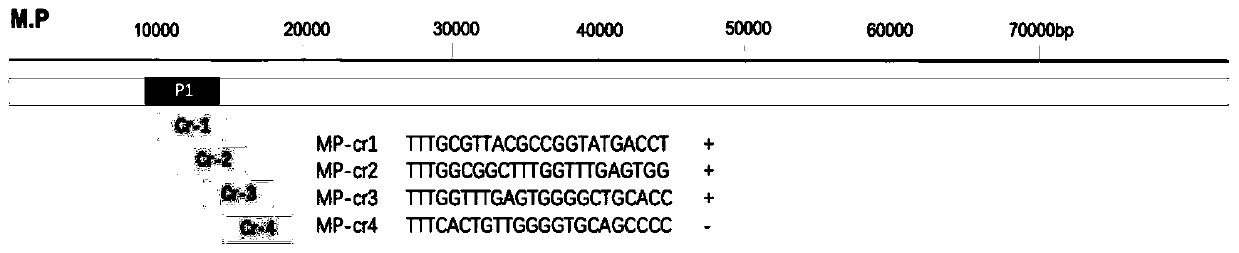
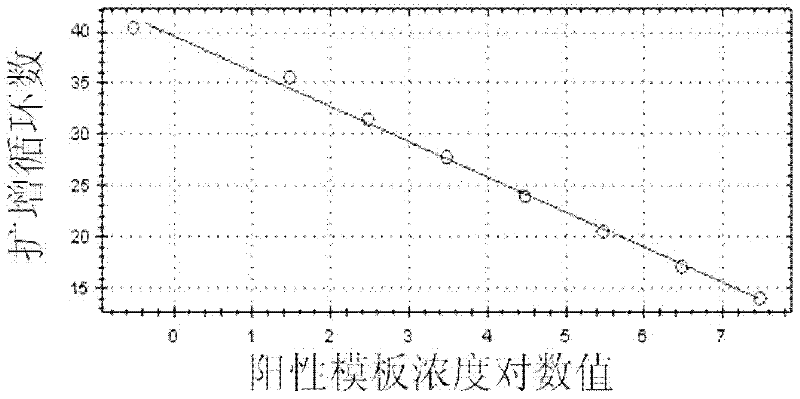
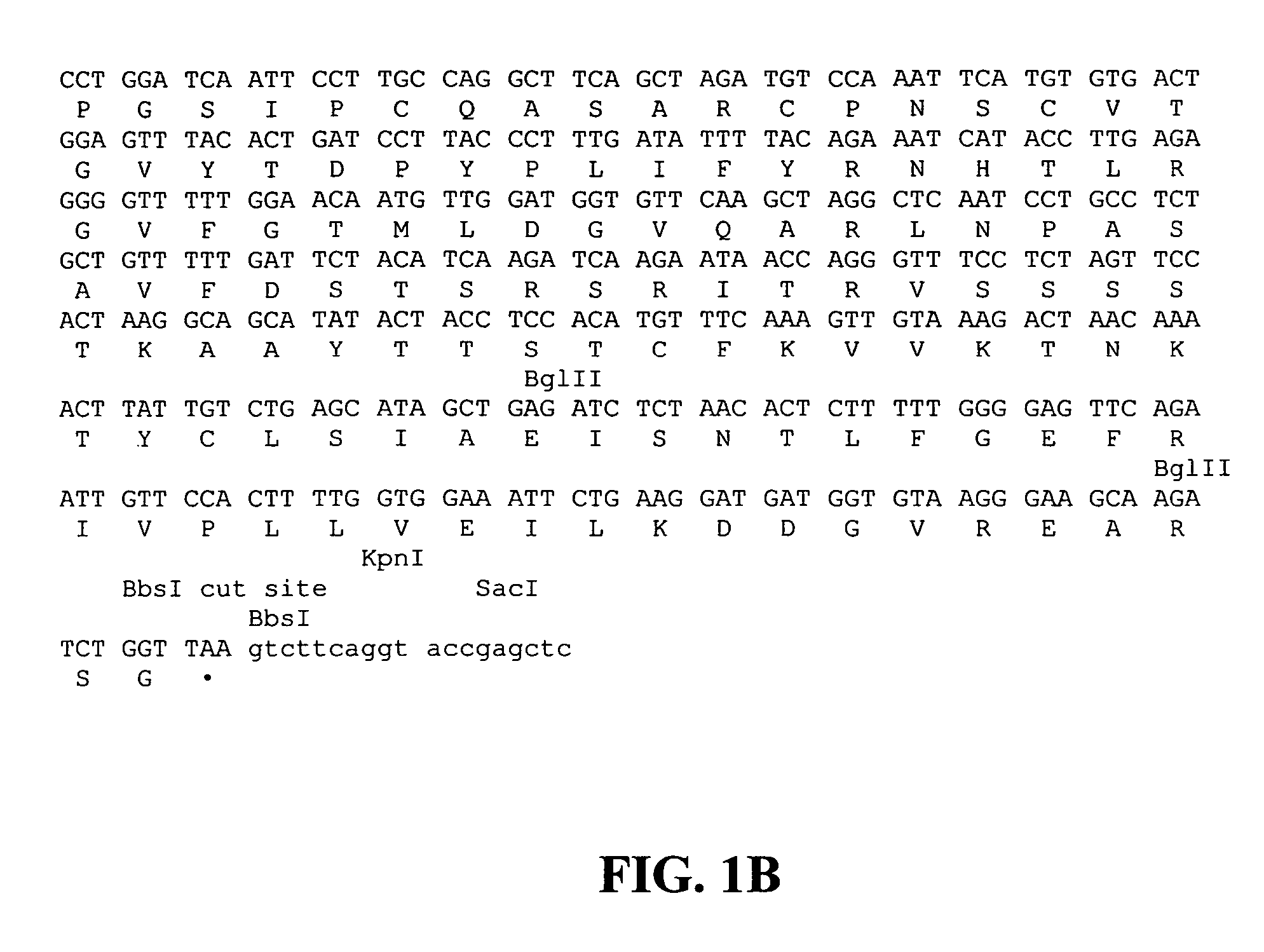
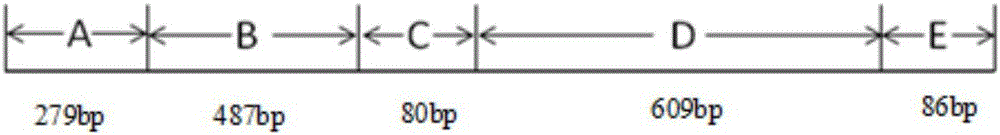
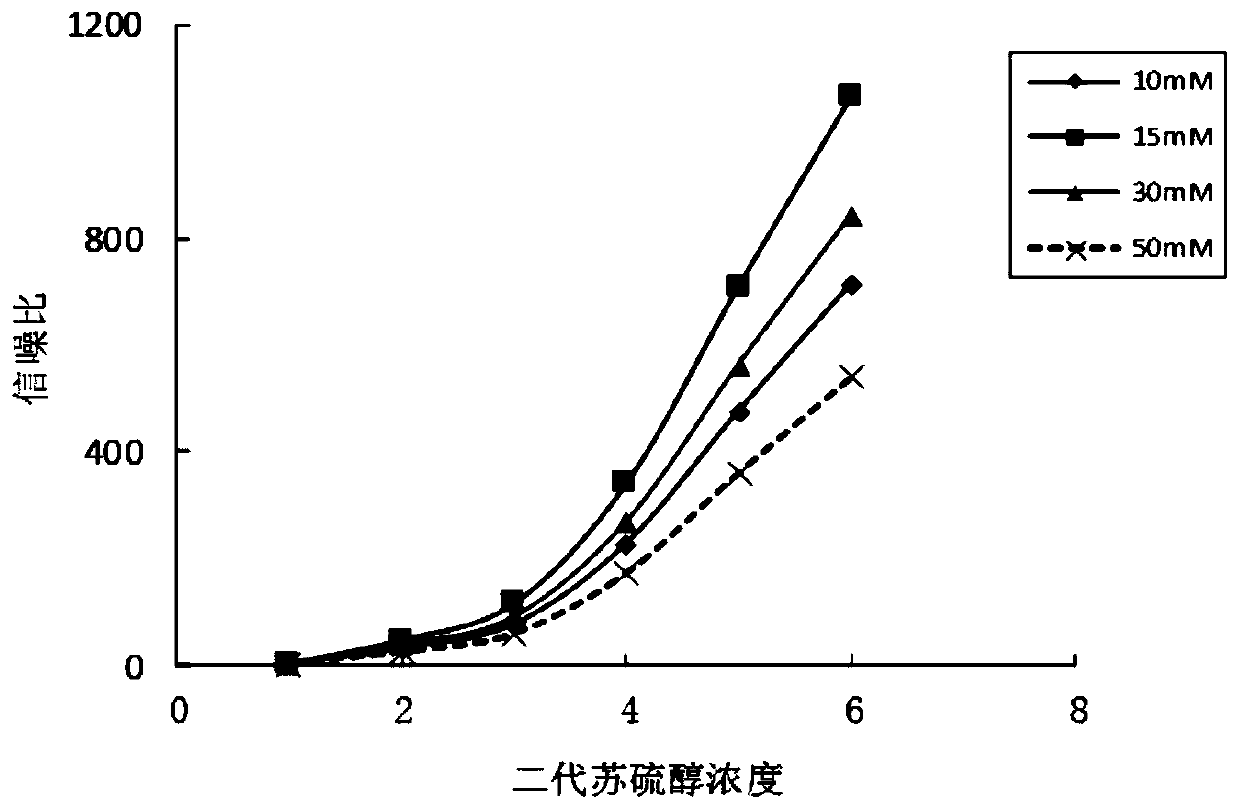
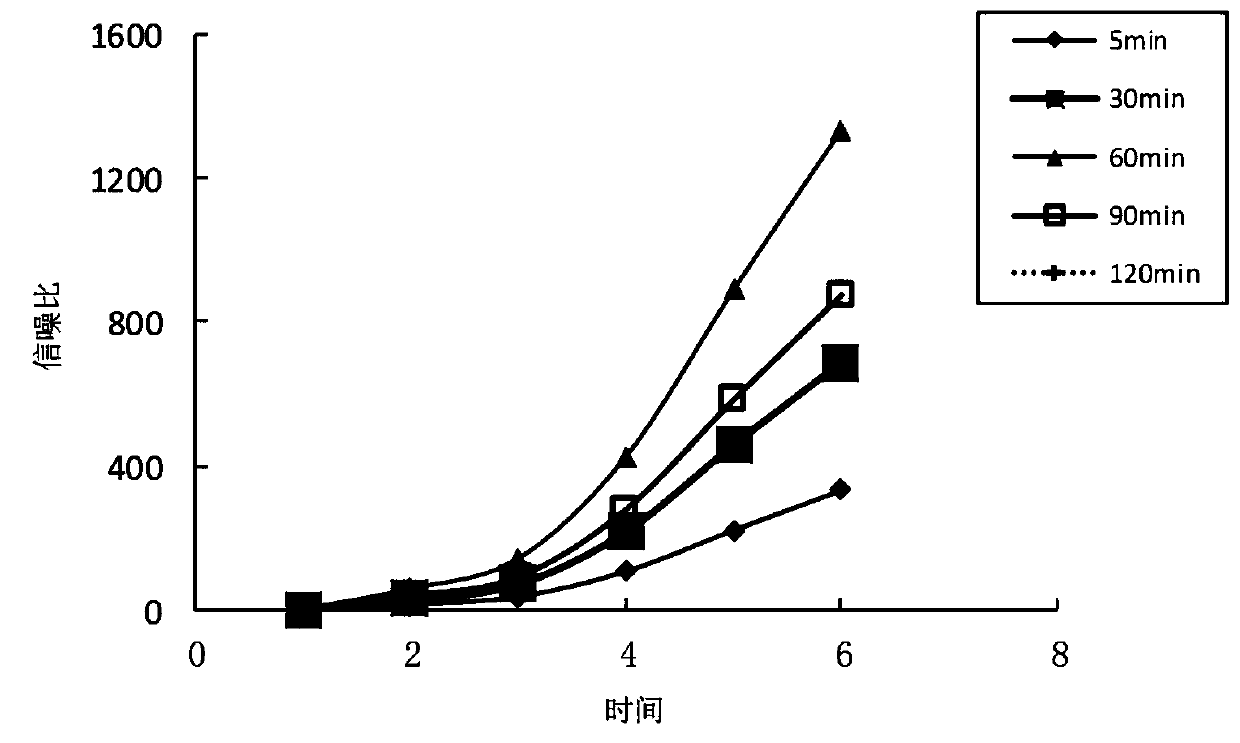
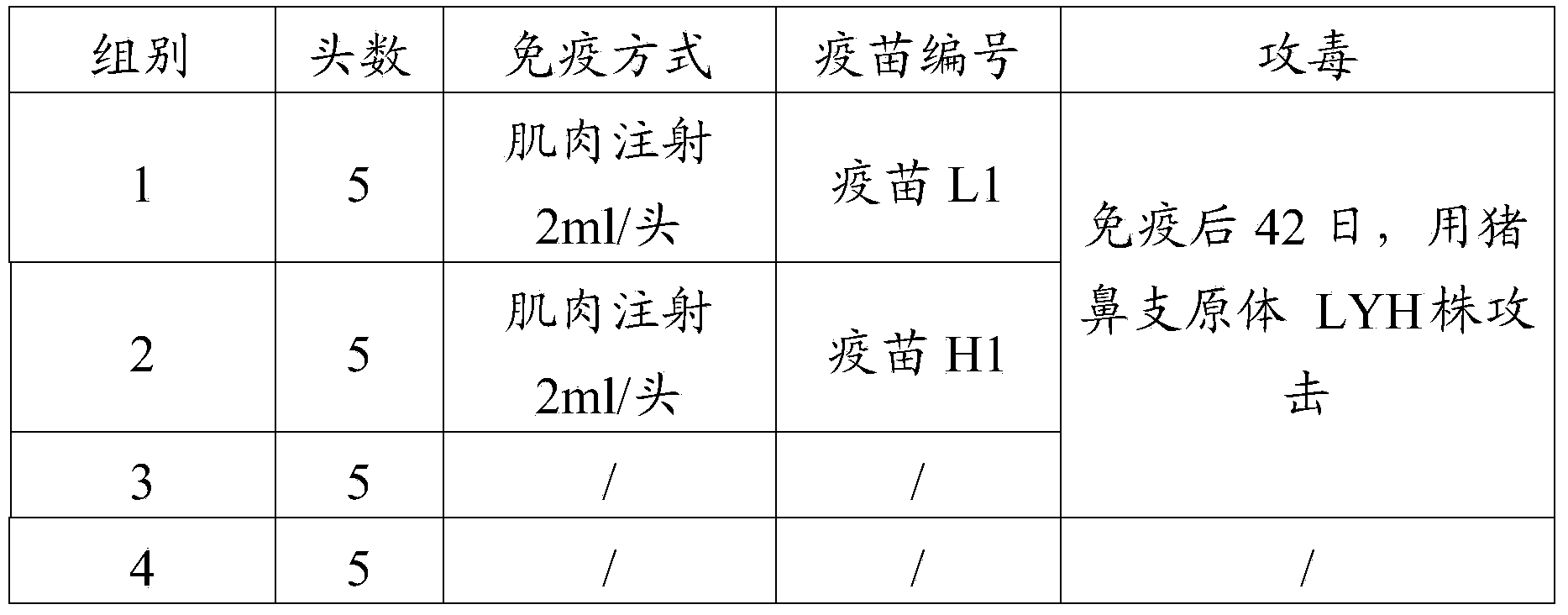
Patents
Literature
112 results about "Mycoplasmata" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bifidobacterium breve CCFM1025, fermented food and application thereof
ActiveCN108949640ARelieve depression-like behaviorImprove the level ofNervous disorderBacteriaGut floraEnteropathy
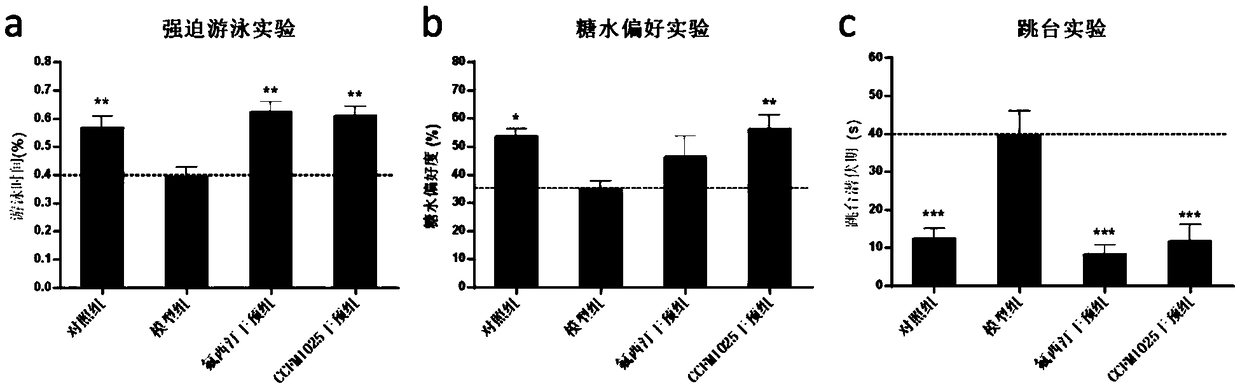
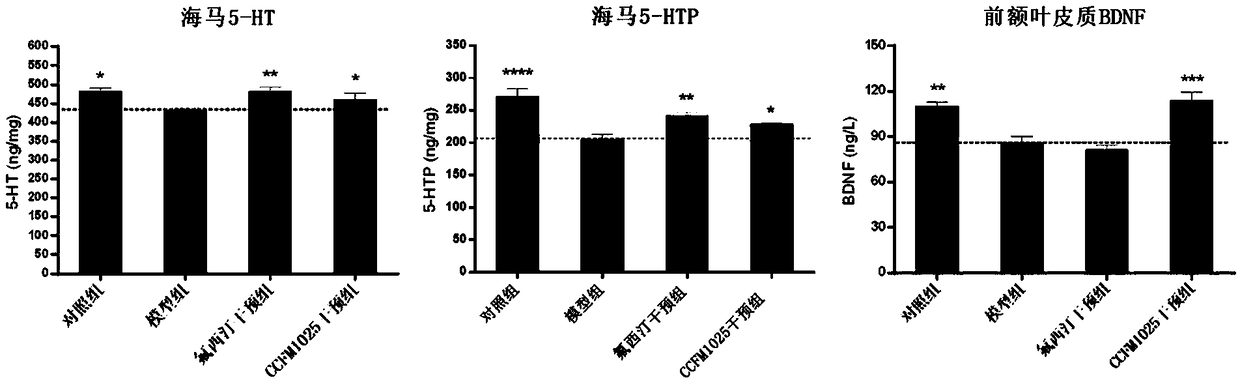
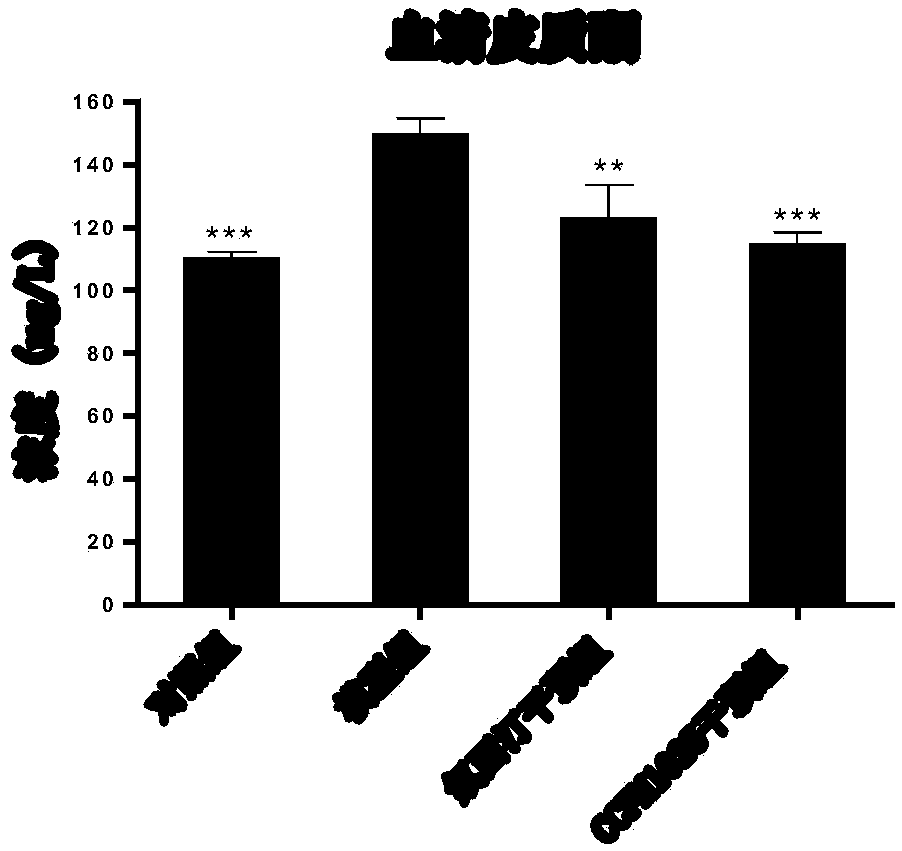
The invention relates to bifidobacterium breve CCFM1025, a fermented food and application thereof. The bifidobacterium breve CCFM1025 can be used for improving the depression-like behavior of a depression mouse, improving 5-hydroxytryptamine in the brain of the depression mouse, improving the level of 5-hydroxytryptamine and a brain-derived neurotrophic factor, reducing the level of corticosteronein serum of the depression mouse, improving the level of 5-hydroxytryptamine in the serum of the depression mouse, improving intestinal flora disturbance of the depression mouse, reducing the abundance of intestinal veillonella, improving the abundance of bifidobacterium and mycoplasmataceae, improving alpha-diversity of intestinal flora and reducing occurrence of inflammatory bowel disease and obesity. By adopting the bifidobacterium breve CCFM1025, the mRNA level of tryptophan hydroxylase in a simulated entero-chromaffin cell can be improved, the secretion volume of 5-hydroxytryptophane ofthe cell can be improved, and a precursor substance is specifically provided to synthesis of 5-hydroxytryptamine in the brain. The bifidobacterium breve CCFM1025 has a wide application prospect.
