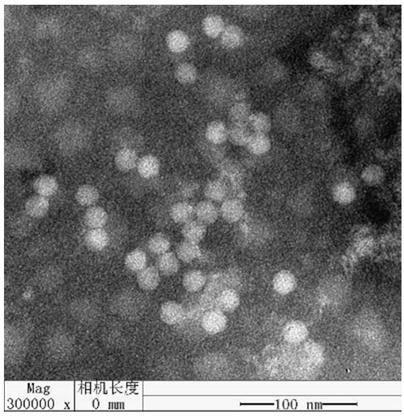
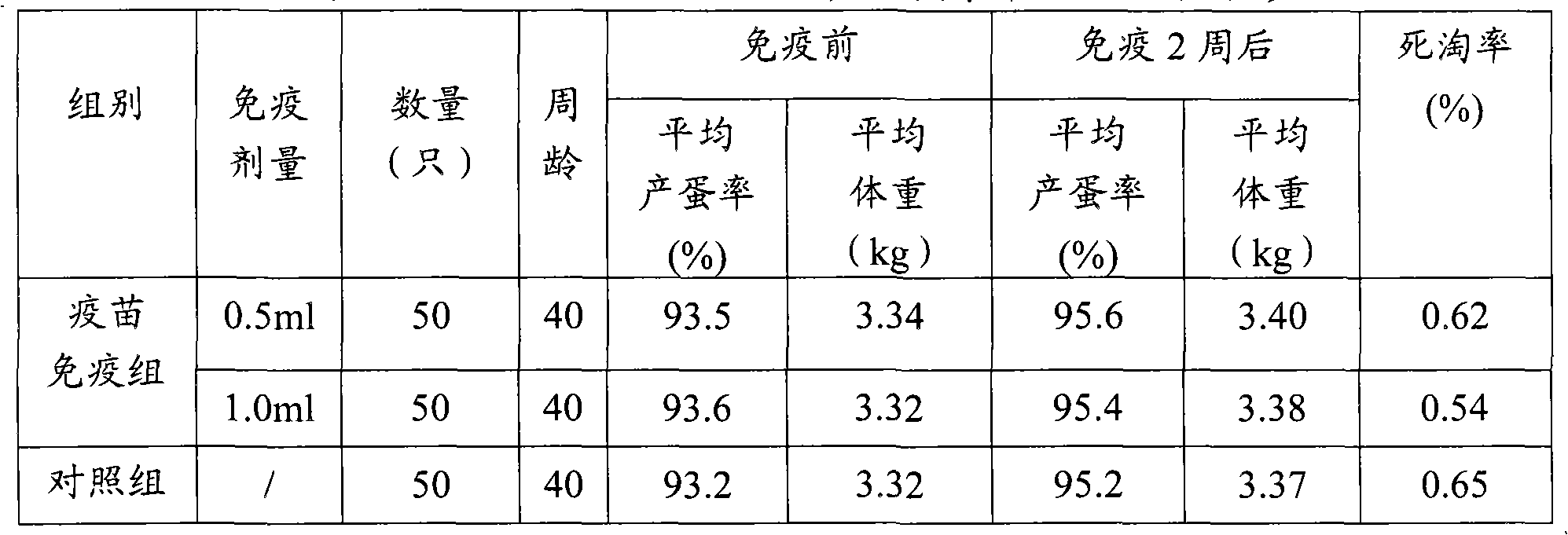
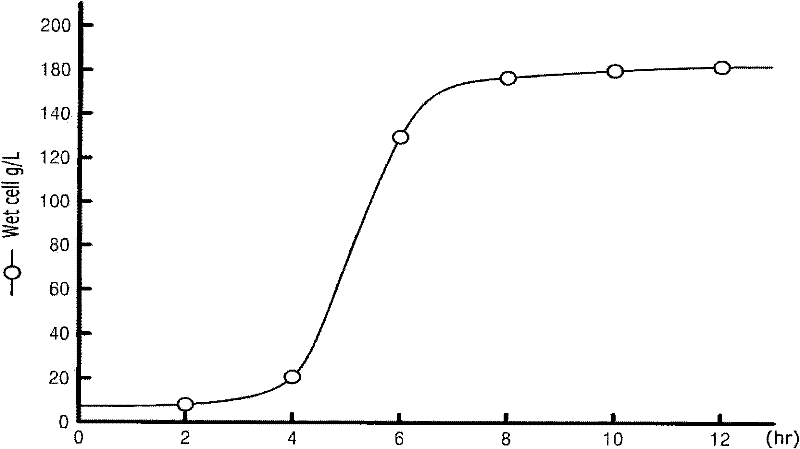
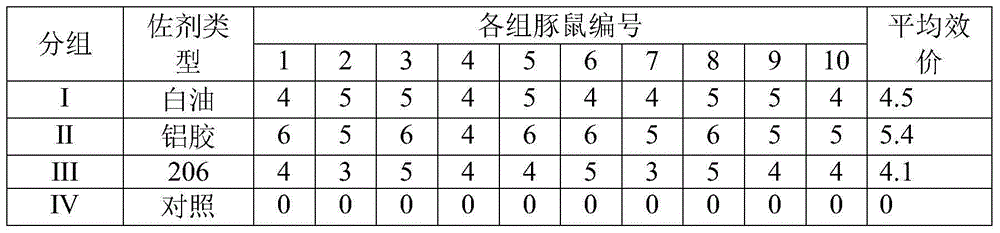
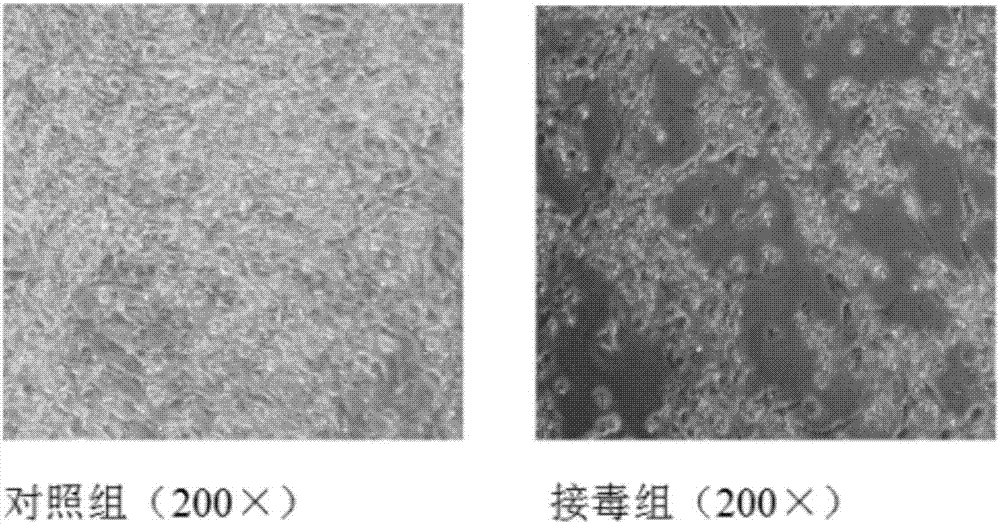
Patents
Literature
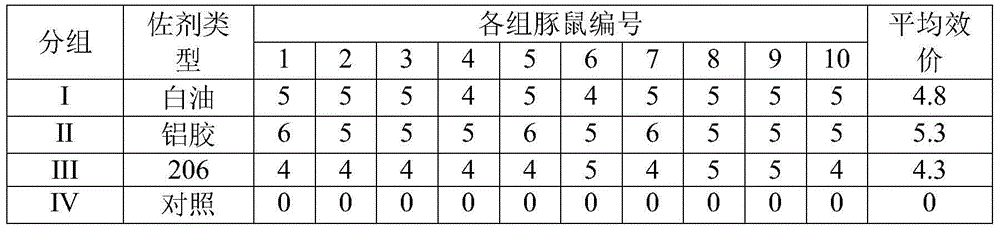
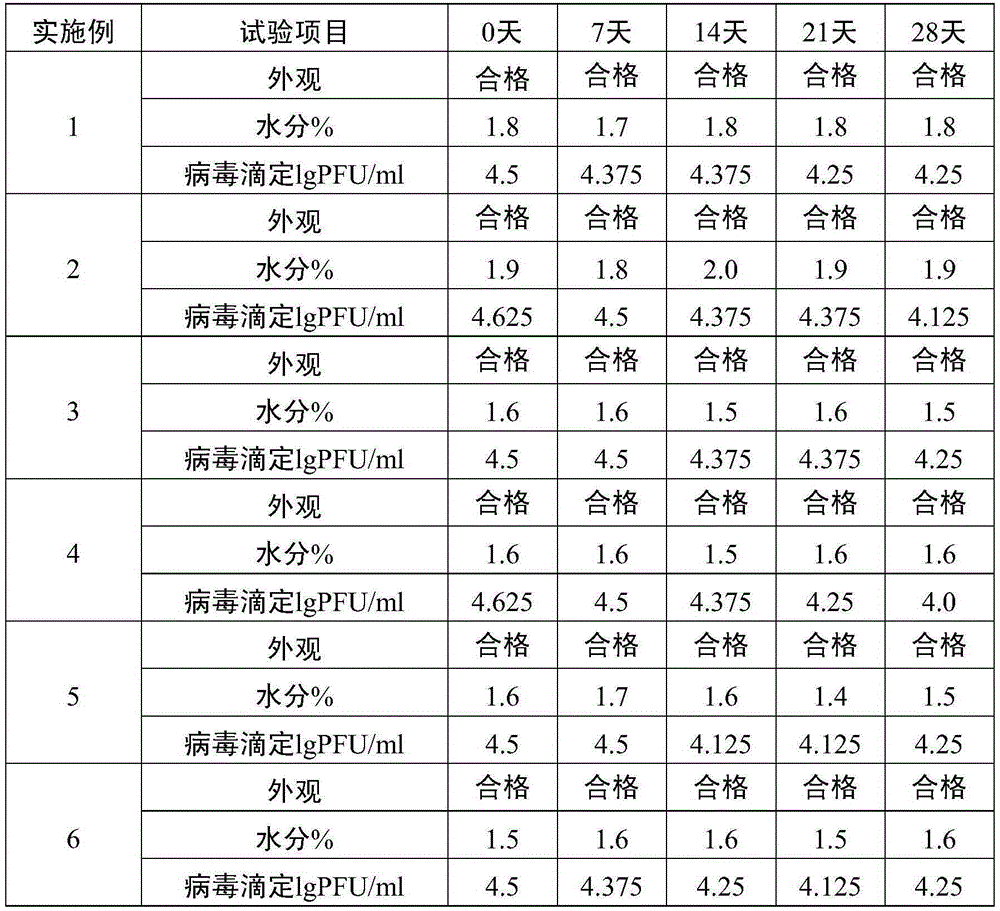
181 results about "Vaccine safety" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The United States’ long-standing vaccine safety program closely and constantly monitors the safety of vaccines. A critical part of the program, CDC’s Immunization Safety Office identifies possible vaccine side effects and conducts studies to determine whether health problems are caused by vaccines. Data show...
