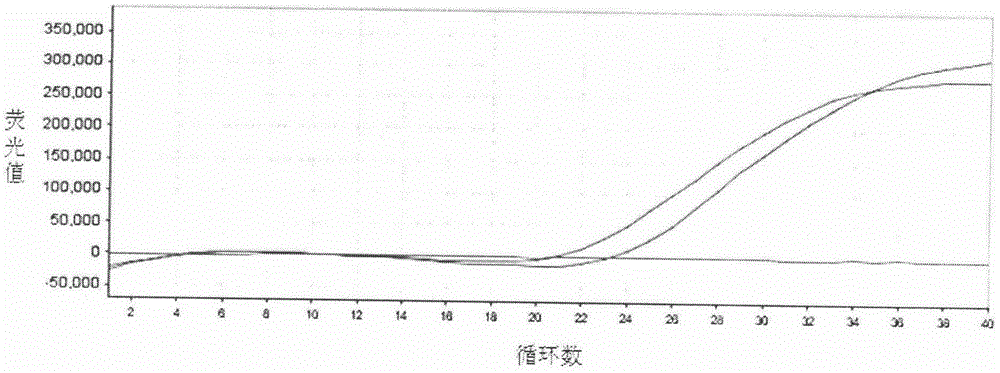
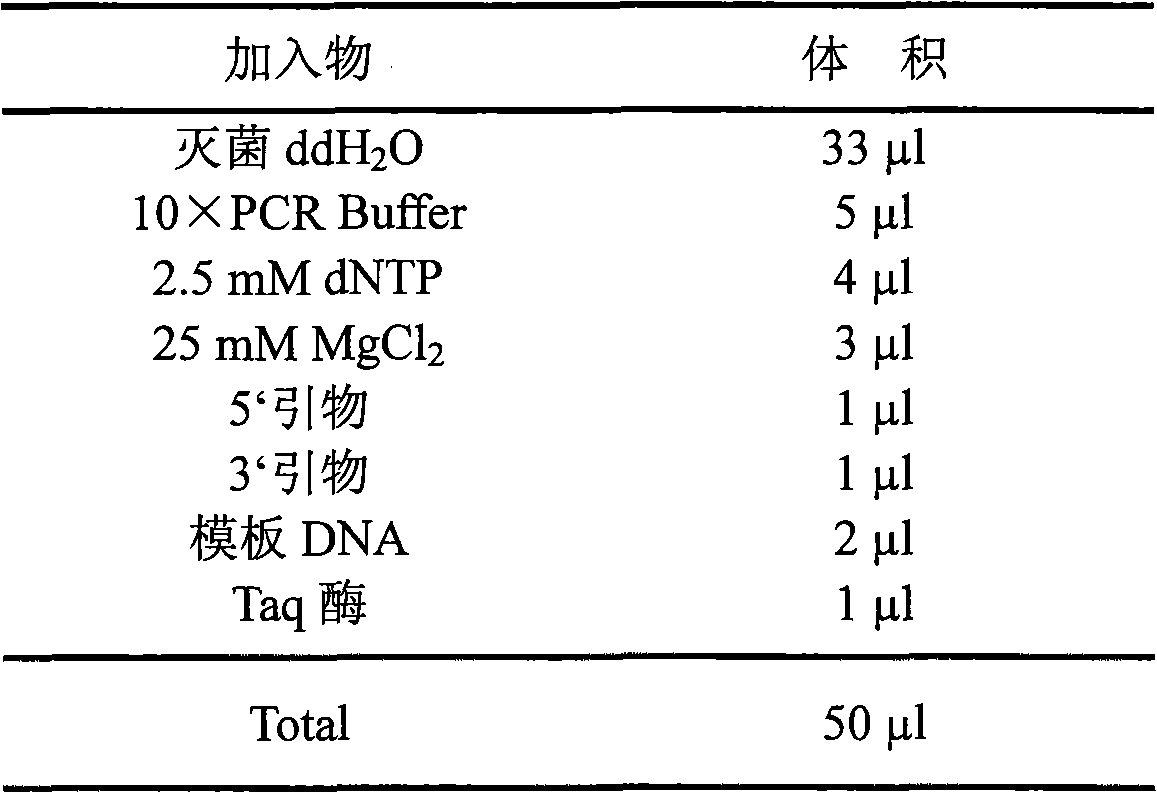
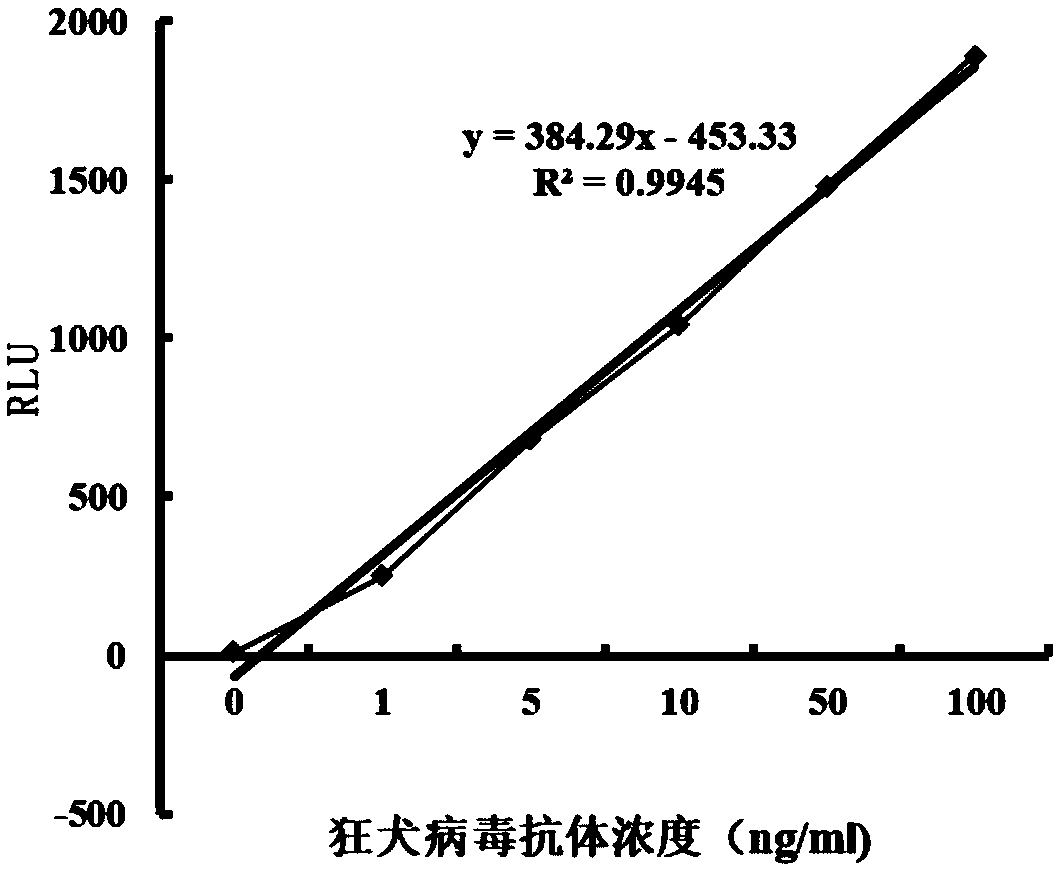
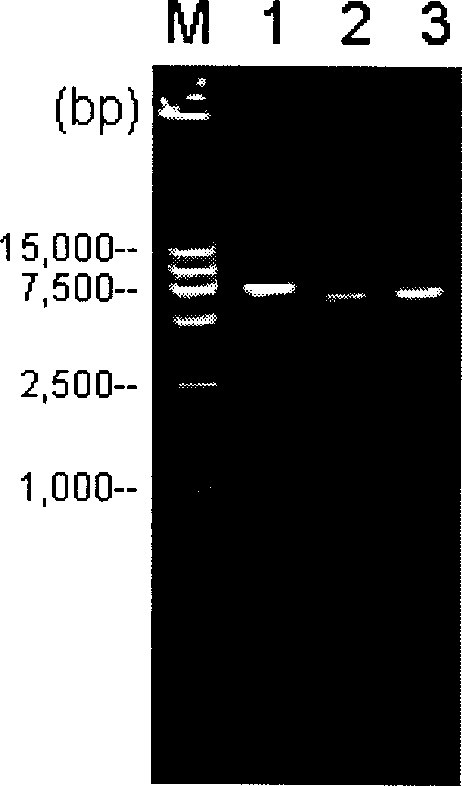Patents
Literature
245 results about "Furovirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Furovirus is a genus of viruses, in the family Virgaviridae. Graminae, winter wheat, wheat, triticale, oat, sorghum bicolor, and plants serve as natural hosts. There are currently six species in this genus including the type species Soil-borne wheat mosaic virus. Diseases associated with this genus include: (SBWMV): green and yellow mosaic.
