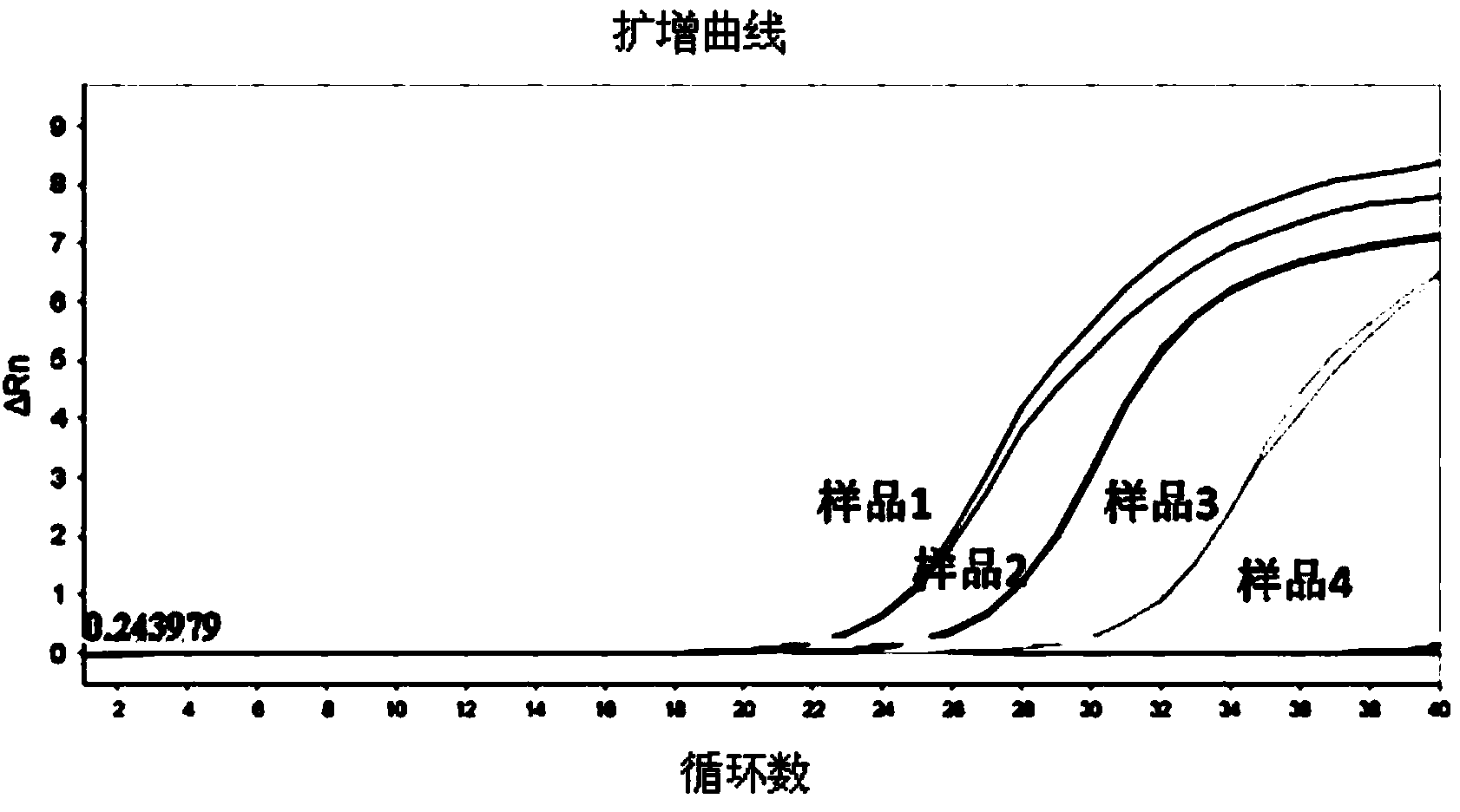
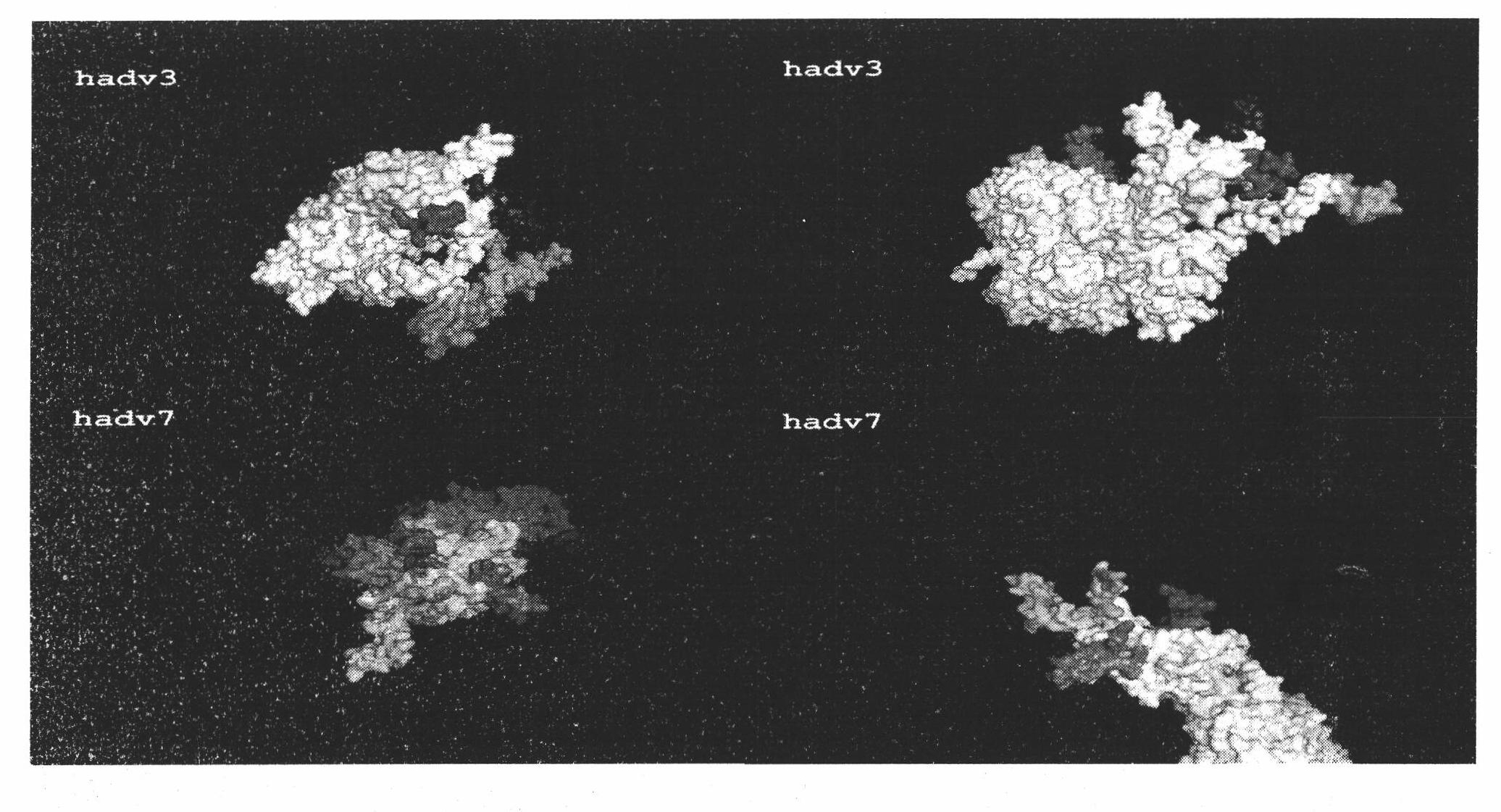
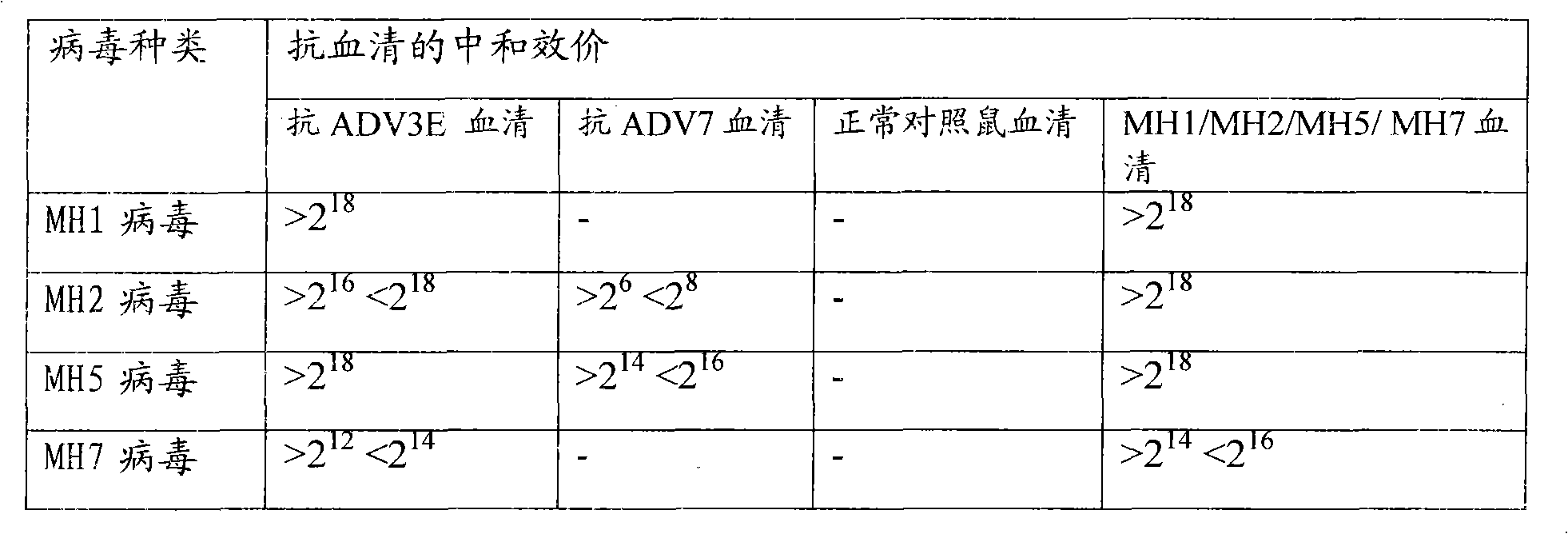
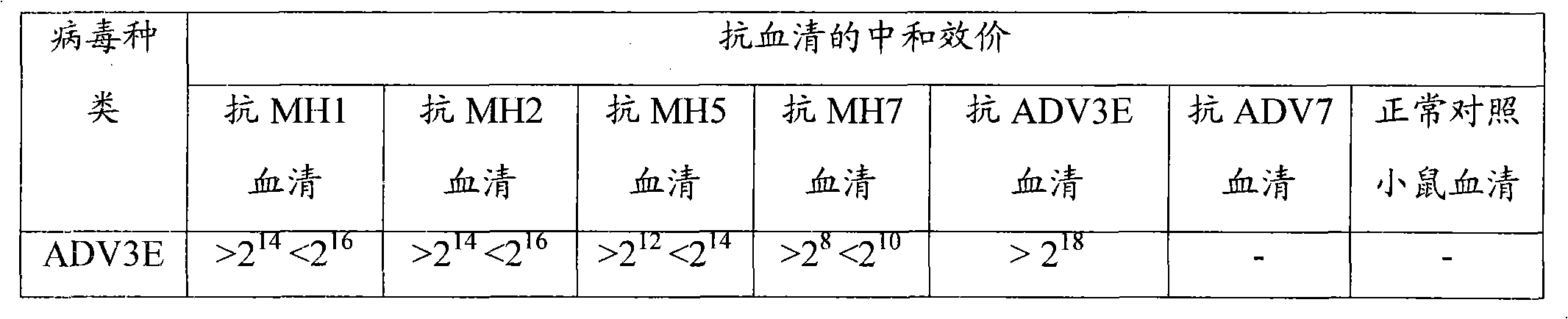
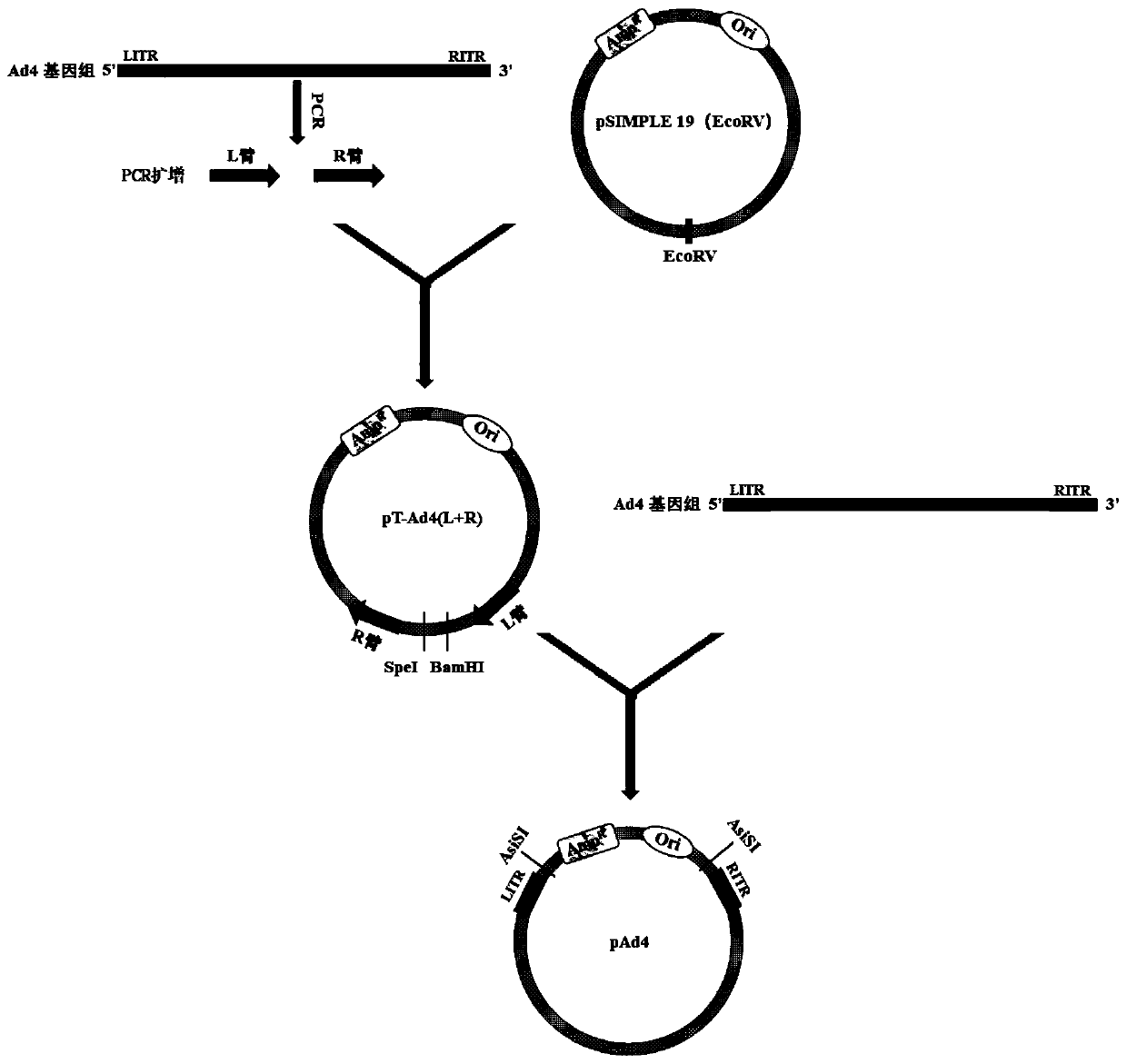
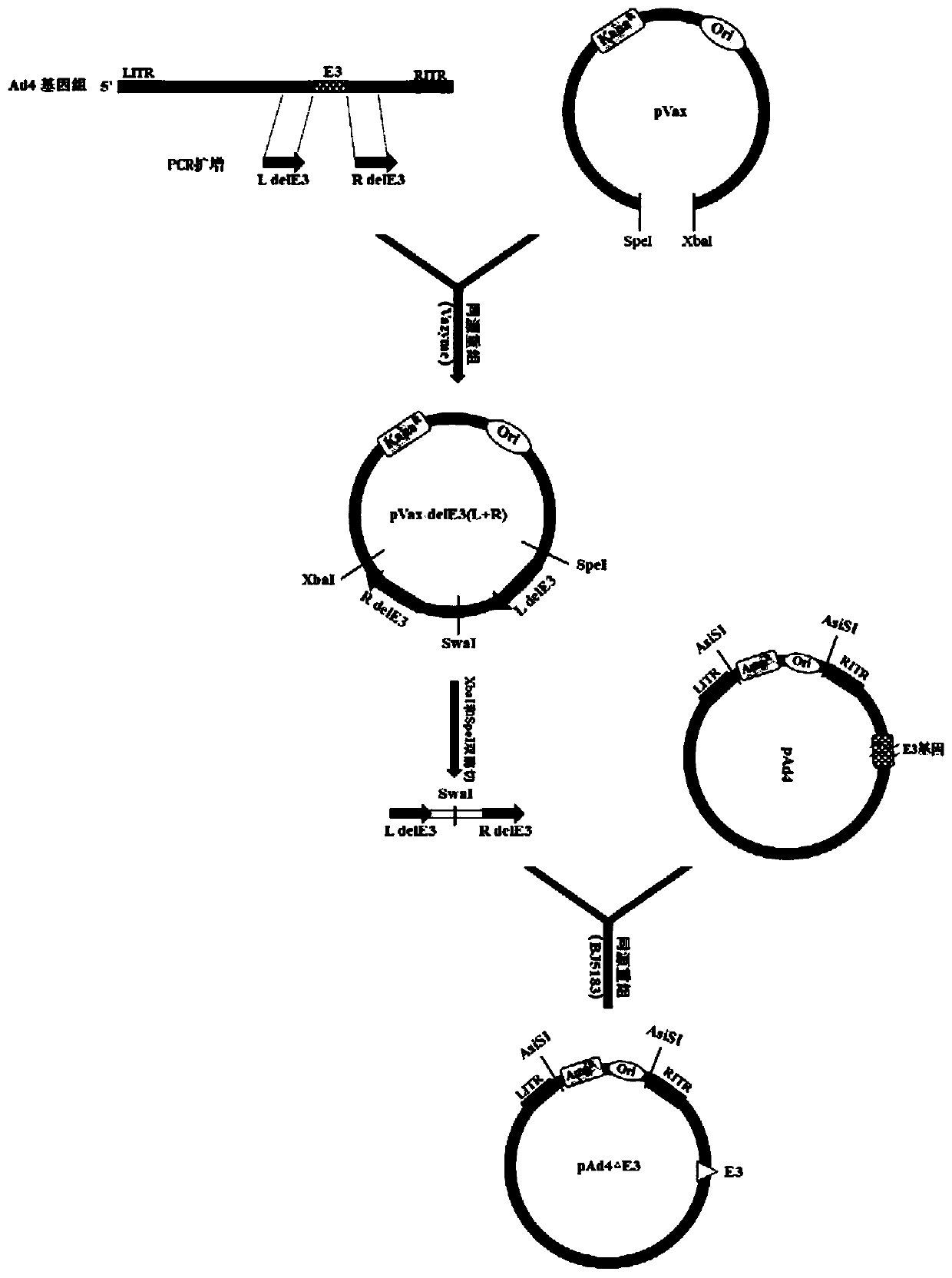
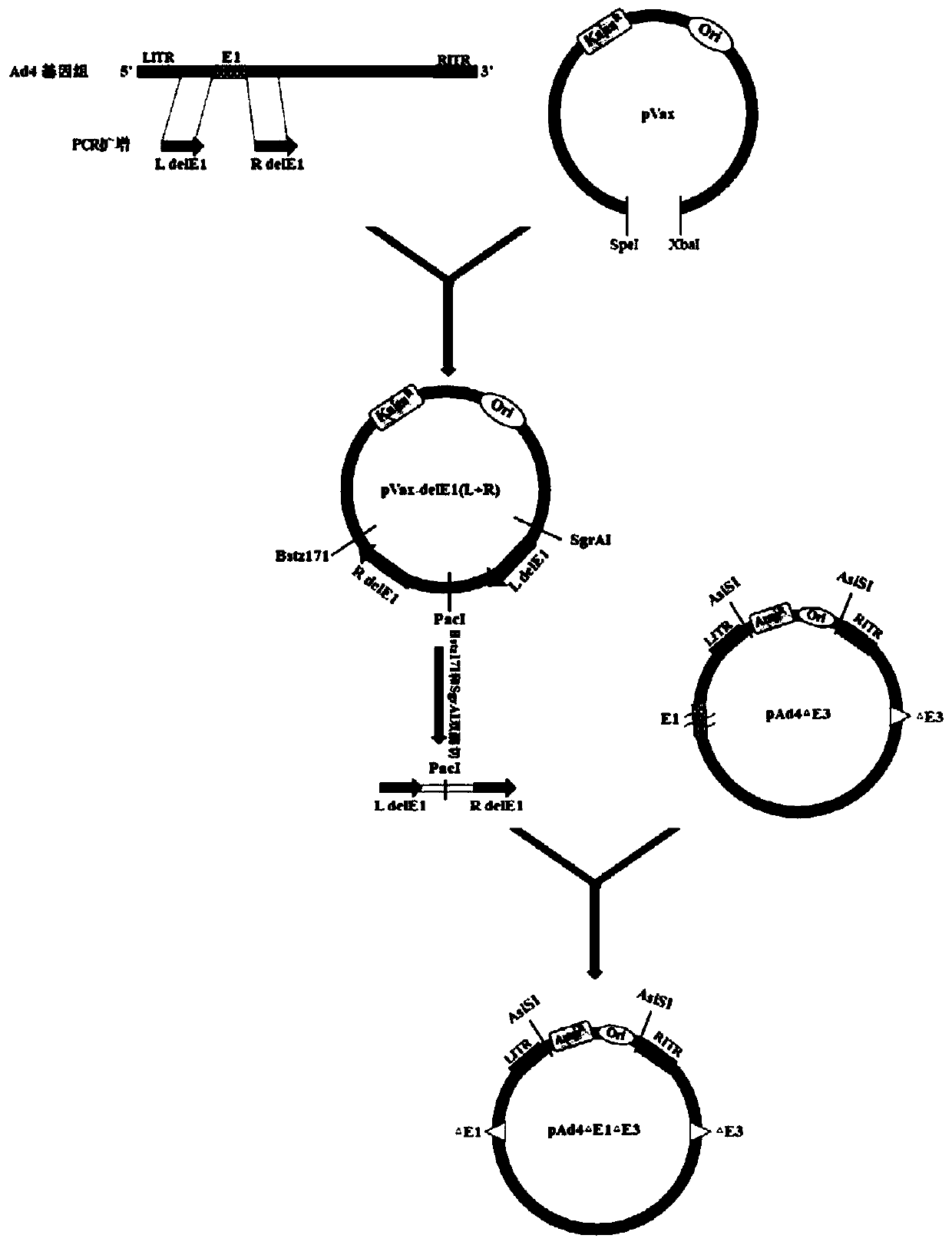
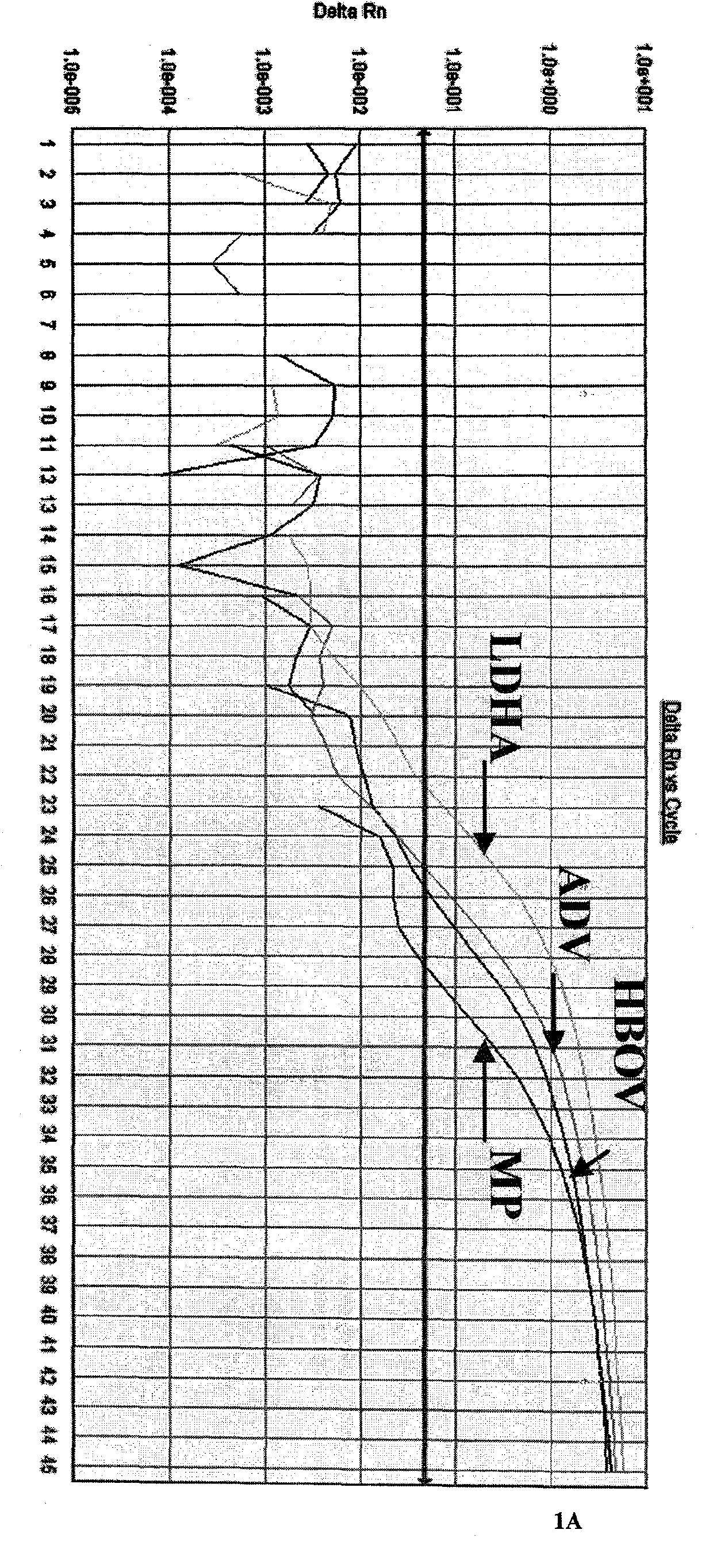
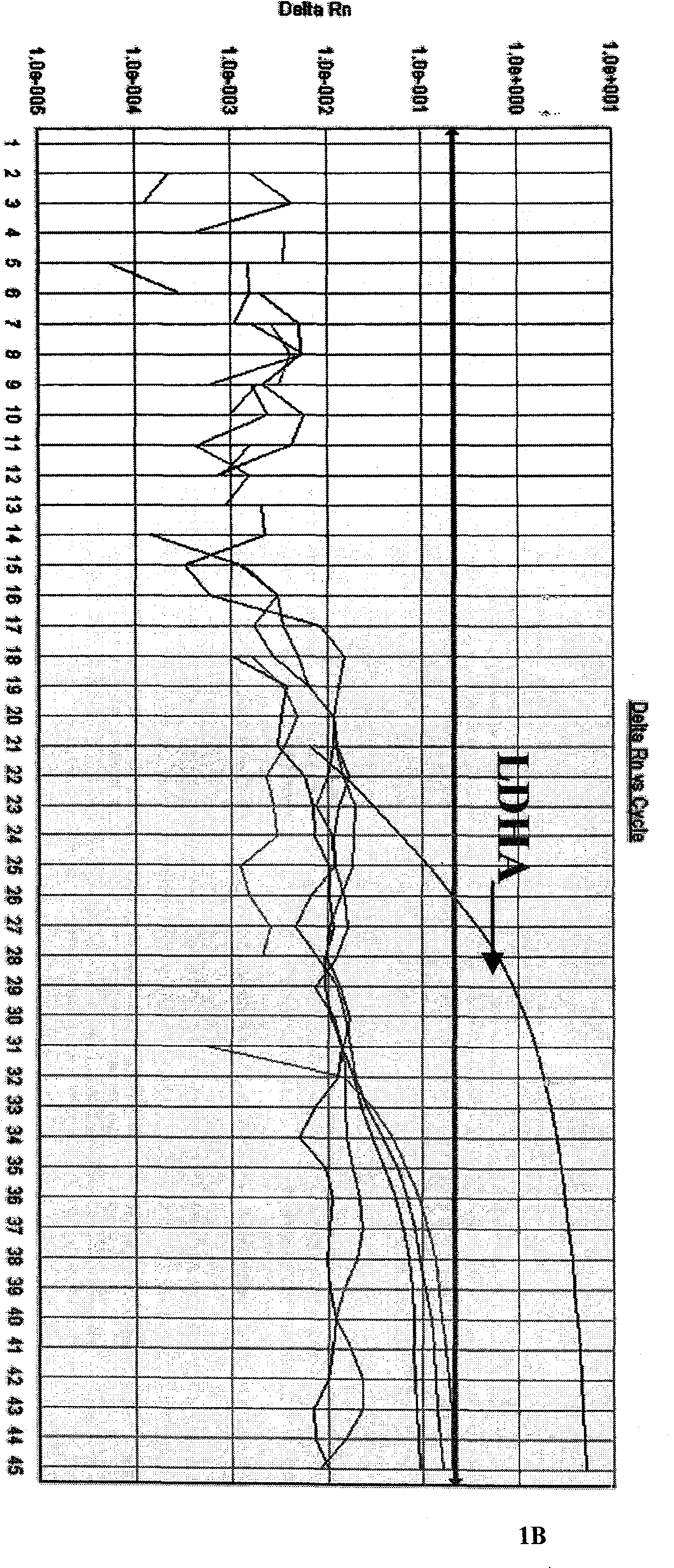
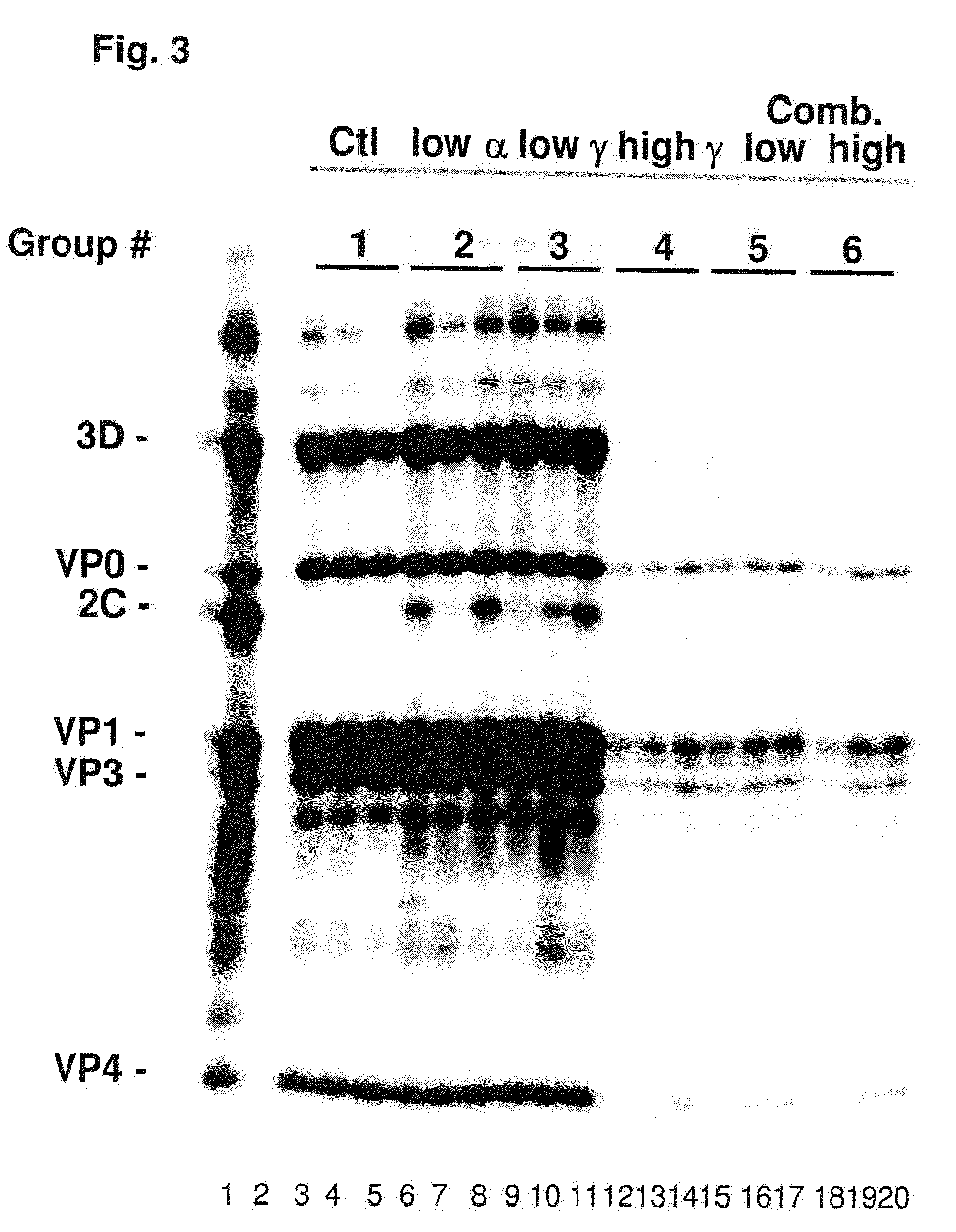
Patents
Literature
142 results about "Human Adenoviruses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Definition of adenovirus. : any of a family (Adenoviridae) of double-stranded DNA viruses originally identified in human adenoid tissue, causing infections of the respiratory system, conjunctiva, and gastrointestinal tract, and including some capable of inducing malignant tumors in experimental animals.
