Kit and method for detecting human leucocyte antigen HLA-B*1502 genetype
A technology of leukocyte antigen and HLA-B, which is applied in the field of kits for detecting the genotype of human leukocyte antigen HLA-B*1502, can solve the problems of many sequencing methods, many steps, expensive chip method equipment, etc., and achieve good specificity Effect with sensitivity, easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
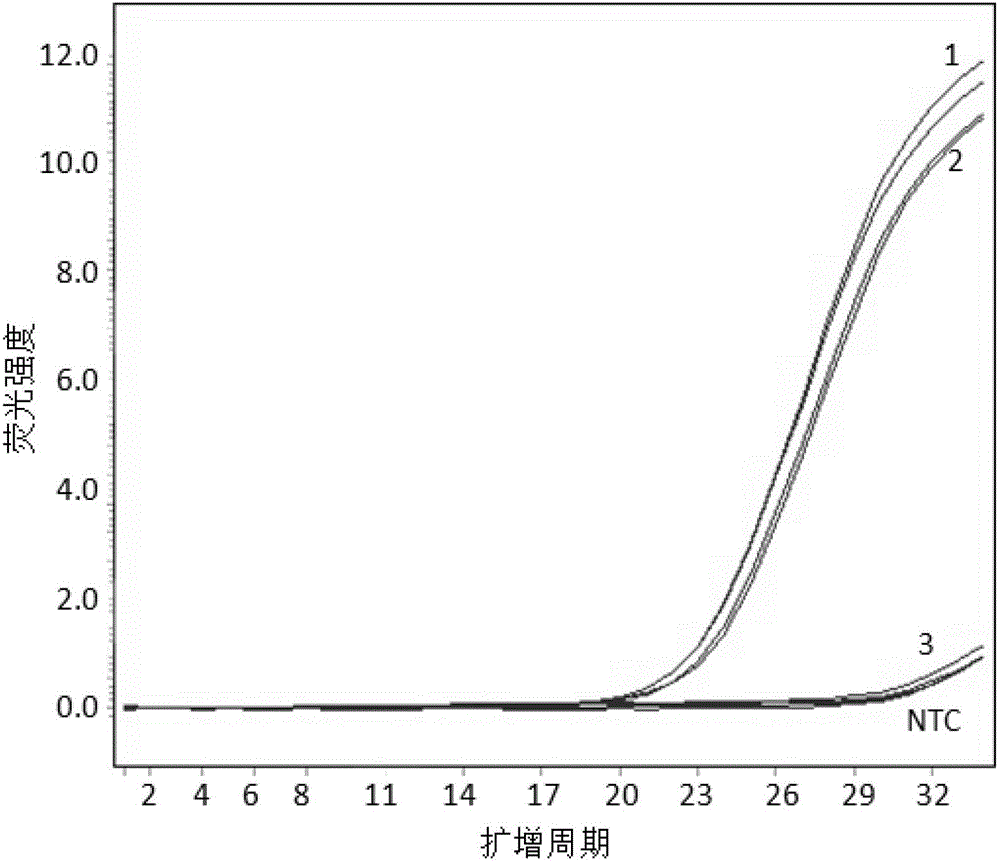
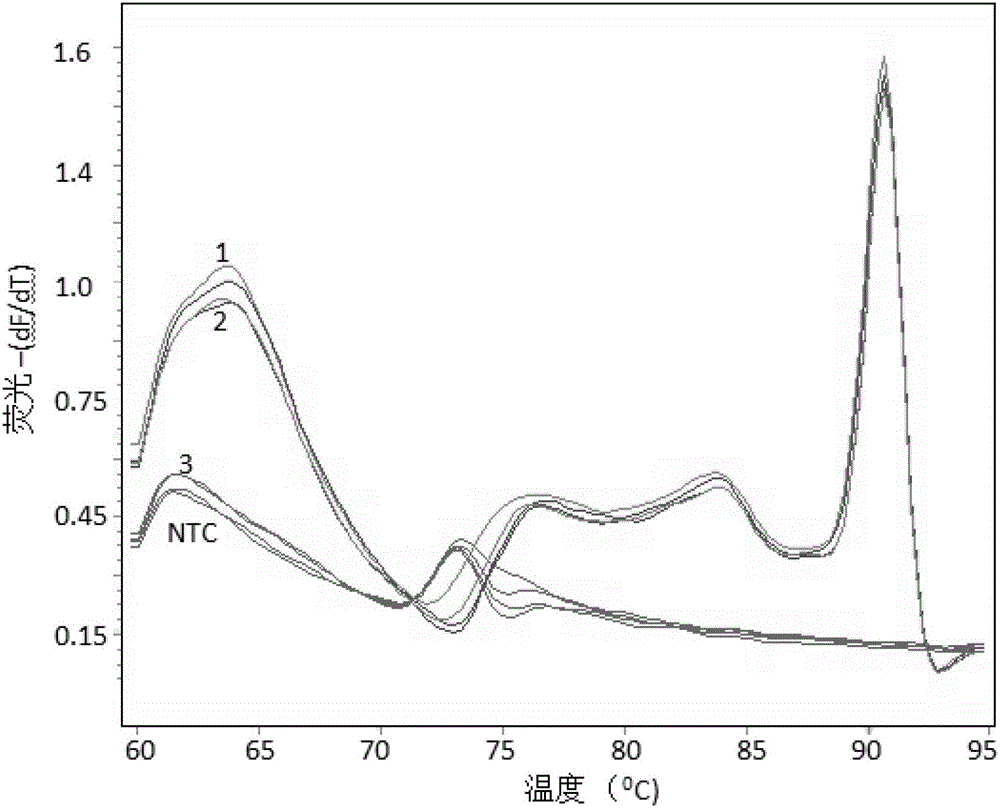
[0028] Embodiment 1, human HLA-B*1502 genotype-specific detection
[0029] 1) Preparation of human peripheral blood genomic DNA.
[0030] According to the routine of the hospital, 1-5ml of peripheral blood was drawn from the subjects, and stored in EDTA blood collection tubes at 4°C for no more than 7 days. Genomic DNA used QIAamp DNA Blood Mini Kit from Qiagen (China). Use 0.2mL peripheral blood for each sample, and dissolve the final product DNA in 100uL sterilized TE buffer and store in a refrigerator at -20°C. Using the above method, the test samples 1, 2 and 3 were respectively obtained; at the same time, no template control (NTC) was set. Sequence analysis confirmed that samples 1 and 2 were positive.
