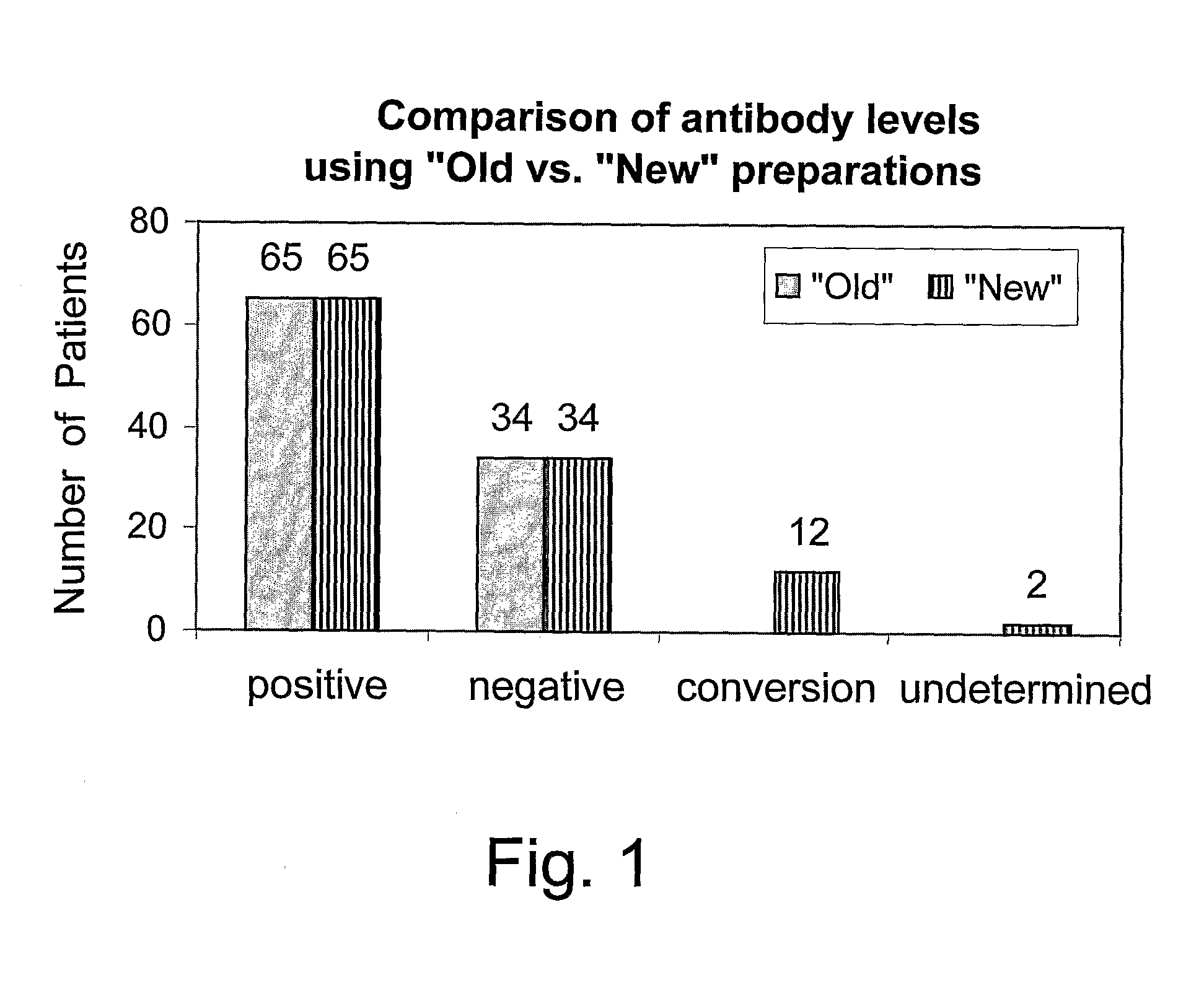
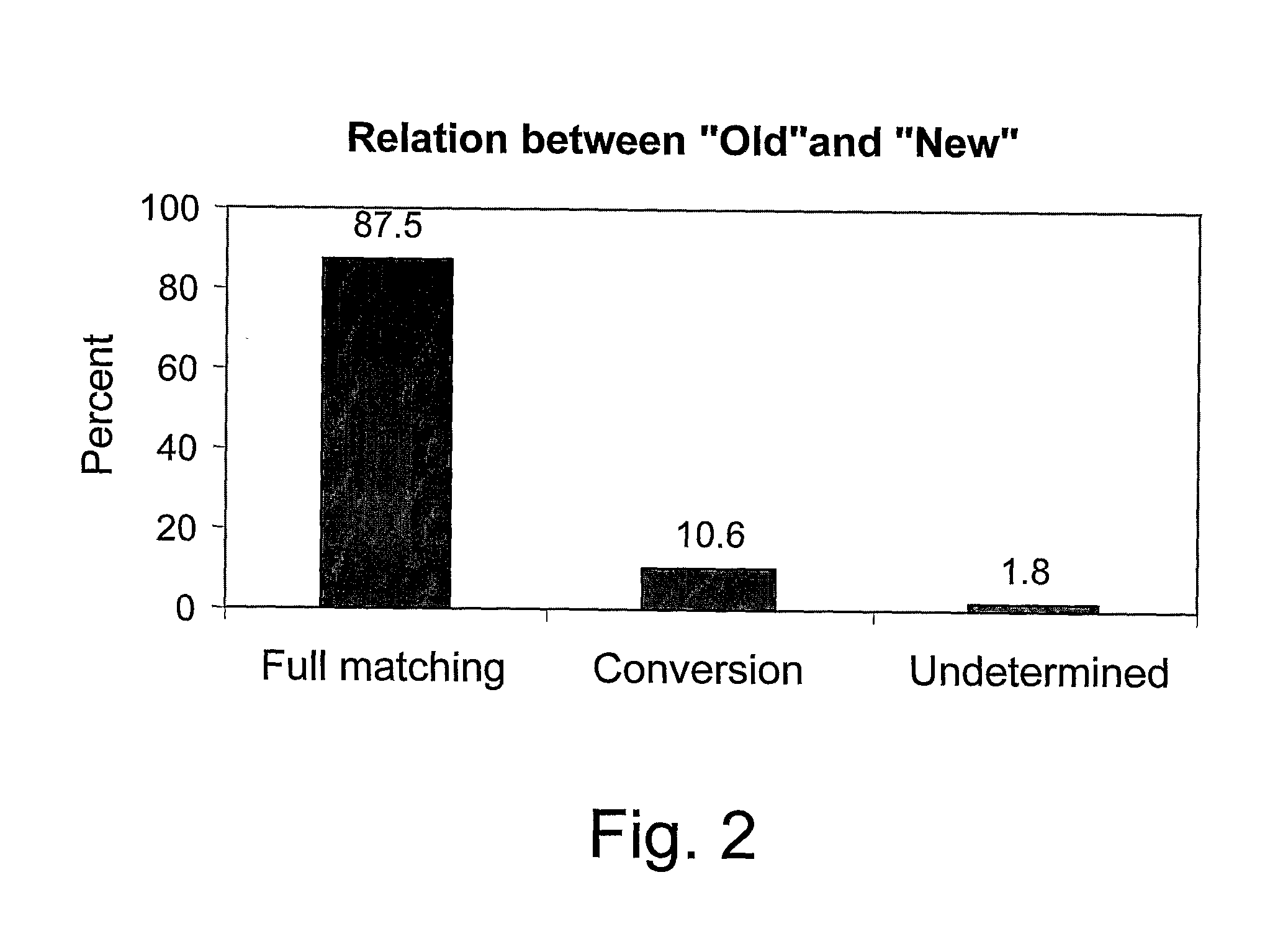
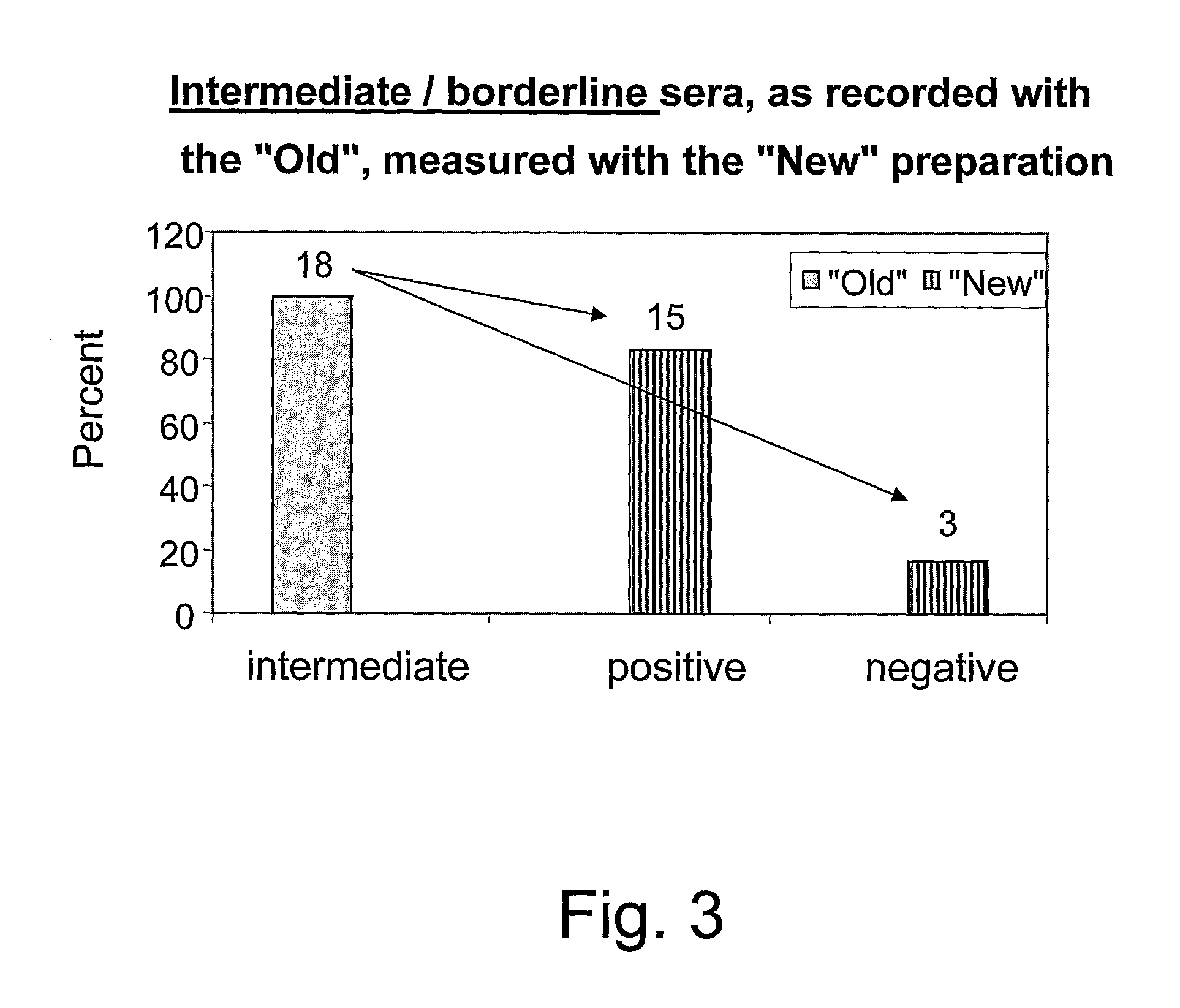
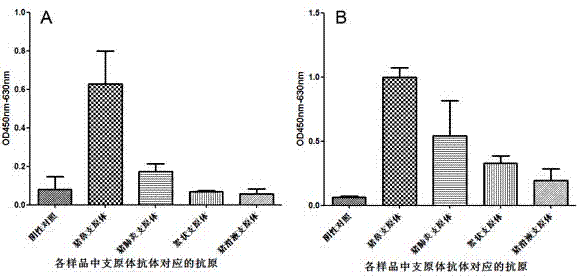
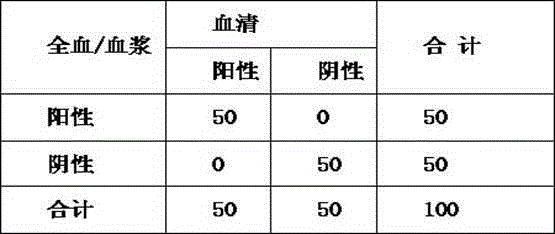
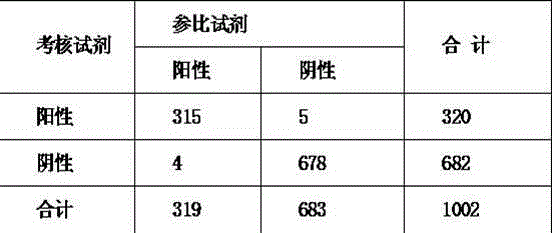
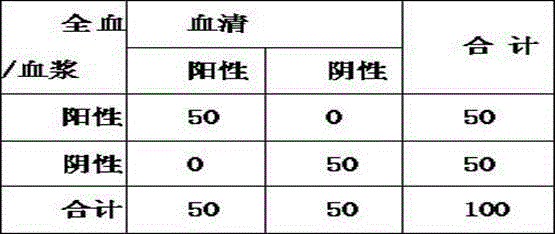
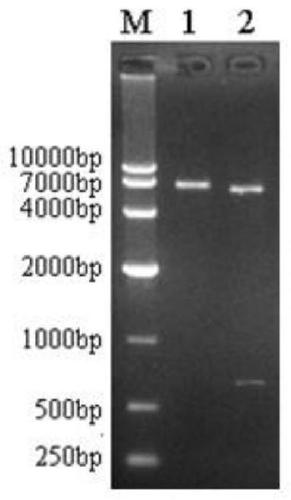
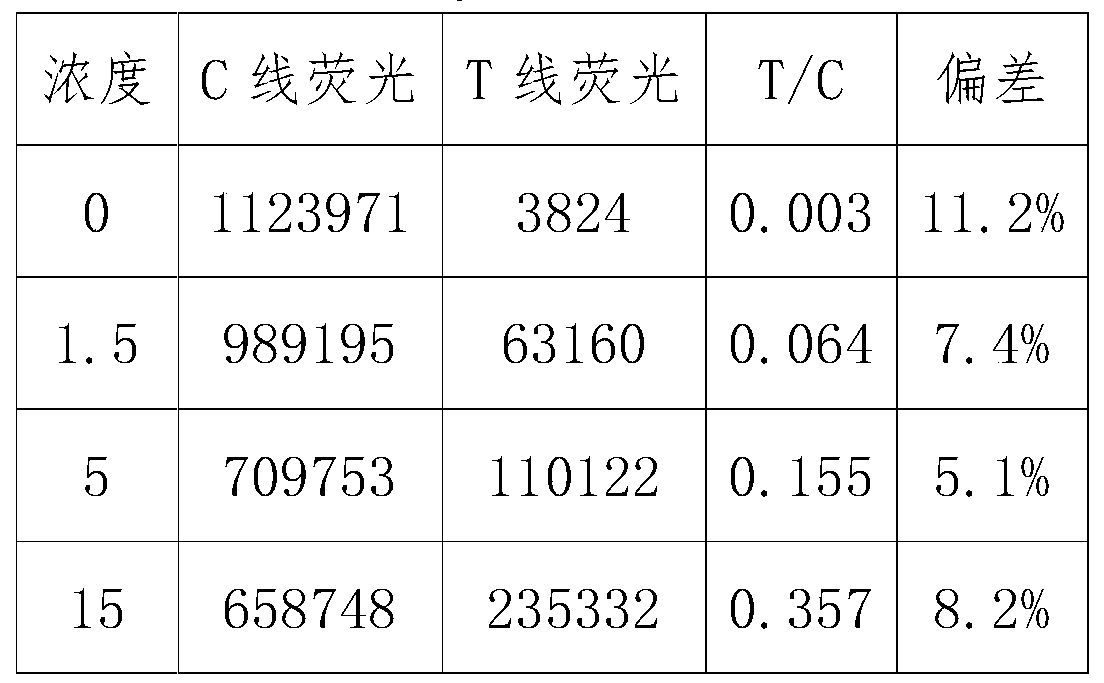
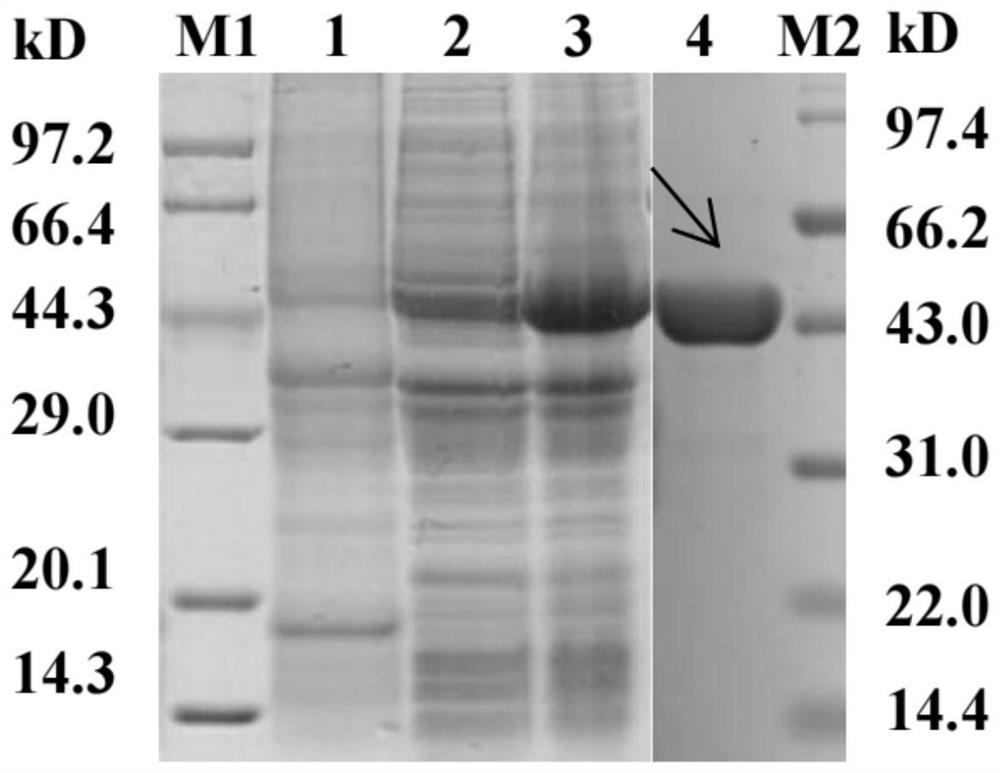
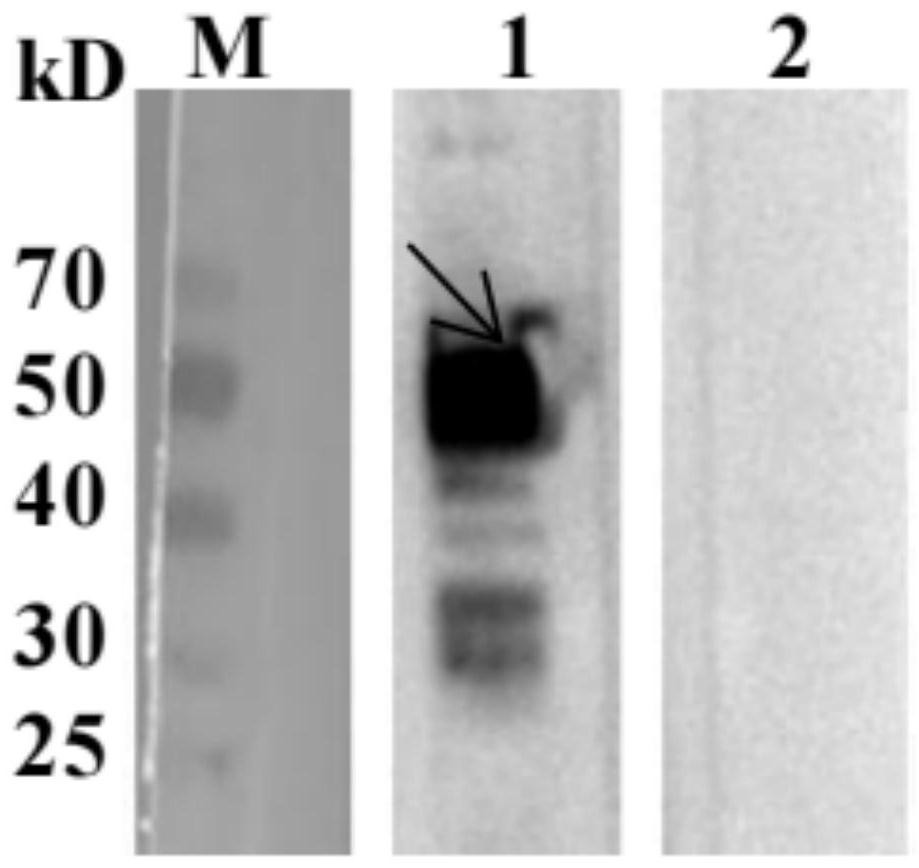
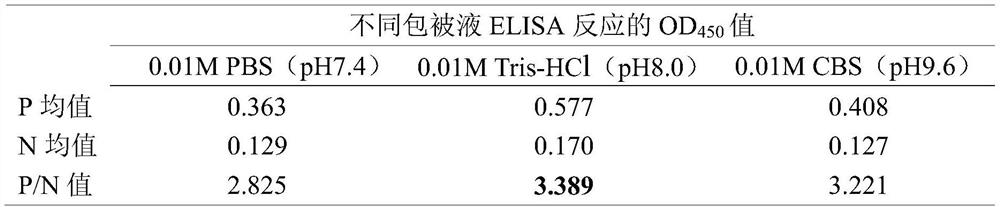
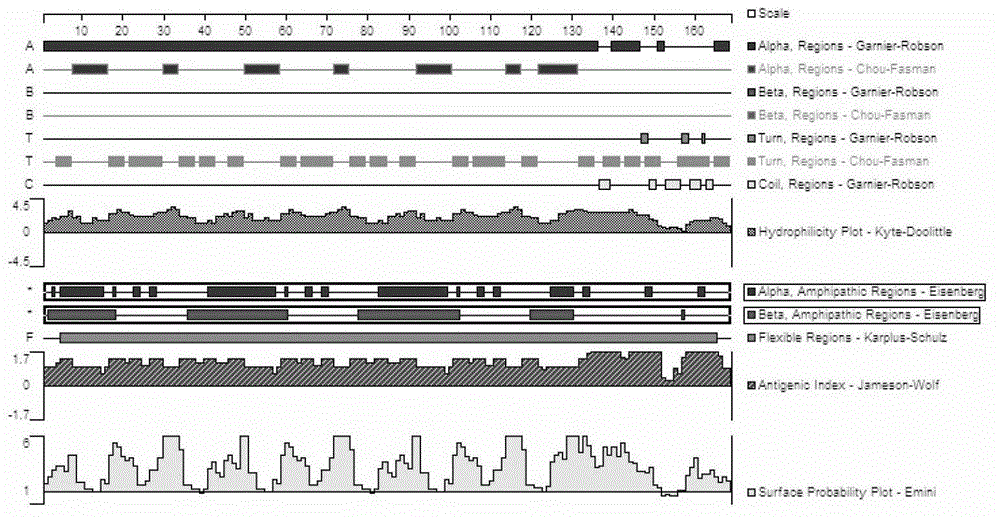
Patents
Literature
30 results about "Mycoplasma antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mycoplasma bovis diagnosis reagent and its application
InactiveCN103172752AReduce manufacturing costSimple and fast operationBiological testingHybrid peptidesMycoplasma antibodySpecific antibody
The invention relates to the diagnostic medicine of animals, especially relates to a mycoplasma bovis diagnosis technology, and concretely relates to a multi-epitope fusion antigen having an amino acid sequence represented by SEQ ID NO:1 or SEQ ID NO:2, and its application in the preparation of a mycoplasma bovis diagnosis reagent. The diagnosis reagent can be used as a solid phase vector coating antigen of an indirect ELISA kit and is combined with its specific antibody, a horseradish peroxidase coupled anti-cattle IgG antibody is added and incubated, and a color development reaction is carried out, and the color development degree is proportional to the amount of the anti-mycoplasma bovis antibody in a sample to be measured. The technology has the advantages of simple operation, no need of complex equipment, low technical requirements on the laboratorial conditions and experiment personals, low detection cost, and suitableness for the large-scale development in the basic level and the culture farm; the multi-epitope fusion antigen has a low making cost and is suitable for large-scale application; and has the advantages of high sensitivity and specificity, small batch difference, and high detection result consistence because of the adoption of multi-epitope as a target.
