Genetically engineered rabies virus vaccine with recombination-defective adenovirus carrier
A genetically engineered vaccine, replication-deficient technology, applied in the field of recombinant vaccines, can solve the problems of unclear efficacy of pathological changes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The present invention will be further described below in conjunction with the accompanying drawings and embodiments. Example 1 Construction of rabies virus CVS-N2c GP gene recombinant replication-defective adenovirus of the present invention A. Immunogen
[0018] The brain tissue of Kunming mice with typical rabies symptoms was taken, and Trizol reagent (Gibco BRL) was added to extract total RNA, and the GP gene cDNA of rabies virus was obtained by reverse transcription polymerase chain reaction, which was cloned into the vector pUC18 and sequenced for double-stranded DNA. For its sequence, see SEQ ID NO: 1 in the sequence listing. B. Build process
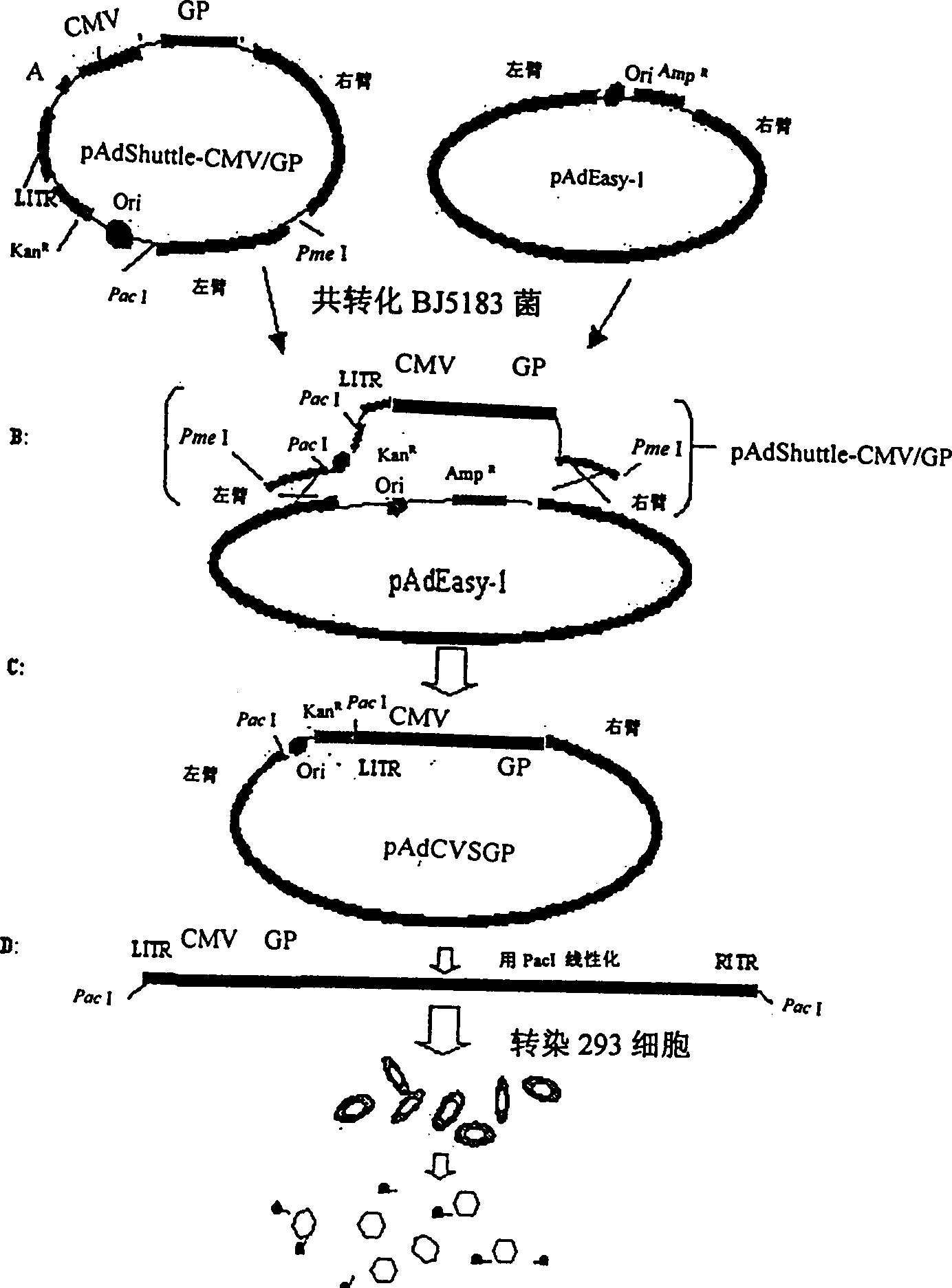
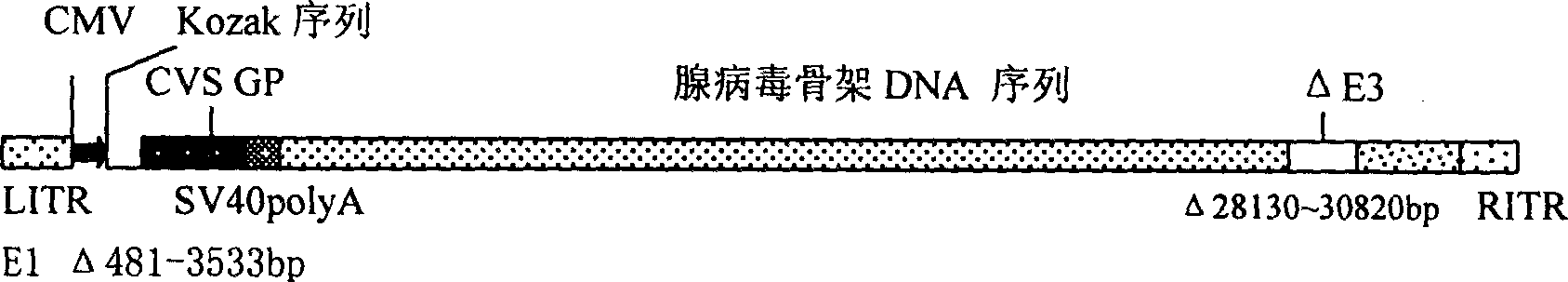
[0019] The GP gene of the CVS-N2c strain was subcloned from its cloning vector into the adenovirus shuttle vector pAdShuttle-CMV to obtain a recombinant shuttle plasmid, such as figure 1 (A). It was confirmed by restriction analysis that a single copy of the foreign gene was inserted. Subsequently, this recombinant shuttl...
Embodiment 2
[0022] When the 293 cells grew to 90% saturation, the recombinant virus rAdCVSGP was inoculated at a multiplicity of infection m.o.i=5-10. After 3-4 days, when the cell lesion reaches 70-90%, the cell solution is collected in a centrifuge tube, centrifuged