Agents and methods for detecting human adenoviruses
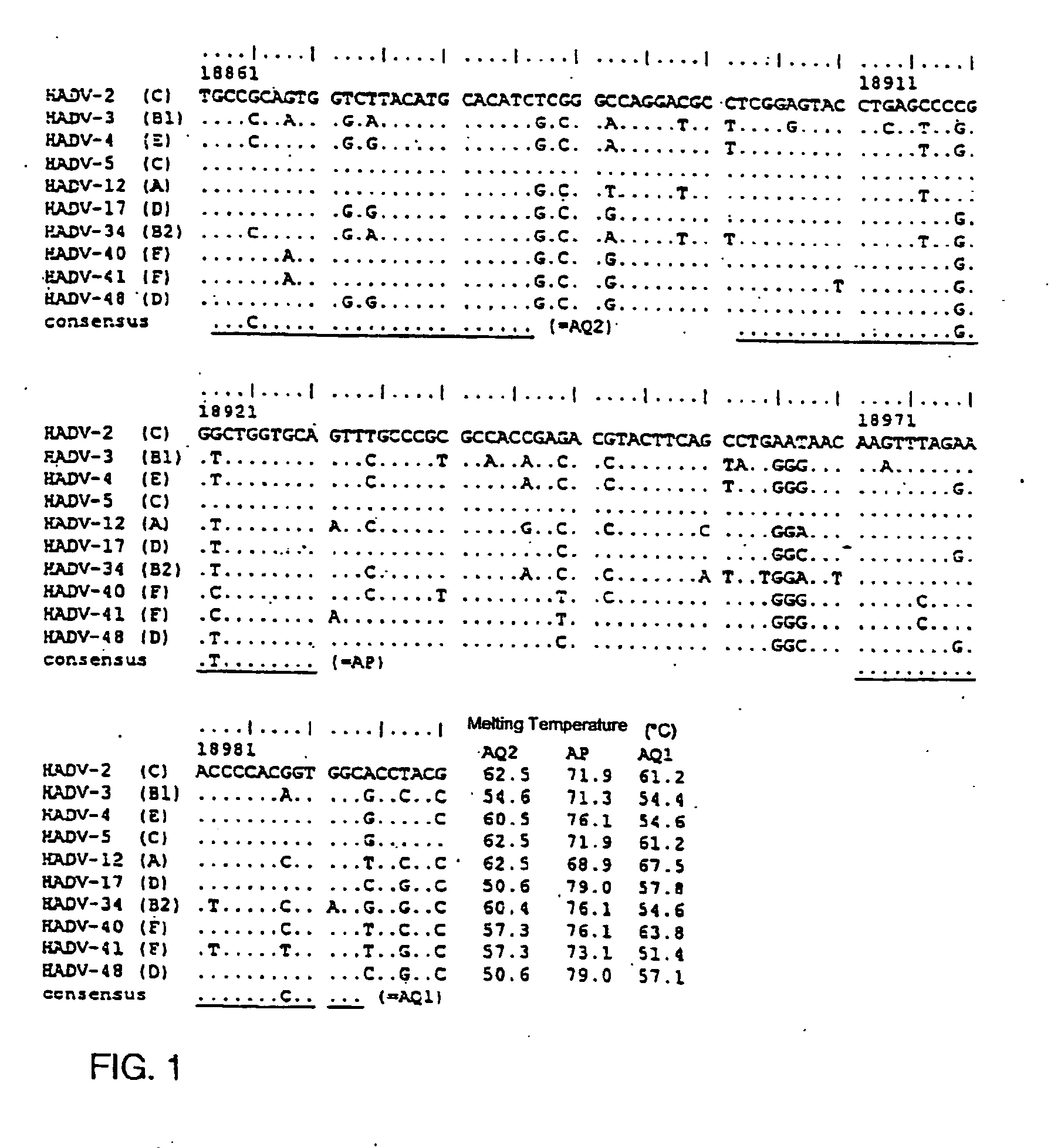
The invention relates to labeled or unlabelled nucleic acids to specifically bind to DNA of human adenoviruses (HAdV DNA), whereby the nucleic acid a) possesses the sequence SEQ ID NO. 1, SEQ ID NO. 2, or SEQ ID NO. 3, b) possesses a sequence with a homology of greater than 78% with respect to SEQ ID NO. 1, SEQ ID NO. 2, or SEQ ID NO. 3, or c) is complementary with respect to a nucleic acid according to a) or b). Also described are methods for the detection of HAdV DNA.
Owner:MEDIZINISCHE HOCHSCHULE HANNOVER
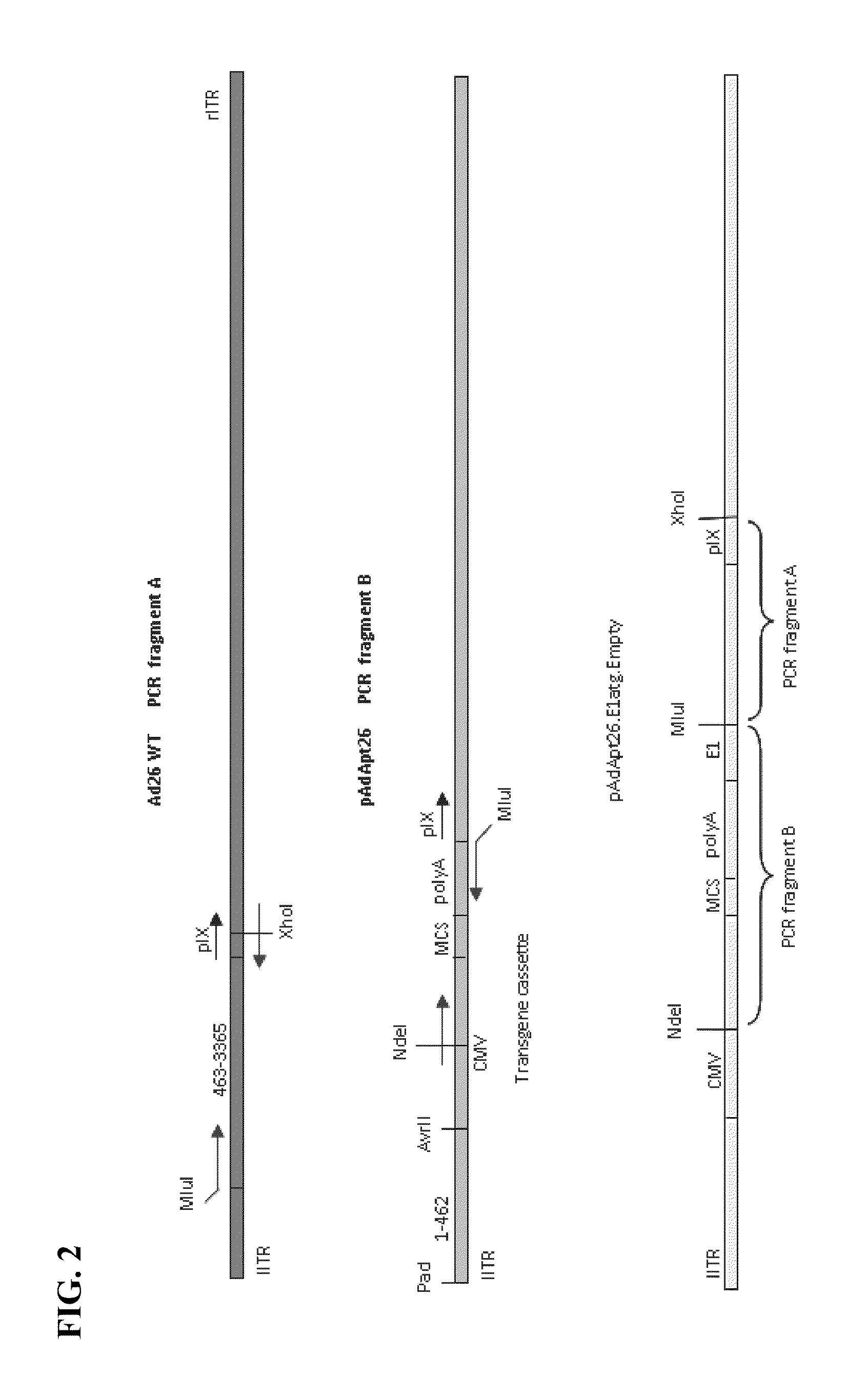
Adenoviral vector system and recombinant adenovirus construction method
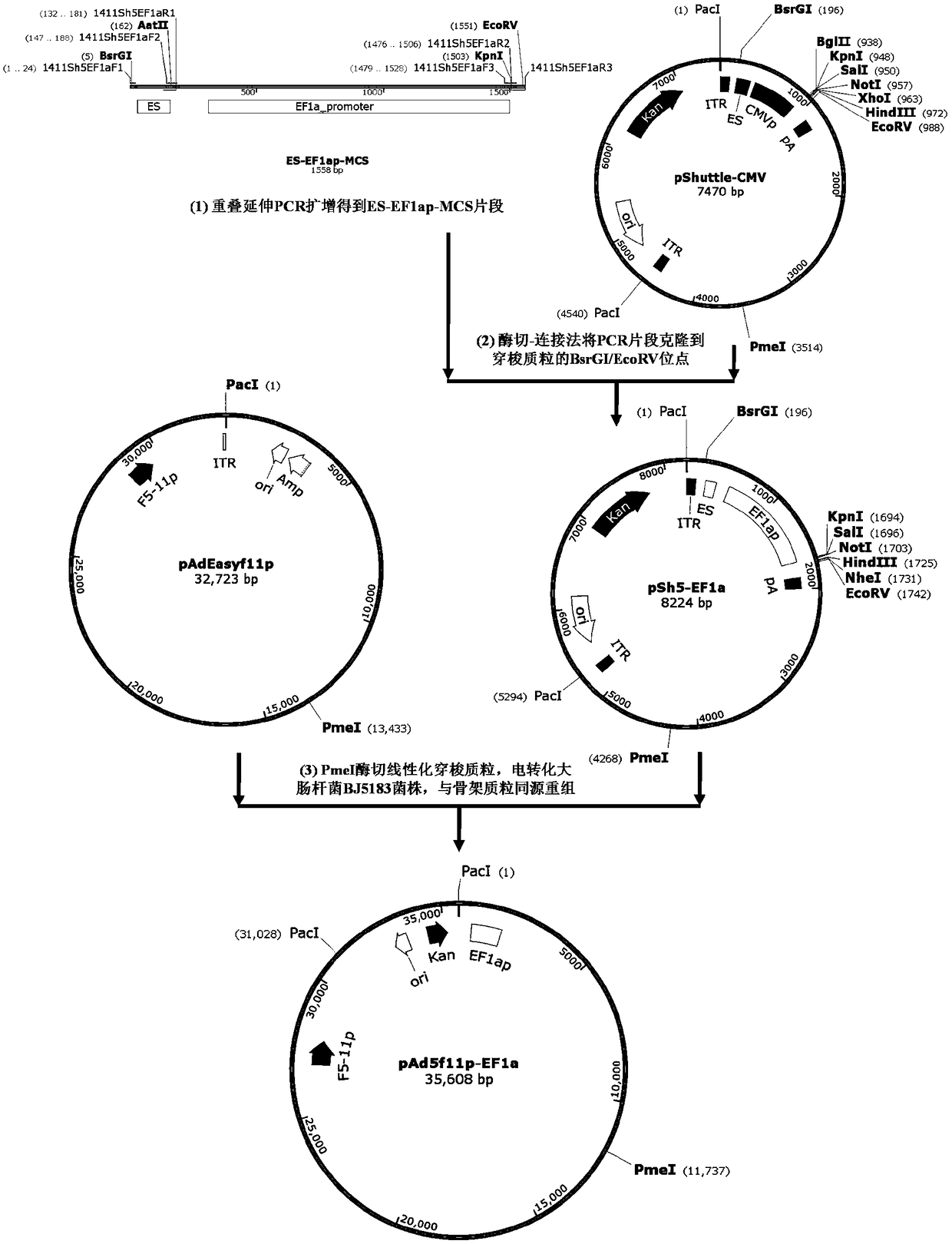
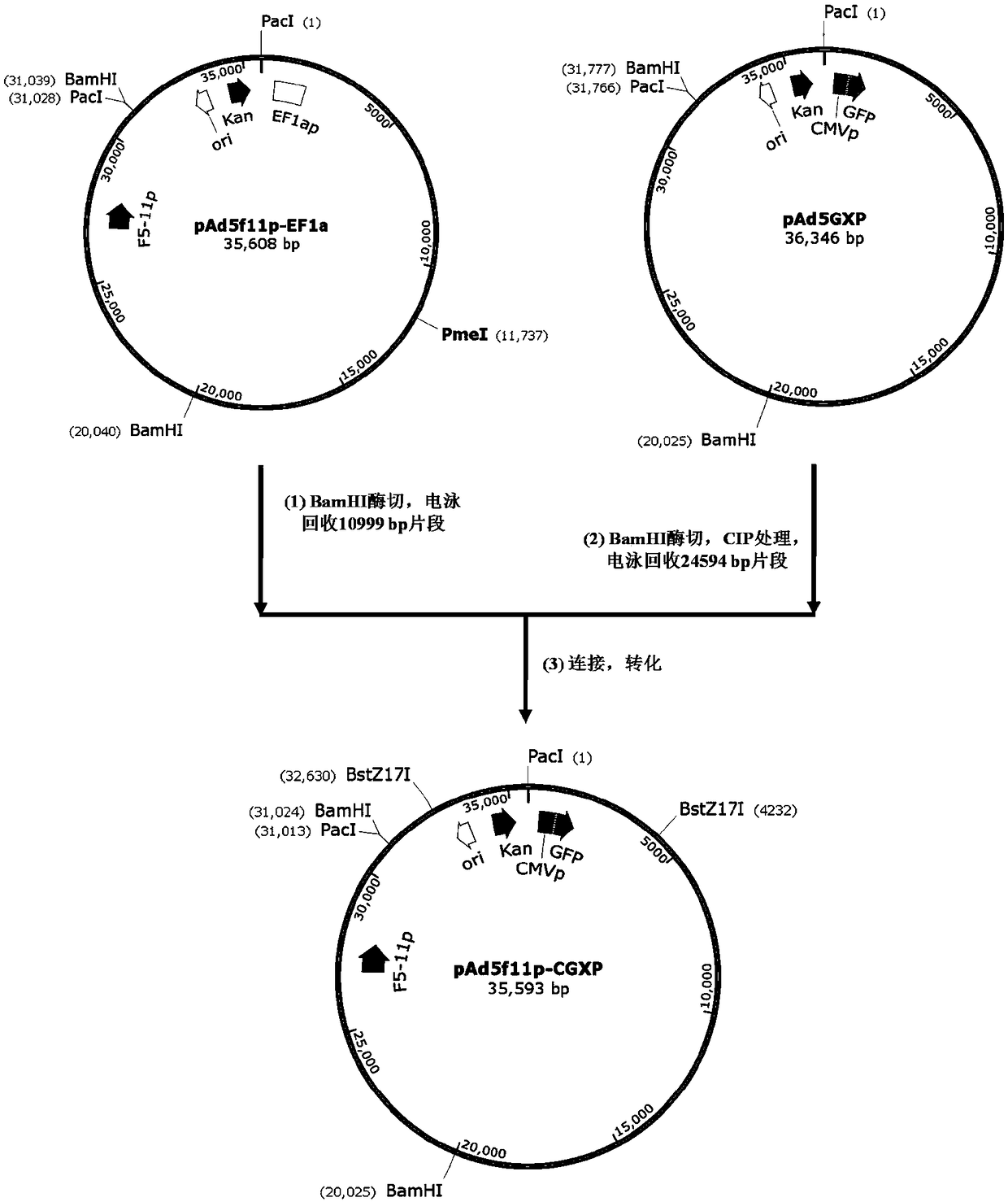
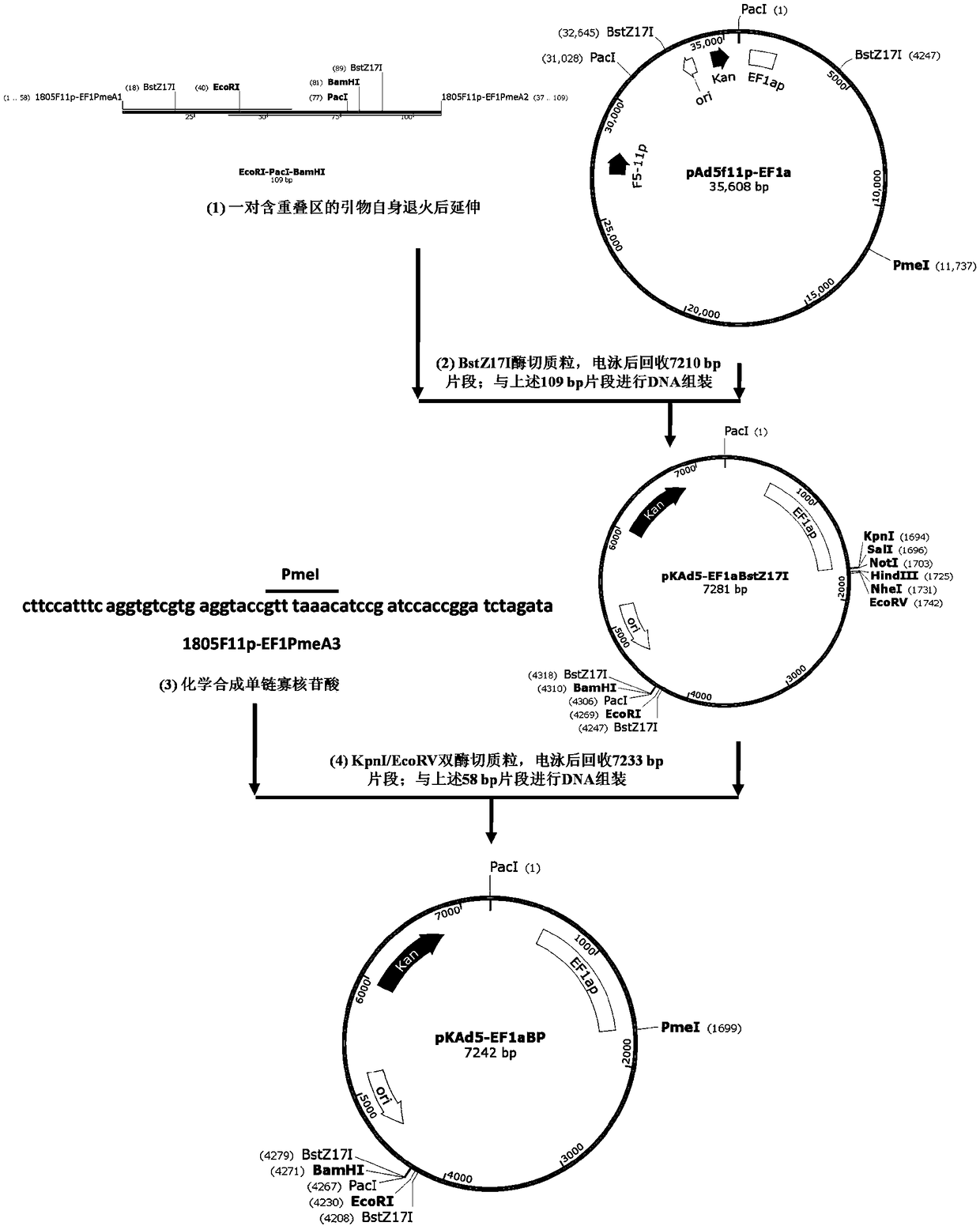
The invention provides an adenoviral vector system and a recombinant adenovirus construction method. The vector system comprises adenoviral plasmids pKAd5f11p-EF1aP and pKAd5f11pES-PmeI and shuttle plasmids pUC19-PM. The adenoviral plasmid contains an E1 / E3 deleted human adenovirus type 5 (HAdV-5) genome and the original HAdV-5 fiber gene is replaced by a fusion gene F5-11p of HAdV-5 and HAdV-11p.A PmeI site is an exogenous gene insertion site. Plasmids pKAd5f11p-EF1aP contain a human EF1a promoter in the original E1 region. The shuttle plasmids pUC19-PM are matched with the plasmids pKAd5f11pES-PmeI. The recombinant adenovirus construction method comprises: carrying out PCR amplification to obtain a desired gene fragment containing homologous overlapping regions on both sides and carrying out DNA assembling on the desired gene fragment and PmeI-linearized adenoviral plasmids to obtain adenovirus plasmids containing the desired exogenous gene, or cloning the multiple gene fragments tothe shuttle plasmids, shearing all the desired gene fragments through a restriction endonuclease and carrying out DNA assembling on the desired gene fragments and PmeI linearized pKAd5f11pES-PmeI toobtain the adenovirus plasmids containing the desired exogenous gene.
Owner:中国疾病预防控制中心病毒病预防控制所 +1
Vaccine for inducing specific immunity of tumor and application thereof
InactiveCN103110939AEnhance tumor immunityStrong immune responseViral antigen ingredientsUnknown materialsOncolytic adenovirusSpecific immunity
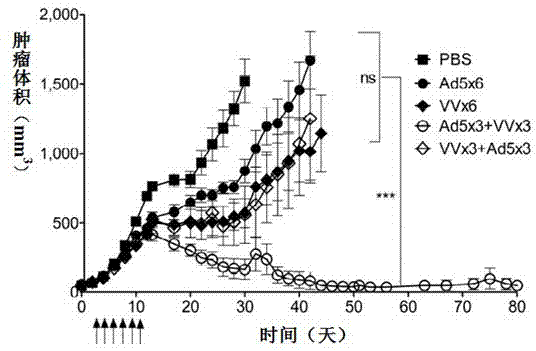
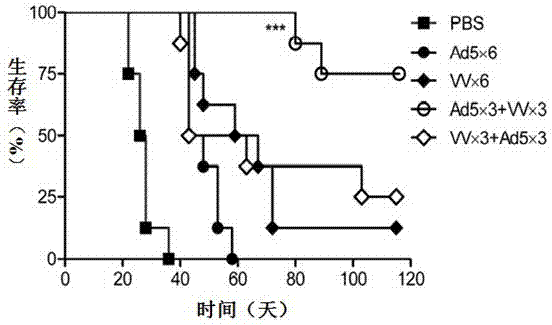
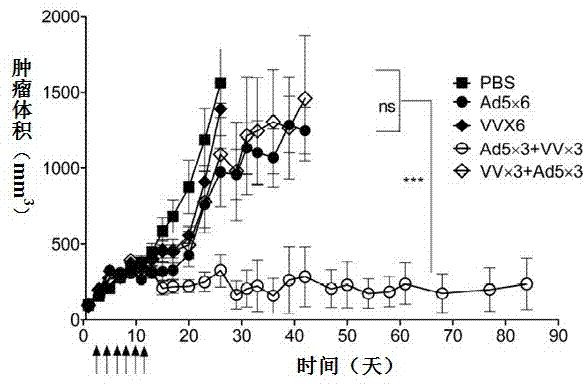
The invention relates to a vaccine comprising an adenovirus vector and a vaccinia virus vector and an application of the vaccine in treating tumor. The vaccine comprises oncolytic adenovirus and vaccinia virus; the two viruses are used in sequence, wherein the oncolytic adenovirus is the reproductive human adenovirus type 5 and the vaccinia virus is the vaccinia virus for smallpox vaccine. According to the vaccine, the oncolytic adenovirus and the vaccinia virus are prepared into the vaccine by sequential combination, so the antineoplastic activity can be improved, the stronger cellular immune response can be generated compared with the effect of reversely associated virus or separate virus, the using effect is better and the long-term specific immunity of tumor is shown. The tumor vaccine can eradicate the tumor and generate the long-term specific immunity of tumor and can be used in the treatment of various tumors of the human or animals; and meanwhile, the vaccine can effectively prevent the tumor from relapsing and has a wide application prospect.