Owner:重庆市动物疫病预防控制中心 +1
Mycoplasma pneumonia antigen, preparation method and immunodetection kit
ActiveCN103059109AAchieve recombinant expressionHigh sensitivityDepsipeptidesBiological testingMycoplasma antibodyIgm antibody
The invention provides a mycoplasma pneumonia (MP) antigen. The antigen fragment of the antigen is MP371 or MP661, wherein the locus of MP 371 is at an N terminal 371-480aa of MP adhesion protein P1, and the locus of MP661 is at an N terminal 661-772aa of MP adhesion protein P1. The invention also relates to a kit comprising any antigen or antigen composition and the application of the kit in detection of a mycoplasma pneumonia antibody. The mycoplasma pneumonia antigen is a recombinant antigen cloned and expressed by a codon-optimized gene and has stronger antigen specificity than that of the antigen cultivated and extracted by MP. The kit can be used for specific detection of an anti-MP-IgM (immunoglobulin m) antibody in clinical samples, thus identifying and diagnosing the infection of mycoplasma pneumonia at early stage.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Collaurum immunochromatography test strip detecting mycoplasma pneumoniae and preparing method thereof
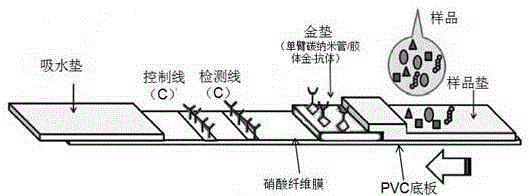
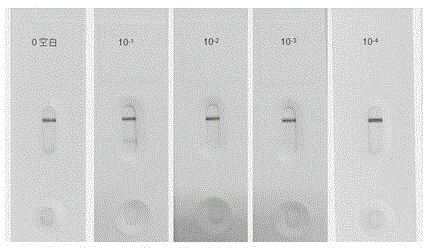
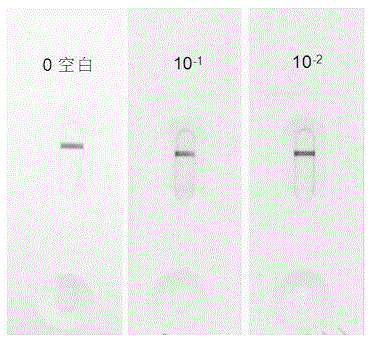
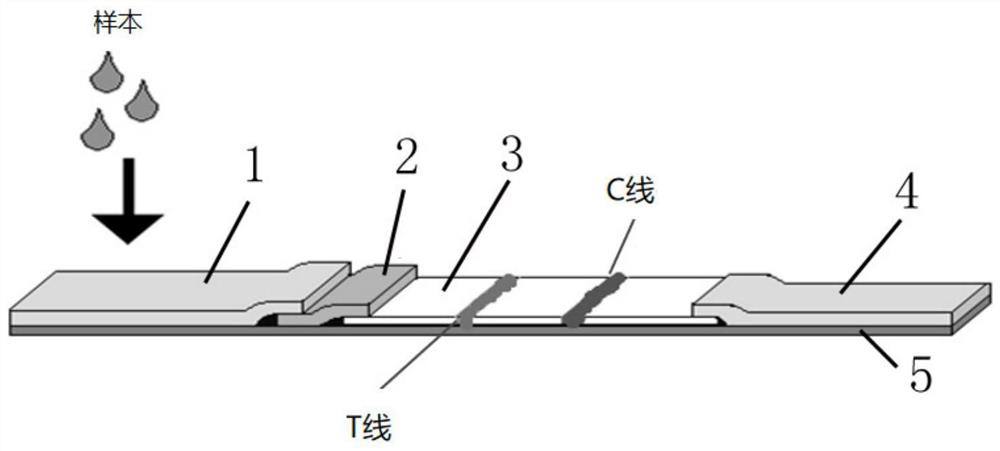
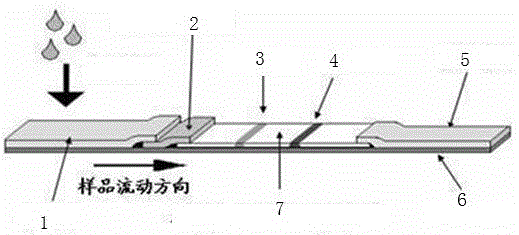
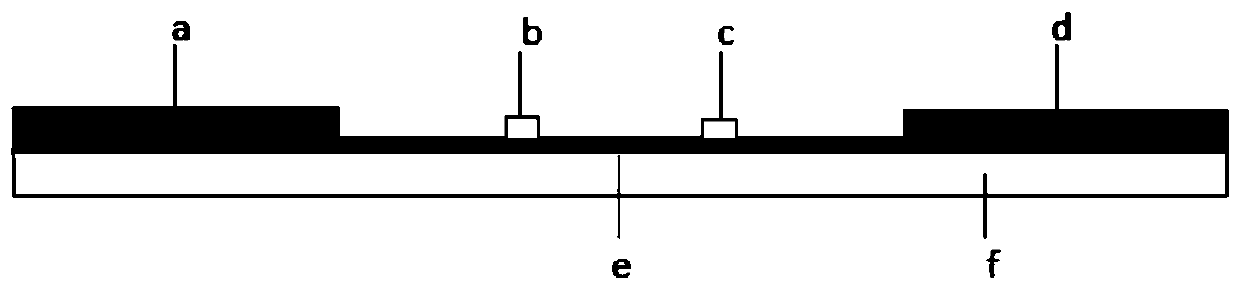
The invention belongs to a collaurum immunochromatography test strip detecting mycoplasma pneumoniae, comprising a sample pad, a combining pad, a nitrocellulose coating film, a water absorption pad and a PVC bottom plate; the sample pad, the combining pad, the nitrocellulose coating film and the water absorption pad are overlapped on the PVC bottom plate in sequence; the combining pad is coated with a mycoplasma pneumoniae antibody MPh2-collaurum-carbon nanotube marker; the nitrocellulose coating film is provided with a detection line and a quality control line; the detection line is coated with a mycoplasma pneumoniae antibody MPh1 and the quality control line is coated with a goat anti mouse IgG. The invention further comprises a preparing method of the collaurum immunochromatography test strip, comprising the steps of preparing of collaurum, preparing of a gold marker antibody, purifying and assembling of the test strip. The collaurum immunochromatography test strip has the characteristics of high specificity, high sensitivity, simple and convenient operation, fast detection, accuracy and suitability for field use.
