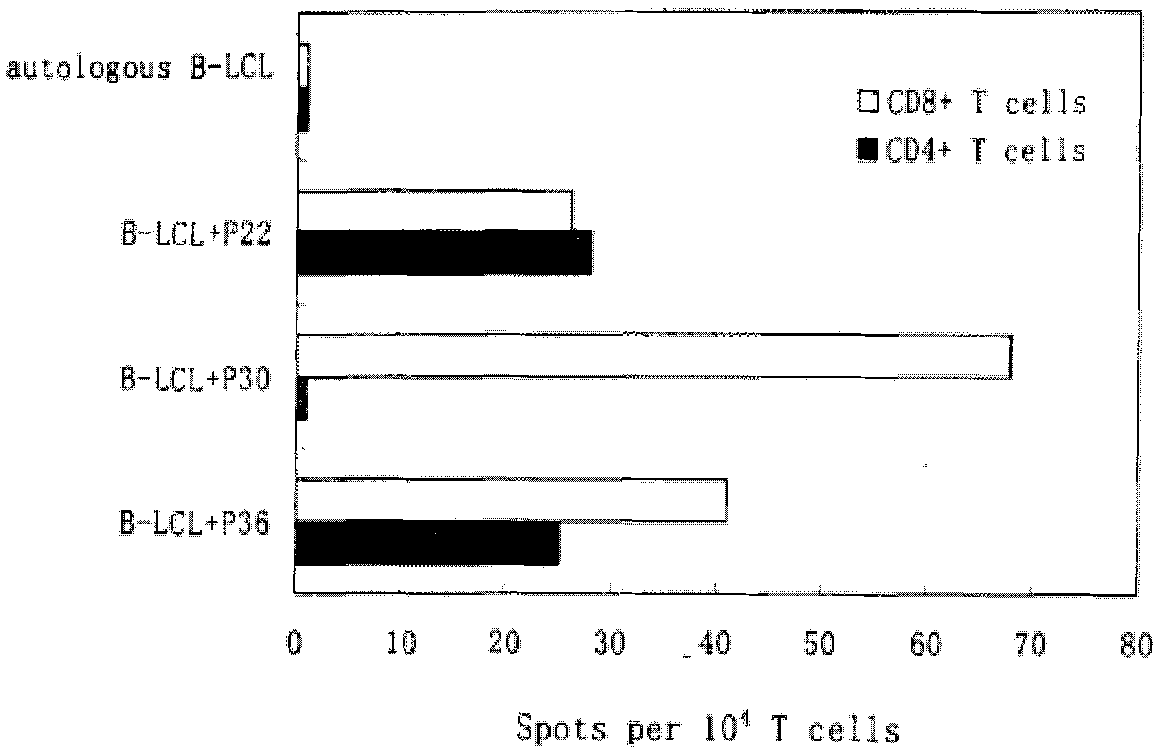Patents
Literature
32 results about "Human leucocyte antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gene panel used for detecting breast cancer gene mutation, detection method used for detecting breast cancer gene mutation and application of gene panel
InactiveCN111647648AComprehensive immunotherapyComprehensive treatmentMicrobiological testing/measurementSequence analysisHuman DNA sequencingGenome human
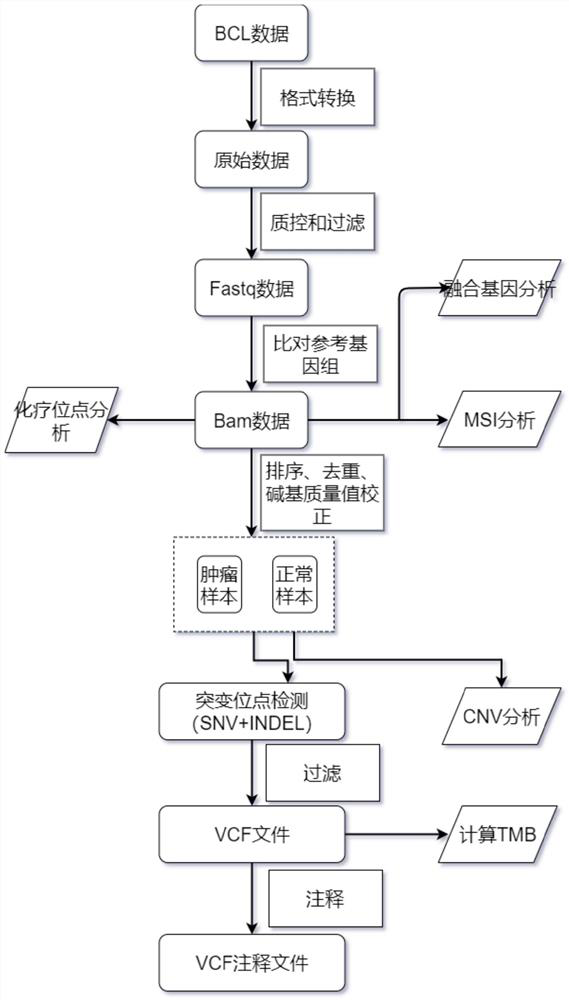
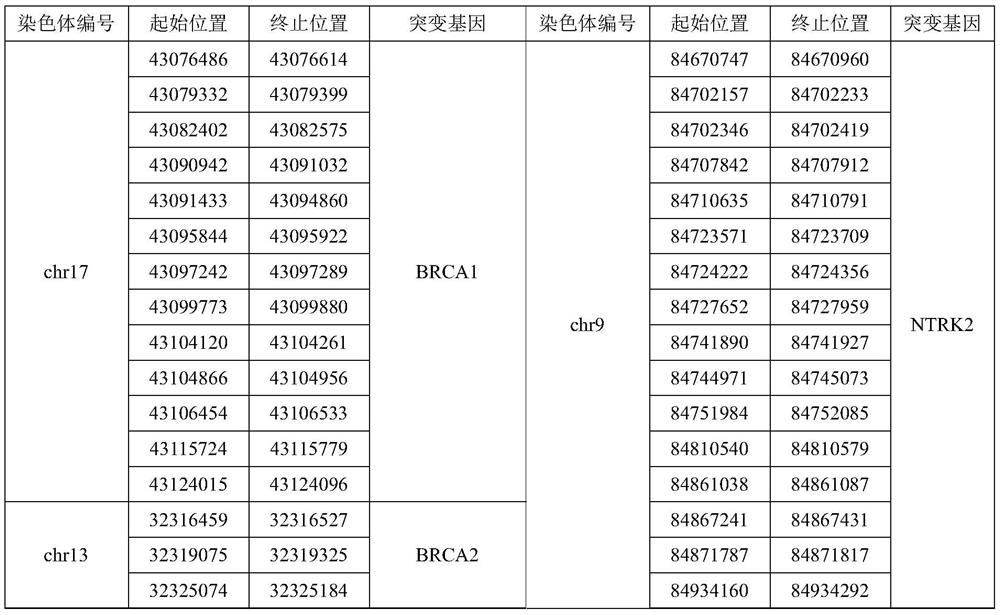
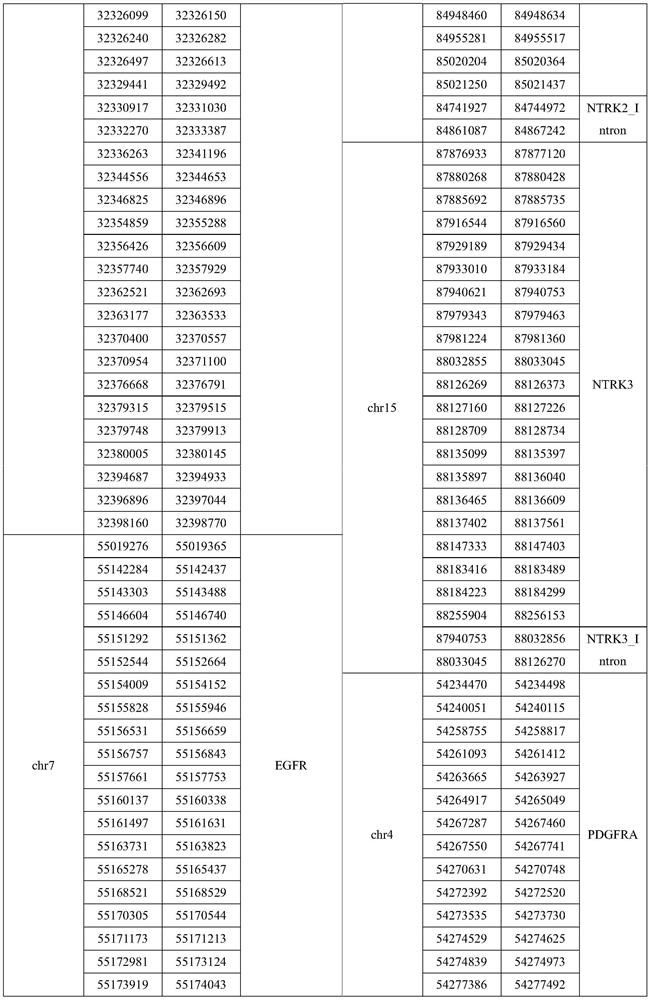
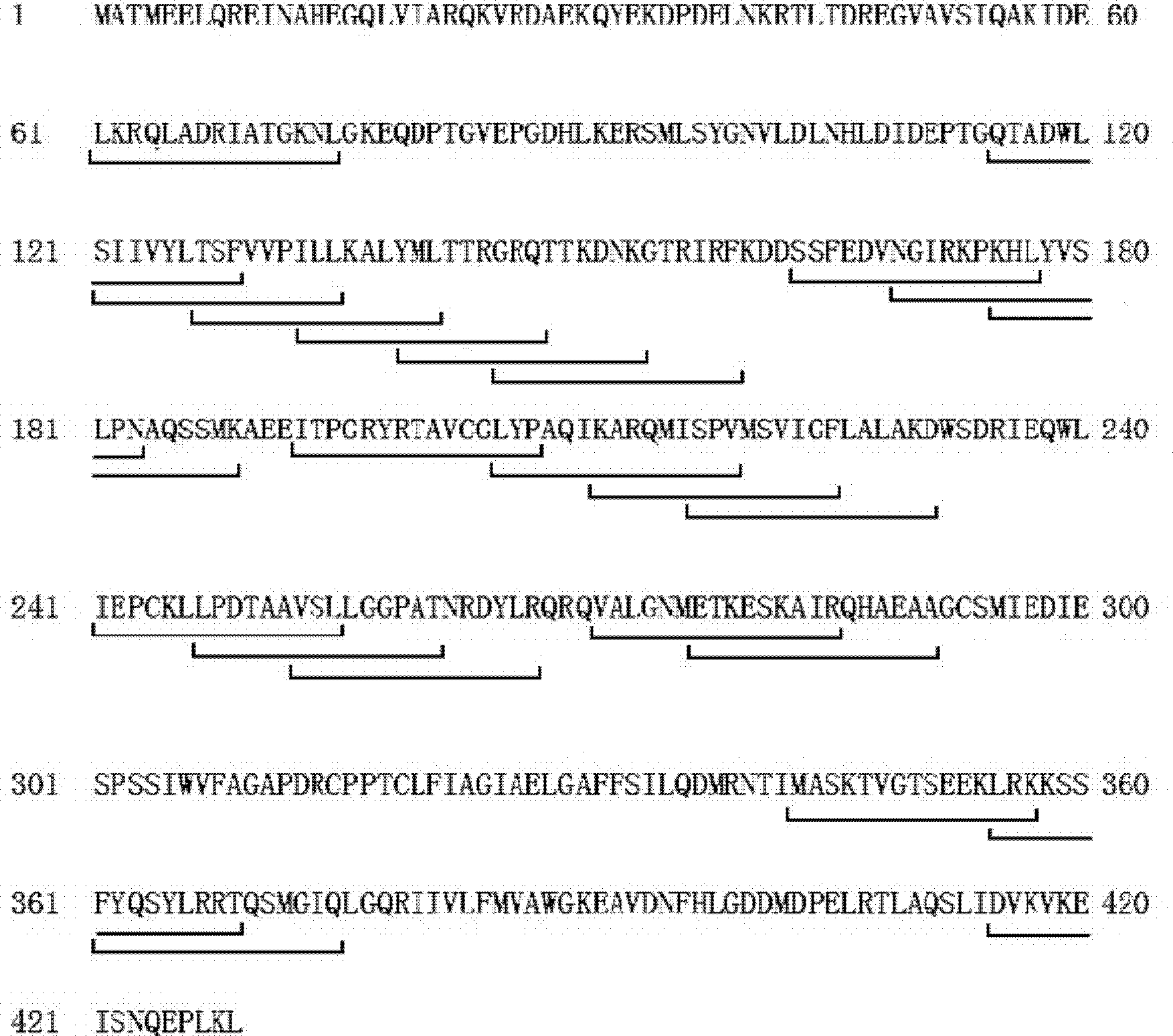
The invention relates to a gene panel used for detecting breast cancer gene mutation, and a detection method and application of the gene panel. The panel disclosed by the invention comprises 54419 pieces of targeted DNA (deoxyribonucleic acid) probes. The targeted DNA comprises the exon regions of 445 pieces of genes on the human genomes, 2573 pieces of MSI (microsatellite instability) sites and 566 position intervals used for detecting gene fusion. The detection method in the invention can be used for detecting SNV (single nucleotide variation), Indel (Insertion and Deletion), CNV (Copy Number Variation), Fusion, MSI, TMB (Tumor Mutational Burden), HLA (human leucocyte antigen) parting and the like of a tumor somatic cell multi-site DNA mutation. An optimal individual treatment medicine and scheme can be conveniently selected according to the genome features of patients in a breast cancer immunotherapy process.
Owner:北斗生命科学(广州)有限公司 +1
Primers, probe, fluorescent PCR kit and method for detecting human HLA-B*1301 gene
InactiveCN104450912ALow efficiencyLow costMicrobiological testing/measurementDNA/RNA fragmentationHuman leucocyte antigenHLA-B
The invention provides primers, a probe, a fluorescent PCR kit and a detection method for detecting a human HLA-B*1301 allele. According to the theory of 'allele specific PCR', a human leucocyte antigen HLA-B*1301 allele can be detected on a real-time fluorescent quantitative PCR technical platform.
