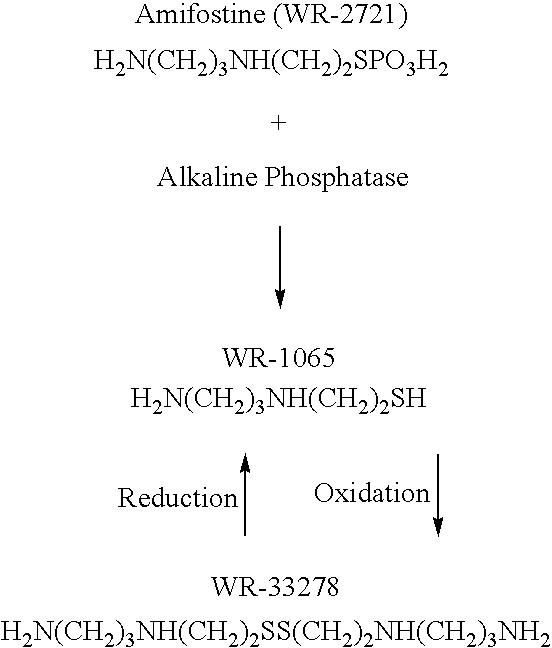
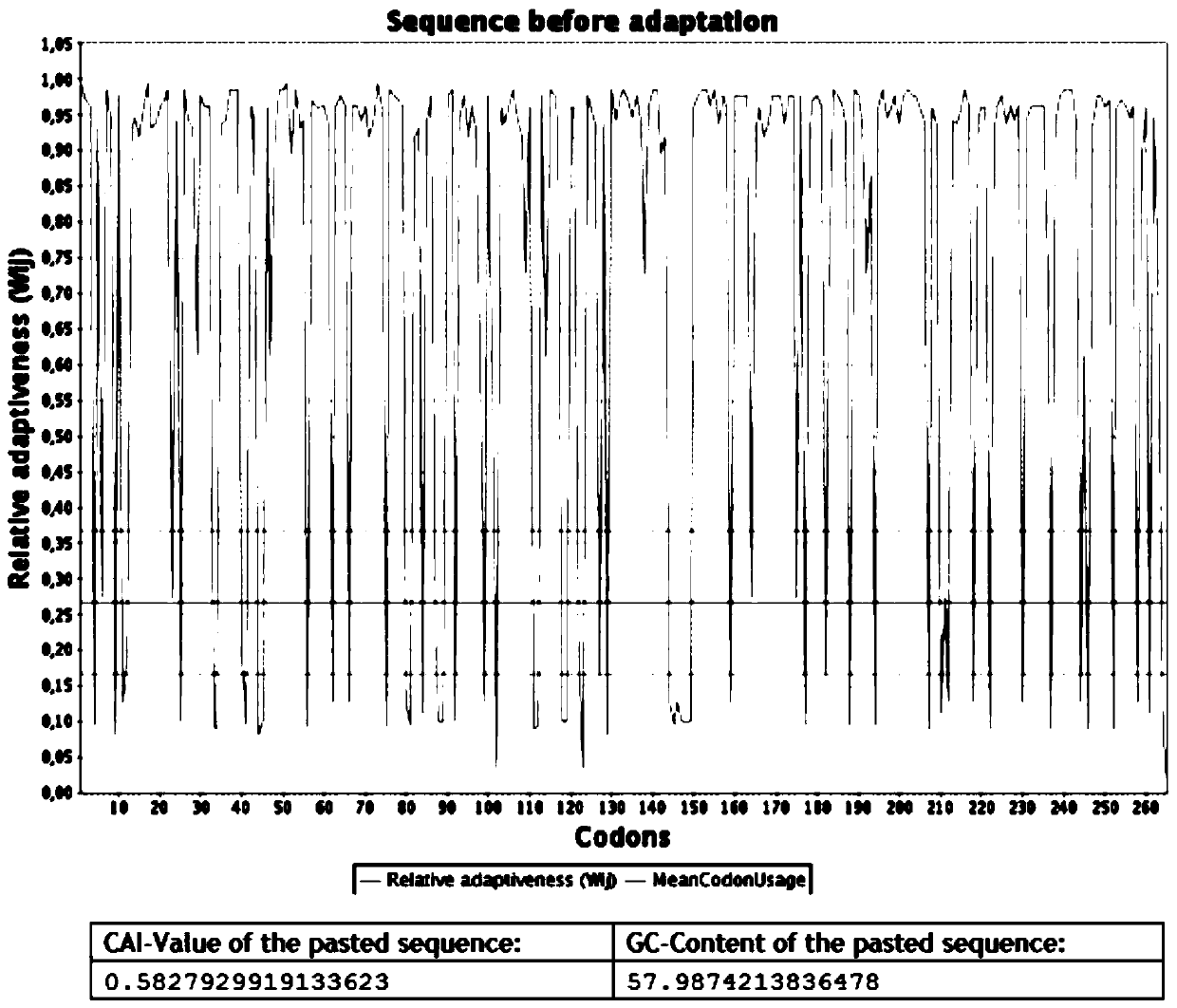
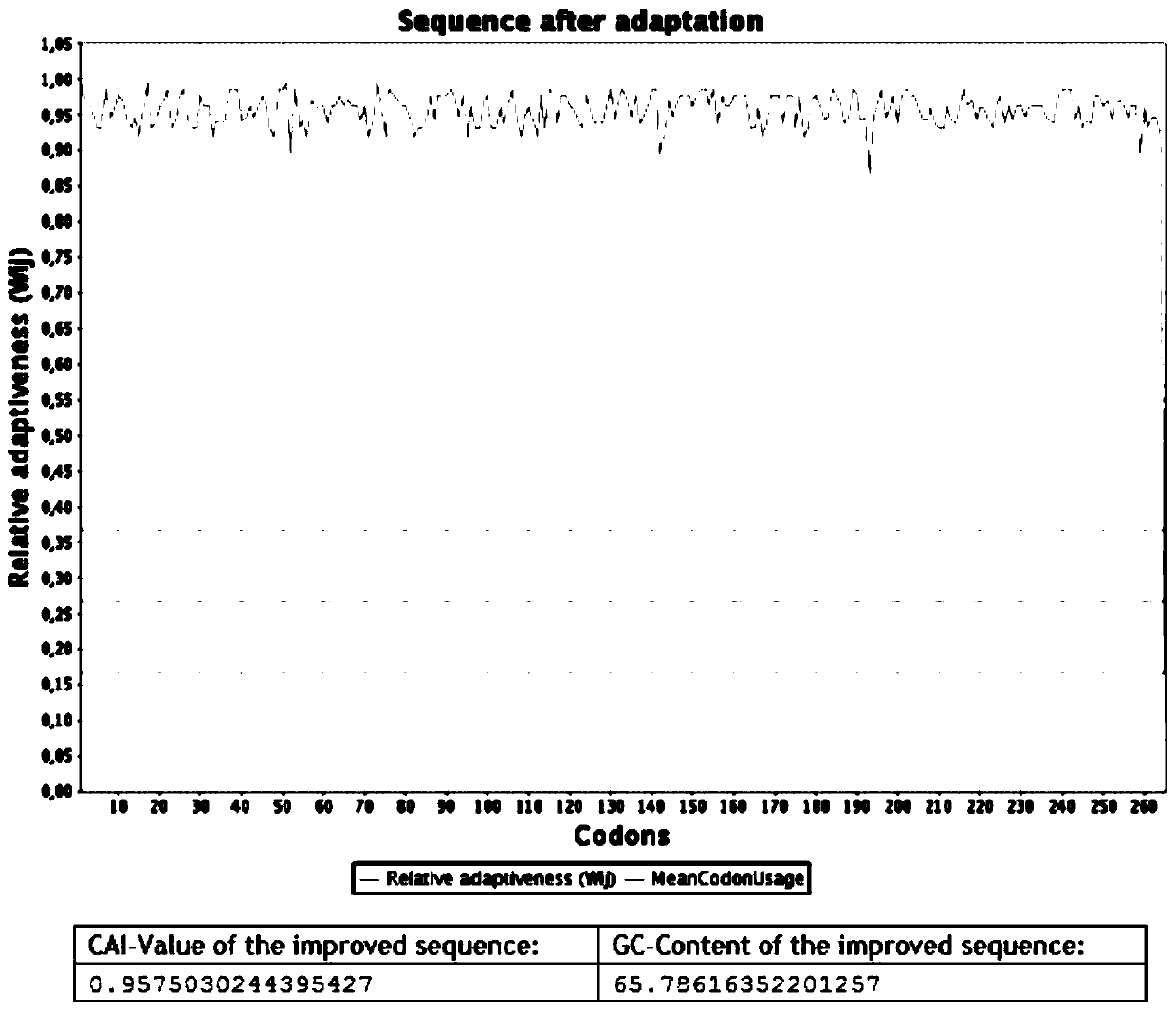
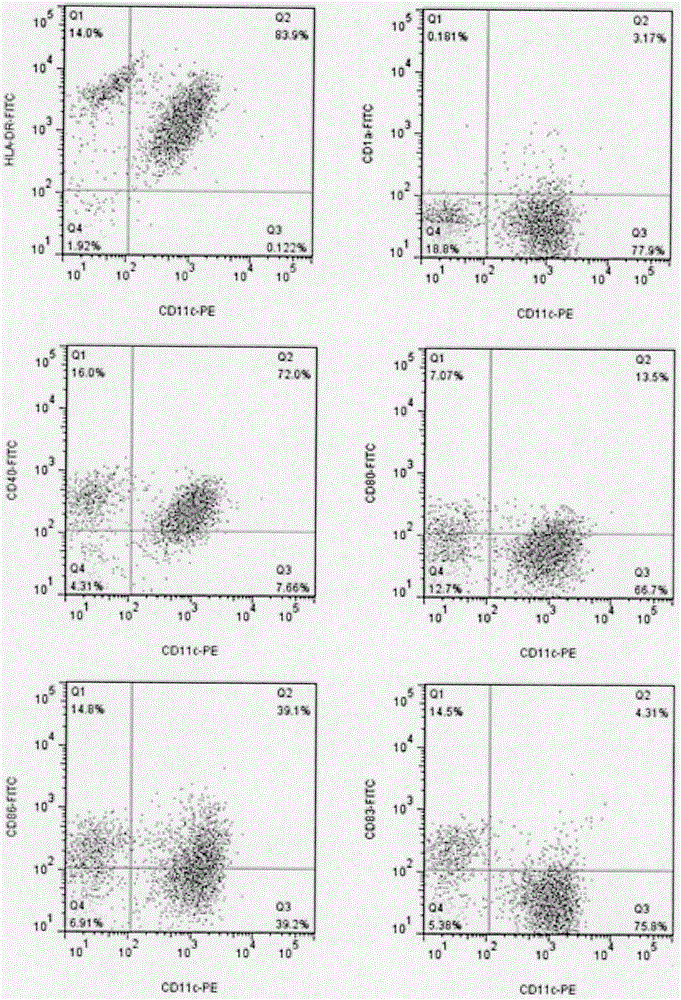
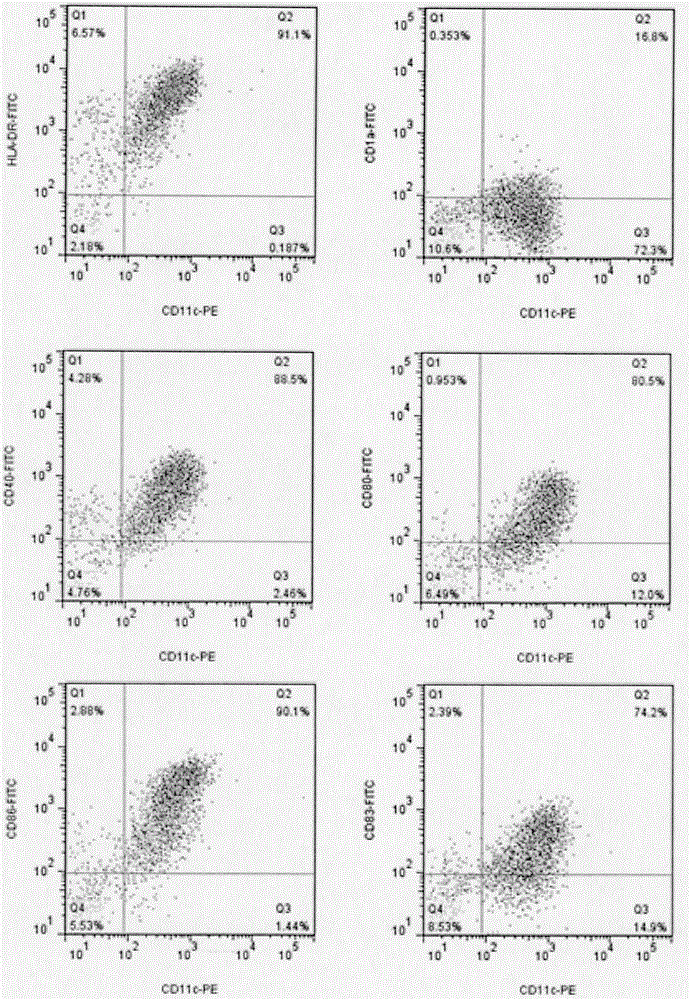
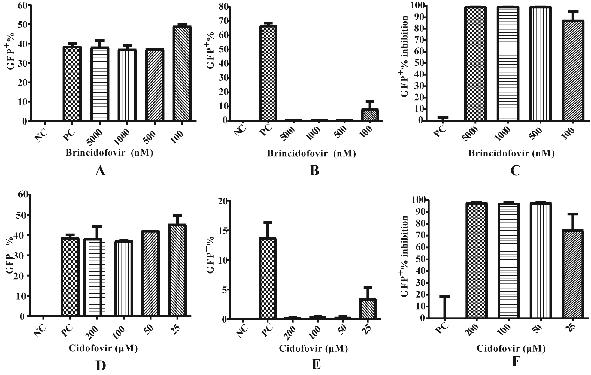
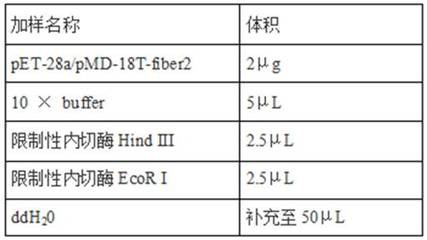
Patents
Literature
59 results about "Adenovirus infection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Adenovirus infections most commonly cause illness of the respiratory system; however, depending on the infecting serotype, they may also cause various other illnesses and presentations.