Owner:姜竹泉 +1
Mycoplasma bovis detection test strip and preparation method thereof
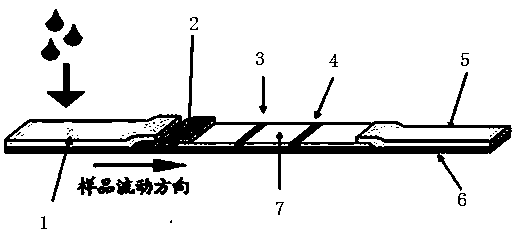
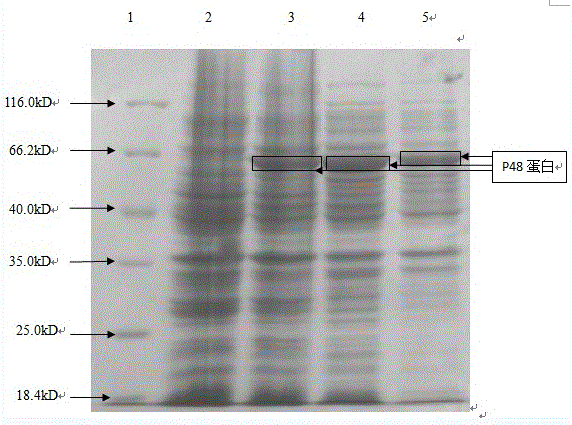
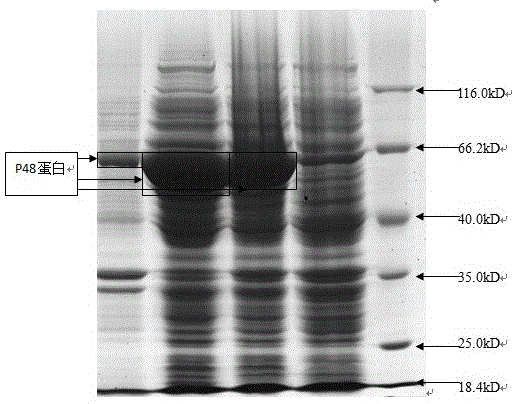
The invention discloses a mycoplasma bovis antibody detection test strip and a preparation method thereof. The test strip provided by the invention comprises a bottom plate and a sample absorption pad, a colloidal gold pad coated with an antigen, a nitrocellulose membrane and a water absorption pad which are all arrayed on the bottom plate in sequence, wherein the nitrocellulose membrane is coated with a quality control line and a detection line; the detection line is coated with a Goat Anti-Bovine Secondary antibody IgG; the quality control line is a rabbit derived P48 fusion protein. The test strip provided by the invention can detect mycoplasma bovis antibody, including blood serum antibody and milk serum antibody, and has the advantages that the test strip is low in cost, simple to operate, convenient and quick to detect, requires no special equipment and apparatus, is high in detection susceptibility, strong in specificity, wide in application range and the like.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Method of detecting infection with urogenital mycoplasmas in humans and a kit for diagnosing same
A method and a kit are provided for detecting an infection by mycoplasma, particularly an urogenital infection in humans. The presence of specific anti-mycoplasma antibodies in a biological sample ofa diagnosed subject is detected by reaction with an immobilized mixture of various antigenic determinants associated with a variety of pathological states.
Owner:MOR RES APPL LTD
Method of detecting infection with urogenital mycoplasmas in humans and a kit for diagnosing same
InactiveUS20100227333A1High affinityDepsipeptidesPeptide preparation methodsAntigenMycoplasma antibody
A method and a kit are provided for detecting an infection by mycoplasma, particularly an urogenital infection in humans. The presence of specific anti-mycoplasma antibodies in a biological sample of a diagnosed subject is detected by reaction with an immobilized mixture of various antigenic determinants associated with a variety of pathological states.
Owner:MOR RES APPL LTD
Combinant for detecting mycoplasma antibody and application of combinant
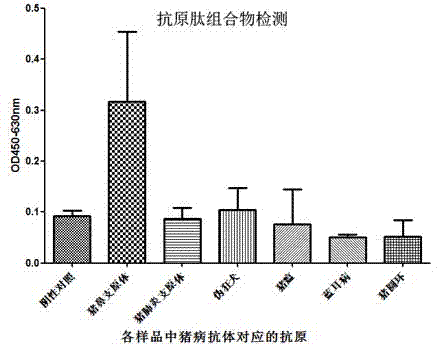
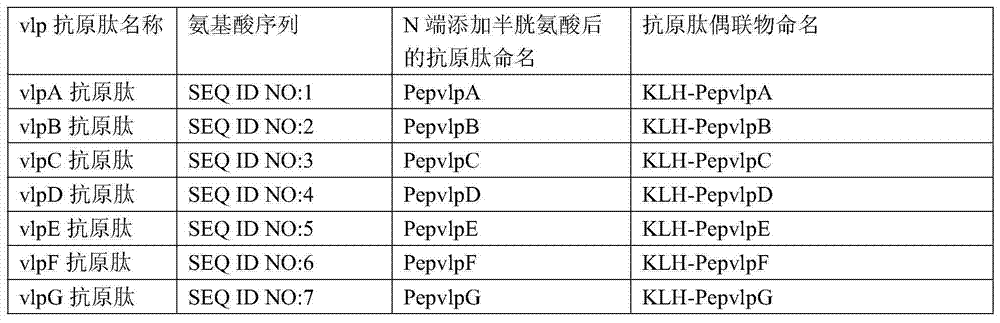
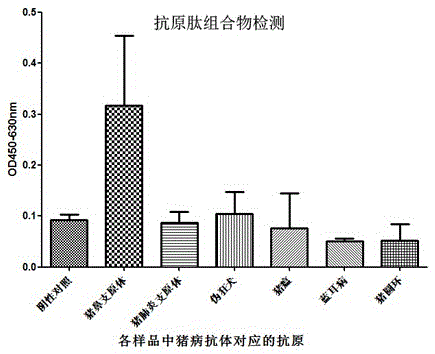
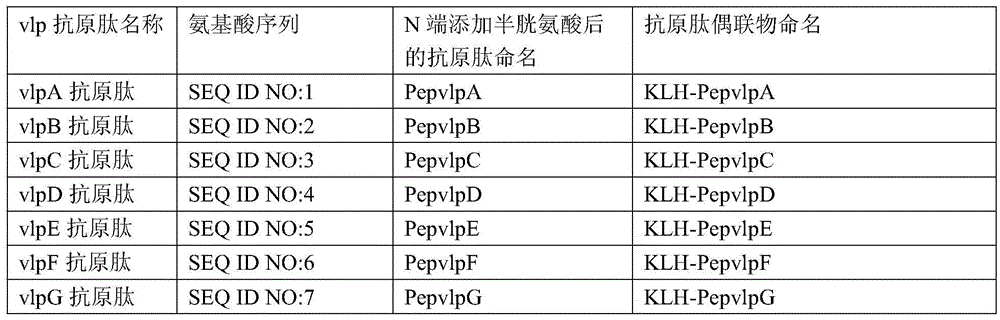
ActiveCN104730256AEasy to getWill not affect normal feeding managementBiological material analysisPeptidesMycoplasma antibodyPig farms
The invention provides a combinant for detecting a mycoplasma antibody and application of the combinant, and belongs to the technical field of biology. The combinant comprises seven polypeptides, wherein amino acid sequences of the polypeptides are shown in SEQ ID NO:1, SEQ ID NO:2, SEQ ID NO:3, SEQ ID NO:4, SEQ ID NO:5, SEQ ID NO:6 and SEQ ID NO:7 respectively. The combinant can be used for specifically detecting the mycoplasma antibody of a pig nose, and the cross reaction on the mycoplasma antibody and other mycoplasmas or other pathogenic infection samples which are commonly seen on the pig body is avoided, and the false positive rate is extremely low. Detection samples are easily available, and the normal feeding management of a pig farm cannot be influenced.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Reagent device and method for detecting mycoplasma pneumonia antibody
ActiveCN103185784AStrong specificityHigh sensitivityColor/spectral properties measurementsMycoplasma antibodyEnzyme linked immunoassay
The invention provides a reagent device and a method used for detecting mycoplasma pneumonia antibodies. The reagent device has a long strip shape, and has a base body comprising 8 hole positions and a handle positioned on one end of the base body. From the end proximal to the handle, the 8 hole positions are sequentially a sample hole, an auxiliary agent hole, an enzyme conjugate hole, a substrate hole, a termination liquid hole, a dilution liquid hole, a reaction hole, and a dilution hole. According to the invention, based on a principle of enzyme-linked immunoassay, mycoplasma pneumonia antibody is detected by using the reagent device. The method is an independent single-person analysis and detection method. The device can be used in cooperation with a corresponding specific analysis instrument. During a detection process, detection reagents or samples are injected by using a full-automatic precise dosing device. The device and the method have the advantages of automatic operation, precise dosing, high detection result accuracy, high detection result precision, and wide application prospect.
Owner:SHENZHEN YHLO BIOTECH
Immunoassay kit by using light initiated chemiluminescent assay for bovine mycoplasma antibody and detection method thereof
InactiveCN109724965AEasy to operateStrong specificityChemiluminescene/bioluminescenceBiological testingMycoplasma antibodyMagnetic bead
The invention discloses an immunoassay kit by using a light-initiated chemiluminescent assay for a bovine mycoplasma antibody and a detection method thereof. The kit comprises bovine mycoplasma antibody P30 protein monoclonal antibodies, goat anti-mouse IgG conjugated acceptor microbeads, His-tagged bovine mycoplasma antibody P30 protein antigens, and nickel chelated donor magnetic beads. The invention prepares outer membrane protein of bovine mycoplasma antibody P30 by using the genetic engineering technology, prepares bovine mycoplasma antibody P30 protein monoclonal antibodies by using thePEG1500 cell fusion technology, and the purified recombinant P30 protein is used as a product of interaction between the antigen and the prepared monoclonal antibody, competing with the monoclonal antibody by the antibody in the sample to bind and recombine the P30 protein, and establishing a method for detecting competitive bovine mycoplasma antibody AlphaLISA. According to the immunoassay kit byusing light initiated chemiluminescent assay for the bovine mycoplasma antibody and the detection method thereof, the detection method has the advantages of short detection time, good specificity, good sensitivity, no need for washing, simple operation and low detection cost.