Owner:ZHENGZHOU UNIV +1
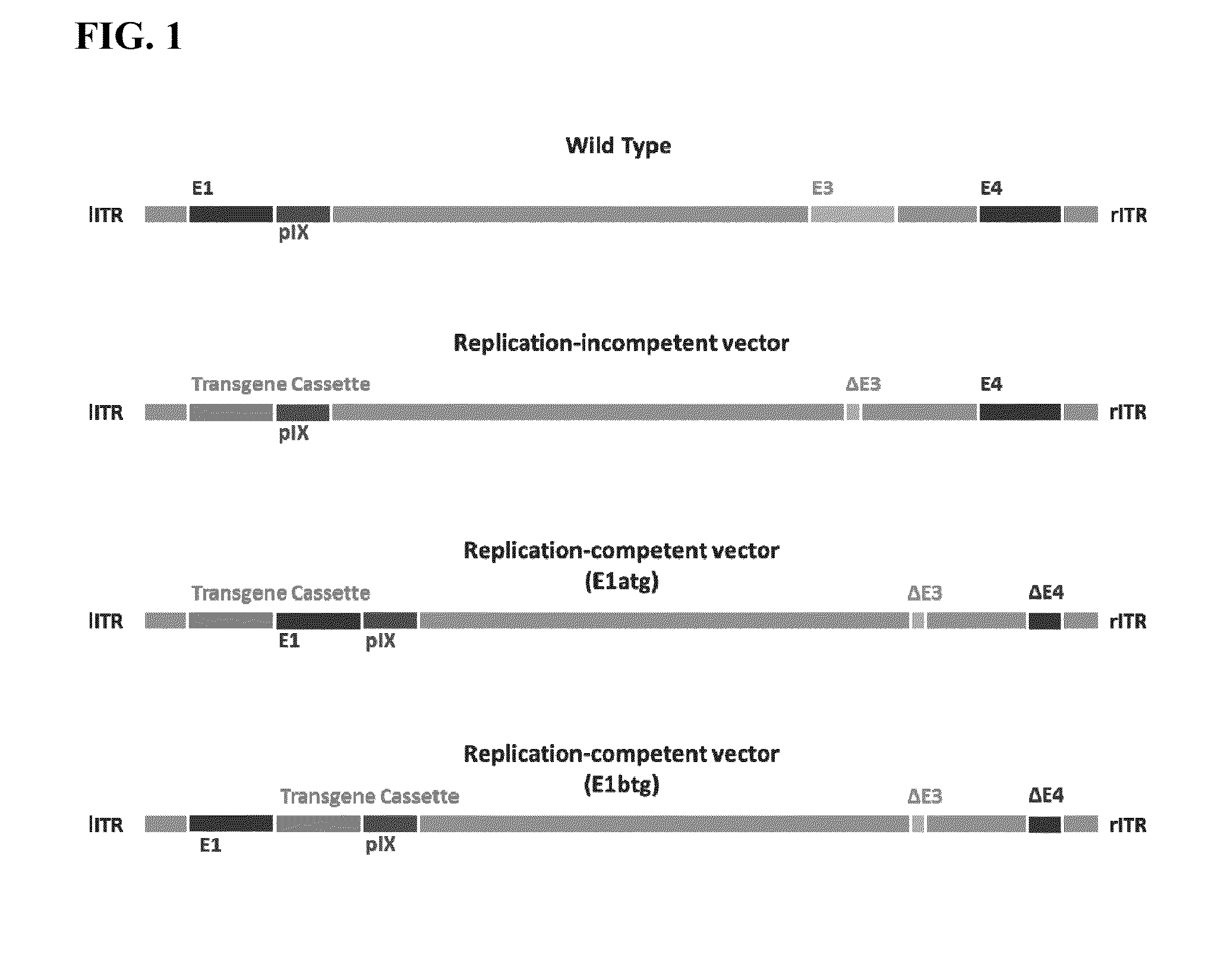
Replicating recombinant adenovirus vectors, compositions, and methods of use thereof
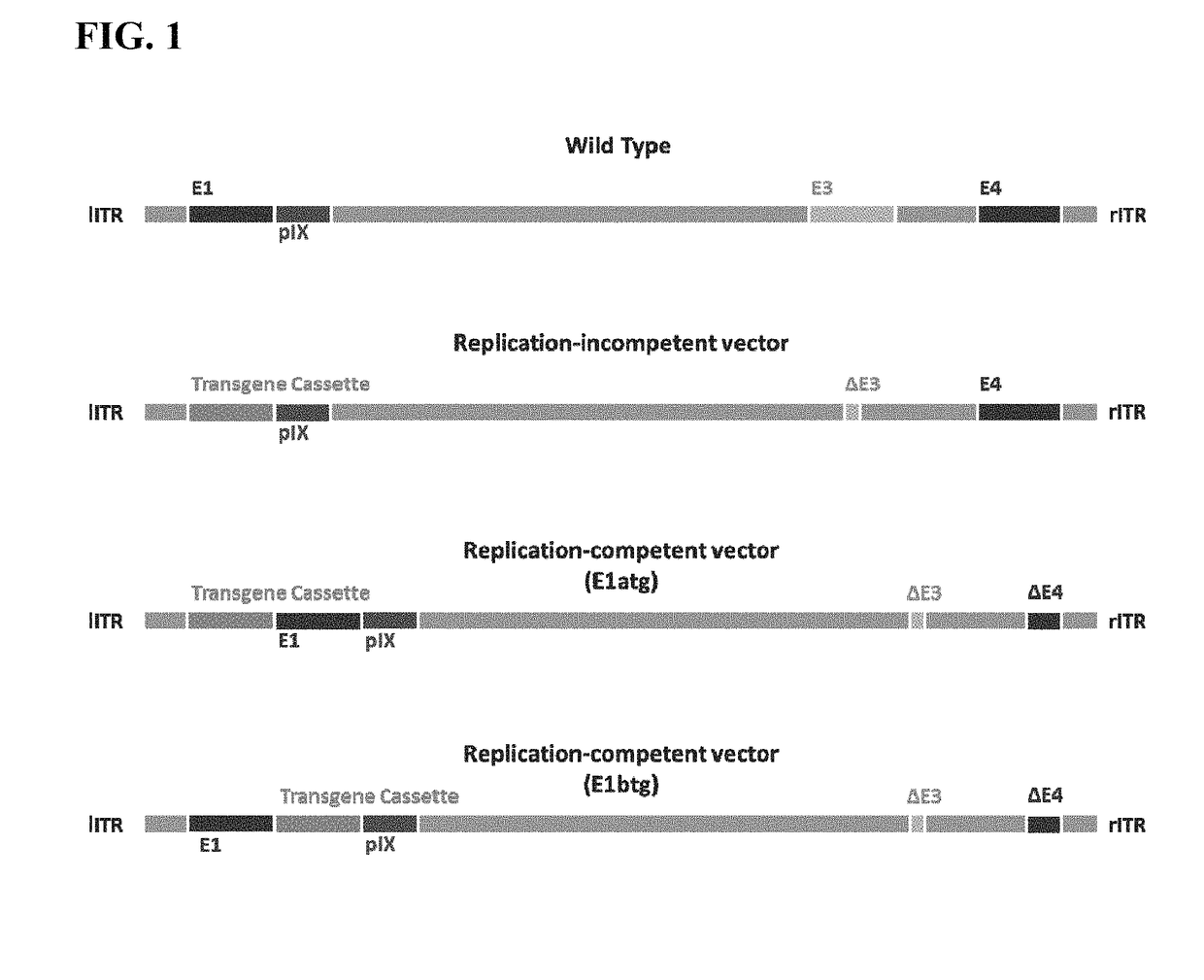
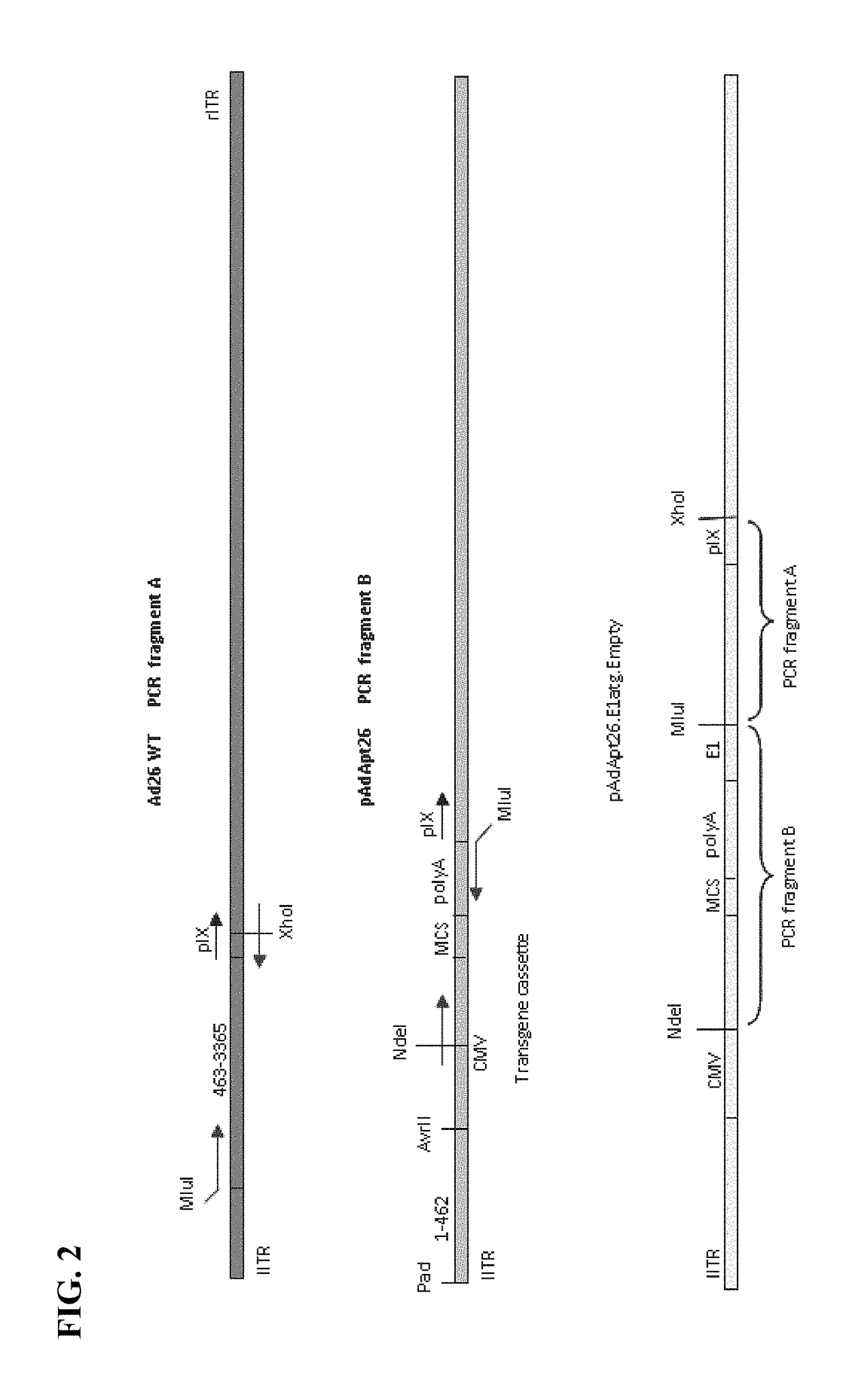
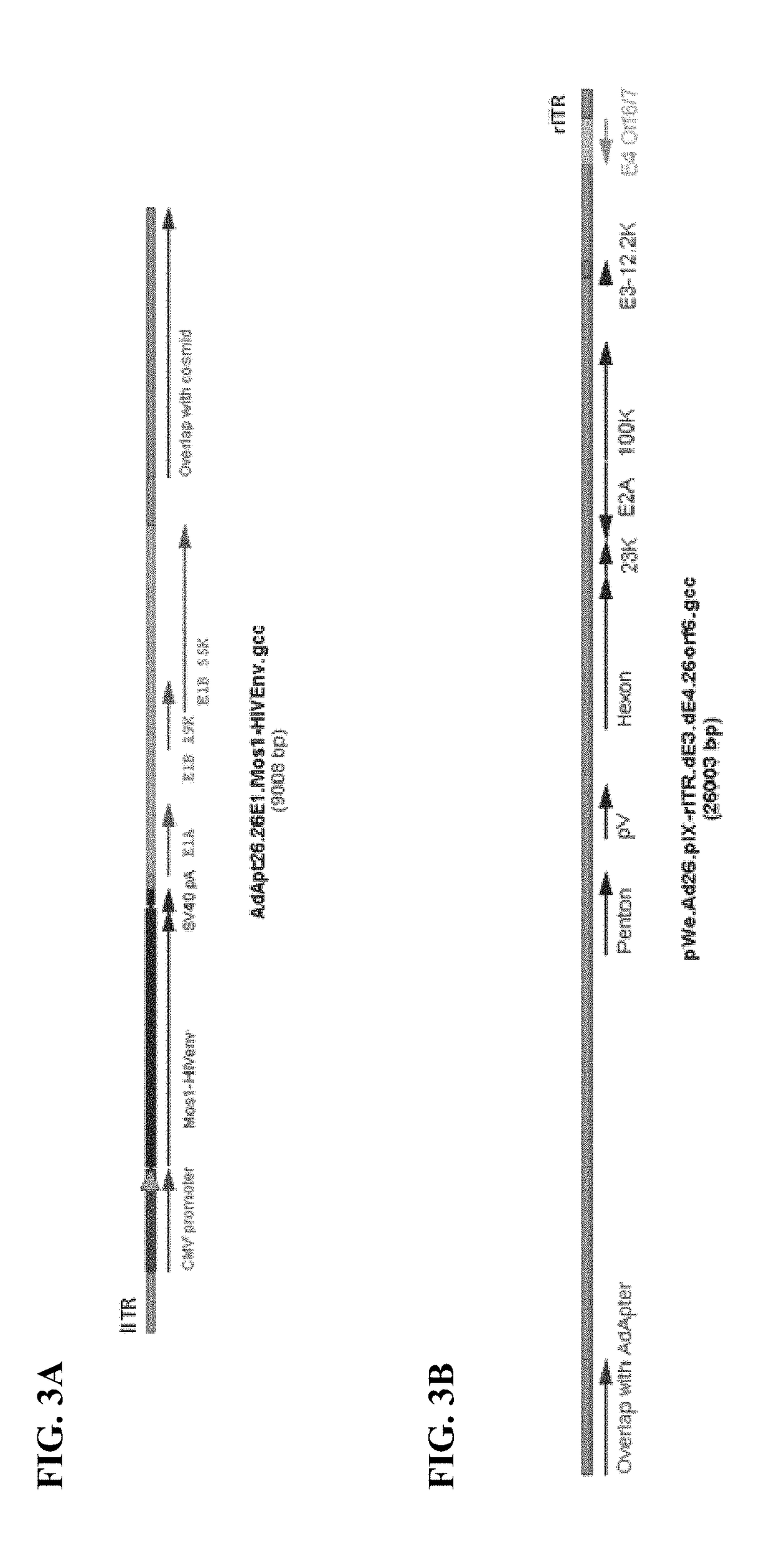
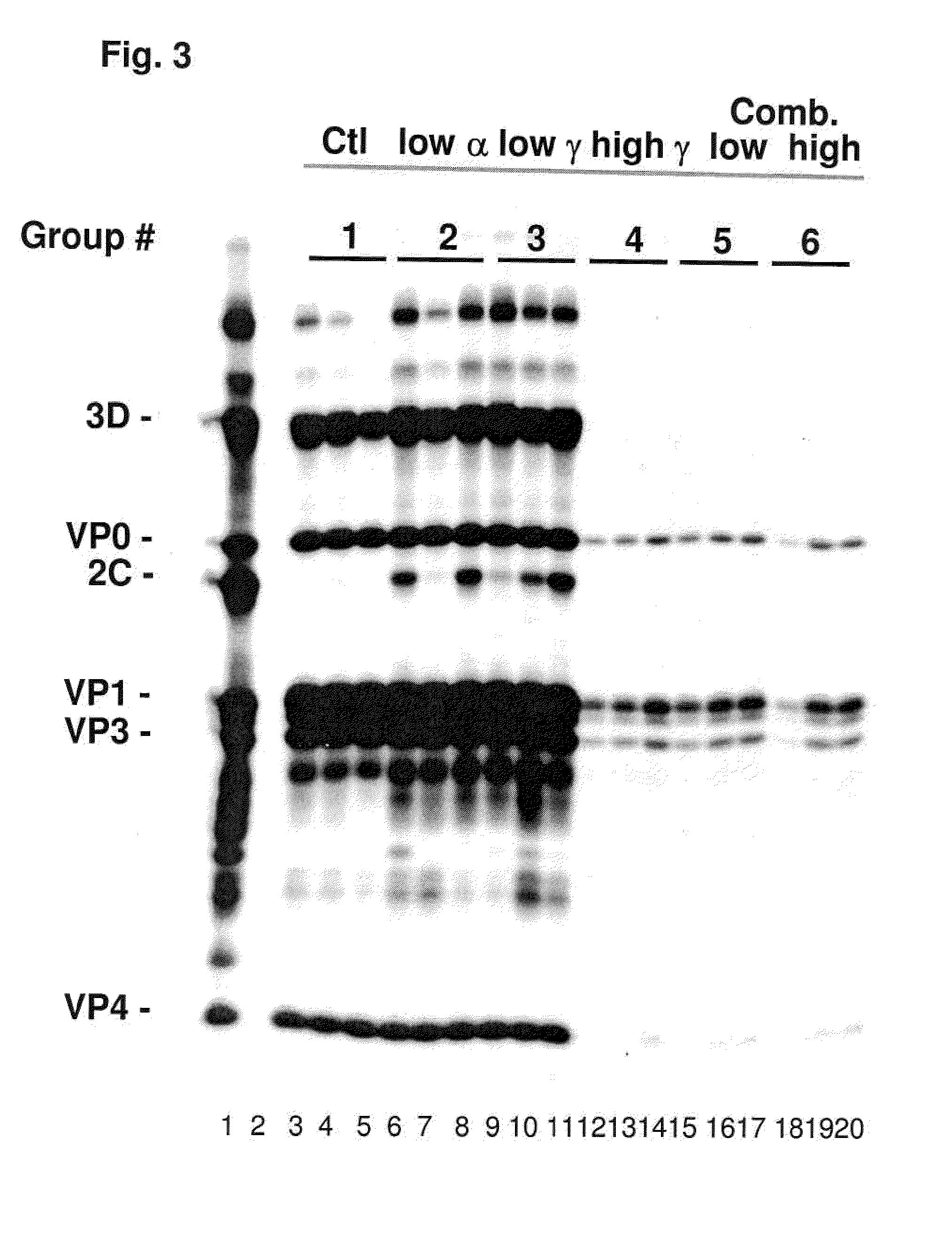
Replicating recombinant adenovirus vectors derived from human adenovirus serotype 26 or human adenovirus serotype 35 are described. The replicating recombinant adenovirus vectors have attenuated replicative capacity as compared to that of the corresponding wild-type adenovirus. They can be used for stable expression of heterologous genes in vivo. Also described are compositions and methods of using these recombinant adenovirus vectors to induce an immune response in a subject, and vaccinate a subject against an immunogenic human immunodeficiency virus (HIV) infection.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC +1
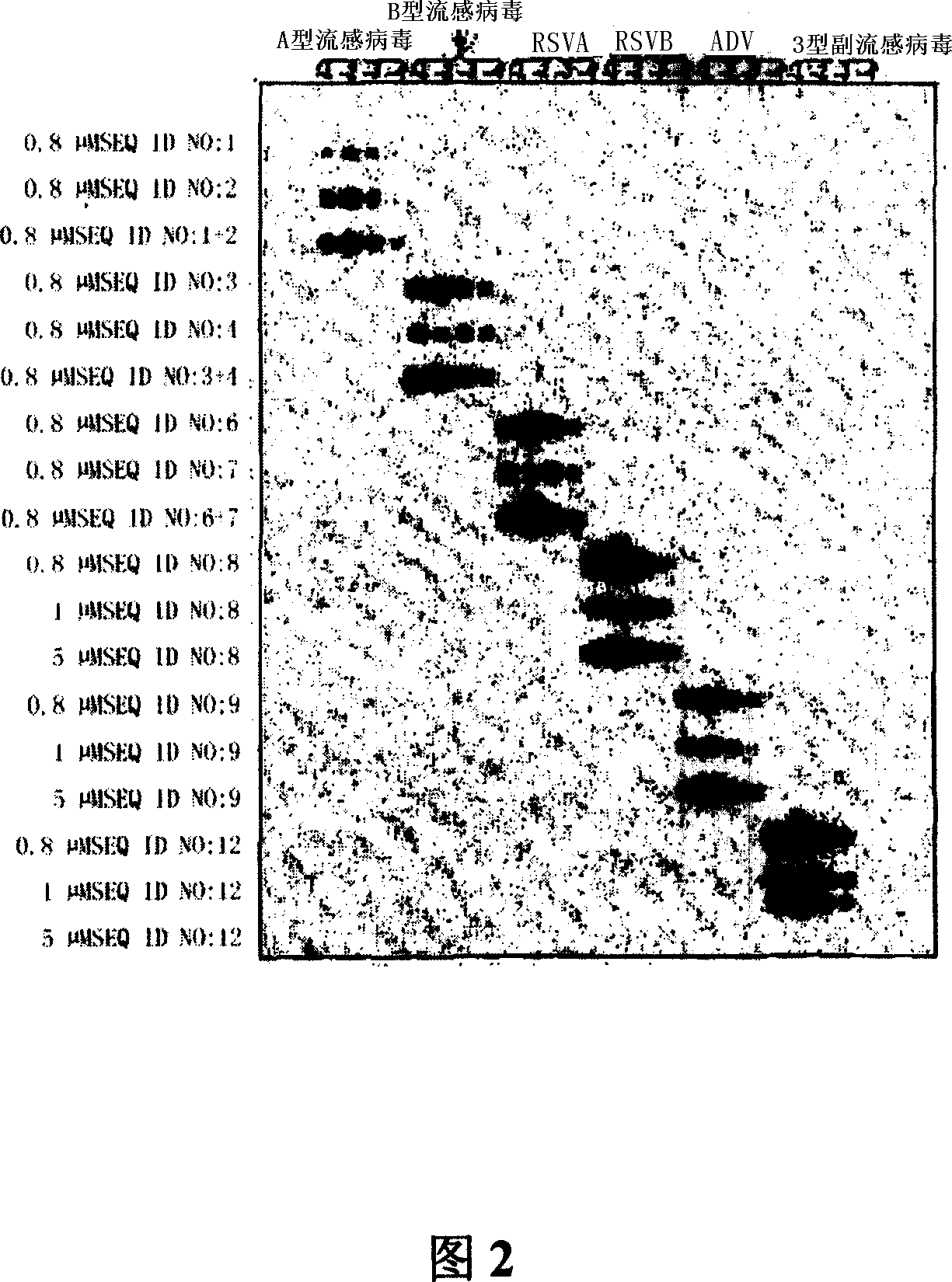
Probes and methods for the simultaneous detection and identification of multiple viruses that cause respiratory infections in humans
InactiveCN101107366AShorten the lengthSynthetic economyMicrobiological testing/measurementAgainst vector-borne diseasesEnterovirusSevere acute respiratory syndrome
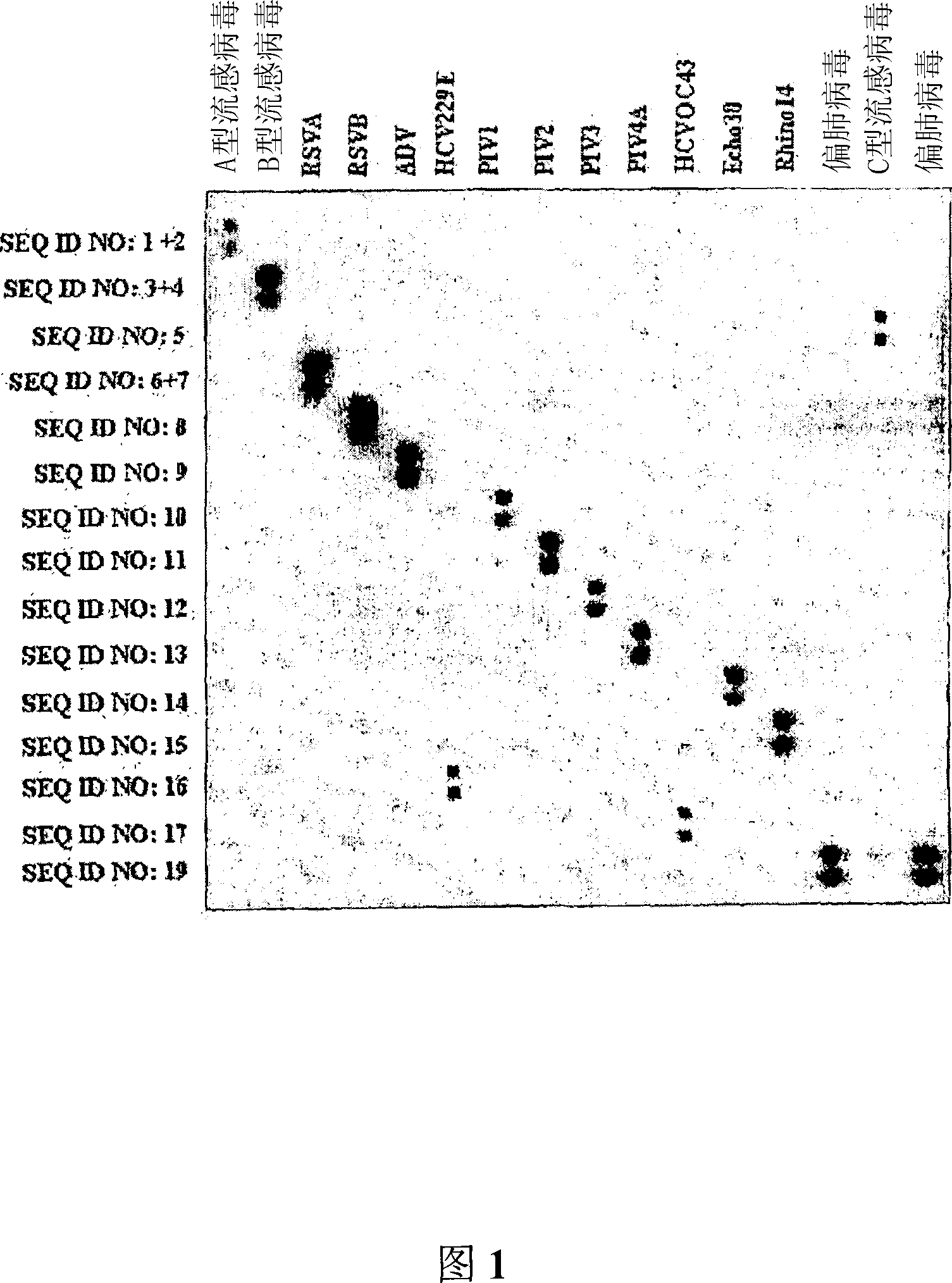
The invention relates to probes and assays which are used for the simultaneous detection, in a single assay sample, of a plurality of nucleic acid sequences of viruses that cause respiratory infections in humans, selected from among influenza virus type A, influenza virus type B, influenza virus type C, human respiratory syncytial virus type A, human respiratory syncytial virus type B, human adenovirus, human parainfluenza virus type 1, human parainfluenza virus type 2, human parainfluenza virus type 3, human parainfluenza virus types 4A and 4B, enterovirus, rhinovirus, human coronavirus type 229E, human coronavirus type OC43, coronavirus that causes severe acute respiratory syndrome (SARS), human metapneumovirus and combinations thereof.