Method for preparing vaccine by editing pseudorabies virus genomes based on CRISPR/Cas9 and Cre/lox systems and application of method
ActiveCN104894075AReduce disease lossEasy to operateAntiviralsViruses/bacteriophagesMCherry fluorescent proteinBiology
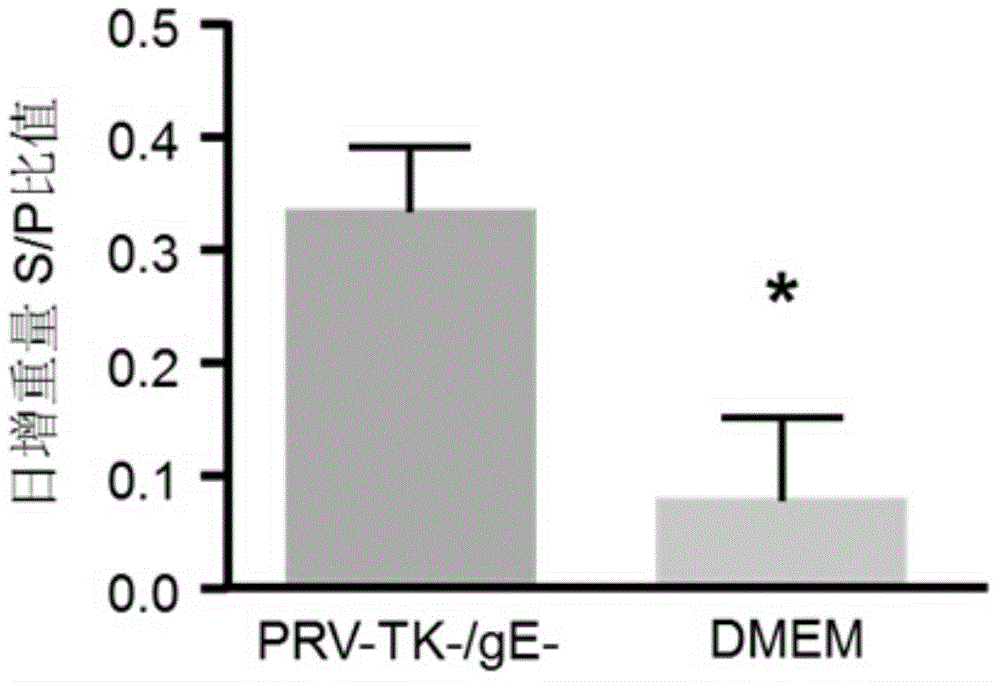
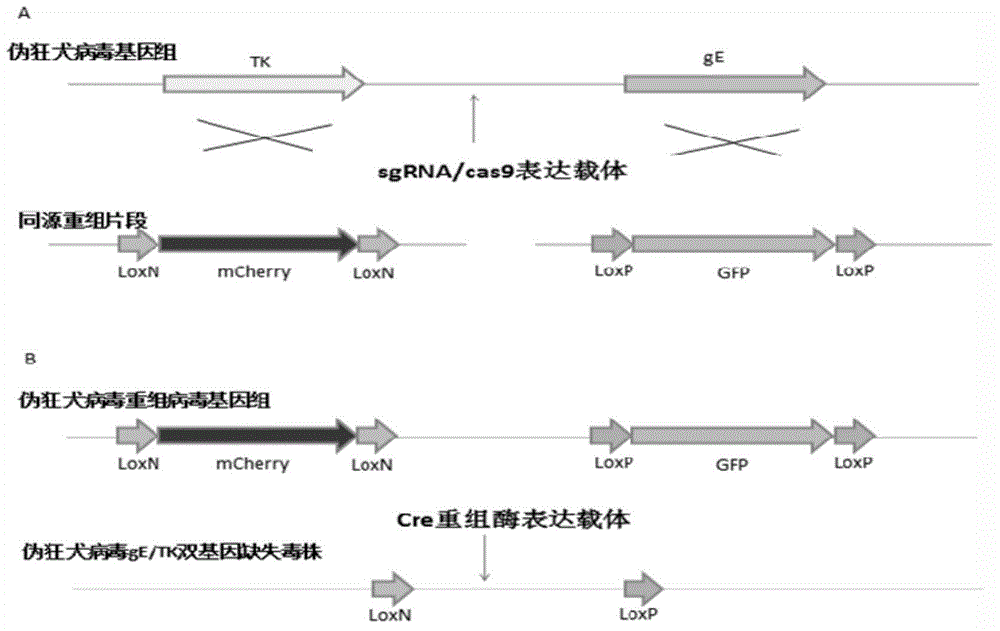
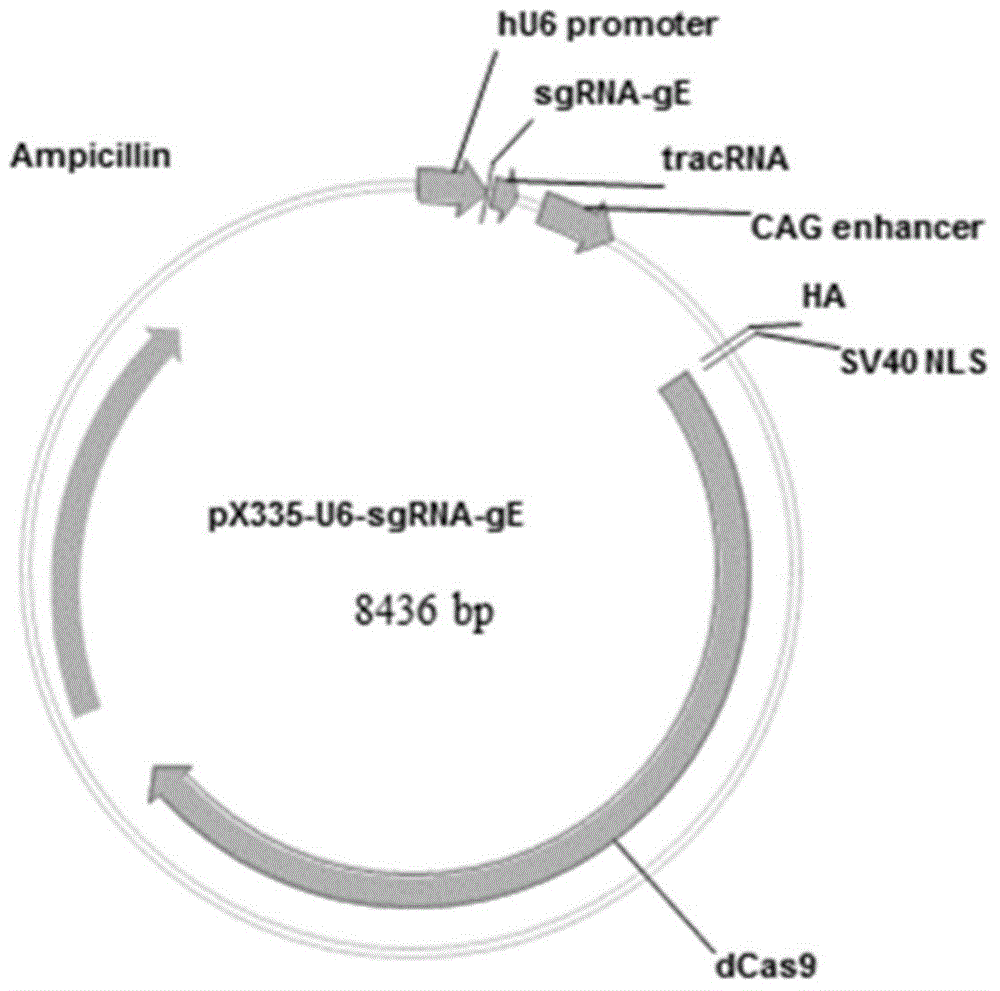
The invention discloses a method for quickly preparing a vaccine by editing pseudorabies virus genomes based on a CRISPR / Cas9 gene editing system and a Cre / lox recombination system and an application of the method. According to the method, the CRISPR / Cas9 gene editing system is used for synchronously and efficiently recombining a GFP gene and a mCherry gene to a pseudorabies virus gE gene site and a TK gene site respectively to obtain conditional deletion strains of a gE gene and a TK gene; after purification, the Cre / lox system is used for cutting off extraneous GFP and mCherry genes in the pseudorabies virus recombinant virus genome so as to perform purification quickly to obtain a live pseudorabies virus vaccine lack of gE / TK genes; multiple genes are operated at the same time, so that multiple rounds of flows for knocking out multiple genes in the conventional method are reduced to one round; and meanwhile, the efficient edition of virus genes by using the CRISPR / Cas9 and Cre / lox systems simplifies about thirty generations of plaque purification processes into 3-4 generations, so that the preparation efficiency of the virus vaccine is greatly improved, and the method provides a strong guarantee for effectively preventing and controlling the larger-range popularization of variant pseudorabies viruses and reducing heavy economic losses.
Owner:武汉都为康生物科技有限公司
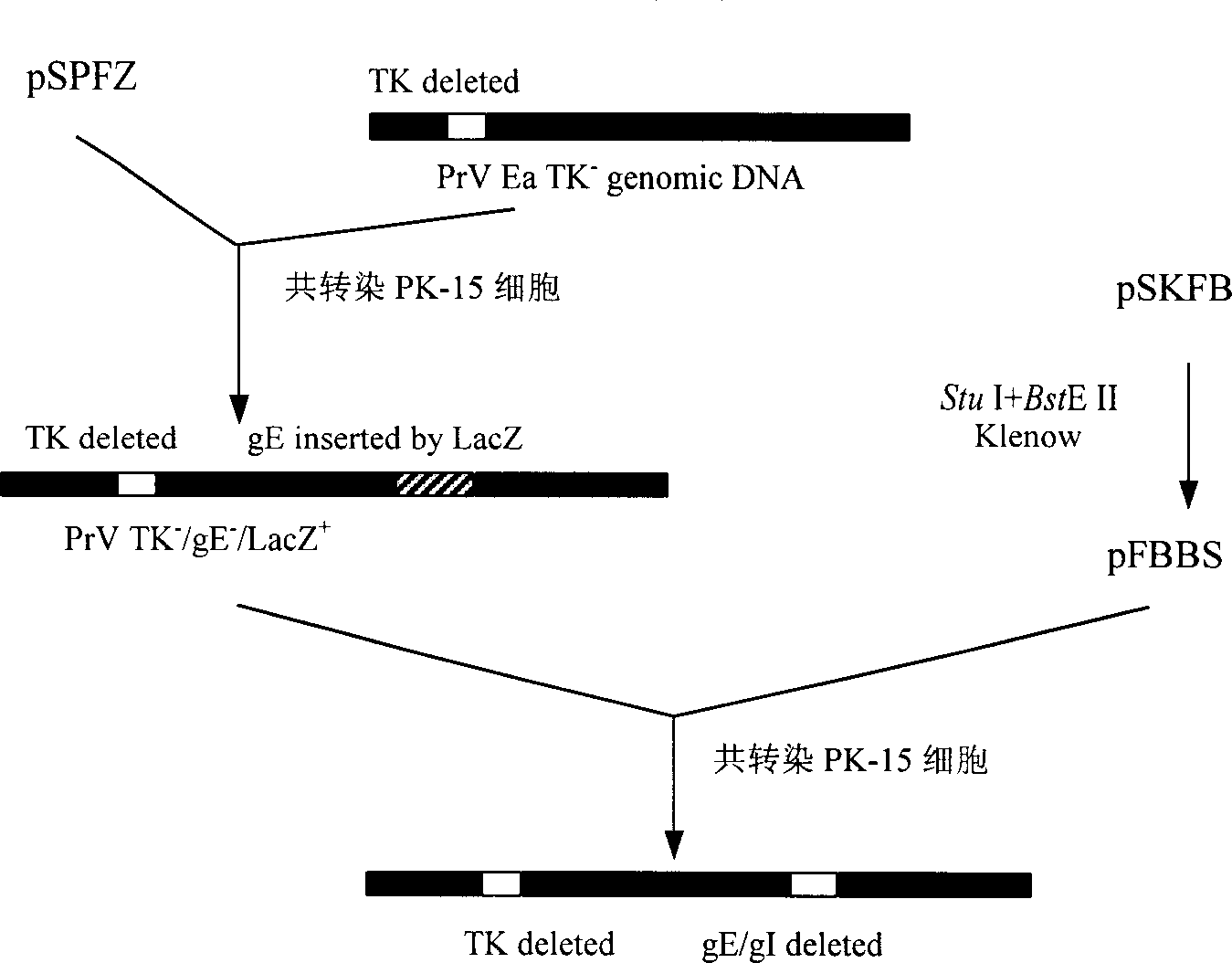
Pseudorabies TK*/gE*/gI* gene dificiency mark live vaccine and preparation method thereof
The present invention discloses a construction of three-gene defected recombinant pseudorabies virus (PrV) strain, vaccine prepared by said strain, method for constructing said strain and method for preparing said vaccine. The described recombinant pseudorabies virus strain defects genes of TK.gE ang gI, and contains no exogenous gene. The described vacine belongs to the freeze-dried live vaccine made up by virus liquid containing said invention and gelatin. Said invented live vaccine can be inoculated on the piglet, slaughter pig and sow with farrow, and can obtain obious immune effect. Said vaccine has good biological safety, and can be used for preventing and curing pseudorabies.