Owner:重庆市动物疫病预防控制中心 +1
Optimized DNA sequence, recombinant plasmid, strain, recombinant protein, mycoplasma gallisepticum antibody colloidal gold detection test paper and detection card
InactiveCN110058017AHigh expressionSuitable for production applicationsMaterial analysisMycoplasma antibodyNitrocellulose
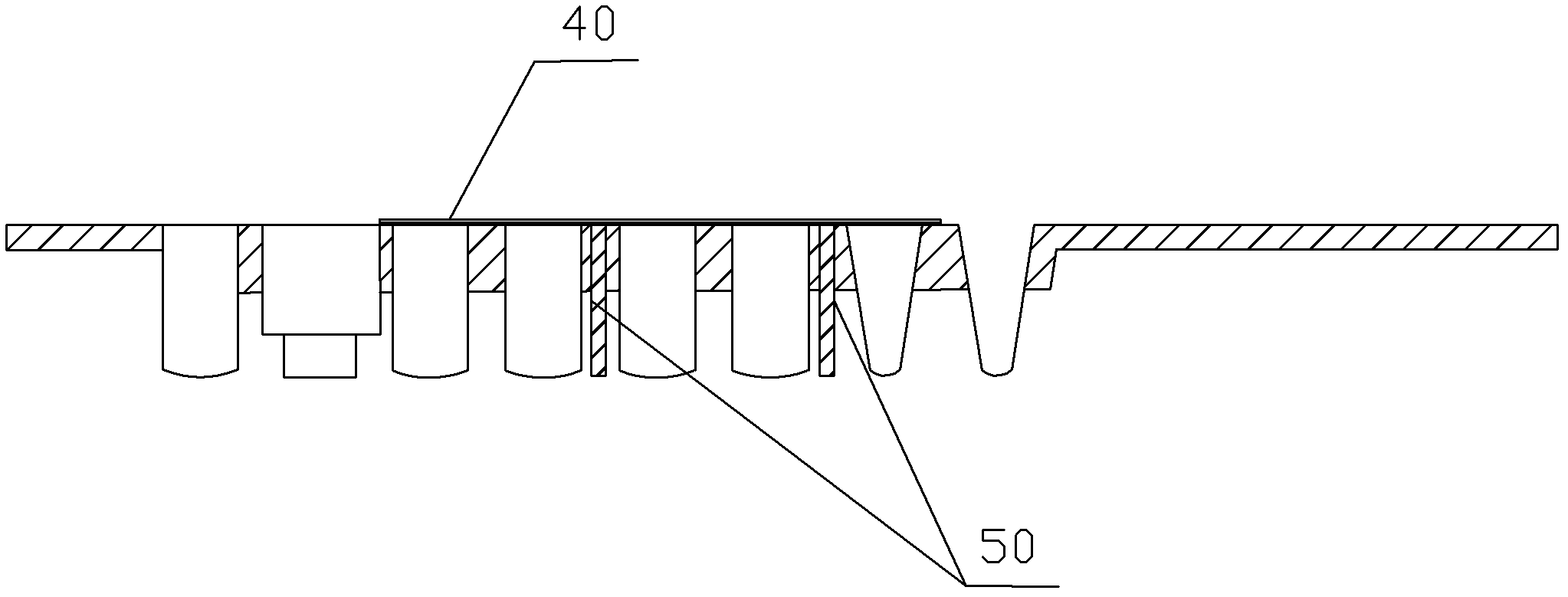
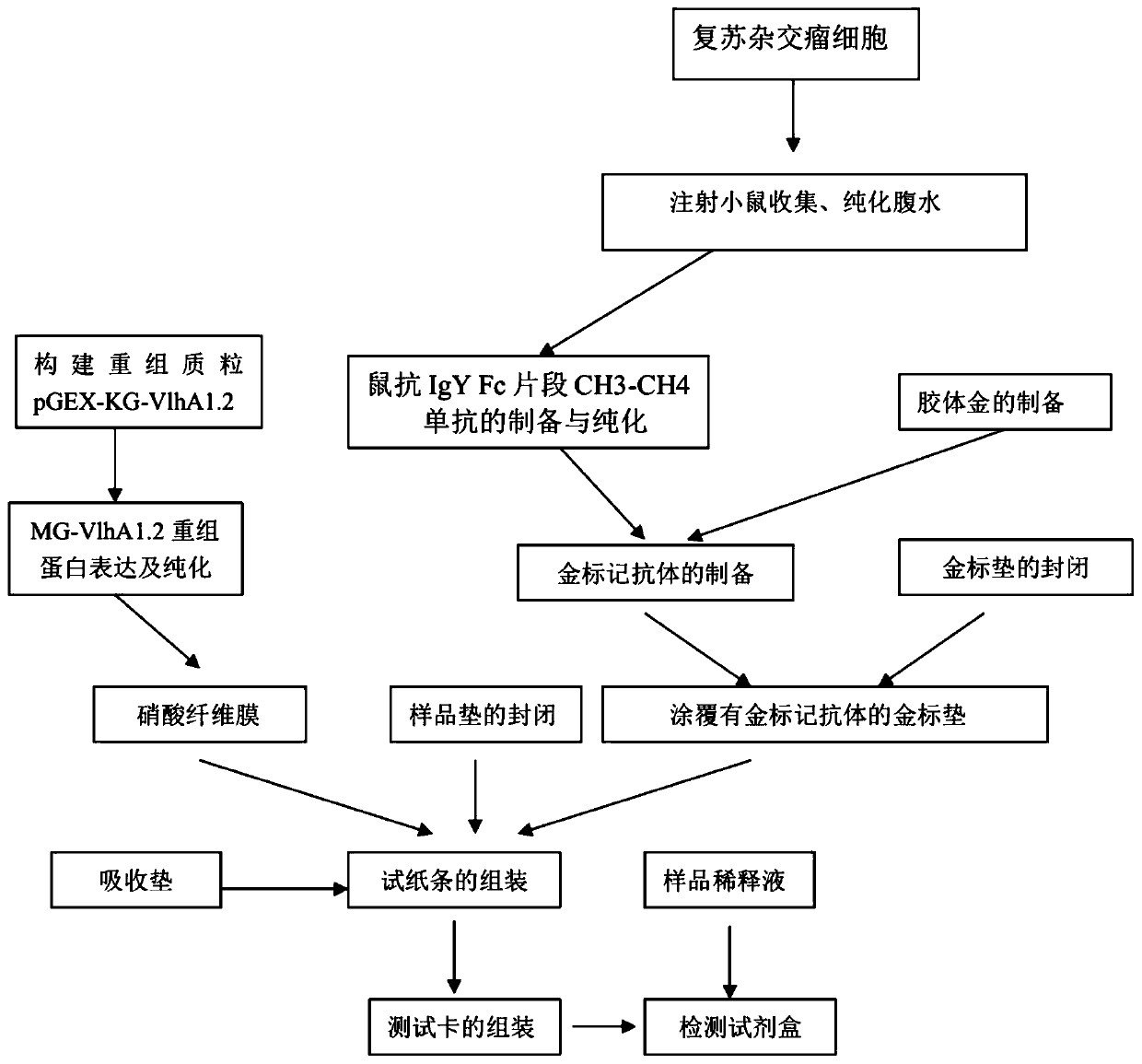
The invention provides an optimized DNA sequence, a recombinant plasmid, a strain, a recombinant protein, mycoplasma gallisepticum antibody colloidal gold detection test paper containing the recombinant protein and a detection card based on mycoplasma gallisepticum VlhA1.2 protein, wherein the test paper can be used for quickly detecting diagnosis of poultry after mycoplasma gallisepticum infection and antibody level detection after immunization. The optimized DNA sequence is shown as SEQ ID NO.1. The optimized DNA sequence is combined with a pGEX-KG vector to obtain the recombinant plasmid pGEX-KG-VlhA1.2, and transforming and expressing is carried out to obtain the MG-VlhA1.2 recombinant protein. The test paper comprises a gold-labeled pad and a nitrocellulose membrane which are sequentially connected, wherein the gold-labeled pad is coated with a colloidal gold label of a monoclonal antibody mouse anti-IgY Fc CH3-CH4; and one end of the nitrocellulose membrane, which is close to thegold-labeled pad, is a detection line coated with MG-VlhA1.2 recombinant protein.
Owner:HUAZHONG AGRI UNIV
Method for detecting mycoplasma gallisepticum antibody of enzyme-linked nucleic acid aptamer and kit special for mycoplasma gallisepticum antibody
ActiveCN109762825AIncreased sensitivityImprove featuresMaterial analysisDNA/RNA fragmentationMycoplasma antibodyMycoplasma gallisepticum antibody
The invention discloses a method for detecting a mycoplasma gallisepticum antibody of an enzyme-linked nucleic acid aptamer and a kit special for the mycoplasma gallisepticum antibody. The nucleic acid aptamer is shown as sequence 3 of a sequence list. The method for detecting the mycoplasma gallisepticum antibody of the enzyme-linked nucleic acid aptamer applies the nucleic acid aptamer. The method for detecting the mycoplasma gallisepticum antibody of the enzyme-linked nucleic acid aptamer has good sensitivity and specificity and is suitable for rapid detection of a large number of samples in the field. The method can effectively detect the antibody infected by MG, the reliable detection method is provided for identifying MG infection, and scientific guidance is also provided for prevention and control of MG epidemic situations.
Owner:CHINA AGRI UNIV
Mycoplasma gallisepticum antibody detection reagent, preparation method and application thereof
InactiveCN111707822AIn line with the domestic marketProductiveImmunoassaysMycoplasma antibodyMethyl violet
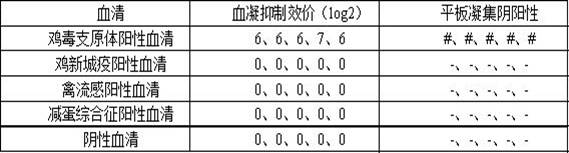
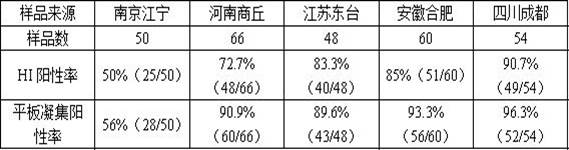
The invention relates to the technical field of poultry antibody detection reagents, particularly to a mycoplasma gallisepticum antibody detection reagent, a preparation method and application thereof. According to the invention, a mycoplasma gallisepticum culture solution is subjected to a series of treatment on, inactivating is performed, the inactivated antigen bacteria solution and a freeze-drying protective agent are uniformly mixed, and freeze drying is performed to obtain the mycoplasma gallisepticum antibody detection reagent; the reagent can be directly used for a hemagglutination inhibition test; a plate agglutination test can be carried out by adding any one coloring agent of methyl violet, amber red and crystal violet into the reagent containing 4-8HA unit of antigen; the hemagglutination value of the detection reagent reaches 26; and the detection reagent not only improves the hemagglutination titer of the mycoplasma gallisepticum antigen, but also has two purposes, is stable, sensitive, strong in specificity, long in storage life, simple to operate, convenient and fast, does not need special equipment and instruments, and can be used for efficacy test, epidemiologicalinvestigation, clinical sample detection and the like of mycoplasma gallisepticum vaccines.
