Common donor stem cell and preparation method thereof
A technology of stem cells and donors, applied in the field of stem cells, can solve the problems of iPSCs such as expensive, immune rejection, and variability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 HLA Class I Project
[0065] Since HLA-I is a dimer composed of α chain and β chain, and all HLA-I share the same β chain, knocking out the B2M gene encoding the β chain can achieve the knockout of HLA-I; However, for some cells, HLA-I knocked out cells will be lysed by NK cells. In order to overcome this "missing self" reaction, the inventors knocked out the HLA single chain while knocking in the heavy chain of the non-polymorphic HLA-E gene to inhibit the lysis of NK cells.
[0066] 1. Design of sgRNA
[0067] 1.1 Selection of sgRNA design location
[0068] According to the gene name B2M searched in NCBI, B2M has four exons. In order to completely knock out B2M without affecting the expression of other proteins, the inventor designed sgRNA on the first exon. The search can be found in NCBI, in the UCSC database, or in geneious software. In the future, SgRNA can be designed on the transcript shared by this gene. Generally, the gene-specific sgRNA template sequenc...
Embodiment 2
[0155] Example 2 HLAⅡ Project
[0156] Unlike HLA class I, class II proteins lack common subunits that can be edited to prevent surface expression. MHC class II deficiency is a combined immunodeficiency caused by defects in the four regulatory factors CIITA, RFXANK, RFX5 and rfxp, which control the expression of MHC II at the transcriptional level. The RFXANK gene encodes a subunit of the heterotrimeric RFX complex, which is involved in the assembly of several transcription factors on the MHC II promoter. Therefore, the present invention edits two copies of the RFXANK transcription factor gene required for MHC class II expression. Patients with RFXANK mutations do not express HLA class II molecules on their antigen presenting cells. And transferred to iCaspase to enhance its safety.
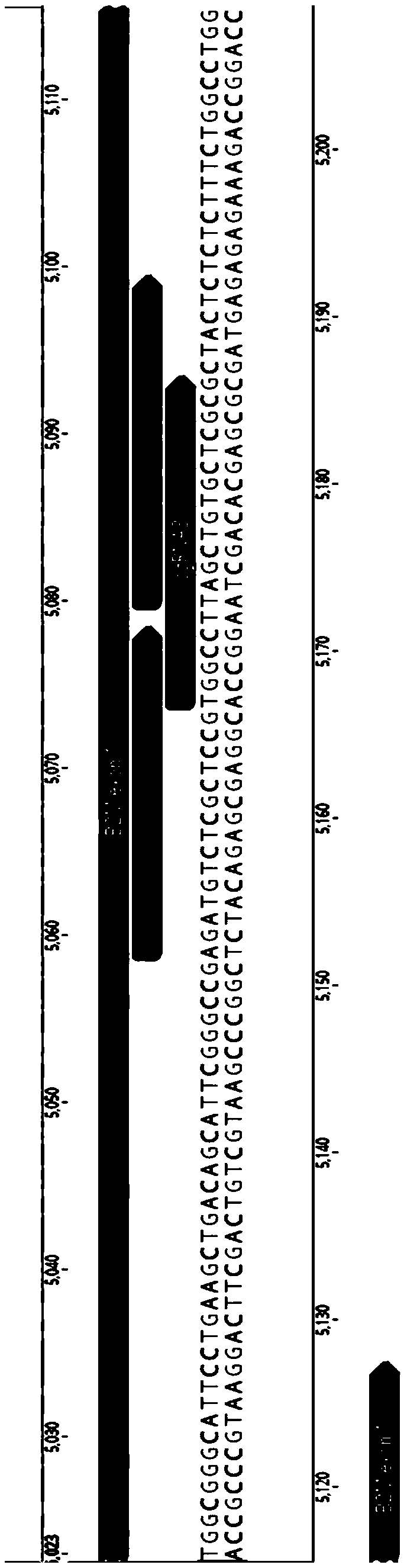
[0157] The relative positions of the three sgRNAs targeting the RFXANK site are as follows Picture 10 As shown, the sequences are as follows:
[0158] GCAGAAGACCTCATCCAGAC (SEQ ID NO: 22)
[0159] T...
Embodiment 3
[0184] Example 3 Differentiation of modified embryonic stem cells
[0185] Modified embryonic stem cells can differentiate into various cell types with reduced or absent HLA expression. Examples of these cell types include mesenchymal progenitor cells (MPC), low immunogenic cardiomyocytes, endothelial cells (EC), macrophages, hepatocytes, leukocytes (e.g. pancreatic leukocytes) or neural progenitor cells (NPC).
[0186] Initially, the inventors reduced MHC-I expression in B2M- / - and B2M- / -RFXANK- / - human ES cells. The inventors then examined the expression of MHC-I in various differentiated cell types. For example, the expression of MHC-I and MHC-II decreased in B2M- / - and B2M- / -RFXANK- / - human mesenchymal progenitor cells (MPC) ( Figure 14 , Figure 15 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com