Methods and compositions for the production of adenoviral vectors
InactiveUS20050158283A1Maximize productionProduced in advanceBiocideGenetic material ingredientsAdenovirus infectionMicrobiology
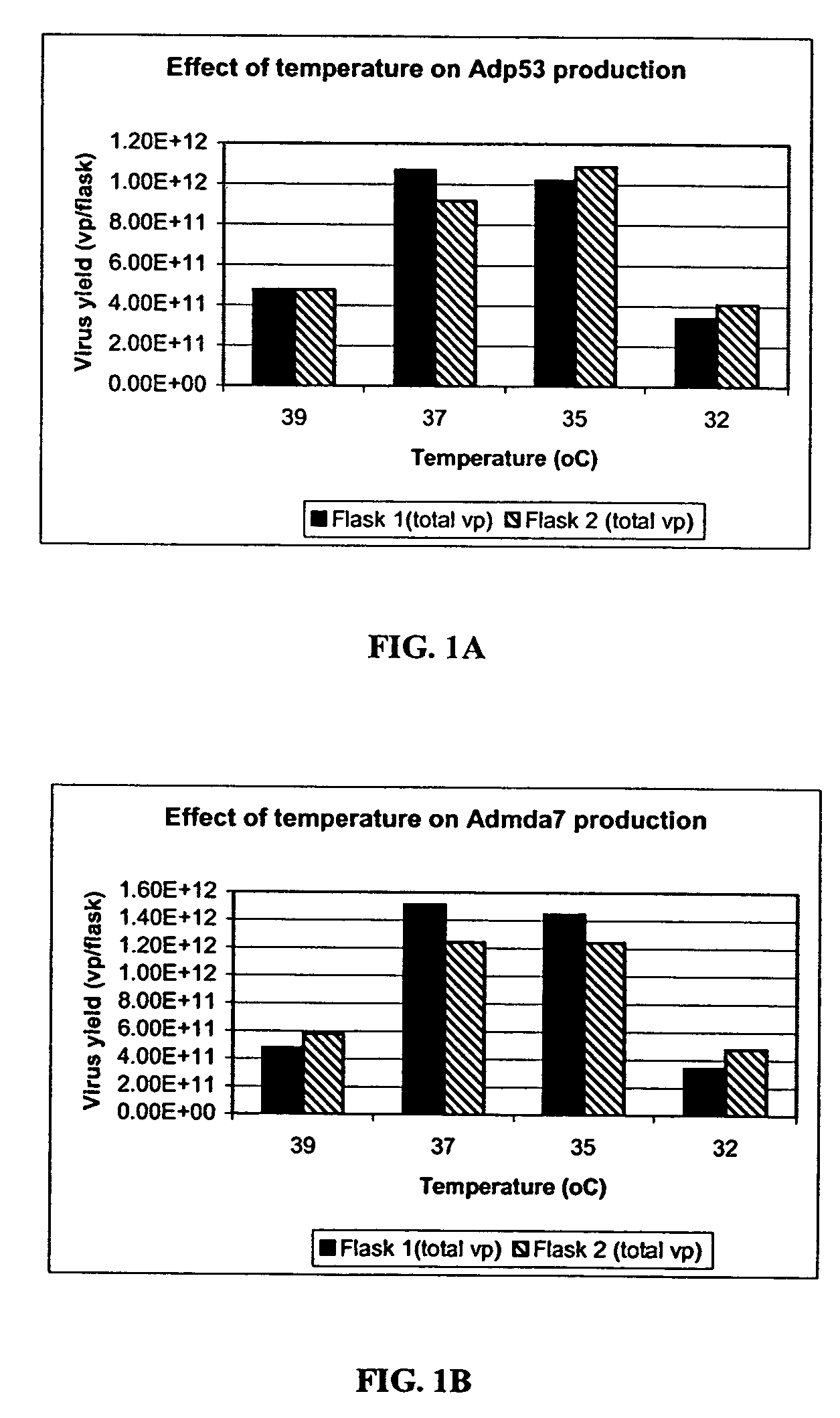
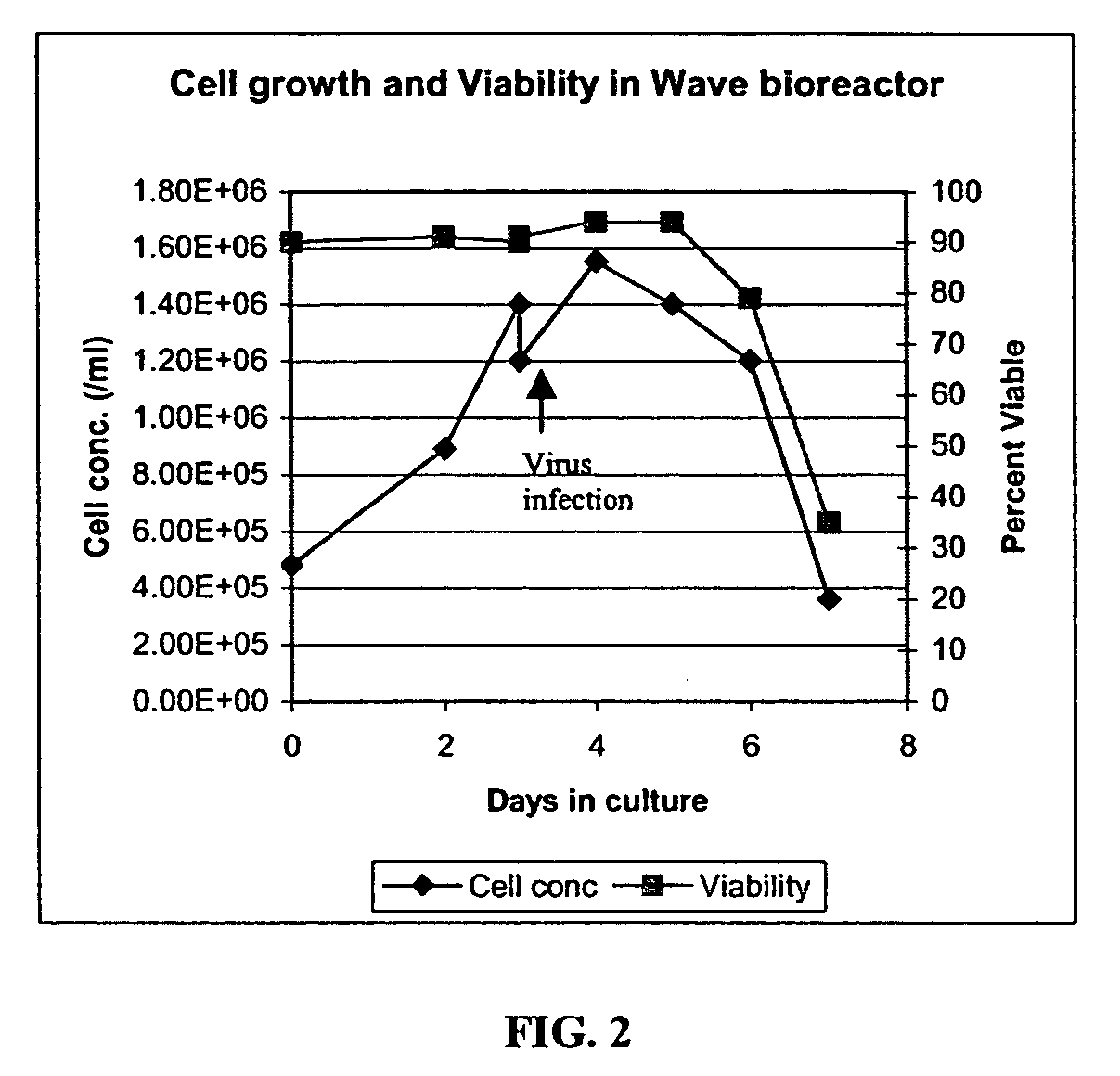
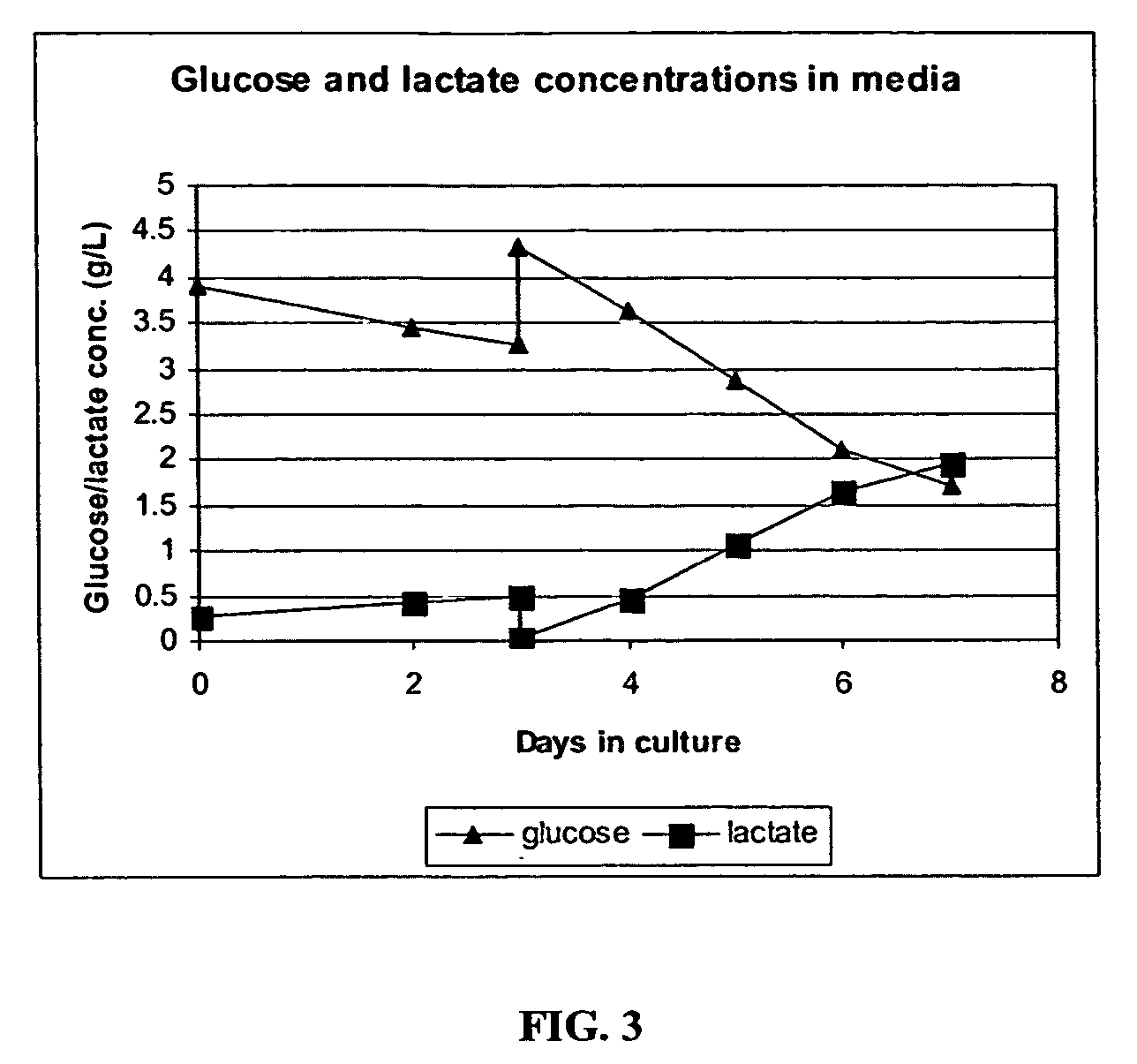
The present invention addresses the need to improve the yield of adenovirus when grown in cell culture systems. In particular, it has been demonstrated that for adenovirus, the use of infection temperatures lower than 37° C. in a cell culture system results in improved yields of adenovirus. In addition, it has been demonstrated that when host cells are grow in a bioreactor, initiating adenovirus infection by diluting the host cells with fresh media and adenovirus results in improved yield of adenovirus. Methods of adenoviral production and purification using infection temperatures less than 37° C. are disclosed. Methods of adenoviral production and purification wherein the host cells are grown in a bioreactor and adenovirus infection is initiated by diluting the host cells with fresh media and adenovirus are also disclosed.
Owner:JANSSEN VACCINES & PREVENTION BV
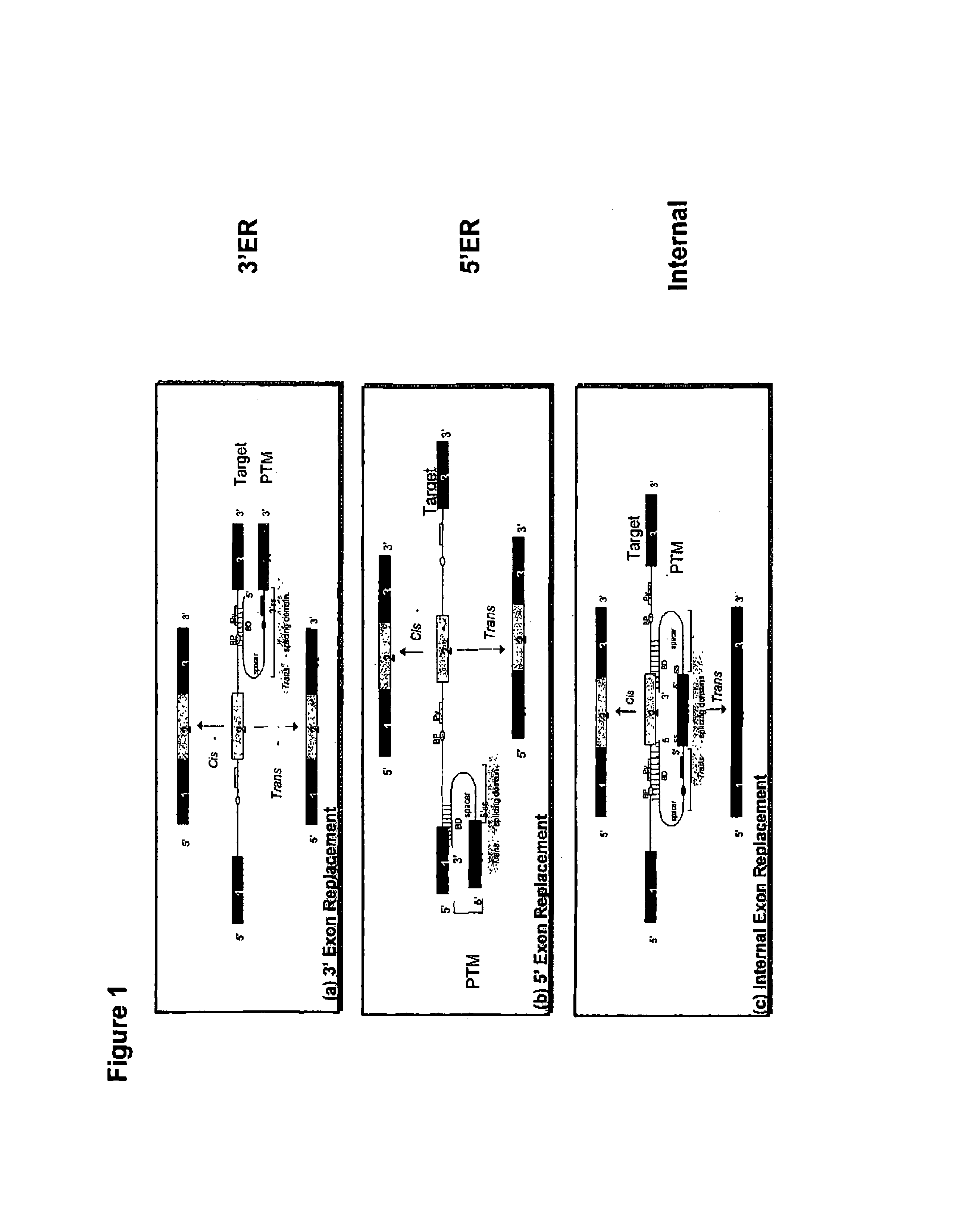
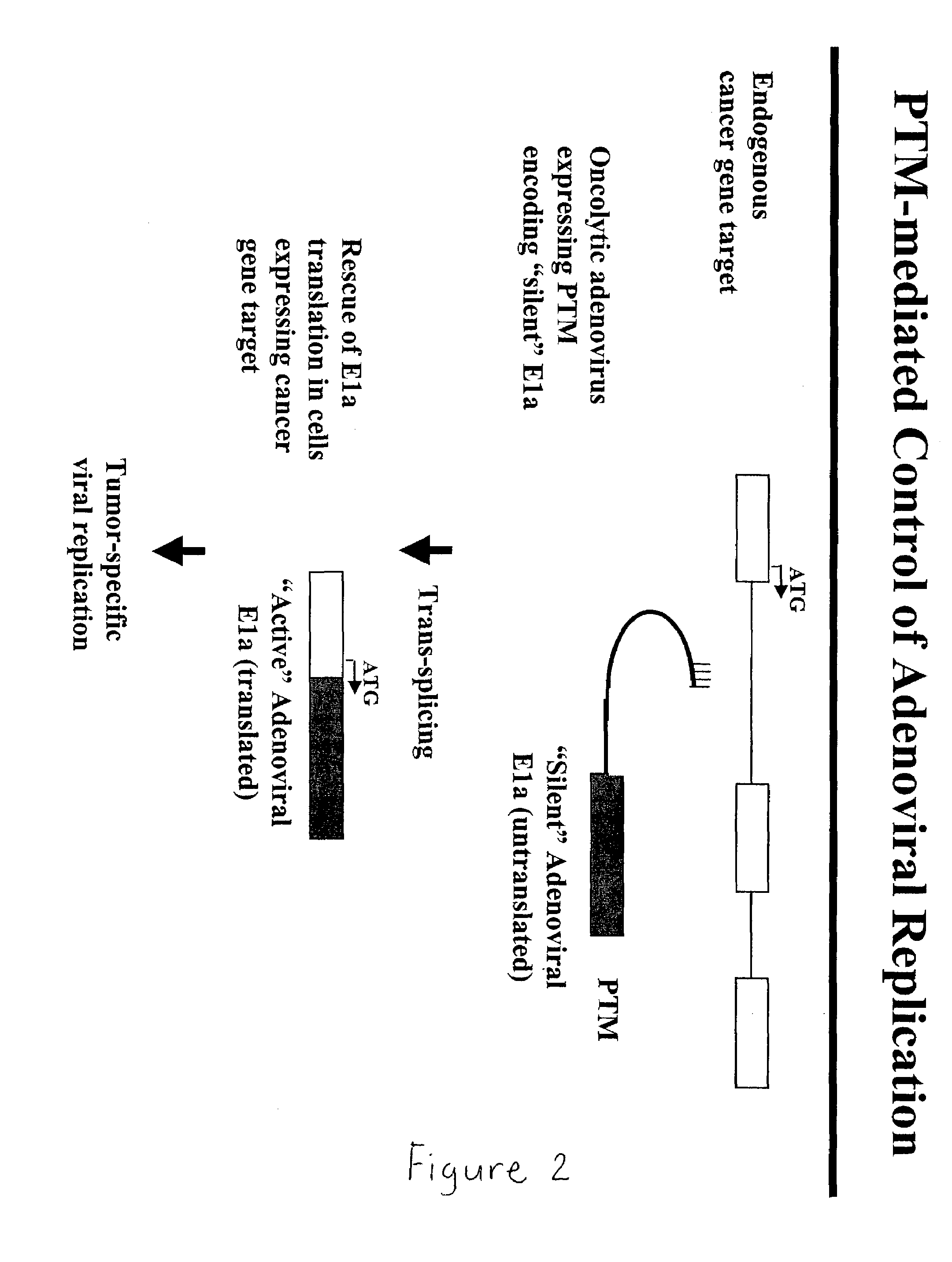
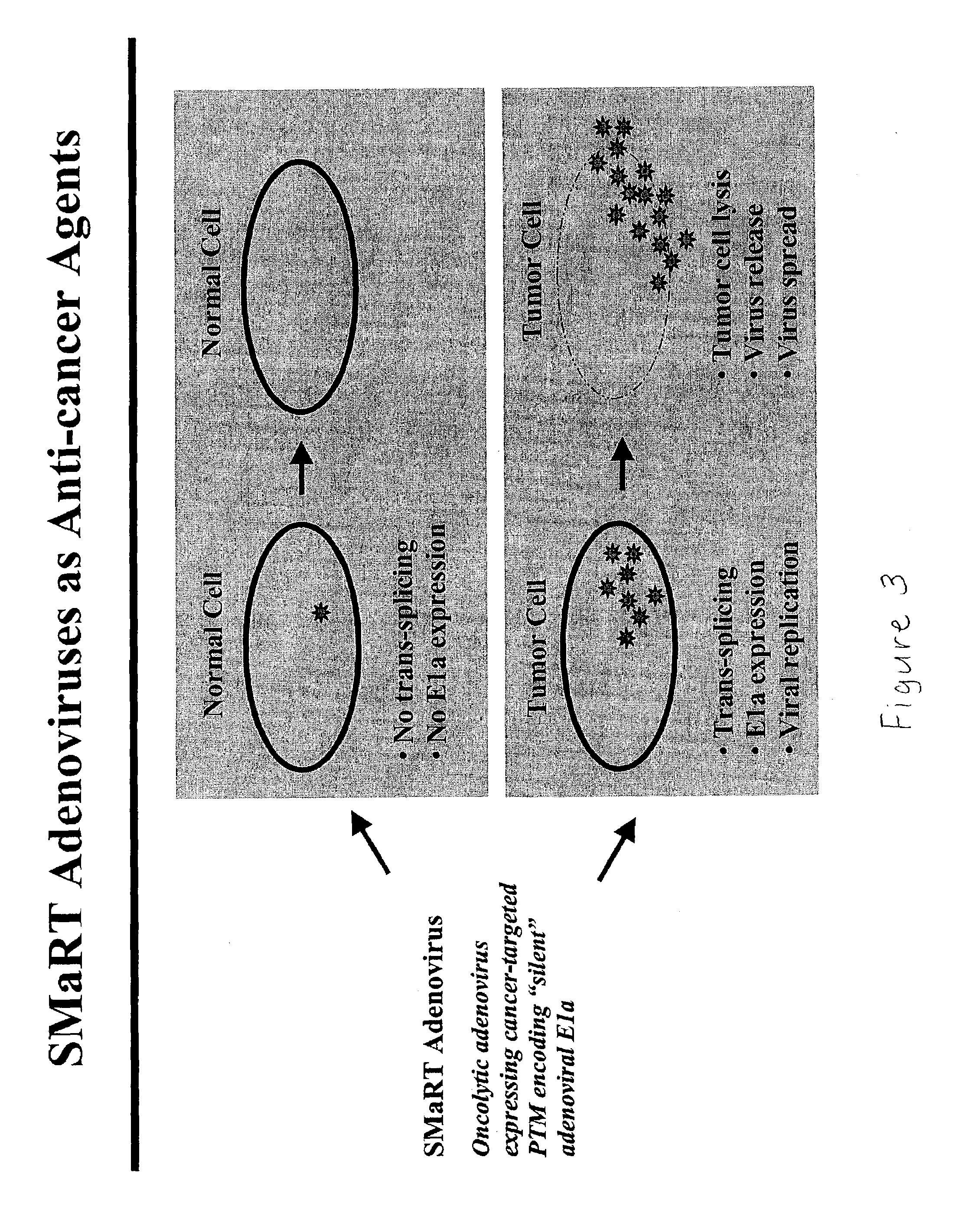
Use of spliceosome mediated RNA trans-splicing to confer cell selective replication to adenoviruses
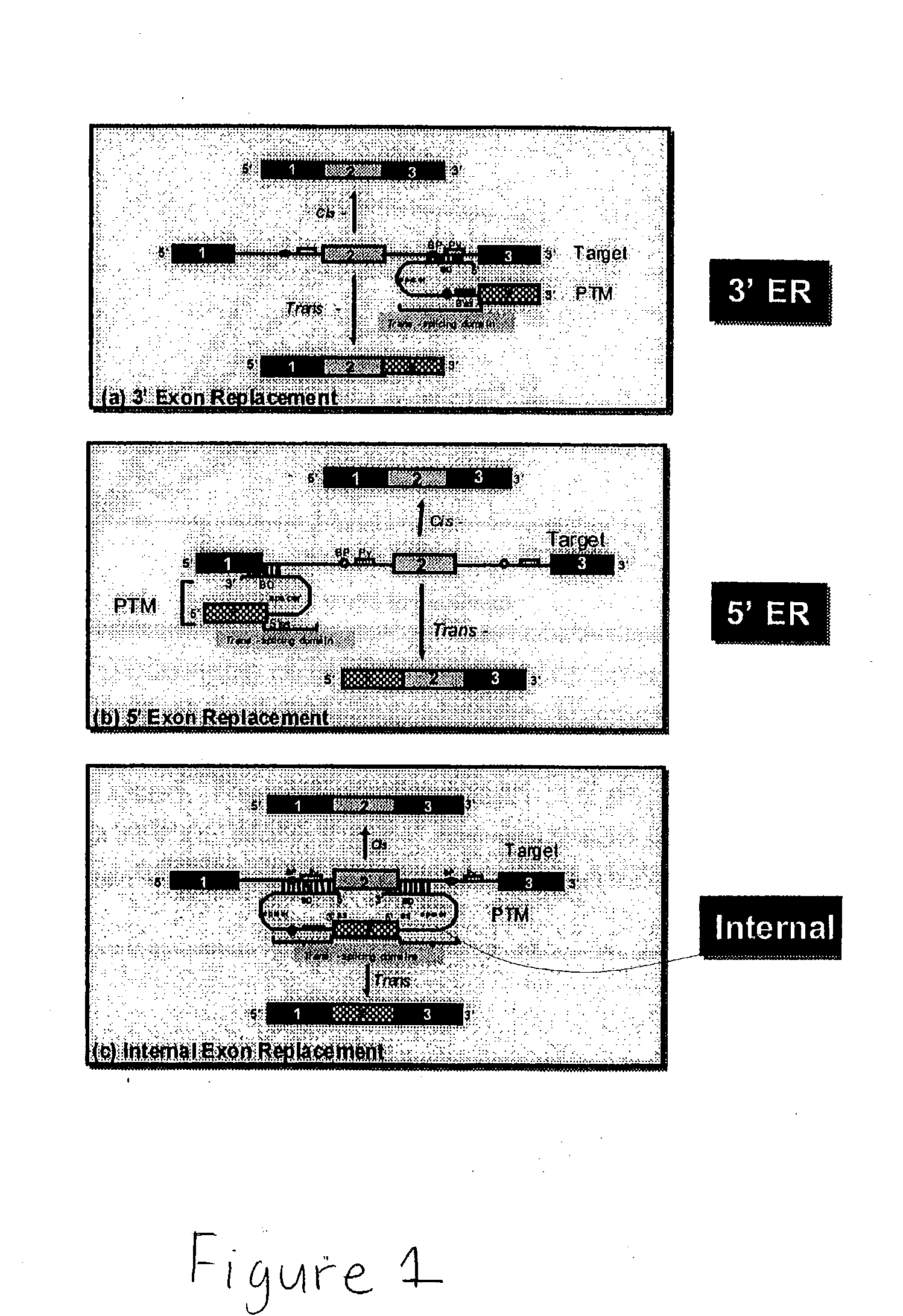
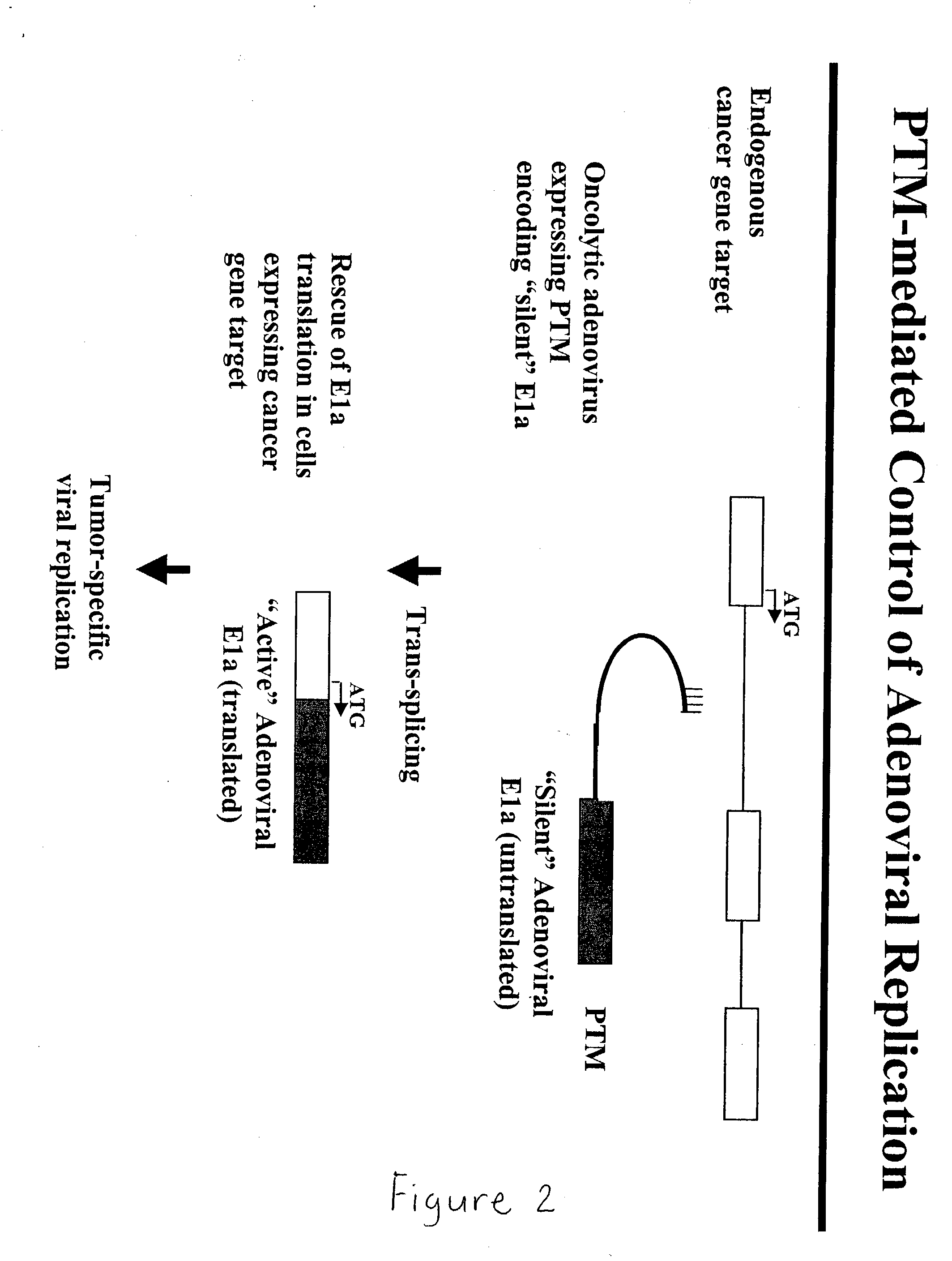
The present invention provides methods and compositions for conferring tumor selective cell death on cancer cells expressing specific target precursor messenger RNA molecules (cancer cell selective target pre-mRNAs). The compositions of the invention include conditionally replicative adenoviruses that have been genetically engineered to express one or more pre-trans-splicing molecules (PTMs) designed to interact with one or more cancer cell target pre-mRNA and mediate a trans-splicing reaction resulting in the generation of novel chimeric RNA molecules (chimeric RNA) capable of encoding adenovirus specific protein(s). Adenovirus specific proteins include those proteins complementing an essential activity necessary for replication of a defective adenovirus. The methods and compositions of the invention may be used to target a lytic adenovirus infection to cancer cells thereby providing a method for selective destruction of cancer cells. In addition, the adenoviruses of the invention may be engineered to encode PTMs designed to interact with target pre-mRNAs encoded by infectious agents within a cell, thereby targeting selective destruction of cells infected with such agents.
Owner:VIRXSYS
Preparation and application of adenovirus parting gene chip
InactiveCN105671212AImprove throughputStrong specificityNucleotide librariesMicrobiological testing/measurementOligonucleotide chipEpidemiologic survey
The invention relates to preparation and application of a gene chip capable of detecting adenoviruses in a parting mode.A preparation method comprises the steps of preparing specific primers and probes for different types of adenoviruses, preparing oligonucleotides chips, establishing a plurality of PCR systems, and establishing a hybridization system.The gene chip prepared through the method can screen different types of adenoviruses including a 3-type adenovirus, a 7-type adenovirus, a 14-type adenovirus, an 11-type adenovirus and a 55-type adenovirus.The gene chip has the advantages of quickness, accuracy, high throughput and high specificity.A new detection means is provided for clinical diagnoses of different types of adenovirus infections and epidemiological surveys.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Use of spliceosome mediated RNA trans-splicing to confer cell selective replication to adenoviruses