Porcine pseudorabies virus (PRV) variant PRV-ZJ01 and application thereof
ActiveCN103627678AImprove securityImproving immunogenicityMicroorganism based processesAntiviralsRabiesEngineering
The invention relates to the technical field of porcine pseudorabies viruses (PRVs) and in particular relates to a porcine PRV variant PRV-ZJ01 with collection number of CGMCCNo.8170 and an application of the porcine PRV variant PRV-ZJ01 in preparation of vaccines. The porcine PRV variant PRV-ZJ01 has the beneficial effects that a water-soluble inactivated vaccine is prepared by adopting a PRV-ZJ01 variant virus solution and is subjected to a swine immune protection test with live vaccines of Bartha-K61, Bucharest and HB-98 strains and the results show that the inactivated vaccine of the ZJ01 strain has relatively high safety and has the immune protection efficiency obviously higher than that of immunity groups of the live vaccines of the Bartha-K61, Bucharest and HB-98 strains, and the live vaccines of the Bartha-K61, Bucharest and HB-98 strains can not provide full protection for the ZJ01 very virulent strain; the inactivated vaccine of ZJ01 has relatively good immune protection effects on the PRV variant and the traditional strains; infected with 10<6.0>TCID50 (Tissue culture infectious dose 50) / ml nasal drops of the PRV-ZJ01 variant, all the 85-day-old non-immune swine can become ill and die; results prove that the virulence of the virus strain is obviously enhanced, the antigenicity is varied and the virus strain has relatively good immunogenicity after being inactivated and can be used for research and development of the vaccine of the virus strain and the diagnostic methods.
Owner:NANJING AGRICULTURAL UNIVERSITY