Owner:SUZHOU KUANGYUAN MOLECULAR BIOTECH
HTNV-NP (Hantaan virus nucleoprotein)-specific CTL (cytotoxic T lymphocyte) epitope peptides and application thereof
The invention discloses HTNV-NP (Hantaan virus nucleoprotein)-specific CTL (cytotoxic T lymphocyte) epitope peptides and application thereof. The CTL epitope peptides have amino acid sequences shown in SEQ ID NO:1-12. Especially, HLA-I (human leucocyte antigen-I) molecule restricted epitope polypeptide in the HTNV-NP-specific CTL epitope peptides can induce CD8+T lymphocyte to generate strong cellular immune response and secrete high-level IFN-gamma. The HTNV-NP-specific CTL epitope peptides can be used for preparing CTL epitope peptide vaccines or for inducing the generation of CTL epitope peptide-specific CTL, or for preparing CTL epitope peptide-sensitized antigen presenting cells and have bright development and application prospects in the field of specific immunization therapy of HFRS (hemorrhagic fever with renal syndrome).
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Special primer and kit for detecting human leucocyte antigen-B27 (HLA-B27) gene
InactiveCN102337334AStrong specificityHigh sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceHuman leucocyte antigenWhite blood cell
The invention discloses a special primer for detecting a human leucocyte antigen-B27 (HLA-B27) gene. The special primer is characterized by comprising an upstream primer B27-1, a downstream primer B27-2 and a probe B27-probe which are used for detecting target genes, and an upstream primer Gapdh-1, a downstream primer Gapdh-2 and a probe Gapdh-probe which are used for detecting an internal control gene, namely glyceraldehyde-3-phosphate dehydrogenase (GAPDH), wherein the B27-1 is shown as GGGTCTCACACCCTCCAGAAT, the B27-2 is shown as CGGCGGTCCAGGAGCT, and the B27-probe is shown as FAM-TACCACCAGGACGCCTAC-TAMRA; and the Gapdh-1 is shown as CAGGTGGAGCGAGGCTAG, the Gapdh-2 is shown as CTACCCATGACTCAGCTTCTCC, and the Gapdh-probe is shown as HEX-ACCATGCCACAGCCACCACACCTC-BHQ1. The invention also provides a kit for detecting the HLA-B27 gene. A method for detecting the gene by using one pipe replaces the original method for detecting the gene by using two pipes, the amount of samples which can be detected by a machine at a time is increased, and the detection efficiency is improved to a great extent.
Owner:FUZHOU ADICON CLINICAL LAB INC
Kit and method for detecting human leucocyte antigen HLA-B*1502 genetype
InactiveCN103114130AEasy to operateAccurate operationMicrobiological testing/measurementHuman leucocyte antigenForward primer
The invention discloses a kit and a method for detecting human leucocyte antigen HLA-B*1502 genetype. The kit disclosed by the invention is characterized by comprising the following primer sequences: a forward primer FP: 5'-CGACGCCGCGAGTCCCAGG-3'; and a reverse primer RP: 5'-CGTCGTAGGCGGACTGGTCATA-3'. The kit further comprises the following probe sequence: a probe PR: 5'-Cy5-AACACACAGATCTCCAAGACCAACACAC-p-3'. The kit disclosed by the invention has good specificity and sensitivity for carrying out PCR (Polymerase Chain Reaction) detection of the human leucocyte antigen HLA-B*1502. The PCR detection method disclosed by the invention has the advantages of being simple for operation (closed pipe), rapid, accurate and the like and is applied to clinical molecular diagnosis.
Owner:瀚吉康生物科技(北京)有限公司
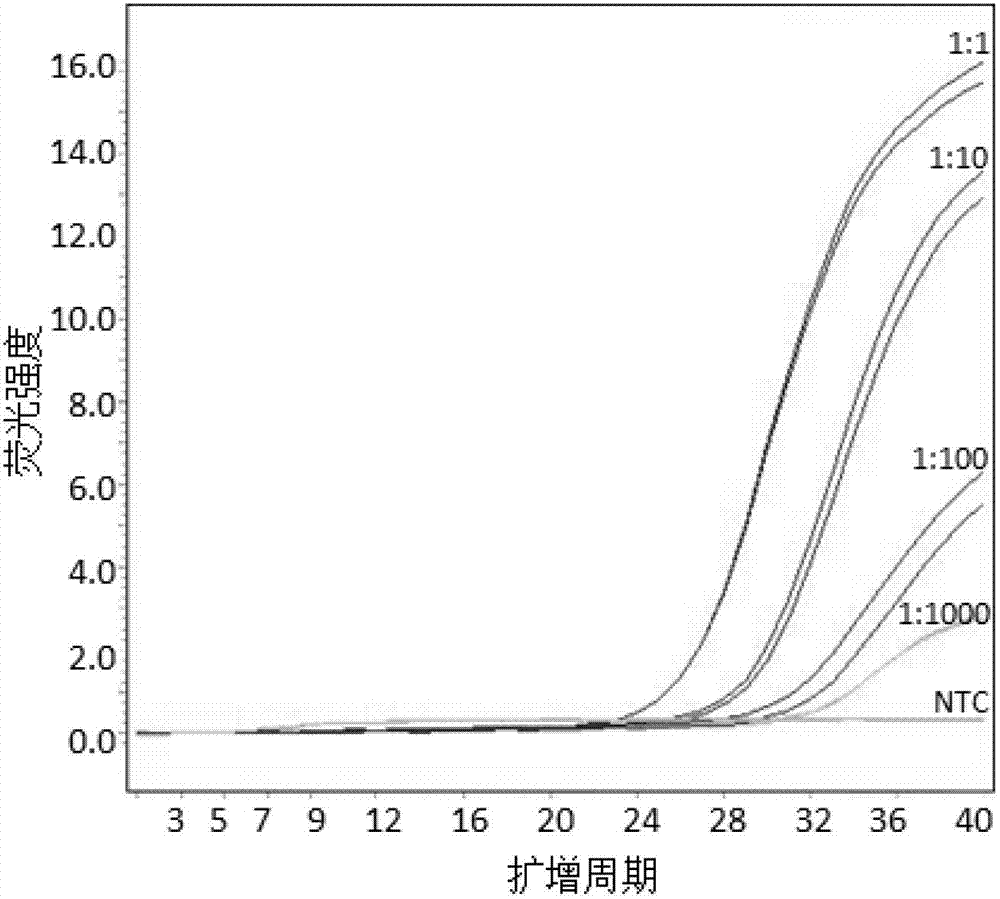
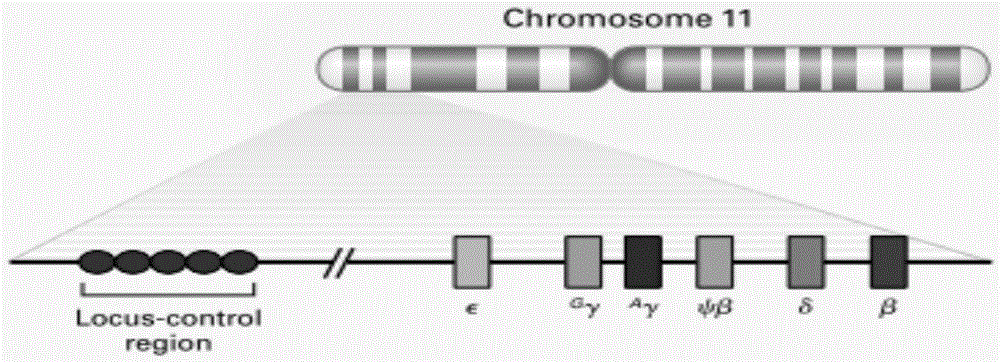
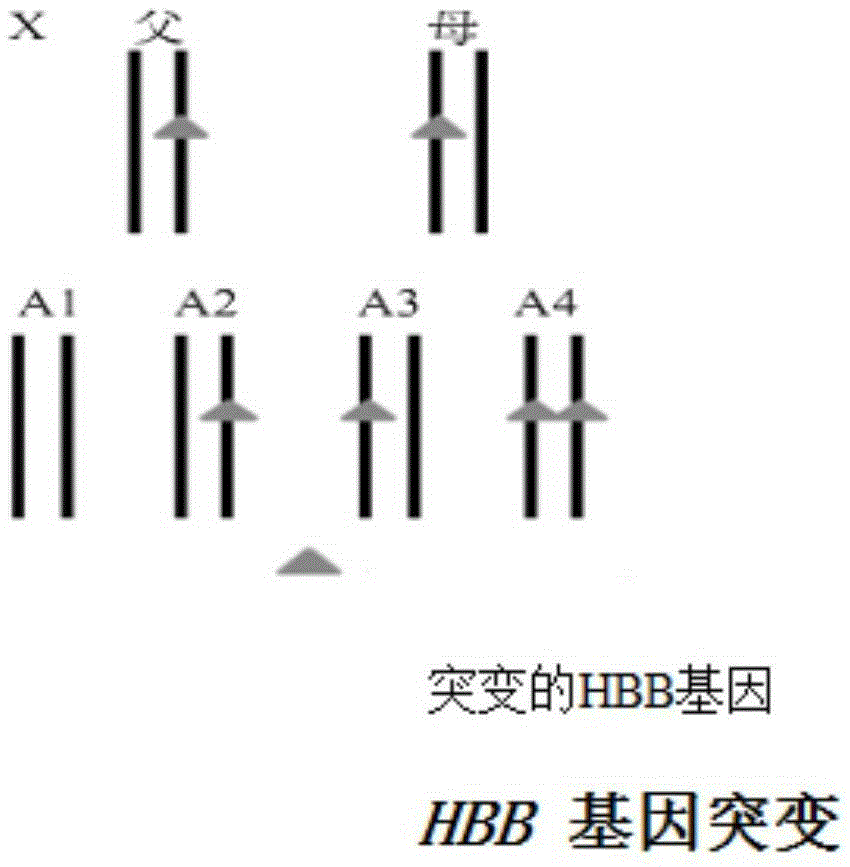
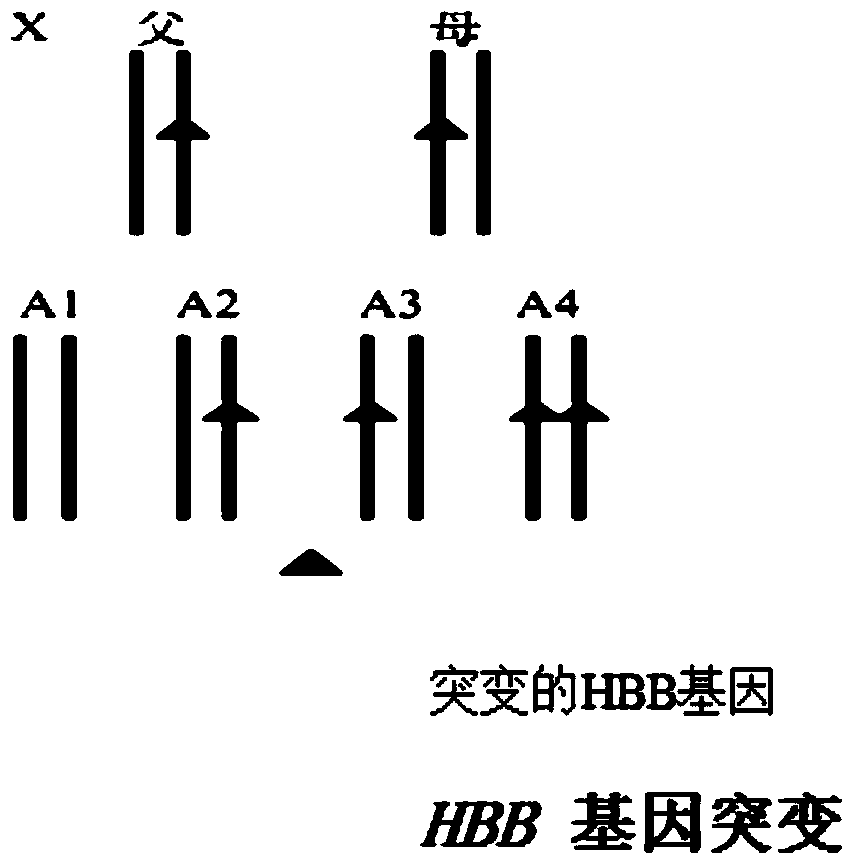
Reagent kit for detecting HBB gene mutation and HLA genotyping
ActiveCN105420233AImprove throughputLow costMicrobiological testing/measurementDNA/RNA fragmentationBeta globinBeta thalassemia
The invention provides a method for detecting HBB gene mutation and HLA genotyping based on the high throughput sequencing technology and a corresponding reagent kit. An adopted primer composition comprises a primer of closely-linked single nucleotide polymorphisms (SNP) within the 1 Mb range of the up stream and the down stream of the specific amplification human embryo beta-thalassemia HBB gene and primers of the closely-linked single nucleotide polymorphisms (SNP) within the ranges at the up stream of the LHA-A gene, between the HLA-A gene and the HLA-B gene, between the HLA-B gene and the HLA-DRA gene, between the HLA-DRA gene and the HLA-DQB1 gene and at the downstream of the HLA-DQB1 gene of the specific amplification human leucocyte antigen system. The method has the advantages of university, single nucleotide polymorphisms (SNP) sequencing, high throughput, low cost, high flexibility and strong specificity.