Owner:HUAZHONG AGRI UNIV
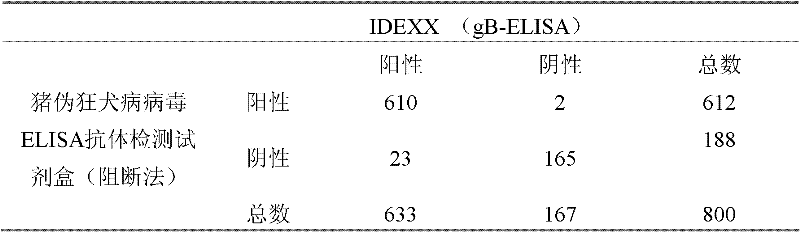
Kit for detecting pig pseudorabies virus antibodies and block enzyme-linked immuno sorbent assay (ELISA) method
The invention discloses a kit for detecting pig pseudorabies virus antibodies and a block enzyme-linked immuno sorbent assay (ELISA) method. The kit for detecting pig pseudorabies virus antibodies comprises pig pseudorabies virus monoclonal antibodies which are labelled by horseradish peroxidase, wherein the pig pseudorabies virus monoclonal antibodies are monoclonal antibodies obtained by pig pseudorabies viruses as immunogens and the pig pseudorabies viruses are pseudorabies virus strain Ea. The kit for detecting pig pseudorabies virus antibodies also comprises an enzyme label plate, a sample diluent, negative and positive contrasts, a coloured solution, a washing solution, and a stopping solution. The block ELISA method comprises the following steps of 1, taking out a detection plate pre-coated with virus antigens from the kit for detecting pig pseudorabies virus antibodies, adding diluted blood serum needing to be detected into the detection plate pre-coated with the virus antigens, and simultaneously, setting negative and positive contrast apertures, 2, shaking up the diluted blood serum in the negative and the positive contrast apertures, shaking off a solution in the negative and the positive contrast apertures, and washing the detection plate by the washing solution, and 3, adding the pig pseudorabies virus monoclonal antibodies labelled by horseradish peroxidase into the negative and the positive contrast apertures, washing, adding the colored solution into the negative and the positive contrast apertures to carry out room-temperature coloration in the dark, adding the stopping solution into the negative and the positive contrast apertures, and determining OD630nm values of the negative and the positive contrast apertures by an ELISA apparatus. The block ELISA method has the advantages of good singularity, high sensitivity, short detection time, and high accuracy because of utilization of an S / N ratio method in result determination.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Anti-rabies virus monoclonal antibody and preparation method and application
InactiveCN101560255AExperimental costs are highEasy to operateImmunoglobulins against virusesTissue cultureImmunoblot AnalysisHybridoma cell
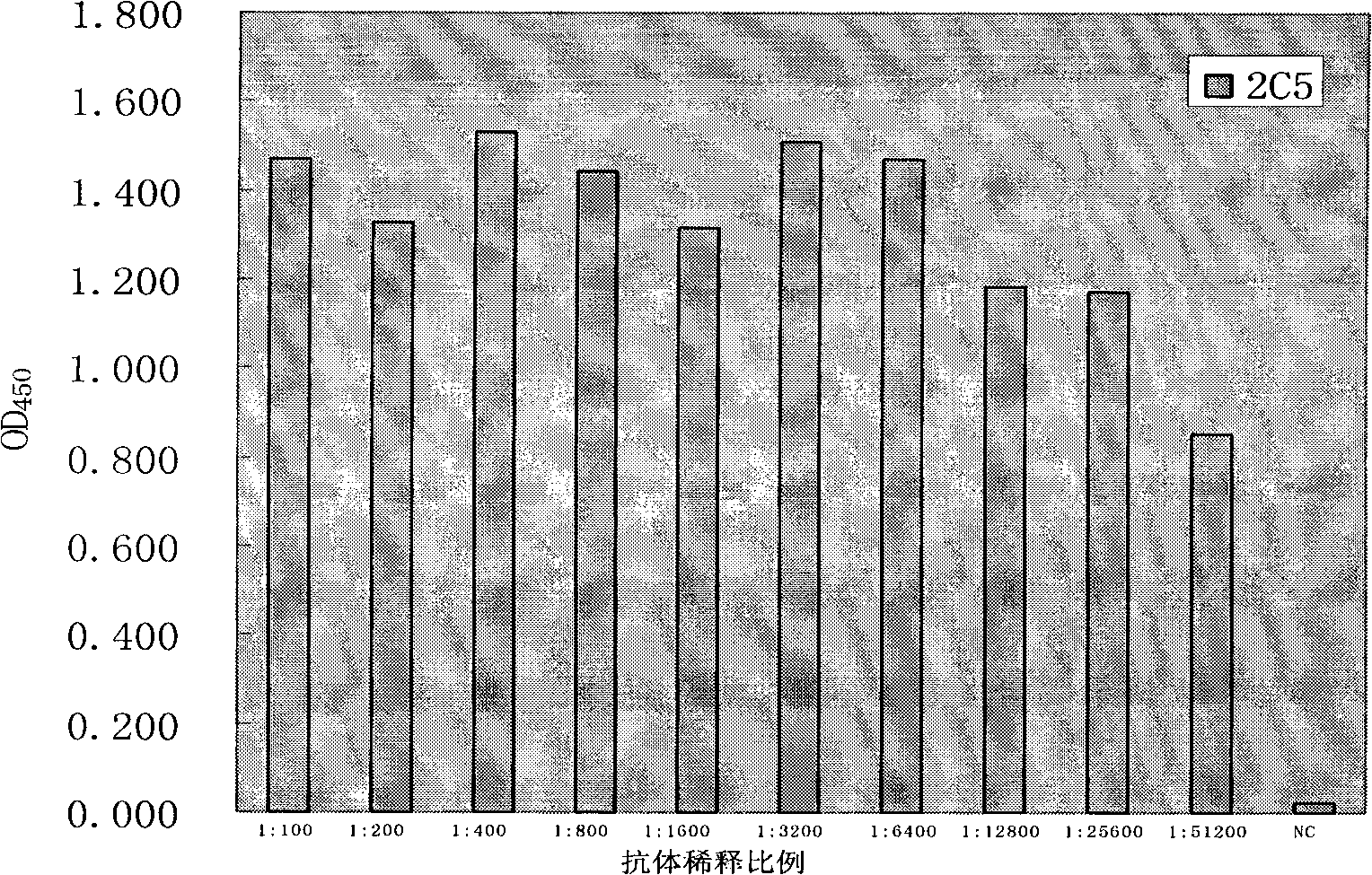
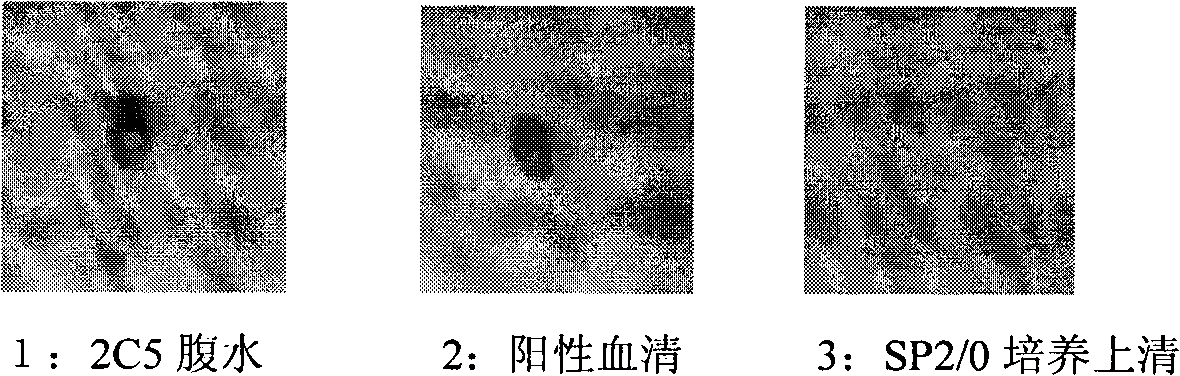
The invention discloses an anti-rabies virus monoclonal antibody and a preparation method and an application, belonging to the field of biomedicine and particularly relating to the preparation of a monoclonal antibody capable of identifying rabies virus and the application. The monoclonal antibody of the invention is screened by indirect Enzyme-linked immunosorbent assay (ELISA), and specificity and affinity thereof combined with antigen are identified by methods such as polyacrylamide gel electrophoresis analysis, speckle ELISA, immunoblot analysis and the like. The anti-rabies virus monoclonal antibody of the invention can be applied in multiple testing methods of antigen of rabies virus and can be also applied in the preparation of rabies virus detecting kit. The anti-rabies virus monoclonal antibody is secreted by anti-rabies virus monoclonal antibody hybridoma cell strain 2C5 with the preservation number being CGMCC No.3014.
Owner:NANJING MEDICAL UNIV +1
Taqman-MGB fluorescent quantitative PCR kit and method for detecting 12 common viruses and bacteria of pig at same time
ActiveCN105624330AQuick checkSensitive detectionMicrobiological testing/measurementPorcine reproductive and respiratory syndrome virusPorcine circovirus
The invention provides a Taqman-MGB fluorescent quantitative PCR kit and a method for detecting 12 common viruses and bacteria of pigs at the same time. The kit comprises PCR reaction liquids A / B / C, wherein the PCR liquids comprise primer pairs and Taqman probes for porcine parvovirus (PPV), type-II streptococcus suis (SS-II), a porcine pseudorabies virus (PRV), type-II porcine circovirus (PCV-2), a hog cholera virus (CSFV), a pig foot and mouth disease virus (FMDV), a porcine reproductive and respiratory syndrome virus (PRRSV), a high pathogenicity porcine reproductive and respiratory syndrome virus strain (Hp-PRRSV), a transmissible gastroenteritis virus (TGEV), an epidemic diarrhea virus (PEDV), rotavirus (PRTV) and a swine influenza virus (SIV) respectively. 12 pathogens of pigs can be detected rapidly and effectively at the same time, the detection method is high in accuracy, specificity and sensitivity and is good in stability, and rapid diagnosis and effective detection on pathogens to be detected can be achieved.
Owner:BEIJING YISEN BIOTECH
Rabies antibody gold immunochromatography assay testing indicator paper and preparation technique
This invention provides one dog rabies virus antigen glue gold immune chromatography test paper and its process, which comprises the following steps: according to antigen antibody immune combination basic principle to label the sheep IgG by glue gold label covering on the glass fiber film as combination pad; covering the purification rabies virus antigen and gene engineer expressed antigen and rabit IgG covering on the NC film as test line and quality control line; when testing the antibody, forming antibody combination to expose red band.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Preparing method for recombinant human anti-rabies monoclonal antibodies
Owner:NCPC NEW DRUG RES & DEV
Method for preparing rabies virus antigen
ActiveCN101307317AReduce consumptionImprove securityAntiviralsDepsipeptidesAntigenBaculovirus expression
The invention provides a method for making rabies virus antigen. The method comprises the following steps that: the antigen gene of rabies virus or the combined expression combination of the antigen gene is respectively cloned in a baculovirus carrier so as to obtain a transfer expression carrier; the transfer expression carrier and baculovirus undergo cotransfection so as to carry out homologous recombination or transposition, thereby obtaining recombined baculovirus; the recombined baculovirus is used to infect insect host and cell; the infected insect host is cultured to express corresponding rabies antigen; and the expressed antigen is ingathered and purified so as to obtain rabies virus antigen. The method adopts a baculovirus expression system to make safe and efficient rabies virus antigen in a domestic silkworm bioreactor; moreover, due to having extremely high safety, the made antigen can be directly used to make injection vaccine and oral vaccine used for animal immunization. The method can substantially reduce the production cost of rabies virus antigen, and has the advantages of safety, high efficiency, less energy consumption and low cost, etc.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI +1
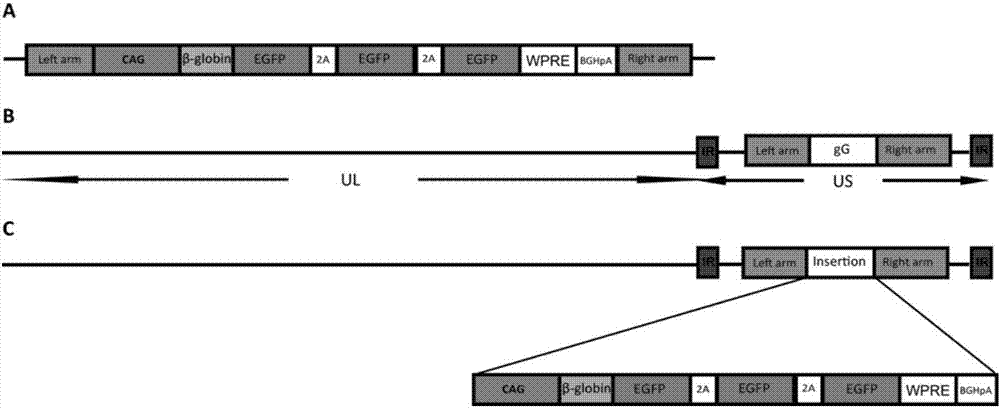
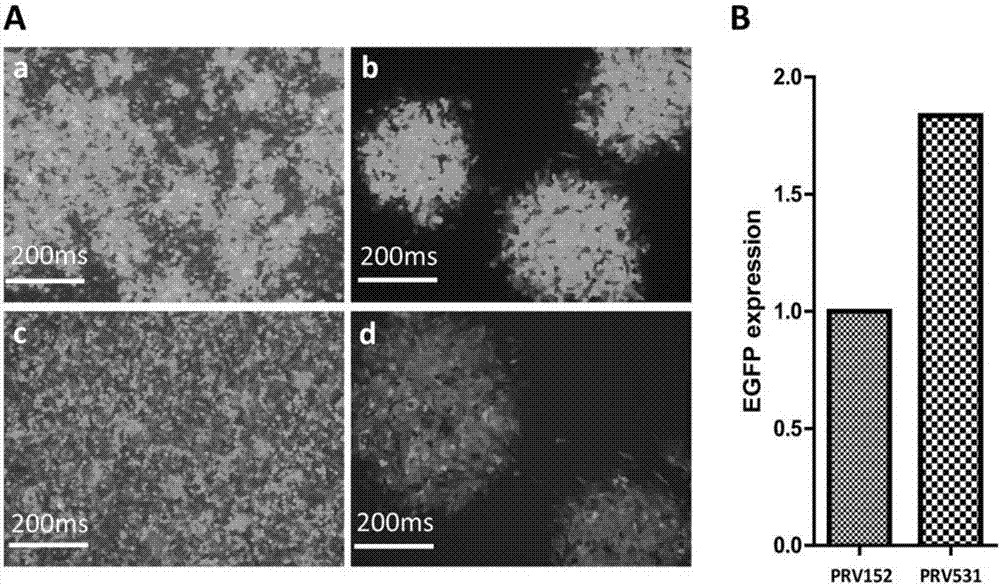
Preparation method of recombinant pseudorabies virus for expressing reverse neural circuit tracing of green fluorescin with high sensitivity, and application
ActiveCN106995824AImprove efficiencyRealize visualizationMicrobiological testing/measurementPreparing sample for investigationGreen fluorescent proteinViral Vaccine
The invention discloses a preparation method of a recombinant pseudorabies virus for expressing reverse neural circuit tracing of green fluorescin with high sensitivity, and application, including (1) the preparation of the recombinant pseudorabies virus for expressing green fluorescin with high sensitivity; (2) the application to marking of a neural circuit. The recombinant pseudorabies virus for expressing the green fluorescin with high sensitivity is successfully prepared by using a platform. The recombinant pseudorabies virus for expressing reverse neural circuit tracing of green fluorescin with high sensitivity is successfully obtained. Wide application value is realized in aspects of neural circuit marking, medicine screening platform building, medicine inhibition virus action mechanism, viral vaccine and diagnostic reagent invention and development, animal model building, virus replication, pathogenic mechanism analysis and the like.