Method for producing swine fever live vaccine with cell line
ActiveCN101181637AGuaranteed to be pureEnsure safetyAntiviralsAntibody medical ingredientsQuality controlSeedling
The invention discloses a method for producing a live swine fever vaccine by using a cell line. The present invention comprises the following technical steps: (1) selecting a cell line as the cells for making seedlings; (2) subculture and cultivation of cells for making seedlings; (3) breeding of cytotoxic species; (4) breeding of venom for making seedlings; 5) Mixing seedlings, subpackaging and freeze-drying. The invention has the advantages of simple and stable production process, easy operation, high virus content, small difference between batches, easy quality control, and can significantly improve the yield and quality of vaccines. The live swine fever vaccine produced by the invention has good safety and high immune efficacy, and has complete immune protection against the virulent attack of swine fever.
Owner:CHINA INST OF VETERINARY DRUG CONTROL +1
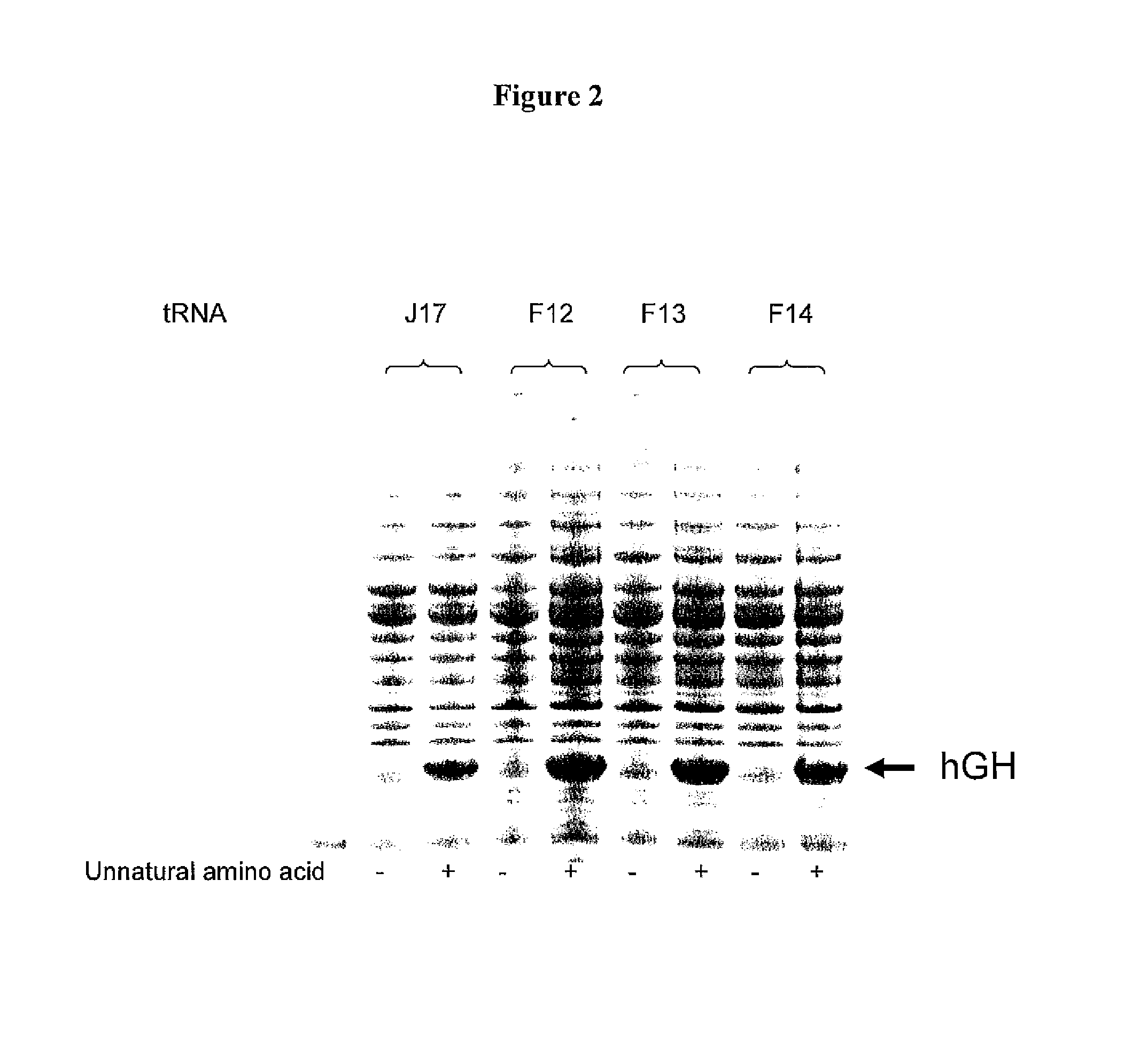
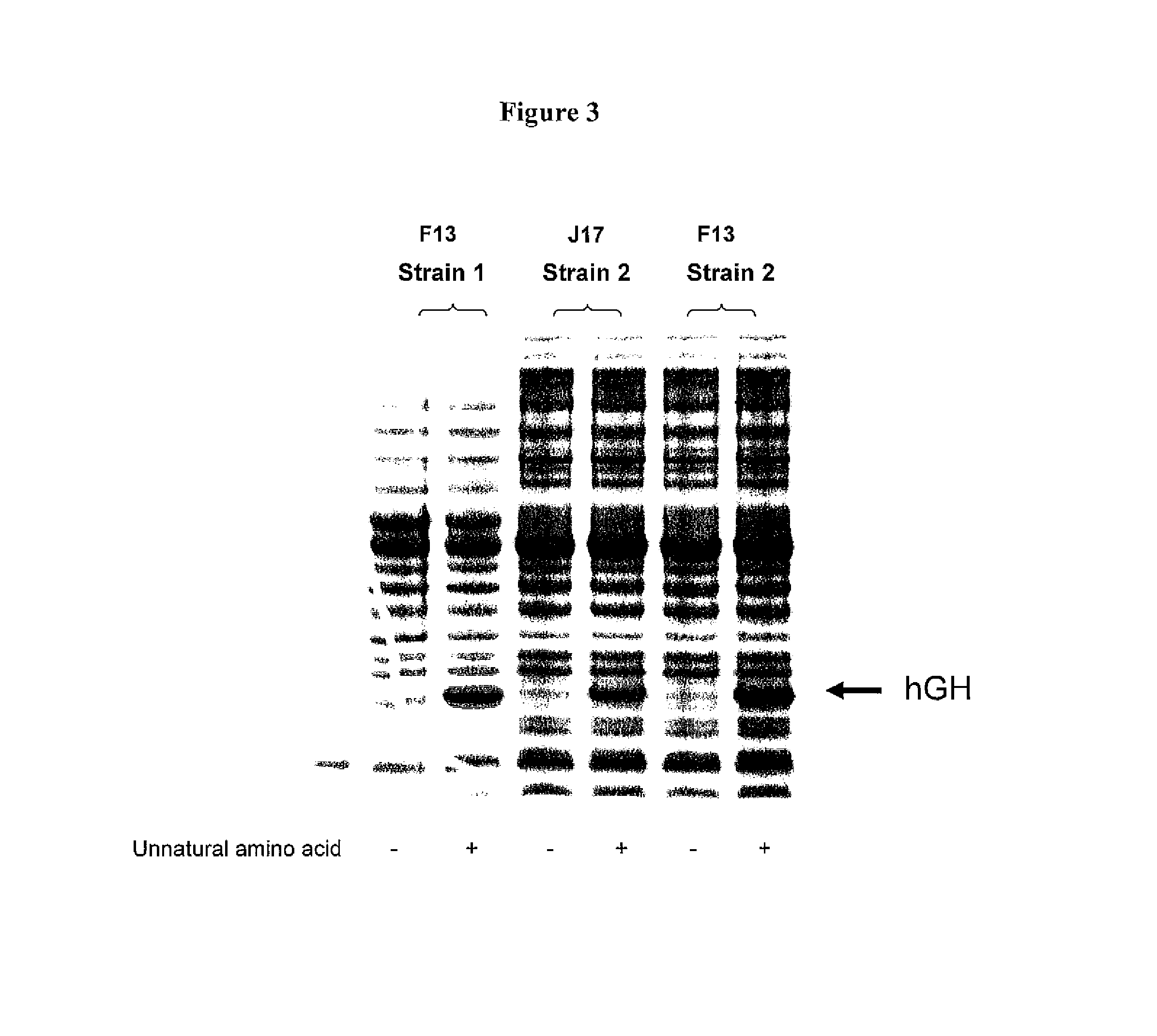
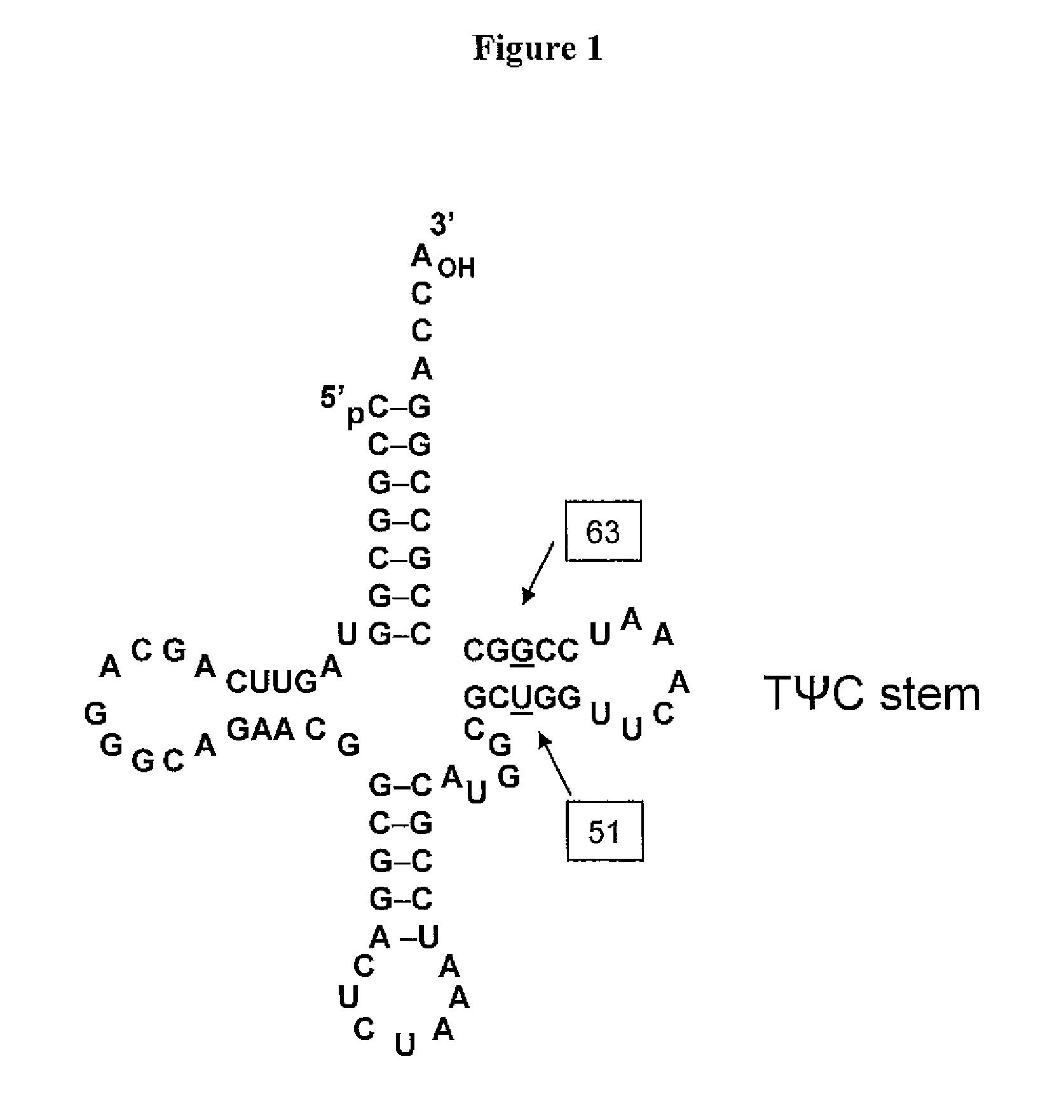
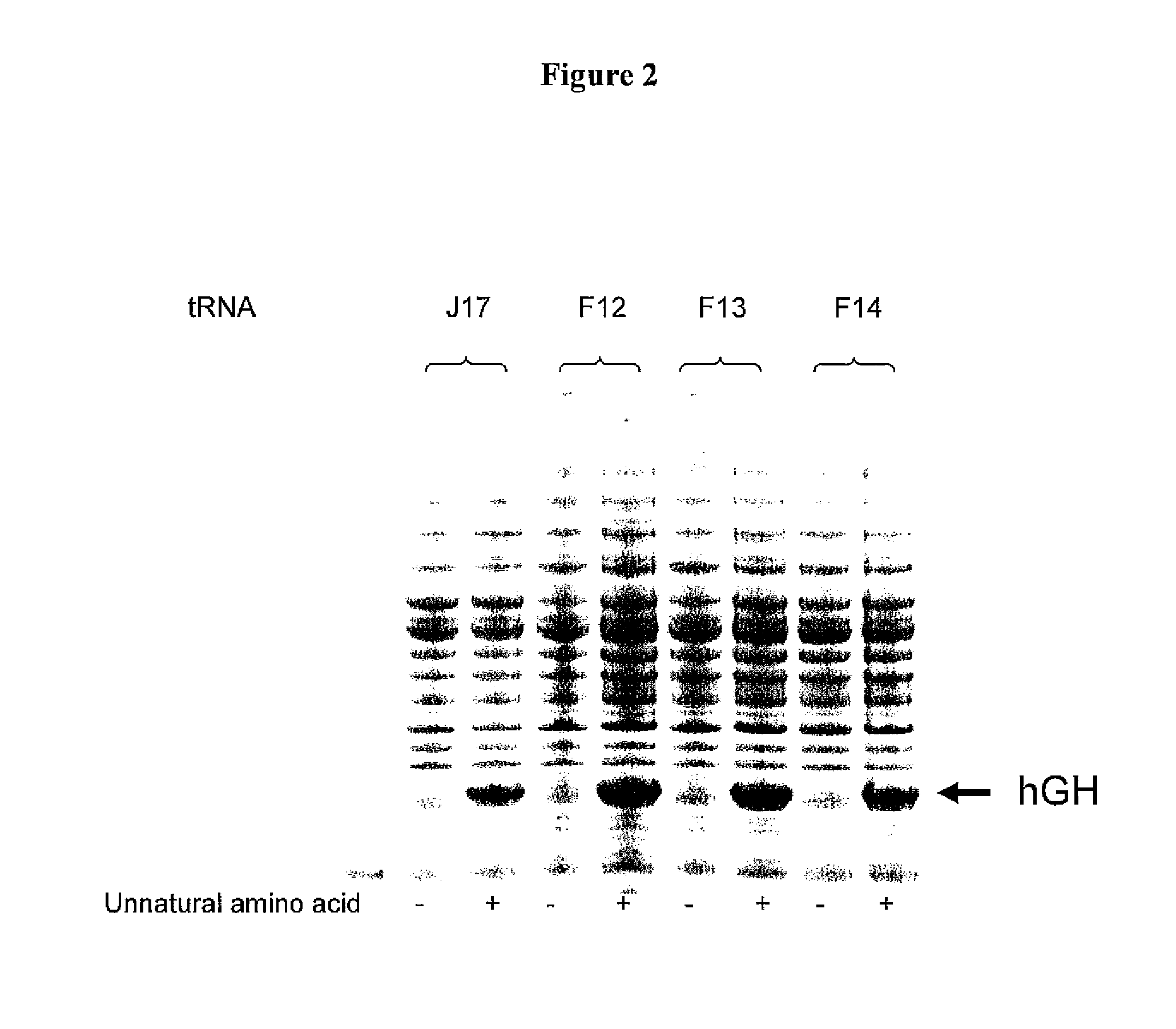
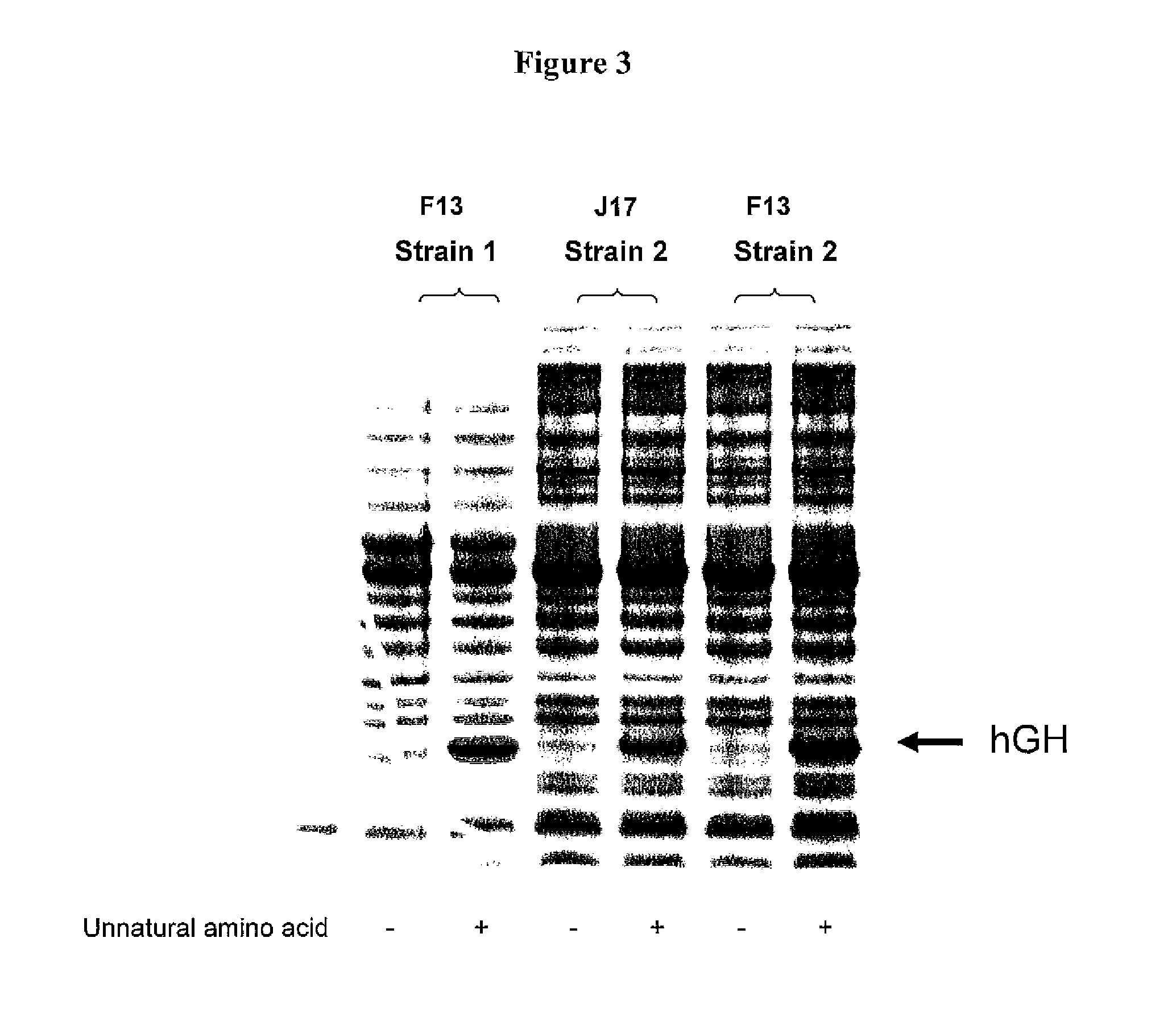
Non-Natural Amino Acid Replication-Dependent Microorganisms and Vaccines
ActiveUS20130323821A1Low efficacyEnhance antigen presentationAntibacterial agentsBacterial antigen ingredientsMicroorganismBiological body
Compositions and methods of producing vaccines, including methods wherein whole organism vaccines are provided with limited replication abilities, thereby increasing vaccine safety and efficacy, through the use of non-natural, unnatural, or non-naturally encoded amino acids.
