Freeze-dried rabies vaccine for humans and preparation method of vaccine
A technology of rabies vaccine and rabies virus, which is applied in the field of freeze-dried human rabies vaccine and its preparation, can solve the problems of insufficient vaccine yield and quality, low antigen expression content, and high incidence of side effects in vaccinators, and achieve saving Raw materials, simple operation, and the effect of improving space utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
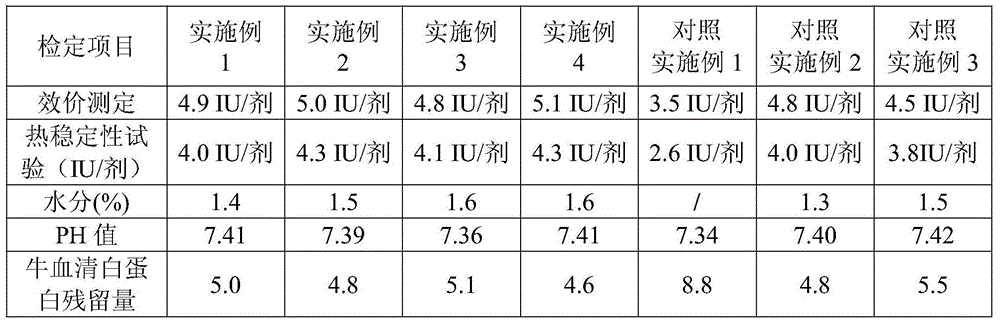
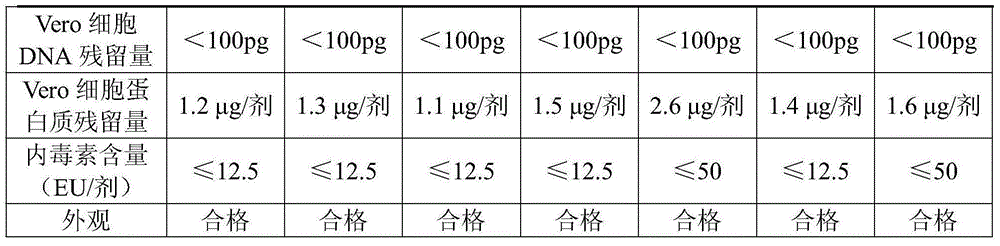
Examples
Embodiment 1
[0034] Embodiment 1 prepares freeze-dried human rabies vaccine
[0035] (1) The virus seed adopts aG strain rabies virus, and the culture substrate adopts Vero cells;
[0036] (2) Digest and disperse the resuscitated Vero cells with 0.25% trypsin, add 199 culture medium and mix evenly, then add to the cell factory (Cell Factories, Nunc or CORNING, USA) and culture at 37°C until a uniform monolayer Vero cells;
[0037] (3) Wash and replace the PBS solution in the bioreactor (BIOFLO CELLIGEN 310 type, produced by NBS Company of the United States) with 199 culture medium containing 10% newborn bovine serum. .0, dissolved oxygen 50%, after each parameter is stable, inoculate the monolayer Vero cells that have been digested and dispersed uniformly, and the number of inoculated cells (working volume) is controlled at 2×10 5 A / ml is appropriate;
[0038] (4) According to the growth and reproduction of Vero cells, the perfusion amount of the bioreactor was adjusted appropriately, w...
Embodiment 2
[0046] Embodiment 2 prepares freeze-dried human rabies vaccine
[0047] (1) The virus seed adopts aG strain rabies virus, and the culture substrate adopts Vero cells;
[0048] (2) Digest and disperse the resuscitated Vero cells with 0.25% trypsin, add 199 culture medium and mix evenly, then add to the cell factory (Cell Factories, Nunc or CORNING, USA) and culture at 37°C until a uniform monolayer Vero cells;
[0049] (3) Wash and replace the PBS solution in the bioreactor (BIOFLO CELLIGEN 310 type, produced by NBS Company of the United States) with 199 culture medium containing 10% newborn bovine serum. .0, dissolved oxygen 50%, after each parameter is stable, inoculate the monolayer Vero cells that have been digested and dispersed uniformly, and the number of inoculated cells (working volume) is controlled at 3×10 5 It is advisable to be within the range of / ml;
[0050] (4) According to the growth and reproduction of Vero cells, the perfusion amount of the bioreactor was...
Embodiment 3
[0058] Embodiment 3 prepares freeze-dried human rabies vaccine
[0059] (1) The virus seed adopts aG strain rabies virus, and the culture substrate adopts Vero cells;
[0060] (2) Digest and disperse the resuscitated Vero cells with 0.25% trypsin, add 199 culture medium and mix evenly, then add to the cell factory (Cell Factories, Nunc or CORNING, USA) and culture at 37°C until a uniform monolayer Vero cells;
[0061] (3) Wash and replace the PBS solution in the bioreactor (BIOFLO CELLIGEN 310 type, produced by NBS Company of the United States) with 199 culture medium containing 10% newborn bovine serum. .0, dissolved oxygen 50%, after each parameter is stable, inoculate the monolayer Vero cells that have been digested and dispersed uniformly, and the number of inoculated cells (working volume) is controlled at 4×10 5 It is advisable to be within the range of / ml;
[0062] (4) According to the growth and reproduction of Vero cells, the perfusion volume of the bioreactor was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com