Owner:INST DE SALUD CARLOS III
Kit for detecting B and E type human adenovirus nucleic acid and detection method thereof
ActiveCN105586439ARapid responseHigh sensitivityMicrobiological testing/measurementFreeze-dryingMagnesium salt
The invention discloses a kit for detecting B and E type human adenovirus nucleic acid and a detection method thereof. The kit for detecting the B and E type human adenovirus nucleic acid comprises RPA freeze-dried particles, an RPA reaction primer, an RPA reaction probe, an RPA buffer solution, a magnesium salt solution, a transverse flow test strip, a buffer solution, double distilled water and an adenovirus genome DNA. The kit for detecting the B and E type human adenovirus nucleic acid has the advantages that the transverse flow test strip is utilized for carrying out visual qualitative detection on an RPA nucleic acid amplification product, the whole reaction is carried out at the constant temperature of 38 DEG C, a stripe on the test strip can be observed by virtue of naked eyes within 5-20 minutes, results are read and discriminated, and a special detecting instrument is not needed during detection. The kit disclosed by the invention has the characteristics of high sensitivity, strong specificity, easiness in operation, quick reaction and portability of instruments and is especially applicable to bedside rapid diagnosis of adenovirus, epidemic prevention and disease control.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
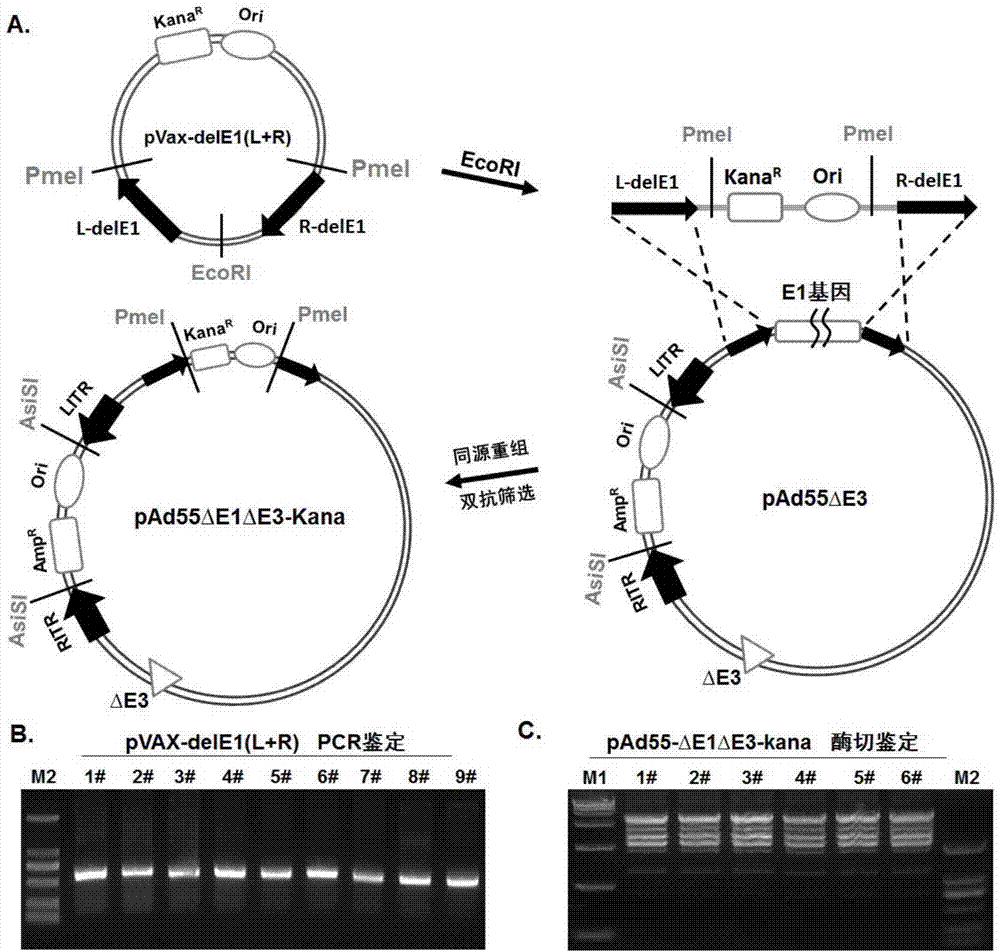
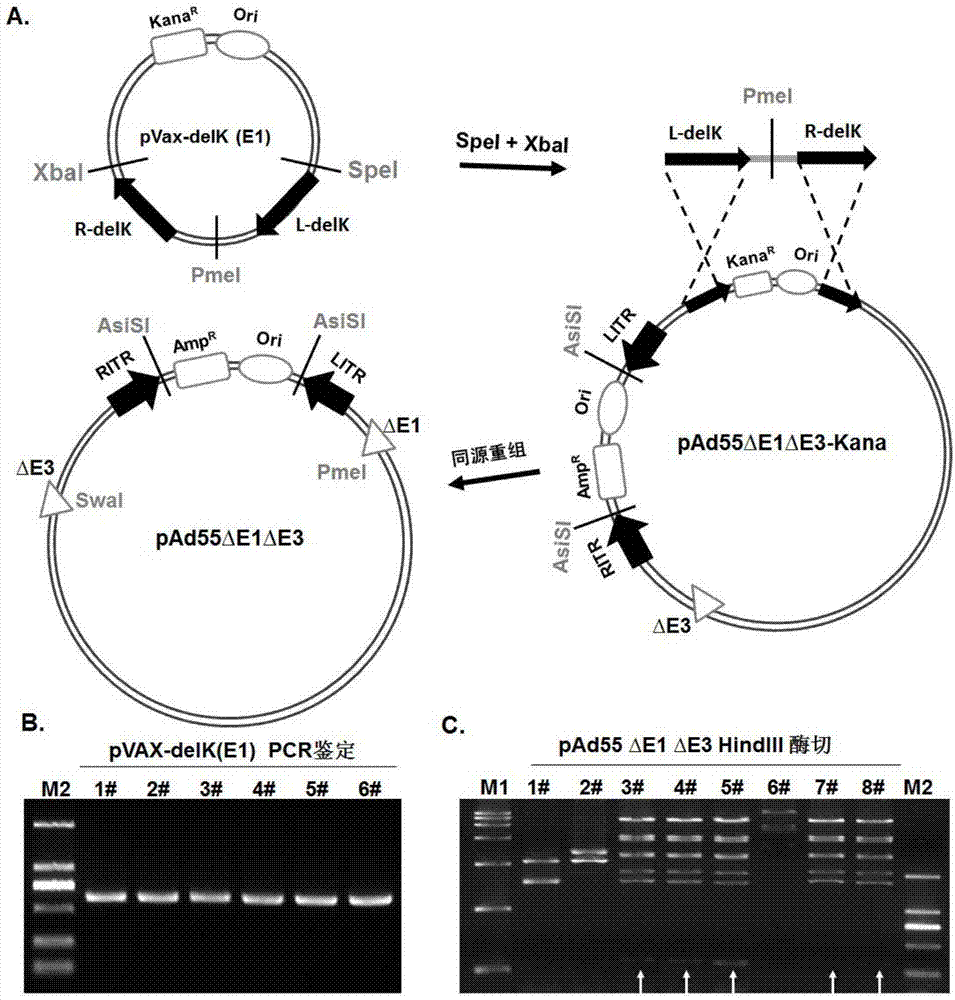
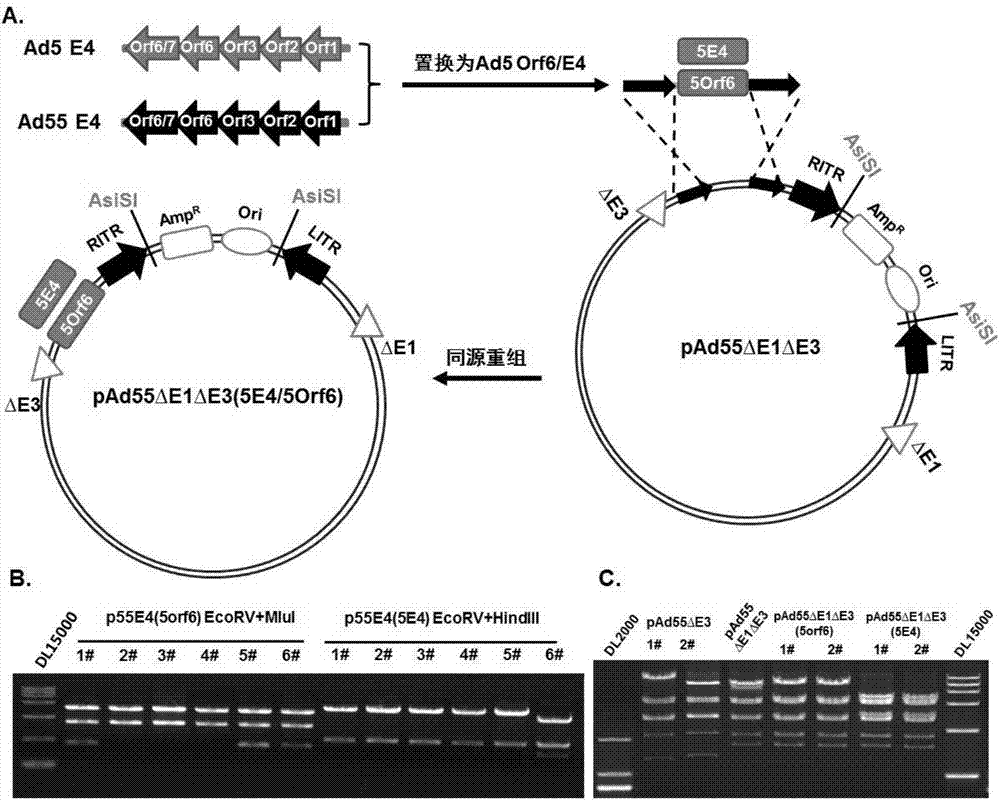
Replication-defective human adenovirus type 55 vector and preparation method and application thereof
ActiveCN104846013ASsRNA viruses negative-senseViral antigen ingredientsNeutralizing antibodyReporting system
The invention relates to the biological technical field, and particularly discloses a replication-defective human adenovirus type 55 vector and a preparation method and an application thereof. The replication-defective human adenovirus type 55 vector is prepared by the following method: E1 and E3 genes of Ad55 are knocked out, an open reading frame 6 or open reading frames 2, 3, 4, 6 and 6 / 7 of an E4 gene in an Ad55 genome are changed into corresponding reading frames of an Ad5 genome, and in addition, an exogenous gene expression frame is integrated in an E1 gene region of the Ad55. The vector can be produced in large quantities in 293, PerC6 and other auxiliary cell lines, and can be concentrated and purified by density gradient centrifugation; normal human cells do not have the replication capacity, thereby having an attenuated phenotype; in addition, the vector can efficiently express an exogenous gene in target cells. The vector can be used as a vaccine or a gene therapy vector, and can also be applied in development of drugs and neutralizing antibodies and in a trace reporting system.
Owner:GUANGZHOU N BIOMED LTD
Methods and reagents for efficient and targeted gene transfer to monocytes and macrophages
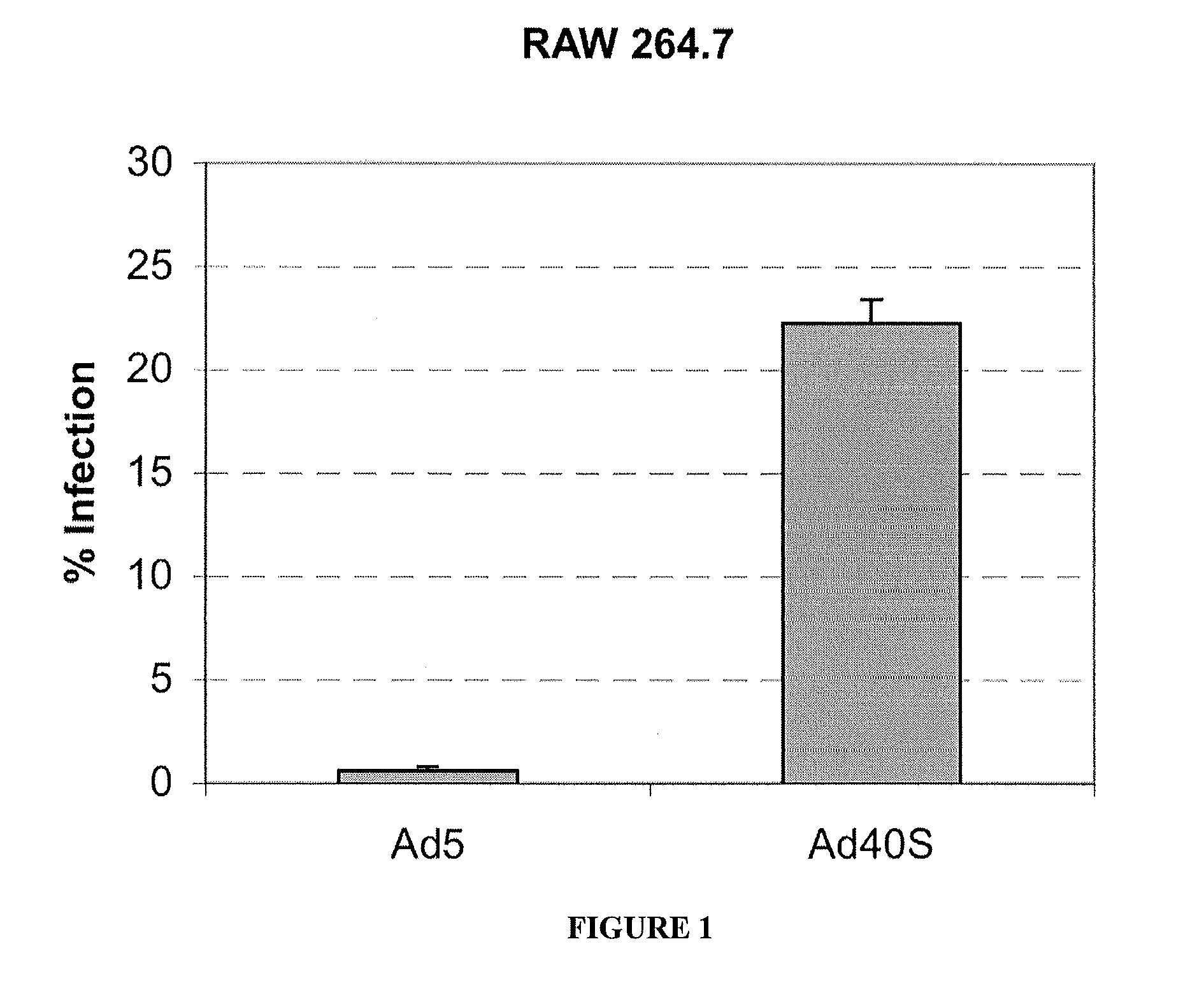
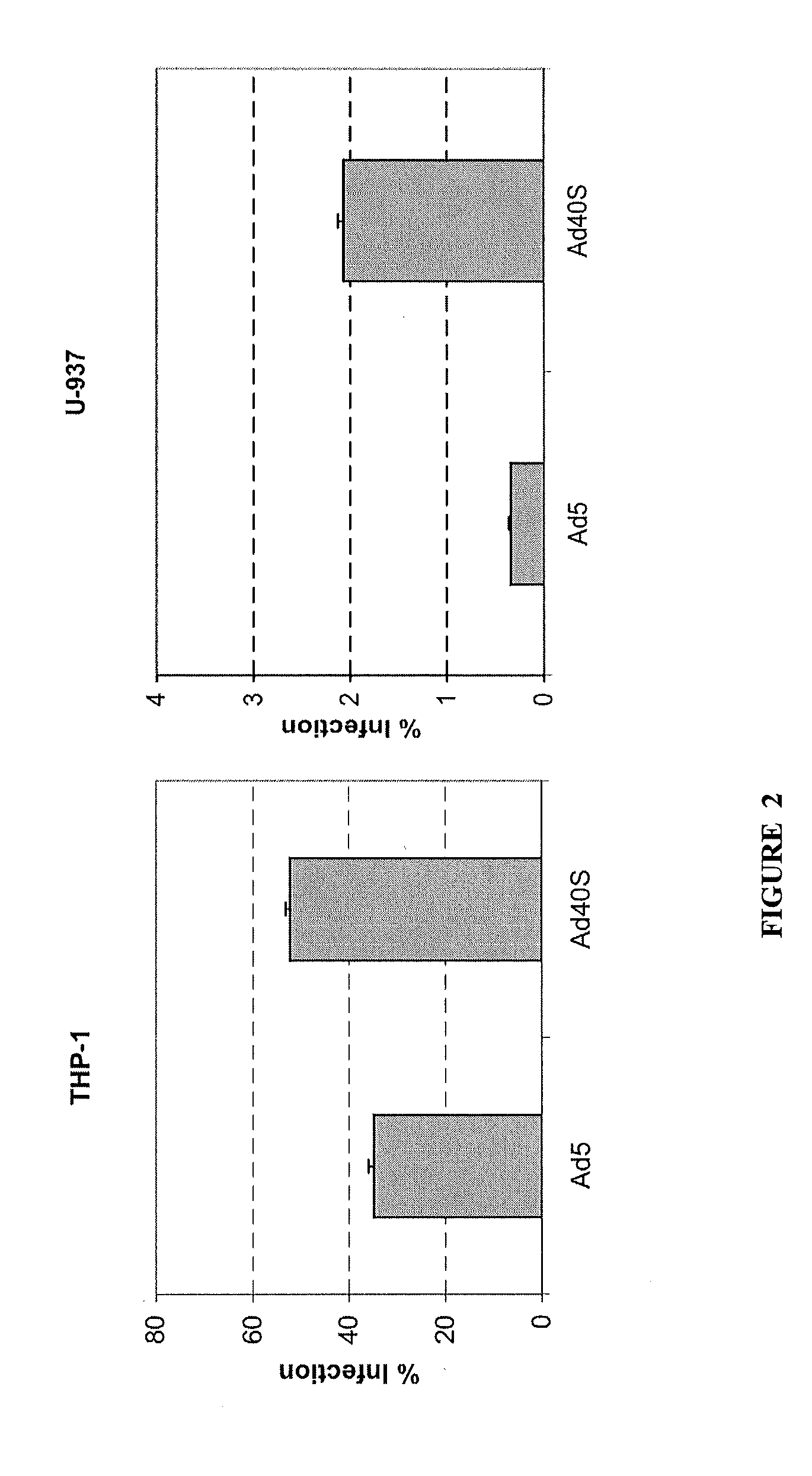
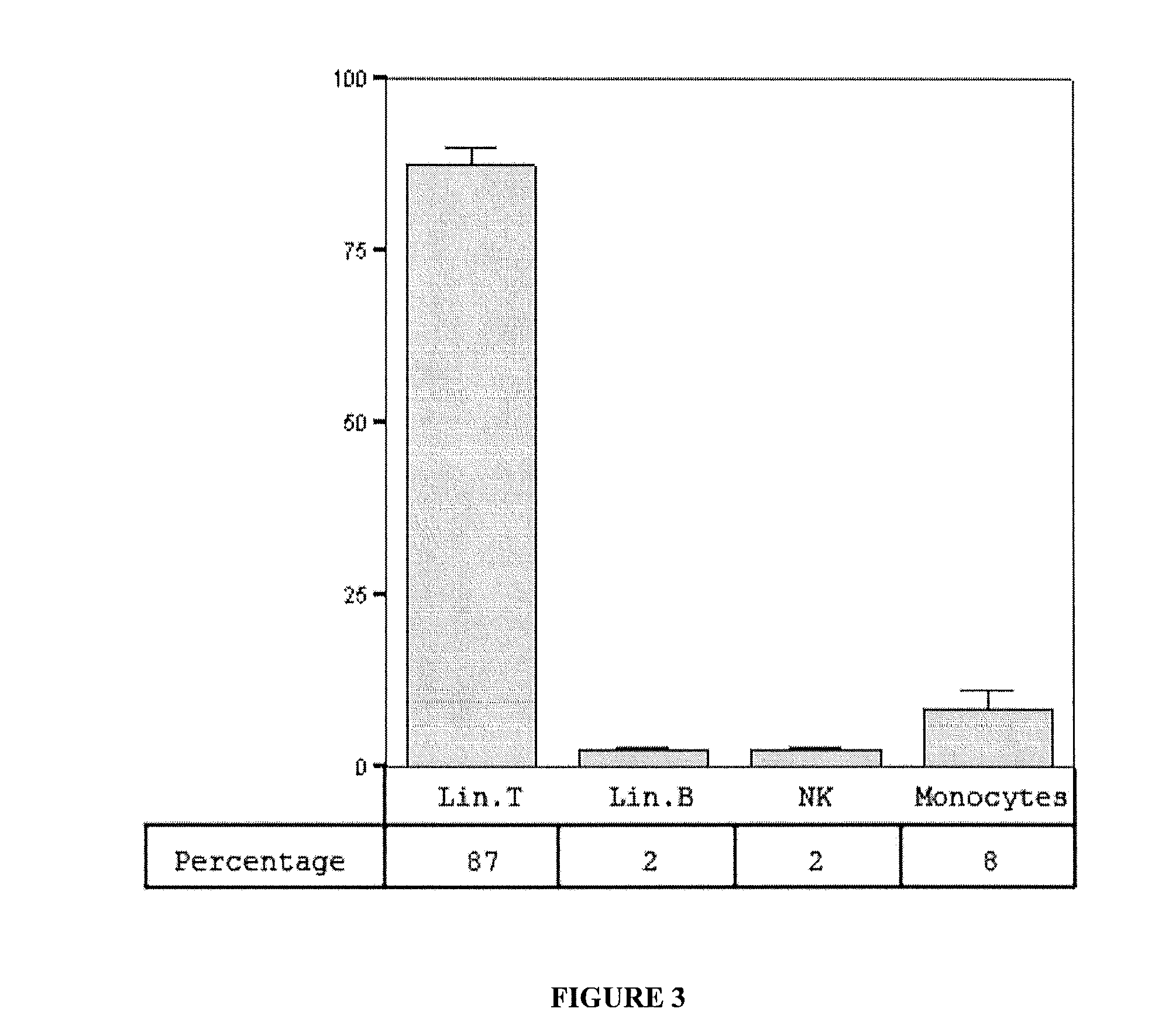
The present invention provides a biosafe and useful vector to transfer genetic material to CD14+ mononuclear cells (monocytes and monocyte-derived macrophages) in an efficient and specific manner. The embodiment of the invention makes use of the chimeric human adenovirus vectors 5 carrying the short fiber of enterotropic Ad40 to transfer genetic material to the target CD14+ mononuclear cells.
Owner:AUTONOMOUS UNIVERSITY OF BARCELONA +3
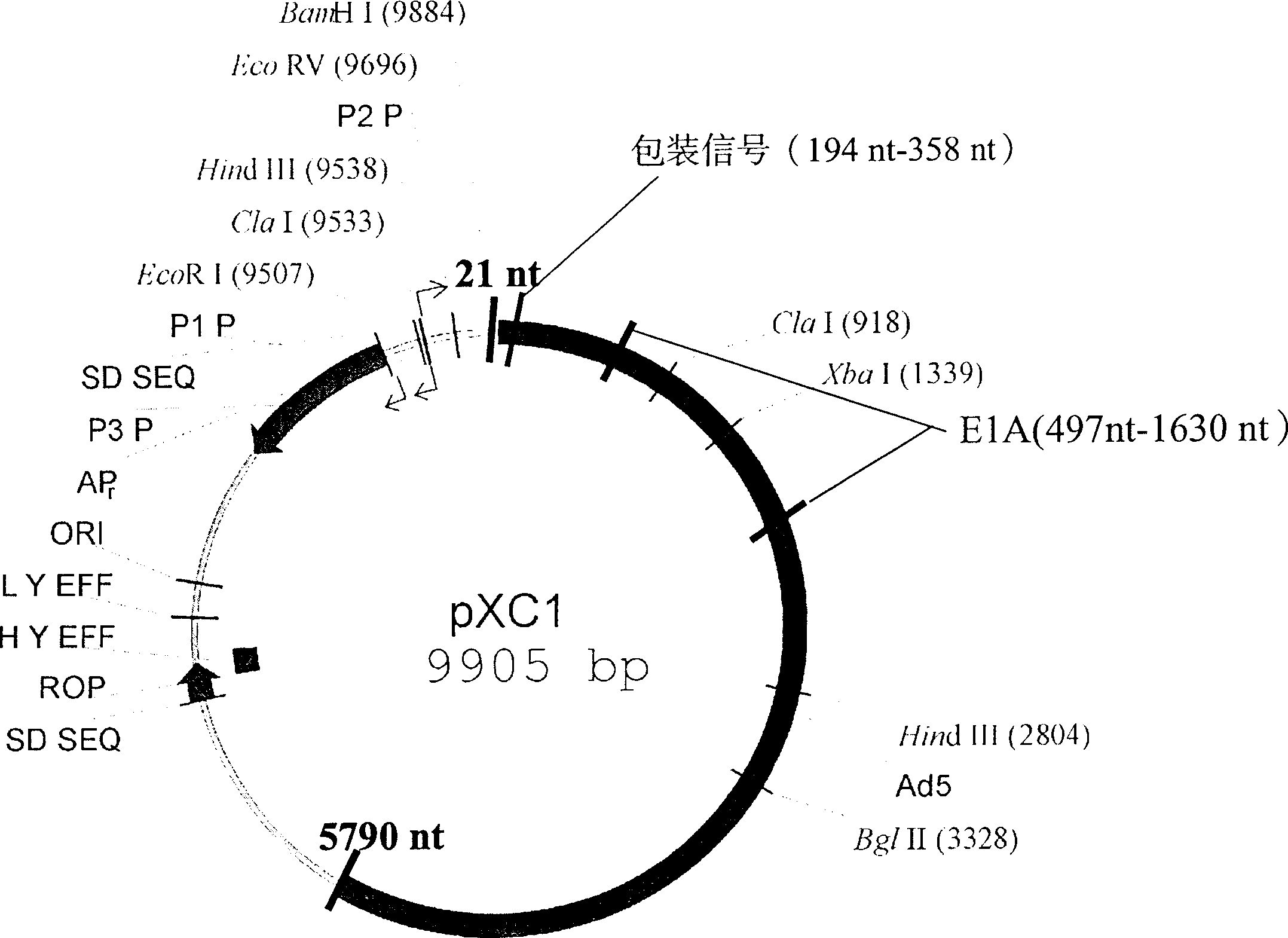
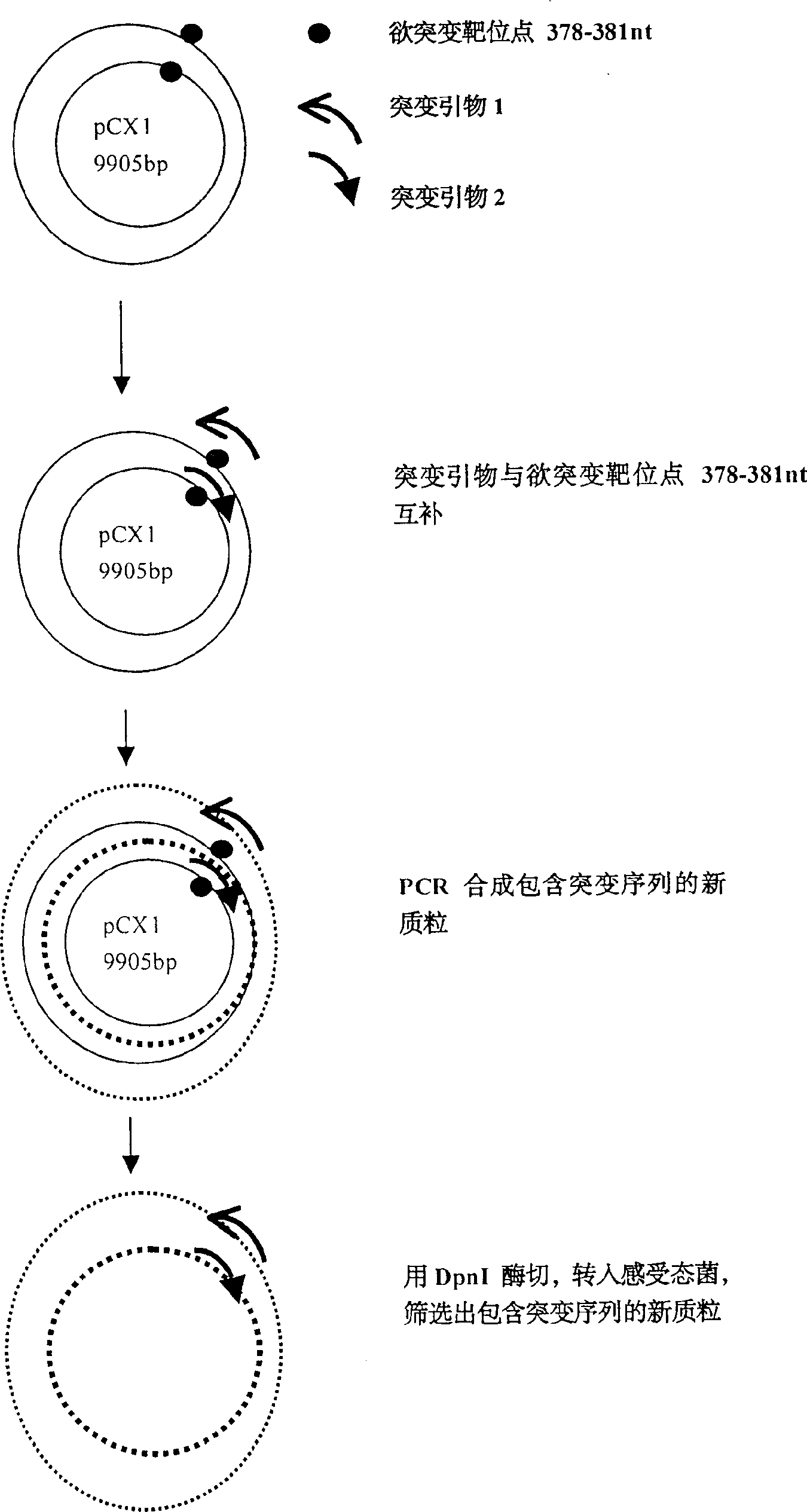
Recombination adenovirus construction body with deletion E1A code sequence and its use
InactiveCN1427075AReliable diagnosisNo significant effect on activityMicrobiological testing/measurementGenetic material ingredientsTumor cellsMutation
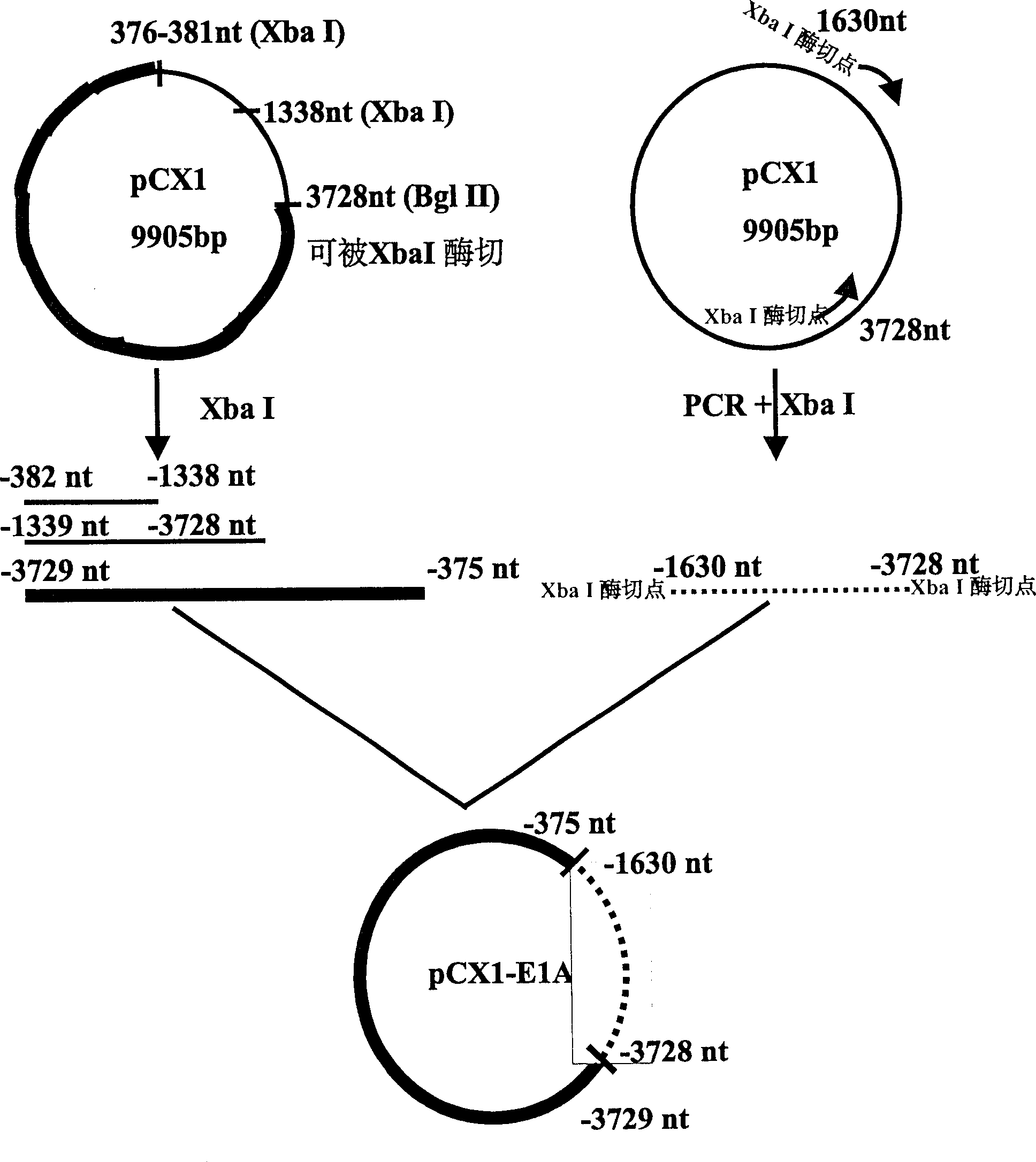
A recombinant adenovirus configurator deleted EIA coding sequence in human adenovirus type 5 genom and its application to diagnosing and treating tumor are disclosed. The site-directed mutation, PCR augmentation, enzyme severing, linking, subcloning, transfection, and single-cloning purification of recombinant adenovirus technologies, and the deleted EIA (382-1630 nt) sequence are used to screen the recombinant adenovirus configurator of being unable to express the EIA function protein. It can specifically kill tumor cells, but not normal cells, and has synergestic action to chemicotherapeutic medicines.