Owner:衠奥生物技术(武汉)有限公司
Porcine pseudorabies virus strain and porcine pseudorabies inactivated vaccine prepared by using same
ActiveCN102344912APromote rapid proliferationHigh titerMicroorganism based processesAntiviralsPig farmsTonsil
The invention discloses a porcine pseudorabies virus strain and a porcine pseudorabies inactivated vaccine prepared by using the same. The porcine pseudorabies virus strain is separated from the brain, tonsil and other tissues of a still birth of a sow on a pig farm by virtue of subculture adaptation and has a microbial preservation number of GCMCC (China General Microbiological Culture Collection Center) No.5013. The virus strain separated in the invention has quick proliferation and high titer on ST (scheduled tribe) cells, can induce an animal to generate a high-titer neutralizing antibody, and has excellent immunogenicity. The invention also discloses a preparation method of the porcine pseudorabies inactivated vaccine, and the method comprises the following steps of: culturing the porcine pseudorabies virus strain to obtain a virus solution; adding an inactivator to inactivate the virus solution; and preparing an oil phase and a water phase, and emulsifying, thus obtaining the porcine pseudorabies inactivated vaccine. In the invention, each process parameter of the inactivated vaccine is optimized. Immune protective efficacy and safety tests prove that the inactivated vaccine has excellent immunogenicity and safety.
Owner:哈药集团生物疫苗有限公司
Porcine pseudorabies virus vaccine composition and preparation method and application thereof
ActiveCN104248757AShort timeEase of mass productionAntiviralsAntibody medical ingredientsDiseaseImmune effects
The invention provides a porcine pseudorabies virus vaccine composition. The porcine pseudorabies virus vaccine composition contains a porcine pseudorabies virus subunit antigen, or a recombinant Newcastle disease virus, namely a porcine pseudorabies virus vector. The invention further provides the preparation method and application of the vaccine composition. The vaccine composition can effectively prevent related diseases of a porcine pseudorabies virus and related diseases infected by the porcine pseudorabies virus. The combination of immunogenicity antigens in the porcine pseudorabies virus vaccine composition can be induced to generate a synergetic immune effect, thereby having good immune effect, and further lowering the immune usage amount to lower the immunization cost.
Owner:PU LIKE BIO ENG
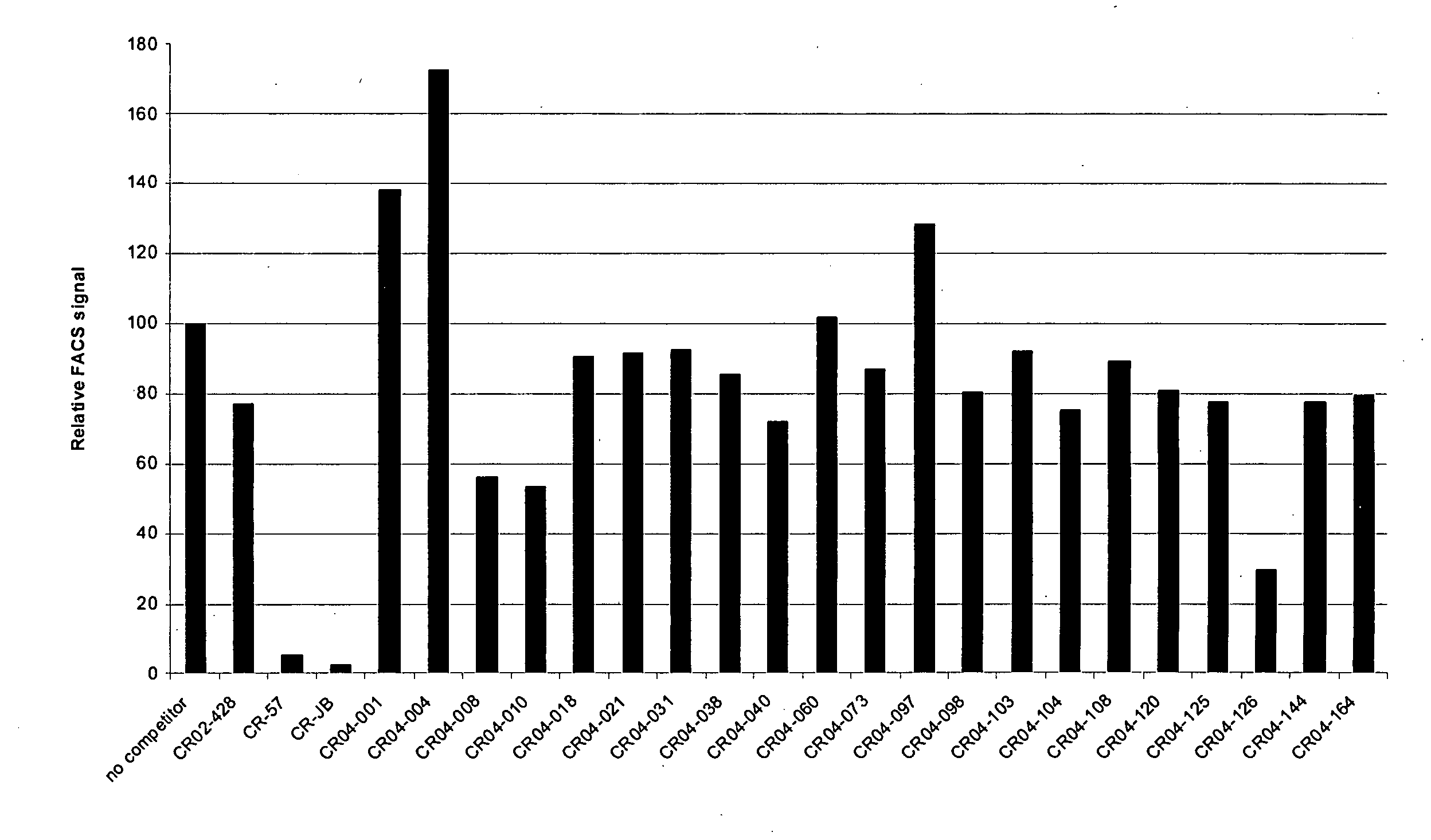
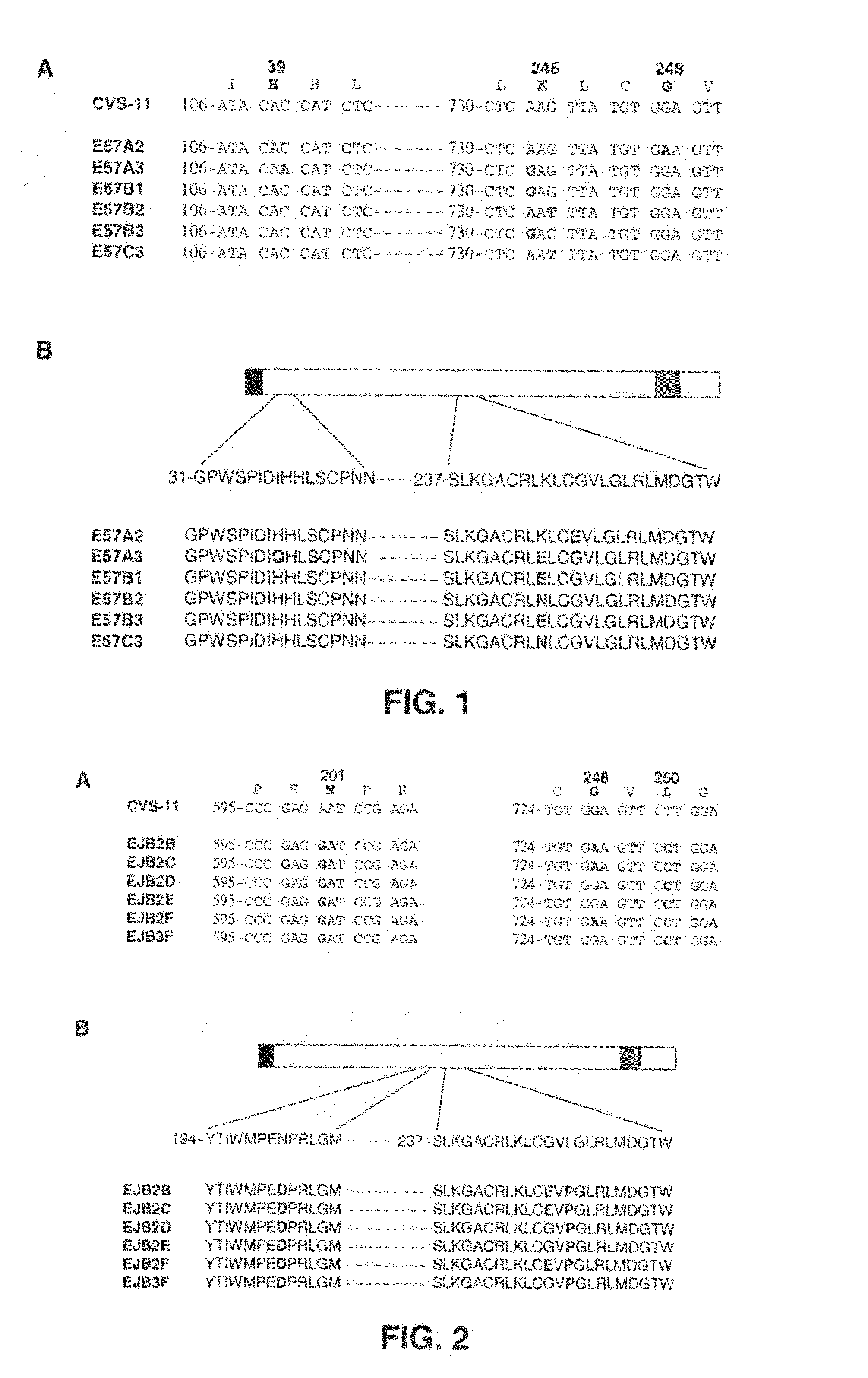
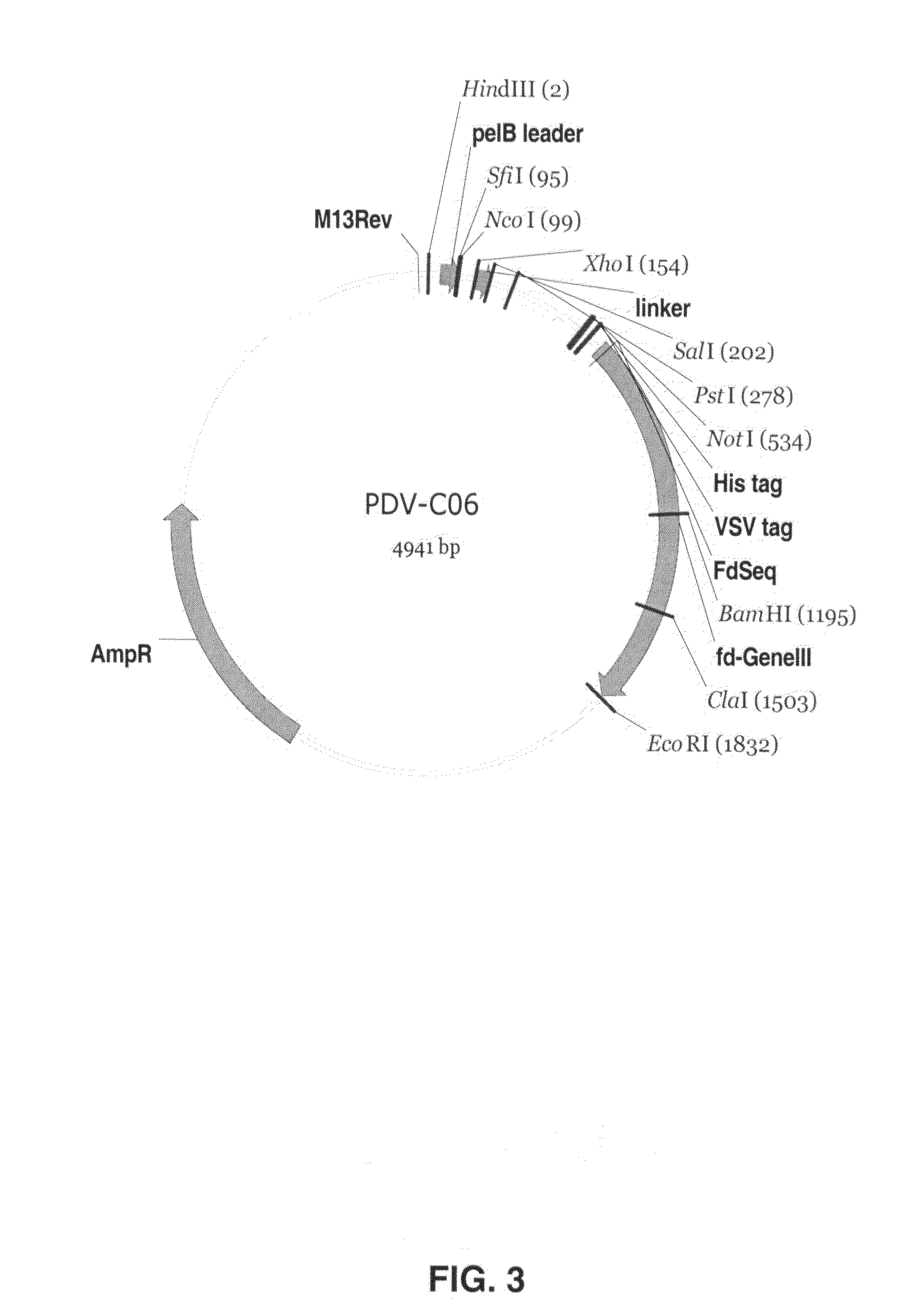
Binding molecules capable of neutralizing rabies virus and uses thereof
InactiveUS20080226652A1SsRNA viruses negative-senseVirus peptidesPost-exposure prophylaxisRabies virus