Owner:海南医学院附属医院 +1
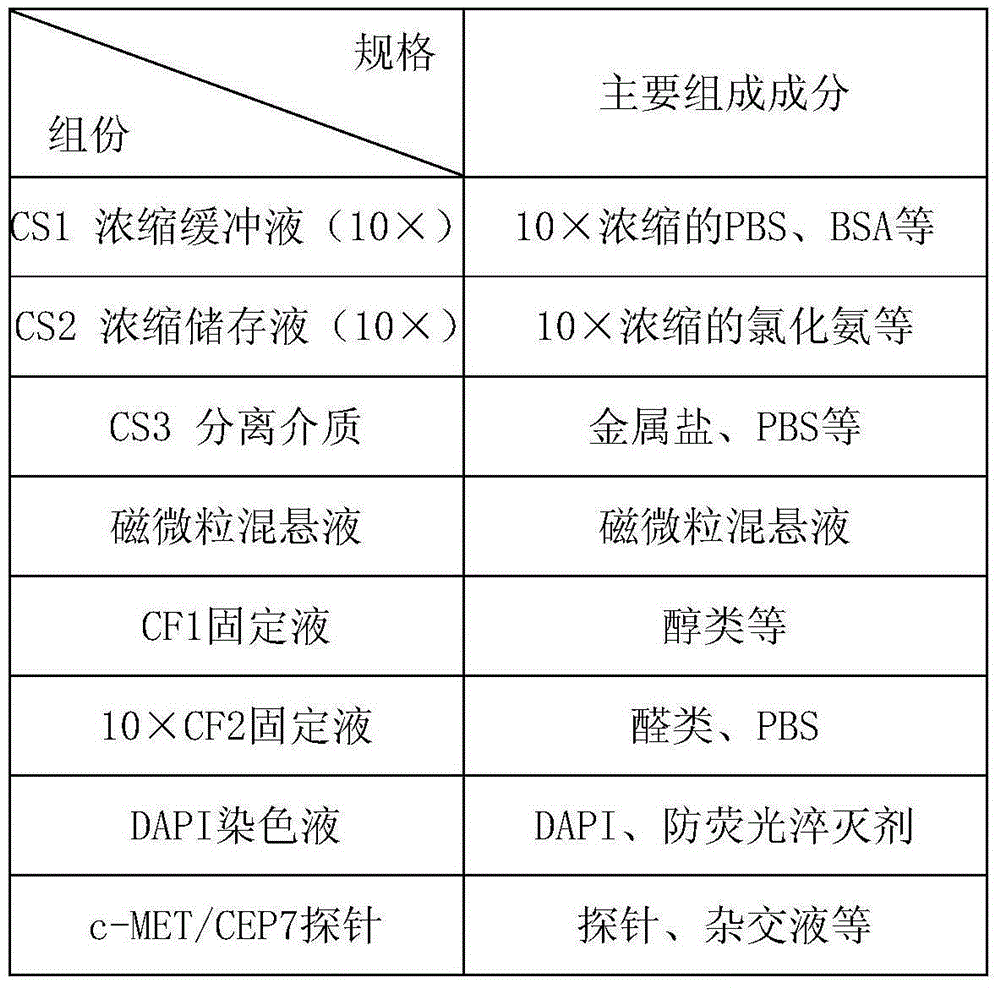
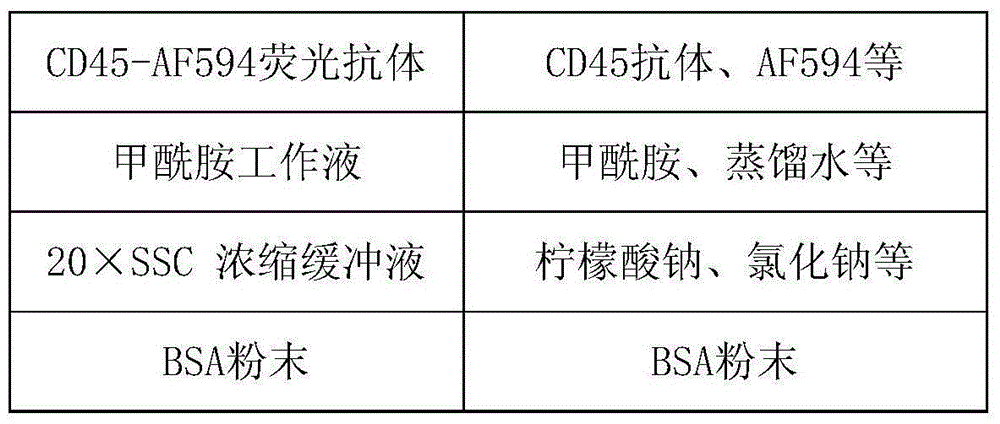
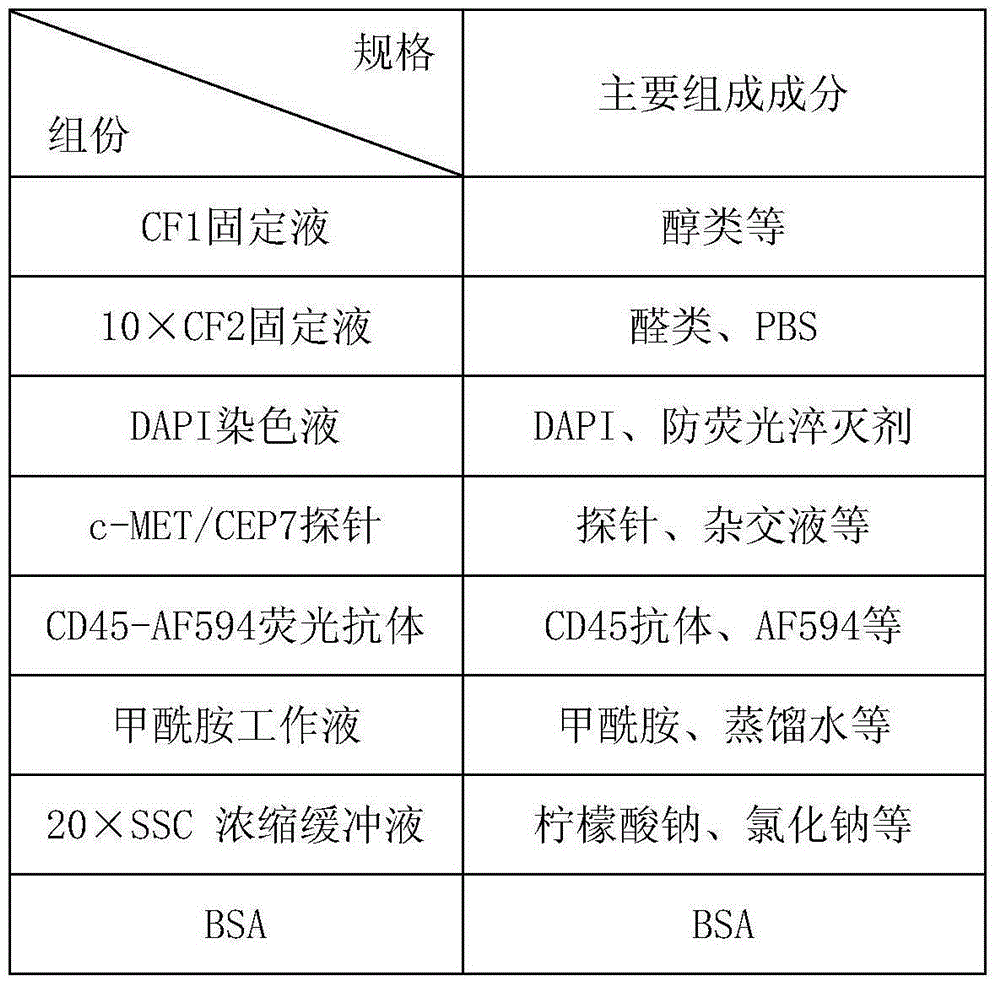
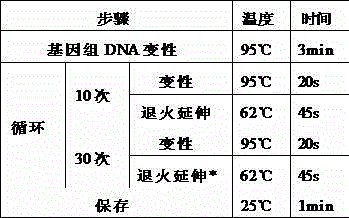
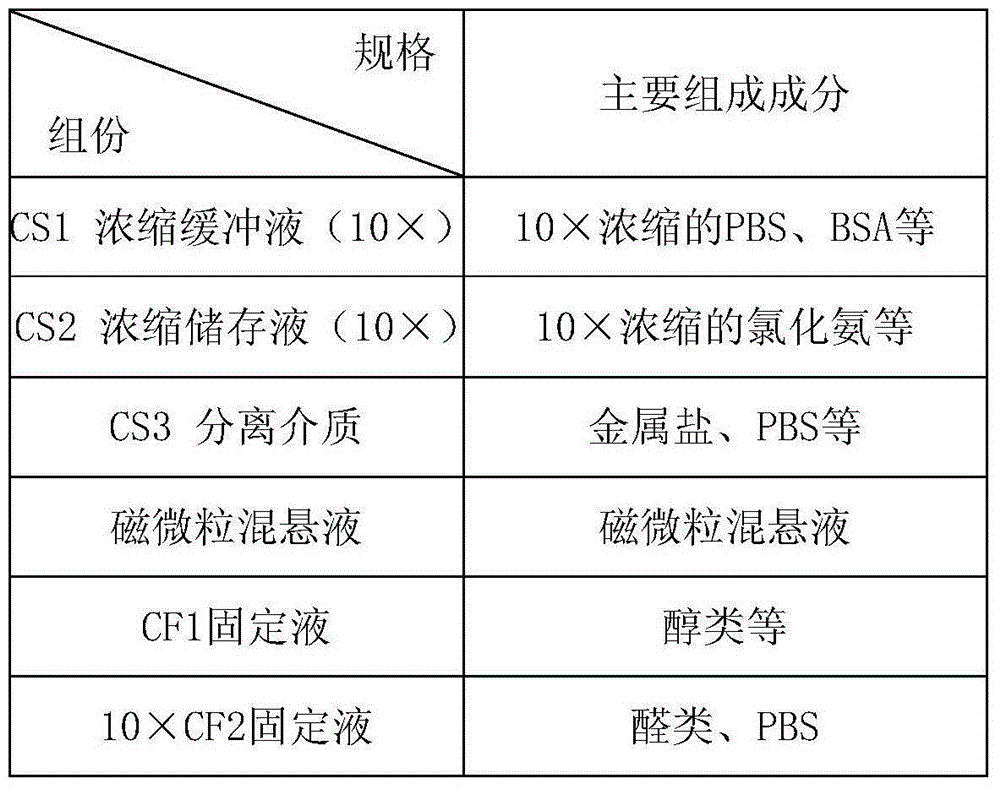
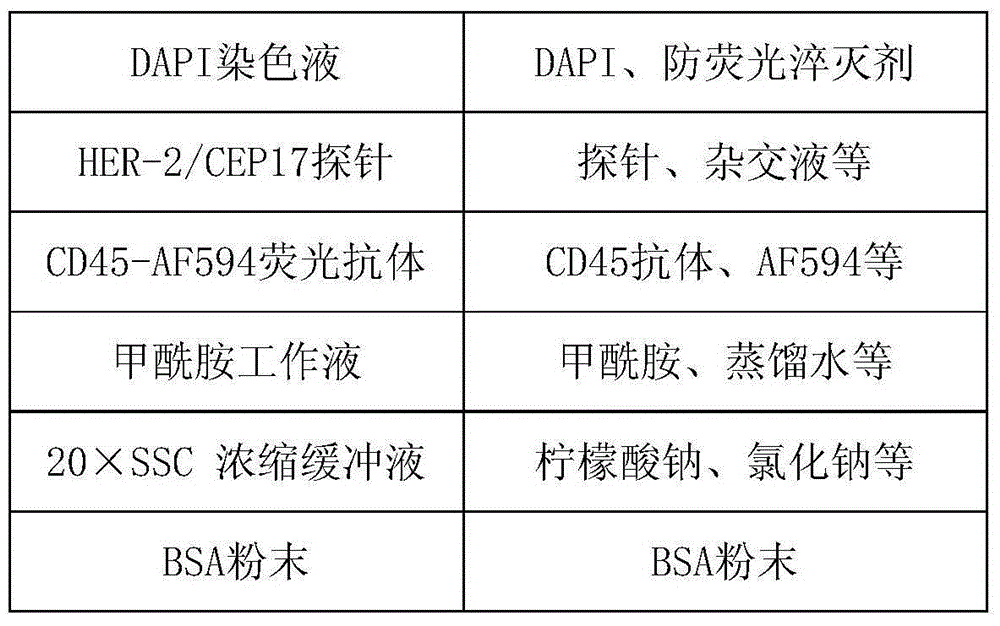
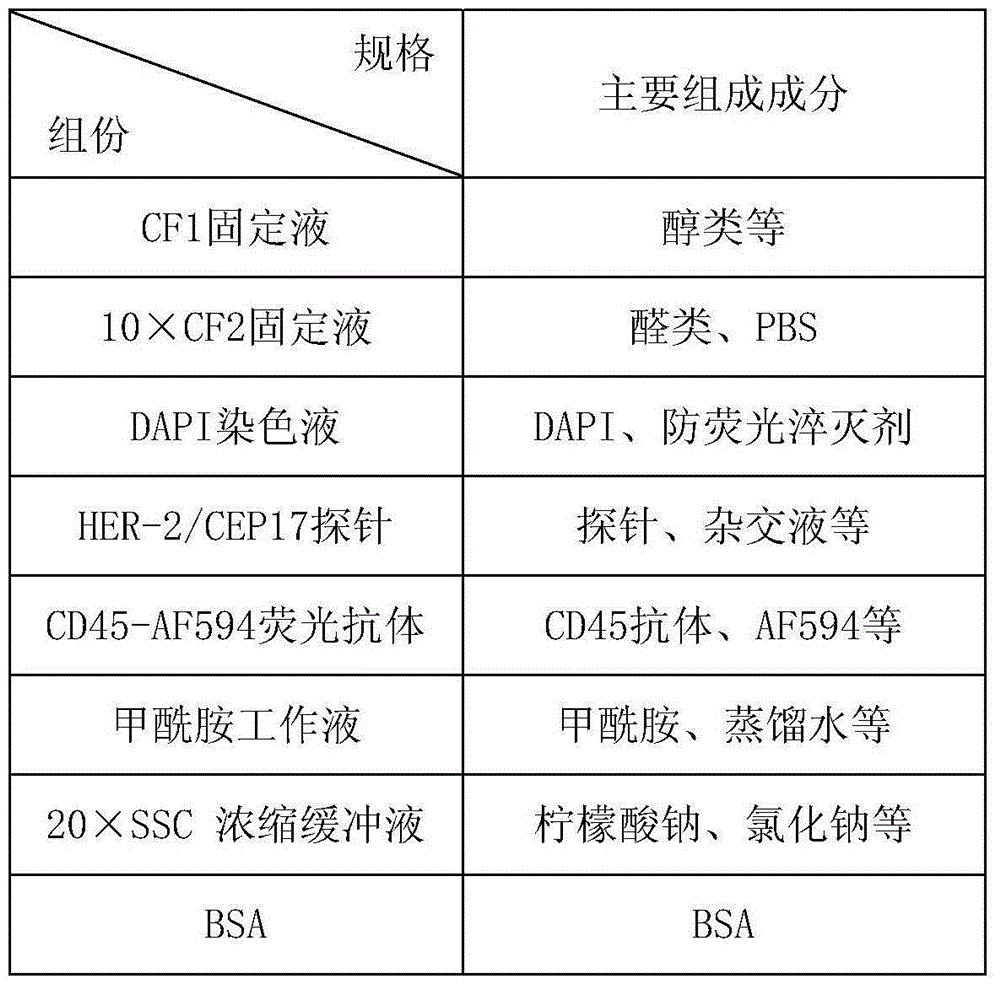
Method and related kit for detecting c-MET/CEP7 gene status based on rare cells
The invention relates to a method and a related kit for detecting c-MET / CEP7 gene status based on rare cells, wherein the method comprises the following steps: 1. acquiring a blood sample or a body fluid sample, wherein the sample includes a mixed cell population of circulating tumor cells or other rare cells and white blood cells; 2. diluting the sample by virtue of a buffer solution, removing plasma and recovering CTC or other rare cells; 3. cracking by virtue of a red blood cell lysis buffer, removing red blood cells and recovering CTC or other rare cells; 4. recovering cells with magnetic particles coated with related anti-human leucocyte antigens and antibodies, uniformly mixing and breeding, and fully combining so as to form a magnetic particle-white blood cell mixture; 5. isolating the magnetic particle-white blood cell mixture from other cells so as to obtain a mixed cell population containing CTC or other rare cells and a few of white blood cells; and 6. by virtue of a method combining immunofluorescence cytochemistry and fluorescence in-situ hybridization, determining the status of c-MET / CEP7, and simultaneously identifying CTC or other rare cells.
