Mycoplasma bovis diagnosis reagent and its application
A technology of Mycoplasma bovis and diagnostic reagents, applied in the field of animal diagnostic medicine, can solve problems such as inability to accurately reflect pathogen infection, lag in basic research on Mycoplasma bovis, long duration of antibodies, etc. The effect of small difference and low detection cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1 multi-epitope fusion antigen
[0061] Construction of a gene vector for pathogenicity-associated protein P33 and specific membrane protein P30 of Mycoplasma bovis
[0062] 1pMD18-T-P33 and pMD18-T-P30 constructs
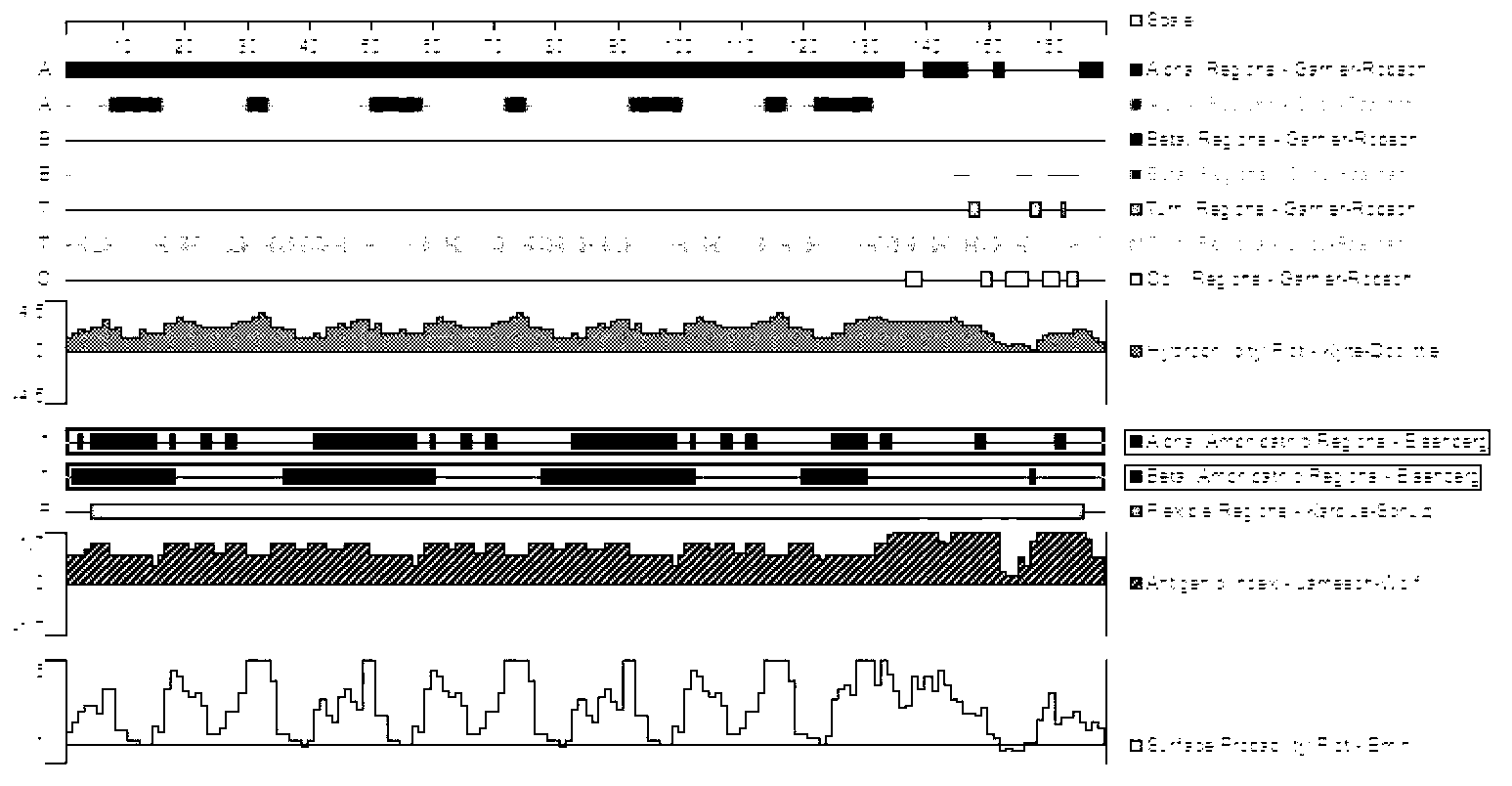
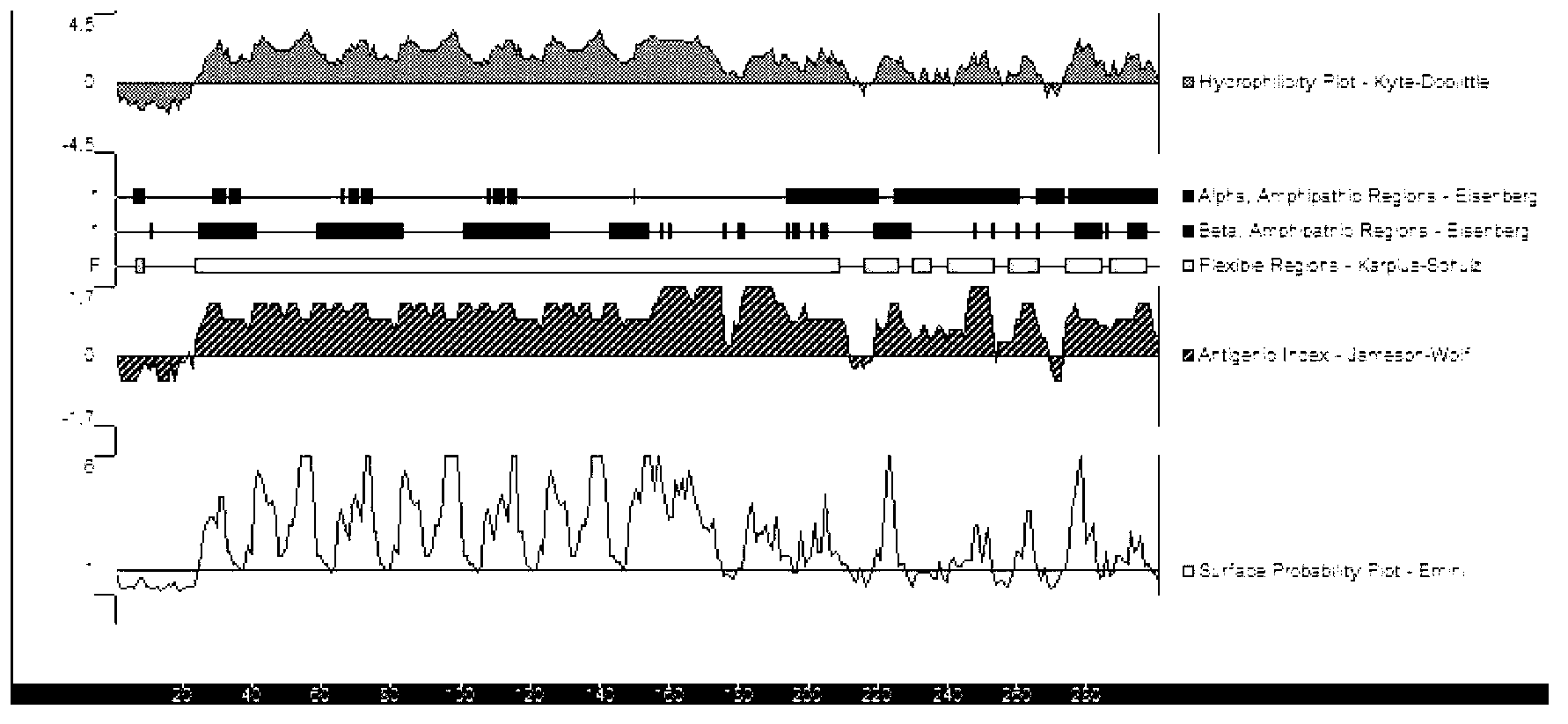
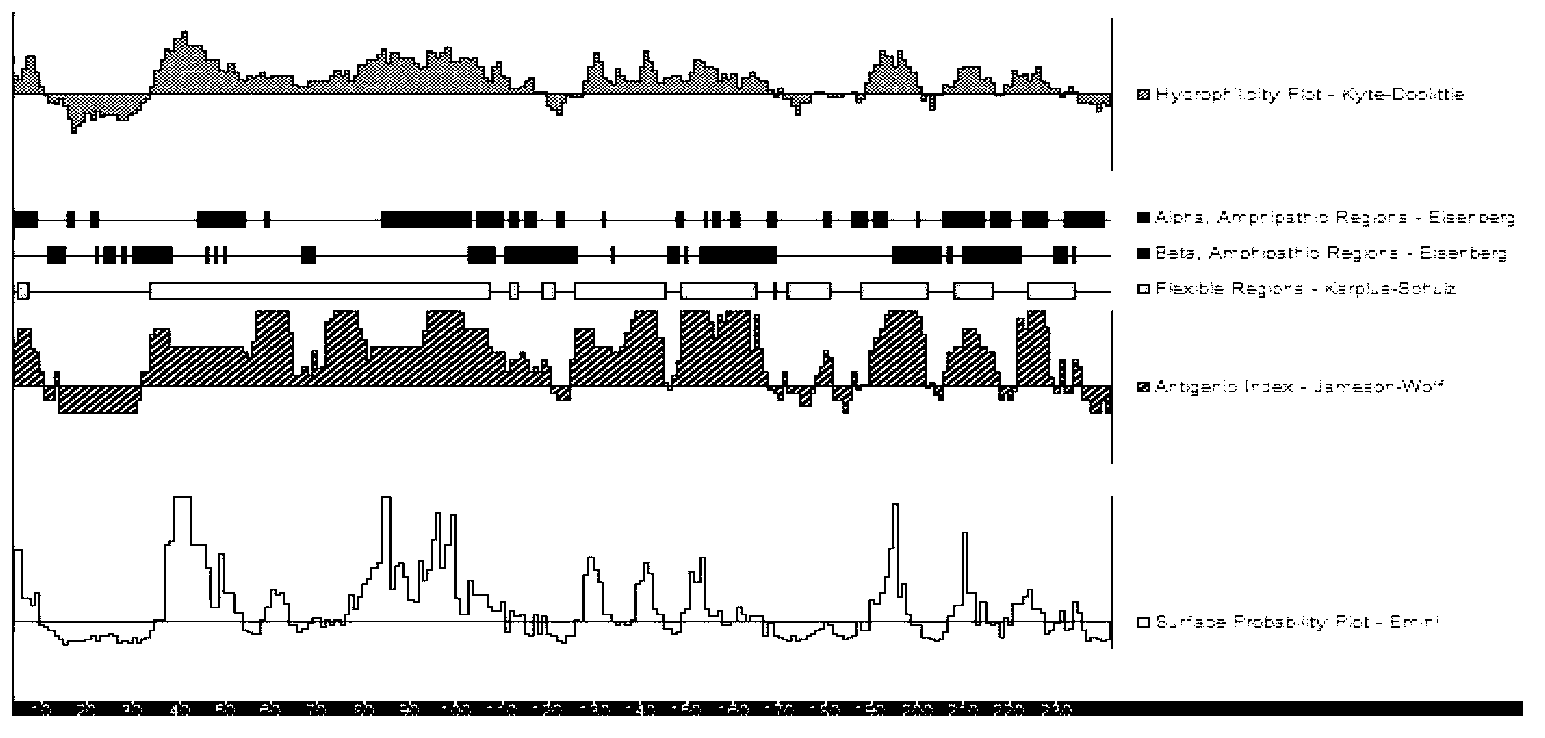
