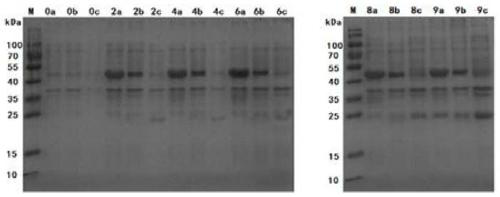
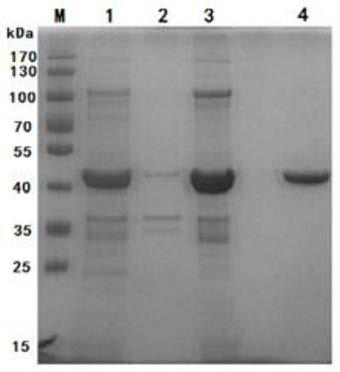
Mycoplasma ovipneumoniae antibody indirect ELISA detection kit
A technology of Mycoplasma pneumoniae and detection kit, which is applied in biological tests, measuring devices, immunoassays, etc., can solve problems such as cross-reaction of whole bacterial antigen coating, unsuitable for serological diagnosis technology, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of Mycoplasma pneumoniae Antibody Indirect ELISA Antigen and Detection of Immunogenicity
[0030] 1. Materials
[0031] (1) Strains and plasmids: positive serum of Mycoplasma ovis pneumoniae and negative serum of Mycoplasma ovis pneumoniae, positive serum of Pasteurella, Staphylococcus aureus, Mannella haemolyticus and Mycoplasma mycoplasma goat subspecies serum, positive serum of Mycoplasma hyopneumoniae, Serum of Mycoplasma filamentous subspecies were all existing materials, which were stored at -20°C by the Veterinary Laboratory of Southwest University for Nationalities; Escherichia coli and Salmonella factor serum were purchased from Sichuan Institute of Biological Products; clinical isolate of Mycoplasma pneumoniae SC01 was obtained from this Separated and stored in the laboratory; the prokaryotic expression vector pET-32a(+) is the product of Novagen; the competent cells DH5ɑ and Rosetta (DE3) are the products of TaKaRa;
[0032] (2) Main re...
Embodiment 2
[0061] Example 2 Establishment of Indirect ELISA Method for Antibody of Ovine Mycoplasma Pneumoniae and Condition Optimization
[0062] ① Determination of the optimal working concentration of antigen and antibody
[0063] The optimum working concentration of antigen and antibody was determined by Founder titration. According to the prepared recombinant protein concentration, the antigen was diluted to 4 μg / ml, 2 μg / ml, 1 μg / ml, 0.5 μg / ml, 0.25 μg / ml in sequence using coating buffer, and each dilution was repeated three times. Use the serum diluent to dilute the negative and positive serum of Mycoplasma pneumoniae in sequence 1:100 times, 1:200 times, 1:400 times and 1:800 times, and seal with 2% BSA. The rest of the steps follow the method of 2.3.1, read Take the results, calculate the P / N value, and select the antigen concentration when the average positive OD450 is around 1 and the P / N is the largest as the optimal concentration of the antigen. The serum dilution correspon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com