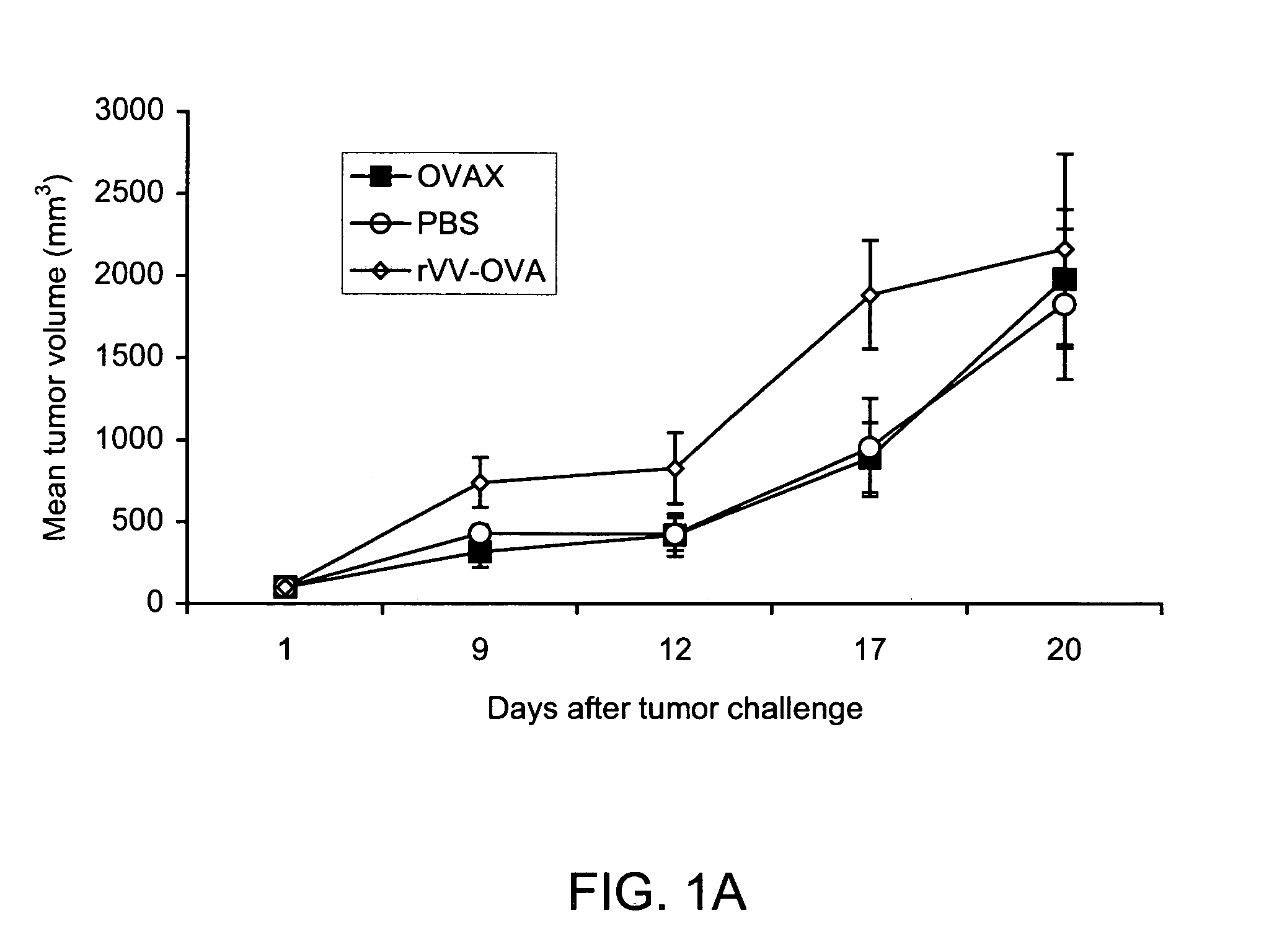
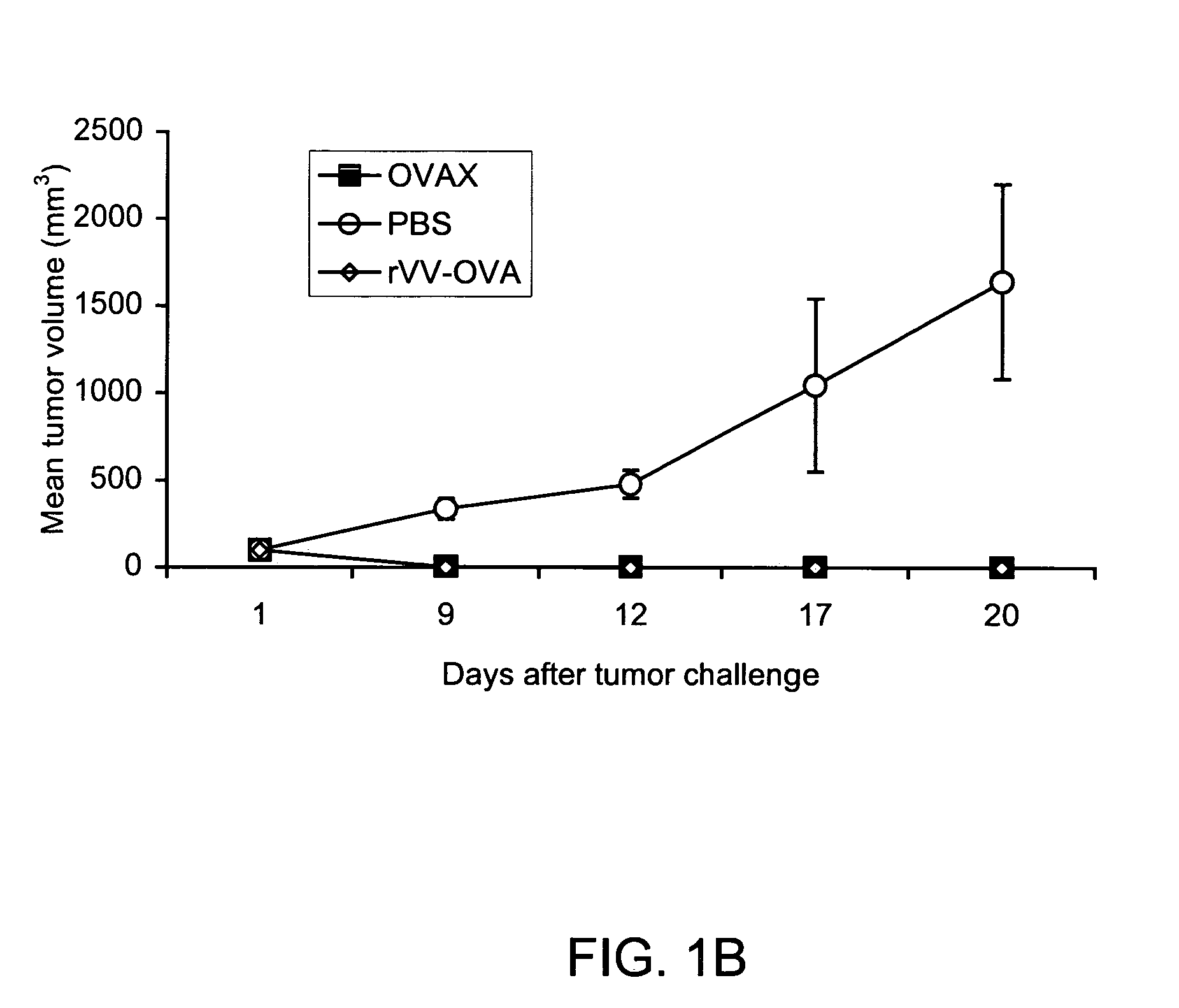
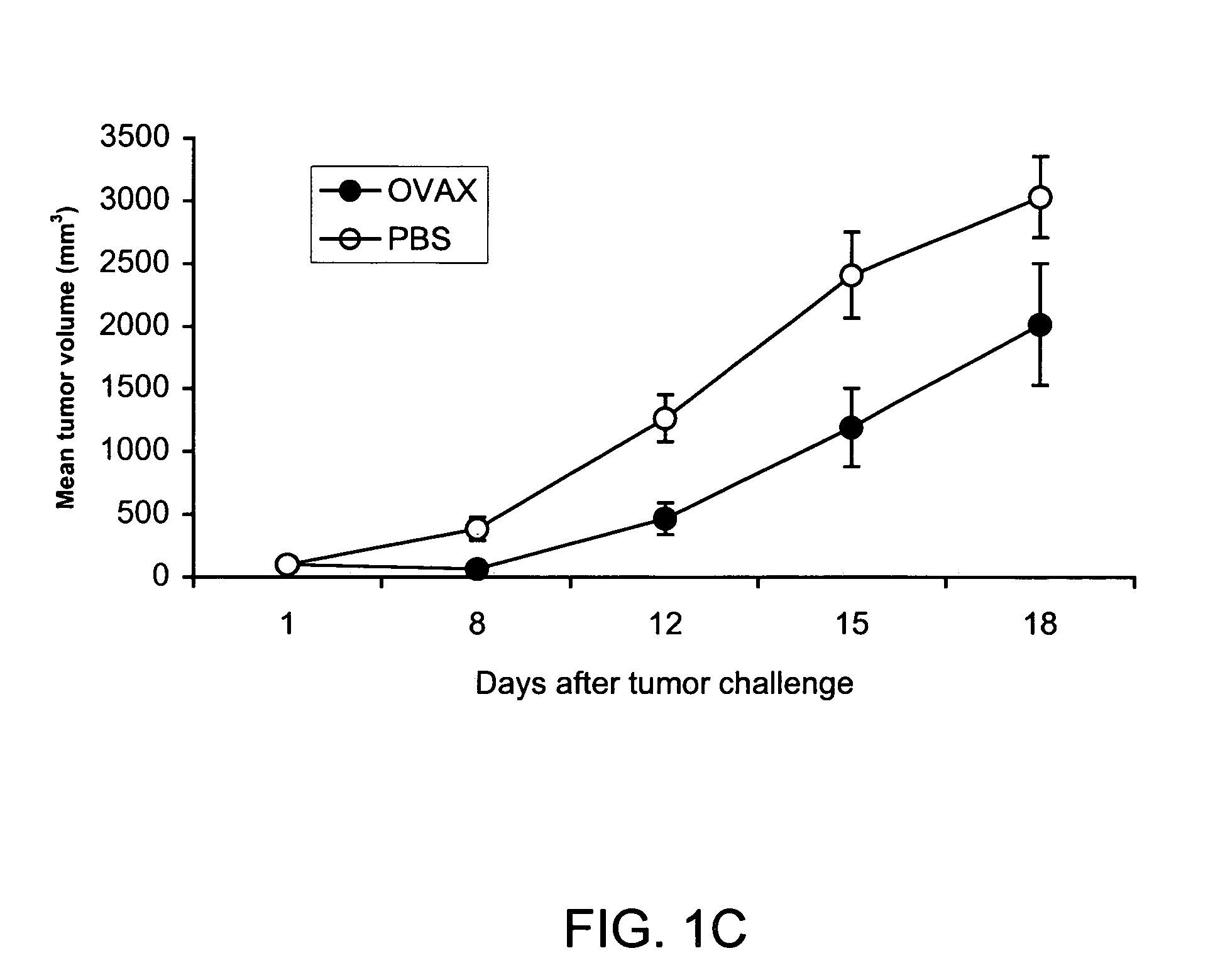
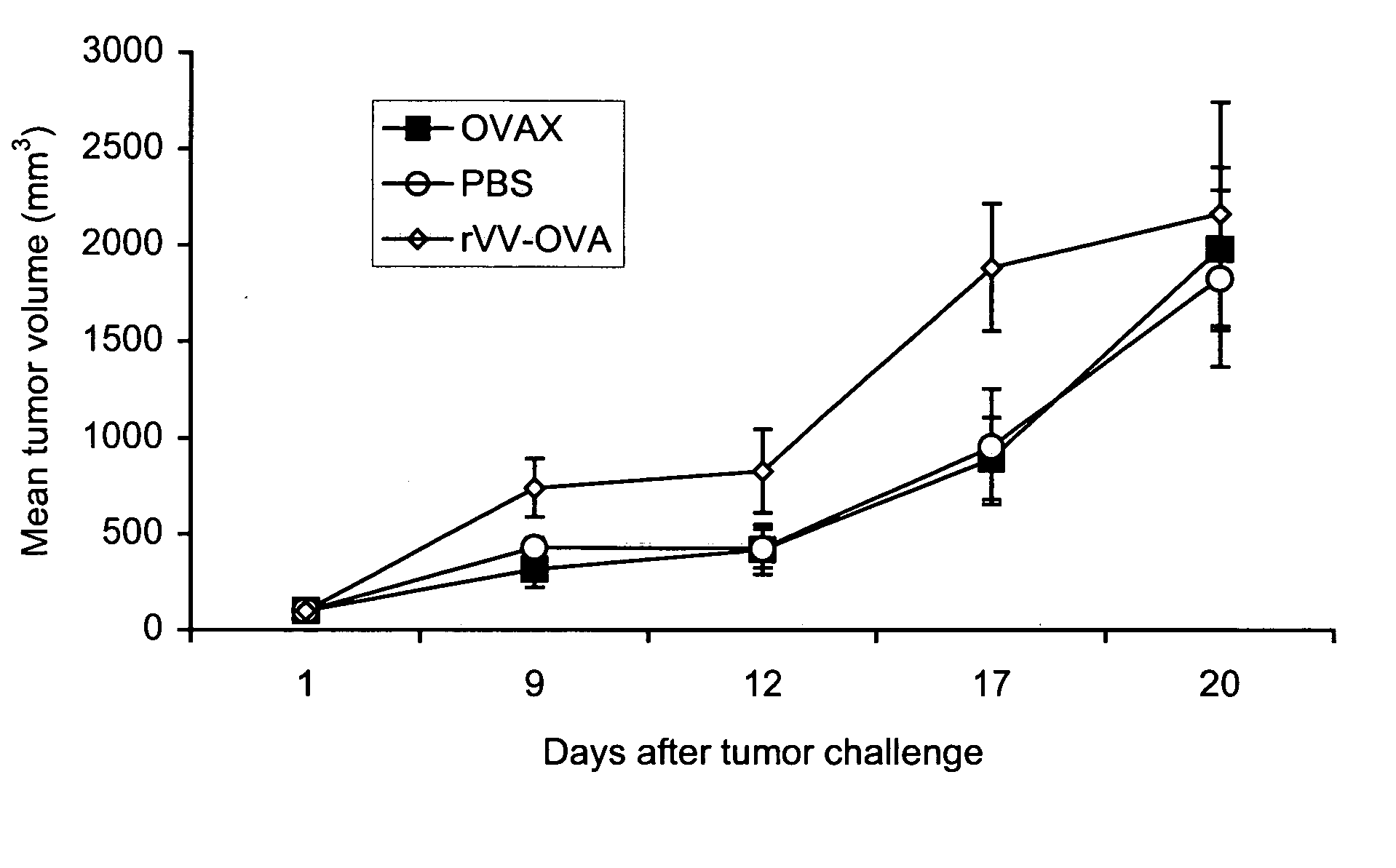
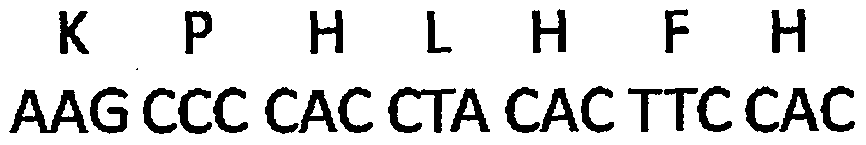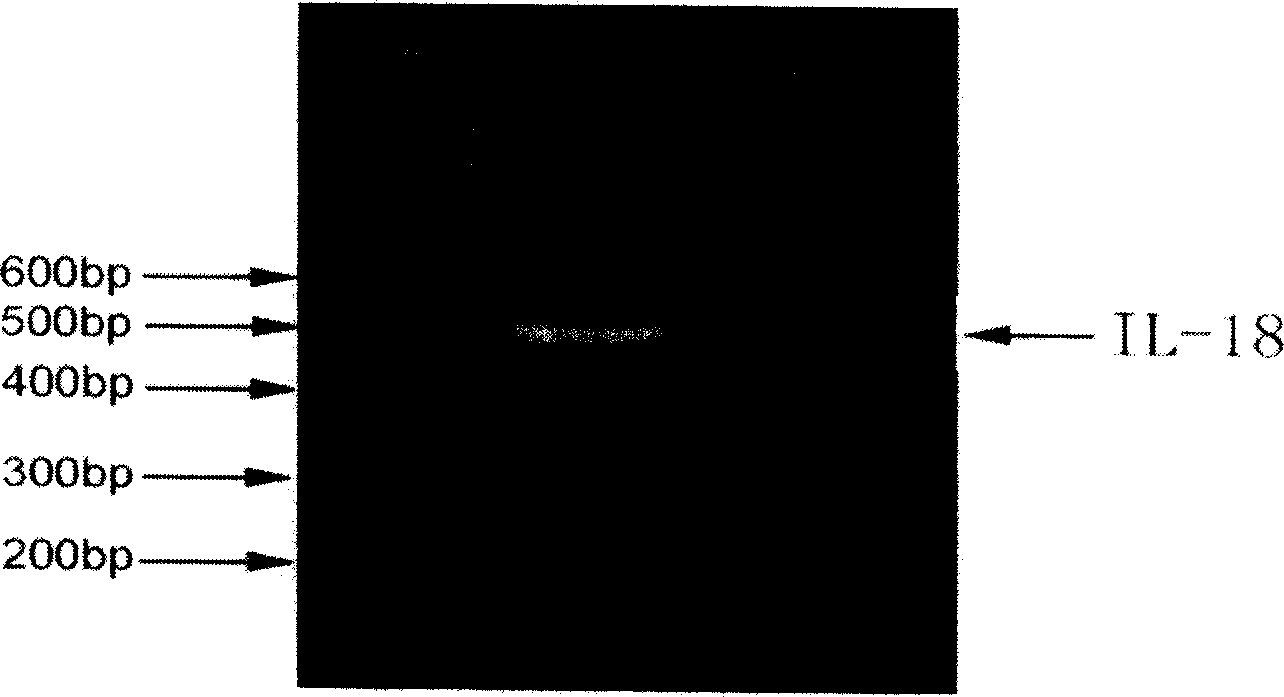Patents
Literature
85 results about "Dendritic cell vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dendritic cell vaccine. An anticancer vaccine made by extracting dendritic (antigen-presenting) cells from a patient with cancer, stimulating those cells to reproduce themselves, and then exposing them to antigens taken from the patient's cancer.