at 2000rpm for 15min to harvest the cells, and the supernatant is frozen for future use. The cells were freeze-thawed and lyzed four times in a dry ice / 37°C water bath, centrifuged at 5000rpm for 15min, and the supernatant of the lysate was added to the upper layer of the CsCl density gradient centrifugation medium. Centrifuge at 35000rpm for 2h. Carefully suck out the virus band with a pipette, add it to the dialysis band and dialyze against PBS with stirring. After dialysis was complete, freeze at -20°C. Example 3 Mice intracerebral challenge-immune protection experiment
Embodiment 3
[0022] When the 293 cells grew to 90% saturation, the recombinant virus rAdCVSGP was inoculated at a multiplicity of infection m.o.i=5-10. After 3-4 days, when the cell lesion reaches 70-90%, the cell solution is collected in a centrifuge tube, centrifuged at 2000rpm for 15min to harvest the cells, and the supernatant is frozen for future use. The cells were freeze-thawed and lyzed four times in a dry ice / 37°C water bath, centrifuged at 5000rpm for 15min, and the supernatant of the lysate was added to the upper layer of the CsCl density gradient centrifugation medium. Centrifuge at 35000rpm for 2h. Carefully suck out the virus band with a pipette, add it to the dialysis band and dialyze against PBS with stirring. After dialysis was complete, freeze at -20°C. Example 3 Mice intracerebral challenge-immune protection experiment
[0023] 12-14g of clean or ordinary Kunming mice were centrifuged with CsCl density gradient to obtain purified virus for intraperitoneal or subcutane...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com