Owner:无锡食生臻选生物科技有限公司
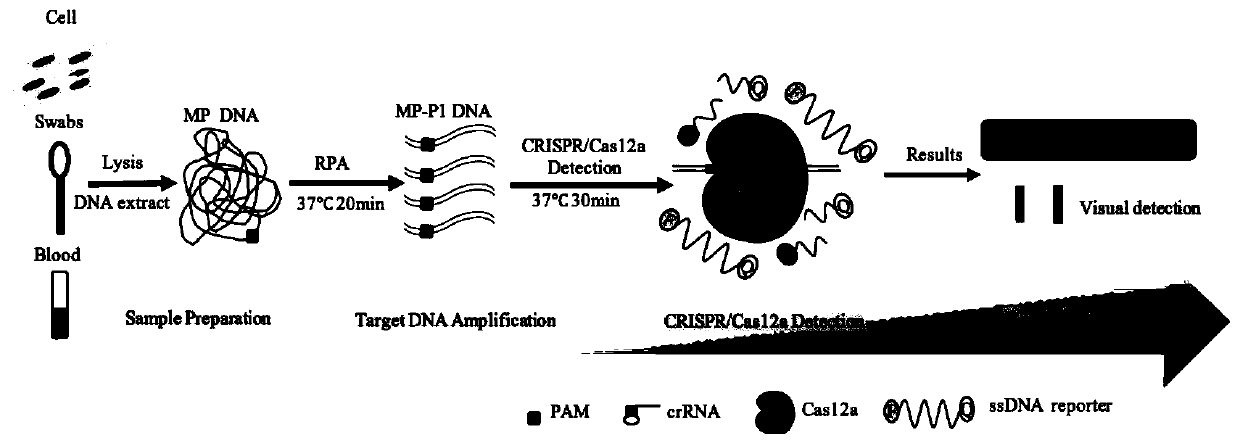
Kit and detection method for rapid detection of nucleic acid of mycoplasma pneumonia on basis of CRISPR/Cas12a
InactiveCN111187804AHigh sensitivityHighly conservativeMicrobiological testing/measurementMicroorganism based processesSingle strandNucleic acid sequencing
Owner:国家卫生健康委科学技术研究所
Mycoplasma bovis diagnosis reagent and its application
InactiveCN103172752AReduce manufacturing costSimple and fast operationBiological testingHybrid peptidesMycoplasma antibodySpecific antibody
The invention relates to the diagnostic medicine of animals, especially relates to a mycoplasma bovis diagnosis technology, and concretely relates to a multi-epitope fusion antigen having an amino acid sequence represented by SEQ ID NO:1 or SEQ ID NO:2, and its application in the preparation of a mycoplasma bovis diagnosis reagent. The diagnosis reagent can be used as a solid phase vector coating antigen of an indirect ELISA kit and is combined with its specific antibody, a horseradish peroxidase coupled anti-cattle IgG antibody is added and incubated, and a color development reaction is carried out, and the color development degree is proportional to the amount of the anti-mycoplasma bovis antibody in a sample to be measured. The technology has the advantages of simple operation, no need of complex equipment, low technical requirements on the laboratorial conditions and experiment personals, low detection cost, and suitableness for the large-scale development in the basic level and the culture farm; the multi-epitope fusion antigen has a low making cost and is suitable for large-scale application; and has the advantages of high sensitivity and specificity, small batch difference, and high detection result consistence because of the adoption of multi-epitope as a target.
Owner:重庆市动物疫病预防控制中心 +1
Preparation technology of mycoplasma ovipneumoniae inactivated vaccine
InactiveCN103110583ASimple preparation processInfection controlAntibacterial agentsBacterial antigen ingredientsTiterTGE VACCINE
The invention discloses a preparation technology of a mycoplasma ovipneumoniae inactivated vaccine, which is based on the improvement on the preparation technology of the mycoplasma ovipneumoniae inactivated vaccine, and provides a preparation method of the mycoplasma ovipneumoniae inactivated vaccine. Through the application of the method provided by the invention, an ideal efficient mycoplasma ovipneumoniae inactivated vaccine adjuvant is screened, the content of toxin in antigen is greatly reduced, and the vaccine safety is improved. After the vaccine is applied to sheep, the antibody is generated early, the titer is high, the duration is long, and the mycoplasma ovipneumoniae infection can be effectively prevented and controlled.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
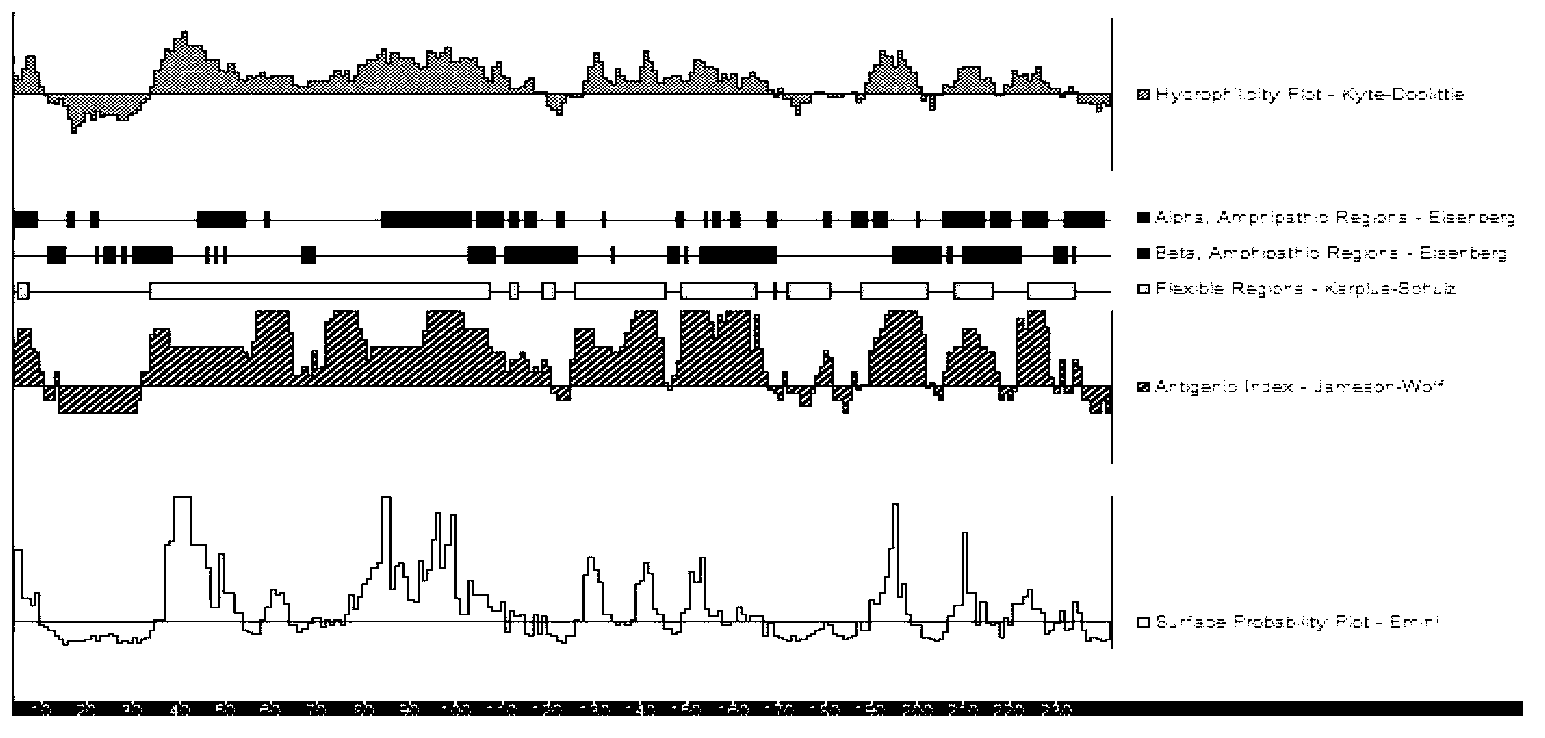