Owner:北京莱尔生物医药科技有限公司
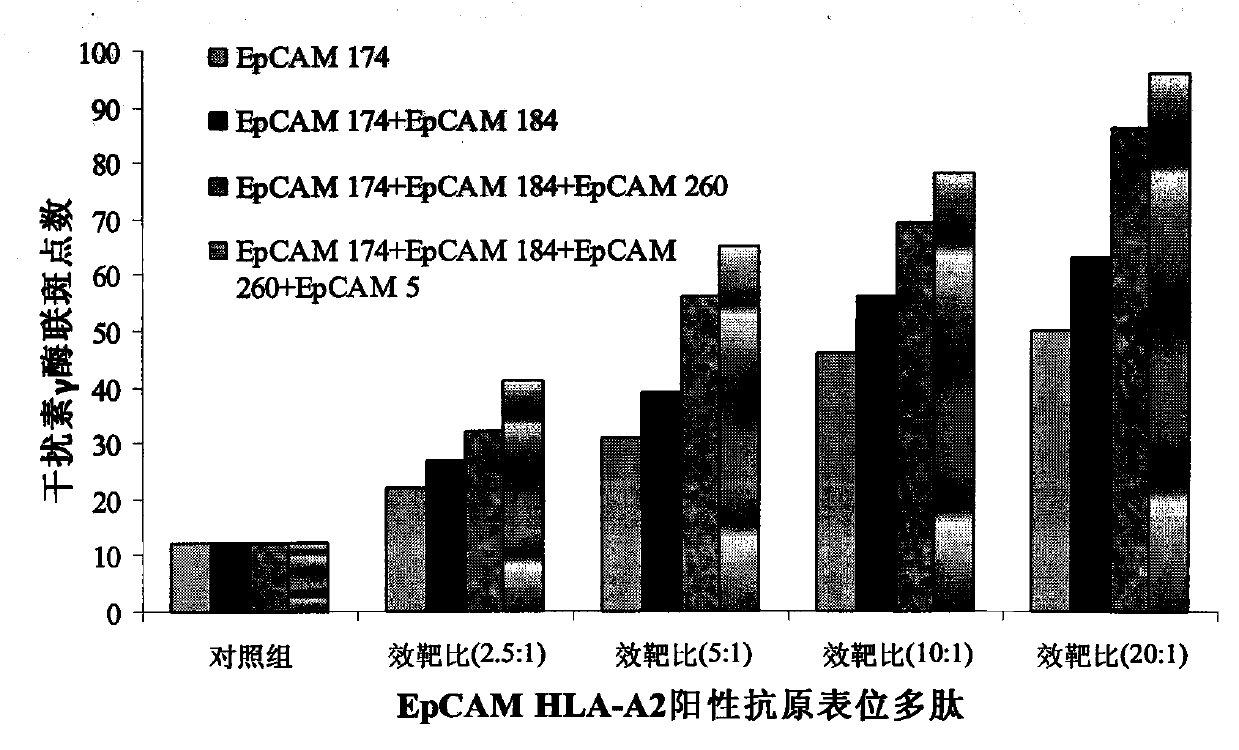
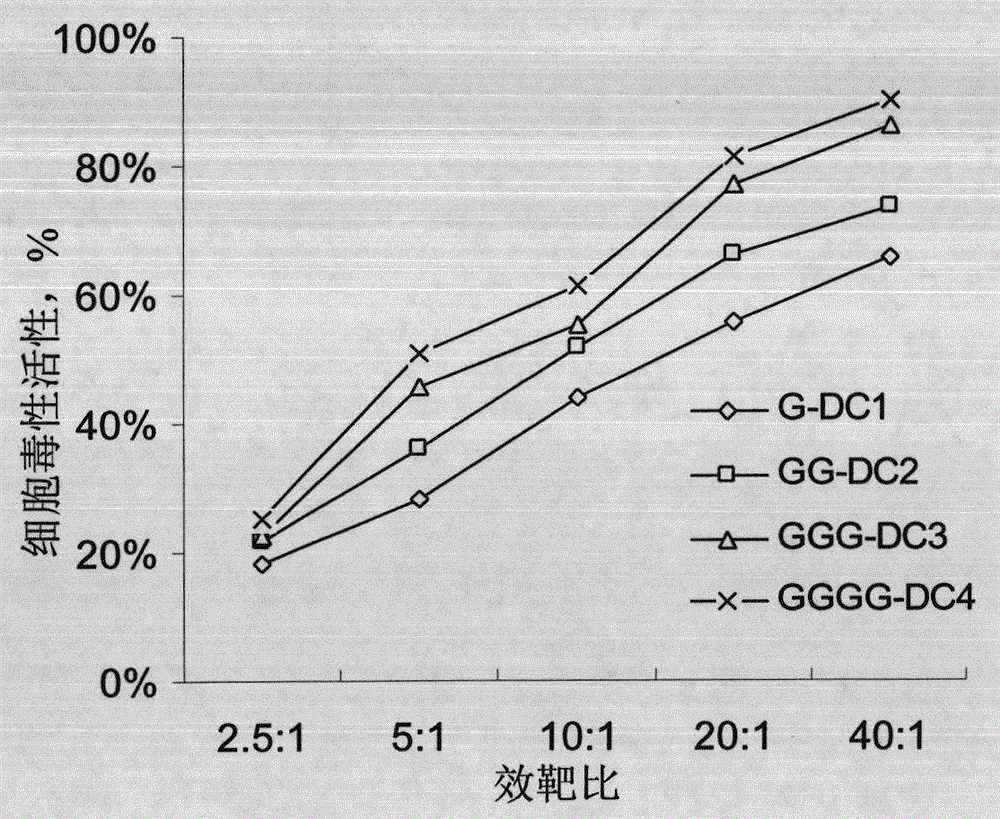
Preparation method and kit for dendritic cell vaccine loaded by tumor specific antigenic epitope polypeptide
InactiveCN103800897ACure recurrenceCure metastasesAntibody medical ingredientsMacromolecular non-active ingredientsHuman leucocyte antigenEpitope
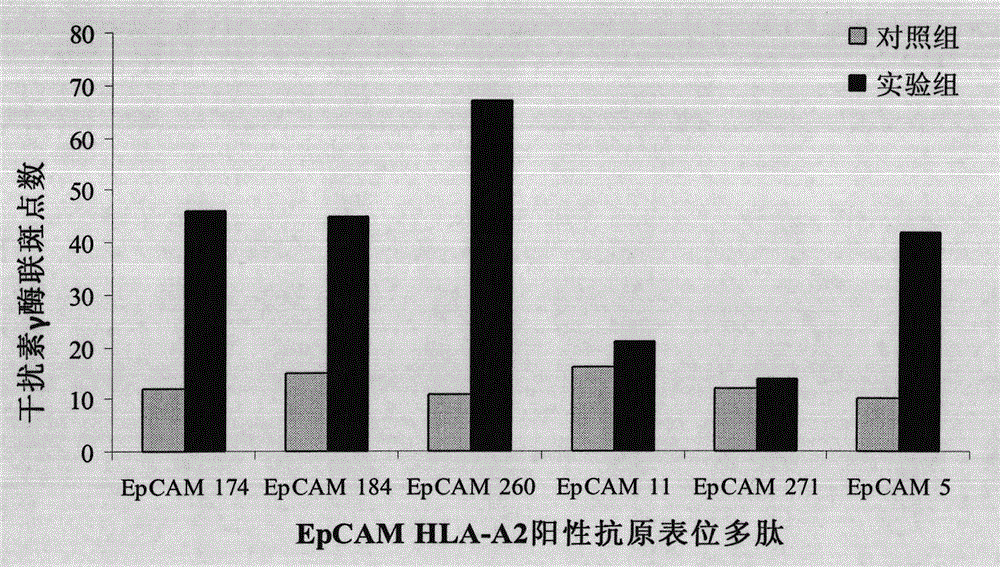
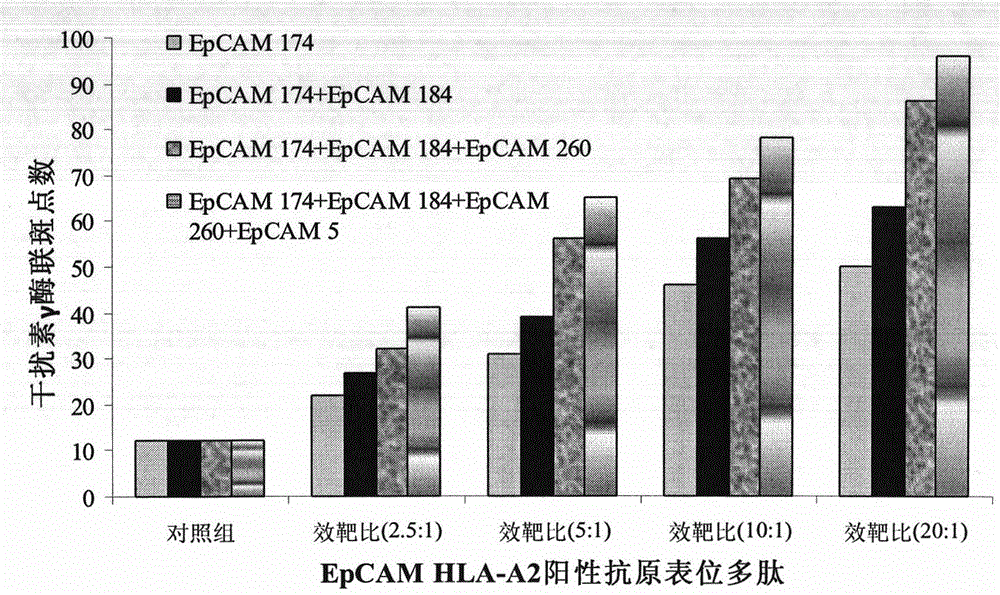
The invention provides a preparation method for a dendritic cell vaccine loaded by a tumor specific antigenic epitope polypeptide. The method comprises the following steps: adopting a human leucocyte antigen A201 positive epitope of an epithelial cell adhesion molecule identified by tumor specific expression, namely a polypeptide composed of amino acid sequences represented by any one of SEQ ID NO.1-6; loading dendritic cells by using the polypeptide of any one of the SEQ ID NO.1-6 to prepare the dendritic cell vaccine. The dendritic cell vaccine can be used for initiating a very strong target anti-tumor cytotoxic T lymphocyte effect. The invention further provides a kit for preparing the dendritic cell vaccine loaded by the tumor specific antigenic epitope polypeptide.
Owner:李金珍
Preparation and use of micro-array chip for HLA-B27 genotyping
ActiveCN101353693AReliability advantageMicrobiological testing/measurementHuman leucocyte antigenDNA Microarray Chip
The invention relates to a gene detection kit used for clinical detection, in particular to the preparation and the usage of a micro-array chip used for HLA-B27 genotyping. A DNA micro-array chip technology is adopted, and the kit can carry out partign to human leucocyte antigen B27, with high pass, high efficiency and high specificity.
Owner:GUANGZHOU DARUI BIOTECH
Method for rapidly detecting human leucocyte antigen B27 (HLA-B27) and kit thereof
ActiveCN102443625AStrong specificityIncreased sensitivityMicrobiological testing/measurementAntigenFluorescence
The invention relates to a method for rapidly detecting a human leucocyte antigen B27 (HLA-B27) and an in-vitro diagnosis kit, which belong to the technical field of medical biology. In the kit, a pair of HLA-B27 specific primers and a specific fluorescent probe are adopted for rapidly detecting the HLA-B27. In the kit, the pair of HLA-B27 specific primer and the specific fluorescent probe can beused for rapidly and accurately detecting HLA-B27 genes in samples such as human peripheral blood and the like, and the kit has the advantages of high specificity, high sensitivity, time saving, labor saving, low cost, high flux and the like, and can be applied to auxiliary diagnosis of multiple spondyloarthropathies which severely endanger human health such as clinical ankylosing spondylitis, Reiter syndrome, psoriatic arthritis, ulcerative colitis accompanying arthropathy, acute anterior tract uveal, and the like.
Owner:浙江夸克生物科技有限公司
Primer, probe, fluorescence PCR (Polymerase Chain Reaction) kit and method for detecting HLA (Human Leucocyte Antigen)-B*5801 genes
InactiveCN104404159AEasy to readHigh specificity of PCR detectionMicrobiological testing/measurementDNA/RNA fragmentationHuman leucocyte antigenFluorescence
The invention provides a primer, a probe, a fluorescence PCR (Polymerase Chain Reaction) kit and a method for detecting HLA (Human Leucocyte Antigen)-B*5801 alleles. Human leucocyte antigen (HLA)-B*5801 alleles are detected on a real-time fluorescent quantitative PCR technical platform according to the principle of 'allele specific PCR'.
Owner:SUZHOU KUANGYUAN MOLECULAR BIOTECH
Method and related kit for detecting HER-2/CEP17 gene status based on rare cells
InactiveCN105087778AMicrobiological testing/measurementBiological testingCytochemistryWhite blood cell
The invention relates to a method and a related kit for detecting HER-2 / CEP17 gene status based on rare cells, wherein the method comprises the following steps: 1. acquiring a blood sample or a body fluid sample, wherein the sample includes a mixed cell population of circulating tumor cells or other rare cells and white blood cells; 2. diluting the sample by virtue of a buffer solution, removing plasma and recovering CTC or other rare cells; 3. cracking by virtue of a red blood cell lysis buffer, removing red blood cells and recovering CTC or other rare cells; 4. uniformly mixing and breeding magnetic particles coated with related anti-human leucocyte antigens and antibodies with the recovered cells, and combining so as to form a magnetic particle-white blood cell mixture; 5. isolating the magnetic particle-white blood cell mixture from other cells so as to obtain a mixed cell population containing CTC or other rare cells and a few of white blood cells; and 6. by virtue of a method combining immunofluorescence cytochemistry and fluorescence in-situ hybridization, determining the status of HER-2 / CEP17, and simultaneously identifying CTC or other rare cells.