The present invention provides methods and compositions for conferring tumor selective cell death on cancer cells expressing specific target precursor messenger RNA molecules (cancer cell selective target pre-mRNAs). The compositions of the invention include conditionally replicative adenoviruses that have been genetically engineered to express one or more pre-trans-splicing molecules (PTMs) designed to interact with one or more cancer cell target pre-mRNA and mediate a trans-splicing reaction resulting in the generation of novel chimeric RNA molecules (chimeric RNA) capable of encoding adenovirus specific protein(s). Adenovirus specific proteins include those proteins complementing an essential activity necessary for replication of a defective adenovirus. The methods and compositions of the invention may be used to target a lytic adenovirus infection to cancer cells thereby providing a method for selective destruction of cancer cells. In addition, the adenoviruses of the invention may be engineered to encode PTMs designed to interact with target pre-mRNAs encoded by infectious agents within a cell, thereby targeting selective destruction of cells infected with such agents.
Owner:VIRXSYS
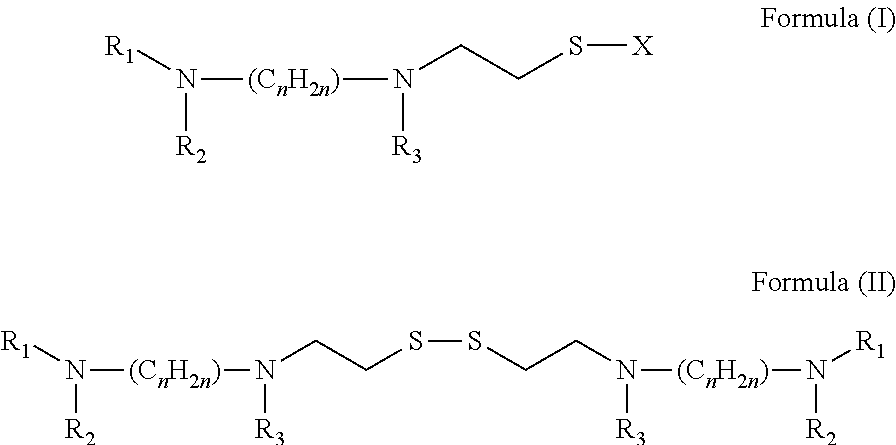
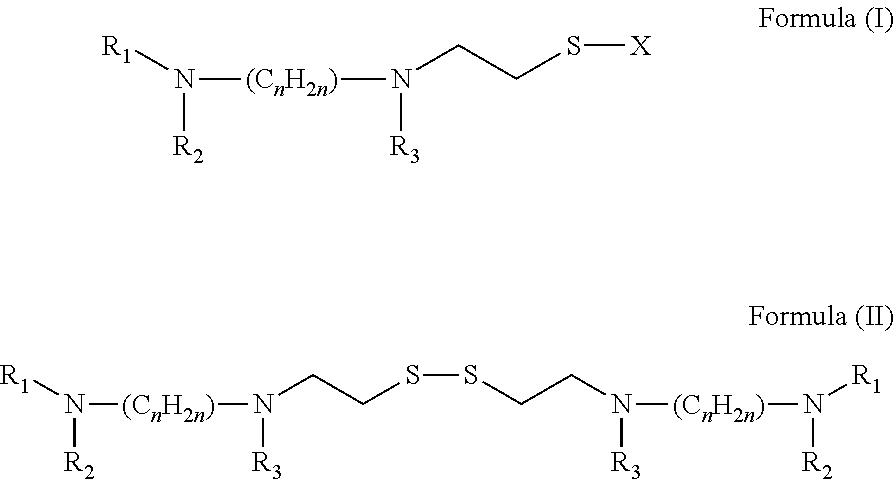
Broad Spectrum Antiviral and Methods of Use
ActiveUS20110053894A1High level of retentionEfficient mechanismBiocidePhosphorous compound active ingredientsAdenovirus infectionBroad spectrum
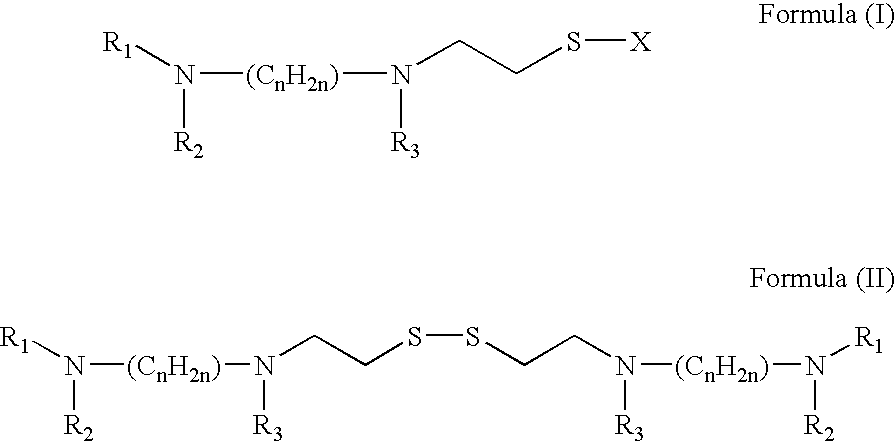
A method for the prevention or treatment of Influenza virus infection or Adenovirus infection by administering an effective amount of a compound of Formula (I), Formula (II), or similar compound to an individual in need is provided.
Owner:THE BURLINGTON HC RES GRP INC
Rapid detection kit for human adenoviruses
InactiveCN104131006AKeep abreast ofIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesPositive controlAdenovirus DNA
The invention belongs to the technical field of virus detection and provides an extracting solution for extracting human adenovirus DNA (deoxyribonucleic acid), a PCR (polymerase chain reaction) reaction solution for detecting human adenoviruses, a PCR primer sequence for detecting human adenovirus DNA, a rapid detection kit for human adenoviruses and a method for quantitatively detecting the quantity of human adenoviruses. The rapid detection kit for human adenoviruses comprises the extracting solution for extracting human adenovirus DNA, the PCR reaction solution for detecting human adenoviruses and the PCR primer sequence for detecting human adenovirus DNA, and further comprises an adenovirus positive control and an amplification primer sequence for detecting the positive control. The adenovirus detection sensitivity of the kit disclosed by the invention can reach 500copies / ml. The quantitative linear range is 500-5.0E+08copies / ml, so that the kit is suitable for detecting the adenoviruses in a trace sample and provides a reliable basis for early diagram of adenovirus infection.