[0063] The full length of the P33 gene fragment is 903bp, and its nucleotide sequence is shown in SEQ ID NO: 3, which contains 5 TGA codons encoding tryptophan. For the B cell epitope analysis of P33, see figure 2 ;
[0064] SEQ ID NO.3:
[0065] “ggatccatattattgacaacactatcgccaattatctcattgccatttttatctgctagttg catcaccgaagcaaaatcagataacaaaatggaaaaagatattaagataaacgaaaatacagatgaaa aaaattcttctgaaacaatgaataacaaacaaaaacaagataaaagcagtatagattcaaagatggaa gaaaaagcagataacaaaacggaaaaagatattaagataaacgaaaatacagatgaaaaaaattcttc tgaaacaatgaataacaaacaaaaacaagataaaagcagtatagattcaaagatggaagaaaaagcag ataacaaaacggaaaaagatattaagataaacgaaaatacagatgaaaaaaattcttctgaaacaatg aataacaaacaaaaacaagataaaagcagtatagaatcgaaaatgaaagaaaaaacagaaaagcaaga ttcaaaaactaactcagaaaaacaagatt...
Embodiment 2
[0086] Example 2 Preparation of multi-epitope fusion antigen P39 kit for detecting Mycoplasma bovis antibodies
[0087] The composition of an indirect ELISA detection kit
[0088] 1 Antigen-coated plate:
[0089] 1) Coating: Multi-epitope fusion antigen was diluted to 5 μg / mL with 50 mM carbonate buffer (pH 9.3), 100 μL was added to each well, and incubated overnight at 4°C.
[0090] ●Carbonate buffer (5×, pH9.3) 1L
[0091] Component Amount and Final Concentration
[0092] Na2CO3 (MW105.99) points 7.949g (75mM)
[0093] Pure analysis
[0094] Analysis of NaHCO3 (MW84.01) 14.702g (175mM)
[0095] pure
[0096] Dissolve in ultrapure water, dilute to 1L, sterilize by filtration, and store at 2-8°C. Dilute 5 times with ultrapure water before use.
[0097] 2) Blocking: 1% casein, 300 μL / well, incubate at 37°C for 2 hours, seal and store at 2-8°C.
[0098] 2 Sample diluent: 0.5% casein, prepared with PBS (1×, pH 7.4). For the preparation method of PBS, refer to page 1570 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com