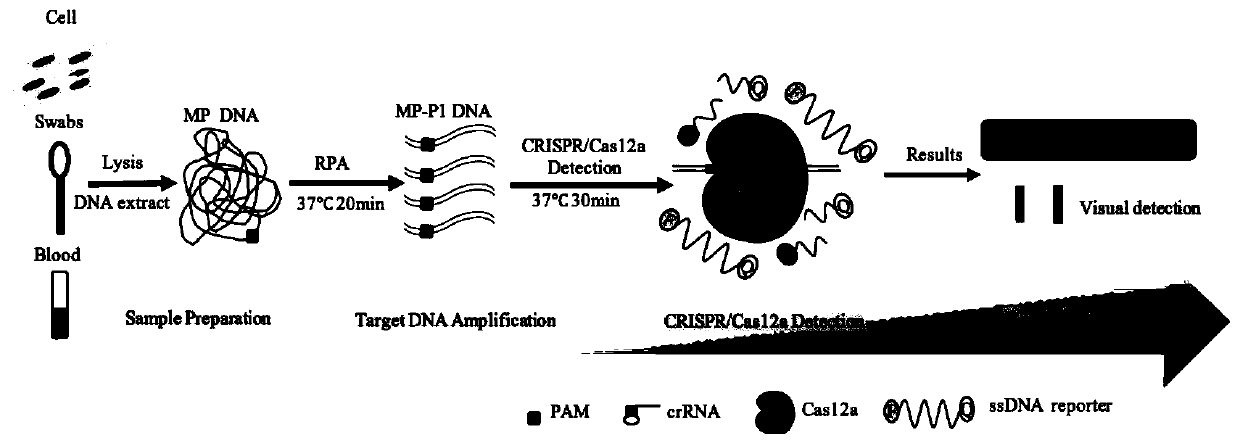
Kit and detection method for rapid detection of nucleic acid of mycoplasma pneumonia on basis of CRISPR/Cas12a
A technology of mycoplasma pneumoniae and detection method, which is applied in the directions of microorganism-based methods, biochemical equipment and methods, and the determination/inspection of microorganisms, can solve problems such as high price, and achieve simple detection methods, high-sensitivity visual detection, and convenience. The effect of quick result interpretation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Rapid and sensitive detection of Mycoplasma pneumoniae nucleic acid fragments
[0040] 1.1 Nucleic acid preparation
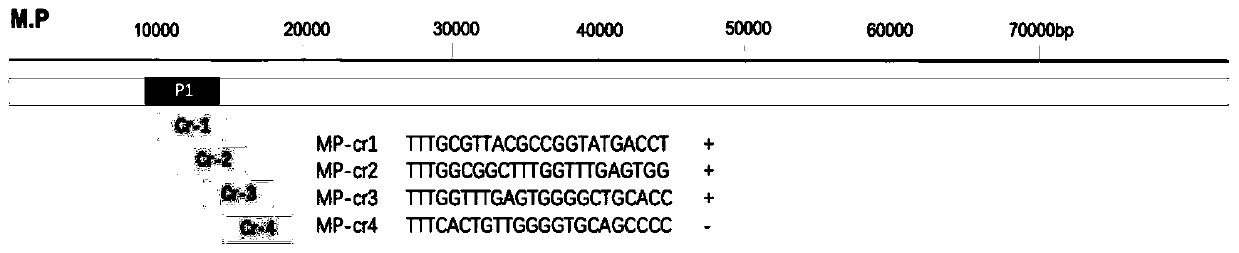
[0041] The MP gene fragment in this case refers to the P1 gene fragment of MP in the NCBI database. The 399bp SEQ NO.1 of the P1 part gene was synthesized by Nanjing GenScript and constructed into the pUC57 vector, named pUC57-MP-P1.
[0042] Using the RPA amplification primers RPA-F1 SEQ NO.2 and RPA-R1 SEQ NO.3 of the present invention, referring to the RPA isothermal amplification operation steps, amplified to obtain the sample to be detected. The specific operation is as follows:
[0043] 1μL (10ng) pUC57-MP-P1 sample was subjected to RPA amplification reaction: 25μL 2*Buffer, 2μL RPA-F1, 2μL RPA-R1 and 2.5μL magnesium acetate, mixed well, reacted at 37°C for 20min, obtained the sample for The next step is nucleic acid testing.
