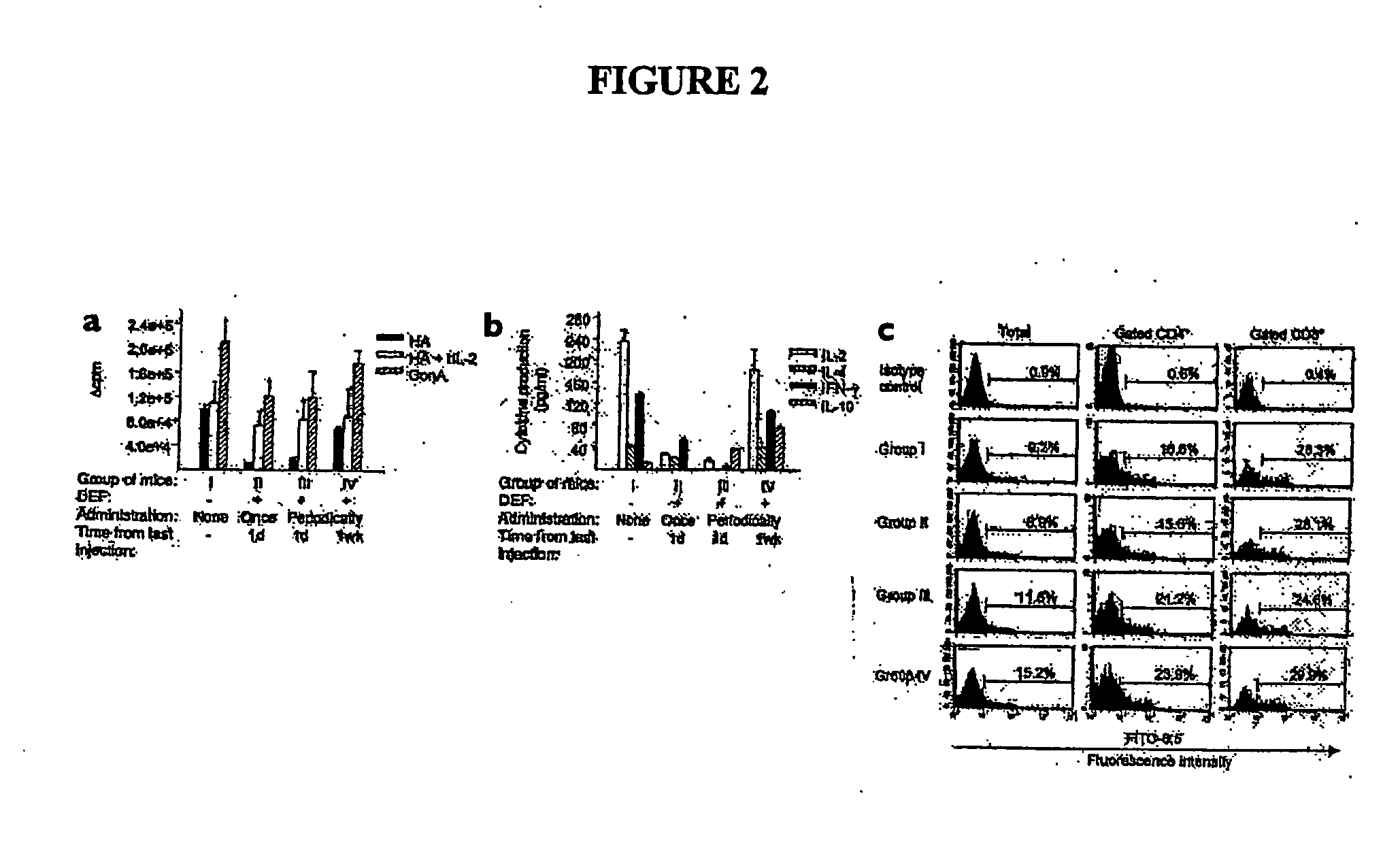
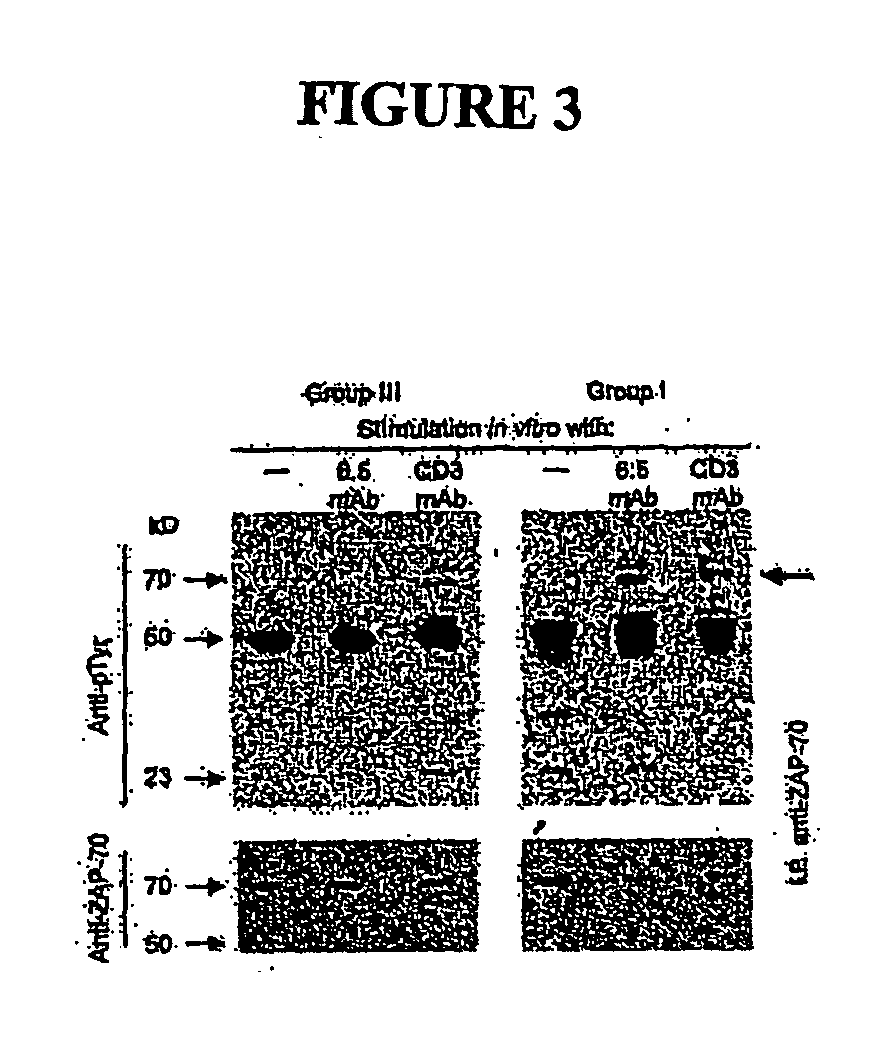
Chimeric human leukocyte antigen and epitope-bearing molecules having immunosuppressant activity
a human leukocyte antigen and immunosuppressant technology, applied in the field of chimeric molecules, can solve the problems of inability to absorb, store and utilize carbohydrates such as glucose, lack of protection by peptide-based therapies, and symptomatic weakness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0040] For clarity of description, and not by way of limitation, the detailed description of the invention is divided into the following subsections:
[0041] (i) general structure of HLA / epitope chimeric molecules;
[0042] (ii) specific HLA / epitope chimeric molecules;
[0043] (iii) complexes of HLA / epitope chimeric molecules and
[0044] (iv) clinical uses of HLA / epitope chimeric molecules.
5.1 General Structure of HLA / Epitope Chimeric Molecules
[0045] The basic structure of an HLA / epitope chimera of the invention comprises the following elements: (i) a class II HLA element; (ii) an epitope of interest; and (iii) a means of linking the HLA element and epitope together in an orientation whereby the epitope is presented to a TCR in the context of HLA. Each of these components is more thoroughly discussed in the sections that follow.
[0046] The chimeric molecule of the invention is referred to herein as a protein, however, such "chimeric proteins" as defined herein may comprise non-protein compone...
PUM
| Property | Measurement | Unit |
|---|---|---|
| half life | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com