Owner:北京莱尔生物医药科技有限公司
Treatment of hepatitis d virus infections by redirection of t cells
InactiveUS20160228531A1Avoid infectionInhibition of replicationSsRNA viruses negative-senseImmunoglobulin superfamilyHuman leucocyte antigenInfected cell
Several approaches to treat, inhibit, or prevent HDV infections by redirecting T cells to HDV and HDV infected cells are described. In several alternatives, a gene encoding a molecule that specifically binds to human leucocyte antigen (HLA) loaded with a HDV derived peptide are introduced into the T cells of individuals. Upon receiving the modified T cells, the modified T cells, derived from the individual, will then recognise the individual's cells that are infected by HDV and / or the HDV virus, blocking HDV replication and / or killing the HDV infected cells thereby preventing and / or treating or inhibiting the HDV infection in the individual.
Owner:CHRONTECH PHARMA
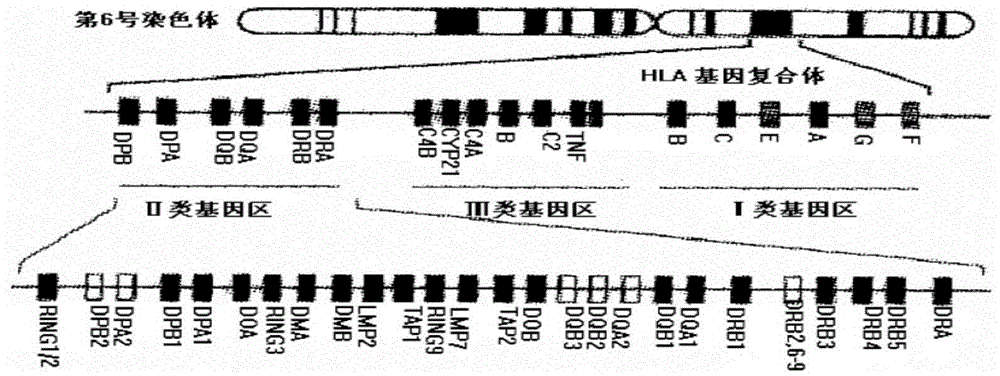
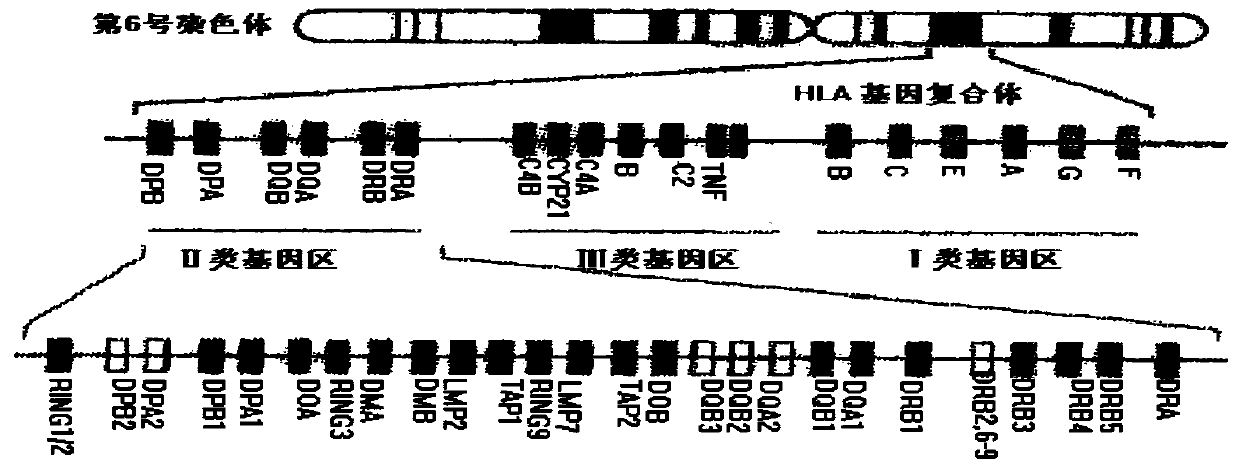
Human leucocyte antigen genotyping method and reagent thereof
InactiveCN105296618ASolve the problem that cannot be effectively typedHigh resolutionMicrobiological testing/measurementAntigenHuman leucocyte antigen
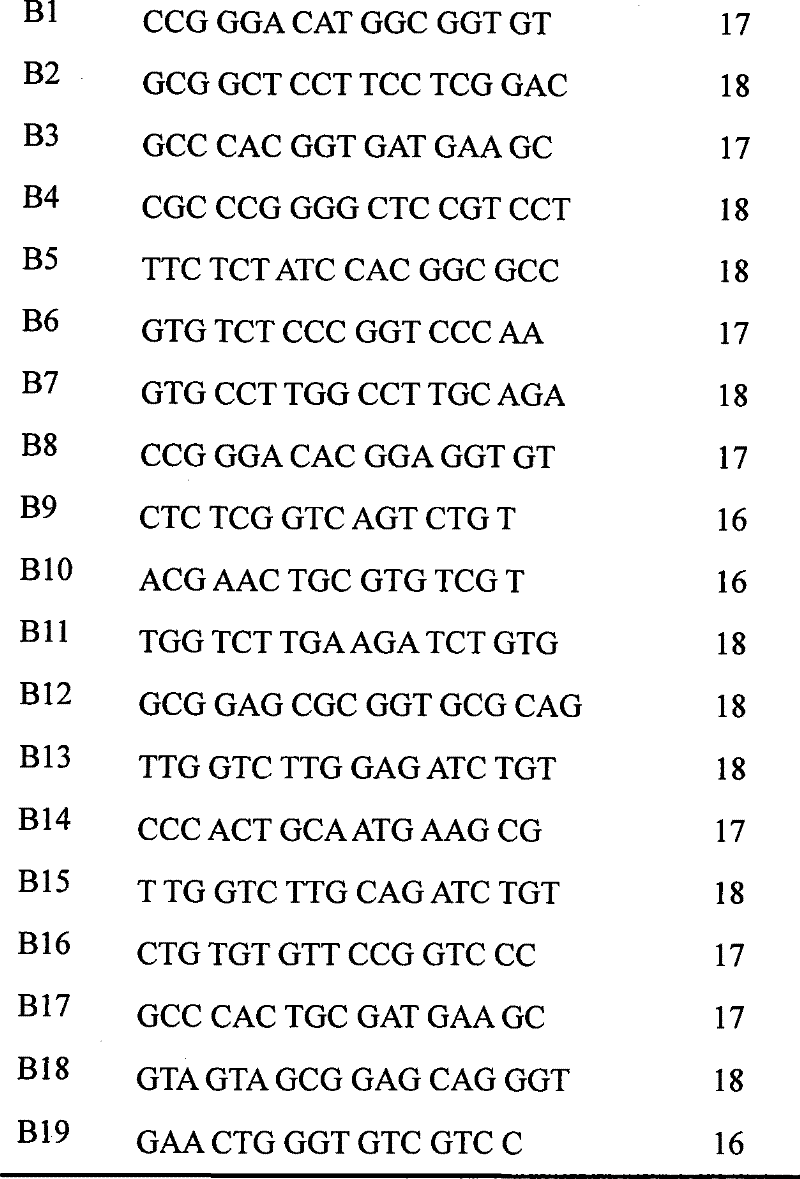
The invention discloses a human leucocyte antigen genotyping method and a reagent thereof. The method comprises the following steps: firstly, implementing PCR amplification to HLA-B and HLA-C site genes of to-be-detected genome DNA; and then, conducting a sequencing reaction to amplification products by virtue of 12 sequencing primers so as to determine a genotype, wherein the sequencing primers include 5th, 6th and 7th sequencing primers of an HLA-B exon and 5th, 6th and 7th sequencing primers of an HLA-C exon. The method disclosed by the invention, by virtue of additionally exon sequencing in an irregular detection area, can effectively solve the problem that many and many ambiguous results appear during HLA high-resolution genotyping. The obtained sequence is free from a background signal and a miscellaneous peak; and the sequence, which can be introduced into analysis software which is matched with the commercial reagent, is easy for recognition and result interpretation. The primers disclosed by the invention can undergo synchronous amplification and sequencing with a commercial kit, so as to improve the working efficiency and to significantly reduce the cost of the reagent; therefore, the invention is of very significant meaning in the improvement of the detection efficiency.
Owner:SHENZHEN BLOOD CENT
Primers, probe, fluorescent PCR kit and method for detecting human HLA-B*27 gene
InactiveCN104450913AGuarantee authenticityGuaranteed accuracyMicrobiological testing/measurementDNA/RNA fragmentationHuman leucocyte antigenHLA-B
The invention provides primers, a probe, a fluorescent PCR kit and a detection method for detecting a human HLA-B*27 allele. According to the theory of 'allele specific PCR', a human leucocyte antigen HLA-B*27 allele can be detected on a real-time fluorescent quantitative PCR technical platform.
Owner:SUZHOU KUANGYUAN MOLECULAR BIOTECH
Kit and method for detecting human leucocyte antigen HLA-B*1502 genetype
InactiveCN103114130BEasy to operateAccurate operationMicrobiological testing/measurementForward primerHuman leucocyte antigen
The invention discloses a kit and a method for detecting human leucocyte antigen HLA-B*1502 genetype. The kit disclosed by the invention is characterized by comprising the following primer sequences: a forward primer FP: 5'-CGACGCCGCGAGTCCCAGG-3'; and a reverse primer RP: 5'-CGTCGTAGGCGGACTGGTCATA-3'. The kit further comprises the following probe sequence: a probe PR: 5'-Cy5-AACACACAGATCTCCAAGACCAACACAC-p-3'. The kit disclosed by the invention has good specificity and sensitivity for carrying out PCR (Polymerase Chain Reaction) detection of the human leucocyte antigen HLA-B*1502. The PCR detection method disclosed by the invention has the advantages of being simple for operation (closed pipe), rapid, accurate and the like and is applied to clinical molecular diagnosis.
Owner:瀚吉康生物科技(北京)有限公司
Method of determining susceptibility to prion disease
InactiveUS20040265820A1Lower latencyGood lookingMicrobiological testing/measurementAnimal husbandryHuman leucocyte antigenDisease
This invention relates to a method of determining the susceptibility of a subject to prion disease comprising the steps of: (a) providing a sample from said subject and in determining the human leucocyte antigen specificity of said sample, wherein if said sample has DQ7 human leucocyte antigen specificity, this indicates that said subject has a decreased susceptibility to prion disease, and if said sample does not have DQ7 human leucocyte antigen specificity, this indicates that said subject has an increased susceptibility to prion disease.
Owner:MEDICAL RESEARCH COUNCIL
Human leucocyte antigen DR4 subtype protein agonist
InactiveCN1935133AHigh antagonistic activityEasy to degradeOrganic active ingredientsAntipyreticHuman leucocyte antigenAgonist
The present invention discloses an antagonist of human leukocyte antigen DR4 subtype protein. Said invention also provides its chemical structure formula and its concrete application range.
Owner:PEKING UNIV
Common donor stem cell and preparation method thereof
InactiveCN110819592APluripotentGenetically modified cellsOther foreign material introduction processesHuman leucocyte antigenImmunogenicity
The invention discloses a common donor stem cell and a preparation method thereof. The common donor stem cell disclosed by the invention can be used for overcoming immunological rejection in a transplantation therapy based on cells, specifically, the common donor stem cell disclosed by the invention does not express MHC-I (major histocompatibility complex-I) or MHC-II (or HLA-I (human leucocyte antigen-I) or HLA-II), and thus the stem cell has low immunogenicity.
Owner:赛元生物科技(杭州)有限公司
Methods and kits for generating and selecting a variant of a binding protein with increased binding affinity and/or specificity
PendingUS20220106584A1High binding affinityStrong specificityHydrolasesScreening processHEK 293 cellsWhite blood cell
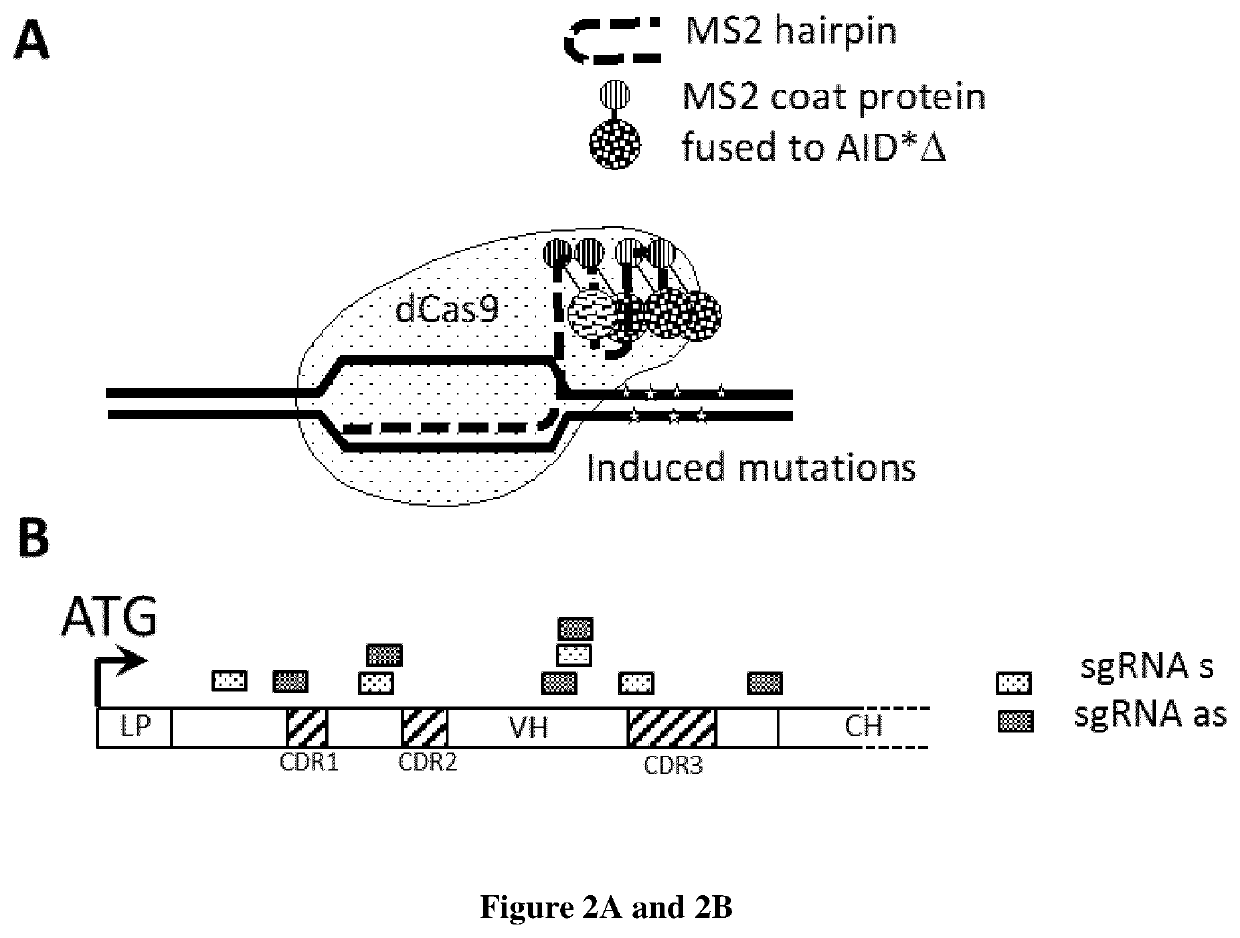
Somatic hypermutation promotes affinity maturation of antibodies by targeting the cytidine deaminase AID to antibody genes, followed by antigen-based selection of matured antibodies. Given the importance of antibodies in medicine and research, developing approaches to reproduce this natural phenomenon in cell culture is of some interest. The inventors use here the CRISPR-Cas 9 based CRISPR-X approach to target AID to antibody genes carried by expression vectors in HEK 293 cells. This directed mutagenesis approach, combined with a highly sensitive antigen-associated magnetic enrichment process, allowed rapid progressive evolution of a human antibody against the Human Leucocyte Antigen A*0201 allele. Starting from a low affinity monoclonal antibody expressed on Ag-specific naïve blood circulating B cells, they obtained in approximately 6 weeks antibodies with a two log increase in affinity and which retained their specificity. The strategy for in vitro affinity maturation of antibodies is applicable to virtually any antigen. It not only allows to tap into the vast naive B cell repertoire but could also be useful when dealing with antigens that only elicit low affinity antibodies after immunization. Accordingly as defined by the claims, the present invention relates to methods and kits for generating and selecting a variant of antibody binding protein with increased binding affinity and / or specificity.