Owner:NANJING AGRICULTURAL UNIVERSITY
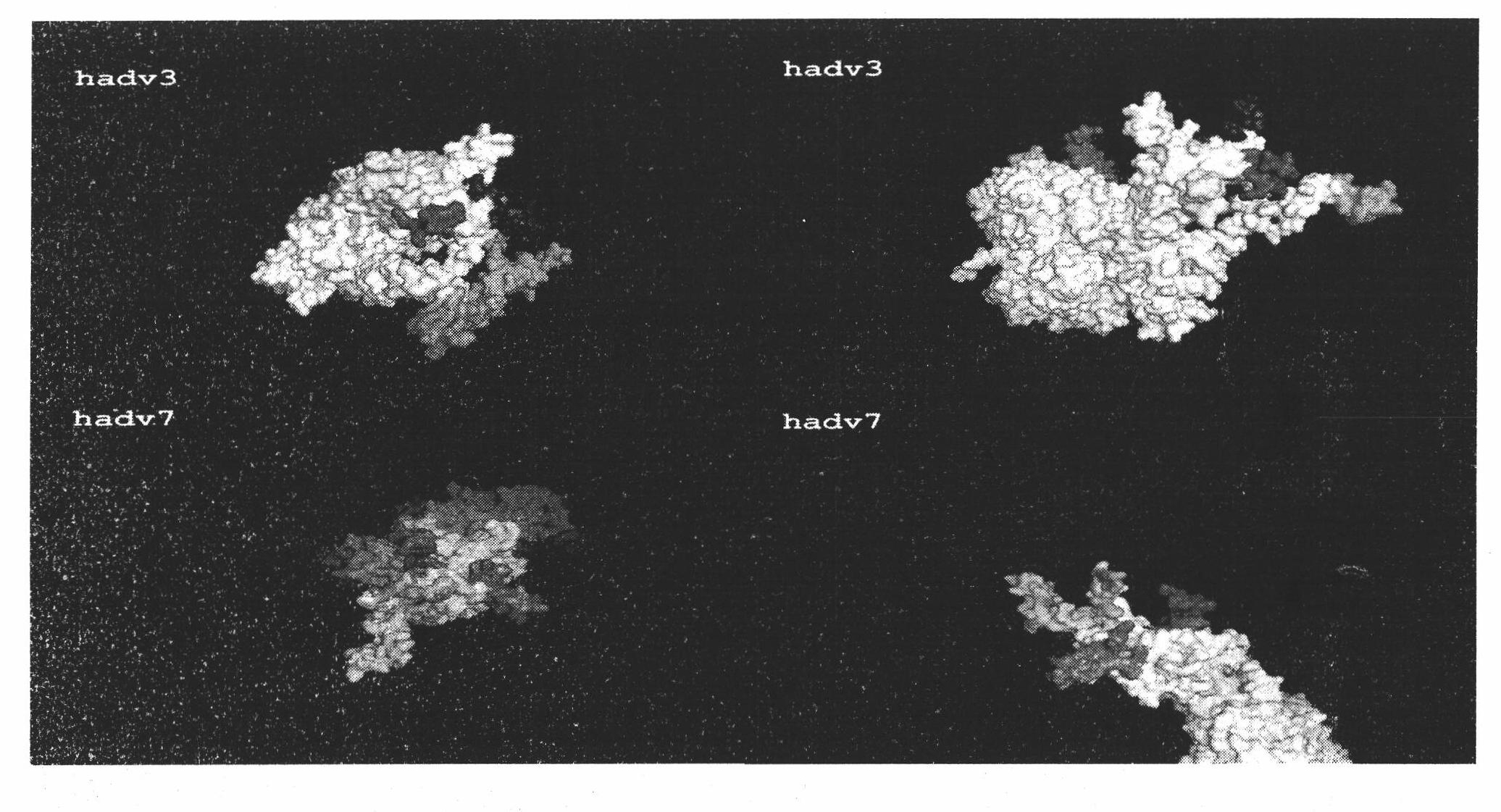
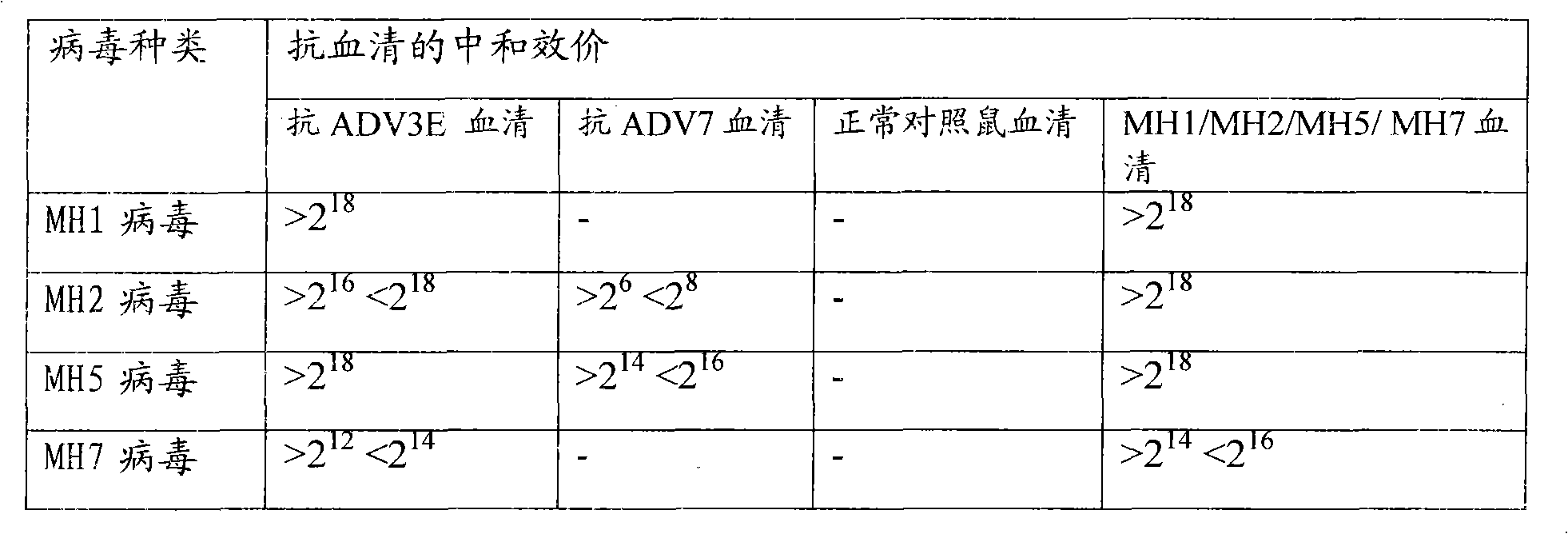
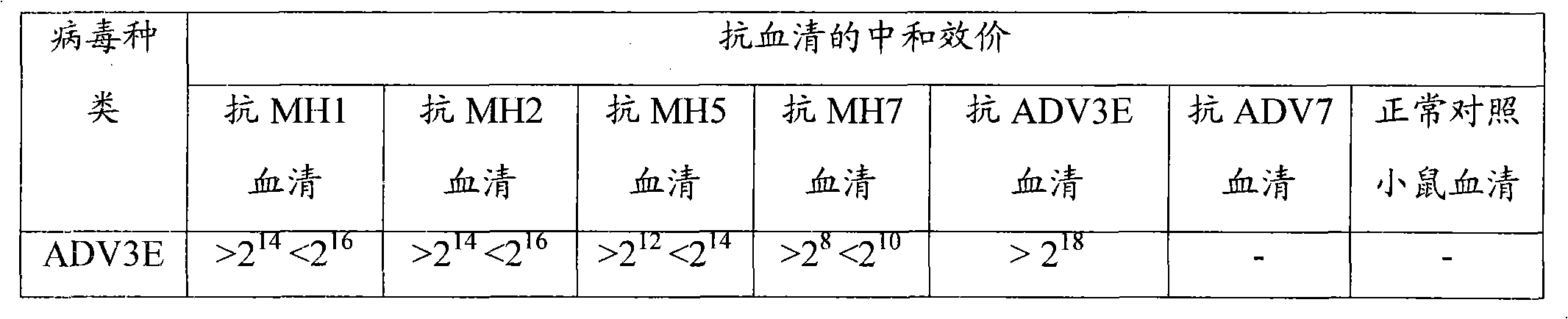
Neutralizing epitope of human adenovirus type 3 (HAdV-3) and type 7 (HAdV-7) and application thereof
InactiveCN101942011AStimulate immune responseEasily exposedImmunoglobulins against virusesAntiviralsProtein targetNucleotide
The invention discloses three neutralizing epitope of human adenovirus type 3 (HAdV-3) and type 7 (HAdV-7) and an application thereof. The amino acid sequence of the neutralizing epitope of the HAdV-3 and HAdV-7 is selected from the amino acid sequence shown in SEQ ID NO: 1, 2, 3, and the nucleotide sequence of the neutralizing epitope of HAdV-3 and HAdV-7 is selected from the nucleotide sequence shown in the SEQ ID NO: 4, 5, 6. The neutralizing epitopes of HAdV-3 and HAdV-7 can be used for preparing vaccines or antibody and antigen binding fragments for preventing infection of human adenovirus type 3 and human adenovirus type 7, and the antibody and the antigen binding fragments can be used for preparing drugs for preventing or curing adenovirus infection. The drugs contain the antibody or antigen binding fragments. The invention also provides a method for preventing and curing adenovirus infection, i.e. the vaccines or drugs with immune effective quantity are applied. The three neutralized epitopes of HAdV-3 and HAdV-7 can be served as target protein for developing a diagnostic kit.
Owner:STATE KEY LAB OF RESPIRATORY DISEASE
Anti-human adenovirus type 7 antibody 2-1H and application thereof
The invention discloses an anti-human adenovirus type 7 antibody 2-1H and an application thereof. The invention provides a monoclonal antibody, and the monoclonal antibody comprises a heavy chain variable region and a light chain variable region; the heavy chain variable region comprises three complementary determining regions HCDR1, HCDR2 and HCDR3; the light chain variable region comprises threecomplementary determining regions LCDR1, LCDR2 and LCDR3; the HCDR1, the HCDR2 and the HCDR3 are sequentially shown as a 26th-33th position, a 51th-58th position and a 97th-117th position of a sequence 2 of a sequence table from an N end; and the LCDR1, the LCDR2 and the LCDR3 are sequentially shown as a 27th-32th position, a 50th-52th position and a 89th-97th position of a sequence 4 of the sequence table from the N end. Based on clinical needs, the anti-human adenovirus type 7 antibody 2-1H is found, is used for prevention and treatment of adenovirus infection, and has important biologicaland medical significance.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Recombinant adenovirus carrier for expressing African swine fever virus B646L gene, construction method and preparation method of recombinant adenovirus
InactiveCN108342414ATo achieve the purpose of normal expressionVirus peptidesFermentationD'Aguilar virusVector vaccine
The invention provides a recombinant adenovirus carrier for expressing an African swine fever virus B646L gene, a construction method and a preparation method of the recombinant adenovirus, belongingto the technical field of genetic engineering. According to the method, adenovirus shuttle vectors pKO-FH and pAD-EF1a-GFP are utilized to be subjected to a series of intermediate processes to obtaina recombinant adenovirus expression plasmid pAD-B646L. An HEK293 cell is transfected to an obtained linear recombinant adenovirus vector plasmid; according to cytopathy caused by adenovirus infection,a recombinant virus is screened to finally realize an adenovirus packaging process, the recombinant adenovirus capable of directly infecting eukaryocyte can be obtained through amplification, concentration and authentication, so that the purpose of normally expressing a B646L gene in the eukaryocyte is realized, and the foundation is laid for researching an adenovirus vector vaccine based on expression of B646L.
Owner:YANGZHOU UNIV
Fluorescence quantitative PCR kit for detecting type-3 cow adenovirus and application
ActiveCN101560573AAccurate determination of starting copy numberIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesFluorescenceBasic research
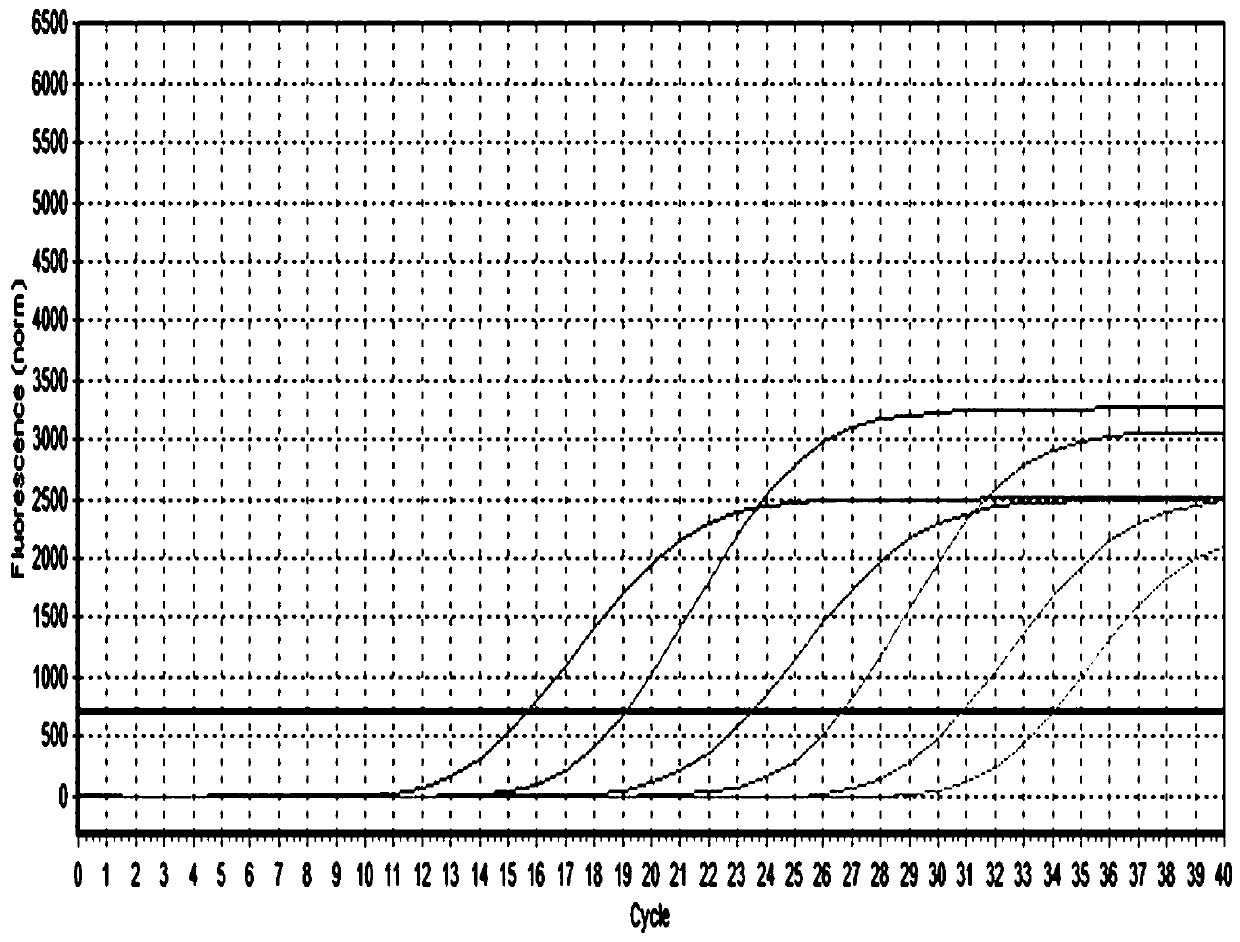
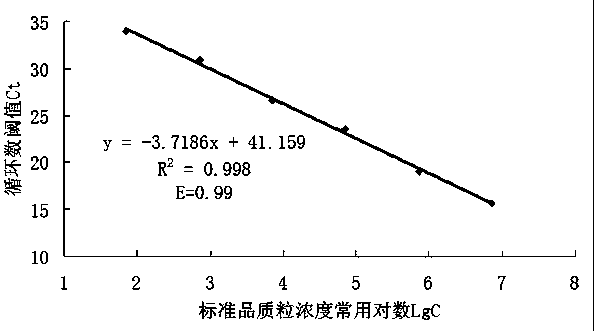
The invention discloses a fluorescence quantitative PCR kit for detecting type-3 cow adenovirus and application. The kit comprises a) DNA extraction reagent, b) hot starting Taq DNA polymerase, c) primers and TaqMan probe, d) standard positive DNA template, and e) PCR fluorescence quantitative reaction liquid. The kit is characterized in that the sequence of a positive primer is 5'-CCTGAATTCTCTTGCAGCCAGA-3', the sequence of a negative primer is 5'-CCTACCGAACCGACGCAGAT-3', the size of an amplicon is 100bp, the sequence of a fluorescence probe is 5'-FAM-TGAGAAGGTACTCCTCGTCGCTGGACCA-TAMRA-3', a 5' end of the probe marks a fluorescence emitting group FAM, a near 3' end of the probe marks a fluorescence quenching group TAMRA, the standard positive DNA template converts colon bacillus DH5a by a pGEM-T carrier inserted into a type-3 adenovirus pol protein 100bp fragment, plasmids are extracted after multiplication to prepare the kit, and A260 is measured by an ultraviolet spectrophotometer to definite quantity and is diluted by 10 times of gradient. The kit efficiently and conveniently monitors type-3 cow adenovirus pollution in a serum product in real time, can be widely applied to epidemiology research on adenovirus infection, can provide technical support for related basic research, and has wide application prospect.
