Novel HER2/neu gene-modified dendritic cell vaccine
A technology of dendritic cells and genetic modification, applied in the fields of biology and medicine, can solve the problems of short expression time, insufficient persistence and strength of immune effect, weakening DC stimulation of immunity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
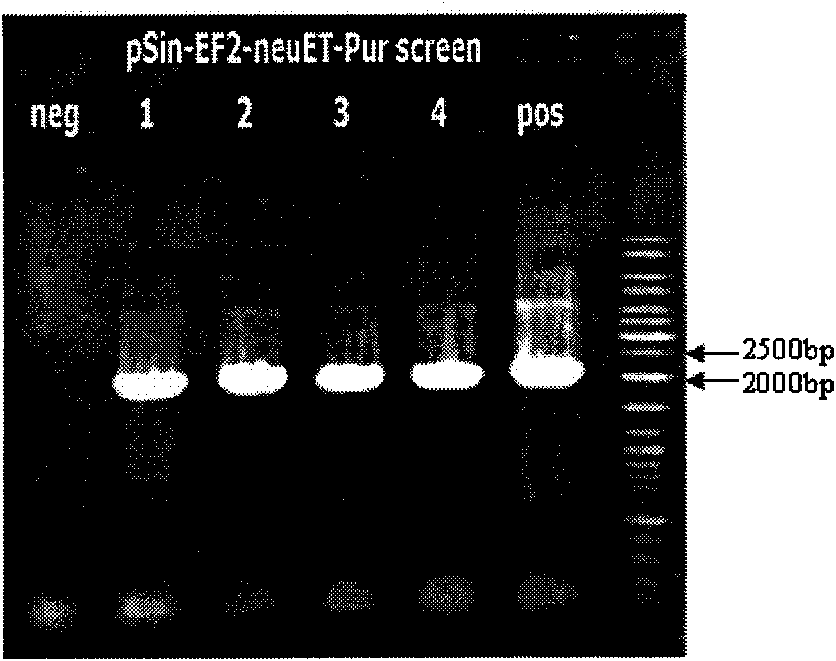
[0026] Example 1 Construction of recombinant lentiviral vectors carrying HER2 / neu gene fragments
[0027] 1. PCR amplification and purification of Neu gene fragment
[0028] Primer sequence upstream 5'-ggaattccagcctggtccagcctgag-3'; downstream 5'-gactagttcactgtctccttcgtttgatt-3'. The reaction template is the plasmid pMMTV-neu carrying the full-length cDNA of neu, which is constructed in this unit according to the method in the prior art. The amplification reaction used the Phusion ultra-fidelity PCR kit from NEB Company. The reaction system is as follows (volume unit is μl):
[0029]
[0030]
[0031] A total of two tubes were amplified. The program is set as follows: 98 degrees for 30s→95 degrees for 10s→62 degrees for 30s→72 degrees for 40s→72 degrees for 5min→4 degrees, repeat 30 cycles. PCR instrument is the product of eppendorf company.
[0032] The PCR product was run on a gel, and the product was purified with a gel recovery and purification kit from TaKaRa C...
Embodiment 2
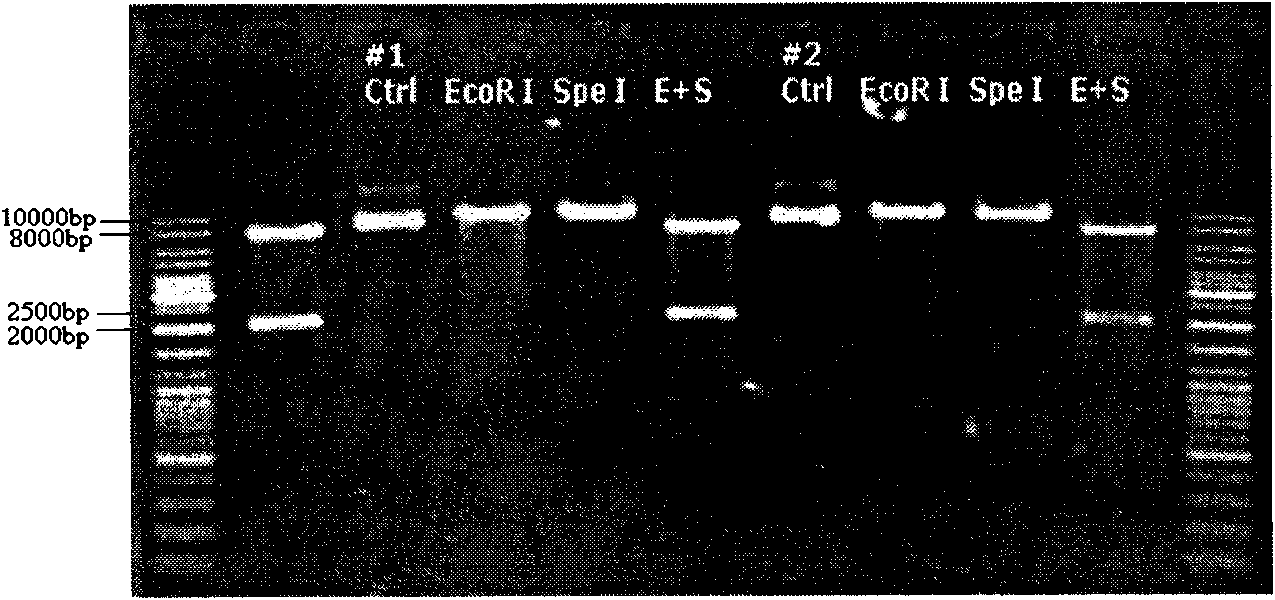
[0046] React at 37 degrees for 30 minutes. For the results of rubber running identification, see figure 2 . The recombinant vector was named pSin-EF2-neu-Pur. Example 2 Packaging Preparation and Concentration of Recombinant Lentivirus (rLVneu) Carrying HER2 / neu Gene Fragment
[0047] 1. Preparation of Plasmids
[0048] Including pSin-EF2-neuET-Pur, pCMV8.91 and pMD.G three kinds of plasmids. After large-scale culture of the bacteria containing the plasmid, the plasmid was prepared using the Invitrogen company's large-scale extraction kit.