Yeast-dendritic cell vaccines and uses thereof
Owner:UNIV OF COLORADO THE REGENTS OF +1
Yeast-dendritic cell vaccines and uses thereof
InactiveUS20060104986A1Enhance immune responseExtended half-lifeBiocideViral antigen ingredientsBody fluidHumoral immune reaction
Disclosed is a vaccine that includes a dendritic cell loaded with a yeast vehicle and antigen. Also disclosed are methods of making the vaccine and using the vaccine to elicit cellular and humoral immune responses in a mammal. Additionally, a method to elicit an immune response by administration of a yeast vehicle and an antigen that is not complexed to the yeast vehicle is disclosed.
Owner:UNIV OF COLORADO THE REGENTS OF +1
Preparation method of dendritic cell (DC) vaccine loaded with autologous tumor associated holoantigen
ActiveCN102091327ASignificant technological progressConvenient for clinical operationBlood/immune system cellsAntibody medical ingredientsCytotoxicityT lymphocyte
The invention belongs to preparation of biological cell formulations, and in particular relates to a preparation method of dendritic cell (DC) vaccine loaded with autologous tumor associated holoantigen. The preparation method comprises the following steps: preparing the autologous tumor associated holoantigen, collecting and separately culturing DCs, impacting the DCs by the autologous tumor associated holoantigen, maturing the DCs and preparing autologous tumor antigen specific DC vaccine. The invention solves the problems in the prior art that the immunogenicity of tumor antigen is not strong enough, the antigen target spots are incomplete, the tumor antigens of most tumor patients are difficult to acquire, and the like. The DC vaccine provided by the invention has the advantages of effectively inducing tumor antigen specific cytotoxic T lymphocyte (CTL) in vitro and in vivo, efficiently generating specific cytotoxicity on tumors, having high overall effective rate and no obvious toxic side effects in clinical application, and the like.
Owner:玥特农生物科技河北有限责任公司
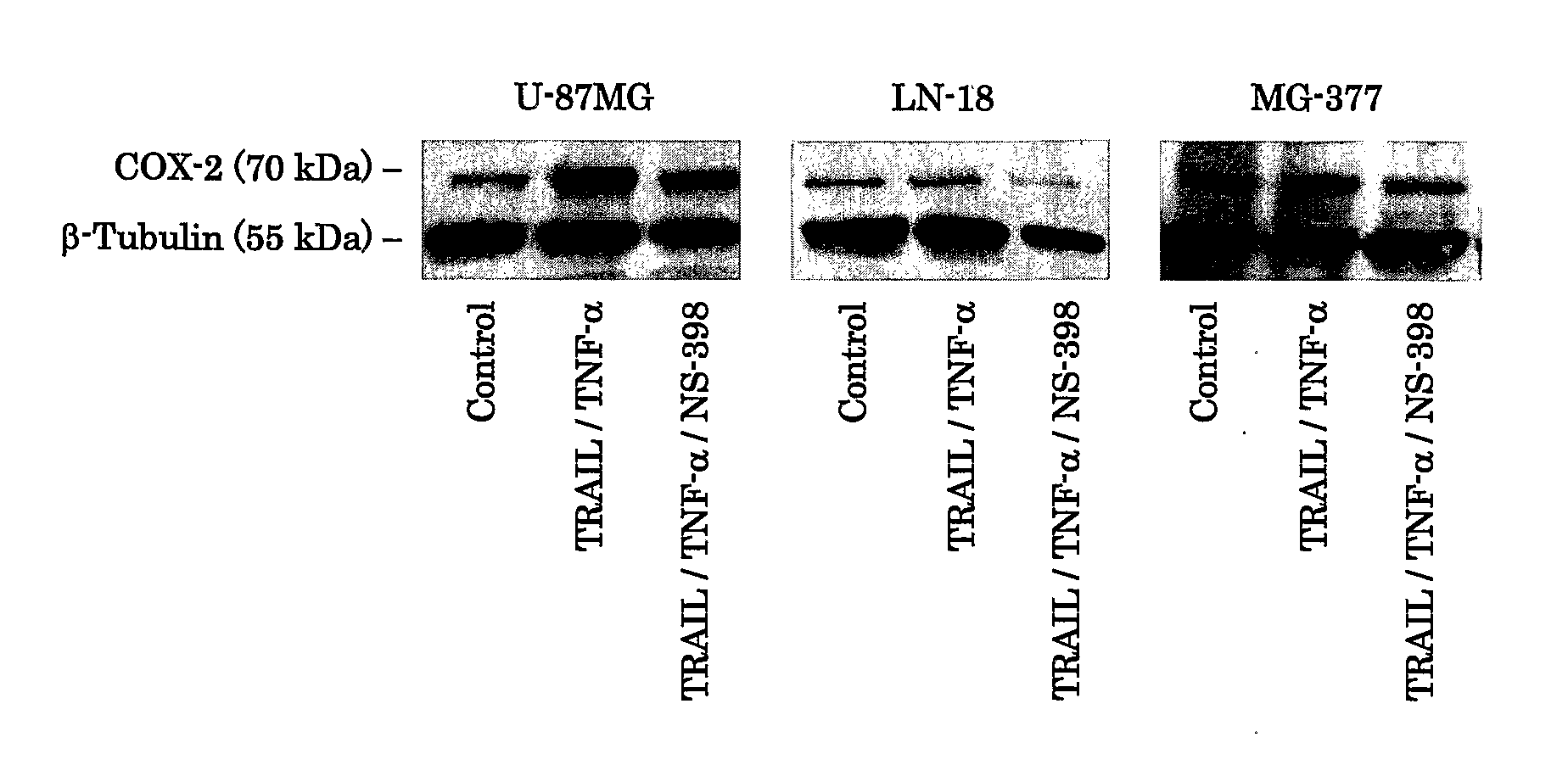
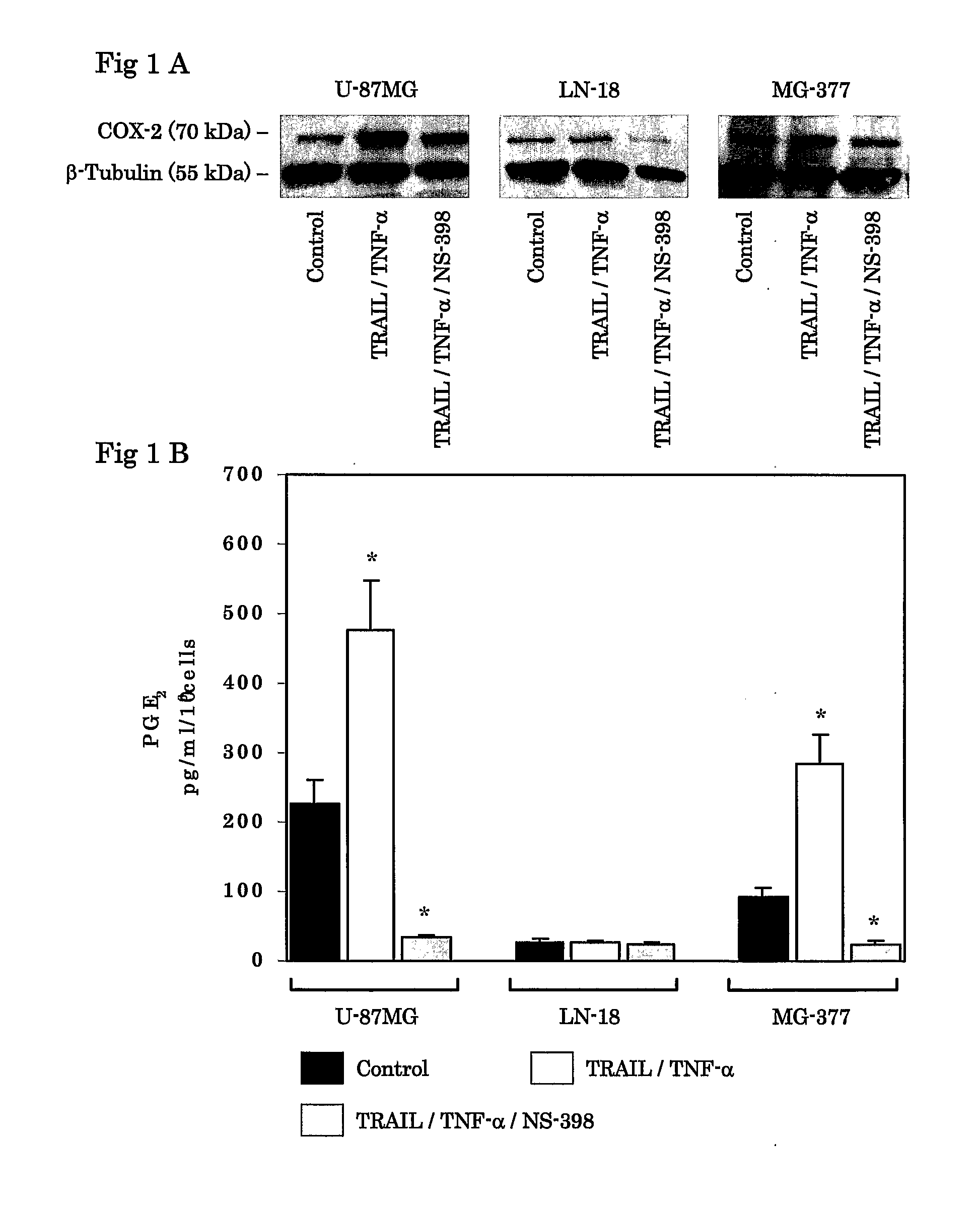
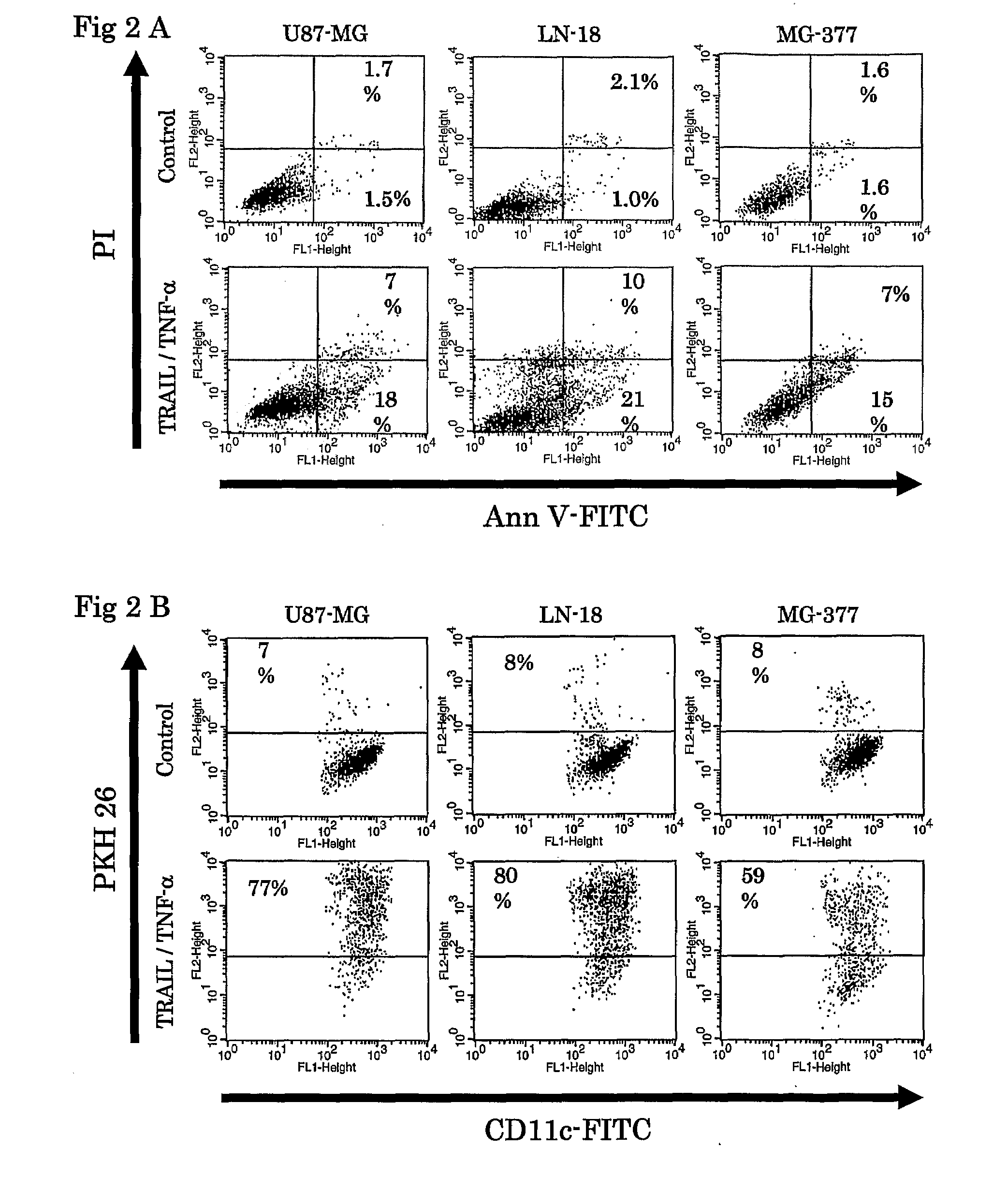
Use Of Cox-2 Inhibitor to Prevent T-Cell Anergy Induced By Dendritic Cell Therapy
InactiveUS20080199484A1Good treatment effectOrganic active ingredientsSnake antigen ingredientsT lymphocyteWilms' tumor
The present invention relates to a method and combination therapy useful in the treatment of cancer. More specifically, the invention relates to the use of COX-2 inhibitors in combination with a therapeutic dendritic cell vaccine for treating cancer. The COX-2 inhibitors of the present invention are believed to inhibit the enzymatic activity of prostaglandineE2 (PGE2); thereby preventing COX-2 overexpressing tumors from evading immune surveillance by antigen-specific cytotoxic T lymphocytes (CTLs). COX-2 inhibitors are believed to suppress PGE2 that COX-2 overexpressing glioma produce, allowing tumor-infiltrating DCs to polarize Th cells toward Th-subset-1 (Th1).
Owner:CEDARS SINAI MEDICAL CENT
Special-purpose kit for preparing human dendritic cell vaccines
InactiveCN103638517AHigh selectivityAchieve the purpose of preventionAntibody medical ingredientsAntineoplastic agentsHigh risk populationsMicrobiology
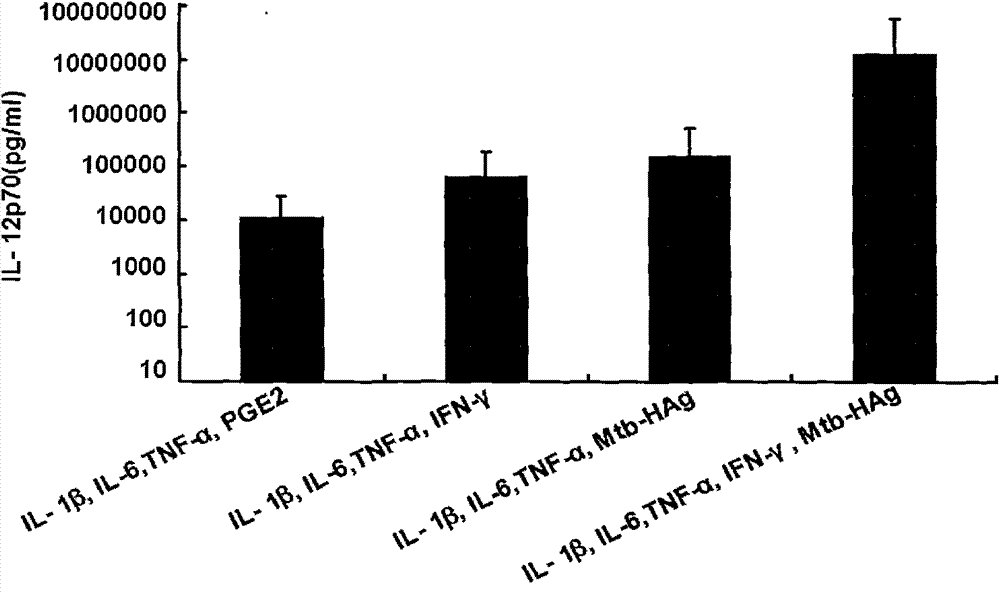
The invention belongs to the technical field of cellular immunology and specifically discloses a special-purpose kit for preparing human dendritic cell vaccines capable of largely secreting IL(Interleukin)-12. The kit consists of a monocyte separating-obtaining culture medium, a culture medium for promoting DC (Dendritic Cell) to induce and differentiate, an agent for promoting the DC to mature and a tumor antigen. The DC prepared by the special-purpose kit disclosed by the invention is mainly applied to treating cancer patients or preventing cancer high-risk population. The special-purpose kit disclosed by the invention has the advantage that the prepared DC can secrete a great deal of IL-12, so that T cells are promoted to differentiate towards Th1 type immune response direction; and meanwhile, the special-purpose kit has the advantages of being simple in preparation process, low in cost, easy for large-scale production, and the like.