Owner:深圳市天达康基因工程有限公司
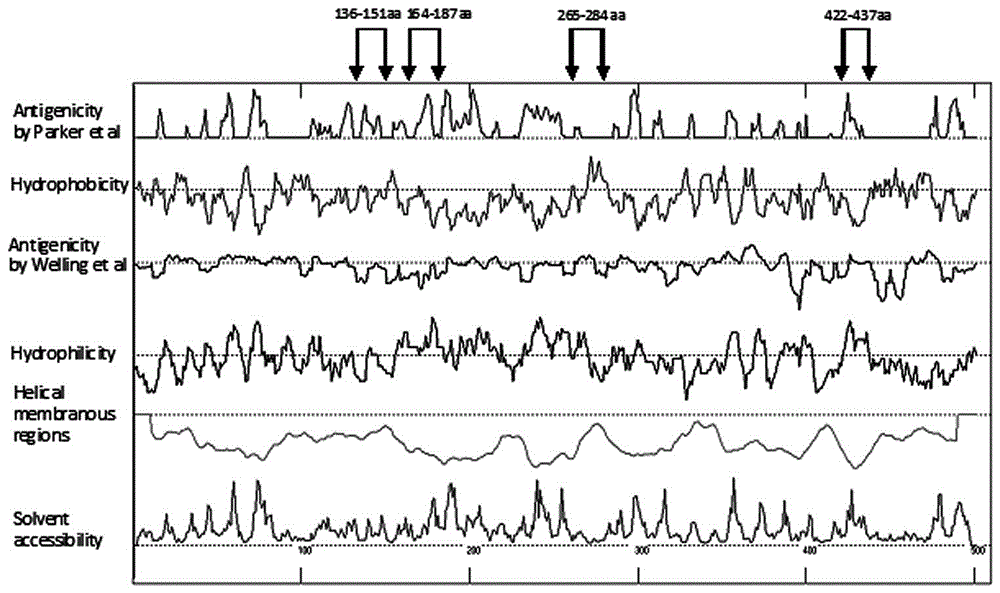
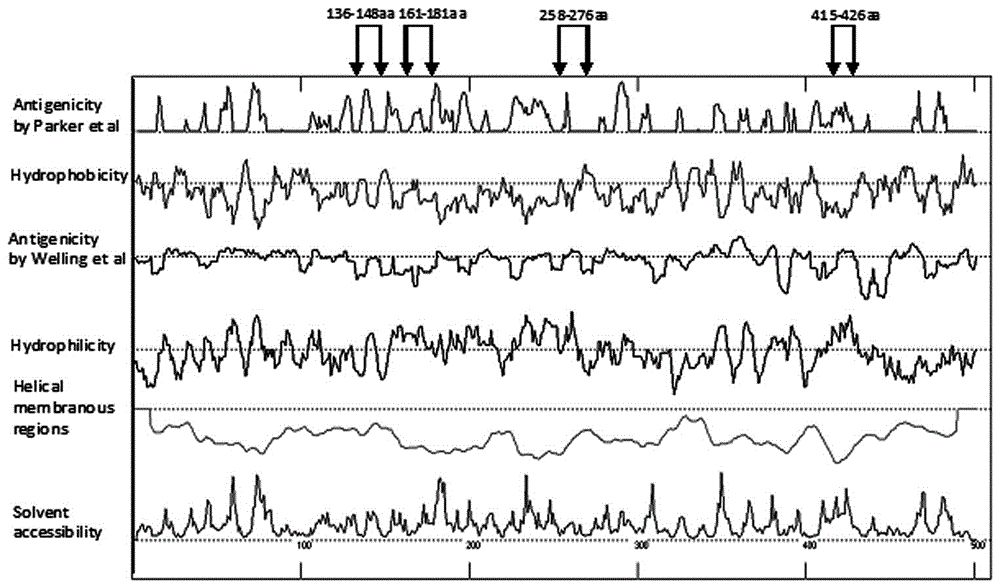
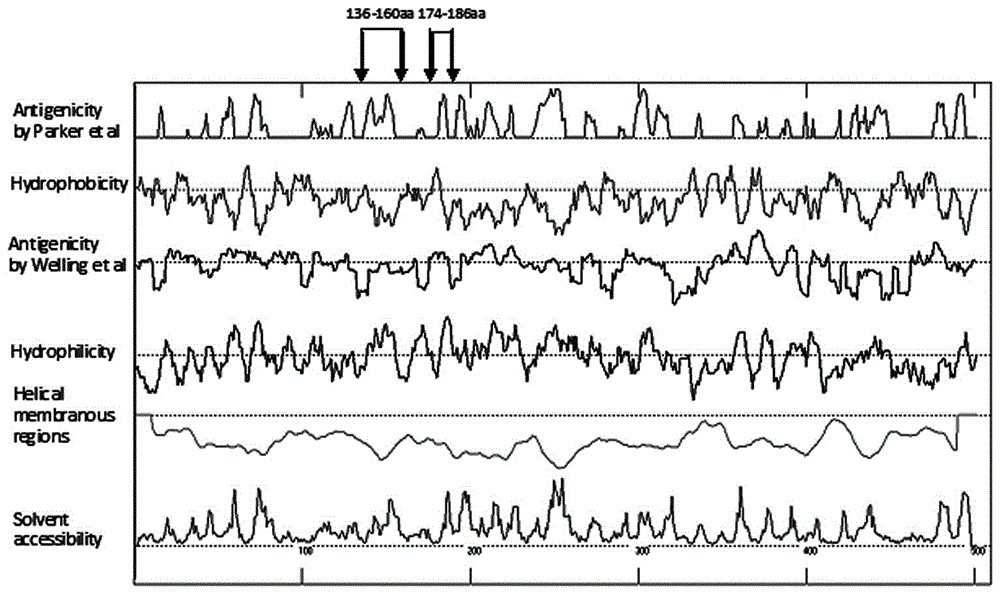
Human adenovirus antigen epitope chimeric protein as well as preparation and application thereof
ActiveCN105820257ALow costEasy to purifyAntibody mimetics/scaffoldsVirus peptidesChemical synthesisNucleotide
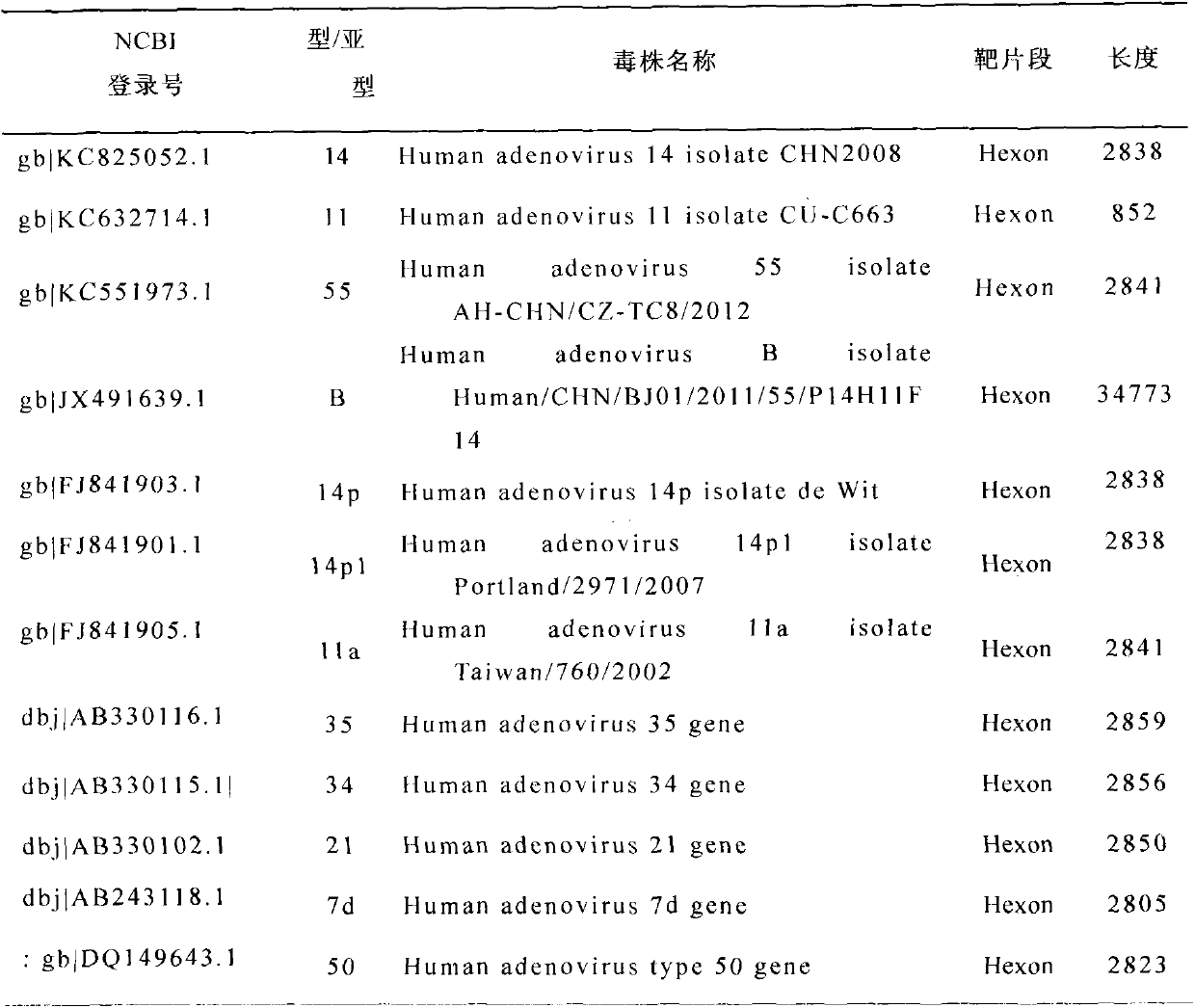
The invention discloses human adenovirus antigen epitope chimeric protein as well as preparation and application thereof, and relates to fields of gene engineering techniques, vaccines and diagnostic reagents. The amino acid sequences of hexon protein of type 3, type 7, type 11, type 14 and type 55 of adenovirus are analyzed through computer analysis, protein fragments with good antigenicity can be screened respectively, the fragments are connected by using two glycine and one serine, then chimeric protein with multiple antigen fragments in serial connection can be formed, pronucleus preferred codons are selected and interpreted into corresponding nucleotide sequences, and full-length genes can be synthesized in a chemical manner. The chimeric protein can be obtained through expression purification by using a gene engineering technique, and the chimeric protein comprises 363 amino acids in whole length. The expressed chimeric protein can be applied to vaccine research, HAdV antibody or antigen detection and the like, and is related to fields such as gene engineering techniques, vaccines and diagnostic reagents.
Owner:中国人民解放军东部战区疾病预防控制中心
Screening tool for antiviral agents
A method is provided for screening anti-adenovirus agents. The method includes reducing the activation of the immune system of a small mammal, administering a human adenovirus vector to the small mammal, monitoring the tumor cells in the mammal, and analyzing infectious virus units within the tumor cells and the organs of the small mammal. Specifically, the immune system of the small mammal is suppressed using cyclophosphamide. The small mammal may be, but is not limited to, one of the following: mice, rabbits, cotton rats, hamsters, rats, and other small rodents.
Owner:SAINT LOUIS UNIVERSITY
Single-chain antibody KGH-R1-ScFv for resisting p21Ras protein and application thereof
The invention provides a single-chain antibody KGH-R1-ScFv for resisting p21Ras protein and application of the single-chain antibody KGH-R1-ScFv, belonging to the field of medical biology, and particularly relates to a recombination human adenovirus vector Ad-hrGFP-ScFv with a single-chain antibody coding gene KGH-R1-ScFv. The single-chain antibody provided by the invention can specifically recognise and perform antagonism to p21Ras proteins with different sources, different tissues and different cell strains in a broad-spectrum way, and also can inhibit the proliferation capacity and invasion capacity of human tumor cell of p21Ras protein with high expression in vitro, reduce the ratio of cells in cell division period, and inhibit the growth of transplantation tumor of human tumor cell of p21Ras protein with high expression in mice.
Owner:杨举伦
Neutralizing monoclonal antibody in human adenovirus 7 and preparation method and application thereof
ActiveCN104086650AEffective displayEnhance immune responseMicroorganism based processesImmunoglobulins against virusesResponse effectInfective disorder
The invention discloses a method for preparing a neutralizing monoclonal antibody in a human adenovirus 7. The hexon fifth hypervariable region of a human adenovirus 3 vector is substituted by a hexon fifth hypervariable region gene of the human adenovirus 7 to construct a chimeric virus which is taken as an immunogen. A murine human adenovirus 7 neutralizing monoclonal antibody and a hybridoma cell strain 5MH5 / 3G5 prepared by using the method are further provided. A humanized and chimeric monoclonal antibody prepared by using the method and a medicinal composition comprising the humanized or human-mouse chimeric monoclonal antibody serving as an effective component are further provided. An exogenous neutralizing epitope is embedded into the hypervariable region of the human adenovirus 3 vector to construct a recombinant adenovirus showing the exogenous neutralizing epitope. By using the recombinant adenovirus as an immunogen, the exogenous neutralizing epitope can be shown effectively, the immune response effect is enhanced, and the research of a monoclonal antibody for treating infectious diseases is facilitated.
Owner:广州瑞发一号健康投资中心(有限合伙)
Recombinant human adenovirus 3, and preparation method and application thereof
InactiveCN103966263ARetain major antigenic activityAvoid infectionMicroorganism based processesFermentationEnterovirus 71Genome
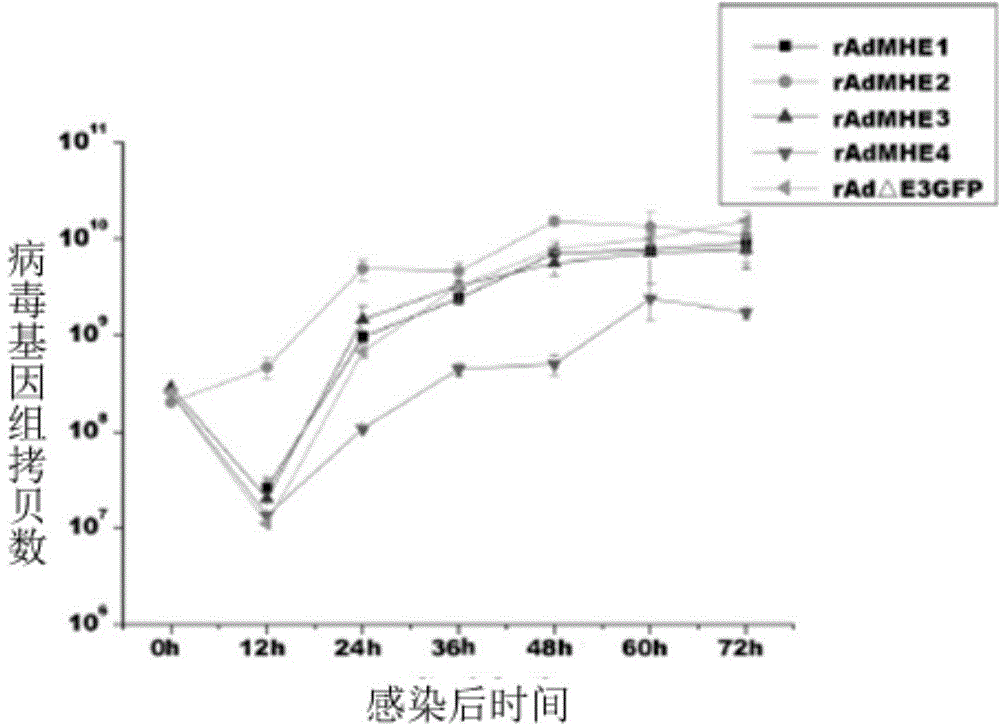
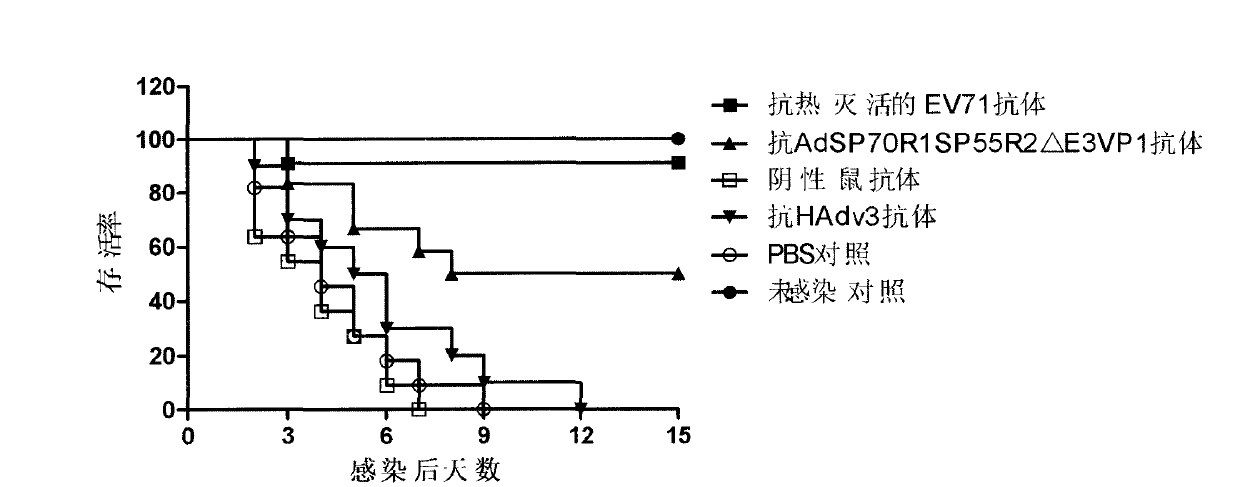
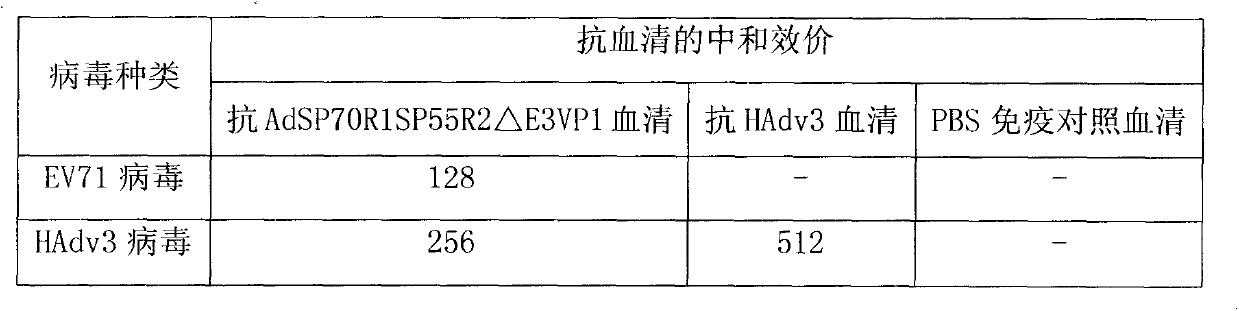
The invention discloses a novel enterovirus 71-recombinant human adenovirus 3 vaccine candidate strain with human adenovirus 3 (HAdv3) as a carrier, and a preparation method thereof. Two EV71 neutralizing epitopes are embedded to the hexon of the human adenovirus 3, and the VP1 protein cassette of EV71 is inserted to the genome E3 region of the human adenovirus 3. The vaccine candidate strain can induce a strong anti-EV71 infection and anti-HAdv3 infection immunization reaction, and can be used for making bivalent vaccines for preventing the EV71 infection and the HAdv3 infection.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV (GUANGZHOU RESPIRATORY CENT) +1
Rapid detection kit for human adenoviruses
InactiveCN104131006AKeep abreast ofIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesPositive controlAdenovirus DNA
The invention belongs to the technical field of virus detection and provides an extracting solution for extracting human adenovirus DNA (deoxyribonucleic acid), a PCR (polymerase chain reaction) reaction solution for detecting human adenoviruses, a PCR primer sequence for detecting human adenovirus DNA, a rapid detection kit for human adenoviruses and a method for quantitatively detecting the quantity of human adenoviruses. The rapid detection kit for human adenoviruses comprises the extracting solution for extracting human adenovirus DNA, the PCR reaction solution for detecting human adenoviruses and the PCR primer sequence for detecting human adenovirus DNA, and further comprises an adenovirus positive control and an amplification primer sequence for detecting the positive control. The adenovirus detection sensitivity of the kit disclosed by the invention can reach 500copies / ml. The quantitative linear range is 500-5.0E+08copies / ml, so that the kit is suitable for detecting the adenoviruses in a trace sample and provides a reliable basis for early diagram of adenovirus infection.