Owner:兆丰华生物科技(南京)有限公司
A kind of Mycoplasma bovis antibody detection reagent and preparation method thereof
ActiveCN104062439BDetect trueReliable detectionDepsipeptidesBiological testingMycoplasma antibodyBlood coagulations
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Indirect ELISA detection kit and detection method of mycoplasma gallisepticum antibody
InactiveCN111537736AStrong specificityImprove featuresBiological testingImmunoassaysMycoplasma antibodyMG infection
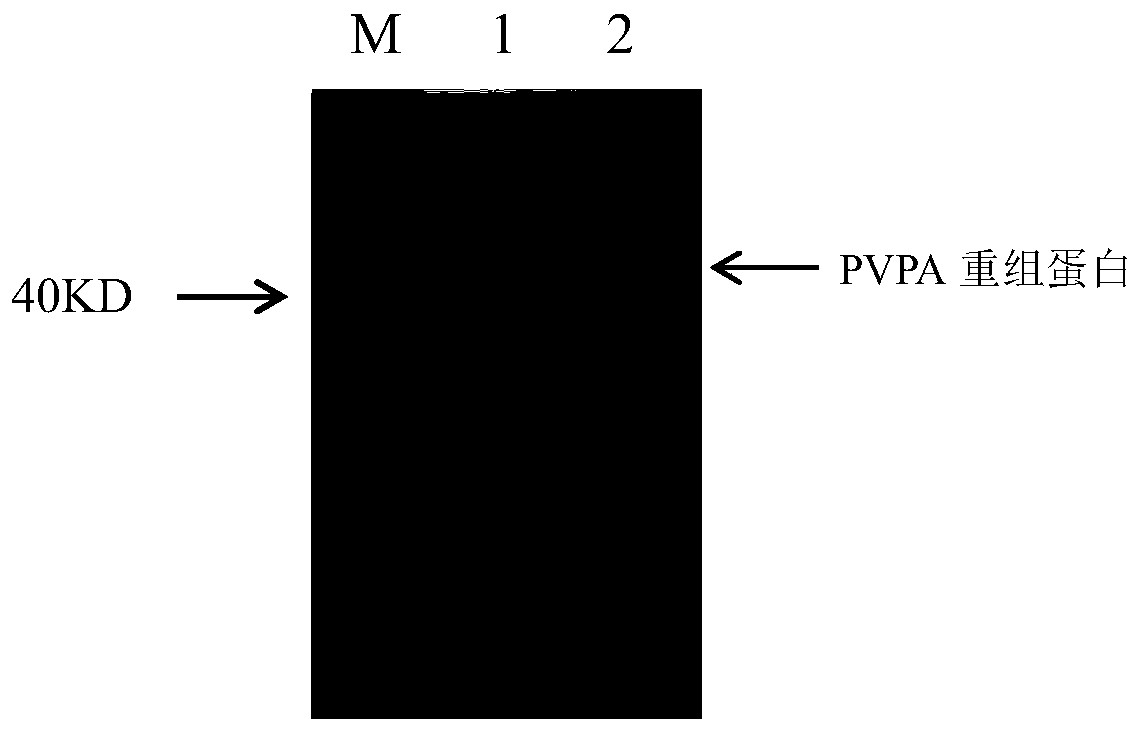
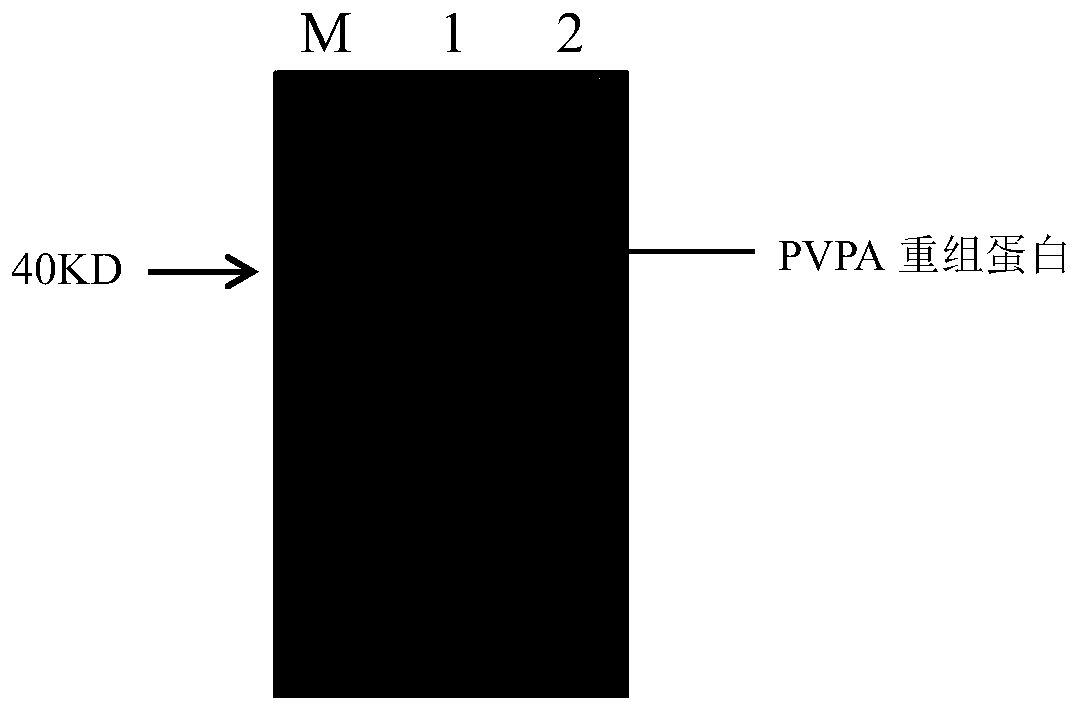
The invention discloses an indirect ELISA detection kit and a detection method of a mycoplasma gallisepticum antibody. The indirect ELISA detection kit of the mycoplasma gallisepticum antibody comprises a PVPA protein, 2.5% skim milk powder, 5% BSA, a coating stabilizer, MG positive serum, MG negative serum, 0.01 M PBS (pH 7.2-7.4), horse radish peroxidase labeled rabbit anti-chicken IgY, a microwell plate, a washing solution, a chromogenic substrate and a stop solution. The PVPA protein is a protein of which the amino acid sequence is 35th-408th sites of a sequence 2 or a protein of the sequence 2. The kit can be used for detecting whether serum of an animal to be detected contains the mycoplasma gallisepticum antibody or not and judging whether the animal to be detected is infected withmycoplasma gallisepticum or not, a reliable detection method is provided for identifying MG infection, and scientific guidance is provided for MG epidemic prevention and control.
Owner:CHINA AGRI UNIV
Mycoplasma pneumoniae antibody detection kit and application thereof
PendingCN114791489ARealize quantitative detectionImprove thermal stabilityChemiluminescene/bioluminescenceBiological testingMycoplasma antibodyDioxyethylene Ether
The invention provides a mycoplasma pneumoniae antibody detection kit and application thereof, and relates to the technical field of biology. The kit comprises a mycoplasma pneumoniae fusion antigen working solution, wherein the mycoplasma pneumoniae fusion antigen working solution contains a mycoplasma pneumoniae fusion antigen and a diluent; the tracer-labeled antibody working solution contains an antibody IgG, IgM or IgA and a diluent; wherein the mycoplasma pneumoniae fusion antigen has an amino acid sequence as shown in SEQ ID No.3; the diluent is a phosphate buffer solution containing 0.02 to 1 weight percent of fatty alcohol polyoxyethylene ether sulfate salt. The kit disclosed by the invention adopts the mycoplasma pneumoniae fusion antigen, so that the specificity is stronger, the sensitivity is higher, and the thermal stability is better; besides, a sulfate salt type anionic surfactant fatty alcohol polyoxyethylene ether sulfate salt is added into the diluent, so that a stable and good microenvironment is provided for the fusion antigen, and the thermal stability of the kit antigen is further improved.
Owner:ZHUHAI LIVZON DIAGNOSTICS
Sample loading method of pneumonia mycoplasma and pneumonia chlamydia antibody detection
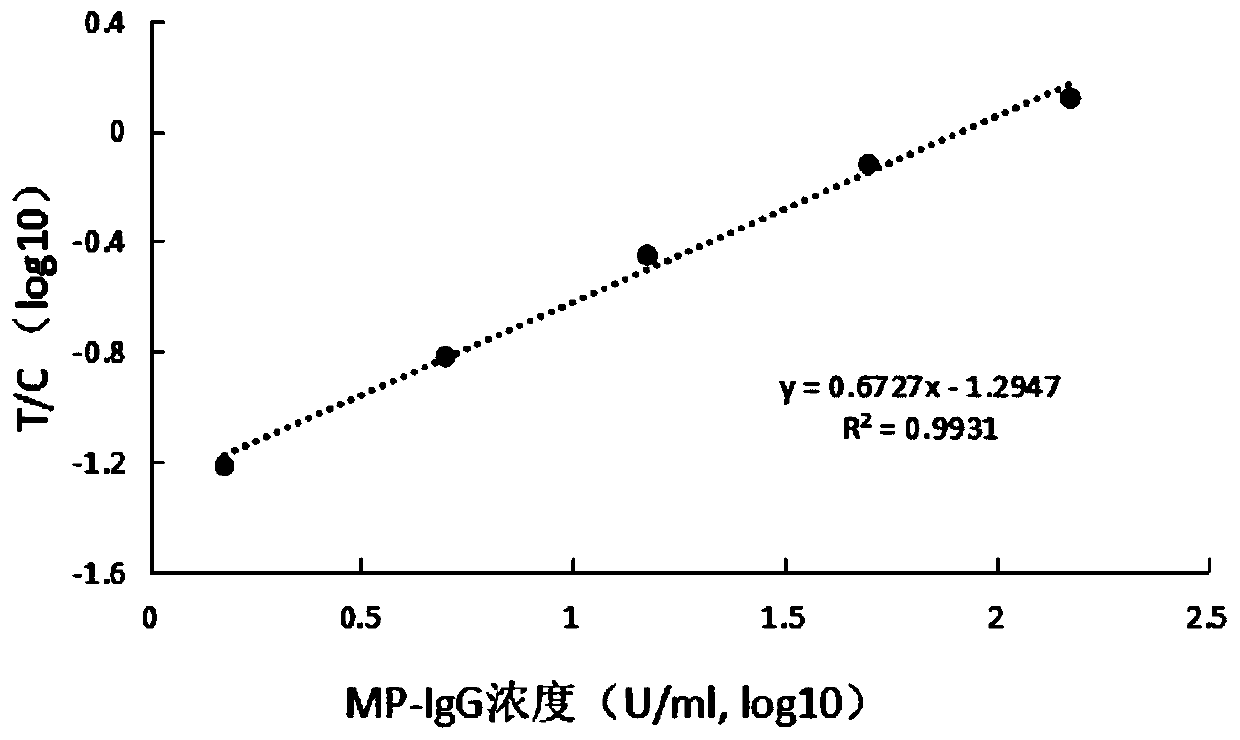
InactiveCN104991062AReduce dosageEasy to take bloodMaterial analysisMycoplasma antibodyBlood collection
The invention provides a sample loading method of pneumonia mycoplasma and pneumonia chlamydia antibody detection. A membrane sample loading method is adopted in detection of pneumonia chlamydia IgG(Cpn-IgG) antibody and pneumonia mycoplasma IgG(MP-IgG) antibody; in the membrane sample loading method, 1.5 [mu]L serum / blood plasma or 2 [mu]L of whole blood is vertically loaded into a sample loading opening by a pipette, and during the sample loading process, the head of the pipette slightly touch the membrane so as to complete the sample loading successfully. If a disposable plastic sample loading ring is used, the annular body should be completely immersed into the sample; then the annular body is vertically contacted with the surface of the membrane of the sample loading opening, thus the membrane can absorb the sample, and when the membrane surface is wetted, the sample loading is successful. The provided method has the advantages that the using amount of sample is largely reduced, the peripheral blood detection is achieved, and the blood collection becomes easier for the patients; serum, blood plasma, and whole blood samples can all be detected, and the requirements of different people and detection environment can all be met.