Owner:SHENZHEN HORNETCORN BIOTECH
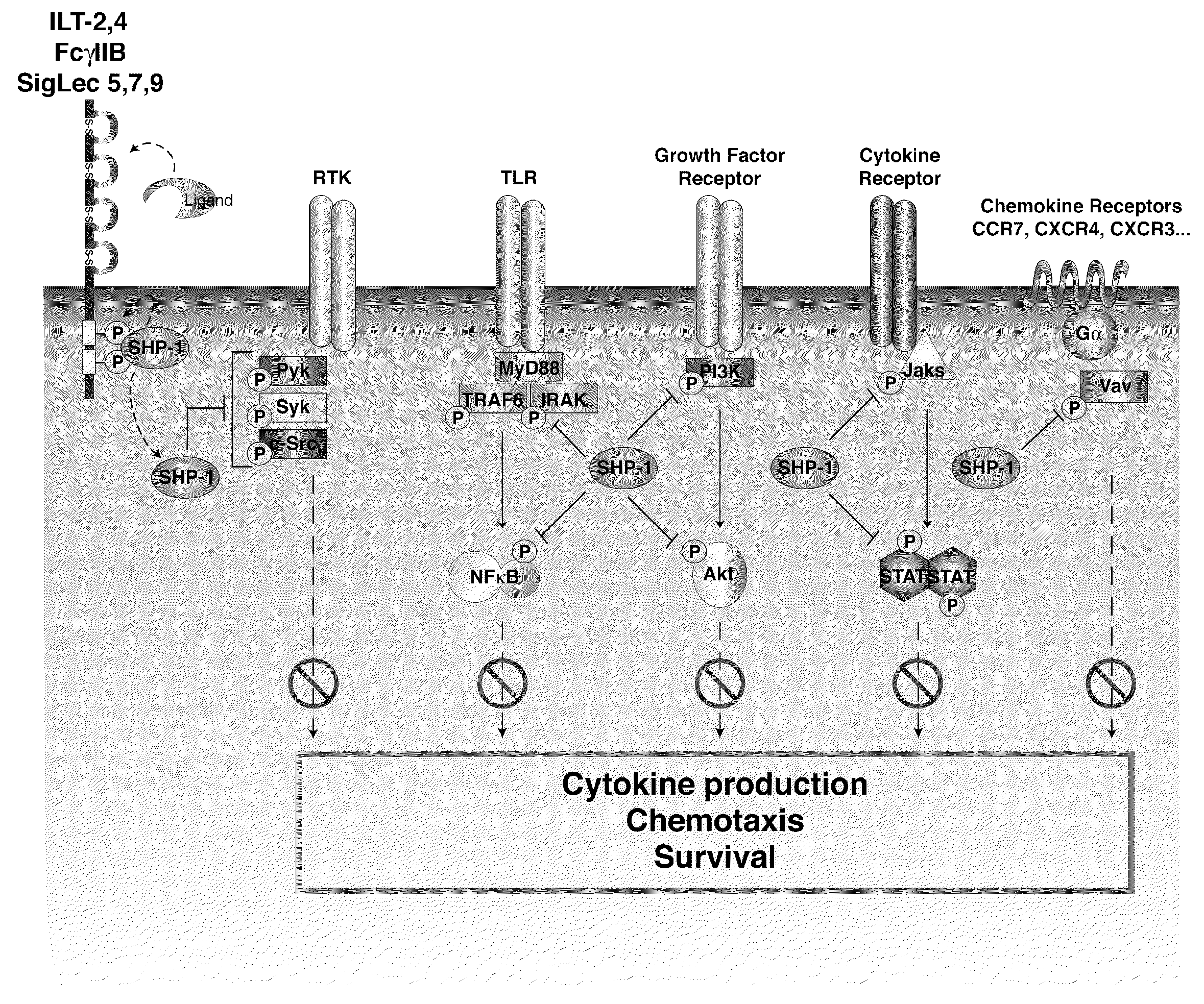
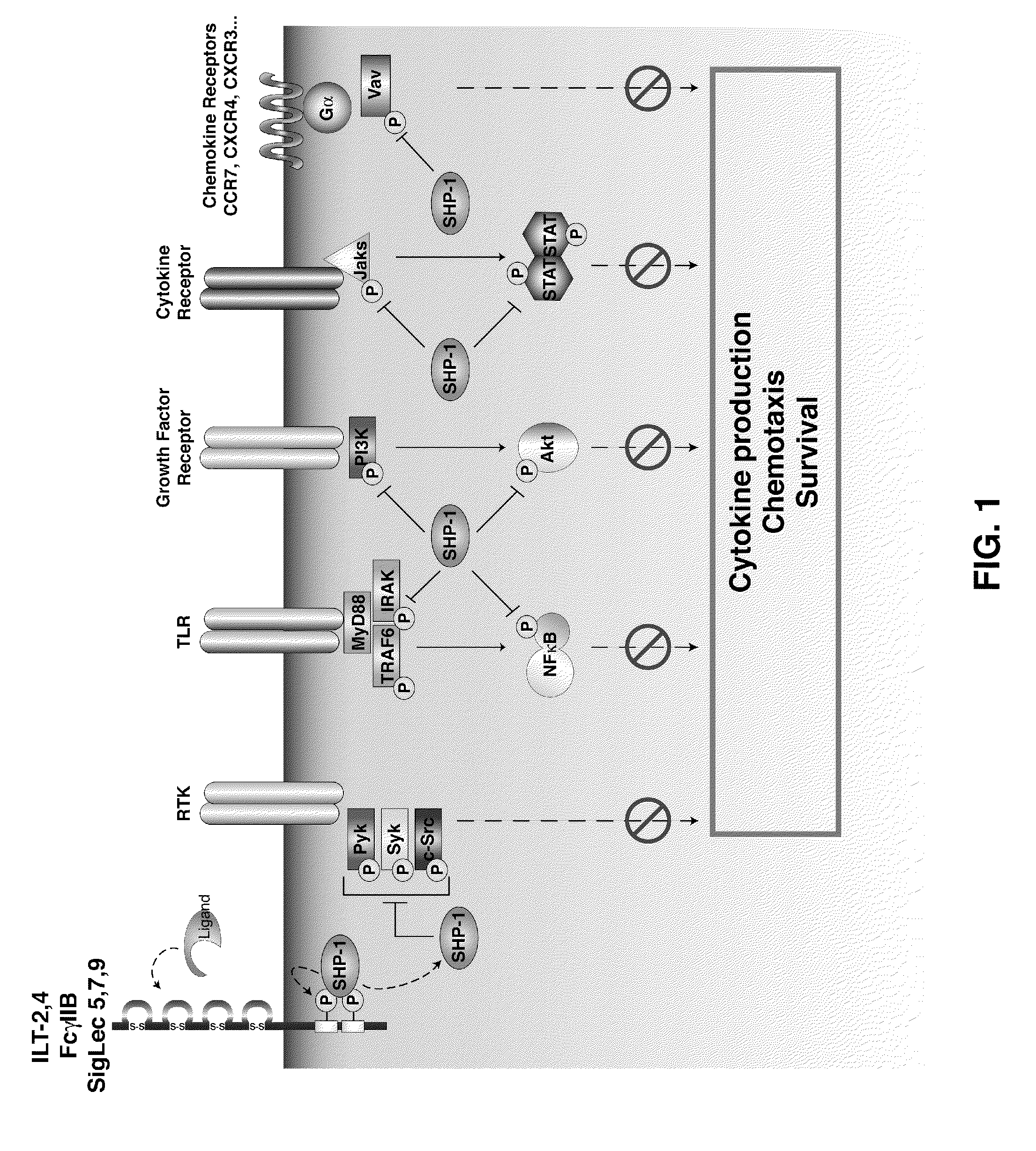
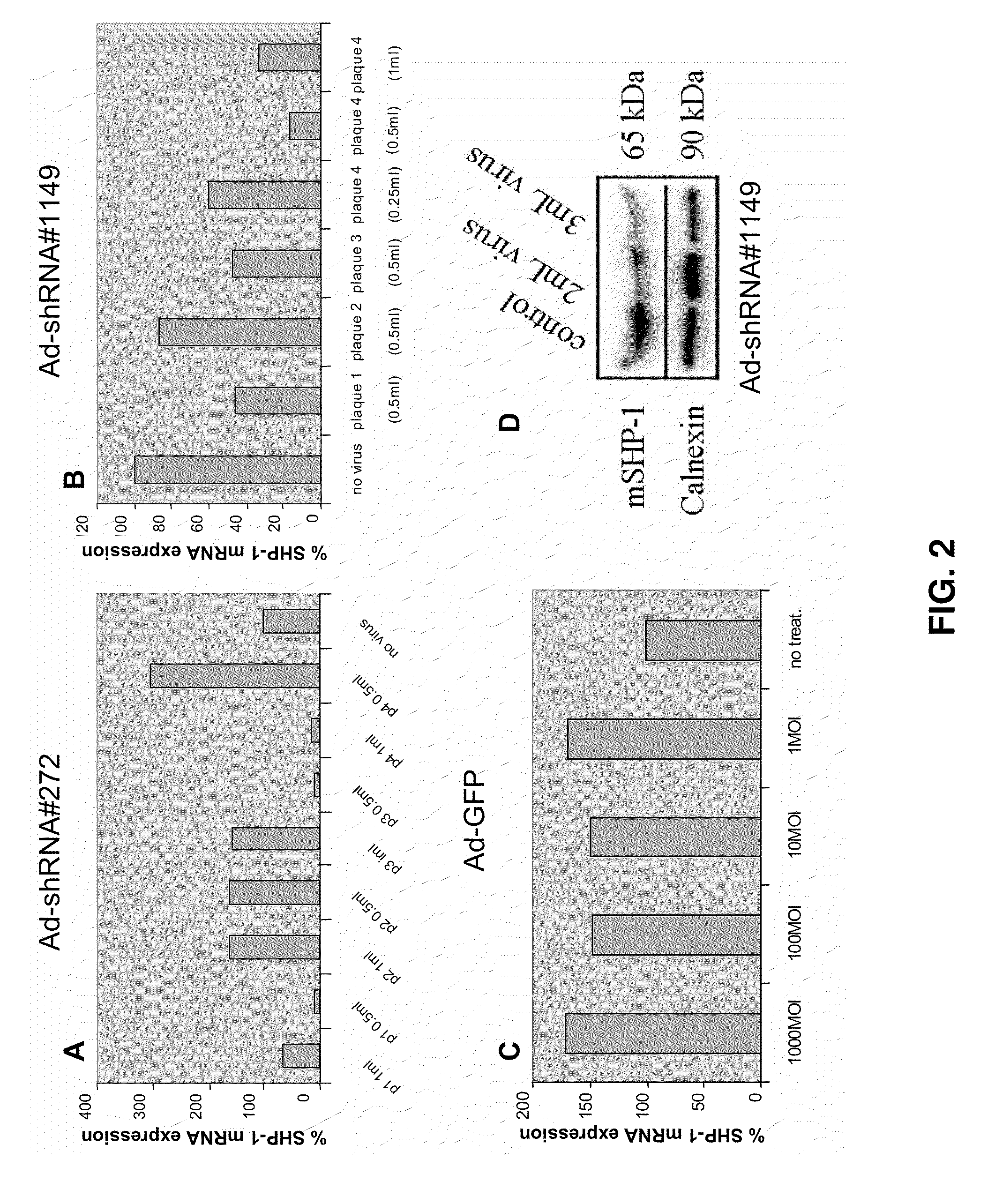
Inhibition of the sh2-domain containing protein tyr-phosphatase, shp-1, to enhance vaccines
The invention describes the use of dendritic cell vaccines, wherein SHP-1 expression or activity is modulated in the dendritic cell. In particular, the invention provides dendritic cells (DC) transduced with an SHP1-shRNA adenovirus, or dominant negative (dn-SHP-1) or constitutively active (ca-SHP-1), and pulsed with an antigen. The methods and compositions of the invention are used for the prevention and / or treatment of cancers, other cell proliferation diseases and conditions, diseases caused by a pathogen, or autoimmune disorders.
Owner:BAYLOR COLLEGE OF MEDICINE
Preparation method for dendritic cell of umbilical cord blood source and dendritic cell vaccine
ActiveCN102676455ABlood/immune system cellsAntibody medical ingredientsHematopoietic cellCell culture media
The invention discloses a preparation method for the dendritic cell (DC) of an umbilical cord blood source and a dendritic cell (DC) vaccine, which relates to a preparation method for the dendritic cell. According to the method, various cell factors are adopted to induce DC obtained by umbilical cord blood separation, and then the DC is stimulated by a tumor specific antigen so as to improve the specific antigen presentation capability of the DC; and a stem cell growth factor and Flt3-L are added into a cell culture medium so as to effectively accelerate a hematopoietic cell in the umbilical cord blood to induce and proliferate to an immune cell. The DC vaccine prepared with the method has the specific antigen presentation capability, can be combined with a CIK (cytokine induced killer) cell to mutually treat the malignant tumor when being used as a tumor immunotherapy product, and is used as an important adjuvant therapy after operations and chemoradiotherapy. Recurrence and metastasis after the operations can be effectively prevented, and toxic and side effects caused by the chemoradiotherapy on patients are lowered so as to improve the treatment effect.
Owner:北京和泽普瑞生物科技有限公司 +1
Preparation method and application of a new tumor dendritic cell therapeutic vaccine
ActiveCN102258772APrevent relapseAvoid diversionBlood/immune system cellsAntibody medical ingredientsAbnormal tissue growthTumor therapy
The invention provides a preparation method and application of a new therapeutic vaccine for dendritic tumor cells. The therapeutic tumor vaccine is chemotherapeutic drug induced tumor antigen, wherein, the dendritic cells are loaded and activated. The chemotherapeutic medicament induced tumor antigen contains a plurality of immunostimulation molecules as well as tumor-associated and specific antigen, which can remarkably carry out chemotaxis and activation on immunocytes such as the dendritic cells, T-cells (thymus-dependent lymphocytes) and the like, stimulate the dendritic cells to be mature and express a plurality of cell factors and chemotactic factors, effectively induce immune response reaction with antigenic specificity and non-specificity and strengthen body immune function. The therapeutic vaccine provided by the invention can be used for preventing and treating tumors and has the characteristics of simple preparation process, low cost, strong specificity, obvious curative effect and the like.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Preparation method of large-scale culture dendritic cell vaccine and application thereof
InactiveCN103301449ABlood/immune system cellsAntibody medical ingredientsWhite blood cellTumor necrosis factor alpha
The invention provides a preparation method of a large-scale culture dendritic cell vaccine. The preparation method comprises the following process steps of: performing in-vitro separation and purification on collected white blood cells in human peripheral blood by adopting a blood cell separation machine to obtain a large number of mononuclear cells, wherein the order of magnitude can be above 10<9>; using a human granulocyte-macrophage colony stimulating factor and interleukin 4 to induce the culture to obtain a large number of immature dendritic cells, wherein the order of magnitude can be above 10<8>; and loading a tumor specific antigen in the immature dendritic cells, and then obtaining the mature dendritic cell vaccine with an anti-tumor effect under the induction culture conditions of a tumor necrosis factor alpha and lipopolysaccharides. The invention further provides a multi-time and multi-treatment course clinical application of the dendritic cell vaccine obtained through the method.
Owner:泰州市数康生物科技有限公司
Dendritic cell tumor vaccine and its preparation and use
ActiveCN1607247AImprove efficiencyEasy to manufactureAntibody medical ingredientsAntineoplastic agentsSpecific immunityTumor antigen
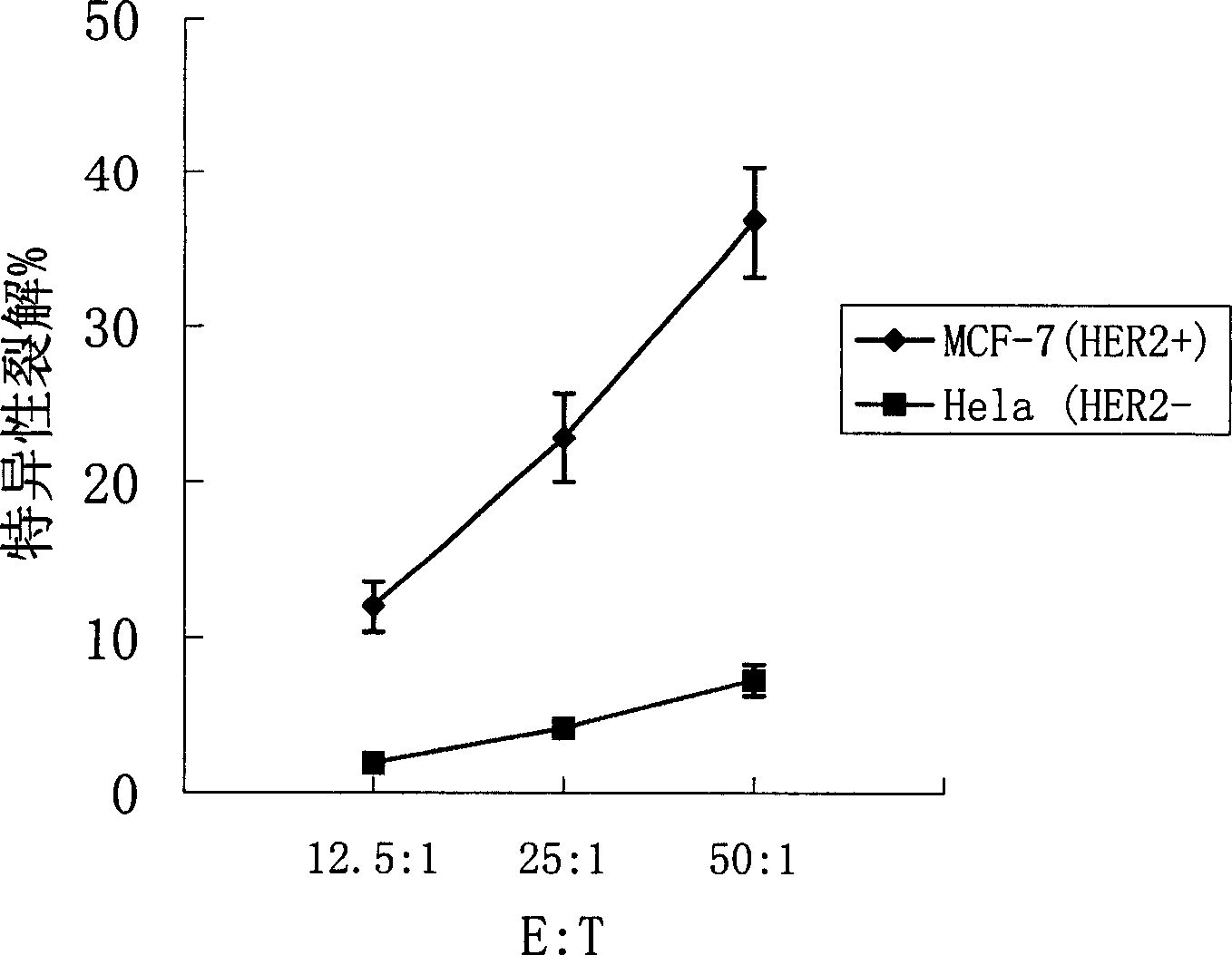
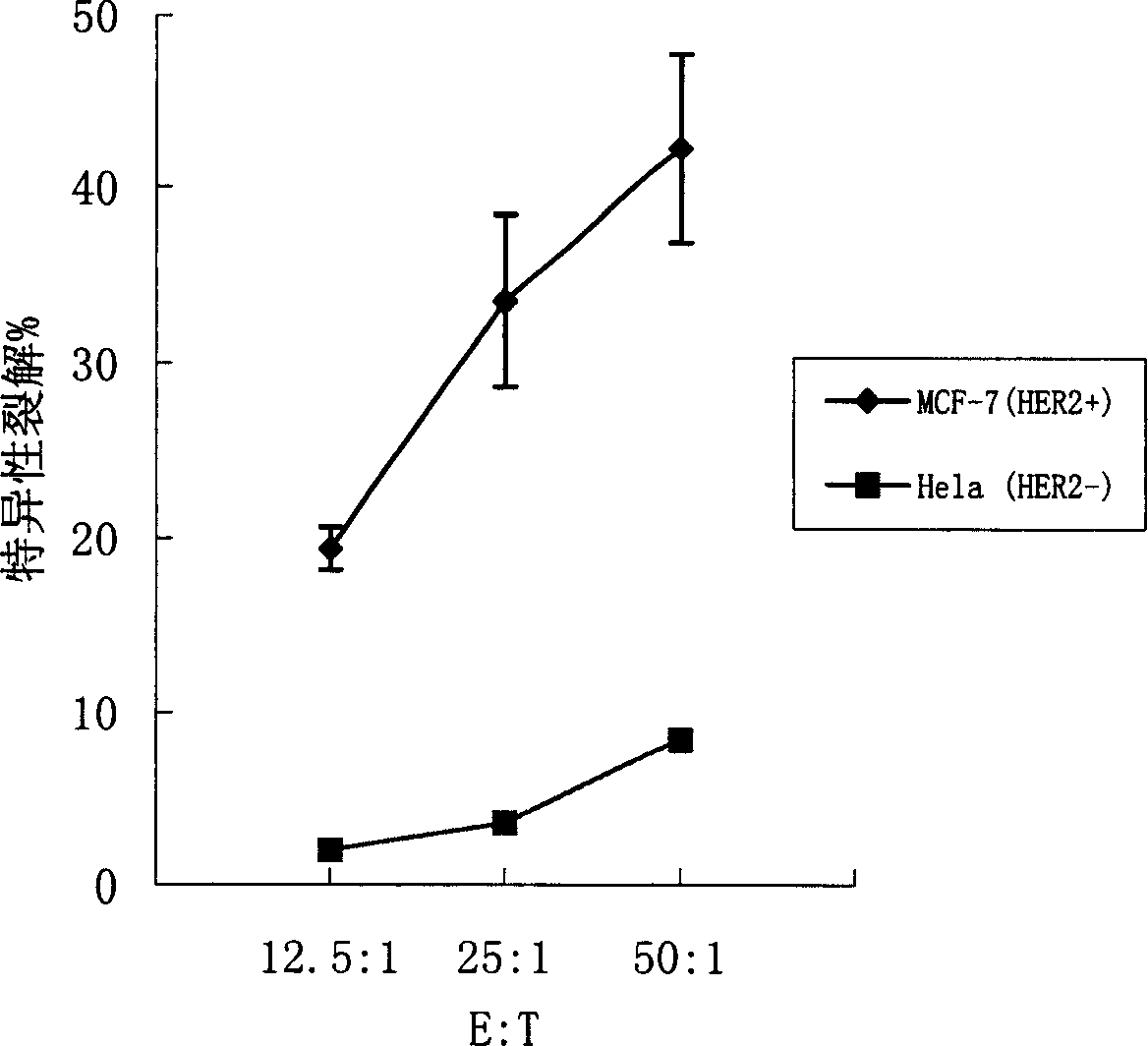
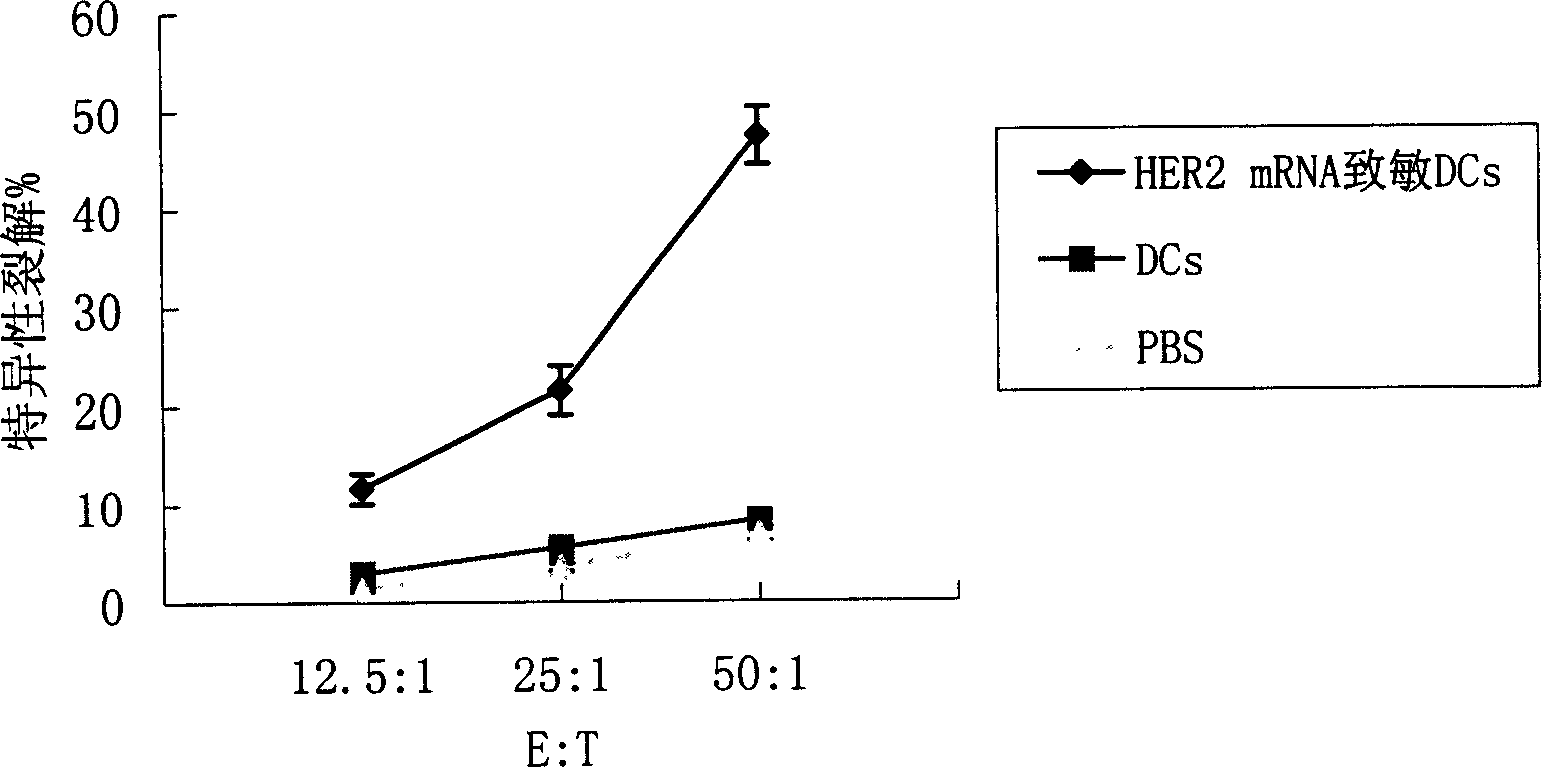
Said invention provides tumor antigen mRNA sensitized dendritic cell tumor vaccine, preparation and use thereof, and medicinal composition of dendritic cell. Said tumor vaccine is specific mRNA modified dendritic cell vaccine which can stimulate body to produce specific immune response aiming at HER2 positive tumor, so it can be used in preventing and curing HER2 positive tumor such as breast cancer etc. said invention has high transfection efficiency, wide anti tumor gammarayspectrum and strong specificity.
Owner:上海海欣生物技术有限公司
Preparation method of breast cancer-specific epitope polypeptide-loaded dendritic cell vaccine and kit thereof