Owner:AMBRX
Seneca valley virus strain and application thereof
ActiveCN109182278AImproving immunogenicityDistant relationshipSsRNA viruses positive-senseViral antigen ingredientsDiseaseVaccine Immunogenicity
The invention discloses a Seneca valley virus strain and an application thereof, wherein the Seneca valley virus SVV-HeNXX / swine / 2017 is deposited in China Center For Type Culture Collection with theserial number of CCTCCNO: V201767. The Seneca valley virus SVV-HeNXX / swine / 2017 has good immunogenicity. It can induce immune animals to produce higher level of immune protection and can be used for preparing vaccine against Seneca Valley Virus Disease. The vaccine has high safety and no detoxification after immunization, and can induce animals to produce higher level of antibody quickly, so as toachieve high-efficient prevention of Porcine Seneca Valley Virus Disease, and has good popularization and application value.
Owner:HENAN CENT FOR ANIMAL DISEASE CONTROL & PREVENTION
Duck virus hepatitis strains and inactivated vaccine
ActiveCN102086447AHigh poison priceRaise antibody levelsDigestive systemMicroorganism based processesDuck hepatitis A virusFreeze thawing
The invention discloses methods for preparing a duck virus hepatitis strain and an inactivated vaccine. The strain is obtained with the method comprising the following steps of: collecting duck hepatitis material with obvious pathological changes in a duck farm where duck virus hepatitis prevails, washing with bi-anti-sterilization PBS, diluting and grinding, freeze thawing twice and performing centrifugal separation; diluting virus separated by sterilization PBS, vaccinating chick embryo, selecting chick embryo with classic pathological changes that is dead 48-96h after vaccination, respectively gaining chick embryo liquid, transferring for 18-25 generations continuously to obtain a duck virus hepatitis virus chick embryo suitable strain. The strain can be in production, vaccinated in the chick embryo to gain chick embryo liquid, and is emulsified after vaccination to prepare the inactivated vaccine. The inactivated vaccine is high in safety, has good immune efficacy to young ducks, long immune time for breeding ducks and high level of antibody of breeding ducks, and the young ducks bred by the breeding ducks can resist duck virus hepatitis strains.
Owner:PU LIKE BIO ENG
Method for producing pseudorabies living vaccines by using subculture cell source and product thereof
ActiveCN101695573AImprove securityImprove immune efficiencyAntiviralsViruses/bacteriophagesPig kidneyAntibiotic Y
The invention provides a method for producing pseudorabies living vaccines by using a subculture cell source and a pseudorabies living vaccine product thereof. The method comprises the following steps: culturing pseudorabies virus low-virulent strains by using subculture cells; harvesting the strains to obtain cell culture venom; and then adding a stabilizing agent and an antibiotic into the cellculture venom, and freezing and vacuum-drying the mixture to obtain the pseudorabies living vaccines of the subculture cell source. The subculture cells are subculture cells ST of pig testicle or subculture cells PK15 or IBRS-2 of pig kidney. The method for producing the pseudorabies living vaccines by using the subculture cell source has the advantages of simple and stable production process, easy operation, high virus content, little batch difference and controllable quality, can remarkably improve the yield and quality of the vaccines and reduce the anaphylactic reaction and the like. The pseudorabies living vaccines obtained by using the production method of the invention have good safety and high immune efficacy, and have better immune protection effect on pseudorabies virulent attack.
Owner:广东永顺生物制药股份有限公司
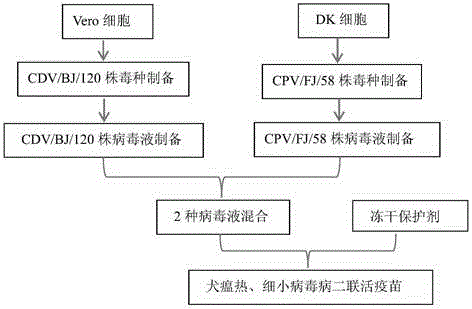
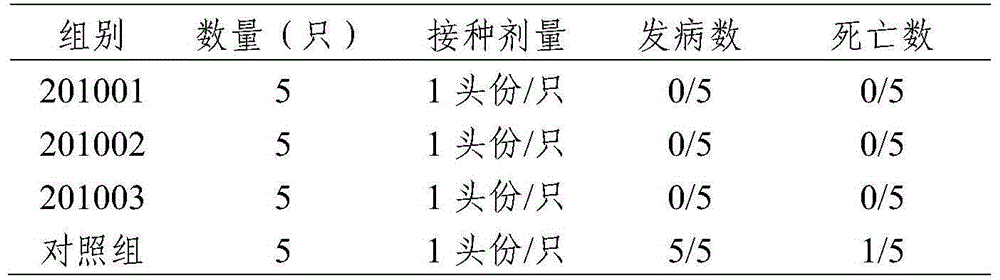
Bivalent live vaccine against canine distemper and parvovirus diseases, and preparation method thereof
ActiveCN106399260AQuality is easy to controlSuitable for industrial productionMicroorganism based processesAntiviralsCanine distemper virus CDVCanine parvovirus
The invention discloses a bivalent live vaccine against canine distemper and parvovirus diseases, and a preparation method thereof. The bivalent live vaccine contains an attenuated anti-canine distemper vaccine strain with a microbial accession number of CGMCC No. 10980 and an attenuated anti-parvovirus distemper vaccine strain with a microbial accession number of CGMCC No. 7408. According to the invention, a canine distemper virus and a canine parvovirus separated from wild viruses of diseased Chinese dogs and having undergone attenuation via domestication are used for preparation of a bivalent vaccine together, and a freeze-drying protective agent is added so as to prepare the bivalent live vaccine against canine distemper and parvovirus diseases. The bivalent live vaccine is reliable in security, good in immune effect, strong in targeting performance, suitable for prevention of diseased caused by diseased Chinese dogs and applicable to prevention of canine distemper and parvovirus diseases.
Owner:兆丰华生物科技(南京)有限公司 +3
Serum-12 type haemophilus lus paradis vaccine strain and application thereof
InactiveCN104450556AStrong pathogenicityLong durationAntibacterial agentsBacteriaSerum igeHaemophilus
The invention relates to a serum-12 type haemophilus lus paradis vaccine strain. The classified name of the vaccine strain is haemophilus lus paradis, the strain name is SHCM10 and the vaccine strain is preserved in the China Center for Type Culture Collection on June 15, 2014 with the preservation number of CCTCC NO:M 2014261. The invention further relates to an application of the serum-12 type haemophilus lus paradis vaccine strain in preparation of a haemophilus lus paradis inactivated vaccine. The serum-12 type haemophilus lus paradis SHCM10 strain is stable in biology, has a strong pathogenicity to a piglet, and has a good immunogenicity when being inactivated and vaccinated on the piglet. A univalent vaccine prepared from the vaccine strain serving as a vaccine candidate strain has good safety, can produce a relatively high antibody on the piglet, has long duration and good immune potency, and can be used for resisting attack of homotype wild strains. After a pig group is immunized, the morbidity and death rate are remarkably reduced, the economic loss of a piggery is reduced, and the immunizing effect of the vaccine strain is the same as or superior to that of existing commercial vaccines on the market.