[0044] 1.2 Design and preparation of MP-specific crRNA
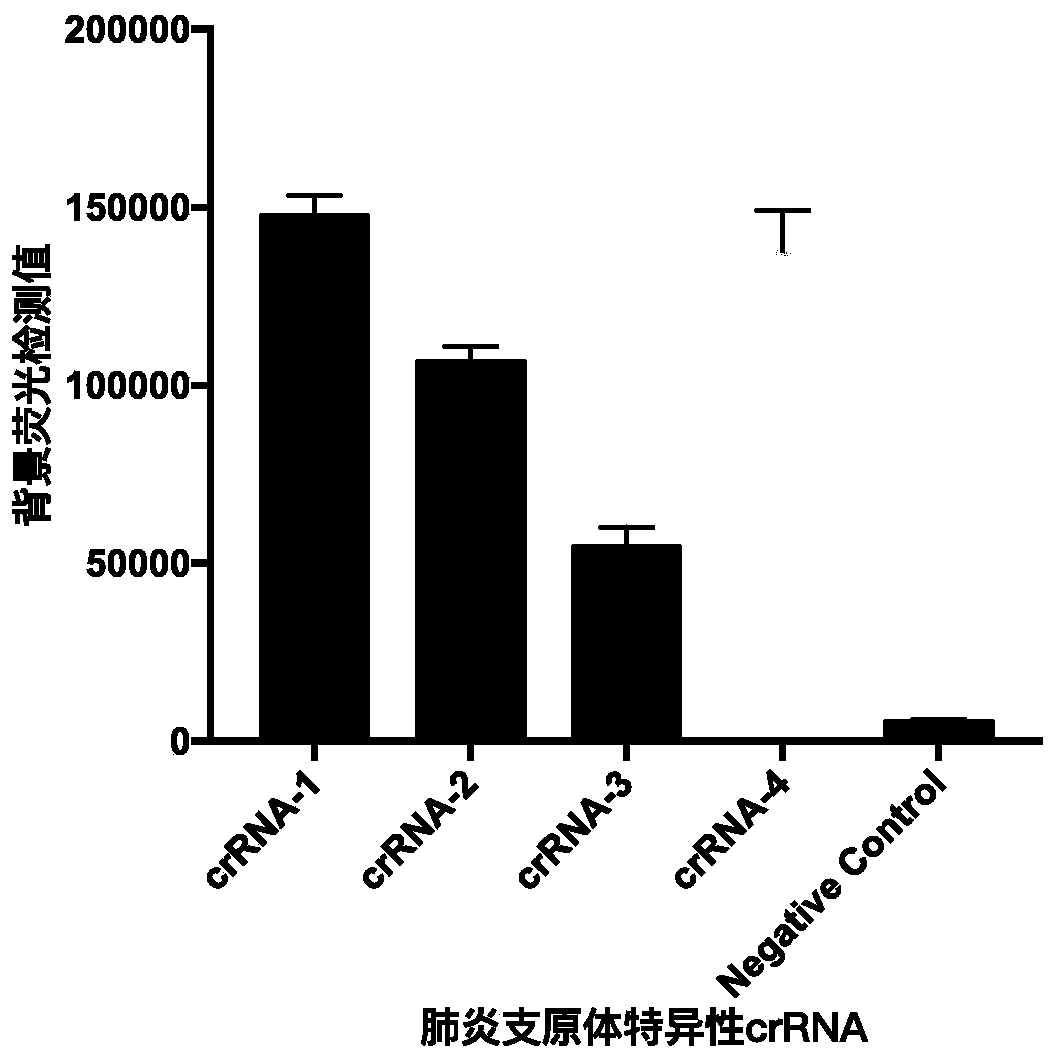
[0045] Such as figure 2 As shown, the preparatio...
Embodiment 2
[0060] Embodiment 2: CRISPR / Cas12a detects Mycoplasma pneumoniae nucleic acid sensitivity
[0061] In the case of sensitivity detection, the pUC57-MP-P1 plasmid was converted to copy number according to the molecular weight, and a 10-fold serial dilution was performed to obtain 2*e7, 2*e6, 2*e5, 2*e4, 2*e3 per microliter , 2*e2, 2*e1 and 2*e0 copy number (copy / μL). 1 μL of gradient dilution samples were subjected to RPA amplification reaction: 25 μL 2*Buffer, 2 μL RPA-F1, 2 μL RPA-R1 and 2.5 μL magnesium acetate, mixed well, reacted at 37°C for 20 minutes, and obtained samples for the next step of nucleic acid detection.
[0062] According to the results obtained in Example 1, crRNA has a high sensitivity for detecting the P1 gene of Mycoplasma pneumoniae, so crRNA1 is used for subsequent detection. This test uses a 20 μL system as shown in Table 3, but is not limited to this, including the adjustment of the proportion of the corresponding components:
[0063] Table 3. Mycop...
Embodiment 3
[0069] Example 3: Rapid detection of Mycoplasma pneumoniae nucleic acid on clinical sample nucleic acid
[0070] In this example, the rapid detection of nucleic acid in clinical samples is performed. All samples and operations are completed in the laboratory. In this case, the sample is DNA obtained from oral swabs for CRISPR / Cas12a detection. In this example, a rapid nucleic acid release agent purchased from Novizyme was used to obtain pretreated nucleic acid. The steps are as follows: wash the oral swab with PBS and centrifuge, take 2 μL of centrifuged supernatant and add 20 μL of nucleic acid lysate, let it stand at room temperature for 3 minutes, add 20 μL of neutralizing solution, mix well and proceed to the next step of detection.
[0071] Using the RPA amplification primers SEQ NO.2 and SEQ NO.3 in the present invention, referring to the RPA isothermal amplification operation steps, take 2 μl of each sample to be tested for RPA pre-amplification to obtain the sample to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com