InactiveCN103784950ACure recurrenceCure metastasesBlood/immune system cellsAntibody medical ingredientsWhite blood cellT lymphocyte
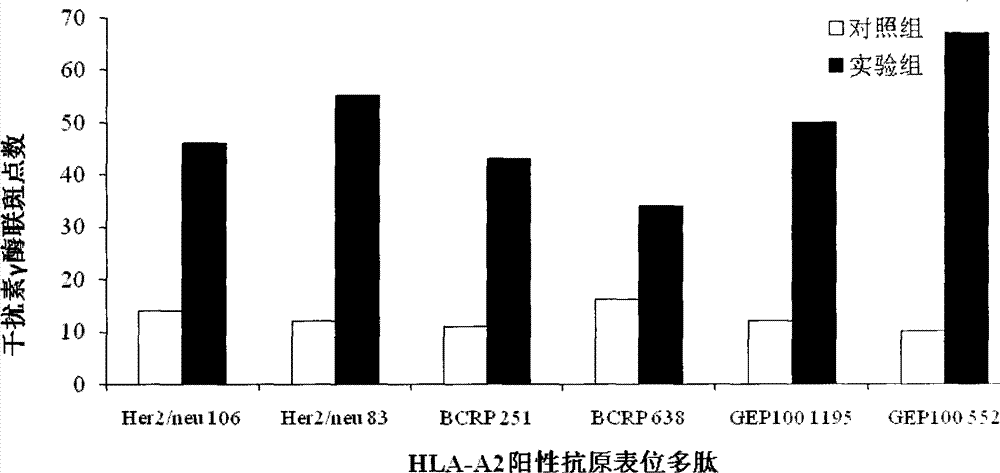
The invention provides a preparation method of a breast cancer-specific epitope polypeptide-loaded dendritic cell vaccine, and the method comprises the following steps: loading dendritic cells with identified human leukocyte antigen A201 positive epitope polypeptide of human epidermal growth factor receptor 2, breast cancer drug resistance protein and IQ motif and Sec7 structural domain 1 protein which specifically express in breast cancer (namely polypeptide comprising the amino acid sequence shown in any of SEQ ID No.1-6) to prepare into the dendritic cell vaccine which can induce strong breast cancer-targeting cytotoxic T lymphocyte response. The invention also provides a kit comprising the breast cancer-specific epitope polypeptide-loaded dendritic cell vaccine.
Owner:BEIJING HONGRUNYUAN BIOLOGICAL TECH
Method for preparing dendritic cell vaccine
InactiveCN103948917AThe ingredients are clear and singleHigh purityBlood/immune system cellsAntibody medical ingredientsCord blood stem cellCD8
The invention belongs to the cellular immunity field, and concretely relates to a preparation method of dendritic cell vaccine. The method comprises the following steps: separating cord blood to obtain cord blood monocyte; screening DC cell from the cord blood monocyte, culturing and performing amplification, then performing single epitope multiple antigen peptide (SEA-MVP) for loading, and then incubating overnight to obtain the DC vaccine. The method has the beneficial effect that the single epitope multiple antigen peptide (SEA-MVP) is used for loading DC cells, the single epitope multiple antigen peptide (SEA-MVP) is obtained by in vitro synthesis, the composition is clear and single, the purity is high; alkaline amino acid is taken as a frame for connecting the single epitope, so that the orientation of the connected epitope peptide has consistency and the epitope peptide is easily recognized and presented by DC cells, the sensitivity and singularity are strong, the loading efficiency is high, and immune tolerance is not generated; compared with the current method, the antineoplastic cell capability of the dendritic cell vaccine for activating the CD4+ and CD8+ cells is increased by more than ten thousand times.
Owner:江苏和泽生物科技有限公司
Multi-epitope peptide-loaded DC (dendritic cell) therapeutic vaccine for HCV (hepatitis C viruses)
The invention discloses a multi-epitope peptide-loaded DC (dendritic cell) therapeutic vaccine for HCV (hepatitis C viruses), relating to the technical field of virology and immunology. The multi-epitope peptide-loaded DC (dendritic cell) therapeutic vaccine is characterized in that two CTL epitopes (namely, a NS4B (1793-1801) SMMAFSAAL and a P7 (774-782) AAWYIKGRL) are used for constructing recombinant adenoviruses, then the recombinant adenoviruses are used for infecting human dendritic cells so as to prepare a multi-epitope DC vaccine. Detection results indicate that the multi-epitope peptide-loaded DC (dendritic cell) therapeutic vaccine for HCV (hepatitis C viruses) disclosed by the invention has an immunogenicity.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
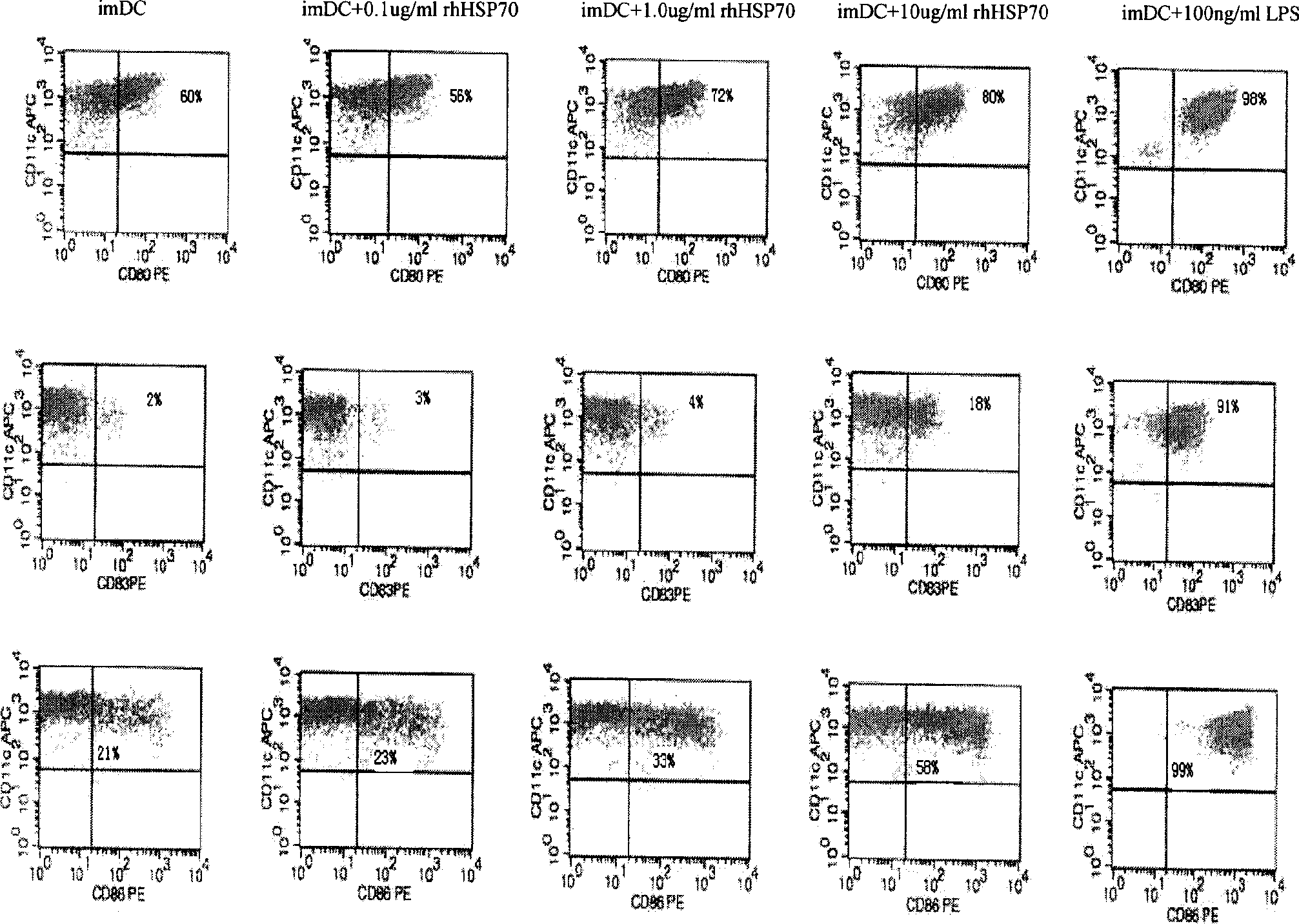
Dendritic cell vaccine carrying recombinant human HSP70 polypeptide complexes, preparation method and application
ActiveCN101461942AConvenient sourceHigh activityBacterial antigen ingredientsCarrier-bound antigen/hapten ingredientsAdjuvantWilms' tumor
The invention discloses a dendrite-shaped cell vaccine for loading a recombination human heat shock protein 70 polypeptide composite, a method for preparing the same and application thereof. The preparation method comprises: preparing a dendrite-shaped cell by designing and synthesizing an antigen polypeptide, forming the composition by the antigen polypeptide and the recombination human heat shock protein 70(rhHSP70), and loading the composite on the dendritic cell (DC) to obtain the vaccine. The vaccine has the advantages of multi-target points, double adjuvants and high efficacy, and can be used for resisting tumors and treating infectious diseases. The preparation process is simple and fast, and low in cost.
Owner:江苏得康生物科技有限公司
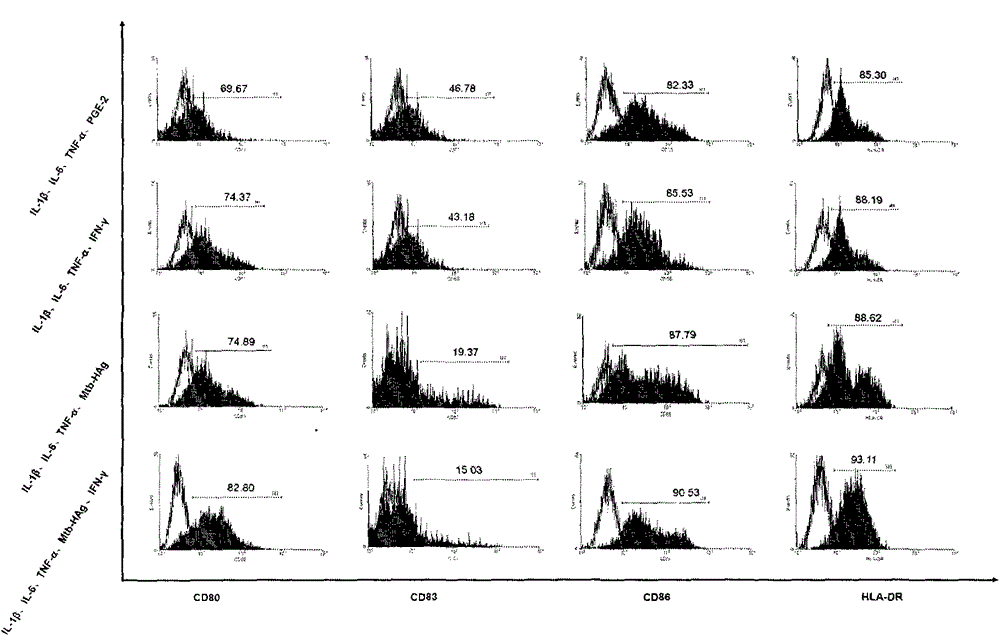
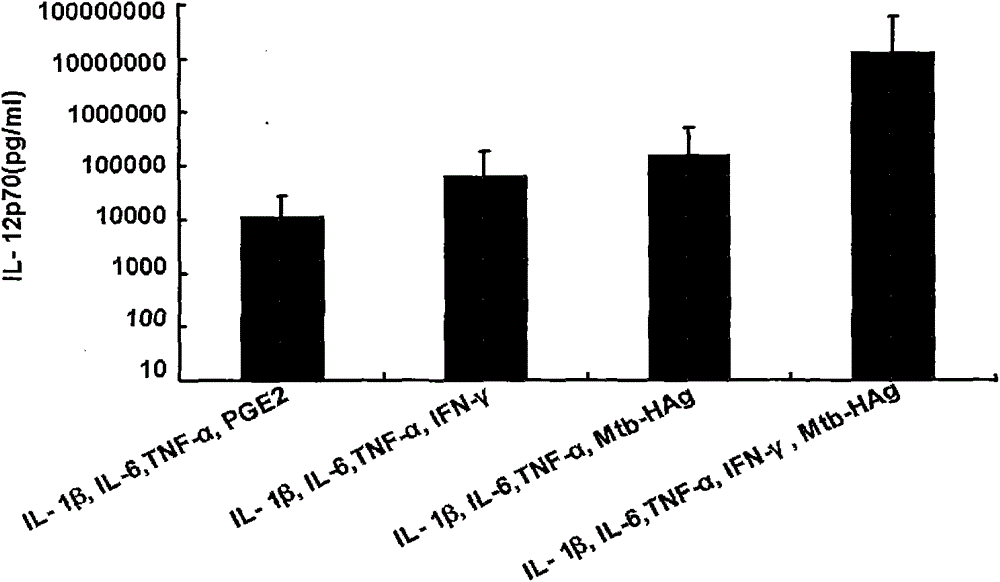
Method for preparing human dendritic cell vaccine
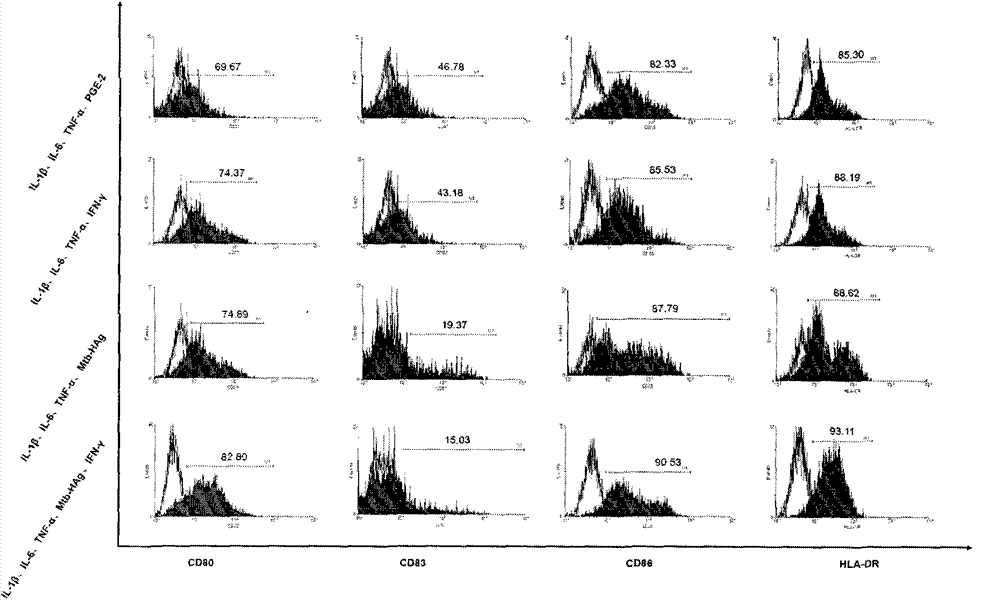
ActiveCN103599528ASimple processLow costBlood/immune system cellsAntibody medical ingredientsPeripheral blood mononuclear cellHigh-risk cancer
The invention belongs to the technical field of cellular immunology, and particularly relates to a method for preparing a human dendritic cell vaccine. The method comprises the following steps: (1) separating a monocyte from peripheral blood mononuclear cells by an adherent method; (2) inducing the monocyte to differentiate into DC by using a DC culture medium, and adding a tumor antigen on the third day; (3) on the fifth day, adding a combined type DC maturity promoting agent of IL-1beta, IL-6, TNF-alpha, IFN-gamma and Mtb-HAg; and (4) on the sixth-seventh day, detecting the maturity degree of the DC and the secretion amount of IL-12. The prepared DC is mainly applied in treatment of cancer patients or prevention of high-risk cancer groups. The method has the advantages that the prepared DC can secrete a large amount of IL-12, thereby prompting T cells to differentiate in the direction of Th1-type immune response; and moreover, the method has the superiorities of simple preparation technology, low cost, easy large-scale production and the like.