Owner:WUHAN SANLI BIO TECH
Broad spectrum antiviral and methods of use
ActiveUS9585849B2High levelEfficient mechanismPhosphorous compound active ingredientsAntiviralsAdenovirus infectionBroad spectrum
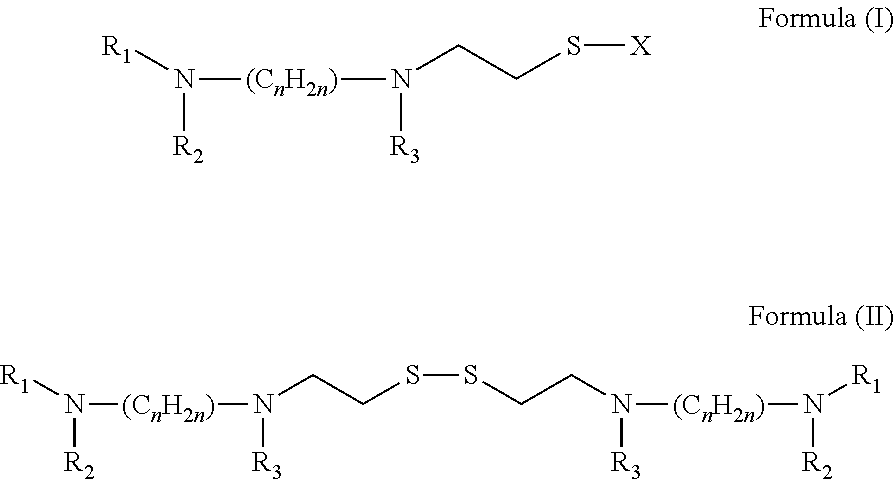
A method for the prevention or treatment of Influenza virus infection or Adenovirus infection by administering an effective amount of a compound of Formula (I), Formula (II), or similar compound to an individual in need is provided.
Owner:THE BURLINGTON HC RES GRP INC
Primers for real-time fluorescent quantitative PCR (polymerase chain reaction) assay of novel pigeon adenovirus EvaGreen
InactiveCN107586889ASimplify operating proceduresLow costMicrobiological testing/measurementDNA/RNA fragmentationAssayAdenovirus infection
The invention provides primers for the real-time fluorescent quantitative PCR (polymerase chain reaction) assay of novel pigeon adenovirus EvaGreen, the sequences of which are shown as SEQ ID NO.1-2 and which have high specificity and sensitivity. An established method can assay the infection of the novel pigeon adenovirus in a pigeon flock, the operation procedure is simplified, and the cost is reduced. After real-time fluorescent quantitative PCR is complete, by observing solubility curve peaks (Tm value), a result can be directly judged. The establishment of the invention can fill the gap of the related field at home and abroad.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Methods and reagents for the detection of antibodies to adenovirus
InactiveUS6964843B1Viral antigen ingredientsMicrobiological testing/measurementAssayAdenovirus infection
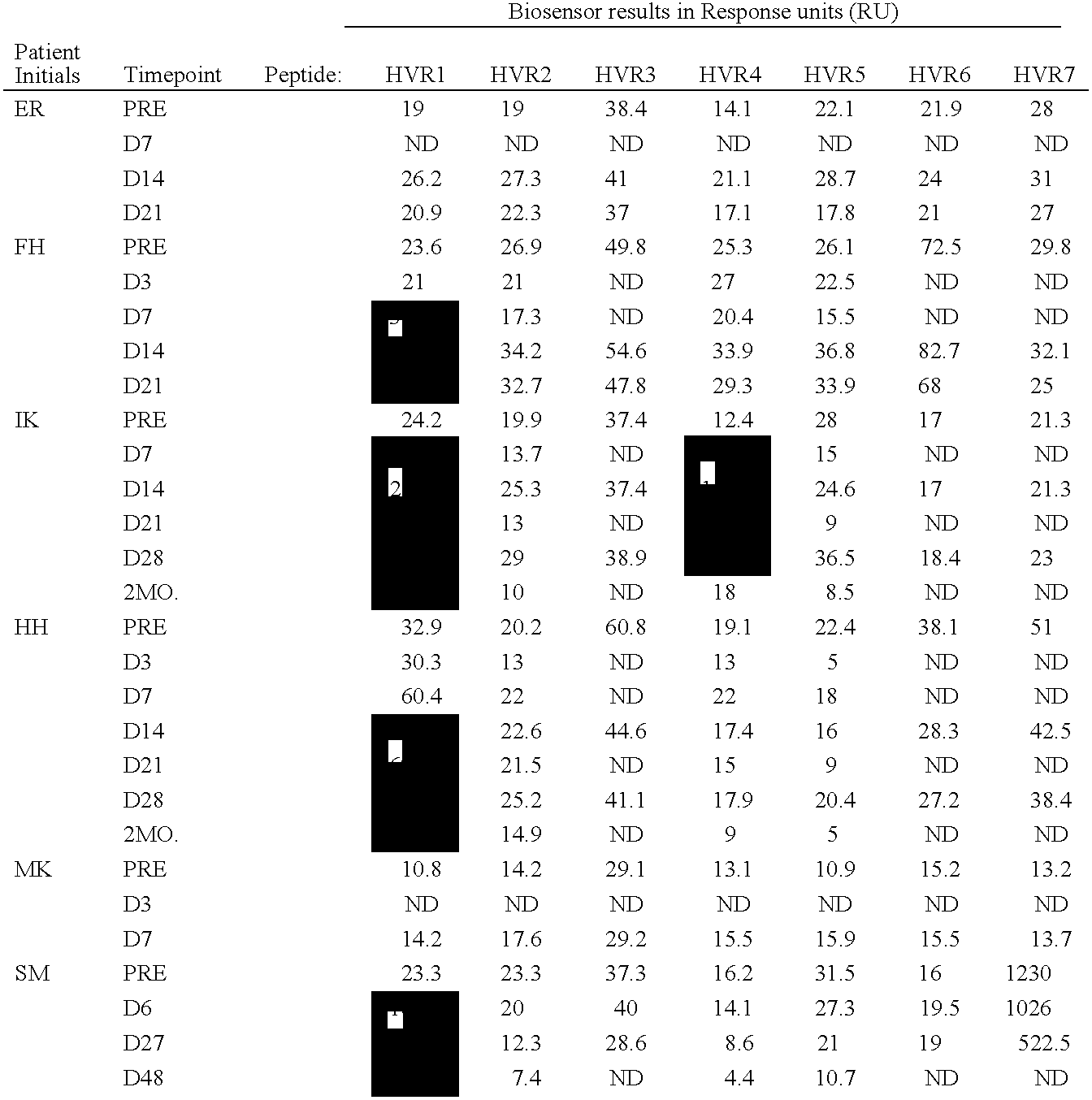
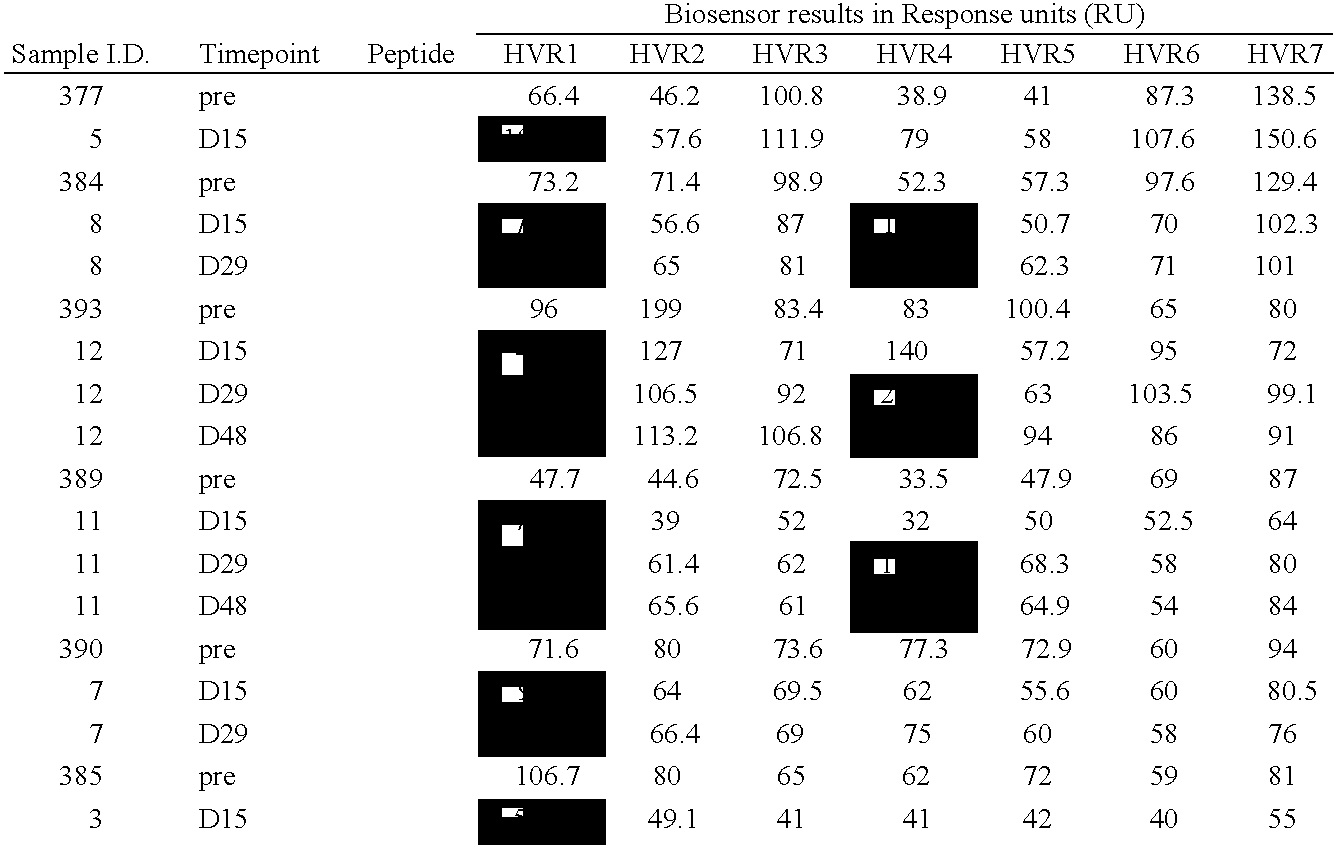
The present invention provides methods and reagents for detecting antibodies to adenovirus. In a preferred embodiment, the present invention is useful for detecting antibodies to adenovirus serotype 5. In a further preferred embodiment, the present invention can be used in a biosensor-based assay. It is contemplated that the present invention is useful to detect antibodies for ascertaining adenovirus infection, evaluating patient response to gene therapy using adenovirus vectors, developing vaccines to adenovirus infection, developing therapeutics for inducing passive immunity to adenovirus infection, as well as other uses.
Owner:SCHERING CORP
Production method of recombinant adenovirus gene vaccine for preventing novel coronavirus
PendingCN111494615AAchieve large-scale industrializationSsRNA viruses positive-senseViral antigen ingredientsPerfusion CultureAdenovirus infection
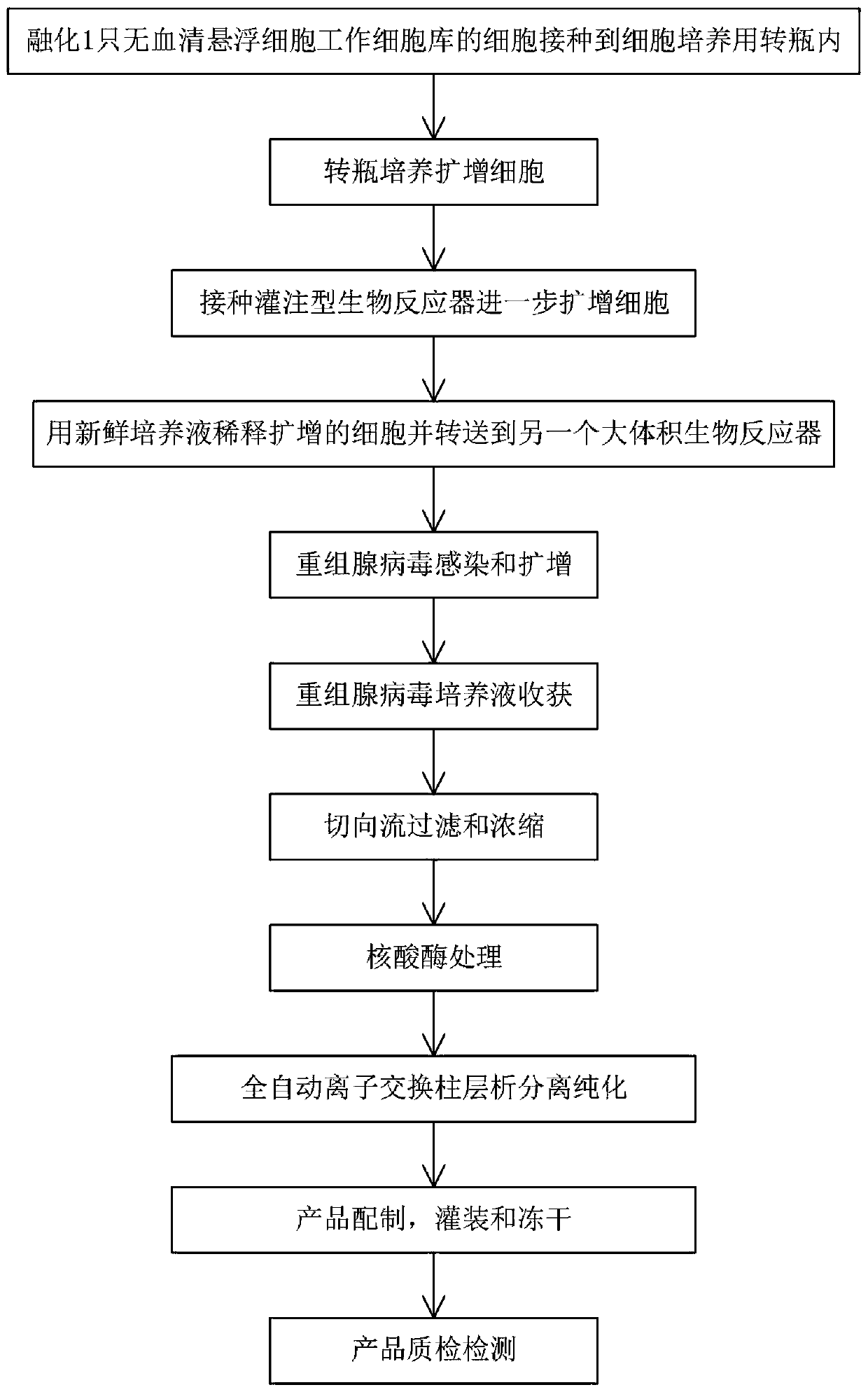
The invention relates to a production method of a recombinant adenovirus gene vaccine for preventing the novel coronavirus. The production method comprises the following main steps: amplifying cells;performing inoculating in a bioreactor, carrying out perfusion culturing in a culture solution, and carrying out cell re-amplification; diluting the cells and transferring the processed cells to a large-volume bioreactor; infecting and amplifying the recombinant adenovirus; harvesting the recombinant adenovirus culture solution and then performing filtering and concentrating; performing nuclease treatment; carrying out full-automatic ion exchange column chromatography separation and purification; and preparing, filling and freeze-drying the product. The method provided by the invention has good amplification and economy, can meet various technical requirements of medical monitoring organizations on GMP production, and fully ensures the quality and biological activity of the product.
Owner:SYNO SHENZHEN BIOMEDICAL RES CO LTD
Method for constructing ribosome inactivating protein gene virus vector and expressing active proteins in tumor cell through ribosome inactivated protein gene virus vector
PendingCN110747231AHas RNAN-glycosidase activityInhibit synthesisPeptide/protein ingredientsNucleic acid vectorPlant virusPlant cell
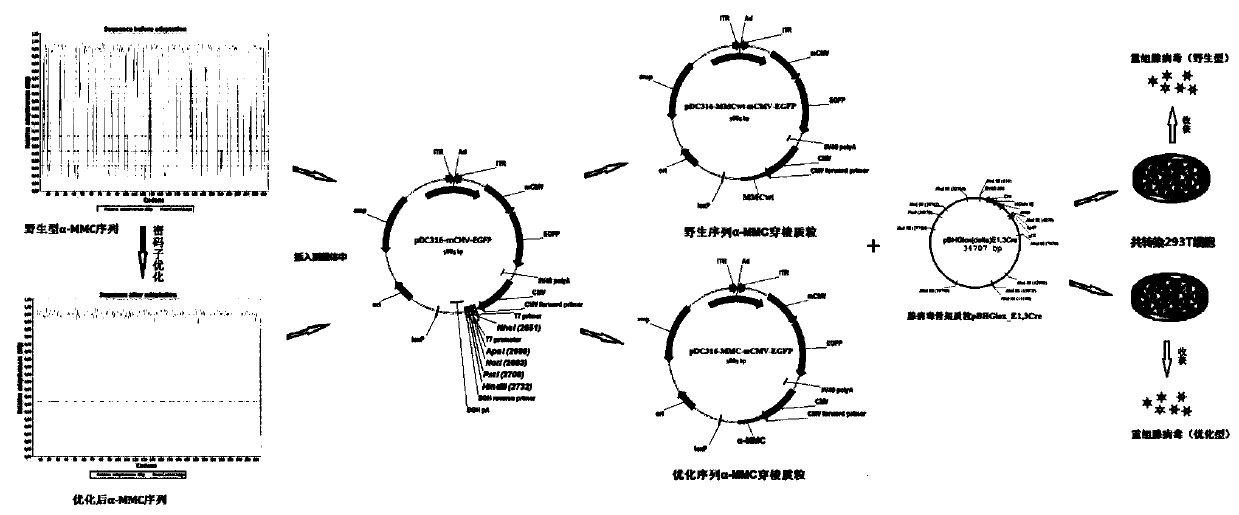
The invention belongs to the technical field of bioengineering and specifically discloses a method for constructing a ribosome inactivating protein gene virus vector and expressing active proteins intumor cells through the ribosome inactivated protein gene virus vector. The method comprises the following steps that first, suitable ribosome inactivated proteins are selected, and codon optimizationfor humanized expression is conducted on mature region genes of the ribosome inactivated proteins; second, an adenovirus carrier of wild type and optimized genes is constructed, and recombinant viruses are packaged; and third, recombinant adenoviruses infect the tumor cells, the recombinant adenoviruses express proteins in the tumor cells through detection, and the anti-tumor effect of the recombinant adenoviruses is detected. According to the method, an alpha-charantin gene is subjected to codon optimization, an expression vector system for the adenoviruses capable of infecting the tumor cells of mammals is constructed so as to be suitable for expressing the active proteins in the tumor cells, and the defects that in the prior art, a prokaryotic cell expression system can only be expressed in escherichia coli when being used, and a plant virus vector can only be expressed in plant cells but cannot be expressed in the tumor cells and kill tumors when being used are overcome.