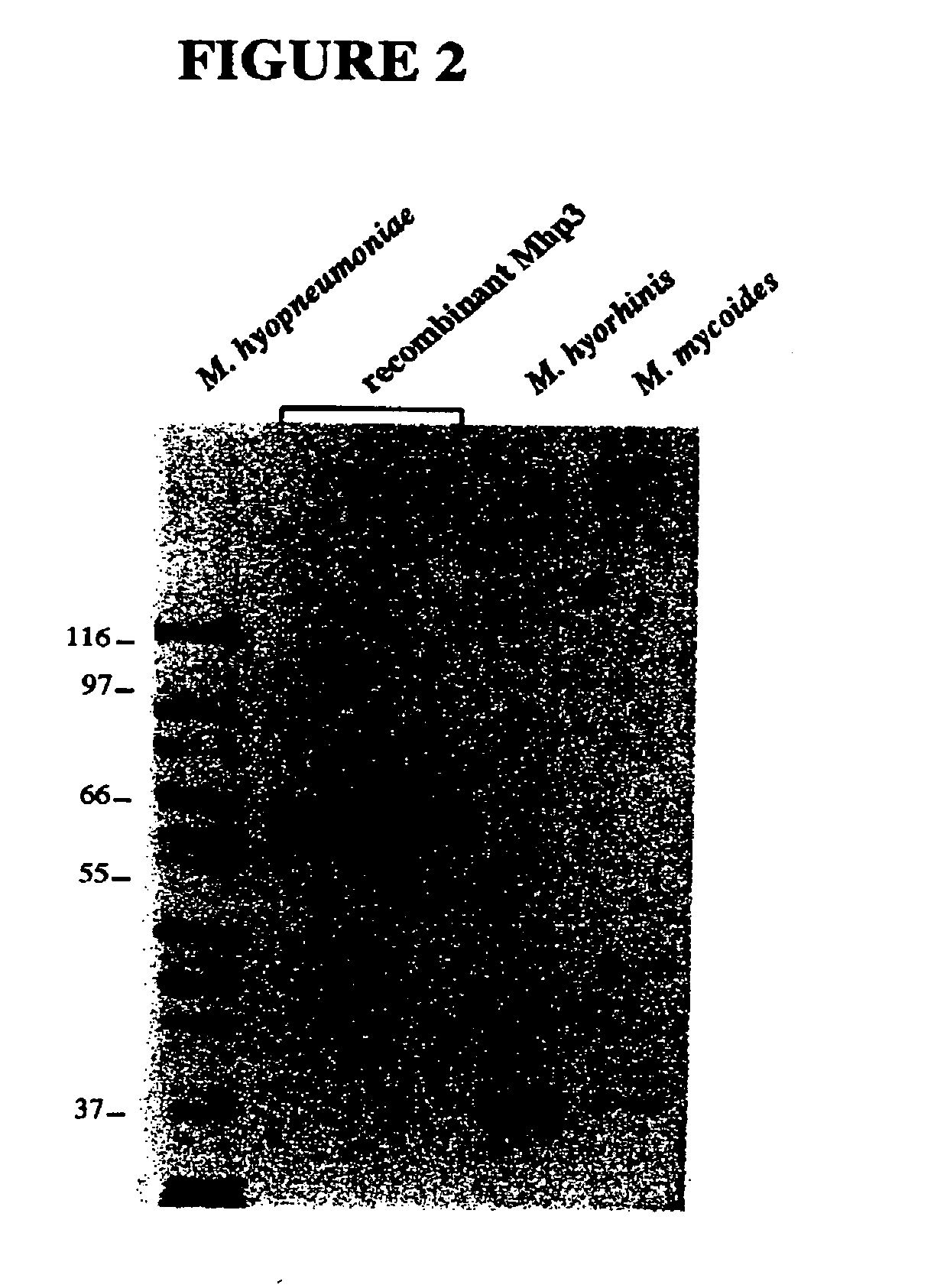
Nucleic acids and proteins of the mycoplasma hyopneumoniae mhp3 gene and uses thereof
InactiveUS20060233823A1Increase stringencyAntibacterial agentsBacteriaAntigenMycoplasma hyopneumoniae
The present invention relates to mhp3 nucleic acids and proteins encoded by the foregoing. The present invention further relates to novel apoprotein antigens encoded by mhp3 for use in vaccines to prevent and treat diseases caused by infection with Mycoplasma hyopneumoniae. The invention further relates to methods for the recombinant production of such antigens.
Owner:KING KENDALL +2
Mycoplasma pneumonia mosaic antigen, antigen detection reagent, and preparation method of both
InactiveCN107573417AStrong specificityEasy to culture and purifyBiological testingHybrid peptidesAntigenMycoplasma pneumonia
The invention provides a mycoplasma pneumonia mosaic antigen amino acid sequence containing an amino acid sequence as shown in SEQ ID NO:1 as well as a full-gene synthesized mycoplasma pneumonia mosaic antigen full-gene sequence containing an amino acid sequence as shown in SEQ ID NO:2. The invention also provides a method for constructing the two gene sequences. The invention also provides a preparation method of the mycoplasma pneumonia mosaic antigen containing full-gene synthesis, a mycoplasma pneumonia detection kit and a preparation method thereof. Mp recombinant mosaic antigen is selected as a mark material and is applied to a gold immunochromatography system, and the detection system is directly marked and captured, so the sensitivity is greatly improved, the specificity of the antigen is high, the antigen is easy in cultivation and purification, and cost is reduced; and a new method for detecting mycoplasma pneumoniae IgG rapidly and accurately is provided for clinical use, and a good market prospect is achieved.
Owner:HANGZHOU CLONGENE BIOTECH
Target sequence, primer, probe and kit for detecting Mycoplasma pneumonia
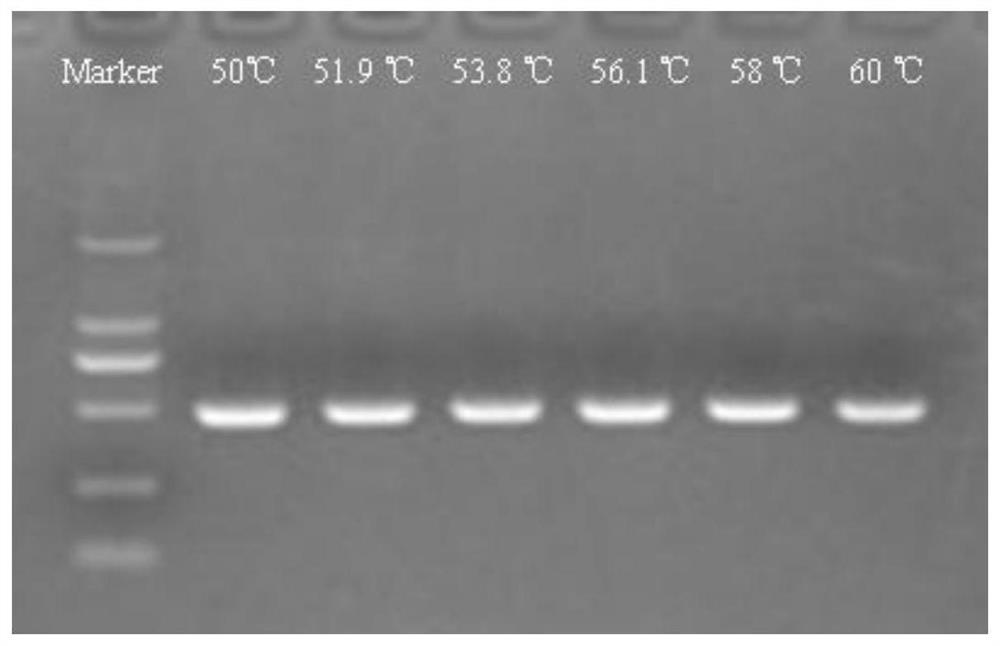
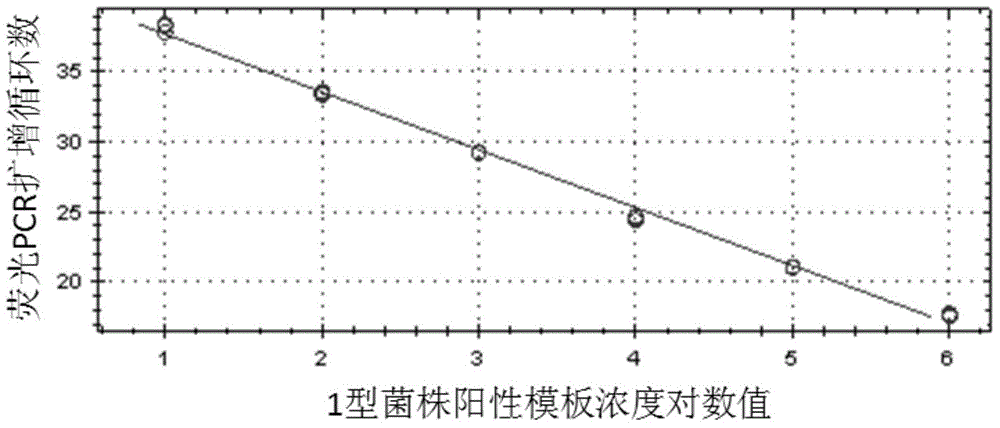
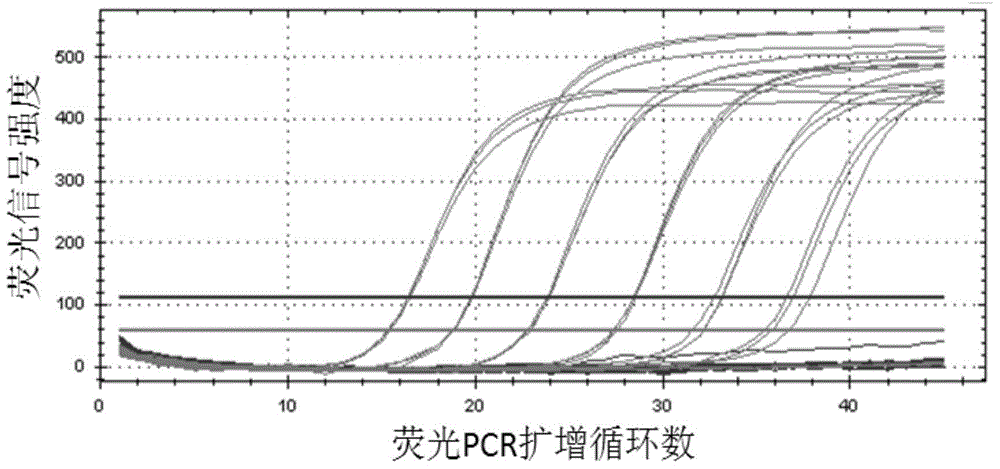
ActiveCN102230013ASimplify testing proceduresShort detection cycleMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceNucleotide sequencing
The invention provides a genetic marker, real-time fluorescence quantitative PCR (polymerase chain reaction) primers and probe for detecting Mycoplasma pneumonia, by sequencing and comparing of Mycoplasma pneumonia genes, which have the nucleotide sequences shown in SEQ ID No.1, SEQ ID No.2, SEQ ID No.3 and SEQ ID No.4 respectively. The invention also provides a method and kit for quantitatively detecting Mycoplasma pneumonia. The detection method has the advantages of accuracy in detection, high sensitivity and strong specificity, is simple and rapid in operation, and is superior in sample detection capacity.