Owner:扬州优邦生物药品有限公司
Non-Natural Amino Acid Replication-Dependent Microorganisms and Vaccines
ActiveUS20130280301A1Low efficacyEnhance antigen presentationAntibacterial agentsBacterial antigen ingredientsMicroorganismBiological body
Compositions and methods of producing vaccines, including methods wherein whole organism vaccines are provided with limited replication abilities, thereby increasing vaccine safety and efficacy, through the use of non-natural, unnatural, or non-naturally encoded amino acids.
Owner:AMBRX
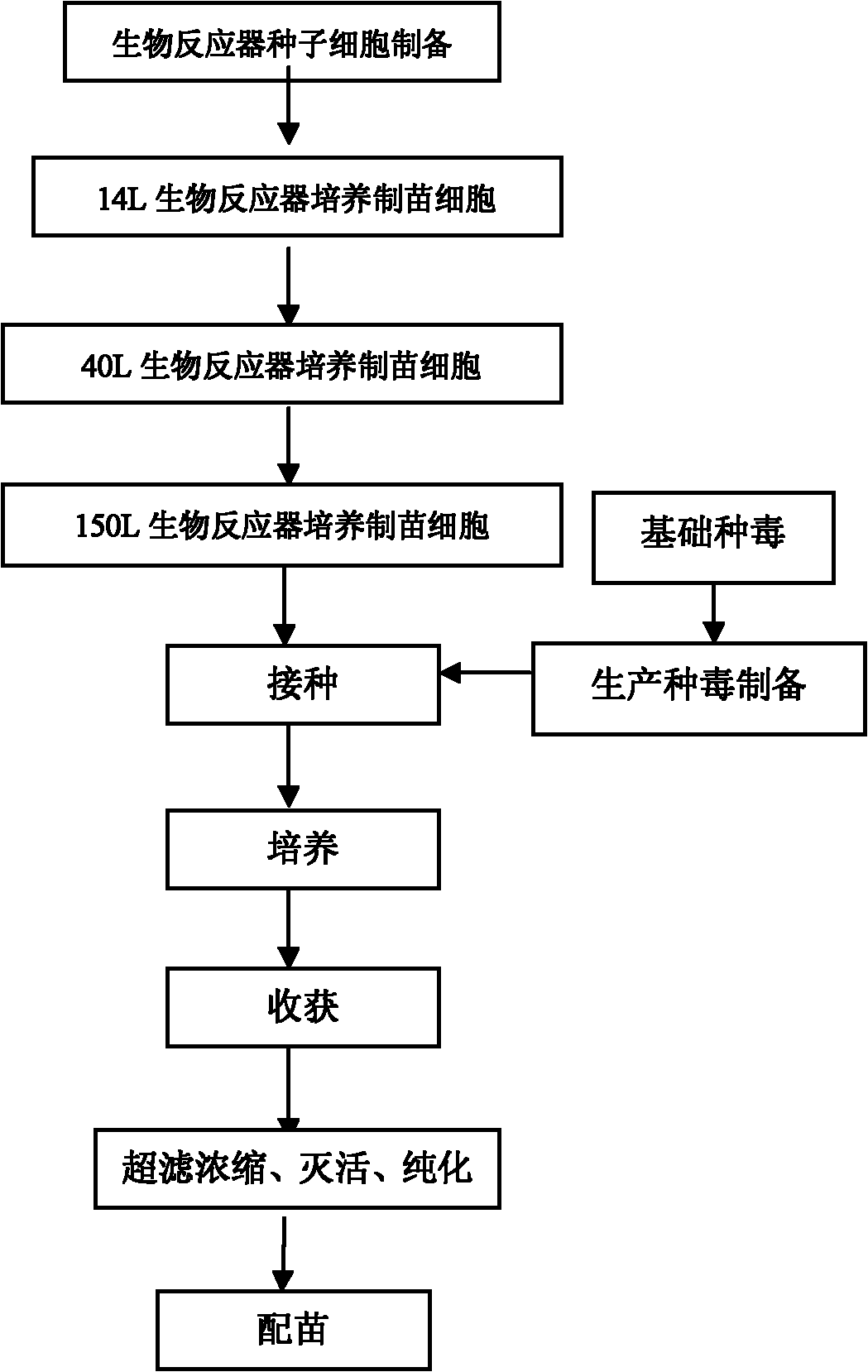
Method for producing pseudorabies attenuated vaccine by using bioreactor and pseudorabies attenuated vaccine product
ActiveCN101695572AImprove immune efficiencyIncrease growth densityMicroorganism based processesAntiviralsVaccine ProductionAntibiotic Y
The invention provides a method for producing a pseudorabies attenuated vaccine by using a bioreactor and a pseudorabies attenuated vaccine product. After being sterilized, the bioreactor and a micro carrier are inoculated with cells for producing the vaccine, and a cell growth medium is added for culture. A maintenance medium containing attenuated strains of pseudorabies viruses are inoculated into the bioreactor to continue culturing the cells. 2 to 3 days after virus inoculation, cell culture virus liquid is obtained and added with a stabilizer and antibiotics, and the cell culture virus liquid is refrigerated and dried under vacuum to obtain the pseudorabies attenuated vaccine. In the method, the cell density and virus concentration are improved greatly, the titer of the vaccine is improved, the side reactions, labor intensity and product cost are reduced, the monitoring performance of vaccine production is improved and uniform and stable product quality is guaranteed. The pseudorabies attenuated vaccine produced by the method has high safety, immune efficacy and good immune and protective effect against the attack by the virulent pseudorabies viruses.
Owner:广东永顺生物制药股份有限公司
Polypeptide vaccine of anti Asiatic I virus of foot-and-mouth disease and its preparing method
The invention is a kind of polypeptide vaccine and the manufacturing method which can resist Asia one foot and mouth disease virus. The vaccine is prepared by that DNA array of B , T cell form location amino acid on VP1 and VP4 contact with coding form location albumen alone, or link with macromolecule of the carrier coding schedule location merged protein. The invention uses chemical method to synthesize the peptide gene of B cell and T cell form location on coding Asia1 type VP1 and VP4, and they are contacted, the gene is inserted with particle carrier alone or connecting with the carrier molecular, and transferred into bacteria express, and yeasted and gets the product.
Owner:FUDAN UNIV
Preparation technology of mycoplasma ovipneumoniae inactivated vaccine
InactiveCN103110583ASimple preparation processInfection controlAntibacterial agentsBacterial antigen ingredientsTiterTGE VACCINE
The invention discloses a preparation technology of a mycoplasma ovipneumoniae inactivated vaccine, which is based on the improvement on the preparation technology of the mycoplasma ovipneumoniae inactivated vaccine, and provides a preparation method of the mycoplasma ovipneumoniae inactivated vaccine. Through the application of the method provided by the invention, an ideal efficient mycoplasma ovipneumoniae inactivated vaccine adjuvant is screened, the content of toxin in antigen is greatly reduced, and the vaccine safety is improved. After the vaccine is applied to sheep, the antibody is generated early, the titer is high, the duration is long, and the mycoplasma ovipneumoniae infection can be effectively prevented and controlled.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
Porcine epidemic diarrhea virus s protein and subunit vaccine thereof as well as method for preparing subunit vaccine and application thereof
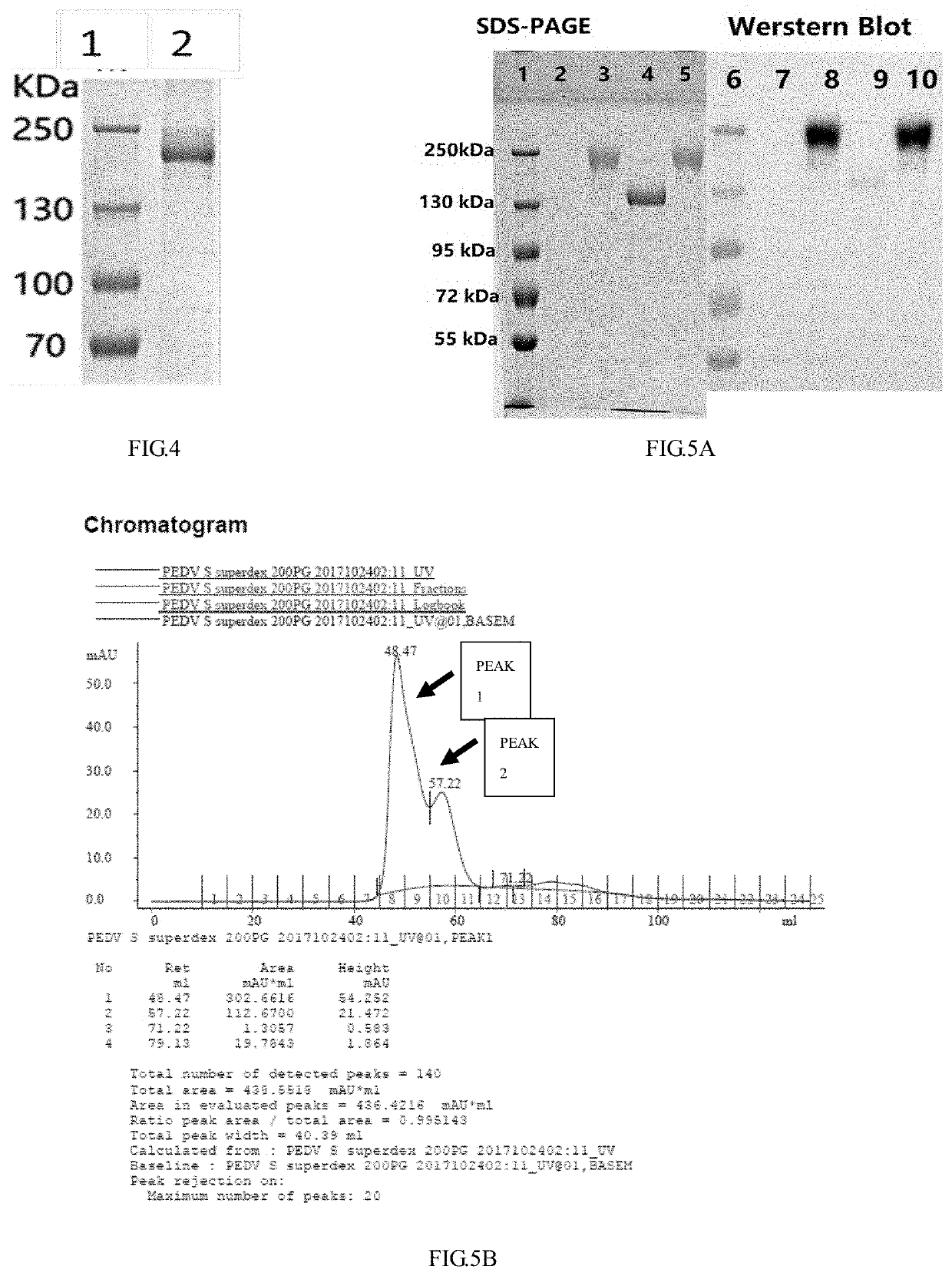
ActiveUS20200188508A1Improve securityImproving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsEpidemic diarrheaAdjuvant
The disclosure discloses a porcine epidemic diarrhea virus S protein and a subunit vaccine thereof as well as a method for preparing the subunit vaccine and application thereof. The vaccine contains 30˜220 μg of a recombinant porcine epidemic diarrhea virus S protein and a pharmaceutically acceptable ISA 201 VG adjuvant. A method for preparing the subunit vaccine comprises the following steps: (1) cloning the recombinant porcine epidemic diarrhea virus S protein; (2) expressing and purifying the recombinant porcine epidemic diarrhea virus S protein; (3) preparing the recombinant porcine epidemic diarrhea virus S protein prepared in (2) into a water phase; (4) emulsifying the water phase and the ISA 201 VG adjuvant in a volume ratio of 46:54 to obtain a vaccine. The vaccine is high in safety, good in immunogenicity, stable in batches, low in production cost and strong in immunogenicity. On the other hand, CHO cell strains suspending and stably and efficiently expressing the PEDV-S protein are successfully constructed and screened for the first time. The CHO cell strain can express the PEDV-S protein in high yield, the yield can reach 1 g / L, and the expressed PEDV-S protein is easy to purify.