Owner:成都富岱生物医药有限公司
Culturing method of adenovirus gene-modifying tumor specificity cytotoxic T lymphocyte
InactiveCN105602900AAvoid restrictionsReduce the burden onBlood/immune system cellsCell culture active agentsLymphocyte cultureCytotoxicity
The invention relates to a lymphocyte culture method, and in particular discloses a culturing method of adenovirus gene-modifying tumor specificity cytotoxic T lymphocyte. The method comprises: taking 150-200ml of bleeding of the umbilicus or autologous peripheral blood for single karyocyte separation, separately culturing DC cells by adhering to a wall for 2h, and performing T lymphocyte preculture on the non-adhered cells in an IL-2-contained culture medium; collecting mature DC after the DC cells are mature through induction of iAPA factor adenovirus infection, stimulating the autologous primary T lymphocyte to differentiate into cytotoxic T lymphocyte according to an effect target ratio of DC to T being 1 to 10, and observing and recording multiplication capacity of CTL and detecting the cytotoxicity of the CTL. The limitation of a blood component single sampling machine is solved, collected blood volume is less, burden of a patient is reduced, and iAPA-DC can be observed in vitro to stimulate and differentiate the primary T lymphocyte into tumor specificity cytotoxic T lymphocyte.
Owner:山东省齐鲁细胞治疗工程技术有限公司
Islet cells differentiated from stem cells, method, compound and application
PendingCN113174408AConvenient treatmentImprove efficiencyPancreatic cellsPeptidesIslet cellsPancreatic hormone
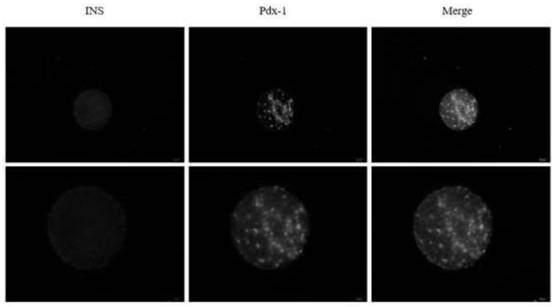
The invention is applicable to the technical field of biomedicine, and provides islet cells differentiated from stem cells, a method, a compound and application. The method for differentiating the stem cells into the islet cells comprises the following steps: conducting extracting and separating to obtain high-purity adipose-derived mesenchymal stem cells with differentiation capacity; transferring the PDX-1 gene into the adipose-derived mesenchymal stem cells by adopting an adenovirus infection method; and directionally differentiating the adipose-derived stem cells into islet cells by using an inducer. The adipose-derived mesenchymal stem cells are directionally differentiated into the islet cells by combining an adenovirus infection method with an inducer, the insulin (INS) recombinant protein is highly expressed in the obtained islet cell cluster, and the PDX-1 protein is still highly expressed in most cells after induction is finished, so that the induced cells have the capability of secreting the insulin (INS) recombinant protein and are high in efficiency; and an islet cell carrying cytoskeleton compound is transplanted to a skeletal muscle part, and diabetes can be well treated.
Owner:JILIN UNIV
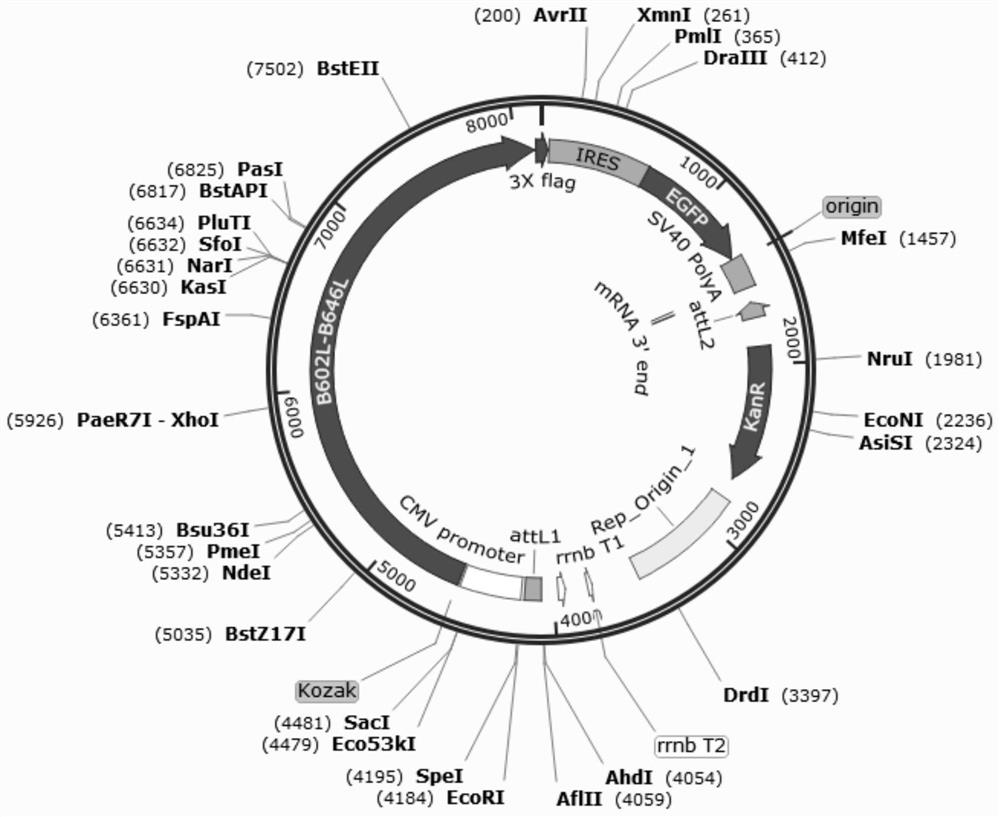
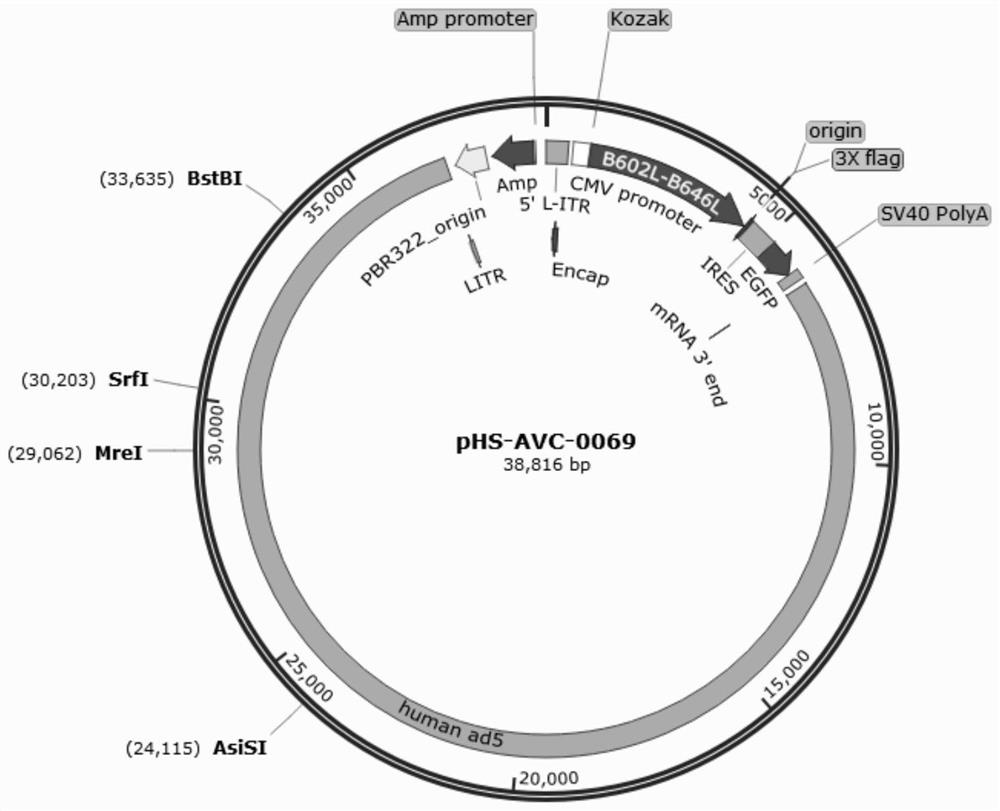
Recombinant adenovirus for expressing African swine fever virus B602L-B646L protein and construction method thereof
PendingCN114107389AViral antigen ingredientsVirus peptidesClassical swine fever virus CSFVCytopathic effect
The invention discloses a recombinant adenovirus for expressing African swine fever virus B602L-B646L protein and a construction method thereof, and belongs to the technical field of genetic engineering. A recombinant adenovirus shuttle vector pENTRE-EGFP-TOPO is utilized, and a recombinant adenovirus vector pAD-CMV-EGFP-B602L-B646L is obtained through a series of intermediate processes; according to the present invention, the African swine fever virus B602L-B646L protein expression recombinant adenovirus is constructed, the African swine fever virus B602L-B646L protein expression recombinant adenovirus is linearized and transfected with AD293 cells, the recombinant virus is screened according to the cytopathic effect formed by adenovirus infection, the adenovirus packaging process is achieved, the recombinant adenovirus expressing the African swine fever virus B602L-B646L protein is obtained, and the foundation is laid for the construction of the recombinant adenovirus vaccine expressing the African swine fever virus
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
High-throughput screening method and application of drug for inhibiting human adenovirus proliferation
PendingCN112592953AContribute to research and developmentImprove throughputMicrobiological testing/measurementMicroorganism based processesInfected cellHigh-Throughput Screening Methods
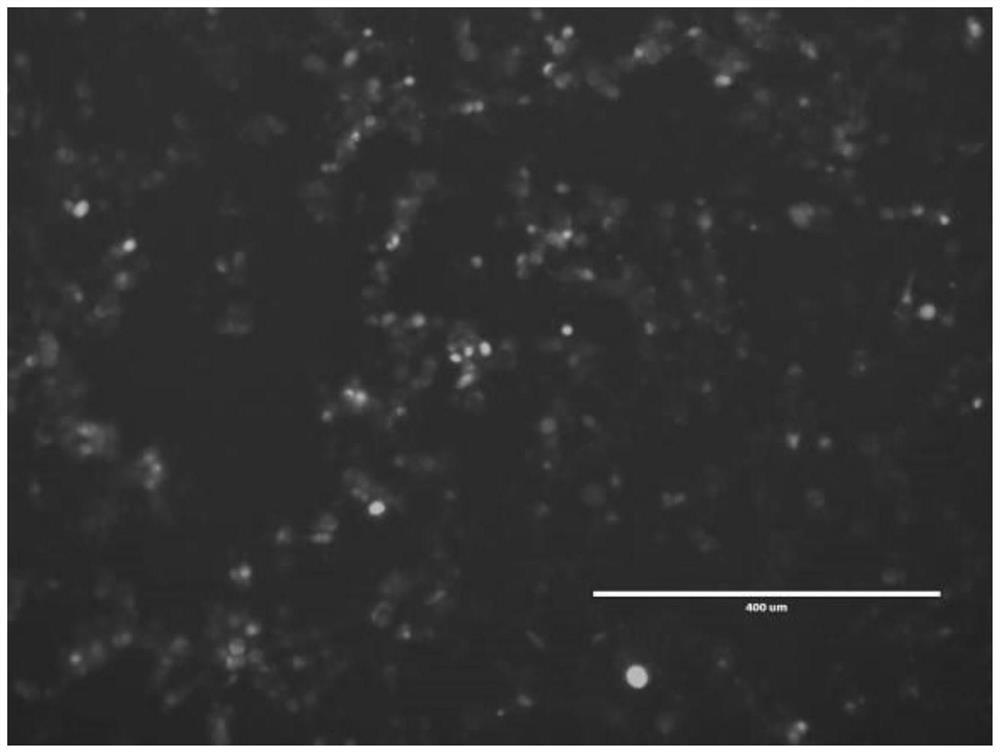
The invention discloses a high-throughput screening method and application of a drug for inhibiting human adenovirus proliferation. A human adenovirus marked with GFP in the patent can express green fluorescent protein after infecting with HEK-293 cells, so that the infected cells are green under a fluorescence microscope. The anti-human adenovirus drug activity is realized through the following steps: detecting the change of the adenovirus content in the supernatant of a culture medium after the anti-adenovirus drug and the human adenovirus are added into the HEK-293 cells by using flow cytometry. The specific operation comprises the following steps: taking a proper amount of the virus supernatant, adding the virus supernatant into a certain quantity of the HEK-293 cells again, and determining the ratio (cells with green fluorescence) of the adenovirus infected cells in the cells by flow cytometry so as to evaluate whether the added drug has an obvious inhibition effect on adenovirusproliferation or not. According to the method, whether candidate drug molecules have the effect of inhibiting human adenoviruses or not can be screened quickly, simply, conveniently and effectively invitro in a low-cost and high-throughput manner. According to the method, the research and development of novel anti-human adenovirus drugs can be accelerated.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Monoclonal antibody 10G12 and application thereof
The invention discloses a monoclonal antibody 10G12 and application thereof. The monoclonal antibody comprises a heavy chain variable region and a light chain variable region, wherein the heavy chainvariable region comprises three complementary determining regions HCDR1, HCDR2 and HCDR3; the light chain variable region comprises three complementary determining regions LCDR1, LCDR2 and LCDR3; theHCDR1, HCDR2 and HCDR3 are sequentially shown as the positions 26-33, 51-66 and 97-109 from the N end of a sequence 2 in a sequence table; the LCDR1, LCDR2 and LCDR3 are sequentially shown as the positions 27-37, 55-57 and 94-102 of the N end of a sequence 4 in the sequence table. Based on clinical requirements, an inventor of the invention finds that the anti-human adenovirus type 7 antibody 10G12 is used for preventing and treating adenovirus infection and has important biological and medical significance.
Owner:ACADEMY OF MILITARY MEDICAL SCI
High-officient production of recombinant adenovirus carrier
InactiveCN1778933AIncrease production capacityEasy to harvestGenetic engineeringFermentationDepolymerizationCell mass
The invention is about a method that can produce recombination adenovirus vector highly, and belongs to the field of cell engineering technology. The cell, which puts the HEK cell as the recombination adenovirus vector, can produce the dissociative cell into cell mass under the Suspension Culture, or depolymerization cell mass as dissociative cell, using the free settling of cell mass or rotary filter interception cell mass and replacing substrate or affusing substrate continuously, it can recombinate the adenovirus infection HEK cell mass and culture it to amplify in the cell. Its advantages are:óƒThe activity of the cell is greater or equal to 90%, the consistency of live cell is greater or equal to 1í‡10 to the power 7 cells / ml.óIt uses the recombination adenovirus vector to infect HEK cell mass directly and enlarge breed in the cell, and can carry into execution substrate replacing or continuous perfusion with the free settling of HEK cell mass or intraoral rotary filter.ó�The titer of pAd-TH-GFP can reach 1-5í‡10 to the power 13 GTU / ml, the product capacity of 5L stirred tank bioreactor can reach 1-2í‡10 to the power 17 GTU / ml, the gain of recombination adenovirus vector is convenient.óœThe producing process is tight and unhindered, and the size can be enlarged easily.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Group I type 4 fowl adenovirus fiber-2 protein antigen as well as method for preparing genetic engineering subunit vaccine and application of group I type 4 fowl adenovirus fiber-2 protein antigen
PendingCN114149493AGood prospects for commercial developmentAvoid infectionViral antigen ingredientsVirus peptidesNucleotideGenetic engineering
The invention relates to the technical field of genetic engineering vaccines, and particularly discloses an I-group 4-type fowl adenovirus fiber-2 protein antigen as well as a method for preparing a genetic engineering subunit vaccine and application of the I-group 4-type fowl adenovirus fiber-2 protein antigen. The method comprises the following steps: adding a non-coding gene in front of a fiber-2 gene of the fowl adenovirus FAdV-4, cloning the non-coding gene to a prokaryotic expression vector pET-28a, transforming the prokaryotic expression vector pET-28a to escherichia coli to construct an engineering bacterium, and inducing the engineering bacterium to express to obtain the soluble fiber-2 protein. Wherein the amino acid sequence of the aviadenovirus fiber-2 antigen protein is SEQ ID NO.2, the nucleotide sequence of a gene coded by the aviadenovirus fiber-2 antigen protein is SEQ ID NO.1, and soluble protein with high expression quantity is obtained through non-coding gene regulation and control. The fiber-2 protein of the fowl adenovirus is expressed through escherichia coli, a soluble expression product is obtained, the subunit vaccine is prepared, safety and effectiveness are achieved, and fowl I-group 4-type adenovirus infection can be prevented.