Owner:UNIV DE NANTES
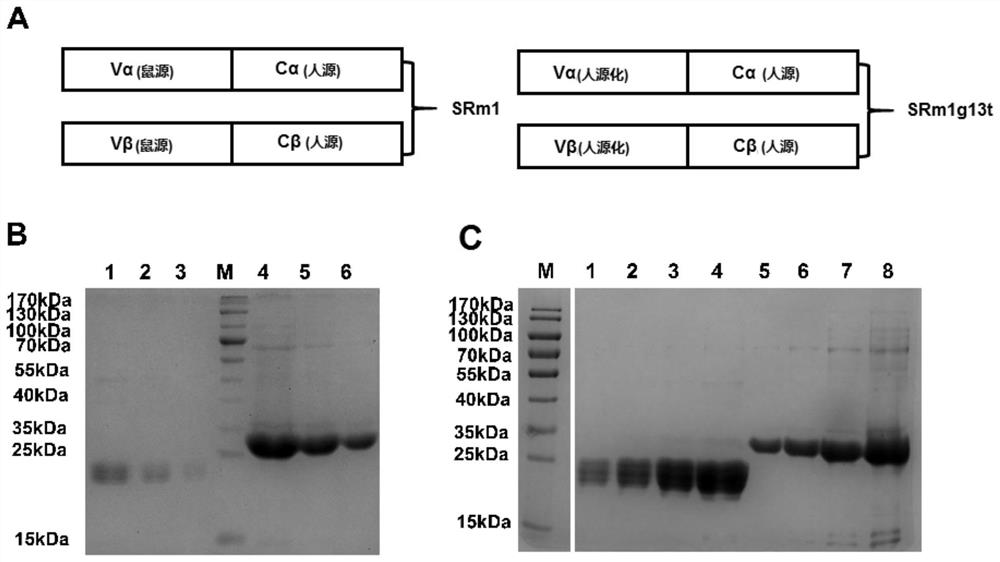
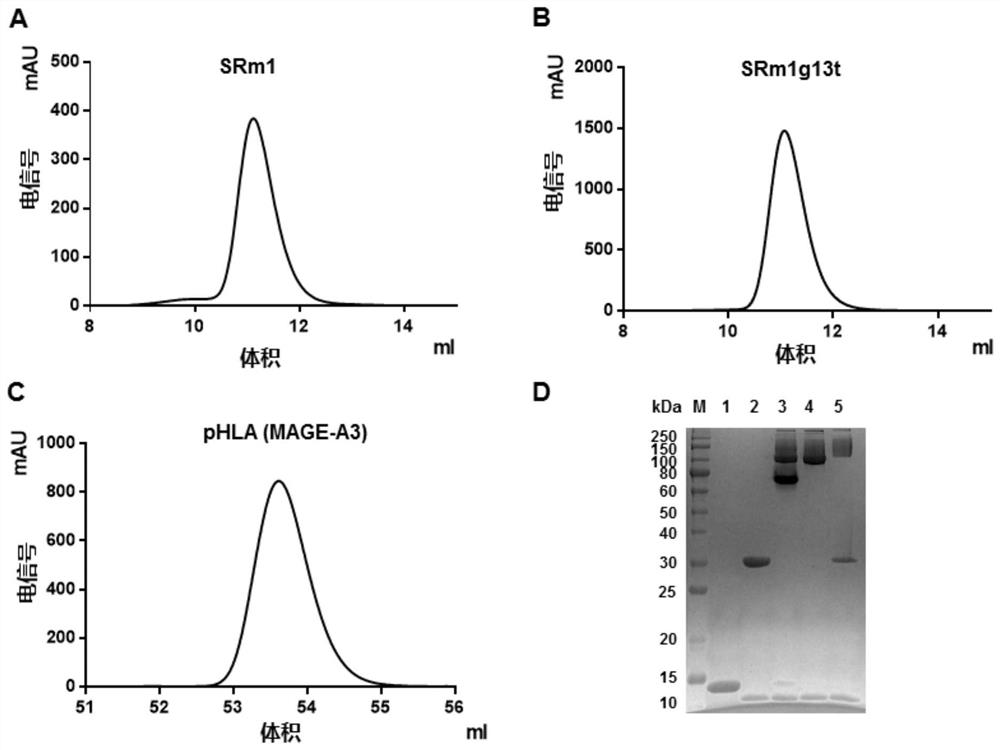
mage-a3 humanized T cell receptor
ActiveCN110016074BImmunoglobulin superfamilyMammal material medical ingredientsAntigenHuman leucocyte antigen
The invention provides a humanized transformed T cell receptor (TCR) SRm1g13t. The humanized T cell receptor has the characteristic of combining human antigen MAGE-A3:112-120(KVAELVHFL) and human leucocyte antigen (HLA) compound: pHLA(MAGE-A3), wherein the combination affinity of SRm1g13t for the pHLA(MAGE-A3) compound is at least 2 times of that of a corresponding mouse TCR SRm1 for the pHLA(MAGE-A3) compound. The invention further provides a fused molecule of TCR and a therapeutical agent. The TCR can be singly used, and can also be combined with the therapeutical agent for use.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
hbb gene mutation and hla typing detection kit
ActiveCN105420233BImprove throughputLow costMicrobiological testing/measurementDNA/RNA fragmentationAntigenHuman leucocyte antigen
The invention provides a method for detecting HBB gene mutation and HLA genotyping based on the high throughput sequencing technology and a corresponding reagent kit. An adopted primer composition comprises a primer of closely-linked single nucleotide polymorphisms (SNP) within the 1 Mb range of the up stream and the down stream of the specific amplification human embryo beta-thalassemia HBB gene and primers of the closely-linked single nucleotide polymorphisms (SNP) within the ranges at the up stream of the LHA-A gene, between the HLA-A gene and the HLA-B gene, between the HLA-B gene and the HLA-DRA gene, between the HLA-DRA gene and the HLA-DQB1 gene and at the downstream of the HLA-DQB1 gene of the specific amplification human leucocyte antigen system. The method has the advantages of university, single nucleotide polymorphisms (SNP) sequencing, high throughput, low cost, high flexibility and strong specificity.