Owner:SHENZHEN HORNETCORN BIOTECH
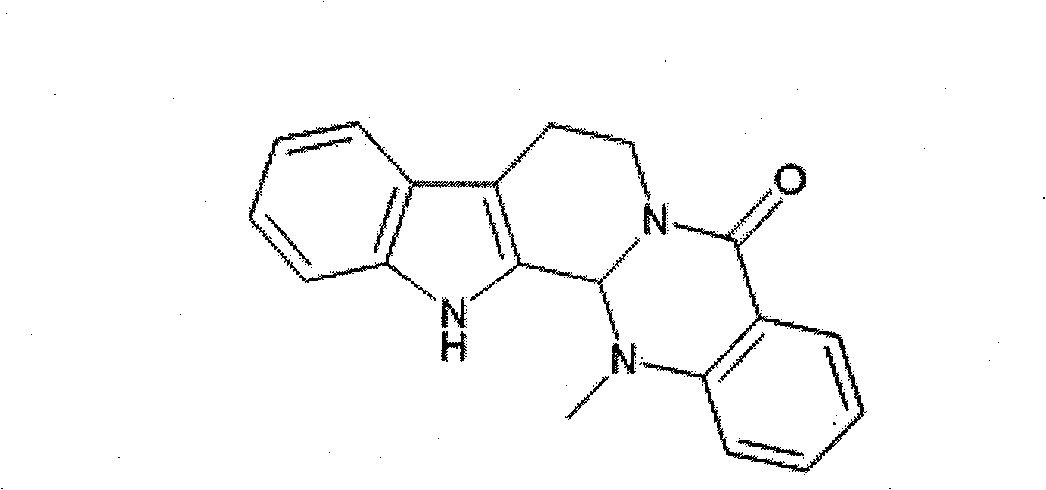
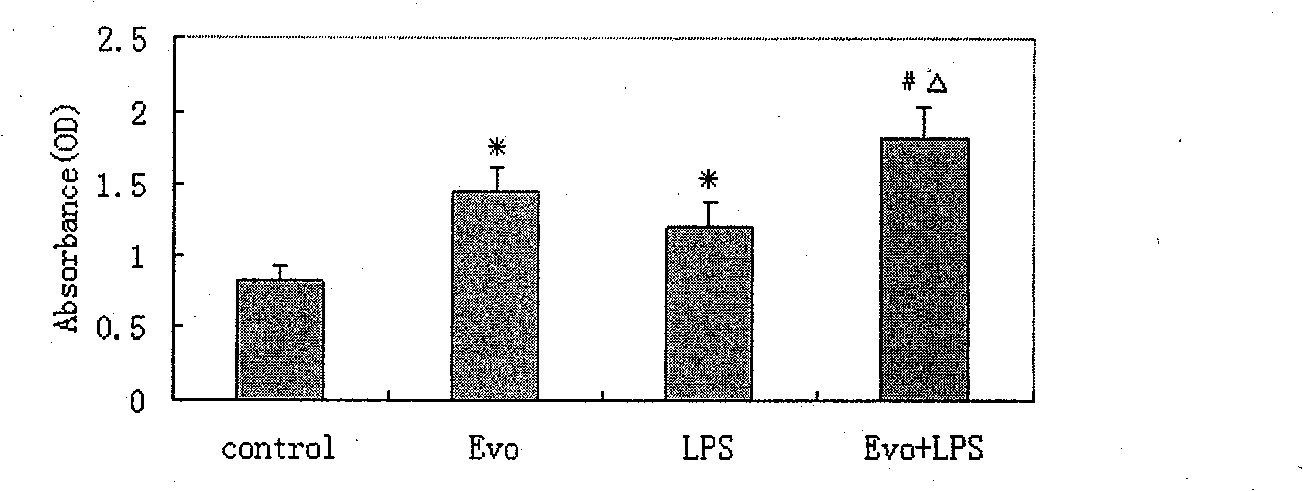
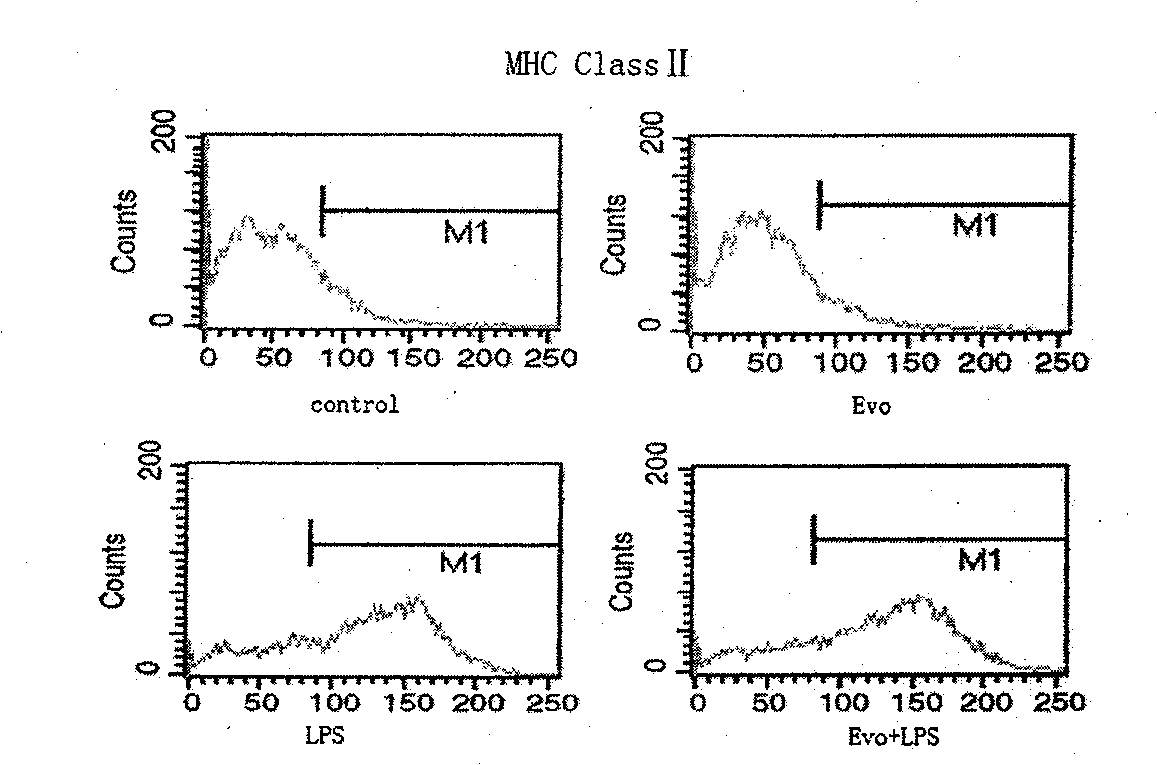
Adjuvant capable of improving dendritic cell vaccine efficiency
The invention relates to an adjuvant for improving the effectiveness of a dendritic cell vaccine, which is a traditional Chinese medicine monomer, namely evodiamine.
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA +1
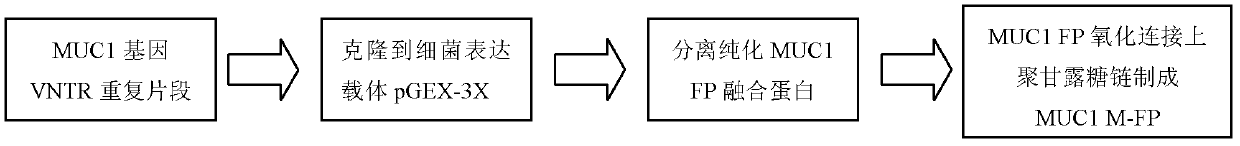
Preparation method of tumor dendritic cell vaccine sensitized by glycosylated MUC1 (mucoprotein 1) antigen
InactiveCN103372205AEfficient activationIncrease intakeBlood/immune system cellsAntibody medical ingredientsSide effectImmune tolerance
The invention provides a preparation method of a tumor dendritic cell vaccine sensitized by glycosylated antigen MUC1 (mucoprotein 1). Glycosylation modified tumor antigen peptide is used for sensitizing externally cultured DC (Dendritic Cell) and then the activated DC cells are transfused to a tumor patient. The invention further provides an application of the tumor DC cell vaccine sensitized by the glycosylated MUC1 antigen in treatment of breast cancer and pancreatic cancer. The preparation method provided by the invention has the following advantages (1) the DC cell vaccine amplification efficiency is high; (2) the DC cells have high antigen activity; (3) the DC cells have high maturity degree, and side effects such as immune tolerance are prevented; and (4) small side effects are caused.
Owner:SHANGHAI FUHETAI BIOLOGICAL TECH CO LTD
Dendritic cell vaccines for asparaginyl-beta-hydroxylase expressing tumors
ActiveUS20110076290A1Enhance anti-tumorImproved anti-metastatic activityCell receptors/surface-antigens/surface-determinantsSnake antigen ingredientsCytokineWilms' tumor
A vaccine containing AAH-loaded mature dendritic cells for treatment of AAH-expressing tumors in mammalian subjects. A method of producing primed dendritic cells is carried out by contacting isolated dendritic cells with an antigen such as AAH. Following the antigen-contacting step, the dendritic cells are contacted with a combination of cytokines such as GM-CSF and IFN-γ.
Owner:RHODE ISLAND HOSPITAL
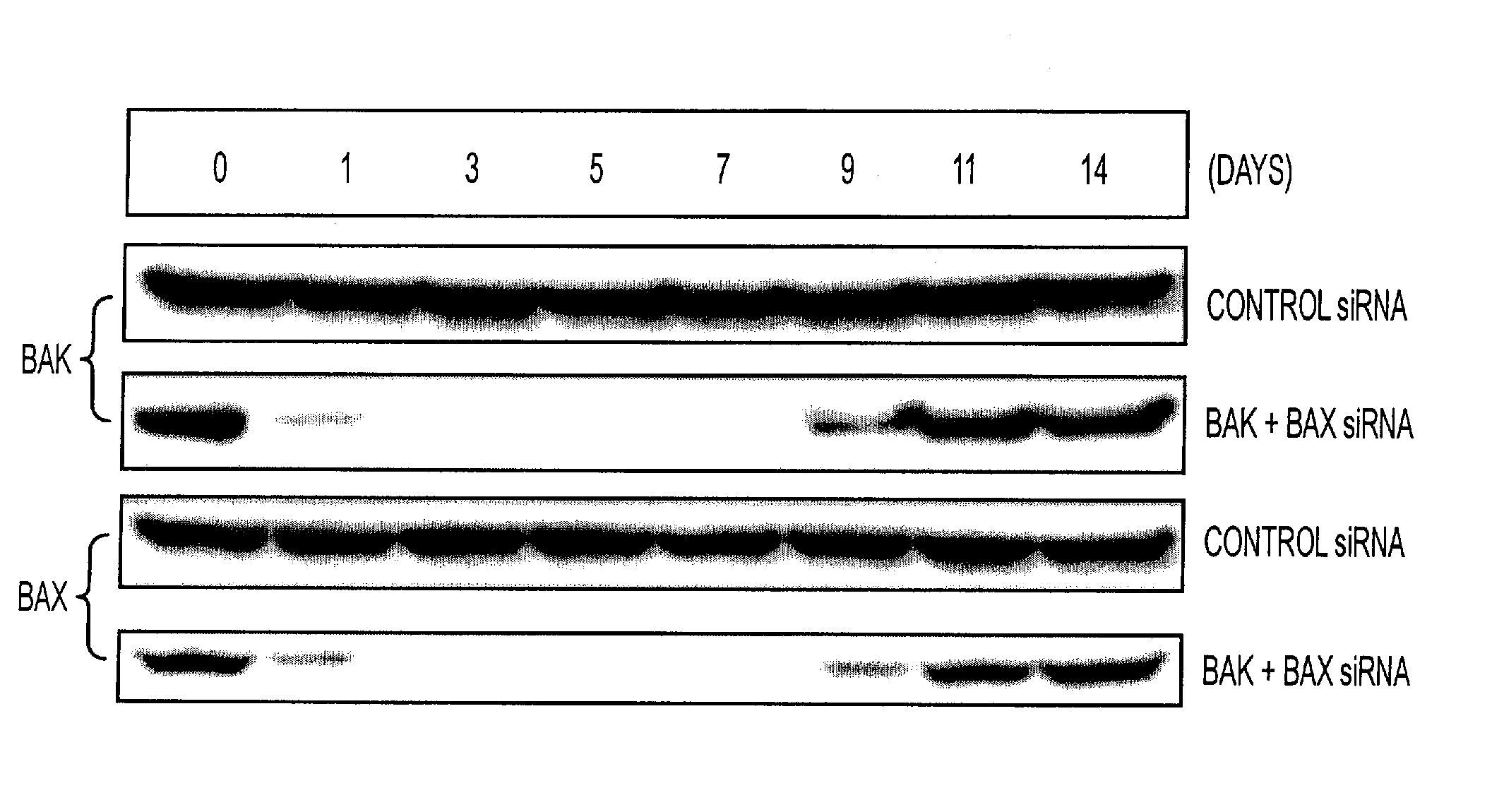
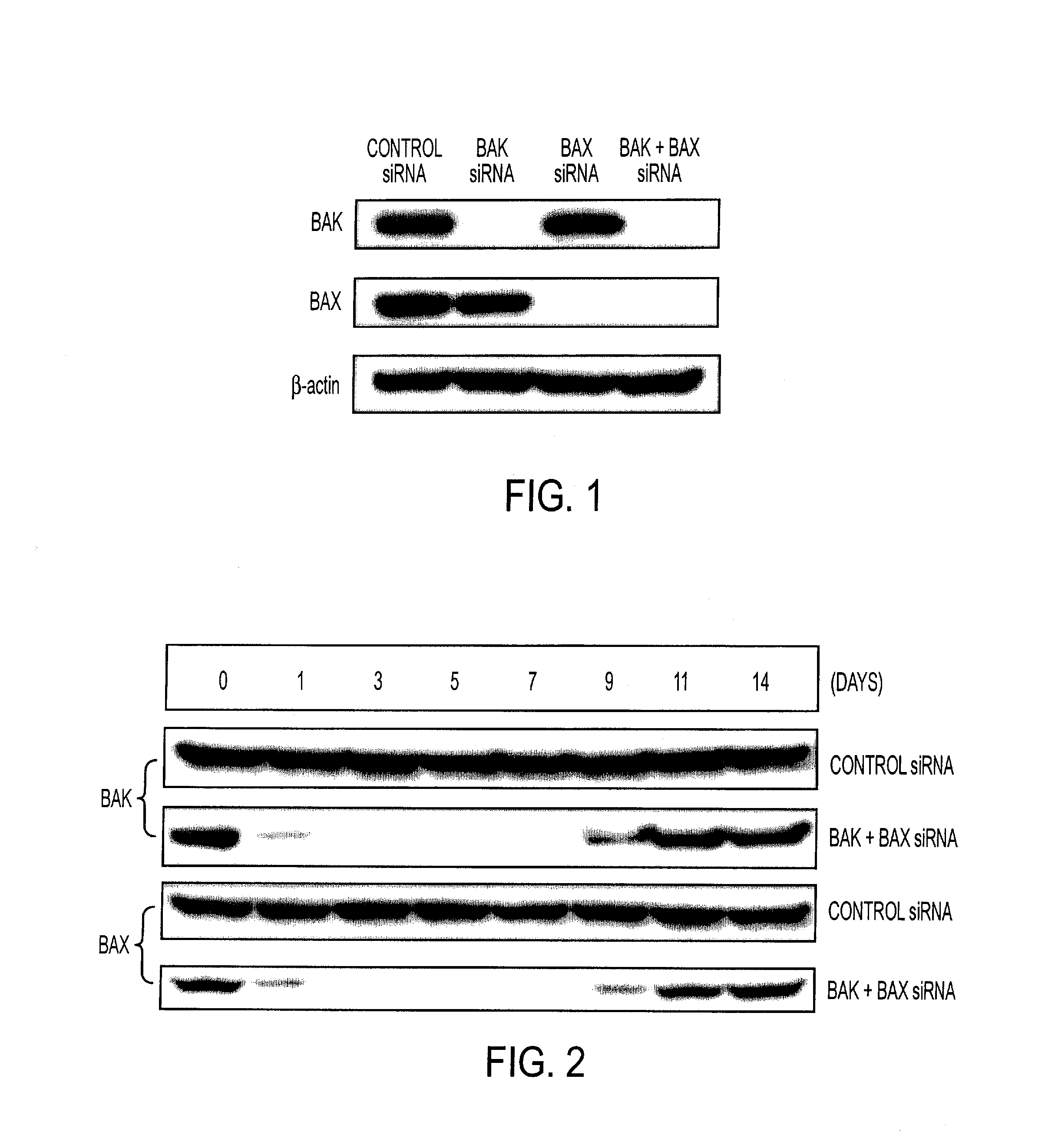
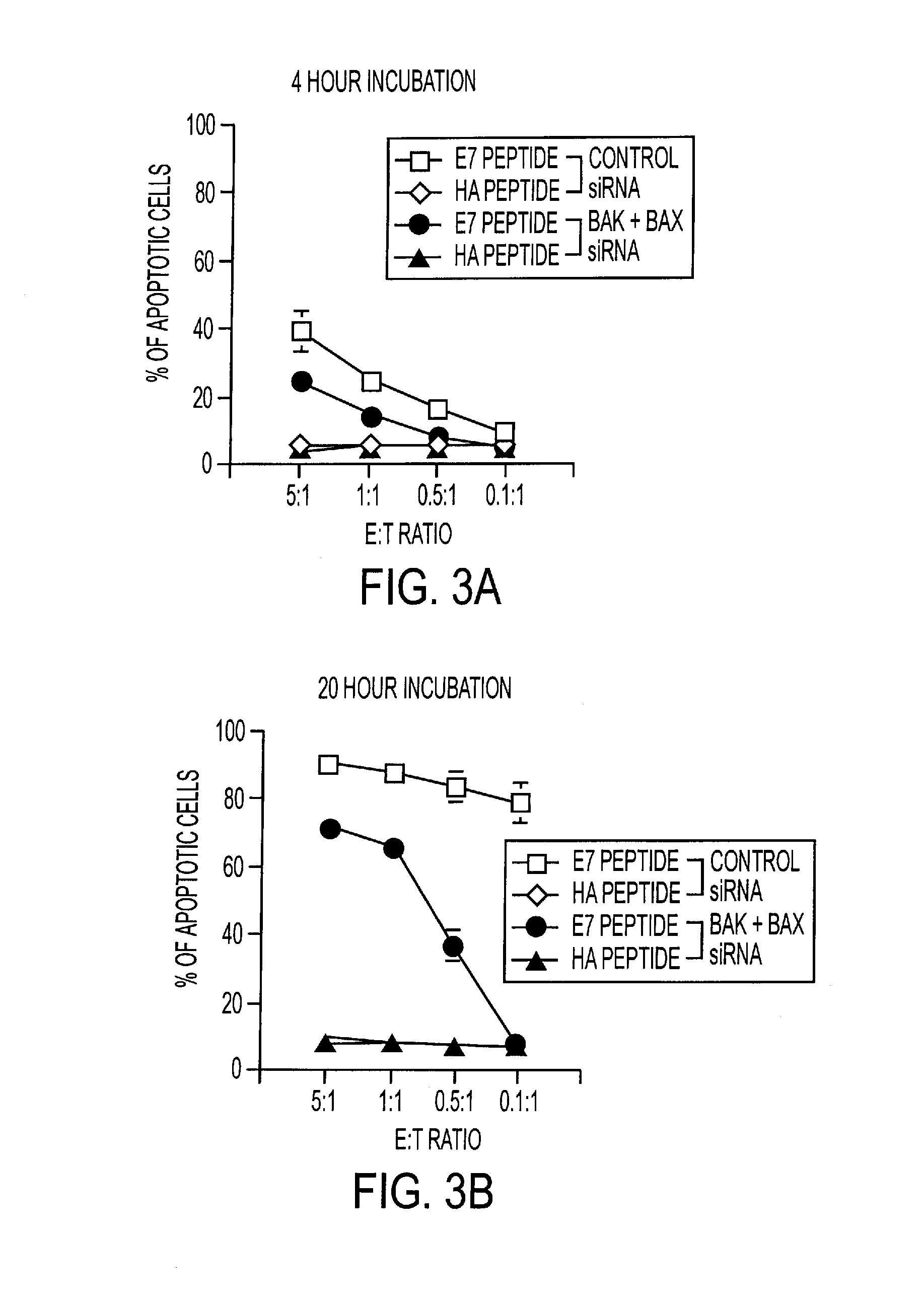
RNA Interference That Blocks Expression of Pro-Apoptotic Proteins Potentiates Immunity Induced by DNA and Transfected Dendritic Cell Vaccines
InactiveUS20080069840A1Enhance antigen presentationConducive to survivalOrganic active ingredientsBiocideAbnormal tissue growthImmunotherapeutic agent
An immunotherapeutic strategy is disclosed that combines antigen-encoding DNA vaccine compositions combined with siRNA directed to pro-apoptotic genes, primarily Bak and Bax, the products of which are known to lead to apoptotic death. Gene gun delivery (particle bombardment) of siRNA specific for Bak and / or Bax to antigen-expressing DCs prolongs the lives of such DCs and lead to enhanced generation of antigen-specific CD8+ T cell-mediated immune responses in vivo. Similarly, antigen-loaded DC's transfected with siRNA targeting Bak and / or Bax serve as improved immunogens and tumor immunotherapeutic agents.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Method for the preparation of dendritic cell vaccines
The present invention relates to a process for obtaining an antigen- loaded dendritic cell showing higher viability and migratory capacity towards lymphatic nodes. The invention also relates to vaccines containing said dendritic cells as well as to the use thereof for the treatment of infectious diseases, especially AIDS.
Owner:LAB DEL DR ESTEVE SA +1
Preparation method and kit for dendritic cell vaccine loaded by tumor specific antigenic epitope polypeptide
InactiveCN103800897ACure recurrenceCure metastasesAntibody medical ingredientsMacromolecular non-active ingredientsHuman leucocyte antigenEpitope
The invention provides a preparation method for a dendritic cell vaccine loaded by a tumor specific antigenic epitope polypeptide. The method comprises the following steps: adopting a human leucocyte antigen A201 positive epitope of an epithelial cell adhesion molecule identified by tumor specific expression, namely a polypeptide composed of amino acid sequences represented by any one of SEQ ID NO.1-6; loading dendritic cells by using the polypeptide of any one of the SEQ ID NO.1-6 to prepare the dendritic cell vaccine. The dendritic cell vaccine can be used for initiating a very strong target anti-tumor cytotoxic T lymphocyte effect. The invention further provides a kit for preparing the dendritic cell vaccine loaded by the tumor specific antigenic epitope polypeptide.