Owner:ICDC CHINA CDC
Preparation of vaccine master cell lines using recombinant plant suspension cultures
InactiveUS20070107086A1Overcome the lack of robustnessImprove stabilitySsRNA viruses negative-senseVirus peptidesCell culture mediaCryopreservation
The subject invention provides a plant cell culture for producing proteinaceous agents comprising a plant cell line stably transformed to express a transgene encoding a proteinaceous agent and a growth medium which supports the growth of said plant cell culture but which does not support the growth of Mycoplasmataceae and contains no materials of animal origin. The plant cell line is capable of being continuously passaged such that consistent transgene expression is maintained during passaging. The plant cell line is also capable of being cryopreserved such that consistent transgene expression is recovered upon recovery from cryopreservation.
Owner:DOW AGROSCIENCES LLC
Mycoplasma gallisepticum formulation
ActiveUS20120021005A1Reduce sensitivitySsRNA viruses negative-senseAntibacterial agentsMedicineMycoplasma gallisepticum
The present invention provides a formulation that prevents virulent Mycoplasma galHsepticum infection in birds of the order GaIHf ormes. The formulation comprises live Mycoplasma galHsepticum strain K5831 or derivatives thereof in a pharmaceutically acceptable carrier. A vaccine that prevents virulent Mycoplasma galHsepticum infection in birds of the order Galliformes is also presented. Methods for administering the formulation and vaccine are also presented.
Owner:UNIV OF GEORGIA RES FOUND INC
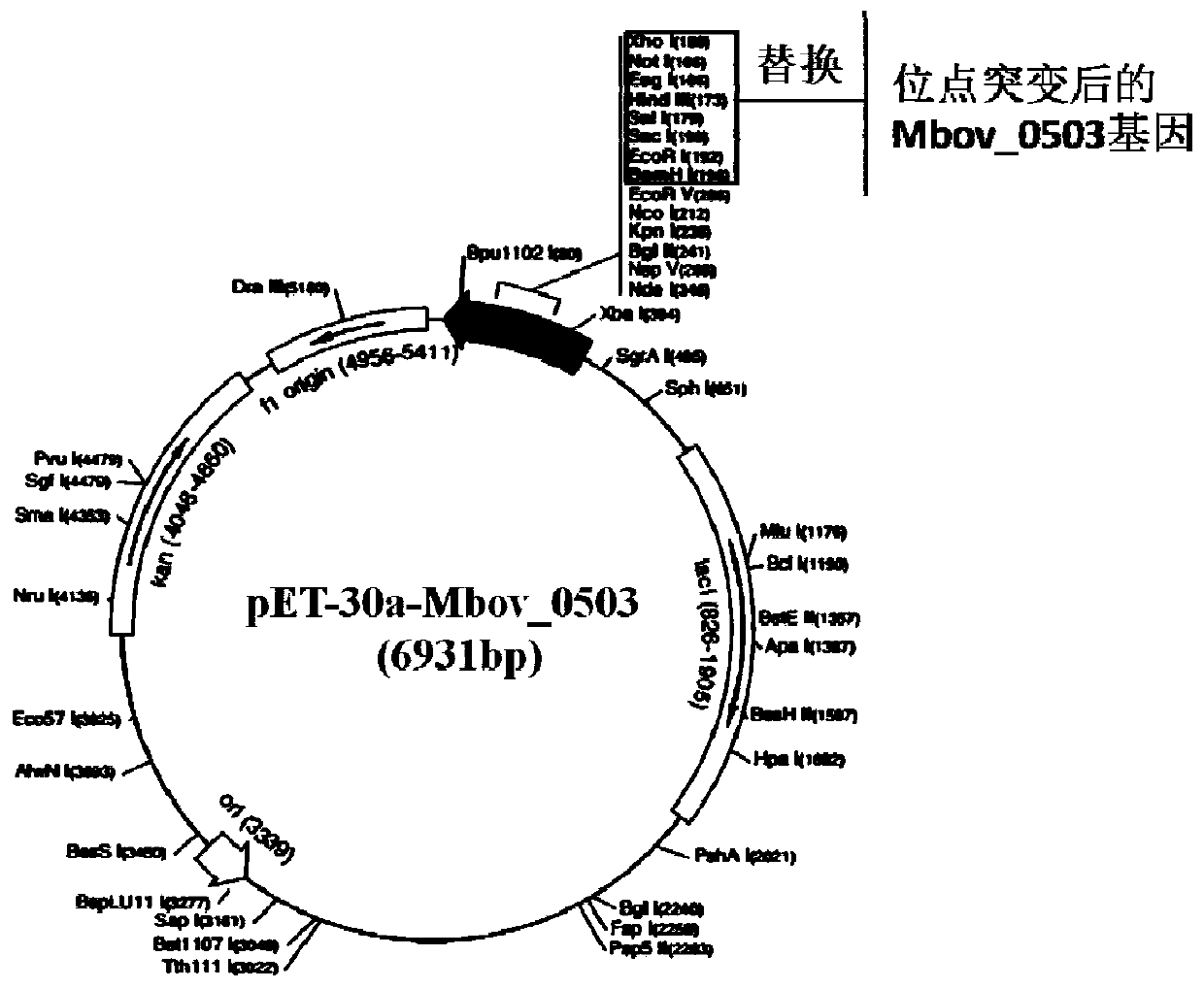
Mutant having reduced adhesive ability and with deleted mycoplasma bovis gene
ActiveCN111235082AReduce adhesionDemonstrated adhesionBacteriaMicrobiological testing/measurementEscherichia coliTransmissible disease
The invention belongs to the field of control of animal borne diseases, and relates to a mutant having reduced adhesive ability and with a deleted mycoplasma bovis gene. The protein gene Mbov_0503 isobtained by cloning from a mycoplasma bovis HB0801 genome. According to the partiality of escherichia coli for codons, the Mbov_0503 gene is modified, and mycoplasma bovis tryptophan codon UGA is mutated into a codon UGG for coding tryptophan in the escherichia coli to obtain a recombinant protein Mbov0503. The sequence of the protein gene which is cloned is shown as SEQID NO:13, and the sequenceof the coded protein is shown as SEQID NO:14. The mutant provided by the invention is an adhesion deficient strain screened from a mutant bank. The mutant has adhesive ability for host EBL cells, andcompared with a wild strain, for the mutant disclosed by the invention, the transmembrane transmission capacity for MDBK cells and the destructivity for tight connection between cells are notably reduced. The mutant can be applied to pathopoiesis and control of the mycoplasma bovis.
Owner:HUAZHONG AGRI UNIV
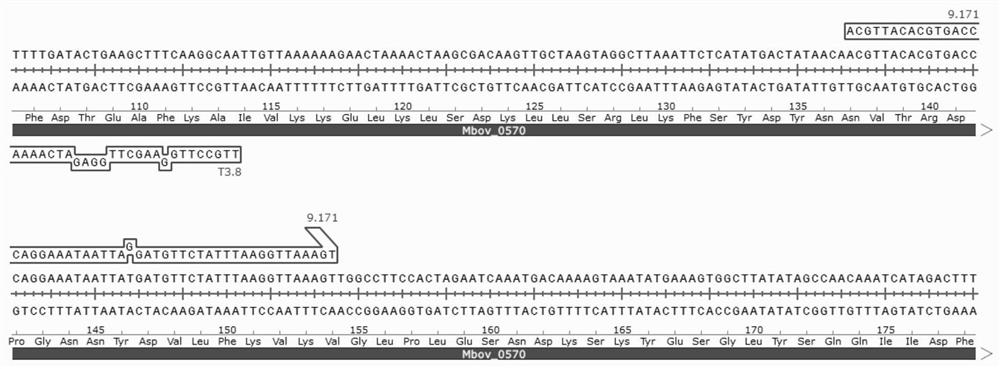
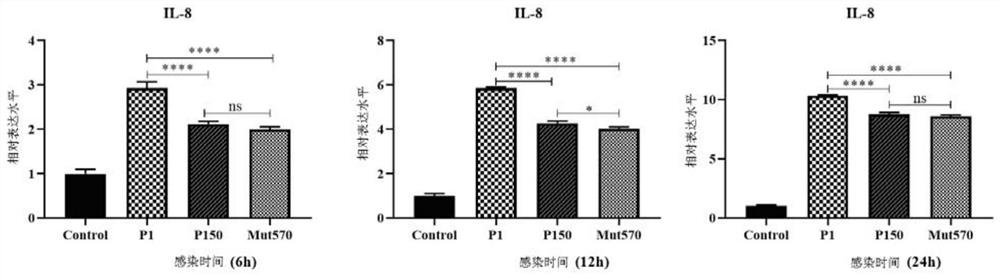
Mycoplasma bovis Mbov_0570 gene mutant strain and application thereof
The invention discloses a Mycoplasma bovis Mbov_0570 gene mutant strain, which belongs to the technical fields of prevention and treatment of animal infectious diseases, is named as Mycoplasma bovis T9.171, and is preserved in China Center for Type Culture Collection (CCTCC). The preservation number of the Mbov_0570 gene is CCTCC NO: M 2020083, and the nucleotide sequence of the Mbov_0570 gene isshown as SEQ ID NO: 1. According to the invention, qRT-PCR is used for detecting the capacity of T9.171 strain for stimulating BoMac cells to express IL-8, which shows that the capacity of the mutantstrain for inducing the BoMac cells to express cytokines is reduced, so that the toxicity of the mutant strain is reduced, and the mutant strain is expected to play an important role in the fields ofmycoplasma bovis immune prevention and treatment as a vaccine strain.