Owner:NOVO BIOTECH CORP
Freeze-dried rabies vaccine for humans and preparation method of vaccine
ActiveCN104826101AThe process steps are simpleEasy to operateInactivation/attenuationAntiviralsHuman useSide effect
The invention relates to a freeze-dried rabies vaccine for humans and a preparation method of the vaccine, relates to the field of vaccine production preparation technologies and aims at solving the problems that effective virus antigen expression content is low, the side effect rate of a vaccinator is high and vaccine yield and quality can not meet standard requirements as only a biological reactor is adopted for producing a rabies vaccine. The freeze-dried rabies vaccine for humans is obtained by inoculating aG strain rabies virus on Vero cells and sequentially carrying out ultrafiltration and concentration, separation and purification as well as freeze drying, wherein the packing volume of the freezed-dried rabies vaccine for human use is 0.5ml / dose, and during freeze drying, the adopted vaccine freeze-drying protecting agent comprises the following ingredients: 60-90g / l of trehalos, 6-14g / l of sodium glutamate, 3-6g / l of urea, 2-3g / l of L-arginine and 10g / l of 199 culture medium, and the vaccine freeze-drying protecting agent does not contain gelatin, human serum albumin or dextran. The freeze-dried rabies vaccine for humans has the advantages that cost is low, operation is easy, pollution is hardly produced, vaccine quality and yield are greatly improved, the content of impurities in a vaccine is reduced, allergy reactions are hardly caused, and vaccine safety is greatly improved.
Owner:江生(深圳)生物技术研发中心有限公司
Inactivated vaccine for preventing and treating novel goose astroviruses and preparation method thereof
ActiveCN108567974AImproving immunogenicityConducive to the prevention and control of infectionSsRNA viruses positive-senseViral antigen ingredientsWhole bodyOutbreak
The invention discloses an inactivated vaccine for preventing and treating novel goose astroviruses and a preparation method thereof. The inactivated vaccine is prepared from the effective dose of inactivated goose astrovirus strain virus liquid in prevention or treatment. A preservation number of the goose astrovirus strain is CCTCC NO: V201808. The prepared novel goose astrovirus inactivated vaccine is good in safety, any local or general adverse effects caused by the vaccine do not appear, and the detection of each index is stable and effective. After the inactivated vaccine is used for immunizing a young goose, the young goose can acquire the higher antibody level, and a persistent period is long. The infection and outbreak of the novel goose astroviruses can be prevented and treated specifically, and the effective immune protection is provided for a goose group. In addition, a production technology for the vaccine is simplified, and the large-scale production can be realized.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
A kind of o/asia type I foot-and-mouth disease virus bivalent genetic engineering polypeptide vaccine and its preparation method and application
InactiveCN102274496AEffective controlNo pollution in the processBacteriaMicroorganism based processesInclusion bodiesAdjuvant
The invention relates to an O / Asia I type foot and mouth disease virus bivalent genetic engineering polypeptide vaccine, its preparation method and its purpose. The method comprises the following steps: selecting two serotypes of O type and Asia I type, taking B cell determinant 15 amino acid fragments of VP1 and T-cell helper of VP4, performing a series connection, cloning without containing carrier protein, constructing O / Asia I gene engineering bacteria. An antigen protein product can be obtained after passing through the processes of high density fermenting, cell disrupting, inclusion body renaturating, fusion protein separating, and is homogenized with an adjuvant to form the O / Asia I type foot and mouth disease virus bivalent genetic engineering polypeptide vaccine. The vaccine of the present invention contains 2<n-1> polypeptide connected in series which is coded by a nucleic acid sequence shown in SEQ ID, wherein, n is an integer of 1-5. The invention has the advantages of good security and high immune efficacy, and can be used once in half year for immunization; and is suitable for large scale production and convenient preservation and transportation; and is capable of effectively preventing and controlling two serotypes foot and mouth disease of O type and Asia I type which is useful in our country; foot and mouth disease virus non-structural protein 3A.B. will not generate, so that the infective animals can be differentiated easily.
Owner:吴晓琰 +2
Riemerella anatipestifer infection attenuated live virus vaccine and preparation method thereof
InactiveCN107513510AImprove protectionImprove securityAntibacterial agentsBacterial antigen ingredientsImmune effectsSocial benefits
The invention discloses a riemerella anatipestifer infection attenuated live virus vaccine and a preparation method thereof. The riemerella anatipestifer infection attenuated live virus vaccine disclosed by the invention contains a riemerella anatipestifer GD strain freeze-drying protection agent and a riemerella anatipestifer GD strain is preserved in China Center for Type Culture Collection (CCTCC) on April 1, 2016, with the preservation number of CCTCC NO: M2016161. The riemerella anatipestifer infection attenuated live virus vaccine disclosed by the invention has good safety and does not have any adverse effect on ducks; the riemerella anatipestifer infection attenuated live virus vaccine has a good immune effect, can be used for remarkably improving the resistance of young ducks on riemerella anatipestifer, can be used for clinically preventing riemerella anatipestifer infection and has important economic and social benefits.