Owner:山东滨州沃华生物工程有限公司
Pigeon ttv and pigeon new adenovirus double Evagreen real-time fluorescent quantitative PCR detection kit
InactiveCN107604100BSimplify operating proceduresLow costMicrobiological testing/measurementMicroorganism based processesAdenovirus infectionAdenovirus diseases
The invention provides a double EvaGreen real-time fluorescent quantitative PCR detection kit for pigeon TTV and new pigeon adenovirus, the kit includes primers whose sequence is shown in SEQ ID NO.1‑4, and has high specificity and sensitivity. The established method can simultaneously detect pigeon TTV and pigeon new adenovirus infection in pigeon flocks, which simplifies operating procedures and saves costs. After the real-time fluorescent quantitative PCR reaction is completed, the result can be directly judged by observing the peak value of the melting curve (Tm value). The establishment of the present invention can fill in the gaps in related fields at home and abroad.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Method for detecting type-5, type-7 and type-55 adenoviruses by using mass spectrum multiple reaction monitoring technology
PendingCN113156133AThe result is accurateSimple and fast operationBiological material analysisBiological testingSerum samplesAdenovirus infection
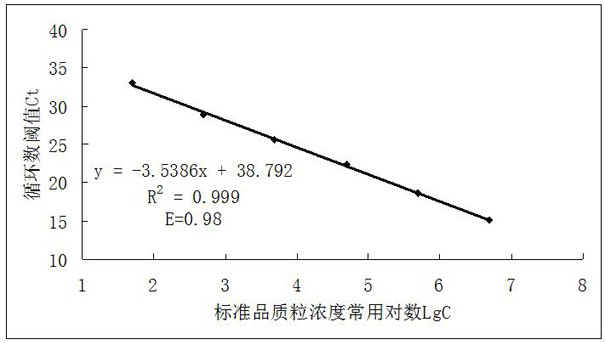
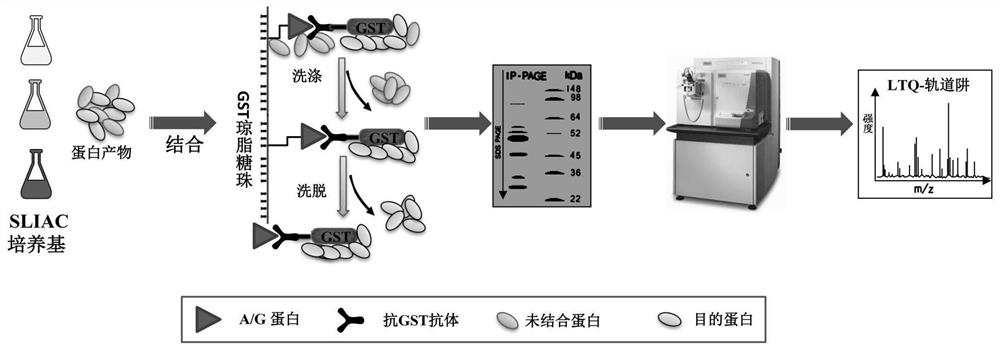
The invention relates to a method for detecting type-5, type-7 and type-55 adenoviruses by using a mass spectrum multiple reaction monitoring technology. The method comprises the following specific steps: synthesizing specific peptide fragment genes of hexon proteins of type-5, type-7 and type-55 adenoviruses; preparing a labeled peptide fragment mixed reagent; determining an ion peak value of the diagnostic marker; preparing the kit; and detecting the sample. The invention provides a new marker for early warning and diagnosis of adenovirus type, type 7 and type 55 infection, and has important clinical value. A mass spectrum result is utilized to confirm that mass spectrum ion peaks consistent with the standard and the heavy standard of preset adenovirus type 5, type 7 and type 55 hexon protein symbolic peptide fragments appear in a serum sample of a patient in the early stage of adenovirus infection. The SILAC-labeled winning and heavy-labeled hexon protein standard mixed reagent of adenovirus type 5, type 7 and type 55 can be used as an early warning and diagnosis marker for adenovirus type, type 7 and type 55 infection, and the result is accurate.
Owner:LOGISTICS UNIV OF CAPF
A method for constructing recombinant adenovirus using anp or iganp gene, recombinant adenovirus and application
ActiveCN107828819BGrowth inhibitionThe role and effect are obviousPeptide/protein ingredientsDigestive systemIntravenous gammaglobulinTongue Carcinoma
The invention discloses a method for constructing a recombinant adenovirus by utilizing an ANP (Atrial Natriuretic Peptide) or IgANP gene, the recombinant adenovirus and application. A recombinant adenovirus vector is constructed by taking the ANP as a target gene; the vector is used for expressing an ANP polypeptide in a tongue carcinoma cell SCC9; meanwhile, a signal peptide sequence of immune globulin is added in front of an ANP sequence to construct the secretory type ANP polypeptide. The tongue carcinoma cell is infected by the recombinant adenovirus; RT-PCR (Reverse Transcription-Polymerase Chain Reaction) proves that the ANP or IgANP can be expressed in the tongue carcinoma cell SCC9; MTT (Methyl Thiazolyl Tetrazolium) cell relative activity detection and a cell scratch experiment prove that the ANP has the effect of inhibiting the tongue carcinoma cell SCC9 activity and migration in an in vitro experiment, and the acting effect of the IgANP is more obvious, so that the growth of the tongue carcinoma cell is inhibited. The method has the advantages of high efficiency and large yield; the invention provides an unreported gene treatment system for resisting tumor cell migration and growth.
Owner:WUHAN INST OF BIOENG
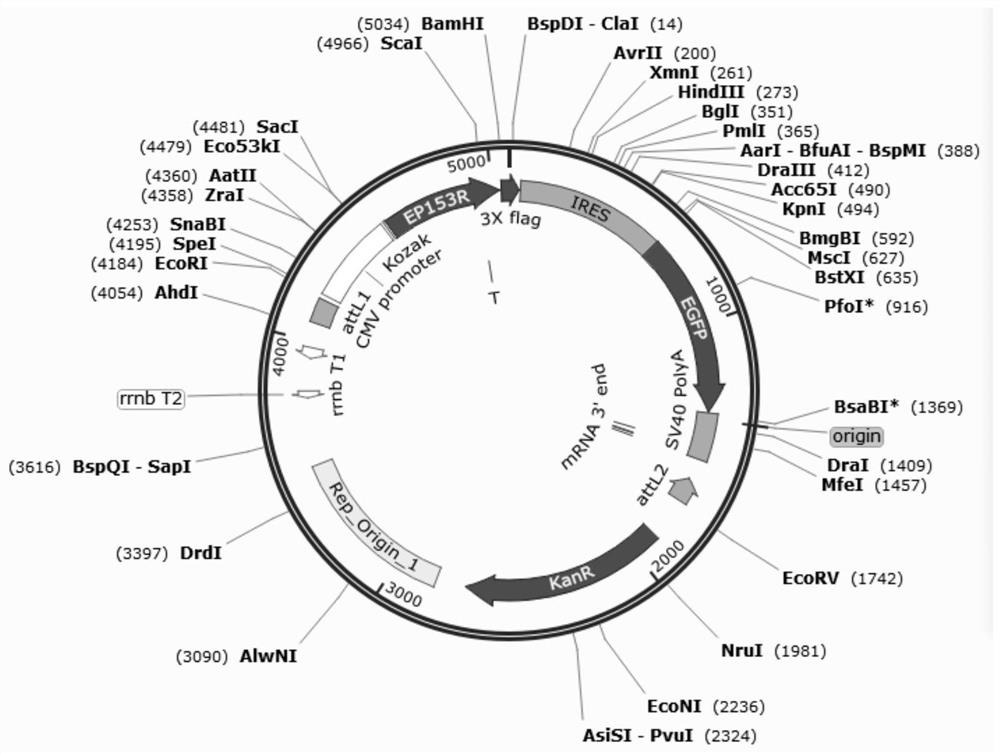
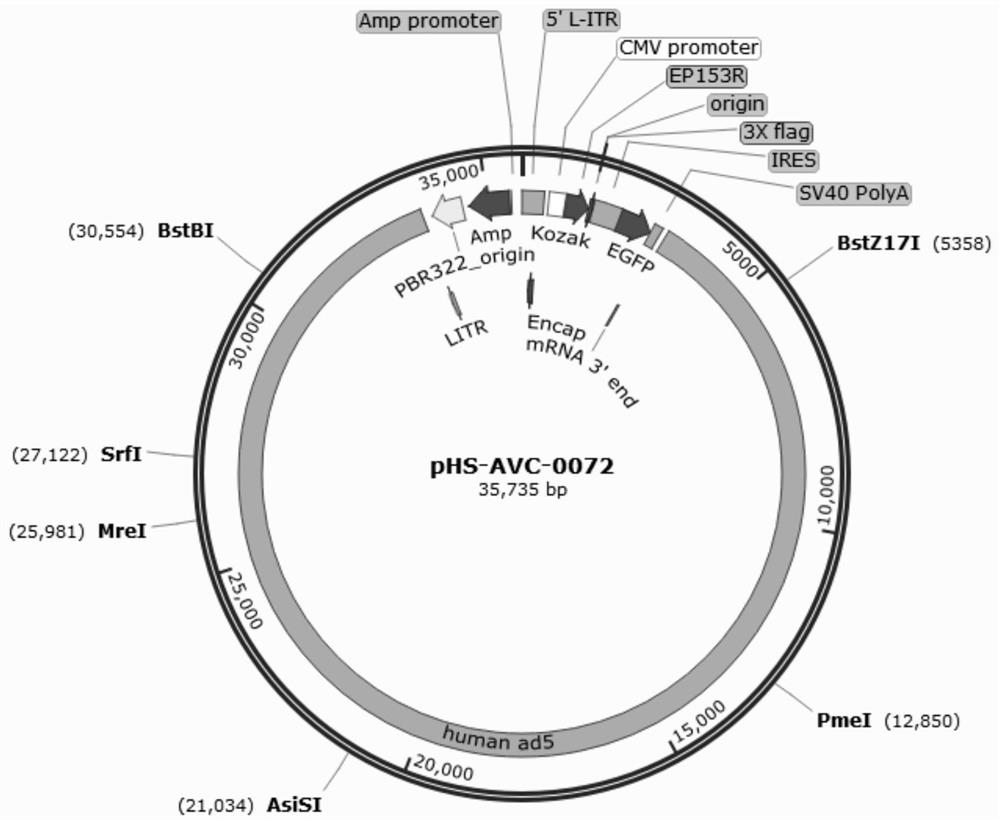
Recombinant adenovirus for expressing African swine fever virus EP153R-EP402R protein and construction method of recombinant adenovirus
PendingCN114058642AImplement packagingViral antigen ingredientsVirus peptidesClassical swine fever virus CSFVShuttle vector
The invention discloses a recombinant adenovirus for expressing African swine fever virus EP153R-EP402R protein and a construction method of the recombinant adenovirus, and belongs to the technical field of genetic engineering. A recombinant adenovirus shuttle vector pENTRE-EGFP-TOPO is utilized, a recombinant adenovirus vector pAD-CMV-EGFP-EP153R-EP402R is obtained through a series of intermediate processes, and the recombinant adenovirus vector is linearized to transfect AD293 cells, the recombinant virus is screened according to the cytopathy formed by adenovirus infection, and the adenovirus packaging process is achieved, the recombinant adenovirus for expressing African swine fever virus EP153R-EP402R protein is obtained, and the foundation is laid for the construction of the African swine fever virus EP153R-EP402R protein expression.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Recombinant adenovirus for expressing African swine fever virus EP153R protein and construction method thereof
PendingCN113913462AViral antigen ingredientsVirus peptidesClassical swine fever virus CSFVShuttle vector
The invention discloses a recombinant adenovirus for expressing African swine fever virus EP153R protein and a construction method thereof, and belongs to the technical field of genetic engineering, the recombinant adenovirus shuttle vector pENTRE-EGFP-TOPO is utilized, and a recombinant adenovirus vector pAD-CMV-EGFP-EP153R is obtained through a series of intermediate processes, and after being linearized, the recombinant adenovirus vector pAD-CMV-EGFP-EP153R is transfected into an AD293 cell; the recombinant virus is screened according to cytopathy formed by adenovirus infection, the adenovirus packaging process is realized, the recombinant adenovirus for expressing the African swine fever virus EP153R protein is obtained, and a foundation is laid for constructing a recombinant adenovirus vaccine for expressing the African swine fever virus EP153R protein.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
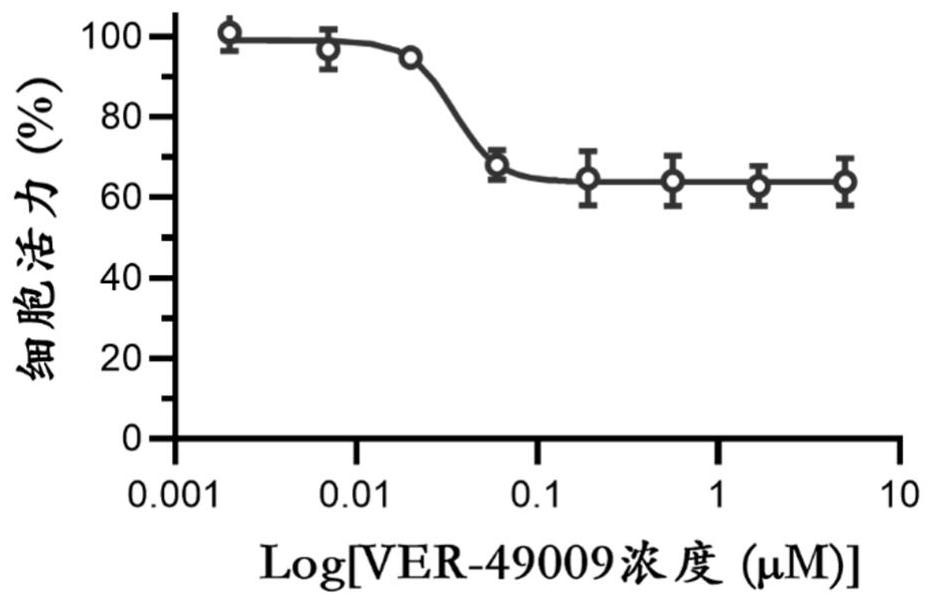
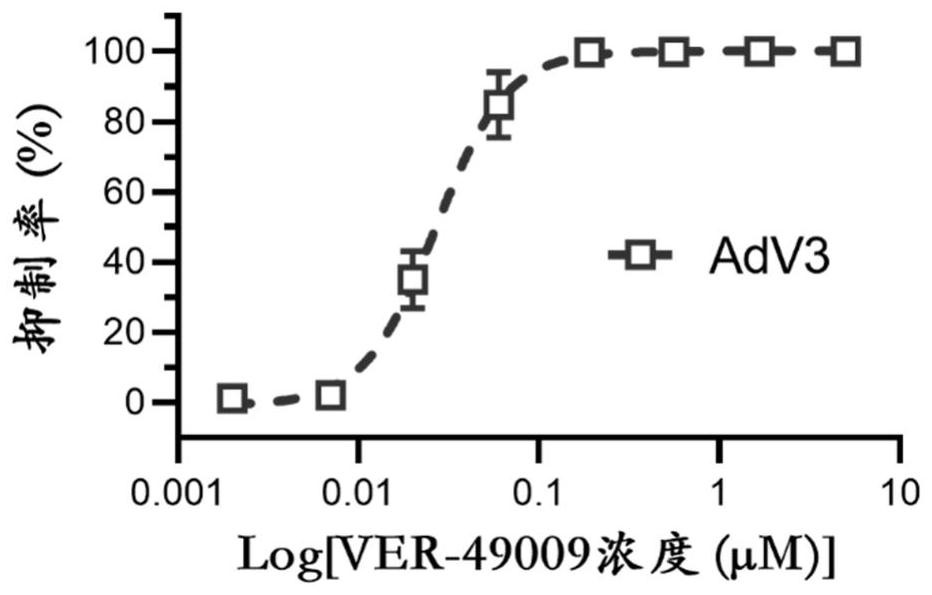
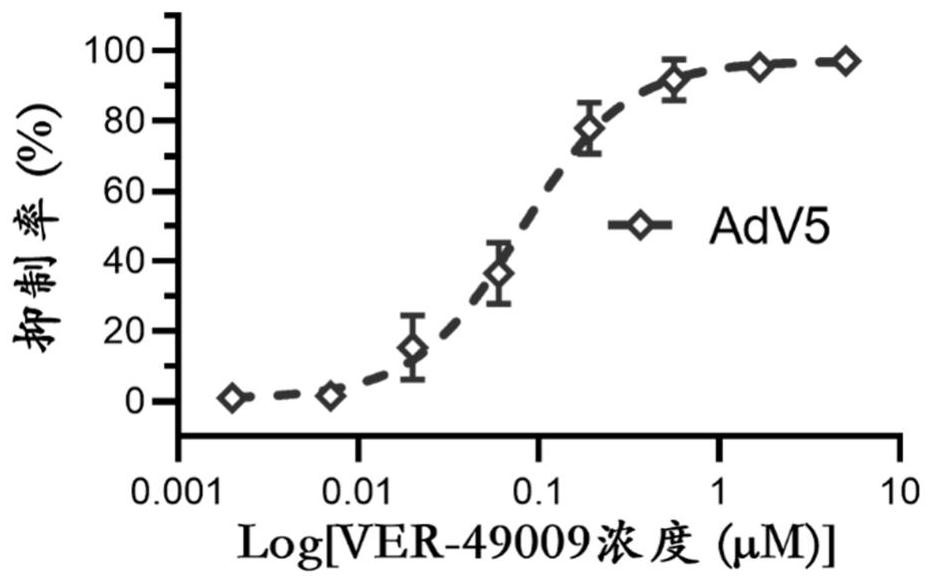
Application of VER-49009 in preparation of medicine for preventing and/or treating adenovirus infection
PendingCN114767671AInhibition of replicationBroad spectrum activityOrganic active ingredientsAntiviralsAdenovirus infectionPharmaceutical drug
The invention belongs to the technical field of medicines, and particularly relates to application of VER-49009 in preparation of a medicine for preventing and / or treating adenovirus infection. The invention discloses an application of VER-49009 in preparation of a medicine for inhibiting adenovirus and / or a medicine for preventing and / or treating adenovirus infection, so that a safe and effective small molecular compound is provided for clinically treating the adenovirus. The VER-49009 can effectively inhibit the replication of the adenovirus in a non-toxic range, can be further developed into a medicine for treating or preventing diseases caused by adenovirus infection, and has a wide application prospect.
