Method and related kit for detecting HER-2/CEP17 gene status based on rare cells
A HER-2 and rare cell technology, applied in the field of medical diagnostics, can solve the problems of inconsistent HER-2 expression, dynamic changes in molecular information, and difficulty in specimen collection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
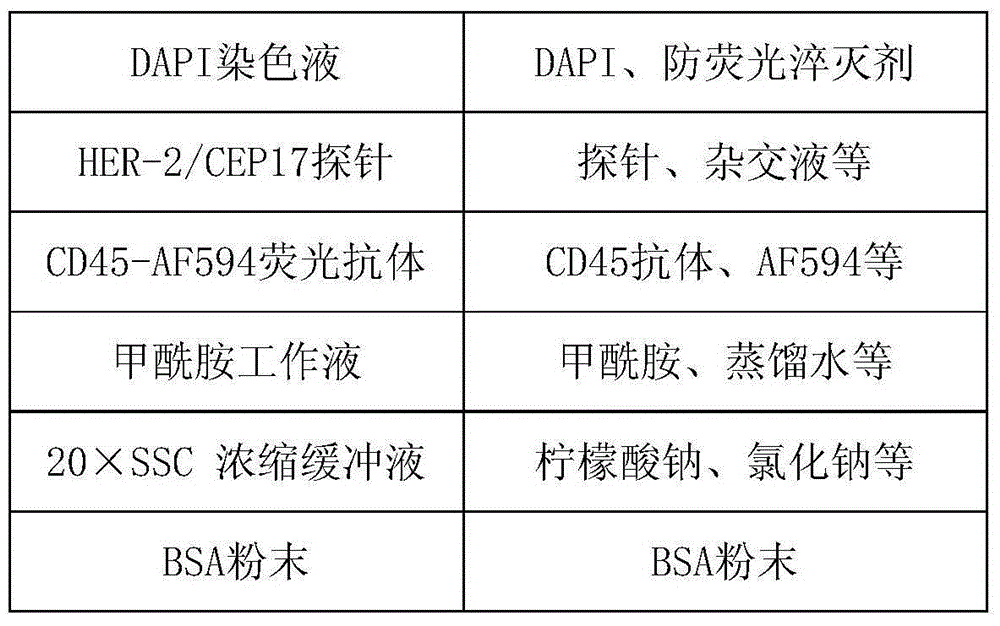
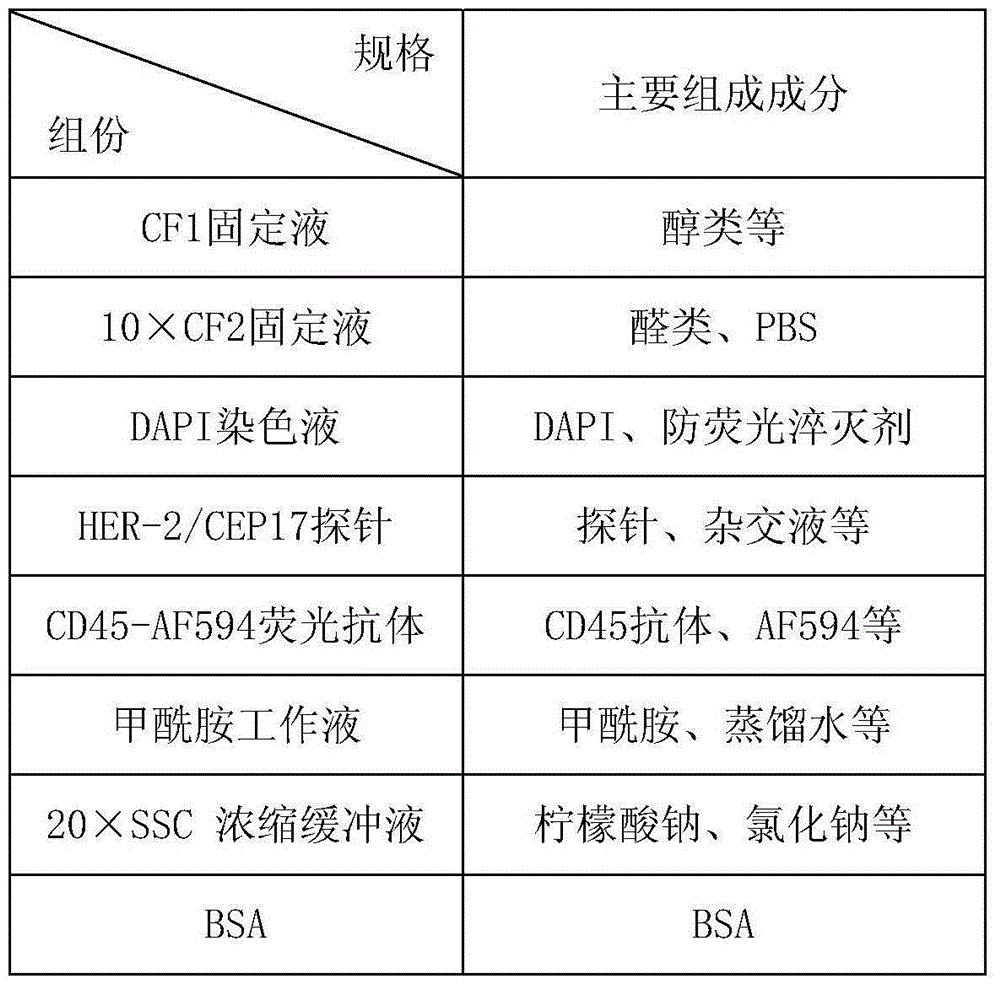
[0122] Detection of a pair of blood samples using the kit
[0123] Test the blood samples of 20 cases of normal people, 20 cases of benign breast diseases, and 20 cases of breast cancer patients. The method is to add 3.2mL blood samples to a 50mL centrifuge tube, add CS1 working solution, centrifuge at 650×g for 5 minutes, and discard the supernatant ; Add CS2 working solution to lyse for 8 minutes, centrifuge at 650×g for 5 minutes, and discard the supernatant; add a certain amount of CS1 working solution again, add 200uL of magnetic particle suspension, and shake well for 20 minutes; absorb all the mixed liquid, Superimpose on the CS3 separation medium, centrifuge at 300×g for 5 minutes; pipette the liquid except the magnetic particle precipitation into a 15mL centrifuge tube, add CS1 working solution to 15mL, centrifuge at 950×g for 5 minutes, discard the supernatant; add 1mL of CS1 for working solution, mixed by pipetting and added to a new 2mL centrifuge tube, magnetic se...