Provided are binding molecules that specifically bind to rabies virus and are capable of neutralizing the virus. Further provided are nucleic acid molecules encoding the binding molecules, compositions comprising the binding molecules and methods of identifying or producing the binding molecules. The binding molecules can be used in the diagnosis, prophylaxis and / or treatment of a condition resulting from rabies virus. In certain embodiments, they can be used in the post-exposure prophylaxis of rabies.
Owner:JANSSEN VACCINES & PREVENTION BV
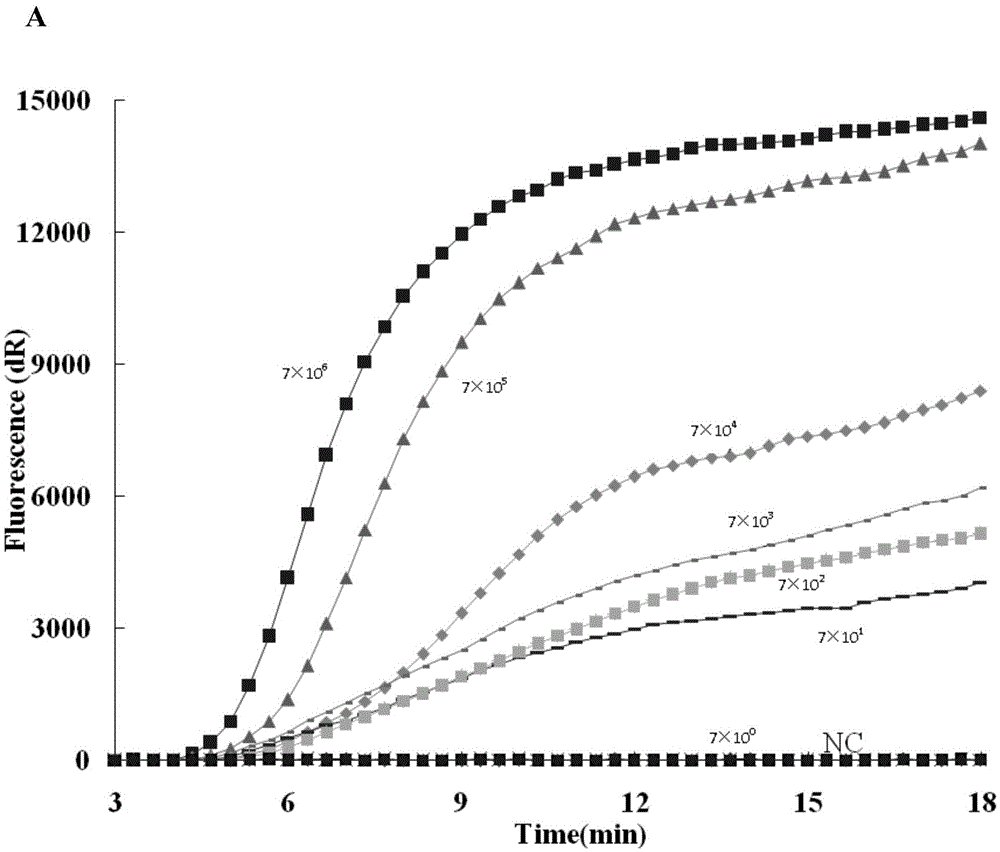
RT-RPA (reverse transcription recombinase polymerase amplification) detection kit for fast detecting high-pathogenicity porcine reproductive and respiratory syndrome virus and application thereof
ActiveCN105567871AShorten test timeLow reaction temperatureMicrobiological testing/measurementMicroorganism based processesBiologyDifferential diagnosis
The invention discloses an RT-RPA (reverse transcription recombinase polymerase amplification) detection kit for fast detecting a high-pathogenicity porcine reproductive and respiratory syndrome virus and application thereof. The kit comprises a pair of primers and a probe, the sequences of the primers are shown as SEQ ID NO.1 and SEQ ID NO.2, and the sequence of the probe is shown as SEQ ID NO.3. It is proved through experiments that the kit can detect adverse effects of the high-pathogenicity porcine reproductive and respiratory syndrome virus (HP-PRRSV), a hog cholera virus, a C-type porcine reproductive and respiratory syndrome virus, a porcine circovirus type II, a porcine pseudorabies virus and a foot and mouth disease virus in a specificity mode. It is proved through experiments that the kit can detect out templates of at least 70 copies at the temperature of 40 DEG C on the condition of 20 min amplification, and the conformity between the kit and RT-qPCR is high. This shows that the kit can detect HP-PRRSV fast, efficiently and sensitively and provides an effective technological means for differential diagnosis of HP-PRRSV.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Method for conferring resistance or tolerance aganist furovirus, potyvirus, tospovirus, and cucomovirus to plant cells
InactiveUS7019195B1Reduce sensitivityAccumulation and reduced and preventedStable introduction of DNAFermentationAntisense RNAPotyvirus
The present invention relates to a method to confer resistance or tolerance to more than one virus from the group consisting of furovirus, potyvirus, tospovirus, and cucomovirus, using sense and antisense RNA fragments of a sequence from their genomes. The sense and antisense RNA fragments are capable of pairing and forming a double-stranded RNA molecule, thereby reducing expression of the viral genome.