Owner:HUAZHONG AGRI UNIV
Preparing method and application of mycoplasma bovis inactivated vaccine
The invention discloses a preparing method of a mycoplasma bovis inactivated vaccine. By studying the formaldehyde inactivation condition for preparing the mycoplasma bovis inactivated vaccine, the immunizing dose of the mycoplasma bovis inactivated vaccine, the immunization way of the mycoplasma bovis inactivated vaccine, the adjuvant adopted by the mycoplasma bovis inactivated vaccine, and clinical application of the mycoplasma bovis inactivated vaccine, the optimal mycoplasma bovis formaldehyde inactivation condition, the optimal immunizing dose of the mycoplasma bovis inactivated vaccine, the optimal immunization way of the mycoplasma bovis inactivated vaccine, and the optimal immunization adjuvant are found out, then clinical animal experiments are conducted, and the results show that the mycoplasma bovis inactivated vaccine has a good effect on prevention of mycoplasma bovis diseases in clinical application, can be used as a clinically popularized vaccine, and lays a scientific foundation for prevention of mycoplasma bovis diseases.
Owner:NINGXIA UNIVERSITY
Product, method and application for simultaneously detecting mycoplasma gallisepticum and mycoplasma synoviae
ActiveCN112048566AStrong specificityHigh sensitivityMicrobiological testing/measurementBiological material analysisMycoplasma synoviaeAntigen
The invention discloses a product, method and application for simultaneously detecting mycoplasma gallisepticum and mycoplasma synoviae. According to the product and the method, a common specific protein antigen of mycoplasma gallisepticum and mycoplasma synoviae is preferably an outer membrane protein WP-041352022.1 as a detection object, a PCR primer pair S1 is designed and screened on the genome DNA level of the protein, and the PCR primer pair has the advantages of strong specificity, high sensitivity and no primer dimer generation. The invention provides a product and a method for simultaneously detecting two pathogens by using one pair of PCR primers, mutual interference between multiple PCR primers and the product is avoided, the detection rate is improved, and the method has the advantages of economy, simplicity, convenience and high efficiency.
Owner:SHANDONG LVDU BIO SICIENCE & TECH
Mycoplasma pneumoniae recombinant antigen and application thereof
ActiveCN108314710AReduced pair screening effortImprove the detection rateBacteriaDepsipeptidesEpitopeBinding peptide
The invention relates to a mycoplasma pneumoniae recombinant antigen and application thereof. The mycoplasma pneumoniae recombinant antigen comprises a PIA fragment, a first binding peptide, a PIB fragment, a second binding peptide and a P30 fragment. Experiment results show that the recombinant antigen can obtain different types of antigen dominant epitopes after being purified in different ways,and the various types of antigen dominant epitopes are stable in expression, namely the different types of antigen dominant epitopes can be stably expressed as required. The matching and screening works of the antigen can be greatly reduced during detection; the detection rate is high, and the specificity is high during the detection of mycoplasma pneumoniae.
Owner:GUANGDONG WESAIL BIOTECH CO LTD
Hybridoma cell strain capable of secreting anti-mycoplasma bovis monoclonal antibody and application thereof
InactiveCN105695417AEfficient identificationStrong specificityImmunoglobulins against bacteriaTissue cultureAntiendomysial antibodiesMicrobiology
The invention provides a hybridoma cell strain capable of secreting an anti-mycoplasma bovis monoclonal antibody and application thereof.The hybridoma cell strain can produce the anti-mycoplasma bovis monoclonal antibody and is used for detecting mycoplasma bovis.The preservation number of the hybridoma cell strain is CCTCC C2015183, and the preservation date is November 8, 2015.The hybridoma cell strain has the advantages that the hybridoma cell strain can specifically secrete the anti-mycoplasma bovis monoclonal antibody and has better sensitivity and higher specificity.The hybridoma cell strain further has the advantages that the antibody produced by the cell strain can be applied in a variety of ways and has a good application value during actual production.
Owner:GANSU AGRI UNIV
Method for coating mycoplasma pneumoniae membrane protein antigens with magnetic beads
PendingCN111579773AAlleviate agglutinationReduce precipitationImmunoassaysAntigenCell Membrane Proteins
The invention provides a method for coating mycoplasma pneumoniae membrane protein antigens with magnetic beads, and relates to the technical field of biology. The mycoplasma pneumoniae membrane protein antigens coated by the method are natural mycoplasma pneumoniae protein. The method comprises the following steps: treating the mycoplasma pneumoniae membrane protein antigens by using a reducing agent, and coupling the mycoplasma pneumoniae membrane protein antigens with magnetic beads. According to the method, the technical problem that the efficiency of coating the magnetic beads with the natural mycoplasma pneumoniae membrane protein antigen is low is alleviated.
Owner:ZHUHAI LIVZON DIAGNOSTICS
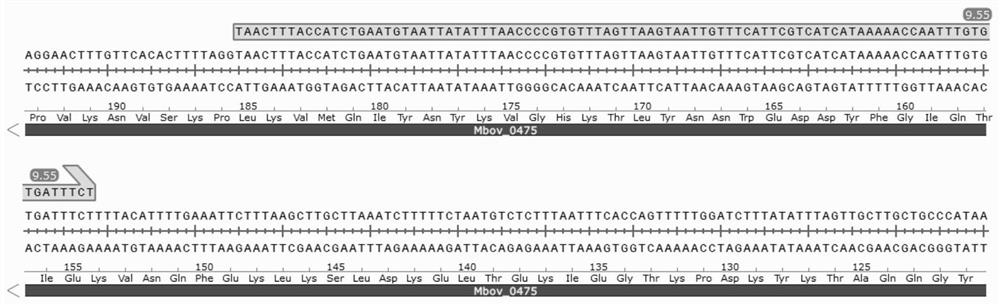
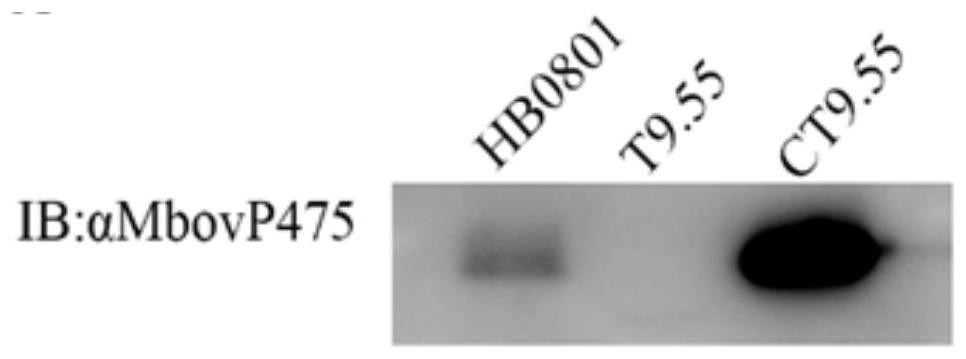
Mycoplasma bovis Mbov_0475 gene mutant strain and application thereof
ActiveCN111748507AIncrease vitalityAntibacterial agentsBacterial antigen ingredientsInfectious DisorderNucleotide
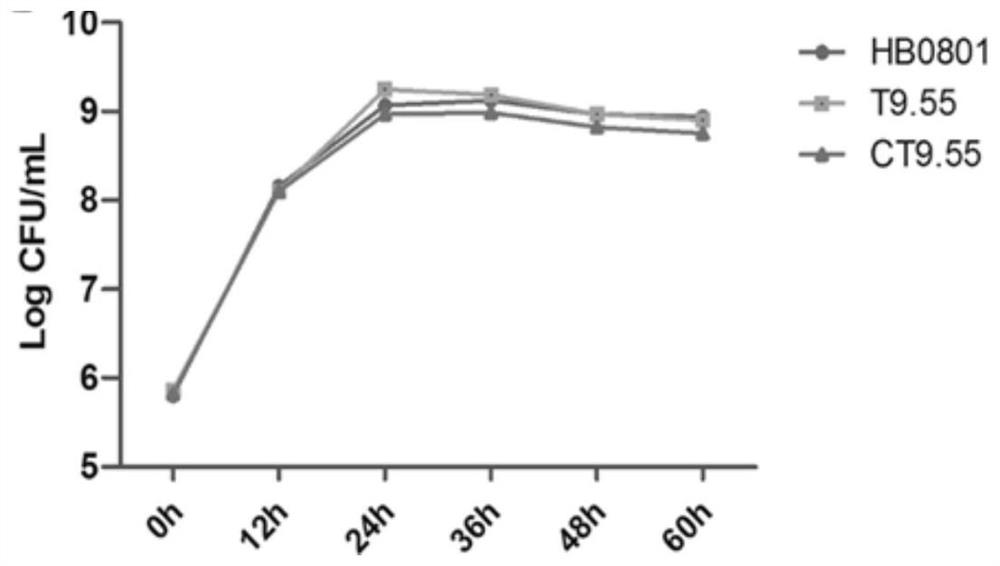
The invention discloses a mutant strain of a mycoplasma bovis Mbov_0475 gene, which belongs to the technical field of animal infectious disease prevention. The mutant strain is named as Mycoplasma bovis T9.55, and is preserved in China Center for Type Culture Collection, a preservation number is CCTCC NO: M 2020082, and the nucleotide sequence of the Mbov_0475 gene is shown as SEQ ID NO: 1. The strain has the effects of inducing macrophage proliferation and improving macrophage activity, and because bovine macrophages are important immune cells of a host for resisting pathogenic infection, themutant strain is expected to play an important role in the field of mycoplasma bovis immune prevention and treatment.
Owner:HUAZHONG AGRI UNIV
Mutant strains of mycoplasma hyopneumoniae
ActiveCN104685057ABacterial antigen ingredientsMicroorganism based processesVector vaccinePorcine enzootic pneumonia
The present invention relates to mutant strains of Mycoplasma hyopneumoniae and to a method for preparing said strains. It also relates to vectors used in said method, to vaccine compositions and to vaccine kits which comprise said strains for use against porcine enzootic pneumonia and other porcine diseases. The invention also relates to the use of M. hyopneumoniae as a host for the expression of recombinant proteins and other DNA sequences of interest.