Owner:JINAN UNIVERSITY
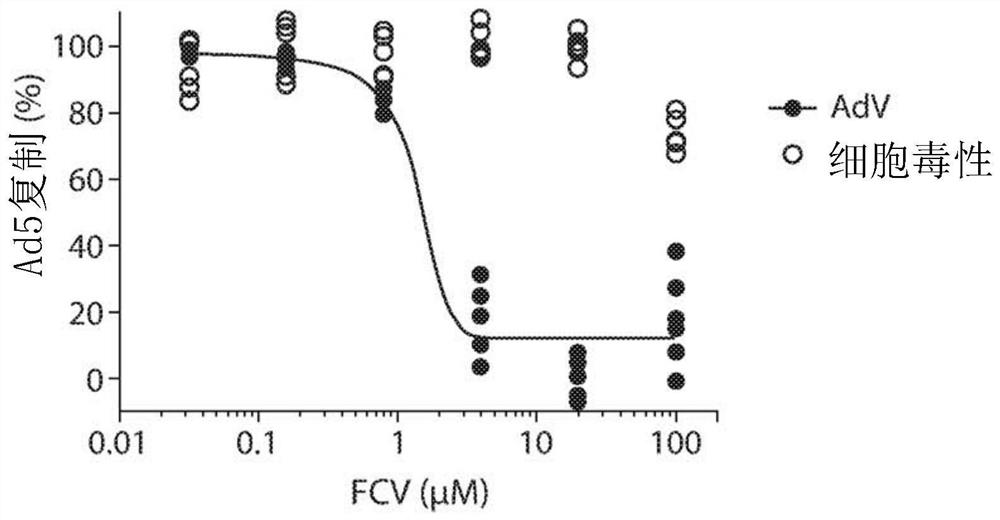
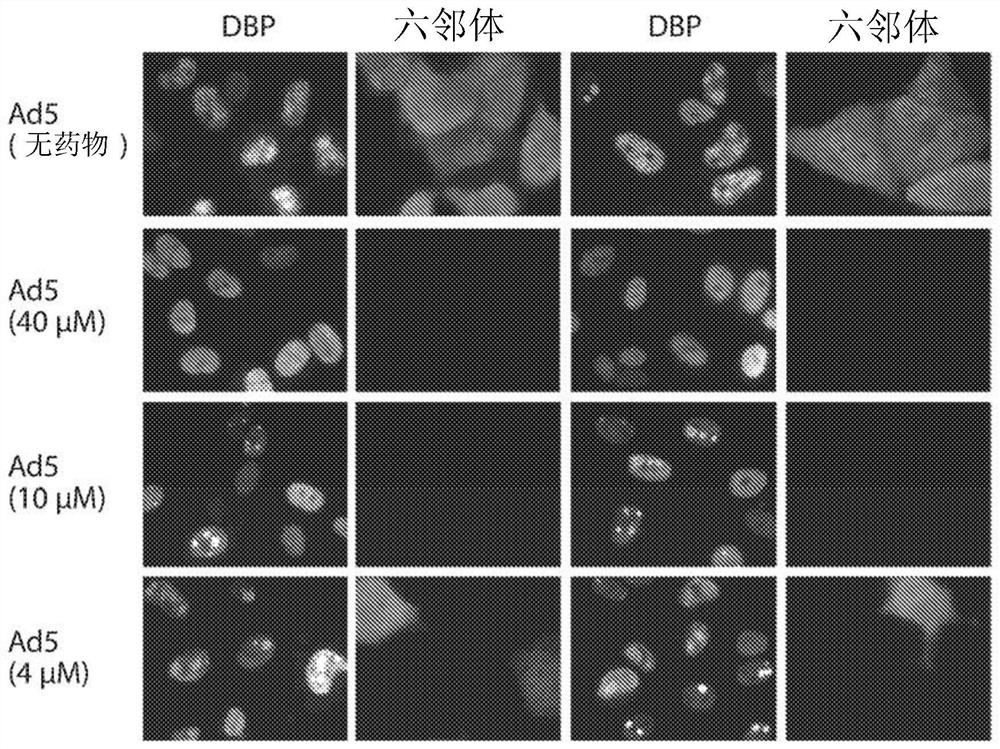
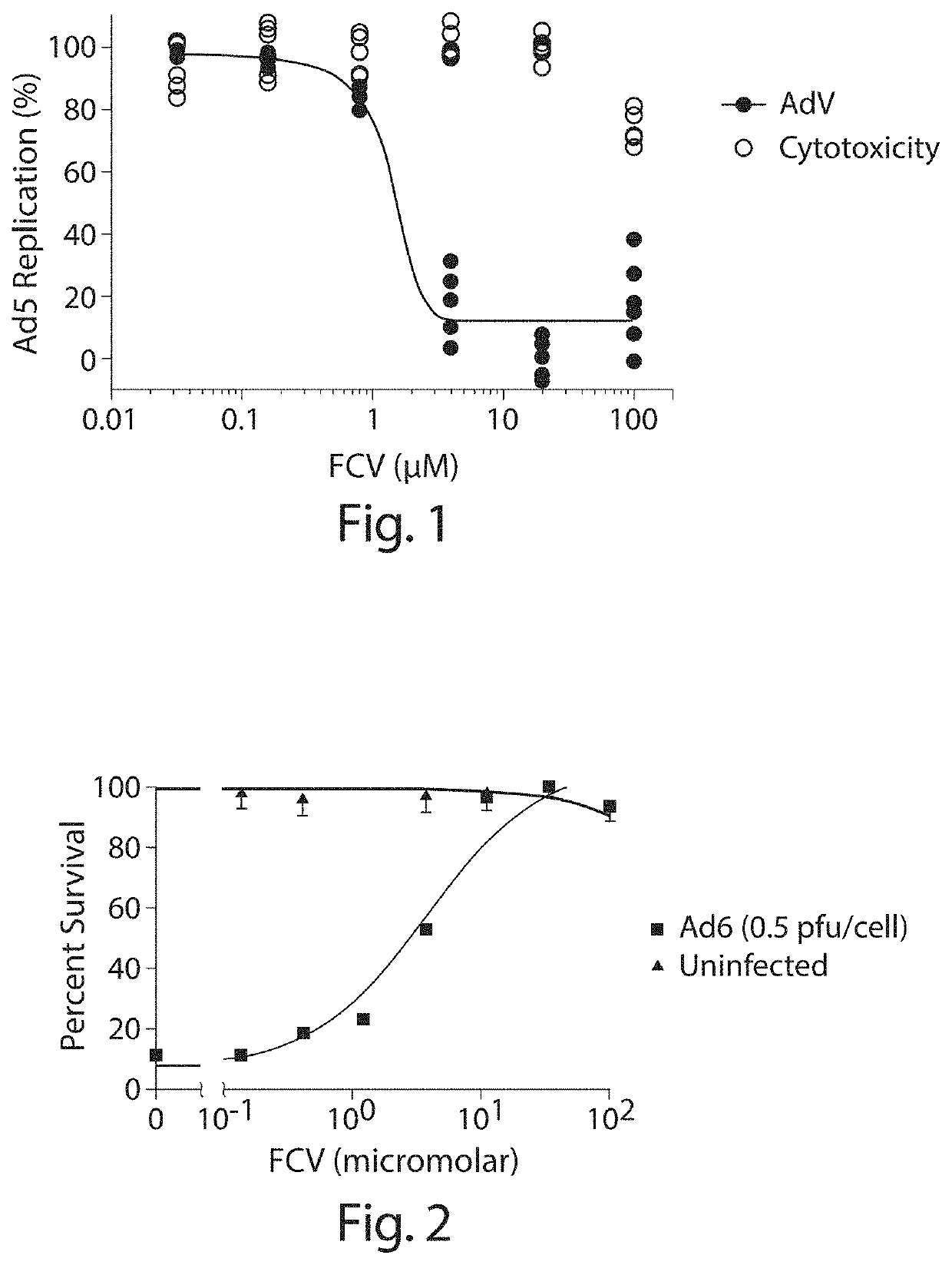
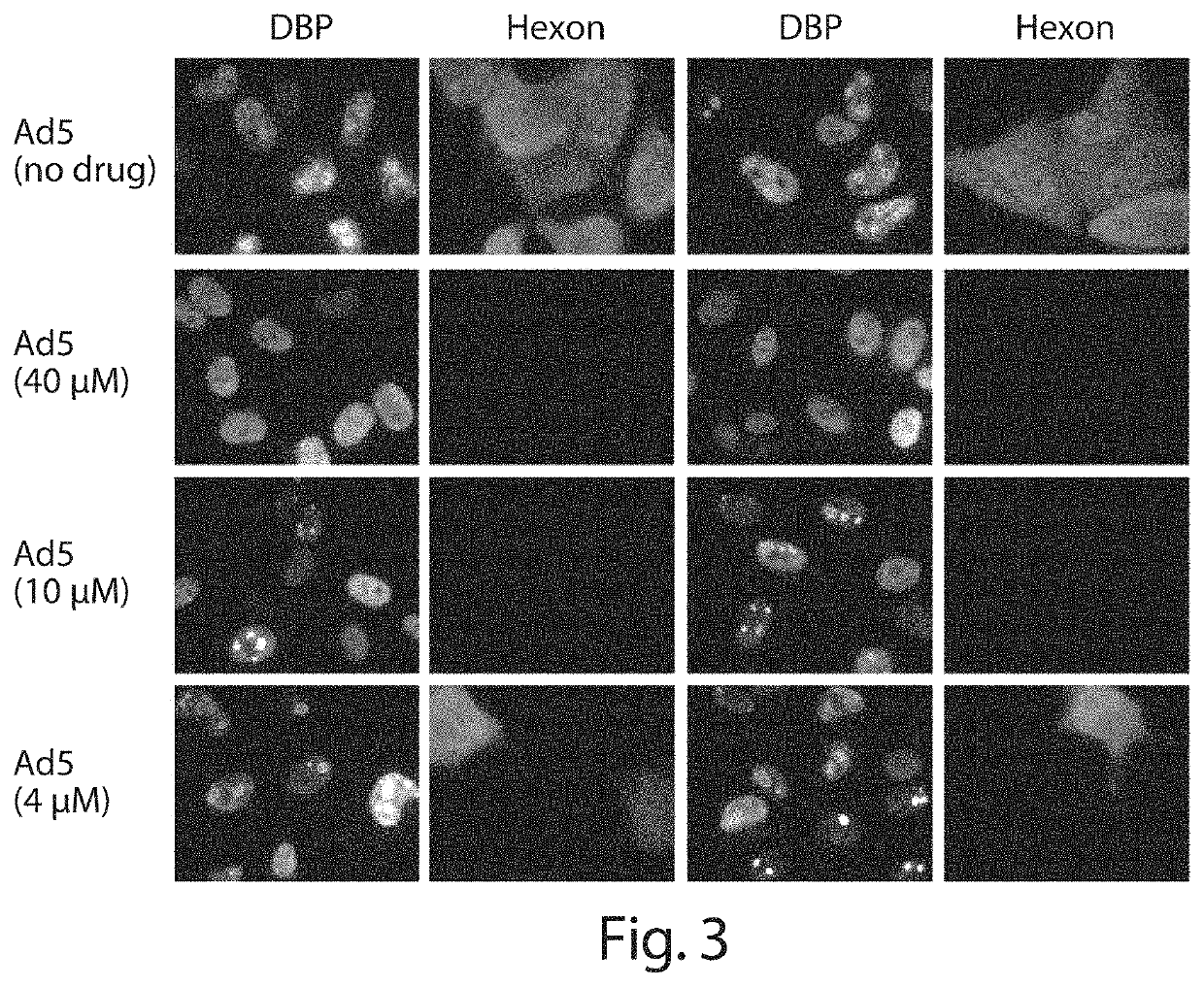
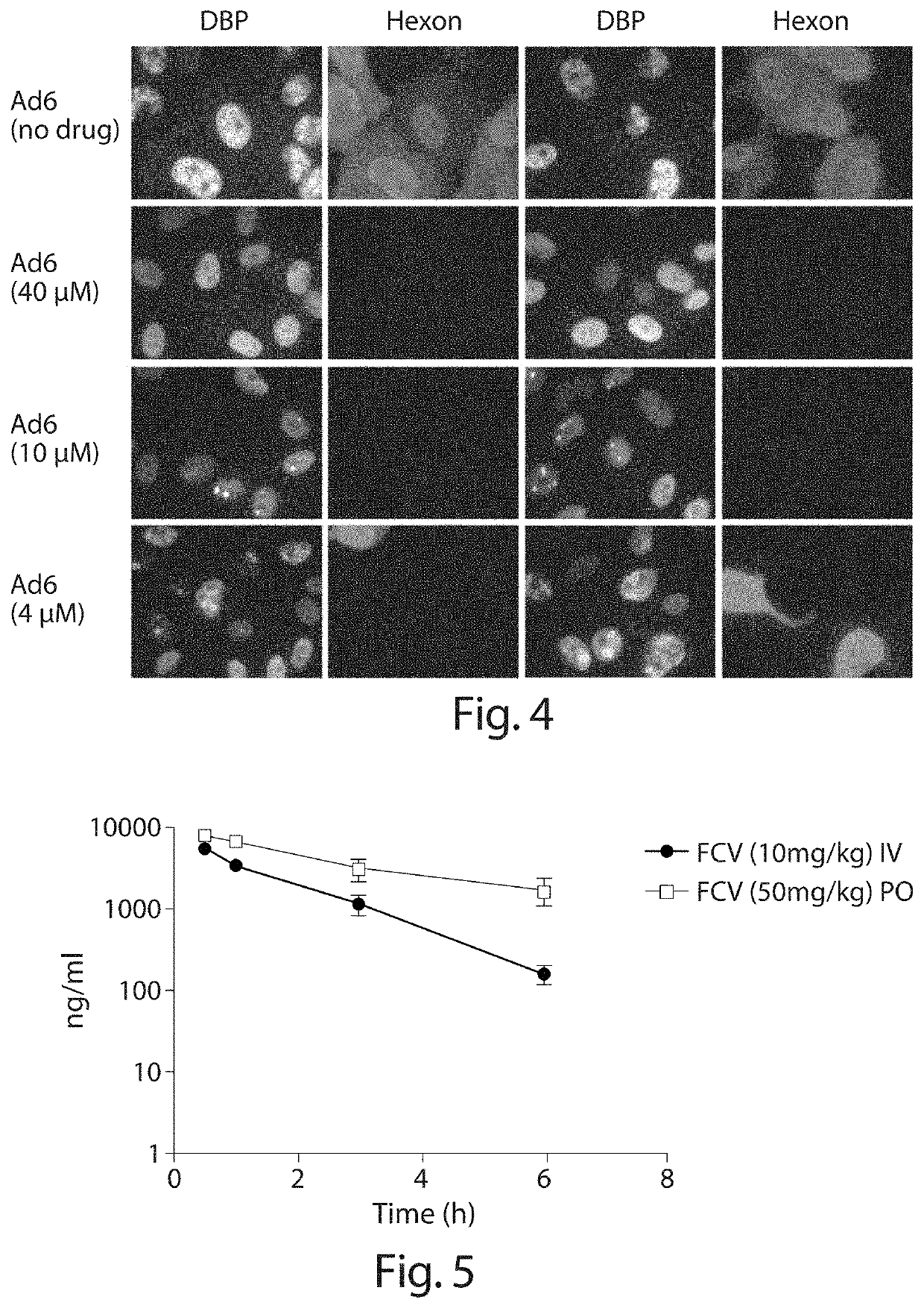
Inhibition of adenoviruses with felociclovir
PendingCN114206446AOrganic active ingredientsPeptide/protein ingredientsAdenoviral infectionsAdenovirus infection
The present invention relates to the development of therapeutic and prophylactic agents for the treatment and / or prophylaxis of adenovirus infection in humans and other mammals. Methods of treating or preventing an adenoviral infection in a mammal by administering an effective amount of felociclovir (FCV) are disclosed.
Owner:MICROBION +1
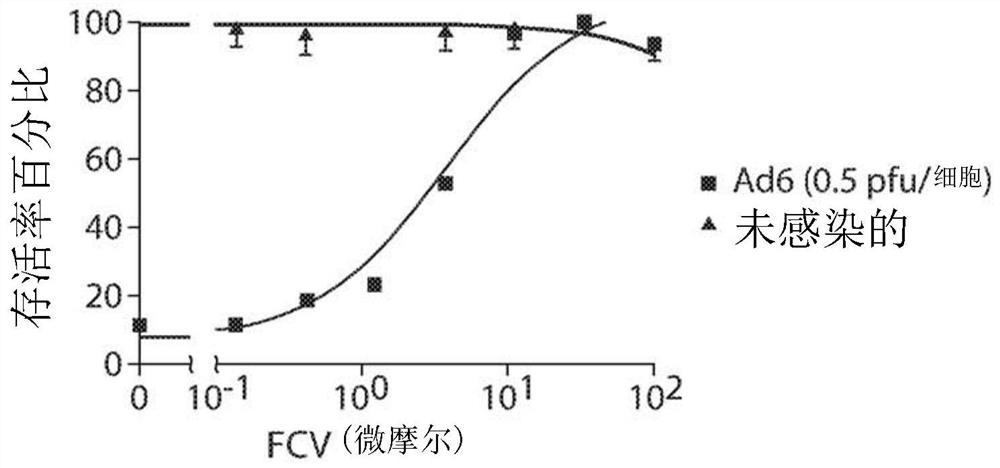
Inhibition of adenovirus with filociclovir
PendingUS20220168310A1AntiviralsHeterocyclic compound active ingredientsAdenovirus infectionAdenovirus diseases
The present invention is related to the development of therapeutics and prophylactics for the treatment and / or prevention of adenovirus infection in humans and other mammals. Disclosed are methods of treating or preventing adenovirus infections in mammals by administration of an effective amount of filociclovir (FCV).
Owner:MICROBIOTIX
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com