Owner:SYNGENTA PARTICIPATIONS AG
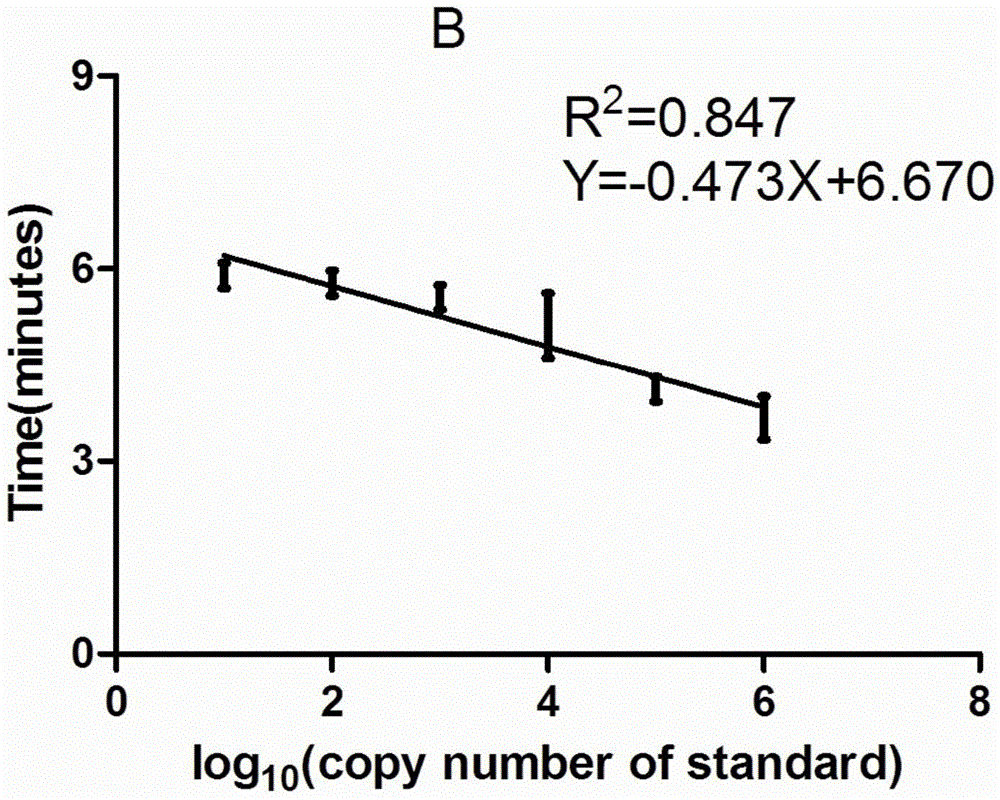
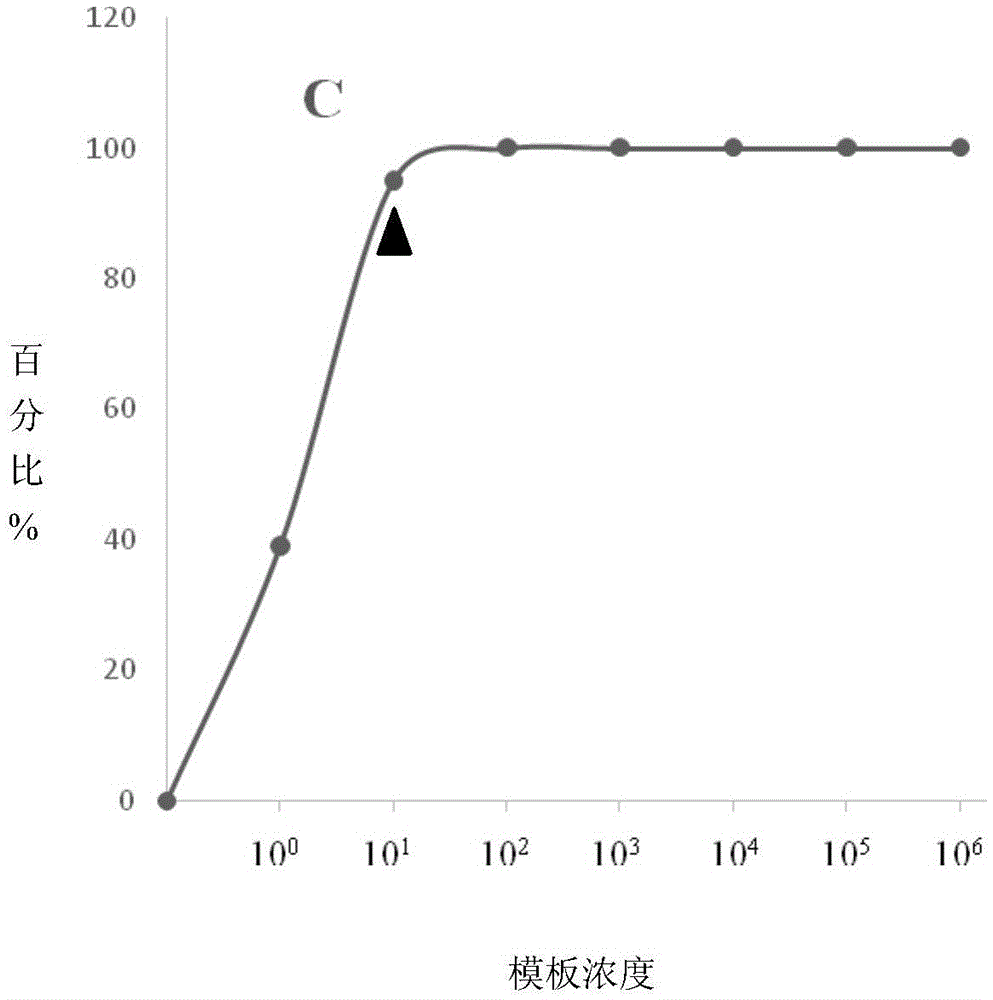
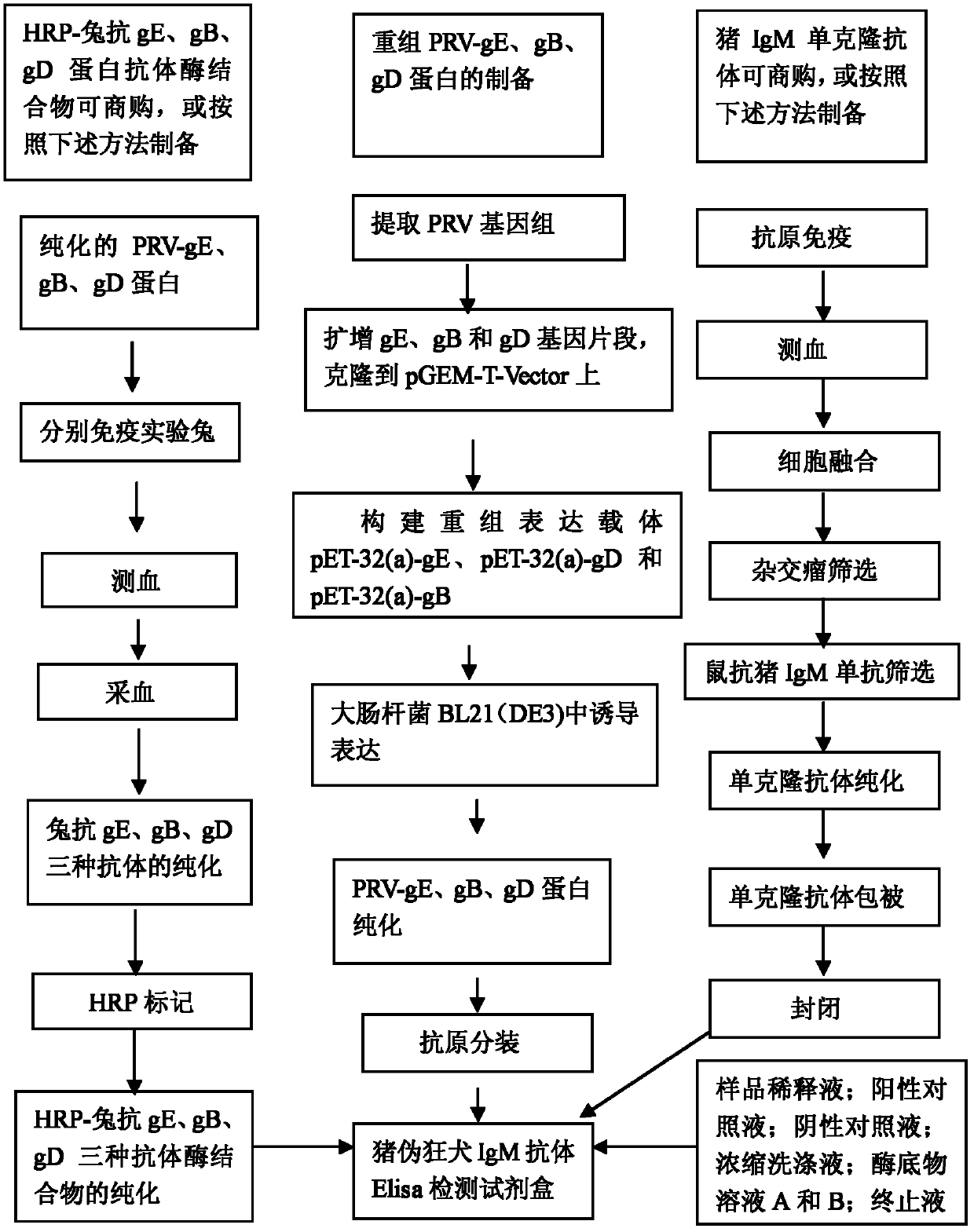
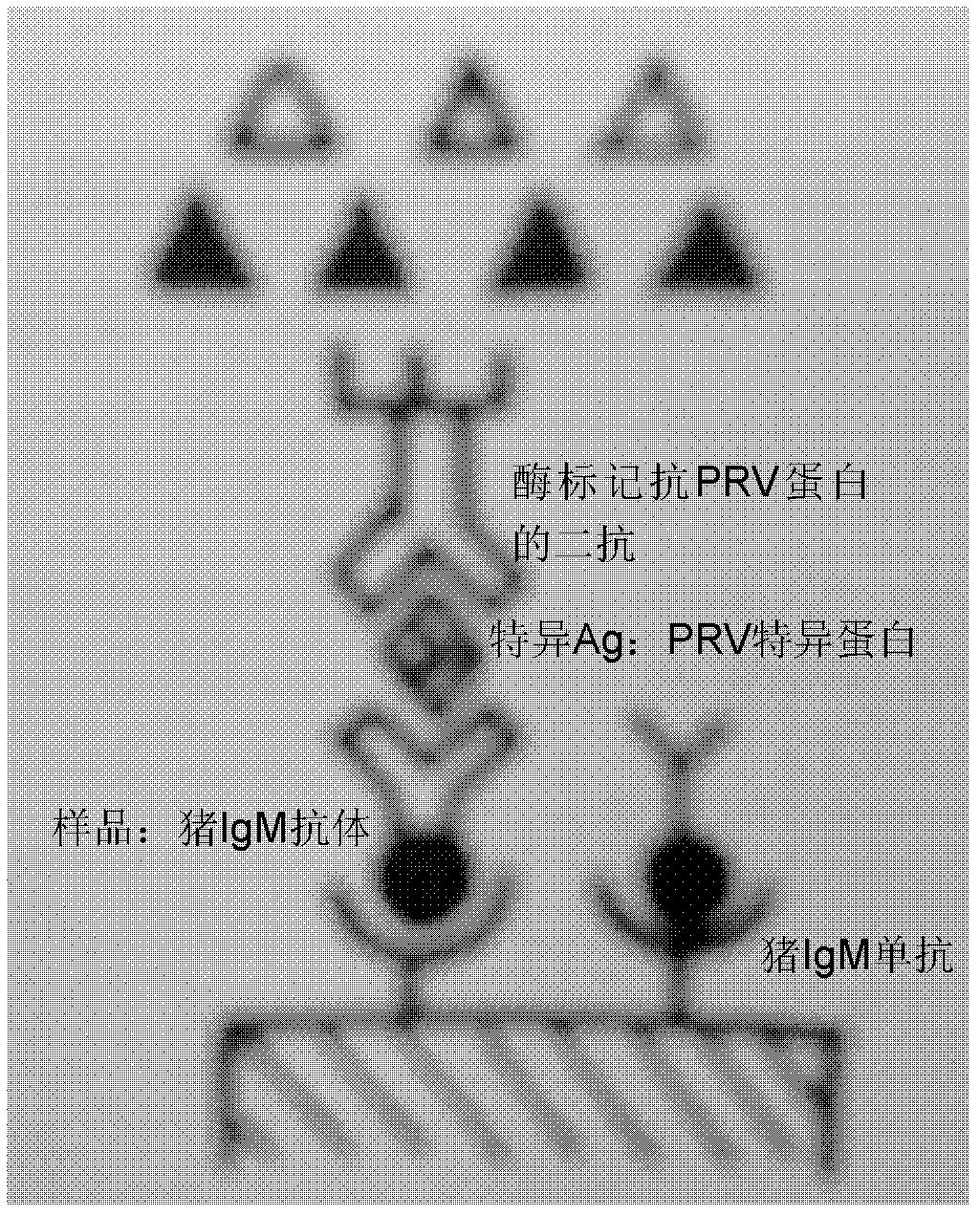
ELISA (enzyme-linked immuno sorbent assay) detection kit of porcine pseudorabies virus IgM antibody
InactiveCN103308684AStrong specificityHigh sensitivityVirus peptidesFermentationIgm antibodyHorseradish peroxidase
The invention relates to an ELISA (enzyme-linked immuno sorbent assay) detection kit of a porcine pseudorabies virus IgM antibody, and a preparation method and application thereof. The ELISA detection kit of the porcine pseudorabies virus IgM antibody disclosed by the invention comprises an elisa plate of enveloping an IgM monoclonal antibody, sealing liquid, sample diluting liquid, detecting antigen, enzyme conjugate, concentrated scrubbing solution, enzyme substrate A solution, enzyme substrate B solution and stop buffer; the detecting antigen is prepared from purified porcine pseudorabies virus gE protein, structure protein gB and structure protein gD; and the enzyme conjugate is horse radish peroxidase-anti-gE, gB or gD protein enzyme compound. The specificity of the kit disclosed by the invention can be up to 100%; the sensitivity is 1:640; and the kit can be used for early diagnosis of porcine pseudorabies virus infection.
