Preparation technology of mycoplasma ovipneumoniae inactivated vaccine
A Mycoplasma pneumoniae, preparation technology, applied in the direction of medical preparations containing active ingredients, bacterial antigen components, antibody medical components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0012] (1) Preparation of medium
[0013] Modified Thiaucourt's medium was used. The medium components are as follows: 21.0 g of PPLO broth, 100 mL of fresh yeast extract, 200 mL of inactivated horse serum, 2 mL of 0.5 g / ml glucose, 8 mL of 0.5 g / mL sodium pyruvate, 3.2 mL of 0.5% phenol red, 10 ml of 10000U / mL penicillin, 0.1 g of thallium acetate, make up to 1000 ml with deionized water, adjust the pH to 7.6-7.8, filter and sterilize with a 0.22 μm bacterial filter, aliquot and store at 4°C for later use.
[0014] (2) Proliferation of Mycoplasma pneumoniae
[0015] Add 1 mL of modified Thiaucourt's medium to dissolve the lyophilized bacteria of Mycoplasma ovis pneumoniae, add 1 mL of bacterial liquid to 10 mL of modified Thiaucourt's medium, and culture on a shaker at 37 C°160 rpm for 48 h (the color of the medium gradually changes from red to (Orange yellow, clear and translucent bacterial liquid, sterile film, no turbidity); inoculate the first-generation culture into...
Embodiment 1
[0031] Example 1 Determination of Antigen Endotoxin, Vaccine Safety Test and Effectiveness Test
[0032] (1) Determination of endotoxin
[0033] The content of endotoxin in inactivated antigen of Mycoplasma ovis pneumoniae was determined by dynamic turbidity method, and the results showed that the content of endotoxin in antigen decreased greatly after potassium dichromate treatment. The endotoxin content of the antigen not treated with potassium dichromate was 912 EU / mL, and the endotoxin content of the antigen treated with potassium dichromate was only 38 EU / mL, that is to say, after being treated with potassium dichromate, the antigen endotoxin content The toxin content was reduced by 24 times, improving the safety of the vaccine.
[0034] (2) Safety inspection
[0035] 5 healthy Chengdu Ma sheep around 5 months old (use MO indirect hemagglutination diagnostic kit to detect no antibodies, use nasal swabs to extract DNA, and PCR identified no MO), neck muscle injection o...
Embodiment 2
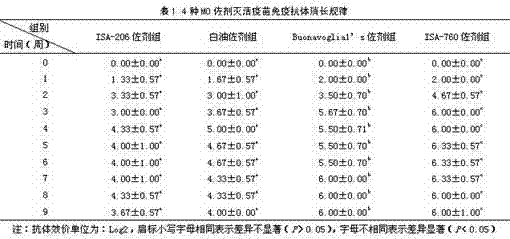
[0038] Example 2 Screening of vaccine adjuvants
[0039] Select adjuvants commonly used in inactivated vaccines: white oil adjuvant, ISA-206 adjuvant, Buonavoglial's adjuvant, and ISA-760 adjuvant to prepare vaccines with inactivated antigens respectively, and immunize healthy adult rabbits by detecting their serum antibody levels , to evaluate the adjuvant effect of the adjuvant on the vaccine, and to screen out the ideal inactivated vaccine adjuvant.
[0040] method:
[0041] 1. Antigen preparation: Antigen preparation was carried out according to the method described in the specific embodiment.
[0042] 2. Preparation of inactivated vaccine:
[0043] (1) Preparation of inactivated vaccine with white oil adjuvant: Add sterilized Tween-80 with a final concentration of 3% according to the volume of antigen, stir while adding, until completely dissolved, and make the aqueous phase of inactivated vaccine. The volume ratio of water phase and white oil adjuvant is 1:1. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com