Owner:李金珍
Genetically modified dendritic cell vaccine
ActiveCN109957548AEfficient DCGenetically modified cellsCancer antigen ingredientsHigh concentrationBULK ACTIVE INGREDIENT
The invention relates to the fields of biotechnology and medicine, and provides a modified dendritic cell (DC); a dendritic cell is infected with MG-7Ag antigen mimic epitope tandem sequences loaded with a lentivirus vector, and a target antigen sequence is integrated into a dendritic cell genome. The invention provides a rapid culture scheme of the dendritic cell derived from a peripheral blood monocyte in vitro, and also provides a vaccine with an active ingredient of the modified dendritic cell. The vaccine is used for tumor prevention and active immunotherapy. The MG-7Ag antigen sequence-modified DC vaccine can obtain high-purity CTL cells and efficient target cell killing ability after DC-CTL co-culture, and the co-culture supernatant contains high-concentration IFN[gamma] secretion.After tumor attack, the tumorigenic volume of mice in a DC-CTL group is significantly smaller than that of mice in a control group, and the DC vaccine has great potential value in immunotherapy of MG-7Ag positive tumor.
Owner:上海尚泰生物技术有限公司
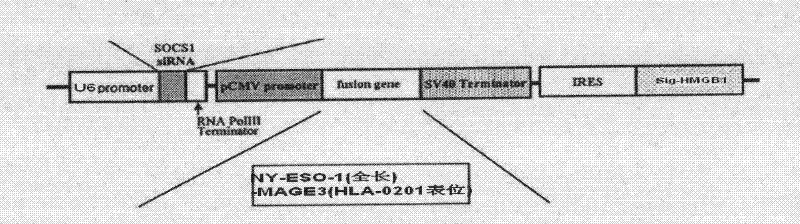
Plasmid for silencing cytokine signal inhibitory factor 1 and expressing high mobility group B1 protein and tumor associated antigen and preparation method thereof
InactiveCN102234659AStable and efficient transfectionExtensive treatmentVector-based foreign material introductionSide effectHigh-mobility group
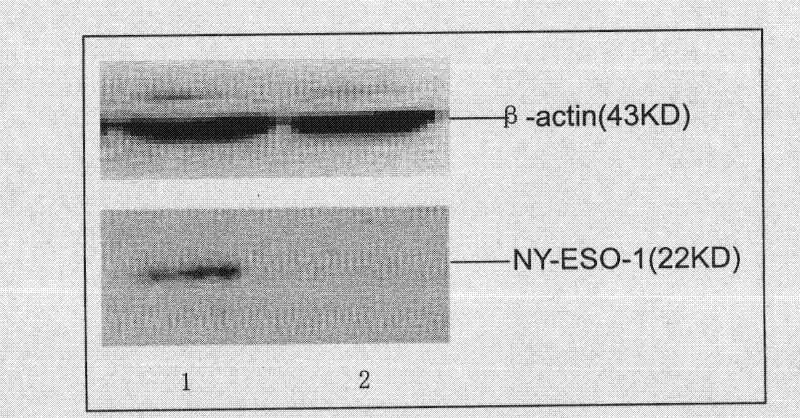
The invention discloses a plasmid for silencing cytokine signal inhibitory factor 1 and expressing high mobility group B1 protein and tumor associated antigen and a preparation method thereof, which belongs to genetic engineering technology. According to the invention, based on a gene recombination method, a cytokine signal inhibitory factor 1 small interfering RNA fragment shSOCS1, a fusion genecoding sequence of NY-ESO-1and MAGE3, and a coding sequence of HMGB1 are connected in the same vector of pIRES-EGFP; respective expression is guaranteed to be correct; and a plasmid coexpressing shSOCS1, HMGB1 and tumor associated antigen is constructed. A new-generation dendritic cell vaccine prepared by the plasmid of the invention can induce high-efficiency immune response of CTL cells with almost no side effects, and can become safe and effective immunotherapy.
Owner:TIANJIN MEDICAL UNIV CANCER HOSPITAL
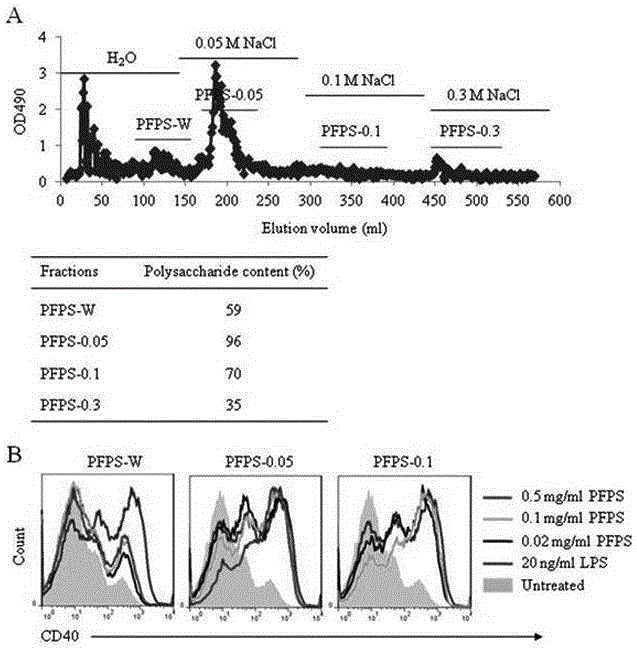
Application of pleurotus ferulae polysaccharide (PFPS) in preparing dendritic cell vaccine adjuvant
The invention discloses an application of pleurotus ferulae polysaccharide (PFPS) in preparing a dendritic cell vaccine adjuvant. The PFPS has an effect of stimulating dendritic cell maturation so as to promote the immunoreaction of dendritic cell vaccines and improve the anti-tumor effect.
Owner:新疆鼎聚医学检验所有限公司
Method for preparing dendritic cell vaccine
InactiveCN102847145AIncrease multipleStrong specific lethalityBlood/immune system cellsAntibody medical ingredientsLiquid ChangeHuman lymphocyte
The invention relates to the technical field of biology, in particular to a method for preparing dendritic cell vaccine. The method is characterized by comprising the following preparation steps: (1) taking autologous blood of a patient, and separating red blood cells, mononuclear cells and plasma from separating medium of human lymphocyte; (2) adjusting concentration of the mononuclear cells; (3) adding the solution into a six-pore plate and performing adherence for 16 hours; (4) sucking and discharging non-adherent cells on the upper layer of each pore of the six-pore plate; (5) adding 3ml of dendritic cell (DC) culture medium, placing the solution in a carbon dioxide cultivating box with the concentration of carbon dioxide of 5% at the temperature of 37 DEG C to perform cultivation for 2-3 days; (6) performing half-quantity liquid changing; (7) enabling the concentration of the autologous tumor antigen in each pore of the six-pore plate to be 20 mu g / ml by loading the autologous tumor antigen in the sixth day; and (8) detecting the quantity and maturity of the matured DC cells after 24 hours. Compared with the prior art, the number of the matured DC cells at least reaches 1*107, and the maturity is larger than 85%.