Owner:WUHAN CHOPPER BIOLOGY
Multiplex real-time fluorescence PCR (polymerase chain reaction) detection primer and method for porcine rabies virus, porcine parvovirus and porcine circovirus type 2
InactiveCN102071259AEasy to identifyEasy diagnosisMicrobiological testing/measurementMicroorganism based processesAgricultural scienceFluorescence
The invention discloses a multiplex SYBR Green I real-time fluorescence PCR (polymerase chain reaction) detection primer and method for porcine rabies virus, porcine parvovirus and porcine circovirus type 2. The primer is obtained through synthesis according to design. The multiplex SYBR Green I real-time fluorescence PCR detection method for detecting porcine rabies virus, porcine parvovirus and porcine circovirus type 2 by utilizing the primer comprises the following steps: extracting the DNA of a sample, and then, detecting the sample by utilizing a SYBR Green I real-time fluorescence PCR reaction system and a SYBR Green I real-time fluorescence PCR amplification program. The invention has the beneficial effects that three types of viruses, namely the porcine rabies virus, the porcine parvovirus and the porcine circovirus type 2, can be simultaneously and effectively diagnosed and detected; non-specific swine fever virus, porcine reproductive and respiratory syndrome virus and swine influenza virus can not be detected; and the invention is beneficial to identification and diagnosis of the breeding disorder virus of a pregnant swine, and has better sensitivity, repeatability and stability.
Owner:HENAN AGRICULTURAL UNIVERSITY
Anthropogenic antivirulin glycosidoprotein neutralizing genetic engineering antibody RD9 and preparation and application thereof
InactiveCN101550189AOvercoming the disadvantages of instabilityHigh affinityMicrobiological testing/measurementGenetic material ingredientsSingle strandGenetic engineering
The invention relates to an anthropogenic antivirulin glycosidoprotein neutralizing genetic engineering antibody RD9 and a preparation and an application thereof. The antibody is VH-connecting peptide-VK of a three-structural single-stranded antibody comprising a variable region of heavy chain and a variable region of light chain by using 12 sequences of amino acid of the 1 (CH1) 5' end of a constant region of a heavy chain as the connecting peptide AKTTAPSVYPL, and the antibody realizes efficient expression in a prokaryotic system. The biological characteristic studies show that the RD9 is an anthropogenic genetic engineering antibody which has high appetency and better stability and can specially centralize rabies virus as well as genes and gene products of the antibody can be used for preparing clinical drugs for preventing and treating the rabies; the preparation method of the antibody is easy for massive industrial production. The invention solves the problem in the other technology of the application of genes of the variable region of the heavy chain and the variable region of a light chain of the antibody and polypeptide coded by the genes in the drugs for preventing and treating the rabies.
Owner:INST OF HYGIENE & ENVIRONMENTAL MEDICINE PLA ACAD OF MILITARY MEDICAL
Method for producing rabies vaccine for human
The invention relates to a method for producing a rabies vaccine for human, comprising the following steps of: culturing human diploid cell lines by adopting a linear amplification technique of three or more levels of bioreactors; after the human diploid cell line in each level of bioreactor reaches 106 / ml, carrying out the vaccination on the next level of bioreactor; after the human diploid cell line in the last level of bioreactor grows on a microcarrier until the density reaches 106 / ml, vaccinating rabies virus strains; propagating viruses on cells by vaccinating the rabies virus strains,harvesting virus stock solutions, and inactivating, concentrating and purifying the harvested virus stock solutions to obtain the rabies vaccine for human. By using the linear amplification techniqueof the bioreactor to culture the human diploid cell lines, the cells do not contain exogenous pollution factors and tumorigenicity, and the residual DNA of the cells has no danger, and the rabies vaccine for human has the advantages of good immunizing effect and high safety and meets the requirement of large-scale industrial production of the rabies vaccine.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD +1
Immunomagnetic bead purification kit and method for 12 kinds of swine common viruses and germs
InactiveCN105779398AEfficient separationSimple separation and purificationBacteriaMicrobiological testing/measurementBiotinStreptomycin
The invention provides an immunomagnetic bead purification kit and method for 12 kinds of swine common viruses and germs. The kit comprises a reagent A, a reagent B and a reagent C, wherein the reagent A is streptomycin immunomagnetic suspension; the reagent B is a 12-biotinylation monoclonal antibody mixed solution; and the reagent C is a 10* immunomagnetic bead separation and purification system buffer solution. The method comprises the following steps of (1) performing a pathogen suspension preparation process; (2) performing a biotin antibody preparation process; and (3) performing a pathogen immunological purification process. The immunomagnetic bead purification kit and method for 12 kinds of swine common viruses and germs provided by the invention have the advantages that 12 kinds of viruses and germs such as PPV (Porcine Parvovirus), SS-II (Streptococcus Suis Type 2), PRV (Porcine Pseudorabies Virus) and the like in a sample can be simultaneously, fast and effectively purified. The detection method has the advantages of high accuracy, high specificity, high sensitivity and high stability, and the subsequent fast diagnosis and effective detection work is facilitated.
Owner:BEIJING YISEN BIOTECH
Kit for detecting pig pseudorabies and application thereof
The invention discloses a kit for detecting pig pseudorabies. The kit comprises an assay plate coated with pig pseudorabies virus gE antigen, a rabbit anti-pig IgG antibody marked by HRP (horseradish peroxidase), fast coating buffer, fast blocking buffer, sample diluents, TMB substrate, stop buffer, 20 times wash solution, negative control, and a positive control. Through adopting pig pseudorabies virus specificity gE antigen with high purity and high activity expressed through gene engineering to coat the assay plate, and through adopting HRP-conjugate of rabbit anti-pig IgG monoclonal antibody containing HRP, the kit for detecting pig pseudorabies, provided by the invention, has the advantages of strong specificity, high sensibility, less susceptibility to misjudge of false positive or false negative and the like; moreover, the kit is simple in structure, low in detection cost, convenient and fast in operation and strong in timeliness, has a wide market prospect, and can create larger social benefits.
Owner:HENAN FENGHUA BREEDING SHARE
Pig pseudorabies virus natural low-virulent C strain and heatproof preservation method thereof
ActiveCN102399755AImprove stabilityImprove securityMicroorganism based processesViruses/bacteriophagesBiotechnologyMicroorganism
The invention relates to a pig pseudorabies virus natural low-virulent C strain and a heatproof preservation method thereof. The microbe collection registry number of the strain is CCTCC NO: V201114. According to the invention, various cryoprotectant substrates are mixed and blended according to a certain ratio; the cryoprotectants are respectively subject to aseptic processing; cryoprotectant components that can be subject to high-pressure sterilization are dissolved in bi-distilled water, and are sterilized for 15-30min under a temperature of 108-121 DEG C; cryoprotectant components that can not be subject to high-pressure sterilization are dissolved in bi-distilled water according to a certain formula, and are sterilized by using a filter membrane with a size of 0.22mum; all the cryoprotectant components are then mixed into a heatproof cryoprotectant; the heatproof cryoprotectant is mixed with a pseudorabies virus liquid according to a ratio of 1:1-1.2; and the mixture is lyophilized with a corresponding lyophilization curve. Compared to prior arts, the C strain provided by the invention has good stability. In a fifth generation animal of continuous back-procreation, the genetic sequence is stable, and no mutation is found. Therefore, the strain provided by the invention provides a good resource for the researches of pig pseudorabies low-virulent vaccines. The strain can bepreserved for 24 months under a temperature of 2-8 DEG C, and the virus titer is reduced by no more than 1 titer.
Owner:SHANGHAI CHUANG HONG BIOTECH
Method and application for fast retrograde transsynaptic labeling of nerve cells
The invention belongs to the construction field of auxiliary viruses, and discloses a method and an application of fast retrograde transsynaptic labeling of nerve cells. The invention uses double-stranded adeno-associated virus (SCAAV) as a helper virus vector to load TVA receptor and rabies virus outer membrane glycoprotein (RVG) respectively, and packaged into virus for specific recognition andretrograde transsynaptic marker of ENVA outer membrane wrapped defective recombinant rabies virus. As shown by that live test result, the recombinant defective rabies virus RV-[delta]G-X-ENVA and helper virus SCAAV combined system, can realize fast retrograde transsynaptic labeling, which saves 1-2 week experiment time, saves material resources and manpower, provides a better research tool for theapplication of defective rabies virus in neural network reverse transsynaptic marker, and lays a good technical support for the structure and function analysis of neural network.
Owner:WUHAN INST OF PHYSICS & MATHEMATICS CHINESE ACADEMY OF SCI
Multiplex RT-PCR detection primer for porcine delta coronavirus, porcine epidemic diarrhea virus and porcine transmissible gastroenteritis virus
ActiveCN105483291ARapid differential diagnosisMicrobiological testing/measurementMicroorganism based processesPorcine parvovirusBovine parvovirus
The invention discloses a multiplex RT-PCR detection primer for a porcine delta coronavirus, a porcine epidemic diarrhea virus and a porcine transmissible gastroenteritis virus. The minimum detection capacity of the multiplex RT-PCR for the three viruses is 4.05*10<1> copies / microliter, 4.52*10<3> copies / microliter and 5.47*10<3> copies / microliter respectively. The amplification results for a porcine parvovirus (PPV) and a porcine pseudorabies virus (PRV) are both negative. The multiplex RT-PCR detection results of 57 clinical samples show that one sample is infected with the three viruses at the same time, 11 samples are infected with the PDCoV, 15 samples are infected with the PEDV, one sample is infected with the TGEV, five samples are infected with the PDCoV and the PEDV, and one sample is infected with the PDCoV and the TGEV.
Owner:HENAN AGRICULTURAL UNIVERSITY
Method for large-scale production of high-purity porcine pseudorabies virus
ActiveCN107254449ASolve the technical problems that are difficult to achieve large-scale production under high purityEfficient productionDsDNA virusesAntigenFiber
The invention belongs to the technical field of vaccines and in particular relates to a method for large-scale production of a high-purity porcine pseudorabies virus. The method comprises processes of continuous flow centrifugation, hollow fiber clarification filtering, ultrafiltration and concentration, and molecular sieve purification. The virus recycling rate is increased to the maximum extent, and the content of impurity proteins is reduced. A porcine pseudorabies virus concentrated solution and purified antigen produced by using the method are particularly applicable to vaccine preparation, and compared with a porcine pseudorabies virus inactivated vaccine produced according to the prior art, the high-purity porcine pseudorabies virus is relatively high in safety, relatively high in uniformity and relatively good in immune effect, and side reactions of vaccines are fundamentally reduced.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Kit for detecting rabies virus antibody
ActiveCN108872572AImprove featuresIncreased sensitivityBiological material analysisAntigenMagnetic bead
The invention discloses a kit for detecting a rabies virus antibody, which belongs to the technical field of biological diagnosis. The kit comprises a magnetic bead coupled with a rabies virus antigen, a second antibody combined with the rabies virus antibody and provided with a marker, a rabies virus antibody standard product, a rabies virus antibody quality control product, a sample dilute solution, and a washing solution, wherein the DNA sequence of the rabies virus antigen is as shown by Seq No. 1, and an amino acid sequence of the rabies virus antigen is as shown by Seq No. 2. By adoptingthe kit, a to-be-detected substance can be separated by adopting a tubular magnetic ball, then the detection is performed by adopting a chemical luminescent method, the interference of impurities canbe avoided, the possibility of false positive can be reduced, and the detection sensitivity can be improved.