Owner:QINGDAO HIGHTOP BIOTECH
Mycoplasma ovipneumoniae antibody indirect ELISA detection kit
The invention relates to the technical field of veterinary biological product detection, in particular to a mycoplasma ovipneumoniae antibody indirect ELISA detection kit. Wherein the coating antigenis mycoplasma ovipneumoniae adhesin gene P113 recombinant protein rP113 (C); the primer sequence of the primer is as follows: P113 (c) F: 5 to CGCGGATCCGAAGGTGCTCAAGACCAAGGTA-3, and P113 (c) R: 5 to CCGCTCCGTTGTTGTTGTTGAGGTGGTGTATCAGGT-3. The invention provides the indirect ELSIA detection kit for the mycoplasma ovipneumoniae antibody, which is established by taking purified P113 recombinant protein rP113 (C) as a coating antigen, and a powerful tool is provided for epidemiological investigation, disease diagnosis and vaccine immune effect evaluation.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
Mycoplasma pneumoniae igm antibody colloidal gold immunochromatography detection kit and preparation method thereof
ActiveCN113533736BImprove detection accuracyLow costBiological testingImmunoassaysMycoplasma antibodyIgm antibody
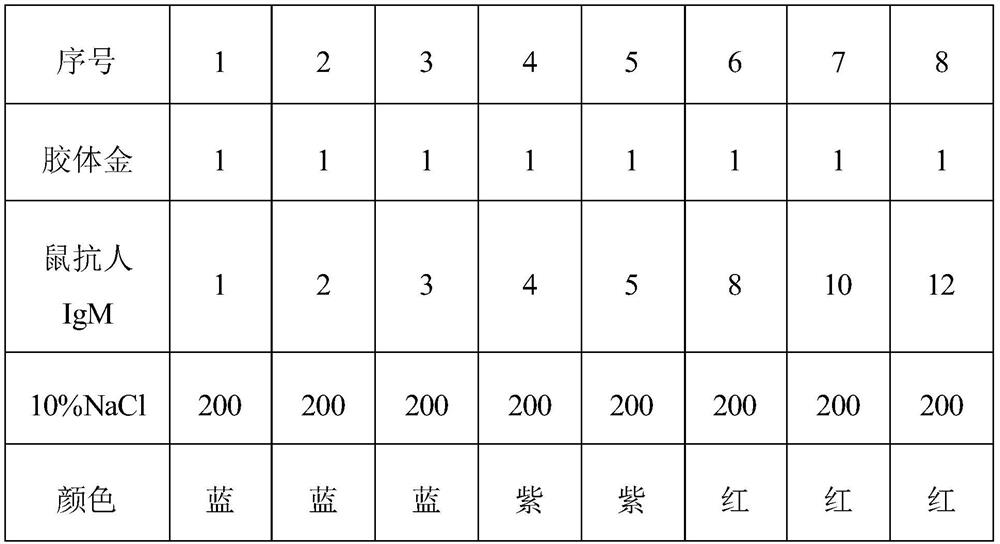
The invention relates to the field of immunochromatography, and specifically provides a colloidal gold immunochromatography detection kit for mycoplasma pneumoniae IgM antibody and a preparation method thereof. The Mycoplasma pneumoniae IgM antibody colloidal gold immunochromatography detection kit of the present invention comprises a detection test strip and a sample diluent to be detected, wherein the detection test strip comprises a bottom plate and a sample pad, a binding pad, Detection pads and absorbent pads, the kit uses the principle of immunochromatography to detect the samples to be tested, with low cost and short time consumption. The diluent of 0.5(w / v)% S17 and 10‑100mM NaCl dilutes the sample to be tested, which can significantly improve the sensitivity and specificity of detection, thereby improving the detection accuracy of Mycoplasma pneumoniae.
Owner:SHANGHAI BIOGERM MEDICAL TECH CO LTD BEIJING BRANCH +1
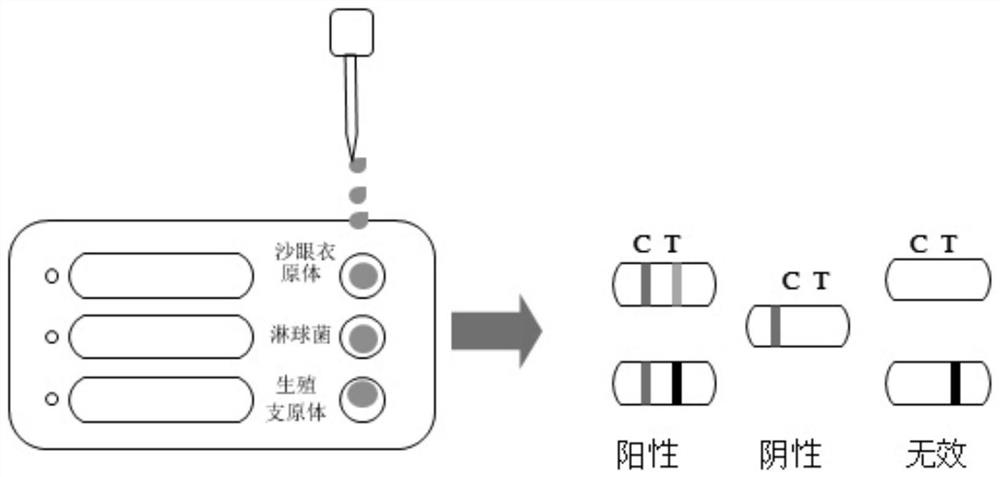
Chlamydia trachomatis/gonococcus/mycoplasma genitalium antigen combined detection kit and preparation method thereof
PendingCN114414798ARapid qualitative detection and judgmentStrong specificityImmunoassaysMycoplasma antibodyCellulose
The invention discloses a chlamydia trachomatis / gonococcus / mycoplasma genitalium antigen combined detection kit and a preparation method thereof, and relates to the field of detection kits. Comprising a detection card, the detection card comprises a bottom plate, a sample adding plate, a latex combination pad, a nitrocellulose membrane and absorbent paper, and the sample adding plate, the latex combination pad, the nitrocellulose membrane and the absorbent paper are sequentially connected end to end and are fixed on the bottom plate; a first latex microsphere-labeled chlamydia trachomatis / gonococcus / mycoplasma genitalium specific antibody and a second latex microsphere-labeled streptavidin are fixed on the latex combination pad, and the nitrocellulose membrane is provided with a detection line coated with a chlamydia trachomatis / gonococcus / mycoplasma genitalium antibody and a quality control line coated with biotin-BSA (Bovine Serum Albumin). The detection kit provided by the invention can simultaneously complete antigen detection of chlamydia trachomatis / gonococcus / mycoplasma genitalium under the condition of only one-time sampling and sample treatment process, and is high in efficiency, strong in specificity and high in sensitivity.
Owner:北京泰格科信生物科技有限公司
A kind of mycoplasma bovis detection test strip and preparation method thereof
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Method for detecting mycoplasma pneumoniae antibody, kit for detection and preparation method of kit
ActiveCN103033615BRapid Simultaneous DetectionNot easy to interfereMaterial analysisMycoplasma antibodySerum ige
Owner:QINGDAO HIGHTOP BIOTECH
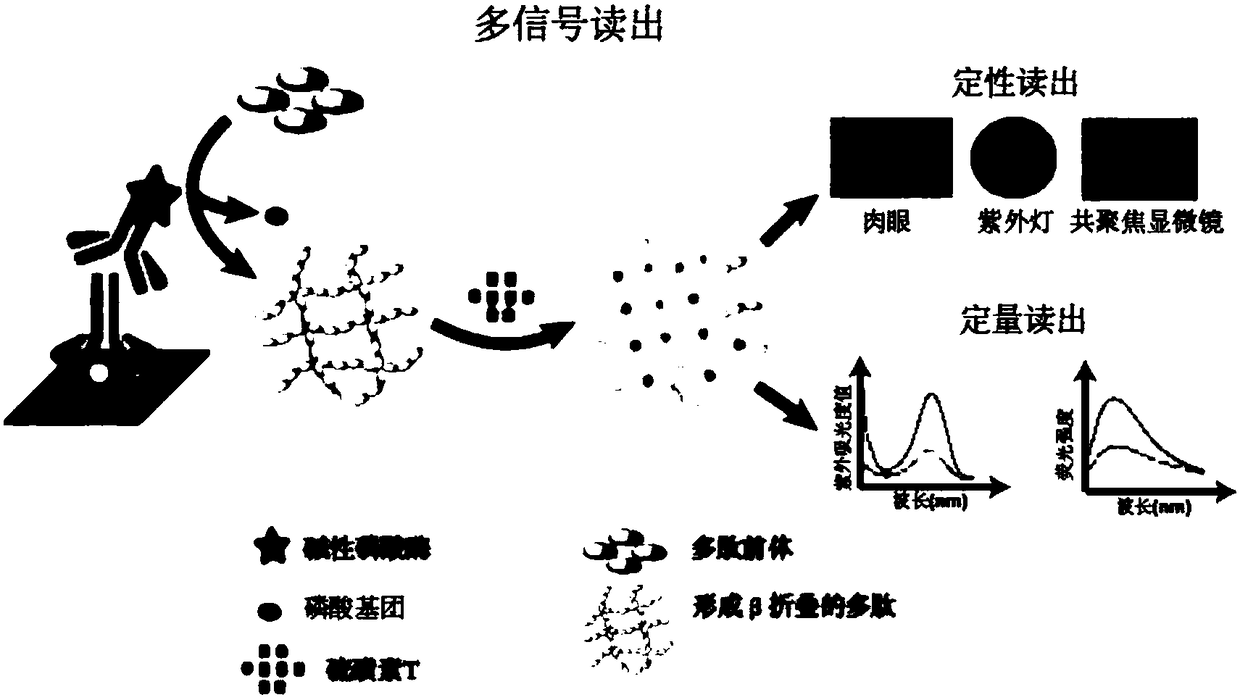
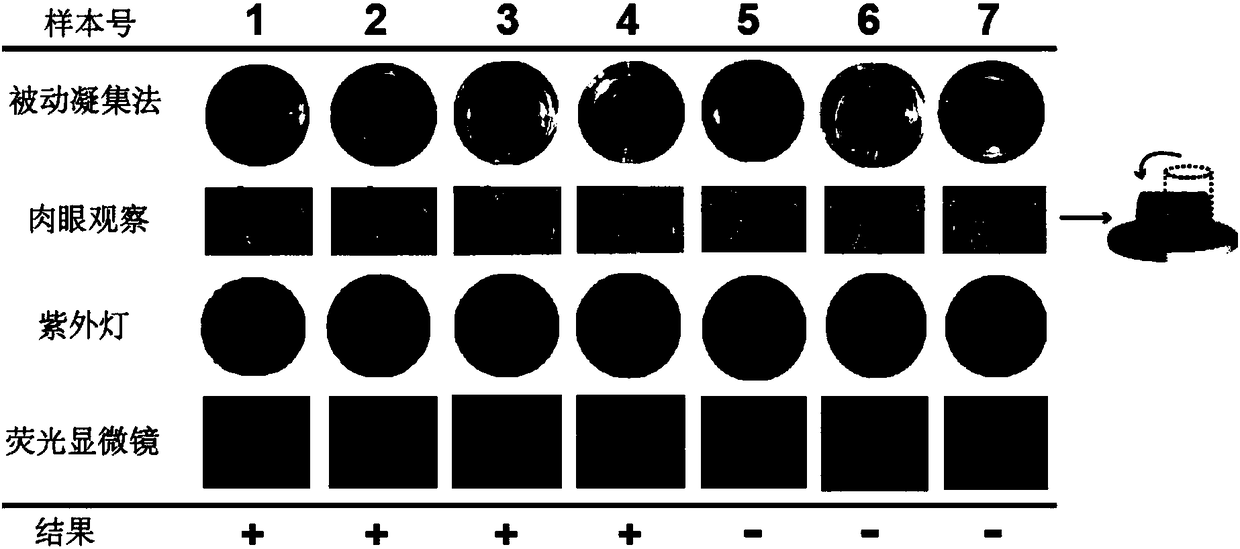
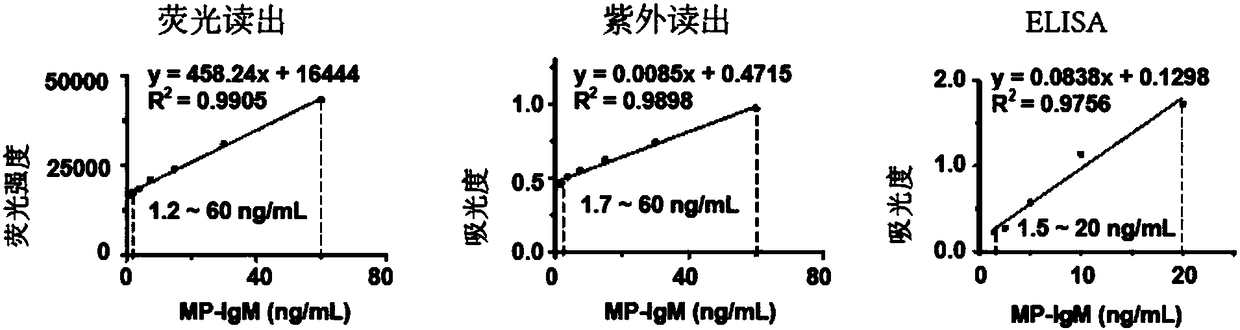
Immunoassay method based on thioflavin T
ActiveCN108872571AHelps to rule outConvenient guidanceMaterial analysisMycoplasma antibodyThioflavin
The invention provides an immunoassay method based on thioflavin T and application thereof. The method comprises the following steps of adding enzyme-responsive polypeptides on the basis of a conventional alkaline phosphatase reaction system ELISA to be used as a reaction substrate so that polypeptides form beta-folding structure under the enzyme catalysis; then, introducing fluorescent dye of thioflavin T; performing detection by using signals generated when the thioflavin T is combined with beta-folding polypeptides. Five kinds of read-out modes are provided for detecting mycoplasma pneumoniae antibodies; more selectivity is shown; an excellent method for detecting mycoplasma pneumoniae IgM antibodies is provided.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Reagent device and method for detecting mycoplasma pneumonia antibody
ActiveCN103185784BStrong specificityHigh sensitivityColor/spectral properties measurementsMycoplasma antibodyImmuno detection
The invention provides a reagent device and a method used for detecting mycoplasma pneumonia antibodies. The reagent device has a long strip shape, and has a base body comprising 8 hole positions and a handle positioned on one end of the base body. From the end proximal to the handle, the 8 hole positions are sequentially a sample hole, an auxiliary agent hole, an enzyme conjugate hole, a substrate hole, a termination liquid hole, a dilution liquid hole, a reaction hole, and a dilution hole. According to the invention, based on a principle of enzyme-linked immunoassay, mycoplasma pneumonia antibody is detected by using the reagent device. The method is an independent single-person analysis and detection method. The device can be used in cooperation with a corresponding specific analysis instrument. During a detection process, detection reagents or samples are injected by using a full-automatic precise dosing device. The device and the method have the advantages of automatic operation, precise dosing, high detection result accuracy, high detection result precision, and wide application prospect.
Owner:SHENZHEN YHLO BIOTECH
Mycoplasma pneumoniae IgM antibody colloidal gold immunochromatography detection kit and preparation method thereof
ActiveCN113533736AImprove detection accuracyLow costBiological testingImmunoassaysMycoplasma antibodyMycoplasma
The invention relates to the field of immunochromatography, and particularly provides a mycoplasma pneumoniae IgM antibody colloidal gold immunochromatography detection kit and a preparation method thereof. The mycoplasma pneumoniae IgM antibody colloidal gold immunochromatography detection kit comprises a detection test strip and a to-be-detected sample diluent, the detection test strip comprises a bottom plate and a sample pad, a combination pad, a detection pad and a water absorption pad which are sequentially arranged on the bottom plate. The kit detects a to-be-detected sample according to the immunochromatography principle, is low in cost and short in time consumption, the to-be-detected sample is diluted by the diluent containing 10-100 mM of Tris-HCl, 0.1-0.5 (w / v)% of S9, 0.1-0.5 (w / v)% of S17 and 10-100 mM of NaCl, the detection sensitivity and specificity can be remarkably improved, and the detection accuracy of mycoplasma pneumoniae is improved.