Owner:INST OF ANIMAL HEALTH GUANGDONG ACADEMY OF AGRI SCI
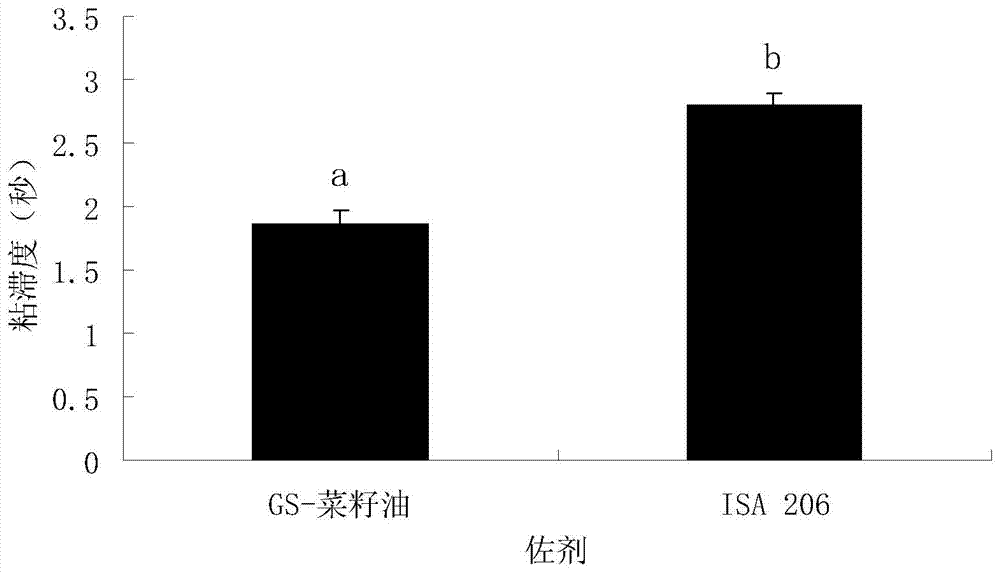
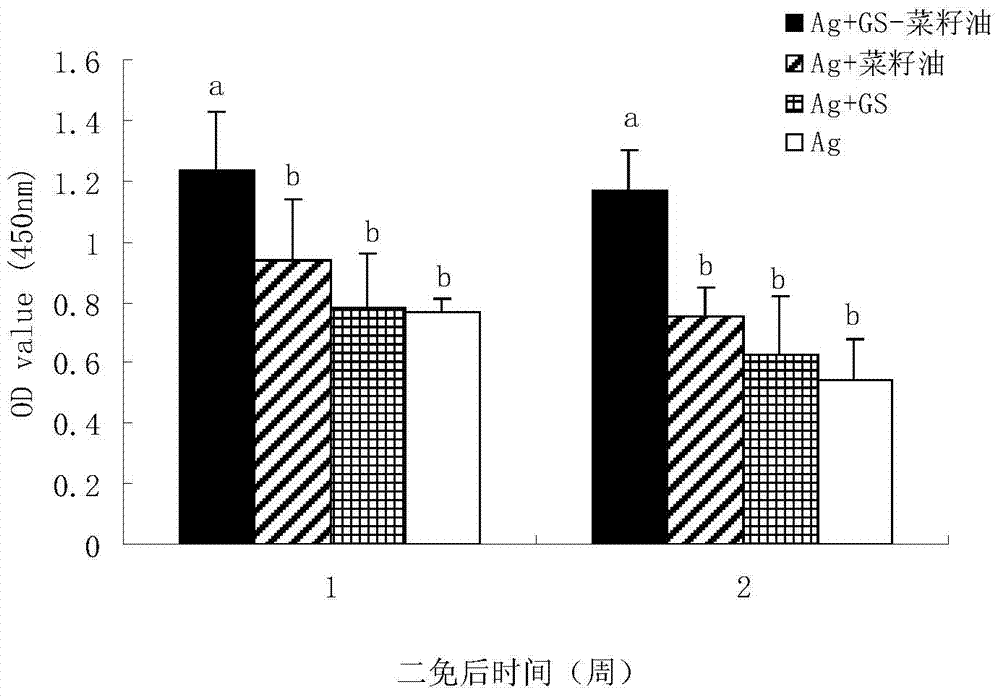
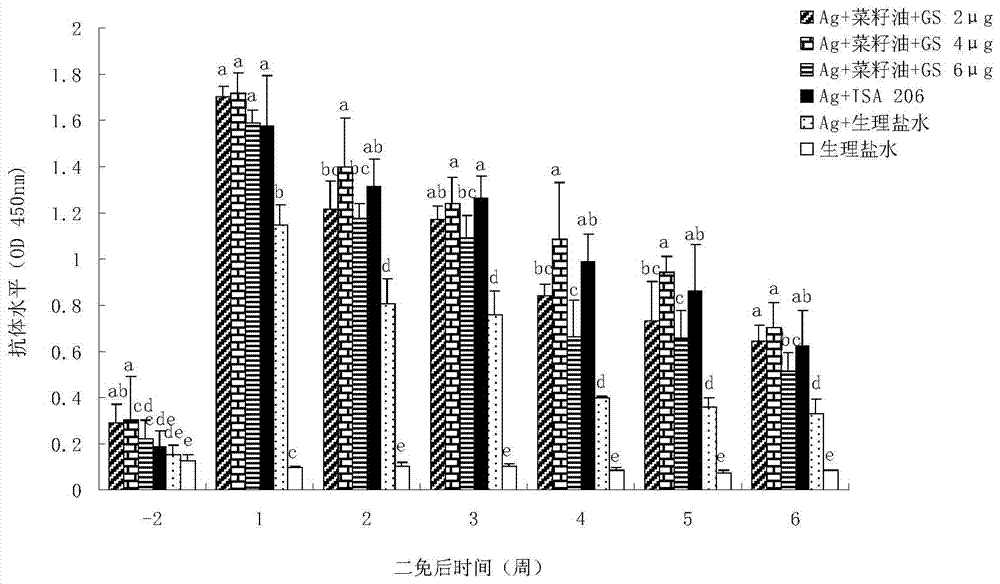
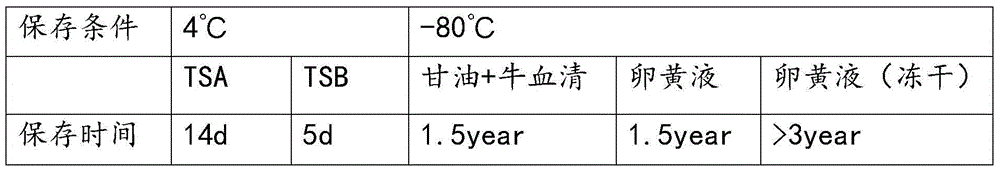
Ginsenoside-containing vegetable oil adjuvant and preparation method and application thereof
InactiveCN103751775ARaise antibody levelsHigh activityImmunological disordersAntibody medical ingredientsOil adjuvantVegetable oil
Owner:ZHEJIANG UNIV
Serum-13 type haemophilus lus paradis vaccine strain and application thereof
InactiveCN104450555AStrong pathogenicityLong durationAntibacterial agentsBacteriaSerum igeHaemophilus
The invention relates to a serum-13 type haemophilus lus paradis vaccine strain. The classified name of the vaccine strain is haemophilus lus paradis, the strain name is FJMH10 and the vaccine strain is preserved in the China Center for Type Culture Collection on June 15, 2014 with the preservation number of CCTCC NO:M 2014262. The invention further relates to an application of the serum-13 type haemophilus lus paradis vaccine strain in preparation of a haemophilus lus paradis inactivated vaccine. The serum-13 type haemophilus lus paradis FJMH10 strain is stable in biology, has a strong pathogenicity to a piglet, and has a good immunogenicity when being inactivated and vaccinated on the piglet. A univalent vaccine prepared from the vaccine strain serving as a vaccine candidate strain has good safety, can produce a relatively high antibody on the piglet, has long duration and good immune potency, and can be used for resisting attack of homotype wild strains. After a pig group is immunized, the morbidity and death rate are remarkably reduced, the economic loss of a piggery is reduced, and the immunizing effect of the vaccine strain is the same as or superior to that of existing commercial vaccines on the market.
Owner:扬州优邦生物药品有限公司
Vaccine freeze-drying protective agent containing no gelatin and human albumin
ActiveCN105267971AImprove securityReduce adverse reactionsPowder deliveryAntiviralsVaccine StabilitySucrose
The present invention relates to the field of biological products, and in particular relates to a vaccine freeze-drying protective agent containing no gelatin and human albumin. The vaccine freeze-drying protective agent comprises 1.5%-10% of sucrose, 1.5%-3.5% of dextran, 1%-2% of sorbitol, 0.8%-1.2% of sodium glutamate and 0.2% to 1% of L-arginine in a vaccine semi-finished product, a matrix liquid for preparation of the vaccine freeze-drying protective agent is 199 culture medium or PBS buffer solution or water for injection, the vaccine freeze-drying protective agent contains no gelatin and human albumin, and the vaccine semi-finished product is a liquid vaccine before freeze-drying. The present invention also provides the use of the vaccine freeze-drying protective agent. When the vaccine freeze-drying protective agent is used for the preparation of a freeze-dried vaccine, the vaccine freeze-drying protective agent can improve the vaccine stability during freeze-drying and storage processes, greatly improves the freeze-dried vaccine safety for human, and reduces adverse effects of the vaccine.
Owner:SINOVAC DALIAN VACCINE TECH
Method of preparing pig replication and respiration syndrome deactivation concentrated vaccine 'SD1 strain'
InactiveCN101234198ANo side effectsImprove immunityViral antigen ingredientsInactivation/attenuationALUMINUM STEARATESPorcine reproductive and respiratory syndrome virus
The invention provides a preparation method of an inactivated and concentrated vaccine 'SD1 strain' for a Porcine reproductive and respiratory syndrome, which is prepared with a water phase and an oil phased according to the following weight percentage content: the water phase is prepared by fully mixing 96 shares of 'SD1 strain' virus culture solution, which is American Porcine reproductive and respiratory syndrome that is inactivated for 20 hours and concentrated 2 times, with 4 shares of Tween minus 80, which occupies 33 percent of the vaccine; the oil phase is prepared by mixing 94 shares of No. 10 white oil with 6 shares of Span minus 80 and then adding a 2 percent aluminum stearate according to the total amount to stir to be transparent, and sterilizing with a high pressure at a temperature of 116 DEG C, which occupies 67 percent of the total amount of the vaccine. The vaccine, with a preservation period of 12 months, is safe and reliable to the Porcine reproductive and respiratory syndrome easily infected animals, and is suitable for pigs of different species and various day old, the immunity protection rate of which reaches 80 percent above, the immunity period of validity of which continues more than 6 months. The safety and immunity efficacy of the vaccine reaches an advanced level among the similar products in the world.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Method for industrially producing swine parvovirus vaccine by utilizing bioreactor
ActiveCN102038945AHigh titerHigh degree of automation controlMicroorganism based processesAntiviralsHigh cellUltrafiltration
The invention provides a method for industrially producing a swine parvovirus vaccine by utilizing a bioreactor, comprising the following steps of: (1) sterilizing a micro-carrier and the bioreactor, adding a cell growth solution, inoculating, preparing the vaccine and culturing with cells, inoculating a swine parvovirus after the cell on the micro-carrier forms a compact single layer, and continuously culturing to propagate the virus; (2) stopping culturing until the cytopathy reaches more than 80%, and harvesting a virus solution; (3) carrying out ultrafiltration concentration and virus inactivation on the harvested virus solution; and (4) purifying and inactivating the virus through a column chromatography method to prepare the vaccine. The invention has the advantages of favorable controllability of processing parameters, high cell density and virus titer, favorable vaccine safety, stable and reliable quality, high production efficiency, and the like.
Owner:WUHAN CHOPPER BIOLOGY
Serum-5 type haemophilus lus paradis vaccine strain and application thereof
InactiveCN104450557ABiologically stableStrong pathogenicityAntibacterial agentsBacteriaSerum igeHaemophilus
The invention relates to a serum-5 type haemophilus lus paradis vaccine strain. The classified name of the vaccine strain is haemophilus lus paradis, the strain name is JSYZ10 and the vaccine strain is preserved in the China Center for Type Culture Collection on June 15, 2014 with the preservation number of CCTCC NO:M 2014260. The invention further relates to an application of the serum-5 type haemophilus lus paradis vaccine strain in preparation of a haemophilus lus paradis inactivated vaccine. The serum-5 type haemophilus lus paradis JSYZ10 strain is stable in biology, has a strong pathogenicity to a piglet, and has a good immunogenicity when being inactivated and vaccinated on the piglet. A univalent vaccine prepared from the vaccine strain serving as a vaccine candidate strain has good safety, can produce a relatively high antibody on the piglet, has long duration and good immune potency, and can be used for resisting attack of homotype wild strains. After a pig group is immunized, the morbidity and death rate are remarkably reduced, the economic loss of a piggery is reduced, and the immunizing effect of the vaccine strain is the same as or superior to that of existing commercial vaccines on the market.