Owner:GUANGZHOU YOUDI BIOTECH CO LTD
Method for detecting canine rabies virus antibody and detection kit
The invention provides a method for detecting canine rabies virus antibody (IgG) and a detection kit. The kit is composed of a coated plate and a reagent reaction system, and comprises a rabies virus antigen-coated reaction plate, a standard substance, positive contrast serum, a washing lotion (20X), a sample weak solution, an enzyme-marked combination substance, a developer A, a developer B and a stopping solution. The method is characterized in that the method uses an ELISA method to determine that the content of the canine rabies virus antibody (IgG) reaches a absorbance corresponding to a protective level by detecting the standard substance of the kit, and by compared the absorbance of the sample to be detected with the absorbance of the standard substance, the content of the canine rabies virus antibody (IgG) contained in the sample to be detected is judged whether to reach an immunization protective level. The method and the kit can simultaneously detect a lot of samples, and a detection result is remarkably with a result by a neutralization experiment, has high accuracy degree, is suitable for monitoring an immunization inoculation effect and determining an individual immunization state, and can be used for investigating animal eqpidemic diseases.
Owner:ZHENGZHOU ZHONGDAO BIOTECHNOLOGY CO LTD
Fusion protein of porcine pseudorabies virus and preparation method, application and vaccine of fusion protein
ActiveCN109134669AImprove antigen broad spectrumGood broad-spectrum antigenAntibody mimetics/scaffoldsVirus peptidesAntigenResearch Object
The invention relates to the technical field of biology, in particular to a fusion protein of porcine pseudorabies virus and a preparation method, application and vaccine of the fusion protein. The fusion protein of porcine pseudorabies virus comprises a gB section and a gD section, wherein the gB section is expressed by the nucleotide sequence shown in SEQ ID NO.1; and the gD section is expressedby the nucleotide sequence shown in SEQ ID NO.2. The sequences shown in SEQ ID NO.1 and SEQ ID NO.2 are the sequences obtained through contrast and analysis by selecting genes of classical strains and current epidemic strains as research objects, and codon optimization and modification are preformed on the sequences, so that the broad spectrum of fusion protein antigen is further improved and theantigen expression amount is increased. The invention further provides a preparation method and application of the fusion protein, and the vaccine for preparation.
Owner:天康生物制药有限公司
Freeze-dried rabies vaccine for humans and preparation method of vaccine
ActiveCN104826101AThe process steps are simpleEasy to operateInactivation/attenuationAntiviralsHuman useSide effect
The invention relates to a freeze-dried rabies vaccine for humans and a preparation method of the vaccine, relates to the field of vaccine production preparation technologies and aims at solving the problems that effective virus antigen expression content is low, the side effect rate of a vaccinator is high and vaccine yield and quality can not meet standard requirements as only a biological reactor is adopted for producing a rabies vaccine. The freeze-dried rabies vaccine for humans is obtained by inoculating aG strain rabies virus on Vero cells and sequentially carrying out ultrafiltration and concentration, separation and purification as well as freeze drying, wherein the packing volume of the freezed-dried rabies vaccine for human use is 0.5ml / dose, and during freeze drying, the adopted vaccine freeze-drying protecting agent comprises the following ingredients: 60-90g / l of trehalos, 6-14g / l of sodium glutamate, 3-6g / l of urea, 2-3g / l of L-arginine and 10g / l of 199 culture medium, and the vaccine freeze-drying protecting agent does not contain gelatin, human serum albumin or dextran. The freeze-dried rabies vaccine for humans has the advantages that cost is low, operation is easy, pollution is hardly produced, vaccine quality and yield are greatly improved, the content of impurities in a vaccine is reduced, allergy reactions are hardly caused, and vaccine safety is greatly improved.
Owner:江生(深圳)生物技术研发中心有限公司
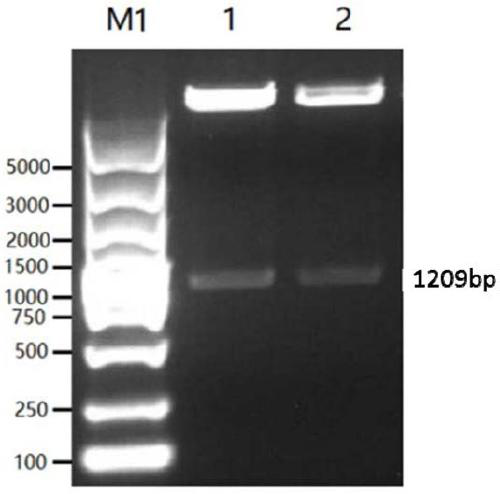
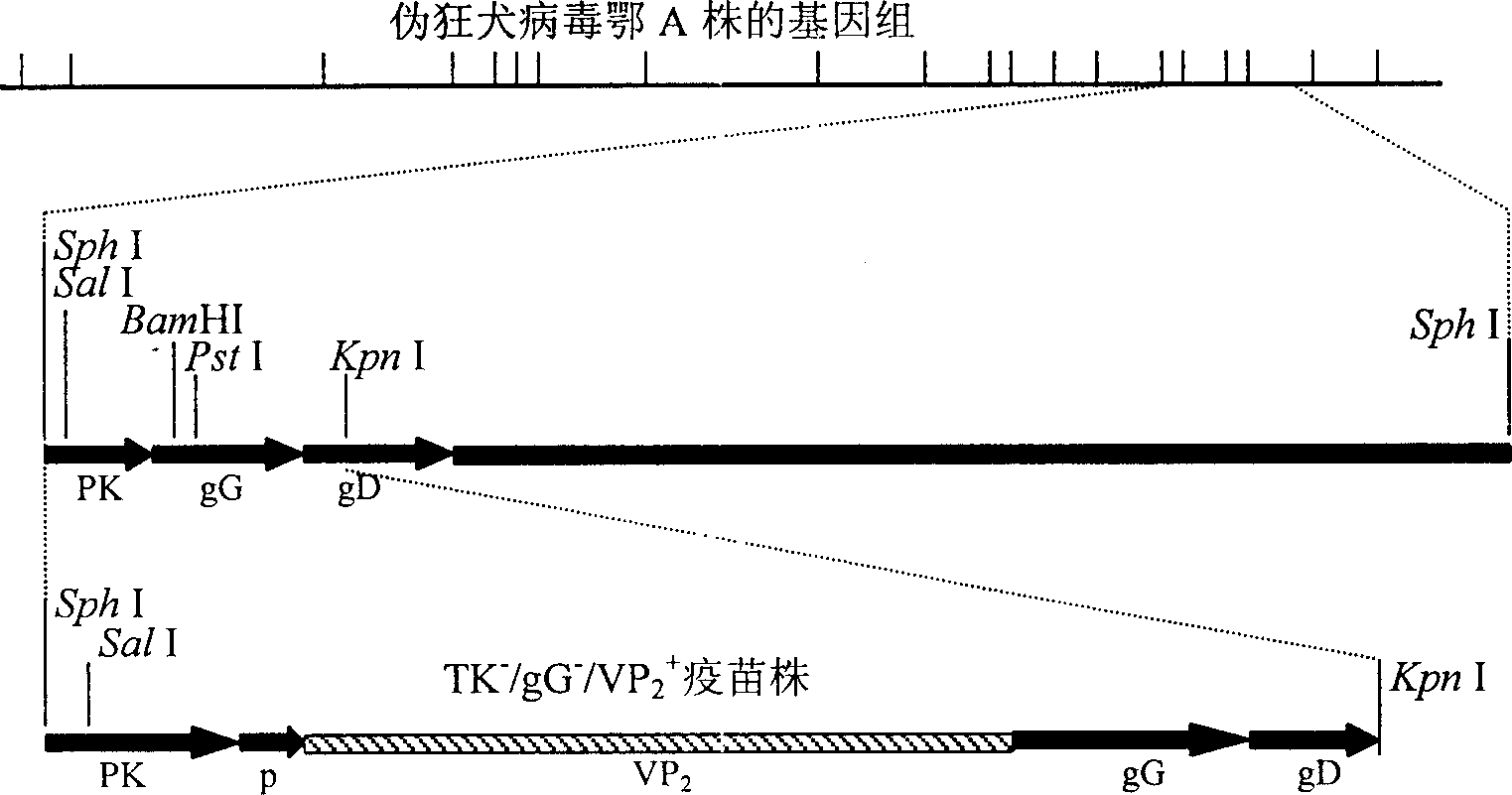
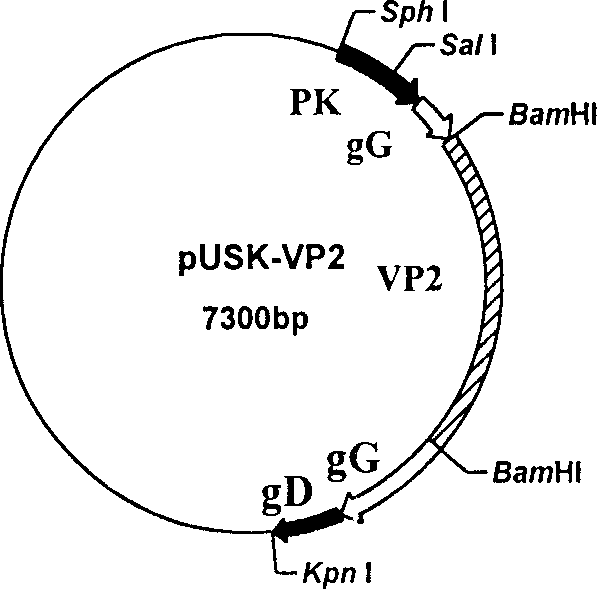
Recombinant pseudo-rabies virus expressing swine parvovirus VP2 gene and vacine and its preparation method
The present invention relates to main structure gene VP2 of artificial constructed pseudorabies virus, PrV and porcine parvovirus, PPV. In the pseudorabies virus genome in which the main toxicity gene (TK) and virus generation nonessential gene (gG) are deleted the VP2 gene of porcine parvovirus can be site-specifically inserted to make it be positioned in strong late promoter downstream of pseudorabies virus, and the inserted exogenous gene coded protein has good immunogenicity, and can stimulate swine to produce protective immune reaction for resisting two virulent challenges of porcine parvovirus and pseudorabies virus. Said invention also includes recombinant pseudorabies virus, Hzau AVL-PRppvV-VP2, vaccine prepared by using it and its preparation method.
Owner:HUAZHONG AGRI UNIV
Dual SYBR Green I real-time fluorescence PCR detection primer and method for porcine pseudorabies virus and porcine circovirus type 2
InactiveCN102071256AEasy to identifyEasy diagnosisMicrobiological testing/measurementMicroorganism based processesFluorescencePorcine circovirus
The invention discloses a dual SYBR Green I real-time fluorescence polymerase chain reaction (PCR) detection primer and a dual SYBR Green I real-time fluorescence PCR detection method for porcine pseudorabies virus and porcine circovirus type 2. The primer is designed and synthesized, and the dual SYBR Green I real-time fluorescence PCR detection method for the porcine pseudorabies virus and the porcine circovirus type 2 by using the primer comprises the following steps of: extracting deoxyribose nucleic acid (DNA) of a sample; and detecting the sample by using an SYBR Green I real-time fluorescence PCR reaction system and an SYBR Green I real-time fluorescence PCR amplification program. By the primer and the method, two viruses, namely the porcine pseudorabies virus and the porcine circovirus type 2 can be detected simultaneously, and porcine parvovirus, classical swine fever virus, porcine reproductive and respiratory syndrome virus and swine influenza virus cannot be detected. The primer and the method have the characteristics of higher sensitivity, repeatability and stability, and contribute to the identification and the diagnosis of pregnant sow reproductive disturbance virus disease.
Owner:HENAN AGRICULTURAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com