Owner:FUDAN UNIV
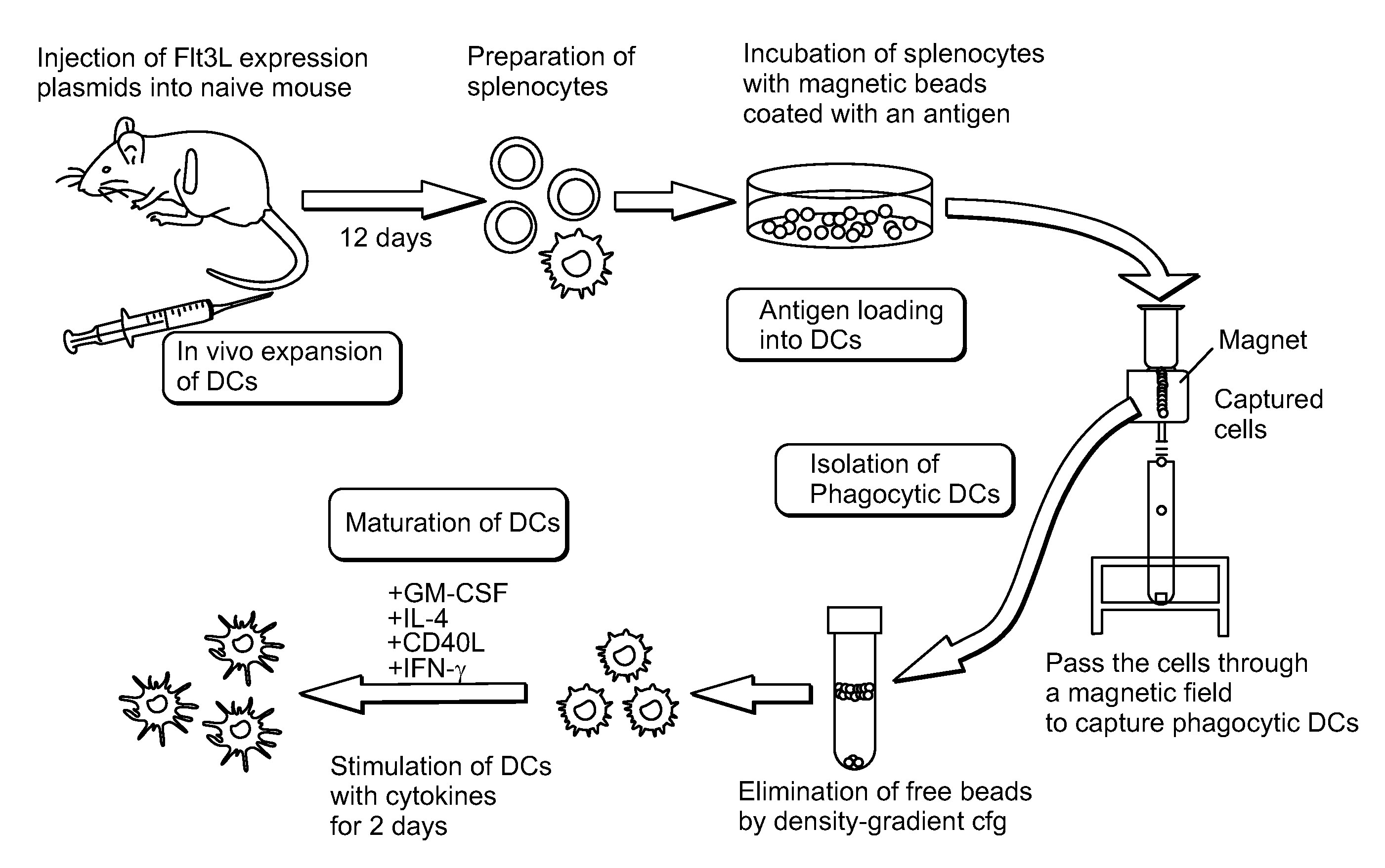
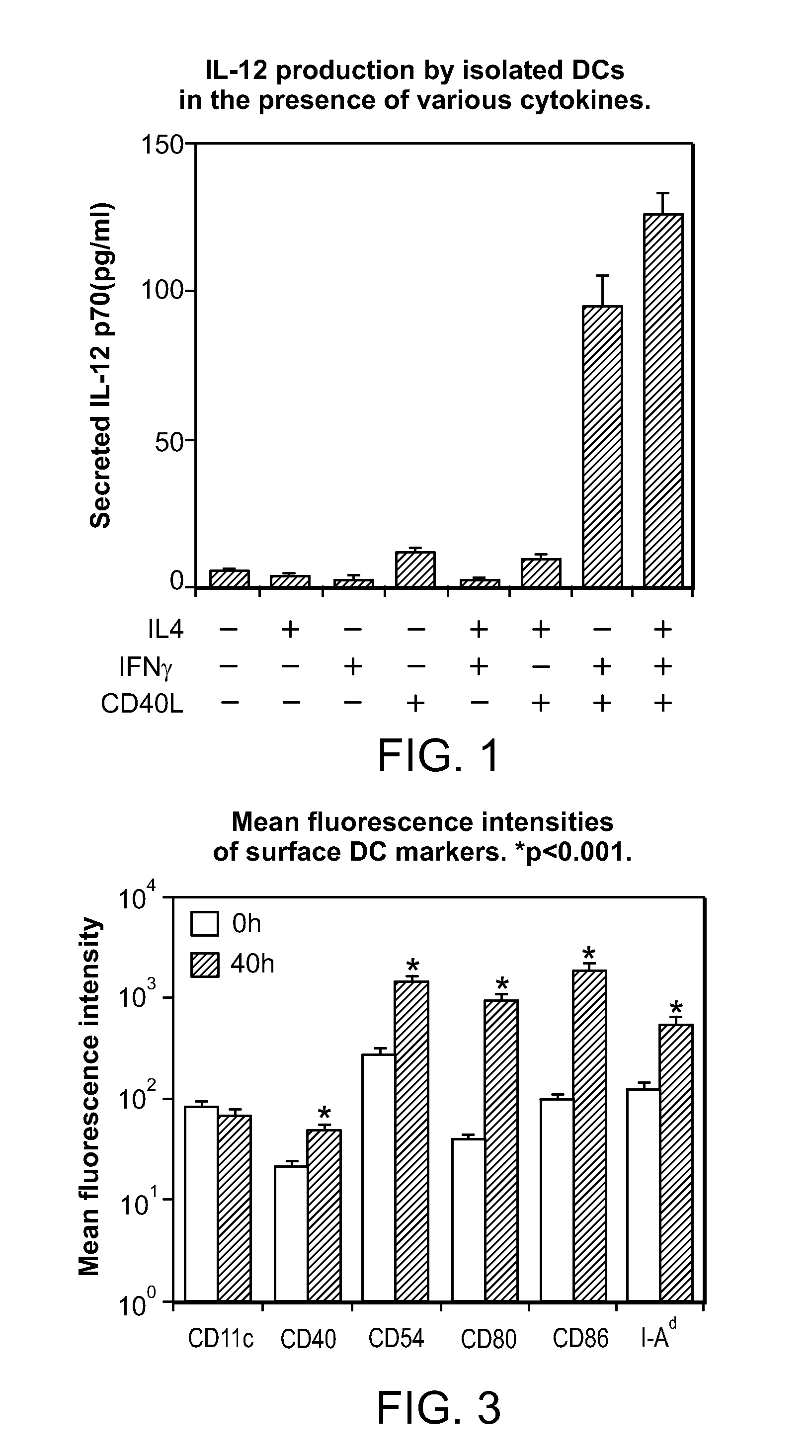
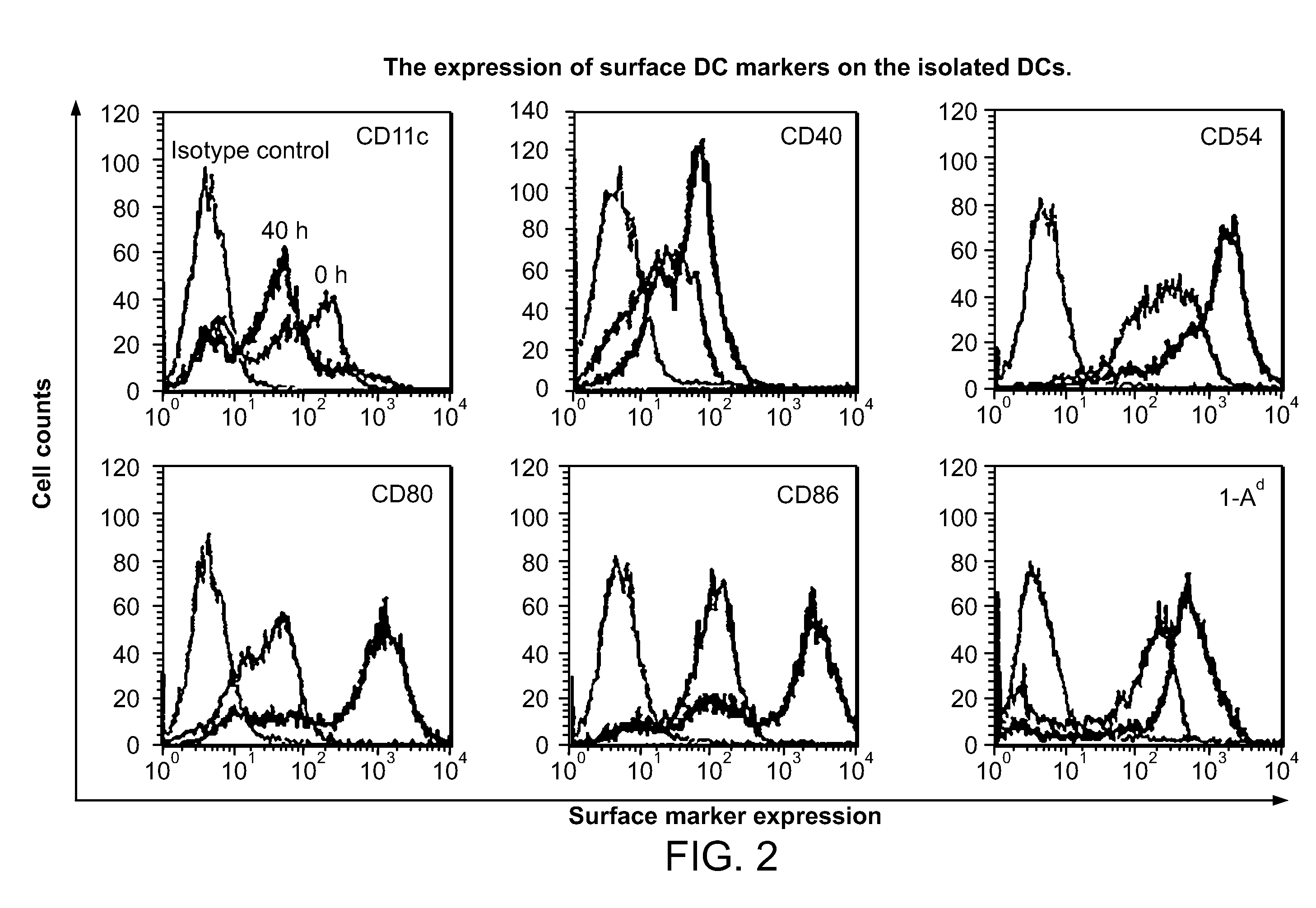
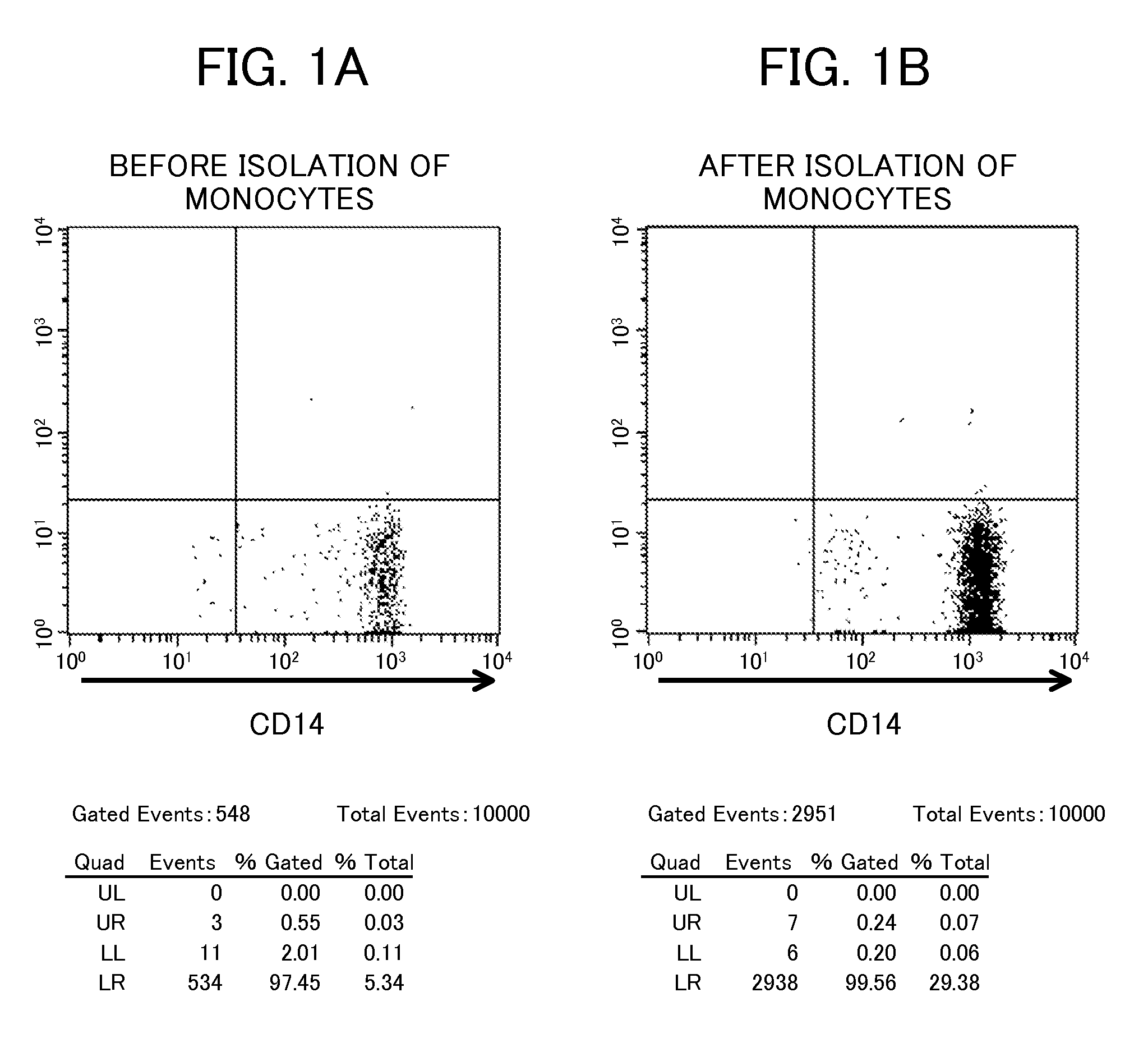
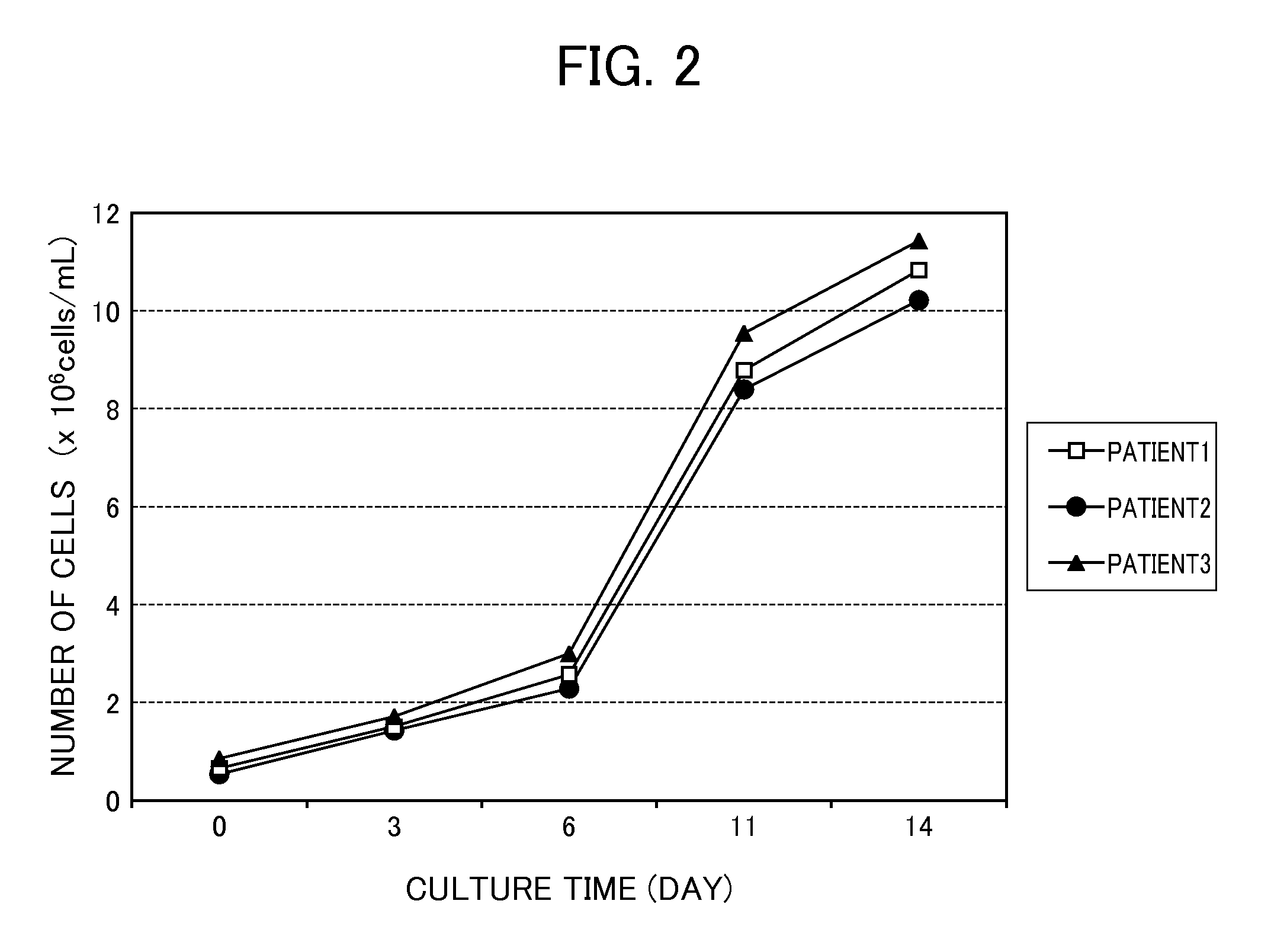
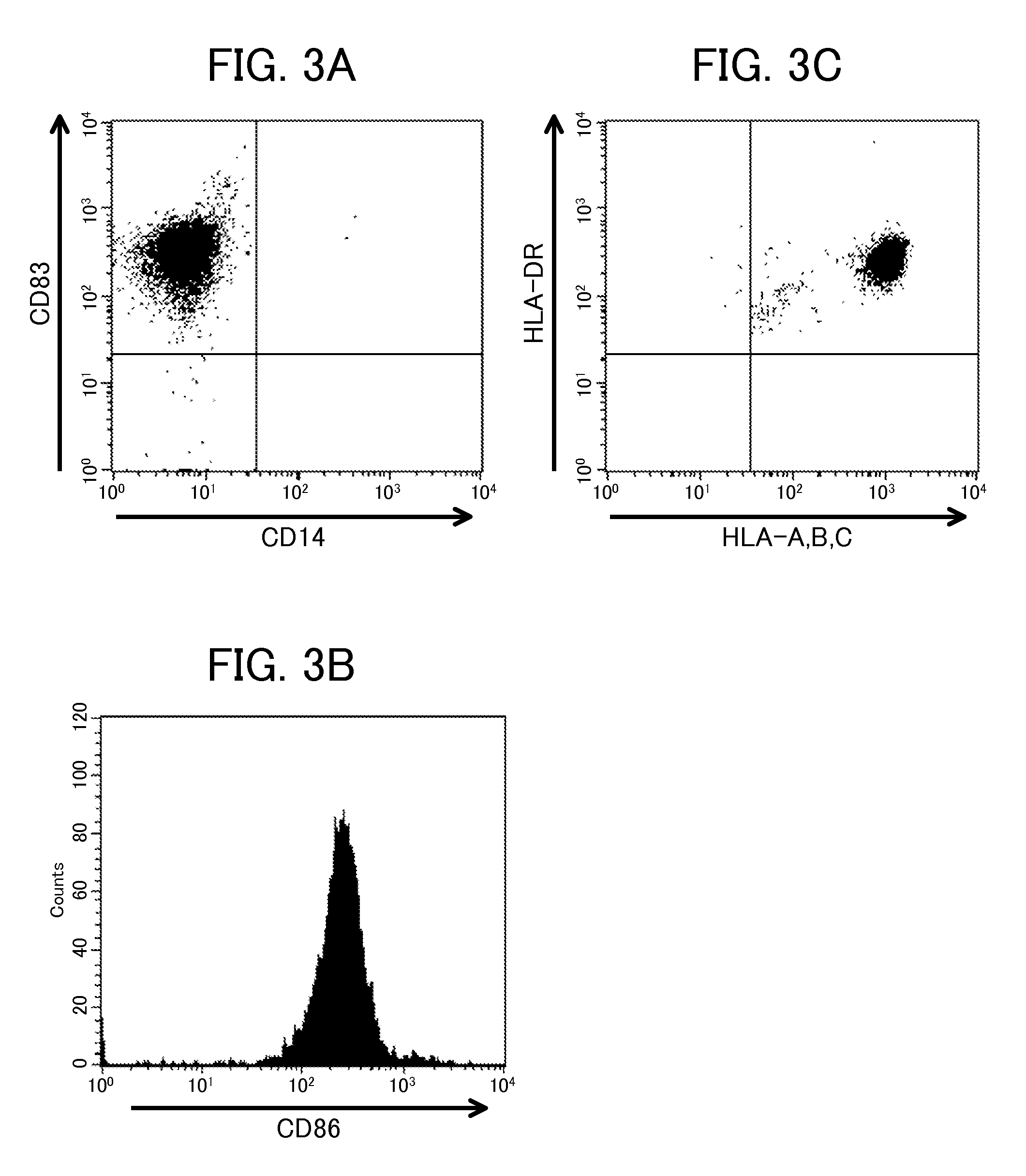
Proliferating agent for monocyte, culture medium for proliferating monocyte, method for producing monocyte, method for producing dendritic cell, and method for producing dendritic cell vaccine
ActiveUS9303247B2Highly efficient and simple proliferationCell receptors/surface-antigens/surface-determinantsColony-stimulating factorMicrobiologyMonocyte
The purpose of the present invention is to provide a means for proliferating a monocyte with high efficiency and in a simple manner. The present invention provides a proliferating agent for a monocyte, which consists of at least one component selected from Flt-3L, IL-3 and IFN-γ and can be used before a treatment for differentiation of a monocyte into a dendritic cell. The present invention also provides a culture medium for use in the proliferation of a monocyte, which contains at least one component selected from Flt-3L, IL-3 and IFN-γ and can be used before a treatment for differentiation of a monocyte into a dendritic cell. The culture medium for use in the proliferation of a monocyte according to the present invention may contain GM-CSF.