Owner:SHANGHAI BIOGERM MEDICAL TECH CO LTD BEIJING BRANCH +1
Mycoplasma pneumonia antigen, preparation method and immunodetection kit
ActiveCN103059109BAchieve recombinant expressionHigh sensitivityDepsipeptidesBiological testingMycoplasma antibodyIgm antibody
The invention provides a mycoplasma pneumonia (MP) antigen. The antigen fragment of the antigen is MP371 or MP661, wherein the locus of MP 371 is at an N terminal 371-480aa of MP adhesion protein P1, and the locus of MP661 is at an N terminal 661-772aa of MP adhesion protein P1. The invention also relates to a kit comprising any antigen or antigen composition and the application of the kit in detection of a mycoplasma pneumonia antibody. The mycoplasma pneumonia antigen is a recombinant antigen cloned and expressed by a codon-optimized gene and has stronger antigen specificity than that of the antigen cultivated and extracted by MP. The kit can be used for specific detection of an anti-MP-IgM (immunoglobulin m) antibody in clinical samples, thus identifying and diagnosing the infection of mycoplasma pneumonia at early stage.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Composition for detecting mycoplasma antibody and application thereof
ActiveCN104730256BEasy to getWill not affect normal feeding managementBiological material analysisBiological testingPig farmsMycoplasma antibody
The invention provides a combinant for detecting a mycoplasma antibody and application of the combinant, and belongs to the technical field of biology. The combinant comprises seven polypeptides, wherein amino acid sequences of the polypeptides are shown in SEQ ID NO:1, SEQ ID NO:2, SEQ ID NO:3, SEQ ID NO:4, SEQ ID NO:5, SEQ ID NO:6 and SEQ ID NO:7 respectively. The combinant can be used for specifically detecting the mycoplasma antibody of a pig nose, and the cross reaction on the mycoplasma antibody and other mycoplasmas or other pathogenic infection samples which are commonly seen on the pig body is avoided, and the false positive rate is extremely low. Detection samples are easily available, and the normal feeding management of a pig farm cannot be influenced.
Owner:JIANGSU ACAD OF AGRI SCI
Immunodetection kit for mycoplasma pneumoniae antibody IgG, and preparation method and use method thereof
PendingCN111239411AHigh strengthHigh sensitivityBiological testingImmunoassaysMycoplasma antibodyAntigen
The invention discloses an immunodetection kit for mycoplasma pneumoniae antibody IgG, which comprises a fluorescence immunochromatography test strip, a microsphere solution and a sample diluent, thefluorescence immunochromatography test strip comprises absorbent paper, a chromatography membrane, a sample pad and a supporting bottom plate, a detection line and a quality control line are arrangedon the chromatography membrane, and the detection line is coated with MP antigen; in the microsphere solution, microspheres of the microsphere solution are microspheres coupled with anti-human IgG antibodies. By adopting the immunochromatography test strip, the detection rate is increased. Quantitative detection is realized by adopting a fluorescent substance. The structure of thetest strip is changed on the basis of an existing test strip, and a combination pad is removed; different detection steps are adopted, so that the intensity and the sensitivity of specific fluorescence signals are remarkably improved, the measurement range is enlarged, the measurement accuracy is improved, and the detection background noise is reduced.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
An ELISA kit for rapid detection of antibodies against Mycoplasma ovine pneumonia
ActiveCN110596402BImproving immunogenicityStrong specificityBiological testingMycoplasma antibodyElisa kit
Owner:GUANGXI VETERINARY RES INST
Recombinant Mh-PGK protein and application thereof in detection of swine haemophilus mycoplasma
ActiveCN114350636AImprove featuresGood repeatabilityBiological material analysisTransferasesDiseaseMycoplasma antibody
The invention discloses a recombinant Mh-PGK protein and an application of the recombinant Mh-PGK protein in detection of swine haemophilus mycoplasma. The invention firstly discloses a recombinant Mh-PGK protein with an amino acid sequence as shown in SEQ ID NO.1. The recombinant Mh-PGK protein disclosed by the invention has the The invention further discloses an indirect ELISA (enzyme-linked immunosorbent assay) detection kit and a detection method for the swine haemophilus mycoplasma antibody, which take the recombinant Mh-PGK protein as an elisa plate of a coating antigen. The swine haemophilus mycoplasma antibody indirect ELISA detection method established based on the recombinant Mh-PGK protein is used for detecting the swine haemophilus mycoplasma antibody level, has no cross reaction with part of other swine disease serum, has good specificity and repeatability, and provides an effective means for clinical diagnosis, epidemiological research and immunodetection of Mycoplasma suis, Mycoplasma parvum and Mycoplasma haemosuis.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Mycoplasma bovis diagnosis reagent and its application
InactiveCN103172752BReduce manufacturing costSimple and fast operationBiological testingHybrid peptidesMycoplasma antibodyMycoplasma spumans
The invention relates to the diagnostic medicine of animals, especially relates to a mycoplasma bovis diagnosis technology, and concretely relates to a multi-epitope fusion antigen having an amino acid sequence represented by SEQ ID NO:1 or SEQ ID NO:2, and its application in the preparation of a mycoplasma bovis diagnosis reagent. The diagnosis reagent can be used as a solid phase vector coating antigen of an indirect ELISA kit and is combined with its specific antibody, a horseradish peroxidase coupled anti-cattle IgG antibody is added and incubated, and a color development reaction is carried out, and the color development degree is proportional to the amount of the anti-mycoplasma bovis antibody in a sample to be measured. The technology has the advantages of simple operation, no need of complex equipment, low technical requirements on the laboratorial conditions and experiment personals, low detection cost, and suitableness for the large-scale development in the basic level and the culture farm; the multi-epitope fusion antigen has a low making cost and is suitable for large-scale application; and has the advantages of high sensitivity and specificity, small batch difference, and high detection result consistence because of the adoption of multi-epitope as a target.
Owner:重庆市动物疫病预防控制中心 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com