Owner:NANJING AGRICULTURAL UNIVERSITY
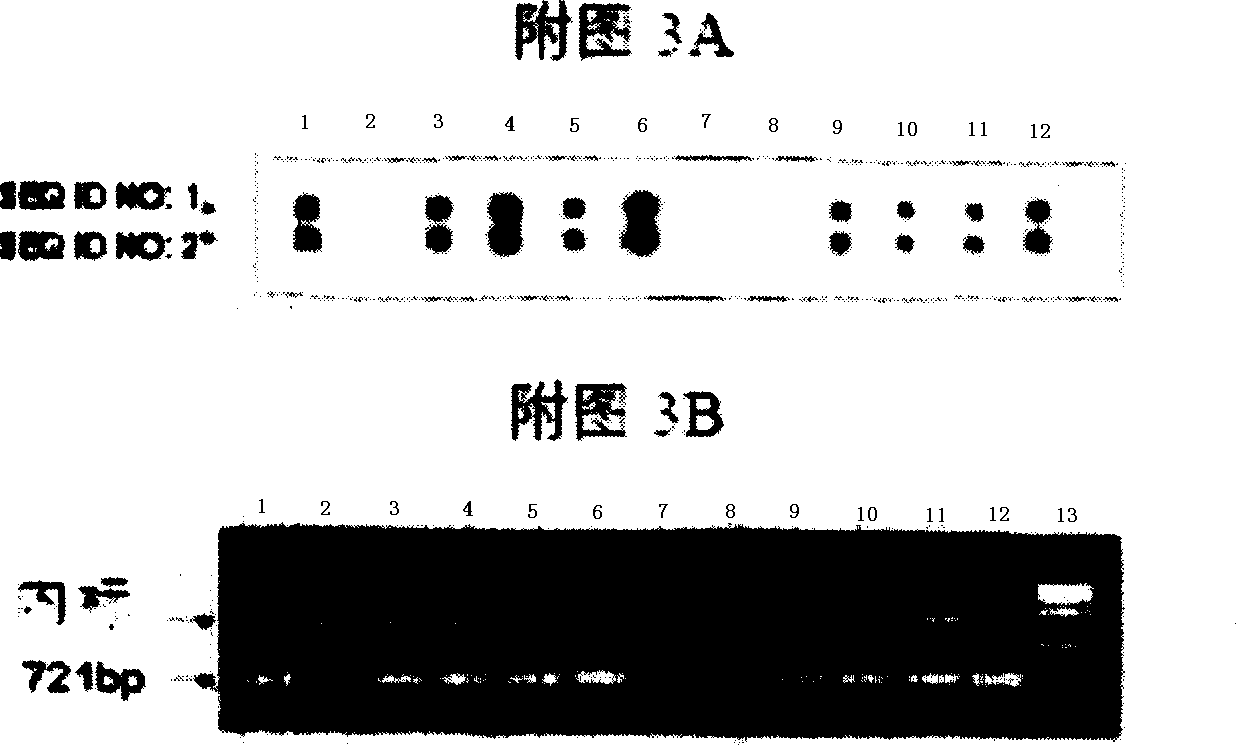
Neutralizing epitope of human adenovirus type 3 (HAdV-3) and type 7 (HAdV-7) and application thereof
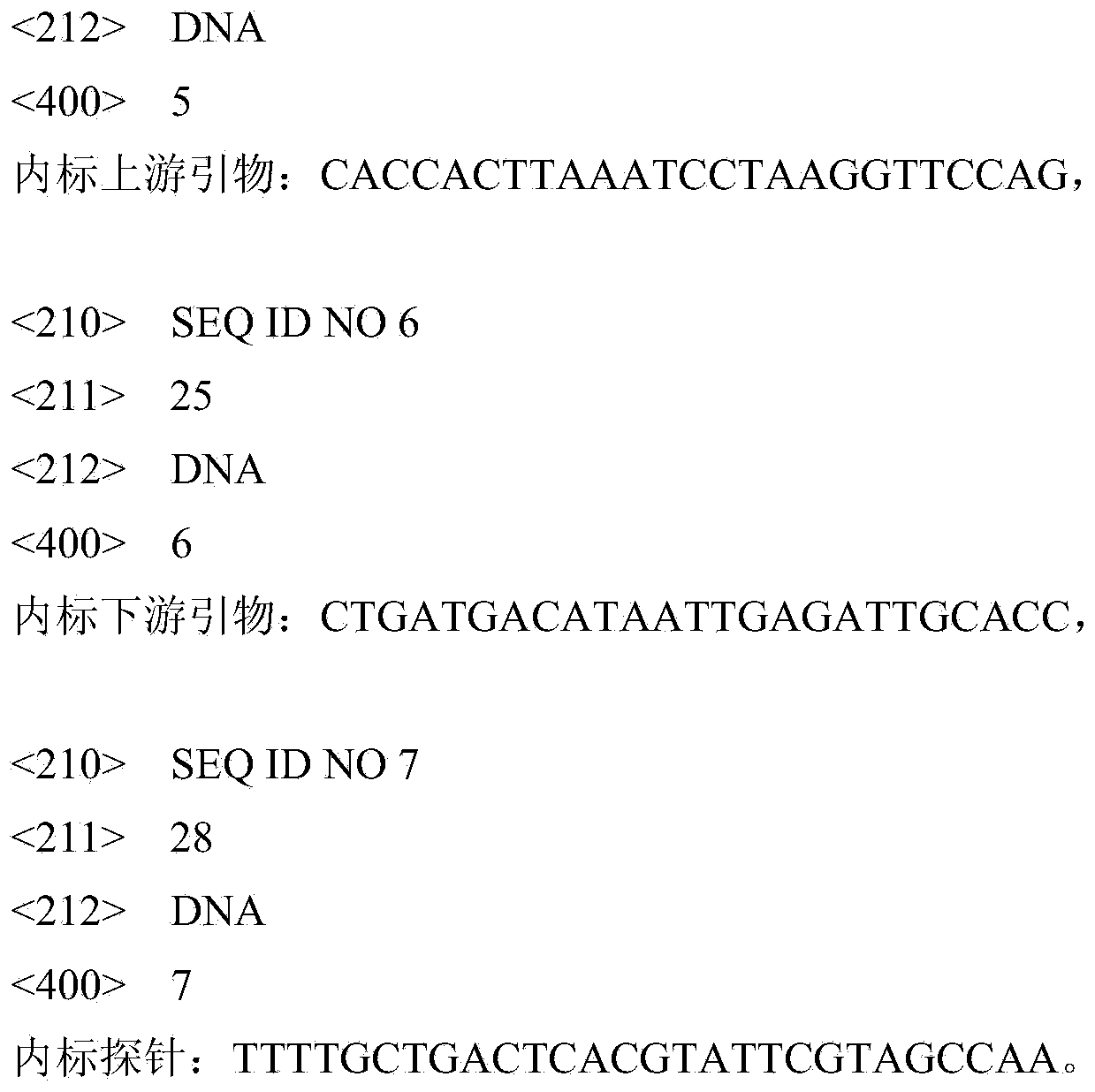
InactiveCN101942011AStimulate immune responseEasily exposedImmunoglobulins against virusesAntiviralsProtein targetNucleotide
The invention discloses three neutralizing epitope of human adenovirus type 3 (HAdV-3) and type 7 (HAdV-7) and an application thereof. The amino acid sequence of the neutralizing epitope of the HAdV-3 and HAdV-7 is selected from the amino acid sequence shown in SEQ ID NO: 1, 2, 3, and the nucleotide sequence of the neutralizing epitope of HAdV-3 and HAdV-7 is selected from the nucleotide sequence shown in the SEQ ID NO: 4, 5, 6. The neutralizing epitopes of HAdV-3 and HAdV-7 can be used for preparing vaccines or antibody and antigen binding fragments for preventing infection of human adenovirus type 3 and human adenovirus type 7, and the antibody and the antigen binding fragments can be used for preparing drugs for preventing or curing adenovirus infection. The drugs contain the antibody or antigen binding fragments. The invention also provides a method for preventing and curing adenovirus infection, i.e. the vaccines or drugs with immune effective quantity are applied. The three neutralized epitopes of HAdV-3 and HAdV-7 can be served as target protein for developing a diagnostic kit.
Owner:STATE KEY LAB OF RESPIRATORY DISEASE
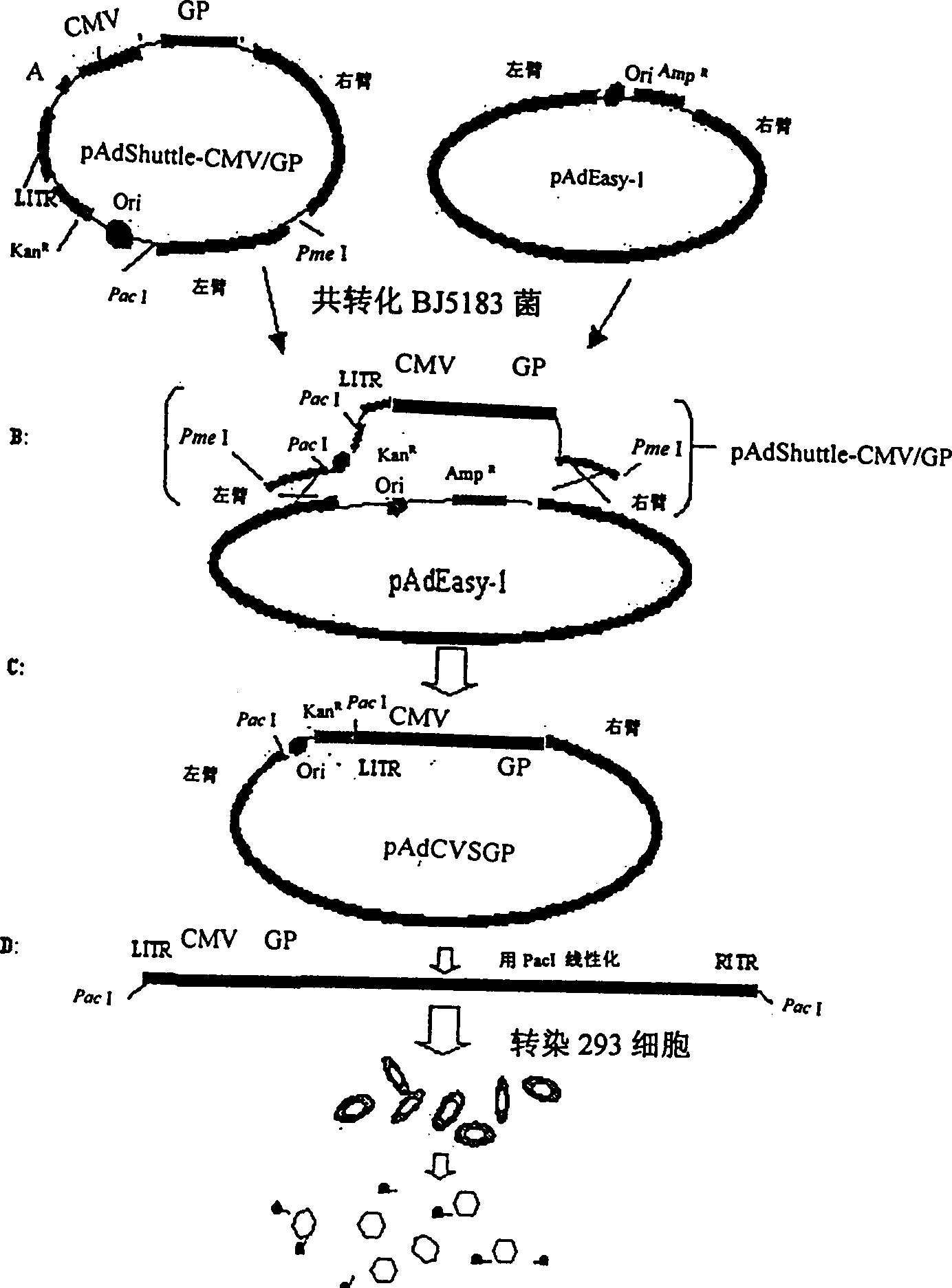
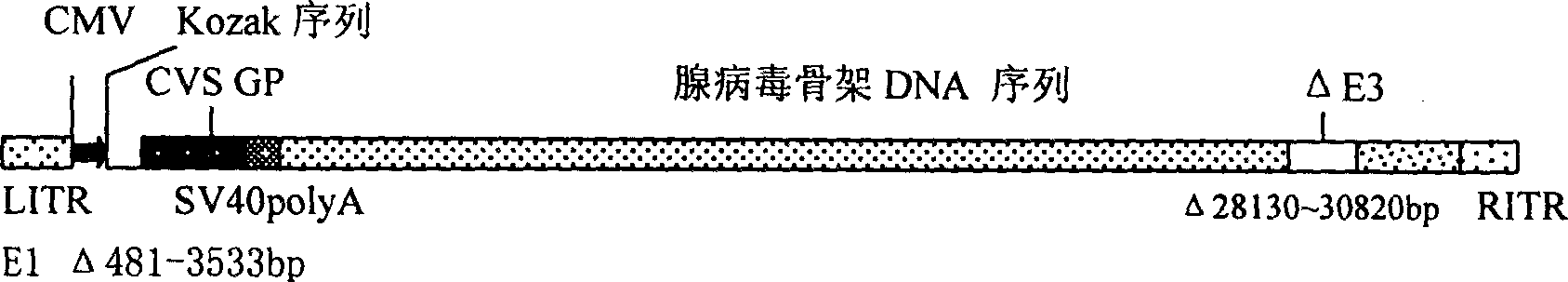
Genetically engineered rabies virus vaccine with recombination-defective adenovirus carrier
A genetically engineered rabies virus vaccine with the E1 and E3 deficient recombinant defective human adenovirus as carrier is disclosed. The rabies virus coated glucoprotein encoding gene and the element to regulate it for efficient expression are carried in the E1 region. The said encoding gene comes from the rabies virus CVS-N2C with strong neurovirulence.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
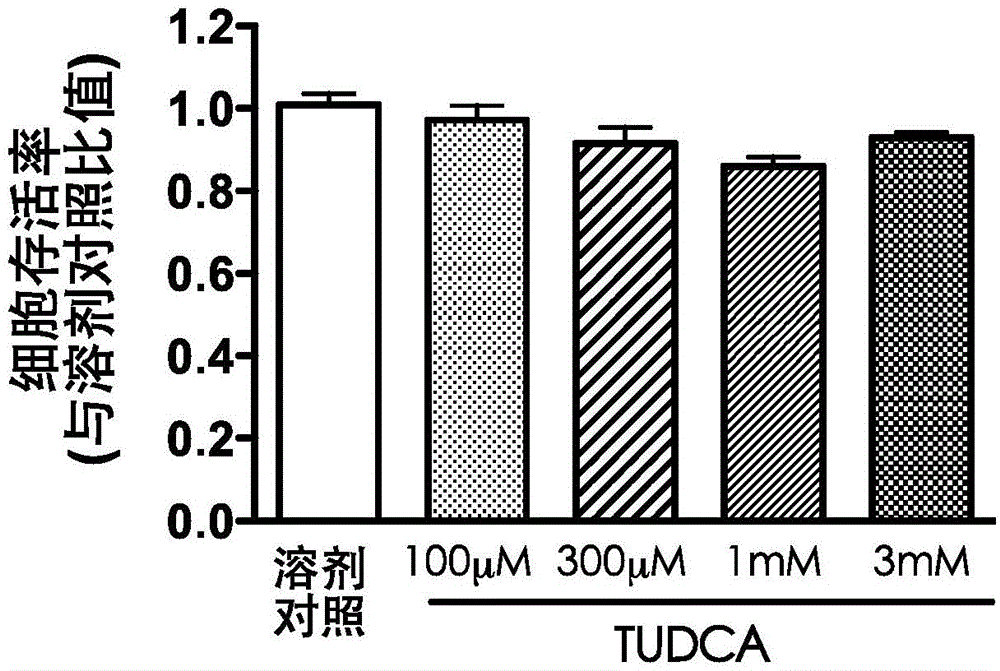
New uses of tauroursodeoxycholic acid
InactiveCN106031731AEffective in preventing pulmonary fibrosisOrganic active ingredientsAntiviralsBiomedicinePulmonary fibrosis
The present invention relates to the technical field of biomedicine, and mainly provides new uses of tauroursodeoxycholic acid, wherein the new uses comprise that the tauroursodeoxycholic acid inhibits the virus-entry-host cells adopting endocytosis so as to achieve prevention and treatment of virus infection, anti-injury, and anti-fibrosis. The present invention provides applications of the tauroursodeoxycholic acid in preparation of drugs for treatment of acute lung injury and pulmonary fibrosis, such that the application range of the tauroursodeoxycholic acid is broadened, and the new drug selection is provided for the virus infection patients. According to the present invention, it is confirmed that the tauroursodeoxycholic acid can effectively prevent and treat H5N1 avian influenza virus, human adenovirus and Zaire-type Ebola virus, and it is cleared that the pulmonary fibrosis prevention and control effect of the tauroursodeoxycholic acid is superior to the pulmonary fibrosis prevention and control effect of the chloroquine.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Antiviral Activity of Bovine Type III Interferon Against Foot-and-Mouth Disease Virus
ActiveUS20120164171A1Reduce degreeReduce rateOrganic active ingredientsSugar derivativesVaccinationInterferon alpha
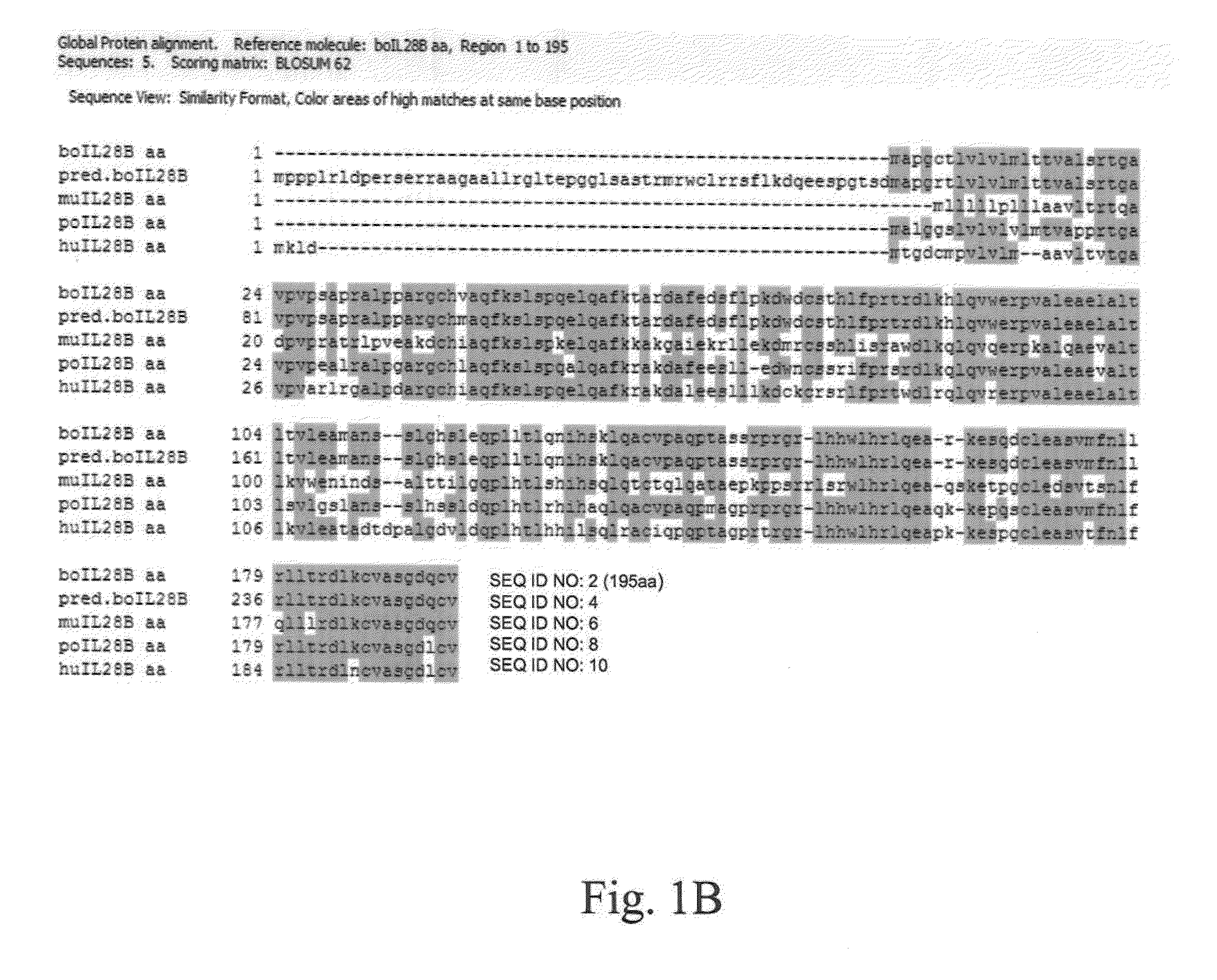
Interferons are the first line of defense against viral infections and administration of interferons as biotherapeutics has been demonstrated to be effective in controlling several viral infections. Here we report for the first time the identification and characterization of a member of the bovine type III IFN family, boIFN-λ3. We have expressed boIFN-λ3 using a recombinant replication defective human adenovirus type 5 (Ad5) and demonstrated antiviral activity against foot-and-mouth disease virus (FMDV) and vesicular stomatitis virus (VSV) in bovine cells in vitro. Furthermore, we have tested the efficacy of boIFN-λ3 against FMDV in vivo by inoculation of cattle with Ad5-boIFN-λ3 followed by intradermolingual or aerosol virus challenge. Our results demonstrate that the type III IFN family is conserved in bovines and that treatment of cattle with boIFN-λ3 alone or in combination with IFN-α is able to confer delayed and reduced severity of FMD. Furthermore inoculation with Ad5-boIFN-λ3 alone conferred full protection against aerosol challenge for at least 7 days after administration suggesting that type III IFN used in combination with FMD vaccines could fill one of the current gaps in emergency vaccination against FMDV.
Owner:US SEC AGRI
Enhanced Antiviral Activity Against Foot and Mouth Disease
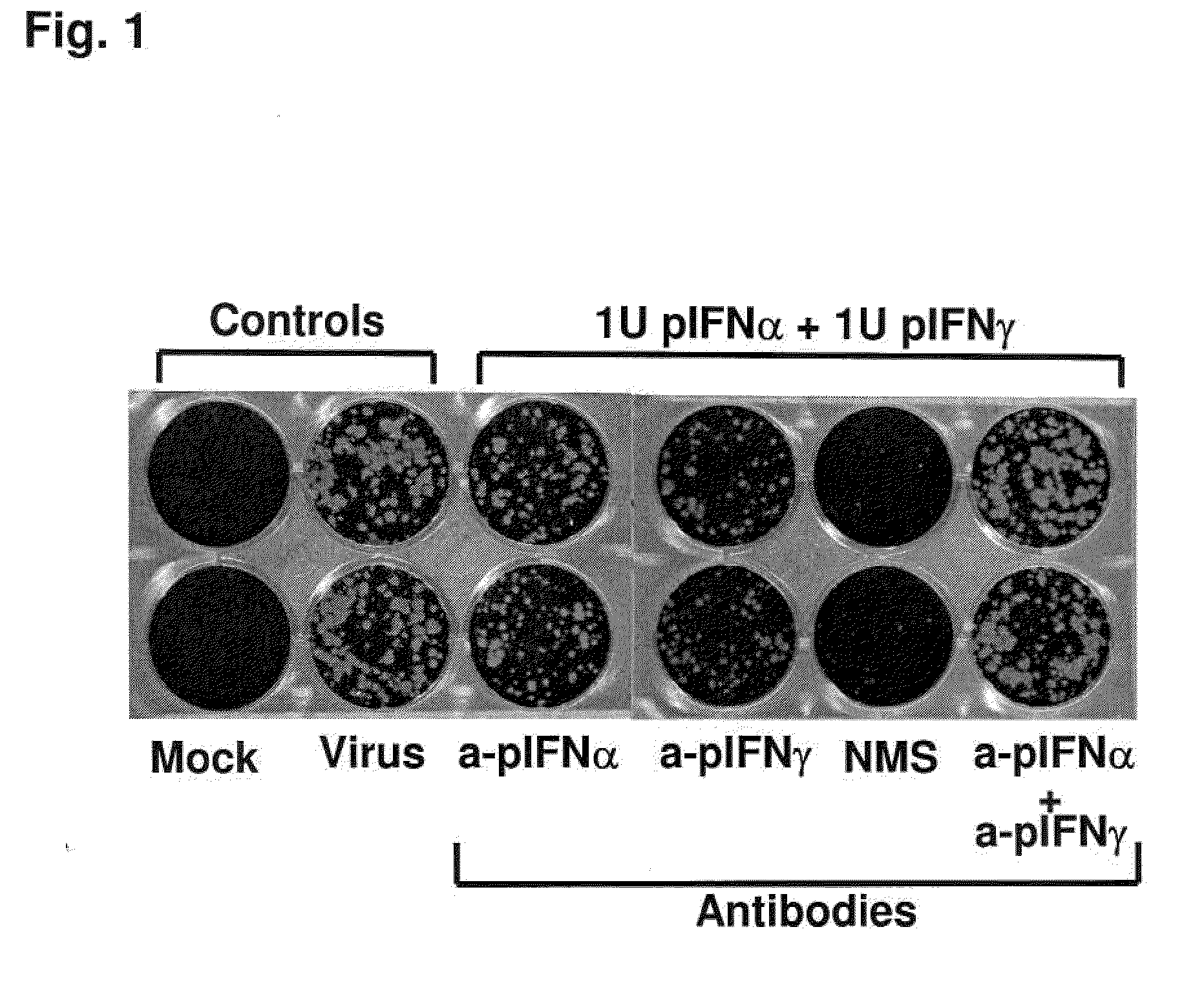
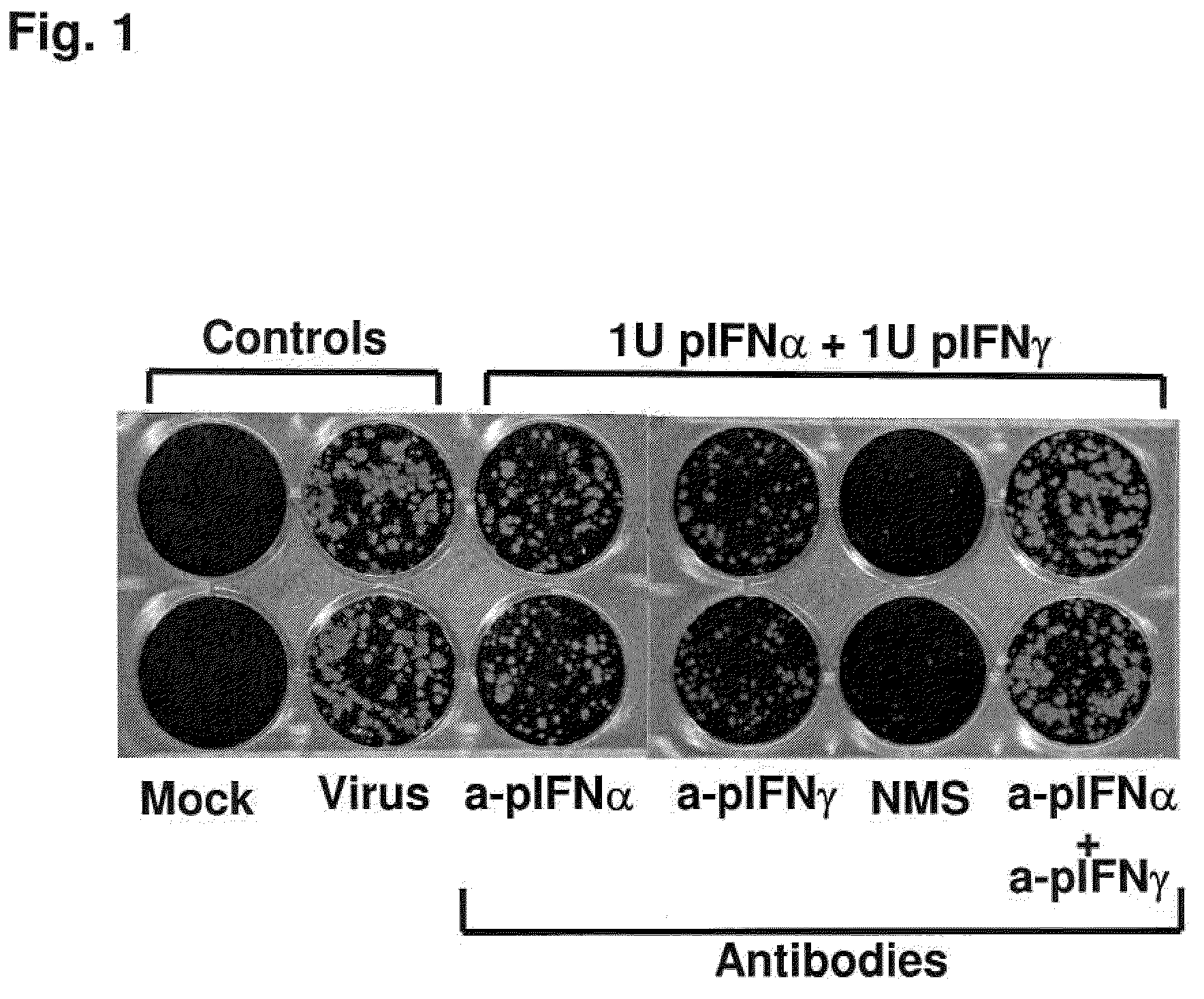
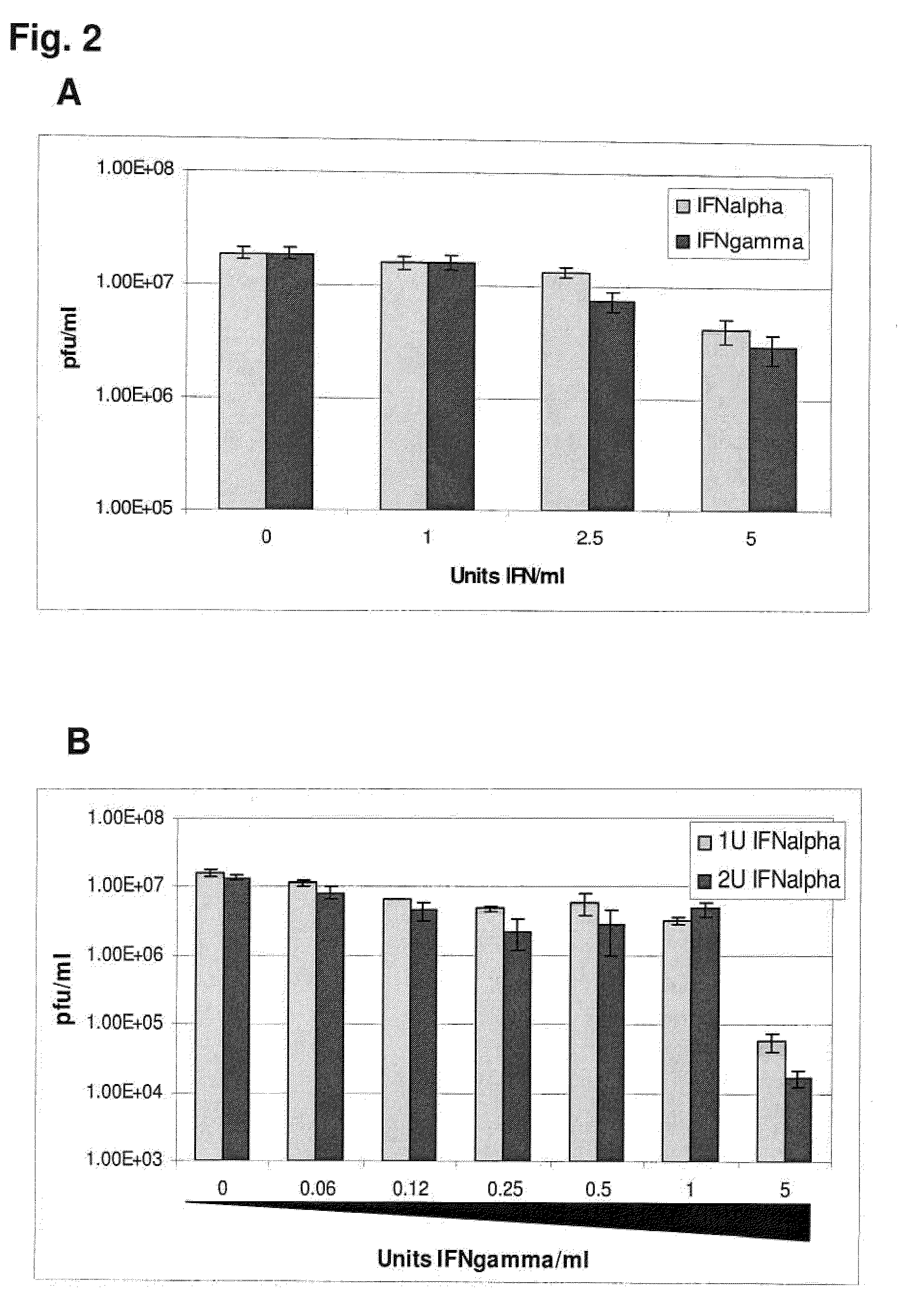
InactiveUS20090269372A1Effectively ensureEarly protection against FMDVSsRNA viruses positive-sensePeptide/protein ingredientsHigh dosesIn vivo
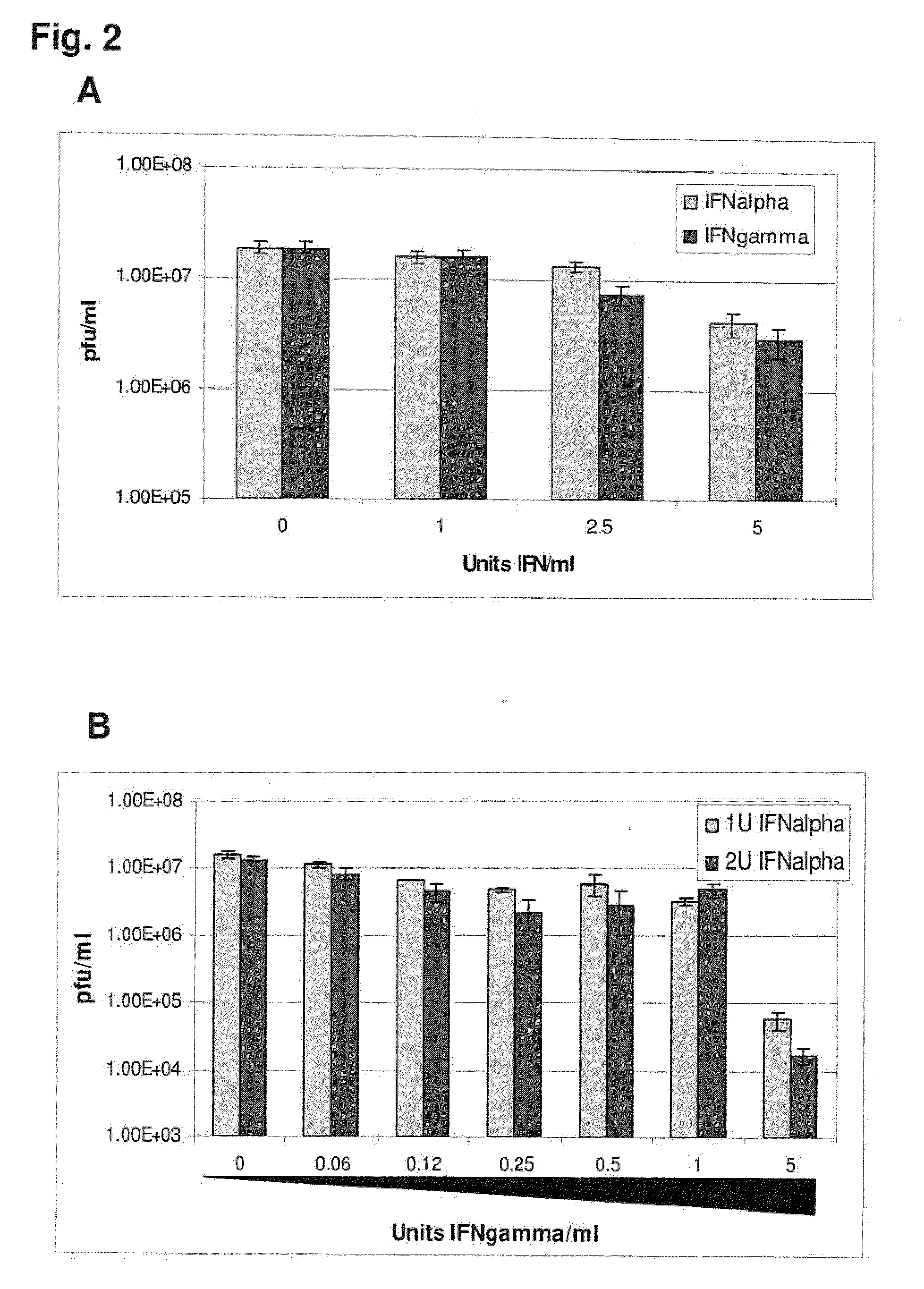
Previously, we showed that type I interferon (alpha / beta interferon [IFN-α / β]) can inhibit foot-and-mouth disease virus (FMDV) replication in cell culture, and swine inoculated with 109 PFU of human adenovirus type 5 expressing porcine IFN-α (Ad5-pIFN-α) were protected when challenged 1 day later. In this study, we found that type II pIFN (pIFN-γ) also has antiviral activity against FMDV in cell culture and that, in combination with pIFN-α, it has a synergistic antiviral effect. We also observed that while each IFN alone induced a number of IFN-stimulated genes (ISGs), the combination resulted in a synergistic induction of some ISGs. To extend these studies to susceptible animals, we inoculated groups of swine with a control Ad5, 108 PFU of Ad5-pIFN-α, low- or high-dose Ad5-pIFN-γ, or a combination of Ad5-pIFN-α and low- or high-dose Ad5-pIFN-γ and challenged all groups with FMDV 1 day later. The control group and the groups inoculated with either Ad5-pIFN-α or a low dose of Ad5-pIFN-γ developed clinical disease and viremia. However, the group that received the combination of both Ad5-IFNs with the low dose of Ad5-pIFN-γ was completely protected from challenge and had no viremia. Similarly the groups inoculated with the combination of Ad5s with the higher dose of Ad5-pIFN-γ or with only high-dose Ad5-pIFN-γ were protected. The protected animals did not develop antibodies against viral nonstructural (NS) proteins, while all infected animals were NS protein seropositive. No antiviral activity or significant levels of IFNs were detected in the protected groups, but there was an induction of some ISGs. The results indicate that the combination of type I and II IFNs act synergistically to inhibit FMDV replication in vitro and in vivo.