Embodiment 2
[0127] The preparation of kit of the present invention
[0128] Kit one:
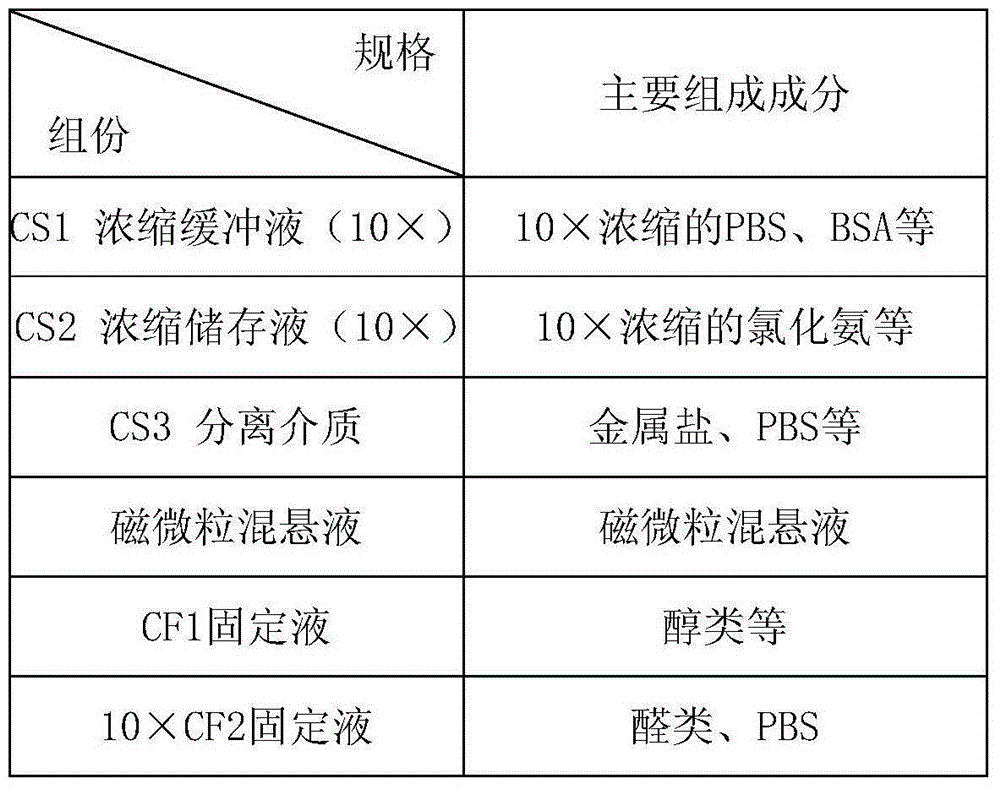
[0129] CS1 Concentrated Buffer (10×):
[0130] Each 1000mL water contains 60gBSA, 5 packs of PBS powder (2L / packet), 100mL0.5M EDTA, 0.8mLProclin300.
[0131] CS2 concentrated stock solution (10×):
[0132] Weigh 82.9gNH per 1000mL of water 4 Cl, 10gKHCO 3 , 0.37gEDTA, water and 0.8mLProclin300, fully stirred and dissolved, constant volume, and prepared as a 10X concentrated solution.
[0133] CS3 separation medium:
[0134] Dilute the gradient centrifugate with a density of 1.077, and test the density during the dilution process to make the density between 1.070-1.075.
[0135] Magnetic particle suspension:
[0136] Adjust the concentration of CD45 antibody to 1mg / mL, and incubate with streptavidin immunomagnetic beads at a ratio of 100uL:1mL for 1h to prepare a suspension of magnetic particles.
[0137] CF1 fixative:
[0138] Mix PEG and absolute ethanol so that the final concentration of PEG...
Embodiment 3
[0155] Use the test kit of the present invention to detect a pair of other body fluid samples
[0156] Note: This kit is not only suitable for blood, but also for the detection of rare cells in other body fluids, such as pleural effusion, ascites, toilet fluid, amniotic fluid, etc., but not limited to these types of body fluids.
[0157] The ascites of 6 cases of gastric cancer were detected by using this kit, and CEP17 amplified positive cells were all found, with a median value of 9.5 (range 4-15). Among them, HER-2 amplified positive cells were found in 2 cases, the numbers were 2 and 5 respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com