Owner:HAKUSHINKOUSEIKAI FOUND +1
Canine tumor cell and allogeneic dendritic cell fused vaccine and method for preparing the same
The present invention provides a dendritic cell-based vaccine by fusing a canine tumor cell and an allogeneic dendritic cell, and a method for preparing the same. The fusion cells expressing canine tumor antigens are generated by fusing canine bone marrow-derived dendritic cells and canine tumor cells. The canine immune system can be induced to produce tumor specific T lymphocytes and natural killer cells when the fusion cells used as a vaccine is injected into a canine body.
Owner:NAT TAIWAN UNIV
Dendritic cell vaccine for promoting spinal cord injury function recovery
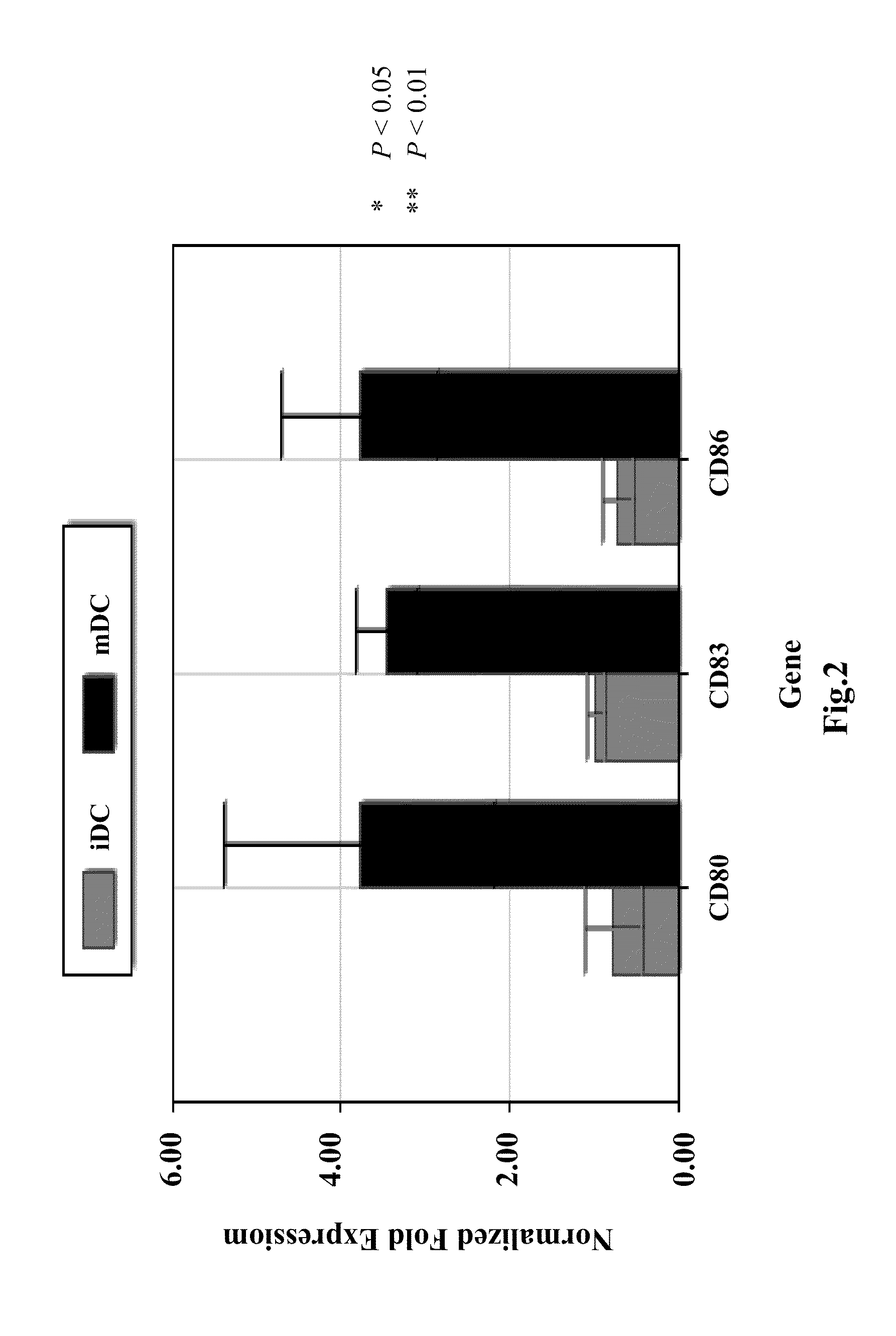
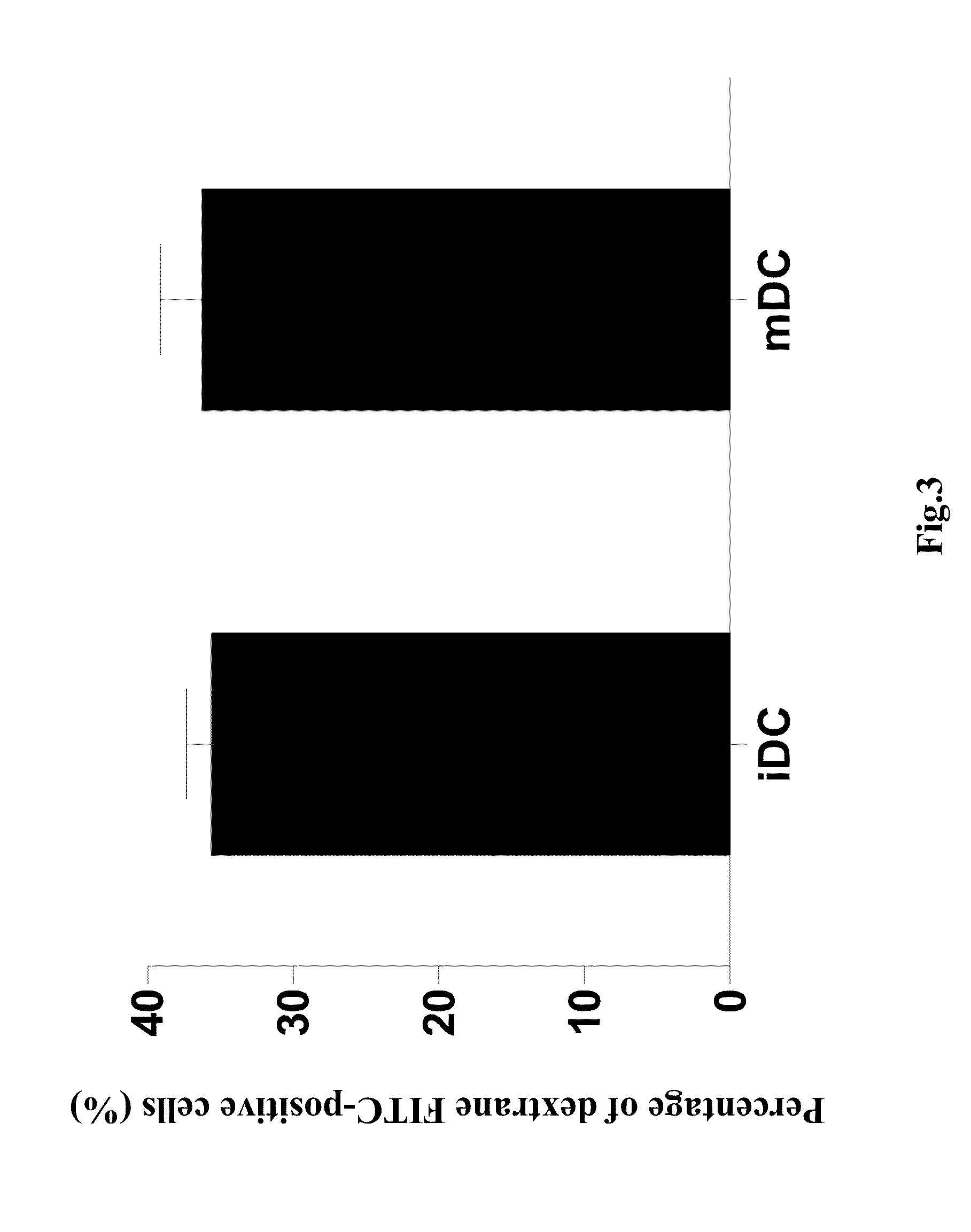
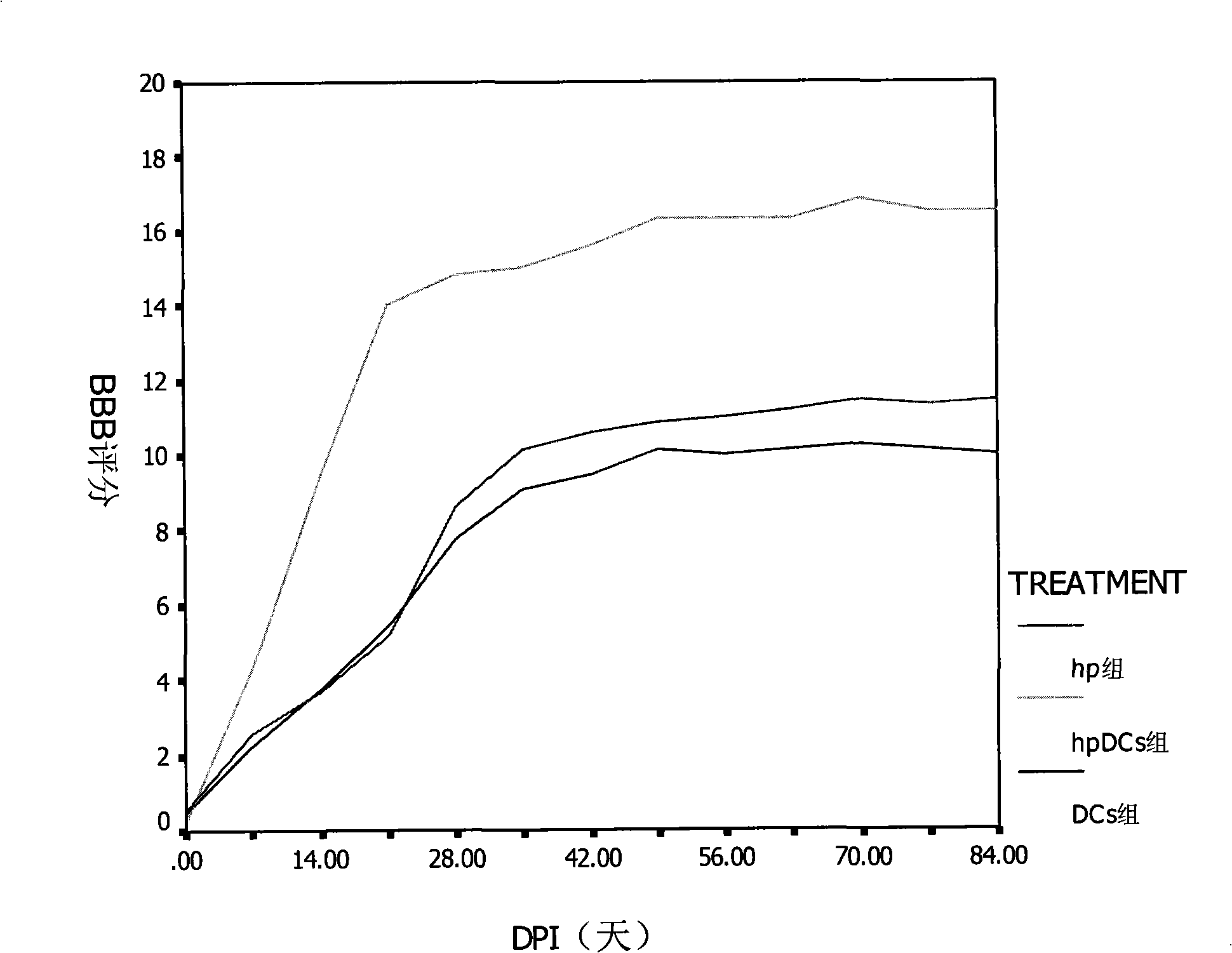
InactiveCN101332296AReduce reactive hyperplasiaLower physical barriersSkeletal disorderArtificial cell constructsFunction recoveryDendritic cell vaccine
The invention relates to a dendritic cell bacterin, in particular to a dendritic cell bacterin which can effectively promote the functional recovery of spinal cord injury and a manufacturing method thereof.
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
Construction expression of fusion gene carrier and its application
InactiveCN1757736ALow costLower medical costsPeptide/protein ingredientsAntineoplastic agentsGackstroemiaReverse transcriptase
A human telomerase reverse-transcriptase (hTERT) / human interleukin 18 (hIL 18) fusion protein with composite function is prepared from 2 cell factors with similar or complementary functions through artificial reforming of their linking terminals and configuring. The expression of its carrier is created for increasing its target killing power to tumor cells and the dendritic cell mediated immune-effect. It can be used to prepare the dendritic cell vaccine for treating cancer.
Owner:ZHEJIANG UNIV
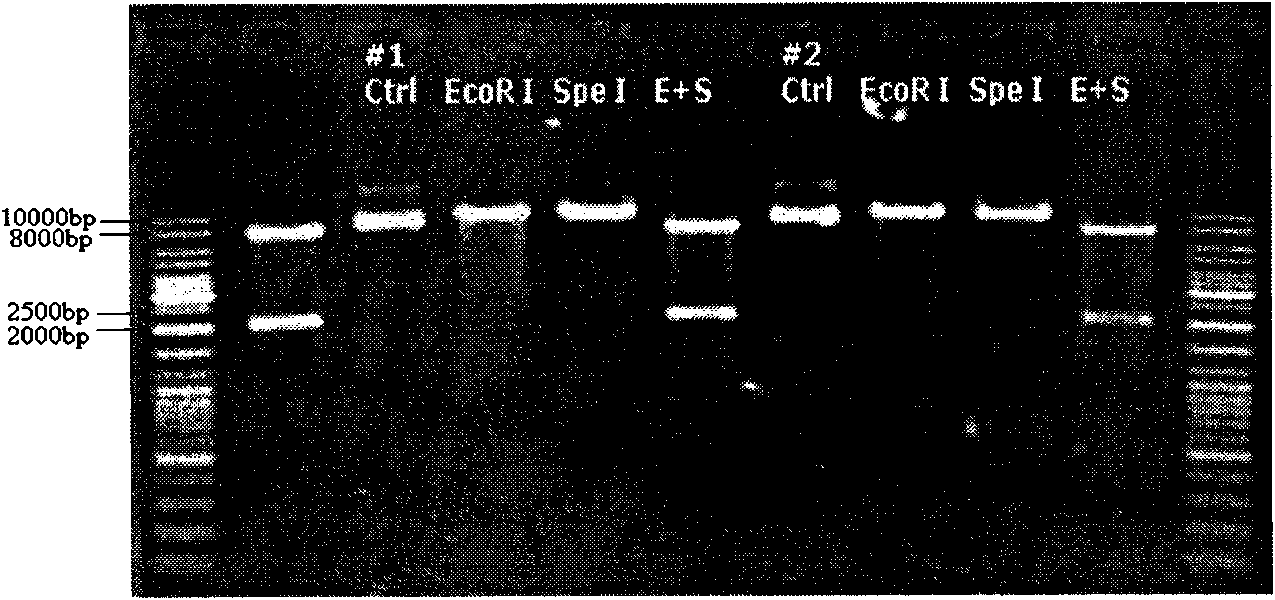
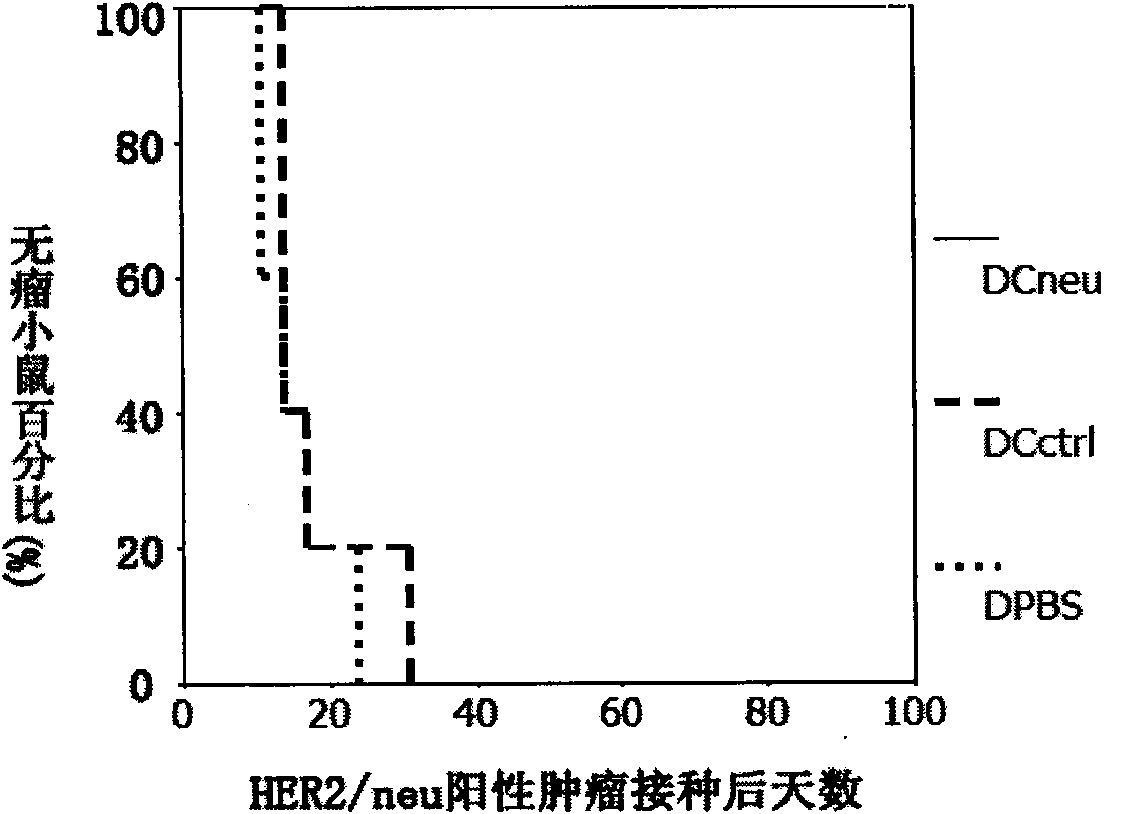
Novel HER2/neu gene-modified dendritic cell vaccine
The invention provides a preparation scheme of a novel HER2 / neu gene-modified dendritic cell vaccine, which comprises: 1, constructing a lentivirus vector carrying the HER2 / neu gene fragment; 2, preparing recombinant lentiviruses carrying the HER2 / neu gene fragment by using the vector; and 3, infecting dendritic cells with the recombinant lentiviruses and obtaining the vaccine. Animal experiment proves the novel HER2 / neu gene-modified dendritic cell vaccine can effectively prevent and treat HER2 / neu positive tumors.
Owner:CHANGSHA HUI LIN LIFE TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com