Owner:海南医学院附属医院 +1
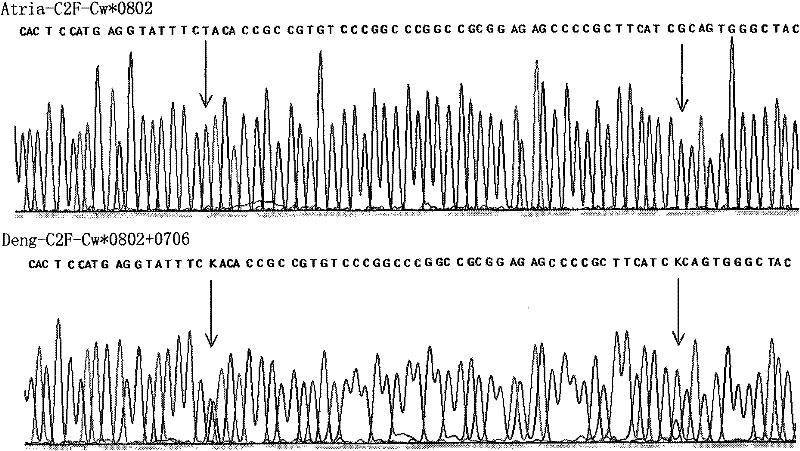
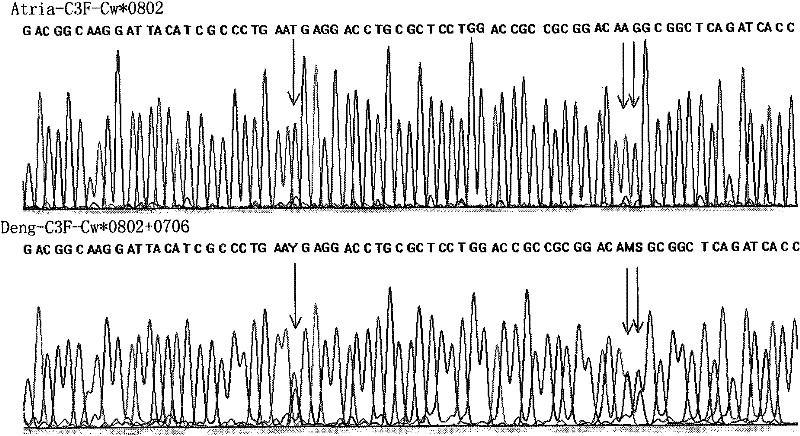
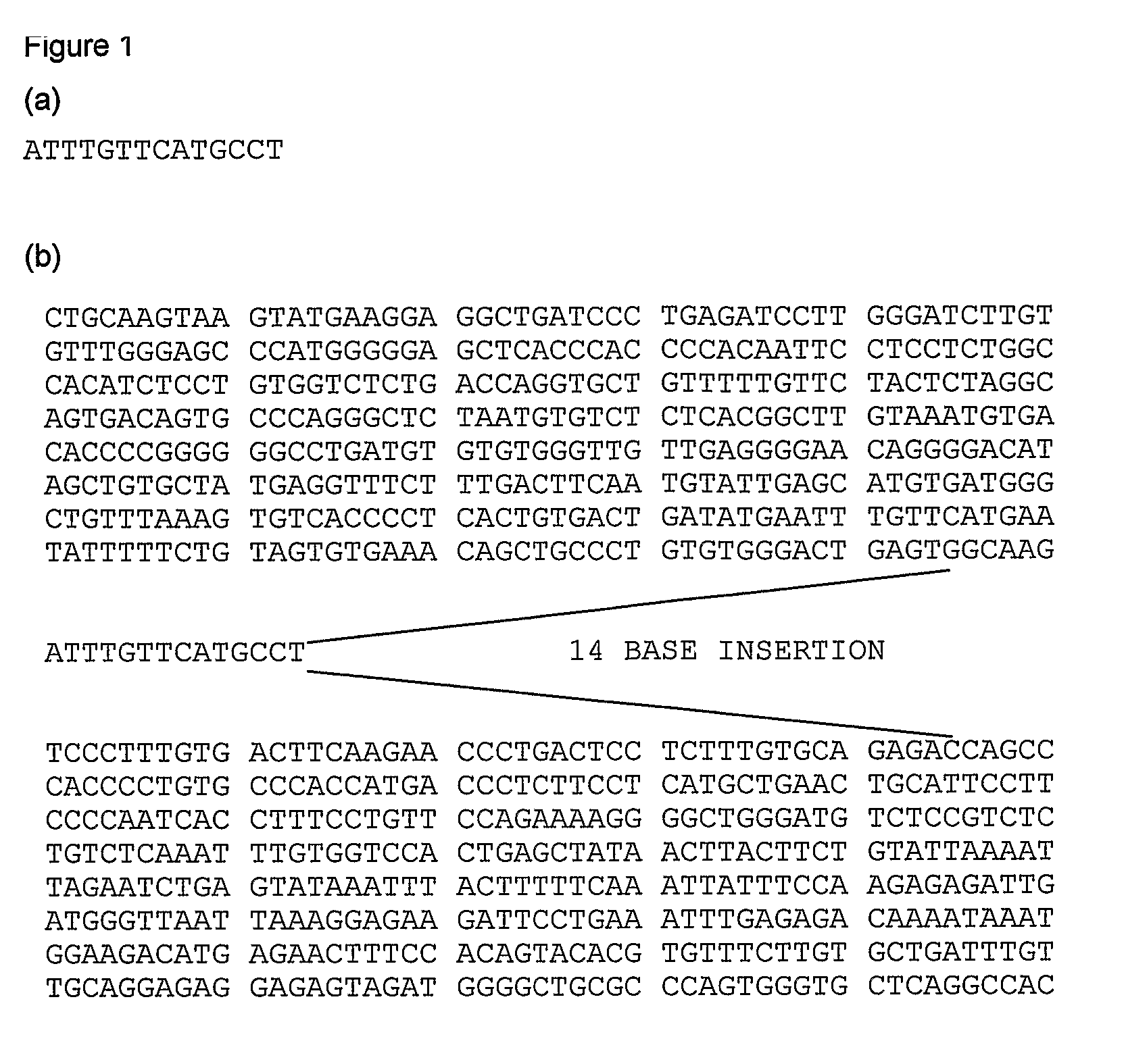
Sequencing-based typing method of human leucocyte antigen (HLA)-Cw gene
InactiveCN101928770BSolve missing detectionAvoid missing detectionMicrobiological testing/measurementHuman leucocyte antigenWhite blood cell
Owner:SHENZHEN BLOOD CENT
Vaccine for Malignant Tumor Treatment
PendingUS20200129602A1Stimulate immune responsePreventing the binding of immune system-side receptorsMacromolecular librariesDisease diagnosisWhite blood cellSpecific immunity
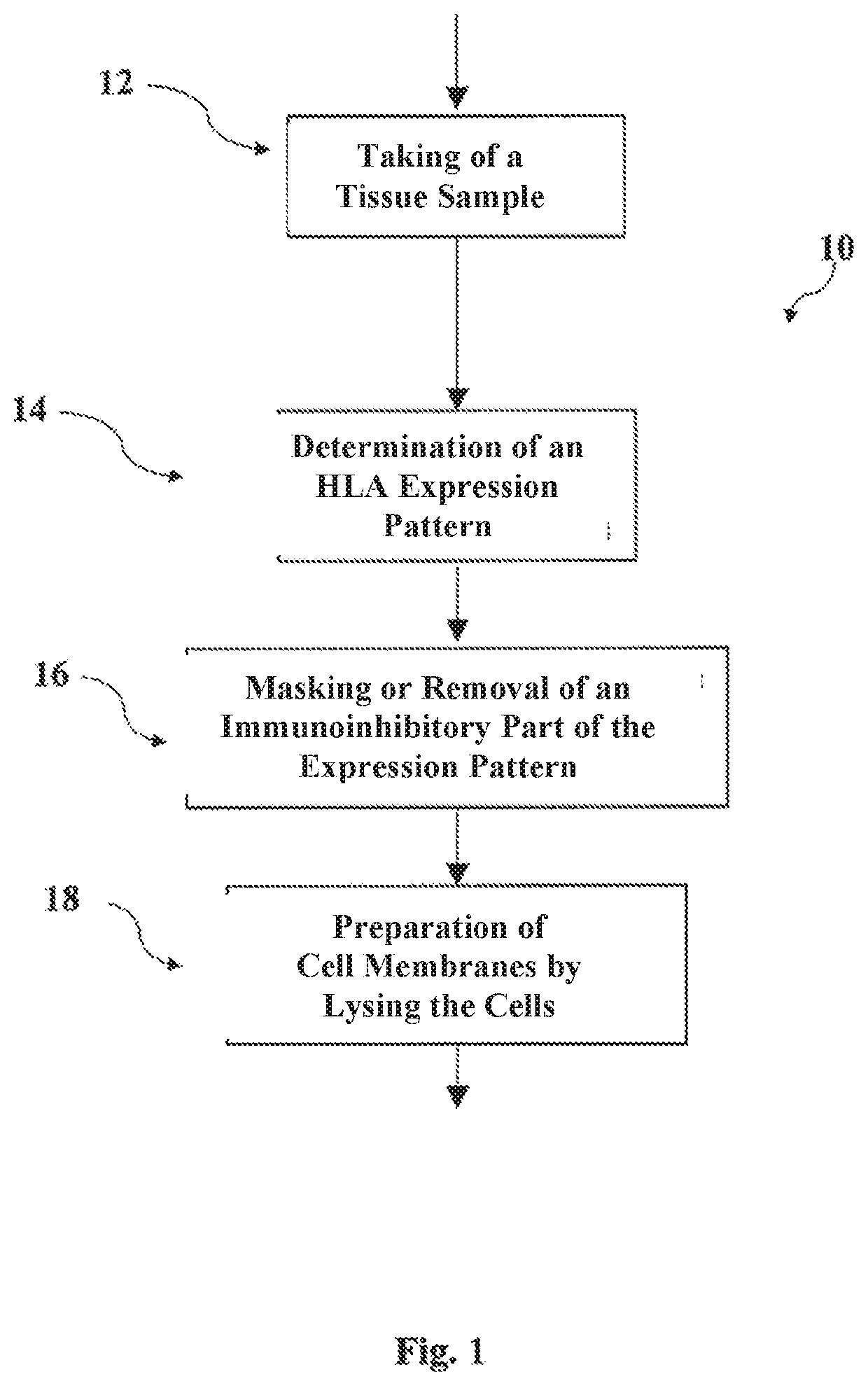
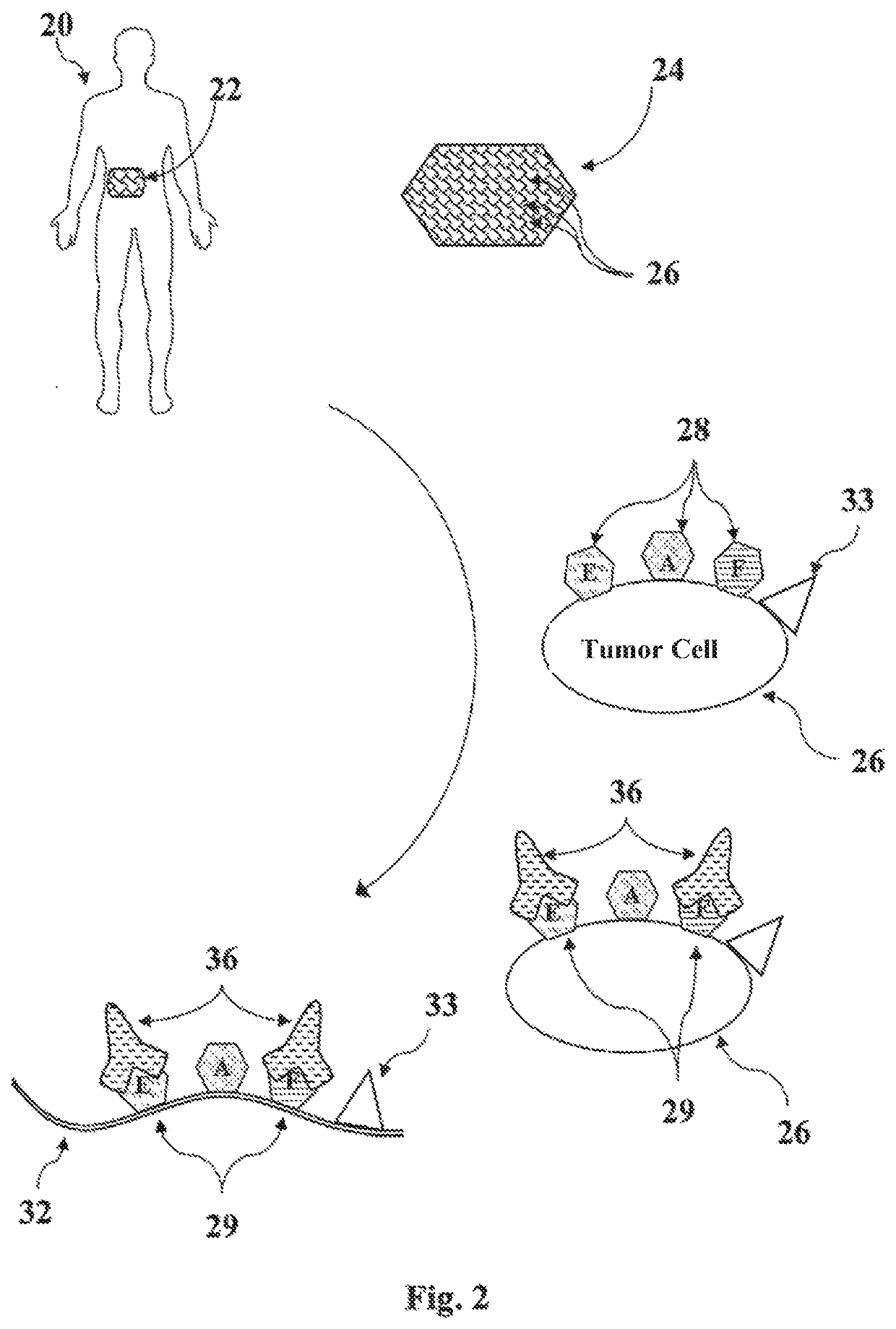
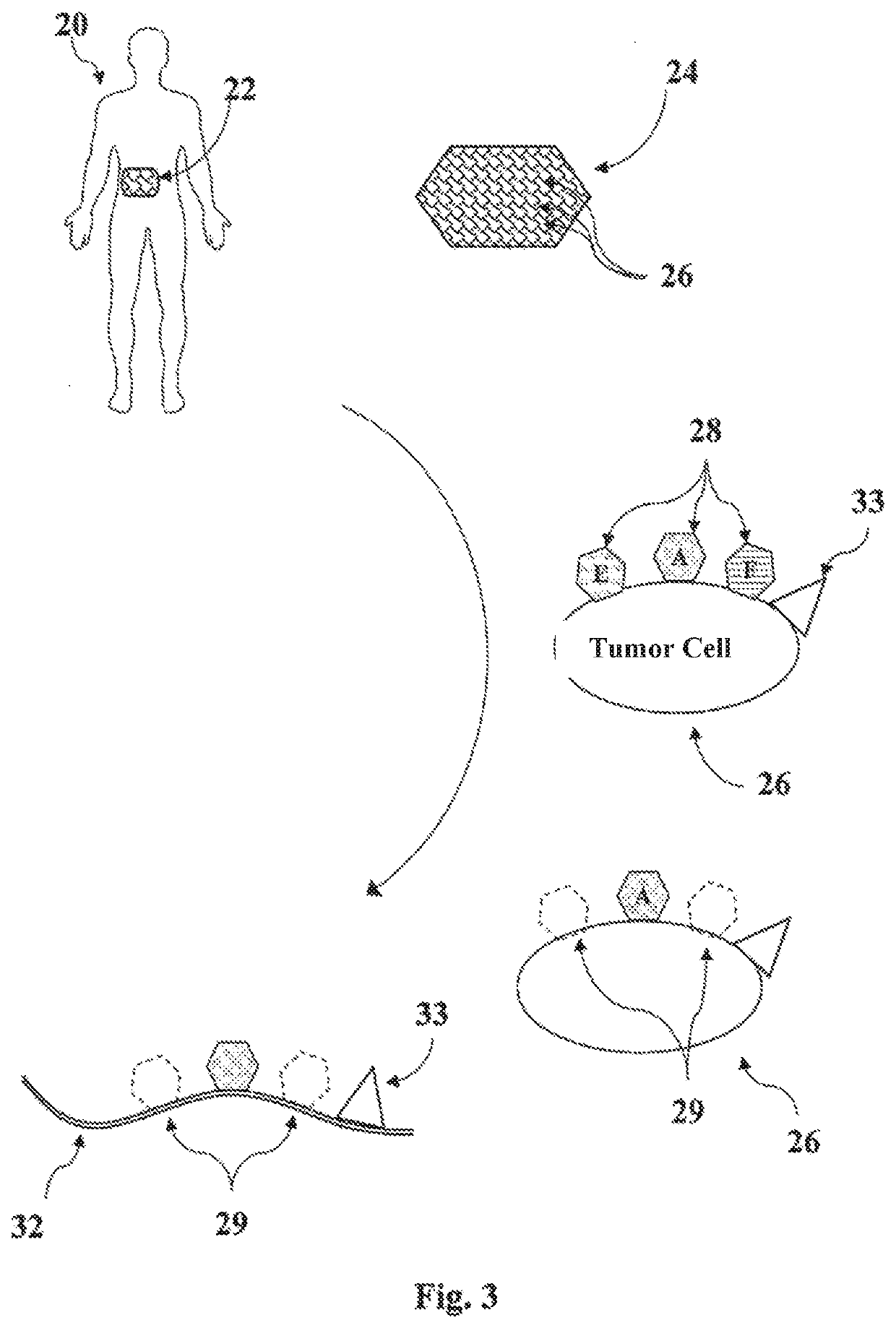
Disclosed are methods for preparing a medicament for treating a malignant tumor, along with cell membranes prepared by such methods and the use thereof, in which the individual communication structure between the malignant tumor and the immune system is ascertained based upon a tissue sample containing cells of the malignant tumor by determining a malignant tumor-specific expression pattern of histocompatibility antigens (Human Leucocyte Antigen, HLA) on said tissue sample, masking or removing at least a part of the expression pattern present on the cells of the tissue sample that is capable of exerting an inhibitory effect on immunocompetent cells, and preparing an individual vaccine for eliciting a specific immunological response by lysing those cells on which a part of the expression pattern has been masked or removed.