[0031] 2) Specific primers for detecting human HLA-B*1502 genotype:
[0032] a. Upstream primer FP: 5'-CGACGCCGCGAGTCCCAGG-3' (sequence 2 in the sequence listing);
[0033] b. Downstream primer RP: 5'-CGTCGTAGGCGGACTGGTCATA-3' (sequence 3 in the sequence listing);
...
Embodiment 2
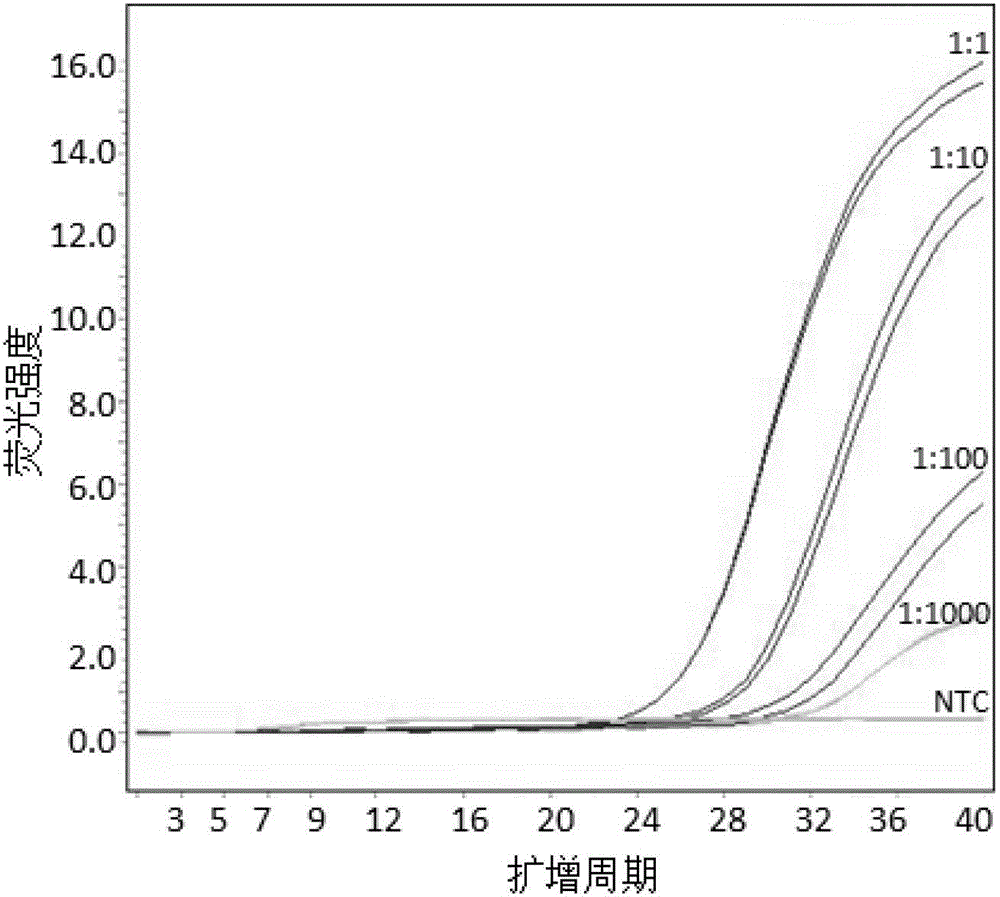
[0042] Example 2, sensitivity detection of human HLA-B*1502 genotype-specific primers
[0043] 1) Preparation of human peripheral blood genomic DNA.
[0044] Take the above-mentioned test sample 1 DNA as a test sample, and its concentration is 20ng / uL. Use sterile TE buffer to dilute the sample 10 times serially, namely 1:1, 1; 10, 1:100, 1:1000; the DNA concentrations are: 20ng / uL, 2ng / uL, 0.2ng / uL, 0.02 ng / uL.
[0045] 2) Specific primers for detecting human HLA-B*1502 genotype:
[0046] a. Upstream primer FP: 5'-CGACGCCGCGAGTCCCAGG-3'
[0047] b. Downstream primer RP: 5'-CGTCGTAGGCGGACTGGTCATA-3'
[0048] c. Probe PR: 5'-Cy5-AACACACAGATCTCCAAGACCAACACAC-p-3'
[0049] Entrust Beijing Aoke Dingsheng Company to synthesize.