Owner:海博莱科学有限公司
Traditional Chinese medicine composition for preventing and treating swine enzootic pneumonia and preparation method and application thereof
ActiveCN104784465AEffective controlGood control effectAnimal feeding stuffRespiratory disorderFritillaria thunbergiiRadix Ophiopogonis
The invention discloses a traditional Chinese medicine composition for preventing and treating swine enzootic pneumonia. The traditional Chinese medicine composition is prepared from the following raw material medicines in parts by weight: 6 parts of radix ophiopogonis, 6 parts of thunberg fritillary bulb, 6 parts of astragalus membranaceus, 6 parts of aristolochia debilis, 4 parts of scutellaria, 4 parts of cortex mori radicis, 4 parts of schisandra chinensis, 3 parts of honeysuckle, 3 parts of rhizoma anemarrhenae, 3 parts of bighead atractylodes rhizome, 3 parts of semen lepidii and 2 parts of radix glycyrrhizae. The invention further provides a preparation method and application of the traditional Chinese medicine composition. The traditional Chinese medicine composition for preventing and treating swine enzootic pneumonia has the benefits and advantages that the swine enzootic pneumonia can be effectively prevented and treated, and the preventing and treating effects are good; (2) the medicine can be fed by adopting water drinking or feed mixing, the operation is simple and the cost is low; (3) as the traditional Chinese medicine composition is Chinese herbal medicine preparation, the problems of drug tolerance and the like are avoided, and the probability of preventing and treating failure is greatly reduced; (4) the traditional Chinese medicine composition is pure traditional Chinese medicine compound, and has the advantages of multiple target, low residue in swine and good biosecurity when being acted on mycoplasma.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
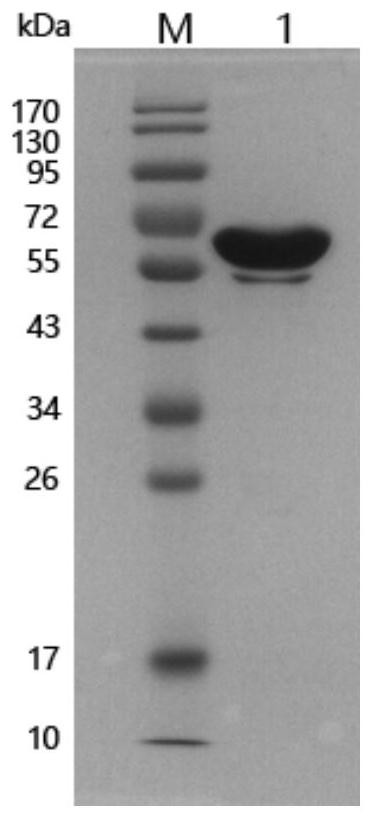
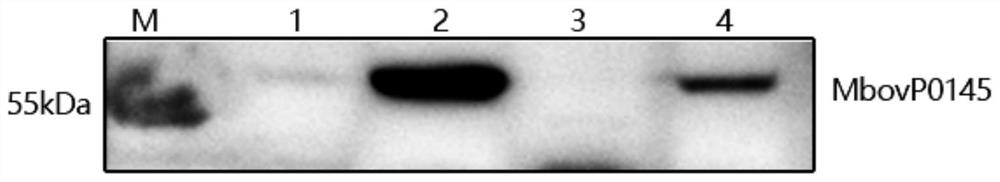
Mycoplasma bovis secretory protein MbovP0145 and application thereof
ActiveCN111621506AHave secretory propertiesImproving immunogenicityImmunoglobulins against bacteriaMicroorganism based processesInfectious DisorderNucleotide
The invention discloses a protein encoded by a mycoplasma bovis Mbov-0145 gene. The mycoplasma bovis Mbov-0145 gene has a nucleotide sequence as shown in SEQ ID NO: 1. The invention also discloses anantibody of the gene, and an application of the protein and the antibody in preparation of a mycoplasma bovis detection kit, and belongs to the technical field of prevention and treatment of animal infectious diseases. The protein can generate specific reaction with positive serum of naturally infected and artificially infected cattle of a mycoplasma bovis virulence strain M.bovis HB0801, and canidentify animals infected with wild virus after vaccine immunization. The protein is expected to be used as a molecular target for diagnosis reagent and vaccine of mycoplasma bovis infection and drugresearch and development, and plays an important role in prevention and control of mycoplasma bovis.
Owner:HUAZHONG AGRI UNIV
Fusion protein containing mycoplasma hyopneumoniae antigen, vaccine composition and application
ActiveCN107868130AImprove protectionImprove purification effectAntibacterial agentsBacterial antigen ingredientsImmune effectsMycoplasma
The invention relates to a fusion protein. The fusion protein comprises enterotoxigenic escherichia coil heat-labile enterotoxin B subunit and mycoplasma hyopneumoniae antigen protein in a sequence from an N end to a C end. The invention also relates to a vaccine composition prepared from the fusion protein and an application thereof. The vaccine composition provided by the invention can achieve the immune effect of the commercial inactivated vaccine or better immune effect after one-time immunization.
Owner:PU LIKE BIO ENG
Traditional Chinese medicine for treating non-gonococcal urethritis
ActiveCN103705818ACompatibility scienceGood curative effectInanimate material medical ingredientsAntiinfectivesMedicinal herbsLiver and kidney
The invention relates to a traditional Chinese medicine for treating non-gonococcal urethritis, and is used for effectively solving the problem of medicines for treating the non-gonococcal urethritis. A method is as follows: the traditional Chinese medicine is prepared from the following Chinese medicinal herbs of wine fried felwort, lichen of parmelia saxitilis, Chinese mahonia, rheum officinale, stir-baked squama manitis, fried semen plantaginis, rhizoma alismatis, radix bupleuri, wine fried angelica sinensis, fried cape jasmine, wine fried scutellaria baicalensis, dianthus superbus, polygonum aviculare, broom cypress fruit, golden cypress, ural licorice root tip, rhizoma anemarrhenae, pseudo-ginseng, cordyceps sinensis, poria cocos, trollflower, fried bighead atractylodes rhizome, ligusticum wallichii, dipsacus root, medicinal cyathula root, radix pseudostellariae, the root bark of the peony tree, amber, rhizoma atractylodis, eucommia bark, morinda meat, radix stemonae, lychee seed and common peony root. The traditional Chinese medicine is scientific in compatibility, good in treatment effect, safe and reliable, integrates a nourishing function into a removing function, integrates a releasing function in the nourishing function so as not to hurt healthy qi and attach pathogenic qi, has the functions of clearing away heat and toxic materials, sterilizing and diminishing inflammation, inducing diuresis for treating strangurtia, promoting blood circulation to remove blood stasis, resolving hard lump, tonifying liver and kidney, strengthening the spleen and stomach and improving the immunity, and is mainly used for treating non-gonococcal mycoplasma and chlamydia microbial infection, male mixed prostatitis and orchitis, female mixed cervicitis and pelvic inflammation, immunodeficiency, and the like.
Owner:张云刚
Tylosin in situ gel drug composition for livestock and preparation method thereof
InactiveCN103705443AAntibacterial agentsOrganic active ingredientsCurative effectHaemophilus influenzae
The invention provides a tylosin in situ gel drug composition which is used for solving the problem that animals, especially piglets, are infected with mycoplasmata, treponematosis, staphylococcus, streptococcus, pasteurella and haemophilus influenzae. Before the piglets suck, the tylosin in situ gel drug composition is directly sprayed or smeared on nipples of sows and can quickly become gel with relatively high viscosity so as to be retained locally, so that the piglets take in tylosin while sucking. The tylosin in situ gel drug composition is convenient to use and ensures the curative effect.
Owner:佟丽 +1
Method for culturing mycoplasma contamination-free cells and method for removing mycoplasma contamination of cells
ActiveUS20120244614A1Prevent mycoplasma contaminationRemove mycoplasma contaminationFungiOrganic chemistryBiotechnologyMycoplasma contamination
Owner:CELLSAFE
Kit for rapid detection and genotyping of mycoplasma pneumoniae
ActiveCN104946769ASave operating timeImprove efficiencyMicrobiological testing/measurementDNA/RNA fragmentationMicroorganismNucleotide
The invention provides a kit for rapid detection and genotyping of mycoplasma pneumoniae, belonging to the technical field of microbiological detection. The kit contains 2 specific primer pairs and 2 probes matched with the primer pairs, and the nucleotide sequences are shown by SEQ ID No.1-6 respectively; rapid detection and genotyping of mycoplasma pneumoniae are realized by a duplex fluorescent quantitative PCR (polymerase chain reaction) method, the detection sensitivity is 3CFU, the coincident rate between the genotyping accuracy and that of common genotyping methods reaches 100%, the genotyping sensitivity is much higher than that of common culture-dependent genotyping technologies, the genotyping time is greatly shortened, and the genotyping can be performed at the time of detection on mycoplasma pneumonia in a sample; and moreover, the shortcomings of low sensitivity, time and labor consuming and relatively high cost of the existing mycoplasma pneumoniae genotyping method are overcome, and the kit provided by the invention has good practical sample detection and genotyping ability and is suitable for popularization and application.
Owner:ICDC CHINA CDC
Mycoplasma pneumoniae fusion antigen as well as preparation method and application thereof
ActiveCN111548423AStrong specificityHigh sensitivityBacteriaAntibody mimetics/scaffoldsSerologyBiomedical engineering
The invention provides a mycoplasma pneumoniae fusion antigen as well as a preparation method and application thereof, and relates to the technical field of biology. The mycoplasma pneumoniae fusion antigen is a fusion protein containing a P1M antigen fragment and a P30A antigen fragment. The mycoplasma pneumoniae fusion antigen provided by the invention is verified by an immunoserological detection technology, and compared with an existing mycoplasma pneumoniae antigen, the mycoplasma pneumoniae fusion antigen is stronger in specificity, higher in sensitivity, easy to culture and purify, morebeneficial to industrial production and lower in cost. The mycoplasma pneumoniae fusion antigen is suitable for preparation of MP antibody detection products, can be processed into products in any form in the field of in-vitro immunodiagnosis, and has a wide market prospect.