Owner:UNITED STATES OF AMERICA
Vaccine against rsv
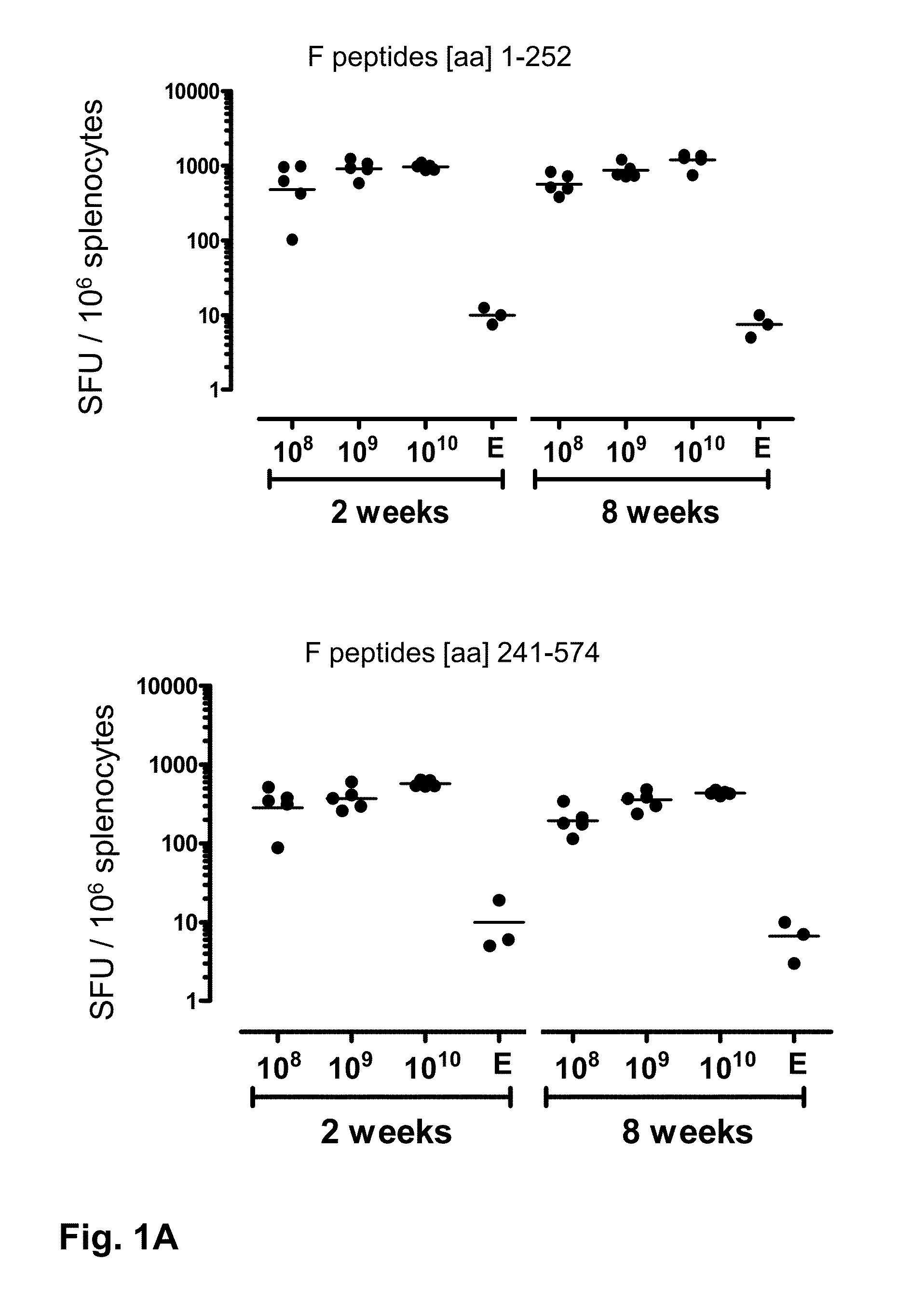
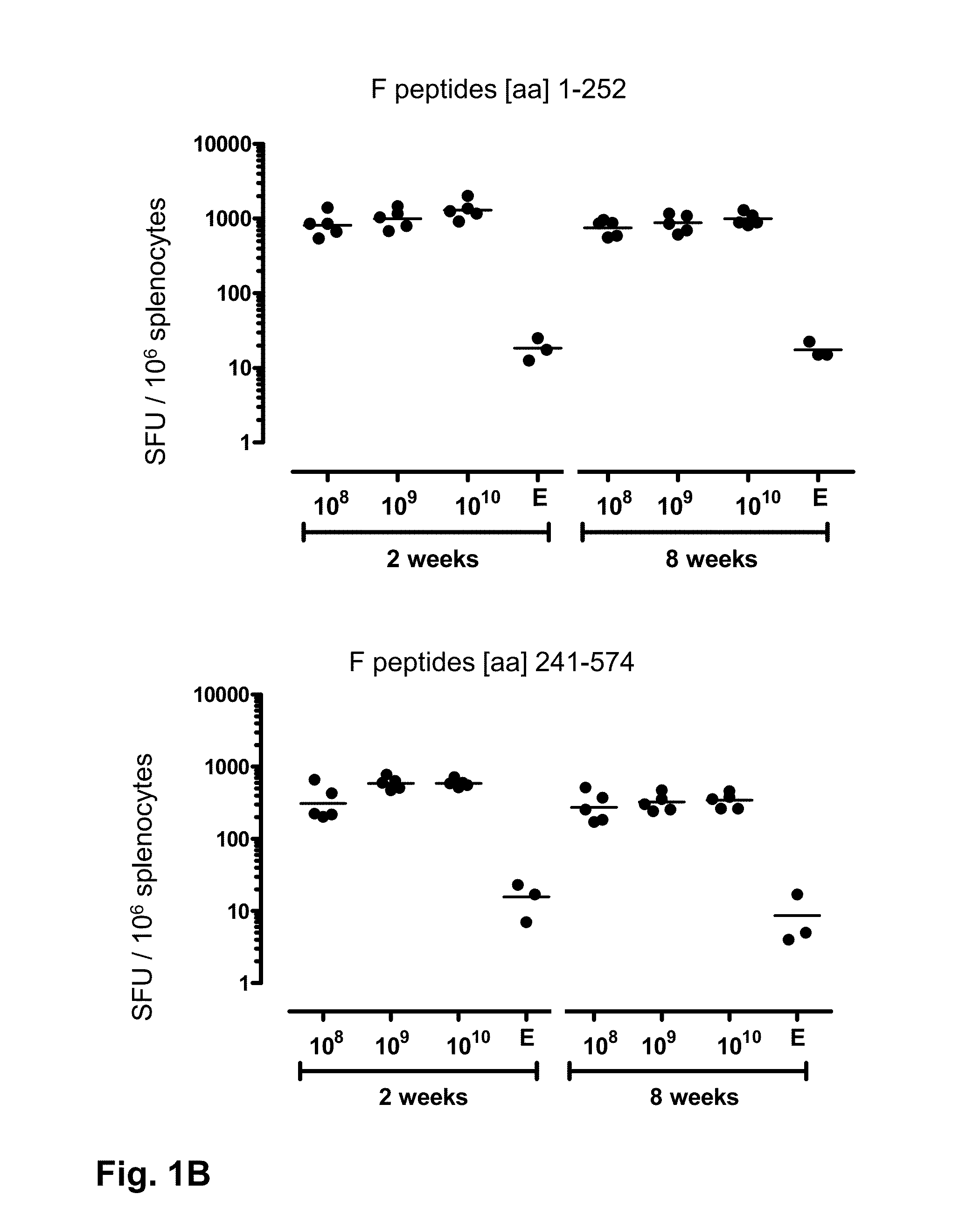
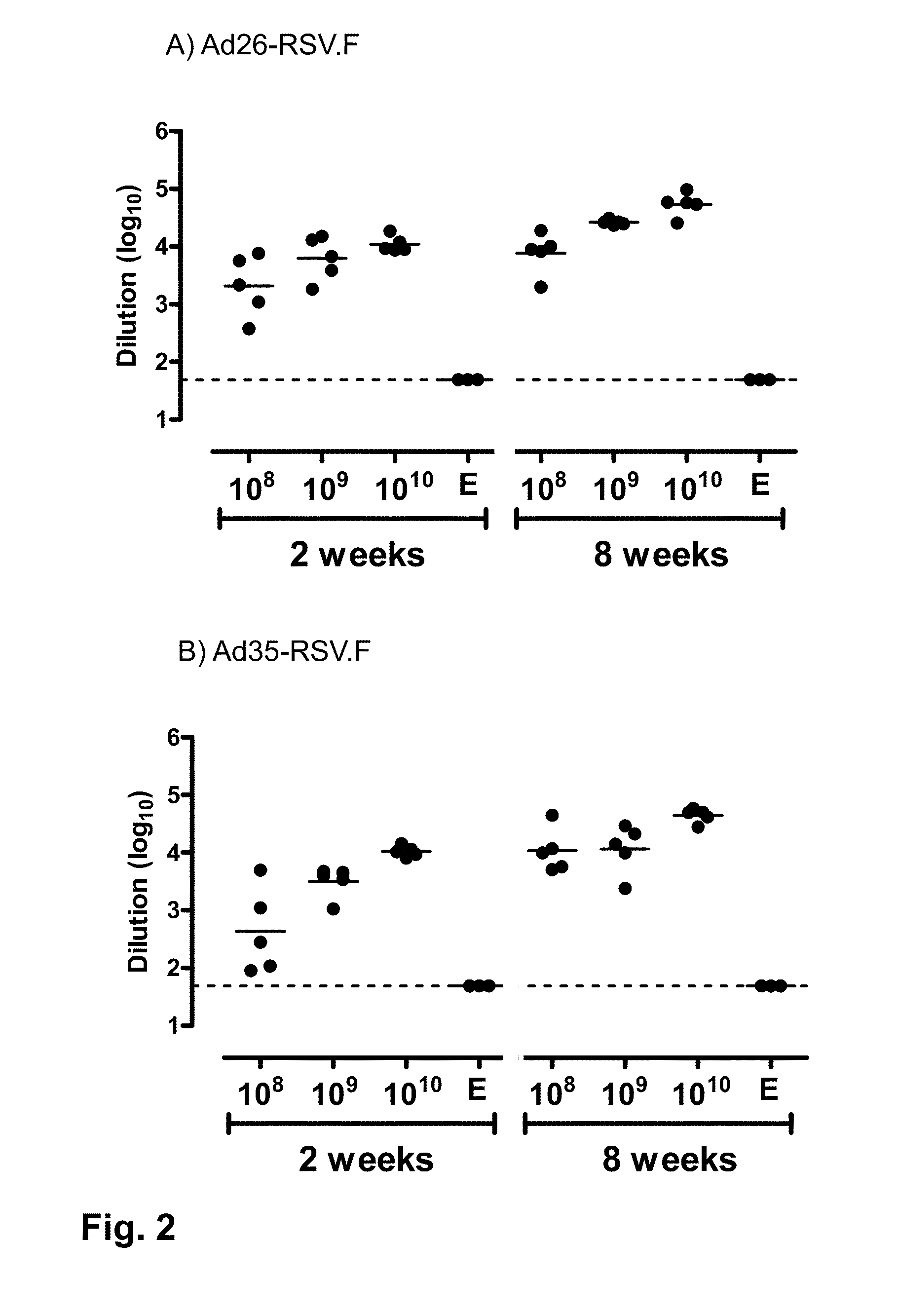
ActiveUS20140147463A1Good curative effectEffective vaccineSsRNA viruses negative-senseSugar derivativesF proteinSerotype
Provided is a vaccine against respiratory syncytial virus (RSV), comprising a recombinant human adenovirus of serotype 26 that comprises nucleic acid encoding a RSV F protein or immunologically active part thereof.
Owner:JANSSEN VACCINES & PREVENTION BV
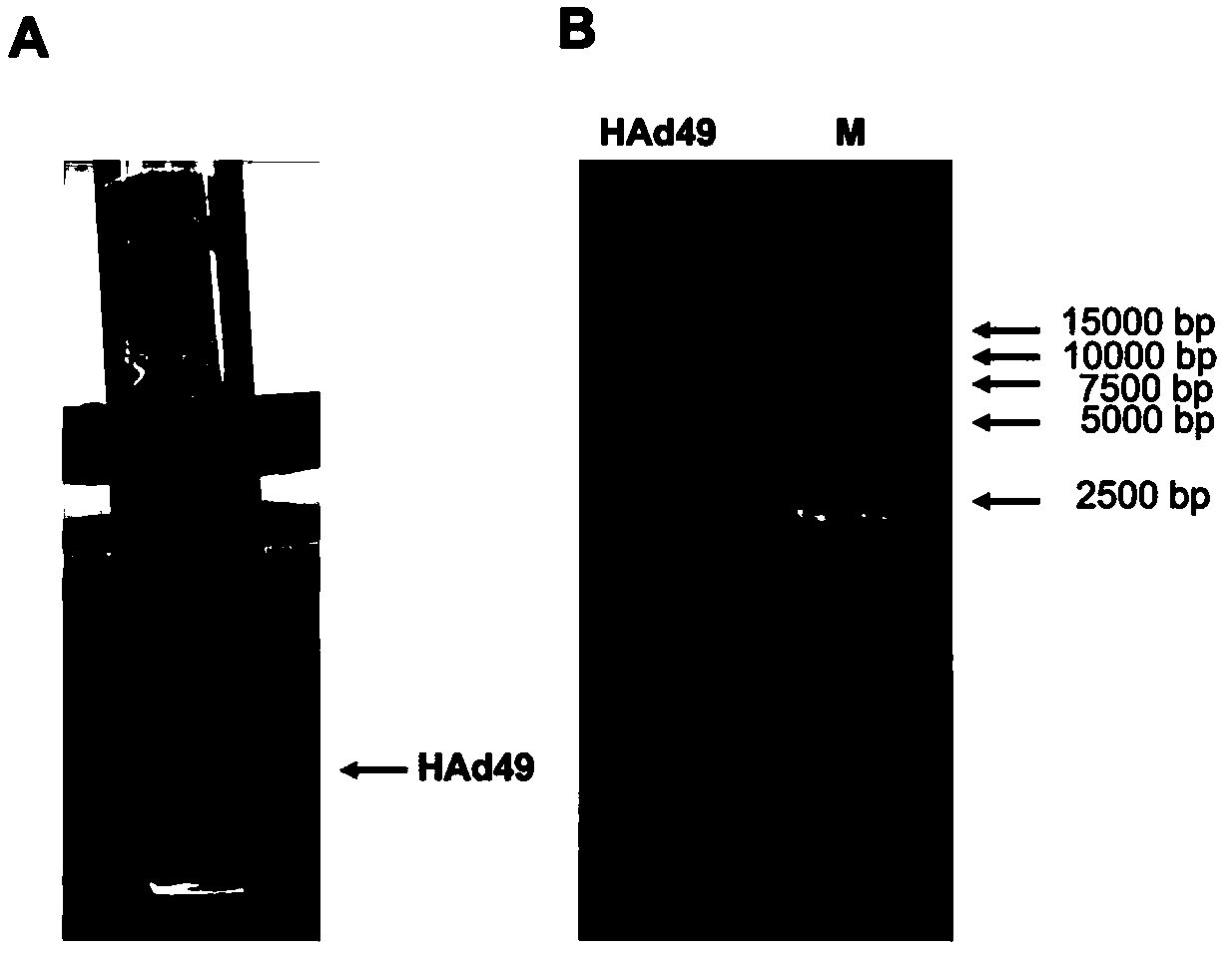
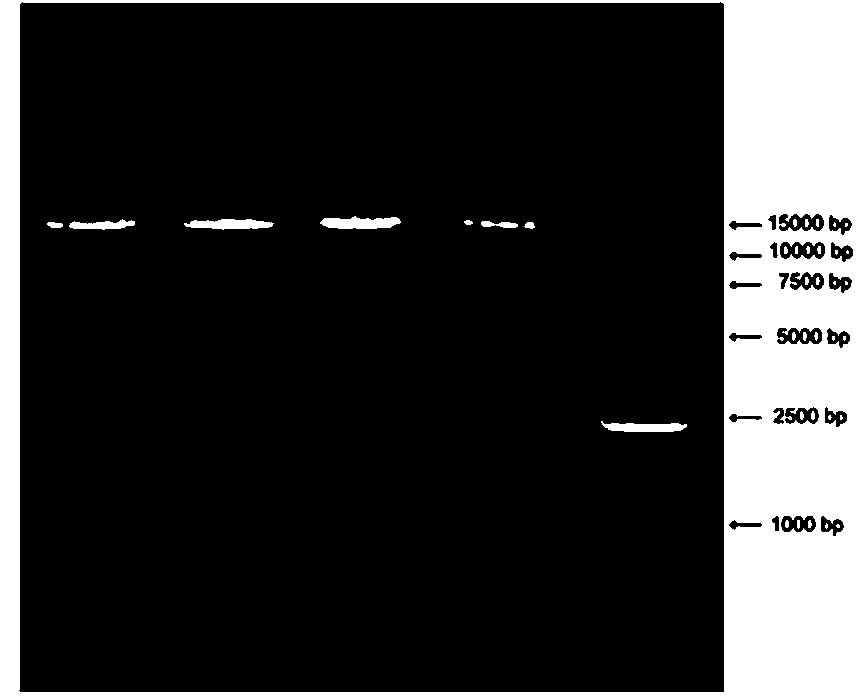
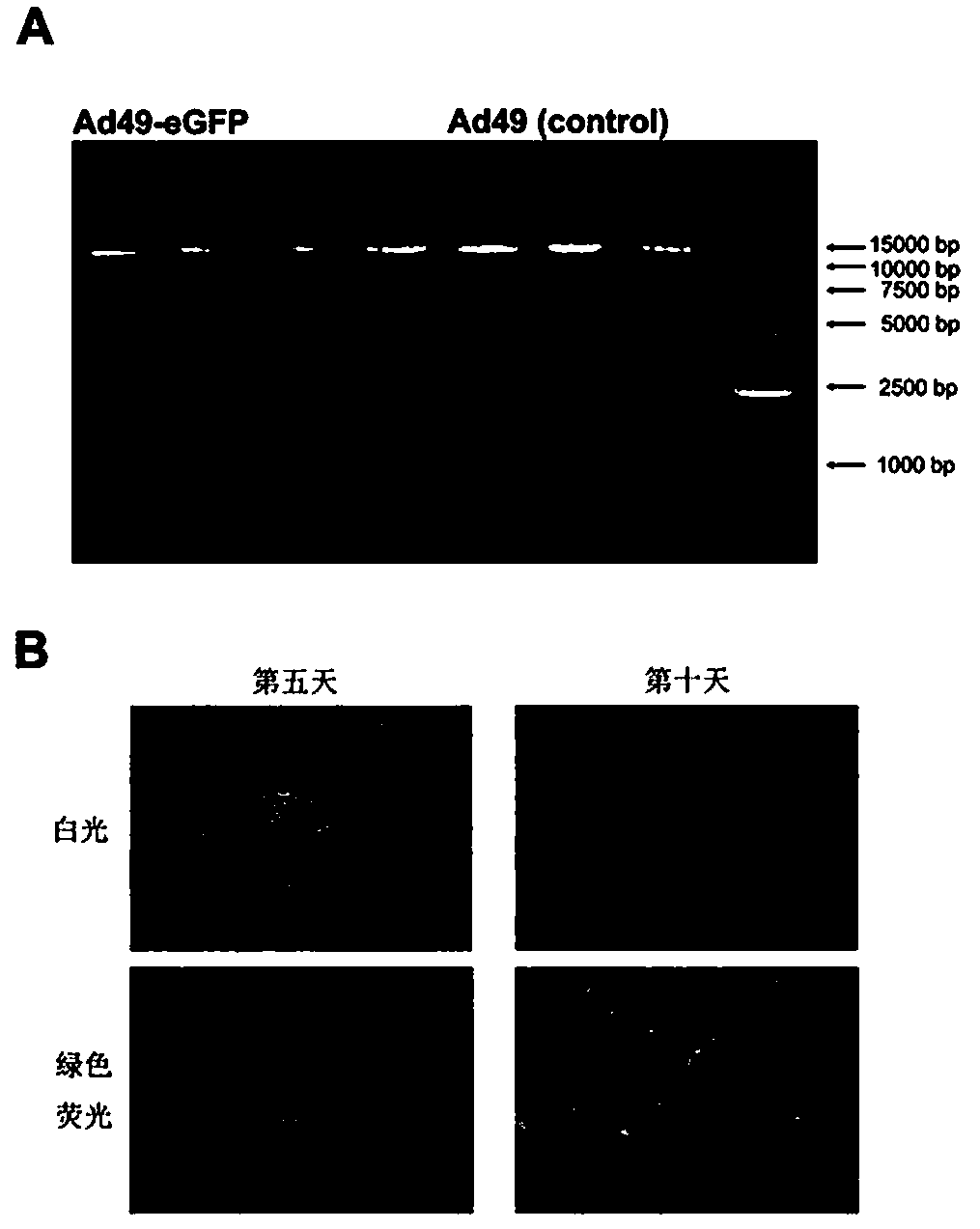
Recombinant adenovirus expression vector based on adenovirus HAd49 and construction method thereof
InactiveCN110564768AHigh titerIncrease success rateSsRNA viruses positive-senseViral antigen ingredientsAntigenSerotype
The invention discloses a recombinant adenovirus expression vector based on human rare serotype adenovirus HAd49 and a construction method of the recombinant adenovirus expression vector. According tothe invention, a genome of wild adenovirus HAd49 is used as a basis, through a direct cloning method by genome, an E1 coding region and an E3 coding region are deleted, an I-CeuI enzyme cutting siteand a PI-SceI enzyme cutting site are inserted into an E1 deletion region, meanwhile, the E4orf6 of the human adenovirus Ad5 is used for replacing the E4orf6 of the HAd49, so that the success rate ofpackaging the recombinant virus in a human kidney embryo cell (HEK293) and the titer of the packaged recombinant virus are improved, and the recombinant adenovirus expression vector Ad49 with replication defects is obtained. The expression vector can be used for expressing various antigens, and provides a basis for research and development of vaccines and biological medicines.