[0050] 3) Reaction system for detecting human HLA-B*1502 genotype: 10xPCR buffer 2uL, 25mM MgCl21.2uL, 10mM dNTPs 0.4uL, 0.8uM upstream primer FP 2uL, 8uM downstream primer RP 2uL, 4uM probe PR 2uL, Taq enzyme 5U / uL 0.2uL, 20x SYBR fluorescent dye 1...
Embodiment 3
[0056] Embodiment 3, the comparison of human HLA-B*1502 genotype detection method and gold standard
[0057] 1. The preparation of 100 cases of normal human peripheral blood genomic DNA is the same as in Example 1.
[0058] 2. Using a 96-well special PCR plate, high-throughput HLA-B*1502 genotype detection can be performed simultaneously.
[0059] The reaction system of the HLA-B*1502 genotype, the amplification procedure, and the identification of the PCR product are the same as in Example 1.
[0060] 3. HLA-B*1502 nucleic acid sequence analysis
[0061] a) Specific primers for amplifying exon 2 of human HLA-B*1502:
[0062] a. Upstream primer FP1: 5'-CCCAGGCTCCCACTCCATGA-3' (sequence 5 in the sequence listing),
[0063] b. Downstream primer RP1: 5'-CGGCCTCGCTCTGGTTGTAGT-3' (sequence 6 in the sequence listing),
[0064] Entrust Beijing Aoke Dingsheng Company to synthesize.
[0065] b) Sequencing-specific primers for HLA-B*1502 exon 2:
[0066] a.5'-CCCAGGCTCCCACTCCATGA-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com