Owner:扬州优邦生物药品有限公司
Duck adenovirus type 2 inactivated vaccine
ActiveCN107137704AImprove securityGood prospects for commercial developmentViral antigen ingredientsAntiviralsImmune effectsAntigen
The invention aims at providing a duck adenovirus type 2 inactivated vaccine, wherein an antigen is an inactivated GD strain virus; and the GD strain virus is preserved in China Center for Type Culture Collection in Wuhan University on June 5, 2016 with preservation number of CCTCC No: V201633. The duck adenovirus type 2 inactivated vaccine prepared by the invention is good in safety and free from any local and systemic adverse reactions caused by the vaccine. Based upon analysis on characters, safety test and efficacy test data in a preservation period test, various indexes are stable and effective; and a result of assessing an immune effect of the vaccine by virtue of a serological method and an immune attack method shows that the inactivated vaccine prepared by the invention can achieve effective immune protection on ducks, and the inactivated vaccine has a good commercial development prospect.
Owner:SHANDONG SINDER TECH +1
Method of preparing hepatitis A inactivated vaccine
ActiveCN102058882AIncrease production capacityHigh purityAntiviralsViruses/bacteriophagesAdjuvantCell factory
The invention provides a method of preparing a hepatitis A inactivated vaccine, which comprises the following steps of inoculating mixed and absorbed hepatitis A virus strain SH and a human embryo diploid cell MRC-5 to hepatitis A virus propagated in a cell factory, digesting the cell in the virus propagation peak to obtain cell virus liquid, removing impurity proteins of the cell by ultrasonication, chloroform extraction and ultrafiltration through ultrafiltration membranes, degerming and filtering by gel filtration chromatography and purification, and absorbing by an aluminium hydroxide adjuvant so that the hepatitis A inactivated vaccine is prepared. The result of in vivo effectiveness experiments performed on a mouse shows that the hepatitis A inactivated vaccine prepared by the method of the invention has higher ED50 and better immunogenicity than the contract strain. The method of the invention can improve the safety of the vaccine, simplify the technique, shorten the productionperiod, reduce the production cost, and realize the scale production of the hepatitis A inactivated vaccine.
Owner:SHENZHEN KANGTAI BIOLOGICAL PROD
Aeromonas hydrophila micro-capsular oral vaccine
InactiveCN101862308AWide variety of sourcesLow priceAntibacterial agentsPharmaceutical non-active ingredientsBacteroidesCytokine
The invention relates to an aeromonas hydrophila micro-capsular oral vaccine, which belongs to the technical field of biological pharmacy. The aeromonas hydrophila micro-capsular oral vaccine is prepared by using sodium alga acid and chitosan as wall materials, using the inactivated bacteria of aeromonas hydrophila as a core material and by using improved atomization and ion crosslinking technology. When the aeromonas hydrophila sodium alga acid-chitosan micro-capsular oral vaccine is used for immunizing a mouse and crucian, the phagocytosis activities of macrophages, the conversion efficiency of the splenic lymphocytes and the expression amount of cell factors such as IFN-gamma and Il-4 of the mouse and the crucian can be improved obviously. The relative protection rate of the vaccine for the crucian is 39.3 percent. A microcapsule is stable in a simulated gastrointestinal environment and shows high burst release and slow release; and the bacterial antigenicity of the microcapsule is well maintained and the vaccine has high safety and high storage stability.
Owner:NANJING AGRICULTURAL UNIVERSITY
Method for producing porcine circovirus type 2 antigens in large scale with high density
ActiveCN104004720AIncrease productionQuality improvementViral antigen ingredientsAntiviralsAntigenHigh density
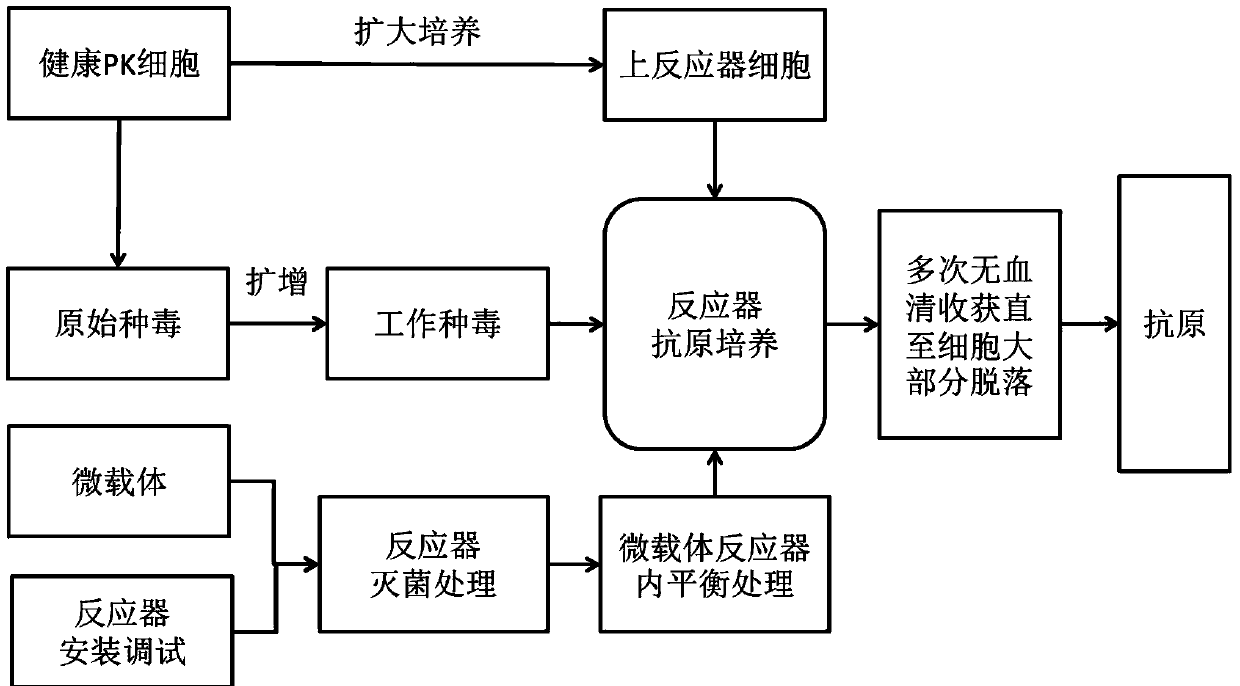
The invention relates to a method for producing porcine circovirus type 2 antigens in large scale with high density. A bioreactor microcarrier suspension culture technology used for replacing an existing spinner bottle culture technology for producing the porcine circovirus type 2 antigens. The method can greatly reduce the production cost and improve the yield. Compared with the spinner bottle technology, the unit antigen cost is reduced by 80% to 90%, the production period is reduced by 7 days, and the yield is improved by 3 times to 10 times; due to the fact that the antigens can be obtained repeatedly, compared with a traditional reactor culture technology, the unit antigen cost is reduced by 40% to 50%, and the yield is improved by 3 times to 5 times. The antigens produced through the method has no serum residues, the produced vaccine is higher in safety and small in batch difference, the quality is stable and easy to control, and the yield and quality of the produced vaccine can be obviously improved.
Owner:JIANGSU NANNONG HI TECH
Foot and mouth disease bivalent polypeptide vaccine and its preparation method and use
ActiveCN1589901AImprove securityAvoid confusionSsRNA viruses positive-senseViral antigen ingredientsMicrobiologyNucleic acid sequencing
A bivalence polypeptide vaccine for foot-and-mouth disease contains 2 to the power n-1 polypeptides coded by the nucleic acid sequences shown by SEQ ID No.1 or 2, where n=1-5. Its preparing process is also disclosed.
Owner:SHANGHAI HUAYI BIO LAB CO LTD
Aeromonas hydrophila and aeromonas veronii duplex oral sustained-release microsphere vaccine and preparation method thereof
ActiveCN104689310AWide variety of sourcesLow priceAntibacterial agentsPharmaceutical non-active ingredientsBacteroidesMicrosphere
The invention provides an aeromonas hydrophila and aeromonas veronii duplex oral sustained-release microsphere vaccine and a preparation method thereof, and belongs to the field of biological pharmacy. With sodium alga acid as a wall material, and inactivated whole bacteria of aeromonas hydrophila and aeromonas veronii as a core material, the aeromonas hydrophila and aeromonas veronii duplex oral sustained-release microsphere vaccine is prepared by an emulsification method. Prussian carp is immunized by the aeromonas hydrophila and aeromonas veronii-calcium alginate microcapsule oral vaccine, so that the serum enzyme activity and the leukocyte phagocytic activity can be significantly improved; the relative protection rate for the prussian carp is 46.7%; the microcapsule is stable in a simulated gastrointestinal condition; relatively good sudden release and slow release are displayed; the bacteria antigenicity in the microcapsule is kept well; and the vaccine safety and the storage stability are relatively good.
Owner:XINXIANG MEDICAL UNIV
Swine flu H1N1 and H3N2 subtype bivalent inactivated vaccine
ActiveCN103468647AImprove securityMicroorganism based processesAntiviralsAnimal virusInactivated vaccine
The invention belongs to the technical field of animal virology and in particular relates to a swine flu H1N1 and H3N2 subtype bivalent inactivated vaccine and an application thereof. The bivalent inactivated vaccine is prepared from H1N1 subtype swine flu viruses, namely H1N1 SIV TJ strains with a preservation number of CCTCC (China Center For Type Culture Collection) NO:V201107 and H3N2 subtype swine flu viruses, namely HuN-1 strains with a preservation number of CCTCC NO:V201308. The swine flu H1N1 and H3N2 subtype bivalent inactivated vaccine is good in safety and has an immunity protection effect reaching more than 80%.
Owner:HUAZHONG AGRI UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com