Owner:INTELLEXON GMBH
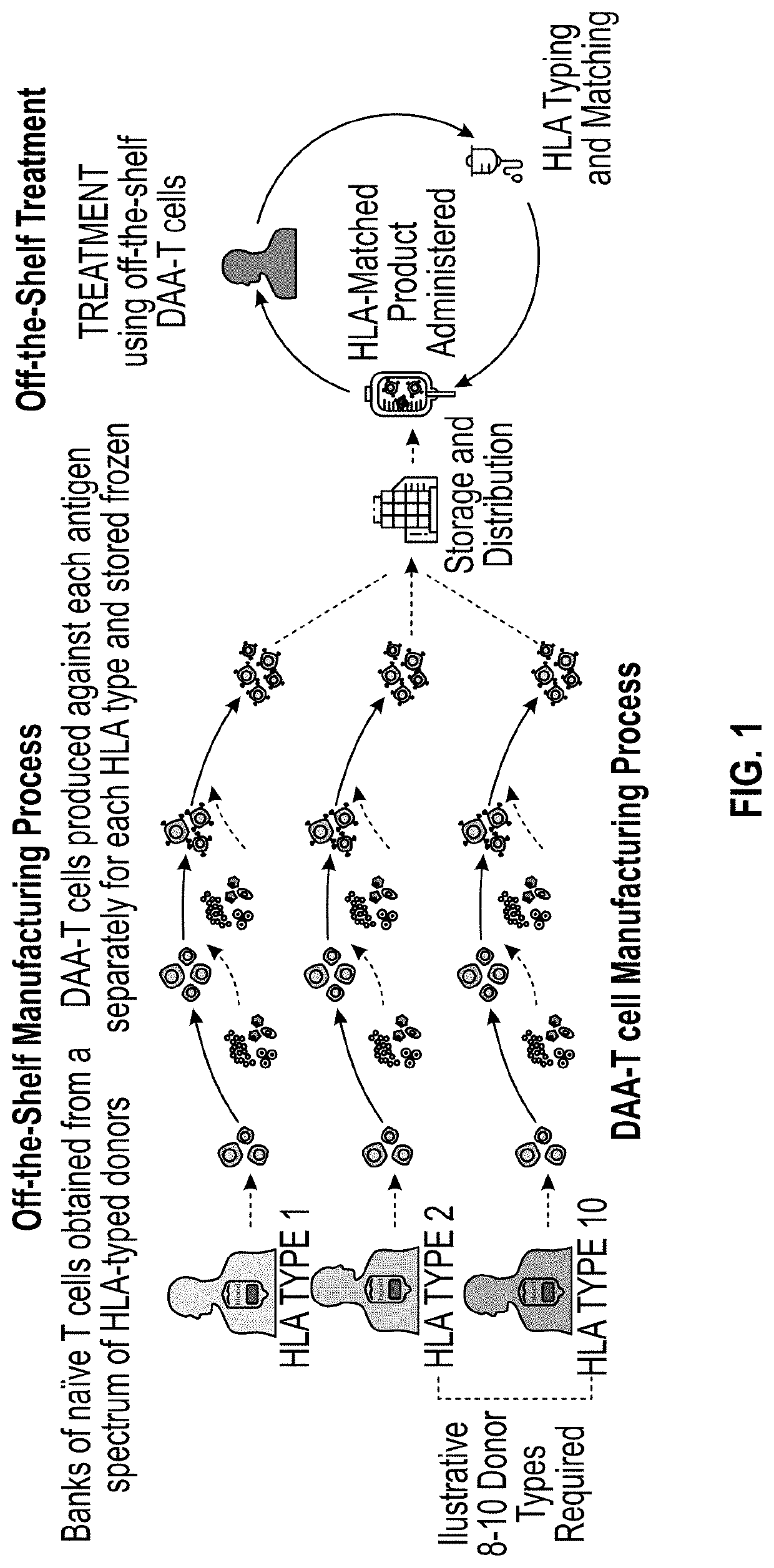
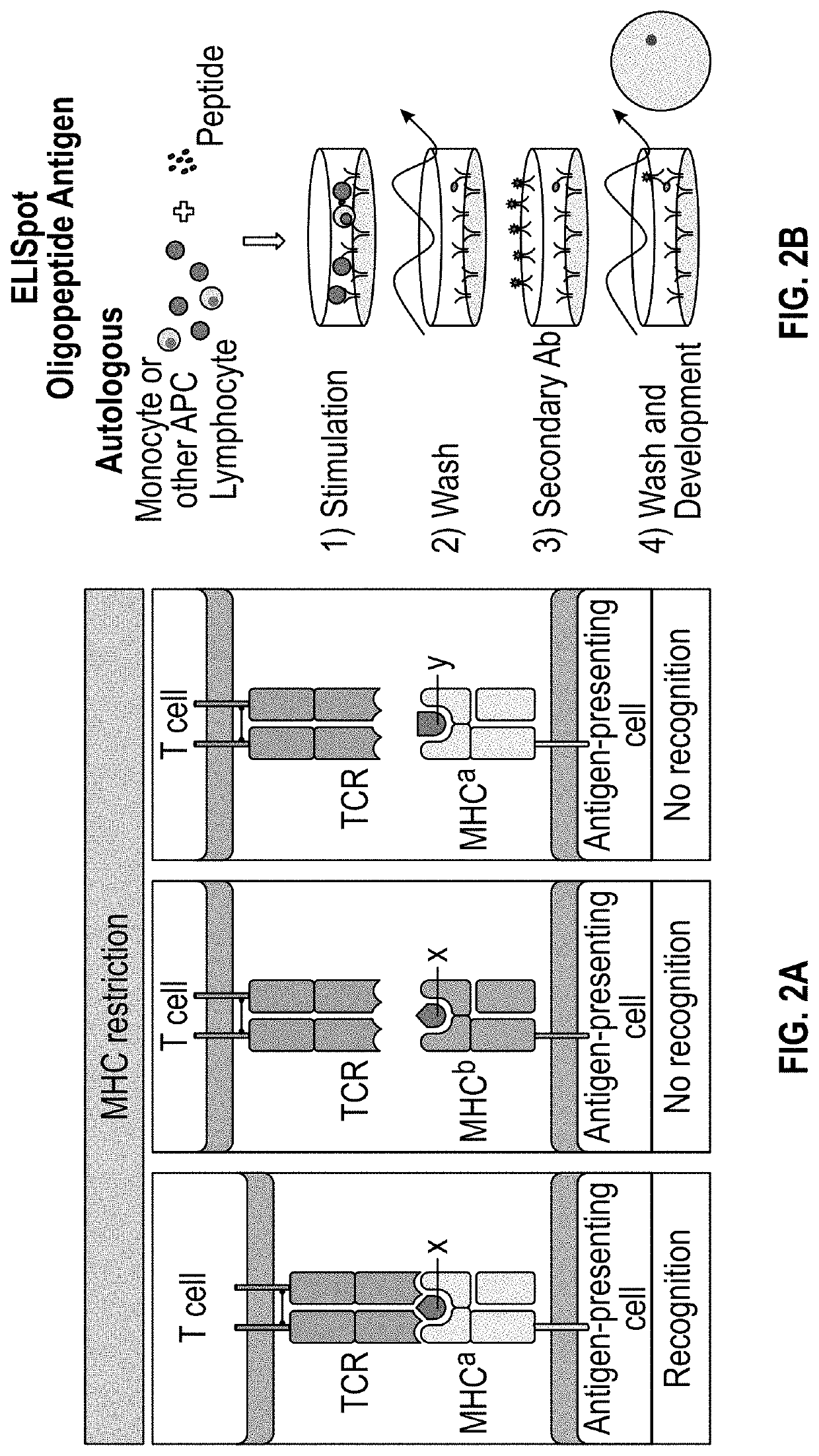
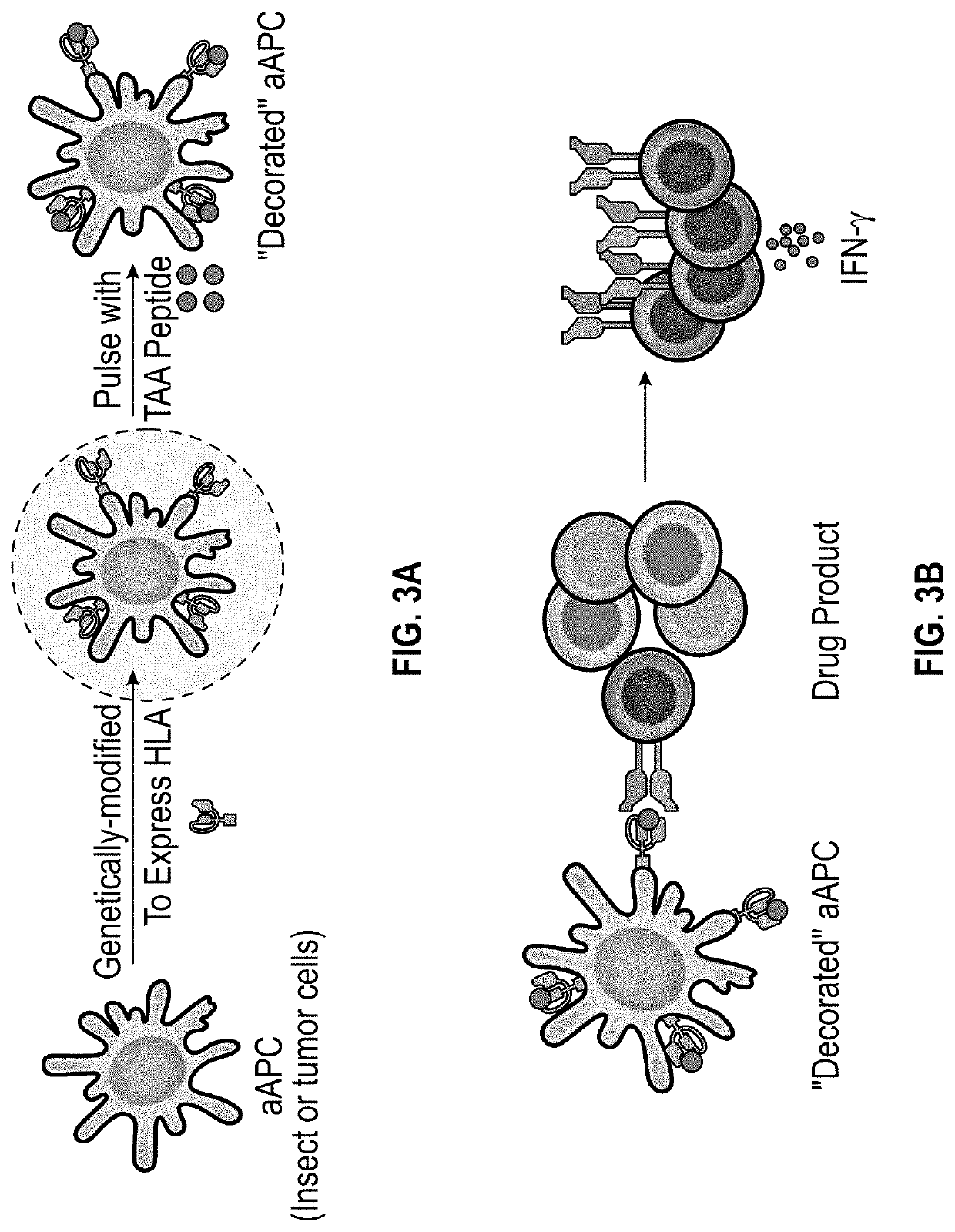
Methods and delivery of allogeneic cell products
The present invention relates to methods for selecting at least one drug product from a cell bank that recognizes a combination of at least one specific disease-associated antigenic peptide with at least one unique human leucocyte antigen (HLA) molecule which closely matches the HLA allele-specific expression profile of a subject. The invention also provides methods for treating a diseased cell expressing at least one disease-associated antigen which comprises the determination of the HLA allele-specific expression profile of the disease cell and the , and the selection and administration of at least one drug product. The invention also relates to a cell bank comprising a plurality of disease-associated antigen-specific T cell subpopulations, each subpopulation of T cells being primed by a plurality of subpopulations of antigen presenting cells (APCs), each subpopulation of APCs being genetically modified to express a unique HLA molecule which presents a specific disease-associated antigenic peptide.
Owner:MANA THERAPEUTICS
HTNV-NP (Hantaan virus nucleoprotein)-specific CTL (cytotoxic T lymphocyte) epitope peptides and application thereof
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
HLA-B27 genotyping detection kit
The invention relates to a gene detection kit used for clinical detection, in particular to the preparation and the usage of a micro-array chip used for HLA-B27 genotyping. A DNA micro-array chip technology is adopted, and the kit can carry out partign to human leucocyte antigen B27, with high pass, high efficiency and high specificity.
Owner:GUANGZHOU DARUI BIOTECH
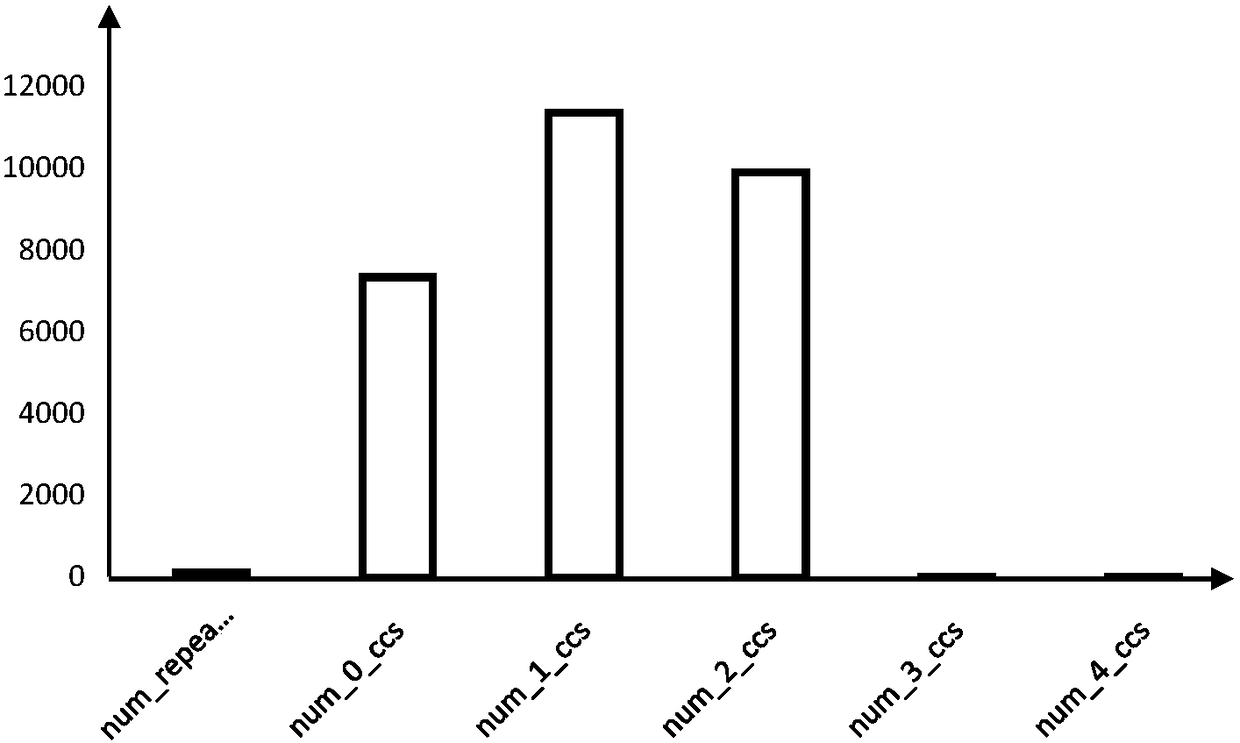
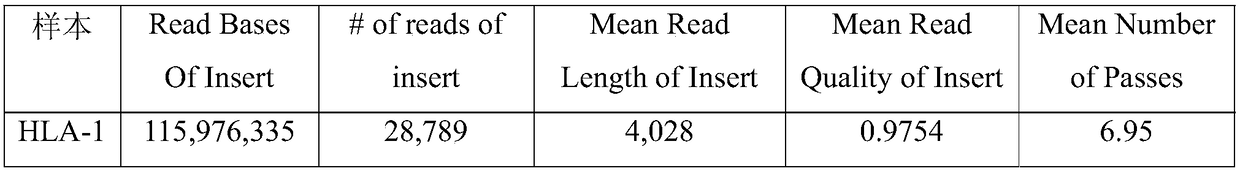
A HLA typing method based on pacbio RS II sequencing platform
Owner:BEIJING GRANDOMICS BIOTECH
Preparation method and kit of dendritic cell vaccine loaded with tumor-specific antigen epitope polypeptide
InactiveCN103800897BOvercoming malignancy resistanceAntibody medical ingredientsMacromolecular non-active ingredientsHuman leucocyte antigenEpitope
Owner:李金珍
Drug response markers
The present invention relates to a method for predicting the response of a disease in a subject, preferably a human, to a drug providing 6-mercaptopurine, the method comprising the step of: (i) determining the presence or absence of a variant allele at a polymorphic site in a human leucocyte antigen (HLA)-G gene wherein the presence of said variant allele at said polymorphic site is indicative of clinical response or tolerance to said drug. The invention also provides a method of treating a subject suffering from a condition that would benefit from a drug providing 6-mercaptopurine.
Owner:GUYS & ST THOMASS NHS FOUND TRUST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com