Owner:ZHUHAI LIVZON DIAGNOSTICS
Nasal cavity protective agent
InactiveCN103083404AFast absorptionEliminate allergic stress reactionAntipyreticAnalgesicsVegetable oilAcute rhinitis
The invention relates to an external nasal cavity protective agent used for allergic rhinitis, acute rhinitis and other nasal cavity and respiratory mucosa inflammations. The using method of the protective agent comprises the step of daubing the protective agent on the front section of the nasal cavity. The nasal cavity protective agent comprises the following components in percentage by weight: 2-30 percent of eucalyptus oil, 2-30 percent of tea tree oil, 2-30 percent of peppermint oil, 2-30 percent of lavender oil, 1-2 percent of enteromorpha oil and 40-90 percent of edible plant oil. The external nasal cavity protective agent has the functions that 1, the protective agent has the antihistamine function, has high effect on allergic rhinitis, acute rhinitis and the like and is fast in effect and durable in effect; 2, the nasal cavity and respiratory mucosa can be activated, and the capacity of the respiratory mucosa for resisting microbial infection is improved; 3, the nasal cavity biological environment is improved, the other symptoms of the nasal cavity are relieved, and the mucosa wound healing is promoted; 4, the protective agent has the functions of inhibiting bacteria, moulds, mycoplasma, viruses and other microbes; 5, the protective agent has the protective functions of delaying water evaporation and isolating sensitizing and pathogenic factors on the nasal cavity mucosa; 6, the protective agent is highly safe to human mucosa tissue and has a health-care function; and 7, the protective agent is convenient to use and high in economical efficiency.
Owner:章迅
Mycoplasma hyorhinis strain, vaccine composition, preparation method and application thereof
ActiveCN104250623AImprove the effect of prevention and controlImmunity overAntibacterial agentsBacterial antigen ingredientsImmune effectsMycoplasma
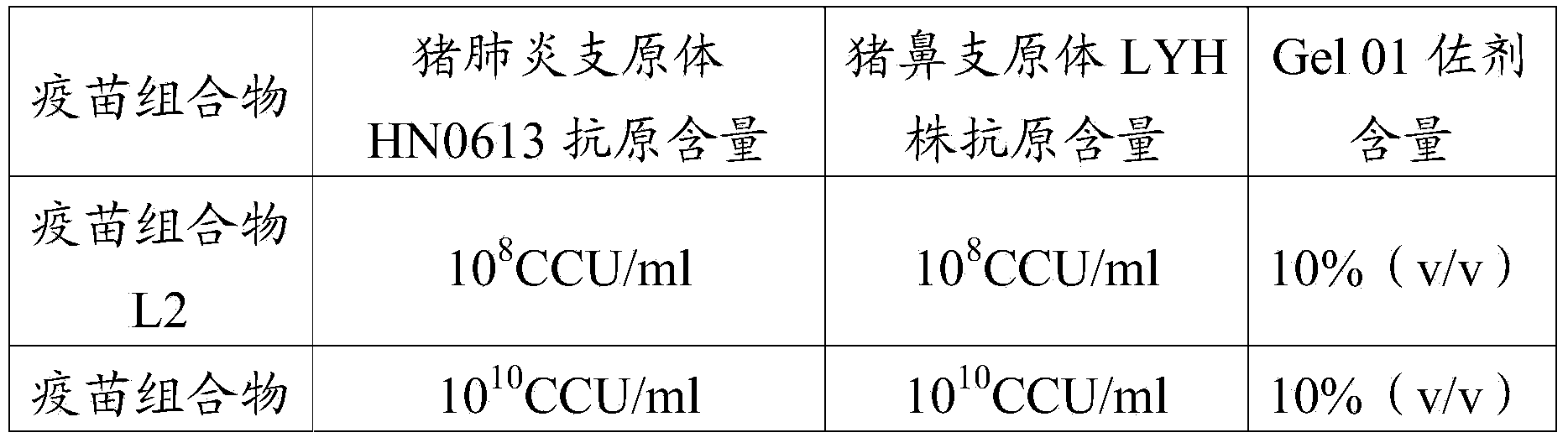
The invention provides a Mycoplasma hyorhinis strain LYH, and a vaccine composition prepared from the Mycoplasma hyorhinis strain LYH, in particular to a vaccine composition comprising the Mycoplasma hyorhinis and Mycoplasma hyopneumoniae. The vaccine composition can be effective in prevention and treatment of swine enzootic pneumonia caused by Mycoplasma hyorhinis, Mycoplasma hyopneumoniae single infection or mixed infection. Especially in the circumstance of mixed infection, immune effect of the vaccine composition significantly exceeds that of each single vaccine.
Owner:PU LIKE BIO ENG
Mycoplasma hyopneumoniae genetic engineering subunit vaccine as well as preparation method and application thereof
ActiveCN111925452ANot pathogenicReduce manufacturing costAntibacterial agentsAntibody mimetics/scaffoldsProtein compositionImmunogenicity
The invention discloses a mycoplasma hyopneumoniae genetic engineering subunit vaccine as well as a preparation method and an application thereof. The vaccine comprises a protein composition and a pharmaceutically acceptable carrier, wherein the protein composition comprises two fusion proteins with sequences shown in SEQ ID NO: 2 and SEQ ID NO: 6 respectively. The vaccine provided by the invention has no toxicity; the vaccine is high in safety, good in immunogenicity and capable of generating strong humoral immunity in pig bodies, immunized animals can resist poison attacking of strong poisonand are comprehensively protected, and the vaccine can be prepared through large-scale serum-free suspension culture of a bioreactor and has the advantages of being easy to control in quality, stablebetween batches, low in production cost and the like.
Owner:苏州世诺生物技术有限公司 +1
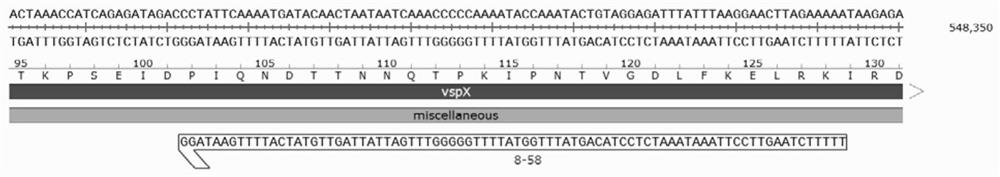
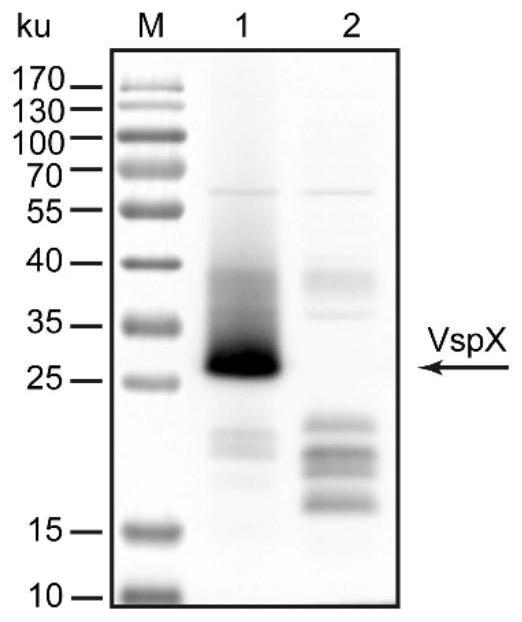
Mycoplasma bovis VspX gene mutant strain, and construction method and application thereof
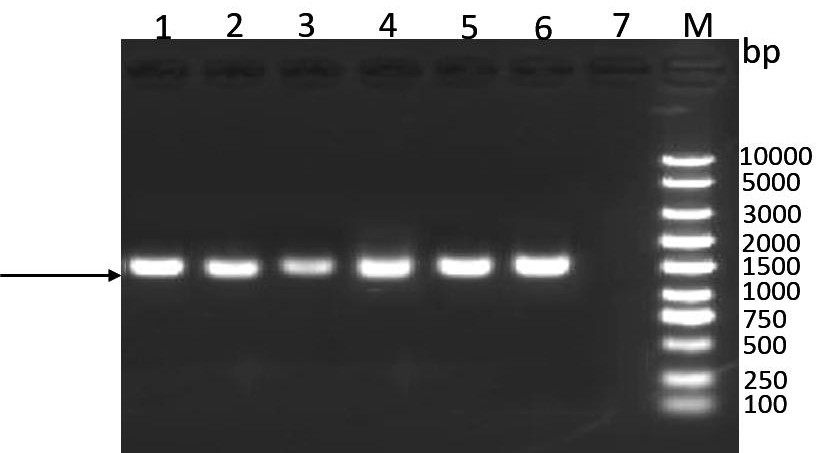
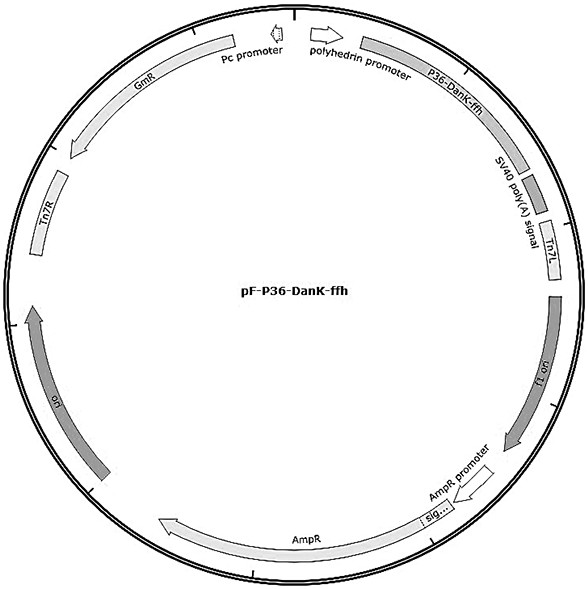
ActiveCN111635880ALow ability to adhere to cellsDecreased adhesion of FnAntibacterial agentsBacterial antigen ingredientsThelial cellMutant strain
The invention discloses a mycoplasma bovis VspX gene mutant strain. The mycoplasma bovis VspX gene mutant strain is named as Mycoplasma bovis T8.101, is preserved in the China Center for Type CultureCollection (CCTCC), and has a preservation number of CCTCC NO: M 20191038, and the VspX gene has a nucleotide sequence shown as SEQ ID NO:1. The invention also discloses a construction method of the mutant strain and an application of the mutant strain in the fields of mycoplasma bovis pathogenic mechanism and immune prevention and control. The mutant strain does not express protein coded by the mutant gene, and the growth curve, colony morphology and size of the mutant strain are not obviously different from those of a wild strain. However, the binding capacity of the mutant strain to bovinepulmonary epithelial cell lines and fibronectin is remarkably reduced, so that the mutant strain has the potential of being applied to the fields of bovine mycoplasma pathogenic mechanism research andprevention and treatment drug research and development as a virulence attenuating strain.
Owner:HUAZHONG AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com