[0049] 2. Preparation of transfection reagent
[0050] 0.3M CaCl 2 : 11.025g CaCl 2 2H 2 Dissolve O into 240ml Milli-Q water, dilute to 250ml, sterilize by 0.22μm filter, and store at 4°C.
[0051] 2×HBS : Add 14ml 5M NaCl solution, 12.5ml 1M HEPES buffer and 250μl 1.5M NaCl to 200ml Milli-Q water 2 HPO 4 Solution, adjust the pH value to 7.10 with NaOH or HCl, dilute to 250ml, sterilize by 0.22μm filter, and store at 4°C. ...
Embodiment 3
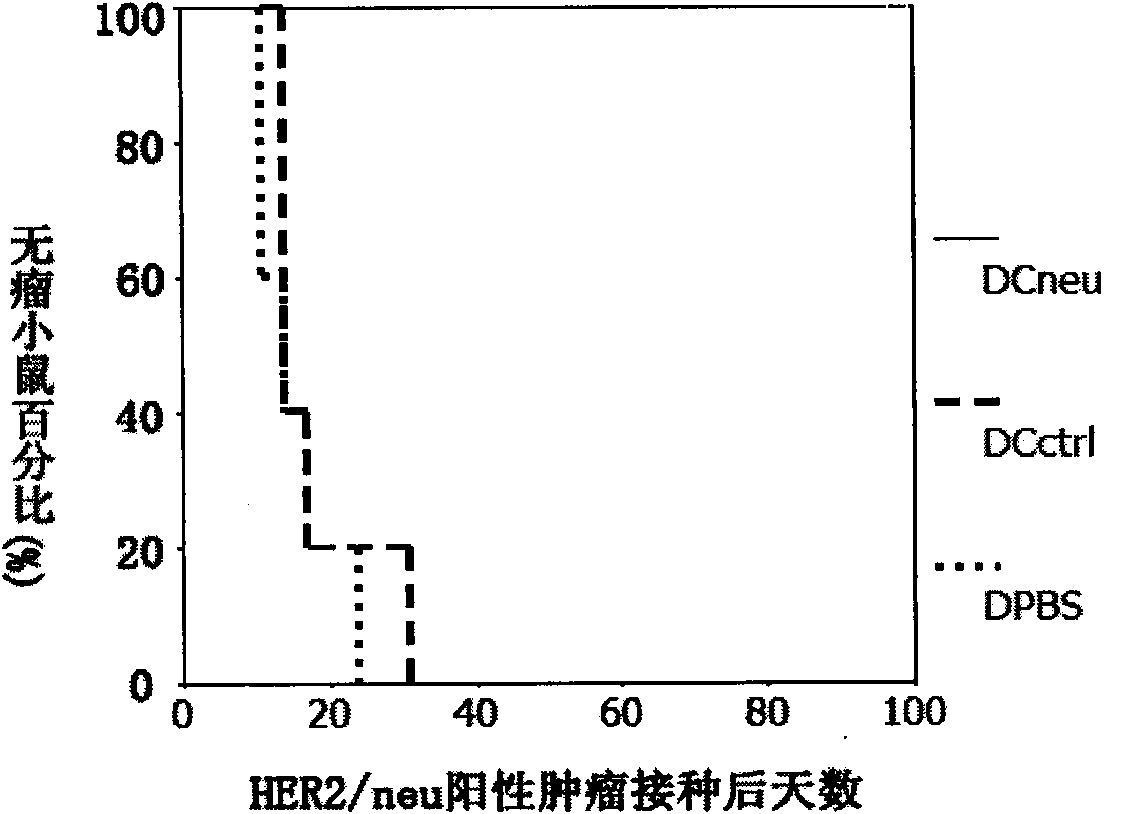
[0071] Example 3 Using rLVneu to infect mouse bone marrow-derived dendritic cells to prepare new DCs HER2 Vaccine (culture scale for 1 6-well plate)
[0072]The complete medium of DC is RPMI1640 medium containing 10% FBS, 2mM L-glutamine, 50μm β-mercaptoethanol and 20ng / ml GM-CSF. GM-CSF is a powder. After rehydration, it can be divided into 100ng / μl, 10μl / tube and frozen at -20 degrees. Avoid repeated freezing and thawing, and add it before use. The medium added with GM-CSF should be stored at 4°C and must be used within 7 days.
[0073] Except for separating the bones, the rest were strictly aseptic. Bacteriological or tissue culture-untreated dishes / plates are best used for DC culture.
[0074] (1) Take the femur and tibia of the mouse, soak them in alcohol for disinfection, and bring them into the ultra-clean bench for operation.
[0075] (2) Soak and wash the bones in RPMI1640, cut off the epiphysis, aspirate 10ml of RPMI1640 with a syringe, and use a 0.45mm injection...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com