Owner:广州佰芮慷生物科技有限公司
Human adenovirus detection kit
ActiveCN103725800AStrong specificityEasy to operateMicrobiological testing/measurementMicroorganism based processesPotassiumBiology
The invention provides a human adenovirus (HAdV) detection kit. The kit comprises a nucleic releaser and PCR (polymerase chain reaction) liquid, wherein the nucleic releaser contains 0.01mM / L to 0.5mM / L of surfactin, 20mM / L to 300mM / L of potassium chloride, 0.01 to 2 percent of sodium dodecyl sulfate and 0.05 to 1 percent of ethanol; the PCR reaction liquid contains primers for HAdV detection and a probe sequence, wherein the HAdV upstream primer is AGTGTAACATGACCAAAGACTGGTTC, the HAdV downstream primer is AAGAAGGAGTACATGCGRTCCTT, and the HAdV probe is ACTACAACATTGGCTACCAGGGCTTCTA. The sensitivity of the kit for detecting the HAdV can reach 400copies / ml, and the quantitative linear range is 400 to 4.00 E+09copies / ml; by utilizing the kit, the HAdV-DNA (deoxyribonucleic acid) in unknown samples such as sputum and throat swab can be rapidly and accurately detected, and reliable experimental reference can be provided for the early diagnosis of the infection of the HAdV.
Owner:SANSURE BIOTECH
LAMP (loop-mediated isothermal amplification) primer combination for detecting six respiratory viruses, and application thereof
ActiveCN106884012AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationInfluenza A virus subtype H3N2Avian influenza virus
The invention discloses LAMP (loop-mediated isothermal amplification) primer combination for detecting six respiratory viruses, and the application thereof. The primer combination provided by the invention consists of 36 DNA molecules as shown in the sequence 1 to the sequence 36. The primer combination can be used for detecting whether a to-be-detected virus is influenza A virus subtype H1N1, influenza A virus subtype H3N2, avian influenza virus subtype H5N1, avian influenza virus subtype H7N9, influenza B virus or human adenovirus, and can be used for detecting whether a to-be-detected sample contains influenza A virus subtype H1N1 and / or influenza A virus subtype H3N2 and / or avian influenza virus subtype H5N1 and / or avian influenza virus subtype H7N9 and / or influenza B virus and / or human adenovirus. The primer combination provided by the invention is used for detecting six respiratory viruses, has high specificity and high sensitivity, and can realize simple, convenient, rapid and accurate detection. A great promotional value is achieved.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
Adenovirus bivalent vaccine
InactiveCN111166875AImprove securityStrong replicabilityViral antigen ingredientsAntiviralsViral typeTGE VACCINE
The invention discloses an adenovirus bivalent vaccine. The vaccine comprises a replication-deficient human adenovirus type 4 and a replication-deficient human adenovirus type 7. E1 and E3 genes of the replication-deficient human adenovirus type 4 and the replication-deficient human adenovirus type 7 are deleted, and partial coding frames of the E4 gene are replaced by corresponding coding framesof E4 gene of human adenovirus type 5. The vaccine can effectively stimulate an organism to generate a humoral immune response and a cellular immune response, generate specific neutralizing antibodieswith high titer, and is used for preventing infection of pathogens.
Owner:GUANGZHOU N BIOMED LTD
Multiple fluorescent PCR method and kit for simultaneous detection of human adenovirus, human mycoplasma pneumonia and bocavirus
The invention belongs to the field of biological technical application, and relates to a multiple fluorescent PCR method and a kit for simultaneous detection of human adenovirus, human mycoplasma pneumonia and bocavirus and containing internal quality control. The invention designs specific primers and probes aiming at conserved sequences of human adenovirus HP (hexonprotein) gene, human mycoplasma pneumonia RP gene (repetitive element RepMP5e) and bocavirus NP1 (Nucleoprotein) gene, and establishes a one-step multiple fluorescent RT-PCR rapid detection method containing internal quality control; the method has simple and quick operations, overcomes the complexity of single hole single detection in a conventional single fluorescent RT-PCR method, simplifies the operation process, saves test cost, and provides a powerful technical support for on-site virus detection, health assessment and clinical diagnosis by using high specificity, sensitivity, efficiency and stability.
Owner:BEIJING CENT FOR DISEASE PREVENTION & CONTROL
Replicating recombinant adenovirus vectors, compositions, and methods of use thereof
Replicating recombinant adenovirus vectors derived from human adenovirus serotype 26 or human adenovirus serotype 35 are described. The replicating recombinant adenovirus vectors have attenuated replicative capacity as compared to that of the corresponding wild-type adenovirus. They can be used for stable expression of heterologous genes in vivo. Also described are compositions and methods of using these recombinant adenovirus vectors to induce an immune response in a subject, and vaccinate a subject against an immunogenic human immunodeficiency virus (HIV) infection.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC +1
Acquisition and use of tumor-selective replicative adenovirus - thymidine kinase gene construct
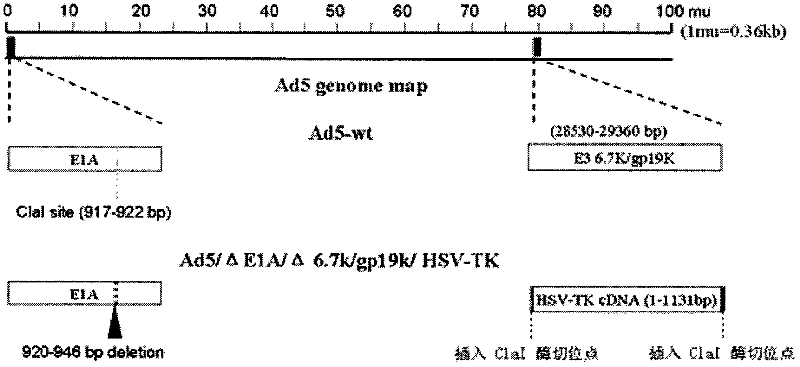
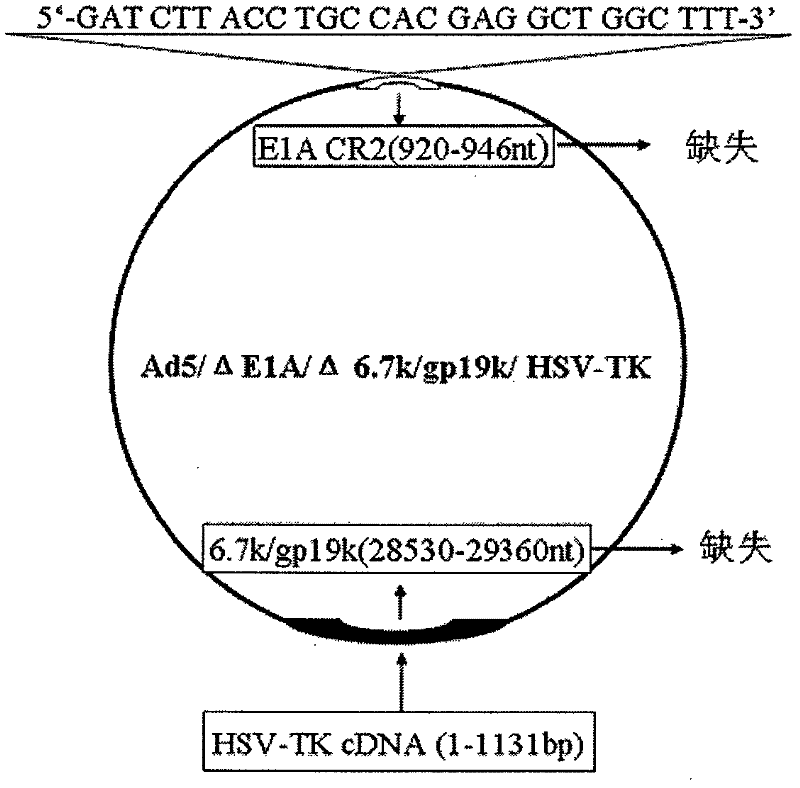
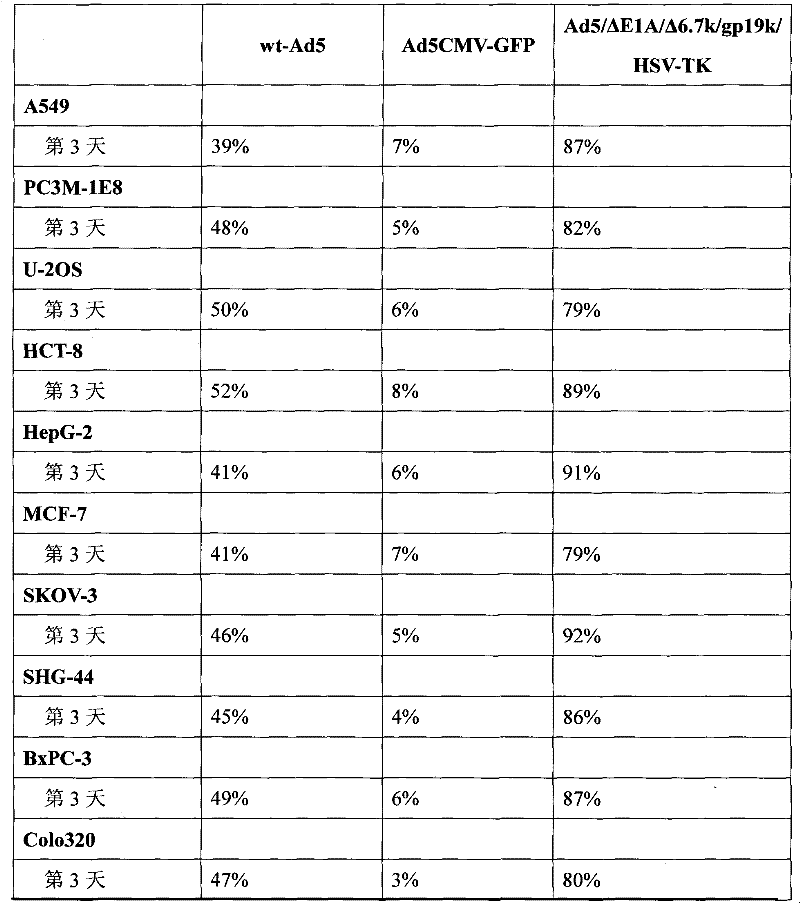
InactiveCN102206613AEfficient amplificationInhibit transferGenetic material ingredientsViruses/bacteriophagesConserved sequenceOncolytic adenovirus
Belonging to the genetic engineering field in medicine, the invention discloses a construction scheme for artificially modified human adenovirus type 5 (Ad5), and specific application of a tumor-selective replicative adenovirus construct in tumor treatment. With the techniques of PCR (polymerase chain reaction) amplifying fixed point deletion, enzyme cutting, connection, cloning, homologous recombination, transfection, single cloning purification of adenovirus and the like, a recombinant adenovirus construct can be acquired. Acquisition of the construct is characterized in that: 27 bases are deleted in 2(CR2) region of E1A conserved sequence of Ad5 genome; 28530-29360nt in 6.7k / gp19k gene in E3 region are deleted, and meanwhile a fragment of full-length cDNA(1131bp) of HSV-TK (herpes simplex virus thymidine kinase) is inserted into the deletion region. The construct of the invention is a novel oncolytic adenovirus carrier with high tumor-selective replication capability. With the suicide gene of HSV-TK and dissolubility of tumor cells by oncolytic viruses, the dual killing effects have unique utility value in tumor biotherapy.
Owner:周剑峰
Enhanced antiviral activity against foot and mouth disease
InactiveUS7833533B2Early protection against FMDVEffectively ensureSsRNA viruses positive-sensePeptide/protein ingredientsHigh dosesIn vivo
Previously, we showed that type I interferon (alpha / beta interferon [IFN-α / β]) can inhibit foot-and-mouth disease virus (FMDV) replication in cell culture, and swine inoculated with 109 PFU of human adenovirus type 5 expressing porcine IFN-α (Ad5-pIFN-α) were protected when challenged 1 day later. In this study, we found that type II pIFN (pIFN-γ) also has antiviral activity against FMDV in cell culture and that, in combination with pIFN-α, it has a synergistic antiviral effect. We also observed that while each IFN alone induced a number of IFN-stimulated genes (ISGs), the combination resulted in a synergistic induction of some ISGs. To extend these studies to susceptible animals, we inoculated groups of swine with a control Ad5, 108 PFU of Ad5-pIFN-α, low- or high-dose Ad5-pIFN-γ, or a combination of Ad5-pIFN-α and low- or high-dose Ad5-pIFN-γ and challenged all groups with FMDV 1 day later. The control group and the groups inoculated with either Ad5-pIFN-α or a low dose of Ad5-pIFN-γ developed clinical disease and viremia. However, the group that received the combination of both Ad5-IFNs with the low dose of Ad5-pIFN-γ was completely protected from challenge and had no viremia. Similarly the groups inoculated with the combination of Ad5s with the higher dose of Ad5-pIFN-γ or with only high-dose Ad5-pIFN-γ were protected. The protected animals did not develop antibodies against viral nonstructural (NS) proteins, while all infected animals were NS protein seropositive. No antiviral activity or significant levels of IFNs were detected in the protected groups, but there was an induction of some ISGs. The results indicate that the combination of type I and II IFNs act synergistically to inhibit FMDV replication in vitro and in vivo.
Owner:UNITED STATES OF AMERICA
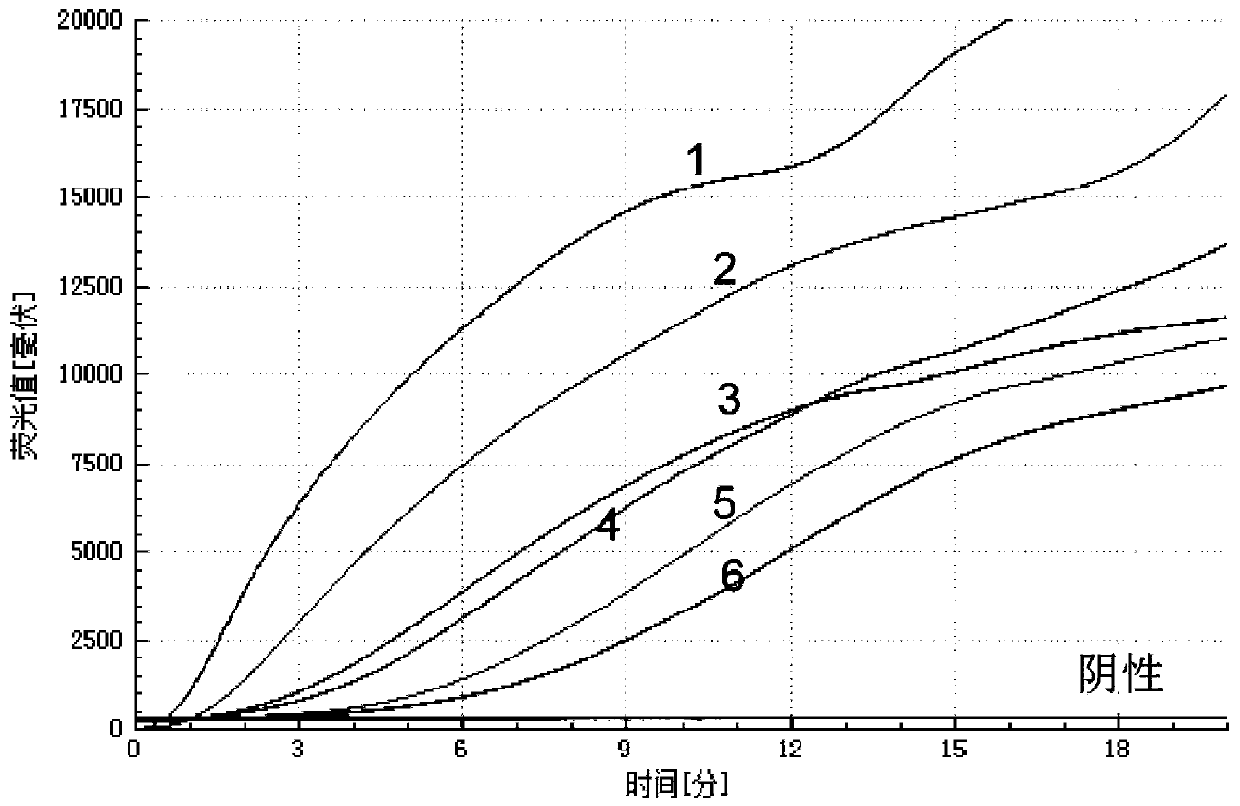
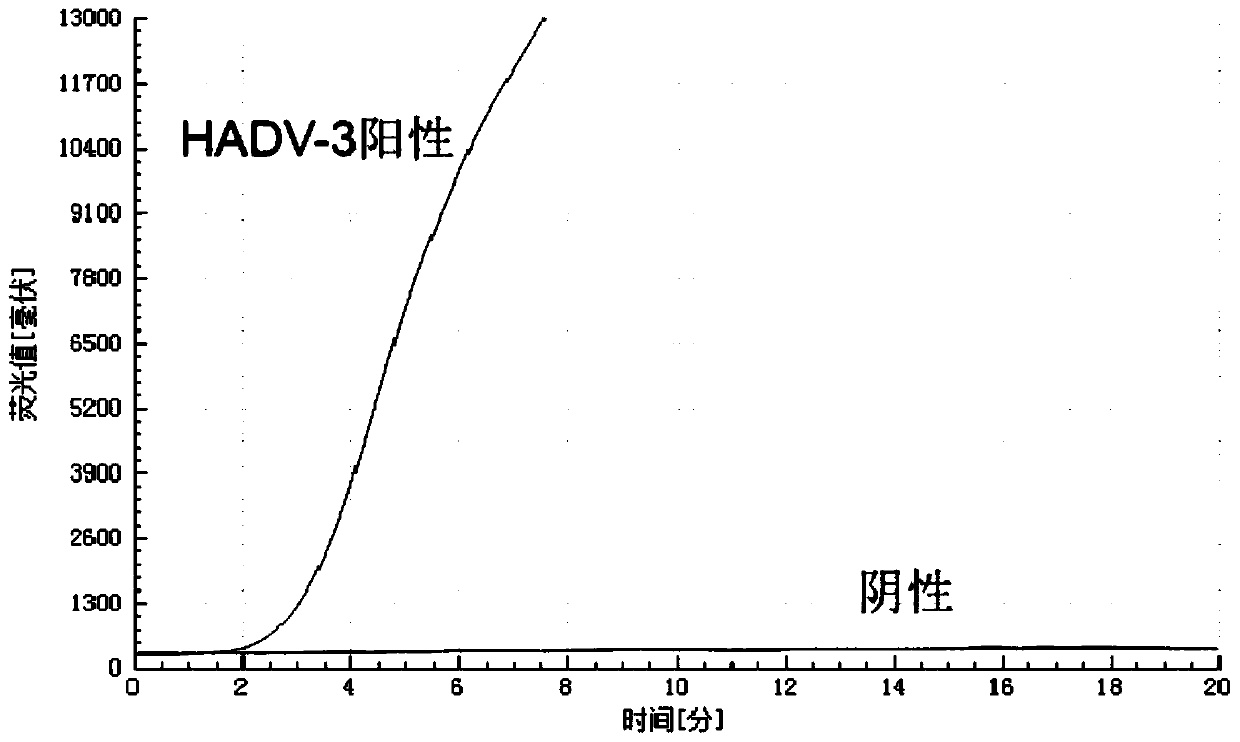
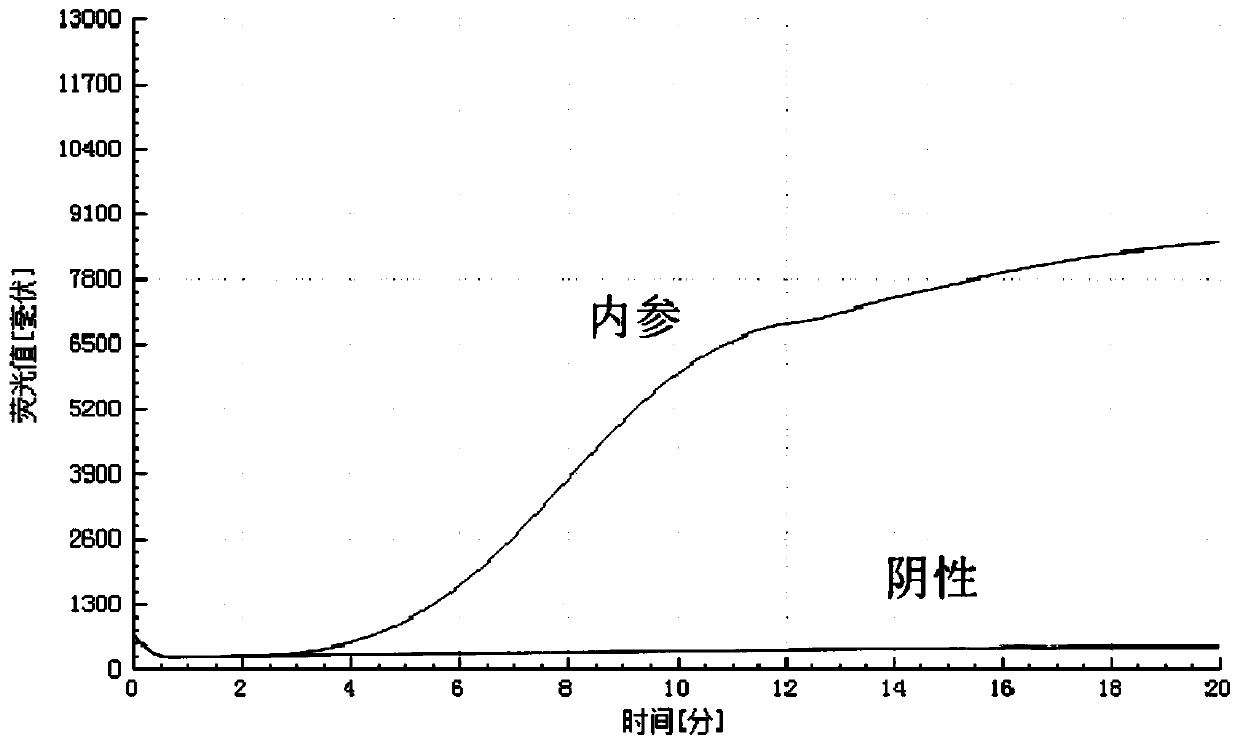
B-actin-contained dual isothermal nucleic acid amplification method for rapidly detecting type-3 human adenovirus
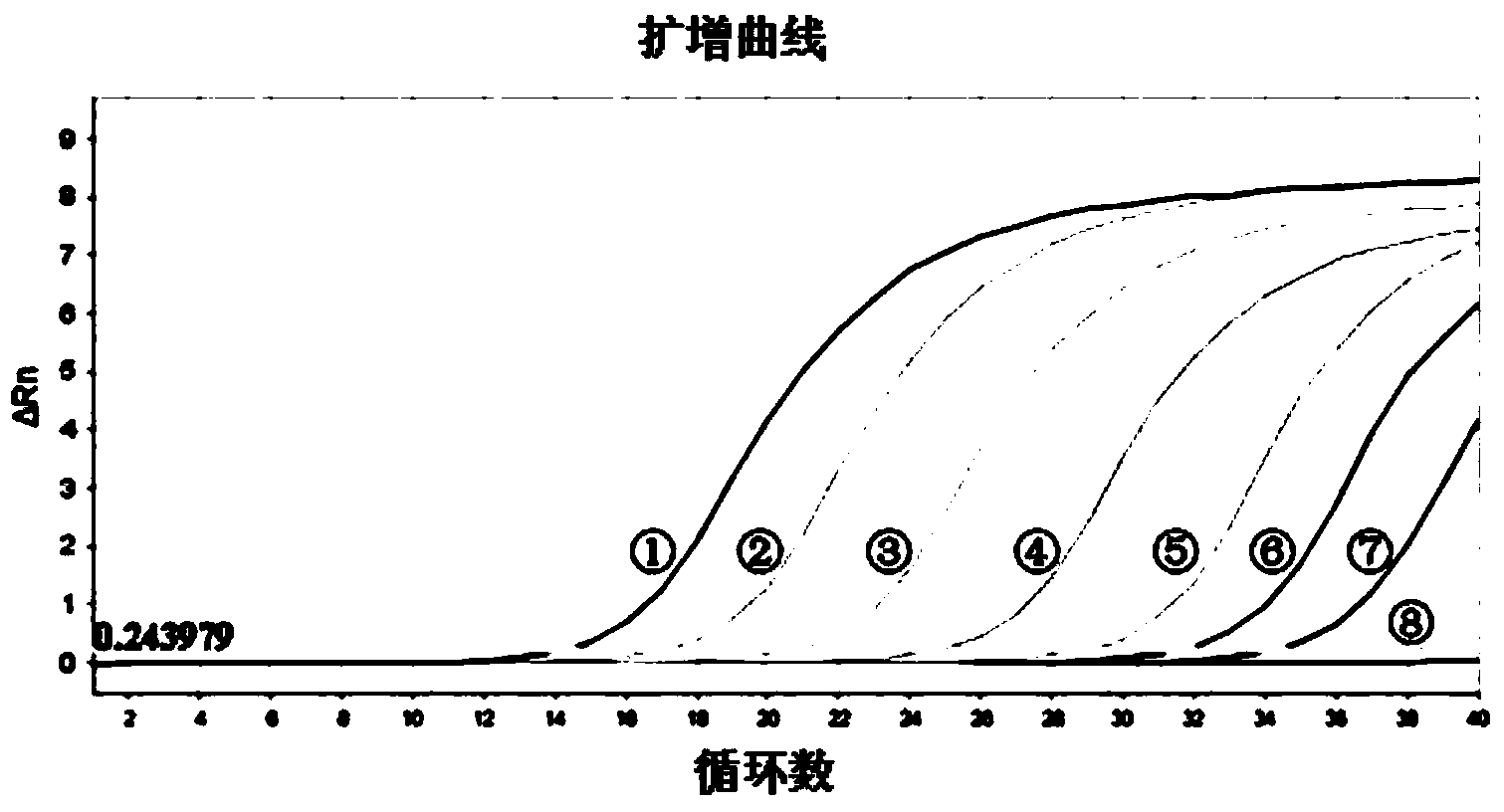
ActiveCN110106285ANo cross reactionStrong specificityMicrobiological testing/measurementMicroorganism based processesNucleotideNucleotide sequencing
The invention provides a b-actin-contained dual isothermal nucleic acid amplification method for rapidly detecting type-3 human adenovirus, and belongs to the technical field of isothermal nucleic acid amplification. The method comprises the steps that firstly, due to type-3 human adenovirus (HAdV-3) gene sequencing and comparing, a specific primer pair and probe for detecting HAdV-3 are designed,a b-actin probe is designed, the nucleotide sequences are shown in SEQ ID NO.1-4, on the basis, a b-actin-contained dual isothermal nucleic acid amplification system is built, and a kit for detectingthe type-3 adenovirus is constructed. The method is implemented under the isothermal condition, type-3 adenovirus and b-actin DNA amplification can be achieved at the same time within 5-20 min, sensitivity is high, specificity is good, the false negative and valid effects are removed by adding b-actin, the method is more suitable for being applied to detection of a large number of samples, clinicapplication is convenient, and the method is applicable to rapid detection of the type-3 adenovirus.
Owner:中国疾病预防控制中心病毒病预防控制所 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com